Abstract
Modulating the neuroinflammatory response is an emerging and interesting approach for treating Parkinson’s disease (PD). In this study, we used an adeno-associated virus (AAV9) to overexpress alpha-synuclein (αSyn) in the substantia nigra pars compacta of mice, inducing a dopaminergic degeneration in a dose-dependent manner. αSyn overexpression was associated with CD4+ T cell infiltration exhibiting a Th1 (IFNγ+TNFα+) phenotype in the ventral midbrain. In a chronic model induced with a low viral dose, treatment with either fingolimod (FTY720), which prevents T cell infiltration, or XPro1595, a selective inhibitor of soluble TNF signaling, led to improved motor function and resulted in partially protected dopaminergic cell bodies. These effects were not observed when an acute lesion was induced with a high viral dose. Our results support the contribution of CD4+ T cells to αSyn-induced neurodegeneration and suggest that immune modulation can provide neuroprotection in chronic neurodegenerative conditions, offering a wider therapeutic window. Further studies are needed to determine the optimal timing and conditions for implementing immunomodulatory strategies in PD.
Similar content being viewed by others
Introduction
Alpha-synuclein (αSyn) plays a central role in the pathophysiology of Parkinson’s disease (PD) by aggregating into toxic oligomers and fibrils that disrupt neuronal function and promote neuroinflammation1. The genetic link between αSyn and PD was established with the identification of the first mutation in the αSyn gene in familial forms of PD2. Additional mutations, duplications, and triplications of this gene correlate with an early onset and severe disease progression, suggesting a dosage effect3,4. Several polymorphisms in the αSyn gene increase the risk of developing sporadic forms of PD5. Neuroinflammatory mechanisms have been implicated in the neurodegeneration associated with αSyn accumulation6,7 and are strongly supported by genetics8. Polymorphisms in the major histocompatibility complex (MHC) are associated with increased risk of developing PD9, while anti-tumor necrosis factor (anti-TNF) treatment reduces the risk of PD10. Further analysis has demonstrated an increase in peripheral inflammatory cytokines that strengthens the clinical evidence that PD is accompanied by an inflammatory response11. αSyn-reactive T cells have been found in the blood of PD patients12,13. Functional T cell dysregulation appears before the manifestation of the cardinal motor symptoms of PD and has been proposed as a potential biomarker for early diagnosis12. However, their role in dopaminergic degeneration still remains unclear.
In postmortem tissue, Lewy pathology and neuronal death in the substantia nigra pars compacta (SNpc) are accompanied by increased expression of MHC class II (MHC-II), TNF, and interleukin-6 (IL6) in microglia14,15,16 and elevated levels of cytokines, such as TNF, IL1β, IL6, and transforming growth factor-β17,18. The increased CD4 and CD8 T cell infiltration in the human SN19,20 has been reproduced in experimental models of PD19,21,22,23,24,25. Data from these models suggest that CD4 T cells may play a more relevant role in dopaminergic degeneration than CD8 T cells19,26. Mouse models generated to overexpress αSyn with an adeno-associated virus (AAV) show an increased expression of MHC-II in myeloid cells in the midbrain25,27, which is accompanied by an anti-inflammatory and phagocytic phenotype27. Astrocytes are the major contributors to the pro-inflammatory response at this stage27. During inflammatory processes, there are multiple cytokines and time courses of the inflammatory response. Further research is required to understand the timing and location of inflammatory signals during disease progression in PD. Once characterized the innate immune response in mice overexpressing αSyn27, in this study, we aim to investigate the effect of T cell infiltration on dopaminergic degeneration and to assess the therapeutic effect of pharmacological immunomodulatory treatments. Controlling the dose of AAV9-Syn delivered to the SNpc allows control over the kinetics of dopaminergic degeneration. In the degenerating midbrain, infiltrating CD4 T cells exhibited a Th1 phenotype. Administration of fingolimod (FTY720), to prevent lymphocyte extravasation into the brain, or XPro1595, to neutralize soluble TNF signaling, provides long-term improvement in motor behavior and partially protects neuronal cell bodies in the SNpc. The data presented here support the relevant role of CD4 T cells in dopaminergic degeneration and the potential of targeting neuroinflammation for the treatment of PD.
Results
Alpha-synuclein-dependent neuronal degeneration promotes a specific Th1 CD4 T cell infiltration into the brain parenchyma
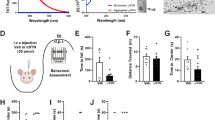
In a previous study, we characterized the innate immune response generated in the ventral midbrain and striatum following αSyn-dependent nigrostriatal degeneration, at 2 weeks after AAV9 administration, at the time of appearance of motor symptoms27. The aim of this study was to investigate the adaptive immune response generated under these conditions using the same AAV9, which simultaneously expresses αSyn and mCherry under the control of the CAG promoter (AAV9-Syn). The internal ribosome entry site (IRES) allows for the independent translation of both proteins. The control AAV9 lacks αSyn but retains the IRES and mCherry sequences (AAV9-Control) (Fig. 1A). Bilateral stereotactic administration of a high dose of AAV9-Syn (1.29 × 1013 vg/mL) into the SNpc induces 70% progressive dopaminergic degeneration over 4 weeks27. To study T cell infiltration in the early stages of the degenerative process, the animals were sacrificed at 2 weeks, when motor deficits became evident in the pole and bar tests, and the immune response was characterized27 (Fig. 1B). A cell suspension was prepared from the ventral midbrain and striatum, and immune cells were analyzed by flow cytometry. The gating strategy used to identify CD4+ and CD8+ T cells is shown in Fig. 1C, and the full gating strategy in Supplementary Fig. 1. The ratio of CD4+ T cells (Fig. 1D) was significantly increased in the ventral midbrain and striatum of parkinsonian mice, while no changes in infiltrating CD8+ T cells were detected (Fig. 1E). We then generated a new set of animals with equivalent motor impairment as the previous group (Fig. 1F) to examine the effector functions of infiltrating T lymphocytes. Representative dot plots for IFNγ, TNFα, and IL17 in CD4+ gated cells are shown in Fig. 1G. Only data from the ventral midbrain are shown because the absolute numbers of infiltrating T cells in the striatum were too low to carry out these experiments. CD4 T helper 17 (Th17) cells were present in the ventral midbrain of control animals, and the percentage of these lymphocytes decreased significantly under parkinsonian conditions (Fig. 1H). The proportion of IFNγ−TNFα+ CD4+ T cells was reduced in the ventral midbrain of parkinsonian animals. Conversely, the most abundant subset, IFNγ+TNFα+ CD4+ T cells, increased, while the least abundant subset, IFNγ+TNFα− cells, remained unchanged (Fig. 1I). Production of IFNγ and/or TNFα remained constant in CD8+ T cells (Fig. 1J). These results show a specific infiltration of CD4+ T cells that polarized toward Th1 effector T cells (IFNγ+TNFα+) to the detriment of a Th17 response in the αSyn mouse model of PD. While the polarization of immune cells was similar in the midbrain and in the striatum, the highest immune cell infiltration in the midbrain suggests that the death of neuronal cell bodies may induce a more pronounced response than the loss of dopaminergic terminals.
A AAV9s used to generate the experimental model. The AAV9-Control overexpresses the protein mCherry and the AAV9-Syn independently overexpresses αSyn and mCherry in the infected neurons. B Scheme of AAV9 injection and motor behavior evaluation 24 h prior to sacrifice. Two weeks after AAV9 injection into the SNpc, motor behavior was evaluated, and the animals were sacrificed. Motor performance was evaluated in the pole and bar tests. In the pole test, the time required to turn down and descend the pole was quantified. Cataleptic behavior was evaluated in the bar test by measuring the time to recover the position of the upper paws. C The ventral midbrain and the striatum were dissociated to prepare cell suspensions for the analysis of immune cell infiltration by flow cytometry. A representative gating strategy for CD8+ and CD4+ T cells in control and αSyn mice is shown. D Fold change of CD4+ and E CD8+ T cells relative to animals injected with the AAV9-Control. F Assessment of motor behavior of the new set of animals prepared for intracellular cytokine labeling. G Gating strategy for the analysis of TNFα, IFNγ, and IL17 production by T cells. H Percentage of CD4+ T cells labeled with IL17. I Analysis of IFNγ-TNFα+, IFNγ+TNFα+, and IFNγ+TNFα− cell subsets in CD4+ and J CD8+ T cells in the ventral midbrain. Data are the mean ± 95% CI from 5 to 6 animals/group. Statistical analysis: t test for data with normal distribution with Welch correction for significantly different variances. Mann–Whitney test for data not following a normal distribution. *p < 0.05, **p < 0.01, ***p < 0.001.
The localization of CD3, CD4, and CD8 T cells was analyzed by immunohistochemistry (Fig. 2A). Consistent with the flow cytometry results, the number of CD3+ and CD4+ T cells increased significantly in the midbrain of parkinsonian animals, and an upward trend was also observed for CD8+ T cells (Fig. 2B). CD3+ T cells were localized in the brain parenchyma and appeared to infiltrate the brain through several pathways: lateral ventricles, choroid plexus, blood vessels traversing the midbrain, and from vessels in the interpeduncular fossa (Fig. 2A). The different T cell subsets were present in all these areas but were not detectable in the hippocampus or in the cortex, suggesting that they are specifically driven to the damaged midbrain. In the striatum, scattered lymphocytes were detected in some sections, whereas no cells were observed in the lateral ventricle or in the choroid plexus of mice overexpressing αSyn (Supplementary Fig. 2). This is probably due to the low number and density of T cells in this region. Given the high concentration of T cells in close proximity to midbrain blood vessels, we asked whether the permeability of the blood–brain barrier (BBB) was affected by the neuronal damage induced by αSyn. Therefore, we administered Evans Blue to the mice on the day of sacrifice, and we observed that the dye was absent from the midbrain but was able to penetrate into the liver (Fig. 2C), indicating that the integrity of the BBB was not altered at the time of sacrifice.
A Representative images of CD3+, CD4+, and CD8+ immunohistochemistry of the midbrain of animals receiving the AAV9-Control and AAV9-Syn. The frames indicate different sites of lymphocyte entry into the brain: through the lateral ventricle (frame 1), the choroid plexus (frame 2), blood vessels (frame 3), and the interpeduncular fossa (frame 4). B Quantification of the number of infiltrated CD3+, CD4+, and CD8+ T cells per midbrain section in control and αSyn groups. C BBB permeability was analyzed by the administration of Evans Blue 3 h before sacrifice. The Evans Blue signal was not detected in the midbrain and the striatum. Liver was used as a positive control. Data are the mean ± 95% CI from 3 to 4 animals/group. Statistical analysis: B Mann–Whitney test. *p < 0.05. Magnification bar: 1 mm in the first column of images and 10 µm for the remaining columns (frames 1–4).
Modulation of T cell infiltration and inhibition of soluble TNF as a therapeutic strategy to prevent degeneration of the nigrostriatal pathway
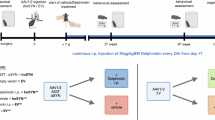
Next, we evaluated the effect of inhibiting T cell entry into the brain parenchyma using fingolimod (FTY720). FTY720 is an approved oral drug for treating relapsing-remitting multiple sclerosis. It retains lymphocytes in lymphoid organs, preventing them from entering the brain28. FTY720 (1 mg/kg) was administered daily to mice, starting 24 h after surgery and continuing until sacrificed 2 weeks later (Fig. 3A). This regimen induces lymphocyte retention in secondary lymphoid organs29 and has been used in the 6-OHDA mouse model of PD30. Parkinsonian mice showed motor deficits in the pole and bar tests (Fig. 3B). FTY720 treatment significantly improved the outcome of αSyn mice in the pole test, but not in the bar test (Fig. 3B). No effect was observed at the level of dopaminergic terminals (Fig. 3C) or on the number of dopaminergic cell bodies in the SNpc (Fig. 3D), although FTY720 effectively reduced CD4 (Fig. 3E) and CD8 T cell infiltration (Fig. 3F).
A Schematic of the experimental design. AAV9-Control and AAV9-αSyn were injected into the SNpc by stereotactic surgery. FTY720 treatment started 24 h later and was administered daily until the time of sacrifice at 2 weeks. B Motor behavior was assessed using the pole test and the bar test. A subset of animals was processed for histological techniques and another for flow cytometry. TH immunohistochemistry was used to analyze the status of the nigrostriatal pathway. C Representative images showing TH immunoreactivity in the striatum and the optical density analysis of the TH immunopositive signal. D Representative images of TH+ neurons in the SNpc and their quantification by unbiased stereology. Cell suspensions from the ventral midbrain and the striatum were prepared from a new cohort of animals to determine immune cell infiltration in these regions E CD4+ and F CD8+ T lymphocytes. Data are the mean ± 95% CI from B 12 animals/group and C–F 5–7 animals/group. Statistical analysis: two-way ANOVA with Tukey’s multiple comparison post hoc test; *p < 0.05, ***p < 0.001. Magnification bar: C, D 1 mm.
To extend the therapeutic window, and considering that the extent of neuronal death is dose dependent31, we reduced the viral dose to 5.47 × 1012 vg/mL to slow its progression. The lower dose resulted in a 35% progressive lesion in 8 weeks (Fig. 4D) compared to a 55% obtained in 2 weeks with the higher dose (Fig. 3D), without compromising animal survival. Viral transduced protein expression was stable after 8 weeks as mCherry was present in TH+ neurons in animals receiving the AAV9-Control (Supplementary Fig. 3A). Total αSyn detection showed its expression in the midbrain and effective transport to striatal dopaminergic terminals. An uneven distribution of αSyn was observed in the cytosol of the remaining cells expressing the protein in the midbrain (Supplementary Fig. 3B). Double immunohistochemistry was performed to detect NeuN and TH in order to discriminate between dopaminergic cell bodies loss and TH downregulation. In the same image, a decrease in the number of TH+ neurons was paralleled by a decrease in the number of NeuN+ cells (Supplementary Fig. 3C), suggesting that the loss of TH+ cell bodies is due to neurodegeneration.
A To induce the experimental parkinsonism, a low dose of AAV9-Control and AAV9-Syn (5.47 × 1012 vg/mL) was injected into the SNpc. FTY720 treatment started 24 h after surgery and it was administered daily until the time of sacrifice 8 weeks later. B Motor behavior was assessed using the pole test and the bar test. A subset of animals was processed for histological techniques and another for flow cytometry. TH immunohistochemistry was used to analyze the status of the nigrostriatal pathway. C Representative images showing TH immunoreactivity in the striatum and the optical density analysis of the TH immunopositive signal. D Representative images of TH+ neurons in the SNpc and their quantification by unbiased stereology. Cell suspensions were prepared from the ventral midbrain and the striatum to determine immune cell infiltration in these regions. Fold increase in E CD4+ and F CD8+ T cells compared to animals injected with the AAV9-Control and treated with the FTY720 vehicle. Data are the mean ± 95% CI from (B) 12 animals/group and C–F 5–6 animals/group. Statistical analysis: two-way ANOVA with Tukey’s multiple comparison post hoc test; *p < 0.05, ***p < 0.001. Magnification bar: C, D 1 mm.
Animals receiving a low viral dose AAV9-Syn or AAV9-Control were treated daily with FTY720 for 8 weeks (Fig. 4A). This treatment prevented αSyn overexpression-induced motor deficits in the pole and bar tests (Fig. 4B). Due to the mild nature of the lesion, the immunohistochemical analysis of the nigrostriatal pathway did not reveal differences at the level of dopaminergic terminals (Fig. 4C). Interestingly, a significant increase in the number of TH+ cell bodies in the SNpc was observed in FTY720-treated mice (Fig. 4D). The specific decrease in the infiltration of CD4+ (Fig. 4E), but not CD8+ (Fig. 4F) T cells in the midbrain (Fig. 4E) could explain the neuroprotective effect of FTY720 in this region.
Our flow cytometry analysis showed that infiltrating CD4+ T cells polarize toward Th1 effector T cells producing TNF and IFNγ in the midbrain of parkinsonian animals. Next, we explored whether blockade of TNF signaling could provide neuroprotection. We selected XPro1595, a drug that binds to sTNF and prevents its interaction with the type 1 TNF receptor32. Mice received subcutaneous injections of XPro1595 (10 mg/kg) every 72 h, starting 3 days after surgery and continuing for 8 weeks (Fig. 5A). This dose and regimen were selected based on pharmacokinetic and pharmacodynamic studies, as well as evidence indicating reduced dopaminergic neurodegeneration in a rat model of PD33 and improved outcomes in other central nervous system (CNS) disease models34,35. Prior to sacrifice, motor behavior was assessed using the pole and bar tests. Treatment with XPro1595 reduced motor deficits in parkinsonian animals (Fig. 5B). Immunohistochemical analysis of the nigrostriatal pathway showed a significant improvement in the extent of TH-immunopositive terminals in the striatum (Fig. 5C) and an increased density of TH+ cell bodies in the SNpc (Fig. 5D). Flow cytometry analysis of immune cell infiltration revealed that parkinsonian mice treated with XPro1595 showed a significant reduction in both CD4+ (Fig. 5E) and CD8+ (Fig. 5F) T cells in the midbrain. Collectively, these results indicate that both targeting T cell infiltration with FTY720 and blocking TNF signaling with XPro1595 protect the nigrostriatal pathway and ameliorate motor symptoms in the αSyn-induced degeneration model of PD. Interestingly, both compounds, FTY720 and XPro1595, prevent CD4 T cell infiltration into the midbrain, suggesting that part of the neuroprotective effect may be mediated by the inhibition of CD4 T cell infiltration.
A To induce the experimental parkinsonism, a low dose of AAV9-Control and AAV9-Syn (5.47 × 1012 vg/mL) was injected into the SNpc. After 3 days, mice were treated with XPro1595 every 72 h until sacrificed 8 weeks later. B Motor coordination was assessed in the pole and bar tests. C Representative images showing TH immunoreactivity in the striatum and optical density analysis of the TH immunopositive signal. D Representative images of TH+ neurons in the SNpc and their quantification by unbiased stereology. E Cell suspensions were prepared from the ventral midbrain and the striatum to determine immune cell infiltration in these regions. Fold increase in CD4+ and F CD8+ T cells compared to animals injected with the AAV9-Control and treated with FTY720 vehicle. Data are mean ± 95% CI from B 12 animals/group and C–F 5–6 animals/group. Statistical analysis: two-way ANOVA with Tukey’s multiple comparison post hoc test; *p < 0.05, **p < 0.01, ***p < 0.001. Magnification bar: C, D 1 mm.
Discussion
In this study, we show that αSyn overexpression in the SNpc using an AAV9 vector induces dopaminergic cell body loss in a dose-dependent manner, which is accompanied by an increase in CD4+ T cells. In the ventral midbrain of parkinsonian mice, infiltrating CD4+ T cells exhibit a TNFα+IFNγ+ Th1 phenotype. Administration of FTY720, which prevents T cell infiltration into the brain, or XPro1595, which blocks soluble TNF interaction with its receptor, improves motor behavior and partially prevents loss of dopaminergic cell bodies in the chronic αSyn overexpression mouse model. These treatments also significantly reduce CD4+ T cells in the midbrain.
As previously described, we generated an AAV9 vector with predominant neuronal tropism following intraparenchymal administration36,37. The AAV9-Syn construct co-expressed αSyn and mCherry under the control of the CAG promoter. In contrast, the AAV9-Control vector lacked the αSyn sequence but retained the IRES and the mCherry, enabling mCherry expression in transduced neurons. The number of transduced cells is proportional to the viral dose, and individual neurons are typically infected by multiple viral particles. Our findings are consistent with previous reports31. Each AAV9 preparation is titrated by PCR to determine the viral genome concentration (vg/mL), the standard method for estimating viral particle content. However, this approach does not assess the efficiency of αSyn packaging. Therefore, we routinely perform in vivo titration using two or three logarithmic doses to establish the high- and low-dose ranges for each batch. A “high dose” is defined as the amount that induces motor symptoms within 2 weeks, leads to 55% loss of TH+ cell bodies in the SNpc, and results in severe impairment by 4 weeks post-injection27. Conversely, a “low dose” is defined as the amount that first induces motor symptoms at 6 weeks and results in a 35% loss of TH+ cell bodies by 8 weeks. Total αSyn is expressed in TH+ cell bodies of the SNpc and is efficiently transported to the dopaminergic terminals in the striatum. The heterogeneous distribution pattern of αSyn suggests the presence of aggregated species. Further characterization, including detection of phosphorylated αSyn at Ser129, would provide additional insight.
In mice with an acute dopaminergic lesion induced by a high viral dose, microglia and infiltrating myeloid cells cooperated in the clearance of dopaminergic debris from the midbrain and adopted an anti-inflammatory phenotype. Meanwhile, astrocytes exhibited a pro-inflammatory profile27. In the present study, we observed increased infiltration of CD4+ T cells in the midbrain, which could be linked to the previously reported upregulation of MHC-II and CD80 in myeloid cells27. These infiltrating T cells localize in the brain parenchyma, choroid plexus, and blood vessels within the midbrain and the interpeduncular fossa. The absence of Evans Blue extravasation into the brain suggests selective permeability of the BBB, and T cell infiltration is likely mediated by chemokine signaling. This may be related to the altered migratory phenotype observed in CD4 T cells derived from PD patients38. Although both CD4 and CD8 T cells contribute to the adaptive immune response in PD, CD4 T cells have been shown to be crucial for promoting neurodegeneration12,19,26,39. CD4−/−, but not CD8−/−, mice are protected against neurodegeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and αSyn overexpression19,26. In addition, MHC-II deficiency reduces microgliosis and prevents dopaminergic degeneration in vivo, highlighting the importance of myeloid-CD4 T interactions in mediating neuronal death25.
To deplete T cells pharmacologically, we used FTY720, a drug approved for the treatment of multiple sclerosis. FTY720 binds to the sphingosine 1-phosphate receptor, acting as a functional antagonist that promotes receptor internalization. This mechanism sequesters lymphocytes in the lymph nodes and prevents their migration into the brain28,40. In αSyn overexpressing mice, FTY720 treatment has been shown to reduce MHC-II expression in myeloid cells in the midbrain26. In the present study, we demonstrate that FTY720 administration effectively prevents T cell infiltration into the midbrain. However, when administered in the context of an acute lesion induced by a high viral dose, FTY720 did not protect the nigrostriatal pathway, though a modest improvement in motor performance was observed in the pole test. To determine whether this lack of neuroprotection reflected an actual absence of therapeutic efficacy or was instead due to a narrow therapeutic window under acute conditions, we reduced the viral dose to generate a milder and chronic lesion, thereby extending the therapeutic window. Under these conditions, FTY720 treatment restored motor behavior and protected dopaminergic cell bodies in the SNpc. The absence of detectable effects at the striatal level did not correlate with the observed behavioral improvement. These results suggest that TH immunohistochemistry may lack the sensitivity to detect subtle changes in dopamine biosynthesis or release that could underlie the motor recovery. Alternatively, the different glial responses elicited during acute vs chronic dopaminergic degeneration may also account for the differential effects between the two conditions41. Notably, the chronic model more accurately mimics the pattern of glial activation observed in the human brain, emphasizing the relevance of the long-term efficacy of the treatment41. Conflicting neuroprotective effects of FTY720 have been reported in the subacute MPTP mouse model42,43. Our results further support the lack of protective activity of FTY720 under acute degenerative conditions43. When assessing the therapeutic activity of FTY720 in the CNS, it is also important to consider its beneficial effects independent of immune trafficking44,45. To dissociate immunosuppressive actions from neurotrophic properties, FTY720-mitoxy was developed46,47. To date, no evidence supports a neuroprotective effect of FTY720-mitoxy in experimental models of PD, suggesting that the adaptive immune system plays a critical role in neurodegeneration and highlighting it as a relevant therapeutic target. Additionally, FTY720 acts as a competitive antagonist of cannabinoid type 1 receptors (CB1R)48. CB1R antagonists improve motor behavior in the rat model of PD49,50, which may explain the behavioral improvements observed following FTY720 treatment in AAV-Syn-injected mice, despite the absence of changes at the level of striatal dopaminergic terminals.
During the active phase of the neurodegenerative process, infiltrating CD4 T cells polarize towards a Th1 phenotype, which is characterized by an increase in the number and proportion of CD4+ IFNγ+TNFα+ cells in the ventral midbrain. In addition to lymphocytes, astrocytes also contribute to the early proinflammatory response during αSyn-induced neurodegeneration by upregulating the expression of transcripts associated with IFNγ, TNF, and IL627. TNF levels are elevated in the brain and in the cerebrospinal fluid of PD patients and are recognized as key mediators of neurotoxicity16,17,51. In this study, we investigated the therapeutic potential of XPro1595, a dominant negative TNF inhibitor that sequesters soluble TNF, thereby preventing its interaction with TNF receptor 132. XPro1595 crosses the BBB and is neuroprotective in the 6-OHDA rat model of PD αSyn33,52. In a rat model of unilateral αSyn-induced dopaminergic degeneration, XPro1595 produced only a mild immunomodulatory effect and failed to confer neuroprotection53. It remains unclear whether the relatively mild nigrostriatal lesion induced in this model elicits a TNF-mediated inflammatory response that could be effectively targeted by XPro1595. Following the FTY720 experiments, we optimized the model to more accurately assess neuroprotection; therefore, the XPro1595 was tested directly using a low viral dose. In this context, we demonstrate that XPro1595 is effective in the long-term αSyn overexpression mouse model of PD, as evidenced by improved motor behavior and preservation of the nigrostriatal pathway. The observed neuroprotective effect may result from the inhibition of the neurotoxic TNF/TNFR1 signaling in neurons or from the reduction of T cell infiltration into the midbrain54. Notably, this therapeutic approach enabled the detection of neuroprotection at the level of striatal dopaminergic terminals.
The therapeutic efficacy of immunomodulators in the long-term αSyn-induced experimental mouse model highlights the potential of targeting neuroinflammation as a disease-modifying strategy for PD. In this study, we demonstrate that two treatments with potential translational relevance, FTY720 and XPro1595, exert sustained and well-tolerated neuroprotective effects in a preclinical model of PD. FTY720 is already approved for treating multiple sclerosis, while XPro1595 is currently undergoing phase II clinical trials for Alzheimer’s disease. Collectively, these findings emphasize the feasibility of targeting neuroinflammation as a therapeutic approach for PD and support the advancement of these candidates toward clinical application. However, further studies to define the optimal therapeutic window for intervention after the onset of neurodegeneration are needed.
Methods
Animals
Male and female 3-month-old C57BL6JRccHsd mice (20–30 g) were obtained from Envigo (Barcelona, Spain). Mice were housed at 21 °C in a humidity-controlled environment with a 12 h light/dark cycle, fed a standard rodent pellet diet (Envigo) ad libitum, and provided with free access to water. All animal procedures were performed in accordance with the Spanish National Research Council’s guide for the Care and Use of Laboratory Animals following protocols approved by the Animal Experimentation Ethics Committee of the University of Navarra (ref. 064-19).
Virus generation
AAV9-Syn-IRES-mCherry and AAV9-IRES-mCherry viruses were generated as previously described. The AAV9-Syn-IRES-mCherry virus simultaneously expresses human αSyn and mCherry under the control of the CAG promoter, which consists of the cytomegalovirus major immediate-early enhancer combined with the chicken beta-actin promoter. The presence of an IRES enables the independent translation of both proteins. The control virus (AAV9-IRES-mCherry) lacks the αSyn sequence but retains the IRES and mCherry elements, enabling the expression of mCherry alone. Viral titers (viral genomes (vg)/mL) were determined by quantitative PCR of viral genome copies extracted from DNase-treated virus particles (High Pure Viral Nucleic Acid Kit, Roche, Basel, Switzerland). Quantitative PCR was performed in triplicate using primers for the ITR region of AAV55.
Stereotactic surgery
Mice were deeply anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). Animals were injected bilaterally with 1 µL of AAV9-Control or 1 µL of AAV9-Syn (high dose: 1.29 × 1013 vg/mL; low dose: 5.47 × 1012 vg/mL) at a rate of 0.2 μL/min into SNpc at the following coordinates from bregma56: anteroposterior −3.5 mm, mediolateral +/−1.3 mm, and dorsoventral −4 mm. Animals with the high dose of AAV9 were sacrificed at 2 weeks, and those with the low dose at 8 weeks. Vectors used for this study came from two different batches that were selected by an in vivo titration.
Pharmacological treatments
Fingolimod (FTY720; Sigma-Aldrich, Saint Louis, MO, USA) was dissolved in saline containing 3% dimethyl sulfoxide (Sigma-Aldrich). Mice were treated with daily intraperitoneal (i.p.) injections of FTY720 (1 mg/kg)29 or its vehicle, starting 24 h after surgery and continuing until sacrifice at 2 or 8 weeks post-surgery. XPro1595 (kindly provided by INmune Bio, Inc.) is a pegylated human TNF variant that forms heterotrimers with native soluble TNF (sTNF) but not membrane-bound TNF (tmTNF), thereby sequestering sTNF and preventing its interaction with TNF receptors32. Starting 3 days after the surgery, mice were treated with subcutaneous injections of XPro1595 (10 mg/kg in saline), or its vehicle, every 3 days until their sacrifice.
Motor behavior
Behavioral tests were performed 24 h prior to sacrifice under dim light conditions. For the pole test, animals were placed upright on the top of a vertical wooden pole, 50 cm high and 1 cm in diameter, covered with a bandage. Animals were pre-trained to be able to turn their head down and to descend the pole in less than 5 s. The mean time to turn their head down and to completely descend the pole was measured in 3 trials with a rest period of 15 min between each trial. For the bar test, mice were placed with their forepaws on a bar oriented parallel to the floor at a height of 4 cm. The mean time to correct the posture was measured in three trials.
Histological techniques
For immunohistochemistry on free-floating sections, animals were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) and transcardially perfused for 5 min with Ringer’s solution (145.4 mM NaCl, 3.4 mM KCl, 2.4 mM NaHCO3, pH 7.4) at a rate of 9.5 mL/min. This was followed by perfusion with 4% paraformaldehyde (PFA; Panreac, Barcelona, Spain) in 0.125 M phosphate-buffered saline (PBS, pH 7.4) for 10 min at the same rate, and an additional 3 min at 16 mL/min. The brain was removed, postfixed in 4% PFA overnight, and stored in 30% sucrose/PBS. Coronal 40 µm-thick sections were cut using a Leica SM2000R sliding microtome (Leica, Wetzlar, Germany). Free-floating sections were washed with PBS, and endogenous peroxidase activity was inactivated by incubation in 0.03% H2O2 (Sigma-Aldrich)/methanol (Panreac) for 30 min. After 3 washes with PBS, the tissue was incubated, first with the blocking solution [4% normal goat serum, 0.05% Triton X-100 (Sigma-Aldrich), and 4% bovine serum albumin (Merck, Darmstadt, Germany) in PBS] for 40 min, and then with primary antibodies diluted in blocking solution at room temperature (RT). The primary antibodies used are indicated in Table 1. For colorimetric immunostaining, sections were incubated with the biotinylated secondary antibodies goat anti-rabbit (1:500; Jackson ImmunoResearch, Ely, UK) and donkey anti-mouse (1:500; Jackson ImmunoResearch) in blocking solution for 2 h at RT, followed by incubation with peroxidase-conjugated avidin in PBS (1:5000; Sigma-Aldrich) for 90 min at RT. After washing with PBS, all sections were simultaneously incubated in the same batch of 0.05% diaminobenzidine (Sigma-Aldrich), 0.03% H2O2, and Trizma-HCl buffer (pH 7.6) for the same time. For immunofluorescence staining, sections were incubated with secondary antibodies Alexa Fluor 568 donkey anti-mouse (1:500; Thermo Fisher Scientific, Waltham, MA, USA) and Alexa Fluor 488 donkey anti-rabbit (1:250; Invitrogen, Waltham, MA, USA) for 2 h at RT and finally stained with DAPI (1:50,000; Sigma-Aldrich). Sections were mounted on glass slides in a 0.2% solution of gelatin in 0.05 M Tris-HCl buffer (pH 7.6) (Sigma-Aldrich), dried, and dehydrated in toluene (Panreac) for 12 min before coverslipping with DPX (BDH Chemicals, Poole, UK).
For immunohistochemistry on paraffin-embedded sections, animals were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) and transcardially perfused with ice-cold PBS for 5 min. The brain was then removed and fixed for 24 h in 4% formaldehyde, followed by an incubation of 48 h in 70% ethanol. The tissue was embedded in paraffin and sectioned into 4 µm-thick slices. Sections were immunostained using conventional procedures with the following antibodies: anti-CD3, anti-CD4, anti-CD8a (Table 1). Immunodetection was performed with biotinylated secondary antibodies and streptavidin–HRP complex (Envision complex, Dako, Glostrup, Denmark), and immune complexes were visualized with DAB substrate chromogen (Dako), followed by counterstaining with hematoxylin.
Image analysis
Images for immunostained sections of TH, total αSyn, CD3, CD4 and CD8 were captured on an Aperio CS2 digital pathology slide scanner (Leica) at ×20 or ×40 magnification. Optical density values of striatal TH and αSyn immunoreactivity were obtained using ImageJ (National Institutes of Health, MD, USA). For total αSyn staining analysis, a random region of the cortex was used as a blank, and its value was subtracted from the average intensity of both hemispheres. For the analysis of TH, a random region of the cortex was used as a blank, and its value was subtracted from the intensity of the most degenerated hemisphere. The total number of CD3+, CD4+, and CD8+ cells infiltrating the midbrain was counted using a cell counter. Confocal images of TH/mCherry immunofluorescence were captured on an LSM 510 confocal microscope (Zeiss, Jena, Germany) using a ×63 oil objective.
Stereology
The number of TH+ neurons present in the SNpc was determined by unbiased design-based stereology using a Bx61 microscope (Olympus, Hicksville, NY, USA) equipped with a DP71 camera (Olympus), a stage connected to a xyz stepper (H101BX, PRIOR), and Stereo Investigator software (version 2021.1.1; MBF Bioscience, Williston, VT). Stereological counting was performed in 7 coronal SNpc sections (40 µm thick) taken at uniform intervals (160 µm) covering the entire rostrocaudal extent of the nucleus between −2.92 and −3.64 mm relative to bregma56. The reference volume (Vr) of the SNpc was calculated from images taken with the ×2 objective using a point count array according to Cavalieri principles57. The cross-sectional area of the nucleus was measured and the Vr for the whole SNpc was estimated using the following equation:
where T is the section thickness, a/p is the area of each point, and Pi is the number of points falling within the SNpc. The SNpc was outlined with the ×10 objective to estimate the area. The number of labeled neurons was obtained at ×100 magnification under oil immersion, using randomized meander sampling and the optical dissector methods. The height of the optical dissector was 11 µm, with an upper guard zone of 2 μm, to count 100–150 cells per animal using a sampling frame of 4900 µm2 and sampling steps of 140 µm × 140 µm (dx, dy). Counting was performed blindly, and the total number of TH-positive neurons (N) was calculated using the following formula:
where ƩQ− is the total number of particles counted, t is the mean section thickness, h is the height of the optical dissector, asf is the area sampling fraction, and ssf is the section sampling fraction. The neuronal density (D) was determined by the following formula: D = N/Vr. Gunderson’s coefficients of error were <0.1 for all stereological quantifications.
Isolation of immune cells for flow cytometry
Mice were anesthetized with ketamine/xylazine and perfused transcardially with ice-cold PBS. The striatum and midbrain were dissected on ice and incubated with 400 units/mL of collagenase D (Roche, Mannheim, Germany), containing 50 µg/mL of DNase I (Sigma-Aldrich) in Dulbecco’s PBS (Lonza, Basel, Switzerland) for 15 min at 37 °C with rotation. After enzymatic digestion, the tissue was mechanically processed with a glass Pasteur pipette, filtered through a 70 µm nylon cell strainer and centrifuged at 300 × g for 15 min. A 25% Percoll gradient was used to remove cell debris and myelin by centrifugation at 1000 × g for 10 min at RT58. The cell pellet was resuspended in cytometry buffer (CB) to perform flow cytometry. Blood was also collected from the tail vein of the animals. A volume of 50 µL of blood was incubated with 750 µL of red blood lysis buffer (BioLegend) for 15 min at RT. After the incubation, the samples were centrifuged at 3500 r.p.m. for 5 min at RT and the cell pellet was resuspended in 1 mL of CB. The samples were centrifuged again under the same conditions and the pellet was resuspended in CB for flow cytometry.
For the analysis of cell surface markers, brain and blood cell pellets were resuspended in 100 µL of CB: 5 mM EDTA (Thermo Fisher Scientific), 0.5% fetal bovine serum (FBS) (Gibco, Paisley, UK), 100 U/mL penicillin G (Gibco), 100 μg/mL streptomycin (Gibco) in PBS and were incubated with Zombie NIR Dye (1:2000; BioLegend, San Diego, CA, USA) in PBS for 5 min at RT to assess viability. The Zombie NIR Dye was quenched with CB and the cells were centrifuged at 2000 r.p.m. for 1 min. Samples were then incubated with different panels of fluorescent antibodies (Table 2) and the FcR blocking reagent (1:50; Miltenyi Biotec, Bergisch Gladbach, Germany) during 15 min at 4 °C. For intracellular cytokine staining, cell pellets were resuspended in 100 µL of RPMI-1640 medium (Invitrogen) supplemented with 10% FBS, 100 U/mL penicillin G, 100 μg/mL streptomycin, 50 μM β-mercaptoethanol (Thermo Fisher Scientific), 10 ng/mL gentamicin solution, and 12.5 mM HEPES buffer (Sigma-Aldrich). To stimulate cytokine T cell production, phorbol 12-myristate 13-acetate (0.05 μg/mL; Sigma-Aldrich) and ionomycin (0.5 μg/mL; Sigma-Aldrich) were added to the cell suspension. To block cytokine secretion, cells were incubated with brefeldin-A 1X (BioLegend) for 4 h at 37 °C. After the incubation, samples were centrifuged at 300 × g for 1 min and washed with PBS before flow cytometry analysis. For the intracellular staining of T lymphocytes, cells were fixed and permeabilized with the Foxp3 transcription factor buffer set (Invitrogen), and then incubated for 15 min at 4 °C with the intracellular panel of fluorescent antibodies diluted in PermWASH solution (Table 2). Once labeled, samples were washed with CB, acquired on a CytoFLEX LX flow cytometer (Beckman Coulter, Brea, CA, USA) and analyzed using the CytExpert 2.3 (Beckman Coulter) and FlowJo 10.0.7r2 (BD Biosciences, Franklin Lakes, NJ, USA) softwares.
BBB permeability assay
Two weeks after stereotactic surgery, Evans Blue (2% in 0.9% saline) (Sigma-Aldrich) was injected i.p. (80 mg/kg), and the animals were sacrificed 3 h later. Mice were deeply anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) and transcardially perfused with ice-cold PBS at 9.5 mL/min for 5 min. The striatum, the midbrain, and the liver were dissected on ice and stored at −80 °C until processing. To homogenize the tissue, equal volumes of 0.9% saline and 100% TCA (5 µL/mg of tissue) (Sigma-Aldrich) were added to the samples to give a final concentration of 50% TCA. After homogenization, samples were incubated overnight at 4 °C in the dark and centrifuged at 10,000 × g for 20 min at 4 °C. The supernatants were added to a 96-well plate, and the concentration of Evans Blue was determined spectrophotometrically at 620 nm.
Statistics
GraphPad Prism version 7.0 was used to generate graphs. All data are presented as mean values with 95% confidence intervals. The normal distribution of the data was analyzed using the Shapiro–Wilk test. Pairwise comparisons of data following a normal distribution were analyzed using a Student’s t test (two-tailed) for equal variances. If the variances were significantly different, Welch’s correction was applied. Data not following a normal distribution were analyzed using the Mann–Whitney U-test. Two-way ANOVA followed by Bonferroni’s test was used for multiple comparisons.
Data availability
No datasets were generated or analyzed during the current study.
References
Burré, J., Sharma, M. & Südhof, T. C. Cell Biology and pathophysiology of α-synuclein. Cold Spring Harb. Perspect. Med. 8, a024091 (2018).
Polymeropoulos, M. H. et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047 (1997).
Singleton, A. B., Farrer, M. J. & Bonifati, V. The genetics of Parkinson’s disease: progress and therapeutic implications. Mov. Disord. 28, 14–23 (2013).
Fuchs, J. et al. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology 68, 916–922 (2007).
Edwards, T. L. et al. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann. Hum. Genet. 74, 97–109 (2010).
Schonhoff, A. M., Williams, G. P., Wallen, Z. D., Standaert, D. G. & Harms, A. S. Innate and adaptive immune responses in Parkinson’s disease. Prog. Brain Res. 252, 169–216 (2020).
Tansey, M. G. et al. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 22, 657–673 (2022).
Kline, E. M. et al. Genetic and environmental factors in Parkinson’s disease converge on immune function and inflammation. Mov. Disord. 36, 25–36 (2021).
Hamza, T. H. et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet. 42, 781–785 (2010).
Peter, I. et al. Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol. 75, 939–946 (2018).
Qin, X.-Y., Zhang, S.-P., Cao, C., Loh, Y. P. & Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 73, 1316–1324 (2016).
Lindestam Arlehamn, C. S. et al. α-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat. Commun. 11, 1–11 (2020).
Sulzer, D. et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546, 656–661 (2017).
McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38, 1285–1291 (1988).
Imamura, K. et al. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 106, 518–526 (2003).
Boka, G. et al. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 172, 151–154 (1994).
Mogi, M. et al. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 165, 208–210 (1994).
Mogi, M. et al. Interleukin-1β, interleukin-6, epidermal growth factor and transforming growth factor-α are elevated in the brain from parkinsonian patients. Neurosci. Lett. 180, 147–150 (1994).
Brochard, V. et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest 119, 182–192 (2009).
Galiano-Landeira, J., Torra, A., Vila, M. & Bové, J. CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain 143, 3717–3733 (2020).
Theodore, S., Cao, S., McLean, P. J. & Standaert, D. G. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol. 67, 1149–1158 (2008).
Sanchez-Guajardo, V., Febbraro, F., Kirik, D. & Romero-Ramos, M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS ONE 5, e8784 (2010).
Chandra, G., Rangasamy, S. B., Roy, A., Kordower, J. H. & Pahan, K. Neutralization of RANTES and eotaxin prevents the loss of dopaminergic neurons in a mouse model of Parkinson disease. J. Biol. Chem. 291, 15267–15281 (2016).
Harms, A. S. et al. α-Synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathol. Commun. 5, 85 (2017).
Harms, A. S. et al. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci. 33, 9592–9600 (2013).
Williams, G. P. et al. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 144, 2047–2059 (2021).
Basurco, L. et al. Microglia and astrocyte activation is region-dependent in the α-synuclein mouse model of Parkinson’s disease. Glia 71, 571–587 (2023).
Pelletier, D. & Hafler, D. A. Fingolimod for multiple sclerosis. N. Engl. J. Med. 366, 339–347 (2012).
Yagi, H. et al. Immunosuppressant FTY720 inhibits thymocyte emigration. Eur. J. Immunol. 30, 1435–1444 (2000).
Zhao, P. et al. Neuroprotective effects of fingolimod in mouse models of Parkinson’s disease. FASEB J. 31, 172–179 (2017).
Oliveras-Salvá, M. et al. rAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol. Neurodegener. 8, 44 (2013).
Steed, P. M. et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science 301, 1895–1898 (2003).
Barnum, C. J. et al. Peripheral administration of the selective inhibitor of soluble tumor necrosis factor (TNF) XPro®1595 attenuates nigral cell loss and glial activation in 6-OHDA hemiparkinsonian rats. J. Parkinsons Dis. 4, 349–360 (2014).
Taoufik, E. et al. Transmembrane tumour necrosis factor is neuroprotective and regulates experimental autoimmune encephalomyelitis via neuronal nuclear factor-kappaB. Brain 134, 2722–2735 (2011).
Brambilla, R. et al. Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain 134, 2736–2754 (2011).
Castle, M. J., Turunen, H. T., Vandenberghe, L. H. & Wolfe, J. H. Controlling AAV tropism in the nervous system with natural and engineered capsids. Methods Mol. Biol. 1382, 133–149 (2016).
Cearley, C. N. & Wolfe, J. H. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 13, 528–537 (2006).
Mamula, D., Khosousi, S., He, Y., Lazarevic, V. & Svenningsson, P. Impaired migratory phenotype of CD4+ T cells in Parkinson’s disease. npj Parkinsons Dis. 8, 171 (2022).
Sun, X., Gu, R. & Bai, J. Differentiation and regulation of CD4+ T cell subsets in Parkinson’s disease. Cell. Mol. Life Sci. 81, 352 (2024).
Brinkmann, V. et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 9, 883–897 (2010).
Ayerra, L. et al. Nigrostriatal degeneration determines dynamics of glial inflammatory and phagocytic activity. J. Neuroinflammation 21, 92 (2024).
Pépin, É, Jalinier, T., Lemieux, G. L., Massicotte, G. & Cyr, M. Sphingosine-1-phosphate receptors modulators decrease signs of neuroinflammation and prevent Parkinson’s disease symptoms in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model. Front. Pharmacol. 11, 77 (2020).
Komnig, D. et al. Fingolimod (FTY720) is not protective in the subacute MPTP mouse model of Parkinson’s disease and does not lead to a sustainable increase of brain-derived neurotrophic factor. J. Neurochem. 147, 678–691 (2018).
Hait, N. C. et al. Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat. Neurosci. 17, 971–980 (2014).
Deogracias, R. et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 109, 14230–14235 (2012).
Vargas-Medrano, J. et al. FTY720-Mitoxy reduces toxicity associated with MSA-like α-synuclein and oxidative stress by increasing trophic factor expression and myelin protein in OLN-93 oligodendroglia cell cultures. Neuropharmacology 158, 107701 (2019).
Segura-Ulate, I., Belcher, T. K., Vidal-Martinez, G., Vargas-Medrano, J. & Perez, R. G. FTY720-derivatives do not induce FTY720-like lymphopenia. J. Pharm. Sci. 133, 187–189 (2017).
Paugh, S. W. et al. Sphingosine and its analog, the immunosuppressant 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol, interact with the CB1 cannabinoid receptor. Mol. Pharm. 70, 41–50 (2006).
González, S. et al. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson’s disease. Brain Res. 1073–1074, 209–219 (2006).
Fernandez-Espejo, E. et al. Cannabinoid CB1 antagonists possess antiparkinsonian efficacy only in rats with very severe nigral lesion in experimental parkinsonism. Neurobiol. Dis. 18, 591–601 (2005).
Aloe, L. & Fiore, M. TNF-alpha expressed in the brain of transgenic mice lowers central tyroxine hydroxylase immunoreactivity and alters grooming behavior. Neurosci. Lett. 238, 65–68 (1997).
Harms, A. S. et al. Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson’s disease. Mol. Ther. 19, 46–52 (2011).
Christiansen, J. R. et al. Peripherally administered TNF inhibitor is not protective against α-synuclein-induced dopaminergic neuronal death in rats. Neurobiol. Dis. 206, 106803 (2025).
Papazian, I. et al. Fundamentally different roles of neuronal TNF receptors in CNS pathology: TNFR1 and IKKβ promote microglial responses and tissue injury in demyelination while TNFR2 protects against excitotoxicity in mice. J. Neuroinflammation 18, 222 (2021).
D’Costa, S. et al. Practical utilization of recombinant AAV vector reference standards: focus on vector genomes titration by free ITR qPCR. Mol. Ther. Methods Clin. Dev. 5, 16019 (2016).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Sterotaxic Coordinates (Academic Press, 2001).
Gundersen, H. J. G. & Jensen, E. B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 147, 229–263 (1987).
Juan, W.-S. et al. Optimal Percoll concentration facilitates flow cytometric analysis for annexin V/propidium iodine-stained ischemic brain tissues. Cytom. A 81, 400–408 (2012).
Acknowledgements
We acknowledge the support of Diego Alignani from the flow cytometry facility for his guidance with the FACS analysis. We acknowledge the technical support of Beatriz Paternain. This work was supported by a grant PID2023-151392OB-I00 funded by MICIU/AEI/10.13039/501100011033 and by the “European UnionNextGenerationEU/PRTR”, grant PI20/01063 funded by ISCIII-FEDER and grants PC 060-061 and PC 192-193 funded by the Government of Navarra and Fundación Gangoiti. L.B., L.A. and A.T. were funded by Ministerio de Universidades (FPU018/02244, FPU19/03255, FPU21/01545, respectively).
Author information
Authors and Affiliations
Contributions
A.T. and L.B. generated the animal models and performed motor behavior, flow cytometry, and histological experiments. M.A.A., L.A. and C.V. collaborated on the animal manipulation and flow cytometry experiments. E.L. and E.M. were involved in stereological counting. A.V. and G.G.-A. prepared the AAV9s. L.M. and M.M.A. performed the paraffin immunohistochemistry. M.G.T. provided background on the XPro1595 treatment. S.H. and M.S.A. designed the experiments. A.T., L.B. and M.S.A. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.G.T. has patent #US 11,365,229 B2 “Methods of treating neurological diseases,” issued to XENCOR, INC, Monrovia, CA (US). M.G.T. has patent #US7,144,987 B1 issued to Xencor, Inc., Monrovia, CA. M.G.T. has patent #US7,244,823 B2 issued to Xencor, Inc., Monrovia, CA. M.G.T. is an ex-employee of Xencor Inc., where she co-invented the DN-TNFs. She is a consultant to INmune Bio, which licensed XPro1595 for neurological indications and holds stock in the company. M.G.T. is Editor-in-Chief of npj Parkinson’s disease. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tavira, A., Basurco, L., Abellanas, M.A. et al. Inhibition of T cell infiltration and soluble TNF signaling is neuroprotective in the alpha-synuclein overexpressing mouse model of Parkinson’s disease. npj Parkinsons Dis. 11, 315 (2025). https://doi.org/10.1038/s41531-025-01158-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01158-x