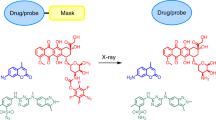
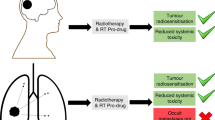
Abstract
Pt(II) drugs are a widely used chemotherapeutic, yet their side effects can be severe. Here we show that the radiation-induced reduction of Pt(IV) complexes to cytotoxic Pt(II) drugs is rapid, efficient and applicable in water, that it is mediated by hydrated electrons from water radiolysis and that the X-ray-induced release of Pt(II) drugs from an oxaliplatin prodrug in tumours inhibits their growth, as we show with nearly complete tumour regression in mice with subcutaneous human tumour xenografts. The combination of low-dose radiotherapy with a Pt(IV)-based antibody–trastuzumab conjugate led to the tumour-selective release of the chemotherapeutic in mice and to substantial therapeutic benefits. The radiation-induced local reduction of platinum prodrugs in the reductive tumour microenvironment may expand the utility of radiotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. All data generated in this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Huynh, E. et al. Artificial intelligence in radiation oncology. Nat. Rev. Clin. Oncol. 17, 771–781 (2020).
De Ruysscher, D. et al. Radiotherapy toxicity. Nat. Rev. Dis. Primers 5, 13 (2019).
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4, 437–447 (2004).
Barker, H. E., Paget, J. T. E., Khan, A. A. & Harrington, K. J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 15, 409–425 (2015).
Forastiere, A. A. et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N. Engl. J. Med. 349, 2091–2098 (2003).
Paci, A. et al. Review of therapeutic drug monitoring of anticancer drugs part 1—cytotoxics. Eur. J. Cancer 50, 2010–2019 (2014).
Rautio, J., Meanwell, N. A., Di, L. & Hageman, M. J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 17, 559–587 (2018).
Johnstone, T. C., Suntharalingam, K. & Lippard, S. J. The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 116, 3436–3486 (2016).
Klein, A. V. & Hambley, T. W. Platinum drug distribution in cancer cells and tumors. Chem. Rev. 109, 4911–4920 (2009).
Wang, X., Wang, X., Jin, S., Muhammad, N. & Guo, Z. Stimuli-responsive therapeutic metallodrugs. Chem. Rev. 119, 1138–1192 (2019).
Fu, Q. et al. External-radiation-induced local hydroxylation enables remote release of functional molecules in tumors. Angew. Chem. Int. Ed. 59, 21546–21552 (2020).
Tanabe, K., Ishizaki, J., Ando, Y., Ito, T. & Nishimoto, S.-i Reductive activation of 5-fluorodeoxyuridine prodrug possessing azide methyl group by hypoxic X-irradiation. Bioorg. Med. Chem. Lett. 22, 1682–1685 (2012).
Petit, M. et al. X-ray photolysis to release ligands from caged reagents by an intramolecular antenna sensitive to magnetic resonance imaging. Angew. Chem. Int. Ed. 50, 9708–9711 (2011).
Geng, J. et al. Switching on prodrugs using radiotherapy. Nat. Chem. 13, 805–810 (2021).
Ding, Z. et al. Radiotherapy reduces N-oxides for prodrug activation in tumors. J. Am. Chem. Soc. 144, 9458–9464 (2022).
Guo, Z. et al. Radiotherapy-induced cleavage of quaternary ammonium groups activates prodrugs in tumors. Angew. Chem. Int. Ed. 61, e202205014 (2022).
Le Caër, S. Water radiolysis: influence of oxide surfaces on H2 production under ionizing radiation. Water 3, 235–253 (2011).
Loh, Z. H. et al. Observation of the fastest chemical processes in the radiolysis of water. Science 367, 179–182 (2020).
Jiang, L. & Iwahashi, H. Current research on high-energy ionizing radiation for wastewater treatment and material synthesis. Environ. Prog. Sustain. Energy 39, 13294 (2020).
Spinks, J. W. T. & Woods, R. J. An Introduction to Radiation Chemistry (Wiley, 1990).
Fukumura, T., Akaike, S., Yoshida, Y. & Suzuki, K. Decomposition of an aqueous solution of [11C]Ro 15-4513: implication of hydrated electrons in the radiolysis of [11C]Ro 15-4513. Nucl. Med. Biol. 30, 389–395 (2003).
Fan, W., Yung, B., Huang, P. & Chen, X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 117, 13566–13638 (2017).
Wang, X. & Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 42, 202–224 (2013).
Deng, Z. et al. A photocaged, water-oxidizing, and nucleolus-targeted Pt(IV) complex with a distinct anticancer mechanism. J. Am. Chem. Soc. 142, 7803–7812 (2020).
Wang, Z. et al. Phorbiplatin, a highly potent Pt(IV) antitumor prodrug that can be controllably activated by red light. Chem 5, 3151–3165 (2019).
Tolbatov, I., Coletti, C., Marrone, A. & Re, N. Insight into the electrochemical reduction mechanism of Pt(IV) anticancer complexes. Inorg. Chem. 57, 3411–3419 (2018).
McCormick, M. C., Keijzer, K., Polavarapu, A., Schultz, F. A. & Baik, M.-H. Understanding intrinsically irreversible, non-nernstian, two-electron redox processes: a combined experimental and computational study of the electrochemical activation of platinum(IV) antitumor prodrugs. J. Am. Chem. Soc. 136, 8992–9000 (2014).
Ji, X. et al. Click and release: a chemical strategy toward developing gasotransmitter prodrugs by using an intramolecular Diels–Alder reaction. Angew. Chem. Int. Ed. 55, 15846–15851 (2016).
Allegra, C. J. et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J. Natl. Cancer Inst. 107, djv248 (2015).
McKeown, S. R. Defining normoxia, physoxia and hypoxia in tumours—implications for treatment response. Br. J. Radiol. 87, 20130676 (2014).
Buxton, G. V., Greenstock, C. L., Helman, W. P. & Ross, A. B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O-) in aqueous solution. J. Phys. Chem. Ref. Data 17, 513–886 (1988).
Tippayamontri, T., Kotb, R., Paquette, B. & Sanche, L. Cellular uptake and cytoplasm / DNA distribution of cisplatin and oxaliplatin and their liposomal formulation in human colorectal cancer cell HCT116. Invest. New Drugs 29, 1321–1327 (2011).
Richard, S. & Martinez Marignac, V. Sensitization to oxaliplatin in HCT116 and HT29 cell lines by metformin and ribavirin and differences in response to mitochondrial glutaminase inhibition. J. Cancer Res. Ther. 11, 336–340 (2015).
Babu, T., Sarkar, A., Karmakar, S., Schmidt, C. & Gibson, D. Multiaction Pt(IV) carbamate complexes can codeliver Pt(II) drugs and amine containing bioactive molecules. Inorg. Chem. 59, 5182–5193 (2020).
Beck, A., Goetsch, L., Dumontet, C. & Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 (2017).
Trail, P. A. et al. Cure of xenografted human carcinomas by BR96–doxorubicin immunoconjugates. Science 261, 212–215 (1993).
Han, Y. et al. Antitumor effects and persistence of a novel HER2 CAR T cells directed to gastric cancer in preclinical models. Am. J. Cancer Res. 8, 106–119 (2018).
Wang, Q. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 (2020).
Xu, Z. et al. Nanoscale metal–organic framework with an X-ray triggerable prodrug for synergistic radiotherapy and chemotherapy. J. Am. Chem. Soc. 145, 18698–18704 (2023).
Acknowledgements
We thank G. Zhu and J. Zhang for reagents; J. Li and M. Zhai for the 60Co source; J. Lin for the in vivo imaging system; and Z. Liu, P. Chen, Y. Li and F. Shao for advice. The measurements of NMR, high-resolution mass spectrometry and confocal imaging were performed at the Analytical Instrumentation Center of Peking University. This study was funded by the National Nature Science Foundation of China (grant no. 22225603), the Ministry of Science and Technology of the People’s Republic of China (grant no. 2021YFA1601400), the Beijing Municipal Natural Science Foundation (grant no. Z200018) and Changping Laboratory to Z.L., and the National Natural Science Foundation of China (grants 21731004 and 91953201), the Natural Science Foundation of Jiangsu Province (BK20202004) and the Excellent Research Program of Nanjing University (ZYJH004) to Z.G.
Author information
Authors and Affiliations
Contributions
Z.L. and Z.G. conceived the study. Q.F., assisted by S.Z., S.S., Z.G., J.C. and C.W., performed material synthesis, characterization and chemical analysis. Q.F., assisted by J.C. and C.W., performed radiosynthesis, PET imaging and data analysis. Q.F., assisted by Z.G., performed cell viability assay. D.S. analysed the NMR spectra. Y.X. and Y.Q.G. performed theoretical calculations. Q.F., assisted by S.S., Z.G., J.C. and P.S., performed all other experiments. S.Z provided technical assistance and valuable suggestions. Q.F., Z.G. and Z.L. analysed the data. Z.L. and Z.G. wrote the manuscript with inputs from all authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Shaohua Gou, Martin Pruschy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Radiation-induced reduction of metal complexes for controlled release in tumours.
a, The water radiolysis by ionizing radiation. The G-value of hydrated electron is 2.63 (G-value means the number of molecules formed by absorbing 100 eV energy in the system). b, The hydrated electrons generated by radiation can reduce metal ions and metal complexes. c, Pt(IV) complexes can be reduced by radiation to readily release Pt(II) drugs and biological function axial ligands (for example fluorophores or anti-cancer drugs).
Extended Data Fig. 2 A proposed two-electron reduction process of radiation-induced reduction of the Pt(IV) complex.
It is initiated by a one-electron reduction of the Pt(IV) complex to give a metastable hexacoordinated Pt(III) intermediate. This is followed by a subsequent acetate ligand detachment with a low activation free energy to form a pentacoordinated Pt(III) species, which is then reduced by the second electron through a barrierless concerted process. Values calculated at the SMD/M06-2X/ aug-cc-pVTZ level of theory.
Extended Data Fig. 3 Radiation-induced release of the FDA-approved Pt(II) drugs are efficient and generally applicable for Pt(IV) complexes.
Nuclear Magnetic Resonance (NMR) study to investigate the Pt(II) drugs release from Pt(IV) complexes. a, 195Pt NMR spectrum showed that the peak of carboPt(IV)-(Suc)2 (1883 ppm, top panels) {ctc-[Pt(NH3)2(Suc)2(CBDCA)]} almost disappears after radiation and a new peak arises at -1707 ppm (middle panels), which is the peak of carboplatin according to external standard (bottom panels). b, 95Pt NMR spectrum showed that the peak of cisPt(IV)-(Suc)2 (1082 ppm, top panels) {ctc-[Pt(NH3)2(Suc)2Cl2]} almost disappears after radiation and a new peak arises at -2150 ppm (middle panels), which is the peak of cisplatin according to external standard (bottom panels). 195Pt NMR spectrum showed that the radiation-induced release of the FDA-approved Pt(II) drugs are efficient and generally applicable for Pt(IV) complexes.
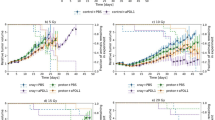
Extended Data Fig. 4 Radiotherapy-induced controlled release of oxaliplatin inhibits the growth of cancer cells.
a, Schematic representation of radiotherapy-induced release of oxaliplatin. b, Cell viability assay of radiation-induced controlled release of oxaliplatin in HCT116, Ls513, LoVo and HT29 cancer cells (n = 6; mean ± s.d., two-tailed unpaired Student’s t-test). Control, without treatment; X-ray, 4 Gy; OxaliPt(IV)-(Suc)2, 10 μM; OxaliPt(IV)-(Suc)2 + X-ray, 10 μM + 4 Gy. c, Pt distribution in different organs in the time span of 48 h after oxaliPt(IV)-(Suc)2 tail vein injection (n = 3, mean ± s.d.).
Extended Data Fig. 5 Radiation-induced near-infra-red fluorophore release from oxaliPt(IV)-HD in vitro and selectively in tumours in mice.
a, Schematic representation of radiation-induced reduction that can release the near-infra-red fluorescence from oxaliPt(IV)-HD. b, Representative confocal fluorescence images of MC38, HeLa, BGC823 cell lines. The cells were pre-treated with oxaliPt(IV)-HD (10 μM in Hank’s balanced salt solution, pH 7.4, 0.1% DMSO) under hypoxic conditions for 30 min, followed by different amounts of radiation. c, Average near-infra-red fluorescence intensities in NIR-positive cells per unit area in the examined field of view by confocal microscopy. Eight filed were chosen randomly (n = 8). d, Schematic diagram of the radiation-induced reduction of oxaliPt(IV)-HD to release axial near-infra-red fluorescent ligands in tumour-bearing mice was verified by non-invasive fluorescence imaging. e, In vivo imaging of radiation-induced fluorescence release in tumour-bearing nude mice. f, Normalized average near-infra-red fluorescence intensities in the tumour (n = 3). c, f, mean ± s.d., two-tailed unpaired Student’s t-test, ***P < 0.0001. a-f, 225 kV X-ray was used as the radiation source.
Extended Data Fig. 6 Characterization of ADCs and evaluation of the possible side effects of treatment with ADC and X-ray in mice.
a, Purity of the oxaliPt(IV)-ADC conjugate determined by HIC HPLC. b, MALDI-TOF analyses reveal that the major peak of the drug-to-antibody ratio of oxaliPt(IV)-ADC is approximately 4. c, Cell viability assay of BGC823 cells treated by X-ray, oxaliPt(IV)-ADC under different conditions and oxaliPt(IV)-ADC + X-ray under different conditions (n = 5, mean ± s.d.). Control, without treatment; X-ray, 8 Gy; OxaliPt(IV)-ADC, 10 nM; OxaliPt(IV)-ADC + hypoxia, 10 nM, 1% O2; OxaliPt(IV)-ADC + X-ray, 10 nM + 8 Gy; OxaliPt(IV)-ADC + hypoxia + X-ray, 10 nM + 8 Gy, 1% O2. d, Design of the NC-ADC. Non-cleavable linker cannot react with e−aq to release the free MMAE. e, Purity of the NC-ADC conjugate determined by HIC HPLC. f, MALDI-TOF analyses reveal that the major peak of the drug-to-antibody ratio of NC-ADC is 4. g, Records of percent survival after the indicated treatments in BGC823 tumour-bearing mice. n = 6. h, Mouse body weight after the indicated treatments in BGC823 tumour-bearing mice (n = 6, mean ± s.d.). i, Representative haematoxylin and eosin (H&E) staining of heart, liver, spleen, lung and kidney tissues from mice with indicated treatments. Data are representative of 6 mice. j, Mouse body weight after the indicated treatments in MC38 tumour-bearing mice (n = 6, mean ± s.d.).
Extended Data Fig. 7 Synthetic routes of related compounds.
a–c, Synthetic route of the carbonate Pt(IV) complexes. d, Synthetic route of the payload of NC-ADC. e, Synthetic route of the payload of oxaliPt(IV)-ADC.
Supplementary information
Supplementary Information
Supplementary methods, discussion, figures and tables.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, Q., Zhang, S., Shen, S. et al. Radiotherapy-triggered reduction of platinum-based chemotherapeutic prodrugs in tumours. Nat. Biomed. Eng 8, 1425–1435 (2024). https://doi.org/10.1038/s41551-024-01239-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-024-01239-x
This article is cited by
-
Single atom engineering for radiotherapy-activated immune agonist prodrugs
Nature Communications (2025)
-
The role of ionizing radiation-initiated reactions in targeted activation of chemotherapeutics
Nature Reviews Chemistry (2025)
-
Ultrasound-induced Activation of Tetravalent Platinum Polyglutamate Nanoprodrug Accelerated by Coumarin Derivatives
Chinese Journal of Polymer Science (2025)