Abstract
The prevalence of Alzheimer’s disease neuropathological changes (ADNCs), the leading cause of cognitive impairment, remains uncertain. Recent blood-based biomarkers enable scalable assessment of ADNCs1. Here we measured phosphorylated tau at threonine 217 in 11,486 plasma samples from a Norwegian population-based cohort of individuals over 57 years of age as a surrogate marker for ADNCs. The estimated prevalence of ADNCs increased with age, from less than 8% in people 58–69.9 years of age to 65.2% in those over 90 years of age. Among participants aged 70 years or older, 10% had preclinical Alzheimer’s disease, 10.4% had prodromal Alzheimer’s disease and 9.8% had Alzheimer’s disease dementia. Furthermore, among those 70 years of age or older, ADNCs were present in 60% of people with dementia, in 32.6% of those with mild cognitive impairment and in 23.5% of the cognitively unimpaired group. Our findings suggest a higher prevalence of Alzheimer’s disease dementia in older individuals and a lower prevalence of preclinical Alzheimer’s disease in younger groups than previously estimated2.
Similar content being viewed by others
Main
Dementia is a growing global challenge, with Alzheimer’s disease (AD) being the most common cause, characterized by ADNCs, encompassing brain deposits of amyloid-β (Aβ) plaque and neurofibrillary tau tangles. The prevalence of dementia and mild cognitive impairment (MCI) is well established2,3, but the prevalence of ADNCs in general populations remains uncertain. With the advent of drugs capable of reducing Aβ plaque pathology and slowing cognitive decline4,5, accurate knowledge of ADNC prevalence is essential for anticipating the number of individuals eligible for treatment and estimating future health-care demands and associated costs. A recent review has reported an overall prevalence of 22% ADNCs in all people 50 years of age and older globally2. However, studies examining the prevalence of ADNCs are typically enriched, including relatively small clinic-based samples, which tend to differ regarding important clinical and demographic features compared with general populations. Such studies may thus report inflated or deflated rates of AD pathology.
Until recently, ADNCs could only be verified in vivo using cerebrospinal fluid analysis or molecular positron emission tomography (PET), substantially hindering its evaluation in large population-based studies. Minimally invasive blood-based markers, particularly plasma phosphorylated tau at threonine 217 (pTau217), that have high accuracy for ADNCs have recently become available but have not yet been used in large community-based studies6. In this study, we capitalized on the large Norwegian population-based Trøndelag Health (HUNT) study7,8, with 11,486 blood samples of participants 58 years of age and older, to explore the following research questions: (1) what the prevalence of ADNCs in the population 58 years of age and older across age and sex groups is; (2) what the association between ADNCs and demographics, cognition, educational level, apolipoprotein E (APOE) ε2, ε3 or ε4 status and comorbidities is; and (3) what proportion of those 70 years of age or older is eligible for disease-modifying therapies (DMTs) according to current recommendations9,10.
The HUNT study has been ongoing for four decades, with a new wave taking place in the same population every 10 years, thus four waves exist so far. In this nested cross-sectional study, we included 2,537 individuals from HUNT3 (age range of 58–69.9 years, 51.2% women) and 8,949 from HUNT4 (age range of 70 years and older (hereafter the 70+ group), 53.6% women). Subsequent diagnostic history was considered when deciding who to approach for inclusion in HUNT3. Although a blood sample was provided in both surveys, the HUNT4 70+ cohort also underwent a standardized clinical assessment for a diagnosis of dementia and MCI11 (Extended Data Fig. 1 and Supplementary Information, ‘Assessment of cognition, physical performance, anxiety, depression, neuropsychiatric symptoms and activities of daily living’). The presence of ADNCs was established by measuring plasma pTau217 levels with a previously validated commercial kit (ALZpath p-Tau 217 Advantage PLUS, Quanterix)1. We used a two cut-off approach as recommended by the Global CEO Initiative on Alzheimer’s Disease12 to categorize individuals as ADNC negative (less than 0.40 pg ml−1), intermediate or positive (0.63 pg ml−1 or more), as previously described1. The agreement between elevated plasma pTau217 concentration and the presence of notable amounts of plaques and tangles at post-mortem examination has been previously found to be very high13. For terminological clarity, in the remainder of this article, the term ‘ADNC’ refers specifically to the presence of elevated plasma pTau217 concentration, used as a surrogate marker for ADNCs.
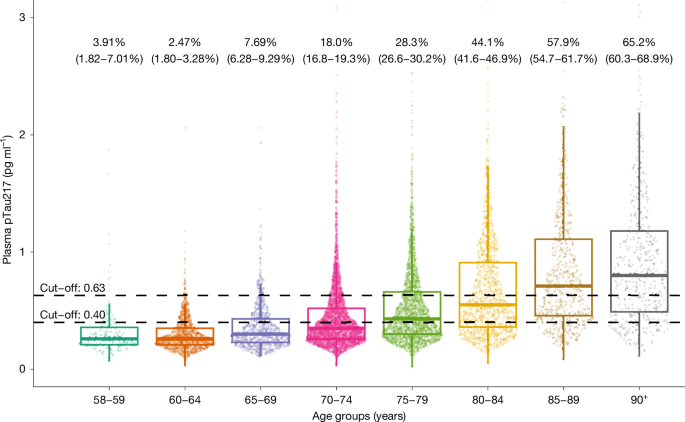
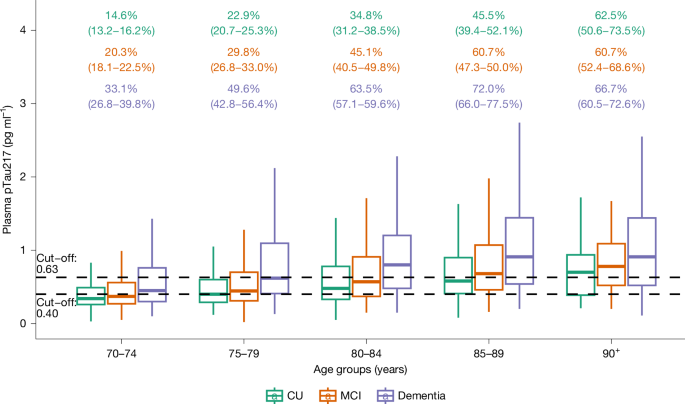
The demographic and clinical characteristics of the cohort are shown in Extended Data Table 1. The estimated proportion of people with and without ADNCs in different age groups is shown in Fig. 1 and Extended Data Table 2. There was a stepwise increase in the proportion of ADNCs across age groups; the proportion was 33.4% in the 70+ group. There was a significant association between ADNCs and cognitive diagnosis. Among individuals with dementia, 60% had ADNCs (that is, AD dementia), compared with 32.6% of those with MCI (that is, prodromal AD) and 23.5% in the cognitively unimpaired group (that is, preclinical AD). The proportion with ADNCs increased with age in each of the cognitive groups. ADNCs were ruled out based on plasma pTau217 concentrations being below the lower cut-off in 19.4% of the dementia group, 41% of the MCI group and 50.1% of the cognitively unimpaired group (Fig. 2 and Extended Data Table 3). Depending on age, 13.5–27.6% of participants had plasma pTau217 concentrations in the intermediate range, with only minor differences between the cognitively unimpaired, MCI and dementia groups (Extended Data Tables 2 and 3). Weighted estimated proportions of ADNCs in the respective clinical cognitive subgroups are shown in Extended Data Table 4.
Individual dots represent plasma pTau217 concentrations (n = 2,537 participants from HUNT3 and n = 8,949 participants from HUNT4 70+). Percentages (95% confidence interval) are estimates of how many in each age group have AD neuropathology, defined by plasma pTau217 concentration of 0.63 pg ml−1 or more. The lower cut-off of 0.40 pg ml−1 is also shown. The horizontal line in each box represents the median, and bottom and top edges delineate the second and third quartiles. The bottom whisker represents the first quartile, and the top whisker denotes the fourth quartile. Concentrations above 3 pg ml−1 are not shown.
Percentages (with 95% confidence intervals; colour-coded to match the box plots) are estimates of how many in each cognitive group have AD neuropathology, defined by plasma pTau217 concentration ≥ 0.63 pg ml−1. The lower cut-off of 0.40 pg ml−1 is also shown. n = 8,949 participants from HUNT4 70+. The horizontal line in each box represents the median, and top and bottom edges delineate the second and third quartiles. The bottom whisker represents the first quartile, and the top whisker denotes the fourth quartile. CU, cognitively unimpaired.
The estimated prevalence of preclinical AD, prodromal AD and AD dementia in the 70+ study population is shown in Fig. 3 and Extended Data Table 5. The estimated prevalence of AD dementia consistently increased with age, whereas the prevalence of preclinical AD increased from the 70–74 year age group to the 80–84 year age group before decreasing in the oldest old (those aged 85 years or older (the 85+ group)). The prevalence of prodromal AD remained stable after 80 years of age (Extended Data Fig. 2).
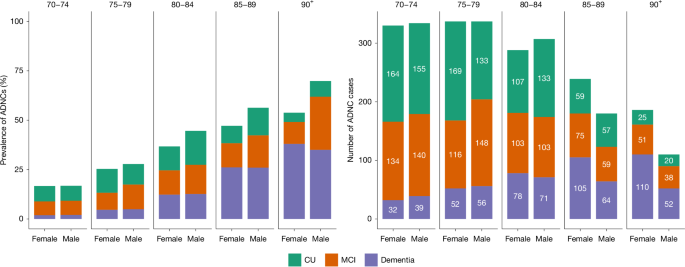
Left, the percentage of participants with ADNCs, defined as plasma pTau217 concentration ≥ 0.63 pg ml−1. Stacked bars represent the estimated proportions of ADNCs. Right, absolute numbers of study participants with ADNCs. Colours represent different levels of cognitive effects. The values are stratified, on the x axis, by sex. The numbers displayed at the top of the graphs are age groups in years.
In the 80–89 year age group, men had a slightly higher estimated prevalence of ADNCs than women, mainly due to a higher prevalence of early-stage AD (preclinical and prodromal AD), although cognitive subgroup differences were not statistically significant. There was no sex difference in the estimated prevalence of AD dementia in any of the age groups. Details can be found in Extended Data Table 5 and Extended Data Fig. 2.
Estimated ADNC prevalence was higher among individuals with one (46.4%) or two (64.6%) APOE ε4 alleles than in those with none (27.1%; Extended Data Table 6). Estimated glomerular filtration rate (eGFR) was inversely associated with plasma pTau217 concentration in individuals with eGFR < 51 ml min−1 per 1.73 m2 (Extended Data Fig. 3). There was no significant association between ADNCs and self-reported cardiovascular and cerebrovascular disease, chronic obstructive pulmonary disease, diabetes, cancer, migraine, psoriasis, kidney disease, rheumatoid arthritis and gout, when adjusting for age, sex, APOE ε4 allele count, cognition, serum creatinine levels and education level (Extended Data Table 7).
Estimated ADNC prevalence was lowest among individuals with tertiary education, highest among those with primary education and intermediate in those with secondary education, with differences becoming more pronounced with age. For those with primary education, ADNC prevalence was higher in women than in men, whereas in those with secondary and tertiary education, men had a higher estimated ADNC prevalence than women (Extended Data Fig. 4).
On the basis of the current eligibility criteria for DMTs4,5, out of a total of 8,949 participants in the 70+ group, 909 (10.2%) fulfilled the eligibility criteria for DMTs (Extended Data Fig. 5), whereas the weighted estimate for the entire 70+ population was 11.1%.
Positive and negative predictive values (PPVs and NPVs, respectively) were examined in an exploratory analysis based on previously reported sensitivity and specificity metrics for pTau217 for ADNCs. The PPV increased with age-specific prevalence, from 59.9% (70–74 years of age) to 92.6% (90 years of age or older (the 90+ group)), whereas the NPV decreased from 97.9% to 84.9%. Overall, the PPV and NPV were 77.4% and 95.4%, respectively. Under optimism correction, the PPV and NPV were attenuated to 71.9% and 92.9% overall, with ranges of 52.9–90.4% (for PPV) and 78.3–96.8% (for NPV) across age strata, consistent with higher PPV and lower NPV at higher prevalence (Extended Data Table 8).
In this large, Norwegian population-based cohort study, the proportion of individuals with ADNCs, as measured with plasma pTau217 concentration, increased with age, from under 8% in people 58–69 years of age to 65% in those over 90 years of age. Among the 70+ population, 10% had preclinical AD, 10.4% had prodromal AD and 9.8% had AD dementia. ADNCs were more prevalent in individuals with lower education and those with APOE ε4 alleles. ADNCs were inversely associated with eGFR < 51 ml min−1 per 1.73 m2. It has previously been shown that age, APOE ε4 status and renal dysfunction are associated with pTau217 concentrations, but that these factors only marginally alter the clinical performance of pTau217 as a marker for ADNCs14,15. The two plasma pTau217 cut-offs used to assess the prevalence of ADNCs (lower cut-off with 95% sensitivity and upper cut-off with 95% specificity) were applied independent of age1. Age-dependent increases in plasma pTau, independent of ADNCs, have been discussed in the literature; however, current evidence does not support this association6.
Although the prevalence of AD dementia among the youngest cognitively assessed age group in our study (70–74 years) was similar to that reported in a recent literature review (2% versus 1.7%)2, we found a higher prevalence of AD dementia in the older age groups (for example, 85–89 years: 25.2% in our study, compared with 7.1% in the review). By contrast, the prevalence of preclinical AD in the youngest age group (70–74 years) was much higher in the review (22.4% for men and 22.2% for women) than in our study (7.6% for men and 7.9% for women). It is possible that, compared with the unselected community-based cohort in our study, the more selected cohorts included in the review over-recruited cognitively healthy people at high risk for AD, and under-recruited older people with dementia. Participants under 70 years of age were not cognitively assessed; thus, their clinical status remains undetermined.
Blood-based AD biomarkers are increasingly being utilized for considering people eligible for treatment with the new anti-AD drugs, which have been recently approved in several countries, including the USA and Europe. On the basis of the current eligibility recommendations for these drugs9,10, we found that 11% of people 70 years of age or older in the study population would potentially be eligible. Such treatments carry risks that would have to be carefully weighed against any potential benefits in each individual16.
On the basis of having plasma pTau217 concentrations below the lower cut-off, ADNCs were ruled out in 41% of the MCI group and 19.4% of the dementia group. Thus, in these groups, cognitive impairment is probably due to causes other than AD. The higher prevalence of ADNCs in people with one or two than with none APOE ε4 alleles is in line with previous findings17,18. The prevalence of ADNCs was slightly higher in men than in women in the 80–89 year age group. There was no sex difference in the prevalence of plasma pTau217-verified AD dementia in any age group. Several clinical studies have reported a female predominance of dementia19, and previous HUNT data have reported a slightly higher prevalence of dementia in women than in men, but only in those over 85 years of age, whereas MCI was more common in men than in women11. The recent review also reported a higher proportion of preclinical AD in men, but a higher prevalence of AD dementia in women2. Thus, the most recent evidence does not seem to support previous reports of higher ADNC prevalence in women.
Having a lower level of education was clearly associated with higher ADNC prevalence, especially in the older age groups. This supports the theory of a protective effect of education, for example, by means of increasing cognitive reserve20. We did not assess potential confounding factors such as smoking, obesity, physical inactivity and excessive alcohol consumption21, which may attenuate the association between education level and ADNCs.
There was no association between self-reported somatic morbidities and plasma pTau217 concentrations above the upper cut-off when adjusting for confounding factors. Previous research has shown that vascular disease can both cause dementia and increase the odds that AD pathology manifests as AD dementia22,23. We have not studied the possible synergistic effect of ADNCs and comorbidities on cognition.
This study has several strengths. It is the largest population-based study of ADNCs. The clinical diagnosis of MCI and dementia was based on a prospective standardized clinical assessment. Analysis of ADNCs was based on an accurate assay performed in a laboratory with extensive experience and expertise using state-of-the art technologies. Until recently, the absence of robust and scalable pTau217 assays meant that research cut-offs for blood-based markers were specific to each cohort and did not generalize effectively during external validation. However, the assay used in this study has shown strong performance in external validation across independent cohorts1.
Plasma pTau217 reflects both phosphorylated, soluble tau in the context of Aβ pathology24 and aggregated tau pathology. Elevated concentrations of soluble pTau217 can occur decades before the onset of aggregated tau25, correlate strongly with the severity of AD pathology and with clinical progression, and are considered specific for AD1. Thus, plasma pTau217 has shown good discriminative accuracy for distinguishing between pathology-confirmed AD and other tauopathies such as frontotemporal lobar degeneration26,27, traumatic encephalopathy syndrome28, primary age-related tauopathy29 and progressive supranuclear palsy13.
Plasma pTau217 concentration has been shown to have the highest predictive values of the blood-based AD markers30. Although recent studies indicate that the ratio of plasma pTau217 to the 42-amino-acid-long form of Aβ (Aβ42) can reduce intermediate test results and may slightly increase diagnostic accuracy in well-controlled research cohorts31,32,33, these findings do not directly translate to large population-based studies such as HUNT. Plasma Aβ42 concentration is highly sensitive to pre-analytical variables, which often deviate from Alzheimer’s Association guidelines in population cohorts34, leading to falsely increased pTau217:Aβ42 ratios35.
This study used a two-cut-off method1. Depending on age, 13.5–27.6% of the study population had plasma pTau217 concentrations in the intermediate range, here defined as plasma pTau217 values between 0.40 and 0.63 pg ml−1 and would thus require further examination to clarify their ADNC status. In an ideal scenario, individuals in the intermediate group would undergo cerebrospinal fluid analysis or PET imaging to obtain a more definitive diagnosis, although these methods also can yield intermediate, ‘grey zone’ results36,37. Regional molecular PET patterns can be an important indicator of observed or expected clinical symptoms38. However, recognizing that such evaluations are often not routinely available, a feasible follow-up strategy would be to repeat plasma biomarker testing after, for example, 1 year12. The presence of an intermediate zone is an expected and intrinsic feature of continuous biomarker distributions when applied to binary clinical outcomes such as ADNCs. Rather than a limitation, this zone reflects biological and clinical heterogeneity, particularly in population-based cohorts. A substantial proportion of individuals in the intermediate range probably exhibit ADNCs.
The participation rate in HUNT4 70+ was 51.1%, and we are unaware of other similarly large population-based studies with such a high participation rate. Because of limited funding, we were unable to analyse blood samples from all participants 58–69 years of age in HUNT3, but we attempted to adjust for a potential selection bias (see Extended Data Table 9). The cross-sectional design might underestimate amyloid abnormality as opposed to lifetime risk estimates. Furthermore, although nearly 90% of HUNT4 70+ participants provided a blood sample, 10% did not. The proportion not providing a blood sample was higher in the dementia group, but we adjusted for this in our analysis, as well as for participation bias in HUNT4 (see Extended Data Table 9).
This study has some limitations. Medical diseases were self-reported and thus might not accurately reflect the degree of comorbidities. Previous findings indicate a high NPV and moderate PPV for self-reported diseases in the HUNT study compared with diagnoses recorded in regular clinical care7. The HUNT study does not collect data on ethnicity, but the population in this region in 2017 included less than 5% of individuals who are immigrants or Norwegian born to parents who immigrated from Africa, Asia, the Middle East or South America, and thus the findings are relevant for a mainly white Norwegian population. Dementia varies in different ethnic groups and thus the prevalence of ADNCs may also differ in other populations2.
The predictive value of a diagnostic test varies with the disease prevalence. Thus, as has been demonstrated, when the likelihood of ADNCs is high, for example, in older individuals, the PPV is high, whereas the NPV is lower, that is, there is a risk of false-negative results30. This analysis is not a substitute for internal validation and has key limitations: (1) the commutability of sensitivity and/or specificity from external cohorts is assumed; (2) circularity is possible because prevalence is estimated from the same biomarker; and (3) we do not model age-specific shifts in sensitivity and/or specificity or spectrum effects.
In conclusion, we present prevalence estimates of ADNCs in a large, Norwegian population-based cohort. Among individuals 70 years of age or older, 33.4% exhibited ADNCs, with 10% classified as preclinical AD, 10.4% as prodromal AD and 9.8% as AD dementia. Compared with previous studies with smaller, less representative cohorts, our findings indicate a higher prevalence of AD dementia in older individuals and a lower prevalence of preclinical AD in younger age groups.
Methods
Ethics statement
This study was approved by the Regional Committee for Medical and Health Research Ethics in Norway (REC Southeast C 565876) as well as according to the General Data Protection Regulation by the Norwegian Agency for Shared Services in Education and Research (SIKT 585403). Participation in HUNT required informed consent, which was provided after receiving oral and written information about the health survey. In participants with reduced capacity to consent, their next of kin gave consent. The clinical trial registration number is NCT06719453.
Cohort selection and study design
The HUNT study is a population-based health study conducted in the Trøndelag region of Central Norway. The key demographic and health indicators of the region mirror Norwegian national averages closely. The HUNT study has so far spanned four waves: HUNT1 (1984–1986), HUNT2 (1995–1997), HUNT3 (2006–2008) and HUNT4 (2017–2019), achieving high participation rates. An updated cohort profile has been previously published7. Participants for this study were recruited from HUNT3 and HUNT4.
In HUNT4, all residents 70 years of age or older (n = 19,463) were invited to the substudy HUNT4 70+ for a standardized cognitive assessment and diagnosis11,39 (see below and Supplementary Information). Of the invited, 9,956 people (51.1%) participated, and 8,949 (46%) people provided a blood sample. To also cover younger ages, we additionally included 2,537 out of 12,243 people (20.1%) 58–69.9 years of age from HUNT3. Of these, 2,391 people (94.2%) participated in both HUNT3 and HUNT4 70+. Subsequent cognitive status from HUNT4 70+ and whether they had provided a blood sample for HUNT4 70+ was considered when deciding who to include from HUNT3. We actively selected those from HUNT3 with a diagnosis of dementia in HUNT4 70+ and included twice as many participants with an MCI diagnosis than a cognitively unimpaired status in the subsequent HUNT4 70+. We thereafter accounted for this selection bias in our analysis (see ‘Selection bias and weighting’). Thus, the majority of those participants included in the HUNT3 analysis of this study provided two blood biospecimen: one for HUNT3 and one for HUNT4. No cognitive assessment was performed at the time of HUNT3.
Among those not providing a blood sample in HUNT4 70+, there were relatively more from the group with dementia (25.4%) than 8.6% with MCI and 6% from the cognitively unimpaired group. This was also statistically accounted for in our analysis (see ‘Selection bias and weighting’).
Comorbidities and educational level were self-reported, using a standardized questionnaire.
Cognitive assessment and diagnosis in HUNT4 70+
Trained health personnel assessed the cognitive, neuropsychiatric and functional status of participants using standardized clinical scales at a field station, at homes or in nursing homes. A structured carer questionnaire was obtained by interview in participants suspected of having substantial cognitive impairment. Clinical and research experts (geriatricians, neurologists and old-age psychiatrists) made diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria by clinical consensus method. Participants were categorized into cognitively unimpaired, MCI (minor neurocognitive disorder in the DSM-5) or dementia (major neurocognitive disorder in the DSM-5)11. Those with MCI were further classified as amnestic MCI or non-amnestic MCI, whereas dementia was further classified as mild, moderate or severe dementia and according to clinical subtype. The detailed diagnostic procedure has been previously published11. A detailed overview over the diagnostic assessment can be found in the Supplementary Information.
Blood sample collection and handling procedures
Blood samples were collected non-fasting, handled and stored according to standardized procedures8. Samples were either collected at field stations, in the homes or in nursing homes of participants. Samples were collected in Vacuette ethylene diamine tetra acetic Acid (EDTA)-plasma 9 ml tubes in HUNT4 and BD Vacutainer EDTA-plasma 10 ml tubes in HUNT3, which were gently inverted (6–8 times after sampling in HUNT4, and 10 times in HUNT3). After a maximum of 45–120 min in room temperature, samples were placed in a refrigerator with a temperature of 2–8 °C before transportation at 4 °C to a central laboratory the same day (until no later than 08:00 the next day in HUNT3). The next day (in HUNT3 on Monday, if blood collection was on a Friday), plasma samples were centrifuged and underwent automated fractioning (Tecan200). Plasma aliquots (tube size of 1.4 ml containing 200 µl plasma) were frozen to −80 °C and stored until transportation for future analyses. Thus, phlebotomy-to-freezing time ensured plasma pTau217 stability35. Transportation from the HUNT Biobank to the Clinical Neurochemistry Laboratory, University of Gothenburg (Sweden) was conducted with temperature-regulated dry-ice transport at −80 °C. Samples were further stored at −80 °C until analysis. Before immunoassay analyses, plasma-EDTA samples were thawed, vortexed and centrifuged at 4,000g for 10 min at 20 °C.
Analysis of plasma pTau217
Using the Simoa HD-X instrument (Quanterix), plasma pTau217 was quantified with a previously validated commercial kit (ALZpath p-Tau 217 Advantage PLUS, Quanterix)1. As recommended by the Global CEO Initiative on AD, we used a two cut-off approach to categorize individuals as ADNC negative, intermediate or positive, using recently validated cut-offs: lower cut-off of 0.40 pg ml−1 (95% sensitivity) and an upper cut-off of ≥0.63 pg ml−1 (95% specificity)1,12. We used the upper cut-off to determine with a high specificity those individuals with the presence of ADNC, and the lower cut-off to identify those with a high likelihood of not having ADNC. Plasma analysis was conducted January to August 2024. A summary of the analytical performance of the assay can be found in the Supplementary Information.
Statistical analyses
Descriptive analysis
Descriptive statistics were used to summarize the data. Means and standard deviations were calculated for continuous variables, whereas frequencies were reported for categorical variables. For non-symmetrically distributed data, medians and interquartile ranges were also calculated. All the estimations were weighted for selection bias as explained below. Differences in proportions were assessed using a chi-squared test. Education levels were self-reported and merged into three categories: ‘primary’ included up to 10 years of compulsory primary and lower secondary education; ‘secondary’ combined 1–2 years of academic or vocational school, 3 years of academic or vocational school, and 3–4 years of vocational training or apprenticeship (upper secondary education); and ‘tertiary’ referred to college or university education of less than 4 years or 4 years or more.
Selection bias and weighting
To adjust for potential selection bias in participation and data availability, we applied a multi-stage inverse probability weighting strategy. First, we used participation weights developed by Skirbekk et al.39, which account for differential participation in HUNT4 70+ based on age, sex and educational level. Second, we estimated the probability of donating a blood sample for pTau217 analysis using a logistic regression model that included age, sex, education, APOE ε4 status, cognitive diagnosis and self-reported medical disorders. Third, for HUNT3, participants included in this nested study, we modelled the probability of them having been selected among all enrolled HUNT3 participants, based on the cognitive diagnosis established in HUNT4 70+, age, sex, education, APOE ε4 status and self-reported medical disorders. Missing categories in APOE ε4 status and education were retained as separate levels in the models.
For each individual, the final weight was calculated as the inverse of the product of the relevant probabilities: three components for participants from HUNT3 (probability of participation in HUNT4, probability of donating a blood sample in HUNT4 70+ and probability of being selected into HUNT3 from HUNT4 70+) and two components for those from HUNT4 70+ (probability of participation in HUNT4 70+ and probability of donating a blood sample in HUNT4 70+). Although covariates were shared across models, each probability addressed a distinct stage of non-random inclusion. Weights were trimmed using the median ± 3 × interquartile range to reduce the influence of outliers40. Details of the estimations from the logistic regression models are provided in Extended Data Table 9.
Imputation of missing covariates for treatment eligibility analyses
When evaluating the eligible population for novel anti-amyloid immunotherapies in HUNT4 70+ participants with MCI or mild dementia, we performed multiple imputation for missing data for body mass index, APOE ε4 status and previous history of stroke or brain haemorrhage, using the mice package in R. The multiple imputation process was conducted using logistic regression for categorical variables (APOE ε4 status and stroke or haemorrhage history) and random forest for continuous variables (body mass index), based on a dataset including these and other key demographic variables to inform the imputation models. To estimate the population-representative proportion of eligible individuals, we applied the above-mentioned sampling weights to our eligibility calculations. The final weighted proportion was derived by weighting the sum of all eligible participants from the population (those meeting all clinical and biomarker criteria for either MCI or dementia groups) and dividing them by the weighted sum of the study population. This approach provides an estimate of the true population prevalence of treatment eligibility, accounting for the complex sampling design and differential participation probabilities across the HUNT4 70+ study.
Model evaluating association between plasma pTau217 and kidney function
To investigate the relationship between plasma pTau217 concentrations and kidney function, eGFR (ml min−1 per 1.73 m2) was calculated using the CKD-EPI 2021 creatinine-based equation41. Plasma pTau217 was log-transformed, and a weighted linear regression model was fitted with eGFR as a predictor. A piecewise (segmented) regression approach was then applied to allow different slopes on either side of an estimated breakpoint in eGFR. The final model was visualized on the original (non-logarithmic) scale, showing the fitted regression lines for each segment and indicating the estimated inflection point.
Predictive value sensitivity analysis
In an exploratory analysis, we estimated age-stratum-specific PPV and NPV for plasma pTau217, with externally derived sensitivity and specificity ranges for the ability of plasma pTau217 to detect ADNC as reported by Ashton et al.1. For each age stratum (70–74, 75–79, 80–84, 85–89, 90 and older, and overall), prevalence was set to the weighted prevalence of plasma pTau217 positivity (≥0.63 pg ml−1) from HUNT4 70+, using inverse-probability weights reflecting participation and selection into the plasma pTau217 subsample. We enumerated all combinations of sensitivity (0.850, 0.982) and specificity (0.745, 0.986) in 0.001 increments and, for each triplet (sensitivity (Se), specificity (Sp) and prevalence (Prev)), computed PPV and NPV via Bayes’ formulas: PPV = (Se × Prev)/(Se × Prev + (1 − Sp) × (1 − Prev)); NPV = (Sp × (1 − Prev))/((1 − Se) × Prev + Sp × (1 − Prev)). For each age stratum, we summarized the empirical distributions with the median and the 2.5th–97.5th percentiles (reported as 95% confidence interval). Given this exploratory analysis assumes sensitivity and specificity commutability from Ashton et al.1 and that a certain degree of worsened performance is generally expected upon external validation studies, we repeated the analyses above at a sensitivity and specificity shrinkage level of 0.9, to correct for optimism.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
To protect the privacy of participants, the HUNT Research Centre aims to limit storage of data outside the HUNT databank and cannot deposit data in open repositories. The HUNT databank has precise information on all data exported to different projects and can reproduce them on request. There are no restrictions regarding data export given approval of applications to the HUNT Research Centre. Researchers can apply for data at the NTNU (https://www.ntnu.edu/hunt/research).
Code availability
All analyses, statistical models and figures were performed using the open-source statistical software environment and programming language R (v4.5.0; R Core Team). All codes for data processing and analysis are publicly available on GitHub (https://github.com/dalejo643/Nature-Aarsland2025.git). All models relied on publicly available R packages and functions.
References
Ashton, N. J. et al. Diagnostic accuracy of a plasma phosphorylated tau 217 immunoassay for Alzheimer disease pathology. JAMA Neurol. 81, 255–263 (2024).
Gustavsson, A. et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 19, 658–670 (2023).
Fang, M. et al. Lifetime risk and projected burden of dementia. Nat. Med. 31, 772–776 (2025).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527 (2023).
Mielke, M. M. et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat. Med. 28, 1398–1405 (2022).
Asvold, B. O. et al. Cohort profile update: the HUNT study, Norway. Int. J. Epidemiol. 52, e80–e91 (2023).
Naess, M. et al. Data resource profile: the HUNT Biobank. Int. J. Epidemiol. 53, dyae073 (2024).
Cummings, J. et al. Lecanemab: appropriate use recommendations. J. Prev. Alzheimers Dis. 10, 362–377 (2023).
Rabinovici, G. et al. Donanemab: appropriate use recommendation. J. Prev. Alzheimers Dis. 12, 100150 (2025).
GjØra, L. et al. Current and future prevalence estimates of mild cognitive impairment, dementia, and its subtypes in a population-based sample of people 70 years and older in Norway: the HUNT study. J. Alzheimers Dis. 79, 1213–1226 (2021).
Schindler, S. E. et al. Acceptable performance of blood biomarker tests of amyloid pathology — recommendations from the Global CEO Initiative on Alzheimer’s Disease. Nat. Rev. Neurol. 20, 426–439 (2024).
Palmqvist, S. et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 324, 772–781 (2020).
Arslan, B. et al. The impact of kidney function on Alzheimer’s disease blood biomarkers: implications for predicting amyloid-β positivity. Alzheimers Res. Ther. 17, 48 (2025).
Lehmann, S. et al. Clinical value of plasma ALZpath pTau217 immunoassay for assessing mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 95, 1046–1053 (2024).
Tonegawa-Kuji, R. et al. Efficacy and safety of passive immunotherapies targeting amyloid β in Alzheimer’s disease: a systematic review and meta-analysis. PLoS Med. 22, e1004568 (2025).
Schmechel, D. E. et al. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl Acad. Sci. USA 90, 9649–9653 (1993).
Ghebremedhin, E., Schultz, C., Braak, E. & Braak, H. High frequency of apolipoprotein E ε4 allele in young individuals with very mild Alzheimer’s disease-related neurofibrillary changes. Exp. Neurol. 153, 152–155 (1998).
Beam, C. R. et al. Differences between women and men in incidence rates of dementia and Alzheimer’s disease. J. Alzheimers Dis. 64, 1077–1083 (2018).
Stern, Y. et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 16, 1305–1311 (2020).
Livingston, G. et al. Dementia prevention, intervention, and care: 2024 report of the Lancet Standing Commission. Lancet 404, 572–628 (2024).
Akomolafe, A. et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch. Neurol. 63, 1551–1555 (2006).
Kivipelto, M. et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 62, 1556–1560 (2005).
Jack, C. R. Jr et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 20, 5143–5169 (2024).
Ossenkoppele, R., van der Kant, R. & Hansson, O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 21, 726–734 (2022).
VandeVrede, L. et al. Detection of Alzheimer neuropathology in Alzheimer and non-Alzheimer clinical syndromes with blood-based biomarkers. JAMA Neurol. 82, 344–354 (2025).
Thijssen, E. H. et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol 20, 739–752 (2021).
Asken, B. M. et al. Plasma P-tau181 and P-tau217 in patients with traumatic encephalopathy syndrome with and without evidence of Alzheimer disease pathology. Neurology 99, e594–e604 (2022).
Yu, L. et al. Plasma p-tau181 and p-tau217 in discriminating PART, AD and other key neuropathologies in older adults. Acta Neuropathol. 146, 1–11 (2023).
Therriault, J. et al. Diagnosis of Alzheimer’s disease using plasma biomarkers adjusted to clinical probability. Nat Aging 4, 1529–1537 (2024).
Palmqvist, S. et al. Plasma phospho-tau217 for Alzheimer’s disease diagnosis in primary and secondary care using a fully automated platform. Nat. Med. 31, 2036–2043 (2025).
Wang, J. et al. Diagnostic accuracy of plasma p-tau217/Aβ42 for Alzheimer’s disease in clinical and community cohorts. Alzheimers Dement. 21, e70038 (2025).
Lehmann, S. et al. Comparative performance of plasma pTau181/Aβ42, pTau217/Aβ42 ratios, and individual measurements in detecting brain amyloidosis. eBioMedicine 117, 105805 (2025).
Sunde, A. L. et al. Preanalytical stability of plasma biomarkers for Alzheimer’s disease pathology. Alzheimers Dement. 15, e12439 (2023).
Figdore, D. J. et al. Differences in Alzheimer’s disease blood biomarker stability: implications for the use of tau/amyloid ratios. Alzheimers Dement. 21, e70173 (2025).
Brum, W. S. et al. A three-range approach enhances the prognostic utility of CSF biomarkers in Alzheimer’s disease. Alzheimers Dement. 8, e12270 (2022).
Hazan, J., Liu, K. Y., Isaacs, J. D. & Howard, R. Cut-points and gray zones: the challenges of integrating Alzheimer’s disease plasma biomarkers into clinical practice. Alzheimers Dement. 21, e70113 (2025).
Crary, J. F. et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–766 (2014).
Skirbekk, V. et al. Marital histories and associations with later-life dementia and mild cognitive impairment risk in the HUNT4 70+ study in Norway. J. Aging Health 35, 543–555 (2023).
Valliant, R. & Dever, J. A. Survey Weights: a Step-by-Step Guide to Calculation (Stata Press, 2017).
Inker, L. A. et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 385, 1737–1749 (2021).
Acknowledgements
The study was funded by the Norwegian Helse Vest trust (F-12850 and F-13067). The HUNT study is a collaboration between the HUNT Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology NTNU), Trøndelag County Council, Central Norway Regional Health Authority and the Norwegian Institute of Public Health.
Author information
Authors and Affiliations
Contributions
D.A., A.L.S., D.A.T.R., H.K.S., G.S. and N.J.A. designed the study. D.A., A.L.S., D.A.T.R., T.F., H.K.S. and G.S. participated in data collection and/or management. H.Z., K.B., K.T., G.D.S., Y.Y., B.A., H.H., I.P., L.G., G.D.M. and N.J.A. coordinated and/or performed the blood biomarker quantification. D.A.T.R. and A.L. performed the data analyses. D.A., A.L.S., D.A.T.R. and A.L. wrote the initial draft of the manuscript. All authors contributed to the interpretation of the results and towards subsequent manuscript drafts.
Corresponding authors
Ethics declarations
Competing interests
D.A. has received research support and/or honoraria from AstraZeneca, H. Lundbeck, Novartis Pharmaceuticals, Evonik, Roche Diagnostics, GE Health, Bioarctic and Sanofi; and served as a paid consultant for H. Lundbeck, Eisai, Heptares, Mentis Cura, Eli Lilly, Cognetivity, Enterin, Acadia, EIP Pharma, Biogen and Takeda. A.L. has acted as a consultant for Enigma Biomedical Group. H.Z. has served on scientific advisory boards and/or as a consultant for AbbVie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Enigma, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave; has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche and WebMD; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside the submitted work). K.B. has served as a consultant and on advisory boards for AbbVie, AC Immune, ALZPath, AriBio, Beckman-Coulter, BioArctic, Biogen, Eisai, Lilly, Moleac, Neurimmune, Novartis, Ono Pharma, Prothena, Quanterix, Roche Diagnostics, Sanofi and Siemens Healthineers; has served on data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. G.S. has participated in advisory board meetings for Roche, Eli-Lilly and Eisai regarding disease-modifying drugs for AD; and has received honoraria for delivering lectures at symposia sponsored by Eisai and Eli-Lilly. N.J.A. has given lectures, produced educational materials and participated in educational programs for Eli-Lily, BioArtic and Quanterix. A.L.S., D.A.T.R., T.F., K.T., G.D.S., L.G., Y.Y., B.A., H.H., I.P., G.D.M. and H.K.S. declare no competing interests.
Peer review
Peer review information
Nature thanks Richard Mayeux and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Study overview.
HUNT = The Trøndelag Health Study, Wave 3 and 4; yr = years; DSM-5 = The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; APOE = apolipoprotein E; p-Tau 217 = tau phosphorylated at threonine 217; pg/mL = picograms per millilitre. Plasma pTau217 was measured with ALZpath p-Tau 217 Advantage PLUS, Quanterix. The figure was created using BioRender (https://biorender.com).
Extended Data Fig. 2 Association between the prevalence of ADNC, age and sex in different cognitive groups.
ADNC = Alzheimer’s disease neuropathological changes, defined as plasma pTau217 ≥ 0.63 picograms per millilitre; Preclin = Preclinical; Prodro = Prodromal. N = 8,949 participants from HUNT4 70+. Dots represent the weight-estimated ADNC prevalence for each sex in 5-year age groups, shown for each cognitive group and for the total. The whiskers are 95% confidence intervals for each estimation.
Extended Data Fig. 3 Piecewise regression analysis of plasma pTau217 and eGFR, showing fitted regression lines and identified cut-off point.
pTau217 = tau phosphorylated at threonine 217; pg/mL = picograms per millilitre; eGFR = estimated glomerular filtration rate. N = 8942 participants from HUNT4 70+. Black dots represent individual data points of plasma pTau217 concentrations and corresponding estimated glomerular filtration rates. The blue solid line represents the fitted piecewise regression model, which allows for different slopes on either side of a threshold. The red dashed vertical line indicates the inflection point (cutoff) at eGFR 50.76 ml/min/1.73 m2, where the slope of the regression changes.
Extended Data Fig. 4 Estimated prevalence of ADNC across age and educational groups.
ADNC = Alzheimer’s disease neuropathological changes, defined as plasma pTau217 ≥ 0.63 picograms per millilitre; Primary Education = up to 10 years of compulsory primary and lower secondary education; Secondary Education = 1–2 years of academic or vocational school, 3 years of academic or vocational school, or 3–4 years of vocational training or apprenticeship; Tertiary Education = college or university education of less than four years or four years or more. N = 8949 participants from HUNT4 70+. Dots represent the weight-estimated ADNC prevalence for each sex in every age group for every education level. The whiskers are 95% confidence intervals for each estimation.
Extended Data Fig. 5 Algorithm for identifying people eligible for treatment with disease-modifying drugs against Alzheimer’s disease according to recommendations.
HUNT = The Trøndelag Health Study; MCI = mild cognitive impairment; pTau217 = tau phosphorylated at threonine 217; pg/mL = picograms per millilitre; APOE = apolipoprotein E; BMI = Body Mass Index; DMT = disease-modifying treatment.
Supplementary information
Supplementary Information
Supplementary Methods and references
Supplementary Information 2
Summary of the analytical performance – ALZpath p-Tau 217 HUNT
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aarsland, D., Sunde, A.L., Tovar-Rios, D.A. et al. Prevalence of Alzheimer’s disease pathology in the community. Nature 650, 182–186 (2026). https://doi.org/10.1038/s41586-025-09841-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-09841-y