Abstract
Obstructive sleep apnea (OSA) is associated with obesity and cardiovascular risk. The SURMOUNT-OSA master protocol comprised two, 52-week, randomized, double-blind, placebo-controlled phase 3 studies (study 1 and study 2) and demonstrated a significant reduction of a number of cardiometabolic risk measures in participants with OSA and obesity following treatment with tirzepatide. Here we report prespecified analysis of cardiometabolic risk measures in SURMOUNT-OSA. Post hoc analyses include changes in a homeostatic model assessment for insulin resistance and mediation analysis to determine the proportion of observed changes attributable to reductions in body weight, apnea–hypopnea index and sleep apnea-specific hypoxic burden. In both study 1 and study 2 of SURMOUNT-OSA, tirzepatide treatment was associated with greater alleviation of cardiometabolic risk factors than placebo. Independent mediation effect of changes in OSA metrics was observed on high-sensitivity C-reactive protein, homeostatic model assessment for insulin resistance and triglycerides. The combination of changes in weight and OSA metrics, as well as weight alone, had a significant mediation effect on systolic blood pressure, but there was no significant mediation effect of weight or OSA metrics observed on diastolic blood pressure. Based on the mediation analysis, treating both sleep-disordered breathing and obesity is likely required to optimize the treatment effect on cardiometabolic benefits for patients with moderate-to-severe OSA and obesity. The ClinicalTrials.gov registration number for this study is NCT05412004.
Similar content being viewed by others
Main
Obstructive sleep apnea (OSA) is a common disorder with major cardiometabolic and neurocognitive sequelae1. Excess adiposity is a major reversible etiologic risk factor for OSA and its complications2,3. Nasal continuous positive airway pressure (PAP) to this point has been first line therapy for OSA but is not always well tolerated4,5. Moreover, PAP has not shown consistent benefits in patients with OSA from the perspective of cardiovascular outcomes6. The reason for these findings is unclear but may relate to underlying risk factors such as obesity, which are not directly addressed by PAP7.
Despite advances in obesity pharmacotherapy, the obesity epidemic is predicted to continue to increase over time, leading to a rising prevalence of OSA8,9. Weight reduction in patients with OSA and obesity yields notable improvements in OSA severity measured by apnea–hypopnea index (AHI)9,10. Considerable interest has been accumulated regarding the use of tirzepatide, a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, which has previously been shown to improve body weight as well as glucose control11,12. The results of the SURMOUNT-OSA program studies were recently published, demonstrating tirzepatide treatment to be superior to placebo in participants not using PAP therapy (study 1) as well as those using PAP therapy (study 2)13. The primary outcome of the SURMOUNT-OSA studies was the change in AHI, which is the traditional marker of sleep apnea severity13. However, the studies also reported key secondary outcomes that were controlled for multiplicity including high-sensitivity C-reactive protein (hsCRP), systolic blood pressure (SBP), sleep apnea-specific hypoxic burden (SASHB) and body weight, as well as patient reported outcomes (PROMIS)13. The results lead to questions about how much of the cardiometabolic benefits associated with tirzepatide are related to improvement in OSA severity per se versus improvement in body weight. In addition, other cardiometabolic outcomes were not reported, leading to questions about the overall benefits of tirzepatide treatment on patients with OSA and obesity13.
Based on this conceptual framework and including a complete set of study prespecified cardiometabolic endpoints, we sought to test the hypothesis that tirzepatide treatment in the SURMOUNT-OSA studies would yield improvement in cardiometabolic risk measures as compared to placebo. We include the previously reported effects of tirzepatide on hsCRP, systolic and diastolic blood pressure (DBP)13. We report the effects of tirzepatide on high-density lipoprotein-cholesterol (HDL-C), non-HDL-C and fasting insulin that were prespecified in the study protocol14. We further report the effects of tirzepatide on low-density lipoprotein-cholesterol (LDL-C), very low-density lipoprotein-cholesterol (VLDL-C) and homeostatic model assessment for insulin resistance (HOMA-IR) and a mediation analysis in the pooled sample of SURMOUNT-OSA participants, which seeks to determine how much of any observed improvement in analyzed cardiometabolic risk measures is related to changes in OSA severity factors versus changes in weight versus interaction between these variables.
Results
Participants
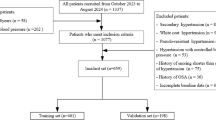
A total of 469 participants were randomized to receive either tirzepatide or placebo across study 1 (Fig. 1; 234 participants; tirzepatide n = 114, placebo n = 120) and study 2 (Fig. 1; 235 participants; tirzepatide n = 120, placebo n = 115). The full participant disposition can be found in Fig. 1. The baseline characteristics of investigated cardiometabolic risk measures were, in general, comparable between the tirzepatide and placebo groups across both study 1 and study 2 at baseline. The details of the baseline demographics and clinical characteristics can be found in Table 1, some of which have been previously reported13.
The data from a previously published study protocol13. HbA1c, hemoglobin A1C; MTD, maximum tolerated dose; TZP, tirzepatide.
Changes in cardiometabolic risk measures
The SURMOUNT-OSA statistical analysis plan prespecified assessment of changes in SBP and DBP, hsCRP, HDL-C, non-HDL-C, LDL-C, VLDL-C and fasting insulin. The changes in HOMA-IR were analyzed post hoc.
Changes in SBP and DBP were assessed at week 48 to prevent confounding effect of PAP discontinuation at week 52 in study 2, all other changes were assessed at week 52.
There was a significant estimated treatment difference (ETD) between tirzepatide and placebo for change in SBP from baseline at week 48 both in study 1 (−7.9 mmHg; 95% confidence interval (CI) −11.0 to −4.9; P < 0.001) and in study 2 (−4.3 mmHg; 95% CI −7.3 to −1.2; P = 0.007). The ETD between tirzepatide and placebo of change in DBP from baseline at week 48 was significant in study 1 (−3.2 mmHg; 95% CI −5.4 to −1.0; P = 0.005) but not in study 2 (Table 2).
The ETD between tirzepatide and placebo for percent change in hsCRP from baseline at week 52 was significant both in study 1 (−28.9% ± 8.2%; P = 0.003) and in study 2 (−45.1% ± 8.0%; P <0.001) (Table 2).
The ETD between tirzepatide and placebo for percent change in HDL-C from baseline at week 52 was significant both in study 1 (7.2% ± 2.1%; P < 0.001) and in study 2 (10.0% ± 2.8%; P < 0.001). The ETD between tirzepatide and placebo for percent change in non-HDL-C from baseline at week 52 was significant both in study 1 (−13.0% ± 3.1%; P < 0.001) and in study 2 (−14.3% ± 2.5%; P < 0.001). The ETD between tirzepatide and placebo for percent change in triglycerides from baseline at week 52 was significant both in study 1 (−32.2% ± 3.8%; P < 0.001) and in study 2 (−31.5% ± 3.7%; P < 0.001) (Table 2).
The ETD between tirzepatide and placebo for percent change in LDL-C from baseline at week 52 was not significant in study 1 but was significant in study 2 (−10.3% ± 3.0%; P = 0.001). The ETD between tirzepatide and placebo for percent change in VLDL-C from baseline at week 52 was significant both in study 1 (−32.6% ± 3.7%; P < 0.001) and in study 2 (−31.4% ± 3.7%; P < 0.001) (Table 2).
The ETD between tirzepatide and placebo for percent change in fasting insulin from baseline at week 52 was significant both in study 1 (−41.4% ± 5.0%; P < 0.001) and in study 2 (−45.4% ± 5.4%; P < 0.001). The ETD between tirzepatide and placebo for percent change in HOMA-IR from baseline at week 52 was significant both in study 1 (−48.0% ± 4.9%; P < 0.001) and in study 2 (−54.5% ± 5.0%; P < 0.001) (Table 2).
Mediation analysis
Mediation analysis was performed to estimate the proportion of tirzepatide treatment-associated changes in cardiometabolic risk measures due to either an effect of weight reduction or an effect of changes in sleep-disordered breathing measured by AHI and SASHB.
In a pooled study 1 and study 2 population, the tirzepatide effect on SBP was significantly mediated by combined effect of changes in body weight, AHI and SASHB, and by change in body weight alone. However, the proportion mediated by changes in OSA metrics, AHI and SASHB alone, was not statistically significant (Fig. 2).
The data are presented as model-based estimate for the mean (95% CI). Statistical analyses were performed using the bootstrap method. All hypothesis testing was conducted using a two-sided framework, and the results are summarized with 95% CIs, which were derived from the corresponding 2.5th and 97.5th percentiles of the bootstrapped estimates (HOMA-IR n = 336; non-HDL-C n = 341; HDL-C n = 342; triglycerides n = 341; hsCRP n = 342; DBP n = 364; SBP n = 364). *P < 0.05, **P < 0.01, ***P < 0.001 representing significant proportion mediated.
The proportions of tirzepatide-associated change in DBP mediated by combined effect of changes in body weight, AHI and SASHB or effects of any of the mediators alone were not statistically significant (Fig. 2).
The tirzepatide-associated change in hsCRP was significantly mediated by combined effect of changes in body weight, AHI and SASHB, by change in body weight alone and by changes in OSA metrics AHI and SASHB (Fig. 2).
The tirzepatide-associated change in triglycerides was significantly mediated by combined effect of changes in body weight, AHI and SASHB, by change in body weight alone and by changes in OSA metrics AHI and SASHB (Fig. 2).
The tirzepatide-associated change in HDL-C was significantly mediated by combined effect of changes in body weight, AHI and SASHB and by change in body weight alone. However, the proportion mediated by change in OSA metrics AHI and SASHB was not statistically significant (Fig. 2).
The tirzepatide-associated change in non-HDL-C was significantly mediated by combined effect of changes in body weight, AHI and SASHB. However, the proportions mediated by change in body weight alone, or mediated by change in OSA metrics, AHI and SASHB, were not statistically significant (Fig. 2).
The tirzepatide-associated change in HOMA-IR was significantly mediated by combined effect of changes in body weight, AHI and SASHB, by change in body weight alone and by change in OSA metrics AHI and SASHB (Fig. 2).
Discussion
In this study, we observe marked improvements in cardiometabolic risk measures with tirzepatide therapy compared with placebo. These included previously reported improvements in SBP, DBP and hsCRP13, as well as improvements in the lipid profile, fasting insulin and HOMA-IR. The improvements in cardiometabolic risk measures were both statistically significant and clinically meaningful and, therefore, tirzepatide treatment may lead to improvement in overall cardiometabolic risk in patients with OSA and obesity. The mediation analysis determined that changes in AHI and SASHB significantly mediated reductions in hsCRP, HOMA-IR and triglycerides, but notably, there was no positive mediation of AHI and SASHB observed on SBP and DBP.
The optimal treatment for patients with OSA and obesity has been unclear. Chirinos et al. reported a comparison of the impact of PAP therapy versus lifestyle weight-loss intervention versus the combination of both treatment approaches7. The results, in aggregate, support that treating both OSA as well as obesity may be the optimal treatment, with the awareness and acknowledgment that weight reduction in and of itself mitigates OSA. Of note, randomized trials so far have not demonstrated significant effect of PAP therapy on major cardiovascular outcomes6,15,16,17,18,19. The reasons for these findings are unclear, but several potential explanations have been proposed. First, adherence to PAP therapy can be quite variable with major improvements expected only in those with excellent adherence to PAP therapy. Indeed, a recent meta-analysis reported improved cardiometabolic outcomes in patients with OSA who had optimal PAP adherence20. Second, the stratification of patients with OSA for participation in randomized controlled trials may be needed to account for cardiometabolic risk. Cluster analyses have suggested that only a subset of patients with OSA may be at major cardiometabolic risk, and thus, randomized controlled trials may need to identify high risk patients a priori rather than the typical one-size-fits-all approach21,22. Ongoing efforts using endotyping techniques may help to identify patients most likely to benefit from various interventions in the design of future randomized trials23. Third, the lack of observed improvement in outcomes in randomized trials of PAP therapy for OSA in the light of success of bariatric surgery in improving cardiovascular outcomes24 may also reflect the failure of some trials to address underlying causes of OSA, such as obesity. In fact, some randomized studies have shown small but significant increases in body weight with PAP therapy, which emphasizes the need to address both OSA and obesity rather than either condition alone25. The results from our analyses of SURMOUNT-OSA provide a compelling rationale that improvements in OSA metrics appear to be associated with benefits in addition to those observed by weight reduction. We observed statistically significant independently mediated reductions by OSA metrics in hsCRP, HOMA-IR and triglycerides. In addition, the combination of weight with OSA metrics, but not weight or OSA improvements alone, significantly mediated reductions in non-HDL cholesterol. Of note, the mediation effect of weight on SBP appears to be larger than that for weight and OSA combined, and AHI/SASHB did not show any mediation effect alone. None of our variables were significant mediators of the improvement seen in DBP, suggesting that there may be other unrecognized variables contributing to cardiometabolic benefit with tirzepatide therapy for comorbid obesity and OSA. The implication for clinical practice is that treatment of OSA in patients with OSA and obesity should always include obesity treatment, and medications such as tirzepatide may represent a therapeutic option for such patients. Therapy of OSA in patients with obesity may be initiated by PAP and tirzepatide, with evaluation of PAP therapy continuation being done based on the treatment effect of tirzepatide therapy26.
Notably, the finding that the observed improvement in SBP with tirzepatide therapy was significantly mediated only via obesity or the combined effect of body mass index/AHI/SASHB changes but not by independent improvement in OSA was surprising, given the extensive literature on the impact of OSA on SBP. Considerable data from large epidemiological studies and human interventional studies have shown a modest but significant impact of OSA on SBP27,28,29,30. One potential explanation may be that the point estimate for weight may have less variance than the estimate for AHI, given the night-to-night variability in sleep apnea31,32. Indeed, a recent study suggested that multinight recordings of sleep apnea were required to capture a large percentage of the variance in blood pressure33. Though study 1 and study 2 were not designed for direct comparison between PAP and non-PAP participants, the results indicate that for participants in study 2, who were on PAP, the improvement in OSA with tirzepatide did not yield the same benefits on both SBP and DBP as for participants who were not on PAP, as PAP may have already improved SBP at baseline. Thus, the findings should not weaken the perception of an important role of OSA treatment in blood pressure control confirmed by previous studies34,35.
The mediation analysis compared the effect of tirzepatide on cardiometabolic risk factors through its impact on weight and on OSA severity without reflecting on a possible direct effect of tirzepatide on these factors. The metabolic effect of GLP-1 and GLP-1/GIP receptor agonists appears to integrate their direct effect on pancreatic islet function with long-term weight reduction that increases insulin sensitivity and reduces beta-cell workload in persons with prediabetes and obesity36. Animal models have shown that GLP-1 receptor agonists may ameliorate excessive sympathetic activity in the hypertensive-diabetic condition37. In addition, GIP receptor agonism, specific for tirzepatide, has been shown to have a direct effect on insulin sensitivity and lipid metabolism38. Future research is needed to explore direct effects of tirzepatide on cardiometabolic risk in the realms of OSA as well as mechanisms of its effect on AHI, SASHB and other measures attributed to OSA.
The studies and analysis have a number of strengths. The studies bring results of prespecified endpoints from prospective, controlled, randomized trials. The existing evidence of the relationship between OSA and CVD is largely based on retrospective analyses and, therefore, prospective randomized trial data are necessary to prove some of the concepts created. The mediation analysis of the pooled data from these prospective studies investigates how weight reduction and OSA alleviation improved cardiometabolic risk measures independently, controlling for confounders. This analysis has also provided the size of these effects, and the findings may represent important information for clinical decision making on therapy of patients with OSA and obesity. Despite the strengths of the studies, we acknowledge a number of limitations. First, the studies did not have sufficient duration of follow-up or statistical power to examine the impact of tirzepatide on cardiometabolic outcomes such as myocardial infarction or stroke. Larger studies with longer follow-up may be required to prove that tirzepatide has benefits on these outcomes of interest in patients with OSA and obesity. Second, the SURMOUNT-OSA study excluded people with mild OSA, as well as those with diabetes, and with body mass index <30 kg m−2. Emerging data suggest that treatment with tirzepatide or GLP-1 receptor agonists results in less weight reduction in patients with type 2 diabetes than in those without diabetes12,39. More data are required to determine which patients with OSA are most likely to benefit from tirzepatide.
In SURMOUNT-OSA, tirzepatide mitigated cardiometabolic risk for patients with moderate-to-severe OSA and obesity. Presented results provide support for the concept that treating both OSA and obesity in patients with a combination of both diseases is required to optimize cardiometabolic benefits. Further work is required to determine which patients with OSA benefit the most from different therapeutic approaches.
Methods
Study design, procedures and participants
The SURMOUNT-OSA program included two 52-week, randomized, placebo-controlled phase 3 studies. Study 1 included participants who were unwilling or unable to use PAP therapy, and study 2 included participants who had been using PAP therapy for at least 3 months at screening, and who planned to continue PAP for the duration of the trial. Participants were randomly assigned in a 1:1 ratio to receive either tirzepatide at the maximum tolerated dose (10 or 15 mg) or placebo once weekly. The SURMOUNT-OSA program was registered at ClinicalTrials.gov (NCT05412004), and the two studies’ full design, key eligibility criteria, procedures and primary efficacy and safety results have been published previously13,14. Participants were screened and enrolled irrespective of their sex. Sex was self-reported by participants.
Objectives
First, this analysis investigated the association of tirzepatide with changes in cardiometabolic risk markers in participants with moderate-to-severe OSA and obesity. Second, the analysis estimated the relative contribution of weight loss, AHI and SASHB reduction to the improvement in cardiometabolic characteristics of subjects treated with tirzepatide.
Parameters measured included change from baseline in SBP, DBP and levels of hsCRP, HDL-C, non-HDL-C, triglycerides, LDL-C, VLDL-C, fasting insulin and HOMA-IR, in tirzepatide versus placebo.
Mediation analysis was performed to estimate the proportion of tirzepatide treatment-associated changes in cardiometabolic risk measures due to either an effect of weight reduction or an effect of changes in sleep-disordered breathing measured by AHI and SASHB. SASHB is a cumulative measure of oxygen desaturation associated with apneas and hypopneas which, in observational studies, predicted OSA-related cardiovascular mortality and morbidity better than AHI40,41.
For each cardiometabolic risk measure, three analyses were performed: estimation of the proportion of efficacy mediated by change from baseline in body weight, change from baseline in AHI and SASHB and, lastly, change from baseline in all three mediators combined.
Improvements in these mediators with tirzepatide treatment compared with placebo from SURMOUNT-OSA studies have been previously published and are as follows:
ETD between tirzepatide and placebo in study 1 was −16.1% (95% CI −18.0 to −14.2) for body weight, −20.0 events per hour (95% CI −25.8 to −14.2) for AHI and −70.1%min h−1 (95% CI −90.9 to −49.3) for SASHB13. ETD between tirzepatide and placebo in study 2 was −17.3% (95% CI −19.3 to −15.3) for body weight, −23.8 events per hour (95% CI −29.6 to −17.9) for AHI and −61.3%min h−1 (95% CI −84.7 to −37.9) for SASHB13.
Statistical analysis
Changes in SBP, DBP and hsCRP from SURMOUNT-OSA have already been reported using treatment-regimen estimand analysis13. The analyses reported here were guided by the ‘efficacy’ estimand; the analysis included data collected before permanent discontinuation of study intervention and was conducted using the efficacy analysis set. For efficacy analysis, missing values were imputed using multiple imputation based on the reason of intercurrent events. All results are reported using the efficacy estimand unless otherwise specified.
Unless otherwise noted, all tests of treatment effects were conducted at a two-sided alpha level of 0.05, and the CIs were calculated at 95%. In statistical summaries and analyses, participants were analyzed as randomized.
The mixed model repeated measures analysis, a restricted-maximum-likelihood-based model, was used to analyze continuous longitudinal variables. All the longitudinal observations at each scheduled postbaseline visit were included in the analysis. The model includes the fixed class effects of treatment, strata (pooled country/geographic region, baseline OSA severity and gender), visit and treatment-by-visit interaction, as well as the continuous, fixed covariate of baseline value. The significance tests were based on least-squares means and type III tests. As all of these endpoints were exploratory in nature, the P values were not adjusted for multiplicity. Statistical analyses were performed using SAS version 9.3.
Mediation analyses were conducted to decompose the total effect of tirzepatide on cardiometabolic risk markers into direct effect and indirect effect. The direct effect quantified the effect of tirzepatide versus placebo on change in cardiometabolic outcomes, independent of changes in mediators. Conversely, the indirect effect captured the effect of tirzepatide versus placebo on change in cardiometabolic outcomes associated with changes in mediators. Because these comparisons involve non-observable counterfactual scenarios, natural effect models were employed to estimate the direct and indirect effects42. The total effect was defined as the sum of these two components. The proportion mediated was calculated as the indirect effect divided by the total effect then multiplying by 100%, and the standard errors were derived using the bootstrap method. The mediation analyses were performed on the pooled study 1 and study 2 populations. The outcome was the change in cardiometabolic risk measure from baseline to the end of the study. Analyses on SBP and DBP were performed on the original scale, and analyses on hsCRP, HDL-C, non-HDL-C, triglycerides and HOMA-IR were performed on the log scale. Baseline covariates included region, sex, baseline cardiometabolic risk measure, AHI, body weight and log-transformed SASHB. We investigated the effects of mediators for three scenarios: first, a single mediator of change from baseline in body weight; second, two independent mediators of change from baseline in AHI and change from baseline in SASHB to represent changes attributable to OSA severity improvement; and lastly, a combination of the effect of changes in body weight, AHI and SASHB as three parallel mediators. The post-treatment confounders were controlled for and included change in AHI and SASHB when the mediator was change from baseline in body weight and change in body weight when the mediator was change from baseline in AHI and SASHB. No post-treatment confounders were controlled for when all of the change from baseline in body weight, AHI and SASHB were mediators. Log transformation was always applied for SASHB. The mediation analyses were performed using the ‘CMAverse’ package version 0.1.0 in R version 4.1.2 (ref. 42).
Pearson’s correlation coefficients were also calculated between the cardiometabolic risk measures and the sleep-disordered breathing parameters on the pooled study 1 and study 2 populations. Analyses on SBP and DBP were performed on the original scale, and analyses on hsCRP, HDL-C, non-HDL-C, triglycerides and HOMA-IR were performed on the log scale. These results are presented in Extended Data Table 1.
Ethics and informed consent
The study was approved by ethics review boards at each site, presented in Supplementary Table 1.
This study was conducted in accordance with consensus ethical principles derived from international guidelines including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethics Guidelines, applicable ICH GCP Guidelines, International Organization for Standardization (ISO) 14155 and applicable laws and regulations.
Participants or their legally authorized representatives signed a statement of informed consent that meets the requirements of 21 Code of Federal Regulations 50, local regulations, ICH guidelines, privacy and data protection requirements, where applicable, and the IRB/IEC or study center.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this Article.
Data availability
Data from the analyses in this study cannot be shared publicly due to the sponsor’s (Eli Lilly and Company) contractual obligations. Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org. Contact the corresponding author for details on submitting a request. Source data are provided with this paper.
Code availability
No novel code was used for data analysis in the current study.
References
Jordan, A. S., McSharry, D. G. & Malhotra, A. Adult obstructive sleep apnoea. Lancet 383, 736–747 (2014).
Hudgel, D. W. et al. The role of weight management in the treatment of adult obstructive sleep apnea. An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 198, e70–e87 (2018).
Morgenthaler, T. I. et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep 29, 1031–1035 (2006).
Jenkinson, C., Davies, R. J., Mullins, R. & Stradling, J. R. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 353, 2100–2105 (1999).
Weaver, T. E. et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am. J. Respir. Crit. Care Med. 186, 677–683 (2012).
McEvoy, R. D. et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N. Engl. J. Med. 375, 919–931 (2016).
Chirinos, J. A. et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 370, 2265–2275 (2014).
McTigue K. & Kuller L. Cardiovascular risk factors, mortality, and overweight. JAMA 299, 1260–1261 (2008).
Kuna, S. T. et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD Study. Am. J. Respir. Crit. Care Med. 203, 221–229 (2021).
Malhotra, A. et al. Weight reduction and the impact on apnea-hypopnea index: a systematic meta-analysis. Sleep. Med. 121, 26–31 (2024).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Garvey, W. T. et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 402, 613–626 (2023).
Malhotra, A. et al. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N. Engl. J. Med. 391, 1193–1205 (2024).
Malhotra, A. et al. Tirzepatide for the treatment of obstructive sleep apnea: rationale, design, and sample baseline characteristics of the SURMOUNT-OSA phase 3 trial. Contemp. Clin. Trials 141, 107516 (2024).
Barbé, F. et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 307, 2161–2168 (2012).
Sánchez-de-la-Torre, M. et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: blood pressure response to continuous positive airway pressure treatment. J. Am. Coll. Cardiol. 66, 1023–1032 (2015).
Sánchez-de-la-Torre, M. et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir. Med. 8, 359–367 (2020).
Peker, Y. et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 194, 613–620 (2016).
Peker, Y., Hedner, J., Norum, J., Kraiczi, H. & Carlson, J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am. J. Respir. Crit. Care Med. 166, 159–165 (2002).
Sánchez-de-la-Torre, M. et al. Adherence to CPAP treatment and the risk of recurrent cardiovascular events: a meta-analysis. JAMA 330, 1255–1265 (2023).
Ye, L. et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur. Respir. J. 44, 1600–1607 (2014).
Mazzotti, D. R. et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am. J. Respir. Crit. Care Med. 200, 493–506 (2019).
Malhotra, A., Mesarwi, O., Pepin, J. L. & Owens, R. L. Endotypes and phenotypes in obstructive sleep apnea. Curr. Opin. Pulm. Med. 26, 609–614 (2020).
Aminian, A. et al. Adverse cardiovascular outcomes in patients with obstructive sleep apnea and obesity: metabolic surgery vs usual care. J. Am. Coll. Cardiol. 84, 1047–1060 (2024).
Drager, L. F. et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax 70, 258–264 (2015).
Pack, A. et al. What will the impact be of use of tirzepatide in patients with obstructive sleep apnea (OSA)?. Sleep 48, zsaf045 (2025).
Pepperell, J. C. et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 359, 204–210 (2002).
Montesi, S. B., Edwards, B. A., Malhotra, A. & Bakker, J. P. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Sleep. Med. 8, 587–596 (2012).
Logan, A. G. et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur. Respir. J. 21, 241–247 (2003).
Bakker, J. P. et al. Blood pressure improvement with continuous positive airway pressure is independent of obstructive sleep apnea severity. J. Clin. Sleep. Med. 10, 365–369 (2014).
Lechat, B. et al. High night-to-night variability in sleep apnea severity is associated with uncontrolled hypertension. npj Digit. Med. 6, 57 (2023).
Lechat, B. et al. Single-night diagnosis of sleep apnea contributes to inconsistent cardiovascular outcome findings. Chest 164, 231–240 (2023).
Punjabi, N. M., Patil, S., Crainiceanu, C. & Aurora, R. N. Variability and misclassification of sleep apnea severity based on multi-night testing. Chest 158, 365–373 (2020).
Bratton, D. J., Stradling, J. R., Barbé, F. & Kohler, M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax 69, 1128–1135 (2014).
Asgari, A., Soltaninejad, F., Farajzadegan, Z. & Amra, B. Effect of CPAP therapy on serum lipids and blood pressure in patients with obstructive sleep apnea syndrome. Tanaffos 18, 126–132 (2019).
Jastreboff, A. M. et al. Tirzepatide for obesity treatment and diabetes prevention. N. Engl. J. Med. 392, 958–971 (2024).
Pauza, A. G. et al. GLP1R attenuates sympathetic response to high glucose via carotid body inhibition. Circ. Res. 130, 694–707 (2022).
Samms, R. J., Coghlan, M. P. & Sloop, K. W. How may GIP enhance the therapeutic efficacy of GLP-1?. Trends Endocrinol. Metab. 31, 410–421 (2020).
Davies, M. et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 397, 971–984 (2021).
Azarbarzin, A. et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 40, 1149–1157 (2019).
Azarbarzin, A., Sands, S. A., Taranto-Montemurro, L., Redline, S. & Wellman, A. Hypoxic burden captures sleep apnoea-specific nocturnal hypoxaemia. Eur. Heart J. 40, 2989–2990 (2019).
Shi, B., Choirat, C., Coull, B. A., VanderWeele, T. J. & Valeri, L. CMAverse: a suite of functions for reproducible causal mediation analyses. Epidemiology 32, e20–e22 (2021).
Acknowledgements
We thank the participants, the study teams and the investigators. We thank S. Curley, PhD (Eli Lilly and Company) for editorial assistance. This study was funded by Eli Lilly and Company. The study sponsor had a role in the study design, data collection, data analysis, interpretation of data, writing of the report and the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
A.M., A.A., V.K.S., J.P.D. and M.C.B. contributed to the conception of the study. A.M., R.G., A.A., S.C. and M.C.B. contributed to the design of the study. R.G. was a trial investigator. A.M., R.G., A.A. and J.B. contributed to data acquisition. A.M., A.A., S.S., V.K.S., J.L., S.C. and J.B. contributed to data analysis. S.C. and J.L. were responsible for the statistical analysis. A.M. wrote the first draft of the paper. All authors participated in interpretation of data and critical review of the paper and gave approval for the final version to be published.
Corresponding authors
Ethics declarations
Competing interests
A.M. is funded by the NIH. A.M. reports income from Eli Lilly, Livanova, Zoll and Powell Mansfield. ResMed gave a philanthropic donation to USCD. R.G. reports income from serving on the advisory board for Apnimed, the steering committee for SURMOUNT-OSA, Eli Lilly and lecture fees from Somnomed Department. He conducts sponsored studies with Eli Lilly, Alkermes, Takeda and Bod Science: Lambert Initiative. A.A. serves as a consultant for Respicardia, Eli Lilly, Inspire, Cerebra and Apnimed. Apnimed is developing pharmacological treatments for Obstructive Sleep Apnea. A.A.’s interests were reviewed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their institutional policies. S.S. received grant support from Apnimed, Prosomnus and Dynaflex and has served as a consultant for Apnimed, Nox Medical, Inspire Medical Systems, Eli Lilly, Respicardia, LinguaFlex and Achaemenid. S.S. receives royalties for intellectual property pertaining to combination pharmacotherapy for sleep apnea via his Institution. S.S. is also the co-inventor of intellectual property pertaining to wearable sleep apnea phenotyping also via his Institution. He has received equity in Achaemenid, a company commercializing biosensor technology for monitoring oral appliance treatment efficacy. S.S. is also co-inventor of intellectual property pertaining to wearable sleep apnea phenotyping also via his Institution. His industry interactions are actively managed by his Institution. V.K.S. serves as a consultant for Eli Lilly, ApniMed, Axsome and Jazz Pharmaceuticals and on the Scientific Advisory Board for Sleep Number. L.J.A. reports receiving consulting fees from/and serving on advisory boards for Altimmune, Atria, Boehringer Ingelheim, Carmot Therapeutics, CinFina Pharma, Corteria, Currax Pharma, Eli Lilly, Enterin, Helicore Biopharma, Jamieson Wellness, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Juvena Therapeutics, Kallyope, Morphic Medic/GI Dynamics, Novartis, Novo Nordisk, Pfizer, Prosciento, Senda Biosciences, Skye Bio/Cbeyond, Summit Clinical, Syntis Bio, Versanis, Veru Pharmaceuticals and Zealand Pharmaceuticals; receiving research funding from Amgen, Eli Lilly, Janssen Pharmaceuticals and Novo Nordisk; having equity interests in ERX Pharmaceuticals, Intellihealth, Jamieson Wellness, Kallyope, Mediflix, Morphic Medic/GI Dynamics, Summit Clinical, Syntis Bio and Veru Pharmaceuticals; and serving on a board of directors for ERX Pharmaceuticals, Intellihealth and Jamieson Wellness. A.M.J. conducts multicenter trials with Amgen, Eli Lilly, Novo Nordisk and Rhythm Pharmaceuticals; serves on scientific advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim, Biohaven, Eli Lilly, Intellihealth, Novo Nordisk, Pfizer, Regeneron, Rhythm Pharmaceuticals, Scholar Rock, Structure Therapeutics, Syntis Bio, Terns Pharmaceuticals, WeightWatchers and Zealand Pharmaceuticals; and receives institutional grant funding from the NIH/NIDDK. J.L., S.C., J.P.D. and M.C.B. are employees and shareholders of Eli Lilly and Company and declare no competing interests. J.B. contributed to the paper as an employee of Eli Lilly and Company.
Peer review
Peer review information
Nature Medicine thanks Anne-Laure Borel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Ashley Castellanos-Jankiewicz and Sonia Muliyil, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Supplementary Information
Supplementary Table 1.
Source data
Source Data Fig. 1
Mediation analyses.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Malhotra, A., Grunstein, R., Azarbarzin, A. et al. Tirzepatide on obstructive sleep apnea-related cardiometabolic risk: secondary outcomes of the SURMOUNT-OSA randomized trial. Nat Med (2026). https://doi.org/10.1038/s41591-025-04071-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41591-025-04071-1