Abstract
The progressive loss of cerebral white matter during aging contributes to cognitive decline, but whether reduced blood flow is a cause or a consequence remains debatable. Using deep multi-photon imaging in mice, we examined microvascular networks perfusing myelinated tissues in cortical layer 6 and the corpus callosum. We identified sparse, wide-reaching venules, termed principal cortical venules, which exclusively drain deep tissues and resemble the vasculature at the human cortex and U-fiber interface. Aging led to selective constriction and rarefaction of capillaries in deep branches of principal cortical venules. This resulted in mild hypoperfusion that was associated with microgliosis, astrogliosis and demyelination in deep tissues, but not the upper cortex. Induction of comparable hypoperfusion in adult mice using carotid artery stenosis triggered a similar tissue pathology specific to layer 6 and the corpus callosum. Thus, impaired capillary–venous drainage is a contributor to hypoperfusion and a potential therapeutic target for preserving blood flow to white matter during aging.
Similar content being viewed by others
Main
The gradual loss of cerebral white matter during aging contributes to cognitive decline1. This degeneration involves axon degeneration, demyelination, microgliosis and astrogliosis, which impair neurotransmission and produce an inflammatory environment detrimental to white matter maintenance2,3. Oligodendrocytes are uniquely vulnerable to ischemia and myelin repair is energy intensive4,5. As a result, age-related deficits in cerebral blood flow6,7 and vascular rarefaction8,9,10 are often implicated in white matter deterioration. However, a direct causal link between cerebral hypoperfusion and age-related white matter loss has not been definitively established11. An alternative possibility is that vascular changes arise secondary to white matter loss due to mechanisms unrelated to blood flow. Understanding this relationship is crucial for designing effective therapeutic strategies to preserve white matter integrity. Key questions remain about the microvascular changes that drive age-related hypoperfusion and whether its severity is sufficient to cause white matter damage. Addressing these gaps in humans is challenging, because clinical studies are typically correlative and noninvasive imaging cannot resolve microvascular changes. This underscores the need for preclinical studies that can visualize white matter microvasculature in vivo and manipulate blood flow to establish causality.
In rodents, the corpus callosum (CC) is the largest white matter tract and a primary focus for white matter research12,13. It consists of neuronal projections that serve local crosstalk between neighboring cortical regions and long-range projections for cortical–subcortical communication. The anatomy and vascular architecture of the rodent CC more closely resemble those of the human superficial white matter (or U-fibers) that lies adjacent to the cortical gray matter, as opposed to the human CC. Superficial white matter facilitates both short-range communication between neighboring gyri and longer-range communication with deeper subcortical white matter, which are essential for complex cognitive processes14. Similar to deep subcortical white matter, superficial white matter undergoes age- and disease-related degeneration and these changes are increasingly recognized as an early biomarker for cognitive decline15. Furthermore, vascular contributions to loss of superficial white matter have been implicated in chronic conditions, such as Alzheimer’s disease16, cerebral small vessel disease17 and multiple sclerosis18.
To better understand the microvascular architecture of the human cerebral cortex and superficial white matter, Duvernoy and colleagues used vascular casts in postmortem tissues to reveal large, horizontally projecting branches of principal cortical venules (PCVs) within deep cortical layers and the U-fiber tracts19. Their findings suggested that the quality of blood drainage through PCVs substantially influences blood flow in these regions. However, their structure, function and perfusion territories remain largely unexamined. To address this knowledge gap, we leveraged recent advances in deep multi-photon imaging to extend in vivo imaging to the deep cortex and CC of adult and aged mice20,21. This allowed us to ask whether murine white matter is drained by an equivalent of the human PCV. These in vivo studies of vascular physiology were complemented by light-sheet imaging and in silico modeling to provide a comprehensive view of how age-related vascular changes affect cerebral perfusion. Our studies revealed specific microvascular alterations in PCV branching networks that contribute to age-related hypoperfusion, offering potential vascular targets to improve cerebral blood flow and mitigate age-related white matter decline.
Results
The murine cortex contains an equivalent to human PCVs
In the human brain, PCVs exhibit a conical structure, with a large trunk spanning all cortical layers and deeper branches, extending over long distances at the interface between the cortical gray matter and underlying U-fiber white matter19 (Fig. 1a,b). Individual PCVs are surrounded by rings of penetrating arterioles, suggesting that each serves as the sole output for multiple sources of blood input to relatively large tissue regions (Fig. 1c). This implicates PCVs as bottlenecks in perfusion of the deep cortex and superficial white matter in humans.
a, PCV visualized in India ink-filled human brain sections from ref. 19. b,c, Schematics from ref. 19 show the PCV structure from the side (b) and the top (c). d, Schematic summary of in vivo multi-photon imaging set-up. The image on the right shows pial vessels in the chronic cranial window with one PCV trunk marked by a cyan circle. e, The side view of the microvasculature in adult mouse somatosensory cortex showing a 900 × 900 × 1,000 µm3 (x, y, z) volume collected by deep two-photon imaging in d (yellow dotted square). f, Imaris reconstruction of the PCV in e, together with branching networks (cyan). All other ascending venules (blue) are also depicted. The dotted lines mark the depth below the pial surface. g,h, The same PCV alongside all penetrating arterioles (red), viewed from the side (g) and the top (h). i, Composite of all segmented vessel types. j, Schematic with mouse calvarial landmarks. The red circle marks the location of the implanted cranial windows and cortical sensory regions accessible within the window are shown on the right. k, PVC trunk locations demarcated by colored circles in an example cranial window. l, The positions of PCV trunk locations collectively overlaid for n = 23 mice. All the PVCs are verified to have reached the CC using in vivo imaging. Cyan-colored circles mark the locations of PCVs analyzed in this study. C, caudal; L, lateral; M, medial; R, rostral. Credit: panel a reproduced with permission from ref. 19, Elsevier.
To examine whether there is a structural correlate to the human PCV in the murine brain, we performed deep in vivo two-photon imaging20. This approach used a far-red intravenous dye, Alexa Fluor-680-dextran, and long-wavelength excitation to achieve deeper tissue penetration than conventional two-photon microscopy (Fig. 1d,e). Imaging was performed on Thy1-YFP mice with neuronal labeling to provide reference to cortical layers. Within large cranial windows, we collected high-resolution datasets, averaging 900 µm (x) × 900 µm (y) × 1,000 µm (z) in size, which centered around PCVs in the forelimb, hindlimb, trunk region (FL/HL/Tr) of the primary sensory cortex (S1) (Fig. 1e and Supplementary Video 1). Segmentation of penetrating vessels within these volumes confirmed a vascular structure similar to that in human PCVs (Fig. 1f, cyan vessel, and Supplementary Video 2). Murine PCVs were also conically shaped and extended progressively longer branches with increasing tissue depth. The deeper branches were connected to elaborate capillary networks spanning the gray–white matter interface.
Although only one PCV was collected in each imaging volume, the same volume contained 23 ± 3 (mean ± s.d.) other ascending venules (Fig. 1f, dark blue vessels; n = 9 mice). However, these other venules terminated at or before cortical layer 5. As such, PCVs represented <4% of all ascending cortical venules and were the only venules that reached the deep cortex and CC. The same tissue volume also contained 15 ± 2 penetrating arterioles, oriented in a ring around the trunk of the PCV, again similar to the human cortex (Fig. 1g–i). Approximately 50% of these penetrating arterioles had branches that reached deep cortical layers, ramifying on reaching the CC. Thus, deeper tissues are served by multiple arteriolar inputs, but drained by rare PCV outputs in mice and humans.
PCVs are sparse but vital for drainage of deeper tissues
To gain a broader view of PCV drainage territories, we surveyed their positions within the cranial imaging window (Fig. 1j–l). The main trunk of murine PCVs ascended to the brain surface either at the end of large-diameter pial venules (Fig. 1k, red and green circles) or at locations hidden beneath large pial venules as they coursed along the surface (Fig. 1k, cyan circle). Individual PCVs tended to be centered within major representations of primary somatosensory cortex, FL/HL/Tr and barrel field (BF) and the visual cortex (V1) (Fig. 1l), with one PCV per region, suggesting that they may be strategically placed to serve major hubs in cortical and white matter function.
To better understand the proportion of penetrating vessel types, we examined light-sheet imaging data collected from optically cleared postmortem mouse brains with all vasculature labeled by fluorescent lectin and arterioles with α-smooth muscle actin (α-SMA) and SM22 (Extended Data Fig. 1a,b). The number and penetration depth of all penetrating arterioles, PCVs and other ascending venules were examined in 2 mm (x) × 2 mm (y) × 1.2 mm (z) regions of interest (ROIs) in the primary somatosensory cortex, which were about 6× larger in volume than the subregions examined in vivo (Extended Data Fig. 1c). This volume contained, on average, 55 ± 10 (mean ± s.d.) penetrating arterioles (n = 4 mice), with a homogeneous distribution of termination points across the cortex (Extended Data Fig. 1d,e). There were 105 ± 12 non-PCV ascending venules that terminated with the highest density in the upper cortical layers, but with some reaching ~900 µm in depth. However, we found only 3.2 ± 0.5 vessels with PCV structure, corresponding to ~3% of total ascending venules, consistent with in vivo data and confirming their sparsity relative to other vessel types.
PCV trunk diameters were on average 57.5 ± 15 µm (mean ± s.d.) in diameter (n = 23), whereas other ascending venules averaged 19.8 ± 7.9 µm in diameter (n = 90)22. This suggests that each PCV could support at least 8× more blood flow than other individual ascending venules, facilitating drainage in broad regions of the deep cortex and CC.
Defining the structure and nomenclature of mouse PCVs
To understand age-related changes in PCV structure, we imaged adult (age 5–7 months) and aged (age 22–24 months) Thy1-YFP mice using deep in vivo two-photon imaging. With both age groups, the PCV structure could be consistently visualized up to ~1,000 µm of depth (Fig. 2a–d). We developed a system to reliably identify the same vascular zones between animals and in different cortical layers (Fig. 2e). The main ascending vessel was denoted the ‘trunk’ of the vessel and larger vessels directly connected to the trunk were termed ‘branches’. Branches were numerous and of different diameters, with the largest diameter branches extending from the PVC trunk at greater cortical depths (Supplementary Fig. 1). This core branching structure of PCVs did not differ across age groups.
a–d, Maximum projection images of deep two-photon imaging stacks showing PCVs in a 6-month-old adult mouse (a,b) and a 22-month-old aged mouse (c,d). e,f, Schematic of vessel types and their nomenclature in PCV networks. e, Structure of PCV drainage network. f, Anatomy of PCV branch networks. g, Side projection of deep in vivo two-photon imaging stack showing a PCV in an adult mouse, overlaid with Imaris reconstructions of PCV branch vascular networks in layers 2/3, 4 and 6/CC. h–j, Top-down views of Imaris reconstructions of PCV branch vascular networks in layers 2/3 (h), 4 (i) and 6/CC (j). The red circles correspond to the point where the main branch joins the trunk of the PCV. The gray-colored vessels correspond to pre-convergence capillaries, violet-colored vessels represent other tributary vessels and cyan-colored segments correspond to the main branch segments. k,l, Three-photon imaging to define the gray–white matter boundary. Side projections of image stacks showing the 3HG signal fluorescence from myelin (k) and the vasculature labeled with the FITC-dextran intravenous dye (l) in an awake adult mouse (aged 6 months). The transition from gray to white matter is visible in the 3HG signal image (white dotted line). m, Maximum projection image showing a top-down view of the layer 6/CC branch (750–1,000 µm depth) from l. The white matter is a striated 3HG signal coming from axon bundles of the CC. n, Side view of reconstructed PCV branch in m. The vascular segments draining layer 6 and the CC are labeled in cyan and yellow, respectively. The dotted white line marks the location of the gray–white matter transition.
Each PCV branch received blood from dense networks of surrounding capillaries, which were characterized in detail. The common strategy of counting vascular branch order from penetrating vessel trunks in the upper cortex was insufficient to categorize these vessels23. This was due to the difficulty in defining a ‘zero-order’ vessel because PCVs ramified into large, horizontally oriented branches. Instead, we relied on analysis of both microvascular branching structure and blood flow direction discerned by line scanning. Capillaries closer to arterioles receive blood flow from divergent bifurcations (Fig. 2e, red circles and left insets). On the venular side, capillaries drain into convergent bifurcations as blood flow merges (Fig. 2e, blue circles and right insets). Our initial characterization focused on vessels downstream of the last divergent bifurcation. We defined a capillary subtype termed the ‘pre-convergence capillary’, found between the last divergent and first convergent bifurcations (Fig. 2f). Pre-convergence capillaries lie in the middle of the arteriovenous circuit. Downstream of pre-convergence capillaries were other capillary-sized vessels that we collectively termed ‘other tributary vessels’, which were connected exclusively by points of convergence. Together, pre-convergence capillaries and other tributary vessels formed vascular units termed ‘tributaries’ and multiple tributaries drain into PCV branches.
We analyzed larger PCV branches and their tributaries in layers 2/3, 4 and 6/CC (gray–white matter interface) because these networks were well separated from each other (Fig. 2g). Cortical layers were estimated based on the endogenous Thy1-YFP fluorescence of pyramidal cortical neurons in layers 2/3 and 5 (Supplementary Fig. 2a). As expected, there was a greater complexity in PCV branch structure with increasing cortical depth, with 6.1 ± 2.2, 21.7 ± 5.3 and 24.7 ± 5.6 (all mean ± s.d.) tributaries per PCV branch in layers 2/3, 4 and 6/CC, respectively (n = 23 mice, aged groups pooled) (Fig. 2h–j).
During deep two-photon imaging, the gray–white matter transition could be estimated based on a reduction in vascular density and shift to more planar capillary orientations (Supplementary Fig. 2b). However, to clearly identify the gray–white matter transition, we also conducted in vivo three-photon microscopy in a subset of mice. Third harmonic generation (3HG) fluorescence allowed visualization of myelin and vascular structure could be labeled with intravenously injected FITC-dextran (Fig. 2k,l). This imaging confirmed that layer 6/CC PCV branches were located at the gray–white matter interface, where vertically oriented myelin fibers in the cortex transition into horizontally oriented fibers of the CC (Fig. 2m). Thus, layer 6/CC PCV branches drain blood from cortical layer 6 and the CC (Fig. 2n) and deep multi-photon imaging can be used to study their structure and physiology in vivo.
Capillaries in deep tributaries of PCVs regress with age
We next characterized the architecture of PCV tributaries. Pre-convergence capillaries were the predominant vessel type in PCV tributaries, representing 60.12 ± 1.47% (mean ± s.d.) of the total vessel segments (n = 23 mice) and 81.60 ± 4.95% of the total vessel length (n = 23 mice) (Fig. 3a,b). It is interesting that the percentage of total vessel segments and total vessel length represented by pre-convergence capillaries increased with age in PCV tributaries across all cortical layers (Fig. 3c and Supplementary Fig. 3a,b). Yet, in deeper layers, there was a reduction in vascular length density (Fig. 3d,e and Supplementary Fig. 3c,d) and a reduction in PCV tributary complexity (Fig. 3f and Supplementary Fig. 3e–g). This suggested that age-related regression of pre-convergence capillaries resulted in simplified PCV tributaries, affecting all cortical layers, but layer 6/CC most strongly.
a, Reconstruction of adult layer 4 PCV branch network with (left) and without (right) pre-convergence capillaries. b, Pie charts showing percentage of total vascular segments (left) and total vascular length (right) represented by pre-convergence capillaries and other tributary vessels in all PCV tributaries across layers and age. c, Age-related change in percentage of pre-convergence capillary segments (**P = 0.008) and length in PCV tributaries (**P = 0.005). d, Reconstruction of adult layer 6/CC PCV tributary with example ROI for vascular length density measurements. The inset shows raw data and segmented vessels in an ROI. e, Vascular length density across layers in adult and aged mice: layer 6/CC, ***P < 0.001. f, Average number of vascular segments in tributaries of adult and aged mice across layers: *P = 0.016. g, Hypothetical model for change in tributary complexity of aged mice involving pre-convergence capillary regression (also see Supplementary Fig. 4). h, Measurement of structural properties of individual pre-convergence capillaries. i, Length of individual pre-convergence capillaries: layer 2/3, ***P < 0.001; layer 6/CC, **P = 0.007. j, Tortuosity of pre-convergence capillaries: layer 6/CC, ***P < 0.001. k, Example of a stalled blood flow in pre-convergence capillary observed in vivo (encircled by the dotted red line). The plugging cell is marked with a white arrow. l, Pie chart showing vessel types experiencing stalled flow across all layers and both ages, as a percentage. m, Examples of pre-convergence capillaries in adult mice exhibiting very low (yellow dotted lines) or stalled (red dotted lines) blood flow (upper row images) that regressed over time (bottom row images). For graphs in panels e, f, i and j, the adult group includes n = 12 mice for layers 2/3 and 4 and n = 11 mice for layer 6/CC, and the aged group includes n = 11 mice for all layers. The data are shown as mean ± s.e.m. and are analyzed by two-way ANOVA with Holm–Šídák’s post hoc comparison testing (see Supplementary Table 1 for details on statistics). E, Euclidean distance; L, length; T, tortuosity.
We developed a hypothesis for how simplification of the tributary structure may be occurring (Fig. 3g and Supplementary Fig. 4). Age-related regression of pre-convergence capillaries (Fig. 3g, black asterisks) reduces vascular density and the number of vessels forming the tributaries. The neighboring pre-convergence capillary becomes longer and more tortuous because a branchpoint is lost and the downstream tributary vessel merges into the same segment (Fig. 3g, red asterisk). The observed increase in percentage of pre-convergence capillary segments and length within PCV tributaries is consistent with this postulation (Fig. 3c). Also consistent with our hypothesis, we detected increases in both the length and the tortuosity of individual pre-convergence capillary segments in aged mice (Fig. 3h–j). As PCV tributaries represent a smaller subset of total vessels in upper cortical layers, their age-related changes were less apparent in general quantifications of vascular density. However, the overall data indicated that PCV tributary rarefaction was occurring across all cortical layers.
We next considered potential causes of age-related, pre-convergence capillary regression. Given their central location in the arteriovenous circuit, they are the capillaries with the lowest blood flow, making them more susceptible to plugging by leukocytes24 and capillary regression with prolonged flow arrest9,25. In the in vivo image stacks, we occasionally observed blood flow stalls, that is, capillaries lacking movement of blood cell shadows (Fig. 3k, white arrow). Our observations of flow stalls were low in number across all tributaries, because each capillary was only imaged for brief periods (0.57 ± 0.16% of all vessels examined from both age groups (mean ± s.d.); n = 23 mice). However, nearly all observed flow stalls (92.5%) occurred in pre-convergence capillaries, even after normalization to total vessel length (Fig. 3l and Supplementary Fig. 3h). Furthermore, instances of pre-convergence capillary regression were observed in separate imaging sessions, 7–14 d apart, and affected vessels exhibited flow stalls or supported very low blood flow during prior imaging (Fig. 3m). Although observations of flow stalls were too low for statistical comparison, their prevalence appeared similar across cortical layers and age groups (Supplementary Fig 3i). It is expected that stalls and regressions would have more deleterious effects in the sparser networks of layer 6/CC.
Deep capillaries constrict and become hypoperfused with age
Capillary diameter is a major determinant for blood flow resistance26,27. We next measured vessel diameters and blood flow using line scans in both isoflurane-anesthetized and awake mice (Fig. 4a–c). In a subset of mice, the same vessels were imaged under both awake and anesthetized conditions for a direct comparison. This revealed significant reductions in pre-convergence capillary diameter in aged animals, with the most pronounced reductions in layer 6/CC under both anesthetized and awake conditions (Fig. 4d,h). Some diameter changes were small in magnitude (layer 4, anesthetized) and may not have caused meaningful flow resistance. It is interesting that, in awake mice, capillaries in layers 2/3 and 4 increased in diameter and diameter reductions in layer 6/CC were larger (Fig. 4h). This difference may be attributed to isoflurane’s vasodilatory effects, because slight capillary dilatation can obscure the true physiological differences in diameter between ages.
a, Imaging performed in anesthetized and awake mice. For a subset of mice (Supplementary Fig. 11), the same vessels were imaged in both states. b,c, Assessment of capillary diameter (b) and acquisition of line scans for blood flow metrics (c). d–g, Diameter and blood flow parameters in pre-convergence capillaries in anesthetized mice: diameter (d): layer 4, P = 0.006; layer 6/CC, P < 0.001; RBC flux (e): layer 2/3, P = 0.001, layer 4, P < 0.001, layer 6/CC, P < 0.001; RBC velocity (f): P < 0.001, layer 2/3, P < 0.001, layer 4, P < 0.001, layer 6/CC, P = 0.001; RBC linear density (g): layer 4, P = 0.002, layer 6/CC, P < 0.001. The adult group includes n = 12 mice for layers 2/3 and 4 and n = 11 mice for layer 6/CC; the aged group includes n = 11 mice for all layers. The data are shown as mean ± s.e.m. h–k, Diameter and blood flow parameters of pre-convergence capillaries in awake mice: diameter (h): layer 2/3, P = 0.019, layer 4, P = 0.003, layer 6/CC, P < 0.001; RBC flux (i): layer 2/3, P < 0.001, layer 6/CC, P < 0.001; RBC velocity (j): layer 2/3, P < 0.001, layer 4, P < 0.001; RBC linear density (k): layer 2/3, P = 0.001, layer 4, P < 0.001, layer 6/CC, P < 0.001. The adult group includes n = 5 mice for all layers and the aged group includes n = 6 mice for layers 2/3 and 4, and 5 mice for layer 6/CC. For h, the adult group includes n = 3 mice per layer and the aged group includes n = 2 mice per layer. The data are shown as mean ± s.e.m. For graphs in d–k, data were analyzed by two-way ANOVA with Holm–Šídák’s post hoc comparison testing (see Supplementary Table 1 for details on statistics). FWHM, full-width at half-maximum.
Critically, the reduced diameter of capillaries was associated with pronounced age- and layer-specific reduction of red blood cell (RBC) flux in layer 6/CC pre-convergence capillaries, irrespective of awake or anesthetized conditions (Fig. 4e,f,i,j) and sex (Supplementary Fig. 5). Furthermore, RBC flux and velocity increased in layers 2/3 and 4 of aged mice (Fig. 4e,f,i,j), suggesting a redistribution of blood flow from deeper tissues to more superficial layers (Supplementary Figs. 6 and 7). The increase in capillary diameter and flow is consistent with prior imaging studies that have focused on the upper cortex28,29. Finally, aging may also be associated with reduced hematocrit, because the linear density of RBCs was reduced across all layers in aged animals29 (Fig. 4g,k). These data emphasize the importance of measuring blood flow across cortical depth to understand the extent and location of age-related cerebral hypoperfusion.
We detected no age-related changes in the length and tortuosity in other tributary vessels, likely due to the shorter lengths of these vessel segments (Supplementary Fig. 8a–d). However, age-dependent reduction of vessel diameter (Supplementary Fig. 8e,f) and redistribution of blood over cortical layers were also evident in other tributary vessels (Supplementary Fig. 9). The observed blood flow differences in PCV tributaries were not due to altered cardiovascular status in aged mice, because we detected no major differences in heart rate measured from blood flow line scans (Supplementary Fig. 10).
Reduced blood flow in deeper tissues could also be caused by constriction of upstream penetrating arterioles or their initial branches. However, we did not detect age-related difference in these upstream vessels in any cortical layer (Extended Data Fig. 2), supporting the idea that vasoconstriction and capillary rarefaction in PCV tributaries were the primary drivers of reduced blood flow.
Finally, in mice examined under both anesthetized and awake states, we leveraged isoflurane’s vasodilatory effect to probe for age-related differences in capillary reactivity. Isoflurane increased RBC flux and velocity over levels seen in awake mice in all cortical layers, with the largest increases in layer 6/CC (Supplementary Fig. 11). A similar magnitude of isoflurane-induced blood flow increase was seen with both age groups, suggesting a general preservation of reactivity. However, aging led to a reduced capacity to dilate capillaries in layer 6/CC, yet an increased capacity in layer 2/3, which may contribute to the abnormal redistribution of blood flow across layers.
Capillary diameter is the key determinant of blood flow
To examine which vascular changes were the strongest drivers of hemodynamic change, we performed Pearson’s correlation analyses between the structural and functional properties of pre-convergence capillaries in the anesthetized (Supplementary Fig. 12 and Supplementary Table 3) and awake states (Supplementary Fig. 13 and Supplementary Table 4). Vessel tortuosity was not correlated with RBC flux, but both capillary segment length and capillary segment diameter were correlated. Of these two parameters, capillary diameter held the strongest relationship with RBC flux, suggesting that local capillary diameter changes substantially influence network perfusion.
In silico models capture age-related hemodynamic changes
To determine whether the degree of capillary constriction and rarefaction observed in vivo was sufficient to explain blood flow deficits seen with aging, we performed blood flow simulations in realistic microvascular networks derived from mouse barrel cortex30. These vascular networks did not contain PCV trunks, but have been widely used and provided a close approximation to cortical regions studied in vivo. The in silico model based on these data is well established and uses Poiseuille’s law and the continuity equation to compute the pressure field and flow rates for all vessels30. Moreover, the model accounts for the presence of RBCs and their effect on the flow field (see Methods for additional details and model limitations)31.
Four cases were studied over two microvascular networks, mimicking key changes seen in awake aged mice, where capillary diameter was increased in the upper layers but decreased in layer 6/CC (Fig. 5a). Capillary density in layer 6/CC was also reduced to the levels seen in aging. These modifications were sufficient to induce layer-specific blood flow changes. Most capillaries in layer 6/CC reduced in blood flow, whereas a small proportion of intermingled capillaries showed increased flow (Fig. 5b). The upper cortical layers exhibited scattered capillaries with increased capillary flow. On average, layer 6/CC showed a significant reduction of RBC flux, velocity and linear density with magnitudes exceeding in vivo levels (Fig. 5c–e). Total blood flow through arteriolar and venular branches across layers followed a similar pattern (Fig. 5f,g). Furthermore, when capillary diameter change and density reduction were applied separately, the parameters exhibited independent influences on RBC flux and velocity, but to a lesser extent than the factors combined (Supplementary Fig. 14).
a, Image of an in silico microvascular network with labeled penetrating arterioles (red) and ascending venules (blue). Age-related structural changes from awake mice were implemented in the model, including capillary dilatation (yellow segments), constriction (light-green segments) and removed segments to mimic capillary regression (dark-green segments). b, Image showing the effect of age-related structural changes on microvascular network flow, with highlighted segments exhibiting reduced (left) or increased (right) RBC velocity. Only vessels with an absolute change >10% are displayed. a and b are in an MVN1-open configuration (Methods). c–e, Changes in average capillary RBC flux (c), RBC velocity (d) and RBC linear density (e) across different cortical layers (n = 4 network configurations, that is, 2 microvascular networks × 2 boundary conditions). f,g, Changes in total blood inflow into the capillary network through arteriolar branches (f) and total blood outflow through venular branches (g) across different cortical layers (n = 4 network configurations, that is, 2 microvascular networks × 2 boundary conditions). All configuration average plots show the median ± s.d.
We also modeled capillary diameter reductions seen in anesthetized aged mice, which were specific to layer 6/CC. This yielded blood flow reductions similar to those observed in vivo, but did not induce blood flow increases in the upper cortex (Supplementary Fig. 15). This suggests that impaired flow to deep tissues does not simply reroute flow to upper layers and that capillary dilatations in the upper layers may actively contribute to abnormal flow redistribution across layers. Overall, these simulations demonstrate that the magnitude of capillary changes seen in vivo are sufficient to produce marked changes in blood flow.
Deep capillary networks are more vulnerable to regression
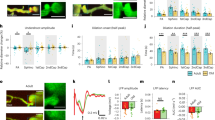
As pre-convergence capillaries regress with age, we next mimicked this change in adult mice to explore its effect on local blood flow. In a previous study, we had shown that optically induced capillary regression in layer 2/3 caused upstream vasoconstriction and reduced capillary flow32. Building on this, we conducted similar studies in deep PCV tributaries of awake adult mice using in vivo three-photon microscopy. Ablative line scans were used to rupture and induce the regression of pre-convergence capillaries in layers 4 and 6/CC. These regions were imaged before and at 3 d, 7 d and 21 d after laser irradiation (Fig. 6a). Sham line scans were performed as controls, which involved irradiating the parenchyma in the immediate vicinity of the vessel (Fig. 6b).
a,b, Laser-induced ablation of pre-convergence capillaries in layer 6/CC and off-target sham controls. The insets show changes evoked in a neighboring capillary before (a) and 21 d after irradiation (b). c,d, Vascular network schematics showing blood flow changes in a (c) and b (d). e,f, RBC flux of neighboring pre-convergence capillaries before and 21 d after capillary ablation in layer 4 (e, left) and layer 6/CC (e, right), or after sham irradiation (f) (layer 4, n = 40 vessels from 8 ROIs; layer 6/CC, n = 48 vessels from 9 ROIs; sham irradiation, n = 24 vessels from 4 ROIs). The data are shown as the mean ± s.e.m. Two-sided, paired Student’s t-test: layer 4, ***P = 0.0003; layer 6/CC, ****P < 0.0001; sham, P = 0.2998. g, Percentage change from baseline for pre-convergence capillary RBC flux at 21 d after laser irradiation. The data are shown as the mean ± s.d. One-way ANOVA with Dunnett’s post hoc comparison tests: *P < 0.05. h, Proportion of total capillaries with no flow at 21 d after ablation. i,j, Pre-convergence capillary diameter before and 21 d after capillary ablation in layer 4 (left) and layer 6/CC (right) (i) or after sham irradiation (j) (layer 4, n = 40 vessels from 8 ROIs; layer 6/CC, n = 45 vessels from 9 ROIs; sham irradiation, n = 24 vessels from 4 ROIs). The data are shown as the mean ± s.e.m. Two-sided, paired Student’s t-test: layer 4, ***P = 0.0003; layer 6/CC, ****P = 0.000013; sham, P = 0.8694. k, Percentage change from baseline for pre-convergence capillary diameter at 21 d after laser irradiation. The data are shown as the mean ± s.d. One-way ANOVA with Tukey’s post hoc comparison tests: *P < 0.05. l, Change in RBC flux as a function of change in diameter across all capillaries examined. The shaded area represents the 95% confidence bands of the best-fit line. For all experiments, n = 5 adult mice. See Supplementary Table 2 for details of statistics.
At day 21, regressions of single pre-convergence capillaries in layer 6/CC led to blood flow cessation or hypoperfusion in the surrounding capillaries served by the same arteriolar branch (Fig. 6a,c,e). Blood flow reductions were more severe in layer 6/CC than layer 4 (70% versus 50% reduction from baseline), with a gradual worsening in deeper tissues (Fig. 6e,g,h and Supplementary Fig. 16). On average, sham irradiations performed across layers 4 and 6/CC did not affect blood flow, suggesting that these outcomes were not a result of nonspecific laser damage (Fig. 6f–h and Supplementary Fig. 16).
Consistent with our prior studies in layer 2/3, capillary ablation resulted in general vasoconstriction in neighboring capillaries, whereas shams exhibited no change (Fig. 6i–k and Supplementary Fig. 16). Relatively small capillary constrictions (~10% from baseline) could explain the range of blood flow changes observed (Fig. 6l), aligning with our observations in aged mice and in silico studies. Thus, capillary regression is a trigger for broader capillary constriction and hypoperfusion, with worsened effects in deeper tissues.
Age-related hypoperfusion is associated with tissue pathology
We next determined whether age-related loss of vascular density and hypoperfusion in layer 6/CC was associated with tissue pathology. In Thy1-YFP mice that we had imaged in vivo, we also extracted brain tissues and immunostained for markers of microglia (Iba1), astrogliosis (glial fibrillary acidic protein (GFAP)) and myelin (myelin basic protein (MBP)) (Supplementary Fig. 17). Consistent with prior studies33, aged mice exhibited increased microgliosis and astrogliosis, as well as demyelination and reduced YFP fluorescence of axons in the CC (Supplementary Fig. 18). These histological indices of tissue pathology correlated with the average RBC flux in layer 6/CC when examining both ages together (Supplementary Fig. 18c,f,i,l).
In a second cohort of mice examined histologically, but not imaged in vivo, we included adult (5–7 months), mid-aged (16–18 months) and aged (24 months) groups to better understand the temporal sequence of events in the mid-cortex and layer 6/CC. We again examined markers of gliosis and myelin, but also added markers to assess vascular structure, inflammation and tissue hypoxia. Age-dependent increase in gliosis and demyelination was observed in layer 6/CC, but not the mid-cortex (Fig. 7a–c), reproducing outcomes from the first cohort and confirming that tissue changes were not an effect of cranial window surgery or in vivo imaging. Critically, a significant loss of vascular density was seen in both the mid-cortex and layer 6/CC by mid-age, suggesting that it occurs early enough to be a potential contributor to age-related gliosis and demyelination in layer 6/CC (Fig. 7d). Vascular cell adhesion molecule 1 (VCAM1), a leukocyte adhesion molecule, was strongly upregulated in the CC of aged mice, with little to no staining in adult or mid-aged groups (Fig. 7e). Prominent staining was observed in PCV trunks and layer 6/CC capillary networks (Fig. 7f,g), suggesting a contribution to capillary flow stalling in white matter.
a–e, Epifluorescent images of adult (left), mid-aged (center) and aged (right) mouse somatosensory cortex from sagittal brain sections with accompanying analyses across age and brain regions. Tissues were stained with anti-Iba1 antibody for microglia (a), anti-GFAP antibody for astrocytes (b), anti-MBP antibody for myelin (c), tomato lectin for labeling all blood vessels (d) and anti-VCAM1 antibody for VCAM1 expression (e). Bar plots show assessment of immunostaining levels in the mid-cortex (Mid-Ctx) and layer 6/CC, with analysis ROIs similar to those shown in a. For plots in a–c, n = 4 adult, n = 4 mid-aged and n = 4 aged mice. For plots in d and e, n = 3 adult, n = 3 mid-aged and n = 3 aged mice. For all plots, data are shown as mean ± s.d. Two-way ANOVA with Holm–Šídák post hoc testing: *P < 0.05, **P < 0.01, ***P < 0.001. See Supplementary Table 2 for details on statistics. f,g, Magnified insets from e showing VCAM1 staining in PCV trunk (f) and branching networks of layer 6/CC (g).
The vascular deficits observed with aging in layer 6/CC did not cause notable hypoxia or induce hypoxic responses. A pimonidazole-HCl probe (Hypoxyprobe), which is sensitive to hypoxia (<10 mm Hg partial oxygen pressure (pO2)), was injected in vivo for postmortem immunostaining and showed no elevation in aged mice (Supplementary Fig. 19a,b). Furthermore, quantitative PCR analyses in mid-aged and aged mice revealed no increase in expression of hypoxia-responsive genes with aging (slc2a1, ldha, vegfa and pdk1), which aligns with prior studies8 (Supplementary Fig. 19c–f). On the contrary, some of these genes trended toward reduced expression, possibly reflecting an age-related dampening of hypoxia-inducible factor 1α activity. Thus, deeper tissues do not become overtly hypoxic with age, but experience a mild but chronic restriction in metabolic supply.
Loss of pericytes in deep capillary networks during aging
Pericytes are mural cells that line brain capillaries and their contractile tone modulates basal capillary flow23. Pericytes are lost at higher levels than endothelial cells during aging10,34 and experimentally induced pericyte loss in adulthood causes capillary blood flow stalling, reduction in capillary structure and leukocyte adhesion35,36. We therefore examined how aging affected pericyte density in the somatosensory cortex in our light-sheet imaging data (Extended Data Fig. 3). Aging was associated with a reduction in vascular length density and pericyte numbers in somatosensory cortex, starting from 400 µm of intracortical depth, which is approximately where layer 4 PCV branching networks are located (Extended Data Fig. 3k,l). When pericyte numbers were normalized to the total vascular length, a specific age-related reduction in pericyte density was detected at 800–1,000 µm of depth, corresponding to layer 6/CC (Extended Data Fig. 3m). These data suggest that pericyte loss or dysfunction could be a contributor to age-related capillary changes.
Inducing mild hypoperfusion in adult mice leads to white matter pathology
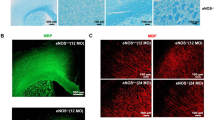
Our in vivo two-photon imaging revealed that relatively mild levels of blood flow reduction (~20%) were associated with age-related tissue pathology in layer 6/CC. To investigate whether this degree of hypoperfusion is sufficient to cause tissue pathology, we induced a similar level of hypoperfusion in adult mice (5–7 months) and examined markers of gliosis and myelin integrity. Mild hypoperfusion in one brain hemisphere was achieved through unilateral common carotid artery stenosis (UCCAS; Fig. 8a,b) using a 0.20-mm-radius microcoil implanted around the left common carotid artery. Mice were pre-implanted with cranial windows to assess capillary RBC flux changes in layers 4 and 6/CC at baseline and 3 d, 7 d, 14 d and 21 d after UCCAS initiation. Brain tissues were then collected for histological assessment, with the contralateral hemisphere serving as an internal control.
a, Schematic showing experimental timeline in adult mice, aged 5–7 months. b, Left: schematic showing the placement of a 0.20-mm microcoil around the common carotid artery (CCA) to induce mild unilateral hypoperfusion. Right: images showing longitudinal deep in vivo two-photon imaging performed on ipsilateral somatosensory cortex layer 4 and layer 6/CC PCV branching networks (top), and microcoil placement during UCCAS surgery (bottom). c,d, Average RBC flux over time in layer 4 (c) and layer 6/CC (d), collected in pre-convergence capillaries of PCVs in animals that underwent UCCAS surgery. The data are presented as mean ± s.d. Each dot in the graphs represents an average RBC flux of a population of pre-convergence capillaries from a PCV branch in a single animal. e–h, Epifluorescent images of contralateral (Contra) and ipsilateral (Ipsi) layers 4 (left) and 6/CC (right) from somatosensory cortex, stained with anti-Iba1 antibody for microglia (e), anti-GFAP antibody for astrocytes (f), anti-MBP antibody for myelin (g) and anti-Hypoxyprobe for labeling hypoxic tissue (h). i–l, Corresponding plots showing analyses of Iba1+ area (i), GFAP+ area (j), MBP signal intensity (k) and Hypoxyprobe signal intensity (l). For all plots, n = 6 mice. The data are shown as mean ± s.d. Two-way ANOVA with Holm–Šídák post hoc testing: ***P < 0.001; Iba1: hemisphere × layer interaction effect, F(1,5) = 27.48, P = 0.0033; GFAP: hemisphere × layer interaction effect, F(1,5) = 25.82, P = 0.0038; MBP: hemisphere effect, F(1,5) = 25.77, P = 0.0038; in l, the layer effect, F(1,5) = 0.1858, P = 0.6844.
On average, both layer 4 and layer 6/CC exhibited relatively stable, ~20% reductions in capillary RBC flux over 3 weeks of imaging after UCCAS (Fig. 8c,d), comparable to the magnitude of blood flow decrease observed in layer 6/CC of aged mice (Fig. 4e,i). Histological analyses revealed increased Iba1 and GFAP staining in the ipsilateral layer 6/CC, whereas layer 4 was unaffected (Fig. 8e,f,i,j). Mild hypoperfusion also produced a strong trend toward reduced myelination in layer 6/CC (Fig. 8g,k). No differences were seen with Hypoxyprobe staining, indicating that mild hypoperfusion caused tissue oxygen changes subthreshold for probe detection, as seen with aging. These findings confirm that the degree of blood flow reduction occurring with aging is sufficient to drive tissue pathologies in layer 6/CC and is, thus, a key contributor to white matter injury and gliotic reactions.
Discussion
Cerebral white matter is highly vulnerable to hypoperfusion during aging and neurological disease, yet its supporting microvascular networks remained poorly defined. Using deep multi-photon imaging, we show that cortical layer 6 and the CC in mice are exclusively drained by rare, wide-reaching, penetrating venules called PCVs. Age-related capillary constriction and rarefaction within the deep branches of PCVs lead to mild hypoperfusion (~20% decrease) in layer 6/CC, which is strongly linked to microgliosis, astrogliosis and myelin loss. Moreover, inducing a comparable level of hypoperfusion in adult mice using common carotid artery stenosis resulted in similar tissue pathologies in layer 6/CC. These findings identify PCVs as critical bottlenecks and sources of blood flow impedance during aging. Given that murine cortical CC vascular architecture mirrors that of the human cortical U-fiber interface19, similar mechanisms may contribute to age- and disease-driven degeneration of superficial white matter in humans.
Pre-convergence capillaries in PCV tributaries were the most vulnerable to blood flow disturbance and rarefaction. Regressed capillaries tended to be those that had previously stalled or supported very low blood flow, reinforcing a growing link between imbalanced capillary flow and the progressive loss of capillary network structure during aging9,25,35. The abnormal contraction or death of pericytes in layer 6/CC can explain these flow disturbances23,35,37, although the triggers of age-related pericyte pathology remain unclear. Concurrently, the endothelium and surrounding glia show heightened expression of immune- and inflammation-related genes, particularly in the white matter13,34,38 Consistent with endothelial inflammation, we find that VCAM1 expression and pericyte loss are most prominent in layer 6/CC. Previous studies suggest that pericyte loss could drive endothelial inflammation and increased leukocyte adherence36. Furthermore, the white matter vasculature appears to be enriched in venous-associated vessels, where endothelial–leukocyte interactions are likely to be heightened. Thus, a cycle of capillary constriction and obstruction, metabolic insufficiency, pericyte loss and vascular inflammation may perpetuate white matter deterioration. Future research should focus on identifying the specific molecular changes in white matter vasculature, leveraging emerging techniques such as cellular-resolution spatial transcriptomics.
Pericyte loss has been shown in clinical neuropathological studies of the aging brain39 and its effects have been broadly studied in experimental models involving either blockade of pericyte survival signals or direct pericyte ablation in adulthood35,40,41. However, the pathophysiological signals that trigger abnormal pericyte contraction are underexplored. More research is needed to understand the mechanism of capillary-level vasoconstriction, which may differ from arterial signaling, and to determine effective strategies to curtail this process42. Pericyte contraction could be driven by membrane depolarization and calcium influx or by increased production of vasoconstrictive agonists such as endothelin-1 or thromboxane A2 (refs. 43,44,45). Other potential factors also cannot be ruled out, including changes in the structure of the endothelium, basement membrane or other perivascular components. Our findings indicate that deep capillaries in the aged brain remain responsive to vasodilatory stimuli such as isoflurane, suggesting that clinically approved therapeutics with known vasodilatory effects on pericytes and capillaries may still be effective23,46. Recent studies have also shown that exercise can restore age-related vascular deficits in the CC47.
We found that capillary flux increases with cortical depth in the adult brain and this gradient is dampened during aging, consistent with earlier work47. The maintenance of higher blood flow rates in deeper tissue is likely necessary to compensate for the reduced oxygen content of RBCs because they travel further from the arterial source and encounter sparser capillary networks. Supporting this idea, an in vivo study reported depth-dependent decreases in pO2 in penetrating arterioles and ascending venules48. We postulate that the ~20% reduction in RBC flux in layer 6/CC of aged mice lowers pO2 to levels that compromise metabolic function in white matter, but insufficient to trigger compensatory responses to hypoxia and are undetectable by Hypoxyprobe. Consequently, even modest age-related declines in blood flow can contribute insidiously to white matter dysfunction and tissue degradation. When compounded by age-related capillary rarefaction, localized regions of low pO2 become more pronounced, increasing susceptibility to secondary insults49,50. For example, in a mouse model of increased Alzheimer’s disease risk (Apoe4), additional vascular insufficiencies in the CC further reduce tissue pO2 to levels detectable by Hypoxyprobe (<10 mm Hg)12. The development of in vivo multi-photon-based techniques to measure pO2 at depth will be an important advancement for testing these concepts.
Finally, deep in vivo multi-photon imaging is poised to reveal new insights into the etiology of cerebrovascular disease and will complement clinical studies. Our study establishes a framework for navigating the complex microvascular networks of the deep cortex and the CC in mice, which is essential for consistent measurements within and across animals. We established a baseline to understand microvascular change in disease models that incorporate aging as a factor. When combined with the broad-scale visualization of microvasculature enabled by light-sheet microscopy10,51,52, in vivo studies can now focus on the specific brain regions that are most vulnerable during aging and disease.
Methods
Mice
All procedures in this study were approved by the Institutional Animal Care and Use Committee at the Seattle Children’s Research Institute and the Allen Institute. Both institutions have accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care and all experiments were performed within the guidelines. Deep in vivo two-photon imaging studies comparing age differences were performed on adult (n = 12; 6 male and 6 female, 5–7 months old) and aged (n = 12; 9 male and 3 female, 22–24 months old) Thy1-YFP mice53 bred on the C57Bl/6 background (B6.Cg-Tg(Thy1-YFP)HJrs/J; Jax ID 003782). The number and sex of mice for other experiments are listed in each relevant section. Age group ranges were determined by the Jax ‘Life Span as a Biomarker’ criterion, which defines 3–6 months as ‘mature adult’ stage and 18–24 months as ‘old’ stage in mice. Aged mice were visually checked for general health and any overt signs of illness such as tumors or weight loss resulted in exclusion from the study. All mice were housed on a 12-h light (06:00–18:00) to 12-h dark cycle, with free access to chow and water. Housing temperatures were ~21 °C with ~40% humidity. The use of adult and aged mice was interleaved over the entire period of study.
Deep in vivo two-photon imaging
For chronic cranial window implantation, dexamethasone (2 μg per g of body weight; Patterson Veterinary) was subcutaneously administered to animals 4 h before the surgery, which helped to reduce brain swelling during the craniotomy. Anesthesia was induced with a cocktail consisting of fentanyl citrate (0.05 mg kg−1), midazolam (5 mg kg−1) and dexmedetomidine hydrochloride (0.5 mg kg−1; Patterson Veterinary). Under sterile conditions, the scalp was excised and the periosteum cleaned from the skull surface. An aluminum flange for head fixation was attached to the right side of the skull surface using the C&B-Metabond quick adhesive cement (Parkell, cat. no. S380). A circular craniotomy (dura left intact), ~4 mm in diameter, was created over the left hemisphere and centered over 2 mm posterior and 3 mm lateral to bregma. The craniotomy was sealed with a glass coverslip plug consisting of a round 4-mm glass coverslip (Warner Instruments, cat. no. 64-0724) glued to a round 5-mm coverslip (Warner Instruments, cat. no. 64-0731) with ultraviolet light-cured optical glue (Edmund Optics, cat. no. 37-322). The coverslip was positioned with the 4-mm side placed directly over the craniotomy, whereas the 5-mm coverslip was laid on the skull surface at the edges of the craniotomy. Loctite Instant Adhesive 401 was carefully dispensed along the edge of the 5-mm coverslip to secure it to the skull. The area around the cranial window was then sealed with dental cement. Throughout the surgery, the body temperature was maintained at 37 °C with a feedback-regulated heat pad (FHC Inc.) and the mice were provided medical air through a nose cone (20–22% oxygen and 78% nitrogen, moisturized by bubbling through water; AirGas Inc.). Imaging was initiated after a 3-week recovery period.
Mice implanted with cranial windows were maintained under light isoflurane anesthesia (~1.5% minimum alveolar concentration) delivered by medical air (20–22% oxygen and 78% nitrogen, moisturized by bubbling through water) and body temperature was maintained at 37 °C with a feedback-regulated heat pad throughout the imaging period. To label the vasculature, 100 μl of 5% (w/v in sterile saline) 2 MDa Alexa Fluor-680-dextran was injected through the retro-orbital vein. Alexa Fluor-680-dextran was custom conjugated using a previously published protocol20. In vivo two-photon imaging was conducted using a Bruker Investigator microscope (run by Prairie View 5.5 software) coupled to a Spectra-Physics Insight X3 laser source. Endogenous YFP fluorescence and Alexa Fluor-680-labeled microvasculature were imaged at 900-nm and 1,210-nm excitation and emission was collected through 525/70-nm and 700/75-nm bandpass filters, respectively. During imaging, laser power ranged between 4 mW and 145 mW, exiting the microscope objective, with higher powers required for greater cortical depth (Supplementary Fig. 20).
Imaging timeline
Each mouse underwent five sessions of deep, in vivo, two-photon imaging under anesthesia with <2.5 h of imaging time per session and 2–3 d between the sessions. During the first imaging session, low-resolution maps of the cranial window were collected using a ×4, 0.16 numerical aperture (NA) objective lens (Olympus; UPlanSAPO) for navigational purposes and to identify PCV locations. Once the appropriate PCV had been located, high-resolution imaging of PCV branches and associated microvascular networks was performed through the entire cortical depth. Image stacks were collected using a ×25, 1.05 NA water-immersion objective lens (XLPlan N, Olympus) across a 483 μm × 483 μm field of view with lateral sampling resolution (x and y) of 0.943 µm per pixel and axial sampling resolution (z) of 1 µm per pixel. Two- to four-image stacks were often collected and stitched using an ImageJ/FIJI ‘Pairwise stitching’ plugin to cover the ROI54.
We analyzed PCV branches originating from the main PCV trunk across 200–400 µm, 400–600 µm and 800–1,000 µm of intracortical depth, roughly corresponding to cortical layers 2/3, 4 and 6/CC, respectively (Fig. 2g). Branches at these depths drained blood from nonoverlapping regions and generally covered the upper, mid and deep layers of the cortex. To confirm the location of cortical layers 2/3 and 4, we examined endogenous fluorescence in pyramidal neurons of layers 2/3 and 5 (Supplementary Fig. 2). Three-dimensional (3D) visualization of high-resolution z-stacks was performed using Imaris software v.7.7.2 (Bitplane, Oxford Instruments). Vascular segments presumed to belong to a PCV branch network were identified based on the evaluation of vascular structure from the image stacks. To ensure that we were including the correct starting points of the networks, all vessel segments starting from one branch order upstream of the putative starting points of the PCV network and downstream of the main branches of the PCV were marked. Then, the location of the marked vascular segments was labeled in the original z-stack using ImageJ or FIJI to provide a map of where line scanning was to be performed in subsequent imaging sessions.
Line-scan data acquisition
During deep in vivo two-photon imaging sessions 2, 3 and 4 under anesthesia, line-scan data were collected from vessel segments in line-scan maps from layers 2/3, 4 and 6/CC (one cortical layer per session). Line scanning was performed using the ×25, 1.05 NA water-immersion objective lens and a ×3 digital zoom was used to guide accurate placement of the line scan. Vessel segments were sampled individually with line-scan duration set to ~1.2 s at a sampling frequency of ~2 kHz. During acquisition of line-scan data, we also followed the number of stalled vessel segments (see below). To ensure that the laser powers used for line scans did not directly induce damage or alter blood flow, we longitudinally imaged the same population of layer 6/CC vessels weekly for 5 weeks in a cohort of mice (Supplementary Fig. 21). We observed no overt signs of vascular damage, that is, dye extravasation indicative of blood–brain barrier damage or vascular regression. Blood flow was consistent and unperturbed across all time points examined.
Analysis of the PCV branches over depth
During deep in vivo two-photon imaging session 5, we again collected high-resolution z-stacks through the entire cortex, starting at the pia mater and ending at ~1,000 µm of cortical depth. As mentioned above, to capture the entire branching structure of the PCV, we collected and stitched four adjacent and partially overlapping z-stacks arranged in a 2 × 2 square formation, with the main PCV trunk set in the center. Using the stitched z-stacks, we analyzed the overall branching structure of PCVs in adult and aged mice. We measured the diameter of each PCV branch segment next to the point where it joined the main trunk of the PCV, as well as the cortical depth at which the branch originated. Furthermore, we analyzed the number of PCV branches through cortical depth, after binning in 200-µm depth groups.
Awake imaging
A subset of Thy1-YFP mice (n = 5 adult, 2 male and 3 female, and n = 6 aged, 5 male and 1 female) was imaged both under isoflurane anesthesia (as described above) and in the awake state. During awake imaging, mice were head fixed and allowed to freely move on a low resistance treadmill (Phenosys SpeedBelt). All mice were habituated for head fixation over three training sessions (each ~2 d apart), where mice were head fixed on the treadmill and placed in the light-shielded microscope cage with the objective over the cranial window to imitate the conditions during imaging. The mice were then held in the imaging set-up for 20 min before being returned to the home cage. After training, each mouse underwent seven imaging sessions over 3.5 weeks (two imaging sessions per week, 3 d apart). The first session was performed under isoflurane anesthesia to collect low-resolution maps of the cranial window for navigational purposes, as well as to identify the location of PCVs, as described above. Once the appropriate PCV had been located, high-resolution imaging was performed throughout the entire depth around the PCV. In the following six imaging sessions, line-scan data were collected from vessel segments from layers 2/3, 4 and 6/CC (one cortical layer per session). We alternated imaging of the same cortical layer in the awake state and under anesthesia on separate sessions in immediate succession to obtain hemodynamic data from the same population of vessels under both conditions. At the end of each awake imaging session, a z-stack of the imaged PCV vascular network was collected for analysis of the vessel diameter.
Defining the vascular networks of a PCV branch
We used the definition that PCV branch networks should include all vessel segments that converge blood flow toward that branch. Therefore, we considered the last points of blood flow divergence within the capillary network as the starting points for the PCV branch vascular network. Using structural information from our image stacks and blood flow directionality extracted from line-scan data, we were able to reliably identify the last points of convergence in the analyzed PCV vascular networks.
The first vessel segments in the PCV branch vascular network are located between the points of flow divergence and points of flow convergence, termed ‘pre-convergence’ capillaries. Pre-convergence capillaries merge and form a system of downstream convergent vessel segments, termed ‘other tributary vessels’, which eventually lead to a larger-diameter PCV branch. Pre-convergence capillaries and other tributary vessels were together termed ‘tributaries’. PCV branches were generally larger-diameter offshoots from the PCV trunk and multiple tributaries of differing complexity could flow into branches, collectively forming PCV branching networks (Fig. 2e,f).
Analysis of tributary structure
As the capillary network is predominantly composed of bifurcating branchpoints, the number of vessel segments in tributaries is almost always an odd number (Fig. 3g). To analyze tributary structure, we measured the average number of vessel segments per tributary and separated tributaries into three groups: low (one or three vessels), medium (five or seven vessels) and high (nine or more vessels) complexity. In the case of a tributary containing a trifurcation point and consisting of an even number of vessel segments, it was categorized as the first higher odd number tributary (that is, four as five, six as seven, and so on). For each analyzed PCV vascular network, we calculated the total number of tributaries belonging to a certain complexity group (low, medium, high) and expressed it in relation to the total number of tributaries in the PCV network (Supplementary Fig. 3e–g).
Segmentation of PCV vascular networks
Vascular networks of PCV branches in z-stacks were reconstructed in 3D using Imaris, v.7.7.2 (Bitplane, Oxford Instruments). Vessel segmentation was performed manually using the ‘Auto Depth’ tracing option in the Filament Tracer module of Imaris. ‘Center’ and ‘Smooth’ options in the Edit tab of the Filament tracer module were used to correct for any tracing mistakes. After segmentation of the networks, the length and straightness of each reconstructed vessel segment were extracted from the Statistics tab of the Filament tracer module. The straightness values reported by the software are reciprocal to tortuosity.
Vessel diameter measurement
The lumen diameter of different vessel types was measured in maximally projected images from high-resolution z-stacks. Maximum projections of 10–40 µm in thickness were used for analysis, dependent on the size of the vessels. To reduce the bias of measurement location, we used a customized ImageJ/Fiji macro called VasoMetrics to analyze lumen full-width at half-maximum diameter at multiple, equidistant locations (spaced 1 µm) along each vessel segment of interest55. The values across each vessel segment were used to calculate the average diameter of the vessel and the s.d. of the diameter along the measured region. For analysis of the vessel diameter in the arteriole–capillary transition zone, three to four vessels were analyzed per layer per animal for branch order 1, n = 6–8 vessels were analyzed per layer per animal for branch order 2 and n = 12–16 vessels were analyzed per layer per animal for branch order 3. Average vessel diameter was then calculated for each layer and animal, and these average values were used for statistical analysis.
Quantification of hemodynamic parameters and stalled vessels
For each line scan captured, we calculated RBC flux by manually counting the number of blood cell shadows over the total duration of the line scan and represented the values as cells per second. The angle of the streaks in relation to the direction of the scan was used to determine RBC flow direction. Analysis of RBC velocity and heart rate was performed using a previously published MATLAB algorithm56. RBC linear density values for each vessel segment were calculated by dividing the RBC flux by the RBC velocity. Blood flow stalls were identified as capillaries lacking moving blood cell shadows for at least a period of 20 min during repeated observations within the same imaging session.
Analysis of vascular density
Analysis of vascular density was performed in two ways using the Imaris software. First, we assessed vascular density (total vascular length per volume) in regions enriched with pre-convergence capillaries. This involved placing six small ROIs (100 × 100 × 100 µm3 (x, y and z)) within each reconstructed PCV network (Fig. 3d,e). Second, vascular density was measured more broadly in cortical layers by assessing larger ROIs (300 × 300 × 100 µm3 (x, y and z)) within layers 2/3, 4 and 6/CC (Supplementary Fig. 3c,d). Two ROIs were analyzed per each layer, for a total 300 × 300 × 200 µm3 (x, y and z) ROI volume.
In vivo three-photon imaging
To identify the gray–white matter transition, adult C57BL/6 mice (n = 3, 2 male and 1 female) were imaged using in vivo three-photon microscopy. A chronic cranial window was implanted over the sensory cortex. Briefly, under 0.5–2% isoflurane anesthesia, a head restraint bar was attached to the skull using C & B-Metabond (Parkell) and a circular craniotomy 5 mm in diameter was opened over the left visual cortex at coordinates 2.7 mm lateral, 3 mm posterior to bregma. A durotomy was performed and the craniotomy was sealed with a plug consisting of a stack of three no. 1 coverslips (two round 5-mm coverslips and one round 6-mm coverslip), attached to each other using optical adhesive and then attached to the skull with Metabond.
Imaging was performed on a three-photon microscope built around a Coherent Monaco/Opera-F laser source (≤2 μJ, 50-fs pulses at 1 MHz; Coherent Inc.). The microscope head was based on a Movable Objective Microscope with 3D objective translation, provided by Sutter Instrument. The scan lens and tube lens were a Thorlabs SL50-3P and Thorlabs TTL200MP, respectively, to enhance transmission at 1,300 nm. An Olympus ×25, 1.05 NA water-immersion objective lens (XLPlan N; 75% transmission at 1,300 nm) was used and image acquisition was controlled by ScanImage (MBF Bioscience) with acquisition gating for low-repetition rate lasers. Animals were imaged in the awake state. To label the vasculature, before imaging mice were injected with 100 μl of 5% (w/v in sterile saline) 2 MDa dextran–FITC through the retro-orbital vein under a brief period of isoflurane anesthesia. Emitted green fluorescence was separated from incoming excitation light by a primary dichroic beam splitter (Semrock, cat. no. FF735-Di02), then filtered by a bandpass filter (Chroma, cat. no. ET525-70m-2p). A third harmonic-generated (3HG) signal produced by blood vessels and myelinated axons was detected using a secondary dichroic beam splitter (Semrock, cat. no. Di02-R488) and filtered by a bandpass filter (Chroma, cat. no. ET434/32m) into a separate blue channel, which allowed us to clearly discern the gray–white matter boundary57.
A separate batch of adult C57BL/6 mice (n = 5, 3 male and 2 female) was used for in vivo three-photon capillary ablation experiments (Fig. 6). After chronic cranial window implantation, using the procedure described above, mice were imaged to identify and map microvascular networks in layers 4 and 6/CC to be studied in greater detail. After 1 week, day 0 baseline imaging was performed, when z-stacks of the chosen networks were collected to obtain baseline vessel diameter data and line scans for determination of baseline RBC flux. This was immediately followed by laser irradiation to induce pre-convergence capillary ablation or to perform sham line scans. Ablative line scans were performed by placing the line-scan path of laser irradiation on the vessel lumen. Line-scan laser power used to achieve ablation was 35–48 mW in layer 4 and 61–77 mW in layer 6/CC, with a 0.5-ms line-scan period and 10,000 repetitions. Sham line scans were performed with the same settings for the corresponding layer and animal, but the line-scan path was placed on the parenchyma in the immediate vicinity of the vessel lumen. Post-irradiation imaging was performed at days 3, 7 and 21. During each post-irradiation imaging day, line scans were performed to determine the blood flow in the same population of vessels, whereas z-stacks of analyzed networks were collected at day 21 for measurement of the vessel diameter. In each session, animals were imaged in the awake state. The vasculature was labeled by 2 MDa dextran–FITC, as described above.
Tissue clearing and 3D immunolabeling for light-sheet imaging
Whole-brain 3D light-sheet fluorescence microscopy imaging was conducted at Pennsylvania State University and approved by the Institutional Animal Care and Use Committee of Pennsylvania State University. Four (two male and two female) 2-month-old (young) and four (two male and two female) 24-month-old (old) C57BL/6J mice were used in the study (Extended Data Figs. 1 and 3). The iDISCO+ tissue-clearing protocol was used with modifications10,51. Brain samples were delipidated in SBiP buffer, consisting of ice-cold water, 50 mM Na2HPO4, 4% sodium dodecylsulfate, 2-methyl-2-butanol and 2-propanol. This buffer becomes activated at room temperature and was therefore made and stored at 4 °C before use. Each sample was submerged in 10 ml of SBiP buffer, rotated at room temperature with buffer changes at 3 and 6 h, followed by incubation with fresh SBiP buffer overnight. For adequate delipidation, particularly with aged samples, each brain was then washed with SBiP buffer for a total of 6 d, with daily buffer changes. After delipidation, brain samples were washed with B1n buffer, which consisted of 0.1% Triton X-100, 1 g of glycine, 0.01% 10 N NaOH and 20% NaN3. Brain samples were washed with 10 ml of B1n buffer at room temperature for 2 d.
To begin immunolabeling, brains were rinsed 3× for 1 h each with PTwH buffer, consisting of 1× phosphate-buffered saline (PBS), 0.2% Tween-20, 10 mg of heparin and 2 g of NaN3. For primary antibody incubations, antibodies were diluted in antibody solution consisting of PTwH buffer with 5% dimethyl sulfoxide and 3% normal donkey serum. Antibodies to α-SMA (rabbit anti-α-SMA, Abcam, cat. no. ab5694, 1:1,000) and transgelin (Sm22) (rabbit anti-Sm22, Abcam, cat. no. ab14106, 1:1,500) were combined to label the artery wall, as previously described10. Pan-vascular labeling was achieved through staining with DyLight 594-labeled Lycopersicon Esculentum (Tomato) Lectin (VectorLabs, cat. no. DL-1177-1), which was added to both primary and secondary incubations at a 1:100 concentration. Pericytes were labeled by combining platelet-derived growth factor receptor β (PDGFRβ; goat anti-PDGFRβ, R&D Systems, cat. no. F1042, 1:100) and mouse aminopeptidase N/CD13 (goat anti-CD13, R&D Systems, cat. no. AF2335, 1:100). Primary antibodies were incubated for 10 d at 37 °C. After primary incubation, PTwH buffer was changed 4–5× for each sample over the course of 24 h. A fresh antibody solution was used to dilute all secondary antibodies to a concentration of 1:500. For secondary antibodies, Alexa Fluor-488-AffiniPure Fab fragment, donkey anti-rabbit immunoglobulin G (IgG) (H + L) (Jackson ImmunoResearch Laboratories, cat. no. 711-547-003) was used to detect artery staining and Alexa Fluor-647-AffiniPure Fab fragment, donkey anti-goat IgG (H + L) (Jackson ImmunoResearch Laboratories, cat. no. 705-607-003) to detect pericyte staining. After secondary incubation for 10 d at 37 °C, brains were washed 4–5× in PTwH buffer for 24 h. Brain samples were then dehydrated in a series of methanol dilutions in water (1-h washes in 20%, 40%, 60%, 80% and 100%). An additional wash of 100% methanol was conducted overnight to remove any remaining water. The next day, brains were incubated in 66% dichloromethane to 33% methanol for 3 h and subsequently incubated in 100% dichloromethane twice for at least 15 min each. Brains were equilibrated in dibenzyl ether for at least 2 d before transitioning to ethyl cinnamate 1 d before imaging.
Light-sheet imaging
A SmartSPIM light-sheet fluorescence microscope (LifeCanvas Technologies) was used for imaging cleared and stained mouse brains. Brains were supported in the customized sample holder by standardized pieces of dehydrated agarose consisting of 1% agarose in 1× Tris-acetate–EDTA buffer. The sample holder arm was then submerged in ethyl cinnamate for imaging. We used a ×3.6 objective (LifeCanvas, 0.2 NA, 12-mm working distance, 1.8-μm lateral resolution) and three lasers (wavelengths of 488 nm, 560 nm and 642 nm) with a 2-μm step size. Acquired data were stitched using customized MATLAB codes adapted from Wobbly Stitcher10,51.
Analysis of light-sheet imaging data
For the analysis of the abundance and penetration depth of cortical penetrating vessels, a 2 mm (x) × 2 mm (y) × 1.2 mm (z) ROI centered on the primary somatosensory cortex was cropped from whole-brain light-sheet datasets of four adult mice (Extended Data Fig. 1c–e). The pial penetration points of all penetrating vessel subtypes (penetrating arteriole, PCVs and other ascending venules) were identified within the ROI and the distance to vessel ending points within the tissue was recorded. For penetrating arterioles, we defined the ending point as the deepest region where α-SMA or Sm22 staining was still discernible. For ascending venules and PCVs, the ending point was defined as the region where the main trunk ended and ramified into many smaller branches, as visualized by lectin staining. PCVs were clearly distinguished from other ascending venules by their large diameter, weak α-SMA or Sm22 staining of the vessel wall and specific branching pattern in the white matter of the CC. Image cropping and analysis were performed using the Fiji software.
For the analysis of vascular length density and pericyte density across different cortical layers, a 540 µm (x) × 540 µm (y) × 1,000 µm (z) ROI centered on the primary somatosensory cortex was cropped from whole-brain light-sheet datasets of four adult and four aged mice (Extended Data Fig. 3e,f). The ROI volumes were then further divided into 200-µm thick (z) subsections (Extended Data Fig. 3g,h). The 3D segmentation of all vessels labeled with lectin, as well as annotation of all PDGFRβ + CD13-labeled pericytes was performed using the Filament Tracer module of Imaris software (Extended Data Fig. 3i,j). Only mesh and thin strand pericytes on small diameter vessels, characterized by the ‘bump on the log’ morphology, were included in the analysis. Vascular length density (total vascular length per cubic millimeter) and pericyte density (total number of pericyte cell bodies) were measured for each subsection. Then the normalized pericyte density was calculated by dividing the pericyte and vascular length density values for each subsection.
In silico modeling of microvascular networks
Both microvascular networks used for in silico modeling in this work (MVN1 and MVN2) were acquired from the vibrissa primary sensory cortex of C57/BL6 male mice by Blinder et al.58. In these segmented and vectorized networks, each vessel is represented by an edge with a given length and diameter and edges are connected at bifurcations (graph vertices). MVN1 and MVN2 are embedded in a tissue volume of ~1.6 mm3 and ~2.2 mm3 and contain ~12,100 and ~19,300 vessels, respectively. The vessels are labeled as pial arteries, penetrating arterioles, capillaries, ascending venules and pial veins. Moreover, for the penetrating vessels, we differ between the main perforator and off-shooting vessels. The predominant vascular length is composed of capillaries (96% and 94% of total vascular length in MVN1 and MVN2, respectively). As staining of mural cells is not available for the microvascular network reconstructions, the vessel identities are based on topology and diameter58.
Blood flow modeling with discrete RBC tracking
The numerical model to simulate blood flow with discrete RBC tracking in realistic microvascular networks has been described in ref. 30. Below, we briefly summarize the key aspects of the modeling approach, which have been detailed in prior studies30,59. The modeling approach is based on the small Reynolds number (Re < 1.0 for all vessels) in the microvasculature. As such, the flow is laminar and mostly even in the Stokes’ regime, which allows description of the flow in individual vessels by Poiseuille’s law. The flow rate qij in vessel ij between nodes i and j is computed by:
where Dij and Lij are the vessel diameter and the length and pi and pj are the pressure at node i and j, respectively; μ is the dynamic plasma viscosity and \({\mu }_{{{\mathrm{rel}}}}^{{\mathrm{e}}}\) is the relative effective viscosity, which accounts for the presence of RBCs and is computed as a function of local hematocrit and vessel diameter as described in ref. 31 (in vitro formulation).
To compute the local hematocrit, we track individual RBCs through the MVNs. We accounted for the Fahraeus effect (RBC velocity is larger than bulk flow velocity) and the phase separation (RBCs partition with a different ratio to the bulk flow at divergent bifurcations)31. At divergent bifurcations with a diameter >10 µm, the phase separation is described by empirical equations31. At smaller diameters, RBC transit is single file and, consequently, the RBC motion can be approximated by assuming that the RBCs follow the path of the largest pressure force30,60. It is important to note that the RBC distribution and the flow field fluctuate in time and that the RBC distribution impacts the local flow field27,59. In this study, we used the time-averaged flow field to compare changes in response to cortical layer-specific alterations in capillary diameters. The time-averaged flow field was computed by averaging over 14–20 s. The exact averaging interval depended on the vessel-specific turn-over time (vessel length/RBC velocity) and ensured that 90% of all vessels are perfused at least six times.
Two configurations were considered regarding the boundary conditions for in- and outflow vertices below the cortical surface. In configuration 1 (‘open’), fixed pressure values were assigned at all in- and outflows. These values are kept constant for all scenarios mimicking vascular alterations during aging (see below). Further details on assigning suitable pressure boundary conditions can be found in our prior studies30. In configuration 2 (‘trimmed’), we assumed that no blood flow enters or leaves the simulation domain below the cortical surface and that all flow enters or leaves via the pial vessels at the cortical surface. This approach is equivalent to assigning no-flow boundary conditions at in- and outflows below the cortical surface or to removing all in- and outflows below the cortical surface. Configuration 1 (‘open’) has the advantage that it does not under-predict perfusion, as is the case for trimmed networks61. On the other hand, the number of boundary nodes is considerably smaller for configuration 2 (‘trimmed’). As such, this set-up is less sensitive to the assigned boundary values, which is especially desirable for relatively large microvascular alterations. Thus, for the current study, we decided to consider both open and trimmed boundary conditions for a total of four configurations: MVN1-open, MVN1-trimmed, MVN2-open and MVN2-trimmed. The inflow hematocrit of 0.3 is constant for all simulations.
Mimicking vascular alterations observed in aged mice in silico
We mimicked two age-related vascular alterations observed in vivo to understand their effects on microvascular perfusion. As we are interested in perfusion changes with respect to cortical depth, each capillary is assigned to one of the five cortical layers (layer L1, L2/3, L4, L5 and L6). This is done by computing the average depth per capillary from the tortuous vessel coordinates and assigning the capillary to the respective layer based on the minimum and maximum depth for each layer (L1: up to 200 µm; L2/3: 200–400 µm; L4: 400–600 µm; L5: 600–800 µm; and L6: <800 µm). Only capillaries within the 5th to 95th percentiles of all vessel depths and at least two branches from any inflow and outflow were considered for vasoconstriction and subsequent analyses. This was done to avoid confounding effects with respect to boundary conditions. For all configurations, the 95th percentile was at a depth of at least 999 µm and we had 8,200–15,000 capillaries for analyses.
We focused on capillaries toward the venule end of the capillary bed to mimic changes to pre-convergence capillaries. To assess the position of individual capillaries along the path from arteriole to venule, the median distance to the penetrating arteriole trunk (medianDistMainDA) and the median distance to the ascending venule trunk (medianDistMainAV) were calculated for each capillary. Therefore, all paths leading from an individual capillary to the arteriole trunk were recorded. The median of these pathlengths represented the characteristic medianDistMainDA per capillary. To mimic vasoconstriction in the anesthetized state (Supplementary Fig. 16), all capillaries belonging to layer 6 and with a medianDistMainDA > 3 and medianDistMainAV > 1 were constricted by 7%. This corresponded to 1,168, 948, 1,669 and 1,610 for MVN1-open, MVN1-trimmed, MVN2-open and MVN2-trimmed, respectively.
To reduce capillary density in layer 6, data from the current study suggested that shorter capillaries tend to regress more frequently during aging (Supplementary Fig. 22). Consequently, the maximum length for a regressing capillary was set to 70 µm. Moreover, as before, only capillaries with a medianDistMainDA > 3 and medianDistMainAV > 1 were eligible for regression. These two criteria (length and position along the capillary path) yielded 788, 627, 1,165 and 1,131 capillaries potentially affected by regression in MVN1-open, MVN1-trimmed, MVN2-open and MVN2-trimmed networks, respectively. Considering that the total number of capillaries in layer 6 is 1,707, 1,413, 1,789 and 1,737 for these networks, it implied that 170, 141, 178 and 173 capillaries needed to be removed to mimic a density reduction by 10%. For each network, there were more capillaries that fit our selection criterion than needed to be removed from the network. Therefore, for each network, we generated five cases where candidate capillaries were randomly removed to match the target density reduction of 10%. These five cases were then used to compute the average change for a density reduction of 10% per configuration, increasing robustness of blood flow outcomes measured for each network. Capillary regression was modeled by constricting the selected capillaries by 97%. By comparing the average flow rate in the regressed capillaries to all flow rates in the network, we confirmed that the average flow rate in the regressed capillaries was close to zero (<2.5th percentile), effectively removing these capillaries from the perfused network. Microvascular alterations during the awake state were characterized by a 4.5% dilatation in layer 2/3, a 5.1% dilatation in layer 4 and a 14% constriction and 10% reduced density in layer 6 (Fig. 5 and Supplementary Fig. 14). The changes were introduced in an equivalent way, as described for the anesthetized case above.
The time-averaged flow field was computed for all four configurations (MVN1-open, MVN1-trimmed, MVN2-open and MVN2-trimmed) and three scenarios each: (1) capillary vasoconstriction in L6; (2) reduced capillary density in L6; and (3) vasoconstriction and density reduction in L6. Quantities of interest were the flow rate, the RBC velocity, the RBC flux and the RBC linear density. The flow rate was directly obtained from the pressure drop across the vessel and the effective flow resistance. The RBC velocity was computed by \({{{\mathrm{vf}}}\times q}_{{ij}}/{A}_{{ij}}\), where \({A}_{{ij}}\) is the vessel cross-section and vf the velocity factor accounting for the increased velocity of RBCs in comparison to the bulk flow, which was defined as the ratio of discharge to tube hematocrit31. The discharge hematocrit, htd, was calculated as a function of the tube hematocrit and the vessel diameter as defined in ref. 31. The RBC flux \({q}_{{ij}}^{{{\mathrm{RBC}}}}\) was the product of the flow rate \({q}_{{ij}}\) and the discharge hematocrit divided by the RBC volume, \({V}^{{{\mathrm{RBC}}}}\), that is, \({q}_{{ij}}^{{{\mathrm{RBC}}}}={q}_{{ij}}\times {{\mathrm{htd}}}/{V}^{{{\mathrm{RBC}}}}\). The RBC volume was 49 fl. As with the in vivo experiments, the linear density was computed by dividing the RBC flux by the RBC velocity.
In addition to comparing average perfusion quantities per layer, we computed the integral capillary inflow and outflow per cortical layer (Fig. 5f,g). Therefore, all start points and endpoints of the capillary bed were identified. A capillary start point was a bifurcation at which the vessel label changes from arterial vessel to capillary. The transition point between capillary and venule vessels marked a capillary end point. Capillary start and end points were assigned to the different layers based on their cortical depth. To obtain the integral capillary inflow per cortical layer, all arterial inflows into all capillary start points of the respective layer were summed (Fig. 5f). The equivalent computation was performed on the venule side to compute the integral outflow of the capillary bed (Fig. 5g).
There are some limitations of our in silico model in its ability to mimic the in vivo scenario. For example, the in silico model considered microvascular alterations only as an average, that is, all capillaries were constricted by a similar magnitude, whereas in vivo a range of constrictions and dilatations occurred. Moreover, our model did not account for additional age-related changes such as a reduced systemic hematocrit or vessel wall stiffening. Also, from a topological perspective it should be noted that the employed vascular networks do not contain a full PCV trunk. This forces capillary flow to be drained via shorter venules, which increases the flow resistance of deeper layers and could explain why predicted flow reductions are larger for the in silico model than observed in vivo. It is not possible to mimic all aspects of biological complexity in an in silico model. Despite this, the mentioned limitations do not affect the conclusions in the context of how layer-wise alterations in capillaries redistribute blood flow.
Immunohistology
Immunohistological analysis of gliosis, white matter deterioration, hypoxia and vascular inflammation was performed on two batches of animals. The first batch included adult (5–7 months) and aged (22–24 months) Thy1-YFP mice that previously underwent deep in vivo two-photon imaging experiments under isoflurane anesthesia. The second batch included adult (5–7 months), mid-aged (16–18 months) and aged (24 months) Thy1-YFP mice with no cranial windows or imaging performed.
Thy1-YFP mice, aged 5–7 months (n = 4, 2 male and 2 female) and 24 months (n = 5, 3 male and 2 female), were deeply anesthetized with Euthasol and transcardially perfused with PBS followed by 4% paraformaldehyde (PFA). Brains were dissected, post-fixed overnight, cryoprotected in 30% sucrose with 0.001% sodium azide for 24 h, frozen in optimal cutting temperature (OCT) compound and cryosectioned using a Leica cryostat. Brain sections (100 µm) were immunostained to detect microglia (rabbit anti-Iba1, Wako Chemicals, cat. no. 019-19741, 1:250), astrocytes (mouse anti-GFAP, Santa Cruz, cat. no. 2E1, 1:200) or myelin (mouse anti-MBP, BioLegend, cat. no. SMI 99; 1:500) for 48 h at 4 °C. After incubation with primary antibodies, sections were incubated with appropriate Alexa Fluor-conjugated secondary antibodies (Thermo Fisher Scientific, 1:500) with DAPI for 2 h at room temperature. All antibody staining was performed in a solution of 2% Triton X-100, 10% goat serum and 0.1% sodium azide in PBS. Tissue sections were washed 3× for 5 min in PBS after each antibody incubation. After staining, tissue was mounted on slides with Fluoromount-G (Thermo Fisher Scientific, cat. no. 00-4958-02).
Views of the entire sagittal brain section were obtained using an Olympus VS120 Slide Scanner. High-resolution confocal images were taken from the CC using a Zeiss 710 LSM confocal microscope and these images were used for analyses of data in Supplementary Fig. 18. Microglia or Iba1 cell density was determined within the CC underlying the somatosensory cortex by counting the number of Iba1+ cells divided by the total area using FIJI. Fluorescence intensity of GFAP, MBP and Thy1-YFP was determined by outlining the CC and measuring the mean signal intensity using FIJI. All samples were stained and imaged within the same batch. Exposure times and laser powers were kept consistent between all samples.
A separate batch of adult (5–7 months, n = 4, 2 male and 2 female), mid-aged (16–18 months, n = 6, 3 male and 3 female) and aged (24 months, n = 6, 3 male and 3 female) mice was injected with 80 mg kg−1 of pimonidazole-HCl (Hypoxyprobe, cat. no. HP3-1000Kit) via the retro-orbital vein under isoflurane anesthesia. Under deep anesthesia, the mice were then transcardially perfused with PBS at 90 min after Hypoxyprobe administration. Brains were isolated and hemisected at the midline. One hemisection was used for transcriptional analyses (see below) and the other was immersed in 4% PFA for 24 h and then transferred to 30% (w/v) sucrose for cryoprotection. Cryoprotected brain hemisections were embedded in OCT compound (Fisher Healthcare, cat. no. 23-730-571) and cut into 100-μm, sagittal, free-floating sections on a Leica cryostat. Sections were then incubated with primary antibodies in antibody solution (10% goat serum, 0.02% Triton X-100, 0.02% sodium azide in PBS) for 48 h at 4 °C with gentle shaking. Specific antibodies were used to immunostain for astrocytes (mouse anti-GFAP antibody; Sigma-Aldrich, cat. no. C9205-2ML, 1:100), microglia (rabbit anti-Iba1 antibody, Wako Chemicals, cat. no. 019-19741, 1:100), myelin (rat anti-MBP antibody, Abcam, cat. no. ab7349, 1:100), VCAM1 (rat anti-CD106 antibody, BioLegend, cat. no. 105701, 1:200) and Hypoxyprobe (rabbit anti-Hypoxyprobe antibody; Hypoxyprobe, cat. no. PAB2627, 1:100), whereas the vasculature was labeled with Lycopersicon Esculentum Tomato Lectin (VectorLabs, cat. nos. DL-1177-1 for DyLight 594-labeled lectin and DL-1178-1 for DyLight 649). After washing away unbound primary antibodies with PBS, sections were incubated with fluorescently labeled secondary antibodies (goat anti-rabbit IgG (H + L) Alexa Fluor-647, goat anti-rabbit IgG (H + L) Alexa Fluor-488 and goat anti-rat IgG (H + L) Alexa Fluor-594) for 2 h at room temperature. Before mounting, the sections were incubated with DAPI to label nuclei.
Stained brain sections were imaged using an Evident Scientific APX100 microscope. All image processing and quantification were performed in ImageJ (v.1.54f). Three nonoverlapping ROIs (300 × 150 × 24 (x, y, z) μm3) from the somatosensory mid-cortex and three nonoverlapping ROIs (150 × 150 × 24 (x, y, z) μm3) from the somatosensory layer 6/CC were obtained from adult, mid-aged and aged sections (four mice per group). For analysis of microglia and astrocytes, the Iba1+ and GFAP+ areas in each ROI were measured and averaged. Specifically, background was subtracted (rolling ball radius = 50, twice) in maximally projected z-stacks of 16-bit exported images. After despeckling and thresholding (triangle thresholding), images were converted to a mask. The binary images were eroded accordingly to remove noncellular signal. The binarized signal was measured and expressed as the percentage of the total ROI area. For analysis of MBP, VCAM1 and Hypoxyprobe staining, signal intensity was measured by recording the mean gray value of each ROI. For vessel density analysis, ROIs that included the mid-cortex and layer 6/CC of the somatosensory cortex were acquired from lectin-labeled sections. Vessel segmentation was performed in Imaris software v.7.7.2 (Bitplane, Oxford Instruments) from maximally projected z-stacks and conducted in a semi-automated fashion to ensure detection of only vasculature and not lectin-labeled cells, such as microglia.
RNA transcription analyses of adult, mid-aged and aged mice
Mice were anesthetized and transcardially perfused with PBS. The brains were dissected and hemisected at the midline, with the hemisected brains placed on their sides in ice-cold PBS to visualize internal structures. The cortex and underlying CC, above the hippocampus, were extracted. Then, the upper and lower cortices were separated using a scalpel, with lower cortex samples retaining the CC. The dissected tissue was immediately placed on dry ice to preserve RNA quality. RNA was extracted using the QIAGEN RNeasy Mini Kit (cat no. 74104) and complementary DNA was synthesized with the BioRad iScript cDNA Synthesis Kit (cat no. 1708890). RNA and cDNA quantity and quality were assessed using a Nanodrop spectrophotometer. Expression of hypoxia-driven genes, including slc2a1, ldha, pdk1 and vegfa, was analyzed using the following primer sequences: slc2a1 forward: TCAGGCGGAAGCTAGGAAC, slc2a1 reverse: GGAGGGAAACATGCAGTCATC; ldha forward: AGCAGGTGGTTGAGAGTGCT, ldha reverse: GGCCTCTTCCTCAGAAGTCA; pdk1 forward: CCCCGATTCAGGTTCACG, pdk1 reverse: CCCGGTCACTCATCTTCACA; and vegfa forward: CAGGCTGCTGTAACGATGAA, vegfa reverse: TTTGACCCTTTCCCTTTCCT. Ppia was used as a housekeeping gene with the following primer sequences: ppia forward: CCCTGGCACATGAATCCTGG, ppia reverse: GAGCTGTTTGCAGACAAAGTTC. ΔΔCt analysis was employed to assess changes in the expression of hypoxia-driven genes in the upper and lower cortices of adult, mid-aged and aged mice.
UCCAS model
A batch of adult (5–7 months) C57BL/6 mice (n = 7, 3 male and 4 female) was used for UCCAS model studies. Each animal was first implanted with a chronic cranial window centered over the somatosensory cortex, as described above. After a 3-week recovery period, animals underwent baseline deep in vivo two-photon imaging to identify layer 4 and layer 6/CC PCV networks for analysis and to perform line scans for baseline RBC flux measurements in pre-convergence capillaries. After 3 d, mild unilateral cerebral hypoperfusion was induced by microcoil placement around the left common carotid artery (ipsilateral to imaged brain hemisphere). Under isoflurane anesthesia, mice were placed in the supine position on a feedback-regulated heat pad (FHC Inc.) under the surgical microscope. A surgical plane of anesthesia was maintained at ~1.5% minimal alveolar concentration of isoflurane, delivered with medical air (20–22% oxygen and 78% nitrogen, moisturized by bubbling through water; AirGas Inc.). The ventral neck skin was cleaned with alternating swabs of 70% ethanol and 10% povidone–iodine solution. A 1.5-cm incision was made in the midline of the ventral neck. After resecting subcutaneous fat and salivary glands from the surgical field, the common carotid area, superior vena cava and vagal nerve were dissected from the connective tissue surrounding them. Two pieces of silk suture (4/0) were placed around the proximal and distal ends of the common carotid artery for ease of manipulation. A microcoil with inner diameter 0.20 mm (Sawane Spring Co. Ltd.; 0.08 × 0.20 × 0.5 × 2.5 SWP-A) was intertwined with the left common carotid artery to encase it within the center of the microcoil62. The neck incision was sutured with 4/0 monofilament (Ethilon, 662G). Post-surgery imaging was performed at days 3, 7, 14 and 21 to determine blood flow in the same population of pre-convergence capillaries on which baseline imaging was performed. In each imaging session, animals were imaged under 1.5% isoflurane anesthesia. To label the vasculature before imaging, mice were injected with 100 μl of 5% (w/v in sterile saline) 2 MDa dextran-Alexa Fluor-680 through the retro-orbital vein.
On post-surgery day 24, mice received 80 mg kg−1 of Hypoxyprobe (cat. no. HP3-1000Kit) via retro-orbital injection under isoflurane anesthesia. After 90 min, the mice were then perfused transcardially with PBS, followed by 4% PFA under the overdose of Euthasol (Patterson Veterinary, cat. no. 07-805-9296). Brains were removed and immersed in 4% PFA for 24 h and then transferred to 30% (w/v) sucrose for cryoprotection. Cryoprotected brains were embedded using OCT compound and 100-μm coronal free-floating sections were obtained to provide comparison of brain hemispheres ipsilateral and contralateral to the stenosed carotid artery. The contralateral hemisphere was used as an internal control for histological assessment. We immunostained for microglia (rabbit anti-Iba1 antibody, Wako Chemicals, cat. no. 019-19741, 1:100), astrocytes (Cy3-conjugate mouse anti-GFAP antibody, Sigma-Aldrich, cat. no. C9205-2ML, 1:100), myelin (rat anti-MBP antibody, Abcam, cat. no. ab7349, 1:100) and Hypoxyprobe (rabbit anti-Hypoxyprobe antibody; Hypoxyprobe, cat. no. PAB2627, 1:100). Pan-vascular staining was achieved via Lycopersicon Esculentum Tomato Lectin (VectorLabs, cat. nos. DL-1177-1 for DyLight 594 and DL-1178-1 for DyLight 649-labeled Lectin). Free-floating sections were incubated in the above-mentioned primary antibodies in antibody solution (10% goat serum, 0.02% Triton X-100 and 0.02% sodium azide in PBS) for 48 h at 4 °C on gentle shaking. After washing the primary antibodies, the tissues were incubated with fluorescently labeled secondary antibodies (goat anti-rabbit IgG (H + L) Alexa Fluor-647, goat anti-rabbit IgG (H + L) Alexa Fluor-488 and goat anti-rat IgG (H + L) Alexa Fluor-594) for 2 h at room temperature. Before mounting, the sections were incubated with DAPI to label nuclei.
Stained brain sections were imaged using an Evident Scientific APEX100 microscope. All image processing and quantification were performed in ImageJ (v.1.54f). For each brain section, three nonoverlapping ROIs (150 × 150 × 24 (x, y, z) μm3) were placed in ipsilateral mid-cortex, contralateral mid-cortex, ipsilateral layer 6/CC and contralateral layer 6/CC of the somatosensory cortex (12 ROIs per section). Analysis of immunostaining was performed as described above for the examination of tissue changes in adult, mid-aged and aged mice.
Statistics
Statistical analyses for in vivo and light-sheet data were performed using SPSS software. Analysis of all parameters was performed using a repeated-measure analysis of variance (ANOVA) model or a linear mixed-effect model, with age (adult versus aged) and layer (2/3 versus 4 versus 6/CC) set as independent factors and animal as a within-group factor. Therefore, data from each animal were assigned to the corresponding age group and further subdivided into the three analyzed layers. This strategy considered the nested nature of the data, that is, that multiple vessels come from one animal and are thus not independent of each other. Strength and significance of correlation between analyzed parameters were determined using Pearson’s correlation analysis in GraphPad Prism v.9 software. All graphs were made using GraphPad Prism v.9 software.
Analysis of age-dependent histological changes and UCCAS studies was performed using a two-way repeated-measure ANOVA model in GraphPad Prism v.9 software. For histology, two independent factors within the model were age (adult versus mid-aged versus aged) and layer (4 versus 6/CC) for aging sections with the dependent variable being either fluorescent intensity or percentage of positive area of the immunohistochemical marker. For UCCAS studies, hemisphere (ipsilateral versus contralateral to the stenosis) and layer (4 versus 6/CC) were determined as independent variables. Post hoc tests were performed using the Holm–Šídák test to further explore significant interactions. All statistical tests were performed at an α level of 0.05.
No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications23,32,35. No formal randomization was used for selecting animals for surgery and imaging. Surgeries were performed and data were collected in an unblinded manner. However, all analyses were performed blinded to the conditions.
Animal and data exclusion criteria
Out of 12 adult chronic cranial windows, 1 was damaged before the line-scan data for layer 6/CC could be obtained. Out of 12 aged windows, 1 was damaged before the line-scan data for layer 6/CC could be obtained, whereas for another, only the line-scan data for layer 6/CC was obtained. This resulted in 12 complete datasets for layers 2/3 and 4, and 11 complete datasets for layer 6/CC in the adult group, as well as 11 complete datasets for all layers in the aged group.
For analysis of structural and functional parameters of vascular segments, if the signal quality did not allow for reliable analysis of lumen diameter, RBC flux or RBC velocity, these parameters were excluded from the analysis for the corresponding vessel segment, whereas the length and tortuosity data were included. Correlation analyses were performed only on the population of vessels where all analyzed structural and functional parameters could be obtained.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw image files are stored on servers at Seattle Children’s Research Institute owing to their large size. Raw image files used in making all figures, along with details of data sampling within these images, will be available upon request from the corresponding author. Data, such as extracted metrics of vascular structure and blood flow used in figures, are provided as Supplementary Information. There are no restrictions on these data. Source data are provided with this paper.
Code availability
Code for analysis of in silico datasets is available via GitHub at https://github.com/Franculino/vgm.git. Further details are available in the original publications of the in silico model and upon request from F.S.
References
Gunning-Dixon, F. M., Brickman, A. M., Cheng, J. C. & Alexopoulos, G. S. Aging of cerebral white matter: a review of MRI findings. Int. J. Geriatr. Psychiatry 24, 109–117 (2009).
Liu, H. et al. Aging of cerebral white matter. Ageing Res. Rev. 34, 64–76 (2017).
Groh, J. & Simons, M. White matter aging and its impact on brain function. Neuron 113, 127–139 (2025).
Dewar, D., Underhill, S. M. & Goldberg, M. P. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow. Metab. 23, 263–274 (2003).
Rosko, L., Smith, V. N., Yamazaki, R. & Huang, J. K. Oligodendrocyte bioenergetics in health and disease. Neuroscientist 25, 334–343 (2019).
Brickman, A. M. et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 172, 117–120 (2009).
Chen, J. J., Rosas, H. D. & Salat, D. H. The relationship between cortical blood flow and sub-cortical white-matter health across the adult age span. PLoS ONE 8, e56733 (2013).
Murugesan, N., Demarest, T. G., Madri, J. A. & Pachter, J. S. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol. Aging 33, e1–e16 (2012).
Schager, B. & Brown, C. E. Susceptibility to capillary plugging can predict brain region specific vessel loss with aging. J. Cereb. Blood Flow. Metab. 40, 2475–2490 (2020).
Bennett, H. C. et al. Aging drives cerebrovascular network remodeling and functional changes in the mouse brain. Nat. Commun. 15, 6398 (2024).
Salat, D. H. Imaging small vessel-associated white matter changes in aging. Neuroscience 276, 174–186 (2014).
Koizumi, K. et al. Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat. Commun. 9, 3816 (2018).
Hahn, O. et al. Atlas of the aging mouse brain reveals white matter as vulnerable foci. Cell 186, 4117–4133 (2023).
Kirilina, E. et al. Superficial white matter imaging: contrast mechanisms and whole-brain in vivo mapping. Sci. Adv. 6, eaaz9281 (2020).
Nazeri, A. et al. Superficial white matter as a novel substrate of age-related cognitive decline. Neurobiol. Aging 36, 2094–2106 (2015).
Phillips, O. R. et al. The superficial white matter in Alzheimer’s disease. Hum. Brain Mapp. 37, 1321–1334 (2016).
Wang, S. et al. Superficial white matter microstructure affects processing speed in cerebral small vessel disease. Hum. Brain Mapp. 43, 5310–5325 (2022).
Kidd, D. et al. Cortical lesions in multiple sclerosis. Brain 122, 17–26 (1999).
Duvernoy, H. M., Delon, S. & Vannson, J. L. Cortical blood vessels of the human brain. Brain Res. Bull. 7, 519–579 (1981).
Li, B. et al. Two-photon microscopic imaging of capillary red blood cell flux in mouse brain reveals vulnerability of cerebral white matter to hypoperfusion. J. Cereb. Blood Flow. Metab. 40, 501–512 (2019).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 7, 205–209 (2013).
Taylor, Z. J. et al. Microvascular basis for growth of small infarcts following occlusion of single penetrating arterioles in mouse cortex. J. Cereb. Blood Flow. Metab. 36, 1357–1373 (2016).
Hartmann, D. A. et al. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 24, 633–645 (2021).
Cruz Hernández, J. C. et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci. 22, 413–420 (2019).
Reeson, P., Choi, K. & Brown, C. E. VEGF signaling regulates the fate of obstructed capillaries in mouse cortex. eLife 7, e33670 (2018).
Secomb, T. W. Red blood cell mechanics and capillary blood rheology. Cell Biophysics 18, 231–251 (1991).
Schmid, F., Barrett, M. J. P., Obrist, D., Weber, B. & Jenny, P. Red blood cells stabilize flow in brain microvascular networks. PLoS Comput. Biol. 15, e1007231 (2019).
Cai, C. et al. Impaired dynamics of precapillary sphincters and pericytes at first-order capillaries predict reduced neurovascular function in the aging mouse brain. Nat. Aging 3, 173–184 (2023).
Desjardins, M., Berti, R., Lefebvre, J., Dubeau, S. & Lesage, F. Aging-related differences in cerebral capillary blood flow in anesthetized rats. Neurobiol. Aging 35, 1947–1955 (2014).
Schmid, F., Tsai, P. S., Kleinfeld, D., Jenny, P. & Weber, B. Depth-dependent flow and pressure characteristics in cortical microvascular networks. PLoS Comput. Biol. 13, e1005392 (2017).
Pries, A. R. & Secomb, T. W. Microvascular blood viscosity in vivo and the endothelial surface layer. Am. J. Physiol. Heart Circ. Physiol. 289, H2657–H2664 (2005).
Bonney, S. K., Nielson, C. D., Sosa, M. J., Bonnar, O. & Shih, A. Y. Capillary regression leads to sustained local hypoperfusion by inducing constriction of upstream transitional vessels. Proc. Natl Acad. Sci. USA 121, e2321021121 (2024).
Raj, D. et al. Increased white matter inflammation in aging- and Alzheimer’s disease brain. Front. Mol. Neurosci. 10, 206 (2017).
Jin, K. et al. Brain-wide cell-type-specific transcriptomic signatures of healthy ageing in mice. Nature 638, 182–196 (2025).
Berthiaume, A. A. et al. Pericyte remodeling is deficient in the aged brain and contributes to impaired capillary flow and structure. Nat. Commun. 13, 5912 (2022).
Choe, Y. G. et al. Pericyte loss leads to capillary stalling through increased leukocyte-endothelial cell Interaction in the brain. Front. Cell. Neurosci. 16, 848764 (2022).
Hall, C. N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014).
Zhao, L. et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat. Commun. 11, 4413 (2020).
Ding, R. et al. Loss with ageing but preservation of frontal cortical capillary pericytes in post-stroke dementia, vascular dementia and Alzheimer’s disease. Acta Neuropathol. Commun. 9, 130 (2021).
Nikolakopoulou, A. M. et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat. Neurosci. 22, 1089–1098 (2019).
Vazquez-Liebanas, E. et al. Adult-induced genetic ablation distinguishes PDGFB roles in blood-brain barrier maintenance and development. J. Cereb. Blood Flow. Metab. 2, 264–279 (2021).
Klug, N. R. et al. Intraluminal pressure elevates intracellular calcium and contracts CNS pericytes: role of voltage-dependent calcium channels. Proc. Natl Acad. Sci. USA 120, e2216421120 (2023).
Hartmann, D. A., Coelho-Santos, V. & Shih, A. Y. Pericyte control of blood flow across microvascular zones in the central nervous system. Annu. Rev. Physiol. 84, 331–354 (2021).
Nortley, R. et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365, eaav9518 (2019).
Korte, N. et al. Inhibiting Ca2+ channels in Alzheimer’s disease model mice relaxes pericytes, improves cerebral blood flow and reduces immune cell stalling and hypoxia. Nat. Neurosci. 27, 2086–2100 (2024).
Shrouder, J. J. et al. Continued dysfunction of capillary pericytes promotes no-reflow after experimental stroke in vivo. Brain 147, 1057–1074 (2023).
Shin, P. et al. Aerobic exercise reverses aging-induced depth-dependent decline in cerebral microcirculation. eLife 12, e86329 (2023).
Li, B. et al. More homogeneous capillary flow and oxygenation in deeper cortical layers correlate with increased oxygen extraction. eLife 8, e42299 (2019).
Moeini, M. et al. Compromised microvascular oxygen delivery increases brain tissue vulnerability with age. Sci. Rep. 8, 8219 (2018).
Goirand, F., Le Borgne, T. & Lorthois, S. Network-driven anomalous transport is a fundamental component of brain microvascular dysfunction. Nat. Commun. 12, 7295 (2021).
Kirst, C. et al. Mapping the fine-scale organization and plasticity of the brain vasculature. Cell 180, 780–795 (2020).
Ji, X. et al. Brain microvasculature has a common topology with local differences in geometry that match metabolic load. Neuron 109, 1168–1187 (2021).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Preibisch, S., Saalfeld, S. & Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 (2009).
McDowell, K. P., Berthiaume, A. A., Tieu, T., Hartmann, D. A. & Shih, A. Y. VasoMetrics: unbiased spatiotemporal analysis of microvascular diameter in multi-photon imaging applications. Quant. Imaging Med. Surg. 11, 969–982 (2021).
Kim, T. N. et al. Line-scanning particle image velocimetry: an optical approach for quantifying a wide range of blood flow speeds in live animals. PLoS ONE P7, e38590 (2012).
Ouzounov, D. G. et al. In vivo three-photon imaging of activity of GCaMP6-labeled neurons deep in intact mouse brain. Nat. Methods 14, 388–390 (2017).
Blinder, P. et al. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 16, 889–897 (2013).
Schmid, F., Reichold, J., Weber, B. & Jenny, P. The impact of capillary dilation on the distribution of red blood cells in artificial networks. Am. J. Physiol. Heart Circ. Physiol. 308, H733–H742 (2015).
Fung, Y. C. Stochastic flow in capillary blood vessels. Microvasc. Res. 5, 34–48 (1973).
Lorthois, S., Cassot, F. & Lauwers, F. Simulation study of brain blood flow regulation by intra-cortical arterioles in an anatomically accurate large human vascular network. Part II: flow variations induced by global or localized modifications of arteriolar diameters. Neuroimage 54, 2840–2853 (2011).
Ihara, M. & Tomimoto, H. Lessons from a mouse model characterizing features of vascular cognitive impairment with white matter changes. J. Aging Res. 2011, 978761 (2011).
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH)/National Institute on Aging (NIA) (nos. R01AG062738, R21AG069375, RF1AG077731 and R01AG081840) and a pilot award from the Albert Institute for White Matter Research. Y.K. was supported by the NIH/National Institute of Neurological Disorders and Stroke (NINDS) (no. R01NS108407). F.S. received funding from the Swiss National Science Foundation (grant no. 202192). S.K.B. was supported by fellowships from the NIH/National Institute of Neurological Disorders and Stroke (no. F32NS117649) and NIH/NIA (no. K99AG080034). M.J.S. was supported by a Diversity supplement for a grant from the NIH/NIA (grant no. R01AG062738-03S1). We thank T. Figueiredo for creating the artwork used in Fig. 2e,f. We also thank J. Gust, Y. Li and C. Foster for their helpful comments and suggestions during manuscript preparation.
Author information
Authors and Affiliations
Contributions
S.S., F.S., G.G., F.A., Y.K., J.W. and A.Y.S. conceived the study and designed the experiments. S.S., F.A., G.G., N.W., K.T., S.K.B., M.J.S. and H.C.B. conducted the experiments. S.S., G.G., N.W., M.J.S. and S.K.B. performed the data analysis and statistics. F.S. performed the in silico studies and analyses. S.S. and A.Y.S. wrote the manuscript with contributions from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Ali Erturk, Christian Goeritz and Nicolas Renier for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Principal cortical venules in relation to other penetrating vessel types in mouse sensory cortex.
a. Light-sheet imaging data showing dorsal view of a mouse brain with arterioles labeled (α-SMA and Sm22). The dotted yellow square marks the region of interest (ROI), centered on the primary somatosensory cortex, used for analysis of the number and penetration depths of cortical penetrating vessels. b. Maximum projection images showing the side view of the ROI defined by the white dotted lines in panel (a), with labeling of cortical arterioles by Acta-2 and Sm22 (upper panel) and all blood vessels by lectin (lower panel). Pial penetration points of arterioles and venules are marked with arrows. c. Side projection of analyzed volume with colored circles marking the intracortical termination points of different penetrating vessel types. The inset on the right shows an enlarged volume denoted by a white square, with a venule termination point marked by a cyan circle. d. Plot of the average number of penetrating vessel types within the ROIs. N = 4 adult mice. Data shown as mean ± SD. e. Plot of the intracortical termination depth of different penetrating vessel types. Each dot corresponds to a single vessel. Gray dotted line shows approximate depth of gray-white matter transition. N = 218, 422, and 13 vessels for arterioles, other venules, and PCVs, respectively, collected over 4 adult mice.
Extended Data Fig. 2 Penetrating arteriole and arteriole-capillary transition zones of adult and aged mice are similar in diameter.
a. Maximally projected image in layer 6/CC showing a top-down view of the vascular bed surrounding the main trunk of a PCV in an adult mouse. Red arrow marks a penetrating arteriole trunk. b. Inset showing a magnified view around the penetrating arteriole with the first three branch orders of vessel offshoots, referred to as arteriole-capillary transition zone. c. Average diameter of the penetrating arteriole trunk throughout cortical depth in adult and aged mice. Each dot corresponds to an average diameter of arteriole trunks at the corresponding depth in a single animal. One-way ANOVA with Holm-Sidak post-hoc test. Adult, n = 12 mice, with 3-4 vessels per cortical depth per animal; Aged, n = 11 mice, with 3-4 vessels per cortical depth per animal. Data shown as mean ± SD. d-f. Average diameter of vessels in the arteriole-capillary transition zone of adult and aged mice in Layer 2/3 (d), Layer 4 (e), Layer 6/CC (f). Each dot corresponds to an average diameter of vessels of the corresponding branch order in a single animal. One-way ANOVA with Holm-Sidak post-hoc test. Adult, n = 12 mice (n = 11 for Layer 6/CC); Aged, n = 11 mice. Data shown as mean ± SD.
Extended Data Fig. 3 Reduction in vascular length and pericyte cell density in layer 6/CC of aged mice.
a. Maximum intensity projection of the dorsal half of a whole mouse brain with arterioles labeled using α-SMA + Sm22 immunostaining imaged by light-sheet microscopy. b-d. Top-down views of the 540 × 540 × 1000 µm (x, y, z) regions of interest (ROI) centered on the somatosensory cortex marked with a dotted yellow square in (a), with arterioles labeled using α-SMA + Sm22 antibodies (b), all vessels labeled using fluorescent lectin (c), and mural cells labeled using the combination of anti-PDGFRβ and anti-CD13 antibodies (d). e,f. 3D side views from Imaris showing lectin (e) and mural cell (f) staining throughout the entire cortical depth. g, h. Examples of 200 µm thick ROIs from (e) and (f) (marked with dotted yellow lines) with reconstructions of all capillary segments (g, gray lines)) and annotated capillary pericyte cell somata (h, blue circles). i. Top-down view of vessels (cyan) and mural cells (red). j. Magnified regions from (i) (marked with yellow squares) with annotated examples of capillary pericytes exhibiting the ‘bump on the log’ morphology (marked with yellow arrows). k. Vascular length density in analyzed cortical subsections of adult and aged mice. Two-sided unpaired t-tests: 0–200 µm, p = 0.3849; 201–400 µm, p = 0.0541; 401–600 µm, p = 0.0005; 601–800 µm, p = 0.0034; 801–1000 µm, p = 0.0006. l. Pericyte density in analyzed cortical subsections of adult and aged mice. Two-sided unpaired t-tests: 0–200 µm, p = 0.7185; 201–400 µm, p = 0.2007; 401–600 µm, p = 0.0418; 601–800 µm, p = 0.0134; 801–1000 µm, p = 0.0038. m. Pericyte density in analyzed cortical subsections of adult and aged mice. Two-sided unpaired t-tests: 0–200 µm, p = 0.8617; 201–400 µm, p = 0.8043; 401–600 µm, p = 0.8913; 601–800 µm, p = 0.6062; 801–1000 µm, p = 0.0376. For all plots in k-m, n = 4 animals per age group. Data shown as mean ± SEM.
Supplementary information
Supplementary Information
Supplementary Figs. 1–22, Tables 1–4 and Methods.
Supplementary Video 1
Video showing deep in vivo two-photon imaging from the pial surface to the CC, centered on a PCV.
Supplementary Video 2
Video showing segmented PCV and surrounding penetrating arterioles and ascending venules.
Supplementary Data
Supplementary Data 1 – Source data for Supplementary Figs. 1, 3, 5–16 and 18–22.
Source data
Source Data Figs. 3–8 and Extended Data Figs. 1–3
Data values for plot generation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stamenkovic, S., Schmid, F., Gurler, G. et al. Impaired capillary–venous drainage contributes to gliosis and demyelination in mouse white matter during aging. Nat Neurosci 28, 1868–1882 (2025). https://doi.org/10.1038/s41593-025-02023-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41593-025-02023-z
This article is cited by
-
The glymphatic system in neurodegenerative diseases and brain tumors: mechanistic insights, biomarker advances, and therapeutic opportunities
Acta Neuropathologica Communications (2025)
-
The brain drain puts aging myelin in deep trouble
Nature Neuroscience (2025)