Abstract
It appears that the biologically-synthesized nanoparticles (NPs) have potential to perform as effective elicitors for the production of valuable secondary metabolites in plants. Besides, it has been reported that the toxicity of the biologically-synthesized NP is not as much as that of the chemically-synthesized NPs. Therefore, it is necessary to test their advantages aspects. In this study, the physical synthesis of perlite NPs and biologically-synthesis of TiO2/perlite nanocomposites (NCs) were conducted. Subsequently, their effects and explant source influence on the growth characteristics and secondary metabolite profiles of Hypericum perforatum callus cultures were evaluated. According to the obtained results, morphology of the synthesized perlite NPs and TiO2/perlite NCs were mesoporous and spherical with sizes ranging about 14.51–23.34 and 15.50–24.61 nm, respectively. Addition of perlite NPs and TiO2/perlite NCs to the culture medium at the concentration range of 25–200 mg/L showed no adverse impacts on the growth characteristics of H. perforatum calli. According to the GC-MS analysis, the stress caused by perlite NPs and TiO2/perlite NCs led to an increase in the variety, amount and number of volatile compounds. The calli obtained from in vitro grown plants produced more volatile compounds relative to the calli obtained from field grown plants under the nanomaterial stress conditions. The production of hypericin and pseudohypericin were also determined in the callus cultures under desired nanomaterials elicitation. Accordingly, our results suggest that perlite NPs and TiO2/perlite NCs can possibly be considered as effective elicitors for the production of volatile compounds, hypericin, and pseudohypericin in callus cultures of H. perforatum.
Similar content being viewed by others
Introduction
Hypericum perforatum L., also known as St. John’s wort, is an important medicinal plant with diverse bioactive constituents such as naphtodianthrones, acyl-phloroglucinols, flavonoids, and xanthones, which have been reported to have anti-inflammatory, antimicrobial, antitumoral, antidepressant and wound-healing activities1,2. Production of secondary metabolites by in vitro cultures of H. perforatum has been one of the most expansively investigated areas3,4. However, application of these plant cultures is still limited because of the low yield of the desired compounds. It has been reported that elicitation can be an attractive approach employed to improve the productivity of in vitro plant cultures5. Quite a lot of abiotic and biotic elicitors have been applied to explore the accumulation of secondary metabolites in cell and organ cultures of H. perforatum6,7. Recently, nanoparticles (NPs) have been proposed to be a nutrient source and an elicitor, leading to the overproduction of various secondary metabolites8. For instance, Poborilova et al.9 reported the accumulation of phenolics upon the addition of different concentrations of Al2O3 NPs to the tobacco cell suspension cultures which reached to the maximum level (211.7 µg/g FW) at the concentration of 100 µg/ml after 96 h of treatment. Similarly, artemisinin content was augmented in the 20-day-old Artemisia annua hairy root cultures treated with Ag-SiO2 core-shell NPs which was 3.9-fold higher than the control10. Multi-walled carbon nanotubes (MWCNTs) induced the production of secondary metabolites in Satureja khuzestanica callus cultures. The maximum amounts of the total phenolics and flavonoids were determined in the cultures exposed to 100 µg/ml of MWCNTs which were 1.9 and 2.6 times higher in comparison to the control, respectively. Furthermore, the highest content of rosmarinic acid (4.01 mg/g DW) and caffeic acid (2.78 mg/g DW) were obtained at the treatments of 100 and 250 µg/ml in S. khuzestanica callus cultures11. In the case of H. perforatum, an investigation reported that the cultures supplemented with 100 ppb (per 30 ml culture) of zinc nano-oxide showed increased amounts of hypericin (7.87 µg/g DW) and hyperforin (217.45 µg/g DW) when compared with the control (2.07 and 16.27 µg/g DW, respectively). Also, the augmented amounts of hypericin and hyperforin (11.18 and 195.62 µg/g DW, respectively) were observed in H. perforatum cultures treated with 100 ppb of iron nano-oxide12.
The mechanism through which NPs modulate secondary metabolism is not exactly understood. Genomic analyses have been revealed that plants can respond to the internalization of nano-sized materials like biotic or abiotic stress factors13,14. It has been proposed that like the other stressors, NPs can modulate plant secondary metabolism via inducing different cellular signal transduction pathways. Calcium flux, reactive oxygen species (ROS) burst, and mitogen-activated protein kinase (MAPK) phosphorylation can be the primary actions activated by NPs. Generation of ROS has been reported in most of the studies investigating NPs effects on plants15. It has been suggested that NP-induced ROS may act as a signal to induce the plant secondary metabolism16. Induction of the typical stress signaling reactions, mediated by cytosolic Ca2+ and ROS, has been reported in the model plant A. thaliana exposed to nanosilver17. Similar to animal and human cells, it is estimated that plants may also utilize oxidative stress signaling by using MAPK cascade modules14. Therefore, activation of signaling pathways finally leads to gene expression followed by enzymatic reactions, which consecutively change the production of secondary metabolites. Changes in the activities of some enzymes such as phenylalanine ammonia lyase, peroxides, and polyphenol oxidase have been already reported to be related to the biosynthesis of secondary metabolites18.
Perlite is a mineral structure with numerous industrial applications. Because of the exceptional properties such as being an inert, porous, low density and cheap material, perlite has been reported to be a suitable support for the immobilization of different catalysts19,20. Meanwhile, the immobilization of TiO2 NPs on perlite has been suggested to be an appropriate photocatalyst21. However, in spite of the varied scope of the applications of these nanomaterials, there is limited information about their impact on plants. Due to the unique properties, for instance large specific surface area and greater reactivity, these ultrafine particles have been considered favorable for many biological applications. Promising properties of perlite in the culture medium, such as improvement of nutrient uptake and aeration, can be used to improve growth and valuable metabolites production in in vitro cultures22. So far, TiO2 NPs have been revealed to have both beneficial and adverse effects on plants, which depends on the physicochemical properties of NPs and the plant species23,24. It has been reported that TiO2 NPs significantly improved the shoot/root length, chlorophyll content, and total soluble leaf protein of mung bean plant25. On the other hand, the increase in yield was observed after treatment of cowpea with TiO2 NPs26. The highest essential oil content and yield were observed in Salvia officials plants exposed to 200 mg/L of TiO2 NPs which were 1.75 and 2.74-folds higher than those of the control plants. Besides, the maximum contents of total phenolic (35.2 mg/g DW.) and flavonoid (21.9 mg/g DW) were determined in S. officials plants treated with 200 and 100 mg/L of TiO2 NPs, respectively27.
Biologically-synthesis of NPs has been considered as an important method to reduce the destructive effects of physico-chemical synthesis methods28. Moreover, biologically-synthesized NPs are more stable, more effective, and less toxic than chemically-synthesized NPs15,29. The physicochemical characteristics of NPs including size, shape, crystal structure, and elemental composition, as well as their biological behavior, can be affected by the utilization of different synthesis methods and diverse reducing and stabilizing materials. Moreover, the final state of the synthesized NPs can be influenced by the interaction with the surrounding media30. In this context, it is necessary to use the combination of diverse techniques for characterizing NPs to realize their full potential. In the present study, a physical approach for the synthesis of perlite NPs and a green method using an aqueous extract of H. perforatum for the synthesis of TiO2/perlite nanocomposites (NCs) were conducted. The properties of the synthesized nanostructures were analyzed using a combination of characterization techniques. Subsequently, the effects of explant source (explants obtained from the field grown plants and explants obtained from in vitro grown plants), perlite NPs and TiO2/perlite NCs on the growth and secondary metabolite modulation in H. perforatum callus cultures were investigated. To the best of our knowledge, this is the first report on the induction of secondary metabolite production by perlite NPs and biologically-synthesized TiO2/perlite NCs in callus culture of H. perforatum.
Materials and Methods
Perlite NPs preparation
Mineral powder of perlite with the chemical composition of Si, 33.8; Al, 7.2; Na, 3.4; K, 3.5; Fe, 0.6; Ca, 0.6; Mg, 0.2; trace elements, 0.2; O2, 47.5 and H2O, 3.0 (w/w %) provided from Mianeh area, Iran. This commercial powder was used as starting materials and was intermixed and ball-milled (ceramic balls, 10 h under Ar) at a ball/mill ratio of 10: 1.
TiO2/perlite NCs synthesis
The aerial part extract of H. perforatum was used for synthesis of TiO2/perlite NCs. About 10 g of the dried plant material was mixed with 100 ml of deionized water, followed by shaking (150 rpm) for 48 h at 25 °C and sonication for 20 min. Then, the mixture was filtered and centrifuged. In order to synthesize of TiO2/perlite NCs, 1 g of perlite NPs and 3.84 ml of titanium isopropoxy solution were mixed with 50 ml of the plant extract under constant stirring for 2 h. Subsequently, the mixture was adjusted to pH 7 with 1 M NaOH and refluxed for about 9 h at 140 °C. After washing with deionized water for several times, the resulting precipitate was subjected to oven drying for 4 h at 80 °C, followed by heating at 400 °C for 4 h.
Characterization of the synthesized nanomaterial
The characteristics of the synthesized nanomaterials were identified using UV-Vis spectroscopy (Spekol 1500), X-ray diffraction (XRD) (D500, Siemens Diffractometer-Germany), transmission electron microscopy (TEM) (LEO 906), field emission scanning electron microscopy equipped with energy dispersive X-ray spectroscopy (EDX) (MIRA3 FEG-SEM.), dynamic light scattering (DLS) (Nanotrac Wave), and Fourier transform infrared (FT-IR) spectroscopy (TENSOR27–Brucker) techniques. The as-synthesized samples for TEM analysis were prepared as follows. The samples were dispersed in ethanol and the suspensions were treated in ultrasonic bath for 20 min. Then, a drop of the dilute suspension was placed on a carbon-coated grid. Afterward, it was allowed to dry by evaporation at room temperature.
Plant material and callus cultures
For induction of callus, nodal stem explants of H. perforatum were used. The fresh stem explants of field grown plants, collected from the herbarium of Tabriz University of Medical Sciences (East Azarbaijan, Tabriz, Iran), were sterilized by 70% ethanol for 1 min and 20% sodium hypochlorite solution for 15 min, followed by washing with sterile deionized water. In order to obtain H. perforatum seedlings, the surface of the seeds was also sterilized using the method mentioned above. Then the sterilized seeds were incubated on Murashige and Skoog (MS) medium (3% sucrose, 0.8% agar, pH 5.6–5.8). 24-day-old seedlings were used as the source of the explant. In order to induce callus formation, the obtained explants from both in vitro and field grown plants were cut into approximately 1 cm segments and transferred on the solid MS medium containing 1 mg/L of 2,4-D, 1 mg/L of 6-benzyl adenine (BA), and different concentrations of perlite NPs and TiO2/perlite NCs (0, 25, 50, 100, 150 and 200 mg/L). Callus cultures were kept under 16 h light and 8 h dark photoperiod at 24 ± 1 °C. The different experimental steps are schematically presented in Fig. 1.
Biomass measurement
For each treatment, 30-day-old calli were used to measure the final fresh weight (FW) and dry weight (DW, dried at 35 °C for 24 h). Moreover, the number of shoots regenerated on calli was measured.
Chlorophyll and carotenoid contents
An amount of 20 mg of callus was homogenized with 2 mL of dimethyl sulfoxide solvent and centrifuged at 8000 rpm for 15 min. Then, the obtained supernatant was separated and analyzed for Chlorophyll a, Chlorophyll b and total carotenoids (Cx + c) contents by using UV-Vis spectrophotometer at 480, 649 and 665 nm31.
GC-Mass analysis
The volatile compounds of treated and untreated calli (600 mg) were isolated by n-hexane solvent (2 ml *3) and subjected to GC-Mass analysis using a fused silica capillary column (Elite-I, 30 m, 0.25 mm, 0.25 µm, 100% dimethylpolysiloxane) and a mass spectrometer 6890 (NMass selective detector/Agilent). The injection volume was 1 µL and the samples were analyzed under electron ionization energy of 70 eV. High purity (99.999%) helium, at a flow rate of 1 mL/min, was used as the carrier gas. The injector and ion-source temperature were set as 150 and 280 °C, respectively. The oven temperature was initially 50 °C for 3 min, then gradually increased to 120 °C at 10 °C/min (3 min), 150 °C at 10 °C/min (3 min), 220 °C at 7 °C/min (3 min), 290 °C at 7 °C/min (5 min), and finally increased to 300 °C and kept there for 2 min32. The volatile components were identified according to the comparison of their mass spectra with the NIST standard reference database. Standard of n-alkanes containing n and n + 1 carbons (Sigma) was used to calculate the retention indices (RI) using a generalized equation33.
LC-MASS/MASS analysis
The dried powder of H. perforatum calli (20 mg) was extracted using 1 mL of methanol in an ultrasonic bath for 60 min. The obtained extracts were collected by centrifugation (8,000 rpm, 10 min) and filtration through a 0.45-µm filter. Finally, the samples were stored at −20 °C in the dark34. The separation of the components of the extract was done using an Alliance separations module 2695 (Waters, Milford, MA, USA), including a quaternary solvent delivery system, degasser, autosampler, column heater combined with a Quattro Micro API Triple Quadrupole LC-MS/MS (Waters, Micromass, Manchester, UK), and a Gemini column (50 × 2.00 mm, 5 micron). Chromatographic elution was performed using triethylammonium acetate buffer (0.01 M) at pH 7.0 as mobile phase A and the mixture of methanol and acetonitrile (50:50, v/v) as phase B, at a flow rate of 1.5 ml/min. The injection volume was 10 µL and the column temperature was maintained at 40 °C. Separation started with 40% A and 60% B (0–2 min), a linear gradient was applied up to 95% B and 5% A (2–4 min) and held for 5 min in this condition. The initial conditions were held for 1.5 min as a re-equilibration step. The total run time was 10.5 min.
Mass analysis was performed in negative ion mode. The ESI negative source values were: capillary voltage, 3.5 kV; cone, 60 V; extractor, 2 V; RF lens, 0.3 V; source temperature, 110 °C; desolvation temperature, 360 °C; desolvation gas and cone gas (nitrogen 99.99%) flow rates, 600 and 50 L/h, respectively. The analyzer settings were: resolution, 24.0 and 14.0 (unit resolution) for LM1 and LM2 resolution, respectively; 14.0 and 14.0 for HM1 and HM2 resolution, respectively; ion energy 0.5 and 1, respectively; entrance and exit energies, 60 and 60 (V); multiplier, 450 (V); collision gas (argon, 99.995%) pressure 1 × 10−4 mbar. The quantification of hypericin and pseudohypericin was performed by preparing different calibration standard solutions and recording the calibration curve. Hypericin and pseudohypericin (1 mg, Sigma) were dissolved in methanol (2 ml) and used as standard solution27. MassLynx 4.1 software was used to quantify the analyses result35. Since the analysis was performed with only one replicate, the data were qualitatively reported.
Data analysis
All trials were directed under a completely randomized design by means of three replicates with 10 explants per treatment. Statistical analysis was carried out by using a one-way analysis of variance (ANOVA) based on Duncan’s comparison mean test (SPAS16, P ≤ 0.05).
Results and Discussion
Structural characterization of perlite NPs and TiO2/perlite NCs
Structural features of perlite NPs and TiO2/perlite NCs were analyzed using UV-Vis spectroscopy, XRD, FESEM, EDX, TEM and FTIR techniques. UV–vis spectroscopy is an indirect technique to evaluate the fabrication of TiO2/perlite NCs from perlite NPs. Earlier reports stated that the maximum absorbance between 300–350 nm arises from the presence of green synthesized TiO2 NPs36 (Fig. 2A,B). When TiO2 nanoparticles are immobilized on the surface of perlite, the absorption shows maxima at 339 nm. Therefore, it confirms the formation of TiO2/perlite NCs (Fig. 2B).
XRD pattern of perlite NPs indicated a characteristic peak at 2θ = 25° with an amorphous nature (Fig. 3A)22. The additional reflections at 2θ = 25.39° (101), 38.11° (004), 48.00° (200), 54.09° (105), 68.24 (116), 70.20° (220) were observed in the XRD pattern of TiO2/perlite NCs when TiO2 NPs were immobilized on the surface of perlite, which confirmed the anatase crystallite structure of TiO2 NPs and the tetragonal structure of TiO2/perlite NCs (Fig. 3B)37. All peaks in the diffractogram (Fig. 3B) were in good agreement with the standard spectrum (JCPDS no.: 88–1175 and 84–1286). The average crystallite size of perlite NPs and TiO2/perlite NCs were measured by Debye–Scherrer formula as 13.72 and 18.65 nm, respectively.
The SEM and TEM images of as-synthesized perlite NPs and TiO2/perlite NCs are showed in Fig. 4A–D. These images (Fig. 4A,C) show the plate-shape and mesoporous entity for perlite NPs. In addition, as can be seen in Fig. 4D the perlite plates are entirely covered by TiO2 nanoparticles that appear as an aggregation of small spherical particles. Based on SEM image the morphology of as-synthesized TiO2 particles on perlite plate were with sizes ranging about 15.50–24.61 nm (Fig. 4B, Table 1).
According to the EDX spectrum of synthesized perlite NPs (Fig. 5A), it can be concluded that the silicon and aluminium were as the major elements because a higher amount of Si and Al are present in the profile. The presence of both Ti and O elements of the TiO2/perlite NCs was evident (Fig. 5B). The peaks of Ti seen on 0.6, 4.7 and 4.9 Kev. It appears that the presence of non-crystalline phytochemical substances which capped the TiO2/perlite NCs reduced the Ti:Si ratio (please see the discausion about FT-IR spectra and possible mechanism for the synthesis of TiO2/perlite NCs). Similar results for EDX patterns have been referred in literatures38. No other impurities were observed in EDX profile.
According to the DLS analysis, the size distribution and zeta potential of nanomaterials were 85.04–93.76 nm and +30.09 mV for perlite NPs and 168–173.4 nm and +37 mV for TiO2/perlite NCs, respectively (Fig. 6, Table 1). The size measurements using DLS are basically determined by the hydrodynamic diameter of NPs, which depends not only on the core of the NP but also on surface coating and ion concentration in the medium. For that reason, the particle size can be larger than the sizes obtained using the SEM and XRD. Consistent with the DLVO theory, the high repulsive force between the nanostructures, due to the high surface charge, inhibits their agglomeration. Therefore, the high values of zeta potential confirm the high dispersity and stability of the synthesized nanomaterials in the suspension.
The FT-IR spectra of perlite NPs and TiO2/perlite NCs can be seen in Fig. 7. At FT-IR spectra of perlite NPs: The bonds at 457 and 1047 cm−1 corresponded to Al-O and Si-O stretching vibration bond, respectively. These peaks are the main features in perlite and other aluminosilicate phases39. The bands 3621 and 3740 cm−1 can also result from water bound directly to Si-O-H and Al-O-H (strongly bound to a surface as inner sphere complexe39 (Fig. 7A). At FT-IR spectra of TiO2/perlite NCs: the FTIR spectrum of TiO2 NPs clearly shows tree bands. The first band is the peak on 789 cm−1 was assigned to the Ti-O stretching bands. The second band is observed around 1610 and 1742 cm−1, corresponding to C=C and C=O of the aromatic ring and carbonyl functional groups, respectively. These bonds can be resulted from the functional groups of secondary metabolites of the extract22,29. It appears that the secondary metabolites of the extract, such as hyperforin, containing C=C and C=O groups, could be as a capping and stabilizing agent. It is due to the fact that the C=C and C=O groups of these secondary metabolites have a strong affinity to bind metals then can act as encapsulating agent and accordingly prevent the agglomeration of TiO2/perlite NCs29. The third is prominent peaks at 17423621, 3740 and 3846 cm−1 related to water bound directly to Si-O-H, Al-O-H and Ti-O-H, respectively39 (Fig. 7B).
The possible mechanism for the synthesis of TiO2/perlite NCs
According to the available data about the biosynthesis of metal oxide NPs by using different plant extracts, a precise mechanism for the synthesis of NPs has not yet been approved. However, polar groups seem to be possible candidates for the biosynthesis of these NPs29,40,41. Hyperforin is the major of the two acylphloroglucinols that present in H. perforatum42. The adapted mechanism related to the capping effect of the plant extract is depicted in Fig. 8. Apparently, the vacant orbital of Ti4+ can be occupied by the lone pair electrons of the polar groups of molecule I. Afterwards, capping of Ti4+ ion by polar groups of the plant extract organizes a complex composite formation. Finally, calcination led to the synthesis of TiO2/perlite NCs in the reaction.
The effect of perlite NPs and TiO2/perlite NCs on growth parameters
In order to investigate the effects induced by the synthesized nanomaterials on callus growth, we exposed the callus cultures of H. perforatum to different concentrations of perlite NPs and TiO2/perlite NCs. Total biomass of the callus cultures (fresh weight) was measured after 30 days of culture with and without the nanomaterials. In the cultures obtained from in vitro grown plants, as presented in Fig. 9A, both perlite NPs and TiO2/perlite NCs affected H. perforatum callus growth. Perlite NPs at the concentration of 50 mg/L and TiO2/perlite NCs at the concentrations of 100 and 200 mg/L significantly enhanced the callus growth by 104%, 108%, and 52% when compared to the control, respectively. In contrast, the callus cultures obtained from field grown plants showed no significant difference in fresh biomass of treated cultures, compared to the control (Fig. 9B). However, compared to the all treated cultures, highest biomass was observed in the calli under 100 mg/L of TiO2/perlite NCs.
Regarding the number of shoots per callus in the cultures obtained from in vitro grown plants, only 25 mg/L of TiO2/perlite NCs showed the lowest shoot number in comparison to the control callus cultures. Nevertheless, in cultures obtained from field grown plants, both perlite NPs and TiO2/perlite NCs had no effect on the shoot number (Figs 10, 11).
The effect of perlite NPs and TiO2/perlite NCs on shoot number of the calli obtained from in vitro grown (A) and field grown (B) plants. Different letters indicate significant differences at p ≤ 0.05. The error bars represent standard error of the mean. Figure 10B shows statistically non-significant results (p > 0.05).
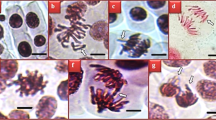
Calli obtained from in vitro grown plants: control (a), callus treated with 50 mg/L of perlite NPs (b), callus treated with 25 mg/L of TiO2/perlite NCs (c). Calli obtained from field grown plants: control (d), callus treated with 50 mg/L of perlite NPs (e), callus treated with 25 mg/L of TiO2/perlite NCs (f). Scale bar = 4 mm.
According to the available data, different plant species show various responses to TiO2 NPs regarding their growth parameters43. Consistent with our results, some studies have indicated that TiO2 NPs have positive effects on plants growth. For instance, the results reported by Dehkourdi and Mosavi44 showed that nano-anatase (TiO2) caused a significant increase in the seed germination and biomass of Petroselinum crispum seedlings. It has also been reported that TiO2 NPs promote the growth at a suitable concentration in wheat seedlings grown in soil45. Nanoperlite at the concentration of 150 mg/L increased the shoot number in Melissa officinalis plant organ cultures which was attributed to the beneficial properties of perlite such as improvement of nutrient uptake and aeration in the culture medium22.
The effects of perlite NPs and TiO2/perlite NCs on photosynthetic pigments content
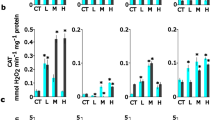
The contents of chlorophyll a, chlorophyll b, and total carotenoids (Cx + c) of H. perforatum calli were measured after treatment with different concentrations of perlite NPs and TiO2/perlite NCs. According to our results, applied nanomaterials had no effect on photosynthetic pigments content of in vitro grown calli (Fig. 12A). Related to the cultures obtained from field grown plants, there were no statistically significant difference in chlorophyll a and chlorophyll b contents between the untreated calli and those treated with perlite NPs and TiO2/perlite NCs. However, total carotenoids total carotenoids (Cx + c) content increased in calli after exposure to 200 mg/L of perlite NPs, as well as 25 and 100 mg/L of TiO2/perlite NCs (Fig. 12B). An increase in the contents of photosynthetic pigments has been reported in plants treated with TiO2 NPs. TiO2 NPs promoted chlorophyll formation, photosynthetic rate, and growth in spinach plants46. Increased photosynthetic rate by TiO2 NPs has also been reported in Vigna unguiculata plant26.
The effect of perlite NPs and TiO2/perlite NCs on the total carotenoids (Cx + c) contents of calli obtained from in vitro grown (A) and field grown (B) plants. Different letters indicate significant differences at p ≤ 0.05. The error bars represent standard error of the mean. Figure 12A shows statistically non-significant results (p > 0.05).
The effects of perlite NPs and TiO2/perlite NCs on volatile compounds
The variability in the composition of the volatile compounds of H. perforatum calli cultures was examined after exposure to perlite NPs and TiO2/perlite NCs. The GC-MS analysis showed 50 and 31 compounds in the extract of the calli obtained from in vitro grown and field grown plants, respectively (Tables 2, 3). Our results revealed the existence of hydrocarbons (aliphatic, aromatic and operating agent), alkaloids, phenolics, fatty acids, steroids and terpenes in the callus extracts. Alkaloids (such as 1,4-Phthalazinedione and 2,3-dihydro-6-nitro) were the main volatile constituents of the calli obtained from in vitro grown and field grown plants (Tables 2, 3). For calli obtained from in vitro grown plants, the control and treatment with 150 mg/L of TiO2/perlite NCs with 5 compounds and the treatment of 100 mg/L of perlite NPs with 20 compounds had the lowest and highest number of volatile compounds, respectively. The volatile compounds of the calli treated with 100 mg/L of perlite NPs contained a high number of aliphatic hydrocarbons (17 types of hydrocarbons). Only the callus cultures of control produced steroid (stigmasta-3,5-diene, 33.9%). A high percentage of alkaloids (84.43%) was determined in the treatment of 25 mg/L perlite NPs. Among the all treatments, 100 and 200 mg/L of perlite NPs significantly increased fatty acids by 4.41% and 12.19%, respectively, but control calli did not have these fatty acids. Only the cultures treated with 100 mg/L of perlite NPs produced sesquiterpene (1.9%) and diterpene (7.2%). Generally, the variety of volatile compounds in both perlite NPs and TiO2/perlite NCs treatments was higher than the control. However, the variety of compounds in perlite NPs treatments was higher than the TiO2/perlite NCs treatments.
The GC-MS results of volatile compounds of the calli obtained from field grown plants (Table 3) indicated that alkaloid and aliphatic hydrocarbon compounds exist in all treatments. The highest number of compounds was observed in 100 mg/L of perlite NPs treatment (15 compounds) and the lowest number of compounds was observed in 200 mg/L of TiO2/perlite NCs treatment (2 compounds). Among the all perlite NPs and TiO2/perlite NCs treatments, only 100 mg/L of perlite NPs induced the production of fatty acid (hexadecanoic acid, methyl ester, 14.34%). Control and treatments with 100 mg/L of perlite NPs and 50 mg/L of TiO2/perlite NCs showed a significant increase in the production of carboxylic acids. The extracts of the calli treated with 50 mg/L of perlite NPs contained a large number of alkaloids (76.11%). All treatments except 200 mg/mL of TiO2/perlite NCs were able to produce alkaloids. Phenolic compounds were observed in all treatments except for 200 mg/L of TiO2/perlite NCs and 100 mg/L of perlite NPs. According to the GC-MS results, calli obtained from in vitro grown plants produced more volatile compounds relative to the calli obtained from field grown plants under the nanomaterial stress conditions. The stress caused by perlite NPs and TiO2/perlite NCs led to an increase in the variety, amount and number of volatile compounds in both calli.
In accordance with our results, some other studies have reported the potential of TiO2 NPs in the modulation of volatile compounds content of plants. Mohammad et al.47 demonstrated that foliar application of TiO2 NPs considerably augmented shoot dry mass and essential oil content of Dracocephalum moldavica L. under normal irrigation and water-deficit stress. The GC–MS revealed that S. officinalis plants enhanced plant dry matter and essential oils content after exposure to nano-TiO227. Regarding the industrial application of essential oils, utilizing effective elicitors can be a beneficial approach to augment the production of useful secondary metabolites.
The effects of perlite NPs and TiO2/perlite NCs on the production of hypericin and pseudohypericin
The effects of perlite NPs and TiO2/perlite NCs on hypericin and pseudohypericin accumulation in callus cultures of H. perforatum were evaluated using LC-MS/MS. We compared the amount of desired compounds in the calli obtained from in vitro grown plants to those obtained from field grown plants. Retention time values (tR) for pseudohypericin and hypericin were 4.7 and 6.2 min, respectively (Fig. 13). The Values of m/z for pseudohypericin and hypericin were 519 and 503, respectively (Fig. 13). According to the results, only pseudohypericin was detected in the extract of control calli obtained from in vitro grown plants, although no hypericin and pseudohypericin were detected in the extract of control calli obtained from field grown plants. The production of hypericin by callus cultures initiated from in vitro grown H. perforatum was observed after treatment with 25 and 100 mg/L of perlite NPs. However, hypericin in callus cultures obtained from field grown H. perforatum was detected in 50, 100, and 200 mg/L of perlite NPs, as well as 25, 50, 150, and 200 mg/L of TiO2/perlite NCs. Pseudohypericin was also evident in the cultures obtained from in vitro grown plants treated with 25, 100, and 150 mg/L of perlite NPs and 50 and 100 mg/L of TiO2/perlite NCs. In the case of cultures initiated from field grown plants, pseudohypericin was observed in 50-200 mg/L perlite NPs and 25, 50, 150, and 200 mg/L TiO2/perlite NCs treatments (Fig. 13).
LC-MS chromatograms of H. perforatum callus extracts. Chromatogram (A) and mass spectrum (B) of hypericin and pseudohypericin standard solution. Chromatogram of the extracts of control calli obtained from in vitro grown (C) and field grown plants (D), calli obtained from in vitro grown plants treated with 100 mg/L of perlite NPs (E), calli obtained from field grown plants treated with 100 mg/L of perlite NPs (F), calli obtained from in vitro grown plants treated with 50 mg/L of TiO2/perlite NCs (G), and calli obtained from field grown plants treated with 50 mg/L of TiO2/perlite NCs (H).
So far, some researches have been conducted to study the potential of chemical elicitors such as NPs on the manipulation of H. perforatum secondary metabolism. It has been revealed that chromium affected the production of protopseudohypericin, hypericin, and pseudohypericin in H. perforatum seedlings48. Supplementation of zinc and iron oxides NPs in H. perforatum cell cultures stimulated the production of hypericin and hyperforin12. In the same way, our results suggest that perlite NPs and TiO2/perlite NCs can possibly be considered as effective elicitors for the induction of hypericin and pseudohypericin production in callus cultures of H. perforatum.
Conclusion
Our results indicated that employing nano-elicitors such as perlite NPs and TiO2/perlite NCs can stimulate the production and accumulation of secondary metabolites without having adverse impacts on the growth of H. perforatum callus cultures. Callus cultures obtained from in vitro grown plants supplemented with perlite NPs and TiO2/perlite NCs produced more volatile compounds than those obtained from field grown plants. Both perlite NPs and TiO2/perlite NCs were able to induce the production of hypericin and pseudohypericin in H. perforatum calli. Therefore, along with numerous well-known biotic and abiotic elicitors, the biosynthesized perlite NPs and TiO2/perlite NCs can be considered as a new class of elicitors. However, little is known about the induction of secondary metabolites in response to biosynthesized nanomaterials and more experimental data are required to provide insights to their application as elicitors.
References
Nahrstedt, A. & Butterweck, V. Lessons learned from herbal medicinal products: the example of St. John’s wort. J. Nat. Prod. 73, 1015–1021 (2010).
Ng, Q. X., Venkatanarayanan, N. & Ho, C. Y. X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 210, 211–221 (2017).
Tusevski, O. et al. Callus cultures of Hypericum perforatum L. a novel and efficient source for xanthone production. Plant Cell Tissue Organ Cult. 125, 309–319 (2016).
Kirakosyan, A., Sirvent, T. M., Gibson, D. M. & Kaufman, P. B. The production of hypericins and hyperforin by in vitro cultures of St. John’s wort (Hypericum perforatum). Biotechnol. Appl. Biochem. 39, 71–81 (2004).
Giri, C. C. & Zaheer, M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult. 126, 1–18 (2016).
Simic, S. G. et al. Fungal elicitor-mediated enhancement in phenylpropanoid and naphtodianthrone contents of Hypericum perforatum L. cell cultures. Plant Cell Tissue Organ Cult. 122, 213–226 (2015).
Gadzovska, S. et al. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 113, 25–39 (2013).
Kim, D. H., Gopal, J. & Sivanesan, I. Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Adv. 7, 36492–36505 (2017).
Poborilova, Z., Opatrilova, R. & Babula, P. Toxicity of aluminium oxide nanoparticles demonstrated using a BY-2 plant cell suspension culture model. Environ. Exp. Bot. 91, 1–11 (2013).
Zhang, B., Zheng, L. P., Yi Li, W. & Wen Wang, J. Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr. Nanosci. 9, 363–370 (2013).
Ghorbanpour, M. & Hadian, J. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 94, 749–759 (2015).
Sharafi, E. et al. Improvement of hypericin and hyperforin production using zinc and iron nano-oxides as elicitors in cell suspension culture of St John’s wort (Hypericum perforatum L.). JMPB 2, 177–184 (2013).
Khodakovskaya, M. V. et al. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proceedings of the National Academy of Sciences 108, 1028–1033 (2011).
Kohan-Baghkheirati, E. & Geisler-Lee, J. Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials 5, 436–467 (2015).
Mahjouri, S., Movafeghi, A., Divband, B. & Kosari-Nasab, M. Toxicity impacts of chemically and biologically synthesized CuO nanoparticles on cell suspension cultures of Nicotiana tabacum. Plant Cell Tissue Organ Cult. 135, 223–234 (2018).
Marslin, G., Sheeba, C. J. & Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 8, 832 (2017).
Sosan, A. et al. Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. The Plant Journal 85, 245–257 (2016).
Hatami, M., Kariman, K. & Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Science of the total environment 571, 275–291 (2016).
Habibi, M. H. & Zendehdel, M. Synthesis and characterization of titania nanoparticles on the surface of microporous perlite using sol–gel method: influence of titania precursor on characteristics. J. Inorg. Organomet. Polym. Mater. 21, 634 (2011).
Thanh, D. N., Singh, M., Ulbrich, P., Strnadova, N. & Štěpánek, F. Perlite incorporating γ-Fe2O3 and α-MnO2 nanomaterials: Preparation and evaluation of a new adsorbent for As (V) removal. Sep. Purif. Technol. 82, 93–101 (2011).
Shavisi, Y., Sharifnia, S., Hosseini, S. & Khadivi, M. Application of TiO2/perlite photocatalysis for degradation of ammonia in wastewater. J. Ind. Eng. Chem. 20, 278–283 (2014).
Rezaei, Z., Jafarirad, S. & Kosari-Nasab, M. Modulation of secondary metabolite profiles by biologically synthesized MgO/perlite nanocomposites in Melissa officinalis plant organ cultures. Journal of Hazardous Materials, 120878 (2019).
Hong, F. et al. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace. Elem. Res. 105, 269–279 (2005).
Clément, L., Hurel, C. & Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants–effects of size and crystalline structure. Chemosphere 90, 1083–1090 (2013).
Raliya, R., Biswas, P. & Tarafdar, J. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 5, 22–26 (2015).
Owolade, O., Ogunleti, D. & Adenekan, M. Titanium dioxide affects disease development and yield of edible cowpea. EJEAF chem. 7, 2942–2947 (2008).
Ghorbanpour, M. Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Indian J. Plant Physiol. 20, 249–256 (2015).
Hussain, I., Singh, N., Singh, A., Singh, H. & Singh, S. Green synthesis of nanoparticles and its potential application. Biotechnology letters 38, 545–560 (2016).
Jafarirad, S., Mehrabi, M., Divband, B. & Kosari-Nasab, M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: a mechanistic approach. Materials Science and Engineering: C 59, 296–302 (2016).
Lapresta-Fernández, A. et al. A general perspective of the characterization and quantification of nanoparticles: imaging, spectroscopic, and separation techniques. Critical Reviews in Solid State and Materials Sciences 39, 423–458 (2014).
Wellburn, A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant physiol. 144, 307–313 (1994).
Guedes, A. P., Amorim, L. R., Vicente, A. M., Ramos, G. & Fernandes-Ferreira, M. Essential oils from plants and in vitro shoots of Hypericum androsaemum L. J. Agric. Food Chem. 51, 1399–1404 (2003).
Hérent, M.-F., De Bie, V. & Tilquin, B. Determination of new retention indices for quick identification of essential oils compounds. Journal of Pharmaceutical and biomedical Analysis 43, 886–892 (2007).
Tatsis, E. C. et al. Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 68, 383–393 (2007).
Gadzovska, S. et al. Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum L. in vitro cultures. Plant Physiol. Biochem. 43, 591–601 (2005).
Khade, G. V., Suwarnkar, M. B., Gavade, K. M. & Garadkar, N. L. Green synthesis of TiO2 and its photocatalytic activity. Journal of Materials Science Materials in Electronics 26, 3309–3315 (2015).
Mallakpour, S. & Aalizadeh, R. A simple and convenient method for the surface coating of TiO2 nanoparticles with bioactive chiral diacids containing different amino acids as the coupling agent. Progress in Organic Coatings 76, 648–653 (2013).
Otari, S. V., Patil, R. M., Ghosh, S. J. & Pawar, S. H. Green phytosynthesis of silver nanoparticles using aqueous extract of Manilkara zapota (L.) seeds and its inhibitory action against Candida species. Materials Letters 116, 367–369 (2014).
Kaufhold, S. et al. Porosity and distribution of water in perlite from the island of Milos, Greece. SpringerPlus 3, 598–607 (2014).
Medina-Ramirez, I., Bashir, S., Luo, Z. & Liu, J. L. Green synthesis and characterization of polymer-stabilized silver nanoparticles. Colloids and Surfaces B: Biointerfaces 73, 185–191 (2009).
Jha, A. K. & Prasad, K. Green synthesis of silver nanoparticles using Cycas leaf. International Journal of Green Nanotechnology: Physics and Chemistry 1, P110–P117 (2010).
Zanoli, P. Role of hyperforin in the pharmacological activities of St. John’s Wort. CNS drug reviews 10, 203–218 (2004).
Cox, A., Venkatachalam, P., Sahi, S. & Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: a review of current research. Plant Physiol. Biochem. 107, 147–163 (2016).
Dehkourdi, E. H. & Mosavi, M. Effect of anatase nanoparticles (TiO2) on parsley seed germination (Petroselinum crispum) in vitro. Biol. Trace. Elem. Res. 155, 283–286 (2013).
Rafique, R. et al. Growth response of wheat to titania nanoparticles application. NUST J. Eng. Sci. 7, 42–46 (2015).
Gao, F. et al. Was improvement of spinach growth by nano-TiO2 treatment related to the changes of Rubisco activase? Biometals 21, 211–217 (2008).
Mohammadi, H., Esmailpour, M. & Gheranpaye, A. Effects of TiO2 nanoparticles and water-deficit stress on morpho-physiological characteristics of dragonhead (Dracocephalum moldavica L.) plants. Acta Agric. Slov. 107, 385–396 (2016).
Tirillini, B., Ricci, A., Pintore, G., Chessa, M. & Sighinolfi, S. Induction of hypericins in Hypericum perforatum in response to chromium. Fitoterapia 77, 164–170 (2006).
Acknowledgements
The authors wish to thank Dr. V. Simmonds for the language assistance and University of Tabriz for financial supports.
Author information
Authors and Affiliations
Contributions
Saeed Jafarirad and Morteza Kosari-Nasab conceived and designed the study. Roghaiieh Ebadollahi, Saeed Jafarirad, Morteza Kosari-Nasab and Sepideh Mahjouri performed the study. The photographs in figure 1 were taken by Roghaiieh Ebadollahi.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ebadollahi, R., Jafarirad, S., Kosari-Nasab, M. et al. Effect of explant source, perlite nanoparticles and TiO2/perlite nanocomposites on phytochemical composition of metabolites in callus cultures of Hypericum perforatum. Sci Rep 9, 12998 (2019). https://doi.org/10.1038/s41598-019-49504-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-019-49504-3
This article is cited by
-
Inspired by taxol: biosynthesis and engineering of hypericin and other phytochemicals in Hypericum
Planta (2026)
-
Interpreting the potential of biogenic TiO2 nanoparticles on enhancing soybean resilience to salinity via maintaining ion homeostasis and minimizing malondialdehyde
Scientific Reports (2025)
-
Titanium dioxide -mediated regulation of enzymatic and non-enzymatic antioxidants, pigments, and diosgenin content promotes cold stress tolerance in Trigonella foenum-graecum L.
Scientific Reports (2025)
-
Physiological mechanisms and sustainable applications of nanotechnology in enhancing secondary metabolite production in plants
Plant Physiology Reports (2025)
-
Macro-fungi mediated nanoparticles for sustainable agriculture: recent advancement and future strategies
Discover Sustainability (2025)