Abstract
Neutrophil extracellular traps (NETs) are DNAs products involved in immune process. Obesity through a low-grade chronic inflammation determines neutrophil activation, but it is still unclear its role in NETs formation. Here we analyzed the NETs levels in healthy and morbid obese, their association with anthropometric and glyco-metabolic parameters and their changes after bariatric surgery. For this study, we enrolled 73 patients with morbid obesity (BMI ≥40 kg/m2 or ≥35 kg/m2 + comorbidity) eligible to sleeve gastrectomy. In parallel, 55 healthy subjects and 21 patients with severe coronary artery disease were studied as controls. We evaluated anthropometric parameters, peripheral blood pressure, biochemical and serum analysis at the enrollment and at twelve months after surgery. Plasmatic levels of MPO-DNA complexes were assessed by ELISA. NETs levels were higher in obese than in control group (p < 0.001) and correlated with the main anthropometric variable (BMI, waist, hip), glyco-metabolic variables and systolic blood pressure. NETs trend after intervention was uneven. The reduction of NETs correlated with the entity of reduction of BMI (ρ = 0.416, p < 0.05), visceral fat area (ρ = 0.351, p < 0.05), and glycemia (ρ = 0.495, p < 0.001). In medical history of patients in whom NETs increased, we observed a higher number of thromboembolic events. Our observations indicate that severe obesity is associated with increased generation of NETs, which in turn could influence the patients’ systemic inflammatory state. Weight loss and in particular, loss of adipose tissue after bariatric surgery does not in itself correct NET’s dysregulated production. Finally, patients in whom NETs accumulation persists after surgery are probably those at the highest risk of cardiovascular events.
Similar content being viewed by others
Introduction
Generation of neutrophil extracellular traps (NETs) contributes to the effector function of neutrophils1,2. NETs are DNA filaments decorated with proteases and citrullinated histones, endowed with a bactericidal action, which is played either directly or via activation of the complement and the coagulation systems2. Thus NETs influence dramatically the extracellular environment and they contribute to systemic inflammation, persistent autoimmune diseases and thromboembolic events2,3,4,5,6.
Obesity increases cardiovascular risk even in the absence of other risk factors such as diabetes, dyslipidaemia, hypertension7 as well as rheumatic diseases8. Currently the extent of the generation of NETs and their role in experimental models of obesity and in obese patients is controversial9,10,11. As far as we know, only few studies have analyzed this topic. Braster et al.9 investigated the effect of NETs (by release inhibition or not) on the development of insulin resistance in a high fat diet mouse model; there was no difference in insulin resistance between treatment groups.
On the contrary, Wang et al.11 observed an effect of NETs on endothelial function (evaluated by functional studies of mesenteric arterioles) in a mouse model.
Finally, Roberts et al.10 studied a human obese patients before and after gastric band surgery observing a reduction in pro-inflammatory state and NETs production.
Bariatric surgery (metabolic surgery) has become a relevant therapeutic tool in obesity, particularly effective on obesity-associated comorbidities such as glycol-metabolic and cardiovascular dysfunctions12, although, as in other interventions, post-procedural weight gain is described13.
Here we have investigated whether byproducts of NETs generation/catabolism accumulate in the plasma of morbid obese patients, whether they correlate with the anthropometric and glyco-metabolic parameters of the patients. We have also compared NETs byproducts before and 12 months after bariatric surgery to verify whether the relative depletion of the adipose mass impacts on neutrophil function.
Patients, Materials and Methods
Patients
The study group consisted of 73 patients (24 males and 49 females) with morbid obesity (mean body mass index 45.5 kg/m2) eligible for sleeve gastrectomy were recruited. Patients with severe obesity were studied immediately before and one year after sleeve gastrectomy.
We choose to re-check these patients after twelve months because weight loss and glyco-metabolic parameters are quite stabilized in that period as documented by our previous publications14,15,16,17,18.
Inclusion criteria19 were BMI ≥40 kg/m2 or ≥35 kg/m2 + comorbidities (every comorbidity that should benefit of bariatric surgery such as metabolic disorders, cardiorespiratory disease, severe joint disease, obesity-related severe psychological problems) and age between 20 and 65 years.
The exclusion criteria were (a) all indicated for sleeve gastrectomy– liver, renal and heart failure, secondary causes of obesity (endocrine - untreated hypothyroidism, Cushing disease, etc. – pharmacologic and genetic causes), and severe psychiatric diseases (as evaluated by a consultant psychiatrist); and (b) patients with autoimmune (e.g. systemic lupus erythematosus, rheumatoid arthritis, small vessel vasculitis, antiphospholipid antibody syndrome and psoriasis), infectious disease or neoplasia emerging before or after surgery19.
We enrolled as control group 55 sex- and aged-matched healthy subjects. Moreover, we also studied a group of 21 patients with severe coronary artery disease (CAD) (with the same exclusion criteria, as described above): nine with previous acute myocardial infarction, and twelve that required angioplasty and stent implantation after coronary angiography (Table 1; Supplementary Table 1).
All patients and controls signed their consent for the study. The ethics committee of the “Santa Maria della Misericordia” Hospital University of Perugia and registered as a clinical trial as NCT03559842, while IRCCS San Raffaele Scientific Institute approved the study protocol. The study was carried out in accordance with the code of ethics of the World Medical Association for human studies (Declaration of Helsinki, 1975). All patients and controls gave their written informed consent to participate to the study.
Anthropometric data assessment
The weight and height were measured and used to calculate body mass index (BMI). Waist and hip circumferences were measured and used to calculate the waist-hip ratio. Peripheral blood pressure was assessed with the patients in supine position by a validated device in the non-dominant arm, after 10 minutes of rest in a quiet environment. Insulin-resistance was determined using the homeostasis model assessment-insulin resistance (HOMA-IR). Visceral fat area (VFA, expressed in cm2) was measured at the end of a normal exhalation by ultrasonography (using a 3.5 MHz convex array probe) according to the Hirooka formula20. Three different distances were measured in order to apply the above-mentioned formula, as follows: VFA = −9.008 + 1.191 × [distance between the internal surface of the abdominal muscle and the splenic vein (mm)] + 0.978 [distance between the internal surface of the abdominal muscle and the posterior wall of the aorta at the umbilicus (mm)] + 3.644 [thickness of the fat layer of the posterior right renal wall (mm)]. The distance between the internal surface of abdominal muscles and the splenic vein was scanned transversely in the midline. None of the patients had dorsal or lumbar spine deformity, nor abdominal aortic aneurysm. Subcutaneous fat thickness (SFT, expressed in mm) refers to the thickness of subcutaneous fat layer as measured by ultrasonography using a 7.5-MHz linear array probe and performing a longitudinal scan 1 cm below xiphoid apophysis. Subcutaneous fat thickness was defined as the distance between the skin and external face of the rectus abdominal muscle. Bioimpedentiometry (50 kHz, amplitude 50 mA, Body Composition Analyzer TBF-410GS; Tanita, Tokyo, Japan), with electrodes applied on the plantar surface of both feet, was used to determine fat mass and free fat mass as a percentage of body weight.
Blood sampling
Venous blood was drawn (in the morning after 13 hour fast) in vacutainers containing clot activator (to prepare serum samples) and containing EDTA (to prepare platelet free plasma). Serum and plasma were retrieved after centrifugation (3000 rpm for 10 minutes). To prepare platelet- free plasma, a second centrifugation of the obtained plasma was performed at 13,000 × g, 5 minutes at 4 °C. Platelet-free plasma retrieved were aliquoted and stored at −80 °C until quantification of NETs.
Blood chemistry measurements
Blood sample was drawn in the morning after a 13-hour fast. Routine auto-analyzers were used to assess hematological parameters and blood chemistry including glycemia, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), apolipoprotein-A1 and –B and insulinemia. Low-density lipoprotein cholesterol (LDL-c) was calculated by Friedewald formula. Serum levels of hs-CRP were measured by colorimetric enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
NETs quantification
Plasmatic levels of MPO-DNA complexes (a bona fide marker of NET formation/catabolism) were identified using a capture ELISA as previously described3,4,6,21. Briefly, multiwell plates were coated with anti-human MPO mAb (capture) overnight at 4 °C, washed and blocked with BSA. After washing, human platelet-free plasma samples were placed in the coated wells with peroxidase-conjugated anti-DNA antibodies (clone MCA-33, from the cell death detection ELISA kit) following the kit instructions. Results are expressed as arbitrary units of optical density (OD).
Statistical analysis
Analyses were performed using SPSS software for Windows (version 22.0; SPSS, Inc, Chicago, Illinois), with significance set at a 2-sided P < 0.05. Values are expressed as mean (±standard deviation). Kolgomorov-Smirnov test was used to determine the normal distribution of the variables. Mann-Whitney U test was used to compare the means of two unmatched groups variables (e.g. healthy and obese subjects). Differences between two paired groups before and after surgery were calculated by Wilcoxon signed-rank test. Variations of the concentration of plasmatic MPO-DNA complexes were analyzed by Mann-Whitney test and the trend in each patient recorded. Chi-Square test statistics was performed to determine if there was a significant relationship between two nominal (categorical) variables. Kruskal-Wallis test was used to compare the values of more than two different groups. Correlation coefficients were calculated with Spearman correlation rank tests, as appropriate. Eta squared analysis was used to study correlations between interval and nominal variables. The difference of different variables at time 0 and after intervention was calculated (delta, Δ).
Results
In the present work, we analyzed 149 adults, 73 patients with severe obesity, 55 healthy controls with normal weight (Table 1) and 21 patients with coronary artery disease (Supplementary Table 1). Patients with severe obesity were studied before and one year after sleeve gastrectomy.
Among patients with severe obesity, a higher prevalence of women was observed (Table 1). Higher blood pressure values and a worse glycol-metabolic profile was observed in patients than in controls. No significantly differences in the two groups were observed in the lipid profile (cholesterol, LDL-c, triglycerides and HDL-c).
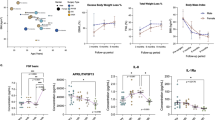
A significantly higher accumulation of DNA fragments associated to MPO was observed in patients with severe obesity than in healthy controls (0.11 ± 0.06 vs 0.46 ± 0.16; p < 0.001, Table 1, Fig. 1a). The concentration of MPO-DNA complexes was significantly associated to weight, body mass index, waist and hip circumferences, systolic and diastolic blood pressure and glycol-metabolic profile (Table 2). Intriguingly, the NETs-associated parameters are those that differ in obese patients compared to healthy donors (Table 1). On the contrary, lipid profile which are similar in obese and control subjects - do not correlate with the plasmatic NETs concentration (Tables 1, 2). There was no statically significant differences in NETs values between male and female obese patients (n = 73, p = 0.787).
The concentration of soluble DNA-MPO complexes (a putative marker of in vivo NET generation/catabolism) was determine by ELISA (see methods) in platelet-free plasma samples of patients and of healthy controls (a) and in patients before and after gastric sleeve (b). Two different groups of subjects were observed among the patients studied: those that reduce (Group 1) (c) and those that increased (Group 2) (d) the fraction of plasmatic MPO-DNA complexes after sleeve gastrectomy. (e) The amount of MPO-DNA complexes observed in obese patients were compared to those observed in patients with history of acute myocardial infarction (AMI) or severe coronary artery disease but without previous AMI or unstable angina. In (a–d), each symbol depict the result of a single subject (patient or control). In (a,b), red lines indicates mean ± SD. In (e) §§P < 0.001; *P < 0.05; ##P ≤ 0.001; °P < 0.05; P values were determined by Kruskal-Wallis and Mann-Whitney Tests.
One year after sleeve gastrectomy, several parameters were significantly reduced in patients (Table 3) including the body mass index, waist and hip circumferences, glycemia, insulinemia, HOMA-IR, triglycerides, VFA. Moreover, the reduction of hs-CRP results statistically significant (19.7 ± 19.5 before vs 10.6 ± 12.6 µg/mL after surgery, p < 0.05). Conversely an increase in HDL-c (p < 0.05) was observed. In 73 patients, the plasmatic concentration of MPO-DNA complexes was determined before and after one year of sleeve gastrectomy (Fig. 1b, Table 3). Unexpectedly, there was no univocal trend and patients could be stratified into two distinct groups, one comprising patients in whom sleeve gastrectomy is associated with a decrease in the accumulation of NET by-products (Group 1, Fig. 1c) and one in which the latter is not affected or it actually increases (Group 2, Fig. 1d). This suggests that weight loss per se is not sufficient to modify the activation state of neutrophils. Of interest, there was no detectable difference in the various parameters analyzed that could be used to predict whether bariatric surgery results influences or not NET accumulation. Before and after surgery of MPO-DNA complexes as well as all other anthropometric or metabolic parameters did not differ in the two groups (Table 4). However, a significant difference is clearly detectable in terms of the fraction of patients with a history of cardiovascular events, including strokes or thromboembolism, which was significantly higher in the group in which NET production did not abate after surgery (Table 5). Surprisingly, the post-surgery values of MPO-DNA complexes in Group 2 results higher than those observed in patients with history of acute myocardial infarction or patients with severe coronary atherosclerosis (p < 0.001, Fig. 1, panel e).
Of interest, we found a statistically significant direct correlation between the entity of reduction of NET byproduct accumulation after surgery (delta NETs, ΔNETs) and reduction of weight (ρ = 0.345, p < 0.05), of BMI (ρ = 0.416, p < 0.05), of VFA (ρ = 0.351, p < 0.05), and of glycemia (ρ = 0.495, p < 0.001) (Table 6). Patients in which NET accumulation persisted after surgery also experienced similar reduction of weight, BMI, VFA and metabolic parameters, which in this group were not associated to variations in NET concentration. This suggests that NET production (and therefore neutrophil activation state) is not per se a consequence of the presence of fat.
In order to deeper analyze the role of systemic inflammation we considered two more parameters, neutrophils blood count and high sensitivity C reactive protein. Obese patients have neutrophil count higher than controls (p < 0.001, Table 7); and neutrophil counts decrease after surgery (p < 0.001, Table 7). Nevertheless, no differences in neutrophil count were observed between the group of patients that increased or reduced the concentration of plasmatic NETs byproducts after surgery (Table 4).
Finally, we clustered obese patients in low and high levels of hs-CRP. No significant difference was found in NETs concentration between groups at high or low level hs-CRP (Table 8).
Discussion
This study provides three main new information. Firstly, severe obesity is associated with increased neutrophil activation and specifically with an increased generation of NETs, which in turn could influence the patients’ systemic inflammatory state. Secondly, weight loss and in particular loss of adipose tissue after bariatric surgery does not in itself correct NET’s dysregulated production. Third, patients in whom NET accumulation persists after surgery are probably those at the highest risk of cardiovascular events.
The accumulation of NETs in obese patients is not per se surprising. Obesity is well-known as an inflammatory condition, possibly because of the accumulation of innate immune cells that on the one hand impact on the sensitivity to insulin and on the other interfere with the function of adipocytes22. Neutrophils directly infiltrate adipose tissue and the vessel wall of various organs in experimental obesity23 while they are functionally activated, as evaluated by phagocytosis, oxidative burst, and release of granular enzymes in at least some obese subjects24,25. The extent of human neutrophils in circulation predicts cardiovascular events even if the mechanisms underlying neutrophil activation have not yet been elucidated26,27. Less information is directly available on NET generation, even if it is known that both insulin and hyperglycaemia, which are hallmarks of severe obesity (see Table 1) have been suggested to facilitate neutrophil activation28. Our data however do not support a direct link between hyperinsulinemia, hyperglycaemia and increased NET generation, since the bariatric surgery, which improves or corrects these metabolic features in virtually all patients, does not necessarily quench NET accumulation in the patients’ plasma. On the other hand our data well agree with the demonstration obtained in experimental models that inhibition of NET generation does not per se influence the accumulation of adipose tissue29. Other signals are likely to be involved in supporting NET generation that are not necessarily modified early after surgery.
Endothelial activation is a promising candidate. Neutrophils interact effectively both with endothelial cells and activated platelets at sites of inflammation and previous studies have correlated soluble markers reflecting endothelial activation in patients and atherosclerosis in experimental animals with NET generation21. Further studies are necessary to verify the association of increased amounts of NETs in obese subjects, with atherosclerosis and increased cardiovascular risks, thrombosis in particular27,30,31. Indeed, NETs were associated with systemic vascular inflammation and endothelial damage26,30,32. Of interest, neutrophils purified from the peripheral blood of obese patients appear to have an impaired capacity to generate NETs in vitro, possibly reflecting previous functional exhaustion in vivo30. The actual site in which neutrophils generate NETs in obese patients, whose byproducts accumulate in the peripheral blood, remain to be identified.
Finally our data indicate a dramatic heterogeneity in the obese population in terms of the regulation of NET generation. A fraction of patients indeed respond nicely to bariatric surgery, with a substantial correction of the accumulation of NETs byproducts (group 1). It is tempting to speculate that neutrophils infiltrating the adipose visceral tissues are the main source of NETs in these subjects (correlations). Conversely, a distinct group of patients fail to respond in terms on NET accumulation to surgery, and this group comprises subjects with an higher incidence of cardiovascular events. The possible role of non-fat associated stimuli supporting neutrophil activation and in particular of the reciprocal activation of neutrophils, platelets and endothelial cells33,34 in this group of patients need to be experimentally verified.
References
Manfredi, A. A., Ramirez, G. A., Rovere-Querini, P. & Maugeri, N. The Neutrophil’s Choice: Phagocytose vs Make Neutrophil Extracellular Traps. Front Immunol 9 (2018).
Boeltz, S. et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ 26, 395–408 (2019).
Kessenbrock, K. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15, 623–625 (2009).
Maugeri, N. et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost 12, 2074–2088 (2014).
Rajagopalan, S. et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood 103, 3677–3683 (2004).
Maugeri, N. et al. Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Sci Transl Med 10 (2018).
Poirier, P. et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss - An update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113, 898–918 (2006).
Nikiphorou, E. & Fragoulis, G. E. Inflammation, obesity and rheumatic disease: common mechanistic links. A narrative review. Ther Adv Musculoskel 10, 157–167 (2018).
Braster, Q. et al. Inhibition of NET Release Fails to Reduce Adipose Tissue Inflammation in Mice. PLoS One 11, e0163922 (2016).
Roberts, H. M. et al. Impact of Bariatric Surgical Intervention on Peripheral Blood Neutrophil (PBN) Function in Obesity. Obes Surg 28, 1611–1621 (2018).
Wang, H. et al. Obesity-induced Endothelial Dysfunction is Prevented by Neutrophil Extracellular Trap Inhibition. Sci Rep 8, 4881 (2018).
Lupoli, R. et al. Effects of bariatric surgery on markers of subclinical atherosclerosis and endothelial function: a meta-analysis of literature studies. Int J Obes (Lond) 40, 395–402 (2016).
Himpens, J., Dobbeleir, J. & Peeters, G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg 252, 319–324 (2010).
Ricci, M. A. et al. Morbid obesity and hypertension: The role of perirenal fat. J Clin Hypertens (Greenwich) 20, 1430–1437 (2018).
Carbone, F. et al. High serum levels of C-reactive protein (CRP) predict beneficial decrease of visceral fat in obese females after sleeve gastrectomy. Nutr Metab Cardiovasc Dis 28, 494–500 (2018).
Ricci, M. A. et al. Sleeve Gastrectomy Efficacy on Metabolic and Cardiovascular Dysfunction With a Focus on the Role of Comorbidities. Angiology 69, 475–482 (2018).
De Vuono, S. et al. Laparoscopic sleeve gastrectomy modifies cholesterol synthesis but not cholesterol absorption. Obes Res Clin Pract 11, 118–122 (2017).
Gentili, A. et al. Circulating Levels of the Adipokines Monocyte Chemotactic Protein-4 (MCP-4), Macrophage Inflammatory Protein-1beta (MIP-1beta), and Eotaxin-3 in Severe Obesity and Following Bariatric Surgery. Horm Metab Res 48, 847–853 (2016).
Fried, M. et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg 24, 42–55 (2014).
Hirooka, M. et al. A technique for the measurement of visceral fat by ultrasonography: comparison of measurements by ultrasonography and computed tomography. Intern Med 44, 794–799 (2005).
Manfredi, A. A., Rovere-Querini, P., D’Angelo, A. & Maugeri, N. Low molecular weight heparins prevent the induction of autophagy of activated neutrophils and the formation of neutrophil extracellular traps. Pharmacol Res 123, 146–156 (2017).
Russo, L. & Lumeng, C. N. Properties and functions of adipose tissue macrophages in obesity. Immunology (2018).
Zernecke, A. et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res 102, 209–217 (2008).
Nijhuis, J. et al. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity (Silver Spring) 17, 2014–2018 (2009).
Shah, T. J., Leik, C. E. & Walsh, S. W. Neutrophil infiltration and systemic vascular inflammation in obese women. Reprod Sci 17, 116–124 (2010).
Maurizi, G., Della Guardia, L., Maurizi, A. & Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J Cell Physiol 233, 88–97 (2018).
Kapoor, S., Opneja, A. & Nayak, L. The role of neutrophils in thrombosis. Thromb Res 170, 87–96 (2018).
Lee, B. C. & Lee, J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta 1842, 446–462 (2014).
Braster, Q. et al. Inhibition of NET Release Fails to Reduce Adipose Tissue Inflammation in Mice. PLoS One 11, e0163922 (2016).
Brill, A. et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 10, 136–144 (2012).
Jimenez-Alcazar, M., Kim, N. & Fuchs, T. A. Circulating Extracellular DNA: Cause or Consequence of Thrombosis? Semin Thromb Hemost 43, 553–561 (2017).
Schreiber, A. et al. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci USA 114, E9618–E9625 (2017).
Gomez-Moreno, D., Adrover, J. M. & Hidalgo, A. Neutrophils as effectors of vascular inflammation. Eur J Clin Invest, e12940 (2018).
Maugeri, N., Baldini, M., Ramirez, G. A., Rovere-Querini, P. & Manfredi, A. A. Platelet-leukocyte deregulated interactions foster sterile inflammation and tissue damage in immune-mediated vessel diseases. Thromb Res 129, 267–273 (2012).
Acknowledgements
Research was financed by “Fondo di Ricerca di Base Anno 2018, University of Perugia, Italy, project title” Ruolo di chemerina nella disfunzione vascolare precoce del grande obeso”.
Author information
Authors and Affiliations
Contributions
M.D’.A. created the database, performed the statistical analysis and wrote the manuscript together with N.M. and G.L. N.M. and G.L. defined the experimental strategy, performed experiments and wrote the manuscript with the vital contribution of E.E.M., M.A.R., S.D.V., E.N.M. and D.S. M.T.P. performed bariatric surgery. C.G. and S.C. selected the patients with coronary artery disease and performed the coronary angiography.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
D’Abbondanza, M., Martorelli, E.E., Ricci, M.A. et al. Increased plasmatic NETs by-products in patients in severe obesity. Sci Rep 9, 14678 (2019). https://doi.org/10.1038/s41598-019-51220-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-019-51220-x
This article is cited by
-
Neutrophil extracellular traps (NETosis) in gynecologic cancers: from pathogenesis to therapeutic opportunities: a narrative review
Clinical and Translational Oncology (2026)
-
Adenosine improves postmenopausal obesity by regulating neutrophil extracellular traps
Scientific Reports (2025)
-
Sepsis-induced coagulopathy: recent insights on the role and clinical application of neutrophil extracellular trap formation
Scientific Reports (2025)
-
Neutrophil extracellular traps in homeostasis and disease
Signal Transduction and Targeted Therapy (2024)
-
Excess of body weight is associated with accelerated T-cell senescence in hospitalized COVID-19 patients
Immunity & Ageing (2024)