Abstract
Hawthorn leave flavonoids (HLF) are widely used as an herb or dietary supplements for cardio-cerebrovascular diseases. However, its gastrointestinal absorption behavior and mechanism have not been disclosed. In this study, gastrointestinal absorption and its regulation of 4’’-O-glucosylvitexin (GLV), 2’’-O-rhamnosylvitexin (RHV), vitexin (VIT), rutin (RUT) and hyperoside (HP) in HLF were investigated using in vitro, in situ and in vivo models. Apparent permeability coefficient (Papp) of five flavonoids were (2.18 ± 0.15) ×10−7~(3.83 ± 0.22) ×10−7 cm·sec−1 across the Caco-2 cells. GLV, RHV, VIT and RUT demonstrated similar and poor absorption in rat stomach (absorption percentage per hour (A), (1.78 ± 0.14 ~ 5.69 ± 0.51)%·h−1) and small intestine (absorption rate (\(\:{k}_{a}^{app}\), (0.012 ± 0.006 ~ 0.055 ± 0.003) h−1), extent (A, (1.28 ± 0.14 ~ 2.82 ± 0.19)% ·h−1) and potential (Papp, (0.31 ± 0.05 ~ 1.41 ± 0.08)×10−7 cm·sec−1)), while HP showed relatively better absorption (Papp, (2.55 ± 0.15 ~ 4.27 ± 0.36)×10−7 cm·sec−1) in rat small intestine. Absorption of five flavonoids exhibited dose-dependence, pH-dependence and region-selectivity. Meanwhile, these flavonoids were excreted via intestine, secreted via bile and metabolized by intestinal microflora. Their absorption was significantly increased by absorption enhancers (cow bile salt and sodium dodecyl sulphate), transporter regulators (verapamil hydrochloride, digoxin and rifampicin). GLV and RHV exhibited enterohepatic circulation after oral administration of HLF. In conclusion, flavonoids in HLF were absorbed via passive diffusion accompanied with active transport, intestinal microflora metabolism, bile secretion and intestinal efflux. They belong to BCS class III (“like” drugs with high solubility and low permeability) and displayed poor oral bioavailability.
Similar content being viewed by others
Introduction
Flavonoids are a class of polyphenolic compounds with 2-phenylchromone, and are frequently found in a variety of plants (e.g., fruits, vegetables, grains and herbs), which mainly exist in the form of glycosides and aglycones1. Currently, more than 10,000 flavonoids have been isolated and identified in nature. They display multiple biological activities, e.g., antitumor, antimicrobial, antiviral, antiangiogenic, antimalarial, antioxidant, anti-proliferative and neuroprotective effects2. However, most flavonoids, especially flavonoid glycosides, demonstrate poor oral absorption. Composition, total quantity of hydroxyl groups and substitution of functional groups on the nuclear structure of flavonoids significantly affect their oral bioavailability (BA)3. It is of great significance to explore absorption behavior and mechanism of flavonoids and improve their oral bioavailability.
Hawthorn (Crataegus sp.), also called Shanzha, is medicinal plant for a long time both in folk and official medicine. It is widely distributed in northern temperate zones, primarily in East Asia, Europe and North America4. The plant name has also been checked with http://www.theplantlist.org. As a well-known traditional Chinese medicine (TCM), fruits and leaves of hawthorn, hawthorn leaf extracts and their preparations were recorded in the Chinese Pharmacopoeia5. Among them, hawthorn leaf is dry leaves of rosaceae plants Crataegus pinnatifida Bge. var. major N. E. Br. or Crataegus pinnatifidaBge. Hawthorn leaf extract (HLE), namely hawthorn leave flavonoids (HLF), is widely used as herbal medicine for the treatment of multiple diseases, especially cardio-cerebrovascular diseases. HLF display multiple pharmacological activity, e.g., antioxidant, anti-inflammatory, anti-ischemic activities and hypolipidemic6,7. Its preparations, e.g., tables and droppills of Yixintong, are used to treat coronary heart disease, angina pectoris, hyperlipidemia and cerebral arterial insufficiency8. The flavonoids 4’’-O-glucosylvitexin (GLV), 2’’-O-rhamnosylvitexin (RHV), vitexin (VIT), rutin (RUT) and hyperoside (HP) are important and representative components in HLF, which collectively contribute to its pharmacological activities9,10,11,12,13 (Fig. 1). Of them, GLV and RHV are the specific components with the highest content in hawthorn leaf and HLE.
The pharmacological activities of HLF and its main components have been well studied, however, behavior and mechanism of their gastrointestinal absorption are hardly been disclosed up to now. Therefore, it is very necessary to comprehensively investigate and disclose the gastrointestinal absorption behavior, mechanism and influence factor of the flavonoids in HLF so as to improve their oral bioavailability. This study will provide important reference for design and development of oral preparations of flavonoids (e.g., HLF and its components) with poor oral bioavailability and their rational clinical use.
Materials and methods
Materials
Extract and reagents
Hawthorn (Crataegus sp.) leave flavonoids (HLF) (i.e., HLE, Batch No. 20230401, consisted of 6.3% GLV, 13.15% RHV, 0.75% VIT, 0.36% RUT, 1.08% HP) were provided by Zhongjin Pharmacy Co., Ltd. (Shanxi, China), which were prepared according to the Chinese Pharmacopoeia (2000 Edition, Volume 1). GLV, RHV, VIT, RUT and HP (Product No. VIP(CT)10078, VIP(X)4016, VIP(X)0886, VIP(CT)900190, VIP(CT)900159, respectively) were provided by National Institutes for Food and Drug Control (Beijing China). Sodium dodecyl sulfate (SDS), pentobarbital sodium, phenol red, cow bile salt (CBS, CAS 345909-26-4), verapamil hydrochloride (VP), digoxin (DIG) and rifampicin (RIF) were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The other chemicals were analytical grade and obtained from standard sources.
Animals
Sprague Dawley (SD) rats (SPF level, 200 ± 20 g, 6–8 weeks old, male and female in equal proportions, Animal qualitative qualification number: 20180006022514) were provided by Laboratory Animal Center, School of Pharmacy, Fudan University (Shanghai, China). All the animals were housed under standard laboratory conditions of temperature (25 ± 2℃), constant humidity (55 ± 5%) and light (12 h dark/light cycle), with freely available food and purified water. After one week of acclimation, these rats were randomly assigned to conduct in situ perfusion, intestinal loop and in vivo pharmacokinetic experiments. Random numbers were generated using the standard = RAND() function in Microsoft Excel.
We confirmed that all experimental procedures in this study were reviewed and approved by the Experimental Animal Ethics Committee of the School of Pharmacy, Fudan University, in strict compliance with the International Code for the Care and Use of Experimental Animals (approval number: 2020-04-LY-MG-01). All the experiments were conducted in accordance with the relevant guidelines and regulations. All the procedure of the study is followed by the ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines. After the experiment, all the rats were euthanised by cervical dislocation under anaesthesia.
Methods
HPLC assay
The High Performance Liquid Chromatography (HPLC) analyses were performed on LC-2010 C HT system (Shimadzu, Japan). Chromatographic separation was carried out with a Diamonsil™ C18 column (Dikma Technologies Inc., USA; 5 μm, 200 mm×4.6 mm). The mobile phase consisted of methanol (A), acetonitrile-tetrahydrofuran (B, 1:19 (v/v)) and 0.5% glacial acetic acid (C) was delivered at a flow rate of 1 mL·min−1. The gradient elution was conducted as follows: 1–21% A and 19–21% B at 0–15 min; 21–65% A and 5% B at 15–20 min; 65 − 1% A and 5–19% B at 20–23 min; 1% A and 19% B at 23–28 min. The column temperature was maintained at 30 °C throughout the entire process. The detection wavelength was 360 nm. The sample volume injected into the HPLC system was 50 µL. This HPLC method was applied to determine the samples from Caco-2 cell, in situ perfusion and intestinal loop, in situ single-pass perfusion and intestinal microflora experiment in the present study.
The samples from in vivo pharmacokinetics and bile excretion experiment were determined using the Shimadzu HPLC system. Chromatographic separation was performed on a Diamonsil™ C18 column (Dikma Technologies Inc., USA, 5 μm, 200 mm×4.6 mm) with a protective column (EasyGuard C18 Kit, 8 mm × 4 mm). The mobile phase consisted of tetrahydrofuran (A), methanol (B), acetonitrile (C) and 0.5% acetic acid (D) (19.4:1:1:78.6, v/v). Detection was carried out at a wavelength of 330 nm using a UV-vis detector. The flow rate was maintained at 0.8 mL·min−1, and the column temperature was set at 30 °C. Sample injection volume was 20 µL.
In the current study, the representative HPLC chromatograms and the regression equations, linear range, limit of detection (LOD), and limit of quantitation (LOQ) of flavonoids in HLF in different biological media have been provided in supplementary information (Fig. S1-S7 and Table S1).
Caco-2 cell experiment
Cell culture and cytotoxicity test
Caco-2 cells (American Type Culture Collection, USA; passage 4–5 times) were cultured with Dulbecco’s modified Eagle medium (DMEM; Gibco BRL, USA) supplemented with fetal calf serum (FCS, 10%; Gibco BRL, USA), non-essential amino acids (1%), L-glutamine (4 mM), penicillin (100 u/mL) and streptomycin (100 u/mL) in a humidified atmosphere of 5% CO2 at 37 °C. After reaching 80% confluence, Caco-2 cells were harvested with trypsin (0.25%)-EDTA (10 mM) and seeded onto 6-well Transwell® plates at a density of 2 × 105/mL. The culture medium was changed once a day until the cells formed a completely dense monolayer (19–25 days). The transepithelial electrical resistance (TEER) of the monolayer was measured using a Millicells®ERS-2 (Millipore, USA), which were more than 400 Ω·cm2.
Cytotoxicity of HLF to Caco-2 cells was evaluated using Cell Counting Kit-8 (CCK-8) assay, measured at 450 nm with a microplate reader (Tecan Trading AG, Switzerland). Cells were seeded in 96-well plates at a density of 1 × 105/mL and cultured for 24 h; then, were treated with GLV, RHV, VIT, RUT and HP (All their concentration were 0, 0.625, 1.25, 2.5, 5, 10 and 20 µM) and HLF (0, 25, 50, 100, 200 and 400 µg·mL−1) for 24 h, respectively. Each experiment was conducted at least three times.
Transport studies of HLF in Caco-2 monolayer
Transwell® inserts with Caco-2 cells grown on it were rinsed twice with prewarmed Hank’s balanced salt solution (HBSS, transport medium) and equilibrated at 37 °C with HBSS for 15 min before the transport experiment. For the transport study, the HBSS with 1% dimethyl sulfoxide (DMSO) and HLF were added either into the AP or BL side as donor chamber (2.5 and 3 mL, respectively), and the corresponding volume of HBSS were added into the receiver chamber. 0.1 mL of samples were collected from the receiver chamber every 30 min, and an equal volume of HBSS was added into the receiver chamber to maintain a constant volume. The samples were added into equal volume methanol, vortexed for 3 min, and centrifuged at 12,000 rpm for 5 min, respectively. The supernatant was analysed by HPLC. At the end of the transport experiment, integrity of the Caco-2 cell monolayer was monitored by TEER value.
Calculation of apparent permeability coefficient
Apparent permeability coefficient (Papp)14 of the flavonoids was calculated according to the following Eq. (1)
where, “dC/dt” (µg·(mL·sec) −1) is the change of drug concentration in the receiver chamber over time; “V” (2.5 or 3 mL) is the volume of the solution in the receiver chamber; “A” (cm2) represents the membrane surface area of Caco-2 monolayer; “C” (µg·mL−1) is the loading drug concentration in the receiver chamber. C0 (µg·mL−1) is the initial concentration of in the donor chamber.
Efflux ratio (ER) was calculated using the following Eq. (2)
where Papp (AP→BL) is the apparent permeability coefficient from AP to BL, and Papp (BL→AP) is the apparent permeability coefficient from BL to AP.
Effect of different factors on transport of HLF across Caco-2 cell monolayer
Effect of HLF concentrations (50, 100 and 200 µg·mL−1), absorption enhancers (10 mg·mL−1 of CBS, 10 mg·mL−1 of SDS) and P-glycoprotein (P-gp) inhibitor/substrate (0.1 mg·mL−1 of VP and 5 ng·mL−1 of DIG) on transepithelial transport of five flavonoids in HLF (100 µg·mL−1) across Caco-2 cell monolayer were investigated in this study. The transport experiments were conducted as described in “2.2.2.2”.
In situ perfusion and intestinal loop experiments
Preparation of solutions
The blank gastric perfusion fluids (i.e., artificial gastric juice, pH 1.2) and the blank intestinal perfusion fluids (i.e., Krebs-Ringer buffers, namely KR buffers) were prepared as described our previous study15. The HLF solutions were prepared by dissolving the HLF into the blank gastric or intestinal perfusion fluids.
In situ gastric perfusion
The SD rats were fasted for 12 h (drank freely), and were anaesthetized by intraperitoneal injection of sodium pentobarbital solution (30 mg·kg−1). Body temperature of the SD rats was maintained at 37 °C. The SD rats were randomly divided into three groups (5, 10 and 20 mg of HLF, n = 5). The gastric perfusion solutions of HLF (1.25, 2.50 and 5.00 mg·mL−1) were prepared by dissolving HLF into blank gastric perfusion solutions. The in situ gastric perfusion experiment was conducted according to our previous study15. The perfusion volume was 4 mL. The perfusion period was 2 h.
Absorption percentage per hour (A) of drugs (i.e., HLF) in the rat stomach were calculated using the following Eq. (3)
where C0 and Ct represent concentration of drugs in the perfusion fluids at the initial and t time; V0 and Vt represent volume of the perfusion fluids at the initial and t time; t represents the perfusion period.
In addition, adsorption and metabolism of HLF in the stomach were investigated by incubating an isolated stomach in the gastric perfusion solution with 2.5 mg·mL−1 of HLF for 2 h. Adsorption and metabolism of HLF were barely found in the stomach (Table S2).
In situ recirculating intestinal perfusion
The in situ recirculating intestinal perfusion experiment (Fig. 2A) was conducted according to our previous study15. HLF intestinal perfusion solutions (0.05, 0.1, 0.2 mg·mL−1) were prepared by dissolving HLF into the K-R solution with phenol red. Herein, phenol red is not absorbed in the intestine and is used to correct volume of the HLF intestinal perfusion solutions. The perfusion fluids in the infusion bottle were mixed well, 1 mL of them was taken every 20 min for 3 h, and the concentrations of GLV, RHV, VIT, RUT and HP in the perfusion fluids were assayed by HPLC.
Absorption percentage per hour (A) of drugs (i.e., flavonoids in HLF) in the rat intestine were calculated using the above Eq. (3). Apparent absorption rate constants (\(\:{k}_{a}^{app}\)) and apparent permeability coefficient (Papp) of drugs were calculated using the Eq. (4) and Eq. (5), respectively.
where, Xt and X0 represent the amount of drugs in intestine at the initial and t time, and t represents the perfusion period.
where, dQ/dt (µg·min−1) represents the change of absorbed amount of drug over time (min). A(cm2) represents the surface area of the test intestinal segments. The value “60” is a conversion factor of 60 s·min−1. C0 represents the initial drug concentration.
In addition, the adsorption and metabolism of HLF in the intestine were investigated by incubating an isolated intestine in the recirculating perfusion solution with three different concentrations of HLF (0.05, 0.1 and 0.2 mg·mL−1) for 3 h. Adsorption and metabolism of HLF were barely found in the intestine (Table S3).
Intestinal Loop
The SD rats were prepared as described in the ‘‘2.2.3.2 In situ gastric perfusion’’. The rat abdomen was opened by a midline longitudinal incision (2 cm). The test intestinal segments were isolated. The common bile duct and the test intestinal segments were ligated. The HLF perfusion solution (0.5, 1.0, 2.0 mg·mL−1) was injected into the intestinal loop (Fig. 2B). The intestinal loop remained in the abdominal cavity for 3 h. Then, the intestinal loop was removed, cut open and rinsed with the K-R solution. The intestinal contents and rinsed solutions were collected, and diluted with the K-R solution. The concentrations of GLV, RHV, VIT, RUT and HP in the test intestinal segments were assayed by HPLC. Absorption percentage per hour (A) of drugs were calculated using the above Eq. (3).
Effect of different factors on absorption of HLF
Effect of different factors (i.e., drug concentration, absorption region, physiological pH, bile secretion, intestinal metabolism, absorption enhancer, transporter regulator) on absorption of GLV, RHV, VIT, RUT and HP in HLF was investigated using the above in situ recirculating intestinal perfusion and intestinal loop (Table S4). Effect of the intestinal efflux on absorption of five flavonoids in HLF was investigated using the rat in situ single-pass intestinal perfusion test. In the study, the K-R solutions with and without 0.2 mg·mL−1 of VP were perfused from the beginning of the duodenum to the end of the ileum of the rat (n = 5) at a flow rate of 0.5 mg·mL−1, respectively. After HLF solution (40 mg·mL−1) was injected into the rat jugular vein, the effluent fluids were collected every 20 min and analysed by HPLC (Fig. 2C).
Pretreatment and analysis of samples
The samples from the in situ perfusion, intestinal loop and in situ single-pass perfusion experiments were added into 3 times volume of methanol, vortexed for 3 min, and centrifuged at 12,000 rpm for 5 min, respectively. The supernatant was analysed by HPLC.
Metabolism of HLF by intestinal microflora
The fresh faeces of SD rats were mixed with physiological saline at a ratio of 1 g : 4 mL to form a suspension. The suspension was centrifuged at 1500 r·min−1 for 10 min. The obtained supernatant (i.e., intestinal microflora solution, 30 mL) was added into 270 mL brain heart infusion (BHI) anaerobic medium, mixed well, and placed it in a 37 °C anaerobic incubator (80% N2, 10% H2 and 10% CO2). Concentration of HLF were 0.25, 0.5 and 1.0 mg·mL−1, and concentration of GLV, RHV, VIT, RUT and HP were 52.96, 112.72, 8.66, 1.16 and 5.0 µg·mL−1 in the anaerobic medium, respectively.
The incubation samples were taken at 0, 1, 2, 3, 4, 6, 8, 10, 12, 20, 24, 30 and 48 h, and added into three times volume of methanol to terminate the metabolism. The mixture was ultrasound extracted for 3 min, and was centrifuged for 5 min at 15,000 r·min−1. The supernatant was used for analysis.
In vivo pharmacokinetics
Pharmacokinetics of HLF via oral administration
The SD rats (fasted for 12 h, drank freely) were randomly assigned to three groups, and were administered intragastrically HLF at the dose of 643, 1285, and 2570 mg·kg−1 of HLF (equivalent to 40.51, 80.96, 161.91 mg·kg−1 of GLV, and 84.55, 168.98, 337.96 mg·kg−1 of RHV, respectively, n = 8), respectively. 0.1 mL of blood were obtained via the rat tail vein at different time within 36 h, and were centrifuged at 5000 rpm for 5 min. The plasma samples were added into 3 times volume of methanol, vortexed for 3 min, and were centrifuged at 12,000 rpm for 5 min. The supernatant (50 µL) was injected into the HPLC-UV system. Mean plasma concentration–time curves of GLV and RHV in rats (n = 8) were established, respectively. MaS Studio PK Software 1.2.0 (Mathematical Pharmacology Professional Committee of China, Shanghai, China) was used to calculate pharmacokinetic parameters.
Bile secretion of HLF
The SD rats were anaesthetized, and their abdomen was opened to intubate common bile duct. HLF solution (40 mg·mL−1) was injected into jugular vein of the rats at the dose of 20 mg·kg−1. The bile was collected at 0, 10, 30, 60, 90, 120, 150, 180, 210, 240, 270, 330, 360, 420 and 480 min. The bile samples were added into 3 times volume of methanol, vortexed for 3 min, and were centrifuged at 12,000 rpm for 5 min. Concentration of GLV and RHV in the samples were determined by HPLC-UV method.
Statistical analysis
In the present study, all the results were presented as the mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 9.0 software for Windows (GraphPad Software, San Diego, CA). The significant differences among different groups were evaluated using one-way or two-way analysis of variance (ANOVA). p < 0.05 was considered to be significant.
Results
Caco-2 cell experiment
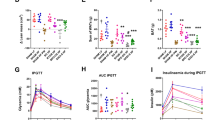
In this study, HLF and its main components (i.e., GLV, RHV, VIT, RUT and HP) showed no cytotoxic effect on Caco-2 cells in the selected drug concentration range (Table S5 and Table S6). Papp of GLV, RHV, VIT, RUT and HP in HLF were in the range of (2.18 ± 0.15) ×10−7cm·sec−1 to (3.83 ± 0.22)×10−7cm·sec−1 (Table 1). This indicated that the five flavonoids in HLF exhibited poor absorption16. Overall, Papp (AP→BL) of five flavonoids first increased (p < 0.05) and then remained unchanged as their concentration increased. Moreover, the values of ER (Papp (BL→AP) /Papp (AP→BL)) were in the range of 0.70–1.56. This indicated that five flavonoids in HLF were absorbed via passive diffusion accompanied with carrier (uptake and efflux transporter)-mediated transport. Papp of five flavonoids increased 7.07% ~ 29.86%, 5.30% ~ 28.29%, 16.78% ~ 34.81% and 10.81% ~ 15.29% in the presence of CBS, SDS, VP and DIG, respectively (Fig. 3 and Table S7). This indicated CBS, SDS, VP and DIG can increase absorption of five flavonoids. Especially, the ER values of five flavonoids in HLF were decreased 25.98% ~ 35.43% by VP and 17.58% ~ 23.86% by DIG (Table S8). This indicated VP and DIG can inhibited P-gp-medicated efflux of five flavonoids.
Papp of GLV, RHV, VIT, RUT and HP in HLF across the Caco-2 cell monolayer in the absence and presence of absorption enhancers and P-gp inhibitor, respectively. (A) Absorption enhancers (CBS and SDS). (B-F) P-gp inhibitors (VP and DIG). Concentration of HLF in the transport medium were all 100 µg·mL−1. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to Control group; #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to CBS or VP group. AP, apical; BL, basal; CBS, cow bile salt; SDS, sodium dodecyl sulfate; VP, verapamil hydrochloride; DIG, digoxin. Data are represented as mean ± SD, n = 3.
In situ perfusion and intestinal loop experiments
Except for HP, absorption of GLV, RHV, VIT, RUT were poor in rat stomach after intragastric administration of HLF, and their A were only (1.78 ± 0.14)%·h−1 ~ (5.69 ± 0.51)%·h−1 (Table 2). Overall, absorption rate of GLV, RHV, VIT, RUT and HP first increased, then decreased with increase of administered dose.
As a whole, GLV, RHV, VIT and RUT showed similar and poor absorption rate (\(\:{k}_{a}^{app}\), 0.012 ± 0.006 h−1 ~ 0.055 ± 0.003 h−1), extent (A, (1.28 ± 0.14)%·h−1 ~ (2.82 ± 0.19)%·h−1) and potential (Papp, (0.31 ± 0.05 ~ 1.41 ± 0.08)×10−7 cm·sec−1), and HP showed relatively better absorption rate (\(\:{k}_{a}^{app}\), 0.166 ± 0.014 h−1), extent (A, (11.40 ± 0.98)%·h−1) and potential (Papp, (4.27 ± 0.36)×10−7 cm·sec−1) in rat small intestine after perfusing different concentrations of HLF (Table 3). Overall, \(\:{k}_{a}^{app}\), A and Papp of GLV, RHV, VIT, RUT and HP in HLF increased significantly (p < 0.05) from low dose to medium dose, and remained unchanged from medium dose to high dose (Table 3), respectively.
\(\:{k}_{a}^{app}\), A and Papp of GLV, RHV, VIT, RUT and HP in HLF were significantly different (p < 0.05) in the duodenum, jejunum, ileum and colon of rats (Table 4). Overall, the optimum absorption segment of five flavonoids was duodenum, which exhibited the maximum \(\:{k}_{a}^{app}\) and Papp, followed by colon, jejunum and ileum.
Compared to that at pH 4 and pH 8, \(\:{k}_{a}^{app}\), A and Papp of GLV, RHV, VIT, RUT and HP in HLF at pH 6 physiological environment in the rat small intestine were significantly higher (p < 0.05) (Table 5). Namely, absorption of five flavonoids exhibited physiological pH dependence in the rat small intestine. This implied that five flavonoids showed superior absorption in near-neutral physiological environments, but poor absorption in acidic and alkaline physiological environments.
In general, \(\:{k}_{a}^{app}\), A and Papp of GLV, RHV, VIT and RUT in HLF were significantly increased by CBS and SDS (p < 0.05) (Fig. 4A-C), which were increased 71.76% ~ 100.00%, 33.50% ~ 165.32% and 71.76% ~ 100.00% by CBS, 140.00% ~ 233.33%, 132.00% ~ 232.52% and 140.00% ~ 233.33% by SDS, respectively (Table S9).
VP and DIG significantly increased \(\:{k}_{a}^{app}\) (54.34% ~ 70.20% by VP and 16.11% ~ 47.81% by DIG), A (52.39% ~ 167.00% by VP and 24.12% ~ 78.23% by DIG) and Papp (54.34% ~ 70.20% by VP and 16.11% ~ 47.81% by DIG) of five flavonoids in HLF (p < 0.05) (Fig. 4D-F and Table S9). RIF significantly increased \(\:{k}_{a}^{app}\) (2.65% ~ 34.48%), A (24.48% ~ 55.72%) and Papp (2.73% ~ 35.06%), and MAN significantly decreased \(\:{k}_{a}^{app}\) (37.95% ~ 78.43%), A (18.99% ~ 41.98%) and Papp (37.92% ~ 78.68%) of five flavonoids in HLF (p < 0.05) (Fig. 5 and Table S9), respectively.
\(\:{k}_{a}^{app}\), A and Papp of GLV, RHV, VIT, RUT and HP in HLF in the absence and presence of absorption enhancers and P-gp inhibitors by the rat in situ recirculating intestinal perfusion, respectively. (A-C) Absorption enhancers (CBS and SDS). (D-F) P-gp inhibitors (VP and DIG). Concentration of HLF in the perfusion solution were all 0.1 mg·mL−1. The perfusion volume of HLF solution were all 100 mL. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to Control group; #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to CBS or VP group. CBS, cow bile salt; SDS, sodium dodecyl sulfate; VP, verapamil hydrochloride; DIG, digoxin. Data are represented as mean ± SD, n = 5.
\(\:{k}_{a}^{app}\), A and Papp of GLV, RHV, VIT, RUT and HP in HLF in the absence and presence of BSEP inhibitor and SGLT1 inhibitor in the rat in situ recirculating intestinal perfusion, respectively. (A-C) BSEP inhibitor (RIF). (D-F) SGLT1 inhibitor (MAN). Concentration of HLF in the perfusion solution were all 0.1 mg·mL−1. The perfusion volume of HLF solution were all 100 mL. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to Control group. RIF, rifampicin; MAN, mannitol. Data are represented as mean ± SD, n = 5.
The A of GLV, RHV, VIT, RUT and HP in HLF in the intestinal loop were higher than that in the in situ recirculating intestinal perfusion at the same dosage (Fig. 6A; Table 3), which meant the five flavonoids were possibly metabolized by intestinal microflora, then their prototypes and metabolites were further absorbed. The A of RHV, VIT and HP was significantly increased (p < 0.05) from low dose to high dose, but only the A of GLV and RUT was significantly increased (p < 0.05) from low dose to medium dose. Moreover, the absorption of each flavonoid in HLF was high at pH 8 (Fig. 6B), which is possibly due to the accelerated metabolism of these flavonoids by intestinal microflora in alkaline environments. Anyway, prototypes of these flavonoids in HLF displayed optimum absorption at pH 6.
The A of each flavonoid in HLF was significantly decreased (p < 0.05) whether the intestine was rinsed or the bile duct was ligatured (Fig. 6C). As a whole, A of GLV, RHV, VIT and RUT in HLF was higher in the lower intestinal segment (i.e., ileum, colon) than that in the upper intestinal segment (i.e., duodenum jejunum) (Table 6), which was possibly due to metabolism of these flavonoids by intestinal microflora. A of HP in HLF in four intestinal segments were similar.
Absorption percentage per hour (A) of GLV, RHV, VIT, RUT and HP in HLF under different conditions in the rat intestinal loop. (A) HLF doses (5, 10 and 20 mg), *p < 0.05, **p < 0.01 and ***p < 0.001 compared to 5 mg group; #p < 0.05, ##p < 0.01and ###p < 0.001 compared to 10 mg group. (B) pH conditions (4, 6 and 8), *p < 0.05, **p < 0.01 and ***p < 0.001 compared to pH 4 group; #p < 0.05, ##p < 0.01and ###p < 0.001 compared to pH 6 group. (C) w or w/o ligation of bile duct and rinsing of intestine, *p < 0.05, **p < 0.01 and ***p < 0.001 compared to Ligation of bile duct + rinsing of intestine group; #p < 0.05, ##p < 0.01and ###p < 0.001 compared to Ligation of bile duct + without rinsing of intestine group. (D-G) Absorption enhancers (CBS and SDS), P-gp inhibitors (VP and DIG), BSEP inhibitor (RIF) and SGLT1 inhibitor (MAN), *p < 0.05, **p < 0.01 and ***p < 0.001 compared to Control group; #p < 0.05, ##p < 0.01 and ###p< 0.001 compared to CBS or VP group. Concentration of HLF in the perfusion solution were all 1 mg·mL−1. The perfusion volume of HLF solution were all 10 mL. CBS, cow bile salt; SDS, sodium dodecyl sulfate; VP, verapamil hydrochloride; DIG, digoxin; RIF, rifampicin; MAN, mannitol. Data are represented as mean ± SD, n = 5.
The A of five flavonoids in HLF was increased 26.48% ~ 80.97% by CBS, 26.32% ~ 89.12% by SDS, 47.25% ~ 128.27% by VP and 20.86% ~ 97.21% by DIG, respectively (Fig. 6D-E and Table S10). The A of GLV, RHV, VIT and RUT were increased 19.32% ~ 43.03% by RIF and decreased 12.07% ~ 49.41% by MAN, respectively (Fig. 6F-G and Table S10).
GLV, RHV and VIT in HLF were detected in the effluent fluid in the rat single-pass intestinal perfusion, while RUT and HP were hardly detected (Table S11). It is noteworthy that, the efflux amount each period and cumulative efflux amount of GLV, RHV and VIT in HLF in the absence of VP were higher than that in the presence of VP in the effluent fluid (Fig. 7), and their cumulative efflux amount were reduced by 53.33%, 45.35%, 47.63%, respectively (Table S11), which meant the VP can significantly reduce the intestinal efflux of GLV, RHV and VIT in HLF.
Efflux of GLV, RHV and VIT via the intestine after the rats were injected into HLF solution via the jugular vein. (A-C) Efflux amount–time curves. (D-F) Cumulative efflux amount–time curves. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the perfusion solution 1 group. Perfusion solution 1 and perfusion solution 2 were K-R solution and K-R solution with VP, respectively. Data are represented as mean ± SD, n = 5.
Metabolism of HLF by intestinal microflora
HLF and its five components (i.e., GLV, RHV, VIT, RUT and HP) were incubated with intestinal microflora, respectively. Their mean concentration–time curves were shown in Fig. 8. The results indicated that, when HLF was incubated with intestinal microflora, mean concentration (i.e., exposure level) of GLV, RHV, RUT and HP decreased with incubation time prolong, but mean concentration of VIT and quercetin (QUE) firstly increased, then decreased in the incubation system. However, mean concentration of GLV, RHV, VIT, RUT and HP decreased with incubation time prolong when the five monomers were incubated with intestinal microflora, respectively. Thus it can be seen, HLF and its five components can be metabolized by intestinal microflora. GLV and RHV in HLF can be metabolized to VIT by the intestinal microflora. RUT and HP can be metabolized to QUE by the intestinal microflora17,18,19.
Mean concentration–time curves of components and metabolite of HLF in incubation medium with intestinal microflora. (A) Concentration of GLV, RHV, VIT, RUT, HP and QUE in the incubation medium when HLF (2.5, 5.0 and 10.0 mg·mL−1) was incubated with intestinal microflora. (B) Concentration of GLV, RHV, VIT, RUT and HP in the incubation medium when these monomers were incubated with intestinal microflora, respectively. Data are represented as mean ± SD, n = 5.
In vivo pharmacokinetics
After intragastric administration of HLF at low, medium and high doses to the SD rats, the mean plasma concentration–time (C-T) curves and pharmacokinetic parameters of GLV and RHV were shown in Fig. 9; Table 7, respectively. The pharmacokinetic behavior of GLV and RHV in HLF was similar. Their mean plasma concentration–time curves exhibited multi-peak phenomenon (i.e., two peaks to four peaks with increase of the HLF dose), which was possibly due to enterohepatic circulation and multiple-site absorption of GLV and RHV in HLF in the gastrointestinal tract. They both demonstrated linear and nonlinear pharmacokinetic characteristics. Their exposure level (AUC(0−t) and AUC(0−∞)) and maximum plasma drug concentration (Cmax1 and Cmax2) in vivo both increased with the increase of dose.
Especially, GLV and RHV were secreted via bile after HLF solution was injected into the jugular vein of the SD rats (Fig. 10). Cumulative secretion percentage of GLV and RHV via the bile were 19.56 ± 0.71% and 28.21 ± 1.52% of the original administration amount within 8 h, respectively (Table S12).
Secretion of GLV and RHV via the bile after the rats were injected into HLF solution via the jugular vein. (A) Secretion amount–time curves. (B) Cumulative secretion amount–time curves. HLF solution (40 mg·mL−1) was injected into jugular vein of the SD rats at the dose of 20 mg·kg−1. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to GLV group. Data are represented as mean ± SD, n = 8.
Discussion
In the present study, gastrointestinal absorption behavior and its regulation of HLF were comprehensively investigated by in vitro, in situ and in vivo methods, and their mechanism was interpreted. The results indicated that five flavonoids in HLF were absorbed via passive diffusion accompanied with active transport (Fig. 11), displayed the optimum absorption at medium dose, physiological pH 6 environment and duodenum region, and metabolism of intestinal microflora, intestinal efflux and bile secretion affected their absorption. However, HLF exhibited poor gastrointestinal absorption and low oral bioavailability. Co-administration of HLF with absorption enhancers (e.g., CBS and SDS), transporter regulators (e.g., VP, DIG and RIF) increased its absorption.
In vitro, in situ and in vivo study indicated that HLF displayed poor gastrointestinal absorption, which can be confirmed by the absorption parameters. Papp (AP→BL) of GLV, RHV, VIT, RUT and HP in HLF were in the range of (2.18 ± 0.15 ~ 3.83 ± 0.22)×10−7 cm·sec−1 in the Caco-2 cell experiment (Table 1). A of five flavonoids were only (1.78 ± 0.14)%·h−1 ~ (5.69 ± 0.51)%·h−1 in the rat stomach (Table 2), which may be due to the small absorption surface area of the rat stomach and the short retention time of drug in the rat stomach. GLV, RHV, VIT and RUT showed poor absorption rate (\(\:{k}_{a}^{app}\), (0.012 ± 0.006)h−1 ~ (0.055 ± 0.003)h−1), extent (A, (1.28 ± 0.14)%·h−1 ~ (2.82 ± 0.19)%·h−1) and potential (Papp, (0.31 ± 0.05 ~ 1.41 ± 0.08)×10−7 cm·sec−1), and HP showed relatively better absorption rate (\(\:{k}_{a}^{app}\), 0.166 ± 0.014 h−1), extent (A, (11.40 ± 0.98)%·h−1) and potential (Papp, (4.27 ± 0.36)×10−7 cm·sec−1) in the rat small intestine. Especially, the oral bioavailability of GLV and RHV via oral administration of HLF at the low, medium and high dose were only 0.81%, 1.36% and 1.63% for GLV, and 1.74%, 3.21% and 2.38% for RHV, respectively, which was calculated with the ratio of the AUC(0−∞) of oral administration of HLF from Table 7 and the AUC(0−∞)of intravenous administration of HLF from our previous study4. All these results indicated that main flavonoids in HLF exhibited poor oral bioavailability, which is possibly due to low intestinal permeability (Papp, (2.18 ± 0.15 ~ 3.83 ± 0.22)×10−7cm·sec− 1 in Caco-2 cell or (0.31 ± 0.05 ~ 4.27 ± 0.36)×10−7 cm·sec−1) in rat intestine) of the flavonoids. Meanwhile, according to 2017 FDA Biopharmaceutical Classification System (BCS) guidance20,21, solubility of HLF in water (2.81 mg·mL−1)22, content (0.36%~13.15%) and solubility (0.04 ~ 100.00 mg·mL−1) of five flavonoids in HLF23,24,25 in the present study, it can speculated that HLF and its five flavonoids belong to BCS class III, namely, they belong to “like” drugs with high solubility and low permeability.
The mechanism of absorption of five flavonoids in HLF. API, apigenin; AT, active transport; BS, bile secretion; F, flavonoids; FM, flavonoids metabolites; GLV, 4’’-O-glucosylvitexin; HLF, hawthorn leave flavonoids; HP, hyperoside; IE, intestinal efflux; QUE, quercetin; RHV, 2’’-O-rhamnosylvitexin; RUT, rutin; SGLT1, sodium-dependent glucose transporter 1; P-gp, P-glycoprotein; PD, passive diffusion; VIT, vitexin.
GLV, RHV, VIT, RUT and HP in HLF were absorbed via passive diffusion accompanied with active transport (i.e., SGLT1 and P-gp-mediated drug transport), which can be confirmed by the change of absorption parameters in vitro and in situ studies26. The Papp (AP→BL) of five flavonoids in HLF first slightly increased, then remained unchanged when their concentrations were increased in Caco-2 cell monolayer (Table 1). Their \(\:{k}_{a}^{app}\), A and Papp were first increased from low dose to medium dose, then kept unchanged from medium dose to high dose in the rat small intestine. Five flavonoids in HLF displayed dose-dependence and saturation phenomena in both in vitro and in situ studies.
Absorption of five flavonoids in HLF displayed certain regioselectivity in the rat intestine (Tables 4 and 6). Overall, the upper part of small intestine (i.e., duodenum) was the major absorptive site of five flavonoids in HLF, which was consistent with the general knowledge that the upper intestine is the main absorptive site of many drugs. It is noteworthy that five flavonoids were also well absorbed in the colon (Table 6). In fact, absorption of five flavonoids in the intestine belonged to apparent absorption, which included absorption of their prototype and metabolites, the latter derived from the metabolism of the flavonoids by the intestinal microflora (Fig. 6C). It should be noted that, on the whole, A of five flavonoids in the intestine ranked as duodenum, colon, jejunum and ileum in the in situ recirculating intestinal perfusion study (Table 4), but their A ranked as colon, ileum, duodenum and jejunum in the intestinal loop study (Table 6). This difference was mainly attributed to effect of intestinal microflora on the absorption of these flavonoids as well as differences in absorption potential of different intestinal segments. The types and quantities of intestinal microflora usually increase from the duodenum, jejunum, ileum to colon in sequence, and the metabolic potential of the intestinal microflora also increases in sequence, which led to increase of apparent absorption (i.e., absorption and metabolism of the prototype in the intestine) of these flavonoids at the far end of the intestine (e.g., colon, ileum). That is to say, the actual absorption of the prototype of five flavonoids was possibly not increased in the colon and ileum, the significant metabolism of these flavonoids by the intestinal microflora in these segments merely appears to increase their absorption.
Absorption of GLV, RHV, VIT, RUT and HP in HLF exhibited pH dependence. Compared to that at pH 4 and pH 8, they displayed the optimal absorption at pH 6 physiological environment in the rat small intestine (Table 5). The reason could be that, compared to those at pH 4 and pH 8, these flavonoids were not easily dissociated at pH 6, and could be absorbed in their non-dissociative forms.
According to the results of the present study and other studies17,18,19,27, the possible metabolic pathway of five flavonoids in HLF by intestinal microflora are as follows: GLV and RHV are metabolized to VIT, subsequently, VIT are further metabolized to apigenin (API), finally, API is metabolized to phenylacetic acid27. Meanwhile, RUT and HP are metabolized to QUE, subsequently, QUE is mainly metabolized to different phenolic acids17,18,19.
Intestinal efflux and bile secretion usually affect intestinal absorption of drugs. In this study, the flavonoids (e.g., GLV, RHV and VIT) in HLF exhibited significant transporter (e.g., P-gp)-mediated intestinal efflux. This was confirmed by transport studies of these flavonoids in HLF in the presence or absence of P-gp inhibitor/substrate (e.g., VP and DIG) in the Caco-2 cell monolayer, in situ single-pass and recirculating intestinal perfusion, and intestinal loop experiments (Figs. 3B-F, 4D-F, 6E and 7). Meanwhile, these flavonoids (e.g., GLV, RHV) in HLF displayed significant bile secretion, which was confirmed by the bile secretion experiment of HLF in the present study (Fig. 10). Intestinal efflux and bile secretion of GLV and RHV in HLF were possibly the main reason for existence of enterohepatic circulation of the two flavonoids, which led to phenomenon of multiple peaks in the concentration–time curves of GLV and RHV (Fig. 9). It should be noted that, only the pharmacokinetic characteristics (C-T curves and pharmacokinetic parameters) of GLV and RHV in HLF were described, and pharmacokinetic characteristics of the other three components (i.e., VIT, RUT and HP) in HLF were not described because the latter cannot be detected in the rat plasma and bile after intragastric administration of HLF due to their low content in HLF and relatively low sensitivity of the analytical instruments in the present study. However, GLV and RHV are the most abundant and representative components in HLF, so their pharmacokinetic behavior can be used to characterize the pharmacokinetic behavior of HLF.
Interestingly, five flavonoids (i.e., GLV, RHV, VIT, RUT and HP) with the same parent nucleus (i.e., 2-phenylchromone) in HLF displayed similar but different absorption characteristics. The similarity was that, as flavonoid glycosides, all of them exhibited poor absorption, similar absorption region, and existences of metabolism of intestinal microflora, intestinal efflux and bile secretion. However, they exhibited different absorption rate (\(\:{k}_{a}^{app})\), degree (A) and potential (Papp), which possibly due to their different chemical structure (e.g., types and numbers of substituents) and physicochemical properties (e.g.,. solubility). Among five flavonoids in HLF, absorption of the flavonoids with monoglycoside (i.e., VIT and HP) were superior to the flavonoids with diglycoside (i.e., GLV, RHV and RUT). For example, \(\:{k}_{a}^{app}\), A and Papp of GLV (vitexin-4’’-O-glucoside) and RHV (vitexin-2’’-O-rhamnoside) were similar, and were lower than that of VIT (apigenin-8-C-glucoside). It is noteworthy that, HP (quercetin-3-O-galactoside) displayed the relatively good gastrointestinal absorption in the five flavonoids. This may be due to their different lipophilicity and hydrophilicity (i.e., log P values) in Table S13. The log Pvalues of HP was the highest among the five flavonoids in HLF, which means that, compared to the other four flavonoids, it has the favorable ratio of lipophilicity to hydrophilicity. In fact, poor absorption of five flavonoids in HLF and their differences in absorption were closely related to their physicochemical properties28(Table S13) which only partially obeyed Lipinski’s rule of five (also known as RO5)29,30, i.e., physicochemical parameters of the drug-like compound were as follows: MW ≤ 500, log P ≤ 5, H-bond donors ≤ 5 and H-bond acceptors ≤ 10.
In the current study, absorption enhancers (e.g., CBS and SDS), P-gp inhibitors/substrate (e.g., VP and DIG) and BSEP inhibitor (RIF) increased absorption five flavonoids in HLF, and SGLT1 inhibitor (MAN) decreased their absorption. The possible mechanisms of absorption enhancement involved in extracting membrane protein or lipids, membrane fluidization, producing reverse micelles in the membrane, creating aqueous channels, disrupting the hemidesmosomes, binding to Ca+2in the regions of tight junctions, influencing or causing damage to the tight junction between the epithelial cells, reducing the viscosity and elasticity of the mucus layer, inhibitory effects on mucosal membrane peptidases, acting as surfactants, induction of membrane wounds and mucoadhesive properties31,32,33,34. P-gp inhibitors35,36increased the absorption of GLV, RHV, VIT, RUT and HP by inhibiting their intestinal efflux. This implied that the five flavonoids in HLF were P-gp substrates. As BSEP inhibitor, RIF37increased the retention of five flavonoids in HLF in vivo by inhibiting BSEP-mediated bile secretion. As SGLT1 inhibitor, MAN38 decreased the absorption of five flavonoids in HLF by inhibiting SGLT1. This implied that, as flavonoid glycosides, five flavonoids in HLF were substrates of SGLT1, and could be transported by SGLT1.
It should be noted that, Papp of HP ((2.26 ± 0.24 ) ~ (3.83 ± 0.22)×10−7 cm·sec−1) in HLF in the present study was similar with the previous study ((1.87 ± 1.11)×10−7 cm·sec−1)14, but Papp of RHV ((0.31 ± 0.05) ~ (0.93 ± 0.13)×10−7 cm·sec−1) and RUT ((2.28 ± 0.27) ~ (3.04 ± 0.22)×10−7 cm·sec−1) were inconsistent with the other two studies ((2.15 ± 0.55) ~ (2.45 ± 0.40)×10−5 cm·sec−1 for RHV and (1.03 ± 0.13)×10−5 cm·sec−1for RUT)39,40, which were 100 folds larger than that in our study. This means RHV and RUT were easily absorbed or transported in their studies. Generally speaking, absorption of flavonoid glycosides is poor because of their strong polar and large molecular weight which is adverse for them to penetrate the intestinal membrane41. So, results of these studies should be further confirmed since they did not conform to general knowledge.
Thus it can be seen that, GLV, RHV, VIT, RUT and HP in HLF can be absorbed via passive diffusion accompanied with active transport in their prototype form, meanwhile, they can also be metabolized into metabolites (e.g., VIT, API, QUE, apigenin, phenylacetic acid, and phenolic acids) in the intestine by intestinal microflora, then be absorbed in their metabolites form. After absorption, GLV, RHV, VIT, RUT and HP in HLF are partially excreted into the intestinal again, while the parts that have not been excreted may be metabolized by enzymes in the liver after entering the liver through the portal vein, then, some of their prototypes and metabolites are further secreted via bile. Poor oral bioavailability of HLF will affect development and clinical application of its oral product. According to the results of this study, it is feasible to improve oral bioavailability of HLF by combining with absorption enhancers (e.g., CBS and SDS), P-gp inhibitors/substrate (e.g., VP and DIG), BSEP inhibitors (e.g., RIF) and/or SGLT1 inducers (e.g., Angiotensin II)42. Moreover, the optimal absorption region, pH physiological environment and intestinal microflora metabolism of HLF found in this study can also provide valuable ideas to design and develop its novel oral preparations, e.g., the controlled-release and targeting preparations (e.g., duodenum or colon-targeted drug delivery systems).
Conclusion
In summary, HLF can be absorbed via passive diffusion accompanied with active transport. Its absorption was affected by multiple factors, e.g., administered dose, absorption region, physiological pH environment, metabolism of intestinal microflora, intestinal efflux and bile secretion. However, its gastrointestinal absorption and oral bioavailability are poor. HLF and its five flavonoids (i.e., GLV, RHV, VIT, RUT and HP) belong to BCS class III, namely “like” drugs with high solubility and low permeability. Absorption and oral bioavailability of HLF can be improved by combining with absorption enhancers, efflux transporter inhibitors and/or uptake transporter inducers.
Data availability
Data will be made available on request. Requests should be directed to corresponding author.
Abbreviations
- A:
-
absorption percentage per hour
- ANOVA:
-
analysis of variance
- AP:
-
apical
- API:
-
apigenin
- AUC:
-
area under curve
- BA:
-
bioavailability
- BCS:
-
biopharmaceutical classification system
- BHI:
-
brain heart infusion
- BL:
-
basal
- C:
-
concentration
- Caco-2 cell:
-
human colon carcinoma cell line
- CCK-8:
-
cell counting kit-8
- CL:
-
total body clearance
- CBS:
-
cow bile salt
- DIG:
-
digoxin
- DMEM:
-
Dulbecco’s modified Eagle medium
- DMSO:
-
dimethyl sulfoxide
- EDTA:
-
Ethylene Diamine Tetraacetic Acid
- ER:
-
efflux ratio
- F:
-
absorption fraction
- GLV:
-
4’’-O-glucosylvitexin
- HBSS:
-
Hank’s balanced salt solution
- HLE:
-
hawthorn leaf extract
- HLF:
-
hawthorn leave flavonoids
- HP:
-
hyperoside
- HPLC:
-
high performance liquid chromatography
- K-R:
-
Krebs-Ringer
- \(k_{a}^{app}\) :
-
apparent absorption rate constant
- LOD:
-
limit of detection
- LOQ:
-
limit of quantitation
- MAN:
-
mannitol
- MRT:
-
mean residence time
- P-gp:
-
P-glycoprotein
- Papp :
-
apparent permeability coefficient
- QUE:
-
quercetin
- RHV:
-
2’’-O-rhamnosylvitexin
- RIF:
-
rifampicin
- RUT:
-
rutin
- SD:
-
Sprague Dawley or standard deviation
- SDS:
-
sodium dodecyl sulfate
- SGLT1:
-
sodium-dependent glucose transporter 1
- t:
-
time
- t1/2z :
-
elimination half-life
- TCM:
-
traditional Chinese medicine
- TEER:
-
transepithelial electrical resistance
- V:
-
apparent volume of distribution
- VIT:
-
vitexin
- VP:
-
verapamil hydrochloride
- VRT:
-
variance of residence time
References
Wen, K. et al. Recent Research on flavonoids and their Biomedical Applications. Curr. Med. Chem. 28, 1042–1066. https://doi.org/10.2174/0929867327666200713184138 (2021).
Ullah, A. et al. Important flavonoids and their role as a therapeutic Agent. Molecules 25 https://doi.org/10.3390/molecules25225243 (2020).
Thilakarathna, S. H. & Rupasinghe, H. V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 5, 3367–3387 (2013).
Ma, G. et al. Simultaneous determination of vitexin-4’’-O-glucoside and vitexin-2’’-O-rhamnoside from hawthorn leaves flavonoids in rat plasma by HPLC method and its application to pharmacokinetic studies. J. Pharm. Biomed. Anal. 44, 243–249. https://doi.org/10.1016/j.jpba.2007.01.033 (2007).
Commission, C. Pharmacopoeia of the people’s Republic of China. China Med. Sci. Press. Beijing. 1, 332 (2020).
Zhang, Q. et al. Total flavonoids of hawthorn leaves promote motor function recovery via inhibition of apoptosis after spinal cord injury. Neural Regen Res. 16, 350–356. https://doi.org/10.4103/1673-5374.286975 (2021).
Dai, H. et al. Dietary hawthorn-leaves flavonoids improves ovarian function and liver lipid metabolism in aged breeder hens. Poult. Sci. 100, 101499. https://doi.org/10.1016/j.psj.2021.101499 (2021).
Li, S. C., Huang, H., Zheng, F., Wen, D. & Mo, S. -w. effects of yixintong on regulating cellular calcium channels. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Materia Med. 28, 754–756 (2003).
Gaitan, E., Cooksey, R. C., Legan, J. & Lindsay, R. H. Antithyroid effects in vivo and in vitro of vitexin: a C-glucosylflavone in millet. J. Clin. Endocrinol. Metabolism. 80, 1144–1147 (1995).
Shahat, A. A. et al. Anti-complementary activity of Crataegus Sinaica. Planta Med. 62, 10–13 (1996).
Negahdari, R. et al. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 35, 1719–1738 (2021).
Wang, S., Sheng, F., Zou, L., Xiao, J. & Li, P. Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism. J. Adv. Res. 34, 109–122 (2021).
Zhao, C. R. et al. Vitexin inhibits APEX1 to counteract the flow-induced endothelial inflammation. Proceedings of the National Academy of Sciences 118, e2115158118 (2021).
Zuo, Z., Zhang, L., Zhou, L., Chang, Q. & Chow, M. Intestinal absorption of hawthorn flavonoids–in vitro, in situ and in vivo correlations. Life Sci. 79, 2455–2462 (2006).
Ma, G. et al. Physicochemical characteristics and gastrointestinal absorption behaviors of S-propargyl-cysteine, a potential new drug candidate for cardiovascular protection and antitumor treatment. Xenobiotica 45, 322–334 (2015).
Artursson, P., Palm, K. & Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 46, 27–43 (2001).
Yang, J. et al. Conversion of rutin to quercetin by acid treatment in relation to biological activities. Prev. Nutr. Food Sci. 24, 313 (2019).
Riva, A. et al. Conversion of rutin, a prevalent dietary flavonol, by the human gut microbiota. Front. Microbiol. 11, 585428 (2020).
Yang, J. et al. Identification of the major metabolites of hyperoside produced by the human intestinal bacteria using the ultra performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. J. Ethnopharmacol. 147, 174–179 (2013).
Charalabidis, A., Sfouni, M., Bergström, C. & Macheras, P. The biopharmaceutics classification system (BCS) and the biopharmaceutics drug disposition classification system (BDDCS): beyond guidelines. Int. J. Pharm. 566, 264–281 (2019).
Chimalakonda, K. T. et al. April;. Current Knowledge Gaps and Regulatory Challenges in the Evaluation of Biowaivers for Biopharmaceutical Classification System (BCS) Class III- like Drugs., (2021). https://www.fda.gov/science-research/fda-science-forum/current-knowledge-gaps-and-regulatory-challenges-evaluation-biowaivers-biopharmaceutical
Hou, G., Gao, M. & Tong, L. Study on the physicochemical properties of total flavonoids in hawthorn leaves. Chin. J. Traditional Chin. Med. 30 (08), 1766–1768. https://doi.org/10.13193/j.archtcm.2012.08.72.hougl.043 (2012).
Frutos, M. J., Rincón-Frutos, L. & Valero-Cases, E. in Nonvitamin and Nonmineral Nutritional Supplements (eds Seyed Mohammad Nabavi & Ana Sanches Silva) 111–117Academic Press, (2019).
Chen, J. et al. Encapsulation of hyperoside with acyclic cucurbiturils: supramolecular binding behavior, water solubility and in vitro antioxidant activity. J. Mol. Struct. 1294, 136342. https://doi.org/10.1016/j.molstruc.2023.136342 (2023).
Wu, J. Y. et al. Enzymatic synthesis of novel vitexin glucosides. Molecules 26, 6274 (2021).
Farrell, T. L., Poquet, L., Dew, T. P., Barber, S. & Williamson, G. Predicting phenolic acid absorption in Caco-2 cells: a theoretical permeability model and mechanistic study. Drug Metab. Dispos. 40, 397–406 (2012).
Peng, Y. et al. Absorption, metabolism, and bioactivity of vitexin: recent advances in understanding the efficacy of an important nutraceutical. Crit. Rev. Food Sci. Nutr. 61, 1049–1064 (2021).
National Center for Biotechnology Information, N & PubChem (2004). https://pubchem.ncbi.nlm.nih.gov/
Leeson, P. Chemical beauty contest. Nature 481, 455–456 (2012).
Lipinski, C. A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technol. 1, 337–341. https://doi.org/10.1016/j.ddtec.2004.11.007 (2004).
Thanou, M., Verhoef, J. C., Nihot, M. T., Verheijden, J. H. & Junginger, H. E. Enhancement of the intestinal absorption of low molecular weight heparin (LMWH) in rats and pigs using Carbopol (R) 934P. Pharm. Res. 18, 1638 (2001).
Yu, Q., Wang, Z., Li, P. & Yang, Q. The effect of various absorption enhancers on tight junction in the human intestinal Caco-2 cell line. Drug Dev. Ind. Pharm. 39, 587–592 (2013).
Moghimipour, E., Ameri, A. & Handali, S. Absorption-enhancing effects of bile salts. Molecules 20, 14451–14473 (2015).
Dahlgren, D., Sjöblom, M., Hedeland, M. & Lennernäs, H. The in vivo effect of transcellular permeation enhancers on the intestinal permeability of two peptide drugs enalaprilat and hexarelin. Pharmaceutics 12, 99 (2020).
Summers, M. A., Moore, J. L. & McAuley, J. W. Use of Verapamil as a potential P-glycoprotein inhibitor in a patient with refractory epilepsy. Ann. Pharmacother. 38, 1631–1634 (2004).
Fromm, M. F., Kim, R. B., Stein, C. M., Wilkinson, G. R. & Roden, D. M. Inhibition of P-glycoprotein–mediated drug transport: a unifying mechanism to explain the interaction between digoxin and quinidine. Circulation 99, 552–557 (1999).
Lai, Y. In Transporters in Drug Discovery and Development (ed Yurong Lai) 327–352 (Woodhead Publishing, 2013).
Zhou, W. et al. Intestinal absorption of forsythoside A in in situ single-pass intestinal perfusion and in vitro Caco-2 cell models. Acta Pharmacol. Sin. 33, 1069–1079 (2012).
Xu, Y., Fan, G., Gao, S. & Hong, Z. Assessment of intestinal absorption of vitexin-2’’-O-rhamnoside in hawthorn leaves flavonoids in rat using in situ and in vitro absorption models. Drug Dev. Ind. Pharm. 34, 164–170 (2008).
Zhang, X. et al. Absorption and metabolism characteristics of rutin in Caco-2 cells. The Scientific World Journal (2013). (2013).
Hu, M. Commentary: bioavailability of flavonoids and polyphenols: call to arms. Mol. Pharm. 4, 803–806 (2007).
Zhao, M., Li, N. & Zhou, H. SGLT1: a potential drug target for cardiovascular disease. Drug. Des. Devel. Ther., 2011–2023 (2023).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 82074109, 82374133, 81873078, and 81374051). Thank Professor Xuehua Jiang, West China School of Pharmacy of Sichuan University, for his guidance and help in completing the study.
Author information
Authors and Affiliations
Contributions
Junwei Chow: Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Xiaolei Yang: Methodology, Formal analysis, Investigation. Jiarong Hu: Formal analysis, Methodology, Investigation, Writing - review & editing. Qiuxia Yu: Methodology, Investigation. Yu Zhong: Methodology, Investigation. Xinyi Hu: Methodology, Investigation. Jingru Liang: Methodology, Investigation. Chenyang Zhu: Methodology, Investigation, Visualization, Software. Shiwei Yan: Methodology, Investigation, Visualization, Software. Longzhou Li: Methodology, Investigation. Haiyang Liu: Methodology, Investigation. Tiandian Wang: Methodology, Investigation. Zihan Wang: Methodology, Investigation. Long Gao: Methodology, Investigation. Guo Ma: Conceptualization, Investigation, Resources, Supervision, Project administration, Writing - review & editing, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The animal experiments were approved by the Institutional Animal Care and Use Committee of School of Pharmacy, Fudan University (2021-08-LY-MG-95). All animal experiments complied with the WMA Statement on animal use in biomedical research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chow, J., Yang, X., Hu, J. et al. Gastrointestinal absorption and its regulation of hawthorn leaves flavonoids. Sci Rep 15, 658 (2025). https://doi.org/10.1038/s41598-024-81823-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81823-y