Abstract
Simvastatin, a 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitor, was used in cardiovascular diseases and could decrease low-density lipoprotein cholesterol, and may have a repurposed role in cancer therapy. However, the effects of simvastatin on endometrial cancer remain controversial. We aimed to elucidate the role and mechanisms of simvastatin in regulating previously identified endometrial cancer-associated mesenchymal stem cells (EmCaMSCs)-mediated immunosuppressive effects and anti-tumor progression. Coculture of EmCaMSCs and peripheral blood mononuclear cells (PBMC) was used to assay the population of CD8 + T cells, natural killer (NK) cells, and cytotoxicity of NK cells. The mechanisms were elucidated by applying recombinant proteins and inhibitors of candidate proteins, transforming growth factor-beta 2 (TGF-β2). Finally, the humanized mouse model was generated to study the effects of simvastatin-mediated immunotherapy in treating endometrial cancer. The protein expressions of TGF-β2, CD56, CD8, and PD-L1 in xenograft tumors were analyzed by Western blot or immunohistochemistry assay. In this study, simvastatin inhibited the proliferation of endometrial cancer cells (HEC-1 A and RL95-2) and EmCaMSCs, and the half-maximal inhibitory concentration (IC50) values of EmCaMSCs were much higher. Simvastatin rescued the proliferation and the population of CD8 + T cells and natural killer (NK) cells from PBMC coculturing with EmCaMSC. Simvastatin treatment reduced the expression of TGF-β2 in EmCaMSCs at both the gene and protein levels. TGF-β2 activated the downstream SMAD2/3 signaling, and their inhibition by simvastatin could enhance the cytotoxicity of NK cells against endometrial cancer cells in vitro. Additionally, a combination of simvastatin and NK cell therapy inhibited xenograft growth, potentially by reducing TGF-β2 expression. In conclusion, simvastatin could rescue the population of CD8 + T cells and NK cells from PBMC cocultured with EmCaMSCs. Furthermore, simvastatin could enhance the cytotoxicity of NK cells in vitro and inhibit tumor growth in vivo in a humanized mouse model. These results suggested that simvastatin may be considered as a repurposed and combination drug for treating endometrial cancer.
Similar content being viewed by others
Introduction
Statins, traditionally applied in cardiovascular diseases to reduce cholesterol1 and can be divided into two groups: type-I derivatives from fermentation, including mevastatin, lovastatin, pravastatin, and simvastatin, and type-II drugs from the synthetic origin, including fluvastatin, atorvastatin, cerivastatin, pitavastatin, and rosuvastatin2. The primary role of statins in the mevalonate pathway is inhibiting 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase (HMGCR), resulting in the depletion of low-density lipoprotein (LDL) cholesterol3. However, recent studies suggested that statins exhibited anti-tumor effects, from meta-analysis and bench, in vitro and in vivo.
Statins could induce cancer cell apoptosis through the traditional caspase cascade and inhibit cell proliferation, migration, invasion, epithelial-mesenchymal transition (EMT), and chemoresistance in various types of cancer, including breast, lung, pancreas, and liver cancer4. Statins induced apoptosis of cancer cells through NFκB and the canonical caspase pathway and reduced proliferation through MEK1/2, ERK1/2, and JNK pathways5. Statins also induced cell cycle arrest of cancer cells by activating AMPK and increasing p21 and p27 expression6. Simvastatin suppresses the invasion of cancer cells by decreasing Pituitary Tumor-Transforming Gene 1 (PTTG1)7. Furthermore, statins could also regulate epigenetic machinery, resulting in cell cycle arrest. DNA methyltransferase (DNMTs) could be the targets of statins, mediating the methylation of the downstream proteins, p16 and p218. In conclusion, statins, especially simvastatin, showed anti-tumor progression in various cancers.
In several meta-analysis studies, statin use improved survival or reduced the risk of endometrial cancer9,10. However, some studies did not support the protective effects of statins11,12,13,14,15. Thus, the impact of statin use remains controversial in endometrial cancer patients. In the in vitro studies, statins exhibited anti-tumorigenic results in endometrial cancer cells when used alone9 or in combination with metformin16. These data suggested that statins could potentially be used for endometrial cancer. However, the detailed mechanisms are largely unknown.
On the other hand, statins may also affect the tumor microenvironment (TME), including immune cells and mesenchymal stem cells (MSCs). Statins mostly showed anti-inflammatory effects and enhanced the number of regulatory T cells (Treg)17 which obtained immunosuppressive effects on immunotherapy and may result in the suppression of the T helper 1 (Th1) immune response17. Besides, treatment of statins showed a reduction in the Th17 population18. On the contrary, a high dose of atorvastatin could reduce the in vitro function of conventional T cells and Treg19. Furthermore, statins were associated with better clinical outcomes in patients treated with PD-1 inhibitors20. Statins plus Th1 cytokines or dendritic cells (DC)-based immunotherapy could suppress breast tumor growth21. Statins could stimulate immunogenicity, promote anti-melanoma immune response22 and enhance the responses to immune checkpoint blockade in non-small cell lung cancer (NSCLC)23 and head and neck cancer cells24.
Statins could enhance the osteogenic differentiation, angiogenic potential, migration, homing, survival, and proliferation of MSCs25 which may have improved therapeutic outcomes in regenerative medicine. Simvastatin could decrease C-C motif chemokine ligand 3 (CCL3) expression from cancer cells and intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM1), interleukin-6 (IL-6), and CCL2 expression from cancer-associated MSCs (CaMSCs), disrupting the crosstalk of the cancer cells and TME and inhibiting tumor progression26. The evidence of statins in regulating CaMSCs is limited.
MSC had a pleiotropic immunosuppressive function, including inhibition of NK cell proliferation, cytotoxicity and cytokine production, suppression of dendritic cell maturation and antigen presentation, and promotion of M2 polarization in macrophages27. Besides, MSC suppressed B cell proliferation and immunoglobulin production, as well as T cell proliferation and effector function28,29. Furthermore, MSC also regulated the expansion and function of myeloid-derived suppressor cells (MDSC) and differentiation of Treg30. These immunosuppressive effects were mediated by IL-6, IL-10, hepatocyte growth factor (HGF), prostaglandin E2 (PGE2), and TGF-β from TME, including MSC or MSC-derived exosomes27. Thus, MSC-mediated immunosuppression may contribute to tumor progression.
Simvastatin exhibits anti-metastatic and anti-tumorigenic effects in endometrial cancer cells through mitogen-activated protein kinase (MAPK)9. Simvastatin, combined with metformin, a drug for diabetes, synergistically inhibited cell growth and induced Bim expression and apoptosis in endometrial cancer cells through AMP-activated protein kinase (AMPK) signaling16. However, the effects of simvastatin in regulating endometrial cancer and TME are largely unknown due to the lack of in vitro and in vivo data.
TME is complex and essential for tumor progression. Among the cell components of TME, CaMSCs and immune cells play critical roles in tumor progression. Thus, we used endometrial cancer-associated MSCs (EmCaMSCs) cocultured with peripheral blood mononuclear cells (PBMC) and NK cells, and together with simvastatin to elucidate the roles and mechanisms of simvastatin in inhibiting the immunosuppressive effects of EmCaMSCs. These data may provide the foundation for the repurposing roles of simvastatin in treating endometrial cancer.
Materials and methods
Ethical declaration
The experimental procedures are approved by the Research Ethics Committee of Hualien Tzu Chi Hospital (IRB number: 109-257-A). This study adhered to relevant ethical standards and guidelines. Written informed consent was obtained from all participants.
Cell culture
Three primary EmCaMSCs (CaMSC1, CaMSC2, and CaMSC3) were obtained from our lab (Stem Cell Lab in Hualien Tzu Chi Hospital). The cells isolated as previously described27and cultured in low-glucose Dulbecco’s Modified Eagle medium (DMEM, Sigma-Aldrich, St Louis, MO, USA), HEC-1 A were cultured in McCoy 5 A medium (Gibco, Waltham, MA, USA), and RL95-2 were cultured in DMEM/F12 medium (Gibco, Waltham, MA, USA). NK-92 cells were cultured in Myelocult H5100 medium (StemCell Technologies) supplemented with 12.5% horse serum (Gibco) and recombinant human IL-2 (500 U/mL, PeproTech, Cranbury, NJ, USA), which obtained better cytotoxicity31. Human peripheral blood mononuclear cells (PBMC) were donated from healthy persons and isolated using Ficoll-Paque (Amersham Biosciences, Piscataway, NJ, USA), following the manufacturer’s instructions. PBMCs were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco). The media were supplemented with 10% fetal bovine serum (FBS, Gibco) and 1× penicillin/streptomycin (PS, Gibco).
Growth inhibition assay
2 × 103 cells were seeded on a 96-well plate. After cell attachment, cells were treated with serially diluted simvastatin (Sigma-Aldrich) and cultured for 3 days. XTT (2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)−2H-Tetrazolium-5-Carboxanilide (Biological Industries Ltd., Beit Haemek, Israel) was used to measure the cell viability. IC50 of simvastatin in each cell was calculated using GraphPad Prism software (version 9, GraphPad Software, Boston, MA, USA). Each experimental group was set up with three replicates, and the experiment was conducted three times independently.
Quantitative reverse transcription polymerase chain reaction (Q-RT-PCR)
Total RNA was purified using the PureLink RNA Mini kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. According to the manufacturer’s instructions, 1 µg total RNA was reverse transcribed into cDNA using SuperScript III enzyme (Invitrogen, Carlsbad, CA, USA). Real-time PCR was performed using Fast SYBR Green Master Mix (Applied Biosystems, Waltham, MA, USA) and analyzed by Quant Studio 5 (Applied Biosystems). GAPDH was measured as an internal control, and all experiments were repeated in triplicate. The primer sequence is listed in Supplement Table 1.
Coculture system and LDH cytotoxicity assay
Briefly, PBMCs or NK-92 cells were cocultured with EmCaMSCs in the presence of IL-2 for 4 days. The ratio of PBMCs or NK-92 to EmCaMSCs was 10:1. For the cytotoxicity test in endometrial cancer cells, 1.5 × 104 RL95-2 and HEC-1 A cells were seeded and cultured overnight in a 96-well plate, respectively. After cell attachment, EmCaMSCs-cocultured PBMCs or NK-92 were harvested and further cocultured with cancer cells at a 10:1 ratio for 6 h in PBS containing 2% FBS and fresh IL-2. The supernatants were collected for the lactate dehydrogenase (LDH) activity test (ThermoFisher, Waltham, MA, USA) following the manufacturer’s instructions. PBMCs or NK-92 cells cultured alone and treated with IL-2 were the control. Recombinant TGF-β2 (3 ng/mL, PeproTech, Cranbury, NJ, USA) and anti-TGF-β2 (300 ng/mL, Abcam, Cambridge, UK) were used in the experiments. All of the experiments were conducted in triplicate.
Flow cytometry analysis
For analysis of the immune cell population, the protein expression of CD56, CD8, and CD4 was analyzed. Briefly, PBMC cells alone or cocultured with EmCaMSCs were harvested and stained with anti-CD56-APC, anti-CD8-APC, and anti-CD4-PE antibodies (all from eBioscience, San Diego, CA, USA) for 30 min. For analysis of the signaling of the TGF-β2/SMAD2/3 axis, NK-92 cells treated with recombinant TGF-β2 and anti-TGFβ2 or simvastatin were harvested. Cells were fixed and stained with anti-TGF-β2 (Abcam, Cambridge, UK), anti-SMAD2/3 (BD Biosciences, Franklin, NJ, USA), and pSMAD2/3 (Cell Signaling Technology, Danvers, MA, USA) in intracellular staining permeabilization wash buffer (BioLegend). After washing, cells were subsequently incubated with FITC-conjugated secondary antibody (Invitrogen). After the washout of the non-specific binding of antibodies, the target protein expression percentage was analyzed by flow cytometry (Lyric, BD Biosciences, Franklin, NJ, USA). Each experimental group was set up with three replicates, and the experiment was conducted three times independently.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of TGF-β2 was determined using an ELISA Kit (ThermoFisher) according to the manufacturer’s instructions. Briefly, the medium derived from CaMSCs or CaMSCs treated with simvastatin was incubated on an antibody-coated plate. After washing, the plate was further incubated with biotin-conjugated secondary antibody, streptavidin–horseradish peroxidase, and chromogen, respectively. The plate was analyzed using an ELISA reader (BioTek Synergy HTX, Agilent, Santa Clara, CA, USA) at 450 nm as the primary wavelength and 620 nm as the reference wavelength. Each experimental group was set up with duplicates, and the experiment was conducted three times independently.
Western blot
Cell or tissue lysates were collected, and proteins were purified using RIPA buffer (ThermoFisher) containing protease and phosphatase inhibitors (Roche Diagnostics, Mannheim, Germany). Proteins were loaded into 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). After blocking with 1% BSA for 1 h, the proteins, including the phosphorylated form, were incubated with primary antibodies, targeting TGF-β2 (Abacm, Cambridge, UK), pSMAD2/3 (SMAD2: Ser465/467; SMAD3: Ser423/425, Cell Signaling Technology, Danvers, MA, USA), SMAD2/3 (BD Biosciences, Franklin, NJ, USA), PD-L1 (Abacm, Cambridge, UK), and Actin (Cell Signaling Technology) at 4℃ overnight and HRP-conjugated secondary antibodies (GeneTex, Irvine, CA, USA) for 1 h. The DAB substrate and ECL (ThermoFisher) were incubated with target proteins and detected by chemiluminescence. Each protein and its phosphorylated form were analyzed and quantified using ImageJ software (version 1.54i, NIH, Bethesda, MD, USA).
Animal study- humanized xenograft mouse model
The animal experiments were approved by the Animal Research and Care Committee of Hualien Tzu Chi Hospital (No. 110 − 28). The reporting of animal experimental protocols in the manuscript follows the recommendations in the ARRIVE guidelines. The animals were housed in standard cages with free access to water and food in a temperature-controlled environment under 12 12-hour light and 12-hour dark cycle. PBMCs were injected at a dose of 1 × 107 cells in an immunocompromised NOD-SCID mouse (strain name: NOD.CB17-Prkdcscid/Jtcu, purchased from Tzu Chi University) via the tail vein (IV) on day 0. Subsequently, 1 × 106 endometrial cancer cells and 1 × 105 EmCaMSCs (10:1) were subcutaneously (SC) co-inoculated into the left flank region on the same days. The mice were noted in the HEC-1 A group (control n = 5, simvastatin, NK, and NK + simvastatin n = 4 each) and in the RL95-2 group (control, simvastatin, NK, and NK + simvastatin, n = 4 each). When the tumor grew to the optimal size, NK-92 cells (1 × 107 cells, IV injections, once) alone and simvastatin (1 mg/kg, PO, 5 times per week) alone, and the combinational treatment were administered. The control mice group received PBS, a replacement for simvastatin, and the tumor size was measured twice weekly. After the tumor size reached 1000 mm³, mice were sacrificed for tumor histopathological assays by CO₂ exposure, followed by decapitation. CO₂ gradually filled the euthanasia chamber, with the flow rate displacing 10–30% of the chamber volume per minute.
Immunohistochemistry analysis
The xenograft tumors were formalin-fixed, paraffin-embedded, and sectioned. The sections were first deparaffinized using xylene and then rehydrated. Antigen retrieval. was performed by heating the sections in citrate buffer (pH 6.0) at 95 °C for 30 min. The sections were treated with 1% hydrogen peroxide and further blocked with 5% bovine serum albumin (Sigma), preventing nonspecific binding. Subsequently, the sections were incubated at 4 °C overnight with primary antibodies targeting TGF-β2 (Abcam), CD56 (Cell Signaling Technology), CD8 (Cell Signaling Technology), and PD-L1 (Abcam) at a 1:100 dilution. The sections were then incubated with HRP-conjugated secondary antibody (GeneTex) for 1 h. Further, they detected the signal by using a DAB substrate kit (Dako, Carpinteria, CA, USA) at room temperature. The sections were counterstained with hematoxylin and photographed under a light microscope (Nikon TE2000-U, Tokyo, Japan).
Statistical methods
The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software, version 22, developed by the SPSS Institute in Chicago, IL, USA. Continuous variables were analyzed using the Student’s t-test to compare the two groups. All values were expressed as mean ± standard deviation (SD). Significance was set at p < 0.05.
Results
Statins could inhibit endometrial cancer cell proliferation
To determine the effect of simvastatin on cancer cells and EmCaMSCs, we evaluated the IC50 of simvastatin on these cells. The IC50 of simvastatin in HEC-1 A cells, RL95-2 cells, EmCaMSC2 (derived from a stage IA patient), and EmCaMSC3 (derived from a stage IIIC patient) were 2.5 µM, 2.8 µM, ~ 50 µM, and ~ 10µM, respectively (Fig. 1). When taken together, simvastatin could inhibit endometrial cancer cell growth more than EmCaMSCs.
Growth inhibition assay. Simvastatin was treated in different doses for 72 h in (A) HEC-1 A cells (n = 3), (B) RL95-2 cells (n = 3), (C) EmCaMSC2 (derived from stage IA patient) (n = 3), and (D) EmCaMSC3 (derived from stage IIIC patient) (n = 3). The IC50 of simvastatin in each cell from (A) to (D) was 2.5 µM, 2.8 µM, ~ 50 µM, and ~ 10µM, respectively. Redline indicated 50% of inhibition.
Simvastatin May increase the proliferation of PBMC by inhibiting EmCaMSC
To know the effect of simvastatin and EmCaMSC on the proliferation of PBMC, we added simvastatin and cocultured EmCaMSC with PBMC. PBMC (CD8, CD56, and CD4 population) reduced proliferation when cocultured with EmCaMSC. Adding simvastatin increased the PBMC proliferation in the cocultured condition (Fig. 2A-C). EmCaMSC-cocultured PBMC with cancer cells could decrease cancer cell death. After adding simvastatin, cancer cell death was increased (Fig. 2D).
The profile and cytotoxicity of PBMC cocultured with EmCaMSCs and simvastatin. PBMCs were cocultured with EmCaMSCs, simvastatin (1 µM), and IL-2 (3,000 U/ml) for 4 days. Two EmCaMSCs (CaMSC2 and CaMSC3) were used for the cocultured experiments. Percentages of (A) CD8 + T cells (n = 3), (B) CD56 + NK cells (n = 3), and (C) CD4 + T cells (n = 3) in PBMCs were analyzed by FACS. The representative flow pattern was shown on the upper panel. (D) Lactate dehydrogenase (LDH) cytotoxicity assay of PBMC cocultured with CaMSCs and simvastatin was calculated by measuring the LDH activity from the culture medium (n = 3). PBMC alone was used for control. PBMC with lysis buffer was performed as a control (100% lyzed), and those without lysis were taken as baseline. Sim: simvastatin. *p < 0.05, **p < 0.01, ***p < 0.001.
Simvastatin decreased TGF-β2 expression in CaMSCs
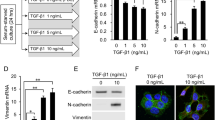
We initially screened several genes—including TNF-α, TGF-β1, TGF-β2, TGF-β3, IL-8 (associated with cell proliferation), PCEAM (angiogenesis-related), EpCAM (involved in EMT and cell motility), LGR5 (a cancer stem cell marker), and PD-L1/PD-L2 (immune-related)—and identified TGF-β2 as showing the most significant change in expression. To know the mechanism of simvastatin on cancer growth, we used Q-RT-PCR to evaluate TGF-β gene expression in EmCaMSCs and endometrial cancer cells. We aimed to assess the effects of clinically relevant concentrations of simvastatin (1 and 5 µM) on EmCaMSCs and cancer cells. TGF-β1 influences the functions of MSCs across various aspects32. However, we found that, in EmCaMSCs, only TGF-β2 was reduced after simvastatin 1 µM or 5 µM treatments (Fig. 3B), but not for TGF-β1 or -β3 (Fig. 3A and C). Moreover, the expression of TGF-β did not alter in RL95-2 and HEC-1 A cells treated with simvastatin (Fig. 3D and F).
Next, we performed an ELISA to evaluate the protein secretion of TGF-β2 in EmCaMSC and cancer cells after simvastatin treatment. Similar to the results of mRNA expression, the protein secretion of TGF-β2 was also reduced after adding simvastatin, but not in cancer cells (Fig. 3G and H). In summary, simvastatin decreased 31.4% (from 22.2 to 11.8 pg/mL) of TGF-β2 protein secretion from EmCaMSC. In summary, simvastatin specifically decreases TGF-β2 gene and protein expressions in EmCaMSCs.
Q-RT-PCR and ELISA analysis of TGFβ genes in EmCaMSCs and endometrial cancer cells. Simvastatin was treated in different doses for 24 h in CaMSC2, CaMSC3, RL95-2, and HEC-1 A cells. mRNA expression of (A) (D) TGFβ1 (n = 3), (B) (E) TGFβ2 (n = 3), and (C) (F) TGFβ3 (n = 3), were analyzed. (G) ELISA of TGFβ2 in CaMSC2 and 3 treated with simvastatin 1 and 5 µM (n = 3). (H) ELISA of TGFβ2 in HEC1A and RL95-2 cells treated with simvastatin 1 µM (n = 3). Two concentrations (1 µM and 5 µM) of simvastatin were used in treating CaMSCs, and 1µM was used in treating endometrial cancer cells. Sim: Simvastatin. * p < 0.05, ** p < 0.01, *** p < 0.001. GAPDH was used for endogenous control. ELISA: enzyme-linked immunosorbent assay.
TGF-β2 decreased PBMC proliferation by activating SMAD2/3
To evaluate the effect of TGF-β2 on the PBMC, we evaluated the proliferation and SMAD2/3 expression of PBMC. After adding recombinant TGF-β2 protein, the sphere formation and proliferation of PBMC were significantly reduced (Fig. 4A-B). Phosphorylation of SMAD2/3 was increased (Fig. 4C). Given their well-characterized cytotoxic profile, NK-92 cells were used to further investigate the cytotoxic effects. NK-92 cells don’t require antigen presentation via MHC to recognize and kill targets, while T cells rely on TCR-mediated and MHC-restricted antigen recognition. Adding TGF-β2 could reduce the cytotoxicity of NK-92 cells in endometrial cancer cells, which could be reversed by treating TGF-β2 antibody and simvastatin (Fig. 4D). Expression of TGF-β2 and phosphorylation of SMAD2/3 in NK92 cells was increased when adding recombinant TGF-β2 but further inhibited by applying anti-TGF-β2 or simvastatin (Fig. 4E-F). We further validated the TGF-β2/SMAD2/3 signaling in NK-92 cells by flow cytometry analysis. Expression of intracellular TGF-β2 remained unchanged in the control and treatment groups (Fig. 5A and B). Simvastatin treatment slightly decreased intracellular SMAD2/3 (Fig. 5A and C). Treatment of TGF-β2 significantly increased the intracellular of pSMAD2/3 and decreased by cotreatment of anti-TGF-β2 and simvastatin (Fig. 5A and D). Taken together, TGF-β2 could reduce the cytotoxicity of NK-92 cells via activating SMAD2/3 in the tumor microenvironment. It could be inhibited by anti-TGF-β2 or simvastatin.
The effects of TGF-β2 on cytotoxicity. (A) The morphology (the bottom panel showed higher magnification than the upper panel) and (B) proliferation of PBMCs cultured with recombinant TGF-β2 alone (n = 3) and with anti-TGF-β2 (αTGF-β2) (n = 3). (C) The downstream signaling of TGF-β2 and SMAD2/3 in PBMC was analyzed by Western blot. (D) Cytotoxicity of NK92 cells against endometrial cancer cells (n = 3) (E) The downstream signaling of TGF-β2 and SMAD2/3 in NK92 cells was analyzed by Western blot. (F) Quantification of proteins listed in (E) (n = 3). Scale bar: 100 μm in the upper panel and 200 μm in the lower panel. * p < 0.05, ** p < 0.01.
The signaling of TGF β−2/SMAD2/3 in NK-92 cells by flow cytometry analysis. NK-92 cells were pretreated with anti-TGF-β2 and simvastatin for 30 min and further treated with recombinant TGF-β2 for 1 h. Untreated NK-92 cells were taken as control (n = 3 for each group). (A) The percentage of intracellular TGF-β2, SMAD2/3, and pSMAD2/3 was shown by flow cytometry. The quantitation of intracellular (B) TGF-β2+ (n = 3), (C) SMAD2/3+ (n = 3), and (D) pSMAD2/3 + cells (n = 3) was shown. * p < 0.05, ** p < 0.01.
Simvastatin and NK cells inhibited xenograft growth
Simvastatin enhanced NK cell cytotoxicity (as observed following anti-TGF-β2 treatment, Fig. 4D) and reduced SMAD2/3 phosphorylation, mimicking the effects of TGF-β2 blockade (Fig. 4E). Based on these findings, a combination therapy using NK-92 cells and simvastatin was conducted. We used a humanized mouse model to determine the NK cells’ and simvastatin’s in vivo inhibitory effect on cancer growth. Human PBMC were first injected into the tail vein of NOD-SCID mice. Subsequently, the endometrial cancer cells (RL95-2 and HEC-1 A) and CaMSCs were injected into the subcutaneous region. After the tumor reached 150 mm3NK-92 cells alone, simvastatin alone, or in combination were given. We found that the NK and NK + simvastatin treatments could inhibit HEC-1 A + CaMSCs cell growth significantly (p < 0.05) than the control and simvastatin alone (Fig. 6A-B). The tumor growth of the NK + simvastatin group was inhibited in RL95-2 + CaMSCs xenografts when compared with the control group (Fig. 6C-D). In summary, simvastatin, in combination with NK-92 cells, could reduce the tumor growth of endometrial cancer cells coinjected with CaMSCs.
Animal study- humanized mouse model. Tumor size was calculated by length x width x width/2 (mm3). (A) HEC-1 A + CaMSCs (C) RL95-2 + CaMSCs. The mice were divided into (i) untreated (n = 5 (HEC-1 A) or 4 (RL95-2)), (ii) treated with NK-92 cells (n = 4), (iii) simvastatin (1 mg/kg) (n = 4), and (iv) combination treatment with NK-92 cells and simvastatin (n = 4). Gross pictures of xenograft tumors formed by (B) HEC-1 A + CaMSCs and (D) RL95-2 + CaMSCs in various groups. *p < 0.05, **p < 0.01, ***p < 0.001.
TGF-β2 and SMAD signaling were decreased in the xenograft tumors of simvastatin and NK cells treatment group
To evaluate the mechanism of simvastatin and NK cell therapy in vivo, we surveyed the expression of TGF-β2, SMAD2/3 signaling, and immune-related molecules programmed cell death 1 ligand 1 (PD-L1) by Western blot and the expression of TGF-β2, CD56, CD8, and PD-L1 by immunohistochemistry analysis. The Western blot showed that TGF-β2 and SMAD2/3 signaling were reduced in the NK + simvastatin group in the HEC-1 A + CaMSC xenograft tumor (Fig. 7A-B). Furthermore, the immunohistochemistry analysis showed that the expression of TGF-β2 was dramatically decreased in the NK + Simvastatin tumors. NK cells (CD56+) were infiltrated into tumor lesions in NK alone and NK + Simvastatin tumors. The cytotoxic T cells (CD8+) were most abundant in the tumor margin in the control group and infiltrated into tumors in the treatment groups (Fig. 6C-D). These results also demonstrated that the humanized mice were successfully generated since the NOD-SCID mice are immunocompromised and devoid of T cells and NK cells. The expression of the immune checkpoint inhibitor, PD-L1, was slightly decreased in treatment groups (Fig. 7A-D). These data suggested that tumor growth inhibition may be associated with TGF-β2-SMAD2/3 signaling and enhanced cytotoxicity of infiltrated NK cells (Fig. 8).
Western blot and immunohistochemistry (IHC) analysis of xenograft tumors. (A) TGF-β2, SMAD2/3 signaling, and PD-L1 in HEC-1 A + CaMSCs were analyzed by Western blot. (B) Quantification of proteins listed in (A) (n = 3). (C) The tumors derived from HEC-1 A + CaMSCs mice were formalin-fixed, paraffin-embedded, and sectioned. The samples were stained with TGF-β2, CD56, CD8, and PD-L1. Hematoxylin and eosin (HE) confirmed cell morphology by staining the nucleus and cytoplasm. Scale bar: 100 μm. (D) The quantitative results of the IHC (n = 3). The positive cells (in brown color) were counted from three fields in each group. Sim: simvastatin. NK + Sim: combination treatment with NK-92 cells and simvastatin. * p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
Simvastatin reversed the immunosuppressive effects of EmCaMSCs, including increases of CD4 + and CD8 + T cells and NK cells and their cytotoxicity against endometrial cancer cells. In EmCaMSCs, TGF-β2 was reduced after simvastatin treatment, but not TGF-β1 and TGF-β3. However, simvastatin treatment did not affect the expression of TGF-βs in endometrial cancer cells. TGF-β2 and activation of SMAD2/3 signaling inhibited the cytotoxicity of NK92 cells and could be rescued by treating with anti-TGF-β2 (αTGF-β2) and simvastatin. Treatment of low-dose simvastatin (1 mg/kg) did not affect the xenograft tumor growth of coinjection of EmCaMSCs-endometrial cancer cells, and the combination of simvastatin treatment and NK cell therapy synergistically reduced the tumor growth in vivo. Expressions of TGF-β2 and SMAD2/3 were downregulated in the simvastatin and NK cells combined treatment group. These results demonstrated that simvastatin could enhance the cytotoxicity of NK cells by inhibiting TGF-β2/SMAD2/3 signaling in the TME.
The MSCs secreted immunomodulation molecules, including anti-inflammatory cytokines, TGFβ1, interleukin (IL)−13 IL-18 binding protein (IL18BP), ciliary neurotrophic factor (CNTF), neurotrophin 3 (NT-3) factor, IL-10, IL-12p70, IL-17E, IL-27, and IL1 receptor antagonist (IL1RA), and proinflammatory cytokines, IL-1b, IL-6, IL-8, and IL933. Among these molecules, the TGFβ family and the downstream signaling have pleiotropic functions in regulating cancer cells as well as immune cells. TGFβ signaling inhibited the proliferation and activation of NK cells and cytotoxic T cells34. Thus, we surveyed the effects of simvastatin on the TGF-β signaling in the CaMSCs and cancer cells.
TGF-β signaling inhibited the activation and function of NK cells, resulting in anti-tumor immunity in solid cancer and B-acute lymphoblastic leukemia (B-ALL), which may be through the downstream SMAD pathway35. Besides, TGF-β could repress mTOR (mammalian target of rapamycin) and further inhibit the activation of NK cells36. NK cells from patients with metastatic breast cancer have reduced activation and function, and dysfunctional mitochondria, but increased expression of TGF-β and glycoprotein-A repetitions predominant (GARP)37. Inhibiting TGF-β signaling by LY2157299 combined with adoptive NK cell therapy obtained better therapeutic efficacy than single treatment in the animal models of colon cancer38. These results suggested that TGF-β, especially TGF-β1, may inhibit NK function through different mechanisms in various cancers. Herein, we found that TGF-β2 impaired the function of NK cells through SMAD2/3 signaling in the TME of endometrial cancer.
Studies have demonstrated that simvastatin inhibits the expression of TGF-β signaling in glioblastoma39. This inhibition occurs through the suppression of the Rho/ROCK and Smad3 signaling pathway39. In lung cancer cells, simvastatin downregulates TGF-β receptor II expression and inhibits cell proliferation via the ERK pathway40. However, no study was noted regarding how statins affected cancer MSCs’ TGF-β expression. In TME, neutralizing TGF-β enhances CD8 + T-cell and NK-cell-mediated anti-tumor immune responses41,42. Inhibiting TGF-β signaling is an effective way to enhance the effector functions of NK cells in cancer. In this study, we discovered that simvastatin suppressed TGF-β2-SMAD2/3 signaling within the TME, specifically affecting EmCaMSCs and NK cells.
Simvastatin induced ferroptosis of gastric cancer cells and activated anti-tumor immunity to sensitize anti-PD-1 immunotherapy43. Both lovastatin and simvastatin enhanced T-cell killing of tumor cells when combined with PD-1 blockade in a head and neck cancer model24. Statin users obtained improved overall survival (OS) and progression-free survival (PFS) than non-users in metastatic renal cell carcinoma patients receiving nivolumab (anti-PD-1)44. Intriguing, post-immune checkpoint blockade (ICB) statin users showed significantly prolonged OS and PFS compared to pre-ICB statin and non-statin users45. Bintrafusp Alfa (BA), a bifunctional fusion protein targeting TGF-β and PD-L1, enhanced the survival in syngeneic ovarian cancer models by promoting T effector and NK cell responses46. BA also advanced into clinical trials in treating various types of cancer, indicating that TGF-β and PD-L1 may have synergistic effects on anti-tumor therapy. In this study, we demonstrated that simvastatin has the same potential in treating endometrial cancer. However, the effects of simvastatin combined with immunotherapeutic targets, such as immune checkpoint inhibitor PD-L1, need further investigation.
Simvastatin was found to reduce the expression of key immunosuppressive molecules—TGF-β2—thereby rescuing immune cell populations and increasing NK cell cytotoxicity. The in vivo effects of simvastatin were confirmed using a humanized mouse model, where it inhibited tumor growth, likely through its immunomodulatory actions. Therefore, the effects of simvastatin combined with other drugs show potential efficacy in treating various types of cancer. For example, simvastatin and metformin, or combined anti-PD-L1, also showed therapeutic effects in many cancers47,48.
There were some limitations in this study. The small sample size limited our study, as each group in the animal model included only four mice, with experiments repeated three times to ensure consistency. Additionally, we used two specific endometrial cancer cell lines, HEC-1 A and RL95-2, which may not fully represent the heterogeneity of endometrial cancer. These factors could limit the generalizability of our findings, and future studies with larger sample sizes and diverse cell lines or patient-derived xenografts are needed to validate our results. Furthermore, the variability in IC50 values between different cell types raises concerns about dose optimization, and the study does not address the long-term sustainability of simvastatin’s effects. The syngeneic mice model of endometrial cancer needs to be generated to investigate the reactions of other immune cells when treated with simvastatin alone or in combination with other drugs.
Statins, including simvastatin, could decrease the PD-L1 expression in melanoma and lung cancer cells49. Herein, we did not find decreases in PD-L1 in the xenograft tumors. We could not rule out that it was due to the low dose of simvastatin or that it was unique in endometrial cancer cells. Further studies were needed to clarify the role of simvastatin in regulating PD-L1 in endometrial cancer. Regardless of the role of PD-L1, simvastatin positively regulated the cytotoxicity of NK cells and reduced tumor growth, indicating that simvastatin may have the potential to enhance the efficacy of immune therapy.
For future research, we suggested investigating the interaction of simvastatin with other cancer treatment drugs (chemotherapy or targeted therapy) to explore potential synergistic effects. Additionally, we emphasized the need for further studies to validate the effects of simvastatin across a broader range of cancer types, using diverse cell lines, animal models, and clinical trials. These future investigations will help elucidate simvastatin’s full therapeutic potential in oncology.
Conclusions
Simvastatin could rescue the population of CD8 + T cells and NK cells from PBMC cocultured with EmCaMSCs. Furthermore, simvastatin could enhance the cytotoxicity of NK cells in vitro and inhibit tumor growth in vivo in a humanized mouse model. These results suggested that simvastatin may be considered as a repurposed and combination drug for treating endometrial cancer. Further research is needed to fully understand its mechanisms, optimize dosing, and confirm its efficacy in more complex clinical settings.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- EmCaMSCs:
-
Endometrial cancer mesenchymal stem cells
- PBMC:
-
Peripheral blood mononuclear cell
- NK:
-
Natural killer
- TGF-β:
-
Transforming growth factor-beta
- HMG-CoA:
-
3-hydroxy-3-methyl-glutaryl-coenzyme A
- HMGCR:
-
HMG-CoA reductase
- LDL:
-
Low-density lipoprotein
- EMT:
-
Epithelial-mesenchymal transition
- TME:
-
Tumor microenvironment
- PD-1:
-
programmed cell death protein 1
- PD-L1:
-
programmed death-ligand 1
References
Tsimikas, S., Gordts, P. L. S. M., Nora, C., Yeang, C. & Witztum, J. L. Statin therapy increases lipoprotein(a) levels. Eur. Heart J. 41, 2275–2284 (2020).
Sadowska, A. et al. Statins-from fungi to pharmacy. Int J. Mol. Sci 25, (2023).
Guerra, B. et al. The mevalonate pathway, a metabolic target in cancer therapy. Front. Oncol. 11, 626971 (2021).
Di Bello, E., Zwergel, C., Mai, A. & Valente, S. The innovative potential of Statins in cancer: new targets for new therapies. Front. Chem. 8, 516 (2020).
Sun, F. R., Wang, S. L., Wang, M. & Sun, L. M. Simvastatin induces apoptosis of nasopharyngeal carcinoma cells through NF-κB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 24, 6726–6734 (2020).
Wang, S. T., Ho, H. J., Lin, J. T., Shieh, J. J. & Wu, C. Y. Simvastatin-induced cell cycle arrest through Inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell. Death Dis. 8, e2626 (2017).
Yin, L., He, Z., Yi, B., Xue, L. & Sun, J. Simvastatin suppresses human breast Cancer cell invasion by decreasing the expression of pituitary Tumor-Transforming gene 1. Front. Pharmacol. 11, 574068 (2020).
Dongoran, R. A., Wang, K. H., Lin, T. J., Yuan, T. C. & Liu, C. H. Anti-Proliferative Effect of Statins Is Mediated by DNMT1 Inhibition and p21 Expression in OSCC Cells. Cancers 12, (2020).
Hafizz, A. M. H. A., Zin, R. R. M., Aziz, N. H. A., Kampan, N. C. & Shafiee, M. N. Beyond lipid-lowering: role of Statins in endometrial cancer. Mol. Biol. Rep. 47, 8199–8207 (2020).
Chen, Y., Han, L. & Zheng, A. Association between Statin use and the risk, prognosis of gynecologic cancer: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 268, 74–81 (2022).
Wang, Y. et al. Statin use and the risk of ovarian and endometrial cancers: a meta-analysis. BMC Cancer. 19, 730 (2019).
Yang, J. et al. Statin use and endometrial cancer risk: a meta-analysis. Oncotarget 8, 62425–62434 (2017).
Sanni, O. B. et al. Commonly used medications and endometrial cancer survival: a population-based cohort study. Br. J. Cancer. 117, 432–438 (2017).
Sperling, C. D., Verdoodt, F., Friis, S., Dehlendorff, C. & Kjaer, S. K. Statin use and risk of endometrial cancer: a nationwide registry-based case-control study. Acta Obstet. Gynecol. Scand. 96, 144–149 (2017).
Yoon, L. S., Goodman, M. T., Rimel, B. J. & Jeon, C. Y. Statin use and survival in elderly patients with endometrial cancer. Gynecol. Oncol. 137, 252–257 (2015).
Kim, J. S., Turbov, J., Rosales, R., Thaete, L. G. & Rodriguez, G. C. Combination Simvastatin and Metformin synergistically inhibits endometrial cancer cell growth. Gynecol. Oncol. 154, 432–440 (2019).
Shahbaz, S. K., Sadeghi, M., Koushki, K., Penson, P. E. & Sahebkar, A. Regulatory T cells: possible mediators for the anti-inflammatory action of Statins. Pharmacol. Res. 149, 104469 (2019).
Ntolkeras, G. et al. On the immunoregulatory role of Statins in multiple sclerosis: the effects on Th17 cells. Immunol. Res. 67, 310–324 (2019).
Rodríguez-Perea, A. L., Rojas, M. & Velilla-Hernández, P. A. High concentrations of Atorvastatin reduce in-vitro function of conventional T and regulatory T cells. Clin. Exp. Immunol. 196, 237–248 (2019).
Cantini, L. et al. High-intensity Statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur. J. Cancer. 144, 41–48 (2021).
Oechsle, C. M., Showalter, L. E., Novak, C. M., Czerniecki, B. J. & Koski, G. K. Statin drugs plus Th1 cytokines potentiate apoptosis and Ras delocalization in human breast Cancer lines and combine with dendritic Cell-Based immunotherapy to suppress tumor growth in a mouse model of HER-2pos disease. Vaccines (Basel) 8, (2020).
L’Orphelin, J. M., Dollalille, C., Akroun, J., Alexandre, J. & Dompmartin, A. Cardiovascular immunotoxicity associated with immune checkpoint inhibitors in metastatic melanoma. Cancers (Basel). 15, 2170 (2023).
Mao, W. et al. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint Blockade in non-small cell lung cancer. JCI Insight 7, (2022).
Kansal, V. et al. Statin drugs enhance responses to immune checkpoint Blockade in head and neck cancer models. J Immunother Cancer 11, (2023).
Gorabi, A. M. et al. Effects of Statins on the biological features of mesenchymal stem cells and therapeutic implications. Heart Fail. Rev. 26, 1259–1272 (2021).
Galland, S., Martin, P., Fregni, G., Letovanec, I. & Stamenkovic, I. Attenuation of the pro-inflammatory signature of lung cancer-derived mesenchymal stromal cells by Statins. Cancer Lett. 484, 50–64 (2020).
Wang, K. H., Chang, Y. H., Harnod, T. & Ding, D. C. Endometrial Cancer-Infiltrating mesenchymal stem cells exhibit immunosuppressive effects. Cell. Transpl. 31, 9636897221104452 (2022).
Liu, J., Liu, Q. & Chen, X. The Immunomodulatory effects of mesenchymal stem cells on regulatory B cells. Front. Immunol. 11, 1843 (2020).
Wei, Z. et al. Regulatory effect of mesenchymal stem cells on T cell phenotypes in autoimmune diseases. Stem Cells Int. 5583994 (2021). (2021).
Kapor, S. & Santibañez, J. Myeloid-derived suppressor cells and mesenchymal stem/stromal cells in myeloid malignancies. J Clin. Med 10, (2021).
Zhang, B., Zhang, J. & Tian, Z. Comparison in the effects of IL-2, IL-12, IL-15 and IFNalpha on gene regulation of granzymes of human NK cell line NK-92. Int. Immunopharmacol. 8, 989–996 (2008).
de Araújo Farias, V., Carrillo-Gálvez, A. B., Martín, F. & Anderson, P. TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor. Rev. 43, 25–37 (2018).
Vizoso, F. J., Eiro, N., Cid, S., Schneider, J. & Perez-Fernandez, R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J. Mol. Sci 18, (2017).
Seoane, J. & Gomis, R. R. TGF-β family signaling in tumor suppression and Cancer progression. Cold Spring Harb Perspect. Biol 9, (2017).
Wong, J. K. M. et al. TGF-β signalling limits effector function capacity of NK cell anti-tumour immunity in human bladder cancer. EBioMedicine 104, 105176 (2024).
Slattery, K. & Gardiner, C. M. NK cell metabolism and TGFβ - implications for immunotherapy. Front. Immunol. 10, 2915 (2019).
Slattery, K. et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J. Immunother Cancer. 9, e002044 (2021).
Otegbeye, F. et al. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS One. 13, e0191358 (2018).
Xiao, A. et al. Statins affect human glioblastoma and other cancers through TGF-β Inhibition. Oncotarget 10, 1716–1728 (2019).
Aftabi, S. et al. Therapeutic targeting of TGF-β in lung cancer. FEBS J. https://doi.org/10.1111/febs.17234 (2024).
Álvarez, M. et al. IL-2 and anti-TGF-β promote NK cell reconstitution and anti-tumor effects after syngeneic hematopoietic stem cell transplantation. Cancers (Basel) 12, (2020).
Shi, X. et al. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J. Hematol. Oncol. 15, 135 (2022).
Ning, Y. et al. Simvastatin induces ferroptosis and activates anti-tumor immunity to sensitize anti-PD-1 immunotherapy in microsatellite stable gastric cancer. Int. Immunopharmacol. 142, 113244 (2024).
Santoni, M. et al. Statin use improves the efficacy of nivolumab in patients with advanced renal cell carcinoma. Eur. J. Cancer. 172, 191–198 (2022).
Wang, Z. et al. The relationship between Statin administration timing and survival outcomes in patients with cancer receiving immune checkpoint Blockade. J. Chemother. 36, 435–440 (2024).
Kment, J. et al. Blockade of TGF-β and PD-L1 by bintrafusp Alfa promotes survival in preclinical ovarian cancer models by promoting T effector and NK cell responses. Br. J. Cancer. 130, 2003–2015 (2024).
Maurya, S. K., Chaudhri, S., Kumar, S. & Gupta, S. Repurposing of metabolic drugs Metformin and Simvastatin as an emerging class of cancer therapeutics. Pharm. Res. 42, 49–67 (2025).
Gao, Y. et al. Lanosterol synthase deficiency promotes tumor progression by orchestrating PDL1-dependent tumor immunosuppressive microenvironment. MedComm 5, e528 (2024).
Lim, W. J. et al. Statins decrease programmed death-ligand 1 (PD-L1) by inhibiting AKT and β-catenin signaling. Cells 10, 2488 (2021).
Funding
This study was supported by Hualien Tzu Chi Hospital (TCRD 111 − 080).
Author information
Authors and Affiliations
Contributions
KHW conceived and designed the study. KHW performed the experiments. DCD, KHW, and YHC analyzed the data. DCD and KHW wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The human cell derivation and experiments were approved by the Research Ethics Committee of Hualien Tzu Chi Hospital (IRB number: 109-257-A).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, KH., Chang, YH. & Ding, DC. Simvastatin inhibits the immunosuppressive effects of endometrial cancer-associated mesenchymal stem cells through TGF-β2/SMAD2/3 signaling and reduces tumor growth. Sci Rep 15, 21533 (2025). https://doi.org/10.1038/s41598-025-08686-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-08686-9