Abstract
Acne vulgaris (AV) is a chronic inflammatory skin disease with a complex pathogenesis, and its association with insulin resistance (IR) remains unclear. This study aimed to identify differential proteins linking AV and IR to improve diagnostic and therapeutic strategies. Plasma proteomic profiling by using LC-MS/MS was performed on 90 AV patients (with IR [n = 40], without IR [n = 50]) and 30 healthy controls (with IR [n = 11], without IR [n = 19]). Differentially expressed proteins were analyzed using bioinformatics tool to identify key molecules and pathways. Candidate molecules were screened based on their positive correlations with both AV severity and insulin levels. In group AV with IR, C4BPA was highly expressed, showed strong positive correlations (Pearson’s R = 0.46, p = 4.21E-6) with insulin levels and were enriched in complement agglutination cascade and B-cell-mediated immune pathways. In group AV, C4BPA (Pearson’s R = 0.23, p = 0.03) also correlated with AV severity (GAGS scores) and was enriched in complement and coagulation cascades and leukocyte-mediated immunity. C4BPA acted as a key molecule, bridging IR and AV pathogenesis. This study offers a highly valuable proteomic resource for AV associated with IR and proposes a mechanistic hypothesis, supported by existing literature, that insulin exacerbates acne by regulating lipid metabolism and inflammatory pathways with C4BPA potentially acting as a central mediator in this process.
Similar content being viewed by others
Background
Acne vulgaris (AV) is a chronic inflammatory disease of the pilosebaceous unit and is among the most prevalent dermatological conditions worldwide. Its complex pathogenesis imposes significant physical and psychological burdens1,2. They manifest as comedones, papules, pustules, and cysts3. Owing to its high incidence, AV affects approximately 95% of teenage boys and 85% of adolescent girls, and almost 50% of individuals continue to experience AV into adulthood1,4. It is a cutaneous disorder resulting from an androgen-induced increase in sebum production, altered keratinization, inflammation, and follicular colonization by Propionibacterium acne (P. acnes) affecting the face, neck, chest, and back5,6. Webster et al.7 indicates that P. acnes can activate the complement cascade. Complement activation is a critical pathway in the pathogenesis of inflammatory skin diseases such as acne, yet its specific mechanisms of action and key regulatory factors remain to be elucidated. However, the molecular events triggering acne vulgaris remain poorly understood. Early diagnosis of acne is crucial for better prognosis.
Insulin resistance (IR) is a systemic disorder that affects multiple organs and insulin-regulated pathways8. Influenced by a multitude of intrinsic and extrinsic factors, IR diminishes the efficacy of glucose uptake and metabolism in human organs9. During puberty, physiological IR leads to hyperinsulinemia, which elevates androgen levels. Both hyperinsulinemia and hyperandrogenemia promote acne onset10. Currently, there is plenty of empirical research investigating the interconnection between AV and IR. Recent studies demonstrate that androgen, insulin, and IGF-1 promote hyperkeratosis and lipid production in various ways, thereby inducing AV3,11. In a previous study involving 88 patients, a significant correlation was observed between acne and IR12. Aikaterini et al. examined the strong relationship between inflammation, IR, and cholesterol levels10. Thus, the diagnosis and management of AV with IR are of paramount importance.
The homeostasis model assessment of IR (HOMA-IR) is an indirect index used to measure IR based on fasting glucose and insulin levels. The simplified formula is: HOMA-IR = fasting blood glucose (mmol/L) * fasting insulin (uU/mL)/22.59. Since all the participants in this study were Chinese, the homeostasis model standard of IR in Chinese populations (i.e., HOMA-IR ≥ 2.14 can be judged as IR) was adopted to evaluate IR13.
In addition to proper everyday care, acne treatment is challenging and involves topical or oral retinoids, antibiotics, benzoyl peroxide, or azelaic acid14. Currently, isotretinoin is the most efficacious treatment available for acne and may be the only medication that can potentially cure this kind of disease2,15. However, isotretinoin is a known teratogen2 and is associated with significant side effects16,17. Furthermore, acne is a persistent dermatological condition characterized by its propensity for relapse. At present, there are few studies on the pathological mechanism of insulin resistance that promotes the occurrence and development of acne and the exacerbation of the disease. Therefore, the development of novel methods for its prevention and treatment of AV with IR is crucial. This study aimed to conduct an unbiased proteomic analysis of plasma proteins in individuals with AV, with and without IR, to comprehensively identify differentially expressed proteins. Detecting protein-level alterations in acne could provide insights into the potential mechanism of the disease and facilitate the development of novel therapeutics.
Methods
Study participants and ethics
The experiment has been approved by the Ethics Committee of the Affiliated Hospital, Southwest Medical University (No.: KY2023426). All research was performed in accordance with relevant guidelines and regulations, and the informed consent was obtained from all participants and/or their legal guardians. Participants were recruited between September 2023 and December 2023, and a total of 120 volunteers were enrolled in the trial, of which 90 were acne patients and 30 were healthy individuals.
Inclusion criteria for the acne group: (1) meet the diagnostic criteria for acne vulgaris in Chinese Clinical Dermatology: Acne vulgaris commonly affects adolescents. The condition primarily manifests on the face, chest, and back, and is characterized by the presence of blackheads, whiteheads, inflammatory papules, and pustules, making it relatively straightforward to diagnose; (2) Age between 14 and 45 years old, gender and course of disease are not limited; (3) The patient has the ability to be autonomous and sign the informed consent form, and is willing to participate in the study; (4) There are no factors included in the exclusion criteria. Exclusion criteria for acne group: (1) other types of acne or other skin diseases and appendage diseases, such as androgenetic alopecia, acanthosis nigricans, psoriasis, etc.; (2) Have used or are using oral contraceptives, isotretinoin, metformin and hormones and other drugs that affect the metabolism level of insulin and androgens in the past three months; (3) Pregnant and lactating patients or patients with polycystic ovary syndrome and adrenal hyperplasia; (4) Patients with diabetes mellitus or other endocrine diseases; (5) Those who meet the inclusion criteria but are unwilling to participate in the study.
Inclusion criteria for the control group: (1) age between 14 and 45 years, gender is not limited; (2) Those who have the ability to be autonomous and sign the informed consent form, and are willing to participate in the study; (3) There are no factors included in the exclusion criteria. Exclusion criteria for the control group: (1) other types of acne or other skin diseases and appendage diseases, such as androgenetic alopecia, acanthosis nigricans, psoriasis, etc.; (2) Have used or are using oral contraceptives, isotretinoin, metformin and hormones and other drugs that affect the metabolism level of insulin and androgens in the past three months; (3) Pregnant and lactating patients or patients with polycystic ovary syndrome and adrenal hyperplasia; (4) Patients with diabetes mellitus or other endocrine diseases; (5) Those who meet the inclusion criteria but are unwilling to participate in the study.
The included volunteers were divided into healthy control group (HC, n = 30) and acne vulgaris group (Acne, n = 90) according to whether they had acne or not. According to HOMA-IR, the included healthy populations were divided into healthy with IR (n = 11) and healthy without IR (n = 19), as well as acne with IR (n = 40) and acne without IR (n = 50). The criteria for IR is: HOMA-IR = fasting blood glucose (mmol/L) * fasting insulin (uU/mL)/22.5. Because all samples in this study are Chinese, the Chinese insulin resistance homeostasis model criteria (i.e., HOMA-IR ≥ 2.14 can be determined as insulin resistance) were used to evaluate IR. In order to make the experimental results more accurate, based on the clinical information and experimental statistics of the recruited volunteers, we selected HOMA-IR < 2.1 as the evaluation criteria for non-insulin resistance and HOMA-IR ≥ 2.67 as the evaluation criteria for insulin resistance on the basis of HOMA-IR ≥ 2.14.
In this trial, we employed the Global Acne Grading System (GAGS) to assess the severity of acne in patients. Originally developed by Doshi et al.18, GAGS is one of the most detailed acne grading systems available. The acne-prone areas are divided into six regions: forehead, right cheek, left cheek, nose, chin, and other areas (chest and upper back). The score for each region is determined based on its surface area (forehead = 2, right cheek = 2; left cheek = 2, nose = 1, chin = 1, chest and upper back = 3), as well as the distribution and density of pilosebaceous units. If no lesions are present in a region, the local lesion score for that area is 0. Blackheads or whiteheads are scored 1 point; papules are scored 2 points; pustules are scored 3 points; and nodules are scored 4 points (if multiple lesion types coexist in the same region, only the most severe type is scored). The local score is calculated as the lesion score multiplied by the regional weighting factor. The total score is the sum of all local scores. Severity is graded as follows: Mild: 1–18 points; Moderate: 19–30 points; Severe: 31–38 points; Very severe: ≥39 points.
Sample preparation
After fasting for 8 h, the volunteers had three tubes of venous blood drawn, 3 mL each. One of the vials was centrifuged at 3000 RPM for 10 min at room temperature, and the supernatant was aliquoted into cryovials, numbered, and stored at −80 °C for DIA analysis. The second tube of blood was used to measure FBG and blood lipids using Beckman Coulter AU5800 automated biochemistry analyzer. The third tube of blood was used to measure sex hormones and insulin.
Protein extraction and trypsin digestion
The body fluid samples were thawed at 4 °C, and 2µL was mixed with 98µL 50mM ammonium bicarbonate solution to the total volume of 100 µL, heated at 95 °C for 3 min for protein denaturation, cooled to room temperature. Then these samples were digested by using trypsin at 37 °C for 16 h, and the peptides were extracted and dried. This was followed by a desalination operation. After lyophilization, the peptides were reconstituted with 100 µL of 0.1% formic acid solution, and the samples were injected at a ratio of 2%.
MS assay for data-independent acquisition
Samples were separated using a high-performance liquid chromatography system EASY-nLC1200 with nanoliter flow rates. Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% acetonitrile formic acid in water (80% acetonitrile). The loading column and analytical column were first equilibrated with 100% mobile phase A. The digested peptides of the sample were transported by the autosampler to the loading column (2 cm, ID100 µm, 3 μm, C18), and then separated by the analytical column (15 cm, ID150 µm, 1.9 μm, C18) at a flow rate of 600 nL/min. Samples were chromatographically separated and analyzed by mass spectrometry using an HFX mass spectrometer. The detection method was positive ion, the precursor ion scanning range was 300–1400 m/z, the first-order mass spectrometry resolution was 60,000 at 200 m/z, the AGC (Automatic gain control) target was 3e6, and the Maximum IT was 20ms. The mass-charge ratio of peptides and peptide fragments was acquired using the following method: 30 DIA Scans were collected after each full scan, HCD fragmentation mode was used, Normalized Collision Energy was 27%, Isolation window was changed according to the isolation window, and the secondary mass spectrometry resolution was 15,000 at 200 m/z.
Statistical analysis
The original data from mass spectrometry analysis are RAW files, and the iProteome one-stop data analysis cloud platform was used for qualitative and quantitative analysis. First, the quantitative information of the target protein set was normalized (z-score). Then, using the pheatmap R package, the expression of the sample and the protein were classified simultaneously (distance algorithm: Euclid, connection: Average linkage), and a hierarchical clustering heat map was generated. GO annotations of genes of the species were obtained from public data (NCBI, Uniprot, or Gene ontology), and interProScan if no annotation information was available. The KEGG annotation information of the gene of the species was obtained through the KEGG database, and if there was no annotation information of the species, the annotation information of the species was indexed to the KEGG database by Blast for annotation. The hypergeometric distribution was used to compare the distribution of each GO class (or KEGG pathway, or Domain) in the target protein set and the overall protein collection, and the enrichment analysis of GO (or KEGG pathway, or Domain) annotation was performed on the target protein collection. Based on the information in the STRING (http://string-db.org/) database, the direct and indirect interactions between the target proteins were found, and the interaction networks were generated and analyzed using CytoScape software (version 3.2.1).
All statistical analysis were performed by SPSS (Version 27.0). The normality of continuous variables was assessed using the Shapiro-Wilk test. Normally distributed variables are presented as Mean ± Standard Deviation (Mean ± SD), while non-normally distributed variables are presented as Median and Interquartile Range (Median [Q1, Q3]). Categorical variables are presented as frequency and percentage (n (%)).
For comparisons of continuous variables between groups: When data were normally distributed and exhibited homogeneity of variance (assessed via Levene’s test), Independent Samples t-tests were used for comparisons between two groups, and One-way Analysis of Variance (One-way ANOVA) was used for comparisons among multiple groups. If the ANOVA result was significant, Tukey’s Honestly Significant Difference (HSD) test was used for post hoc pairwise comparisons. When data were non-normally distributed or variances were unequal, the Mann-Whitney U test was used. Comparisons of categorical variables between groups were performed using the Chi-square test (χ² test) or Fisher’s exact test. Pearson correlation analysis was used to evaluate the correlation between two continuous variables.
All hypothesis tests were two-tailed. In all statistical tests, a P value < 0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics
To map plasma proteome distinctions between individuals with AV with and without IR, we analyzed multi-omics data from 90 patients with AV (Acne with IR [n = 40] and Acne without IR [n = 50]) and 30 healthy controls (HCwith IR [n = 11] and HC without IR [n = 19]) recruited from Hejiang People’s Hospital between November and December 2023. Baseline characteristics of all participants are summarized in Tables 1 and 2.
Proteomic characterization of plasma
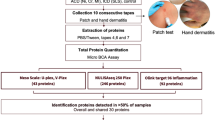
The experimental workflow is illustrated in Fig. 1A. LC-MS/MS analysis was conducted based on the data-independent acquisition (DIA) method for all samples from both groups, and all plasma proteomic data were analyzed. We quantified an average of 2000 proteins per plasma sample, and the number of proteins identified in the two groups was essentially equal, without significant differences (Fig. 1B). There were no outliers, and all samples were viable for further analyses. In all samples, the protein abundance in both healthy controls and patients with acne exhibited a large dynamic range, spanning eight orders of magnitude. The horizontal line in the boxplot represents the median, which reflects the overall uniformity of protein abundance across all samples with minimal bias (Fig. 1C). In the subgroups, protein abundance was comparable between the HC and patients with acne, and the number of identifications for all individual samples was essentially equal (Fig. 1D). In total, 7738 proteins were identified in the entire cohort (Fig. 1E), with 5572 co-identified in HC and patients with acne (Fig. 1F). Meanwhile, a total of 552 specific proteins were identified exclusively in the healthy controls, whereas 1614 proteins were found to be unique to the acne patients.
Proteomic characterization of plasma. A Plasma proteomics workflow using DIA mass spectrometry (HC=30, Acne=90). B An average of 2000 proteins qualified in per plasma sample. C The overall uniformity of protein abundance across all samples with minimal bias. D The number of identifications for all individual samples was essentially equal. E 7738 proteins were identified in the entire cohort. F Venn plots depict the protein identification for the volunteers.
Proteomic subtypes of the entire cohort
Considering the differences between healthy controls and patients with AV, it is important to perform molecular subtyping. Because proteomic data directly reflect cell functions, we performed consensus clustering based on protein expression ranks in 120 individuals. This analysis identified two subgroups (Fig. 2A). Differential protein expression analysis revealed significantly upregulated and downregulated proteins. Through pathway enrichment, we identified characteristic pathways in these two subtypes.
Proteomic subtypes of the entire cohort and immune landscape of the two subtypes. A The entire cohort was proteo-typed, and the cohort was divided into two types, which was a good differentiator between healthy controls and acne patients. And the difference in the clinical information in both subtypes. B Differential protein expression analysis revealed significantly upregulated (FC ≥1.5, P <0.05) and downregulated (FC ≤0.67, P <0.05) proteins and pathway enrichment. C Heatmap analysis showed that proteins associated with molecular features in characteristic pathways of Sub 1 and 2 exhibited significant upregulated and downregulated. D MGST2(P =1.09E-6) and ANGPTL3(P =4.82E-4) were dominantly expressed in the Sub 1 subtype (Wilcoxon sign-rank test). E RELA(P =3.55E-4) and HMOX1(P =7.4E-3) were dominantly expressed in the Sub 1 subtype (Wilcoxon sign-rank test). F Immune microenvironment heterogeneity between two subtypes. The values were z-score transformed. G Molecules that were highly expressed in fibroblasts (P <0.05, n=13) and neutrophils (P <0.05, n=13), which were more active in Sub 1, were presented in the form of line plots and heat maps (ANOVA).H Molecules that were highly expressed in keratinocytes (P <0.05, n=6) and CD8+ T cells (P <0.05, n=4), which were more active in Sub 2, were presented in the form of line plots and heat maps (ANOVA). I Summary schematic integrating key findings from panels A-H.
Sub 1 was characterized by the arachidonic acid metabolic process, fatty acid catabolism, insulin receptor signaling pathway, ketone catabolism, lipid catabolism, nucleoside metabolism, oxidative phosphorylation, and positive regulation of NF-κB signaling. In contrast, Sub 2 was characterized by the ERBB signaling pathway, progesterone metabolism, Ras protein signal transduction, and unsaturated fatty acid metabolism (Fig. 2B).
Further analysis of molecules in representative pathways for specific per-sample expression showed that protein molecules like MGST2, ACAT2, SMPD4, ENPP7, SULT1E1, SESN2, NEU1, NUDT19, GALC, ANGPTL3, PLCB4, and TBL1XR1 involved in the lipid catabolic process and PPP5C, MAVS, TBK1, EEF1D, DHX15, HMOX1, TRIM27, RELA, and TRAF2 involved in the positive regulation of I-kappa B kinase/NF-kappa B signaling were significantly upregulated in Sub 1. Proteins such as KANK2, SDCBP, NCKAP1, GIT2, PPP2CB, PRPF38B, ARHGEF1, and STAMBP involved in Ras protein signal transduction, and TSG101, MVB12B, HIP1R, and MMP9 involved in the ERBB signaling pathway, were significantly upregulated in Sub 2 (Fig. 2C).
Among these, MGST2 in Sub 1 is an important inflammatory mediator involved in the biotransformation of arachidonic acid metabolites, promoting inflammation and contributing to acne development. ANGPTL3 is an angiopoietin-like protein regulating angiogenesis and lipid and glucose metabolism. However, its inductive effect on angiogenesis is weak, and its regulatory impact on lipid metabolism is more significant, making it an important regulator of lipoprotein metabolism. RELA, a transcription factor of NF-κB, is a classical protein molecule involved in the NF-κB signaling pathway that mediates the occurrence of associated inflammation. HMOX1, a key molecule that mediates inflammasome activity, is a heme oxidase with anti-inflammatory properties.
Notably, MGST2, ANGPTL3, RELA, and HMOX1 were dominantly expressed in the Sub 1 subtype (Fig. 2D and E). Their elevated expression promoted acne occurrence and development. Together, these four proteins were more significantly upregulated in Sub 1 than in Sub 2, suggesting that their high expression can promote acne development.
Immune landscape of the two subtypes
Through proteomic profiling, we systematically characterized cell-type-specific protein signatures to delineate immune microenvironment remodeling in acne pathogenesis, identifying molecular alterations across 10 functionally distinct immune cell populations. Based on immune cell enrichment analysis, the Sub 1 subtype showed significantly higher activity of fibroblasts, neutrophils, preadipocytes, and pro-B-cells, while the Sub 2 subtype exhibited higher expression of mature B cells, CD4 + T cells, CD8 + T cells, keratinocytes, macrophages, and memory B cells (Fig. 2F). Specifically, Fig. 2G shows 13 protein molecules highly expressed in fibroblasts and 13 in neutrophils, both of which were more active in Sub 1. Similarly, Fig. 2H identifies six protein molecules highly expressed in keratinocytes and four in CD8 + T cells, which were more active in Sub 2.
In this study, protein subtypes could roughly distinguish the healthy controls from patients with acne. Sub 1 was predominantly comprised of AV patients (Acne, n = 90, 94.7%), but also contained a small number of healthy controls (HC, n = 5, 5.3%). Critically, all AV patients (100%) were assigned to Sub 1. Sub 2 (n = 25) consisted exclusively of healthy controls (HC, 100%). The analysis of its contingency is presented in Supplementary Table 1. These results indicate that acne development may be driven by lipid metabolism and inflammatory processes mediated by fibroblasts and neutrophils (Fig. 2I).
Detection of related proteome in the plasma of patients with acne and healthy individuals
Principal component analysis (PCA) revealed a clear difference between patients with acne and IR and healthy individuals with IR, indicating that patients with acne and IR exhibit plasma proteomic characteristics that are different from those in healthy individuals (Fig. 3A). Furthermore, we identified individual molecular features associated with acne that resulted in significantly upregulated and downregulated proteins in patients with acne (P < 0.05, FC ≥ 2 or ≤ 0.5) (Fig. 3B). We further analyzed these differentially expressed proteins by pathway enrichment, which revealed that 17 upregulated proteins were mainly involved in the regulation of lymphocyte migration, fatty acid metabolism, cell proliferation, and mast cell activation. In contrast, 18 downregulated proteins were primarily involved in epithelial development, epithelial cell differentiation, keratinization, and ECM-receptor interaction. These pathways may be important for the development of IR-associated acne (Fig. 3C). Figure 3D and E demonstrate the difference in the clinical information of patients with acne in the insulin-resistant group compared to the healthy population. We found that the weights and insulin levels of patients with acne were significantly higher than those of healthy individuals, whereas their glucose levels were significantly lower. Furthermore, the dietary habits of patients with acne were characterized by preferences for greasy or spicy foods, or a combination of both, with all those who prefer greasy food being patients with acne. Consistent with established knowledge10,19, our findings demonstrate that excessive lipid accumulation in the sebum of the insulin-resistant group is associated with acne development, further supporting the mechanistic link between insulin resistance and acne pathogenesis.
Detection of related proteome in the plasma of patients with acne and healthy controls. A PCA revealed a clear difference between patients with acne and IR(n=40) and healthy controls with IR(n=11). B Individual molecular features associated with acne resulted in significantly upregulated and downregulated proteins in patients with IR (P < 0.05, FC ≥ 2 or ≤ 0.5). C Heatmap analysis showed expression levels and pathway enrichment of proteins in both patients with acne and IR and healthy controls with IR groups. D and E The difference in the clinical information of patients with acne in the insulin-resistant group compared to the healthy controls. F. PCA revealed a distinct difference between patients with acne without IR(n=50) and healthy controls without IR(n=19). G Individual molecular features associated with acne resulted in significantly upregulated and downregulated proteins in patients without IR (P < 0.05, FC ≥ 2 or ≤ 0.5). H Heatmap analysis showed expression levels and pathway enrichment of proteins in both patients with acne and healthy individuals without IR groups. I and J The difference in the clinical information of patients with acne in the non-insulin-resistant group compared to the healthy population (Dietary Categories: Light -1; Sweet-2; Greasy-3; Spicy-4; Mixed-5).
In addition, PCA revealed a distinct difference between patients with acne and healthy controls without IR, indicating that patients with acne without IR exhibit plasma proteomic characteristics that are different from those in healthy controls (Fig. 3F). We identified significantly upregulated and downregulated proteins in patients with acne from the individual molecular features associated with acne (P < 0.05, and FC ≥ 2 or ≤ 0.5) (Fig. 3G). In this group, 17 proteins were significantly upregulated in the insulin receptor signaling pathway, type I interferon production, NF-κB signaling regulation, and prostaglandin metabolism. Conversely, 19 proteins were significantly downregulated in processes such as epithelial cell differentiation, EMC-receptor interaction, ERBB signaling pathway regulation, and unsaturated fatty acid metabolism. These pathways may be essential for the development of acne without IR (Fig. 3H). From the above analysis, we preliminarily observed that in the non-insulin-resistant group, the mechanism of acne development may be due to the increase in insulin and androgen levels, such as in the prostate, and the downregulation of ERBB signaling and unsaturated fatty acid metabolism. This results in the differentiation of hair follicle cells in the skin and keratinization, which triggers the enhancement of inflammatory responses like NF-κB signaling and type I interferon, leading to the immune response of the skin and the occurrence of acne. Furthermore, Fig. 3I and J demonstrate the difference in the clinical information of patients with acne in the non-insulin-resistant group compared to the healthy controls. It can be concluded that the AST, ALT, HOMA-IR, and age levels of patients with acne in the non-insulin-resistant group were significantly lower than those in healthy controls.
Plasma proteomic characterization of acne with or without IR
Retrospective tissue analysis revealed molecular signatures associated with tissue damage pathways in patients with acne with or without IR (Fig. 4A). They both had commonalities, as both groups of patients had certain damage to small intestine- and kidney-related cells but differed in the specific cell types affected. In patients with IR, CSH1 and CSH2-positive cells in the adrenal gland were damaged, whereas in patients without IR, NKT cells in the liver were damaged.
Plasma proteomic characterization of acne with or without IR. A Retrospective tissue analysis revealed molecular signatures associated with tissue loss in patients with acne with or without IR. B Statistical analysis of the clinical information revealed significant differences in gender(P =1.46E-3, Fisher’s exact test) between the two groups. C Statistical analysis of the clinical information revealed significant differences in testosterone levels (P =4.83E-3, Wilcoxon sign-rank test) between the two groups. D Pearson correlation analysis showed a significant positive correlation between T cells and the arachidonic acid metabolism pathway (Pearson’s R =0.45, P = 9.75E-4), and between T cells and prostaglandin signals(Pearson’s R =0.30,P = 0.36) in patients with acne without IR. E Pearson correlation analysis showed no significant positive correlation between T cells and the arachidonic acid metabolism pathway (Pearson’s R =0.15, P = 0.349), and between T cells and prostaglandin signals (Pearson’s R =0.27,P = 0.09) in patients with acne without IR in patients with acne with IR. F The representative characteristic molecules and molecules of the distinct pathways of tissue damage in patients with acne with or without IR.
By the characteristic pathway analysis, pathways related to endothelial cell proliferation, fatty acid β-oxidation, mast cell-mediated immunity, DNA replication, and mast cell activation were significantly upregulated in patients with acne and IR, whereas TGF-β signaling, cholesterol biosynthesis, arachidonic acid metabolism, and interferon 12 pathways were upregulated in patients with acne without IR (Fig. 4A).
Statistical analysis of the clinical information revealed significant differences in gender and testosterone levels between the two groups (Fig. 4B and C). Therefore, we correlated arachidonic acid metabolism and prostaglandins with testosterone and found that both arachidonic acid and prostaglandin signals had significant positive correlations with testosterone levels in patients with acne without IR (Pearson’s R > 0, P < 0.05), whereas no significant correlations were observed in the other group (Fig. 4D and E).
Figure 4F shows representative plasma-derived molecules and molecular signatures associated with putative tissue damage pathways in acne patients with or without IR. In the acne group, we concluded that there was a significantly higher proportion of males in the non-IR group than in the IR group and that the androgen level in this group was also significantly higher than that in the IR group. The signals of androgen precursors, such as arachidonic acid and cholesterol, were significantly upregulated in the non-IR group. Through gender stratified analysis, Supplemental Tables 2 and Table 3 demonstrate that the testosterone levels of acne patients are mainly influenced by gender (partial η² = 0.87), and the IR status has no independent effect. In male patients, the trend of increased testosterone in the non-IR group (P = 0.083, r = 0.31) requires validation with a large sample size.
Immune landscape of acne with or without IR
To explore the features and differences in the immune microenvironment between acne with and without IR, we obtained characteristic immune cells from these two groups (Fig. 5A). By analyzing the immune cells exhibiting the most significant difference between the two groups, we found that B-cells had significantly higher activity in the IR group and fibroblasts in the non-IR group (Fig. 5B).
Immune landscape of acne with(n = 40) or without IR(n = 50). A Heatmap displays Z-score normalized expression profiles of nine cell populations in acne with IR and acne without IR groups, highlighting immune microenvironmental compositional differences. B Normalized enrichment scores (NES) of B-cells had significantly higher activity in the IR group (P = 7.55 E-3, Wilcoxon sign-rank test) and fibroblasts in the non-IR group (P = 6.85 E-3, Wilcoxon sign-rank test). C Five major signature differential molecules were enriched in B cells and three major signature differential molecules were enriched in fibroblasts. D Molecules positively correlated with B cell activity (Pearson’s R > 0, P < 0.05). E Pathways positively correlated with B cell activity in acne with IR group. F The molecular-specific correlations in representative pathways such as NF-κB signaling and Ras signaling (*P < 0.05, ** P < 0.01). G Molecules positively correlated with fibroblast activity (Pearson’s R > 0, P < 0.05). H Pathways positively correlated with fibroblast activity in the non-IR group. I The molecular-specific correlations in representative pathways such as the actin filament-based processes and platelet activation (* P < 0.05,** P < 0.01). J Summary schematic integrating key findings from panels A-I .
Specifically, five major signature differential molecules were enriched in B cells, namely, SNX2, RRAS2, AFTPH, ARHGAP17, and WDR11. Among these, WDR11 expression was significantly different between the two groups and was significantly upregulated in the IR group. In addition, three major signature differential molecules were enriched in fibroblasts, namely, PRDX4, CSTF2T, and PCDHGA11, of which PCDHGA11 was significantly upregulated in the non-IR group (Fig. 5C). Further correlation analysis of B cells and molecules highly expressed in the IR group screened out those positively correlated with B cell activity (Pearson’s R > 0, P < 0.05) (Fig. 5D). Pathway enrichment analysis revealed that in the IR group, the Ras signaling pathway, nucleotide biosynthesis process, NF-κB signaling, and activation of immune responses were positively correlated with B cell activity (Fig. 5E). Specific correlations in representative pathways such as NF-κB signaling and Ras signaling are shown in Fig. 5F (*P < 0.05, ** P < 0.01).
Similarly, fibroblasts were analyzed for their correlation with molecules highly expressed in the non-IR group. Molecules positively correlated with fibroblast activity (Pearson’s R > 0, P < 0.05) were screened out (Fig. 5G). Pathway enrichment analysis indicated that in the non-IR group, fibroblast activity was positively correlated with pathways associated with pyrimidine metabolism, platelet activation, nucleotide metabolism, mitotic nuclear division, and actin filament-based processes (Fig. 5H). Specific correlations in representative pathways, such as the actin filament-based processes and platelet activation, are presented in Fig. 5I (* P < 0.05, ** P < 0.01). These findings demonstrate that B cells were more active in the IR group, and the pathways that interacted with them were NF-κB signaling and RAS signaling. Conversely, fibroblasts were more active in the non-IR group, and the pathways that interacted with them were actin fiber processing and platelet activation (Fig. 5J).
Analysis of the relationship between acne vulgaris and insulin concentration
To investigate the potential mechanism linking acne with insulin concentration, we found that body weight, menstrual cycle, and insulin concentration were significantly and positively correlated with the red module by subjecting the overall cohort to WGCNA analysis (Pearson’s R > 0, P < 0.05). Similarly, total cholesterol content and prolactin (PRL) were significantly positively correlated with the pink module, and E2 showed a significant positive correlation with the yellow module (Fig. 6A).
Pathway enrichment analysis revealed that the main characteristic pathways presented in the red module were lymphocyte-mediated immunity, complement activation, and B-cell-mediated immunity. The pink module was enriched in pathways related to cell cycle regulation, pyruvate metabolism, and mitotic cell cycle processes. The yellow module was predominantly associated with supramolecular fiber organization, lipid metabolism, and actin filament-based processes (Fig. 6B).
We performed correlation analysis between insulin concentration and molecules within the red module, finding the strongest correlation with complement factor I (CFI) (Fig. 6C). By stratifying insulin concentrations from low to high, pathways enriched among molecules significantly and positively correlated with insulin, as shown in Fig. 6D, included the complement agglutination cascades (the relevant molecules were CFI, FGA, SERPIND1, F10, FGB, F13A1, and CFHR1), regulation of the actin cytoskeleton (involving DIAPH1, FN1, RHOA, and VCL), and B-cell-mediated immunity (involving CRP, APCS, Complement component 4 binding protein alpha [C4BPA], and complement 3 [C3]).
Figure 6J shows the correlation between the relevant protein activity within these pathways and increasing insulin concentrations. Notably, proteins such as CFI, C3, and C4BPA exhibited highly significant correlations (Fig. 6E, 6 F, and 6G). Figure 6H and I present the statistical analysis of differences in clinical characteristics of patients with acne at varying insulin concentrations. In addition, by grouping the high and low insulin groups with the original IR and non-IR groups in this study, we found that the glucose and triglyceride levels of people with high insulin levels were significantly higher (Fig. 6H and I).
Therefore, we conclude that CFI, C3, and C4BPA are significantly overexpressed in plasma samples of patients with acne with increasing insulin levels. Correspondingly, the complement agglutination cascade, actin cytoskeleton regulation, and B- cell-mediated immune pathways are significantly upregulated (Fig. 6K). These findings further indicate that elevated insulin levels may activate the complement system in the patient, thereby mediating inflammatory processes and remodeling of the cell skeleton, further activating the immune process. This may be the mechanism by which insulin causes acne in patients.
Correlation analysis with insulin concentration variations. A Weighted gene co-expression network analysis (WGCNA) of 90 plasma samples showed that the three modules of red, pink and yellow were significantly correlated with clinical information. B Characteristic pathways in three modules. C Molecular correlation analysis of insulin concentration with the red module showed the strongest correlation with complement factor I (CFI). D Pathways enriched among molecules significantly and positively correlated with insulin by stratifying insulin concentrations from low to high. E CFI exhibited highly significant correlation with increasing insulin concentrations (Pearson’s R =0.56, P = 1.37E-8). F C3 exhibited highly significant correlation with increasing insulin concentrations (Pearson’s R =0.48, P = 1.32E-6). G C4BPA exhibited highly significant correlation with increasing insulin concentrations (Pearson’s R =0.46, P = 4.21E-6). H The glucose levels of people with high insulin levels were significantly higher (P = 4.44E-4, Wilcoxon sign-rank test). I The triglyceride levels of people with high insulin levels were significantly higher (P = 2.16E-5, Wilcoxon sign-rank test). J The correlation between the relevant protein activity within these pathways in D and increasing insulin concentrations. K Summary schematic integrating key findings from panels A-J.
We assessed acne patients using the Global Acne Grading System (GAGS) and classified the severity of their condition into four categories: mild, moderate, severe, and very severe. By stratifying GAGS scores from low to high, we found that the molecular pathways enriched were significantly positively correlated with acne severity, as shown in Fig. 7A, including M Phase, Cell Cycle, Complement and coagulation cascades, leukocyte mediated immunity, and B cell mediated immunity. Through correlation analysis between the relevant proteins and disease severity, we observed that the expression levels of Complement C1q B chain (C1QB), C4BPA, and Coagulation factor II (F2) were significantly upregulated and positively correlated with acne severity, as illustrated in Fig. 7B and C, and 7D. These findings suggest that the complement system and coagulation cascades may play important roles in exacerbating acne severity.
Correlation analysis with changes in acne GAGS scores. A Pathways enriched among molecules significantly and positively correlated with GAGS scores by stratifyingscores from low to high. B C1QB exhibited highly significant correlation with increasing GAGS scores (Pearson’s R =0.27, P = 0.01). C C4BPA exhibited highly significant correlation with increasing GAGS scores (Pearson’s R =0.23, P = 0.03). D F2 exhibited highly significant correlation with increasing GAGS scores (Pearson’s R =0.26, P = 0.015).
Therefore, we concluded that as insulin levels increased, CFI, C3, and C4BPA were significantly expressed in plasma samples of acne patients. Similarly, as GAGS scores increased, C1QB, C4BPA, and F2 were significantly expressed. From the analysis of both groups, we obtained that C4BPA as a common molecule, suggesting that C4BPA may serve as a potential candidate protein to reveal the novel mechanism by which IR promotes the development and exacerbation of AV.
Discussion
Acne is a chronic inflammatory skin disease with a high incidence, frequent recurrence, diverse clinical manifestations, and limited treatment efficacy. It is characterized by a distribution corresponding to the highest density of pilosebaceous units5. Despite extensive research, the molecular mechanisms underlying acne vulgaris remain unclear. However, because of patient concerns about appearance, the visual appearance of acne frequently results in psychological and social morbidity20. Therefore, it is necessary to investigate its pathogenesis and potential treatments. This study represents the first comprehensive analysis of specific candidate molecules associated with AV, particularly in the context of IR, offering new insights into its diagnosis and treatment.
Considering that the participants in this study were all college students, the age range was narrow (between 15 and 25 years), and they were not randomly distributed across all age groups in the inclusion criteria; hence, there were certain limitations. Regardless, we screened for specific biomarkers in plasma samples from patients with acne, with or without IR. However, our findings require in vivo and in vitro experiments and a large sample size for validation.
To further investigate the differences between patients with acne and healthy controls, we analyzed the characteristics of differentially expressed protein molecules, enrichment pathways, and immune microenvironments between the two groups using protein phenotyping. In addition, proteomic subtyping of the overall cohort effectively distinguished healthy controls from patients with acne.
By demonstrating the molecules in representative pathways on a case-by-sample basis, proteins involved in fatty acid metabolism and the positive regulation of NF-κB were significantly overexpressed in Sub 1. By reviewing the relevant literature, we found that MGST2, ANGPTL3, RELA, and HMOX1 may promote acne development. MGST2 (microsomal glutathione S-transferase 2) is an important inflammatory mediator with leukotriene C4 synthase activity, involved in the biotransformation of arachidonic acid metabolites. Therefore, MGST2 may promote the synthesis of leukotriene C4 by catalyzing the binding of leukotriene A4 to reduced glutathione, which is key for intracrine signaling of endoplasmic reticulum stress, oxidative DNA damage, and cell death21, further generating leukotriene B4. Leukotriene B4 is an important inflammatory inducer that promotes inflammation22,23,24. Interestingly, O Schröder et al. reported that MGST2 is found in the liver, kidneys, endothelial cells, adrenal glands, small intestine, and human mast cells22. This finding can be linked to the discussion of the immune microenvironment in this study. ANGPTL3 is an angiopoietin-like protein that regulates angiogenesis and lipid and glucose metabolism25,26 and is an important liver-derived regulator of lipoprotein metabolism27. While its induction effect on angiogenesis is weak, its regulatory impact on lipid metabolism is more significant, and it is an important regulator of lipoprotein metabolism28,29. ANGPTL3 binds to fat cells, facilitating the breakdown of fat into fatty acids and glycerol. Excessive fatty acid accumulation has also been reported to be a key factor in acne. Furthermore, several studies have associated ANGPTL3 function with disease phenotypes, such as obesity and diabetes at an observational level26,30. ANGPTL3 expression was also increased in insulin-deficient and insulin-resistant experimental models of diabetic mice, as reported by Kouichi et al.26. Therefore, acne occurrence and development in individuals with IR may be associated with ANGPTL3, leading to excessive fatty acid accumulation.
To investigate the mechanistic differences between acne with IR and acne without IR, we compared the plasma proteomes and demonstrated that lipid accumulation contributes to the occurrence of acne in individuals with IR, while elevated androgen levels promote the development of acne in those without IR31. Acne has been associated with lipids for decades and is characterized by sebum overproduction in the sebaceous glands, leading to comedones formation19,32. Therefore, the pathogenesis of acne in patients with IR may be because of excessive accumulation of fatty acids and lipids in the sebum, resulting in impaired development and differentiation of hair follicle cells in the skin, as well as keratinization and cell-to-cell interactions. Eventually, the skin undergoes an immune response, such as the activation of immune cells like mast cells, leading to an inflammatory response at the hair follicle level, which results in acne occurrence. However, the role of certain sebum components, rather than total sebum levels, as causative factors of acne remains controversial19. The pathogenesis of acne in patients without IR may be because of increased levels of insulin and androgens, such as prostaglandins12,33as well as the downregulation of estrogen signaling and unsaturated fatty acid metabolism. These changes impair keratinocyte proliferation and differentiation, resulting in the differentiation of hair follicle cells in the skin and impaired keratinization and cell-cell interactions31. This triggers an increase in inflammatory signals such as NF-κB signaling and type I interferon, culminating in an immune response in the skin, promoting acne development.
Kaiser et al. proposed that RELA, a transcription factor of NF-κB, is a classical protein molecule involved in NF-κB signaling that mediates inflammatory responses34. In our study, we also found that RELA expression was significantly higher in Sub 1 than in Sub 2. In addition, some studies have shown that HMOX1 is a heme oxidase, a key molecule that mediates inflammasome activity and has anti-inflammatory effects. Nrf2/HMOX1 signaling can activate antioxidant effects35,36. The significant upregulation of HMOX1 exerts a protective effect on cells during inflammatory responses37,38. Notably, HMOX1 is expressed at low levels in the intestine under healthy conditions but is significantly induced upon triggering an inflammatory state39,40. Additionally, carbon monoxide (CO) derived from HMOX1 has been shown to regulate intestinal inflammation, and the administration of low concentrations of exogenous CO has a protective effect against intestinal inflammation40. However, the effectiveness of this method for treating acne inflammation remains to be verified. Therefore, the underlying mechanism of acne development may be related to lipid metabolism and inflammatory processes mediated by fibroblasts and neutrophils with high expression of the above protein molecules14. Our results suggest potential options for enhancing the therapeutic efficacy of acne treatment.
Using tissue traceability analysis, we revealed molecular signatures associated with tissue damage pathways in patients with acne with and without IR. Interestingly, both groups of patients exhibited damage to small intestine- and kidney-related cells. However, each group has distinct damaged cells and related pathways. For instance, in patients with acne and IR, damaged cells were characterized by CSH1 and CSH2-positive cells in the adrenal glands. Our results align with findings by Unluhizarci et al. and Higgs et al., who demonstrated that prolonged hyperinsulinemia triggers adverse anabolic effects and dysfunction of the adrenal glands41,42. The mechanisms behind these occurrences remain unclear and their relationship requires further study. In our clinical analysis, we observed that there were significantly more men than women without IR in the acne group, and the androgen level in this group was also significantly higher than that in the insulin-resistant group. Research has established that high androgen levels can enhance sebum secretion, inducing acne occurrence32. In our study, the signals of androgen precursors, such as arachidonic acid and cholesterol43, were significantly upregulated in the non-insulin-resistant group, indicating that androgens in this group play critical roles in acne occurrence and development. However, our supplementary table indicates that this result is influenced by gender. Whether there is any influence between the two remains to be confirmed through further experiments. Notably, Munir et al. reported that insulin stimulates ovarian androgen production44, suggesting a potential correlation with the gender of the patients with acne.
In addition, an analysis of the immune microenvironment of patients with acne revealed that cells with higher activity in the IR group were B cells, and their significantly marked differential molecule was WDR11 (previously annotated as BRWD2). WDR11 interacts with pathways such as NF-κB and RAS signaling. WDR11, a gene involved in human puberty45, encodes a highly conserved protein; however, its precise function remains unknown. Its proposed functions include tumor suppression, transcription regulation, endocytosed ricin trafficking, and vital assembly control46. Several studies have reported that patients with BRWD3 mutations have undescended testes and minimal facial or axillary hair45,47, which is the first report of the specific role of WD proteins in Kallmann syndrome. These findings suggest that WD repeat-mediated protein interactions of WDR11 are key requirements for normal puberty45,48, corresponding to our findings in young people aged 15–25 years. However, the precise function of WDR11 in puberty requires additional investigation, and its relevance for other age groups remains to be demonstrated. Interestingly, WDR11 is also required for primordial germ cell (PGC) development and regulates both canonical and noncanonical Hedgehog (HH) signaling49. This suggests that WDR11 may act on sex hormones. Sex hormones, in turn, may promote the production of pro-inflammatory cytokines via NF-κB signaling, triggering an inflammatory response50,51. The specific relationship between WDR11 and acne or acne with IR requires further exploration.
In the non-IR group, fibroblasts were more active, and the most significantly differentially expressed molecule was PCDHGA11. PCDHGA11 interacts with actin fiber processing and platelet activation pathways. We found that PCDHGA11 is located within the protocadherin gene cluster of the 5q31 chromosomal region52. In a study by Anke et al., members of the protocadherin-gamma cluster were assumed to play a role in synaptic connections between neurons52. However, there is limited research on PCDHGA11, which has only been mentioned in articles related to depression53. We speculate that PCDHGA11 may mediate actin fiber processing, platelet activation, and other pathways, triggering an immune response in immune cells, mainly fibroblasts, which in turn causes acne. However, specific links between these processes remain unclear.
Based on the above analysis, we believe that these two protein molecules have revealed, to some extent, the possible mechanism behind acne with or without IR. However, a larger sample size is needed to determine whether WDR11 can be used as a specific biomarker for acne with IR or if PCDHGA11 can be used as a specific biomarker for acne without IR. Such findings will facilitate the early screening, clinical diagnosis, and precise treatment of patients with acne.
We performed a correlation analysis to investigate the relationship between insulin concentration and acne development. We found that with an increase in insulin concentration, CFI, C3, C4BPA, and other proteins were significantly expressed in the plasma of patients, which may activate the complement system, mediate the inflammatory process, influence the skeletal remodeling of cells, and further activate the immune process, leading to acne occurrence. CFI is a crucial inhibitor of all complement pathways54. It is a serine protease with a typical trypsin-related serine protease domain54. In addition, C3, the central effector molecule of the complement system, enhances B cell responses to many antigens and mediates its multiple functions through different binding sites and their corresponding receptors55. C3 can be detected in elevated concentrations in individuals with IR and plays an important role in lipid metabolism56. Dahl et al.57 confirmed the presence of C3 in acne lesions and established the tissue-specificity of complement activation. In a study by Monica et al., C4BPA was found to be part of the complement regulator C4BP, which is also closely related to the occurrence of inflammation58. This finding aligns with the high expression levels of these proteins observed in the hyper-insulinemic group.
Regarding AV severity, we analyzed correlation between protein expression and GAGS scores. We found that with the increase of scores, C1QB, F2 and C4BPA were significantly upregulated in plasma and positively correlated with scores. This discovery suggests that these molecules may exacerbate AV by promoting the cell cycle, aggravate the inflammatory response, and further activating the immune reaction, thereby contributing to the onset and worsening of AV. C1q is a target recognition component of the classical pathway and is a fundamental bridge between innate and adaptive immunity59. C1QB, a subunit of the first component of complement C1q, has been known in previous studies that this protein has a protective role in lupus60. Additionally, some studies have indicated that C1QB can increase macrophage numbers, leading to pancreatic β-cell damage and promoting the occurrence of type I diabetes61, which is related to IR in this study. In the research by Wenxing Su et al., C1QB was shown to play an important role in maintaining the stability of the body’s environment, oxidative stress, and glucose-lipid metabolism62, etc. All of the above collectively reveal that C1QB may cause IR by damaging pancreatic β-cells, thereby triggering chronic inflammation63 and oxidative stress, which aggravates the inflammatory response in AV. F2, also known as prothrombin, is a key blood coagulation factor that circulates abundantly in the bloodstream, which is a vitamin K-dependent zymogen64. However, the link between F2 and AV remains to be further studied.
C4BPA was identified as an intersection molecule through correlation analysis of protein molecules with insulin levels and GAGS scores. In the molecular structure of C4BPA, previous studies have confirmed the presence of binding sites for low-density lipoprotein receptor-related protein (LRP) and complement component C3b, both of which play major roles in lipid metabolism and inflammatory responses65. IR can significantly affect systemic lipid metabolic homeostasis and modulate inflammatory response levels. Therefore, C4BPA may be a key molecule mediating the role of insulin in the development and progression of AV, and it holds potential as a target for AV associated with IR, providing a theoretical foundation for precision therapy and prognostic of AV with IR. While our findings suggest a key role of C4BPA in insulin-mediated acne, further studies are needed to elucidate its precise molecular mechanisms, particularly in relation to lipid metabolism and inflammatory responses.
Among the limitations of this study, first, we have a small sample size, and a short recruitment period which will potentially introduce temporal bias and limiting the diversity of the population studied. Second, participants were recruited from a single hospital, which may not represent broader demographic or clinical diversity. Third, the findings are based solely on the study cohort, with no external validation to confirm the reproducibility of the results. Fourth, apart from the C3 molecule, other molecules (such as C4BPA, CFI, and C1QB, etc.) still lack direct histological evidence in the acne-affected skin. Plasma proteomics analysis captured the systemic characteristics of acne, but other non-complement factors may not fully represent the local skin pathological state. However, future research should involve a larger, more diverse cohort from multiple geographic locations to improve the generalizability of the findings. And future studies should validate these findings in skin biopsies from AV lesions to establish direct tissue-pathology correlations.
Conclusion
In conclusion, our study indicated that C4BPA could act as a crucial molecular factor that mediates the influence of insulin on the onset and aggravation of AV. This study employed a proteomic strategy to uncover potential key proteins and evaluate the underlying pathogenesis of AV in patients with IR, as well as the possible mechanisms by which insulin levels contribute to acne development. In addition, this study provides a highly valuable proteomic resource for the research community to better understand AV pathophysiology with or without IR, and provide clues for potential therapeutic strategies for this condition.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AV:
-
Acne vulgaris
- HC:
-
Healthy control
- Acne_IR:
-
Acne in the insulin-resistant group
- Acne_nIR:
-
Acne in the non-insulin-resistant group
- IR:
-
The insulin-resistant group
- nIR:
-
The non-insulin-resistant group
- FBG:
-
Fasting blood glucose
- FINS:
-
Fasting insulin
- HOMA-IR:
-
The homeostasis model assessment of insulin resistance
- DIA:
-
Data-independent acquisition
- PCA:
-
Principal component analysis
- PRL:
-
Prolactin
- CFI:
-
Complement factor I
- C4BPA:
-
Complement component 4 binding protein alpha
- C3:
-
Complement 3
- GAGS:
-
The Global acne grading system
- C1QB:
-
Complement C1q B chain
- F2:
-
Coagulation factor II
References
Tuchayi, S. M. et al. Acne vulgaris. Nat. Rev. Dis. Primers https://doi.org/10.1038/nrdp.2015.29 (2015).
Harper, J. C. Acne vulgaris: what’s new in our 40th year. J. Am. Acad. Dermatol. 82, 526–527. https://doi.org/10.1016/j.jaad.2019.01.092 (2020).
Vora, S., Ovhal, A., Jerajani, H., Nair, N. & Chakrabortty, A. Correlation of facial Sebum to serum insulin-like growth factor-1 in patients with acne. Br. J. Dermatol. 159, 990–991. https://doi.org/10.1111/j.1365-2133.2008.08764.x (2008).
Hazarika, N. Acne vulgaris: new evidence in pathogenesis and future modalities of treatment. J. Dermatological Treat. 32, 277–285. https://doi.org/10.1080/09546634.2019.1654075 (2019).
Williams, H. C., Dellavalle, R. P. & Garner, S. Acne vulgaris. Lancet 379, 361–372. https://doi.org/10.1016/s0140-6736(11)60321-8 (2012).
Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 31, 8–12. https://doi.org/10.1111/jdv.14374 (2017).
Webster, G. F., Leyden, J. J. & Nilsson, U. R. Complement activation in acne vulgaris: consumption of complement by comedones. Infect. Immun. 26, 183–186. https://doi.org/10.1128/iai.26.1.183-186.1979 (1979).
Artunc, F. et al. The impact of insulin resistance on the kidney and vasculature. Nat. Rev. Nephrol. 12, 721–737. https://doi.org/10.1038/nrneph.2016.145 (2016).
Tahapary, D. L. et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metabolic Syndrome: Clin. Res. Reviews https://doi.org/10.1016/j.dsx.2022.102581 (2022).
Andreadi, A. et al. Insulin Resistance and Acne: The Role of Metformin as Alternative Therapy in Men. Pharmaceuticals https://doi.org/10.3390/ph16010027 (2022).
Zouboulis, C. C. Acne as a chronic systemic disease. Clin. Dermatol. 32, 389–396. https://doi.org/10.1016/j.clindermatol.2013.11.005 (2014).
Gruszczyńska, M., Sadowska-Przytocka, A., Szybiak, W., Więckowska, B. & Lacka, K. Insulin Resistance in Patients with Acne Vulgaris. Biomedicines https://doi.org/10.3390/biomedicines11082294 (2023).
Chen, X., Yang, D., Li, L., Feng, S. & Wang, L. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum. Reprod. 21, 2027–2032. https://doi.org/10.1093/humrep/del142 (2006).
Nowowiejska, J., Baran, A. & Flisiak, I. Lipid alterations and metabolism disturbances in selected inflammatory skin diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24087053 (2023).
Huang, Y. C. & Cheng, Y. C. Isotretinoin treatment for acne and risk of depression: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 76, 1068–1076e1069. https://doi.org/10.1016/j.jaad.2016.12.028 (2017).
Steensel, M. A. M. Acne in the 21st century. Br. J. Dermatol. 181, 647–648. https://doi.org/10.1111/bjd.18202 (2019).
Blasiak, R. C., Stamey, C. R., Burkhart, C. N., Lugo-Somolinos, A. & Morrell, D. S. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatology https://doi.org/10.1001/jamadermatol.2013.6746 (2013).
Doshi, A., Zaheer, A. & Stiller, M. J. A comparison of current acne grading systems and proposal of a novel system. Int. J. Dermatol. 36, 416–418. https://doi.org/10.1046/j.1365-4362.1997.00099.x (1997).
Knox, S. & O’Boyle, N. M. Skin lipids in health and disease: A review. Chem. Phys. Lipids https://doi.org/10.1016/j.chemphyslip.2021.105055 (2021).
Layton, A. M. & Ravenscroft, J. Adolescent acne vulgaris: current and emerging treatments. Lancet Child. Adolesc. Health. 7, 136–144. https://doi.org/10.1016/s2352-4642(22)00314-5 (2023).
Thulasingam, M. et al. Crystal structures of human MGST2 reveal synchronized conformational changes regulating catalysis. Nat. Commun. https://doi.org/10.1038/s41467-021-21924-8 (2021).
Schröder, O., Sjöström, M., Qiu, H., Jakobsson, P. J. & Haeggström, J. Z. Microsomal glutathione S-transferases: selective up-regulation of leukotriene C4 synthase during lipopolysaccharide-induced pyresis. Cell. Mol. Life Sci. 62, 87–94. https://doi.org/10.1007/s00018-004-4366-7 (2005).
Higgins, L. G. & Hayes, J. D. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab. Rev. 43, 92–137. https://doi.org/10.3109/03602532.2011.567391 (2011).
Yan, K. L. et al. A novel MGST2 Non-Synonymous mutation in a Chinese pedigree with psoriasis vulgaris. J. Invest. Dermatology. 126, 1003–1005. https://doi.org/10.1038/sj.jid.5700186 (2006).
Akoumianakis, I., Zvintzou, E., Kypreos, K. & Filippatos, T. D. ANGPTL3 and Apolipoprotein C-III as Novel Lipid-Lowering Targets. Curr. Atheroscler. Rep. https://doi.org/10.1007/s11883-021-00914-7 (2021).
Inukai, K. et al. ANGPTL3 is increased in both insulin-deficient and -resistant diabetic States. Biochem. Biophys. Res. Commun. 317, 1075–1079. https://doi.org/10.1016/j.bbrc.2004.03.151 (2004).
Kersten, S. Angiopoietin-like 3 in lipoprotein metabolism. Nat. Reviews Endocrinol. 13, 731–739. https://doi.org/10.1038/nrendo.2017.119 (2017).
Burks, K. H., Basu, D., Goldberg, I. J. & Stitziel, N. O. Angiopoietin-like 3: An important protein in regulating lipoprotein levels. Best Pract. Res. Clin. Endocrinol. Metab. https://doi.org/10.1016/j.beem.2022.101688 (2023).
Sylvers-Davie, K. L. & Davies, B. S. J. Regulation of lipoprotein metabolism by ANGPTL3, ANGPTL4, and ANGPTL8. Am. J. Physiol. Endocrinol. Metab. 321, E493–e508. https://doi.org/10.1152/ajpendo.00195.2021 (2021).
Cinkajzlová, A. et al. Angiopoietin-like protein 3 and 4 in obesity, type 2 diabetes mellitus, and malnutrition: the effect of weight reduction and realimentation. Nutr. Diabetes. 8, 21. https://doi.org/10.1038/s41387-018-0032-2 (2018).
Deplewski, D. & Rosenfield, R. L. Role of hormones in pilosebaceous unit development. Endocr. Rev. 21, 363–392. https://doi.org/10.1210/edrv.21.4.0404 (2000).
Li, X. et al. A review of the role of Sebum in the mechanism of acne pathogenesis. J. Cosmet. Dermatol. 16, 168–173. https://doi.org/10.1111/jocd.12345 (2017).
Bienenfeld, A. et al. Androgens in women. J. Am. Acad. Dermatol. 80, 1497–1506. https://doi.org/10.1016/j.jaad.2018.08.062 (2019).
Bijli, K. M., Fazal, F. & Rahman, A. Regulation of Rela/p65 and endothelial cell inflammation by Proline-Rich tyrosine kinase 2. Am. J. Respir. Cell Mol. Biol. 47, 660–668. https://doi.org/10.1165/rcmb.2012-0047OC (2012).
Uchi, H., Yasumatsu, M., Morino-Koga, S., Mitoma, C. & Furue, M. Inhibition of Aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 85, 36–43. https://doi.org/10.1016/j.jdermsci.2016.10.003 (2017).
Xiong, H. et al. Syringic acid suppresses Cutibacterium acnes-induced inflammation in human keratinocytes via regulating the NLRP3/caspase-1/IL-1β signaling axis by activating PPARγ/Nrf2-antioxidant pathway. Int. Immunopharmacol. https://doi.org/10.1016/j.intimp.2024.112708 (2024).
Luo, J. F. et al. Activation of Nrf2/HO-1 pathway by nardochinoid C inhibits inflammation and oxidative stress in Lipopolysaccharide-Stimulated macrophages. Front. Pharmacol. 9, 911. https://doi.org/10.3389/fphar.2018.00911 (2018).
Chen, Z. et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Therapy. 21, 300. https://doi.org/10.1186/s13075-019-2085-6 (2019).
Sebastián, V. P. et al. Heme Oxygenase-1 as a modulator of intestinal inflammation development and progression. Front. Immunol. https://doi.org/10.3389/fimmu.2018.01956 (2018).
Takagi, T., Naito, Y., Uchiyama, K. & Yoshikawa, T. The role of Heme Oxygenase and carbon monoxide in inflammatory bowel disease. Redox Report: Commun. Free Radical Res. 15, 193–201. https://doi.org/10.1179/174329210x12650506623889 (2010).
Higgs, J. A. et al. Pathophysiological link between insulin resistance and adrenal incidentalomas. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23084340 (2022).
Unluhizarci, K., Karaca, Z. & Kelestimur, F. Role of insulin and insulin resistance in androgen excess disorders. World J. Diabetes. 12, 616–629. https://doi.org/10.4239/wjd.v12.i5.616 (2021).
Nikolakis, G., Stratakis, C. A., Kanaki, T., Slominski, A. & Zouboulis, C. C. Skin steroidogenesis in health and disease. Reviews Endocr. Metabolic Disorders. 17, 247–258. https://doi.org/10.1007/s11154-016-9390-z (2016).
Munir, I. et al. Insulin augmentation of 17alpha-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology 145, 175–183. https://doi.org/10.1210/en.2003-0329 (2004).
Kim, H. G. et al. WDR11, a WD protein that interacts with transcription factor EMX1, is mutated in idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am. J. Hum. Genet. 87, 465–479 (2010).
Navarro Negredo, P., Edgar, J. R., Manna, P. T., Antrobus, R. & Robinson, M. S. The WDR11 complex facilitates the tethering of AP-1-derived vesicles. Nat. Commun. https://doi.org/10.1038/s41467-018-02919-4 (2018).
Field, M. et al. Mutations in the BRWD3 gene cause X-linked mental retardation associated with macrocephaly. Am. J. Hum. Genet. 81, 367–374. https://doi.org/10.1086/520677 (2007).
Kim, H. G. & Layman, L. C. The role of CHD7 and the newly identified WDR11 gene in patients with idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Mol. Cell. Endocrinol. 346, 74–83. https://doi.org/10.1016/j.mce.2011.07.013 (2011).
Lee, J. et al. Coordination of canonical and noncanonical Hedgehog signalling pathways mediated by WDR11 during primordial germ cell development. Sci. Rep. https://doi.org/10.1038/s41598-023-38017-9 (2023).
Kurokawa, I., Layton, A. M. & Ogawa, R. Updated treatment for acne: targeted therapy based on pathogenesis. Dermatology Therapy. 11, 1129–1139. https://doi.org/10.1007/s13555-021-00552-6 (2021).
Ketchem, J. M., Bowman, E. J. & Isales, C. M. Male sex hormones, aging, and inflammation. Biogerontology 24, 1–25. https://doi.org/10.1007/s10522-022-10002-1 (2023).
Waha, A. et al. Epigenetic Silencing of the Protocadherin family member PCDH-γ-All in Astrocytomas. Neoplasia 7, 193–199. https://doi.org/10.1593/neo.04490 (2005).
Garafola, C. S. & Henn, F. A. A change in hippocampal Protocadherin gamma expression in a learned helpless rat. Brain Res. 1593, 55–64. https://doi.org/10.1016/j.brainres.2014.08.071 (2014).
Nilsson, S. C., Sim, R. B., Lea, S. M., Fremeaux-Bacchi, V. & Blom, A. M. Complement factor I in health and disease. Mol. Immunol. 48, 1611–1620. https://doi.org/10.1016/j.molimm.2011.04.004 (2011).
Zarantonello, A., Revel, M., Grunenwald, A. & Roumenina, L. T. C3-dependent effector functions of complement. Immunol. Rev. 313, 120–138. https://doi.org/10.1111/imr.13147 (2022).
van Oostrom, A. J. H. H. M., Alipour, A., Plokker, T. W. M., Sniderman, A. D. & Cabezas, M. C. The metabolic syndrome in relation to complement component 3 and postprandial lipemia in patients from an outpatient lipid clinic and healthy volunteers. Atherosclerosis 190, 167–173. https://doi.org/10.1016/j.atherosclerosis.2006.01.009 (2007).
Dahl, M. G. & McGibbon, D. H. Complement C3 and Immunoglobulin in inflammatory acne vulgaris. Br. J. Dermatol. 101, 633–640. https://doi.org/10.1111/j.1365-2133.1979.tb05641.x (1979).
Olcina, M. M. et al. Intracellular C4BPA levels regulate NF-κB-dependent apoptosis. iScience https://doi.org/10.1016/j.isci.2020.101594 (2020).
Pegoraro, S. et al. Epigenetic regulation of complement C1Q gene expression. Front. Immunol. https://doi.org/10.3389/fimmu.2024.1498097 (2024).
Westra, H. J. et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 45, 1238–1243. https://doi.org/10.1038/ng.2756 (2013).
Ji, L. & Guo, W. Single-cell RNA sequencing highlights the roles of C1QB and NKG7 in the pancreatic islet immune microenvironment in type 1 diabetes mellitus. Pharmacol. Res. 187, 106588. https://doi.org/10.1016/j.phrs.2022.106588 (2023).
Su, W. et al. Exploring the pathogenesis of psoriasis complicated with atherosclerosis via microarray data analysis. Front. Immunol. https://doi.org/10.3389/fimmu.2021.667690 (2021).
Chang, D. C., Xu, X., Ferrante, A. W. & Krakoff, J. Reduced plasma albumin predicts type 2 diabetes and is associated with greater adipose tissue macrophage content and activation. Diabetol. Metab. Syndr. https://doi.org/10.1186/s13098-019-0409-y (2019).
Pozzi, N. & Di Cera, E. Prothrombin structure: unanticipated features and opportunities. Expert Rev. Proteomics. 11, 653–655. https://doi.org/10.1586/14789450.2014.971763 (2014).
Iqbal, A. et al. C4BPA: A novel co-regulator of immunity and fat metabolism in the bovine mammary epithelial cells. Front. Genet. https://doi.org/10.3389/fgene.2021.830566 (2022).
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing. We thank the technical assistance provided by Shanghai iProteome Biotechnology Co., Ltd (Shanghai, China). This work was financially supported by Sichuan Science and Technology Program (2022YFS0631), The Hejiang People’s Hospital-Southwest Medical University Science and Technology Strategic Cooperation Project (2023HJXNYD04), and the Hejiang People’s Hospital Special Research Project (2023HJSRY02).
Funding
This work was supported by Sichuan Science and Technology Program (2022YFS0631), The Hejiang People’s Hospital-Southwest Medical University Science and Technology Strategic Cooperation Project (2023HJXNYD04), and the Hejiang People’s Hospital Special Research Project (2023HJSRY02).
Author information
Authors and Affiliations
Contributions
Y.R., Zheng. L. and S.C. collected plasma samples and wrote the main manuscript. X.Z., Y.X., X.X. and Y.L. performed data curation. X.C. and Q.T. analyzed the data. Z.J., Zong. L. and C.L. provided guidance. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The experiment has been approved by the Ethics Committee of the affiliated Hospital, Southwest Medical University (No.: KY2023426). All participants signed an informed consent form.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rao, Y., Lei, Z., Chen, S. et al. Differential plasma proteome analysis reveals key proteins associated with insulin resistance in acne vulgaris patients. Sci Rep 15, 29342 (2025). https://doi.org/10.1038/s41598-025-12344-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12344-5