Abstract
To characterize the associations between nighttime sleep duration and control of blood pressure (BP) and glycemia in individuals with comorbid hypertension and diabetes, we conducted a cross-sectional analysis of 2794 participants aged ≥ 50 years with confirmed diabetes and hypertension. Participants were categorized into five self-reported nighttime sleep duration groups: < 6 h, 6–7 h, 7–8 h, 8–9 h, and > 9 h. Multivariable logistic regression models were used to evaluate associations between sleep duration and BP/glycemic control, with adjustment for demographic, clinical, and behavioral confounders. We observed that prolonged sleep duration (> 9 h) was independently associated with uncontrolled BP after confounder adjustment. Subgroup analyses revealed that short sleep duration (< 6 h and 6–7 h) was linked to uncontrolled BP among males, whereas prolonged sleep duration was associated with uncontrolled BP among females, participants under 65 years, those with regular medication adherence, and non-nappers. For glycemic control, prolonged sleep duration conferred a protective effect among normal-weight individuals, but this association was not evident in the overall sample. Our results suggested that BP regulation may play a dominant role in competitive metabolic regulation demands compared to glycemic regulation. We recommended that individualized interventions aimed at modifying the habitual sleep duration of patients with comorbidities may be a crucial strategy to enhance BP and blood glucose management.
Similar content being viewed by others
Introduction
Multimorbidity, characterized by the concurrent presence of two or more chronic conditions in an individual, is a prevalent issue within community populations1,2. Among the various comorbidity patterns in China, the coexistence of hypertension and diabetes stands out as particularly significant, with an estimated prevalence of 10.9% among individuals aged 45 years and older3. Diabetes and hypertension can be characterized as “closely intertwined conditions” owing to their shared complications and overlapping pathophysiological mechanisms4. Prospective study indicates that individuals with both hypertension and diabetes have a 2.4-fold risk of developing cardiovascular disease5. Blood pressure control constitutes a critical component of diabetes management. The guideline recommends stricter blood pressure management requirements (130/80mmHg) for people with coexisting hypertension and diabetes6. Thus, from the perspective of disease management, it is of great significance to treat hypertension and diabetes as a whole.
The impact of sleep on health is a widely discussed topic, with evidence linking sleep duration to hypertension, diabetes, cardiovascular disease risk, and overall mortality7,8,9,10. Current studies have explored the potential role of sleep duration in managing blood pressure and blood glucose level. For instance, research based on the National Health and Nutrition Examination Survey (NHANES) found that short sleep duration was associated with uncontrolled hypertension in US adults with hypertension11. Additionally, a meta-analysis revealed a U-shaped relationship between sleep duration and hemoglobin A1c (HbA1c) levels in patients with type 2 diabetes12. These findings suggest that maintaining optimal sleep duration may contribute to the management of both blood pressure and glycemic control. However, there is a lack of studies examining the relationship between sleep duration and the management of hypertension and diabetes in a comorbid population.
This study aims to explore the correlation between sleep duration and the management of patients with comorbid hypertension and diabetes mellitus, and to investigate the potential application value of sleep intervention strategies.
Materials and methods
Ethics statement
The protocol of this study was approved by the Ethics of Community of the Shanghai Center for Disease Control and Prevention (2023-3). All participants provided their written informed consent. Our study was conducted in accordance with the principles of the Declaration of Helsinki.
Study subjects
The Community Healthcare Center (CHC) stands as an institution dedicated to delivering comprehensive medical and health services to the residents within its jurisdiction. This esteemed medical facility is committed to providing free, unwavering, and standardized care to patients aged 35 and above who have been diagnosed with hypertension and/or type 2 diabetes, in strict accordance with the mandates of the National Basic Public Health Services program. The concrete services offered encompass screening, the establishment and update of electronic health records, regular follow-ups, personalized health education and lifestyle interventions (such as meticulous dietary and exercise guidance), medication supervision, and prompt referrals for patients deemed high-risk or unstable13.
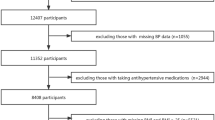
Approximately 600 participants were randomly selected from each of 6 CHCs to participate in our investigation during March and June in Shanghai, China. The sampling method is to generate random numbers in simple random sampling (SRS). These participants are all the objects managed by the CHC. Specifically, each participant has an electronic health record, a contracted family doctor, personalized monitoring plans for blood pressure and glucose, and standardized monitoring services. During the investigation period, 557 participants refused to be investigated or failed to complete all the investigation contents. Overall, 3023 participants completed the investigation. The inclusion criteria of the participants: (1) age ≥ 50 and < 80 years old; (2) participants who appear healthy (exhibited no cognitive impairments, mental health disorders, physical disabilities, visual impairments, or any other conditions that could hinder the completion of this project) (3) both diagnosed with hypertension and type 2 diabetes. The diagnosis criteria of hypertension was the blood pressure measured in the clinic three times on different days, with SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg14. Diabetes was defined as either (1) the presence of classic diabetes symptoms (polyuria, polydipsia, polyphagia, and unexplained weight loss) plus one of the following: FPG ≥ 7.0 mmol/L, 2-hour OGTT ≥ 11.1 mmol/L, random plasma glucose ≥ 11.1 mmol/L, or HbA1c ≥ 6.5%; or (2) in the absence of classic symptoms, two of the above glycemic parameters meeting or exceeding diagnostic thresholds, either at the same time point or on separate occasions15. 229 of them were excluded because of (1) chronic kidney disease (n = 81); (2) missing data on HbA1c measurement (n = 20); (3) extreme values of sleep duration (sleep duration ≤ 3 h, sleep duration > 11 h) (n = 128). A total of 2,794 people were included in the follow-up analysis.
Assessments of sleep characteristics
All investigators received training and assessment organized by Shanghai center for disease control and prevention, and administered questionnaires through face-to-face interviews. The information of sleep duration was self-reported. We systematically inquired into the participants’ sleep characteristics during both diurnal and nocturnal periods, respectively. In the present study, the duration of nighttime sleep among participants was a subject of investigation. Participants were asked “how long is your habitual night sleep duration?”. Whether participants had a habitual napping practice constituted an important confounding factor. To capture this part of information, the investigators will ask the participants, “Do you have the habit of taking a nap?”
Definition of outcomes
According to the standards of medical care for type 2 diabetes in China (2019 version)16. BP control was defined as average SBP < 130 mmHg and DBP < 80 mmHg. Glycemic control was defined as HbA1c < 53 mmol/mol (7.0%).
Assessments of covariates
All participants provided completed questionnaires and peripheral blood samples. The electronic questionnaires involved demographic characteristics (age, gender, marital status, etc.), behavioral risk factors (smoking status, alcohol consumption, physical activity, high fat intake, high salt intake, etc.), medical history, medication history and family history. We used International Physical Activity Questionnaire-Short Form (IPAQ-SF) to assess the physical activity levels of the participants, and according to the standard data processing and analysis procedures, the physical activity levels of the participants were classified into three grades: “low”, “medium” and “high”17. Medication adherence was assessed using the 8-item Morisky Medication Adherence Scale (MMAS-8), with scores < 6 indicating low adherence18. High fat intake was defined as daily intake of cooking oil > 30 g. High salt intake was defined as daily intake of salt > 6 g. Napping habits were categorized as “daytime napping” or “no nap” based on self-reported nap behavior.
Height and weight were measured using an electronic height and weight meter (HNH-219, Omron Company, Dalian, China). Body mass index (BMI) was calculated as weight in kilograms divided by standing height in meters squared (kg/m2). We used standard WHO criteria to define overweight (BMI ≥ 25 kg/m2). Blood pressure measurement was carried out using the automatic office blood pressure measurement (AOBP) with the assistance of medical staff. Participants were required to sit and rest quietly for at least 5 min, then have their upper arm blood pressure measured, with the upper arm placed at the level of the heart. The blood pressure of the upper arm was measured twice consecutively with an interval of one minute using a standard type of blood pressure monitor (HBP-1100U, Omron Company, Dalian, China). If the absolute value of the difference between two systolic blood pressure (SBP) or diastolic blood pressure (DBP) values was ≤ 5 mmHg, the final blood pressure value was the average of the two readings. If the difference between two SBP or DBP was ≥ 5 mmHg, a third blood pressure was measured after an interval of 1 min, and the average of the three readings was calculated as the final blood pressure value. Venous blood samples were collected after an overnight fast for at least 8 h. Plasma glucose, triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured by automated biochemical analyzer (Cobas C501, Roche, the USA). HbA1c was measured by high performance liquid chromatography (HLC-723G8, TOSOH, Japan).
Statistical analysis
Data are presented as mean ± SD or median with interquartile range (IQR) depending on the normality of the continuous variables, or percentages for categorical variables. Analysis of variance (ANOVA) or chi-square tests were used to assess differences in the means or proportions of subject characteristics. Nighttime sleep duration was classified into five categories:< 6 h, 6–7 h, 7–8 h (reference), 8–9 h, > 9 h. To examine the association between habitual sleep duration and control of BP and glycemia, multivariate logistic regression analyses were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs). The adjustment of confounding factors in this study was mainly based on the reports of previous literature, adjusting for confounding factors involved age, gender, BMI, disease duration (hypertension and diabetes), medication compliance, and lifestyle (smoking status, alcohol consumption, physical activity levels, intake of high salt, intake of high fat and daytime napping habits). The variance inflation factor (VIF) was used to test the multicollinearity of the variables. If VIF is greater than 5, this variable is considered to have multicollinearity. Restricted cubic spline was fitted to determine potential non-linear association between sleep duration and control of BP and glycemia19. The number of knots, from 3 to 7, was determined according to Akaike’s information criterion (AIC) to balance best fit and over fitting20. Likelihood ratio tests for models with and without interaction terms were used to assess modification effects of stratified variables. The variables in the subgroup analysis included age (≥ 65 years old, < 65 years old), gender (male, female), disease duration (≥ 10 years, < 10years), overweight (BMI ≥ 25, BMI < 25), medication compliance (high, low) and daytime napping (yes, no). All analyses performed in R (version 4.1.2). All P values were two-tailed with a significant level of < 0.05.
Results
Participant characteristics
A total of 2794 individuals diagnosed with both diabetes and hypertension were incorporated into the current analysis. The participants had a mean age of 67.9 ± 5.8 years, with females accounting for 52.7%. The median duration of diabetes and hypertension was 10.5 years and 14.7 years, respectively. Notably, the rates of effective BP control and glycemic control were suboptimal, standing at 18.9% and 34.8%, respectively. The average duration of nightly sleep across the participants was 7.0 h, with 17.0% reporting less than 6 h and 7.0% indicating 9 or more hours. Baseline characteristics according to sleep duration categories were shown in Table 1.
Sleep duration and control of blood pressure and glycemia
The logistic regression analyses indicated that compared with reference group (7–8 h), long sleep duration (> 9 h) was associated with uncontrolled BP, even after adjusting for age, gender, and the duration of hypertension (OR 1.78, 95% CI 1.10–3.04). Building upon this model, we further adjusted for additional covariates, including smoking status, drinking status, high fat intake, high salt intake, BMI, physical activity, medication compliance and daytime napping habits. This refinement maintained the observed association (OR 1.69, 95% CI 1.04–2.88). However, our study did not detect any significant association between sleep duration and glycemic control. All results were detailed in Table 2.
Subgroup analyses between sleep duration and control of blood pressure and glycemia
Subgroup analyses were further performed according to gender, age, disease duration, BMI status, medication compliance, and daytime napping habits. We observed that compared with reference group (7–8 h), short sleep duration (< 6 h and 6–7 h) was associated with uncontrolled BP among males (for the < 6 h group: OR 1.61, 95% CI 1.04–2.56; for the 6–7 h group: OR 1.54, 95% CI 1.05–2.29) as detailed in Table 3. Conversely, compared with reference group (7–8 h), long sleep duration (> 9 h) was linked to uncontrolled BP among females (OR 3.25, 95% CI 1.31–10.89). Additionally, an effect modification by the gender factor was detected for the association (P for interaction = 0.001). We also found that compared with the reference group (7–8 h), long sleep duration (> 9 h) was associated with uncontrolled BP among under 65 years old (OR 5.79, 95% CI 1.60–10.50), those who took medication regularly (OR 1.70, 95% CI 1.00–3.03), and those who did not take naps (OR 1.94, 95% CI 1.01–4.14). The above detailed results were in Table 3. Similar analyses were performed to explore the relationship between sleep duration and glycemic control; among people with normal weight, compared with the reference group (7–8 h), a long sleep duration (> 9 h) was a protective factor for glycemic control (Table 4).
Dose-response analyses on the relationship between sleep duration and control of blood pressure and glycemia
The restricted cubic spline model did not uncover any significant linear or nonlinear associations between sleep duration and either BP control or glycemic control, even after adjusting for potential confounders (Poverall > 0.05, Pnon−linear>0.05) as depicted in Fig. S1. However, distinct morphological differences in the dose-response curves were apparent across subgroup. Specifically, a linear trend was observed between sleep duration and uncontrolled BP among females (Poverall < 0.001) (Fig. 1. picture l). Non-linear trends were observed between sleep duration and uncontrolled glycemia among under 65 years old (Pnon-linear = 0.041) (Fig. 2. picture b) and normal weight (Pnon-linear = 0.031) (Fig. 2. picture d).
The dose-response relationships between sleep duration and blood pressure control within each subgroup. Overweight indicated a BMI ≥ 25. Normal weight indicated a BMI < 25. Long-term disease course indicated ≥ 10 years. Short-term disease course indicated < 10 years. The dose-response relationship curves indicate that the inflection point of the J-shaped relationship between sleep duration and blood pressure control is approximately at 7 h.
The dose-response relationships between sleep duration and glycemic control within each subgroup. Overweight indicated a BMI ≥ 25. Normal weight indicated a BMI < 25. Long-term disease course indicated ≥ 10 years. Short-term disease course indicated < 10 years. There is a nonlinear relationship between sleep duration and glycemic control in people under 65 years old (Pnon-linear = 0.041) and those with normal weight (Pnon-linear = 0.041). The inflection point of all dose-response relationships is approximately at 7 h.
Discussion
In this community-based cross-sectional study involving 2794 participants diagnosed with both hypertension and diabetes within a Chinese population, we identified that long sleep duration (> 9 h) was associated with uncontrolled BP compared to the reference group (7–8 h), even after adjusting for confounding factors. Subgroup analyses further revealed that compared with reference group (7–8 h), short sleep duration (< 6 h and 6–7 h) was linked to uncontrolled BP control among males, whereas long sleep duration was associated with uncontrolled BP among females. We also observed that compared with the reference group (7–8 h), long sleep duration (> 9 h) was associated with uncontrolled BP among under 65 years old, those who took medication regularly, and those who did not take naps. For glycemic control, prolonged sleep duration conferred a protective effect among normal-weight individuals. Moreover, dose-response analyses elucidated a linear trend between sleep duration and uncontrolled BP control in females and non-linear trends between sleep duration and uncontrolled glycemia among under 65 years old (Pnon-linear = 0.041) and normal weight (Pnon-linear = 0.031).
Direct evidence linking sleep duration to BP control remains limited. However, current evidence suggests that both short (< 6 h) and long (> 9 h) sleep duration may increase the risk of hypertension7,21,22. This aligns with our findings that long sleep duration was associated with uncontrolled BP. From the perspective of hypertension management, our study adds new evidence to relationship between sleep duration and hypertension. Ogugu et al.11 reported that compared with 7–9 h of sleep at night, short sleep duration (< 6 h) was associated with uncontrolled BP, which partially corroborates our results. The discrepancies in findings may be attributed to differences in the subjects. For instance, our study focused on a comorbid population (hypertension and diabetes), whereas their study included individuals diagnosed with hypertension. Additionally, ethnic disparities among the study participants may have contributed to variations in genetic backgrounds and lifestyles, thereby potentially accounting for inconsistent findings. Based on previous studies and our results, we hypothesized that too short or too long sleep duration is a risk behavior for both high-risk population and hypertensive patient. Obtaining 7–8 h of sleep is a beneficial behavior for the prevention and management of hypertension.
In the analysis of the comorbid population, we found long sleep duration (> 9 h) was associated with a protective effect against uncontrolled glycemia among non-overweight individuals. Owing to the absence of subgroup analyses in prior studies, our findings cannot be directly compared. Thus, this observation warrants further validation. In the broader sample, no statistically significant findings were observed when comparing different sleep duration categories with the reference group. Potential explanations for the discrepancy between our findings and prior studies include: (1) the association between sleep and glycemic control is susceptible to confounding by factors such as insulin status23 and duration of diabetes24; (2) BP management may play a central role in the interplay of metabolic regulatory demands among patients with comorbid hypertension and diabetes.
The mechanisms underlying prolonged sleep duration and hypertension are not well understood. A few studies demonstrated that depression25,26obstructive sleep apnea (OSA)27and other factors may confound this association. Nevertheless, an alternative view is that there is reverse causality between long sleep duration and hypertension, in other words, prolonged sleep duration is an epiphenomenon of hypertension28. The evidence of the association between long sleep duration and hypertension is still limited to cross-sectional studies, and there is still a lack of longitudinal studies29. Therefore, the mechanisms of long sleep duration and hypertension still need further investigation. The causal relationship between short sleep duration and hypertension is clear, and previous experimental studies have suggested that sleep deprivation causes autonomic nervous activation, and further lead to an increase in blood pressure30,31. The gender disparity may be attributable to biological factors (e.g., sex-specific prevalence of OSA32hormonal regulation33,34) or sociobehavioral determinants (e.g., caregiving-related sleep disruption35), although elucidating the underlying mechanisms requires further investigation.
The underlying mechanisms through which prolonged sleep duration (≥ 9 h) confers a protective effect against poor glycemic control in normal-weight individuals warrant further investigation. Ohkuma et al.36 have detailed that depression, uncontrolled physical performance, OSA, and restless legs syndrome may play a confounding role in the relationship between long sleep duration and higher glycemic level. Furthermore, considering the robust correlation between obesity and glucose metabolism disorders, and the well-documented evidence linking prolonged sleep duration to obesity and its underlying mechanisms37it is plausible that obesity may serve as a mediator in the association between extended sleep duration and impaired glycemic control.
The primary strength of this study is its investigation into the potential role of sleep duration in managing BP and glycemic control among individuals with coexisting hypertension and diabetes, who are at an elevated risk for cardiovascular disease mortality. Understanding the association between sleep duration and BP, as well as glycemic control within this population, carries significant implications for the management of comorbid conditions. To comprehensively investigate the relationship between sleep duration and the regulation of blood pressure (BP) and glycemic levels, we utilized restricted cubic spline analysis to assess dose-response relationships, thereby mitigating potential information loss associated with categorizing sleep duration.
However, the current study also has certain limitations. Firstly, nighttime sleep duration was assessed through self-report, which may introduce recall bias. For follow-up research, wearable devices will be employed to corroborate our findings. Additionally, we did not collect data on depressive status, the prevalence of OSA, or other potential confounders. These factors are likely to confound the association. Then, due to the limited sample size in our study, the accuracy of the findings may be compromised to some extent. Finally, due to the cross-sectional nature of our data, we cannot establish a causal link between sleep duration and the control of BP and glycemic levels, necessitating careful consideration of the potential inversion of cause and effect. Prospective studies tracking sleep patterns and cardiometabolic outcomes, or trials testing sleep interventions are needed to establish causality.
Conclusion
In conclusion, we found that long sleep duration (> 9 h) was associated with uncontrolled blood pressure (BP) control among individuals with coexisting hypertension and diabetes. Significant gender-differences were evident in the relationship between sleep duration and the control of BP and glycemic levels. During clinical care, recommendations emphasize that clinicians should assess patients’ sleep parameters and implement personalized interventions to enhance blood pressure and glycemic control, particularly among individuals with comorbid hypertension and diabetes, though intervention efficacy must first be validated in trials.
Data availability
De-identified data will be shared upon reasonable request to the corresponding author, pending institutional approvals.
References
Valderas, J. M., Starfield, B., Sibbald, B., Salisbury, C. & Roland, M. Defining comorbidity: implications for understanding health and health services. Ann. Fam. Med. 7, 357–363 (2009).
Skou, S. T. et al. Multimorbidity. Nat. Rev. Dis. Primers. 8, 48 (2022).
Hu, Y. et al. Prevalence and patterns of Multimorbidity in China during 2002–2022: a systematic review and meta-analysis. Ageing Res. Rev. 93, 102165 (2024).
Vu, T. H. L. et al. Comorbidities of diabetes and hypertension in vietnam: current burden, trends over time, and correlated factors. BMC Public Health. 23, 2419 (2023).
Hu, G., Jousilahti, P. & Tuomilehto, J. Joint effects of history of hypertension at baseline and type 2 diabetes at baseline and during follow-up on the risk of coronary heart disease. Eur. Heart J. 28, 3059–3066 (2007).
American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of care in Diabetes-2024. Diabetes Care. 47, S179–S218 (2024).
Guo, X. et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep. Med. 14, 324–332 (2013).
Shan, Z. et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 38, 529–537 (2015).
Cappuccio, F. P., Cooper, D., D’Elia, L., Strazzullo, P. & Miller, M. A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 32, 1484–1492 (2011).
Svensson, T. et al. Association of sleep duration with all- and major-cause mortality among adults in Japan, China, Singapore, and Korea. JAMA Netw. Open. 4, e2122837 (2021).
Eg, O. et al. The association between habitual sleep duration and blood pressure control in united States (US) adults with hypertension. Integr. Blood Press. Control 15, 53–66 (2022).
Lee, S. W. H., Ng, K. Y. & Chin, W. K. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep. Med. Rev. 31, 91–101 (2017).
Chen, Z. Launch of the health-care reform plan in China. Lancet 373, 1322–1324 (2009).
Liu, L. S. Writing group of 2010 Chinese guidelines for the management of hypertension. [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 39, 579–615 (2011).
Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China(2020 edition). Chin. J. Endocrinol. Metabolism. 37, 311–398 (2021).
Jia, W. et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 35, e3158 (2019).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395 (2003).
Moon, S. J., Lee, W. Y., Hwang, J. S., Hong, Y. P. & Morisky, D. E. Accuracy of a screening tool for medication adherence: a systematic review and meta-analysis of the Morisky medication adherence Scale-8. PLoS One. 12, e0187139 (2017).
Durrleman, S. & Simon, R. Flexible regression models with cubic splines. Stat. Med. 8, 551–561 (1989).
Marrie, R. A., Dawson, N. V. & Garland, A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J. Clin. Epidemiol. 62, 511–517e1 (2009).
Fang, J. et al. Association of sleep duration and hypertension among US adults varies by age and sex. Am. J. Hypertens. 25, 335–341 (2012).
Wang, Q., Xi, B., Liu, M., Zhang, Y. & Fu, M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens. Res. 35, 1012–1018 (2012).
Full, K. M. et al. The relationship between sleep duration and glycemic control among Hispanic adults with uncontrolled type 2 diabetes. Diabetes Educ. 43, 519–529 (2017).
Kim, B. K. et al. Sleep duration and glycemic control in patients with diabetes mellitus: Korea National health and nutrition examination survey 2007–2010. J. Korean Med. Sci. 28, 1334–1339 (2013).
Dong, L., Xie, Y. & Zou, X. Association between sleep duration and depression in US adults: a cross-sectional study. J. Affect. Disord. 296, 183–188 (2022).
Chunnan, L., Shaomei, S. & Wannian, L. The association between sleep and depressive symptoms in US adults: data from the NHANES (2007–2014). Epidemiol. Psychiatr Sci. 31, e63 (2022).
Sd, G. & A, S. The relationship between sleep-disordered breathing and hypertension in a nationally representative sample. Sleep Disord. (2015).
Shivashankar, R. et al. Associations of sleep duration and disturbances with hypertension in metropolitan cities of delhi, chennai, and Karachi in South asia: cross-sectional analysis of the CARRS study. Sleep 40, zsx119 (2017).
Jike, M., Itani, O., Watanabe, N., Buysse, D. J. & Kaneita, Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep. Med. Rev. 39, 25–36 (2018).
Lusardi, P. et al. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am. J. Hypertens. 12, 63–68 (1999).
Ogawa, Y. et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep 26, 986–989 (2003).
Senaratna, C. V. et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep. Med. Rev. 34, 70–81 (2017).
Mong, J. A. & Cusmano, D. M. Sex differences in sleep: impact of biological sex and sex steroids. Philos. Trans. R Soc. Lond. B Biol. Sci. 371, 20150110 (2016).
Pan, Z. et al. Different regimens of menopausal hormone therapy for improving sleep quality: a systematic review and meta-analysis. Menopause 29, 627–635 (2022).
van de Straat, V., Willems, B. & Bracke, P. Care to sleep? Daily caregiving and sleep problems in an ageing European population. Health Sociol. Rev. 30, 204–217 (2021).
Ohkuma, T. et al. Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka diabetes registry. Diabetes Care. 36, 611–617 (2013).
Tan, X., Chapman, C. D., Cedernaes, J. & Benedict, C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: A review of possible mechanisms. Sleep. Med. Rev. 40, 127–134 (2018).
Acknowledgements
The authors thank all participants and stuffs from six Community Healthcare Center (CHS) and three Center for Disease Control and Prevention (CDC).
Funding
The Shanghai New Three-year Action Plan for Public Health (Grant No. GWVI-8).
Author information
Authors and Affiliations
Contributions
B.C. analyzed the data and drafted the paper. H.X. analyzed the data. Y.S. and M.C. revised the paper. J.C., X.S. and H.T. carried out quality control for the project. D.P. conceptualized the study. Qinghua Yan conceptualized the study and revised the paper. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, B., Xu, H., Shi, Y. et al. Nighttime sleep duration and dual management of blood pressure and glycemia in Chinese with comorbid hypertension and diabetes. Sci Rep 15, 27017 (2025). https://doi.org/10.1038/s41598-025-12567-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12567-6