Abstract
Dopamine (DA) transmission from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) is strongly associated with depression in Parkinson’s disease (PD). Decreased DA levels due to alpha-synuclein (α-syn) aggregation have been underappreciated in relation to depression in PD. This study investigated the role of VTA-related α-syn aggregation in depression of PD, and whether gial cell line-derived neurotrophic factor (GDNF) can inhibit α-syn aggregation to improve symptoms. Here, α-syn aggregation and GDNF overexpression were induced in the VTA of 8-week-old male mice (C57BL/6J, DAT-cre, D2-cre) using adeno-associated viruses, combined with a cis-tracer virus as well as a chemogenetic virus. Behavioral tests, immunofluorescence (IF), western blot (WB), and enzyme-linked immunosorbent assay (ELISA) were employed for analysis. The mice with increased p-α-syn in VTA exhibited depressive-like behavior, along with reduced DA levels in the VTA and NAc, as well as reduced expression of related proteins in the NAc. However, GDNF overexpression in the VTA, led to a decrease in p-α-syn expression and reversed the aforementioned phenomena. Furthermore, results from cis-tracer virus and Immunofluorescence (IF) assay revealed a reduction in transmission of DA from the VTA to the NAc in mice with α-syn aggregation, which can be reversed by GDNF overexpression. Additionally, chemogenetic virus-induced diminished D2-MSN activation in the NAc significantly ameliorated depression-like behavior due to α-syn aggregation in the VTA. In conclusion, the aggregation of α-syn within the VTA may contribute to depression in PD, while GDNF can alleviate depression by inhibiting α-syn aggregation.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder among middle-aged and elderly individuals1. The current consensus suggests that the main pathological hallmark of PD is the progressive degeneration and diminishment of dopaminergic neurons caused by α-synuclein (α-syn) aggregation in the midbrain, which results in dysfunction in the dopamine projection pathway, followed by typical motor dysfunction (e.g., bradykinesia, resting tremor, muscle rigidity and freezing of gait) as well as non-motor dysfunction (e.g., cognition, emotion, sleep and depression)2. Depression is a prevalent non-motor symptom in PD, and often precedes the onset of motor symptoms3. The presence of depression not only intensifies negative emotions, but also complicates patients’ lives and their medication4. Although aberrant dopamine (DA) transmission has been implicated in the pathophysiology of various psychiatric disorders, including depression5the pathogenesis of depression in PD remains incompletely understood.
The midbrain limbic DA system plays a pivotal role in psychiatric disorders such as depression, with the main brain regions involved including the ventral tegmental area (VTA), nucleus accumbens (NAc), prefrontal cortex (PFC) and the Anterior Cingulate Cortex (ACC)6. Among these regions, the VTA is considered to be one of the major regions where DA neurons are predominantly located7. Optical stimulation of the VTA-NAc pathway can modulate depression-like behaviors in animal models8. The efficacy of antidepressants in alleviating depressive symptoms is attributed to their ability to enhance the activity of DA neurons within the VTA and facilitate DA transmission along the VTA-NAc pathway9,10,11. Furthermore, the repeated deep brain stimulation (DBS) in the NAc led to an augmented activity of DA neurons in the VTA, potentially offering significant therapeutic advantages for individuals with exaggerated depression-like symptoms12.
More than 95% of neurons in NAc are medium spiny neurons (MSN) expressing dopamine D1-type or D2-type receptors (D1R, D2R), and these neurons are output γ-aminobutyric acid (GABA) neurons13,14. GABA is the main inhibitory neurotransmitter in mammalian central nervous system, which can directly play an antidepressant role through GABA type A receptors (GABAAR)11. In an animal model of depression induced by chronic unpredictable stress (CUS), a significant reduction was observed in both the DA levels and D2R expression within the NAc15. A study showed that NE-100, a sigma-1 receptor antagonist, reduced GABAAR, D2R in the NAc, causing depression-behaviors; conversely, quinpirole, a D2R agonist, injected into the NAc restored GABAAR levels and alleviated depressive behaviors in NE-100 mice16. D2R within the NAc may play a predominant role in depression of PD.
Clinical studies have demonstrated a positive correlation between the expression level of α-syn in the peripheral blood and the degree of depression17. α-syn is physiologically involved in the regulation of DA synthesis; and the overexpression of α-syn in DA neurons led to a reduction in both tyrosine hydroxylase (TH) activity and DA levels18. The aggregation of α-syn in PD may potentially impact DA transmission, thereby contributing to the development of depression. In addition, the findings of a study demonstrated that the antidepressant desipramine not only effectively alleviated depression-like behaviors in animal models of PD, but also exhibited a concurrent significant reduction in α-syn expression19. The targeting of α-syn aggregation may potentially offer a promising strategy to mitigate depression of PD.
The neurotrophic factor glial cell line-derived neurotrophic factor (GDNF) enhances the survival of DA neurons20. The serum GDNF level was significantly lower in clinical PD patients compared to the healthy population group21. A clinical retrospective study found that the GDNF level in peripheral blood was significantly lower in patients with major depression compared to the control group22. An increase in GDNF levels in peripheral blood was observed following repetitive transcranial magnetic stimulation (rTMS) treatment in patients with depression23. The addition of GDNF to primary cultured DA neuronal cells has been demonstrated to effectively inhibit the aggregation of α-syn and consequently enhance neurons survival24. Given its effect on a-syn aggregation, GDNF may be a promising option for the treatment of depression in PD.
This study was conducted to verify that pathological α-syn aggregation in the VTA disrupts dopamine transmission from the VTA to the NAc, thereby triggering depression-like behavior, through stereotactic administration of AAV-SNCA into the VTA of mice. Based on the above foundation, the effects of GDNF on depressive symptoms and its potential underlying mechanisms were further investigated. These findings provide an experimental basis for the potential application of GDNF in treating depression in PD.
Results
Aggregation of α-syn in the VTA induced depression-like behavior
Given the effect of α-syn aggregation on DA levels in vitro (Figure S1), an adeno-associated virus of α-syn (AAV-SNCA) was stereotactically injected into the left VTA to establish PD mice model, followed by assessment of depression-like behavior in mice (Fig. 1A).
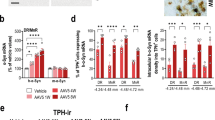
The results of OFT demonstrated that the AAV-SNCA mice exhibited significantly reduced locomotor activity, as evidenced by decreased total distance [F(2, 21) = 76.65, P < 0.0001 vs. AAV-NC1] (Fig. 1B, C), fewer grid crossings [F(2, 21) = 16.98, P = 0.0002 vs. AAV-NC1] (Fig. 1B, D), and decreased time spent in the central area [F(2, 21) = 15.10, P = 0.0002 vs. AAV-NC1] (Fig. 1B, E). In addition, the AAV-SNCA mice displayed a significant prolongation of immobility duration in the TST [F(2, 21) = 17.33, P = 0.0008 vs. AAV-NC1] (Fig. 1F, G), as well as in the FST [F(2, 21) = 23.62, P = 0.0005 vs. AAV-NC1] (Fig. 1F, H). The IF results showed an increase in p-α-syn distribution [F(2, 15) = 13.6, P = 0.0014 vs. AAV-NC1] (Fig. 1I, J) and a decrease in TH distribution in the VTA of the AAV-SNCA mice [F(2, 15) = 22.99, P = 0.0002 vs. AAV-NC1] (Fig. 1K-L).
The utilization of AAV-SNCA resulted in the aggregation of α-syn and a decrease in TH in the VTA, as well as depression-like behaviors. (A) Experimental timeline. (B) OFT trajectories (blue: start; red: end). (C) Moving distance (OFT, n = 8). (D) Grid crossings (OFT, n = 8). (E) Center time (OFT, n = 8). (F) TST/FST images. (G) Immobility time (TST, n = 8). (H) Immobility time (FST, n = 8). (I, J) p-α-syn IF in the VTA (n = 6; scale bars: 200/500 µm). (K, L) TH IF in the VTA (n = 6; scale bars: 200 μm). Data: Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. AAV-NC (one-way ANOVA with Tukey’s test).
Aggregation of α-syn in the VTA resulted in decreased DA levels and reduced synaptic protein in the NAc
Furthermore, in the VTA of mice, WB analysis revealed that the expression levels of α-syn [F(2, 12) = 18.89, P = 0.0002 vs. AAV-NC1] (Fig. 2A, B) and p-α-syn [F(2, 12) = 126, P < 0.0001 vs. AAV-NC1] (Fig. 2A, C) were increased in AAV-SNCA mice, whereas TH expression was decreased [F(2, 12) = 22.93, P = 0.0003 vs. AAV-NC1] (Fig. 2A, D). The ELISA results depicted in Fig. 2E demonstrated a reduction in DA content within both the VTA [F(2, 15) = 11.66, P = 0.0018 vs. AAV-NC1] and NAc [F(2, 15) = 6.88, P = 0.0134 vs. AAV-NC1] of the AAV-SNCA mice. In addition, in the NAc of mcie, WB analysis demonstrated a significant reduction in the expression levels of SNAP-25 [F(2, 12) = 21.68, P = 0.0011 vs. AAV-NC1] (Fig. 2F, G), PSD95 [F (2, 12) = 24.18, P = 0.0004 vs. AAV-NC1] (Fig. 2F, H), DAT [F(2, 12) = 18.42, P = 0.0012 vs. AAV-NC1] (Fig. 2F, I) and D2R [F(2, 12) = 11.7, P = 0.0034 vs. AAV-NC1] (Fig. 2J, K) in the NAc of the AAV-SNCA mice; whereas there was no statistically difference in D1R expression [F(2, 12) = 0.4028, P = 0.6772 vs. AAV-NC1] (Fig. 2J, K).
Effect of AAV-SNCA on levels of DA, as well as the expression levels of protein in both the VTA and the NAc. (A-D) WB analysis of α-syn, p-α-syn, and TH in the VTA (n = 5). (E) DA levels in the VTA and the NAc by ELISA (n = 6; VTA: left Y axis, NAc: right Y axis). (F-I) WB analysis of SNAP-25, PSD95, and DAT in the NAc (n = 5). (J, K) WB analysis of D1R and D2R in NAc (n = 5). Data: Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. AAV-NC1 (one-way ANOVA with Tukey’s test).
GDNF ameliorated depression-like behavior caused by α-syn aggregation
To investigate whether GDNF can improve depression-like behavior by inhibiting α-syn aggregation, an adeno-associated virus of GDNF (AAV-GDNF) was co-injected within the VTA (Fig. 3A). Results from OFT demonstrated that the AAV-SNCA + AAV-GDNF mice exhibited increased total distance traveled [F(3, 28) = 21.48, P = 0.0031 vs. AAV-SNCA + AAV-NC2] (Fig. 3B, C), more grid crossings [F(3, 28) = 14.01, P = 0.0047 vs. AAV-SNCA + AAV-NC2] (Fig. 3B, D), and an extended duration spent in the central area [F(3, 28) = 15.33, P = 0.0003 vs. AAV-SNCA + AAV-NC2] (Fig. 3B, E). Similarly, the AAV-SNCA + AAV-GDNF mice displayed a significant reduction in immobility duration in the TST [F(3, 28) = 24.75, P = 0.0002 vs. AAV-SNCA + AAV-NC2] (Fig. 3F, G) and FST [F (3, 28) = 24.75, P < 0.0001 vs. AAV-SNCA + AAV-NC2] (Fig. 3F, H). ELISA results also showed an increase in DA levels of the AAV-SNCA + AAV-GDNF mice both in the VTA [F(3, 20) = 17.08, P = 0.0004 vs. AAV-SNCA + AAV-NC2] and NAc [F(3, 20) = 15.8, P = 0.0035 vs. AAV-SNCA + AAV-NC2] (Fig. 3I). In addition, the Pearson correlation analysis demonstrated a statistically positive relationship between DA levels in the VTA and the NAc [r = 0.6974, P = 0.0002] (Fig. 3J).
The administration of AAV-GDNF ameliorated depression-like behavior induced by α-syn aggregation. (A) Experimental timeline. (B) OFT trajectories (green: start; red: end). (C) Moving distance (OFT, n = 8). (D) Grid crossings (OFT, n = 8). (E) Center time (OFT, n = 8). (F) TST/FST images. (G) Immobility time (TST, n = 8). (H) Immobility time (FST, n = 8). (I) ELISA results for the level of DA both in the VTA and the NAc of mice (n = 6). Data: Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA with Tukey’s test). (J) Pearson correlation analysis of DA in the VTA and NAc (n = 24, r = 0.6974, P = 0.0002).
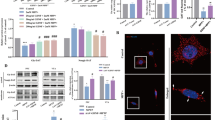
Moreover, results obtained from WB showed reduced p-α-syn [F(3, 16) = 30.23, P = 0.0023 vs. AAV-SNCA + AAV-NC2] (Fig. 4A, B) expression, increased GDNF [F(3, 16) = 11.65, P = 0.0015 vs. AAV-SNCA + AAV-NC2] (Fig. 4A, C) and TH [F(3, 16) = 77.46, P = 0.0039 vs. AAV-SNCA + AAV-NC2] (Fig. 4A, D) expression within the VTA of the AAV-SNCA + AAV-GDNF mice. Additionally, a significant decrease in SNAP-25 [F(3, 16) = 20.53, P = 0.0118 vs. AAV-SNCA + AAV-NC2] (Fig. 4E, F), PSD95 [F(3, 16) = 14.67, P = 0.0047 vs. AAV-SNCA + AAV-NC2] (Fig. 4E, G) and D2R [F (3, 16) = 12.21, P = 0.0089 vs. AAV-SNCA + AAV-NC2] (Fig. 4E, H) levels within the NAc of the AAV-SNCA + AAV-GDNF mice; however, there were no statistically differences in D1R expression [F(3, 16) = 0.9903, P = 0.5205 vs. AAV-SNCA + AAV-NC2] (Fig. 4E, I).
The overexpression of GDNF counteracted the impact of α-syn aggregation on protein expression levels in both the VTA and NAc. (A-D) WB analysis of p-α-syn, GDNF and TH in the VTA (n = 5). (E-I) WB analysis of SNAP-25, PSD95, D2R and D1R in the NAc (n = 5). Data: Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA with Tukey’s test).
GDNF reversed the attenuation of VTA-to-NAc dopamine transmission due to α-syn aggregation
To further substantiate the involvement of GDNF in dopamine transmission from the VTA to the NAc, DAT-cre mice were subjected to stereotactic embedding of cannulae in the VTA, and then sequentially injected with AAV-SNCA, AAV-GDNF, and cis-tracer virus (AAV-hSyn) (Fig. 5A). A significant decrease in fluorescence intensity of cis-tracer virus (marked in green) within the VTA [F(2, 15) = 47.48, P < 0.0001 vs. PBS + hSyn] and NAc [F(2, 15) = 148.2, P < 0.0001 vs. PBS + hSyn] in the AAV-SNCA + hSyn mice compared to the PBS + hSyn mice. However, the fluorescence intensity of cis-tracer viruses in both VTA [F(2, 15) = 47.48, P = 0.002 vs. AAV-SNCA + hSyn] and NAc [F(2, 15) = 148.2, P = 0.0003 vs. AAV-SNCA + hSyn] was significantly increased in AAV-GDNF + hSyn mice compared to AAV-SNCA + hSyn mice (Fig. 5B, C).
Furthermore, in the NAc, IF staining results (Fig. 5D-G) for the co-localization of GAD67 (a biomarker for MSN) and SYN (cis-tracer virus) showed a significant reduction in the AAV-SNCA + hSyn mice compared to the PBS + hSyn mice [F(2, 15) = 31.62, P < 0.0001 vs. PBS + hSyn]; whereas, compared to the AAV-SNCA + hSyn mice, an increase was observed in the AAV-GDNF + hSyn mice [F(2, 15) = 31.62, P = 0.004 vs. AAV-SNCA + hSyn].
The overexpression of GDNF ameliorated the compromised DA transmission from the VTA to the NAc caused by α-syn aggregation. (A) Experimental timeline. (B) The results for the localization of the cis-tracer virus (green) in both the VTA and NAc of mice (scale bars: 100/200/500 µm), as well as the corresponding statistic diagram (C) (n = 6). (D-F) GAD67 (red) and SYN (green) co-localization in the NAc (scale bars: 100 μm), as well as the corresponding statistic diagram (G) (n = 6). Data: Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA with Tukey’s test).
The diminished D2-MSN activation in the NAc alleviates depression-like behaviour due to α-syn aggregation
The D2-cre mice were subjected to stereotaxic injections of AAV-SNCA into the VTA and Cre-dependent chemical genetic virus into the NAc (Fig. 6A). The IF results demonstrated accurate viral injection of chemical genetic virus in the NAc (Fig. 6B). OFT results before and after intraperitoneal injection showed improved locomotor activity in the hM3Di group mice and deteriorated activity in the hM4Dq group mice only after CNO injection, according to total distance [hM3Di vs. hM3Di + CNO: Mean diff = −10.96, P = 0.0094; hM4Dq vs. hM4Dq + CNO: Mean diff = 11.17, P = 0.0021] (Fig. 6C), frequency of grid crossings [hM3Di vs. hM3Di + CNO: Mean diff = −24.33, P = 0.0003; hM4Dq vs. hM4Dq + CNO: Mean diff = 29.33, P = 0.0021] (Fig. 6D), and time spent in the central area [hM3Di vs. hM3Di + CNO: Mean diff = −12.42, P = 0.0039; hM4Dq vs. hM4Dq + CNO: Mean diff = 12.71, P = 0.0003] (Fig. 6E). The findings were observed in the in the TST [hM3Di vs. hM3Di + CNO: Mean diff = 26.63, P = 0.0033; hM4Dq vs. hM4Dq + CNO: Mean diff = −34.47, P = 0.0055] (Fig. 6F) and FST [hM3Di vs. hM3Di + CNO: Mean diff = 30.27, P = 0.0144; hM4Dq vs. hM4Dq + CNO: Mean diff = −28.68, P = 0.0004] (Fig. 6G).
In addition, among different groups following intraperitoneal injection, the OFT results showed that the hM3Di + CNO group mice showed the best performance, while the hM4Dq + CNO group mice exhibited the worst performance, in terms of total distance [F(5, 30) = 14.44; NC + CNO vs. hM3Di + CNO: Mean diff = −12.1, P = 0.0014; NC + CNO vs. hM4Dq + CNO: Mean diff = 10.56, P = 0.0064] (Fig. 6H), frequency of crossing the grid [F(5, 30) = 14.08; NC + CNO vs. hM3Di + CNO: Mean diff = −25.67, P = 0.0025; NC + CNO vs. hM4Dq + CNO: Mean diff = 24.83, P = 0.0036] (Fig. 6I), and time spent in the central region [F(5, 30) = 16.74; NC + CNO vs. hM3Di + CNO: Mean diff = −14.02, P = 0.0002; NC + CNO vs. hM4Dq + CNO: Mean diff = 10.77, P = 0.0053] (Fig. 6J). Furthermore, the similar findings were observed in the TST [F(5, 30) = 16.70; NC + CNO vs. hM3Di + CNO: Mean diff = 28.73, P = 0.0037; NC + CNO vs. hM4Dq + CNO: Mean diff = −34.78, P = 0.0004] (Fig. 6K) and FST [F(5, 30) = 15.65; NC + CNO vs. hM3Di + CNO: Mean diff = 28.4, P = 0.0018; NC + CNO vs. hM4Dq + CNO: Mean diff = −28.65, P = 0.0016] (Fig. 6L).
The inhibition of D2-MSN in the NAc can improved depression-like behavior induced by α-syn aggregation. (A) Experimental timeline. (B) The representative diagram of the chemical genetic virus (red) in the NAc (scale bar = 200 μm). The statistic diagram for self-comparison before and after intraperitoneal injection in the OFT (C-E), TST (F) and FST (G) (n = 6, Data: Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001) (Paired-samples t-test). The statistic diagram for comparative results between different groups after intraperitoneal injection in the OFT (H-J), TST (K) and FST (L) (n = 6, Data: Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001) (one-way ANOVA with Tukey’s test).
Discussion
In this study, we reported that α-syn aggregation in PD leads to a reduction in DA transmission from VTA to NAc by downregulating DA levels, ultimately triggering depression-like behavior due to enhanced D2-MSN activation. Importantly, our findings reveal that GDNF can effectively inhibit α-syn aggregation within the VTA and subsequently alleviate depression-like behaviors in PD mice.
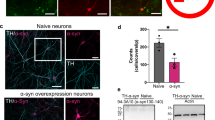
Depression is one of the early non-motor symptoms in patients with PD, and clinical studies have shown a positive correlation between the level of expression of the α-syn mutant gene SNCA in peripheral blood and severity of depression in PD patients25,26. Under physiological conditions, α-syn is believed to play an important role in DA transmission, whereas under pathological conditions α-syn aggregates to form Lewy bodies and loses its normal function27,28. Although, a large number of studies have demonstrated the association between α-syn and the development of mood disorders such as depression, the precise pathogenesis remains unclear29,30. The midbrain VTA is one of the major brain regions that produce DA and is involved in the regulation of mental and emotional changes in PD. In this study, we established a PD model by inducing α-syn aggregation in the VTA of mice. These PD mice exhibited depression-like behaviors, suggesting that SNCA overexpression in the VTA induces the α-syn aggregation and leads to the development of depression-like behaviors (Fig. 1B-H), which is consistent with the reported findings29. The aggregation of α-syn leads to internalization of N-methyl-D-aspartic acid receptor (NMDAR) subunit NR1 on the surface of DA neurons cultured in vitro, which is necessary for the assembly and function of NMDAR31. The impaired NMDAR function caused by α-syn aggregation may reduce the presynaptic calcium influx through the NMDAR and affects the calcium-independent regulation of calmodulin kinase32thereby reducing the synthesis of TH. The aforementioned studies provide robust support for our finding that our PD mice showed a significant aggregation of α-syn as well as a decrease in the expression of TH in the VTA. Similarly, reductions in TH expression and DA levels were observed after addition of pathologically aggregated α-syn to DA neurons in vitro (Supplementary Fig. 1).
The NAc, a brain region downstream of the VTA, contains over 95% of MSN, and its synaptic plasticity is involved in depression. Previous studies have shown that α-syn binds to vesicles located at neuronal synaptic terminals, thereby regulating the assembly of synapse-associated proteins33. Conversely, α-syn aggregation disrupts the assembly of synapse-associated proteins and impairs the transport of synaptic neurotransmitter vesicles34. In this study, the expression levels of synapse-associated proteins (e.g., SNAP-25, DAT and PSD95) in the NAc were significantly decreased after α-syn aggregation in the VTA (Fig. 2F-I), echoing the significant reduction of DA in both regions (Fig. 2E).
The role of DAT protein is to actively transport excessive DA from the synaptic gap back into the intracellular compartment, thereby ensuring the normal physiological function of synapses35. Erica et al. showed that 10-month-old transgenic mice expressing A53T mutation in α-syn exhibited decreased DAT expression and impaired DA uptake in the NAc as well as in the striatum36which is in agreement with the reduction of DAT protein in the NAc after α-syn aggregation in the VTA detected by the present study (Fig. 2A-C, F and I). α-syn may reduce DA uptake by decreasing DAT activity, leading to a further decrease in tissue DA levels. Our results from cis-tracer virus and IF assay showed diminished transmission of DA from the VTA to the NAc in PD mice (Fig. 5B, C).
The role of DA is exerted via its receptors. The majority of MSN in the NAc express D1R and D2R, and numerous studies have shown that DA transmission plays an important role in depression37,38. Consistent with our results, α-syn aggregation in the VTA of PD model mice results in a decrease in DA levels and thus depression-like behaviors. Furthermore, there was a significantly reduction observed in D2R expression within the NAc of PD mice, while no difference was detected in D1R (Fig. 2J, K). D1R and D2R play activating and inhibitory roles respectively, and the balance of the two is disrupted when DA-ergic fibers are depleted of their projections, leading to distinct alterations39. In the present study, we propose that a decrease in DA level in the NAc caused by α-syn aggregation in the VTA, results in enhanced activation of D2-MSN, followed by abnormal neurotransmitter release from the next level of interneurons, ultimately leading to depression-like behaviors. Therefore, we further validated the role of D2-MSN in NAc in regulating depression-like behaviors using D2-cre mice and chemogenetic viruses. In this study, significant behavioral differences were observed in the activation or inhibition of D2-MSN within the NAc only when α-syn aggregation (mice injected with AAV-SNCA into the VTA) was induced in the VTA; while, no such differences were detected in the control mice (mice injected with AAV-NC1 into the VTA) (Supplementary Fig. 2). The results showed that when D2-MSN was inhibited in the NAc of PD mice, depression-like behaviors improved; however, when D2-MSN was activated, depression-like behaviors worsened (Fig. 6). It has been suggested in the literature that inhibition of D2R leads to triggering mood disorders40. Thus, in the NAc, D2-MSN is mainly involved in regulating depression-like behaviors.
Currently, drugs used in the treatment of depression include 5-hydroxytryptamines, tricyclic antidepressants and DA reuptake inhibitors41,42. However, the use of these drugs may exacerbate pre-existing movement disorders in PD patients or lead to other harmful side effects43. The studies have shown that GDNF has a protective effect on DA neurons24,44and lower levels of GDNF have been detected in the serum of PD patients compared to those of normal subjects45,46. Therefore, the present study investigated whether GDNF could improve the depression-like behavior caused by the pathological α-syn aggregation. The behavioral results showed that GDNF could improve the depression-like behaviors in PD mice (Fig. 3B-H), along with an increase in TH expression and a reduction in α-syn aggregation in the VTA (Fig. 4A, D). Consistent with previous findings, the addition of GDNF to in vitro cultured DA neurons inhibited further aggregation of exogenously α-syn pre-formed fibrils (PFF)24. In addition, the expression of synapse-associated proteins, such as SNAP-25 and PSD95, were increased, which could be attributed to GDNF inhibiting α-syn aggregation and thereby improving DA neurons survival (Fig. 4A-G). Furthermore, GDNF significantly enhanced the expression of D2R within the NAc of PD mice, but had no effect on D1R (Fig. 4A, H, I). Moreover, the results from IF showed that overexpression of GDNF increased the number of co-labeling of DA synaptic endings in the NAc with the MSN marker GAD67 (Fig. 5D-G). In summary, GDNF enhanced the transmission of DA originating from the VTA to the NAc and promoted the effect of DA on the D2-MSN in the NAc.
Although the VTA-NAc pathway plays a critical role in the regulation of motivation and reward processing—domains that are central to the experience of anhedonia and apathy, which are hallmark features of depression in PD—it is essential to acknowledge that PD-related depression represents a complex, multifactorial syndrome that cannot be fully explained by mesolimbic dopaminergic dysfunction alone. Notably, prefrontal-limbic dysregulation, which is driven by serotonergic and noradrenergic deficits in the raphe nuclei and locus coeruleus, may underlie affective symptoms such as emotional blunting and cognitive bias47. Furthermore, hyperactivity of the HPA axis, evidenced by elevated cortisol levels, may enhance dopaminergic vulnerability and disrupt the functionality of mood-related brain regions48. Thus, while VTA-NAc disruption may be sufficient to induce depression-like behaviors in animal models, human PD-related depression likely involves the interplay of multiple systems.
In conclusion, our work confirmed that GDNF can improve the depression-like behavior of PD mice by inhibiting α-syn aggregation in the VTA and promoting DA transmission from the VTA to the NAc (Fig. 7). The present study helps us understand the pathological role of α-syn in PD and provides a foundation for the clinical treatment of depression in PD. While this study provides novel insights into α-syn-mediated dopaminergic dysfunction in PD-associated depression, several limitations warrant consideration. The viral vector-based approach for α-syn overexpression, while spatially precise in targeting VTA neurons, lacks comprehensive controls for potential off-target. Restricting pathology to the VTA does not comprehensively replicate the systemic neurodegeneration observed in PD. And the depression-improving effect of GDNF was not further validated using chemogenetic viruses in this study. Notably, this study specifically focused on the VTA-NAc pathway’s role in depression pathophysiology and did not investigate other neural circuits involved in depression neurobiology. In future research, we will refine the above-mentioned studies to conduct further investigations into specific mechanisms, thereby enhancing our understanding of this field.
Materials and methods
Animals
Eight-week-old male C57BL/6J and D2-Cre mice were obtained from Jiangsu Nanjing GemPharmatech Co., Ltd. (License No. SYXK(Su)2018-0008), while DAT-Cre mice were purchased from Jiangsu Taicang Cyagen Biosciences Inc. (License No. SCXK(Su)2018-0003). All mice were maintained in a controlled environment at the Laboratory Animal Center of Xuzhou Medical University, with adjustable ambient temperature (23 ± 1.5 °C) and humidity (45 ± 15%), and a 12 h light-dark cycle (19:00 pm to 7:00 am). Experiments were performed using 8-12-week-old mice. All mice were acclimatized to this environment for a minimum of 7 days prior to the experiment. In the experiment, six or more mice were assigned to each group. Mice were euthanized via inhalation of a lethal dose of CO2. Animal experimentation procedures were conducted in accordance with the guidelines for the Care and Use of Laboratory Animals developed by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Xuzhou Medical University (approval number: 202209S042). All animal experiments were conducted in strict accordance with the “Guiding Opinions on the Treatment of Laboratory Animals” promulgated by the Ministry of Science and Technology of China. All methods were performed in accordance with relevant guidelines and regulations. Animal studies were reported in accordance with ARRIVE guidelines.
Mouse brain stereotactic injection of adeno-associated virus (AAV)
Mice were deeply anesthetized with sodium pentobarbital (45 mg/kg) (merck, cat#57-33-0). According to The Mouse Brain in Stereotaxic Coordinates (Second Edition), the anesthetized mice were immobilized on a stereotaxic apparatus for unilateral injections into the VTA (AP: −2.92 mm, ML: 0.45 mm, DV: −4.4 mm) or NAc (AP: 1.5 mm, ML: 0.75 mm, DV: −4.5 mm) using a trace syringe connected to a micro-injection pump. Mice were unilaterally injected with 0.3 µl of phosphate-buffered saline (PBS), AAV-SNCA (BrainVTA, cat#GT-0072, AAV2/9, 5.63E + 1.3 V.G./ml), AAV-NC1 (BrainVTA, cat#PT-0142, AAV2/9, 5.33E + 1.3 V.G./ml), AAV-GDNF (OBiO, Cat# Y12419, AAV2/9, 1.16E + 1.3 V.G./ml), AAV-NC2 (OBiO, Cat# Y9957, AAV2/9, 1.55E + 1.3 V.G./ml), or a mixed virus solution (in a ratio of 1 : 1) individually. The micro-syringe was carefully inserted into the VTA or NAc, allowing for a stabilization period of 2 min. Then, solution was delivered at a rate of 0.06 µl per minute, allowing for a stabilization period of 5 min. Subsequently, the micro-syringe was gradually withdrawn, and the incision site on the scalp was sutured. After the surgical procedure, the mice were housed in a controlled warm environment and provided with peanuts as nutritional supplements. The behavioral experiments were performed after stable expression of the virus (approximately four weeks), followed by the perfusion and histology procedure.
The use of cis-tracer virus
Firstly, brain stereotaxic cannula was surgically implanted the VTA of DAT-cre mice at coordinates AP: −2.92 mm, ML: 0.45 mm, DV: −4.4 mm; subsequently, AAV-SNCA (α-syn mutant gene) and AAV-NC1 (negative control) were administered via the cannula. On the second day, PBS, AAV-GDNF, and AAV-NC2 were also administered via the cannula. On the third day, anterograde tracer (AAV-hSyn: BrainVTA, Cat# PT-1168) were also administered via the cannula. After a period of 4 weeks, the distribution of enhanced green fluorescent protein (EGFP) in the VTA and NAc were assessed using the Olympus BX43 microscope (Tokyo, Japan).
The use of chemogenetic virus
First, D2-cre mice were brain stereotaxic injection of AAV-SNCA in the VTA (AP: −2.92 mm, ML: 0.45 mm, DV: −4.4 mm), as well as cre-dependent chemogenetic virus AAV-hM3Dq (BrainVTA, cat# PT-0042), AAV-hM4Di (BrainVTA, cat# PT-0043) and control virus AAV-mCherry (BrainVTA, cat# PT-0013) in the NAc region (AP: 1.5 mm, ML: 0.75 mm, DV: −4.5 mm). After 4 weeks, mice in each group were injected with Clozapine N-oxide (CNO) (ApexBio, cat# A3317) or normal saline to directly activate or inhibit D2-MSN in NAc, and depression was evaluated by open field test, forced swimming test and tail suspension test within one hour.
Open field test (OFT)
Mice in Each group were subjected to OFT at postoperative week 4, and the degree of depression in mice was assessed by their distance traveled, number of grid crossing, and time spent in the center of the field. After setting up the experimental box, the ANY-maze software 7.33 (Stoelting Co., Wood Dale, IL, USA) was used to divide the site into 16 small squares, of which the middle 4 squares were the central area and the rest were the peripheral areas. After a 30-minute of acclimatization period, the labeled mice were placed sequentially into the center area of the field (disinfect the box with 75% alcohol after each test), while the video recording was started for 5 min.
Forced swimming test (FST)
Mice in Each group underwent FST at postoperative week 4, and the degree of depression in mice was assessed by the time spent floating in the water. The mice were acclimatized to the environment for 30 min before the experiment. The mice were subsequently placed in a plastic cylindrical tank (25 cm in height and 12 cm in diameter) filled with water (20 cm in height), while the water temperature was maintained at 23–25 °C for a total of 6 min under dim-light conditions (white light). At the same time, time of immobility of the mice in the water was recorded and videotaped using ANY-maze software 7.33 (dry the mice after each test). Mice immobility is defined as that mice cease struggling only exerting effort to keep their heads above the water.
Tail-suspension test (TST)
Mice in each group were subjected to TST at postoperative week 4, the degree of depression in mice was assessed by the time the mice give up struggling to remain immobile. After a 30-minute of acclimatization period, the labeled mice were tied to the end of a wire horizontally positioned, 1 cm from the tail, so that the mice were suspended upside down on the wire with their heads approximately 15 cm above the ground. Each group of mice was separated by a white baffle. The mice were tail-suspended for 6 min, while recorded and videotaped using ANY-maze software.
Protein extraction and western blot (WB)
After the mice were sacrificed, the entire brain was carefully stripped and placed on ice. The VTA and NAc regions were accurately excised unilaterally using a scalpel, with the fontanelle serving as a reference point. Protease inhibitor (KeyGEN, Cat# KGP610) was then added to the lysis buffer (Beyotime, Cat# P0013B) and the tissue was homogenized and then centrifuged (12,000 r/min, 20 min) at 4 °C to obtain the supernatant. Protein concentration in the supernatant was quantified by BCA Protein Assay (KeyGEN, Cat# KGP902). The optical density values were quantified using Multi-functional Microplate Tester (BioTek, Hercules, CA, USA).
Equal quantities of protein from each group were separated by electrophoresis using sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE), followed by transferred onto nitrocellulose membrane (BioTrace, Cat# P/N66485). The membranes were closed for 2 h at room temperature (20–25 °C) with 5% skimmed milk, which subsequently, were incubated overnight at 4 °C with specified primary antibodies (α-syn, Abcam, Cat# ab138501; TH, Santa, Cat# sc-374048; p-α-syn, Thermo Fisher Scientific, Cat# PA5-37740; synaptosomal-associated protein 25 (SNAP-25), Santa, Cat# sc-20038; postsynaptic density protein 95 (PSD95), CST, Cat# 3450 S; D2R, Santa, Cat# sc-5303; D1R, CST, Cat# #79777T; DAT, Santa, Cat# sc-32259; GDNF, Affinity, Cat# DF7727; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), huabio, Cat# ET1601-4; β-actin, Proteintech, Cat# 66009-l-lg). The membranes were washed three times with wash buffer, and then incubated with corresponding secondary antibodies (goat anti-rabbit IgG secondary antibody: Cat# 926-32211, goat anti-mouse IgG secondary antibody: Cat# 926-68070, LI-COR, USA) for 2 h at room temperature. After three washes, protein expression on the membrane was detected using a dual infrared laser imaging system (Odyssey CLX, LI-COR, Inc., Lincoln, NE, USA), and analyzed using ImageJ software.
Freezing slices and immunofluorescence (IF)
Mice were anesthetized with pentobarbital sodium (45 mg/kg) and subsequently perfused with pre-cooled normal saline to thoroughly remove all blood from the circulatory system. Following blood removal, the tissue was perfused with 4% paraformaldehyde for fixation. The brains were subsequently postfixed for 24 h with 4% paraformaldehyde and then transferred to a 30% sucrose solution. Use a cryogenic slicing system (CM1950, Leica, Wetzlar, Germany) to cut the frozen brain into 20 μm coronal sections at −20 °C. Brain sections were glued onto slides, dried at room temperature and stored in a slide box at −20℃. The sections were incubated overnight at 4 °C with the corresponding primary antibodies (TH, Santa, Cat# sc-374048; p-α-syn, Millipore, Cat# MABN826; and glutamate decarboxylase 67 isoforms (GAD67), Abclonal, cat# A2938). The following day, after threes tims washes, the sections were incubated with secondary fluorescent antibodies (Alexa Fluor 488, Abcam, Cat# ab150077;Alexa Fluor 594, Abcam, Cat# ab150120) at room temperature. Then they were washed more three times and stained with 4’,6-diamidino-2-phenylindole (DAPI) for 10 min. All image acquisition is performed using the Olympus BX43 microscope (Tokyo, Japan).
Enzyme-linked immunosorbent assay (ELISA)
The samples for the assay were pre-thawed at room temperature, and the standards were diluted with ddH2O to different concentrations as instructed by the ELISA kit (DA ELISA Kit [mice]: Cat# NM-0355R2, MEIMIAN, Yancheng, Jiangsu, China). The standard wells were each added 50 µL of different concentrations of standards, while the blank and sample wells were prepared separately. The test sample (10 µL) and sample diluent (40 µL) were added to the designated wells, followed by the addition of enzyme reagent (100 µL) to each wel excluding blank wells. The mixtures were then incubated at 37℃ for 60 min. After washing, colorant A (50 µL) and colorant B (50 µL) were added sequentially to each well for the reaction. The reaction was carried out at 37℃ for 15 min, and terminated by adding termination solution (50 µL). The optical density values of each well were measured using Multi-functional Microplate Tester (BioTek, Hercules, CA, USA).
Statistical analysis
The experimental data were analyzed using SPSS 22.0 (IBM SPSS Inc., Chicago, IL, USA), and the quantitative data were presented as mean ± standard errors. The data were assessed for the homogeneity of variance using Levene’s test, as well as for normality using Shapiro-Wilk tests. If the data met the assumptions of the parametric test, comparisons between two groups utilized Student’s t-test, whereas comparisons among multiple groups employed one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. The criterion for determining a statistically significant difference was set at P < 0.05. The statistical graphs were generated using GraphPad 8.0.2 and Adobe Illustrator 23.0.3 (Adobe, San Jose, CA, USA).
Data availability
All data generated or analysed during this study are included in this published article.
References
Hayes, M. Parkinson’s disease and parkinsonism. Am. J. Med. 132, 802–807. https://doi.org/10.1016/j.amjmed.2019.03.001 (2019).
Assogna, F., Cravello, L., Caltagirone, C. & Spalletta, G. Anhedonia in parkinson’s disease: a systematic review of the literature. Mov. Disord. 26, 1825–1834. https://doi.org/10.1002/mds.23815 (2011).
Assogna, F. et al. Drug choices and advancements for managing depression in parkinson’s disease. Curr. Neuropharmacol. 18, 277–287. https://doi.org/10.2174/1570159X17666191016094857 (2020).
Arnaud, A. M. et al. Impact of major depressive disorder on comorbidities: A systematic literature review. J. Clin. Psychiatry. 83, 21r14328. https://doi.org/10.4088/JCP.21r14328 (2022).
Hori, H. & Kunugi, H. Dopamine agonist-responsive depression. Psychogeriatrics 13, 189–195. https://doi.org/10.1111/psyg.12014 (2013).
Lobo, M. & Nestler, E. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front. Neuroanat. 5, 41. https://doi.org/10.3389/fnana.2011.00041 (2011).
Douma, E. & de Kloet, E. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci. Biobehav Rev. 108, 48–77. https://doi.org/10.1016/j.neubiorev.2019.10.015 (2020).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536. https://doi.org/10.1038/nature11713 (2013).
Nieoullon, A. & Coquerel, A. Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr. Opin. Neurol. 16 (Suppl 2), S3–9 (2003).
Renard, C., Fiocco, A., Clenet, F., Hascoet, M. & Bourin, M. Is dopamine implicated in the antidepressant-like effects of selective serotonin reuptake inhibitors in the mouse forced swimming test? Psychopharmacol. (Berl). 159, 42–50. https://doi.org/10.1007/s002130100836 (2001).
Pallis, E., Thermos, K. & Spyraki, C. Chronic Desipramine treatment selectively potentiates somatostatin-induced dopamine release in the nucleus accumbens. Eur. J. Neurosci. 14, 763–767. https://doi.org/10.1046/j.0953-816x.2001.01698.x (2001).
Song, N. et al. NAc-DBS corrects depression-like behaviors in CUMS mouse model via disinhibition of DA neurons in the VTA. Mol. Psychiatr. 29, 1550–1566. https://doi.org/10.1038/s41380-024-02476-x (2024).
Sesack, S. & Grace, A. Cortico-Basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47. https://doi.org/10.1038/npp.2009.93 (2010).
Meredith, G. The synaptic framework for chemical signaling in nucleus accumbens. Ann. N Y Acad. Sci. 877, 140–156. https://doi.org/10.1111/j.1749-6632.1999.tb09266.x (1999).
Qiao, H., Yang, S., Xu, C., Ma, X. & An, S. Involvement of D2 receptor in the NAc in chronic unpredictable stress-induced depression-like behaviors. Stress 23, 318–327. https://doi.org/10.1080/10253890.2019.1673361 (2020).
Qin, Y. et al. Repeated Inhibition of sigma-1 receptor suppresses GABAA receptor expression and long-term depression in the nucleus accumbens leading to depressive-like behaviors. Front. Mol. Neurosci. 15, 959224. https://doi.org/10.3389/fnmol.2022.959224 (2022).
Ishiguro, M. et al. Increased serum levels of α-Synuclein in patients with major depressive disorder. Am. J. Geriatric Psychiatry. 27, 280–286. https://doi.org/10.1016/j.jagp.2018.10.015 (2019).
Lou, H. et al. Serine 129 phosphorylation reduces the ability of α-Synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J. Biol. Chem. 285, 17648–17661. https://doi.org/10.1074/jbc.M110.100867 (2010).
Jeannotte, A., McCarthy, J. & Sidhu, A. Desipramine induced changes in the norepinephrine transporter, alpha- and gamma-synuclein in the hippocampus, amygdala and striatum. Neurosci. Lett. 467, 86–89. https://doi.org/10.1016/j.neulet.2009.10.010 (2009).
Lara-Rodarte, R. et al. Mouse embryonic stem cells expressing GDNF show enhanced dopaminergic differentiation and promote behavioral recovery after grafting in parkinsonian rats. Front. Cell. Dev. Biol. 9, 661656. https://doi.org/10.3389/fcell.2021.661656 (2021).
Tang, C. X. et al. Blunt dopamine transmission due to decreased GDNF in the PFC evokes cognitive impairment in parkinson’s disease. Neural Regen Res. 18, 1107–1117. https://doi.org/10.4103/1673-5374.355816 (2023).
Yang, Y. et al. The association of decreased serum Gdnf level with hyperglycemia and depression in type 2 diabetes mellitus. Endocr. Pract. 25, 951–965. https://doi.org/10.4158/EP-2018-0492 (2019).
Ozkan, B. N. et al. Altered blood parameters in major depression patients receiving repetitive transcranial magnetic stimulation (rTMS) therapy: a randomized case-control study. Transl Psychiatry. 14, 264. https://doi.org/10.1038/s41398-024-02942-8 (2024).
Chmielarz, P. et al. GDNF/RET signaling pathway activation eliminates lewy body pathology in midbrain dopamine neurons. Mov. Disord. 35, 2279–2289. https://doi.org/10.1002/mds.28258 (2020).
Brazdis, R. M., von Zimmermann, C., Lenz, B., Kornhuber, J. & Muhle, C. Peripheral upregulation of parkinson’s Disease-Associated genes encoding alpha-Synuclein, beta-Glucocerebrosidase, and ceramide glucosyltransferase in major depression. Int. J. Mol. Sci. 25, 3219. https://doi.org/10.3390/ijms25063219 (2024).
Rotter, A. et al. Alpha-Synuclein RNA expression is increased in major depression. Int. J. Mol. Sci. 20, 2029. https://doi.org/10.3390/ijms20082029 (2019).
Sulzer, D. & Edwards, R. H. The physiological role of alpha-synuclein and its relationship to parkinson’s disease. J. Neurochem. 150, 475–486. https://doi.org/10.1111/jnc.14810 (2019).
Benskey, M. J., Perez, R. G. & Manfredsson, F. P. The contribution of alpha synuclein to neuronal survival and function - Implications for parkinson’s disease. J. Neurochem. 137, 331–359. https://doi.org/10.1111/jnc.13570 (2016).
Xia, D. et al. Chronic stress induces depression-like behaviors and parkinsonism via upregulating alpha-synuclein. NPJ Parkinsons Dis. 11, 139. https://doi.org/10.1038/s41531-025-00998-x (2025).
Miquel-Rio, L., Sarries-Serrano, U., Pavia-Collado, R., Meana, J. J. & Bortolozzi, A. The role of alpha-Synuclein in the regulation of serotonin system: physiological and pathological features. Biomedicines 11, 541. https://doi.org/10.3390/biomedicines11020541 (2023).
Yu, W., Yang, W., Li, X., Li, X. & Yu, S. Alpha-synuclein oligomerization increases its effect on promoting NMDA receptor internalization. Int. J. Clin. Exp. Pathol. 12, 87–100 (2019).
Desce, J., Godeheu, G., Galli, T., Glowinski, J. & Cheramy, A. Opposite presynaptic regulations by glutamate through NMDA receptors of dopamine synthesis and release in rat striatal synaptosomes. Brain Res. 640, 205–214. https://doi.org/10.1016/0006-8993(94)91874-0 (1994).
Burre, J. et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663–1667. https://doi.org/10.1126/science.1195227 (2010).
Wang, L. et al. alpha-synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr. Biol. 24, 2319–2326. https://doi.org/10.1016/j.cub.2014.08.027 (2014).
Jayaramayya, K. et al. Unraveling correlative roles of dopamine transporter (DAT) and parkin in parkinson’s disease (PD) - A road to discovery? Brain Res. Bull. 157, 169–179. https://doi.org/10.1016/j.brainresbull.2020.02.001 (2020).
Unger, E. et al. Locomotor hyperactivity and alterations in dopamine neurotransmission are associated with overexpression of A53T mutant human alpha-synuclein in mice. Neurobiol. Dis. 21, 431–443. https://doi.org/10.1016/j.nbd.2005.08.005 (2006).
Cabib, S. & Puglisi-Allegra, S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav Rev. 36, 79–89. https://doi.org/10.1016/j.neubiorev.2011.04.012 (2012).
Gershon, A., Vishne, T. & Grunhaus, L. Dopamine D2-like receptors and the antidepressant response. Biol. Psychiatry. 61, 145–153. https://doi.org/10.1016/j.biopsych.2006.05.031 (2007).
Meurers, B. et al. Dopamine depletion induces distinct compensatory gene expression changes in DARPP-32 signal transduction cascades of striatonigral and striatopallidal neurons. J. Neurosci. 29, 6828–6839. https://doi.org/10.1523/JNEUROSCI.5310-08.2009 (2009).
Zhang, T. et al. MPTP-Induced dopamine depletion in basolateral amygdala via decrease of D2R activation suppresses GABA(A) receptors expression and LTD induction leading to Anxiety-Like behaviors. Front. Mol. Neurosci. 10, 247. https://doi.org/10.3389/fnmol.2017.00247 (2017).
Hamon, M. & Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol. Biol. Psychiatry. 45, 54–63. https://doi.org/10.1016/j.pnpbp.2013.04.009 (2013).
Clevenger, S. S., Malhotra, D., Dang, J., Vanle, B. & IsHak, W. W. The role of selective serotonin reuptake inhibitors in preventing relapse of major depressive disorder. Ther. Adv. Psychopharmacol. 8, 49–58. https://doi.org/10.1177/2045125317737264 (2018).
Lee, M. Y., Hong, S., Kim, N., Shin, K. S. & Kang, S. J. Tricyclic antidepressants amitriptyline and Desipramine induced neurotoxicity associated with parkinson’s disease. Mol. Cells. 38, 734–740. https://doi.org/10.14348/molcells.2015.0131 (2015).
Kramer, E. R. & Liss, B. GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett. 589, 3760–3772. https://doi.org/10.1016/j.febslet.2015.11.006 (2015).
Tang, C. et al. Distinct serum GDNF coupling with brain structural and functional changes underlies cognitive status in parkinson’s disease. CNS Neurosci. Ther. 30, e14461. https://doi.org/10.1111/cns.14461 (2024).
Tong, S. Y. et al. Serum glial cell line-derived neurotrophic factor (GDNF) a potential biomarker of executive function in parkinson’s disease. Front. Neurosci. 17, 1136499. https://doi.org/10.3389/fnins.2023.1136499 (2023).
Bennett, M. R. The prefrontal-limbic network in depression: modulation by hypothalamus, basal ganglia and midbrain. Prog Neurobiol. 93, 468–487. https://doi.org/10.1016/j.pneurobio.2011.01.006 (2011).
Knezevic, E., Nenic, K., Milanovic, V. & Knezevic, N. N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells. 12, 2726. https://doi.org/10.3390/cells12232726 (2023).
Acknowledgements
We would like to express our gratitude to all members of the Public Experimental Research Center and Experimental Animal Center of Xuzhou Medical University. Additionally, this work was supported by grants from the National Natural Science Foundation of China (82101263 to Tang CX), Graduate Research and Innovation Projects of Jiangsu Province (KYCX20_2444 to Chen J) and Innovation and Entrepreneurship Training Programme for University Students (202410313036Z).
Author information
Authors and Affiliations
Contributions
T. C. and C.J. conceived the project and designed the study; C.J. and L.Z. wrote the manuscript; C.J., L.Z., L.M., X.W., C.S., S.L., M.Z. and W.C. performed the experiments and acquired animal research data; S.Y. provided scientific input and English- editing work; C.J., S.Y. an T.C. contributed to analysis. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animal experimentation procedures were conducted in accordance with the guidelines for the Care and Use of Laboratory Animals developed by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Xuzhou Medical University (approval number: 202209S042).
Consent for publication
All authors have approved the consent for publication of this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, J., Ling, Z., Lv, M. et al. GDNF attenuates a-synuclein aggregation-induced damage to VTA-NAc dopaminergic transmission and alleviates depression-like behaviors in mice. Sci Rep 15, 30804 (2025). https://doi.org/10.1038/s41598-025-16556-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16556-7