Abstract
Immune checkpoint combination therapy, particularly dual LAG-3/PD-1 blockade, demonstrates superior clinical efficacy over monotherapy in cancers like melanoma, yet its mechanistic synergy requires further validation. In this study, we established a cell co-culture model by co-culturing LAG-3+PD-1+ Jurkat cells induced by phytohemagglutinin (PHA) and human tumor cells with high expression of LAG-3 and PD-1 major ligands to characterize the combination effect of LBL-007 with anti-PD-1 antibodies and the mechanism of action in cancer immunotherapy. The results showed that the combination of LBL-007 and anti-PD-1 antibodies in the cell co-culture model enhanced the ability of activated Jurkat cells to kill tumor cells compared with monotherapy. Furthermore, this combination also inhibited the apoptosis of Jurkat cells and promoted IL-2, IL-10, and TNF secretion from Jurkat cells. Tumor cell death via apoptosis induced by activated Jurkat cells was observed, which was enhanced by combined LBL-007 and anti-PD-1 antibody treatment. The combination of LBL-007 and anti-PD-1 antibodies delayed tumor growth and promoted tumor cell apoptosis compared with monotherapy in human LAG-3 transgenic mice subjected to transplantation with colorectal tumor cells. Taken together, the combination of LBL-007 and anti-PD-1 antibodies plays an enhanced antitumor role by improving T cell viability and activity as well as by promoting T cell-induced apoptosis, thereby suggesting this combination as a potential effective strategy for cancer immunotherapy.
Similar content being viewed by others
Introduction
Immune checkpoint antagonists represent an important milestone in the field of cancer immunotherapy. These antagonists reverse T cell exhaustion in tumors by blocking the binding of immune checkpoints to ligands, and by inducing the reactivation of immune cells that consequently exert an antitumor effect1. Many new clinically effective immune checkpoint targets, such as TIM3, TIGIT, and LAG-3, have been discovered. Of these, LAG-3 has shown good therapeutic effects against various tumors and autoimmune diseases, thus being potentially applicable2,3,4. In March 2022, Opdualag has been a dual approved anti-PD1 and anti-LAG-3 combination which doubles the progression-free survival duration in melanoma5. This marks LAG-3 as the third immune checkpoint therapeutic target after PD-1 and CTLA-4. To date, more than 20 different LAG-3-targeted therapeutic drugs have been evaluated in more than 160 clinical trials worldwide (The data were from the website: https://www.clinicaltrials.gov). Clinical experiments have shown that drugs that block LAG-3 function have a good efficacy and safety profile6, being potentially effective in patients who had histologically confirmed locally advanced, unresectable or metastatic solid tumors and had exhausted available therapeutic options, especially when combined with drugs targeting PD-17. LAG-3 and PD-1 function as non-redundant inhibitory receptors on exhausted T cells, synergistically suppressing T-cell activation. Their co-expression amplifies T-cell dysfunction in tumors beyond what either checkpoint mediates alone. Blocking both receptors simultaneously rescues T-cell function more effectively than single blockade. The mechanisms underlying the synergistic effects of LAG-3 and PD-1 blockage have just recently been published. Unique clinical trial design (NCT03743766) data showed that combination of Relatlimab and Nivolumab enhanced the capacity for CD8+T cell receptor signaling and altered CD8+T cell differentiation, leading to heightened cytotoxicity8. Recently, a study analyzed the multiomic expression profiles obtained from all TCGA databases of cancer immune infiltrates and the proteome difference of PD-1/LAG-3 T-cells and found that CBL ubiquitin ligases can be key immunotherapy targets9. Furthermore, other studies have found that the combination of PD-1 and LAG-3 blockers broadens TCR clonality and enhance the enrichment of effector-like and interferon-responsive genes, resulting in enhanced IFN-γ release, which is indicative of functionality10. However, these mechanisms still need to be verified based on more equivalent data from the experiment on immune checkpoint antagonists.
In our previous work, we showed the novel anti-LAG-3 monoclonal antibody LBL-007, which is an immune checkpoint antagonist isolated from a human antibody phage display library11. LBL-007 has an antitumor effect and is currently used in clinical trials. However, the combination effect of LBL-007 with anti-PD-1 antibody and the mechanism used by these immune checkpoint antagonists to promote the clearance of tumor cells have not been thoroughly studied. In vitro cell co-culture models have been employed in the existing literature to investigate the effects of immune checkpoint antibodies or small molecule inhibitors12,13,14. Therefore, in this work, a cell co-culture model (co-culturing LAG-3+PD-1+ Jurkat cells induced by PHA and human tumor cells with high expression of LAG-3 and PD-1 major ligands) which we used in our previous work was verified the feasibility and then used to assess the antitumor effect of LBL-007 combined with commercially available anti-PD-1 antibodies in vitro, and to further explore its mechanism of action in vivo. The obtained results provide an experimental basis for the future construction of bispecific targeted antibody drugs and the optimization of clinical application strategies of related drugs.
Materials and methods
Cell lines
Human non-small cell lung cancer cell lines A549 and PC-9, human esophagogastric adenocarcinoma cell lines HGC-27 and MGC-803, and the human T-lymphoblastic leukemia Jurkat cell line were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Full details of the cell lines used are described in Supplementary Table 1. All cell lines were cultured in RPMI-1640 medium (Gibco, 8122321) supplemented with 10% fetal bovine serum (FBS) (Excell, 111017) and incubated at 37 °C in a 5% CO2 atmosphere.
Reagents and antibodies
Phytohemagglutinin (PHA) was obtained from Med Chem Express (MCE, L1668). Z-VAD-FMK was purchased from Sellcek (Sellcek, S7023). Antibodies specific for caspase-3 ((rabbit mAb, CST, 9662), cleaved caspase-3 (rabbit mAb, CST, 9664), PARP (rabbit mAb, CST, 9532), cleaved PARP (rabbit mAb, CST, 5625), LAG-3 (rabbit mAb, CST, 15372), and horseradish peroxidase (HRP)-conjugated secondary antibodies (CST, 7074) for Western blot analysis and immunocytochemistry were purchased from Cell Signaling Technology. Antibodies specific for PD-1 (mouse mAb, Abcam, ab52587), MHC-Ⅱ (rabbit mAb, Abcam, ab170867), Alexa Fluor®-647 conjugated goat anti-mouse secondary antibody (Abcam, ab150077), and Alexa Fluor® 488-conjugated goat anti-rabbit secondary antibody (Abcam, ab150115) for immunocytochemistry or flow cytometric analysis were purchased from Abcam (Cambridge, MA, USA). Antibodies specific for β-actin (mouse mAb, Proteintech, 66009) and PD-L1 (mouse mAb, Proteintech, 28076) for Western blot analysis were obtained from Proteintech.
Establishment of cell co-culture model and cell groups
Since LAG-3 and PD-1 are predominantly expressed on activated T cells, Jurkat cells were pretreated with 2 µg/mL PHA for 48 h to induce activation. Tumor cells were seeded in a 96-well plate at a density of 5 × 103 cells per well or in a 6-well plate at a density of 1 × 105 cells per well and incubated overnight until adhesion. Next, tumor cells were co-cultured with activated Jurkat cells at a ratio of 1:10 and stimulated with the antibodies listed below for 48 h as the co-culture model. The validity of this cell co-culture model has been described in our previous report15.
Tumor cells were divided into 5 groups; group 1: only tumor cells without Jurkat cells as a control group; groups 2–5: co-culture of tumor cells with activated Jurkat cells, in which group 2 was treated with isotype human IgG antibody (5 μg/mL); group 3 was treated with the anti-LAG-3 antibody LBL-007 (1 μg/mL); group 4 was treated with anti-PD-1 antibody BE0188 (BioXcell, BE0188) (5 μg/mL); and group 5 was treated with LBL-007 (1 μg/mL) and the anti-PD-1 antibody BE0188 (5 μg/mL).
Immunocytochemistry
The expression of LAG-3 and PD-1 in Jurkat cells and the expression of MHCⅡand PD-L1 in different tumor cell lines were detected by immunocytochemistry. Firstly, cells were seeded on glass slides, fixed with 4% paraformaldehyde for 15 min at room temperature, followed by washing three times (5 min each) in PBS (pH 7.4). The slides were permeabilized with 0.1% Triton X-100 for 5 min, and incubated in 10% normal donkey serum for 1 h to block nonspecific protein–protein interactions. Next, the slides were washed in PBS again and sections were incubated with the primary antibodies (LAG-3 and MHCⅡ were rabbit mAb; PD-1 and PD-L1 were mouse mAb)overnight at 4 °C, followed by the Alexa Fluor® 488-conjugated goat anti-rabbit secondary antibody (Green) and Alexa Fluor-647 conjugated goat anti-mouse secondary antibody (Red) for 1 h. Sections were then incubated in DAPI (Beyotime, C0131) to stain the cell nuclei (blue), sealed with nail polish to prevent drying and movement under the microscope, and visualized and analyzed under an OLYMPUS fv10i confocal laser scanning microscope (Olympus, Japan).
Cell Counting Kit-8 (CCK-8) assay
Tumor cells were seeded into a 96-well plate overnight and co-cultured with activated Jurkat cells at a ratio of 1:10 for 48 h. RPMI-1640 medium (100 µL) in each well was replaced after washing the cells three times with PBS. Cell viability was assessed using CCK-8 (Cat: CK04, Dojindo, Japan) as previously described12.
The antitumor effect of the targeted antibody by blocking the receptor ligand binding was further validated by a cell culture plate with 0.4 μm. Transwell inserts (Cat: #3460, Corning, United Kingdom) to prevent direct cell contact. Briefly, the tumor cells A549 and MGC-803 (1 × 105/well) were seeded overnight on the lower chamber, and activated Jurkat cells (1 × 106/well) were seeded in the upper chamber. Tumor cells were co-cultured with activated Jurkat cells at a ratio of 1:10 for 48 h. Then, tumor cells viability was assessed using the CCK-8 assay.
To evaluate if the loss of viability of tumor cells after the addition of LBL-007 and BE0188 in the co-culture model was due to apoptosis, tumor cells were seeded into a 96-well plate and treated with 50 μM Z-VAD-FMK (Selleck, S7023) overnight. Next, the medium was removed and activated Jurkat cells with fresh medium were added to the co-cultured model for 24 h and in the co-culture model, Jurkat cells were incorporated at a ratio of 20:1 relative to the tumor cells. Then, the viability was assessed using CCK-8 assay.
Wells with only tumor cells were used as a control group and wells with medium only were used as the blank background group. The in vitro cell viability rate was calculated using the formula: (Valuecontrol − Valuetest)/(Valuecontrol − Valueblank) × 100%.
Cytokine enzyme-linked immunosorbent assay (ELISA)
Tumor cells (1 × 105/well) were seeded in a 6-well plate overnight and then co-cultured with activated Jurkat cells (1 × 106/well) at a ratio of 1:10 for 48 h. The supernatant of the co-culture was collected and centrifuged at 1000 rpm for 5 min to remove the cells. Levels of IL-2 (DAKEWE, 1110202), IL-10 (DAKEWE, 1111002), and TNF-α (DAKEWE, 1117202) were measured with ELISA kits according to the manufacturer’s instructions.
Western blot analysis
A549 and MGC-803 cells were washed in PBS, lysed in RIPA lysis buffer (Beyotime, P0013) containing a protease and phosphatase inhibitor cocktail (Beyotime, P1005), and kept on ice for 10 min. The lysates were cleared by centrifugation (20,000g for 20 min at 4 °C) and the extracted total proteins were separated by 12% SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane. Membranes were blocked with 5% skim milk (BD, 232100) for 60 min at room temperature, incubated with primary antibodies (1:1000 dilution) overnight at 4 °C, washed three times with Tris-buffer solution-Tween 20 (10 mM Tris–HCl, pH 7.6, 150 mM NaCl, and 0.05% Tween-20), and incubated with HRP-conjugated secondary antibodies (1:1000 dilution). Immunoblotting and visualization of proteins by enhanced chemiluminescence (ECL) (Thermo Fisher Scientific, 34,080) were performed according to the manufacturer’s instructions.
Flow cytometry analysis
The expression of LAG-3 and PD-1 in Jurkat cells was evaluated by collecting all cells and centrifuged at 1000 rpm for 5 min, washed three times with PBS (pH 7.4), and incubated for 60 min with 100 μL LAG-3 (Rabbit mAb) or PD-1 (Mouse mAb) primary antibody diluted in PBS. Cells were washed twice with PBS to remove excess unbound primary antibody and incubated for 30 min in the dark with 100 μL Alexa Fluor® 488 conjugated secondary antibody or Alexa Fluor® 647 conjugated secondary antibody. Cells were washed twice with PBS and single-cell suspensions were examined using a BD LSR Fortessa X20. The percentage of positive cells was calculated by FlowJo Version 10.9. Unstained and isotype control IgG stains were only used as compensation controls.
The Annexin V/propidium iodide (PI) double-staining assay was employed to detect cell apoptosis. Firstly, collected the cells from the upper layer of the co-culture model and centrifuged at 1000 rpm for 5 min, washed three times with PBS. According to the protocol of the Annexin V-FITC/PI cell apoptosis detection kit (Miltenyi Biotec, 130-092-052), Annexin V-FITC (10 μL) solution and PI (5 μL) dye were added to the cell culture dish and incubated for 30 min in the dark at 37 °C, flow cytometer was used to analyze the Jurkat cells.
RNA extraction and RT-qPCR
Total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, 15596018), reverse transcribed into cDNA using the PrimeScript™ reverse transcription reagent kit (TaKaRa, 639505) and subsequently used for qPCR analysis. qPCR was performed on a LightCycler instrument (Thermal Cycler) using the TB Green® Premix Ex Taq™ qPCR kit (TaKaRa, RR036A). Oligonucleotide primers (Supplementary Table 2) used were collected from PrimerBank (https://pga.mgh.harvard.edu/primerbank/). The cycling conditions were set according to the manufacturer’s instructions. Gene expression was normalized to β-actin expression using the 2−ΔΔCt method and expressed as the fold change compared to the control.
Animals
Fifty-six female C57BL/6-hLAG-3 transgenic mice, 6-week-old, weighing 25–30 g, were purchased from Shanghai Model Organisms Center, Inc. (Shanghai, China).
Animal experiments
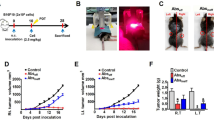
C57BL/6-hLAG-3 transgenic mice expressing human LAG-3 and mouse PD-1 were housed under 12-h light/dark cycles, a controlled temperature (20–26 °C), and humidity (30–70%), with free access to pathogen-free mouse chow and sterile drinking water ad libitum. The colon adenocarcinoma tumor model was obtained by treating female C57BL/6-hLAG-3 transgenic mice with a subcutaneous injection of MC38-OVA cells (1 × 106) into the right flank. A caliper was used to measure the length (L), width (W), and height (H) of the tumor twice a week and the volume was calculated as follows: L × W × H/2. When the average tumor volume reached ~ 80 mm3, mice were randomly divided into 7 groups (n = 8 mice per group) and treated with an intraperitoneal injection of antibodies twice a week. Groups 1 to 7 were treated with the isotype human IgG control (10 mg/kg), LBL-007 (four doses per group of 0.3 mg/kg, 1 mg/kg, 3 mg/kg, and 10 mg/kg), BE0146 (BioXcell, BE0146, anti-mouse PD-1 antibody, 10 mg/kg), and a combination of BE0146 (10 mg/kg) and LBL-007 (10 mg/kg). On day 31, mice were anesthetized via intraperitoneal injection of pentobarbital sodium (40 mg/kg), euthanized by cervical dislocation, and tumors were collected for immunohistochemical analysis.
Statistical analysis
Statistical analysis was performed using SPSS statistical software for Windows, version 26.0. Two groups were compared using a two-tailed Student’s t-test, while multiple groups were compared using one-way analysis of variance (ANOVA) with a Tukey’s post hoc test. All experiments were performed at least in triplicate, and the results were presented as the mean ± standard deviation. P ≤ 0.05 was considered statistically significant.
Results
Establishment of a cell co-culture model
Since T cells are cytotoxic to tumor cells, different cell lines were selected to establish a cell co-culture model to test the combination effect of anti-LAG-3 antibody and anti-PD-1 antibody on the antitumor activity of T cells against tumor cells in vitro. PHA was used to stimulate Jurkat cells and it was evaluated if LAG-3 and PD-1 expression were induced16. The results revealed that Jurkat cells pre-treated with PHA for 48 h effectively induced LAG-3 and PD-1 expression, which remained for more than 48 h after PHA removal (Fig. 1A and B). Moreover, the LAG-3 expression rate in Jurkat cells was more than 80% after PHA treatment and remained approximately 60% for 48 h after PHA removal. In addition, the PD-1 expression rate was more than 80% after PHA treatment and remained approximately 75% for 48 h after PHA removal (Fig. 1C). Taken together, these results demonstrated that LAG-3+PD-1+T cells were successfully obtained by means of PHA treatment. Next, the expression of the major ligand of LAG-3 and PD-1, such as the major histocompatibility complex class II molecules (MHC-II) and PD-L1, respectively, was measured in tumor cells17,18. A549 cells and MGC-803 cells expressed MHC-II and PD-L1, while PC-9 cells and HGC-27 cells did not (Fig. 1D and E, Supplementary Fig. 1, original blots of Supplementary Fig. 1 are presented in Supplementary File 1). Therefore, A549 and MGC-803 were co-cultured with activated Jurkat cells (PHA pretreatment) for 48 h to establish a cell co-culture model for subsequent experiments to test the combination effect of LBL-007 and anti-PD-1 antibodies in vitro.
Establishment of a cell co-culture model. (A) Immunofluorescence staining of Jurkat cells after incubation with PHA (2 µg/mL) as indicated. "Removed PHA 48 h" indicates that after the pretreatment of Jurkat cells with PHA for 48 h, which was replaced by medium without PHA, cells were incubated for another 48 h. (B) Immunoblot of Jurkat cell lysates using the indicated antibodies. Cells were stimulated with PHA (2 µg/mL) as indicated. β-actin protein was used as the internal control. Results are representative of three independent experiments. Original blots are presented in Supplementary File 2. (C) Flow cytometry analysis of Jurkat cells after incubation with PHA (2 µg/mL) as indicated. (D) Immunofluorescence staining of MHC-Ⅱ in tumor cells. (E) Immunofluorescence staining of PD-L1 in tumor cells.
Combination of LBL-007 and anti-PD-1 antibodies enhances the killing effect of activated Jurkat cells on tumor cells compared with monotherapy
The treatment of tumor cells or Jurkat cells with antibodies without being co-cultured did not affect cell viability (Supplementary Fig. 2). However, the activated Jurkat-A549/MGC-803 co-culture model treated with LBL-007 or BE0188 (a human anti-PD-1 antibody) showed a significantly enhanced killing effect of activated Jurkat cells on A549 cells, and MGC-803 cells compared with the IgG group (P < 0.05). In addition, LBL-007 and BE0188 treatment further enhanced the killing effect compared to that exerted only by LBL-007 or BE0188 (P < 0.05). Antibody treatment did not significantly enhance the killing effect of Jurkat cells on HGC-27 and PC-9 cells, since they are without ligand expression, such as MHC-II and PD-L1.
In the co-culture model, Transwell inserts were used to prevent direct contact between tumor cells and Jurkat cells. The data showed that LBL-007 or BE0188 failed to enhance the killing effect of Jurkat cells on A549 or MGC-803 cells when direct contact was prevented (Fig. 2A). A visible difference in cell loss between A549 cells and MGC-803 cells was observed by microscope analysis, which was consistent with the CCK-8 results (Fig. 2B). Thus, these results indicate that combination of LBL-007 and anti-PD-1 antibodies enhanced the killing effect of activated Jurkat cells compared with monotherapy on tumor cells, which was dependent on cell direct contact.
Viability of tumor cells co-cultured with activated Jurkat cells and incubated with different antibodies. (A) Viability of tumor cells co-cultured with Jurkat cells incubated with the indicated antibodies for 48 h. In the "A549 and Transwell" and "MGC-803 and Transwell" group, Jurkat cells were added to the upper compartment of the Transwell inserts and A549/MGC-803 were seeded in the lower compartment to avoid direct contact between cell types. The results are expressed as the mean ± SD from triplicates. *P < 0.05, **P < 0.01. (B) Image of A549 cells and MGC-803 cells co-cultured with Jurkat cells after incubation with the indicated antibodies for 48 h by optical microscopy.
Combination of LBL-007 and anti-PD-1 antibodies protects Jurkat cells from apoptosis compared to monotherapy in the co-culture model
LBL-007 or BE0188 treatment of the activated Jurkat-A549/MGC-803 co-culture model significantly inhibited the apoptosis of Jurkat cells, compared to the IgG group (P < 0.05). In addition, combining the two antibodies further inhibited the apoptosis of Jurkat cells compared to LBL-007 or BE0188 treatment alone (P < 0.05) (Fig. 3). Combined, these results indicated that combination of LBL-007 and anti-PD-1 antibodies enhanced the inhibition of apoptosis of Jurkat cells compared with monotherapy in the co-culture model.
Effect of LBL-007 and BE0188 on apoptosis of Jurkat cells in the co-culture model. Flow cytometry assay of Jurkat cells after incubation with the indicated antibodies for 48 h. Results are expressed as the mean ± SD from triplicates. Control group was showed Jurkat T cell apoptosis data in the absence of co-culturing with cancer cells.*P < 0.05, **P < 0.01.
Combination of LBL-007 and anti-PD-1 antibodies enhances apoptosis of tumor cells compared to monotherapy in the co-culture model
The potential cell death-related signaling pathway was investigated in A549 cells and MGC-803 cells from the antibody-treated co-culture model and the results demonstrated that the co-culture with Jurkat cells significantly upregulated the expression of apoptotic indicators compared with the control group (P < 0.05). LBL-007 or BE0188 antibodies significantly increased the expression of cleaved caspase3/cleaved PARP and decreased the expression of caspase3/PARP in activated Jurkat-A549/MGC-803 co-culture model compared with the IgG group (P < 0.05). Moreover, treatment with a combination of the two antibodies further increased the expression of cleaved caspase3/cleaved PARP and decreased the expression of caspase3/PARP compared to LBL-007 or BE0188 treatment alone (P < 0.05) (Fig. 4A and B). Other cell death modes were investigated, such as pyroptosis, autophagy, and necroptosis and the results revealed no significant change in the expression of related indicators in the co-culture model (data not shown).
Effect of LBL-007 and anti-PD-1 antibodies on T cell-induced apoptosis in tumor cells. (A) Immunoblot of cell lysates from tumor cells co-cultured with Jurkat cells after incubation with the indicated antibodies for 48 h. β-actin protein was used as the internal control. Results are representative of three independent experiments. Original blots are presented in Supplementary File 3. (B) Quantification of cleaved caspase3, cleaved PARP, caspase3 and PARP. The intensity of the bands was quantified using the Tanon Gel Image System. Results are expressed as the ratio of protein to β-actin and to the sample with the lowest expression set as 1 in each type of protein. Results are expressed as the mean ± SD from triplicates. (C) Viability of tumor cells pretreated with Z-VAD-FMK (50 μM) co-cultured with Jurkat cells at a ratio of 1:20 after incubation with the indicated antibodies for 24 h. Results are expressed as the mean ± SD from triplicates. *P < 0.05, **P < 0.01.
To verify that the killing effect of T cells on tumor cells was mainly due to apoptosis, A549 cells and MGC-803 cells were pretreated with the apoptosis inhibitor Z-VAD-FMK before the co-culture with activated Jurkat cells. The results showed that Z-VAD-FMK significantly decreased the killing effect of T cells on tumor cells (P < 0.05). Moreover, no significant difference was observed between the "IgG + DMSO" group and "LBL-007 and BE0188 + Z-VAD-FMK" group (Fig. 4C) in A549 cells and MGC-803 cells, which indicated that the apoptosis inhibitor attenuated the enhanced killing effect of T cells on tumor cells induced by LBL-007 and anti-PD-1 antibodies. Thus, these results suggested that the combination of LBL-007 and anti-PD-1 antibodies enhanced the T cell-induced apoptosis of tumor cells compared with monotherapy.
Combination of LBL-007 and anti-PD-1 antibodies increases the secretion of IL-2, IL-10, and TNF by activated Jurkat cells compared with monotherapy
The secretion of IL-2, IL-10, and TNF was regulated by the LBL-007 and PD-1 antibodies, while the secretion of other cytokines such as IL-6, IFN-γ and IL-17 was not significantly changed (data not shown). LBL-007 or BE0188 antibodies significantly increased the secretion of IL-2, IL-10, and TNF in Jurkat-A549/MGC-803 co-culture model, as compared with the IgG group (P < 0.05). In addition, combining the two antibodies further increased the secretion of IL-2, IL-10, and TNF when compared to LBL-007 or BE0188 treatment alone (P < 0.05) (Fig. 5A). Jurkat cells and tumor cells in the antibodies-treated cell co-culture model were separated and cytokine expression was measured in both cell types. Increased expression of IL-2, IL-10, and TNF mRNA was observed in Jurkat cells (P < 0.05) treated with antibodies, which was consistent with the ELISA results. This expression was not observed in A549 or MGC-803 cells (Fig. 5B). Thus, these results indicated that LBL-007 and anti-PD-1 antibodies increased the secretion of IL-2, IL-10, and TNF-α from active Jurkat cells and these two antibodies had a combination effect.
LBL-007 and anti-PD-1 antibodies promote IL-2, IL-10, and TNF production. (A) ELISA of IL-2, IL-10, and TNF in the supernatants of the co-culture model after incubation with the indicated antibodies for 48 h. “A549” refers to the Jurkat-A549 co-culture model, "MGC-803" refers to the Jurkat-MGC-803 co-culture model. (B) qPCR of IL-2, IL-10, and TNF in cells from the Jurkat-A549 co-culture model (upper layer) or the Jurkat-MGC-803 co-culture model (lower layer) after incubation with the indicated antibodies for 48 h. The results are expressed as the relative expression of the gene normalized to the endogenous control and to the sample with the lowest expression set as 1 in each type of cell. The results are expressed as the mean ± SD from triplicates. *P < 0.05, **P < 0.01, ***P < 0.001.
In vivo antitumor effect of LBL-007 and anti-PD-1 antibodies
The above-mentioned results indicated the enhanced antitumor activity of the combination of LBL-007 and anti-PD-1 antibodies in vitro. Next, these effects were assessed in vivo using human LAG-3 transgenic mice in a transplanted murine colorectal cancer model. Treatment with either LBL-007 or BE0146 alone (≥ 1.0 mg/kg) resulted in a significant delay of tumor growth compared with the IgG control (P < 0.05). Combined treatment of these two antibodies further suppressed tumor growth compared with treatment with LBL-007 or BE0146 alone (P < 0.05) (Fig. 6). These findings were consistent with the results presented in our previous study11 and demonstrated that combination of LBL-007 and anti-PD-1 antibodies enhanced the antitumor activity in vivo compared with monotherapy.
LBL-007 and anti-PD-1 antibodies inhibit mouse colorectal cancer cell growth in vivo. C57BL/6-hLAG-3 mice (n = 56) were randomly divided into 7 groups (n = 8 mice/group) when the MC38 colon adenocarcinoma tumor volume reached approximately 80 mm3. Then, mice were treated with IgG (10 mg/kg), LBL-007 (0.3 mg/kg-10.0 mg/kg), BE0146 (mouse anti-PD-1 antibody, 10 mg/kg), or a combination of LBL-007 (10 mg/kg) and BE0146 (10 mg/kg) twice a week. The tumor volume was calculated at the end of the experiment. Results are expressed as the mean tumor volume ± SD. *P < 0.05, **P < 0.01.
Treatment with the combination of LBL-007 and anti-PD-1 antibodies promotes apoptosis in the tumor cells compared with monotherapy
The potential ability of LBL-007 and anti-PD-1 antibodies to induce apoptosis of tumor cells was investigated through immunohistochemical analysis and the results revealed that LBL-007 or BE0146 treatment significantly increased T cell infiltration (Fig. 7A), which was consistent with the in vitro results (Fig. 3), as well as the expression of apoptosis indicators, cleaved caspase3 (Fig. 7B) and TNF (Fig. 7C) in the tumor, compared to IgG treatment. Treatment with a combination of the two antibodies further increased T cell infiltration and the expression of apoptosis indicators compared to the effect of LBL-007 or BE0146 treatment alone (Fig. 7D). Further, the TUNEL test showed results that were similar to the immunohistochemical analysis (Fig. 7E and F). Thus, these results indicated that in vivo treatment with the combination of LBL-007 and anti-PD-1 antibodies enhanced the Jurkat-induced apoptosis of tumor cells compared with monotherapy.
LBL-007 and anti-PD-1 antibodies promote the expression of apoptotic indicators in the tumor. Immunohistochemical staining of CD3 (A), cleaved caspase3 (B), and TNF (C) expression in the tumor from transplanted murine colorectal cancer model after treatment with the indicated antibodies. ×400 magnification. (D) Quantification of CD3, cleaved-caspase3, and TNF by the Aipathwell® System. Results are expressed as the H-Score (∑(pi × i) = (percentage of weak intensity cells × 1) + (percentage of moderate intensity cells × 2) + (percentage of strong intensity cells × 3), where i denotes the grade of positive cells: 0 points for negative without staining; 1 point for weak positive yellowish; 2 points for moderate positive tan; 3 points for strong positive tan. pi denotes the percentage of positive cells of the corresponding grade). (E) TUNEL staining. Red staining indicates DNA nicks, and blue DNA counterstaining was used for comparison. Scale bar = 50 μm. (F) Quantification of TUNEL staining by the Aipathwell® System. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Immune checkpoint antagonists used alone often result in a low response rate or even drug resistance in some patients, which limits the application of these drugs. For example, a meta-analysis involving more than 800 tumor patients in six trial cohorts showed that the total response rate of anti-PD-1/PD-L1 monotherapy in melanoma and various solid tumors was only approximately 20%19. However, combined medications significantly improve the clinical response rate and the therapeutic effect on patients, without showing a higher side effect rate compared with the treatment with the single drug, and its safety and tolerance are equivalent to single drug treatment20. Therefore, it is of great significance to study whether LBL-007, a novel fully human anti-LAG-3 antibody previously identified and characterized by our group, has an enhanced therapeutic effect with other targeted immune checkpoint drugs, especially anti-PD-1 antibodies both in vivo and in vitro.
Animal models are often used in the basic research of antibody drugs. However, the cell model used in vitro was simpler and easier to obtain, which is optimal for the basic evaluation of antibody drugs in the early stage of cancer immunotherapy studies. Previously, co-cultured cell systems were mostly used to study the function and underlying mechanism of a single immune checkpoint antagonist21,22. In this work, we describe the successful establishment of a cell co-culture model to study the function and underlying mechanism of two immune checkpoint antagonists (an anti-LAG-3 antibody and an anti-PD-1 antibody) working together to regulate the T cell killing effect on tumor cells by stimulating Jurkat cells with PHA to simultaneously induce LAG-3 and PD-1 expression . Activated T cells were co-cultured with tumor cells to avoid the interference of other cells and inflammatory factors in the tumor microenvironment, and the co-culture cell model also imitates the immune escape process of tumor cells interacting with tumor infiltrating lymphocytes (TIL)23. The combination of LBL-007 and BE0188 in the cell co-culture model enhanced the killing effect of activated Jurkat cells on tumor cells, proving the validity of the model. This cell co-culture model may be useful in the exploration of function and mechanism not only of LBL-007 and BE0188 in vitro but also of combining other immune checkpoint antagonists during basic research of cancer immunotherapy.
In addition, Transwell inserts were used to prevent direct contact between tumor cells and Jurkat cells, and the results showed that LBL-007 or BE0188 failed to enhance the killing effect of Jurkat cells on A549 or MGC-803 cells (Fig. 2A) in the Transwell group. This is because of the well-known fact that Mixed Lymphocyte Reaction (MLR) is essential for the detection of antibody potentiation. MLR is also the mechanism through which Jurkat cells establish a physical contact with cancer cells. When there is a mismatched TCR-MHC contact, T cells exert cytotoxic activities. This system is widely used to mimic antigen recognition in vitro. Our experiment verified that the direct contact between the TCR-MHC from Jurkat cells and cancer cells is essential in MLR to detect the effect of the blocking antibodies.
There have recently been new discoveries on the mechanism by which LAG-3 and PD-1 pathways working together to inhibit T cell functions. Researchers compared the effects of PD-1 signaling, LAG-3 signaling, and PD-1 + LAG-3 signaling, identifying all the interactomes in T cells that regulate their activity, and the mechanisms of action of PD-1 and LAG-3 blockers either alone or in combination. The results demonstrated that a major regulatory node corresponds to activation of CBL E3 ubiquitin ligases8. Researchers found that PD-1/LAG-3 co-blockade inhibited CBL-B expression in T cells8, and CBL-B is reported to potentiate the apoptosis24,25. This mechanism is consistent with the result that combination of LBL-007 and anti-PD-1 antibodies inhibits the apoptosis of Jurkat cells in the co-culture model (Fig. 3). Thus, our work provides effective equivalent data for verifying the mechanism recently reported.
Many studies report that the increase secretion of certain cytokines is one of the important mechanisms of the antitumor effect of immune checkpoint antagonists26,27,28. The levels of IL-2, IL-10, and TNF secreted by T cells in our co-culture model significantly increased after the inhibition by PD-1 and LAG-3 by antibodies, thereby suggesting that these cytokines may play an important role in the killing effect of activated Jurkat cells on MGC-803 and A549 cells. IL-2 is mainly secreted by activated T cells, which promote the proliferation and activation of T cells, thus enhancing the killing effect on the tumor29. Moreover, when IL-10 and IL-2 stimulate T cells together, they significantly enhance the killing effect on tumor cells30,31. TNF also kills tumor cells by enhancing T cell function32. IFN-γ is also an important antitumor immune cytokine affected by LAG-3 and PD-1 blockade10. However, IFN-γ is mainly secreted by CD8+ effector T cells, and in our co-culture model, Jurkat T cells, which are CD4+ T cells, secreted very low levels of IFN-γ.
This study has some limitations. First, CD4+ T cells are not effector cytotoxic T cells, and are different from the in vivo immune milieu. Furthermore, stimulation of the polyclonal stimulant PHA using the Jurkat cells to upregulate the expression of PD-1 and LAG-3 is also not identical to the phenomenon observed in tumors in vivo. However, this in vitro co-culture model was adequate to evaluate the effect of the blocking antibody. An increase in the secretion of cytokines such as TNF, IL-10, and IL-2, as well as increased apoptosis of tumor cells and decreased apoptosis of Jurkat cells, were observed after the addition of the blocking antibodies. Our model may reflect that blocking antibodies can exert antitumor effects by restoring CD4+ T cell activity, which in turn enhances the ability to secrete multiple cytokines and induce apoptosis in tumor cells.
The killing effect of T cells in the co-culture model in vitro on tumor cells was mainly due to inducing apoptosis, and antibody treatment played an antitumor role by influencing T cells to induce apoptosis to tumor cells; our in vivo and in vitro results were consistent. In addition, TNF is an important cytokine, which effectively induces apoptosis33, and our results revealed that LBL-007 and an anti-PD-1 antibody improved TNF expression both in vitro and in vivo, thus suggesting that TNF may play a vital role in the process of solid tumors treated with antibodies in clinical practice. Besides, cell cycle regulation is another important mechanism that may contribute to T cell-induced tumor suppression34. T cell-induced tumor cell cycle arrest might partially mediate this suppression, though its specific effects and mechanisms require further investigation.
A recent study demonstrated that Relatlimab + Nivolumab led to enhanced capacity for CD8+ T cell receptor signaling and altered CD8+ T cell differentiation, leading to heightened cytotoxicity despite the retention of exhaustion signatures8. This groundbreaking discovery challenges previous assumptions regarding the behavior of depleted immune cells and will inform future therapeutic strategies. Consequently, based on this finding, future analysis may elucidate whether a comparable phenomenon occurs in the in vitro co-culture model with the assistance of high-throughput sequencing technology. This report also provides a new idea for the further study of our co-culture model.
At present, 10 clinical trials including phase I and phase II are underway, aiming to evaluate the anticancer effect of LBL-007 in combination with different drugs in patients with various tumors. A multi-center and open-label clinical trial in China showed that LBL-007 is safe in 17 patients with advanced solid tumors, and no patients in the six dose groups developed dose-limited killing effects, showing good tolerance35. Evaluation of the clinical therapeutic effect of LBL-007 combined with the anti-PD-1 drug Toripalimab in patients with advanced malignant tumors is currently underway, up to December 2023, out of 75 efficacy evaluable patients, the Objective Response Rate (ORR) and Disease Control Rate (DCR) were 13.3% and 48.0%, respectively36. Other anti-LAG-3 mAbs with a similar clinical trial progress to LBL-007, such as Favezelimab (MK-4280), Fianlimab (REGN3767) and Ieramilimab (LAG525), have been evaluated alone or in combination with anti-PD-1/PD-L1 mAbs in Phase I to Ⅲ trials. Well tolerated and promising antitumor activity in Hodgkin lymphoma was observed in the combination of Favezelimab and Pembrolizumab with an ORR of 29% including 9% complete responses and 21% partial responses (NCT03598608)37. This is comparable to the encouraging anti-tumour activity observed with the combination of LBL-007 and Toripalimab in advanced melanoma, which have an ORR of 23.6% and DCR of 58.2% (NCT04640545)38. Compared to other anti-LAG-3 mAb, the available data of LBL-007 demonstrates the similar or even better clinical efficacy, with further studies on LBL-007 finished, there will be more and more encouraging results. However, the results of clinical trials still lack the support of experimental data in vitro on the pharmacological mechanism. Here the antitumor mechanism of the combination of LBL-007 and anti-PD-1 antibodies was explored by an in vitro co-culture model, which provides an experimental basis and experimental direction, and this work will facilitate the design and evaluation of further clinical trials.
Conclusion
In summary, this work demonstrateds that the combination of LBL-007 and anti-PD-1 antibodies improved the antitumor effect by enhancing T cell viability and activity and promoting T cell-induced apoptosis. These results might help the development of strategies considering the combination of LBL-007 with an anti-PD-1 antibody for cancer immunotherapy.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kraehenbuehl, L., Weng, C., Eghbali, S., Wolchok, J. D. & Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 19(1), 37–50 (2022).
Huo, J., Wang, Y., Fu, W., Lu, N. & Liu, Z. The promising immune checkpoint LAG-3 in cancer immunotherapy: From basic research to clinical application. Front. Immunol. 13, 956090 (2022).
Garcia-Martin, E. et al. Association between LAG3/CD4 gene variants and risk of Parkinson’s disease. Eur. J. Clin. Invest. 52(11), e13847 (2022).
Lecocq, Q., Keyaerts, M., Devoogdt, N. & Breckpot, K. The next-generation immune checkpoint LAG-3 and its therapeutic potential in oncology: Third time’s a charm. Int. J. Mol. Sci. 22(1), 75 (2020).
Tawbi, H. A. et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 386(1), 24–34 (2022).
Dirix, L. & Triebel, F. AIPAC: A phase IIb study of eftilagimod alpha (IMP321 or LAG-3Ig) added to weekly paclitaxel in patients with metastatic breast cancer. Future Oncol. 15(17), 1963–1973 (2019).
Yap, T. A. et al. A phase 1 first-in-human study of FS118, a tetravalent bispecific antibody targeting LAG-3 and PD-l1 in patients with advanced cancer and PD-l1 resistance. Clin. Cancer Res. 29(5), 888–898 (2023).
Cillo, A. R. et al. Blockade of LAG-3 and PD-1 leads to co-expression of cytotoxic and exhaustion gene modules in CD8(+) T cells to promote antitumor immunity. Cell 187(16), 4373–4388 (2024).
Chocarro, L. et al. PD-1/LAG-3 co-signaling profiling uncovers Cbl ubiquitin ligases as key immunotherapy targets. EMBO Mol. Med. 16(8), 1791–1816 (2024).
Andrews, L. P. et al. LAG-3 and PD-1 synergize on CD8(+) T cells to drive T cell exhaustion and hinder autocrine IFN-gamma-dependent anti-tumor immunity. Cell 187(16), 4355–4372 (2024).
Yu, X. et al. Characterization of a novel anti-human lymphocyte activation gene 3 (LAG-3) antibody for cancer immunotherapy. MAbs 11(6), 1139–1148 (2019).
Li, Z. et al. The immunostimulative effect and mechanisms of a novel mouse anti-human PD-1 monoclonal antibody on jurkat lymphocytic cells cocultured with hepatoma cells. OncoTargets Ther. 13, 12225–12241 (2020).
Liu, J. et al. Discovery and crystallography study of novel biphenyl ether and oxadiazole thioether (non-arylmethylamine)-based small-molecule PD-1/PD-l1 inhibitors as immunotherapeutic agents. J. Med. Chem. 66(18), 13172–13188 (2023).
Yang, Y., Wang, K., Chen, H. & Feng, Z. Design, synthesis, evaluation, and SAR of 4-phenylindoline derivatives, a novel class of small-molecule inhibitors of the programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-l1) interaction. Eur. J. Med. Chem. 211, 113001 (2021).
Zhang, C. et al. Preliminary study on the anti-tumor effect and mechanism of a novel fully human anti-LAG3 monoclonal antibody in vitro. Chin. J. Cancer. Biother 29, 419–425 (2022).
Ascione, A. et al. Development of a novel human phage display-derived anti-lag3 scfv antibody targeting cd8(+) t lymphocyte exhaustion. BMC Biotechnol. 19(1), 67 (2019).
Fournel, L. et al. Cisplatin increases pd-l1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. 464, 5–14 (2019).
Li, J. et al. miR-19 regulates the expression of interferon-induced genes and MHC class I genes in human cancer cells. Int. J. Med. Sci. 17(7), 953–964 (2020).
Nagaraju, G. P., Malla, R. R., Basha, R. & Motofei, I. G. Contemporary clinical trials in pancreatic cancer immunotherapy targeting pd-1 and pd-l1. Semin. Cancer. Biol. 86(Pt 3), 616–621 (2022).
Kraman, M. et al. Fs118, a bispecific antibody targeting lag-3 and pd-l1, enhances t-cell activation resulting in potent antitumor activity. Clin. Cancer Res. 26(13), 3333–3344 (2020).
Chakrabarti, J. et al. Mouse-derived gastric organoid and immune cell co-culture for the study of the tumor microenvironment. Methods Mol. Biol. 1817, 157–168 (2018).
Zettl, M. et al. Combination of two novel blocking antibodies, anti-pd-1 antibody ezabenlimab (bi 754091) and anti-lag-3 antibody bi 754111, leads to increased immune cell responses. OncoImmunology 11(1), 2080328 (2022).
Bhagwat, B. et al. Establishment of engineered cell-based assays mediating lag3 and pd1 immune suppression enables potency measurement of blocking antibodies and assessment of signal transduction. J. Immunol. Methods 456, 7–14 (2018).
Mu, X. et al. Ubiquitin ligase Cbl-b is involved in icotinib (BPI-2009H)-induced apoptosis and G1 phase arrest of EGFR mutation-positive non-small-cell lung cancer. Biomed Res. Int. 2013, 726375 (2013).
Qu, D. et al. Cbl-b-regulated extracellular signal-regulated kinase signaling is involved in the shikonin-induced apoptosis of lung cancer cells in vitro. Exp. Ther. Med. 9(4), 1265–1270 (2015).
Datar, I. et al. Expression analysis and significance of pd-1, lag-3, and tim-3 in human non-small cell lung cancer using spatially resolved and multiparametric single-cell analysis. Clin. Cancer Res. 25(15), 4663–4673 (2019).
Sievilainen, M., Saavalainen, J., Adnan-Awad, S., Salo, T. & Al-Samadi, A. Ido1 inhibition reduces immune cell exclusion through inducing cell migration while pd-1 blockage increases il-6 and -8 secretion from t cells in head and neck cancer. Front. Immunol. 13, 812822 (2022).
Okoye, I. S., Xu, L., Walker, J. & Elahi, S. The glucocorticoids prednisone and dexamethasone differentially modulate T cell function in response to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade. Cancer Immunol. Immunother. 69(8), 1423–1436 (2020).
Lichtenegger, F. S. et al. Targeting LAG-3 and PD-1 to enhance T cell activation by antigen-presenting cells. Front. Immunol. 9, 385 (2018).
Kumagai-Takei, N. et al. Effect of asbestos exposure on differentiation and function of cytotoxic T lymphocytes. Environ. Health Prev. 25(1), 59 (2020).
Geginat, J. et al. The light and the dark sides of interleukin-10 in immune-mediated diseases and cancer. Cytokine Growth Factor Rev. 30, 87–93 (2016).
Cervera-Carrascon, V. et al. TNFa and Il-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. OncoImmunology 7(5), e1412902 (2018).
Minchenko, O. H., Tsymbal, D. O., Minchenko, D. O. & Ratushna, O. O. The role of the TNF receptors and apoptosis inducing ligands in tumor growth. Ukr. Biochem. J. 88(5), 18–37 (2016).
Seung, E. et al. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. Nature 603(7900), 328–334 (2022).
Shi, Y. et al. Phase I study of LBL-007, a novel anti-human lymphocyte activation gene 3 (LAG-3) antibody in patients with advanced solid tumors. J. Clin. Oncol. 39(15), 2 (2021).
Yang, Y. et al. Anti-LAG-3 antibody LBL-007 in combination with anti-PD-1 antibody toripalimab, in patients with advanced malignant tumors: A phase Ib/II, open-label, multicenter, dose escalation/expansion study. Cancer Res. 84(7), 2 (2024).
Timmerman, J. et al. A phase 1/2 study of favezelimab in combination with pembrolizumab for heavily pretreated anti-PD-1-refractory classical Hodgkin lymphoma (Chl): An updated analysis. J. Clin. Oncol. 42(16), 1 (2024).
Bai, X. et al. Updated safety and efficacy results from the phase I study of either LBL-007 (an anti-LAG-3 antibody) in combination with toripalimab (an anti-PD-1 antibody) or LBL-007 in combination with toripalimab and axitinib in patients with advanced melanoma. J. Clin. Oncol. 41(16), 1 (2023).
Funding
This work was supported by the National Natural Science Foundation of China [32170933 and 82202382] and the Beijing Municipal Natural Science Foundation [7242135].
Author information
Authors and Affiliations
Contributions
Kei-wei Qin: Writing—original draft, Methodology, Conceptualization, Investigation, Formal analysis, Data curation. Hui-nan Zhou: Writing—original draft, Methodology, Formal analysis, Data curation, Conceptualization. Xiao-jie Yu: Investigation, Formal analysis, Data curation. Jian-fei Liu: Formal analysis, Data curation. Chen-lin Wu: Formal analysis, Data curation. Cheng Zhang: Formal analysis, Data curation. Li-jun Zhou: Supervision, Resources, Formal analysis, Data curation, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures were conducted in full compliance with the ARRIVE guidelines. The animal study was reviewed and approved by the Animal Ethics Committee of the Chinese PLA General Hospital. All methods were carried out in accordance with relevant guidelines and regulations (approval number: 2019X047).
Consent for publication
All authors agree to publish this research article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qin, K., Zhou, H., Yu, X. et al. Novel anti-LAG-3 antibody LBL-007 with anti-PD-1 blockade enhances antitumor immunity by promoting T cell-induced apoptosis. Sci Rep 15, 37515 (2025). https://doi.org/10.1038/s41598-025-21400-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-21400-z