Abstract
Over the past decades, survival outcomes for childhood acute lymphoblastic leukaemia (ALL) have significantly improved. However, adverse drug reactions (ADRs), particularly those related to 6-mercaptopurine (6-MP), remain a major concern. Myelosuppression associated with 6-MP administration can lead to infections or treatment interruptions. Excessive 6-thioguanine (6-TGN) levels worsen neutropenia, while elevated 6-methylmercaptopurine (6-MMP) levels contribute to hepatotoxicity. This study investigates genetic variants influencing 6-MP response in children with ALL. Seventeen tagSNPs in key enzymes involved in 6-MP metabolism, GMPS, IMPDH1, XO, and ITPA, were analysed. Genetic data were correlated with clinical and pharmacological parameters in 280 ALL patients treated under DFCI ALL 05–001, 11–001, and 16–001 protocols at CHU Sainte-Justine. Outcomes included 6-MP dose intensity, 6-TGN and 6-MMP metabolite levels, and hematologic and hepatic toxicities during consolidation II and maintenance phases. Results revealed that the rs6710015 variant allele in the XO gene is linked to lower 6-TGN and higher 6-MMP levels, while rs1884725 variant allele in the same gene is correlated with reduced neutropenia and higher cumulative 6-MP doses. In contrast, two variants in the IMPDH1 gene, rs2228075 and rs2278294, are correlated with more frequent neutropenia. These findings highlight novel genetic variants influencing 6-MP metabolism and toxicity in paediatric ALL patients.
Similar content being viewed by others
Introduction
Thiopurine drugs, notably 6-mercaptopurine (6-MP), play a role as cytotoxic antimetabolites and immunosuppressant in the treatment of acute lymphoblastic leukemia (ALL). ALL is the most common form of pediatric cancers accounting for about 25% of cancer diagnoses in children under 15 years of age1,2,3,4. While 6-MP represents an essential component in the multi-agent multi-phase treatment of ALL, compliance to 6-MP treatment schedule is critical to achieve favorable outcomes1,5. Nevertheless, 6-MP is known to cause myelotoxicity and hepatotoxicity6; the degree of these two specific adverse drug reactions (ADRs) can be severe enough to interrupt or discontinue 6-MP treatment, which contribute to reducing chances of cure and increasing the risk of long-term health problems7. Early identification of susceptible patients can optimize 6-MP monitoring and guide decisions about individualized dose adjustments8.
The susceptibility to 6-MP-related ADRs could be mediated by genetic factors involved in the 6-MP pathway9,10. 6-MP is a pro-drug and requires extensive metabolism to produce its active metabolites, 6-thioguanine nucleotides (6-TGN) and 6-methylmercaptopurine ribonucleotides (6-MMP)11. Previous studies have shown that individual metabolic variability is largely determined by variants in the enzyme thiopurine S-methyltransferase (TPMT), which catalyzes the production of 6-MMP1,12. Patients with reduced TPMT activity accumulate excessive 6-TGN, causing thiopurine-induced myelotoxicity characterized by severe neutropenia predominantly, but also other cytopenias when treated with standard 6-MP dose 1. In 2015, genetic variants in another enzyme, nudix hydrolase 15 (NUDT15), have been identified as risk factors of myelosuppression and 6-MP intolerance13,14,15. NUDT15 converts thioguanine triphosphate (TGTP) to thioguanine monophosphate (TGMP), which limits TGTP incorporation into DNA12,13,14. Loss-of-function NUDT15 variants lead to similar toxic effects as those causing reduced TPMT activity12,13,16. Therefore, pre-emptive 6-MP dose adjustment may be necessary for patients with reduced TPMT and/or NUDT15 enzyme activity, as recommended by the Clinical Pharmacogenetics Implementation Consortium (CPIC)16.
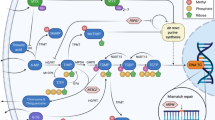
Genetic variants in TPMT and NUDT15 enzymes do not account for all the variability in response to 6-MP. Several other enzymes are involved in the action pathway of 6-MP and in the production of 6-TGN and 6-MMP metabolites (Fig. 1)17. These enzymes include inosine triphosphate pyrophosphatase (ITPA), hypoxanthine–guanine phosphoribosyl transferase (HGPRT), inosine monophosphate dehydrogenase 1 (IMPDH1), guanosine monophosphate synthase (GMPS) and xanthine oxidase (XO). 6-MP is converted by HGPRT into thio-inosine monophosphate (TIMP), which is subsequently metabolized to active 6-TGN by IMPDH1 and GMPS enzymes17. 6-TGN metabolite may either integrate into DNA or be converted into 6-thionosine triphosphate (TITP), a step that can be reversed by ITPA18,19. 6-MP can also be inactivated by XO into 6-thiouric acid (6-TU) or by TPMT into 6-MMP20. The amount of 6-MP conversion to 6-TGN is modulated by the competition between TPMT, XO and HGPRT enzymes20. The 6-TGN to 6-MMP ratio is highly variable, affecting both treatment efficacy and ADRs development, as high 6-TGN can cause neutropenia, while excess 6-MMP may lead to hepatotoxicity21,22.
Metabolic pathways involved in the mechanism of action of thiopurines. Abbreviations: 6-MP, 6-mercaptopurine; 6-Me-MP, 6-methyl-mercaptopurine; 6-Me-TG, 6-methyl-thioguanine; 6-Me-tIMP, 6-methyl-thioinosine-monophosphate; 6-Me-tITP, 6-methyl-thioinosine-triphosphate; 6-TG, thioguanine; 6-TGN, 6-thioguanine nucleotides; 6-tIDP, 6-thio-inosine diphosphate; 6-tIMP, 6-thio-inosine monophosphate; 6-tITP, 6-thio-inosine triphosphate; AZA, azathioprine; GMPS, guanosine monophosphatase synthetase; HGPRT, hypoxanthine guanine phosphoribosyl transferase; IMPDH, inosine monophosphate dehydrogenase; ITPA, inosine triphosphate pyrophosphatase; SAH, S-adenosyl-L-homocysteine; SAM, S-adenosyl-L-methionine; TPMT, thiopurine S-methyltransferase; XO, xanthine oxidase17.
In this study, we assessed the role of genetic variants in other enzymes of 6-MP pathway (beside TPMT and NUDT15), in contributing to 6-MP toxicity in children diagnosed with ALL.
Methods
Patient population
The study population included patients of European descent from 0 to 18 years of age at the time of ALL diagnosis and treated at CHU Sainte-Justine (Montreal, QC) (n = 280). All patients were treated according to the Dana Farber Cancer Institute (DFCI) ALL 05–001 (n = 155), 11–001 (n = 54), and 16–001 (n = 71), receiving at least 6 months of 6-MP therapy23,24,25. 6-MP metabolite levels were available for 140 patients. Table 1 presents a summary of the baseline demographic characteristics of the study cohort.
Identification of polymorphisms and genotyping
Seventeen tagSNPs located in regulatory and exonic regions with a minor allele frequency greater than 5% were selected and analyzed for an association with 6-MP toxicity. The selected polymorphisms include: IMPDH11 (rs2228075, rs2288550, rs2278294, rs2278293), ITPA (rs6139031, rs11591, rs1127354, rs45620433, rs7270101, rs8362), GMPS (rs4679758, rs2063903), and XO (rs1884725, rs2295475, rs6710015, rs6752058, rs207440) (Supplemental Table 1).
The analyses focused on the selected polymorphisms listed above. However, to account for potential bias introduced by previously identified no-function TPMT and NUDT15 variants, additional analyses were conducted while considering these variants. The no-function variants include rs1800462, rs1800460, and rs1142345 in the TPMT gene, which define the *2, *3A, *3B, and *3C alleles, as well as rs116855232 in the NUDT15 gene, which defines the *2 or *3 allele12,26.
Genotypes were obtained from whole-exome sequencing (average n = 42) and for the remaining patients were determined using allele-specific PCR with the introduction of artificial mismatch27. The primer sequences as well as conditions for amplification are presented in Supplemental Table 2. Three SNPs (rs8362 in ITPA, rs2063903 in GMPS, and rs207440 in XO) were genotyped by allele specific oligonucleotide hybridization as previously described28.
Pharmacological and clinical outcomes
6-MP administration
The 6-MP dose intensity was expressed as the ratio between total cumulative received dose and the cumulative intended protocol dose. As per the DFCI protocols for the consolidation II and maintenance phases, the starting 6-MP dose was 50 mg/m2/day, administered for 14 consecutive days in a 3-week cycle23,24,25. Doses that were missed due to 6-MP toxicity were counted in the analysis. Initial 6-MP doses were not modified for the TPMT or NUDT15 genotypes.
6-MP metabolites
Concentrations of 6-TGN and 6-MMP metabolites (picomoles per 8 × 108 red blood cells (RBC)) were available for 140 patients (average 5 measurements, range 1–36, interquartile range, 2–6). The levels were adjusted to the received 6-MP dose.
The therapeutic efficacy of 6-MP is correlated with 6-TGN concentrations ranging from 235 to 450 pmol per 8 × 108 RBC. Higher 6-TGN levels are associated with a higher risk of myelosuppression, whereas elevated 6-MMP levels (≥ 5 700 pmol per 8 × 108 RBC) are associated with hepatotoxicity11,21.
Myelosuppression
As per the DFCI protocol recommendations, myelosuppression was defined based on the absolute phagocyte count (APC), calculated by summing the absolute neutrophil count and absolute monocyte count. Criteria to start a cycle of chemotherapy would require APC˂0.75 × 109 cells/L or would delay start of treatment if insufficient APC count, whereas APC˂0.5 × 109 cells/L warrant treatment interruptions23,24,25. APC frequency during the consolidation II and maintenance phases was calculated as the proportion of days with an APC reduction below defined thresholds during 6-MP treatment.
Hepatotoxicity
The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes were used as indicators of liver function. Following the recommendations outlined in the DFCI protocol23,24,25, hepatotoxicity was defined based on elevation of AST enzyme by eight times. In addition, we used values exceeding five times the normal range categorized as grade 3 per the common terminology criteria for adverse events (CTCAE)29.
Statistical analysis
Association analyses for all the SNPs with 6-MP dose intensity, neutropenia defined by APC reduction, hepatotoxicity defined by ALT and AST elevations, as well as metabolite levels were conducted using non-parametric tests (Mann–Whitney or Kruskal–Wallis test, as applicable) and SPSS statistical software (version 28, SPSS Inc., NY). The genetic model that best explains the data were retained for the analyses. The initial analysis focused on patients with measured 6-MP metabolite data (n = 140). The results were considered significant (p < 0.05) if a variant was associated with at least two 6-MP-related outcomes, or if two variants within the same gene were associated with the same outcome and in both cases were subsequently confirmed in the full cohort (n = 280).
The five-year relapse-free survival (RFS) in relation to XO and IMPDH1 variants was analyzed using Kaplan–Meier survival estimates.
Results
The available data on 6-MP dose administration covered an average of 29 cycles (range 3–34 cycles). The average duration of reduced APC levels of ˂0.5 × 109 cells/L and ˂0.75 × 109 cells/L was 4 days and 2.5 days, respectively (range 0–24 days). The average of elevated AST levels were 0 (range from 0 to 4 days) while elevated ALT levels were present for an average of 4 days (ranging from 0 to 26). Average of elevated AST levels was 0 (range from 0–10 days), while the average of elevated ALT levels was 10 days (ranging from 0 to 50 days).
The variants in three candidate genes were significantly associated with clinical and pharmacological outcomes in the initial analyses conducted in 140 patients and two were subsequently confirmed in the full cohort of 280 patients (Supplemental Table 3).
The rs6710015 in XO gene was associated with 6-TGN and 6-MMP metabolite levels. Patients with at least one copy of the variant allele (n = 77) had a significantly lower mean 6-TGN levels compared to homozygote wild-type patients (n = 60) (p = 0.02, Fig. 2a). In contrast, an opposite and anticipated effect was found for the 6-MMP metabolite levels. The levels of the 6-MMP metabolite were significantly higher in the carriers of the variant allele (p = 0.005, Fig. 2b). According to the Genotype-Tissue Expression (GTEx) database, the rs6710015 variant, located in 3’UTR, is expression quantitative trait loci (eQTL) associated with increased XO mRNA expression in cultured fibroblast cells and skeletal muscle tissue30 (Supplemental Fig. 1a). The rs1884725 variant in the XO gene showed an association with the 6-MP dose intensity. Carriers of the variant A allele (n = 148) received a higher 6-MP dose compared to GG homozygotes (n = 131) (p = 0.01, Fig. 3a). In addition, carriers of the variant A allele had a lower frequency of APC < 0.75 × 109 cells/L reduction during both consolidation II and maintenance phases (p = 0.003, Fig. 3b). These results led us to further examine the potential association between the XO variants and disease outcome. The carriers of the variant XO alleles who all had wild-type TPMT and NUDT15 genotypes were not at a higher risk of relapse. The carriers of the rs6710015 variant had even slightly better RFS (Supplemental Fig. 2).
The impact of rs6710015 variant in the XO gene on 6-MP metabolite levels. Effect of the rs6710015 on 6-TGN levels in (a) and on 6-MMP levels in (b). Average 6-TGN and 6-MMP metabolite levels (pmol/8 × 108 red blood cells) during the consolidation II and maintenance phases are adjusted for 6-MP dose (mg/m2). The box plots, number of individuals and median metabolite levels for TT (wild-type) and TC + CC (variant carriers) genotypes are shown. The statistical significance of the differences between genotypes, assessed using the Mann–Whitney test, is indicated by the corresponding p-values.
The effect of the rs1884725 polymorphism in the XO gene on 6-MP treatment response in pediatric ALL. Effect of rs1884725 on 6-MP dose intensity in (a) and the frequency of APC reduction in (b). APC reduction refers to frequency of APC < 0.75 × 109 cells/L during the consolidation II and maintenance phases of the chemotherapy. Median values for GG (wild-type) and GA + AA (variant carriers) genotypes are presented next to the box plot, and the number of individuals is indicated at the top of the plots. The p-value for the difference between GG and GA + AA genotype, as obtained by Man-Whitney test, is shown.
Two polymorphisms in the IMPDH1 gene, rs2228075 and rs2278294, were in contrast associated with a higher frequency of neutropenia. Homozygote patients (rs2228075, n = 18 and rs2278294, n = 35) for the minor allele had higher frequency of APC < 0.75 × 109 cells/L reduction during consolidation II and maintenance phases (p = 0.038, Fig. 4a; p = 0.046, Fig. 4b, respectively). The effect was more apparent during the maintenance phase (p = 0.015, Fig. 4c; and p = 0.008, Fig. 4d, respectively). We further investigated the distribution of the carriers of IMPDH1 variants in relation to known no-function mutations in the TPMT and NUDT15 genes to exclude potential confounding effects. The results remain significant after adjustment (p = 0.008 and p = 0.005, respectively, Supplemental Fig. 3a,b). The effect seems to be driven by the combination of two polymorphisms, since the significant increase in APC < 0.75 × 109 cells/L reduction (p = 0.009, Fig. 5), was seen only for patients who were homozygotes for rs2228075 and rs2278294 compared to other genotype combinations. In addition, to assess the contribution of IMPDH1 variants to the development of neutropenia, we evaluated their effect in the context of known TPMT and NUDT15 risk alleles. We observed an additional 11.4% increase in neutropenia risk associated with IMPDH1 variants beyond the contribution of TPMT and/or NUDT15 (Supplemental Table 4).
Effect of the variants in the IMPDH1 gene on 6-MP treatment response in pediatric ALL. Effect of the rs2228075 (a, c) and rs2278294 (b, d) polymorphisms on the frequency of APC reduction. APC reduction refers to frequency of APC < 0.75 × 109 cells/L during the consolidation II and maintenance treatment phases in (a and b) and during maintenance phase only in (c and d). The number of individuals with median values for each genotype group is indicated along the p value for the difference between genotype groups.
Effect of the rs2228075 and rs2278294 combination on the frequency of APC reduction. APC reduction refers to frequency of APC < 0.75 × 109 cells/L during maintenance phase of the chemotherapy. All observed genotype combinations are included. Horizontal lines with corresponding p values next to the lines show pairwise comparisons between each genotype group and the wildtype. The number of individuals in each genotype group is displayed at the top of the boxplots.
According to GTEx, both rs2228075 and rs2278294 are eQTLs associated with elevated IMPDH1 mRNA expression in several tissues including EBV-transformed lymphocytes30 (Supplemental Fig. 1b and c).
Our primary analysis in a subset of 140 patients revealed significant associations between the rs4679758 variant in the GMPS gene and the incidence of both neutropenia and hepatic toxicity related to 6-MP administration (Supplemental Table 3). However, these associations were not replicated when assessed in the full cohort of 280 patients.
Discussion
The variability in therapeutic response to thiopurine drugs, such as 6-MP, have been partly explained by the polymorphisms in the genes encoding key enzymes involved in 6-MP metabolism, which affect the formation of active metabolites. The enzymes TPMT and NUDT15 play a crucial role in thiopurine metabolism and the development of ADRs11,12,13,14,15,16. The tolerated 6-MP dose is influenced by the number of non-functional alleles in genes encoding for these enzymes12,16. The degree of 6-MP intolerance is comparable between individuals carrying non-functional alleles in TPMT and those in NUDT1512,13,16.
However, the established pharmacogenetic variants in these genes do not explain all the variability in 6-MP metabolism and other enzymes in the metabolic pathway may also contribute to 6-MP-related ADRs and clinical outcomes. This study aimed to evaluate the associations between specific genetic variants and 6-MP-related toxicity in children with ALL, focusing on factors such as dose intensity, metabolite levels, myelosuppression, and hepatotoxicity. We identified several variants in the XO and IMPDH1 genes that influence 6-MP metabolism and toxicity.
The enzyme XO is an early detoxifying enzyme in the metabolism of thiopurine drugs31. It oxidizes 6-MP into 6-thiouric acid, an inactive metabolite that is excreted in urine17,31. While it is one of the two major enzymes for catabolism, its impact on the metabolic response has not been studied thoroughly. XO plays a significant role in determining the bioavailability of 6-MP. Approximately, two-thirds of a standard 6-MP dose are inactivated by XO’s catabolic pathway32.
A subset of patients treated with thiopurines exhibit abnormal thiopurine metabolism driven by hypermethylation leading to preferential conversion into 6-MMP32,33,34. This shift results in insufficient 6-TGN levels, contributing to treatment resistance. These patients are commonly known as “shunters”32,34. To counteract the overproduction of 6-MMP and reduce the risk of treatment failure or hepatotoxicity from its accumulation, thiopurine is combined in some cases with a xanthine oxidase inhibitor, such as allopurinol32,33,34. The administration of allopurinol helps normalize the metabolic profile by decreasing 6-MMP levels and increasing 6-TGN concentrations. Interestingly, a study investigating 6-MP toxicity in Ethiopian pediatric ALL patients identified the XO gene variant rs2281547 as a genetic risk factor for grade 4 hematologic toxicities in patients treated with 6-MP35. The allele frequency of rs2281547 in the European population is approximately 40%, however, no association between this variant and 6-MP toxicity has been reported in this population. A recent study found, that among Chinese patients with inflammatory bowel disease (IBD), XO activity was significantly lower in those experiencing ADRs, particularly in patients with leukopenia, compared to those without side effects20. Another case report on XO activity in patients with chronic autoimmune pancreatitis showed a negative correlation between XO activity and the formation of 6-TGN31.
Our findings regarding the polymorphisms rs1884725 and rs6710015 suggest that XO genetic variants play a significant role in influencing the metabolism of 6-MP in pediatric ALL patients. Specifically, the rs6710015 variant, which is eQTL with higher expression in some tissues, was associated with lower 6-TGN levels potentially reducing the risk of myelosuppression. We also observed elevated levels of 6-MMP, however no association with hepatotoxicity was found. Additionally, we observed that the variant allele of rs1884725 was associated with higher cumulative 6-MP doses and reduced frequency of neutropenia. These findings suggest a shift in thiopurine metabolism toward the methylation pathway that may reduce the risk of myelotoxicity and improve treatment tolerance. However, the presence of the variant alleles could predispose to hepatotoxicity. Although the present results may suggest lower sensitivity to 6-MP for variant alleles, they were not associated with higher risk of relapse, which might be due to the higher drug dose received. Together, these observations highlight the potential utility of XO genotyping to complement existing TPMT and NUDT15 testing to optimize 6-MP dosing and minimize the risk of ADRs.
Another gene that we identified associated with 6-MP related toxicity is IMPDH1. Since variants in this gene were associated with higher risk of neutropenia, they might have higher pharmacogenetic relevance as compared to those in XO gene. The IMPDH1 enzyme catalyzes the conversion of 6-thio inosine monophosphate to xanthosine monophosphate and is thus essential for synthesis of 6-TGN metabolite. We found that two variants in the IMPDH1 gene, rs2228075 and rs2278294, are linked to an increased risk of myelosuppression particularly during maintenance treatment phase. The effect was present in double homozygotes and was independent of known no-function variants in TPMT and/or NUDT15 genes. In addition, we observed that IMPDH1 variants were associated with an additional 11.4% increase in the risk of neutropenia, beyond the known effects of TPMT and NUDT15 variants.
According to the GTEx, the rs2278294 and rs2228075 variants are associated to elevated IMPDH1 mRNA levels in EBV-transformed lymphocytes and other tissues29. Given that IMPDH1 is a rate-limiting enzyme in the conversion of 6-MP to 6-TGN, its enzymatic activity is expected to positively correlate with 6-TGN concentrations.
A study, which addressed the impact of IMPDH1 genetic polymorphisms on 6-TGN levels and toxicity in pediatric patients with IBD treated with azathioprine, found that the rs2278294 variant was associated with lymphopenia36. Interestingly, another study conducted in pediatric heart transplant patients reported that the same polymorphisms were associated with gastrointestinal intolerance (GI) to immunosuppressive agent mycophenolate mofetil (MMF)37. IMPDH1 is the primary target of mycophenolic acid, the active metabolite of MMF. As a key enzyme in the de novo nucleotide synthesis pathway, IMPDH1 regulates the rate limiting step in this process37,38. Although 6-MP and MMF have different mechanisms of action, they would both interfere with purine synthesis and the genetic variants in IMPDH1 can possibly contribute in both cases to the development of side effects. Moreover, a study on the association of IMPDH1 gene polymorphisms and acute graft-versus-host disease (aGVHD) after hematopoietic stem cell transplantation reported that the rs2278294 polymorphisms was significantly associated with a higher incidence of 2–4 aGVHD in recipients who were treated with MMF39.
ITPA is the most frequently studied enzymes (besides TPMT and NUDT15) of 6-MP pathway. One study conducted in childhood ALL patients found that the ITPA rs1127354 variant influenced RBC 6-MMP levels. Patients with the TPMT wild-type and ITPA variant had the highest concentrations compared to patients with other genotype groups40. A meta-analysis involving mixed pediatric ALL patients demonstrated a significant association between the rs1127354 variant and 6-MP induced neutropenia as well as hepatotoxicity41. It was also shown that ITPA activity was absent in homozygotes for the variant allele and reduced to 25% of wild-type activity in heterozygote patients41. A study investigating the association between polymorphisms in the ITPA gene and ADRs to azathioprine therapy in patients treated for IBD found that variant allele in rs1127354 was significantly associated with ADRs characterized by flu-like symptoms, rash, and pancreatitis42. However, not all studies found the association of the ITPA rs1127354 variant and an increased risk of myelotoxicity or hepatotoxicity from 6-MP therapy43, which is in line with our observation.
One notable limitation of this study is the potential confounding effect of myelosuppression and hepatotoxicity relied on AST/ALT elevations resulting from concurrent chemotherapy. Agents such as doxorubicin, used during consolidation II in high-risk patients, and methotrexate (MTX) during the maintenance phase could both lead to myelosuppressive and hepatotoxic effects. In addition, asparaginase administered during consolidation II phase is also known for its association with hepatotoxicity44. This overlap complicates the ability to isolate the specific contributions of the studied intervention to neutropenia or liver toxicity. In addition, non-chemotherapy-related factors such as infections, inflammatory processes, or inconsistent medication adherence may also influence hematologic and hepatic outcomes, further limiting the clarity of causal associations. Patient characteristics may as well play a role. Patients younger than 10 years were reported as more likely to develop hepatotoxicity during chemotherapy45. Another limitation of this study is that it was restricted to a European population, which may limit the generalizability of the findings to other ethnic groups with different genetic backgrounds. Therefore, the absence of a significant association in our cohort may reflect population-specific genetic factors that influence the metabolism of 6-MP. Additionally, differences in treatment regimens or environmental factors could also contribute to the variability in findings between studies. Moreover, our study cohort was relatively small, which could reduce the statistical power and the ability to detect less common associations. To validate our results, larger studies including more ethnic diverse populations may be necessary.
The identification of variants that increase the risk of ADRs following 6-MP administration holds great potential to better personalize treatment for children with ALL. Such knowledge could help minimize the occurrence of ADRs, improve overall treatment outcomes, and significantly enhance the quality of life for these patients.
Data availability
The datasets used and/or analyzed during the current study are available upon request.
Abbreviations
- 6-MMP:
-
6-methylmercaptopurine ribonucleotides
- 6-MP:
-
6-mercaptopurine
- 6-TGN:
-
6-thioguanine nucleotides
- 6-TU:
-
6-thiouric acid
- aGVHD:
-
Acute graft-versus-host disease
- ADR:
-
Adverse drug reactions
- ALL:
-
Acute lymphoblastic leukemia
- ALT:
-
Alanine aminotransferase
- APC:
-
Absolute phagocyte count
- AST:
-
Aspartate aminotransferase
- CPIC:
-
Clinical pharmacogenetics implementation consortium
- CTCAE:
-
Common terminology criteria for adverse events
- DFCI:
-
Dana farber cancer institute
- eQTL:
-
Expression quantitative trait loci
- GI:
-
Gastrointestinal intolerance
- GMPS:
-
Guanosine monophosphate synthase
- GTEx:
-
Genotype-tissue expression database
- HGPRT:
-
Hypoxanthine–guanine phosphoribosyl transferase
- IBD:
-
Inflammatory bowel disease
- IMPDH1:
-
Monophosphate dehydrogenase 1
- ITPA:
-
Inosine triphosphate pyrophosphatase
- MMF:
-
Mycophenolate mofetil
- MTX:
-
Methotrexate
- NUDT15:
-
Nnudix hydrolase 15
- RBC:
-
Red blood cells
- RFS:
-
Relapse-free survival
- TGMP:
-
Thioguanine monophosphate
- TGTP:
-
Thioguanine triphosphate
- TIMP:
-
Thio-inosine monophosphate
- TITP:
-
Thionosine triphosphate
- TPMT:
-
Thiopurine S-methyltransferase
- XO:
-
Xanthine oxidase
References
Azimi, F., Jafariyan, M., Khatami, S., Mortazavi, Y. & Azad, M. Assessment of thiopurine-based drugs according to thiopurine S-methyltransferase genotype in patients with acute lymphoblastic leukemia. Iran J. Ped. Hematol. Oncol. 4(1), 32–38 (2014).
Oliwia, et al. Cytotoxicity of thiopurine drugs in patients with inflammatory bowel disease. Toxics 10(4), 151.22. https://doi.org/10.3390/toxics10040151 (2022).
Mullighan, C. G. How advanced are we in targeting novel subtypes of ALL?. Best Pract. Res. Clin. Haematol. 32(4), 101095. https://doi.org/10.1016/j.beha.2019.101095 (2019).
Pui, C. H., Robison, L. L. & Look, A. T. Acute lymphoblastic leukaemia. Lancet 371(9617), 1030–1043. https://doi.org/10.1016/S0140-6736(08)60457-2 (2008).
Bhatia, S. et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: a children’s oncology group study. JAMA Oncol. 1(3), 287–295. https://doi.org/10.1001/jamaoncol.2015.0245 (2015).
Zou, Y. et al. Preparation, characterization, pharmacokinetic, and therapeutic potential of novel 6-mercaptopurine-loaded oral nanomedicines for acute lymphoblastic leukemia. Int. J. Nanomedicine. 16, 1127–1141. https://doi.org/10.2147/IJN.S290466 (2021).
Dulucq, S., Laverdière, C., Sinnett, D. & Krajinovic, M. Pharmacogenetic considerations for acute lymphoblastic leukemia therapies. Expert Opin. Drug Metab. Toxicol. 10(5), 699–719. https://doi.org/10.1517/17425255.2014.893294 (2014).
Paugh, S. W., Stocco, G. & Evans, W. E. Pharmacogenomics in pediatric leukemia. Curr. Opin. Pediatr. 22(6), 703–710. https://doi.org/10.1097/MOP.0b013e32833fde85 (2010).
Cecchin, E. & Stocco, G. Pharmacogenomics and personalized medicine. Genes 11(6), 679. https://doi.org/10.3390/genes11060679 (2020).
Roden, D. M. et al. Pharmacogenomics. Lancet (London, England). 394(10197), 521–532. https://doi.org/10.1016/S0140-6736(19)31276-0 (2019).
Bradford, K. & Shih, D. Q. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J. Gastroenterol. 17(37), 4166–4173. https://doi.org/10.3748/wjg.v17.i37.4166 (2011).
Relling, M. V. et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 Update. Clin. Pharmacol. Ther. 105(5), 1095–1105. https://doi.org/10.1002/cpt.1304 (2019).
Yang, J. J. et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J. Clin. Oncol. 33(11), 1235–1242. https://doi.org/10.1200/JCO.2014.59.4671 (2015).
Texis, T. et al. Genotyping NUDT15*3 rs1166855232 reveals higher frequency of potential adverse effects of thiopurines in natives and mestizos from Mexico. Pharmacol. Rep. 74(1), 257–262. https://doi.org/10.1007/s43440-021-00287-3 (2022).
Moriyama, T. et al. Novel variants in NUDT15 and thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry. Blood 130(10), 1209–1212. https://doi.org/10.1182/blood-2017-05-782383 (2017).
Pratt, V. M. et al. TPMT and NUDT15 genotyping recommendations: a joint consensus recommendation of the association for molecular pathology, clinical pharmacogenetics implementation consortium, college of american pathologists, dutch pharmacogenetics working group of the royal dutch pharmacists association, european society for pharmacogenomics and personalized therapy, and pharmacogenomics knowledgebase. J. Mol. Diagn. 24(10), 1051–1063. https://doi.org/10.1016/j.jmoldx.2022.06.007 (2022).
Abaji, R. & Krajinovic, M. Thiopurine S-methyltransferase polymorphisms in acute lymphoblastic leukemia, inflammatory bowel disease and autoimmune disorders: influence on treatment response. Pharmgenomics Pers. Med. 10, 143–156. https://doi.org/10.2147/PGPM.S108123 (2017).
Sahasranaman, S., Howard, D. & Roy, S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur. J. Clin. Pharmacol. 64(8), 753–767. https://doi.org/10.1007/s00228-008-0478-6 (2008).
Gerbek, T. et al. Role of TPMT and ITPA variants in mercaptopurine disposition. Cancer Chemother Pharmacol. 81(3), 579–586. https://doi.org/10.1007/s00280-018-3525-8 (2018).
Ding, L. et al. Xanthine oxidase activity in thiopurine curative Chinese inflammatory bowel disease patients. Pharmacol. Res. Perspect. 9(3), e00764. https://doi.org/10.1002/prp2.764 (2021).
Lampič, K. et al. Determination of 6-thioguanine and 6-methylmercaptopurine in dried blood spots using liquid chromatography-tandem mass spectrometry: Method development, validation and clinical application. Clin. Chim. Acta. 499, 24–33. https://doi.org/10.1016/j.cca.2019.08.024 (2019).
Dervieux, T. et al. Possible implication of thiopurine S-methyltransferase in occurrence of infectious episodes during maintenance therapy for childhood lymphoblastic leukemia with mercaptopurine. Leukemia 15(11), 1706–1712. https://doi.org/10.1038/sj.leu.2402259 (2001).
Vrooman, L. M. et al. Refining risk classification in childhood B acute lymphoblastic leukemia: results of DFCI ALL Consortium Protocol 05–001. Blood Adv. 2(12), 1449–1458. https://doi.org/10.1182/bloodadvances.2018016584 (2018).
Vrooman, L. M. et al. Efficacy and toxicity of pegaspargase and calaspargase pegol in childhood acute lymphoblastic leukemia: results of DFCI 11–001. J. Clin Oncol. 39(31), 3496–3505. https://doi.org/10.1200/JCO.20.03692 (2021).
Tran, T. H. et al. Whole-transcriptome analysis in acute lymphoblastic leukemia: a report from the DFCI ALL Consortium Protocol 16–001. Blood advs. 6(4), 1329–1341. https://doi.org/10.1182/bloodadvances.2021005634 (2022).
Stocco, G. et al. Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia. Clin. Pharmacol. Ther. 85(2), 164–172. https://doi.org/10.1038/clpt.2008.154 (2009).
Sabirova, Z. et al. Novel variant in Nudix hydrolase 15 gene influences 6-mercaptopurine toxicity in childhood acute lymphoblastic leukemia patients. Pharmacogenet Genomics. 34(5), 170–173. https://doi.org/10.1097/FPC.0000000000000533 (2024).
Mootoosamy, C. et al. IL16 and factor V gene variations are associated with asparaginase-related thrombosis in childhood acute lymphoblastic leukemia patients. Pharmacogenomics 24(4), 199–206. https://doi.org/10.2217/pgs-2022-0164 (2023).
Freites-Martinez, A., Santana, N., Arias-Santiago, S. & Viera, A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas. Dermosifiliogr. 112(1), 90–92. https://doi.org/10.1016/j.ad.2019.05.009 (2021).
GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369(6509), 1318–1330. https://doi.org/10.1126/science.aaz1776 (2020).
Wong, D. R., Derijks, L. J., den Dulk, M. O., Gemmeke, E. H. & Hooymans, P. M. The role of xanthine oxidase in thiopurine metabolism: a case report. Ther. Drug Monit. 29(6), 845–848. https://doi.org/10.1097/FTD.0b013e31815bf4dc (2007).
Belhocine, M. et al. Optimizing thiopurine therapy with a xanthine oxidase inhibitor in patients with systemic autoimmune diseases: a single-centre experience. Can J Hosp Pharm. 74(4), 361–369. https://doi.org/10.4212/cjhp.v74i4.3199 (2021).
Mosher, N. et al. Utilization of thiopurine metabolites and allopurinol in pediatric acute lymphoblastic leukemia: consideration for an algorithmic approach. J. Pediatr. Hematol. Oncol. 44(2), e521–e525. https://doi.org/10.1097/MPH.0000000000002313 (2022).
Lim, S. Z. & Chua, E. W. Revisiting the role of thiopurines in inflammatory bowel disease through pharmacogenomics and use of novel methods for therapeutic drug monitoring. Front Pharmacol. 9, 1107. https://doi.org/10.3389/fphar.2018.01107 (2018).
Ali, A. M. et al. Genetic variants of genes involved in thiopurine metabolism pathway are associated with 6-mercaptopurine toxicity in pediatric acute lymphoblastic leukemia patients from Ethiopia. Front Pharmacol. 14, 1159307. https://doi.org/10.3389/fphar.2023.1159307 (2023).
Lee, M. N. et al. Impact of genetic polymorphisms on 6-thioguanine nucleotide levels and toxicity in pediatric patients with IBD treated with azathioprine. Inflamm. Bowel Dis. 21(12), 2897–2908. https://doi.org/10.1097/MIB.0000000000000570 (2015).
Ohmann, E. L. et al. Inosine 5’-monophosphate dehydrogenase 1 haplotypes and association with mycophenolate mofetil gastrointestinal intolerance in pediatric heart transplant patients. Pediatr. Transplant. 14(7), 891–895. https://doi.org/10.1111/j.1399-3046.2010.01367.x (2010).
McCune, J. S. et al. Inosine monophosphate dehydrogenase pharmacogenetics in hematopoietic cell transplantation patients. Biol. Blood Marrow Transplant. 24(9), 1802–1807. https://doi.org/10.1016/j.bbmt.2018.04.006 (2018).
Cao, W. et al. Genetic variations in the mycophenolate mofetil target enzyme are associated with acute GVHD risk after related and unrelated hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 18(2), 273–279. https://doi.org/10.1016/j.bbmt.2011.06.014 (2012).
Adam Beaumais, T. et al. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br. J. Clin. Pharmacol. 71(4), 575–584. https://doi.org/10.1111/j.1365-2125.2010.03867.X (2011).
Lee, Y., Jang, E. J., Yoon, H. Y., Yee, J. & Gwak, H. S. Effect of ITPA polymorphism on adverse drug reactions of 6-mercaptopurine in pediatric patients with acute lymphoblastic leukemia: a systematic review and meta-analysis. Pharmaceuticals (Basel, Switzerland) 15(4), 416. https://doi.org/10.3390/ph15040416 (2022).
Marinaki, A. M. et al. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics 14(3), 181–187. https://doi.org/10.1097/00008571-200403000-00006 (2004).
Milosevic, G. et al. Variants in TPMT, ITPA, ABCC4 and ABCB1 genes as predictors of 6-mercaptopurine induced toxicity in children with acute lymphoblastic leukemia. J. Med. Biochem. 37(3), 320–327. https://doi.org/10.1515/jomb-2017-0060 (2018).
Stock, W. et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk lymphoma. 52(12), 2237–2253. https://doi.org/10.3109/10428194.2011.596963 (2011).
Yang, W. et al. Association of inherited genetic factors with drug-induced hepatic damage among children with acute lymphoblastic leukemia. JAMA netw. open. 5(12), e2248803. https://doi.org/10.1001/jamanetworkopen.2022.48803 (2022).
Acknowledgements
The authors would like to thank all patients and their parents for the participation in the study, as well as all study collaborators for their valuable contribution.
Funding
This investigation was supported by grants from The Cole Foundation, the Foundation of Charles-Bruneau and Network of Applied Genetic Medicine (RMGA).
Author information
Authors and Affiliations
Contributions
M.K. and T-H T. designed the study; Z.S., V.G. M.T.E.B., R.R.B. performed the genetic analyses; D.S. supervised WES analyses; Y.T.,T.N., JM.L and C.L. contributed to patients’ sample and data processing; Z.S., and M.K. executed statistical analysis; Z.S. and M. K. wrote the manuscript, and all authors revised it critically.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent
Written informed consent was obtained from every patient or parent/legal guardian. The study was conducted in accordance with the Declaration of Helsinki and was approved by Research Ethics Board of SJUHC.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sabirova, Z., Boucetta, M.T.E., Bensouyad, R.R. et al. Influence of xanthine oxidase and inosine monophosphate dehydrogenase polymorphisms on 6-mercaptopurine treatment response in pediatric acute lymphoblastic leukemia. Sci Rep 15, 40519 (2025). https://doi.org/10.1038/s41598-025-24355-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-24355-3