Abstract
The global population is rapidly aging, presenting significant public health challenges, particularly with the increased risk of chronic diseases. Biological age acceleration refers to a faster-than-expected aging process, which is associated with an increased risk of age-related chronic diseases. Uric acid to high-density lipoprotein cholesterol ratio (UHR) is a novel biomarker that reflects metabolic disturbances and has been linked to various chronic conditions. Understanding the relationship between UHR and biological age acceleration, as well as its modifiable risk factors, is crucial for developing strategies aimed at promoting healthy aging and managing chronic diseases in older adults. Using data from a cohort study, the ratio of uric acid to high-density cholesterol (UHR), uric acid (UA), and high-density lipoprotein (HDL) were assessed in relation to biological age acceleration, including phenotypic age acceleration (PhAA) and Klemera–Doubal method age acceleration (KDM-AA), through multiple linear and logistic regression models, adjusted for demographic characteristics, lifestyle factors, and medical histories. Restricted cubic spline (RCS), subgroup, and interaction analyses were also conducted. Subgroups were stratified according to age (< 60 vs. ≥60 years), sex (male vs. female), race (Black vs. others), BMI (< 30 vs. ≥30), hypertension (yes/no), cardiovascular disease (yes/no), cancer (yes/no), and physical activity level (< 600, 600–3999, and ≥ 4000 min/week). The results reveal significant associations between elevated UHR, UA levels, reduced HDL cholesterol, and accelerated biological aging. RCS regression analyses revealed a complex relationship between UHR and age acceleration, with a non-linear association with PhAA and a linear relationship with KDM-AA. Subgroup analysis showed that the association between UHR and biological age acceleration remained robust across all groups. Interaction analyses revealed differential effects across subgroups, particularly among individuals with varying physical activity levels, cardiovascular disease, and hypertension status. This study demonstrates a significant positive association between UHR and markers of biological age acceleration. These findings suggest that UHR could serve as a potential biomarker for aging and chronic disease management in older adults, offering insights into strategies to reduce the burden of age-related conditions.
Similar content being viewed by others
Introduction
The rapid aging of the population presents a significant public health challenge, as aging is a key risk factor for various chronic diseases, including cancer, atherosclerotic cardiovascular diseases, neurodegenerative disorders, and metabolic syndrome, with the burden of age-related conditions escalating rapidly1,2,3,4. Aging itself is a universal biological process, characterized by the gradual and irreversible decline in the physiological functions of all organ systems5. While aging is inevitable, the rate at which it occurs varies across individuals6. Biological age, or physiological aging, provides a more accurate measure of the body’s aging process compared to chronological age, as it reflects the actual physiological changes that occur over time7. Biological age acceleration, which is calculated by subtracting chronological age from biological age, indicates whether an individual’s physiological aging is advancing faster than expected. A positive value suggests accelerated aging, which may be associated with an increased risk of age-related diseases and conditions. Recent studies, however, challenge the notion that aging is entirely irreversible, indicating that aging may be modifiable through specific lifestyle interventions and behaviors8,9,10,11. This emerging evidence underscores the importance of exploring clinical indicators of aging, identifying modifiable risk factors, and understanding the underlying biological mechanisms. Such research is crucial not only for reducing the socio-economic and healthcare burdens posed by age-related diseases but also for developing strategies that promote healthy aging12.
Among the clinical indicators of aging, biomarkers such as uric acid (UA) and high-density lipoprotein (HDL) cholesterol levels have gained considerable attention due to their potential roles in age-related diseases. Elevated UA levels are associated with a range of health conditions, including hypertension, cardiovascular diseases, and metabolic syndrome—pathologies that become increasingly prevalent with aging. Although UA is traditionally regarded as a natural antioxidant, it also exhibits pro-oxidant effects that contribute to the production of reactive oxygen species (ROS), lipid peroxidation, DNA damage, and heightened inflammation13,14,15. These pro-oxidant activities exacerbate cellular damage, a hallmark of aging. In fact, studies have shown that elevated serum UA levels correlate with markers of cellular aging, suggesting that elevated UA may accelerate the aging process and associated diseases16,17. Conversely, HDL is known for its anti-atherosclerotic properties, including the removal of cellular lipids, which helps protect against cardiovascular diseases. HDL also plays a crucial role in promoting cell survival and modulating key age-related signaling pathways, such as those involving the longevity factor Klotho18,19. Given these protective functions, HDL is considered a beneficial factor in counteracting age-related pathologies. Recent research has, however, shifted focus to the ratio of uric acid to HDL cholesterol (UHR) as a promising new biomarker for assessing an individual’s inflammatory and metabolic status20,21. While UA and HDL individually provide valuable information about metabolic and cardiovascular health, the UHR has been shown to outperform these single markers by capturing the combined effects of both, offering a more comprehensive measure of an individual’s risk for age-related diseases. Elevated UHR has been correlated with multiple metabolic and cardiovascular conditions, and an increased UHR value may signal a higher risk for cardiovascular events as well as an accelerated aging process. As such, UHR has emerged as a potential marker for both aging and the early detection of age-related diseases. This rationale is supported by previous studies, which highlight the clinical utility of UHR in evaluating age-related risks22. Despite these promising findings, further studies investigating the relationship between UHR and biological age acceleration remain limited.
In this study, we utilized data from the National Health and Nutrition Examination Survey (NHANES) 2009–2018 to investigate the association between the UHR and biological age acceleration, assessed by two validated measures: Phenotypic Age (PhenoAge) and the Klemera–Doubal Method (KDM) biological age. The primary objective of this study was to determine whether UHR is independently associated with accelerated biological aging in the general U.S. adult population. The secondary objective was to explore whether these associations differ across population subgroups defined by demographic, lifestyle, and clinical characteristics. We hypothesized that (1) higher UHR is positively associated with accelerated biological aging, and (2) the associations between UHR and biological age acceleration may vary across subgroups, such as age, sex, and comorbid disease status.
Materials and methods
Study population
NHANES is a comprehensive, nationally representative survey designed to collect detailed health and nutritional data from the U.S. civilian population using a stratified, multistage probability sampling method. This stratified, multistage probability sampling design ensures that the NHANES participants are representative of the civilian, non-institutionalized U.S. population, thereby enhancing the generalizability of study findings23. The study protocol was approved by the Research Ethics Review Board of the National Center for Health Statistics (NCHS) at the Centers for disease control and prevention (CDC), and all participants provided written informed consent. Detailed information regarding the NHANES dataset is publicly accessible on the NHANES website (https://www.cdc.gov/nchs/nhanes).
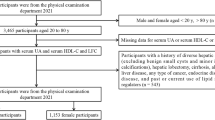
The initial study population consisted of 49,693 individuals. After excluding 18,165 participants with missing UHR data, 7185 with missing biological age data, and 411 individuals who were either pregnant or under the age of 20, the final cohort included 23,932 participants (Fig. 1).
Data collection
Assessment of UHR
HDL and uric acid levels were measured from fasting morning blood samples. HDL was isolated using magnesium sulfate/dextran and converted to HDL cholesterol with polyethylene glycol esterase, followed by colorimetric measurement at 600 nm. Uric acid was measured with a DxC800 analyzer using a peroxidase reaction, with colorimetric detection at 520 nm. UHR (%) was calculated as UA (mg/dL) divided by HDL (mg/dL), multiplied by 100.
Assessment of biological age
Biological age was assessed using two well-validated algorithms: Phenotypic Age (PhenoAge) and the Klemera–Doubal Method (KDM) biological age. While PhenoAge primarily reflects disease risk and mortality prediction, KDM Age is designed to capture physiological aging based on biomarker variability.
PhenoAge was originally developed to predict mortality by integrating chronological age with a panel of clinical biomarkers. We followed the algorithm originally proposed by Levine et al., which estimates mortality risk using chronological age plus selected biomarkers24. In the present study, we applied the algorithm using biomarkers consistently available across all NHANES cycles: albumin, creatinine, glucose, lymphocyte percentage, mean Cell Volume (MCV), red cell distribution width, alkaline phosphatase, and white blood cell count24,25. C-reactive protein (CRP), which is part of the original PhenoAge formulation, was not consistently measured across all five survey cycles and was therefore excluded to ensure comparability. KDM Age was calculated following the method proposed by Klemera and Doubal (2006), which employs a weighted regression of biomarkers on chronological age to minimize error variance26. The biomarkers incorporated were systolic blood pressure, total cholesterol, creatinine, blood urea nitrogen, glycated hemoglobin (HbA1c), serum albumin, and body mass index (BMI)26. Both PhenoAge and KDM Age were computed using the BioAge R package (http://github.com/dayoonkwon/BioAge)27.
To measure aging rate, Phenotypic Age Acceleration (PhAA) and KDM Age Acceleration (KDM-AA) were calculated as residuals from regressing PhenoAge and KDM-BA on chronological age, respectively. Positive residuals indicate accelerated aging, while negative residuals suggest decelerated aging.
Assessment of covariates
We considered several covariates, including demographic factors, lifestyle habits, and metabolic disease status, which were obtained from standardized NHANES household interviews, laboratory tests, and physical examinations. Previous studies have consistently reported that demographic factors (e.g., age, sex, race/ethnicity, education)28,29, lifestyle factors (e.g., smoking, alcohol consumption, physical activity)30,31,32,33,34,35, and clinical conditions (e.g., cardiovascular disease, hypertension, diabetes, cancer)36 influence uric acid metabolism, HDL levels, UHR, or biological aging. These covariates were therefore included to minimize residual confounding. Demographic variables such as age, sex, and race/ethnicity were collected from interviewer-administered questionnaires, and education levels were classified into three categories: less than high school, high school graduates, and more than high school. Lifestyle variables were derived from self-reported survey data: alcohol consumption was categorized as never (≤ 12 drinks in a lifetime), low-to-moderate (≤ 1 drink/day for women, ≤ 2 drinks/day for men), or high (≥ 1 drink/day for women, ≥ 2 drinks/day for men) during the past year; smoking status was defined as never (≤ 100 cigarettes in a lifetime), former (≥ 100 cigarettes in a lifetime but not currently smoking), or current (≥ 100 cigarettes in a lifetime and currently smoking); and physical activity was assessed using the NHANES Physical Activity Questionnaire, with metabolic equivalent (MET) values calculated from standardized energy expenditure and stratified as < 600, 600–3999, or ≥ 4000 min/week. Clinical histories were collected through standardized medical questionnaires and examination data: cardiovascular disease included self-reported physician diagnoses of congestive heart failure, coronary artery disease, angina, or myocardial infarction; hypertension was defined as systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 90 mmHg, a prior diagnosis of hypertension, or current use of antihypertensive medications; diabetes mellitus was defined as a previous diagnosis, current use of diabetes medication or insulin, fasting blood glucose ≥ 126 mg/dL, 2-hour glucose ≥ 200 mg/dL, or HbA1c ≥ 6.5%; and cancer status was identified through a positive response to the relevant survey item.
Statistical analyses
In this study, the NHANES complex sampling design was carefully considered. The full sample 10-year mobile examination center weight was used to calculate estimates for the U.S. non-institutionalized population. Categorical variables were compared between groups using the chi-square test. For continuous variables, distributional assumptions were evaluated using histograms, Q–Q plots, the Shapiro–Wilk test, and distributional indices (skewness and kurtosis). Variables were considered non-normal if the Shapiro–Wilk test p-value was < 0.05 or if absolute skewness > 1 or absolute kurtosis > 1; these were summarized as median (IQR) and compared using the Kruskal–Wallis rank sum test, with the Mann–Whitney U test employed for pairwise comparisons. Potential outliers in UHR, UA, and HDL were screened using Tukey’s rule (values < Q1 − 1.5×IQR or > Q3 + 1.5×IQR) and regression diagnostics (standardized residuals > 3 SD, high leverage, or Cook’s distance > 4/n). Outliers were not excluded from the primary analyses; instead, robust survey-weighted models were employed. As a sensitivity analysis, UHR, UA, and HDL were winsorized at the 1st and 99th percentiles, and the results were consistent with the primary findings. For regression analyses, key model assumptions were evaluated. For multiple linear regression models, multicollinearity was examined using variance inflation factors (VIF), with all covariates demonstrating VIF values below 5, indicating no significant collinearity. Linearity, homoscedasticity, and the normality of residuals were checked using residual-versus-fitted plots and Q–Q plots37. For logistic regression models, the linearity of continuous variables with the logit was confirmed using the Box–Tidwell test, and overall model calibration was assessed by the Hosmer–Lemeshow goodness-of-fit test. For restricted cubic spline models, knot selection was guided by Akaike’s Information Criterion (AIC), and model fit was verified to ensure robust specification.
Weighted multiple linear regression was employed to estimate regression coefficients (β) and 95% confidence intervals (95% CI) for UHR, UA, and HDL in relation to biological age acceleration. Additionally, weighted logistic regression models were applied, using PhAA and KDM-AA values greater than zero as outcome variables. Continuous UHR, UA, and HDL were assessed using standardized methods to ensure consistency across measures. To further explore the associations, UHR, UA, and HDL were categorized into tertiles, and trend p-values were calculated. Three models were utilized in this study: Model 1 (unadjusted), Model 2 (adjusted for age, sex, and race), and Model 3 (further adjusted for education level, alcohol consumption, smoking status, cardiovascular disease, hypertension, diabetes, cancer, and physical activity).
To address missing data in the covariates, multivariate imputation by chained equations was applied, using random forest algorithms to impute the missing values38. Restricted Cubic Spline (RCS) regression was further applied to evaluate potential non-linear associations between UHR, UA, HDL, and biological age acceleration (PhAA and KDM-AA). In these models, PhAA and KDM-AA served as outcomes, while UHR, UA, and HDL were modeled as continuous predictors. Four knots were placed at the 5th, 35th, 65th, and 95th percentiles of the predictor distribution, following recommended practice for spline modeling. All RCS models were adjusted for the same covariates as Model 3, including demographic, lifestyle, and clinical factors. Linearity was tested by comparing models with and without spline terms, and model fit was evaluated using Akaike’s Information Criterion (AIC). Subgroup analyses were performed by stratifying participants according to age (< 60 vs. ≥60 years), sex (male vs. female), race (Black vs. others), BMI (< 30 vs. ≥30), hypertension (yes/no), cardiovascular disease (yes/no), cancer (yes/no), and physical activity level (< 600, 600–3999, or ≥ 4000 min/week). Interaction analyses were further conducted to evaluate whether these covariates modified the observed associations. Given the large NHANES sample size, only adjusted p-values were reported for multiple regression models to reduce the risk of type I error. To better capture the strength of associations, effect size statistics were also provided. For linear regression, standardized β coefficients and Cohen’s f² were reported39; for logistic regression, odds ratios were supplemented with McFadden’s and Nagelkerke’s pseudo R²40,41. In addition, standardized mean differences (SMD) were used to describe baseline group differences. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were carried out using R software, version 4.2.2 (https://www.R-project.org ; R Foundation, Austria).
Results
Characteristics of participants
Table 1 presents the baseline characteristics of the study population (n = 23,932), stratified by accelerated and non-accelerated biological age, defined as whether PhAA was greater than zero. Individuals in the accelerated age group (n = 6,846) were significantly older than those in the non-accelerated group (n = 17,086) (52.12 ± 18.14 vs. 48.69 ± 17.29 years, p < 0.001). Men accounted for 65.6% of the accelerated group compared with 42.8% of the non-accelerated group (p < 0.001), while non-Hispanic Black participants were also more prevalent in the accelerated group (26.1% vs. 18.1%, p < 0.001). Educational attainment differed significantly between groups: 41.6% of the accelerated group had only a high school education, compared with 33.3% in the non-accelerated group (p < 0.001).
With respect to lifestyle factors, current smoking was reported by 29.7% of the accelerated group versus 15.9% of the non-accelerated group (p < 0.001), and physical activity levels < 600 MET-min/week were more common among those with accelerated aging (42.1% vs. 38.2%, p < 0.001). The accelerated group also had a higher proportion of individuals with BMI ≥ 30 kg/m² (mean 31.57 ± 8.04 vs. 28.35 ± 6.29, p < 0.001) and lower poverty income ratio (median 1.79 [1.02–3.45] vs. 2.19 [1.12–4.28], p < 0.001).
In terms of chronic diseases, hypertension was more prevalent in the accelerated group (49.9% vs. 30.6%, p < 0.001), as were cardiovascular disease (15.4% vs. 8.1%, p < 0.001), diabetes (16.2% vs. 9.8%, p < 0.001), cancer (10.8% vs. 8.8%, p < 0.001), and chronic kidney disease (31.7% vs. 11.2%, p < 0.001).
Biochemical and hematologic parameters also showed marked differences. Compared with the non-accelerated group, participants with accelerated aging had higher white blood cell counts (8.46 ± 2.35 vs. 6.65 ± 1.70 × 10³/µL, p < 0.001) and lower serum albumin levels (40.99 ± 3.39 vs. 42.96 ± 3.12 g/L, p < 0.001). They also exhibited higher uric acid concentrations (5.98 ± 1.52 vs. 5.25 ± 1.34 mg/dL, p < 0.001), lower HDL cholesterol levels (median 46.00 [38.00–56.00] vs. 52.00 [43.00–64.00] mg/dL, p < 0.001), and consequently a significantly higher UHR (median 12.68 [9.55–16.67] vs. 9.69 [7.14–13.08], p < 0.001).
Taken together, these results demonstrate that individuals with accelerated biological age were older, more likely to be male and Black, had higher burdens of metabolic and chronic diseases, and displayed a more adverse metabolic profile, including elevated UHR and UA and reduced HDL cholesterol. These findings support the hypothesis that UHR is closely associated with phenotypic age acceleration.
Association between UHR and biological age acceleration
Table 2 summarizes the results of weighted linear regression analyses investigating the associations between UHR and biological age acceleration. In all three models, UHR showed a significant positive association with both PhAA and KDM-AA. For continuous UHR, in Model 3, which adjusted for a wide range of covariates including age, sex, race, education level, alcohol consumption, smoking status, physical activity, cardiovascular disease, hypertension, diabetes, and cancer, demonstrated a significant association with both PhAA (β = 1.00, 95% CI: 0.94–1.06, p < 0.001) and KDM-AA (β = 1.39, 95% CI: 1.28–1.50, p < 0.001). When UHR was categorized into tertiles, participants in the highest tertile showed a stronger association with PhAA (β = 2.18, 95% CI: 2.03–2.32, p < 0.001) and KDM-AA (β = 2.66, 95% CI: 2.39–2.94, p < 0.001), compared to those in the lowest tertile. A clear dose-response relationship was observed with statistically significant trend p-values (< 0.001).
Table 3 presents the results of weighted logistic regression analyses for the risk of biological age acceleration associated with UHR. For continuous UHR in Model 3, the odds ratios (ORs) were 1.45 (95% CI 1.40–1.50, p < 0.001) for PhAA and 1.30 (95% CI 1.26–1.34, p < 0.001) for KDM-AA. Similarly, when categorized into tertiles, the highest UHR tertile was associated with significantly increased odds of accelerated biological aging compared to the lowest tertile, with ORs of 2.40 (95% CI 2.20–2.62, p < 0.001) for PhAA and 1.72 (95% CI 1.59–1.86, p < 0.001) for KDM-AA. The trend analysis indicated a robust dose-response relationship across all tertiles (p for trend < 0.001).
These findings highlight the independent association between higher UHR and accelerated biological age, underscoring its potential as a biomarker for biological aging.
Association of UA and HDL with age acceleration
Tables 4, 5, 6 and 7 summarize the results of weighted linear and logistic regression analyses examining the associations between UA, HDL, and the risk of biological age acceleration. Continuous UA was significantly associated with both PhAA (β = 0.90, 95% CI: 0.84–0.96, p < 0.001) and KDM-AA (β = 1.99, 95% CI: 1.88–2.10, p < 0.001) in Model 3 (Table 4). Tertile analysis revealed that participants in the highest UA tertile exhibited stronger associations with PhAA (β = 1.74, 95% CI: 1.59–1.89, p < 0.001) and KDM-AA (β = 3.68, p < 0.001), with a significant dose-response relationship (p for trend < 0.001). Weighted logistic regression further confirmed that higher UA levels were significantly associated with an increased risk of biological age acceleration (Table 5). In contrast, HDL was inversely associated with biological age acceleration for both PhAA and KDM-AA (Table 6). For PhAA, the adjusted β value in Model 3 was − 0.71 (95% CI: -0.77 to -0.66, p < 0.001), with a stronger inverse association observed in the highest HDL tertile (β = -1.54, 95% CI: -1.68 to -1.40, p < 0.001), demonstrating a dose-response relationship (p for trend < 0.001). For KDM-AA, the adjusted β for continuous HDL was − 0.15 (95% CI: -0.26 to -0.04, p < 0.001), and tertile-based analysis showed that participants in the highest HDL tertile had a β value of -0.45 (95% CI: -0.72 to -0.20, p < 0.001), further supporting this inverse relationship. Table 7 summarizes the logistic regression results for the association of HDL with both PhAA and KDM-AA, showing the odds ratios (ORs) for both continuous HDL levels and HDL tertiles, further confirming the inverse relationship between higher HDL and accelerated biological aging. Figure 2 summarizes the associations between UHR, UA, and HDL levels with biological age acceleration.
Logistic regression analysis for the associations of uric acid (UA), high-density lipoprotein (HDL) and uric acid to high-density cholesterol ratio (UHR) with age acceleration. Regression model was adjusted for age, sex, race, education level, alcohol consumption, smoking status, cardiovascular disease, hypertension, diabetes, cancer, and physical activity. UA, HDL, UHR were standardized. Tertiles were based on the distribution of each independent variable and Tertiles 1 of each was reference.
Restricted cubic spline regression
RCS regression analyses, as shown in Fig. 3, revealed a complex association between UHR and age acceleration. The relationship between UHR and PhAA was highly non-linear (p for overall < 0.001, p for non-linearity < 0.001), indicating that the effect of UHR on age acceleration intensifies at higher UHR levels. Conversely, the relationship between UHR and KDM-AA was linear (p for overall < 0.001, p for non-linearity = 0.0694). Furthermore, analyses for UA and HDL demonstrated significant associations with age acceleration, both exhibiting non-linear relationships.
Restricted cubic spline (RCS) regression was used to analyze the relationship between uric acid (UA), high-density lipoprotein (HDL), and the uric acid-to-high-density lipoprotein ratio (UHR) with the risk of biological age acceleration. The RCS model was adjusted for age, sex, race, education level, alcohol consumption, smoking status, cardiovascular disease, hypertension, diabetes, cancer, and physical activity.
Subgroup analysis and interaction analyses
Figure 4 illustrates that the association between UHR and biological age acceleration remained significant and consistent across all evaluated subgroups. Interaction analyses identified significant modifying effects of age, sex, BMI, diabetes, and cancer for PhAA, and age, HBP, diabetes, CVD, cancer, and physical activity levels for KDM-AA.
Association between uric acid to high-density cholesterol ratio (UHR) and biological age acceleration in subgroup and interactive analyses. The logistic regression model was adjusted for age, sex, race, education level, alcohol consumption, smoking status, cardiovascular disease, hypertension, diabetes, cancer, and physical activity.
Discussion
Our study identifies a significant association between the UHR and accelerated biological aging, thereby expanding the spectrum of biomarkers linked to biological aging. The observed non-linear relationship suggests that the impact of UHR on aging varies at different concentration levels, with stronger effects observed at higher UHR levels. Subgroup analyses further revealed that this association is particularly pronounced among older individuals, women, and those with hypertension or diabetes, highlighting the potential of UHR as a targeted biomarker in these populations. Notably, our study found that the association between UHR and biological age acceleration was non-linear for PhAA but linear for KDM-AA. This divergence can be explained by the distinct methodologies and physiological interpretations of the two measures. PhenoAge was originally developed as a mortality prediction tool, incorporating chronological age and a panel of clinical biomarkers related to systemic inflammation, metabolic dysfunction, and immune dysregulation. As such, PhAA may be particularly sensitive to threshold effects of metabolic stress, leading to the observed non-linear pattern at higher UHR levels. By contrast, KDM Age is derived from a weighted regression model designed to minimize error variance between multiple biomarkers and chronological age. It captures biomarker variability that reflects general physiological aging rather than mortality risk, resulting in a more stable and linear relationship with UHR. These methodological and conceptual differences highlight that PhAA and KDM-AA provide complementary perspectives on biological aging, and they may capture distinct pathways.
Our study also demonstrates a strong positive association between elevated UA levels and biological age acceleration, consistent with previous studies that have linked hyperuricemia to various age-related diseases, including cardiovascular diseases42, neurodegenerative diseases43, and metabolic syndrome44. This relationship aligns with findings that elevated UA exacerbates oxidative stress and inflammation, key processes in aging45. However, while the current study and several others emphasize the risks associated with elevated UA, there is debate in the literature regarding the dual nature of UA in aging. For example, UA may act as an antioxidant in certain contexts46, and it has been shown to enhance lifespan47. This suggests that the relationship between UA and aging might not be entirely negative and could be influenced by the timing and individual health factors. These divergent views suggest that UA may have both harmful and protective roles depending on the context, reinforcing the need for further research into the circumstances under which UA could potentially act as a modifiable factor in aging.
In contrast to UA, our study reveals an inverse association between HDL cholesterol levels and biological age acceleration. Higher levels of HDL are linked to slower aging, which is consistent with numerous studies highlighting HDL’s protective role against cardiovascular diseases and other age-related conditions48,49. HDL not only reduces cardiovascular disease risk but also plays a vital role in mitigating oxidative stress, a critical factor in aging50,51. Our findings echo these observations, reinforcing the protective effects of HDL against biological age acceleration. However, while HDL cholesterol is generally considered protective, this study also highlights certain complexities. For instance, HDL may be dysfunctional in certain populations, particularly those with metabolic syndrome or diabetes52,53. In these individuals, HDL may not exhibit its typical protective effects. Our study further supports this complexity, as we observed significant interaction effects with diabetes, obesity, and physical activity, suggesting that the relationship between HDL and aging is not straightforward and may be influenced by other factors such as genetics and lifestyle.
While previous studies have explored the individual contributions of UA and HDL to aging, our research uniquely investigates the combined effect of UHR on comprehensive measures of biological age, providing a more nuanced understanding of the aging process. One of the strengths of this study is the use of RCS to identify nonlinear associations between biomarkers and biological age acceleration. We found that both UA and HDL exhibit nonlinear relationships with age acceleration, which is consistent with findings that the relationship between biomarkers and aging is complex and cannot be adequately captured by simple linear models54,55. The use of RCS regression in our study provided a more nuanced understanding of how biomarkers like UA and HDL interact with biological age, revealing that their impact intensifies at certain threshold levels. This nonlinear relationship suggests that aging is not a uniform process and that biomarkers may have differential effects depending on their levels and the individual’s health status. It also highlights the importance of personalized medicine in aging, where interventions targeting UA or HDL must consider an individual’s baseline levels and overall health profile.
The subgroup analysis in this study sheds light on the role of modifiable risk factors such as diabetes, cancer, and physical activity in accelerating biological aging. These factors significantly modified the relationship between biomarkers like UA and HDL and biological age. This finding is consistent with previous studies indicating that physical activity can mitigate the effects of aging and inflammatory biomarkers56,57. Moreover, our study supports the hypothesis that lifestyle interventions, such as increasing physical activity and managing chronic conditions like diabetes, could help delay biological age acceleration and reduce the risk of age-related diseases. Interestingly, the effects of lifestyle factors on aging may vary based on genetic predisposition and environmental factors58. As aging is a multifactorial process influenced by both genetic and environmental factors, future research should continue to explore the intricate relationships between biomarkers, lifestyle choices, and aging. By developing a deeper understanding of these interactions, we can better design targeted interventions aimed at promoting healthy aging and reducing the burden of age-related diseases.
Despite the strengths of our study, including the large sample size and the use of robust, nationally representative data from NHANES, several limitations should be acknowledged. First, the cross-sectional design precludes the establishment of causal inferences, and the possibility of residual confounding cannot be excluded despite adjustment for a wide range of covariates. Second, because the study population was predominantly White, the generalizability of our findings to more diverse racial and ethnic groups may be limited. Future longitudinal studies in multi-ethnic cohorts are warranted to validate these associations and to elucidate the underlying mechanisms by which UHR influences biological aging. Third, several potentially relevant covariates were not incorporated into our analyses due to their incomplete or inconsistent availability across NHANES cycles. These include detailed dietary intake data, additional renal function markers beyond creatinine and blood urea nitrogen, and medication use such as diuretics, statins, and uric acid–lowering therapies. The omission of these variables may have introduced residual confounding, as they could plausibly affect both uric acid/HDL metabolism and biological aging processes. Future investigations with more comprehensive clinical, biochemical, and lifestyle data will be essential to validate and extend our observations, and to enhance the clinical utility of UHR as a predictive biomarker for age-related health outcomes. Fourth, genetic variation was not considered in our analysis. Previous studies have shown that genetic and epigenetic factors can influence both uric acid metabolism and aging-related pathways59,60. The absence of genetic information in the NHANES dataset may therefore limit our ability to fully disentangle the biological mechanisms underlying the observed associations. Future research integrating genomic, epigenetic, and environmental data will be essential to provide a more comprehensive understanding of how UHR contributes to biological age acceleration.
Conclusion
The results indicate a significant positive association between UHR and biological age acceleration markers. Specifically, the relationship with PhAA was non-linear, whereas it was linear with KDM-AA. Subgroup analyses confirmed the robustness of this association, and interaction analyses identified key modifiers, including age, sex, BMI, diabetes, cancer, hypertension, cardiovascular disease, and physical activity. Future research should explore the underlying biological mechanisms linking UHR to accelerated aging.
Data availability
The datasets generated and analyzed during the current study are publicly available from the National Center for Health Statistics at https://wwwn.cdc.gov/nchs/nhanes.
References
Rae, M. J. et al. The demographic and biomedical case for late-life interventions in aging. Sci. Transl. Med. 2 (40), 4021. https://doi.org/10.1126/scitranslmed.3000822 (2010).
MacNee, W., Rabinovich, R. A. & Choudhury, G. Ageing and the border between health and disease. Eur. Respir. J. 44 (5), 1332–1352. https://doi.org/10.1183/09031936.00134014 (2014).
Niccoli, T. & Partridge, L. Ageing as a risk factor for disease. Curr. Biology: CB. 22 (17), 741–752. https://doi.org/10.1016/j.cub.2012.07.024 (2012). [published Online First: 2012/09/15].
Cortes-Canteli, M. & Iadecola, C. Alzheimer’s disease and vascular aging: JACC focus seminar. J. Am. Coll. Cardiol. 75 (8), 942–951. https://doi.org/10.1016/j.jacc.2019.10.062 (2020).
Leidal, A. M., Levine, B. & Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 20 (12), 1338–1348. https://doi.org/10.1038/s41556-018-0235-8 (2018).
Xiong, Y. et al. The association between the platelet to white blood cell ratio and chronic kidney disease in an aging population: A four-year follow-up study. J. Clin. Med. 12 (22). https://doi.org/10.3390/jcm12227073 (2023).
Diebel, L. W. M. & Rockwood, K. Determination of biological age: geriatric assessment vs biological biomarkers. Curr. Oncol. Rep. 23 (9), 104. https://doi.org/10.1007/s11912-021-01097-9 (2021).
Bharath, L. P. et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate Aging-Associated inflammation. Cell Metabol. 32 (1), 44–55e6. https://doi.org/10.1016/j.cmet.2020.04.015 (2020).
Lei, J. et al. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis. Protein cell. 13 (3), 220–226. https://doi.org/10.1007/s13238-021-00860-9 (2022).
Son, J. M. & Lee, C. Mitochondria: multifaceted regulators of aging. BMB Rep. 52 (1), 13–23. https://doi.org/10.5483/BMBRep.2019.52.1.300 (2019).
Lu, W. H. Effect of modifiable lifestyle factors on biological aging. JAR Life. 13, 88–92. https://doi.org/10.14283/jarlife.2024.13 (2024).
Scott, A. J., Ellison, M. & Sinclair, D. A. The economic value of targeting aging. Nat. Aging. 1 (7), 616–623. https://doi.org/10.1038/s43587-021-00080-0 (2021).
Grassi, G. et al. Uric Acid, hypertensive Phenotypes, and organ damage: data from the Pamela study. Curr. Hypertens. Rep. 24 (2), 29–35. https://doi.org/10.1007/s11906-022-01174-9 (2022).
Kimura, Y., Tsukui, D. & Kono, H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 22 (22). https://doi.org/10.3390/ijms222212394 (2021).
Wan, Q. L. et al. Uric acid induces stress resistance and extends the life span through activating the stress response factor DAF-16/FOXO and SKN-1/NRF2. Aging 12 (3), 2840-56. https://doi.org/10.18632/aging.102781 (2020).
Yu, J. et al. Negative association of serum URIC acid with peripheral blood cellular aging markers. J. Nutr. Health Aging. 23 (6), 547–551. https://doi.org/10.1007/s12603-019-1200-3 (2019).
Lee, H. J. et al. Association between serum uric acid and α-klotho protein levels in the middle-aged population. Aging 14 (6), 2537–2547. https://doi.org/10.18632/aging.203987 (2022).
Kanda, E. et al. Association of High-Density lipoprotein subclasses with chronic kidney disease Progression, Atherosclerosis, and Klotho. PloS One. 11 (11), e0166459. https://doi.org/10.1371/journal.pone.0166459 (2016).
Jiang, S. et al. The association of serum Klotho concentrations with hyperlipidemia prevalence and lipid levels among US adults: a cross-sectional study. BMC public. Health. 23 (1), 1645. https://doi.org/10.1186/s12889-023-16566-y (2023).
Lai, X. & Chen, T. Association of serum uric acid to high-density lipoprotein cholesterol ratio with all-cause and cardiovascular mortality in patients with diabetes or prediabetes: a prospective cohort study. Front. Endocrinol. 15, 1476336. https://doi.org/10.3389/fendo.2024.1476336 (2024).
Huang, X. et al. Relationship between uric acid to high-density cholesterol ratio (UHR) and Circulating α-klotho: evidence from NHANES 2007–2016. Lipids Health Dis. 23 (1), 244. https://doi.org/10.1186/s12944-024-02234-6 (2024).
Chen, Z. et al. Associations of serum uric acid-to-high density lipoprotein cholesterol ratio with age-related diseases and mortality among older population in the united States. Arch. Gerontol. Geriatr. 130, 105707. https://doi.org/10.1016/j.archger.2024.105707 (2025).
Statistics NCfH. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010 (Department of Health and Human Services Public Health Servic, 2013).
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. 10 (4), 573. (2018).
Ferrucci, L. et al. Time Metrics Aging ;123(7):740–744. (2018).
Klemera, P. Doubal SJMoa, development. A new approach to the concept and computation of biological age. 127 (3), 240–248. (2006).
Kwon, D. & Belsky, D. W. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. GeroScience 43 (6), 2795 – 808. https://doi.org/10.1007/s11357-021-00480-5 (2021).
DeBoer, M. D., Dong, L. & Gurka, M. J. J. M. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National health and nutrition survey 1999–2006. 61 (4), 554–561. (2012).
Freedman, D. S. et al. Education, race, and high-density lipoprotein cholesterol among US adults. 82 (7), 999–1006. (1992).
Williams, P. T. J. T. A. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy. Vigorously Act. Men. 87 (5), 1480–1487 (2008).
Jang, Y. S. et al. Association between smoking behavior and serum uric acid among the adults: findings from a National cross-sectional study. 18 (5), e0285080. (2023).
Čypienė, A. et al. Effect of alcohol consumption habits on early arterial aging in subjects with metabolic syndrome and elevated serum uric acid. 15 (15), 3346. (2023).
Yang, T. et al. Relationship between cigarette smoking and hyperuricemia in middle-aged and elderly population: a cross-sectional study. 37 (1), 131–136. (2017).
Salonen, J. T. et al. Relation of alcohol, physical activity, dietary fat and smoking to serum HDL and total cholesterol in young Finnish men. 13 (3), 99–102. (1985).
Latifovic, L. et al. The influence of alcohol consumption, cigarette smoking, and physical activity on leukocyte telomere length. 25 (2), 374–380. (2016).
Jee, S. H., Lee, S. Y. & Kim, M. T. J. E. J. P. C. Serum uric acid and risk of death from cancer, cardiovascular disease or all causes in men. 11 (3), 185–191. (2004).
Tabachnick, B. G., Fidell, L. S. & Ullman, J. B. Using multivariate statistics: Pearson Boston, MA. (2007).
Xiong, Y. et al. The predictive role of hematological inflammatory markers on the prognosis of kidney injury. Int. J. Surg. (London England). 110 (4), 2453–2454. https://doi.org/10.1097/js9.0000000000001072 (2024).
Cohen, J. Statistical power analysis for the behavioral sciences. (Routledge, 2013).
McFadden, D. Conditional logit analysis of qualitative choice behavior. (1972).
Nagelkerke NJJb. A note on a general definition of the coefficient of determination. 78 (3), 691–692. (1991).
Kuwabara, M. & Hyperuricemia Cardiovascular Disease, and hypertension. Pulse (Basel Switzerland). 3 (3–4), 242–252. https://doi.org/10.1159/000443769 (2016).
Otani, N. et al. Uric acid and neurological disease: a narrative review. Front. Neurol. 14, 1164756. https://doi.org/10.3389/fneur.2023.1164756 (2023).
Raya-Cano, E. et al. Association between metabolic syndrome and uric acid: a systematic review and meta-analysis. Sci. Rep. 12 (1), 18412. https://doi.org/10.1038/s41598-022-22025-2 (2022).
Deng, Y. et al. The key role of uric acid in oxidative Stress, Inflammation, Fibrosis, Apoptosis, and immunity in the pathogenesis of atrial fibrillation. Front. Cardiovasc. Med. 8, 641136. https://doi.org/10.3389/fcvm.2021.641136 (2021).
Bowman, G. L. et al. Uric acid as a CNS antioxidant. J. Alzheimer’s Disease: JAD. 19 (4), 1331–1336. https://doi.org/10.3233/jad-2010-1330 (2010).
Cutler, R. G. et al. Uric acid enhances longevity and endurance and protects the brain against ischemia. Neurobiol. Aging. 75, 159–168. https://doi.org/10.1016/j.neurobiolaging.2018.10.031 (2019).
Navab, M. et al. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat. Reviews Cardiol. 8 (4), 222–232. https://doi.org/10.1038/nrcardio.2010.222 (2011).
Hong, B. V., Zheng, J. & Zivkovic, A. M. HDL function across the lifespan: from childhood, to pregnancy, to old age. Int. J. Mol. Sci. 24 (20). https://doi.org/10.3390/ijms242015305 (2023).
Brites, F. et al. Antioxidative activity of high-density lipoprotein (HDL): mechanistic insights into potential clinical benefit. BBA Clin. 8, 66–77. https://doi.org/10.1016/j.bbacli.2017.07.002 (2017).
Maldonado, E. et al. Aging hallmarks and the role of oxidative stress. Antioxid. (Basel Switzerland). 12 (3). https://doi.org/10.3390/antiox12030651 (2023).
Farbstein, D. & Levy, A. P. HDL dysfunction in diabetes: causes and possible treatments. Expert Rev. Cardiovasc. Ther. 10 (3), 353–361. https://doi.org/10.1586/erc.11.182 (2012).
Hansel, B. et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 89 (10), 4963–4971. https://doi.org/10.1210/jc.2004-0305 (2004).
Monaghan, C. et al. Modelling the Non-linear associations between age and health. Implications Care. 53 (4), afae178 (2024).
Moqri, M. et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell 186 (18), 3758–3775. https://doi.org/10.1016/j.cell.2023.08.003 (2023).
Beavers, K. M. et al. Long-term physical activity and inflammatory biomarkers in older adults. Med. Sci. Sports. Exerc. 42 (12), 2189–2196. https://doi.org/10.1249/MSS.0b013e3181e3ac80 (2010).
Zhu, J. et al. The association of physical activity behaviors and patterns with aging acceleration: evidence from the UK biobank. Journals Gerontology: Ser. A. 78 (5), 753–761. (2023).
Castruita, P. A. et al. Genetic, Social, and lifestyle drivers of healthy aging and longevity. Curr. Genetic Med. Rep. 10 (3), 25–34. https://doi.org/10.1007/s40142-022-00205-w (2022).
Rivera-Paredez, B. et al. Influence of genetic and non-genetic risk factors for serum uric acid levels and hyperuricemia in Mexicans. 11 (6), 1336. (2019).
Pal, S. & Tyler, J. K. J. S. Epigenetics Aging 2 (7), e1600584. (2016).
Acknowledgements
I would like to express my gratitude to Dr. Fengping Zhang for her guidance on the statistical aspects of this article.
Funding
This work was supported by grants from the Sichuan Science and Technology Program to Ying Zhao (2022ZYZFSY01 and 2023YFS0099), and the high quality development of Guang ‘an People’s Hospital to Ying Zhao (21FZ002).
Author information
Authors and Affiliations
Contributions
X. C.: Conceptualization, data analysis, and writing of the manuscript.H. D.: Data collection, statistical analysis, and manuscript writing.F. S.: Data curation, review and editing of the manuscript.F. Q.: Supervision, conceptualization, methodology, and manuscript review.Y. Z.: Study design, supervision, manuscript revision, and final approval of the manuscript.#X. C. and H. D. contributed equally to this work.*F. Q. and Y. Z. are co-corresponding authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This research analyzed de-identified information downloaded from the National Health and Nutrition Examination Survey public database. The National Center for Health Statistics Ethics Review Committee granted ethics approval. All methods were carried out in accordance with relevant guidelines and regulations (declaration of Helsinki). All individuals provided written informed consent before participating in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, X., Dong, H., Shi, F. et al. Association of uric acid to high-density cholesterol ratio (UHR) with biological age acceleration: evidence from NHANES 2009–2018. Sci Rep 15, 41847 (2025). https://doi.org/10.1038/s41598-025-25877-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25877-6