Abstract
Atrial fibrillation (AF) patients frequently require polypharmacy, increasing the risk of drug-drug interactions and hepatotoxicity. We analyzed the use of drugs potentially associated with liver injury in anticoagulated AF patients. We applied the LiverTox classification to AF patients from the START registry, categorizing them based on the use of LiverTox A drugs (high risk of liver toxicity). Logistic regression was used to calculate the odds ratios (ORs) and 95% confidence intervals (95%CIs) for factors associated with the use of these drugs and their impact on liver function tests (LFTs). Median age was 81 years with 46% of women. 8,215 AF patients were taking 40,355 distinct molecules at enrolment, with a median of 5 drugs per patient (IQR 3–7). Overall, 3,416 (41.6%) received at least one LiverTox A drug. These patients were more often male, overweight, smokers, hypertensive, diabetic, with cardiovascular or cerebrovascular history, and on polypharmacy. The most commonly prescribed LiverTox A drugs were statins, amiodarone, and allopurinol, both in the overall population and within subgroups stratified by major cardiovascular conditions. In multivariable logistic regression, among LiverTox A drugs, amiodarone (OR 1.61, 95%CI 1.29–2.00) and methimazole (OR 1.83, 95%CI 1.04–3.03) were associated with elevated LFTs. Among frequently prescribed drugs, warfarin, furosemide, ramipril, lansoprazole, and canrenone were also associated with increased LFTs. Direct oral anticoagulants, compared to warfarin, showed a lower risk of increased LFTs after adjustment for confounders. In conclusion, many AF patients are treated with drugs at high risk of hepatotoxicity.
Similar content being viewed by others
Introduction
Elevation of liver function tests (LFTs) is a common finding in patients with atrial fibrillation (AF)1. This elevation may arise from various causes, including metabolic-associated steatotic liver disease, which is highly prevalent in the AF population due to the burden of cardio-metabolic risk factors, such as obesity and diabetes, that are strongly associated to liver disease2. Furthermore, a significant proportion of AF patients may have undiagnosed liver fibrosis, which can be identified using non-invasive tools like the FIB-4 index3,4,5.
Moreover, AF management often requires polypharmacy, exposing patients to potential drug-drug interactions and drug-induced liver injury (DILI)6. DILI is a relatively rare yet important cause of liver disease, requiring the consideration of all types of medications, even when another cause appears likely. With an estimated incidence of approximately 19 cases per 100,000 individuals7 DILI can be severe, although rarely fatal, and it usually improves upon discontinuation of the offending drug8. The growing number of medications used by patients complicates the detection and diagnosis of DILI, as its presentation can resemble a variety of liver disorders, ranging from acute hepatitis to chronic liver injury, and can occur even months after exposure to certain agents9.
To address these challenges, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Library of Medicine, and the Drug-Induced Liver Injury Network developed the so-called LiverTox10.
LiverTox is a free, open-access website11 that provides comprehensive information on the hepatotoxicity of currently used medications based on a rigorous literature search of published case reports from the previous 50 years. LiverTox uses a five-point likelihood score to classify drugs based on their potential to cause DILI. These classifications range from Class A, indicating well-established hepatotoxicity risk with more than 50 reported cases, to Class E, suggesting no evidence of liver damage despite isolated case reports.
To date, no studies have directly investigated the risk of elevated LFTs and DILI in patients with AF. Given that patients with AF are frequently prescribed a wide range of medications, which may increase the risk of adverse outcomes12, it is necessary to examine whether this population is also at risk for DILI and whether this risk translates into clinical hepatotoxicity.
The aim of this study is to explore the distribution of drugs with a high risk for DILI, as classified by the LiverTox score, in patients with AF enrolled in the START registry. Additionally, association between high-risk medications and clinical outcomes, such as increases in LFTs and hepatotoxicity markers will be evaluated.
Methods
START registry
The START registry is an ongoing, observational, multicenter cohort study enrolling patients aged 18 and older in Italy who begin anticoagulation therapy for AF management. Details of the START registry are described elsewhere13. The study protocol is registered on ClinicalTrials.gov (NCT02219984) and was approved by the Institutional Review Board at each participating center, with informed consent obtained from all patients before enrolment. The protocol adheres to the ethical guidelines of the Helsinki Declaration.
Baseline features
Demographic, clinical, and laboratory data were collected by physicians during clinical examination and were recorded using an internet-based case report form at enrolment during first visit in the outpatient clinic. The collected variables included age, sex, body mass index (BMI), family history of cardiovascular diseases, smoking habits, alcohol consumption, cardiovascular risk factors, concomitant diseases, and laboratory tests such as creatinine, platelets, and transaminases. A threshold of 40 U/L for AST and 45 U/L for ALT was used to define elevated LFTs. Additionally, the FIB-4 score was calculated as a further parameter to assess the liver toxicity profile, with a threshold of 3.25 used to define high levels5.
Inclusion and exclusion criteria
All consecutive adult patients with AF requiring oral anticoagulation were included in the study.
As previously reported14 patients treated with low-molecular-weight heparin were excluded. Patients with a life expectancy < 6 months, not residents in the participant region, or planning on leaving in the next 6 months were not included in the registry, as well as patients already enrolled in phase 2 or 3 clinical studies. Patients enrolled in other observational, or phase 4 studies were considered eligible for the study.
Pharmacological anamnesis and drug classification
Drugs reported in pharmacological anamnesis were considered. Combination drugs containing two different active substances were considered as two different drugs.
High Dose [HD] was defined by LiverTox11 as drugs that could cause liver injury only at high doses (e.g. drug overdose), not when administered at normal or therapeutic doses.
All prescribed medications were evaluated individually and classified according to their mechanism of action. Supplements, and inhaled medications were excluded from the analysis. Polypharmacy was defined as the concomitant use of at least five different medications15. Each drug was then classified according to LiverTox Score. Detailed descriptions of the classifications and levels of evidence are provided in Supplementary Material 1.
Primary and secondary endpoints
The primary objective of this study was to assess the prescription of potentially hepatotoxic drugs in patients with AF, considering both the overall population and stratifying by major comorbidities. Secondly, factors associated with an increased likelihood of prescribing Livertox A drugs were examined.
Following the epidemiological analysis, the impact of Livertox A drug use—both as a class and considering each drug individually—on hepatotoxicity markers was evaluated. Lastly, the association between use of the top 25 most prescribed drugs and increase of hepatic markers was assessed, to provide valuable insights and raise awareness among prescribing physicians.
Statistical analysis
Descriptive statistics were used to summarize patients’ characteristics. Continuous variables were reported as medians and interquartile ranges (IQR), while categorical variables were presented as counts and percentages. To compare patient groups, Wilcoxon rank-sum test was used for continuous variables, and chi-square or Fisher’s exact tests for categorical variables, as appropriate. Univariable and multivariable logistic regression analyses identified predictors of transaminase and FIB-4 elevation. Model coefficients represented the strength and direction of associations between predictor variables (e.g., LiverTox A drug usage, demographic factors, comorbidities) and liver injury risk.
The multivariate model to assess factors associated to Livertox A drugs use included the following variables: sex, age (≥ 90), geographic area, BMI (> 25), family history of cardiovascular disease, cancer, diabetes, hypertension, previous cerebrovascular events, ischemic heart disease, heart failure, peripheral vascular disease, chronic respiratory disease, kidney function class, and elevated transaminases. Multivariate models assessing each drug impact on elevation of hepatotoxicity markers was adjusted for CHA₂DS₂-VASc score, creatinine clearance, BMI, and the presence of cirrhosis with esophageal varices. Data analysis was conducted using R Software (v. 4.2.3) and SPSS (IBM SPSS-25, SPSS Inc.), with a two-tailed significance level of α = 0.05 for all tests.
Results
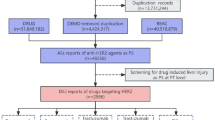
A total of 8,215 patients were included in the study, of whom 3,754 (45.7%) were women, with a median age of 81 years (IQR 75–86).
Descriptive analysis
From a pharmacological perspective, 8,215 AF patients were taking 40,355 distinct molecules at enrolment, with each patient receiving a median of 5 drugs (IQR 3—7). The most commonly prescribed drug classes were antihypertensives, anticoagulants, and diuretics, each accounting for 25.3%, 20.4% and 10.7% of all the prescribed medications, respectively. Each drug was subsequently categorized individually using the LiverTox classification system, with detailed classifications provided in Supplementary Table 2.
Baseline characteristics of the population, stratified by the use of at least one LiverTox A drug, are summarized in Table 1. Patients using at least one LiverTox A drug were more likely to be male, less likely to be aged over 90, a slightly reduced renal function, and exhibited a higher prevalence of overweight status, and cardiovascular risk factors such as smoking, hypertension, diabetes, prior cerebrovascular events, ischemic heart disease, and chronic respiratory disease. Regarding baseline hepatic function, levels of transaminases (AST and ALT) and FIB-4 values were clinically comparable between the groups. Notably, patients using at least one LiverTox A drug were more likely to be on polypharmacy (74% taking five or more drugs, with 8.4% taking ≥ 10 drugs) and more frequently used LiverTox A [HD] or B drugs.
Distribution of LiverTox A drug consumption is illustrated in Fig. 1. Antihyperlipidemic represent the most commonly prescribed drug class, with atorvastatin, simvastatin, and rosuvastatin prescribed to 1,128 (13.7%), 807 (9.8%), and 358 (4.4%) of the population, respectively. Amiodarone is the second most frequently prescribed LiverTox A drug, used by 925 (11.3%) individuals, followed by allopurinol, prescribed to 626 (7.6%) patients. Other LiverTox A drugs, including methimazole (118 patients, 1.4%) and ticlopidine (113 patients, 1.4%), were prescribed to slightly more than 1% of the cohort.
A stratified analysis was conducted to identify the most prescribed LiverTox A and A [HD] drugs in patients with ischemic disease, diabetes, hypertension, cancer, renal impairment, or aged over 75 years (Supplementary Table 3). Overall, prescription trends mirrored those observed in the general population, with atorvastatin, amiodarone, simvastatin, and allopurinol being the most commonly used drugs across all predefined subgroups. Among LiverTox A [HD] drugs, aspirin and insulin were the most frequently prescribed.
Prescription trends for other LiverTox drug classes were analyzed in patients already taking at least one LiverTox A drug (N = 3416) (Table 2). A substantial proportion of these patients were also prescribed one (33.5%) or at least two (2.9%) LiverTox A [HD] drugs. LiverTox B drugs showed even higher rates of co-prescription, with 37.3% taking one and 13.7% taking at least two. Notably, the majority of patients in this cohort (58.9%) were prescribed at least two or more LiverTox C drugs, while 31% were prescribed one.
We further investigated baseline factors associated with the prescription of at least one LiverTox A drug (Table 3). Multivariable analysis revealed that ischemic heart disease (OR: 4.88, 95% CI: 4.15–5.76), severely decreased kidney function (OR: 1.68, 95% CI: 1.27–2.24), diabetes (OR: 1.62, 95% CI: 1.41–1.86), and previous cerebrovascular disease (OR: 1.40, 95% CI: 1.20–1.62) were the most significant independent predictors. Hypertension, mild-to-moderate reductions in kidney function, chronic respiratory disease, and heart failure were also significantly associated with LiverTox A drug use. In contrast, age over 90 years emerged as the only inversely associated factor, with LiverTox A drug prescription (OR: 0.54, 95% CI: 0.45–0.65).
Impact of LiverTox scores on LFTs
The effect of individual LiverTox A drugs on markers of hepatic impairment was also assessed. Among drugs, amiodarone (OR: 1.63, 95% CI: 1.31–2.02, p < 0.001) and methimazole (OR: 1.77, 95% CI: 1.01–2.91, p = 0.033) were significantly associated with an increase in transaminase levels (Table 4). Conversely, simvastatin was associated with a reduced probability of having elevated transaminase levels (OR: 0.61, 95% CI: 0.44–0.82, p = 0.002). All these observations were confirmed after adjusting for CHA₂DS₂-VASc score, creatinine clearance, BMI, and the presence of cirrhosis with esophageal varices, with similar estimates.
We performed a similar analysis for the 25 most prescribed drugs, i.e. administered to at least 5% of patients, in our cohort. After adjustment, several drugs, including warfarin (OR: 2.43, 95% CI: 2.02–2.93, p < 0.001), diuretics (e.g., furosemide, canrenoate), ramipril, amiodarone, and lansoprazole, were significantly associated with an increased probability of elevated transaminase levels (Table 5). In contrast, simvastatin (OR 0.6, 95%CI 0.43–0.82) apixaban (OR 0.53, 95%CI 0.39–0.7), rivaroxaban (OR 0.51, 95%CI 0.37–0.68), and dabigatran (OR 0.52, 95%CI 0.37–0.68) were associated with a lower probability (Table 5).
When the FIB-4 score was used as an outcome, no significant increases were observed for any LiverTox A drug (Supplementary Table 4). Conversely, simvastatin was associated with a reduced probability of having a FIB-4 score > 3.25 (OR: 0.64, 95% CI: 0.46–0.87, p = 0.007).
Regarding the most frequently prescribed drugs and the risk of elevated FIB-4 score > 3.25, warfarin, furosemide, pantoprazole, and canrenoate were associated with an increased probability, whereas simvastatin and flecainide were associated with a reduced probability (Supplementary Table 5).
Discussion
Results from this observational nationwide cohort study that examined the risk of drug-associated hepatotoxicity in patients with AF on oral anticoagulants, showed that the majority of elderly AF patients are exposed to multiple medications, including those with intrinsic hepatotoxicity risks. Indeed > 50% of the population was on polypharmacy and > 40% was prescribed with at least one drug classified as high liver toxicity risk. In particular, AF patients on treatment with LiverTox A drugs were more likely to be male, overweight, smokers, with cardio-cerebrovascular or metabolic comorbidities, chronic respiratory disease, hyperthyroidism and diabetes. The most frequently prescribed LiverTox A drugs were statins, amiodarone, and allopurinol. After adjusting for covariates, amiodarone and methimazole were associated with elevated LFTs. Similar associations were also observed for lower-tier LiverTox drugs, including warfarin, furosemide, ramipril, lansoprazole, and canrenone, while DOACs had a lower hepatotoxicity risk compared to warfarin.
The number of concomitant medications is comparable to data reported in the literature, with percentages ranging from about 30% to more than 70% of patients takes five or more drugs concomitantly16,17. Importantly, half of the enrolled population takes 5 or more drugs at the same time, which is the common definition of polypharmacy, a situation that exposes patients to unnecessary risks12,15,18.
Some of these drugs are often wrongly prescribed or not optimally monitored over time. For example, allopurinol, one of the top five most prescribed drugs in this cohort, has been reported as commonly misprescribed19. This highlights an opportunity for deprescribing in relevant cases 20.
Patients exposed to at least one LiverTox A drug were generally in poorer clinical conditions and affected by more comorbidities. This may represent a ‘confounding by indication’ bias, where patients with multiple conditions inherently require more medications, including LiverTox A drugs. However, this should not justify excessive drug use in this group. Vulnerable patients should be protected from unnecessary exposure to DILI risk, which could further deteriorate their health status, by preferring lower-LiverTox-level drugs over LiverTox A drugs to minimize the potential risks to patients. Of note, very advanced age was associated with a lower probability of receiving a LiverTox A drug. This may be explained by the fact that, in very old patients, most drugs are being deprescribed, either because the risks may outweigh the benefits or because they are no longer required21.
Our findings highlight heterogeneous effects of commonly prescribed drugs on markers of hepatic impairment. Amiodarone and methimazole were confirmed as agents associated with increased hepatocellular injury, consistent with their LiverTox classification. We did not find any association of some others LiverTox A drugs with elevations in LFTs.
On the other side, several non-LiverTox A drugs were identified as being associated with higher LFTs or FIB-4 scores. This highlights the need to raise awareness of the potential hepatotoxicity of these drugs, which should be further investigated and factored into prescribing decisions. Some of these drugs, such as proton pump inhibitors22 and warfarin23, are already known to carry a hepatotoxic risk.
Other factors may also explain the observed associations. For instance, the link between canrenoate and elevated transaminase levels may be due to the fact that it is not the drug itself causing liver impairment, but rather that the drug is commonly prescribed to patients with pre-existing or advanced liver disease24. Additionally, canrenoate is frequently given to patients with heart failure, which significantly affects the prognosis of patients with AF25, and may also contribute to liver damage through heart failure-related hepatic congestion.
Additionally, the inverse association between DOACs and LFTs levels may be of clinical relevance but it warrants further exploration. This could be a result of the drugs’ pharmacological properties26, the presence of unidentified confounders, such as prescribing bias, or the fact that patients on DOACs are not using warfarin.
Similarly, the association between statins and liver toxicity risk is of interest. According to the LiverTox classification, atorvastatin, rosuvastatin, and simvastatin are categorized as high-risk (LiverTox A), while fluvastatin, lovastatin, and pravastatin fall under level B. This classification is only partially supported by a meta-analysis, which found increased odds of hypertransaminasemia for atorvastatin, rosuvastatin, and lovastatin but not for pravastatin, fluvastatin, or simvastatin27. However, in our cohort, the only significant association observed with statins was a reduced risk of LFTs for simvastatin, both for transaminases and the FIB-4 score. These findings are in keeping with a previous metanalysis showing lower levels of LFTs in patients with metabolic liver disease compared to untreated ones28.
Some general limitations affecting the reliability and usefulness of LiverTox have been highlighted10,29. First, it does not assess the quality of the published reports it includes, leading to potential reliance on poorly documented cases without robust causality assessment. Second, its coverage is limited, as it primarily includes drugs approved in the United States, and regular updates are necessary to include newly approved medications. Furthermore, in our cohort of AF patients, the LiverTox A classification did not always accurately predict hepatotoxicity risk, with some lower-graded medications being associated with higher hepatotoxicity markers. Despite these limitations, the LiverTox score remains the most accessible and evidence-based tool for categorizing drug-related liver injury risk. However, it should be used as a reference rather than a definitive measure, with the understanding that exceptions to its classifications may occur, particularly in specific subpopulations.
To our knowledge, this is the first study to assess the drug-related risk of hepatotoxicity in patients with AF. By individually classifying each drug for each patient, rather than generalizing by class, we provide a more precise understanding of the pharmacological effects of each molecule, offering a detailed population characterization and practical insights for clinicians.
However, our study has some limitations. First, as part of a registry, our data is limited to the information provided at enrollment; we lack follow-up data, which would have been valuable for monitoring hepatic marker levels over time. Additionally, we do not have other clinical or imaging data necessary to fully assess the hepatic profiles of the patients. Moreover, it is worth noting that the extent of polypharmacy is likely underestimated, as inhaled medications, vitamins, and supplements—which are widely prescribed—were not included in the analysis. Of note, we excluded inhaled medications due to their low systemic absorption that probably do not allow to reach sufficiently high serum level to have an impact on LFTs, as shown by previous studies30,31,32.
Similarly, temporary or occasional-use medications, such as non-steroidal anti-inflammatory drugs (NSAIDs) and antibiotics, were likely underreported, as patients may not have identified them as routine treatments.
Secondly, although we have coded each drug singularly, we do not have information on therapy duration and neither daily dose, both of which are critical factors in determining variations in hepatic risk.
In conclusion, polypharmacy is a significant concern in patients with AF, likely reflecting their complex comorbidity profiles. Drugs categorized by high LiverTox levels were commonly prescribed within this cohort. While the LiverTox classification remains a valuable tool for assessing hepatotoxicity risk, our findings suggest that also drugs listed as low-medium risk according to the LiverTox category may have an association with increased LFTs, suggesting that liver injury may vary according to the studied population. A comprehensive evaluation, considering also the potential liver injury risk related to cardiovascular and non-cardiovascular drugs is needed to reduce the risk of liver toxicity in this population.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Makar, G. A. et al. Incidence and prevalence of abnormal liver associated enzymes in patients with atrial fibrillation in a routine clinical care population. Pharmacoepidemiol. Drug Saf. 17, 43–51. https://doi.org/10.1002/pds.1514 (2008).
Driessen, S. et al. Metabolic dysfunction-associated steatotic liver disease and the heart. Hepatology 82, 487–503. https://doi.org/10.1097/HEP.0000000000000735 (2025).
Boeckmans, J. et al. Inflammation in liver fibrosis and atrial fibrillation: A prospective population-based proteomic study. JHEP Rep. 6, 101171. https://doi.org/10.1016/j.jhepr.2024.101171 (2024).
Kang, M. K., Park, J. G. & Kim, M. C. Association between Atrial Fibrillation and Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Yonsei. Med. J. 61, 860–867. https://doi.org/10.3349/ymj.2020.61.10.860 (2020).
Sterling, R. K. et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43, 1317–1325. https://doi.org/10.1002/hep.21178 (2006).
Chen, N. et al. Polypharmacy, Adverse Outcomes, and Treatment Effectiveness in Patients >/=75 With Atrial Fibrillation. J. Am. Heart Assoc. 9, e015089. https://doi.org/10.1161/JAHA.119.015089 (2020).
Bjornsson, E. S., Bergmann, O. M., Bjornsson, H. K., Kvaran, R. B. & Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 144, 1419–1425. https://doi.org/10.1053/j.gastro.2013.02.006 (2013).
Fontana, R. J. et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology 77, 1036–1065. https://doi.org/10.1002/hep.32689 (2023).
Chalasani, N. et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 148, 1340–1352. https://doi.org/10.1053/j.gastro.2015.03.006 (2015).
Bjornsson, E. S. & Hoofnagle, J. H. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology 63, 590–603. https://doi.org/10.1002/hep.28323 (2016).
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (2012).
Gallagher, C. et al. Polypharmacy and health outcomes in atrial fibrillation: a systematic review and meta-analysis. Open Heart 7, e001257. https://doi.org/10.1136/openhrt-2020-001257 (2020).
Antonucci, E. et al. The Italian START-Register on Anticoagulation with Focus on Atrial Fibrillation. PLoS ONE 10, e0124719. https://doi.org/10.1371/journal.pone.0124719 (2015).
Menichelli, D. et al. Renin-angiotensin-aldosterone system inhibitors and mortality risk in elderly patients with atrial fibrillation. Insights from the nationwide START registry. Eur. J. Intern Med. 119, 84–92, https://doi.org/10.1016/j.ejim.2023.08.019 (2024).
Masnoon, N., Shakib, S., Kalisch-Ellett, L. & Caughey, G. E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 17, 230. https://doi.org/10.1186/s12877-017-0621-2 (2017).
Eggebrecht, L. et al. Relevance of Polypharmacy for Clinical Outcome in Patients Receiving Vitamin K Antagonists. J. Am. Geriatr. Soc. 67, 463–470. https://doi.org/10.1111/jgs.15712 (2019).
Martinez-Montesinos, L. et al. Polypharmacy and adverse events in atrial fibrillation: Main cause or reflection of multimorbidity?. Biomed. Pharmacother. 158, 114064. https://doi.org/10.1016/j.biopha.2022.114064 (2023).
Menichelli, D. et al. Reviewing the use of antiarrhythmic drugs in elderly patients with atrial fibrillation. Expert Rev Cardiovasc Ther, 1–10, https://doi.org/10.1080/14779072.2025.2561717 (2025).
Pasina, L. et al. Inappropriate prescription of allopurinol and febuxostat and risk of adverse events in the elderly: results from the REPOSI registry. Eur. J. Clin. Pharmacol. 70, 1495–1503. https://doi.org/10.1007/s00228-014-1752-4 (2014).
Carollo, M. et al. Clinical impact of medication review and deprescribing in older inpatients: A systematic review and meta-analysis. J Am. Geriatr. Soc. 72, 3219–3238. https://doi.org/10.1111/jgs.19035 (2024).
Crisafulli, S. et al. Deprescribing as a strategy for improving safety of medicines in older people: Clinical and regulatory perspective. 2 2022, https://doi.org/10.3389/fdsfr.2022.1011701 (2022).
Zeng, Y., Dai, Y., Zhou, Z., Yu, X. & Shi, D. Hepatotoxicity-Related Adverse Effects of Proton Pump Inhibitors: A Cross-Sectional Study of Signal Mining and Analysis of the FDA Adverse Event Report System Database. Front Med (Lausanne) 8, 648164, https://doi.org/10.3389/fmed.2021.648164 (2021).
Arora, N. & Goldhaber, S. Z. Anticoagulants and transaminase elevation. Circulation 113, e698-702. https://doi.org/10.1161/CIRCULATIONAHA.105.603100 (2006).
Aithal, G. P. et al. Guidelines on the management of ascites in cirrhosis. Gut 70, 9–29. https://doi.org/10.1136/gutjnl-2020-321790 (2021).
Menichelli, D. et al. Atrial Fibrillation, Heart Failure Phenotypes, and Mortality Risk in the Nationwide START Registry: A Propensity Score Matching Analysis. J. Am. Heart Assoc. 14, e042586. https://doi.org/10.1161/JAHA.125.042586 (2025).
Caldeira, D. et al. Risk of drug-induced liver injury with the new oral anticoagulants: systematic review and meta-analysis. Heart 100, 550–556. https://doi.org/10.1136/heartjnl-2013-305288 (2014).
Villani, R. et al. Risk of Statin-Induced Hypertransaminasemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Mayo Clin. Proc. Innov. Qual Outcomes 3, 131–140. https://doi.org/10.1016/j.mayocpiqo.2019.01.003 (2019).
Pastori, D. et al. Statin liver safety in non-alcoholic fatty liver disease: A systematic review and metanalysis. Br. J. Clin. Pharmacol. 88, 441–451. https://doi.org/10.1111/bcp.14943 (2022).
Teschke, R. & Danan, G. The LiverTox Paradox-Gaps between Promised Data and Reality Check. Diagnostics (Basel) 11, https://doi.org/10.3390/diagnostics11101754 (2021).
Borghardt, J. M., Kloft, C. & Sharma, A. Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes. Can Respir J 2018, 2732017. https://doi.org/10.1155/2018/2732017 (2018).
Walker, S. R., Evans, M. E., Richards, A. J. & Paterson, J. W. The clinical pharmacology of oral and inhaled salbutamol. Clin Pharmacol Ther 13, 861–867. https://doi.org/10.1002/cpt1972136861 (1972).
Lipworth, B. J. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol 42, 697–705. https://doi.org/10.1046/j.1365-2125.1996.00493.x (1996).
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Consortia
Contributions
Gianluca Gazzaniga: Conceptualization, Formal analysis, Visualization, Writing—original draft. Danilo Menichelli: Conceptualization, Formal analysis, Writing—original draft. Daniela Poli: Data curation, Writing—review & editing. Gualtiero Palareti: Data curation, Writing—review & editing, Supervision. Emilia Antonucci: Data curation, Writing—review & editing. Arianna Pani: Writing—review & editing. Pasquale Pignatelli: Supervision, Writing—review & editing. Daniele Pastori: Conceptualization, Formal analysis, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gazzaniga, G., Menichelli, D., Poli, D. et al. Drug-related hepatotoxicity risk in elderly patients with atrial fibrillation: insights from the nationwide Italian START registry. Sci Rep 15, 45782 (2025). https://doi.org/10.1038/s41598-025-28502-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28502-8
Keywords
This article is cited by
-
Drugs associated with liver injury in elderly patients with AF
Reactions Weekly (2026)