Abstract
Formaldehyde contamination in urban drinking water posed significant health and environmental risks, necessitating effective and sustainable elimination strategies. This applied research investigated the efficacy of the Sonozone process—a hybrid advanced oxidation technique combining ultrasound and ozone—for formaldehyde degradation in water. Laboratory-scale experiments were conducted under varying conditions of pH (3–11), contact time (0–32 min), formaldehyde concentration (110–330 mg/L), ozone dosage (0.2–0.6 mg/min L), and electrical power (50–150 W). Formaldehyde concentrations were measured using spectrophotometry at 400 nm via the chromotropic acid 6252 colorimetric method. The process followed first-order kinetics (R2 = 0.9839), and statistical analysis via one-way ANOVA (p < 0.05) confirmed the significance of operational parameters. Optimal elimination (100% efficiency) was achieved at 110 mg/L formaldehyde, pH 3, 32 min contact time, 0.6 mg/min·L ozone, and 150 W power. The Taguchi model identified formaldehyde concentration as the most influential factor. Energy consumption was calculated at 371 kJ total and 30,601 kJ/kg removed. The enhanced oxidation was attributed to ultrasound-induced cavitation, which promotes ozone decomposition and hydroxyl radical generation. Compared to other AOPs, Sonozone offered superior environmental compatibility and operational simplicity. Based on these findings, Sonozone was recommended as an effective and sustainable method for formaldehyde elimination in urban water treatment applications.
Similar content being viewed by others
Introduction
The discharge of industrial wastewater releases hazardous pollutants into surrounding lakes and rivers. Formaldehyde is one of the most toxic organic refractory compounds widely used in the chemical industry and anatomy laboratories. Therefore, discharging it into aquatic environments can have serious consequences for ecosystems and human health1. According to the Transnational Cancer Research Organization, formaldehyde is classified as a carcinogenic and toxic agent. Formaldehyde is recognized by the US Environmental Protection Agency as a probable human carcinogen. Its widespread use in industries such as synthetic resins, fertilizers, pharmaceuticals, textiles, wood and paper production, and adhesives leads to significant discharge into water bodies2. Concentrations in industrial wastewater often range between 100–1000 mg/L, posing serious risks to human health and the environment3. Exposure can cause respiratory irritation, asthma exacerbation, and long-term cancer risks, making formaldehyde contamination a pressing water pollution issue.
The growing contamination of groundwater and surface water with formaldehyde highlights the urgent need for effective purification technologies. Many countries are tightening permissible exposure limits, increasing demand for advanced treatment methods. Conventional approaches such as biological purification, absorption, and deionization face limitations and often require supplementary physicochemical processes to break down formaldehyde in aqueous solutions1,4. Developing new, green technologies such as advanced oxidation processes (AOPs) that can completely mineralize formaldehyde into harmless products like carbon dioxide (CO₂) and water is essential for protecting public health and ensuring sustainable water recovery5.
Formaldehyde is a resistant organic compound that is difficult to degrade using conventional methods. Biological treatments alone are insufficient, while physicochemical methods such as photocatalysis, Fenton reactions, and hydrogen peroxide oxidation suffer from drawbacks including low mass transfer6, strong pH dependence7, and high costs. These challenges make it difficult to achieve efficient, complete removal of formaldehyde from aqueous environments. Thus, there is a need for innovative processes that overcome these limitations while maintaining efficiency and sustainability.
Although advanced oxidation processes (AOPs) have been explored, most studies have focused on pollutants other than formaldehyde or have not fully investigated the synergistic effects of combined methods. Research on ultrasound (US) and ozone (sonozone) has shown promise for degrading persistent organic pollutants, but systematic studies on formaldehyde removal—considering different US power ranges, ozone concentrations, degradation mechanisms, and kinetic modeling—have not been carried out. This gap in the literature leaves the problem unresolved and highlights the novelty of investigating sonozone specifically for formaldehyde.
This study introduces a specially designed reactor capable of operating across a wide range of US frequencies exceeding the audible limit and ozone flow rates as a new method of water and wastewater purification due to features such as low reaction time, novelty, equipment and simpler system, wide application range, rapid decomposition rate, limited space, increased ozone transfer speed, stimulation of hydroxyl radical ((•OH) production, being clean, and relatively high efficiency in degradation the persistence hard-to-degradable organic compounds by conventional methods of water purification is considered8, enabling detailed investigation of parameter interactions. No produce any carcinogenic byproducts, requires little space for the establishment of ultrasound units, and excellent mass transfer, including the benefits of the sonochemistry technique as a supplementary process9. The remarkable feature of sonication is its ability to create cavitation bubbles in water, which results in high local temperatures and pressure from their explosion along with increased efficiency10. Simultaneous application of ultrasonic (US)-ozone increases the degradation rate of contaminants11. This method combines the characteristics of advanced water treatment technologies such as enhanced oxidation, burning, and supercritical oxidation technology12. A combination of ozonation and other effective processes in the field of decomposition and reduction of organic material from water matrixes is applicable13. Rossi and colleagues14 reported the implementation of the US-ozone process in the reuse of primary effluent. Yargeauro and Danylo8 reported the application of US-ozone process in the reduction of ibuprofen, as an emerging pollutant. The innovation lies in the reactor design, which enables systematic variation of US intensity and ozone flow, and in the kinetic model developed to capture the synergistic effects of ultrasound and ozone. Unlike electrocoagulation, which generates sludge, our method achieves direct mineralization, reducing secondary waste. Previous student works have not investigated the mechanistic pathways, kinetic modeling, or optimization of operational parameters for formaldehyde removal in sonozone systems. Thus, this study fills critical gaps in the literature and establishes a new framework for sustainable water purification technologies.
A kinetic model is developed to capture the synergy between US and ozone in formaldehyde degradation. The reactor design maximizes gas–liquid contact, improving efficiency and reducing energy consumption compared to similar methods. By addressing health risks, environmental impacts, and compliance with international standards, this research establishes the sonozone process as a promising, sustainable technology for formaldehyde removal from water matrices, with novelty in both reactor design and kinetic modeling. In this study, a reactor is designed that is capable of operating over a wide range of US intensities and ozone flow rates. This feature allows for the investigation of the interaction effects of operational parameters. In this study, a developed kinetic model is presented that considers the synergy between ultrasound and ozone in formaldehyde elimination. The geometric design of the reactor and the method of ozone injection and ultrasound application are designed to provide maximum contact between the gas phase and the solution in order to increase the efficiency of formaldehyde removal and reduce energy consumption compared to similar methods. Considering the above, the claim of novelty of the study in reactor design and kinetic modeling can be inferred. Health risks (possible human carcinogenesis, environmental effects (damage to the ozone layer and groundwater pollution), and transnational concentration standards are among the ideas of research. Therefore, due to the role of AOPs in the degradation of various contaminants, including sonozone, this study aims to investigate the effect of US-ozone on the efficiency of removing formaldehyde from water matrixes.
Materials and methods
Chemicals
Materials used in research such as formaldehyde, sulfuric acid (H2SO4), and sodium hydroxide (NaOH) were prepared from Merck, Germany, with analytic purity of more than 99 percent. A 1 N H2SO4 and NaOH solutions were used to regulate pH.
Water sample preparation
Water samples used in sonozone experiments were prepared from the Tehran water network located in the laboratory of Tehran Medical Sciences Islamic Azad University, Tehran. Chemical, petrochemical, preservative production, sterilization, production of synthetic resins, and the production of fiberboard adhesives are among the industries that pollute the aqueous matrix with formaldehyde. Because of the existence of intervening factors, including suspended and pacifying substances, formaldehyde-containing effluent had not been used to prepare the sample. The synthetic formaldehyde solution of 1000 mg/L was prepared through the dissolution of 2.7 ml of formaldehyde 37% to the mark with twice distilled water in a calibrated 1000 ml volumetric flask. By performing dilution (with a ratio of 1 to 9.09, 4.54 and 3.03), formaldehyde concentrations of 110, 220, and 330 mg/L of water were obtained15. Formaldehyde was measured by colorimetric with chromotropic acid16. The efficacy of reduction was assessed by selecting and analyzing the reactor water at the end of each process round. The use of Taguchi design and ANOVA analysis strengthened our research by ensuring both efficiency and rigor. Taguchi’s orthogonal arrays allowed us to optimize multiple parameters with fewer experiments, making the study resource-efficient and robust against variability. ANOVA then validated the statistical significance of these results, confirming which factors truly influence performance. Together, they provided a powerful framework that enhanced the reliability, credibility, and reproducibility of our findings. The design of the experiment technique (DOE) is one of the techniques to improve wastewater degradation quality17. The number of samples was obtained according to a factorial method with repeat twice and design of experiments (DOE) 486 (N = Lm where N, L, and m were number of tests, levels of 3, and variables of 5, respectively) and 15 (Ln (Xy where n, X, and y were number of tests, levels of 3, and variables of 5, respectively), respectively. In other words, L15 = 35, meaning 5 three-level factors could be examined. We tested 486 samples, which were determined to identify effect sizes with 80% power at α = 0.05. This number ensures that our findings are both reliable and generalizable. The sample size chosen balances feasibility with scientific rigor. A full factorial design would require 486 runs, which is impractical. Taguchi’s orthogonal array approach allows us to reduce this to 15 runs while maintaining balanced representation of factor levels and critical interactions. Each factor level appears equally across the reduced set, ensuring unbiased estimation of main effects. Higher-order interactions were assumed negligible, consistent with the sparsity-of-effects principle. To confirm validity, we performed additional checks and found the reduced design adequately captured system behavior. This streamlined plan provides reliable insights without unnecessary repetition of experiments (Table 1). The L15 orthogonal array was selected because it efficiently accommodates the number of factors and levels in our study, reducing the experimental runs from a full factorial design to 15 while maintaining balanced representation. The output data were analyzed using the signal-to-noise (S/N) ratio, where the average S/N values at each factor level were compared to determine the relative importance of parameters. ANOVA was further applied to statistically validate these findings. The calibration curve was prepared with 5 different concentration levels (0.5, 5, 10, 20, and 30 µg/L of formaldehyde with R2 = 0.999. Recoveries at 5 different concentration levels ranged from 95.2 to 101.7%, the limit of detection (LOD), and limit of quantification (LOQ) were 0.3 and 1.0 ng/L, respectively.
Batch sonozone reactor specification
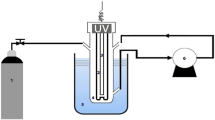
This research was an experimental laboratory study conducted on a pilot scale. 360 ml cylindrical container (a diameter (D) of 68.6 mm; height of 97.5 mm) with an effective volume of 200 ml was made of pyrex (Fig. 1). Thorough trials were completed at laboratory temperature (20 °C). US generator (SONOREX SUPER/Bandelin, Iran) had a frequency of 20 kHz, an electrical power of 50–150 W, and a maximum voltage of 230 V. The cold water bath was a model of GBTC158550 (Grock Engineering Design Company, Iran) for temperature control at 20 °C. COD-OM HACH/(USA) ozone generator had a maximum capacity of 0.5 g/h equipped with an oxygen supply source of the Iranian Leap Model (Iran) with a purity of more than 90 percent. Ozone was injected into the batch reactor through a fine-pore diffuser. The injection was maintained for 10 min under constant stirring to ensure homogeneous distribution. Dissolved ozone concentration was quantified using the indigo colorimetric method (APHA 4500-O3 B), with calibration against standard ozone solutions. Measurements were taken immediately after injection to account for ozone’s rapid decomposition. According to the Taguchi model, 3 levels of removal efficiency were selected due to physical, error reduction, accuracy increase, and efficiency increase, for 5 operational variables based on previous research. Samples with electric power sonication 50, 100, and 1500, ozone concentrations 0.2, 0.4 and 0.6 mg/min L, times 8, 16, and 32 min, pH 3, 7, and 11 were treated. The goal was to evaluate the impact of US waves and ozone on the degradation of formaldehyde concentrations of 110, 220, and 330 mg/L, as a model pollutant. AIKA (Germany) magnetic stirrer at 100 rpm was used to uniform the water samples. We selected pH values of 3, 7, and 11 to represent acidic, neutral, and alkaline conditions, respectively. This range covered the main operational environments encountered in water treatment and allows us to study both direct ozone oxidation (low pH) and indirect •OH radical oxidation (high pH). Reaction time, ozone concentration, and electrical power rangers were chosen to capture the kinetics of pollutant degradation, the dose–response relationship, and the energy–efficiency trade-off. These ranges were consistent with prior studies in AOPs and ensured that our findings were broadly applicable to practical treatment scenarios. The power (Bio-Tek US digital wattmeter, model UW-2 with resolution of 0.1 W and an accuracy of 10%) and time (Stopwatch) calibration of the US device was tested using a continuous waveform and a frequency of 1 MHz at different intensities of 0.5, 1, 1.5, and 2 W/cm2 and the results had been compared with the American standard of ± 20% for output power accuracy and ± 10% for timer accuracy. Ozone analyzers were calibrated or verified every 182 days by the national standard method iodine quantity method, that was, the calibration of potassium iodide method. The ozone was produced by an ozone generator and bubbled in the solution through an agitator of 100 rpm.
Lab methods
Each test was performed three times to determine average data values. Measurement of residual formaldehyde concentration after filtering by Wattman membrane filter (UK) with size 0.45 micron was conducted by Unico UV-2100 (Germany) spectrophotometer at wavelength 400 nm according to the guidelines in the book standard methods for the examination of water and wastewater (6252)16. The measurement of ORP, pH, and heat by ORP meter (CG, Malaysia) and pH meter (Hack, US) model was carried out after the Sonozone process according to the guidelines in the book standard methods for the examination of water and wastewater. The efficacy of formaldehyde reduction in this experimental study was calculated on a laboratory scale by the relationship (1)17:
That: Ct and Ct0 are the concentration of formaldehyde after sonochemical at t time and the concentration of primary formaldehyde at 0 time, respectively. Reaction kinetics models were calculated using relationships (2) and (3)17,18:
Taguchi method was identifying appropriate control factors to obtain optimal process results19. Orthogonal arrays were used to perform a set of tests. The correlation amid the three levels of elimination efficiency was shown as an array and 5 operating variables by the Taguchi model. The levels of selection of each of the parameters examined as Taguchi data were listed in Table 2.
Results
Water features
Table 3 shows the average and standard deviation of water samples characteristics of Tehrans drinking water distribution network before entering the batch reactor.
The impact of US on formaldehyde elimination
Figure 2 shows the effect of time, electrical power, and pH variables on the elimination rate of various formaldehyde concentrations by US. The efficacy of the elimination process in optimal US conditions (pH 3, formaldehyde concentration 110 mg/L) increased by augment the electrical power from 50 to 150 W and the duration from 8 to 32 min (from 14 to 20%). The efficacy of the formaldehyde elimination process under optimal US conditions (pH 3, 150 W, 32 min) at 110 mg/L (20%) is more than any of formaldehyde concentrations of 220 mg/L (13.5%) and 330 mg/L (8%). The efficiency of the elimination process under optimal US conditions (formaldehyde concentration of 110 mg/L, electric power of 150 W, time of 32 min) is reduced by increasing the pH from 3 to 11 (from 8 to 20%) due to the production of •OH radicals from hydrogen peroxide as a result of the reaction of superoxide radicals with hydrogen ions.
The effect of ozone on formaldehyde removal
Figure 3 shows the effect of time, ozone retention, and pH variables on the elimination rate of various formaldehyde concentrations by ozonization method. The efficacy of the elimination process in optimal ozonization conditions (pH3, formaldehyde concentration 110 mg/L) increases with ozone concentration from 0.2 to 0.6 mg/min L and duration increases from 8 to 32 min (from 16 to 22%). The efficacy of formaldehyde reduction process in optimal ozonization conditions (pH 11, ozone concentration 0.6 mg/min.L, time 32 min) at concentrations 110 mg/L (22%) is greater than that of formaldehyde concentrations 220 mg/L (15.5%) and 330 mg/L (10%). The efficacy of the elimination process in optimal ozoniation conditions (formaldehyde concentration 110 mg/L, ozone concentration 0.6 mg/min L, time 32 min) is reduced by increasing pH from 11 to 3 (from 22 to 17.6%).
The effect of sonozone on formaldehyde removal
Figure 4 shows the effect of ozone concentration, time, electrical power, and pH variables at a speed of elimination of various formaldehyde concentrations by the method of sonozone. The efficacy of the elimination process under optimal conditions of sonozone (pH 3, formaldehyde concentration 110 mg/L, ozone concentration 0.6 mg/min.L increases with electrical power from 50 to 150 W and duration from 8 to 32 min (from 94 to 100%). The efficacy of the formaldehyde reduction process under optimal conditions of sonozone (pH 3, electrical power 150 W, ozone concentration 0.6 mg/min L, time 32 min) at concentrations of 110 mg/L (100%) is greater than that of formaldehyde concentrations of 220 mg/L (100%) and 330 mg/L (92%). The efficacy of the elimination process under optimal sonozone conditions (formaldehyde concentration of 110 mg/L, electrical power of 150 W, ozone concentration of 0.6 mg/min.L, time of 32 min) is reduced by increasing pH from 3 to 11. 32 min as the maximum contact time is selected because our elimination figures reach a practical plateau beyond ~ 25–30 min, where further increases in time produces minimal additional removal relative to energy and ozone consumption. At the highest influent formaldehyde (330 mg/L), complete removal was not observed within 32 min, which is consistent with ozone’s limited direct reactivity and the increased radical scavenging at high organic load. Extending time alone brings marginal gains; achieving near-complete elimination at this concentration requires increasing the effective rate constant—via higher ozone dose, enhanced •OH radical formation, optimized pH for radical generation, and improved gas–liquid mass transfer or staged treatment. Sonozone produces short-lived oxidants (sonic cavitation + ozone) that convert formaldehyde to end products; adsorption with deep eutectic solvent can act as a downstream “polishing” step to remove unreacted formaldehyde at high concentrations (330 mg/L) and dissolved CO2 formed from mineralization, with the help of specially designed CO2 adsorbents.
Reaction kinetics and formaldehyde removal optimization
The Fig. 5a and b of the kinetics model shows first-degree (a) and second-degree (b) reactions to the reduction of formaldehyde by the sonozone. The outcomes of the kinetics study of formaldehyde reduction through the batch reactor of the sonozone showed that the reduction of formaldehyde follows the first-degree kinetics (R2 = 0.9839). The rate constant and half-life of the first-order kinetics are measured to be 0.147 min−1 and 4.71 min, respectively.
Model diagrams of first-degree reaction kinetics (a) and second-degree (b) formaldehyde reduction in the batch reactor of sonozone (Laboratory conditions: Temperature = 20 °C, pH = 3, Time = 0–32 min, Electrical power = 50 W, Ozone concentration = 0.6 mg/min.L, Formaldehyde concentration = 110 mg/L).
Optimal values for operating variables are determined using the Taguchi model (Table 4). Effective parameters on the reduction of formaldehyde with sonozone are shown in Fig. 6. According to Fig. 3, it is observed that the percentage of effective factors are concentration, pH, ozone concentration, electrical power and radiation time, respectively. The relative standard deviation or coefficient of variation of 1.87 is a sign of high repeatability of the data obtained during the experiments. Also Low RSD (< 10%) indicates that the data points are relatively close to the mean, and the data has low variability. The measurements are consistent, and the reactor producing the data is considered precise.
One-way variance analysis of sonozone formaldehyde removal and the effect of important water cations and anions on the formaldehyde elimination efficiency by the sonozone process
Table 5 shows the one-way variance of formaldehyde elimination with sonozone. The degree of freedom is a significant indication of the sonozone reactor in the reduction of formaldehyde. Concentration (p-value = 0.001 and correlation coefficient 0.995) has the greatest impact, which is well matched to the test data.
The effect of important water cations and anions on the formaldehyde elimination efficiency by the sonozone process is indicated in Table 6.
Economic evaluation
Economic evaluation is one of the important aspects in choosing the type of purification process. Although operating costs, such as energy costs, are fluctuating and it is difficult to determine the exact operating costs, in this study, to compare the two processes of US and ozonation, the operating costs of these two methods are estimated based on current prices (Table 7). US degradation is energetically costly relative to the chemical energy of formaldehyde because cavitation creates radicals inefficiently; most input power becomes heat and non-productive losses rather than targeted chemistry.
Preliminary experiments to investigate the efficiency of formaldehyde removal with resin effluent
Pre-tests evaluating the resinous wastewater matrix show that parameters such as high chemical oxygen demand (COD), alkalinity (bicarbonate), total suspended solids (TSS), and phenols can reduce the nominal 70% formaldehyde removal efficiency by Sonozone to 80% without adjusting ozone dose, US electrical power, and contact time through •OH radical competition, •OH radical scavenging, and mass transfer limitation. However, by adjusting the pH to 6.5–7.5, reducing alkalinity, removing TSS as a light pretreatment, and increasing the oxidation capacity (ozone dose/US electrical power), the disturbing effects can be minimized and the efficiency can be maintained close to the nominal value. Parameters such as nitrate and sulfate have less impact on performance within normal ranges (Table 8).
Mechanism of the sonozone process for formaldehyde degradation
This schematic illustrates the synergistic interaction between ultrasound and ozone in the sonozone technique. Cavitation bubbles generated by ultrasound collapse violently, producing localized high temperatures and pressures that facilitate the homolytic cleavage of water molecules and accelerate ozone decomposition. These reactions generate reactive species such as hydroxyl radicals (•OH) and hydrogen radicals (•H), which initiate the stepwise oxidation of formaldehyde into formic acid and ultimately mineralize it into carbon dioxide and water. The combined effect of ultrasound and ozone enhances mass transfer, radical yield, and pollutant degradation efficiency (Fig. 7).
Discussion
Impact of US on formaldehyde reduction
pH changes in AOPs through the production of oxidizing factors including radical •OH affect the degradation of organic matter such as formaldehyde20,21. The increased efficiency of the US process at the optimum pH of 3 can be attributed to the production of perhydroxyl radicals (HO2•) resulting from the combination of superoxide radicals (O₂•⁻) with hydrogen ions. Perhydroxylase radicals can produce hydrogen peroxide, which is converted to •OH radicals. Formaldehyde sonolysis at the optimum pH of 3 increases because of the production growth of •OH radicals. At a speed of formaldehyde degradation in acidic (pH 3) and neutral (pH 7) conditions is higher than that in basic (pH 11) conditions, due to the rapid decomposition of •OH radicals in basic conditions. This finding directly addresses the knowledge gap identified in the introduction by clarifying the role of pH in formaldehyde degradation via the sonozone method. The observation that acidic (pH 3) and neutral (pH 7) conditions yield higher decomposition rates than basic conditions (pH 11) demonstrates that •OH radical stability is critical to removal efficiency. This result is consistent with mechanisms proposed in similar studies, such as Huang et al.22, who reported enhanced radical activity under acidic/neutral conditions, and Wang et al.23, who noted rapid OH radical decomposition in alkaline environments. These findings reinforce the conclusion that pH is a decisive parameter governing the degradation pathway and efficiency in advanced oxidation processes. Increasing formaldehyde concentration have a negative effect on elimination efficiency due to a decrease in formaldehyde degradation. Choi and colleagues24 announced the optimal concentration of eosin paint removal with a US process of 5 mg/L. The observed reduction in degradation rate at higher formaldehyde concentrations can be explained by competitive consumption of •OH radicals, a phenomenon widely reported in sonochemical and photooxidative degradation studies. This reduction in the rate of degradation is related to the reduction in the production of •OH radicals. Also, with increasing formaldehyde concentration, its amount in the reactor increases and the resistance of this compound increases as a result of its competition for the •OH radical, ultimately reducing the elimination efficiency. Since the contact time and pH are the same at all concentrations, the amount of •OH radicals produced is also the same at all 3 concentrations. Therefore, formaldehyde elimination is greater in samples with lower concentrations. The reason for the increase in the efficacy of elimination over time is increased cross-sectional area for formaldehyde entry into the bubbles due to the longer life span and the smaller bubbles dimensions. The number of transient cavitation per unit of time in a given volume of the environment and the intensity of cavitation are the keys to achieving maximum formaldehyde cleansing. The degradation of sonolysis of different formaldehyde concentrations is more severe in the first 8 min and then the degradation proceeded slowly. Tran and Associates25 optimal time to remove the carbamazepine with the US process was 116 min. The reason for this difference in reaction time is due to the incomplete mineralization of carbamazepine with a 3-ring structure under optimal conditions and the products produced from carbamazepine that resistance of the intermediate compounds resulting from the primary decomposition increases as a result of the competition of these molecules for the •OH radical, and ultimately the elimination efficiency of the reduction decreases, and complete mineralization requires more removal time. The US effect in formaldehyde oxidation is mainly accomplished through a reaction with •OH radicals produced during water cavitation. The increase in electrical power is accompanied by an enlarge in the speed of reactions of •OH radicals to break down formaldehyde. Lower electrical power leads to lower •OH radical production and less possibility of radicals leaving the cavitation bubbles. Cavitation activity associated with •OH radical production has been increased linearly with electrical power. The effect of electrical power upon the reduction of formaldehyde when holding constant time (32 min) is greater than time. The size, number, longevity, and severity of the subside of the bubbles produced are important factors in the degradation of sonolysis, which in turn depends on the frequency and power of the US. Low-speed bubble mutation occurs at a lower frequency, and the life span of the pitting bubble should be longer. Therefore, the chances of recombining •OH radicals at the interface increase. Increasing the frequency allows access to more broadcasts and the scattered area of cavitation bubbles. Therefore, even if the size of the cavity decreases at a higher frequency, access to the reaction position and many of cavity events increases, and the creation of •OH radical increases. The increase in electrical power leads to an output enlarge of •OH radicals due to an enlarge in the number of oscillations and the longevity of bubbles. Rayaroth et al.26 have reported the optimal frequency and electrical density of elimination of the coomassie brilliant blue with the sonochemical process, respectively, 350 kHz and 19.6 kHz/ml. Villaroel et al.27 announced the optimal electrical power of acetaminophen removal with a sonochemistry process at 60 W. The reason for these differences in electrical powers are due to being coomassie brilliant blue as an amphoteric dye with two sulfonic acid groups and three basic nitrogen groups and being acetaminophen as an antipyretic agent with acetyl and p-aminophenol groups. Both of them are resistance and their more efficient decomposition requires more electrical power.
The effect of ozone on formaldehyde removal
Reaction time is among the parameters affecting AOPs, including ozone. Increasing reaction time gives the ozone oxidizing agent and •OH radical produced from the degradation of the ozone molecule, which is more in contact with the pollutant for a longer time and thus a higher percentage of the pollutant. It degradation s so, the reaction is more efficient. The contact time of 32 min in this study is determined as the optimal time. Zhao and Associates28 announced the optimal time to remove nitrobenzene by 30 min of catalytic ozonization. This may be due to the production of the same amount of •OH radicals within 30 min, despite the difference in the type of pollutant and the selective elimination method. Increased concentration of formaldehyde in the aqueous matrix, increases consumption of oxidants such as •OH radical and ozone molecule. The reduction of elimination efficiency by enlarging the amount of formaldehyde can be interpreted as the initial amount of methanal in situations where all parameters such as the amount of ozone input, pH, and reaction time are constant and the same amount of oxidizer is produced, the initial amount of methanal increases, as a result, the process of formaldehyde degradation at high concentrations does not take place completely and reduces efficiency. Therefore, by increasing the flow of incoming ozone and thus reducing the ozonization time, higher degradation efficiency can be achieved at high concentrations of formaldehyde. This finding addresses the knowledge gap identified in the introduction by clarifying how ozone flow rate and ozonization time influence degradation efficiency at high formaldehyde concentrations. It is consistent with Liu et al.29, who reported enhanced pollutant removal with higher ozone doses, and Gumus and Akbal30, who showed that increased ozone input reduces the need for extended treatment times. These studies reinforce that ozone dosage and contact time are critical parameters governing the efficiency of AOPs and according to the known mechanisms of ozone oxidation, increasing ozone dose increases the molecular concentration of ozone in the liquid phase, increases the production of •OH radical, and improves gas–liquid mass transfer, all of which increase the oxidation rate of pollutants. Factor pH in AOPs, including the ozonization method, due to its effect on formaldehyde structure and the production rate of oxidizing factors including •OH radical, plays an important role. The pH range of formaldehyde is 3–3.5 and formaldehyde is acidic. Protonation and deprotonation of formaldehyde, in other words, ozone hydrolysis is dependent on pH. In other words, the abundance of hydroxyl ions that initiate radical creation and ozonolysis reactions are dependent on pH. This research finding is consistent with research conducted by Bavass and colleagues31. This alignment demonstrates the accuracy and reproducibility of our findings within the framework of existing knowledge The higher elimination at pH 11 reflects greater •OH radical formation from base-catalyzed ozone decomposition and radical attack on hydrated formaldehyde, and progression to formate/CO₂, whereas at pH 3 ozone is more stable and the less effective direct ozonation pathway dominates. Therefore, considering a greater oxidizing power potential of •OH radicals compared to ozone, the improved effectiveness of the ozonization technique in removing formaldehyde can be confirmed in line with other studies. The outcomes revealed that the efficacy of methanal decomposition with increasing time is because of the increase in the occurrence of reactions to produce •OH radicals, which affects the reaction rate. The decomposition of formaldehyde accelerates with time due to the increase in mass transfer. Therefore, the efficacy of methanal reduction increases with increasing time. Derco et al.32 reported an enlarge in the elimination efficacy of BTX components with reaction time in ozonization and ozone/ultraviolet (UV) processes. This agreement indicates the influence of time as a key parameter in AOPs because ozone is a strong oxidizer and as time passes, more opportunity is provided to react with formaldehyde molecules. The amount of ozone concentration used, as an operation parameter, is foremost in the ozonization process. The outcomes of the study showed that changing the concentration of ozone flow led to a change in the degradation efficiency of formaldehyde. Ozone removes formaldehyde 1.1 times faster than US. Kidak et al.33 announced the degradation of amoxicillin by ozone over US. Consistency with the above study is the difference in the elimination mechanisms of ozonation (mainly based on the production of active radicals such as •OH and rapid reaction with the pollutant) and US (mostly due to cavitation and indirect production of radicals). Therefore, the reaction rate in the ozonation process is usually higher. The rate of occurrence of reactions to produce •OH radicals that affect the speed of reaction is under the ozone flow in the reactor. The number of •OH radicals is proportional to the ozone flow, and the degradation efficiency is associated with it, with increasing ozone flow rate, formaldehyde degradation is done more rapidly. Increasing ozone flow helps in the rapid production of products responsible for the decomposition of formaldehyde such as the •OH radical. The effectiveness of formaldehyde removal is proportional to the increase in ozone flow rate. The quantity of •OH radicals created is controlled by the amount of ozone flow applied. •OH radical production has a large share in the further destruction of formaldehyde. Baresel et al.34 announced an enlarge in the efficacy of removing 42 types of drug compounds by increasing ozone concentration. The reason for the synchronization is that ozone has a greater oxidizing capacity at higher concentrations and oxidation reactions occur more quickly and completely.
The effect of sonozone on formaldehyde removal
The integrated technique of sonozone has many advantages over both processes being performed separately. One of its main advantages is the transfer of ozone mass to react with formaldehyde using the mechanical effects of US. Cavitation bubbles are easily decomposed into triple atomic oxygen and oxygen molecules through US. As a result of this combined system, it increases the oxidation of formaldehyde. Ozone concentration is an important parameter when ozone is applied, how it affects formaldehyde degradation efficiency depends on formaldehyde concentration. Increasing contact time increases the concentration of ozone molecules in the reactor to saturation. Therefore, more •OH radicals will be produced and increase elimination efficiency. This finding addresses the knowledge gap identified in the introduction by clarifying the direct relationship between ⋅OH radical generation and elimination efficiency in the sonozone process. The observation that higher ⋅OH radical production enhances formaldehyde removal is consistent with the mechanisms proposed by Aghaeinejad-Meybodi et al.35, who demonstrated that radical yield is the key factor governing pollutant degradation in AOPs. These results strengthen the mechanistic understanding of radical-driven pathways and highlight operational parameters that can be optimized for improved treatment efficiency. Also, the synergy of the elimination mechanisms of the ozonation process (mainly based on the direct production of active radicals such as •OH and rapid reaction with the pollutant) with ultrasound (mostly acting through cavitation and indirect production of radicals). Combination of US and ozone to improve and increase the efficiency range of formaldehyde elimination 0.2–20%, 2.2–22%, and 10–53%, respectively, for the best removal mode (formaldehyde concentration 110 mg/L, pH 3, time 32 min, electric power 50 W, and ozone flow rate 0.6 mg/min.L) and the worst elimination mode (formaldehyde concentration 330 mg/L, pH 11, time 8 min, electric power 150 W, and ozone flow rate 0.2 mg/min.L). The increase in formaldehyde elimination in the integrated of US and ozone can be ascribed to its degradation due to an increase in •OH radicals. The implementation of the integrated of US and ozone emphasizes the favorable conditions for the purification of formaldehyde-contaminated water for the standard drinking water of 10 µg/l. The high efficiency of the combined process is a sign of its excellent ability as an environmentally friendly green process to purify water contaminated with formaldehyde. pH is vital for determining the degradation rate of formaldehyde. The rate of changes in the creation of •OH radicals is subject to changes in pH. When using Sonozone process, formaldehyde degradation increases at optimum pH of 3 due to synergistic increase in •OH radical production. The acidic environment of the sonozone process is a more suitable condition for the electrical creation of •OH radicals. Guo et al.36 the amount of •OH radicals produced and the speed of sulfamethoxazole degradation reactions with US/ozone process in different pH and pH optimal elimination of sulfamethoxazole by US/ozone process 3.2 announced. The linear creation of •OH radicals for US and ozone processes is obtained, unlike sonozone. A slight increase in pH is observed in the range of 0.2–0.8 without a change in behavior and •OH radical production. Malik and Saroha37 declared the ideal pH of phenol removal in acidic conditions after 120 min in US system. The reason for this is consistent with the above study: hydroxyl radicals act as the dominant oxidant in an acidic environment and will have greater oxidizing properties, which will increase the removal efficiency. Increasing the concentration of contaminants in the reactor along with increasing the consumption of oxidizing factors including •OH radical, direct oxidation by sonolysis and ozone. Decreasing process efficiency by increasing pollutant concentration can be ascribed to the constant production of oxidizing factors including •OH radical and hydrogen peroxide (H2O2), ozone flow rate, electrical power, and reaction time to elevated amounts of methanal. Therefore, a complete breakdown of formaldehyde in high concentrations is not possible. In other words, increasing the initial amount of methanal requires more degradation time. A lower concentration of formaldehyde leads to faster removal. Faster de-formaldehyde is ascribed to the mobility of formaldehyde molecules in a dilute solution. Kida and Ziembowicz38 reported that the efficacy elimination of 50 mg/L of Indigo Carmine after 30 min with UV/TiO2, ozone, and US were 95%, 91%, and 15%, respectively. Ayedi et al.39 the optimal concentration of methylene blue color elimination with the hydrodynamic ozone-cavity process was declared 10 mg/L, after 30 min of treatment for obtaining efficacy elimination 100%. Operation time is an important variable of performance in the sonozone process, because the higher operating time is associated with the need for more energy and the lower treatment speed, and therefore should be optimized for cost-effectiveness treatment. The process of removing formaldehyde has a steep slope after 8 min. The application of US with ozone reduces the time to achieve 100 percent formaldehyde removal. Sonozone requires less time to completely break down formaldehyde compared to US. Wen et al.40 announced the optimal time to remove atrazine with UV/ozone/US process by 60 min. Asheghmoalla and Mehrvar41 announced the optimal time range for the elimination of micro-pollutants with ozone processes (70–100% efficiency) and US-assisted photochemical oxidation (90–98% efficiency) as 5–180 min. The reason for increasing the efficacy of the sonozone process by increasing the ozone flow rate can be ascribed to increasing the production of oxidizing factors including •OH radical. Increasing the flow rate of ozone accelerates the rate of reactions. The reduction in ozone flow rate is accompanied by a longer degradation time.
Formaldehyde reaction kinetics and optimization
The reaction rate has a direct and linear relationship with the amount of reactants (formaldehyde amount) in first-order kinetics. The rate constant and half-life of the first-order kinetics are measured to be 0.147 min−1 and 4.71 min, respectively. Raschitor and colleagues42 announced the first-order kinetics of removing contaminated colloidal wastes with US/electro-coagulation. Shojaei et al.43 reported the second-order quadratic model was used to predict the response of each dye. Shojaei et al.44 reported the quadratic model was used to predict the response of each dye. Yang et al.45 announced the quadratic model was used to predict the response of dye and drug. Taguchi method, the statistical model of process quality optimization strategy and one of the powerful, simple, and effective methods of DOE, is based on the strong relationship between inputs and outputs in the field of environmental health engineering. The minimum test, the possibility of examining the effectiveness of parameters, the possibility of analyzing the signal to noise, and determining the optimal levels of the selected levels are among the reasons for the effectiveness of this method46. Using statistical methods, Taguchi identified 15 experiments that have the greatest impact on the five related factors from 405 experiments. Concentration is the most important variable dependent on the efficiency of formaldehyde removal determined by the Taguchi approach. Salman47 declared time as the most important variable dependent on the efficacy of the elimination achieved by the Taguchi model. Abbas and Abbas48 declared time foremost variable based on the efficacy of the elimination achieved by the Taguchi model.
One-way variance analysis of formaldehyde removal sonozone
The predictability of the model at a confidence level of 95 percent is confirmed based on one-way variance analysis. The correlation coefficient range (0.761–0.995) is a sign of strong proportionality. The range of F values highlights the high meaning of purification. The value of F, 50.4 is a significant indication of the process. The range of values P less than 0.05 for each variable emphasizes the significant impact of predictor variable on response variables. Therefore, it follows that, concentration is the important variable for maximum formaldehyde reduction efficacy. Cokay et al.49 reported an F value of 12.18 as a significant indication of the US process for treatment of table olive processing wastewater. The outcomes of the Taguchi experimental design model are consistent with the results of statistical analysis (SPSS). The study of the impact of US on the removal efficiency of different formaldehyde concentrations using the sonozone method in the scope of different electrical powers with different ozone currents, identification of the degradation, mechanism, identification of the level of importance of variables affecting the removal efficiency, comparison of reaction kinetics, and comparison of Taguchi results with one-way analysis of variance are among strong points of the research.
Elimination cost
The sonozone reactor has the lowest cost per kg removed because it actually removes most of the formaldehyde (92%). US alone looks least cost-effective per kg removed due to low efficiency. Ozonation sits in the middle.
Industrial applications
Many AOPs for wastewater have been developed and tested under controlled laboratory conditions using synthetic wastewaters. These synthetic matrices typically contain a single pollutant or simplified mixtures, which allow researchers to demonstrate high removal efficiencies. However, such results often do not translate directly to real industrial wastewaters, which are far more complex in composition. Industrial effluents may contain diverse organic and inorganic contaminants, fluctuating pH, high TSS, and toxic by-products that interfere with radical generation or adsorption processes. For example, studies have shown that photocatalytic and adsorption methods achieve near-complete removal in synthetic solutions but exhibit reduced efficiency when applied to resin, textile, or petrochemical wastewaters due to competitive reactions and matrix effects50. This gap highlights the importance of validating treatment technologies under real industrial conditions before claiming practical applicability. Documented reviews emphasize that while synthetic wastewater studies are valuable for mechanistic understanding, industrial application requires consideration of variability, secondary pollutants, and operational costs51. Therefore, the present work contributes to bridging this gap by evaluating sonozone treatment in conditions that better reflect industrial wastewater complexity, ensuring that the findings are relevant for practical deployment. The sustainability of sonozone-based formaldehyde removal can be further enhanced by considering the regeneration and reuse of the catalyst employed in the process. Catalyst deactivation due to fouling, surface blockage, or structural changes is a common challenge in advanced oxidation systems, and addressing this issue is critical for long-term applicability. Regeneration strategies such as thermal treatment, chemical washing, or ozone-assisted cleaning can restore catalytic activity while minimizing material waste. Moreover, the potential for in situ regeneration through sonication and oxidative reactions offers a promising pathway to reduce operational costs and environmental impact. Incorporating catalyst reuse into the design of sonozone systems not only extends catalyst lifetime but also aligns the technique with principles of sustainable environmental remediation, thereby strengthening its feasibility for large-scale and continuous applications. This study demonstrates that sonozoe treatment can effectively remove formaldehyde from water under controlled laboratory conditions. However, several limitations should be noted. First, experiments were conducted at small scale with synthetic water matrices, which may not fully represent the complexity of industrial wastewater such as organic materials and anion carbonate. In other hands failure to check the efficiency of the system on a real scale due to the overlap of several technical (uneven distribution of ultrasound energy due to fluctuations in electrical energy as result of true sample matrix), analytical (daily and seasonal changes in formaldehyde concentration), operational (inadequate optimal values of US parameters due to power and frequency fluctuations for real samples), and logistic (Insufficient time and budget allocated to conduct and statistically replicate experiments with real samples) factors. Second, the elimination efficiency was evaluated only within a concentration range of 110–330 mg/L; applicability to higher or mixed contaminant loads remains uncertain. Third, equipment, and consumable costs, and scalability of the sonozoe process were not assessed, limiting conclusions about practical implementation. These factors define the scope of validity of our results and highlight the need for further studies under real-world conditions. Bay et al.52 a coupled multiphase flow transport model in unsaturated soils (granular thermodynamic ideology) introduced new concepts such as particle temperature and particle entropy to describe energy dissipation at the mesoscale costs. The findings of this study highlight the potential of the sonozone method as a promising AOPs for the removal of formaldehyde from aqueous systems. In practical terms, this technique could be implemented in industrial wastewater treatment facilities, particularly in sectors such as textiles, resins, and healthcare laboratories, where formaldehyde-containing effluents are prevalent. Its integration into decentralized or portable treatment units also offers opportunities for safeguarding drinking water supplies in regions affected by accidental contamination. Future research should focus on optimizing operational parameters to enhance degradation efficiency while minimizing energy consumption, as well as elucidating the mechanistic pathways of formaldehyde oxidation under sonozone exposure. Comparative studies with other AOPs, alongside scalability and cost-effectiveness assessments, will be essential to determine the feasibility of large-scale applications. Moreover, extending the sonozone approach to other volatile organic compounds and exploring hybrid systems that couple sonozone with biological or membrane-based treatments could broaden its utility as a versatile water purification technology.
Mechanism
Ozone reacts with formaldehyde in two ways: direct oxidation under acidic environments and non-direct oxidation (in which ozone decomposes and produces •OH radicals that react with formaldehyde and destroy it). Two main reactions take place in the ozone process mechanism:
Alternating compression cycles of sound waves under US radiation (16 kHz-100 MHz) can result in triplet successive stages of cavities (i.e., nucleation, growth, and explosive collapse) that are made of vapor and tiny gas-filled bubbles. The collapse of a fine bubble can immediately create high temperatures (500–4200 K) and high pressures (500–200 atm). The gauze form of water molecules collapse inside tiny bubbles amidst harsh conditions to produce •OH radicals:
Electronic beam radiation produces diverse free radicals in water through water fission53:
As ozone concentration increases, the likelihood of US waves hitting ozone molecules increases, resulting in more •OH radicals being produced, which is spent on oxidation of formaldehyde molecules, thereby reducing formaldehyde concentration. Enlarging the concentration of ozone oxidizing agent, •OH radical production also increases, and as a result, the rate of reaction and the speed of formaldehyde decomposition increases. Based on the above mechanism and relationships, by-products resulting from the reaction of formaldehyde with the sonozone reactor are •OH radicals and carbon dioxide. Formic acid measurement test of samples taken from the reactor in the presence of phenolphthalein reagent with 1N NaOH titrant solution shows that the samples are free of formic acid. CO2 measurement test of samples taken from the reactor in the presence of phenolphthalein reagent with 1N NaOH titrant solution shows that the samples have CO2. To detect •OH radicals in the process, tert-butanol and methanol with high solubility in water are used as •OH radical traps. Tert-butanol and methanol react rapidly with •OH radicals, and the decrease in formaldehyde elimination efficiency by tert-butanol (99%) and methanol (83%) indicates the important role of •OH radicals in formaldehyde elimination. According to Eq. 7, complete mineralization occurred in the sonozone reactor, resulting in the production of water and carbon dioxide. The carbonyl groups in formaldehyde contain a highly electron-deficient C atom, which is easily polarized to form a carbonyl carbocation54. The reported gaseous formaldehyde adsorption by plasma-activated porous bamboo carbon microfibers provided a useful mechanistic analogy that adsorption onto activated surfaces and subsequent radical-mediated reactions controlled pollutant elimination. Similarly, in the aqueous sonozone process, adsorption of formaldehyde at bubble interfaces and reactions with •OH radicals at gas–liquid boundaries may represent key pathways for its degradation55. We have now clarified in the manuscript that while advanced studies in formaldehyde elimination focus on mechanisms (such as adsorption of the inner layer onto the kaolinite surface) and adsorbent analysis, they do not examine by-products. We highlight this gap to further justify the importance of our work, which explicitly investigates by-product formation in the sonozone process56. US does not directly disrupt the molecular structure of formaldehyde; rather, acoustic cavitation generates transient hotspots and reactive radicals (•OH) that attack formaldehyde molecules at bubble–liquid interfaces. In this way, US alters the reaction medium by creating highly oxidative microenvironments that facilitate the conversion of formaldehyde into formic acid and ultimately CO₂ and H₂O. Similar to how pH influences adsorption mechanisms in red mud solutions57, our results indicate that pH also alters the degradation pathway of formaldehyde in the sonozone method. At acidic pH, removal is primarily governed by direct ozone attack, whereas at alkaline pH, hydroxyl radical generation dominates, leading to enhanced oxidation efficiency. Thus, pH is a critical parameter that controls the mechanism of formaldehyde removal in advanced oxidation processes. Similar to the findings of Shokri58, who demonstrated that ferrous ions effectively activate peroxydisulphate under UV irradiation to enhance toluene degradation, our results confirm that ferrous ions also play a crucial role in boosting •OH radical generation in the sonozone process. This parallel underscores the broader applicability of Fe2⁺-mediated radical activation across AOPs. The role of ferrous ions in enhancing AOPs has been widely reported. For example, Bayat and Shokri59 demonstrated that Fe2⁺ effectively activates peroxymonosulfate to generate sulfate radicals, achieving efficient degradation of p-nitrotoluene under optimized conditions using a full factorial experimental design. In agreement with these findings, our study shows that Fe2⁺ also promotes •OH radical generation in the sonozone process, thereby accelerating formaldehyde removal. This comparison highlights the broad applicability of Fe2⁺-mediated radical activation across different oxidants (PMS vs. ozone/ultrasound) and pollutants (nitroaromatics vs. aldehydes). Alternative treatment methods such as electrocoagulation have also been investigated for industrial wastewater remediation. For instance, Shokri and Nasernejad60 optimized the electrocoagulation process for spent caustic treatment, analyzing sludge characteristics and economic feasibility. While electrocoagulation achieves pollutant removal through coagulation and precipitation, it generates significant sludge requiring further management. In contrast, our sonozone process with ferrous ion activation promotes direct oxidation and mineralization of formaldehyde, thereby minimizing secondary waste. Both approaches highlight the importance of process optimization and energy efficiency, yet differ in them by-products and long-term sustainability considerations.
Conclusions
In line with the outcomes the statistical analysis of the present study, the optimal amount of time, ozone flow rate, electrical power, concentration of formaldehyde, and pH to achieve the maximum elimination of methanal in the process of sonozone is 32 min, 0.6 mg/min.L, 150 W, 110 mg/L and pH 3, respectively. The outcomes of this survey show that, the efficiency of the removal of the sonozone process is mainly dependent on the variables of operation such as pH, ozone flow rate, time, electrical power, and formaldehyde concentration. Formaldehyde elimination follows first-degree kinetics (R2 = 0.9839) and concentrations are regarded as to be the most important variable based on the effectiveness of formaldehyde elimination obtained by the Taguchi model. Direct oxidation is effective in the reduction of formaldehyde along with the formation of •OH, H· and HO2· radicals. According to the results, this study provides new insights for the study of sonozone purification to remove formaldehyde through direct oxidation (sonolysis) and indirect oxidation. The method of sonozone is very effective for removing formaldehyde at high concentrations and its application in water and wastewater purification is recommended. The competitive advantage of the sonozone method over other AOPs are: environmental compatibility, in which the •OH radical is the main factor, is a clean agent; higher formaldehyde decomposition rate due to continuous and greater production of •OH radicals, which also reduces the volume of sludge produced.
Data availability
The datasets generated and analyzed during the current study were available from the corresponding author on reasonable request.
References
Soltani, R. D. C., Rezaee, A., Safari, M., Khataee, A. & Karimi, B. Photocatalytic degradation of formaldehyde in aqueous solution using ZnO nanoparticles immobilized on glass plates. Desalin. Water Treat. 53, 1613–1620 (2015).
Zhang, Y. et al. Highly selective gas sensors for formaldehyde detection based on ZnO@ ZIF-8 core-shell heterostructures. Sens. Actuators B Chem. 398, 134689 (2024).
Yamada, M., Funaki, S. & Miki, S. Formaldehyde interacts with RNA rather than DNA: Accumulation of formaldehyde by the RNA-inorganic hybrid material. Int. J. Biol. Macromol. 122, 168–173 (2019).
Hosseinzadeh, A. et al. Improving formaldehyde removal from water and wastewater by fenton, photo-fenton and ozonation/fenton processes through optimization and modeling. Water 13, 2754 (2021).
Ahmed, S. N. & Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: a review. Nanotechnology 29, 342001 (2018).
Iervolino, G., Zammit, I., Vaiano, V. & Rizzo, L. Limitations and prospects for wastewater treatment by UV and visible-light-active heterogeneous photocatalysis: A critical review. Heterogeneous Photocatalysis Recent Adv. 225–264 (2020).
Bello, M. M., Raman, A. A. A. & Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 126, 119–140 (2019).
Yargeau, V. & Danylo, F. Removal and transformation products of ibuprofen obtained during ozone-and ultrasound-based oxidative treatment. Water Sci. Technol. 72, 491–500 (2015).
Hu, D., Liu, S. & Zhang, G. Sonochemical treatment for removal of aqueous organic pollutants: principles, overview and prospects. Sep. Purif. Technol. 353, 128264 (2024).
Hu, D., Liu, S., Qi, L., Liang, J. & Zhang, G. A critical review on ultrasound-assisted adsorption and desorption technology: mechanisms, influencing factors, applications, and prospects. J. Environ. Chem. Eng. 12 (6), 114307 (2024).
Alfonso-Muniozguren, P. et al. Tertiary treatment of real abattoir wastewater using combined acoustic cavitation and ozonation. Ultrason. Sonochem. 64, 104986 (2020).
Wang, J. et al. Review on the treatment of organic pollutants in water by ultrasonic technology. Ultrason. Sonochem. 55, 273–278 (2019).
Wang, Y. et al. The formation and control of ozonation by-products during drinking water advanced treatment in a pilot-scale study. Sci. Total Environ. 808, 151921 (2022).
Rossi, G., Mainardis, M., Aneggi, E., Weavers, L. K. & Goi, D. Combined ultrasound-ozone treatment for reutilization of primary effluent—A preliminary study. Environ. Sci. Pollut. Res. 28, 700–710 (2021).
Farhoodi, A. M., Hassani, A. H., Kashi, G., Javid, A. H. & Mansouri, N. Optimization of the electro-photocatalytic process for the removal of formaldehyde from water using the Taguchi model. Heliyon 10, e38442 (2024).
Association, A. P. H. Standard Methods for the Examination of Water and Wastewater Vol. 6 (American Public Health Association, 1926).
Kashi, G. & Hydarian, N. Optimization electrophotocatalytic removal of sulfanilamide from aqueous water by Taguchi model. J. Math. 2015, 86–98 (2015).
Yousefi, M. et al. Adsorption of diazinon from aqueous solution using metal organic framework and functionalized graphene: Comparison of BBD, ANN models. Chemosphere 351, 141222 (2024).
Yousefi, M., Nabizadeh, R., Alimohammadi, M., Mohammadi, A. A. & Mahvi, A. H. Removal of phosphate from aqueous solutions using granular ferric hydroxide process optimization by response surface methodology. Desalin. Water Treat. 158, 290–300 (2019).
Fan, J., Zhang, X., He, N., Song, F. & Wang, X. Investigation on novel deep eutectic solvents with high carbon dioxide adsorption performance. Environ. Chem. Eng. 13(5), 117870 (2025).
Rahmani, A. R., Shabanloo, A., Mehralipour, J., Fazlzadeh, M. & Poureshgh, Y. Degradation of phenol in aqueous solutions using electro-Fenton process. Res. J. Environ. Sci. 9, 332 (2015).
Huang, T. et al. Effects and mechanism of diclofenac degradation in aqueous solution by US/Zn0. Ultrason. Sonochem. 37, 676–685 (2017).
Wang, X. et al. Degradation of Acid Orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation. Sep. Purif. Technol. 122, 41–46 (2014).
Choi, Y. et al. Investigation of the synergistic effect of sonolysis and photocatalysis of titanium dioxide for organic dye degradation. Catalysts 10, 500 (2020).
Tran, N., Drogui, P., Zaviska, F. & Brar, S. K. Sonochemical degradation of the persistent pharmaceutical carbamazepine. J. Environ. Manag. 131, 25–32 (2013).
Rayaroth, M. P., Aravind, U. K. & Aravindakumar, C. T. Sonochemical degradation of Coomassie Brilliant Blue: Effect of frequency, power density, pH and various additives. Chemosphere 119, 848–855 (2015).
Villaroel, E., Silva-Agredo, J., Petrier, C., Taborda, G. & Torres-Palma, R. A. Ultrasonic degradation of acetaminophen in water: Effect of sonochemical parameters and water matrix. Ultrason. Sonochem. 21, 1763–1769 (2014).
Zhao, L. et al. Characteristic mechanism of ceramic honeycomb catalytic ozonation enhanced by ultrasound with triple frequencies for the degradation of nitrobenzene in aqueous solution. Ultrason. Sonochem. 21, 104–112 (2014).
Liu, X., Zhou, Z., Jing, G. & Fang, J. Catalytic ozonation of Acid Red B in aqueous solution over a Fe–Cu–O catalyst. Sep. Purif. Technol. 115, 129–135 (2013).
Gümüş, D. & Akbal, F. A comparative study of ozonation, iron coated zeolite catalyzed ozonation and granular activated carbon catalyzed ozonation of humic acid. Chemosphere 174, 218–231 (2017).
Bavasso, I., Montanaro, D., Di Palma, L. & Petrucci, E. Electrochemically assisted decomposition of ozone for degradation and mineralization of Diuron. Electrochim. Acta 331, 135423 (2020).
Derco, J., Šimovičová, K. & Dudáš, J. Removal of BTX Contaminants with O3 and O3/UV. Physico-Chem. Wastewater Treat. Resour. Recov. 1 (2017).
Kıdak, R. & Doğan, Ş. Medium-high frequency ultrasound and ozone based advanced oxidation for amoxicillin removal in water. Ultrason. Sonochem. 40, 131–139 (2018).
Baresel, C., Malmborg, J., Ek, M. & Sehlén, R. Removal of pharmaceutical residues using ozonation as intermediate process step at Linköping WWTP, Sweden. Water Sci. Technol. 73, 2017–2024 (2016).
Aghaeinejad-Meybodi, A., Ebadi, A., Shafiei, S., Khataee, A. & Rostampour, M. Degradation of antidepressant drug fluoxetine in aqueous media by ozone/H2O2 system: Process optimization using central composite design. Environ. Technol. 36, 1477–1488 (2015).
Guo, W.-Q. et al. Sulfamethoxazole degradation by ultrasound/ozone oxidation process in water: Kinetics, mechanisms, and pathways. Ultrason. Sonochem. 22, 182–187 (2015).
Malik, S. & Saroha, A. K. Removal of phenol from wastewater using ultrasound cavitation. J. Hazard. Toxic Radioact. Waste 29, 04024042 (2025).
Kida, M. & Ziembowicz, S. The influence of ultraviolet radiation, ozonation, and ultrasonic field on the effectiveness of dye removal from aqueous solutions. Appl. Sci. 15, 2373 (2025).
Ayedi, K., Innocenzi, V. & Prisciandaro, M. Application of hybrid oxidative processes based on cavitation for the treatment of methyl blue solutions. Sustain. Water Resour. Manag. 10, 92 (2024).
Wen, D., Chen, B. & Liu, B. An ultrasound/O3 and UV/O3 process for atrazine manufacturing wastewater treatment: a multiple scale experimental study. Water Sci. Technol. 85, 229–243 (2022).
Asheghmoalla, M. & Mehrvar, M. Integrated and hybrid processes for the treatment of actual wastewaters containing micropollutants: A review on recent advances. Processes 12, 339 (2024).
Raschitor, A., Llanos, J., Cañizares, P. & Rodrigo, M. A. Improved electrolysis of colloid-polluted wastes using ultrasounds and electrocoagulation. Sep. Purif. Technol. 231, 115926 (2020).
Shojaei, S., Nouri, A., Baharinikoo, L., Farahani, M. D. & Shojaei, S. Removal of the hazardous dyes through adsorption over nanozeolite-X: Simultaneous model, design and analysis of experiments. Polyhedron 196, 114995 (2021).
Shojaei, S. et al. Application of Taguchi method and response surface methodology into the removal of malachite green and auramine-O by NaX nanozeolites. Sci. Rep. 11, 16054 (2021).
Yang, J., Shojaei, S. & Shojaei, S. Removal of drug and dye from aqueous solutions by graphene oxide: Adsorption studies and chemometrics methods. NPJ Clean. Water 5, 5 (2022).
Hisam, M. W. et al. The versatility of the Taguchi method: Optimizing experiments across diverse disciplines. J. Stat. Theory Appl. 23, 365–389 (2024).
Salman, R. H. Removal of manganese ions (Mn2+) from a simulated wastewater by electrocoagulation/electroflotation technologies with stainless steel mesh electrodes: Process optimization based on Taguchi approach. Iraqi J. Chem. Pet. Eng. 20, 39–48 (2019).
Abbas, R. N. & Abbas, A. S. The Taguchi approach in studying and optimizing the electro-Fenton oxidation to reduce organic contaminants in refinery wastewater using novel electrodes. Eng. Technol. Appl. Sci. Res. 12, 8928–8935 (2022).
Çokay, E., Eker, S. & Taşkın, E. Treatment of table olive processing wastewater with US/UV processes. Heliyon 10, e37484 (2024).
Kato, S. & Kansha, Y. Comprehensive review of industrial wastewater treatment techniques. Environ. Sci. Pollut. Res. 31(39), 51064–51097 (2024).
Li, Y. Technology review and selection guide for industry wastewater treatment. Comput. Water Energy Environ. Eng. 9(02), 22 (2020).
Bai, B., Wu, H., Wu, N. & Zhang, B. Granular thermodynamic ideology on the heavy metal migration in unsaturated soils driven by seepage-temperature. J. Hydrol. 661(Part C), 133738 (2025).
Deng, Y. & Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 1, 167–176 (2015).
Xiong, J. et al. Electrospun biomass carbon-based porous nanofibers modified by green-dry cold plasma for gaseous formaldehyde adsorption. Ind. Crops Products 222(Part C), 119769 (2024).
Su, G. et al. Gaseous formaldehyde adsorption by eco-friendly, porous bamboo carbon microfibers obtained by steam explosion, carbonization, and plasma activation. Chem. Eng. J. 455, 140686 (2023).
Salman, M. et al. Removal of formaldehyde from aqueous solution by adsorption on kaolin and bentonite: a comparative study. Turk. J. Eng. Environ. Sci. 36 (3), 263–270 (2012).
Bai, B. et al. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 28, 102944 (2022).
Shokri, A. Employing UV/peroxydisulphate (PDS) activated by ferrous ion for the removal of toluene in aqueous environment: Electrical consumption and kinetic study. Int. J. Environ. Anal. Chem. 102(16), 4478–4495 (2022).
Bayat, A. & Shokri, A. Degradation of p-Nitrotoluene in aqueous environment by Fe (II)/Peroxymonosulfate using full factorial experimental design. Sep. Sci. Technol. 56(17), 2941–2950 (2021).
Shokri, A. & Nasernejad, B. Electrocoagulation process for spent caustic treatment: optimization, sludge analysis and economic studies. J. Ind. Eng. Chem. 25(135), 471–479 (2025).
Acknowledgements
The authors would like to express their gratitude to the Department of Environmental Health Engineering and Water Purification Research Centre, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran, for their instrumental and financial support.
Funding
The authors would like to express their gratitude to the Department of Environmental Health Engineering and Water Purification Research Centre, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran, for their instrumental and financial support.
Author information
Authors and Affiliations
Contributions
Giti Kashi: Writing-review and editing, Writing-original draft, Supervision, Methodology, Investigation. Amir Mohammad Farhoodi: Writing-review and editing, Writing-original draft, Methodology, Investigation. Fatemeh Aghakasiri: Writing-review and editing, Writing-original draft, Methodology, Investigation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Farhoodi, A.M., Kashi, G. & Aghakasiri, F. Evaluating the efficiency of formaldehyde degradation from aqueous environment by sonozone technique. Sci Rep 16, 3313 (2026). https://doi.org/10.1038/s41598-025-33224-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-33224-y