Abstract
β-Glucan is extensively utilized in the food industry for its functional benefits, including blood glucose and lipid regulation, enhanced food texture, and a prolonged shelf life. In the pharmaceutical field, it serves as a potent immune modulator, anti-tumor agent, and vaccine adjuvant, highlighting its significant biological potential. Despite its wide-ranging applications, standardized methods for detecting β-glucan in Cordyceps species have been lacking. To address this deficiency, the current study developed a Congo Red Ultraviolet Spectrophotometry assay for β-glucan quantification. The optimal conditions for extraction were identified as dimethyl sulfoxide (DMSO) being the solvent, a pH of 7.0, a temperature of 65 °C, and a reaction time of 60 min. Validation tests confirmed the method’s precision, reproducibility, and stability, demonstrating its reliability for β-glucan analysis. The assay was applied to three Cordyceps species, i.e., Cordyceps militaris, C. cicadae, and C. fumosorosea. Results revealed a marginally higher β-glucan content in C. fumosorosea compared to the other species. This efficient and cost-effective method offers a valuable tool for β-glucan analysis, with potential applications in quality control and product development for medicinal and edible fungi. Future research should extend to additional Cordyceps species and compare results with alternative analytical techniques to further enhance standardization and broaden the applicability of this method.
Similar content being viewed by others
Introduction
As a polysaccharide ubiquitously present in the cell walls of fungi, plants, and bacteria, β-glucan has drawn significant attention, owing to its distinctive biological activities and physiological functions1. Edible fungal β-glucan is recognized by the body as a foreign substance, activating immune cells, stimulating cytokine production, and functioning as a potent biological response modifier (BRM)2. Additionally, both edible and medicinal fungal β-glucan have antifungal properties and can combat viral infections such as hepatitis B virus (HBV), human immunodeficiency virus (HIV-1), and influenza virus3. These multifaceted pharmacological effects have positioned β-glucan as a critical target for both scientific research and industrial applications.
Among natural β-glucan sources, Cordyceps fungi hold particular significance. Cordyceps is a group of fungi that parasitize insects, a few fungi, and plant bodies. It is a general term for the fungi of the genus Cordyceps sensu lato4. According to historical records, around 2000 BC, Ophiocordyceps sinensis was prized in ancient China for its bioactivities and health benefits5, used as a medicinal plant. Today, Cordyceps drives a thriving Chinese industry valued at over 30 billion RMB annually6. Within this medicinal fungus, β-glucan serves dual roles—acting as a structural component while demonstrating exceptional medicinal value through well-documented immunomodulatory, antioxidant, and anti-tumor activities. This functional duality underscores the importance of developing precise analytical methods for β-glucan quantification in Cordyceps, a prerequisite for quality control and resource optimization.
Effective quantification begins with efficient extraction. Current β-glucan isolation methods include aqueous7, alkaline8, acid9, and DMSO-based approaches10, with dimethyl sulfoxide (DMSO) emerging as the most effective solvent. Since its synthesis in 186711, DMSO has earned the moniker “universal solvent” due to its unique capacity to dissolve both hydrophilic and hydrophobic compounds12. This property makes DMSO an efficient solvent for extracting β-glucan from Cordyceps, facilitating the separation of insoluble impurities during purification13.
Post-extraction analysis presents its own challenges. Existing detection methodologies—Congo red assay14, fluorescence method15, β-glucan-specific hydrolase assay16, and protein-specific recognition method17—each carry limitations. While fluorescence detection offers high efficiency and accuracy, its reliance on expensive instrumentation and sensitivity to experimental conditions restricts widespread adoption15. Similarly, enzymatic methods suffer from cost barriers and error susceptibility due to enzyme purity issues16. The protein-specific identification method is mainly used in medical diagnosis18, but it is time-consuming due to multiple experimental steps that can affect the final results. Amid these constraints, the Congo red assay stands out for its practical advantages. This technique exploits the specific binding between Congo red and β-glucan’s triple-helix structure through hydrogen and hydrophobic bonds19,20,21, generating a redshift in absorption spectra that enables selective quantification22. The method’s specificity is exemplified in Du et al.’s work23 with oat β-glucan, where interference from starch and proteins has proven negligible—a finding that validates its applicability to complex matrices like Cordyceps24. For industrial settings prioritizing cost-effectiveness and throughput, Congo red-UV spectrophotometry offers distinct advantages: minimal pretreatment, rapid analysis, and compatibility with standard laboratory equipment.
The urgency for such methodology extends beyond academic interest. In pharmaceutical development, β-glucan’s role as an immunomodulatory agent necessitates precise content measurement for dosage optimization and quality control25,26. Similarly, the nutraceutical industry requires accurate quantification to substantiate product claims and guide consumption guidelines27. These intersecting demands highlight the critical need for a standardized detection protocol—a gap this study aims to address.
Herein, we present an optimized Congo Red UV Spectrophotometry for β-glucan quantification in Cordyceps. Using commercially available artificial powders (the raw material, mainly consisting of C. militaris and C. cicadae cultivated with various Chinese medicinal and food homologues, was provided by Yunnan Herbal Laboratory), we systematically investigated sample pretreatment protocols, reaction condition optimization, and detection system development. Our approach uniquely integrates DMSO extraction efficiency with Congo red’s binding specificity, enabling cost-effective analysis without compromising accuracy. Following method validation, we further applied this protocol to compare β-glucan contents across three laboratory-cultivated Cordyceps strains, demonstrating its utility in both quality control and functional research. This work establishes a framework for β-glucan analysis in other medicinal fungi.
Materials and methods
Strains collection and preparation
Sources of Cordyceps samples
The strain of C. militaris was obtained through tissue isolation from wild sample of C. militaris in Dashao Village (2600 m altitude, Pinus amandii forest), Songming County, Kunming City, Yunnan Province, China. The strain was originally numbered DS.
The strain of C. cicadae was obtained through tissue isolation from wild sample of C.cicadae in Dimaluo Village (1680 m altitude, evergreen broad-leaved forest), Bundang Township, Gongshan County, Nujiang Prefecture, Yunnan Province, China. The strain was originally numbered ICI-dmlc19.
The strain of C. fumosorosea was obtained through tissue isolation from wild sample of C. fumosorosea in Dayao County (2300 m altitude, semi-humid evergreen broad-leaved forest), Chuxiong Prefecture, Yunnan Province, China. The strain was originally numbered DYCF.
Pretreatment of strains and preparation of samples
Experimental strains were collected by Yunnan Herbal Laboratory, isolated and identified by Professor Hong Yu’s team at Yunnan University, and preserved in the Yunnan Fungal Culture Collection (YFCC). Specimens were also stored in the Yunnan Herbal Herbarium (YHH).
Sterilization & Isolation: Wild samples of C. militaris, C. cicadae, and C. fumosorosea underwent sterilization with hydrogen peroxide for 5–120 s based on their level of softness or hardness. Harder samples could also be briefly soaked in varying concentrations of ethanol before being washed with sterile water using 3–5 petri dishes each time (with each dish soaked for 2 min). Afterwards, substrates were cut using forceps or a knife, dried on filter paper, and inoculated onto plates containing antibiotics. Grow for a few days and then progressively purify in order to obtain a pure strain. These purified strains were inoculated into slant test tube medium and stored at a low temperature.
Culture & Purification: The strains of C. militaris, C. cicadae, and C. fumosorosea were inoculated on solid PDA medium for 7 days at 25 °C for activation.
Liquid Culture: Activated mycelia (0.5 cm2) were transferred to liquid medium (25 °C, 120 r/min, 5 days).
Solid-State Fermentation: Mycelial suspensions (10% v/v inoculum) were cultured on rice medium (25 °C, 60 days). Harvested biomass was dried (50 °C), milled (80-mesh), and stored for analysis.
Commercial Sample: The Yunbaicao Brand Artificial Cordyceps Powder, produced by Yunnan Herbal Laboratory, is a mixture of artificial C. militaris and C. cicadae, abbreviated as YBC. We utilized this commercial artificial Cordyceps powder for method optimization and experiments. The powder contains Cordyceps and other interfering substances, which increase the complexity of detecting the target component. To address this, we employed standard substances as controls to evaluate the specificity and accuracy of our method. This approach not only ensures the reliability of the detection method but also provides a scientific basis for establishing the quality standard of β-glucan in Cordyceps powder.
Experimental reagents and preparatory procedures prior to experimentation
Experimental reagents
β-Glucan standard (70% purity), potassium hydroxide (KOH), sodium hydroxide (NaOH), hydrogen chloride (HCl), dimethyl sulfoxide (DMSO) and distilled water were used. Additionally, a buffer solution was prepared using sodium hydrogen phosphate (Na2HPO4) and sodium dihydrogen phosphate (NaH2PO4), and Congo red was also utilized.
Preparation of experimental sample solutions
Accurately weigh 50 mg of Cordyceps sample into a centrifuge tube, add 10 mL 90% DMSO to dissolve, and subject to gradient conditions. After water bath treatment, centrifuge at 4000 r/min for 5 min, and collect the supernatant for analysis.
Optimization of extraction parameters
In this experiment, YBC and a 70% β-glucan standard were used as controls. These controls are essential for ensuring the specificity of the Congo Red assay for β-glucan detection. The β-glucan standard, with its defined composition and structure, provides a reliable reference for optimizing experimental conditions and comparing extraction efficiencies, thereby enhancing the study’s validity and precision.
Reaction system
The reaction system was precisely set at a total volume of 8 mL. Using a calibrated pipette, 2 mL of treated samples were accurately measured and transferred into glass test tubes. The volume in each tube was then topped up to 4 mL with a NaH2PO4-Na2HPO4 buffer adjusted to the optimal pH of 7.0, which was determined through gradient optimization experiments. Next, 4 mL of freshly prepared Congo red reagent was added. For calibration, a blank control was set up by replacing the sample with 90% DMSO solution, ensuring accurate absorbance readings for reliable data analysis.
Determine the optimal extraction reagent
A thorough and systematic investigation was conducted to assess the impact of five distinct extraction reagents—potassium hydroxide (1 mol/L), sodium hydroxide (1 mol/L), hydrochloric acid (0.5 mol/L), dimethyl sulfoxide (90%), and distilled water—on the extraction efficiency of β-glucan. Following preliminary experimentation, the optimal extraction parameters for each reagent were precisely defined: a 45 min extraction period at 90 °C in KOH solution, 30 min at 60 °C in NaOH solution, 45 min at 50 °C in HCl solution, 60 min at 65 °C in 90% DMSO solution, and 90 min at 90 °C in distilled water. Concurrently, spectrophotometric measurements at 540 nm were precisely taken to determine the absorbance values. For reliable data acquisition, blank controls were systematically set up by sequentially substituting the sample solution with each extract solution, enabling a detailed comparison of the extraction performances of the different reagents.
Determination of optimum pH
This experiment aimed to assess the impact of NaH2PO4-Na2HPO4 buffers at pH 6.5, 7.0, 7.5, and 8.0 on the reaction system. Prepared matching pH Congo red solutions, set up experimental systems per the “Reaction system” part. Used 90% DMSO solution to extract samples at 65℃ for 60 min, with corresponding pH buffers as blanks, and measured absorbance at 540 nm for analysis.
Determination of optimal extraction temperature
To investigate the effect of extraction temperatures (25–75 °C in steps of 10 °C) on the reaction, set extraction time at 60 min, pH at 7.0, follow “Reaction system”. Use 90% DMSO solution as blank, measure absorbance at 540 nm for analysis.
Determine the optimal extraction time
To study the effect of different extraction times (30–70 min in steps of 10 min) on the reaction, set extraction temperature at 65 °C, follow “Reaction system”. Use 90% DMSO solution as blank, measure absorbance at 540 nm for analysis.
Preparation of β-glucan standard curve
Accurately weigh 50 mg of β-glucan standard, dissolve it completely in 90% DMSO solution via 65 °C water bath agitation. Cool to room temperature, dilute to 10 mL for a 5 mg/mL stock solution. Pipette 0.4, 0.8, 1.2, 1.6, 2.0 mL aliquots into 10 mL test tubes, make up to 4.0 mL with pH 7.0 NaH2PO4–Na2HPO4 buffer. Add 4.0 mL 0.01% Congo red, vortex, incubate 15 min. Use 90% DMSO solution as blank for calibration. Measure the sample absorbance at 540 nm using a spectrophotometer, with a 90% DMSO solution as the blank. Plot concentration (y-axis) vs. absorbance (x-axis) for regression curve and analysis.
Data processing & analysis
Data were analyzed via one-way ANOVA (IBM SPSS 26.0) with Tukey’s post-hoc test (P < 0.01). Graphs were generated using OriginPro.
Validation of the Congo Red UV Spectrophotometry
Except for the part of “Determination of β-glucan content in three different Cordyceps species”, the rest of the test samples in this subsection are YBC and β-glucan standard.
Precision experiments
Pipette 1.0 mL of a 5 mg/mL β-1,3-glucan standard solution into a glass test tube and proceed with the reaction and processing as outlined in the “Reaction System” protocol. Then the absorbance was measured at 540 nm by UV spectrophotometer and repeated 5 times. The results were substituted into the standard equation and RSD was calculated.
Repeatable experiments
Weigh five 50 mg YBC Cordyceps samples and place them into five 15 mL centrifuge tubes labeled I–V. Add 10 mL of 90% DMSO solution to each tube to dissolve the samples, then water-bathe at 65 °C for 60 min. After adding samples and color reagent as per the “Reaction system”, measure the absorbance at 540 nm using a UV spectrophotometer. Calculate the RSD of the result.
Stability experiments
The absorbance was measured at 540 nm after waiting for 0, 5, 10, 15, 20, 25, and 30 min using the β-glucan standard solution and YBC sample solution of the completed reaction. The relative standard deviation was calculated by substituting into the standard equation. Calculate the RSD of the result.
Recovery experiment
Pipette 1 mL of pretreated YBC sample into a glass test tube, then add 0.2, 0.4, 0.6, 0.8, and 1.0 mL of β-glucan standard solution successively. Adhere to the “Reaction system” for the reaction setup. After treatment per the specified method, detect the absorbance at 540 nm using a UV spectrophotometer. Subsequently, calculate the recoveries and RSD for data analysis.
Determination of β-glucan content in three different Cordyceps species
The β-glucan in Cordyceps was extracted from processed powders of C. militaris, C. cicadae, and C. fumosorosea (from “Pretreatment of strains and preparation of samples”).
First, weigh 50 mg of Cordyceps samples precisely in a glass test tube, then add 10 mL of 90% DMSO solution for dissolution. Secondly, during “Optimization of Extraction Parameters”, extraction was done under optimized conditions, also following the “Reaction system” for content determination. A bacteria-free solid cultivation medium served as a blank control, and each group had three parallel replicates for reliable data analysis.
The yield of β-glucan (%) = (the content of extracted glucan / the total mass of the sample) × 100.
Results
Through systematic parameter screening, The experimental results are as follows:
Solvent: DMSO performs best (Fig. 1). pH: Neutral pH (7.0) yields maximum absorbance (Fig. 2; P < 0.001). Temperature: Optimal at 65 °C; higher temperatures degrade β-glucan (Fig. 3; P < 0.001). Time: Peak absorbance at 60 min; longer times reduce content (Fig. 4; P < 0.001).
The comparison of extraction efficiencies among five extraction reagents demonstrates that DMSO exhibits the most effective extraction performance (left image: β-glucan standard; right image: YBC). The differences in extraction efficiency were statistically significant. Significance levels: **P < 0.01, ***P < 0.001.
Comparison of the effects of different pH values on the extraction of β-glucan showed that the best results were obtained at pH 7.0 (left image: β-glucan standard; right image: YBC). The results were represented as the mean value of β-glucan content ± standard deviation (SD) in three parallel experiments (n = 3).
Temperature has a significant effect on the extraction efficiency of β-glucan, and the best extraction effect was achieved at a temperature of 65 °C (left image: β-glucan standard; right image: YBC). The results were represented as the mean value of β-glucan content ± standard deviation (SD) in three parallel experiments (n = 3).
The influence of varying time intervals on the extraction efficiency of β-glucan was investigated. The results demonstrated that an extraction time of 60 min yielded the optimal extraction outcome. (left image: β-glucan standard; right image: YBC). The results were represented as the mean value of β-glucan content ± standard deviation (SD) in three parallel experiments (n = 3).
Impact of diverse extraction solvents on the quantification of β-glucan content
The experimental results demonstrated significant differences among the five reagents in β-glucan extraction efficiency (as depicted in Fig. 1. The comparison of extraction efficiencies among five extraction reagents demonstrates that DMSO exhibits the most effective extraction performance (left image: β-glucan standard; right image: YBC). The differences in extraction efficiency were statistically significant. Significance levels: **P < 0.01, ***P < 0.001.). Among them, DMSO stood out as the most efficient reagent for both the standard and YBC samples, while HCl proved to be the least effective.
The superior performance of DMSO can be attributed to two main factors. Firstly, its strong polarity enhances its interactions with β-glucan. Secondly, it has a greater ability to penetrate the matrix compared to water or acid–base reagents. The fact that DMSO exhibited a similar extraction efficiency in both YBC and standard glucan samples indicates its consistent performance across different substrates.
However, the comparable performance of KOH in the YBC sample is worth noting. It suggests that the chemical structure and composition of YBC may have a significant impact on the extraction process. YBC is a complex mixture containing polysaccharides and other bioactive compounds. These components could potentially interact differently with the solvents, leading to variations in extraction efficiency. This finding further supports the accuracy of our experimental results.
The influence of pH value on the extraction efficacy
During the experiment, at pH 6.5, the Congo red solution was a relatively deep red. As pH rose, it shifted to light red. After background removal, the absorbance of the β-glucan–Congo red reaction increased with pH then dropped rapidly with alkalinity. As per Fig. 2. Comparison of the effects of different pH values on the extraction of β-glucan showed that the best results were obtained at pH 7.0 (left image: β-glucan standard; right image: YBC). The results were represented as the mean value of β-glucan content ± standard deviation (SD) in three parallel experiments (n = 3). , absorbance peaked at pH 7.0 and was lowest at pH 8.0. Low pH over-protonates Congo red’s acidic groups, compacting its structure, and β-glucan’s hydroxyls bind H+, curling its chain, impeding their combination and lowering absorbance. High pH deprotonates Congo red’s groups, stretching it and weakening binding. β-glucan’s functional groups dissociate, increasing chain flexibility and steric hindrance and risking degradation, reducing binding and absorbance. These affect β-glucan content determination. Thus, a neutral pH of 7.0 is preferable for the experiment. The outcomes of the one-way ANOVA examining the impact of diverse pH levels on the extraction of β-glucan manifested that, with a p-value less than 0.001, it was unequivocally proven that the influence of pH on the extraction of glucan is highly significant (Table 1).
The impact of temperature on the extraction effectiveness
As depicted in Fig. 3. Temperature has a significant effect on the extraction efficiency of β-glucan, and the best extraction effect was achieved at a temperature of 65 °C (left image: β-glucan standard; right image: YBC). The results were represented as the mean value of β-glucan content ± standard deviation (SD) in three parallel experiments (n = 3)., there is a progressive enhancement in absorbance concomitant with the elevation of extraction temperature, signifying an escalation in the quantity of β-glucan extracted from the specimens. The absorbance culminates at an optimum temperature of 65 °C. Nonetheless, upon surpassing this threshold, a decrement in absorbance is observed. This phenomenon could be attributed to the thermal-induced structural alterations of β-glucan, encompassing potential chain fragmentation and conformational transitions. Such modifications might impair the affinity of β-glucan for Congo red, consequently diminishing the quantified β-glucan concentration. Hence, it is prudent to maintain the extraction temperature at approximately 65 °C to optimize the extraction yield of β-glucan. The outcomes of the one-way ANOVA, which meticulously investigated the impact of disparate temperature regimes on β-glucan extraction, yielded a p-value below 0.001. This unequivocally demonstrates that the influence of temperature on β-glucan extraction is of utmost significance (Table 2).
The influence of time on the extraction outcome
As shown in Fig. 4, absorbance increased with extraction time,indicating a progressive rise in β-glucan content. The extracted β-glucan content reached its peak at 60 min. Nevertheless, upon further prolongation of time, the absorbance of the colorimetric reaction between Congo red and β-glucan commenced to decline. An overly long extraction time induces the hydrolysis of the glucan’s glycosidic bond, resulting in the shortening of the molecular chain and a reduction in the binding sites with Congo red. Hence, it is advisable to maintain the optimal extraction time around 60 min. The influence of varying time durations on β-glucan extraction was meticulously explored through the application of one-way ANOVA. The resultant p-value were found to be beneath 0.001. The outcomes of this comprehensive analysis unmistakably signified that the impact of extraction time on the extraction efficacy of β-glucan is both substantial and statistically significant (Table 3).
Standard curve for β-glucan
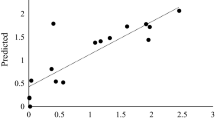
The standard curve was constructed in accordance with the test protocol of “Preparation of β-glucan standard curve”, as illustrated in Fig. 5. According to the curve, within the mass concentration range of 0.7 to 3.5 mg/mL, the β-glucan content and absorbance adhered to Beer’s law. The regression equation for this standard curve was y = 4.6355x + 0.3125, where x denoted the absorbance value and y represented the concentration (mg/mL). The correlation coefficient R2 = 0.9993, which signified a highly significant regression effect.
Method validation: precision, repeatability, stability, and recovery
The method was comprehensively validated for precision, repeatability, stability, and recovery to ensure reliability and reproducibility. All validation criteria met or exceeded the requirements for analytical methods. Key results are summarized below:
Precision
The UV spectrophotometer was used to measure the same β-glucan standard solution five times. As shown in Table 4, the SD and RSD were 0.670 and 0.336%, respectively. This indicates that the experimental results have little variation under the same conditions, suggesting that both the method and the instrument are highly precise.
Repeatability
The absorbance of five YBC sample solutions was measured precisely, and the β-glucan content was calculated accurately. The repeatability test results (Table 5) showed that the SD and RSD were 2.992 and 1.272%, respectively. This indicates that the experimental method has excellent reproducibility in sample processing and measurement.
Stability
The absorbance of five β-glucan standard solution groups and five YBC sample solution groups was measured. Stability experiment results are in Table 6. The standard solutions’ SD was 1.251, with an RSD of 0.638%. The YBC samples’ SD was 1.617, and RSD was 0.687%. These data show the assay had good stability.
Recovery rate
The recovery rate was ascertained through the addition of varying amounts of standard substances into five groups of sample solutions. The experimental outcomes are presented in Table 7. The average recovery reached 100.93%, with a SD of 0.014 and a RSD of 1.391%. The favorable recovery results fulfill the general detection requirements.
Determination of β-glucan content in the three species of Cordyceps
The β-glucan content in three varieties of Cordyceps, as determined using the Congo Red UV Spectrophotometry and presented in Fig. 6, exhibits only minor variations. Specifically, C. militaris, C. cicadae, and C. fumosorosea show no significant differences in their β-glucan content, with C. fumosorosea having a marginally higher β-glucan level than the other two species. The extraction rates, calculated using the appropriate formula, are as follows: C. militaris at (12.23 ± 0.004)%, C. cicadae at (12.12 ± 0.004)%, and C. fumosorosea at (12.83 ± 0.002)%. Furthermore, the statistical analysis depicted in the figure reveals a highly significant difference in β-glucan content between the blank control group and the three Cordyceps varieties. Notably, the β-glucan content in the rice cultivation medium is considerably lower than that found in the Cordyceps samples. This suggests that the β-glucans measured are endogenous to the Cordyceps, being metabolically produced by the fungi themselves rather than derived from the culture medium.
Summary and discussion
In recent years, Cordyceps and its derivatives have garnered significant attention due to their rich nutritional profile and remarkable medicinal properties, with β-glucan from Cordyceps emerging as a focal point of research. As a class of polysaccharides exhibiting diverse bioactivities—including antioxidant and hypoglycemic effects—β-glucans represent critical components of fungal polysaccharides. Studies have demonstrated a strong correlation between the bioactivity of cereal- and fungal-derived polysaccharides and their β-glucan content28,29. For instance, the CMBG-1 fraction isolated from C. militaris exhibits potent inhibitory activity against α-amylase and α-glucosidase, suggesting its potential for development as a functional food targeting postprandial hyperglycemia30, while simultaneously demonstrating robust in vitro antioxidant activity. However, the advancement of Cordyceps-based applications has been hindered by the lack of standardized detection methods for β-glucan. To address this critical gap, the present study employs Congo Red UV spectrophotometry for β-glucan quantification in Cordyceps, systematically optimizing key analytical parameters including detection conditions, accuracy, precision, and stability. Through extensive experimental validation, the optimal conditions were established as follows: dimethyl sulfoxide (DMSO) as the extraction solvent, pH 7.0, 65 °C reaction temperature, and 60 min incubation. Notably, the extraction efficiency of β-glucan varied significantly across solvents, with DMSO demonstrating the highest yield, followed by KOH and NaOH, while HCl exhibited the lowest efficiency—a finding consistent with the experimental outcomes reported by Mirończuk-Chodakowska et al.31 and Khan et al.32.
The Congo Red UV Spectrophotometry demonstrates distinct advantages over existing techniques for β-glucan quantification through three principal aspects. First, regarding precision and cost-effectiveness, Yang et al.33 reported a relative standard deviation (RSD) of 1.86% for fluorescence-based β-glucan detection in edible fungi after optimization, whereas the Congo red method developed in this study achieved superior precision with an RSD of 0.336%. Although enzymatic assays, as investigated by Anderson et al.34, exhibit high accuracy, their reliance on costly high-purity β-glucan hydrolases and prolonged assay durations renders them impractical for large-scale applications. Second, in terms of extraction efficiency, the Congo Red UV Spectrophotometry yielded β-glucan extraction rates of (12.23 ± 0.004)%, (12.12 ± 0.004)%, and (12.83 ± 0.002)% for C. militaris, C. cicadae, and C. fumosorosea, respectively. These values markedly exceed those obtained via subcritical water-assisted enzymatic extraction (4.22%) reported by Sun et al.35, ultrasonic-assisted extraction (2.13%) by Deng et al.36, and microwave-ultrasonic combined extraction (6.3%) by Yu et al.37, underscoring the method’s superior extraction efficiency. Third, the method exhibits exceptional anti-interference capability. Congo red selectively binds to the triple-helix conformation of 1,3–1,6-β-D-glucans without cross-reactivity with other polysaccharides (e.g., chitosan or glycoproteins)31, enabling direct quantification in unpurified extracts and highlighting its practical utility.
In conclusion, compared to alternative approaches, the Congo Red UV Spectrophotometry offers unparalleled advantages, including reagent affordability, operational simplicity, rapid detection , and high accuracy. These features position it as an ideal solution for industrial-scale β-glucan analysis, particularly in scenarios requiring high-throughput screening.
Additionally, this study revealed that the β-glucan content in C. fumosorosea is comparable to that of C. militaris and C. cicadae, suggesting that C. fumosorosea possesses similar potential for food and medicinal applications as C. militaris. However, further evaluation of its practical value requires comprehensive analysis of the levels of other bioactive components in C. fumosorosea.
This study identified several limitations that warrant acknowledgment and resolution. Firstly, the experimental scope was confined to C. militaris, C. cicadae, and C. fumosorosea, excluding other Cordyceps species. While reports indicate that β-glucans in C. militaris and C. fumosorosea primarily consist of β-(1 → 3)-linked backbones with β-(1 → 6)-branches32,38—a structure shared with many fungal species such as Ganoderma and yeast39,40. The theoretical applicability of the Congo Red UV Spectrophotometry to other Cordyceps species requires empirical validation. Additionally, despite the method’s demonstrated advantages in Cordyceps β-glucan quantification, cross-validation with established techniques (e.g., fluorescence or enzymatic assays) is necessary to confirm its absolute superiority.
Research on β-glucans in Cordyceps polysaccharides remains limited compared to other components, with challenges in precise detection, analysis, and extraction hindering deeper exploration. This study innovatively establishes a Congo Red UV Spectrophotometry for β-glucan quantification in Cordyceps, optimized through systematic parameter refinement. This approach not only provides technical support for accurate β-glucan detection but also lays the groundwork for advanced research and industrial applications, such as high-value product development and resource utilization, thereby enhancing the commercial potential and versatility of Cordyceps-based products. Future efforts should prioritize the integration of the Congo Red UV Spectrophotometry into ISO/GB draft standards and the construction of a structure–function relationship database for β-glucans to guide functional product design.
Data availability
The data that support the findings of this study are available from the corresponding author, [Hong Yu], upon reasonable request.
References
Bashir, K. M. I. & Choi, J. S. Clinical and physiological perspectives of β-glucans: The past, present, and future. Int. J. Mol. Sci. 18, 1906 (2017).
Adachi, Y., Okazaki, M., Ohno, N. & Yadomae, T. Enhancement of cytokine production by macrophages stimulated with (1→ 3)-β-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol. Pharm. Bull. 17, 1554–1560 (1994).
Ying, L. & Kuo, S. Clinical study of Beta glucan from edible-medicinal fungi on hyperlipidemia. China Health Care Nutr. 23, 01 (2013).
Sung, G.H. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 57, 1–63 (2007).
Li, S. P., Li, P., Dong, T. T. X. & Tsim, K. W. K. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia. Phytomedicine. 8, 207–212 (2001).
Dong CaiHong, D. C. et al. Cordyceps industry in China: Current status, challenges and perspectives-Jinhu declaration for cordyceps industry development. Mycosystema. 35, 01 (2016).
Cyran, M. R. & Snochowska, K. K. Evidence of intermolecular associations of β-glucan and high-molar mass xylan in a hot water extract of raw oat groat. Carbohydr. Polym. 272, 118463 (2021).
Sousa, P. et al. β-Glucan extracts as high-value multifunctional ingredients for skin health: A review. Carbohydr. Polym. 322, 121329 (2023).
McCleary, B. V. & Draga, A. Measurement of β-glucan in mushrooms and mycelial products. J. AOAC Int. 99, 364–373 (2016).
Young, S. H. et al. Pulmonary inflammation induced by office dust and the relation to 1→ 3-β-glucan using different extraction techniques. Toxicol. Environ. Chem. 93, 806–823 (2011).
Yu, Z. W. & Quinn, P. J. Dimethyl sulphoxide: A review of its applications in cell biology. Biosci. Rep. 14, 259–281 (1994).
Chen, X. R. Zhang, H. Y. & Tian, X. Y. Property and application of dimethyl sulfoxide. Liaoning Chem. Ind. 29, 31–35 (2000).
Prozil, S. O., Costa, E. V., Evtuguin, D. V., Lopes, L. P. C. & Domingues, M. R. M. Structural characterization of polysaccharides isolated from grape stalks of Vitis vinifera L. Carbohydr. Res. 356, 252–259 (2012).
Yan, Z. Research progress in methods for quantitative determination of fungal polysaccharide. Food Mach. 28, 02 (2012).
Wu, J., Deng, X., Tian, B., Wang, L. & Xie, B. Interactions between oat β-glucan and calcofluor characterized by spectroscopic method. J. Agric. Food Chem. 56, 1131–1137 (2008).
Mcclear, B. V. & Glennieholmes, M. Enzymic quantification of (1 to 3) (1 to 4)-beta-d-glucan in barley and malt. J. Inst. Brew. 91, 285–295 (1985).
Miyazaki, T. et al. Limulus test (factor G) and polysaccharides from fungus Kansenshogaku zasshi. J. Jpn. Assoc. Infect. Dis. 66, 1030–1036 (1992).
Gandhi, N. S., Landrieu, I., Byrne, C., Amniai, L. & Lippens, G. The use of phosphorylated peptides to explore the folding properties of the protein tau required for AT8 antibody recognition (2015).
Du, B., Yang, Y., Bian, Z. & Xu, B. Molecular weight and helix conformation determine intestinal anti-inflammatory effects of exopolysaccharide from Schizophyllum commune. Carbohydr. Polym. 172, 68–77 (2017).
Liu, Y. et al. Structure, preparation, modification, and bioactivities of β-glucan and mannan from yeast cell wall: A review. Int. J. Biol. Macromol. 173, 445–456 (2021).
Zheng, Z. et al. Effects and mechanisms of ultrasound-and alkali-assisted enzymolysis on production of water-soluble yeast β-glucan. Bioresour. Technol. 273, 394–403 (2019).
Nitschke, J. et al. A new colorimetric method to quantify β-1, 3–1, 6-glucans in comparison with total β-1, 3-glucans in edible mushrooms. Food Chem. 127, 791–796 (2011).
Juan, Z., Du, X. F. &Rao, Y. Q. Measurement of β-glucan form oats by congo red. J. Anhui Agric. Univ. 34, 01 (2007).
Schmidt, M. Cereal beta-glucans: An underutilized health endorsing food ingredient. Crit. Rev. Food Sci. Nutr. 62, 3281–3300 (2022).
He, L., Zhu, Z. & Qi, C. β-Glucan—A promising immunocyte-targeting drug delivery vehicle: Superiority, applications and future prospects. Carbohydr. Polym. 339, 122252 (2024).
Murphy, E. J. et al. Sustainable production and pharmaceutical applications of β-glucan from microbial sources. Microbiol. Res. 274, 127424 (2023).
Guleria, P., Kumari, S. & Dangi, N. β-glucan: Health benefits and role in food industry—a review. Int. J. Enhanc. Res. Sci. Technol. Eng. 4, 3–7 (2015).
Fernandez-Julia, P. J., Munoz-Munoz, J. & van Sinderen, D. A comprehensive review on the impact of β-glucan metabolism by Bacteroides and Bifidobacterium species as members of the gut microbiota. Int. J. Biol. Macromol. 181, 877–889 (2021).
Chiozzi, V. et al. Biotechnological addition of β-glucans from cereals, mushrooms and yeasts in foods and animal feed. Processes. 9, 1889 (2021).
Lin, X. Y., Yan, G. Y. & Wang, Y. R. Isolation and purification, structural characterization, hypoglycemic activity of soluble β-glucan from Cordyceps cherysanthemi. Sci. Technol. Food Ind. 12, 1–17 (2024).
Mirończuk-Chodakowska, I. & Witkowska, A. M. Evaluation of Polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health. 17, 7299 (2020).
Khan, T., Dong-Hai, H., Zhou, J. N., Yang, Y. L. & Yu, H. Comprehensive analysis of metabolites in the mycelium of Cordyceps fumosorosea cultured on Periplaneta americana. Ann. Microbiol. 74, 9 (2024).
Yang, K. et al. Fluorometry method for quantitative determination of functional β-glucans from edible and medicinal mushrooms. Mycosystema. 28, 03 (2009).
Anderson, M. A., Cook, J. A. & Stone, B. A. Enzymatic determination of 1, 3: 1, 4-β-glucans in barley grain and other cereals. J. Inst. Brew. 84, 233–239 (1978).
Sun, Q. Z., Jiang, C. F. & Cai, X. Q. Optimization of co-extraction of β-glucan from oat bran using subcritical water assisted enzymatic treatment. J. Food Saf. Qual. 15, 171–179 (2024).
Deng, A. H., Yang, P. H. & Liu, Y. J. Optimization of ultrasonic assisted extraction process of β-glucan from highland barley. Food Sci. Technol. Econ. 47, 108–112 (2022).
Liya, Y. U. et al. Effect of microwave-ultrasound treatment on physicochemical and structural properties of highland barley β-glucan. Shipin Kexue. 45, 185–192 (2024).
Bi, S. et al. Structural elucidation and immunostimulatory activity of a new polysaccharide from Cordyceps militaris. Food Funct. 9, 279–293 (2018).
Liu, Y. F. et al. Structural and conformational characterization of Ganoderma lucidum β-glucan and its structure-activity relationship. In Proceedings of the 2018 Annual Meeting of the Mycological Society of China 153 (Edible Fungi Research Institute, 2018).
Liu, L. T., He, Y., Shao, X. L., & Ma, X. The research advance and application of β-glucan from yeast. Food Industry. 38, 238–242 (2017).
Acknowledgements
Thanks to Yunnan Fungal Culture Collection (YFCC), Yunnan Herbal Laboratory (YHL), and College of Ecology and Environment, Yunnan University, was for providing us with all the facilities we needed to do our work as well as financial support.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No.31870017) and supported by Practice Innovation Fund for graduate students of Yunnan University (ZC-23234542).
Author information
Authors and Affiliations
Contributions
Haiwen Xia, Ran Zhang and Prof. Hong Yu (authors) contributed to the study’s conception and design. The paper was written by Haiwen Xia and Ran Zhang, grammar-checked by SOUVANHNACHIT Sisommay, reviewed by Yufan Yin, Yingling Lu and Zihao Liu. Supervised by Prof. Hong Yu and Prof. Wenju Zhang. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xia, H., Zhang, R., Yin, Y. et al. Quantitative detection of β-glucans in Cordyceps species using a validated Congo red assay. Sci Rep 15, 9938 (2025). https://doi.org/10.1038/s41598-025-94217-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94217-5
Keywords
This article is cited by
-
A review on pharmacological insights of edible and medicinal mushroom based β-glucans
Applied Biological Chemistry (2025)