Abstract
Chronic refractory wounds are a common, costly and recurrent complication of diabetes. Platelet-rich plasma (PRP), a new therapy for chronic wounds, is limited by a long treatment period and unstable effects. FG-4592(Roxadustat) is a new prolyl-4-hydroxylation domain(PHD) inhibitor, which can stabilize hypoxia-inducible factor-1α(HIF-1α) effectively. It is a promising drug for wound repair and needs to be demonstrated via various appropriate application scenarios. This study investigated the effects of combining FG-4592 and PRP to promote diabetic wound healing. Diabetic rats were randomly assigned to five groups: nondiabetic control, diabetic untreated, diabetic + PRP, diabetic + FG-4592, and diabetic + PRP + FG-4592. Diabetes was induced with streptozotocin (STZ). A full-thickness skin defect was created, and FG-4592 (peritoneal injection) and PRP (periwound injection) were administered alone or together. Wound healing was assessed by histological analysis (Hematoxylin-Eosin (HE) and Masson staining) and protein expression of HIF-1α, VEGF(vascular endothelial growth factor), α-SMA(α-smooth muscle actin), CoL1α1(collagen type 1 alpha 1), and SDF-1(Stromal Cell-derived Factor 1) via Western blotting and qRT-PCR(Quantitative real time polymerase chain reaction). Immunohistochemistry(IHC) and immunofluorescence(IF) were also used to evaluate CD34 (Cluster of Differentiation 34), CD31(Cluster of Differentiation 31), VEGF(vascular endothelial growth factor), SDF-1(Stromal Cell-derived Factor 1), PCNA(Proliferating Cell Nuclear Antigen), and Integrin-β1 expression. The untreated diabetic group exhibited impaired wound healing, histological damage, and reduced expression of key proteins compared to the nondiabetic control group. Both PRP and FG-4592 alone improved wound healing, reduced damage, and upregulated protein expression. However, the combination of FG-4592 and PRP showed the most significant improvement. This study suggests that FG-4592 combined with PRP accelerates diabetic wound healing by enhancing endothelial progenitor cell recruitment, neovascularization, and cell proliferation and migration via upregulation of HIF-1α and its target genes. This combination therapy may provide a novel approach for treating chronic diabetic wounds.
Similar content being viewed by others
Background
The global impact of diabetes mellitus, including type 2 diabetes mellitus, is substantial, and diabetes mellitus is the most prevalent metabolic disease in the world1. Due to intrinsic metabolic and extrinsic injury-causing factors, many diabetic patients develop diabetic wounds with poor wound healing ability2. Wounds in diabetic patients are characterized by impaired healing, prolonged inflammation and reduced epithelialization kinetics. The wound healing process can be roughly divided into inflammatory, proliferative and remodeling phases according to the time sequence, which are independent of each other and overlap each other3. Vascular regeneration is one of the important processes in the proliferative phase of wound healing. Recruitment of bone marrow-derived endothelial progenitor cells (EPCs) is an important way to form new blood vessels. These endothelial progenitor cells are selectively recruited to areas of ischemia or injury, where hypoxia plays a major role, and are facilitated to enter ischemic and hypoxic tissues through SDF-1, a target gene of HIF-1α4. Currently, diabetic wounds are mainly treated through regular debridement, infection treatment, pressure offloading, and bioengineered replacement tissue products5,6. However, most treatments are only effective for minor or moderate wounds. Therefore, there is an urgent need to develop new drugs or therapies for diabetic wound healing in clinical practice.
Wound healing is regulated by HIF-1, and the HIF-1-mediated hypoxic response is involved in several aspects of wound healing7. Under hypoxic conditions, prolyl hydroxylase activity is inhibited, and HIF-1α is stably expressed and binds to HIF-1β to initiate target gene expression8. Hyperglycaemia decreases the stability of HIF-1α, leading to the suppression of HIF-1α target gene expression, which may be responsible for poor healing and ulcer complications in diabetic patients9. Roxadustat (also known as FG-4592) is an activator of HIF-1α that increases the transcriptional activity of HIF-1α by inhibiting hypoxia-inducible factor prolyl hydroxylase (HIF-PHD)10. Li et al. found that FG-4592 can reduce depression-like symptoms caused by lipopolysaccharide through PI3K signalling11. Ko et al. demonstrated that FG-4592 accelerated lung growth, development, and function in a compensatory lung growth model12. Another study found that FG-4592 can stabilize HIF-1α and HIF-2α in a two-way mixed lymphocyte reaction, inhibit cell proliferation, inhibit the differentiation of CD4 + T cells into Th1 and Th17 subsets, while promoting differentiation into Th2, Treg and Tfh, and inhibiting humoral immunity13. A recent review indicates that FG-4592 accelerates wound healing by initiating and promoting angiogenesis through targeting certain growth factor pathways14. Our group has previously demonstrated that exogenous FG-4592 effectively accelerates skin wound healing and induces epidermal hyperplasia and concluded that HIF-1α is an important target for promoting wound healing15. Reduced HIF-1 expression is one of the reasons why diabetic wounds are difficult to heal16. Collectively, these findings suggest FG-4592 may be a promising therapeutic candidate for wound repair, particularly in addressing diabetes-associated chronic wounds by modulating the pathological transition from sustained inflammatory states to active proliferative phases. To date, the specific therapeutic mechanisms have not been fully elucidated.
In recent years, many studies have reported the beneficial effects of PRP on diabetic wound healing17,18,19. PRP contains high levels of factors that promote wound healing, such as platelet-derived growth factor, transforming growth factor-β, insulin-like growth factor-1, vascular endothelial growth factor, and basic fibroblast growth factor20. However, PRP therapy requires long treatment cycles and yields unstable treatment effects, and therefore, optimizing and improving its therapeutic effect is currently a research hotspot.
In this study, a full-thickness skin defect wound model in diabetic rats was used to evaluate the therapeutic effect of PRP combined with FG-4592 on diabetic wounds. The hypothesis of this study was that FG-4592 optimizes the therapeutic effect of PRP on diabetic wounds by upregulating the expression of HIF-1α and its target genes and thereby reversing the effects of high glucose levels on endothelial progenitor cell (EPC) recruitment, vascular neovascularization, and cell proliferation and migration.
Methods
Preparation of PRP
PRP was prepared using a rat peripheral blood platelet separation kit (TBD, Tianjin, China). In a centrifuge tube, first, anticoagulated blood was added to separation liquid. The ratio of anticoagulated blood to separation solution was 1:2. Then, the sample was centrifuged at 300 × g for 15 min. Finally, a straw was used to carefully draw the first platelet plasma layer, which contains platelet-rich plasma, into a fresh 15-mL centrifuge tube. The platelet concentration was determined using automatic blood cell analyzer with 1 mL of normal plasma (NP) or PRP.
Induction of the diabetic rat model
After thirty 8 ~ 10-week-old SPF Sprague–Dawley (SD) male rats were acclimatized, they were intraperitoneally injected with 60 mg/kg STZ21 (Aladdin, Shanghai, China) to induce diabetes; diabetic rats were identified by the presence of hyperglycaemia (fasting blood glucose > 300 mg/dL or 16.7 mmol/L in tail vein blood samples monitored by a glucose metre) within one week after injection. Blood glucose was measured on day 8 after STZ induction, and diabetic rats with fasting blood glucose levels greater than 16.7 mmol/L were selected.
Construction of a full-thickness skin defect wound model
SD rats were anaesthetized, the dorsal hair was removed, and a round full-thickness skin defect wound with a diameter of 1 cm was made on the dorsal surface of the rats. Then, a metal ring(1 cm in diameter) was sutured around the wound to decrease error due to wound shrinkage.
Animal grouping and corresponding treatment
The experimental animals were randomly divided into five groups (n = 6 in each group): nondiabetic group, diabetic untreated group, diabetic + PRP group, diabetic + FG-4592 group and diabetic + PRP + FG-4592 group. Experimental animals were systematically assigned to designated groups and permanently identified via ear tagging for longitudinal tracking.
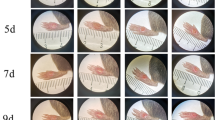
Immediately after wounding, the treatment corresponding to each group was carried out (shown in Fig. 1A). The rats in the diabetic + PRP group were injected with PRP around the wound22,23,24 (200 µL/site, 4 sites, 1 injection per week); the rats in the diabetic + FG-4592 group were injected intraperitoneally with FG-4592 (Aladdin, Shanghai, China) at a dose of 25 mg/kg every other day25; and the rats in the diabetic + PRP + FG-4592 group received both treatments. The rats in the nondiabetic group and the diabetic untreated group were injected with equal amounts of PBS during the same period.
Effect of FG-4592 combined with PRP on wound healing in rats with diabetic skin defects. A: Experimental grouping and corresponding treatment for each group. The figure was created in Fig Draw (www.figdraw.com). B: Confirmation of the diabetic wound model. C: Changes in the body weight of the rats in each group on days 0, 3, 7, and 10, The interaction was significant (p < 0.05), but the time effect was not significant within each group. D-F Changes in the wound contraction rate of rats in each group on days 0, 3, 7, 10, and 12. Since the interaction was not significant, the difference in treatment methods was analyzed by combining all time points (D). #P < 0.05 vs. the nondiabetic group; *P < 0.05 vs. the diabetic untreated group.
Weekly PRP application aligns with the metabolic kinetics of growth factors (peak activity at 48–72 h followed by rapid decline), ensuring sustained signalling for tissue repair, while synchronizing with the proliferative phase’s high growth factor demand and balancing clinical feasibility and patient tolerance. The every-other-day dosing aligns with FG-4592’s 12-15-hour half-life, sustaining HIF-α stabilization to promote wound repair-related gene expression while preventing drug overaccumulation, thereby balancing efficacy and safety.
The body weight of the rats was measured on days 0, 3, 7, and 10 of treatment, and the wound area was photographed at a fixed distance from the wound and quantified using ImageJ software. Wound healing rate was calculated according to the following formula: wound healing rate (%) =(A0-Atd)/A0 × 100, where A0 indicates the wound area on day 0 and Atd indicates the wound area on day t after treatment. On day 12, rats were anaesthetised with 3% sodium pentobarbital 40 mg/kg intraperitoneally, blood was collected from the abdominal aorta, and tissues were rapidly collected from the dorsal wound.
HE staining and Masson staining
Wound tissue was fixed with 10% formaldehyde solution for 48 h and then sequentially dehydrated with an ethanol gradient, cleared with xylene, embedded in paraffin, and sectioned (5 μm). HE staining and Masson’s trichrome staining were performed. Inflammatory cell infiltration, angiogenesis and collagen deposition were observed under a light microscope.
Western blot
A portion of traumatic tissue stored in a -80 °C freezer was cut, proteins were extracted with radio immunoprecipitation assay(RIPA) lysis buffer, protein content was measured by the bicinchoninic acid assay(BCA) method, a protein standard curve was generated, and the amount of protein in the sample was calculated. The extracted proteins were denatured for gel electrophoresis, which was followed by membrane transfer, and membrane blocking and then incubation with anti-HIF-1α (1:1000), anti-VEGFA (1:1000), anti-α-SMA (1:1000), anti-CoL1α1 (1:1000), anti-SDF-1 (1:1000), anti-PCNA (1:1000), anti-integrin-β1 (1:1000), and anti-GAPDH (1:1000) primary antibodies at 4℃ overnight. Then, the membranes were incubated with a goat anti-rabbit IgG secondary antibody (1:20,000), and the bands were analysed with ImageJ software. All antibodies were purchased from Bioswamp (Wuhan, China).
qRT‒PCR
The mRNA expression of VEGFA, α-SMA, CoL1α1, SDF-1, PCNA, and integrin-β1 in wound tissue was measured by qRTPCR. Total RNA was extracted from frozen wound tissue under RNase-free conditions and quantified. The experimental procedures were performed according to the kit instructions, and all the data were analysed using the 2−ΔΔCt method to calculate mRNA expression. The primer sequences are shown in Table 1.
IHC
The expression of CD31, CD34 and VEGF was detected by immunohistochemistry. Wound tissues from each group were routinely embedded in paraffin and sectioned for immunohistochemical analysis. Primary antibodies against CD31 (1:100), CD34 (1:100) and VEGF (1:200) were added dropwise to cover the sections, followed by incubation overnight at 4 °C. After rinsing the sections with PBS buffer, secondary antibodies were added, and the sections were incubated at 37 °C for 50 min. After washing the sections, DAB was added for colour development at room temperature. Three high-magnification view fields of each group were randomly selected, and the number of positive cells in each field of view was counted separately. The results are expressed as mean values.
IF
Prepared paraffin sections were dewaxed in water. Antigen was retrieved by the microwave heat repair method, followed by routine immunofluorescence procedures such as blocking, incubation with primary antibodies (SDF-1, PCNA, Integrin-β1) and incubation with secondary antibodies. Fluorescence was observed under a fluorescence microscope and photographed.
Statistical analysis
SPSS 24.0 software was used for statistical analyses, and GraphPad Prism 8.0 software was used for plotting the data. The results are expressed as the mean ± standard deviation (mean ± SD). Two-factor analysis of variance was used to analyze the treatment method and time. If there is no interaction between the two factors, main effect analysis is performed. If there is an interaction between the two factors, simple effect analysis is performed. One-way analysis of variance and LSD test were used to compare the mean differences between multiple groups, and t-test was used to compare the mean differences between two groups. P < 0.05 indicated that a difference was statistically significant.
Results
Effects of FG-4592 combined with PRP on wound healing in rats with diabetic skin defects
The platelet concentration in our prepared PRP is about 8 times higher than the platelet concentration in plasma. To ensure that the diabetic rat model was reliable, blood glucose levels were measured, and as shown in Fig. 1B, the blood glucose levels in diabetic rats were significantly greater than those in nondiabetic rats and greater than 16.7 mmol/L.
The two-way ANOVA showed a significant interaction between treatment method and time on body weight (P < 0.05). Therefore, we conducted a simple effects analysis and found that the body weight of the rats in the diabetic untreated group was lower than that of rats in the nondiabetic group on days 0, 3, 7, and 10. Compared with those of rats in the diabetic untreated group, the body weights of the rats in the diabetic + PRP group and diabetic + FG-4592 group tended to be higher on days 3, 7 and 10, and the body weights of the rats in the diabetic + PRP + FG-4592 group were significantly higher on days 3, 7 and 10. There was a trend towards increased body weight in the diabetic + PRP + FG-4592 group compared with the diabetic + PRP group and the diabetic + FG-4592 group. It indicates that the combined treatment of PRP and FG4592 shows a better effect on the recovery of body weight in diabetic rats compared to the use of these two drugs individually (Fig. 1C).
The two-way ANOVA showed no significant interaction between treatment method and time on wound contraction rate (P > 0.05). Therefore, we performed main effect analysis. Compared with the nondiabetic group, the diabetic untreated group showed a significantly reduced contraction rate. Compared with the diabetic untreated group, both the diabetic + PRP group and the diabetic + PRP + FG-4592 group exhibited a significantly increased contraction rate (Fig. 1D). And then we did separate analysis of different groups at different time (Fig. 1E). Compared with that in the nondiabetic group, the wound healing rate was consistently lower in the diabetic untreated group, and the difference was statistically significant on days 3 and 7. Compared with that in the diabetic untreated group, the wound healing rate of the diabetic + PRP group was significantly greater on days 3 and 7, that of the diabetic + PRP + FG-4592 group was significantly greater on day 7; on day 10, there was a trend toward an increase in the diabetic + FG-4592 group and the diabetic + PRP + FG-4592 group; on day 12, the wound healing rate was closest in the diabetic + PRP + FG-4592 group compared with the non-diabetic group (Fig. 1E and F). This result suggested that PRP may have better efficacy in the early stage of wound healing and that FG-4592 may have a better tendency to promote proliferation the middle and late stages of wound healing, the benefits of the combination therapy can be sustained after the initial healing.
Effects of FG-4592 combined with PRP on pathological wound damage and HIF-1α expression in rats with diabetic skin defects
HE staining revealed the differences hidden beneath the scab. As shown in Fig. 2A, in the nondiabetic group, the new epithelial tongues on both sides of the wound were almost completely fused. In the untreated diabetic group, there was a large amount of inflammatory cell infiltration, and the distance between the epithelial tongues was the greatest. Compared with those in the diabetic untreated group, a thickened epithelial tongue extending towards the wound centre, less inflammatory cell infiltration, and more dense neovascularization were observed in both the FG-4592 and PRP-treated groups, and healing was more significant in the combined treatment group.
Effect of FG-4592 combined with PRP on pathological wound damage and HIF-1α expression in rats with diabetic skin defects. A: HE staining was used to observe pathological damage to wound tissue; Scale bar = 400 μm, the bidirectional arrows represent the distance between the tongues of the new epithelium; the image in the second row is an enlarged version of the area in the black box in the first row. B and C: The protein expression of HIF-1α in wound tissue was analysed by western blot. *, P < 0.05.
As shown in Fig. 2B and C, the expression of HIF-1α in the diabetic untreated group was significantly lower than that in the nondiabetic group. Compared with that in the diabetic untreated group, the expression of HIF-1α was significantly higher in both the diabetic + PRP and diabetic + FG-4592 groups; and as expected, the expression of HIF-1α in the diabetic + PRP + FG-4592 group was further significantly upregulated.
Effects of FG-4592 combined with PRP on angiogenesis in rats with diabetic skin defects
Compared with those in the nondiabetic group, the protein (Fig. 3A) and mRNA (Fig. 3B) expression levels of VEGF in the diabetic untreated group were significantly lower. Compared with those in the diabetic untreated group, the mRNA and protein expression levels of VEGF in the diabetic + PRP group and the diabetic + FG-4592 group were significantly higher. Compared with those in the diabetic + PRP group and the diabetic + FG-4592 group, the mRNA and protein expression levels of VEGF in the diabetic + PRP + FG-4592 group were significantly higher (Fig. 3A and B).
Effect of FG-4592 combined with PRP on angiogenesis in rats with diabetic skin defects. A: The protein expression of VEGF in wound tissue was analysed by western blot. B: The expression of VEGF mRNA in wound tissue was measured by qRT‒PCR. C-D: The expression of CD34, CD31, and VEGF in wound tissue was detected by immunohistochemistry, Scale bar = 100 μm. *P < 0.05. E: Microvascular regeneration was shown by HE staining, with the black arrow indicating new blood vessels, Scale bar = 100 μm.
Further verification by immunohistochemistry revealed that compared with those in the wound tissue of rats in the nondiabetic group, the expression levels of VEGF, CD31 and CD34 in the wound tissue of rats in the diabetic untreated group were significantly lower. Compared with those in the diabetic untreated group, the expression levels of VEGF, CD31 and CD34 were significantly greater in the diabetic + PRP group and the diabetic + FG-4592 group. Compared with those in the diabetic + PRP group and the diabetic + FG-4592 group, the expression levels of VEGF, CD31 and CD34 were significantly greater in the diabetic + PRP + FG-4592 group (Fig. 3C and D). This phenomenon was consistent with the changes in neovascularization density observed by HE staining (Fig. 3E). These results suggest that both PRP and FG-4592 can promote angiogenesis and that combined intervention with these two agents has the best effect.
Effects of FG-4592 combined with PRP on collagen deposition and myofibroblast differentiation in rats with diabetic skin defects
As shown in Fig. 4A and B, Masson staining revealed that compared with that in the nondiabetic group, the collagen fibre content in the diabetic untreated group was lower, and the distribution was uneven. Compared with those in the untreated diabetic group, the number of collagen fibres in the other groups was higher, and the fibres were thicker and more evenly distributed; PRP combined with FG-4592 had the greatest effect.
Effect of FG-4592 combined with PRP on collagen deposition and myofibroblast differentiation in rats with diabetic skin defects. A and B: Masson staining and semiquantitative analysis were used to assess collagen deposition in the dermis, Scale bar = 100 μm C: The mRNA expression levels of α-SMA and CoL1α1 in wound tissue were measured by qRT‒PCR. D: The protein expression levels of α-SMA and CoL1α1 in wound tissue were analysed by western blot. *P < 0.05.
Type I collagen is an important component in skin tissue repair. α-SMA is a marker of myofibroblasts, which differentiated from fibroblasts and shrink wounds by contraction. Compared with those in the wound tissue of rats in the nondiabetic group, the protein and mRNA expression levels of α-SMA and CoL1α1 in the wound tissue of rats in the diabetic untreated group were significantly lower. Compared with those in the diabetic untreated group, the protein and mRNA expression levels of α-SMA and CoL1α1 in the diabetic + PRP group and the diabetic + FG-4592 group were significantly higher. Compared with those in the diabetic + PRP group and the diabetic + FG-4592 group, the protein and mRNA expression levels of α-SMA and CoL1α1 in the diabetic + PRP + FG-4592 group were significantly greater (Fig. 4C and D).
Effects of FG-4592 combined with PRP on EPC recruitment in rats with diabetic skin defects
SDF-1 plays an important role in EPC recruitment, which is critical for neovascularization. Compared with the nondiabetic group, the protein and mRNA expression of SDF-1 in the wound tissue of rats in the diabetic untreated group was significantly lower. Compared with the diabetic untreated group, the protein and mRNA expression levels of SDF-1 in the wound tissue of rats in the diabetic + PRP group and the diabetic + FG-4592 group were significantly higher. Compared with those in the wound tissue of rats in the diabetic + PRP group and the diabetic + FG-4592 group, the protein and mRNA expression levels of SDF-1 in the wound tissue of rats the diabetic + PRP + FG-4592 group were significantly greater (Fig. 5A and B). In addition, consistent results were found by immunofluorescence; both PRP and FG-4592 upregulated the expression of SDF-1 (Fig. 5C). These results suggest that both PRP and FG-4592 can reverse the decreased recruitment of endothelial cells induced by high glucose levels and that the best effect is achieved when PRP and FG-4592 are combined.
Effect of FG-4592 combined with PRP on the expression of SDF-1 in rats with diabetic skin defects. A: Western blot analysis of SDF-1 protein expression in wound tissue. B: qRT‒PCR analysis of SDF-1 mRNA expression in wound tissue. *P < 0.05. C: Immunofluorescence detection of SDF-1 expression in wound tissue, Scale bar = 50 μm.
Effects of FG-4592 combined with PRP on cell proliferation and migration in rats with diabetic skin defects
PCNA is a marker of proliferating active cells, and integrin-β1 mediates cell migration. Compared with the nondiabetic group, the protein and mRNA expression levels of PCNA and integrin-β1 in the wound tissue of rats in the diabetic untreated group were significantly lower. Compared with the diabetic untreated group, the protein and mRNA expression levels of PCNA and integrin-β1 in the wound tissue of rats in the diabetic + PRP group and the diabetic + FG-4592 group were significantly higher. Compared with the diabetic + PRP group and the diabetic + FG-4592 group, the protein and mRNA expression levels of PCNA and integrin-β1 in the wound tissue of rats in the diabetic + PRP + FG-4592 group were significantly greater (Fig. 6A and B). Then, the results were further verified by immunofluorescence (Fig. 6C and D), and the results were consistent with the above results. The results indicated that both PRP and FG-4592 promoted cell proliferation and migration, and the effect was greatest when PRP and FG-4592 were combined.
Effect of FG-4592 combined with PRP on cell proliferation and migration in rats with diabetic skin defects. A: Western blot analysis of PCNA and integrin-β1 protein expression in wound tissue. B: qRT‒PCR analysis of the mRNA expression of PCNA and integrin-β1 in wound tissue. *P < 0.05. C-D: Immunofluorescence detection of PCNA and integrin-β1 expression in wound tissue, Scale bar = 100 μm.
Discussion
The incidence of diabetic wounds, which consume many medical resources, is increasing annually. It is very important to explore more effective treatment methods to promote the healing of diabetic wounds. In this study, we confirmed that FG-4592 combined with PRP promotes wound healing in diabetic wounds, improves histopathological wound damage, and upregulates the expression of VEGF, CD34, CD31, α-SMA, CoL1α1, SDF-1, PCNA and integrin-β1. Those effects may be related to the upregulation of HIF-1α expression.
Studies have shown that delayed wound healing in diabetic patients is associated with the impaired expression of HIF-1α and VEGF, leading to impaired neovascularization in response to hypoxia25. As an inhibitor of HIF-PHD, FG-4592 can reversibly bind HIF-PHD and significantly reduce its activity, thereby inhibiting the degradation of HIF-1α and increasing the transcriptional activity of HIF-1α and its downstream pathways10,26. In this study, we found that FG-4592 can upregulate the expression of HIF-1α in the wound tissue of diabetic rats and significantly increase the expression of VEGF, CD34, CD31 and SDF-1. We can speculate that FG-4592 promotes the entry of these EPCs into ischemic and hypoxic tissues by acting on the target gene of HIF-1α, SDF-1, and promotes para-angiogenesis, which leads to faster wound healing. Our findings are in accordance with recent studies by Zhu et al.25.
In our study, the combination of PRP and FG-4592 not only accelerated angiogenesis, but also reduced pathological damage, such as decreased inflammatory cell aggregation, more importantly, epithelial tongues were thickened and expanded, and collagen deposition was increased in diabetic wounds. In terms of overall recovery effect on diabetic rats, we found that the combined treatment of PRP and FG4592 shows a better effect on the recovery of body weight in diabetic rats compared to the use of these two drugs individually. Consistent with our findings, another study reported that the hyperbaric oxygen-induced upregulation of HIF-1α expression promoted fibroblast proliferation27. Integrin β1 is a biomarker of epidermal stem cells with proliferative potential; moreover, it mediates the adhesion between cells and the extracellular matrix and plays an important role in cell (keratinocytes and fibroblasts) migration. The expression of integrin-β1 was significantly increased in the diabetic + FG-4592 group, suggesting that FG-4592 is beneficial for the re-epithelialization of diabetic wounds. These findings are consistent with the results of our previous study showing that FG-4592 activates epidermal stem cells during the wound healing process in normal mice15. Recently, Fang et al.. pointed out in a review that FG-4592, an HIF-PHD inhibitor, has potential for treating diabetes-related complications28. In this study, our findings confirm that FG-4592 can effectively upregulate the expression of HIF-1α in diabetic wounds and has great application prospects in the treatment of diabetic wounds.
After activation, PRP can release numerous growth factors and cytokines29. Studies have confirmed that PRP can shorten the wound healing time and improve the healing quality mainly by promoting the wound epithelization through increasing the expression of EGF, VEGF, TGF-β, HIF-1α, Integrin α3, and meanwhile increasing the release of integrin β1 and other mechanisms20. In this study, PRP treatment upregulated the expression of HIF-1α, VEGF, CD34, CD31, α-SMA, CoL1α1, SDF-1 and integrin β1; promoted wound healing. Most importantly, we found that the concurrent intervention of PRP and FG-4592 was more effective than either agent alone, which indicates the potential of clinical promotion of combined PRP and FG-4592 therapy for the treatment of diabetic wounds. It is hypothesised that both PRP and FG-4592 are able to stabilise HIF-1α and promote the expression of HIF-1 target genes. The fibrin in PRP provides the three-dimensional structure needed for cell proliferation and crawling during the wound repair process, which may have a beneficial effect on diabetic wound healing30. There are no studies showing the relationship between PRP and FG-4592, which is worth exploring in the future.
This study still has several limitations. Firstly, the use of PRP gel as a carrier for FG-4592 was our original research idea, but this could not be achieved because FG-4592 would precipitate from PRP due to its poor water solubility, and the current experimental design was adopted. Secondly, the study’s reliance on animal models, which may not fully mirror clinical scenarios (e.g., stress responses induced by ear tagging in animals, potentially interacting with impaired healing mechanisms31), limits its direct clinical applicability. Thirdly, the lack of long-term observational data and insufficient exploration of time-phased combination regimens limit comprehensive evaluation. The PRP group had different effects at different stages of ulcer healing, with better short-term effects and poor long-term effects32, and FG-4592 may benefit chronic wounds by transferring from sustained inflammatory states to proliferation phases, so time-phased combination therapy may further enhance therapeutic outcomes.
In summary, FG-4592 combined with PRP can accelerate the healing of diabetic wounds by upregulating the expression of HIF-1α and its target genes, thereby reversing the effects of high glucose levels on the recruitment of EPCs, angiogenesis, and cell proliferation and migration. Even though these findings are exciting, translating these preclinical findings into clinical practice requires rigorously designed Phase I-III trials to systematically evaluate the safety and synergistic efficacy of PRP combined with FG-4592, focusing on stratified patient cohorts with well-controlled diabetic chronic wounds. Optimization of PRP treatment protocols (e.g., platelet concentration, frequency) and FG-4592 treatment protocols (e.g., start timing and dosing), coupled with dynamic monitoring of angiogenesis (e.g., VEGF) and inflammatory biomarkers, is essential to elucidate mechanistic synergy. Long-term follow-up for recurrence risks and comparative analyses against standard therapies will bridge the evidence gap between experimental models and clinical implementation.
In addition, due to the pathophysiological characteristics of diabetes itself, the complexity of ulcers, and the impact of therapeutic interventions, some complications may arise during the treatment of diabetic ulcers. Research has shown that the combination of cell therapy (such as the activation of mast cells, which affects macrophage behavior and secondary healing efficacy) and gene therapy can prevent secondary inflammatory complications, surpassing regenerative medicine33. The fields of secondary complications and DFU-related recurrence are relatively novel, but the role of immune cell subpopulations in the DFU pathogenesis should be discovered in the near future.
Conclusion
FG-4592 and PRP accelerated diabetic wound healing by reversing the effects of high glucose levels on EPC recruitment, neovascularization, collagen deposition and cell proliferation and migration through the upregulation of the expression of HIF-1α and its target gene expression (Fig. 7). Concurrent intervention with PRP and FG-4592 was more effective than either agent alone. The combined application of PRP and FG-4592 in the treatment of diabetic wounds has the potential of clinical promotion, and can be used as an efficient and accessible treatment option for diabetic wounds, significantly improving the quality of life of patients and reducing the medical burden.
Summary diagram of the mechanism of action of FG-4592 and PRP in diabetic wound healing. The figure was created in Fig Draw (www.figdraw.com). FG-4592 and PRP accelerated diabetic wound healing by upregulation of the expression of HIF-1α and its target gene expression, reversing the effects of high glucose levels on EPC recruitment, neovascularization, collagen deposition and cell proliferation and migration.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- BCA:
-
Bicinchoninic Acid Assay
- CD31:
-
Cluster of Differentiation 31
- CD34:
-
Cluster of Differentiation 34
- CoL1α1:
-
Collagen type 1 alpha 1
- DAB:
-
Diaminobenzidine
- EPC:
-
Endothelial progenitor cell
- FG-4592:
-
Roxadustat
- HE:
-
Hematoxylin-eosin
- HIF-1α:
-
Hypoxia-inducible factor-1α
- HIF-PHD:
-
Hypoxia-inducible factor prolyl hydroxylase
- IF:
-
Immunofluorescence
- IHC:
-
Immunohistochemistry
- LSD:
-
Least Significant Difference
- mean ± SD:
-
Mean ± Standard Deviation
- NP:
-
Normal plasma
- PBS:
-
Phosphate Buffered Saline
- PCNA:
-
Proliferating Cell Nuclear Antigen
- PRP:
-
Platelet-rich plasma
- qRT-PCR:
-
Quantitative real time polymerase chain reaction
- RIPA:
-
Radio Immunoprecipitation Assay
- SD:
-
Sprague–Dawley
- SDF-1:
-
Stromal Cell-derived Factor 1
- STZ:
-
Streptozotocin
- VEGF:
-
Vascular endothelial growth factor
- α-SMA:
-
α-smooth muscle actin
References
IDF releases report of global survey on access to medicines and supplies for people with diabetes. Diabetes Res. Clin. Pract., 129, 224–225 (2017). https://doi.org/10.1016/j.diabres.2017.06.001
Holl, J. et al. Chronic diabetic wounds and their treatment with skin substitutes. Cells 10(3), 655. https://doi.org/10.3390/cells10030655 (2021).
Dasari, N. et al. Updates in diabetic wound healing, inflammation, and scarring. Semin Plast. Surg. 35(3), 153–158. https://doi.org/10.1055/s-0041-1731460 (2021).
Liu, Y. et al. Knockdown of HIF-1α impairs post-ischemic vascular reconstruction in the brain via deficient homing and sprouting BmEPCs. Brain Pathol. 28(6), 860–874. https://doi.org/10.1111/bpa.12628 (2018).
Powers, J. G., Higham, C., Broussard, K. & Phillips, T. J. Wound healing and treating wounds: chronic wound care and management. J. Am. Acad. Dermatol. 74(4), 607–625. https://doi.org/10.1016/j.jaad.2015.08.070 (2016). quiz 625 – 606.
Braun, L. R., Fisk, W. A., Lev-Tov, H., Kirsner, R. S. & Isseroff, R. R. Diabetic foot ulcer: an evidence-based treatment update. Am. J. Clin. Dermatol. 15(3), 267–281. https://doi.org/10.1007/s40257-014-0081-9 (2014).
Ruthenborg, R. J., Ban, J. J., Wazir, A., Takeda, N. & Kim, J. W. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol. Cells. 37(9), 637–643. https://doi.org/10.14348/molcells.2014.0150 (2014).
Stroka, D. M. et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. Faseb J. 15(13), 2445–2453. https://doi.org/10.1096/fj.01-0125com (2001).
Botusan, I. R. et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. U S A. 105(49), 19426–19431. https://doi.org/10.1073/pnas.0805230105 (2008).
Besarab, A. et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of Roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol. Dial Transpl. 30(10), 1665–1673. https://doi.org/10.1093/ndt/gfv302 (2015).
Li, A. et al. Roxadustat (FG-4592) abated lipopolysaccharides-induced depressive-like symptoms via PI3K signaling. Front. Mol. Neurosci. 16, 1048985. https://doi.org/10.3389/fnmol.2023.1048985 (2023).
Ko, V. H. et al. Roxadustat (FG-4592) accelerates pulmonary growth, development, and function in a compensatory lung growth model. Angiogenesis 23(4), 637–649. https://doi.org/10.1007/s10456-020-09735-9 (2020).
Eleftheriadis, T. et al. In mixed lymphocyte reaction, the Hypoxia-Inducible factor Prolyl-Hydroxylase inhibitor Roxadustat suppresses cellular and humoral alloimmunity. Arch. Immunol. Ther. Exp. (Warsz). 68(6), 31. https://doi.org/10.1007/s00005-020-00596-0 (2020).
S, A. K. & Patel, S. S. Possible drug repurposing and accelerated wound healing. Regenerative Eng. Translational Med. https://doi.org/10.1007/s40883-024-00347-z (2024).
Tang, D. et al. FG-4592 accelerates cutaneous wound healing by epidermal stem cell activation via HIF-1α stabilization. Cell. Physiol. Biochem. 46(6), 2460–2470. https://doi.org/10.1159/000489652 (2018).
Catrina, S. B., Okamoto, K., Pereira, T., Brismar, K. & Poellinger, L. Hyperglycemia regulates hypoxia-inducible factor-1 protein stability and function. Diabetes 53(12), 3226–3232 (2017).
Martino, M. M. et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl Med. 3(100), 100ra189. https://doi.org/10.1126/scitranslmed.3002614 (2011).
Losi, P. et al. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 9(8), 7814–7821. https://doi.org/10.1016/j.actbio.2013.04.019 (2013).
Beanes, S. R., Dang, C., Soo, C. & Ting, K. Skin repair and Scar formation: the central role of TGF-beta. Expert Rev. Mol. Med. 5(8), 1–22. https://doi.org/10.1017/s1462399403005817 (2003).
Yuan, T., Guo, S. C., Han, P., Zhang, C. Q. & Zeng, B. F. Applications of leukocyte- and platelet-rich plasma (L-PRP) in trauma surgery. Curr. Pharm. Biotechnol. 13(7), 1173–1184. https://doi.org/10.2174/138920112800624445 (2012).
Mansoub, N. H. et al. The role of PRP and adipose tissue-derived keratinocytes on burn wound healing in diabetic rats. Bioimpacts 8(1), 5–12. https://doi.org/10.15171/bi.2018.02 (2018).
Ghufran, H. et al. Curcumin preconditioned human adipose derived stem cells co-transplanted with platelet rich plasma improve wound healing in diabetic rats. Life Sci. 257, 118091. https://doi.org/10.1016/j.lfs.2020.118091 (2020).
Ni, X. et al. Adipose-derived stem cells combined with platelet-rich plasma enhance wound healing in a rat model of full-thickness skin defects. Stem Cell. Res. Ther. 12(1), 226. https://doi.org/10.1186/s13287-021-02257-1 (2021).
Hosseini Mansoub, N. et al. The role of PRP and adipose tissue-derived keratinocytes on burn wound healing in diabetic rats. Bioimpacts 8(1), 5–12. https://doi.org/10.15171/bi.2018.02 (2018).
Zhu, Y., Wang, Y., Jia, Y., Xu, J. & Chai, Y. Roxadustat promotes angiogenesis through HIF-1α/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats. Wound Repair. Regen. 27(4), 324–334. https://doi.org/10.1111/wrr.12708 (2019).
Jain, I. H. et al. Hypoxia as a therapy for mitochondrial disease. Science 352(6281), 54–61. https://doi.org/10.1126/science.aad9642 (2016).
Huang, X. et al. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 259, 118246. https://doi.org/10.1016/j.lfs.2020.118246 (2020).
Fang, T., Ma, C., Zhang, Z., Sun, L. & Zheng, N. Roxadustat, a HIF-PHD inhibitor with exploitable potential on diabetes-related complications. Front. Pharmacol. 14, 1088288. https://doi.org/10.3389/fphar.2023.1088288 (2023).
Aghajanova, L. et al. In vitro evidence that platelet-rich plasma stimulates cellular processes involved in endometrial regeneration. J. Assist. Reprod. Genet. 35(5), 757–770. https://doi.org/10.1007/s10815-018-1130-8 (2018).
Nourizadeh, N. et al. PRP-Fibrin glue for pain reduction and rapid healing in tonsillectomy. Iran. J. Otorhinolaryngol. 34(125), 289–294. https://doi.org/10.22038/ijorl.2022.40429.2532 (2022).
Klabukov, I. et al. Refinement of animal experiments: replacing traumatic methods of laboratory animal marking with Non-Invasive alternatives. Anim. (Basel). 13(22). https://doi.org/10.3390/ani13223452 (2023).
Shen, Z. et al. Efficacy and safety of platelet-rich plasma in treating cutaneous ulceration: A meta-analysis of randomized controlled trials. J. Cosmet. Dermatol. 18(2), 495–507. https://doi.org/10.1111/jocd.12853 (2019).
Evstratova, E., Yatsenko, E., Baranovskii, D. & Klabukov, I. Effectiveness of stem cell therapy for diabetic foot ulcers: cell therapy alone is not enough for effective management of chronic wounds. Int. J. Low Extrem Wounds. 15347346241295306 https://doi.org/10.1177/15347346241295306 (2024).
Funding
This work was supported by the Hubei Provincial Natural Science Foundation (2021CFB164), the Youth Program of the National Natural Science Foundation of China (82302818), and the Postdoctoral Fund of Central Theater General Hospital (BSH017).
Author information
Authors and Affiliations
Contributions
Di Tang: Writing- Original draft preparation; Formal analysis; Funding acquisition. Qiang Lin: Data curation; Visualization. Kai Xu: Methodology. Song Wang: Investigation. Pei-Wen Li: Validation. Qi-Ping Lu: Supervision; Project administration. Yue-Sheng Huang: Conceptualization; Writing- Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was reviewed and approved by the Ethics Committee of Central Theater General Hospital (2021004) and Wuhan Myhalic Biotechnology Co., Ltd. (HLK-20220816-001). All methods were carried out in accordance with relevant guidelines and regulations, and all methods are reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, D., Lin, Q., Li, PW. et al. FG-4592 combined with PRP significantly accelerates the healing of refractory diabetic wounds by upregulating HIF-1α. Sci Rep 15, 14292 (2025). https://doi.org/10.1038/s41598-025-99356-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99356-3