Abstract
Background
This observational study aimed to assess the impact of the pandemic on the way kidney transplantation, survival, and vaccination evolved in chronic dialysis recipients (CDR) over the course of the COVID-19 pandemic waves and inter-waves.
Methods
Using the French national health claims database, incident persons with end-stage kidney disease in the years 2015 to 2021 treated with dialysis were followed up until 31 December, 2022. Kidney transplantation and survival in the pandemic sub-periods compared to the pre-pandemic period were investigated using longitudinal models with time-dependent covariates. In addition, the impact of cumulative doses of COVID-19 vaccine on hospitalization and survival was studied, comparing CDR and matched controls.
Results
Here, we show that the follow-ups of the 71,583 CDR and the 143,166 controls totalize 639,341 person-years (CDR: 184,909; controls: 454,432). The likelihood of receiving a kidney transplant is lower in all pandemic sub-periods. The 3 waves are associated with a higher risk of death (hazard ratio (HR [95% confidence interval]): 1.19 [1.13–1.27], 1.19 [1.15–1.23], and 1.12 [1.07–1.17], respectively). While vaccine coverage declines with each booster dose, receiving these doses is associated with a lower risk of COVID-19-related hospitalization (0.66 [0.56–0.77], 0.83 [0.72–0.94] for 1st booster versus 2nd dose and 2nd booster versus 1st booster, respectively) and death (corresponding HR: 0.55 [0.51–0.59], 0.88 [0.83–0.95]).
Conclusions
The evolving patterns in mortality and vaccination outcomes are similar in CDR and controls, suggesting that the impact of the pandemic on CDR is not specific to kidney disease per se.
Plain language summary
This study investigated the impact of the COVID-19 pandemic period on chronic dialysis recipients in France. Using national data, 71,000 chronic dialysis recipients and 143,000 matched controls were followed up between 2015 to 2022. We estimate the kidney transplant events and survival rates throughout the pandemic waves, compared to the pre-pandemic period. We found that kidney transplantation was performed less, and death rates increased during the pandemic, mostly linked to COVID-19. Booster vaccinations were associated with significantly lower risks of COVID-19-related hospitalizations and death. Observed trends were similar for chronic dialysis recipients and their controls, suggesting that the pandemic-related risk modifications were not related to kidney disease per se.
Similar content being viewed by others
Introduction
In 2020, the COVID-19 pandemic rapidly plunged the world into an unprecedented systemic crisis. On 30 January 2020, the World Health Organization (WHO) declared COVID-19 a global public health emergency1, leading to multiple lockdown periods worldwide, including France, for mitigating the spread of the virus2. Some early studies highlighted a higher risk of hospitalization and mortality from COVID-19 in persons under maintenance dialysis for end-stage kidney disease (ESKD)3,4. After the first COVID-19 vaccination campaigns, several studies reported lower response to vaccines and a faster waning of immunity in chronic dialysis recipients (CDR) compared to the general population5,6, and additional booster doses 4 to 6 months after initial vaccination have been recommended to protect this more vulnerable population as far as possible7. In addition, CDR’s adherence to or reluctance towards these booster campaigns are still insufficiently documented in the literature. Indeed, a recent systematic review examining the relationship between Health Belief Model constructs and COVID-19 vaccination intentions included 109 studies, but only 13 articles explored booster vaccines8, with only 1 of them exploring reluctance towards booster vaccines in hemodialysis patients8,9. These findings led us to investigate vaccination coverage for booster doses. Another challenging consequence of the COVID-19 pandemic for CDR was the reduction in kidney transplant activity. An overall decrease of 8,560 kidney transplants across 22 countries was reported in an international study, including a decrease of 1041 (-34%) kidney transplants in France from 29 February, 2020 to 31 December, 202010. To the best of our knowledge, despite the well-established impact of COVID-19-related hospitalizations on CDR survival3,4, there has not been any detailed report on the likelihood to receive a kidney transplant over the course of the pandemic.
In order to assess the global impact of the COVID-19 pandemic period on CDR, we undertook a large comprehensive study covering the entire span of the COVID-19 crisis. Adopting a French national perspective, the present study encompassed several objectives. Firstly, it aimed to characterize the evolving risk of mortality and the likelihood of receiving a kidney transplant for CDR in the different pandemic waves and inter-wave periods. Secondly, it aimed to investigate the aforementioned outcomes in CDR who experienced a severe form of COVID-19, compared to CDR who did not. Although great caution towards causal inference is required in the context of this observational study, the underlying idea of this second study objective was to separate as far as possible the impact of severe forms of COVID-19 on patients infected by SARS-CoV-2 (hereafter referred to as the “primary impact” of the pandemic period), and the impact of all other changes that occurred during this period and affected all patients (hereafter referred to as the “secondary impact” of the pandemic period). Thirdly, we investigated the course of COVID-19 vaccinations over time in CDR during the pandemic period and its effect on the risk of COVID-19-related hospitalization and survival. Lastly, analyses on appropriate control subpopulations were conducted simultaneously in order to investigate the extent to which estimates relating to survival and vaccination for CDR were specific to this particular subpopulation.
Study results indicate that, compared to the pre-pandemic period, the likelihood of receiving a kidney transplant in CDR was lower during the pandemic period, especially during the initial wave. In addition, this likelihood was lower in CDR with a history of COVID-19-related hospitalization. The higher risk of death observed during the pandemic waves mainly concerns individuals with a history of COVID-19-related hospitalization. It is worth noting that analyses estimate similar mortality patterns in CDR and their matched controls. Vaccine booster coverage declined with additional doses, but receiving a larger number of doses (including booster doses) is associated with a lower risk of death and COVID-19-related hospitalization for both CDR and controls.
Methods
This observational study was conducted according to the STROBE guidelines11.
Data Sources
The French Système National des Données de Santé (SNDS) was the only source of data used in the study. The SNDS is a national claims database designed to reimburse care via the French Health Insurance System, covering nearly 100% of the population receiving heathcare12. With the growing interest in the scientific medical community for real-world data derived from large administrative healthcare databases, the SNDS has been described and used for pharmaco-epidemiological studies13,14,15, including studies on COVID-1916,17.
Patients included in the study
ESKD incident persons were identified in the SNDS using G10 mapping18, as previously described in a study investigating the primary and secondary impact of the pandemic on the survival of kidney transplant recipients19. Only individuals aged 18 years or over at the time of incidence and whose first treatment related to ESKD was dialysis (peritoneal or hemodialysis), were included in the study. This restriction to ESKD-incident persons treated with dialysis was an essential feature of the study, adopted to guarantee that the age of the disease was duly documented for each individual included in the study. Each CDR included in this study was matched at the beginning of his/her follow-up, i.e. the date of entry into ESKD status, with two controls identified in the SNDS without ESKD but with the following identical characteristics (exact matching): sex, age, history of diabetes, history of chronic cardiovascular diseases, and region of residence.
The pre-pandemic and pandemic periods, the COVID-19 wave and inter-wave sub-periods, and the vaccination rollout
The number of COVID-19 cases reported in France rose from 100 to 1000 from 29 February to 8 March, and lockdown started on 17 March. The pre-pandemic period in this study was therefore defined as the period from 1 January 2015 to 29 February 2020 and the pandemic period began on 1 March 2020. The pandemic period was censored on 31 December 2022, which is considered as the end date of the study. In order to investigate any emerging patterns in the course of the pandemic period, it was divided into 6 sub-periods according to the frequency of COVID-19-related hospital admissions in the SNDS database: three-wave sub-periods during which the rate of COVID-19-related hospitalizations exceeded 5 per 100,000 admissions, (from 1 March to 18 May 2020 for the first wave sub-period, from 7 September 2020 to 10 May 2021 for the second wave sub-period, and from 21 November 2021 to 25 April 2022 for the third wave sub-period), and three inter-wave sub-periods (from 19 May to 6 September 2020 for the first inter-wave, from 11 May to 10 November 2021 for the second inter-wave, and from 26 April to 31 December 2022 for the third inter-wave sub-period). In France, COVID-19 vaccination began on 27 December 2020. The vaccination rollout for CDR allowed them to receive their doses directly at dialysis or vaccination centers, or at home for those with limited mobility. In April 2021. The Directeur Général de la Santé recommended a booster dose for immuno-compromised individuals, including CDR20. The recommended schedule for the corresponding injection was 4 weeks at least after the initial vaccine scheme (the first two doses), or as soon as possible for those exceeding this deadline. In August 2021 recommendations for a second booster injection 3 to 6 months after the first booster were made, and CDR also benefited from specific monitoring enabling an adjustment of their vaccination schedule according to their immune response21.
Statistical analyses
The ESKD incidence date was considered as the starting point (t0) of the corresponding individual follow-up. The individual end of follow-up was censored on 31 December 2022, the end date of the study. Supplementary Fig. 1 describes the follow-up of typical persons included in the study. It is important to note that we chose to investigate CDR because we already had a detailed account of the impact of the pandemic on kidney transplant recipients in another recent study19. Therefore, persons entering ESKD status with a pre-emptive transplantation event were not considered for the present study, and CDR follow-up ended in case of kidney transplant or death. The initial follow-up time of a case and of his/her matched controls was identical, i.e. the date of entry into ESKD status of the case. Furthermore, censoring a case led to the simultaneous censoring of his/her matched controls whenever one of the following events occurred: when a case received a transplant, or when the follow-up duration reached five years. It is worth mentioning that the death of a given case did not result in ending the follow-up of the corresponding matched-controls.
Investigations on the evolving impact of the COVID-19 pandemic on CDR according to the waves of the pandemic and the related sub-periods led us to consider additional covariates that we deemed important to study alongside. COVID-19, as the principal or related code (International Classification of Diseases, 10th revision) for hospitalization was considered as a proxy for a severe COVID-19 episode. Age, sex, diabetes and chronic cardiovascular diseases (defined in the G10 mapping of the SNDS as the presence of at least one of the following features: stroke sequelae, chronic heart failure, coronary disease, peripheral arterial obliterative disease), were also considered as additional covariates. The age variable was transformed into an ordinal variable handled according to the following breakdown: 18–44, 45–54, 55–64, 65–74 (reference), 75–84, and ≥85 years. COVID-19-related hospitalizations were handled as a binary variable, with 0 or ≥1 hospitalization(s). Some covariates could have varied during the longitudinal follow-up of the individuals, and accordingly the following variables were handled as time-dependent variables in the analyses: period (pandemic versus pre-pandemic), pandemic sub-periods (1st wave, 1st inter-wave, 2nd wave, 2nd inter-wave, 3rd wave, and 3rd inter-wave), COVID-19-related hospitalization, and COVID-19 vaccination (0 or 1 dose, 2 doses, 3 doses—first booster dose, hereafter referred to as a third dose, and 4 doses—second booster dose, hereafter referred to as a fourth dose). The absence of data for a diagnosis or an event was considered to be the absence of an actual diagnosis or event, so that there was no missing data for analysis.
Survival models with time-dependent covariates were used to assess how the COVID-19 pandemic affected both the likelihood of receiving a kidney transplant and the risk of death in CDR. Competing risks of death and kidney transplantation were handled using Cox cause-specific regression models. In order to compare the impact of the pandemic period on mortality between CDR and matched controls, a model including an interaction term between populations (CDR versus matched controls) and periods (pre-pandemic versus pandemic) was developed. Furthermore, study investigations extended to analyzing the association between each additional dose of COVID-19 vaccine and two outcomes: COVID-19-related hospitalization and survival. These investigations involved comparing individuals who had received a given dose with those who had received the previous dose, using Cox cause-specific regression models with time-dependent covariates accounting for changes in vaccination status over time. As the choice of cause-specific models to handle competing risks in the presence of time-dependent covariates is debatable, we conducted sensitivity analyses using the Fine and Gray model instead22,23,24. Analyses were performed using R version 4.1.2 and R package survival. A p value < 0.05 was considered statistically significant.
Ethics statement
The SNDS is a set of strictly anonymous databases comprising all mandatory national health insurance reimbursement data. Since 30 June 2021, INSERM has had direct access to the SNDS. This permanent access is provided by French Decree No. 2016-1871 of 26 December, 2016 relating to the processing of personal SNDS data and French legislation Art. R. 1461-1325 and 14. This study was declared prior to its initiation to the CépiDc-INSERM registry of studies requiring the use of the SNDS. The study was registered and approved by CépiDc-INSERM (Ref #SRQ0315448) before initiation, as required for SNDS data use. By national legislation and EU General Data Protection Regulations, written informed consent for participation was not required for this study.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
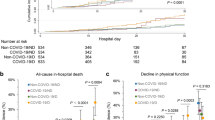
Figure 1 illustrates the study profile: considering the whole population of adults living with ESKD in France each year from 2015 to 2021 (prevalent cases), the selection process for ESKD incident cases undergoing dialysis during this period resulted in a total of 71,583 CDR and 143,166 matched controls (2 matched controls per CDR) with a total follow-up of 639,341 person-years, (184,909 for CDR and 454,432 for matched controls). Table 1 presents the characteristics of the CDR included in the study at the time of incidence. Nearly 83% of the recipients were at least 55 years old, 64% were male, and almost 68% presented either diabetes, or a chronic cardiovascular disease, or both. With regard to the exact matching process, the controls had identical baseline characteristics to those of the CDR. For the CDR, 14,455 deaths (1468 per 10,000 person-years) and 5853 kidney transplants (595 per 10,000 person-years) occurred during the 98,444 person-years of cumulated follow-up in the pre-pandemic period, compared to 13,932 deaths (1573 per 10,000 person-years) and 4172 kidney transplants (471 per 10,000 person-years) that occurred during the 88,603 person-years of cumulated follow-up in the pandemic period. For the matched controls, 10,898 deaths occurred during the 231,632 person-years of cumulated follow-up in the pre-pandemic period (470 deaths per 10,000 person-years) and 11,387 deaths occurred during the 222,760 person-years of cumulated follow-up in the pandemic period (511 deaths per 10,000 person-years).
Transplantation
Details of the decrease in kidney transplantation activity in France during the pandemic period are given in Supplementary Fig. 2. Alongside, considering the whole pandemic period, the likelihood of CDR to receive a kidney transplant was 28% lower than in the pre-pandemic period (hazard ratio (HR) [95% confidence interval] at 0.72 [0.70–0.76], see Table 2, model 1). Most of the corresponding CDR were without a history of COVID-19-related hospitalization. Compared to the latter, those with a history of COVID-19 related hospitalization had an additional 29% lower likelihood of receiving a kidney transplant (HR = 0.71 [0.59–0.85], see Table 2 model 2). In the end, considering the evolving HR for receiving a kidney transplant according to the pandemic wave and inter-wave sub-periods, compared to the pre-pandemic period (Table 2, model 3). CDR were less likely to receive a kidney transplant in all the wave and inter-wave sub-periods, with the greatest reduction observed during the first wave sub-period (HR = 0.16 [0.12–0.20]).
Mortality
During the pandemic period, a higher risk of death was observed for CDR compared to the pre-pandemic period: HR = 1.08 [1.05–1.10] (Table 3, Model 4). However, Model 5 further indicates that compared to the pre-pandemic period, the risk of death in CDR without a history of COVID-19-related hospitalization was similar (HR = 0.98 [0.95–1.00]), while those with a history of COVID-19-related hospitalization presented a substantially higher risk (HR = 3.48 [3.32–3.65]). Regarding the mortality trends over the different pandemic sub-periods (Table 3, model 6), each of the three wave sub-periods was associated with a greater risk of death, compared to the pre-pandemic period, with increments of 19% (HR = 1.19 [1.13–1.27]), 19% (HR = 1.19 [1.15–1.23]), and 12% (HR = 1.12 [1.07–1.17]), respectively, while inter-wave sub-periods were not associated with differing risks of death, except for the first inter-wave (HR = 0.93 [0.88–0.97], 0.98 [0.94–1.02], 1.03 [0.98–1.07] for the 1st, 2nd, and 3rd inter-wave sub-period, respectively). Similar trends of higher risks of death during the wave sub-periods were found in the matched controls. In contrast, the 1st and 2nd inter-wave sub-periods were associated with a lower risk of death in the control group (HR = 0.87 [0.82–0.93], 0.91 [0.87–0.96], and 0.97 [0.93–1.02] for the 1st, 2nd, and 3rd inter-wave sub-period, respectively)
Vaccination
Figure 2 presents the dynamics of COVID-19 vaccination. By 31 December 2022, nearly 90% of CDR had received the two initial doses of vaccine, 82% had received a third dose, and 54% had received a fourth dose. This observed decrease in the vaccination coverage of booster doses was even greater in the matched controls. In CDR, receiving two doses was associated with a 63% (HR = 0.37 [0.32–0.42]) lower risk of COVID-19-related hospital admissions (Table 4), and a 9% (HR = 0.91 [0.86–0.97]) lower risk of all-cause mortality (Table 5). Receiving additional booster doses was associated with lower risks of COVID-19-related hospitalizations (HR = 0.66 [0.56–0.77] and 0.83 [0.72–0.94] for additional third and fourth doses, respectively), and all-cause mortality (HR = 0.55 [0.51–0.59] and 0.88 [0.83–0.95] for additional third and fourth doses, respectively). Almost similar results were found for the matched controls for COVID-19-related hospitalization (HR = 0.28 [0.24–0.32], 0.60 [0.51–0.72], 0.77 [0.62–0.98] for receiving additional second, third and fourth doses, respectively), and all-cause mortality (HR = 0.70 [0.65–0.75], 0.61 [0.56–0.67], 0.81 [0.73–0.90], respectively).
Sensitivity analyses
On the whole, sensitivity analyses using models based on the Fine and Gray model to handle competing risks (Supplementary Tables 2–5) yielded similar results to those relying on cause-specific Cox models (Tables 2–5).
Discussion
The major findings of the study are discussed below and organized according to three main points: transplantation, mortality, and vaccination.
Numerous studies have reported a decline in transplantation activity during the pandemic period compared to the pre-pandemic period25. However, the implications of the reduced likelihood of CDR to receive a kidney transplant during the pandemic sub-periods remain largely unexplored. To our knowledge, the present national study, which is based on real-world data was the first to take time-dependent covariates and the competing risks between transplantation and death into account to assess the impact of the pandemic on the likelihood of CDR to receive a transplant. The study indicates that, considering individuals with equal duration since their ESKD incidence date, the pandemic period was associated with a lower HR for receiving a kidney transplant than in the pre-pandemic period. The lower likelihood to receive a transplant during the pandemic was observed in CDR without a history of COVID-19-related hospitalization (Table 2, model 2), which highlights a secondary impact of the pandemic. However, compared to the CDR without a history of COVID-19-related hospitalization, those with a history of COVID-19-related hospitalization had a lower likelihood of receiving a kidney transplant, suggesting an additional primary impact of the pandemic relating to long-term consequences of COVID-19 in CDR. Our study further indicates that CDR had a lower likelihood to receive a transplant during all wave and inter-wave sub-periods than during the pre-pandemic period (Table 2, model 3).
Our study suggests that despite the challenges faced by the French healthcare system during the pandemic, there was no impact on the survival of CDR without a history of COVID-19-related hospitalization (see Model 5 in Table 3, HR = 0.98 [0.95–1.00]). In contrast, a dramatic higher risk of mortality was observed in the CDR and matched controls who experienced COVID-19-related hospitalizations (see the stratified Cox model 5 in Table 3, HR = 3.48 [3.32–3.65] in CDR, and HR = 7.54 [7.11–8.00] in matched controls). The additional higher risk in matched controls compared to the CDR, likely reflects the fact that the incremental risk relating to COVID-19-related hospitalization for the CDR was somewhat limited because their baseline risk of death was already substantially higher than that of the matched controls (HR = 3.15 [3.07–3.23], see Supplementary Table 1).
Previous studies in the USA and the UK have reported that mortality in CDR observed during the first wave of the COVID-19 pandemic was nearly 30% higher than the corresponding mortality trends over the previous 5 years26,27. The present study also estimates a 19% higher risk of death for CDR in France during the first wave (Model 6 in Table 3), and additionally extended analyses until 31 December, 2022, exploring the evolving pattern of CDR mortality over the pandemic wave and inter-wave sub-periods. The study results (Model 6 in Table 3) indicate in particular that the excess risk of death remained substantial during the 2nd and 3rd pandemic waves, with corresponding HRs at 1.19 [1.15–1.23], and 1.12 [1.07–1.17], respectively. It is important to note that similar higher risks of death were also observed in the matched controls during all wave sub-periods. Therefore, these higher risks were not specific to the kidney disease in the CDR under study, and continued well after the first wave in both vulnerable populations (CDR and matched controls). These results are reinforced by those presented in the Supplementary Table 1, which indicate that globally, the differences in the risk of death between CDR and matched controls during the pre-pandemic and pandemic periods were similar (HR = 1.02 [0.98–1.05] for the interaction term period × population in the Supplementary Table 1).
Before COVID-19 vaccinations were started in France, surveys found that reluctance towards the COVID-19 vaccine in the general population had reached nearly 30%28,29. However, our investigations on the dynamics of the COVID-19 vaccination coverage indicate that almost 90% of CDR received two doses of the COVID-19 vaccine (Fig. 2). Above all, this high percentage reflects the fact that vaccination policy in France was for the whole population, to receive the two first doses was highly recommended. The study analyses on additional (i.e. booster) doses deserve particular attention. Several studies have reported an association between COVID-19 vaccination and lower risks of COVID-19-related hospitalization or death6,7, but because vaccine immunity waned over time, researchers have emphasized the need for additional booster campaigns, particularly for vulnerable populations, such as CDR. To date, only Alobaidi et al. 9 have reported that 22% of hemodialysis patients were reluctant to receive the COVID-19 booster vaccine. French policies recommended that any person with a positive test in the last three months should delay their booster dose20. Whatever the potential contribution of these features – among others – to vaccine coverage, our study is unable to draw clear conclusions on the reasons why booster campaigns did not achieve a vaccine coverage similar to that observed for the two initial doses, with even poorer coverage in the matched control group. Nevertheless, the study indicates a positive association between receiving further doses of COVID-19 vaccine and lower risks of COVID-19-related hospitalization and death. To date, only one study conducted by Cohen-Hagai et al. 30, based on data from 1030 patients, has compared the clinical efficacy of receiving a fourth dose of vaccine versus three doses in dialysis patients, with a corresponding 44% lower risk of COVID-19-related mortality. In our study, receiving a fourth dose versus three doses was associated with a 12% lower risk of all-cause mortality, and a 24% lower risk of COVID-19-related hospitalization. Lastly, the present study evaluated the effectiveness of the COVID-19 vaccine in CDR from 1 January, 2021 to 31 December, 2022. Therefore, the study results also address concerns raised by Rouphael and Bausch-Jurken regarding the lack of reports on vaccine effectiveness after January 2022, when the Omicron variant became predominant among dialysis recipients6.
Our study shares many limitations with a recent study that investigated the impact of the COVID-19 pandemic on persons living with a kidney transplant19. The first limitation concerns SNDS database which does not include detailed medical/clinical and individual socioeconomic data. The first incidence date considered was 1 January, 2015, and consequently, the CDR under study were relatively recent cases. The study results cannot therefore be extended to CDR with more than 5 years under dialysis. However, the limitation on relatively recent incidence dates was a requirement, and allowed us to include persons for whom the onset of the disease and potential comorbidities were documented in the SNDS. Another limitation of the study stems from its observational nature. We attempted to address this limitation by adjusting estimates on the basis of comorbidities identified by the G10 mapping in the SNDS data. Although the matching of CDR and controls was based on relevant characteristics, including the date of ESKD incidence, direct comparisons between these populations should be interpreted with great caution. Indeed, the potential influence of features not taken into account in this observational study remains unknown, and it is worth recalling that the risk of death at baseline was three times higher in the CDR than in matched controls (see Supplementary Table 1). Comparisons between CDR with an incidence date in the pre-pandemic and pandemic periods have similar limitations: Table 1 shows no meaningful differences between these two CDR sub-populations on their measured characteristics (p values reflect the large sample sizes), but one cannot exclude that some unmeasured variables were unbalanced, resulting in different heath conditions for CDR with an ESKD onset during the pandemic. The use of COVID-19-related hospitalizations as a proxy for severe COVID-19 cases could also lead to an underestimation of the primary impact of COVID-19. Factors such as variants, virus exposure, mitigation measures, changes in the healthcare system, vaccination schedules and unidentified confounding factors prevent from a straightforward generalizability of our findings to other countries. However, similar drawbacks are usual in for any observational study. Nevertheless, our methodological framework could be used to plan comparable investigations in other countries, and even to consider other diseases. It is important to note that although this study is among the first to follow CDR for almost two years after the beginning of the pandemic, it is still too short to identify long-term consequences of the pandemic in this population, and this aspect should be documented by further studies. The study has many strong points related to the methodological features of its experimental analysis plan, with four particular elements that are worth recalling. Firstly, the results presented were obtained with a real-world evidence approach, based on exhaustive nationwide data. Secondly, the outcomes during the pre-pandemic and pandemic periods were compared between individuals with similar times lapses since ESKD incidence. Thirdly, the outcomes estimated for the CDR were also estimated for the matched controls, enabling a comprehensive interpretation of the kidney disease contribution per se in the CDR estimates. Fourthly, the results from analyses based on the Fine and Gray model for handling competing risks (see sensitivity analyses in Supplementary Tables 1 to 5) were roughly similar to those based on cause-specific Cox regressions presented as the main analyses, and therefore, the evolving patterns reported in the present study appear particularly robust.
Conclusion
To conclude, this national study provides details on the dynamics of kidney transplantation access, survival, and vaccination over the COVID-19 pandemic sub-periods for CDR having an incident date in the years 2015 to 2021. Considering the pandemic period as a whole, we did not observe higher mortality among CDR without a history of COVID-19-related hospitalization, suggesting that efforts to limit the secondary impact of the pandemic on survival for this vulnerable population were successful. During the three-wave sub-periods, similar excess mortality rates were observed both in the CDR and the matched controls, indicating that this excess mortality was more related to contextual covariates (e.g., comorbidities, age, etc.) than to kidney disease per se. Vaccine coverage decreased with each additional booster dose, although receiving additional booster doses was associated with significant benefits. Therefore, our study results argue in favor of future research on candidate policies aiming to increase adherence to vaccine booster doses.
Data availability
According to the principles of data protection and French regulations, the authors cannot publicly release the data from the French National Health Data System (SNDS). However, any person or structure, public or private, for-profit or non-profit, can access the SNDS data with authorization from the French Data Protection Office (CNIL, Commission Nationale de l’Informatique et des Libertés) to carry out a study, research, or an evaluation of public interest (https://www.snds.gouv.fr/SNDS/Contexte-et-perspectives-reglementaires#). The vaccination data from the SNDS is the source of Supplementary Data 1 (the data used to create Fig. 2), with its access conditions already detailed above.
References
COVID-19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum. https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum (Accessed 06 March 2024).
Info Coronavirus COVID-19—Les actions du Gouvernement. Gouvernement.fr. https://www.gouvernement.fr/info-coronavirus/les-actions-du-gouvernement (Accessed 06 March 2024).
Semenzato, L. et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg. Health Eur. 8, 100158 (2021).
Mahalingasivam, V. et al. COVID-19 and kidney disease: insights from epidemiology to inform clinical practice. Nat. Rev. Nephrol. 18, 485–498 (2022).
Hsu, C. M. et al. Seroresponse to SARS-CoV-2 vaccines among maintenance dialysis patients over 6 months. Clin. J. Am. Soc. Nephrol. 17, 403–413 (2022).
Rouphael, N. & Bausch-Jurken, M. COVID-19 vaccination among patients receiving maintenance renal replacement therapy: immune response, real-world effectiveness, and implications for the future. J. Infect. Dis. 228, S46–S54 (2023).
El Karoui, K. & De Vriese, A. S. COVID-19 in dialysis: clinical impact, immune response, prevention, and treatment. Kidney Int. 101, 883–894 (2022).
Limbu, Y. B. & Gautam, R. K. How well the constructs of health belief model predict vaccination intention: a systematic review on COVID-19 primary series and booster vaccines. Vaccines 11, 816 (2023).
Alobaidi, S. et al. COVID-19 booster vaccine hesitancy among hemodialysis patients in saudi arabia using the health belief model: a multi-centre experience. Vaccines 11, 95 (2022).
Aubert, O. et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health 6, e709–e719 (2021).
Vandenbroucke, J.P. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 4, e297 (2007).
Tuppin, P. et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev. Epidemiol. Sante Publique 65, S149–S167 (2017).
Lam, L. et al. Impact of direct-acting antiviral treatment for hepatitis C on cardiovascular diseases and extrahepatic cancers. Pharmacoepidemiol. Drug Saf. 32, 486–495 (2023).
Billioti de Gage, S., Desplas, D. & Dray-Spira, R. Roll-out of HIV pre-exposure prophylaxis use in France: a nationwide observational study from 2016 to 2021. Lancet Reg. Health Eur. 22, 100486 (2022).
Lam, L., Pol, S., Cohen, A. & Carrat, F. Direct-acting antivirals and the risk of arrhythmias and conduction disorders in patients with chronic hepatitis C: a French nationwide cohort study. Drugs 83, 1207–13 (2023).
Poucineau, J. et al. Hospital admissions and mortality for acute exacerbations of COPD during the COVID-19 pandemic: a nationwide study in France. Front. Med. 9, 995016 (2022).
Schwarzinger, M. et al. Mental disorders, COVID-19-related life-saving measures and mortality in France: a nationwide cohort study. PLoS Med. 20, e1004134 (2023).
Méthode de la cartographie des pathologies et des dépenses de l’Assurance Maladie. https://assurance-maladie.ameli.fr/sites/default/files/2014_etude-algorithmes-definition-pathologies-partie-1_cartographie.pdf (Accessed 06 March 2024).
Leye, E. et al. Direct and indirect impact of the COVID-19 pandemic on the survival of kidney transplant recipients: A national observational study in France. Am. J. Transpl. 24, 479–490 (2024).
DGS urgent n43 vaccination modalités d’administration des rappels. https://sante.gouv.fr/IMG/pdf/dgs_urgent_n43_vaccination_modalites_d_administration_des_rappels.pdf (Accessed 14 August 2024).
DGS urgent 90 rappel vaccinal. https://sante.gouv.fr/IMG/pdf/dgs_urgent_90_rappel_vaccinal.pdf (Accessed 29 August 2024).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94, 496–509 (1999).
Austin, P. C., Latouche, A. & Fine, J. P. A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat. Med. 39, 103–113 (2020).
Poguntke, I., Schumacher, M., Beyersmann, J. & Wolkewitz, M. Simulation shows undesirable results for competing risks analysis with time-dependent covariates for clinical outcomes. BMC Med. Res. Methodol. 18, 79 (2018).
Nimmo, A., Gardiner, D., Ushiro-Lumb, I., Ravanan, R. & Forsythe, J. L. R. The global impact of COVID-19 on solid organ transplantation: two years into a pandemic. Transplantation 106, 1312–1329 (2022).
Savino, M. et al. Outcomes of patients with COVID-19 on kidney replacement therapy: a comparison among modalities in England. Clin. Kidney J. 14, 2573–2581 (2021).
Weinhandl, E. D. et al. Initial effects of COVID-19 on patients with ESKD. J. Am. Soc. Nephrol. 32, 1444–1453 (2021).
Schwarzinger, M., Watson, V., Arwidson, P., Alla, F. & Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health 6, e210–e221 (2021).
Lazarus, J. V. et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 27, 225–258 (2021).
Cohen-Hagai, K. et al. Clinical efficacy of the fourth dose of the BNT162b2 vaccine in maintenance dialysis patients. J. Nephrol. 36, 1957–1964 (2023).
Acknowledgements
The authors thank the editorial department of INED and Angela Verdier for their editorial work. This work was supported by the Initiative Économie de la Santé of Sorbonne Université (Idex Sorbonne Université, programmes Investissements d’Avenir), and by the Ministère de la Solidarité et de la Santé (Programme de Recherche sur la Performance du Système des Soins, PREPS 20-0163). The sponsor and the funders had no role in the study design, data collection and analysis, interpretation of data, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
T.D., G.H., and N.L. initiated the study. GH and NL supervised the study; K.E.K., G.H., N.L., and E.L. designed the experimental plan; E.L. managed the data and performed the analyses; G.H., N.L., and E.L. take responsibility for the integrity of the data and the accuracy of the data analysis, E.L. is the guarantor; G.H., N.L., and E.L. prepared the first draft of the manuscript; all authors (T.D., M.E., K.E.K., G.H., M.K., N.L., S.L.C., and E.L.) contributed to the interpretation of the data, critically revised the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Leye, E., El Karoui, K., Delory, T. et al. Evolving impact of the COVID-19 pandemic in chronic dialysis recipients in France. Commun Med 5, 147 (2025). https://doi.org/10.1038/s43856-025-00848-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-00848-0