Abstract
Congenital heart disease (CHD) represents a significant burden in Sub-Saharan Africa (SSA), where limited healthcare infrastructure, inadequate diagnostic facilities, and financial constraints contribute to delayed diagnosis and suboptimal care. Cardiac magnetic resonance imaging (CMR), recognized internationally for its exceptional anatomical and functional cardiac assessment capabilities, remains underutilized in SSA primarily due to inadequate infrastructure, high operational costs, lack of trained professionals, and maintenance requirements. Artificial intelligence (AI) has the potential to revolutionize the role of MRI in CHD diagnosis by reducing scan times, automating image processing, and improving diagnostic accuracy. Despite its potential for improving diagnosis, AI implementation is limited by a lack of local datasets, technological incompatibility, data privacy concerns, and lack of expertise among healthcare providers. Strategic interventions such as adopting low-field MRI technologies, enhancing public-private partnerships, and establishing dedicated cardiac imaging units at tertiary centers could significantly expand CMR access and improve diagnosis of CHD in Sub-Saharan Africa. Additionally, targeted training initiatives and locally developed AI solutions that address ethical and interoperability concerns are essential. This Review explores these strategies and emphasizes how CMR augmented by AI could substantially improve CHD diagnosis, clinical outcomes, and healthcare equity in resource-constrained African settings.
Similar content being viewed by others
Introduction
Improving the diagnosis and treatment of congenital heart disease (CHD) in resource-limited settings demands innovative solutions. Recent technological advancements indicate that the integration of cardiac magnetic resonance (CMR) imaging with artificial intelligence (AI) may offer a transformative approach to diagnosis of CHD1. Despite this potential, significant challenges hinder widespread adoption in low- and middle-income areas such as Sub-Saharan Africa (SSA), where infrastructural, economic, and technological barriers persist2,3. This narrative review explores the role of CMR and AI in CHD diagnostics and discusses strategies to address current limitations in SSA.
We used a flexible search approach to identify relevant literature across PubMed, Scopus, and Google Scholar. Search terms included combinations of “cardiac magnetic resonance,” “cardiac MRI”, “congenital heart disease,” “artificial intelligence,” “low-field MRI,” “Sub-Saharan Africa,” and “low- and middle-income countries,” using both free-text and MeSH terms where applicable. No publication date limits were applied. Additional sources were identified by reviewing the bibliographies of included articles and key global reports. Articles were included if they addressed cardiac MRI implementation in low-resource settings, AI applications in CMR workflows, diagnostic aspects of congenital heart disease relevant to imaging or AI, or broader policy, ethical, or infrastructural factors affecting the deployment of these technologies. Studies were selected based on relevance and synthesized thematically across infrastructure, workforce, technology, ethics, and economics. No formal quality appraisal or meta-analysis was performed due to the heterogeneity and narrative scope of the review.
Congenital heart disease in Sub-Saharan Africa
In 2016, it was estimated that 19.5% of disability-adjusted life years (DALYs) in the world’s poorest 16 countries (all of which are in Sub-Saharan Africa) were due to congenital heart disease, more than any other cardiovascular disease4. They also estimated that CHD caused 6.5% of deaths due to cardiovascular disease, preceded by stroke, ischemic heart disease, and hypertensive heart disease4. Epidemiologic data on the prevalence of congenital heart disease in low-income countries are significantly limited compared to high-income countries. However, it is known that higher prevalence of malnutrition and maternal infectious diseases such as rubella and syphilis contribute to increased prevalence in the lowest income countries, especially in sub-Saharan Africa5. In 2013, the estimated birth prevalence of CHD was 463 per million population in sub-Saharan Africa compared to 137 per million population in the United States6. The 2017 Global Burden of Disease study demonstrated that the overall prevalence is higher in sub-Saharan African countries due to overall younger populations compared to high-income countries; however, once age-adjusted, the difference in prevalence between SSA and high-income countries (HIC) narrows4. In the 2017 GBD Study, it was estimated that one-third of DALYs due to CHD were concentrated in low socioeconomic demographic regions4. Though preventable pediatric deaths in LMICs have decreased by 50% globally since 1990, mortality rates from non-communicable diseases such as CHD have remained unchanged7; furthermore, 90% of the estimated 1.3 million children born with CHD do not have access to cardiovascular care4.
Current diagnostic standard of care for congenital heart disease in the United States
The 2018 American College of Cardiology/American Heart Association (ACC/AHA) Guidelines recommend CMR as standard of care for congenital heart disease8. CMR’s key advantage lies in its ability to provide detailed anatomic visualization without ionizing radiation, making it particularly useful in complex or atypical cases when no contraindications exist. While 3D echocardiography shows potential to replace CMR or CT for specific applications such as ventricular volume or intracardiac anatomy, its role remains limited8. Overall, the guidelines note that CMR is superior for ventricular function, echocardiography is preferred for valve evaluation, and echocardiography remains the most cost-effective. Cardiac CT and catheterization are less favorable in terms of both cost and diagnostic utility for ventricular and valvular assessment8.
Challenges in CMR accessibility in Sub-Saharan Africa
Infrastructure and equipment distribution constraints
A 2022 study found that the average number of MRI scanners in Africa is one per million people9. MRI access remains limited but is somewhat higher than previous estimates10,11,12. The shortage is most pronounced in West Africa, where 54% of countries had less than one scanner per million people, and in East Africa, where 41% fell below this threshold9. Countries such as Guinea (0.08 ppm) and Côte d’Ivoire (0.08 ppm) have some of the worst MRI availability, with a single MRI unit for over 13 million people2.
Previous reports have estimated even lower MRI availability10,11,12. For example, the Consortium for Advancement of MRI Education and Research in Africa (CAMERA) reported in 2020 that the average MRI density in Africa was 0.8 scanners per million people and noted that 11 countries had no scanners at all10. However, findings from Hasford et al. suggest that MRI access in some of these countries is slightly better than previously reported9. For example, Mali and Niger, previously reported by CAMERA as having no MRI scanners, are now documented to have 3 (0.15 per million people) and 5 (0.21 per million people) scanners, respectively9,10. This is still low, as Romania, which has a similar population to Mali, has between 7 and 10 scanners per million people11. Separate studies conducted in South Africa and Ghana have shown that most MRI services are available only through private and academic institutions, limiting access for the general population13,14. In West Africa, another study found that the entire region has just 84 MRI units, with most concentrated in Nigeria, leaving many countries with very limited access2.
There is no dedicated study on cardiac imaging infrastructure in low-income countries, but research highlights major disparities15. Even in middle-income countries, a 2022 study found that CMR access is limited (54%), and prenatal CHD screening is low (19%), indicating persistent imaging gaps that are likely worse in low-income countries16.
Although specific examples are highlighted based on available data, the challenges and solutions discussed apply broadly across sub-Saharan Africa17. Implementation barriers such as linguistic diversity, regional conflicts, and fragile governance structures remain widespread issues, particularly in central and western regions18,19. Additionally, the lack of standardized referral systems and persistent rural-urban disparities in healthcare access further complicate efforts20. Therefore, context-specific strategies addressing linguistic, political, and infrastructural differences are necessary to ensure effective implementation21.
Power and internet infrastructure limitations
CMR requires stable electricity and connectivity, which are often lacking in Sub-Saharan Africa. Frequent power outages and voltage fluctuations disrupt MRI services2. In one regional survey, 57% of MRI centers reported equipment downtime at least once a week or a few times per month due to power issues10. Although most facilities have backup generators, maintaining a steady power supply remains challenging10. Limited internet bandwidth and unreliable networks further hinder remote consulting and teleradiology, factors crucial for AI-assisted diagnostics22. In some areas, satellite links have been used to transmit MRI data to avoid issues with the reliability of broadband access2. These infrastructure gaps make it difficult to run advanced CMR and any AI support tools consistently.
Low MRI utilization rates
Despite the increasing availability of MRI systems in parts of Africa, utilization rates remain disproportionately low, particularly for specialized applications including CMR2,10. Several factors contribute to this underutilization, including a shortage of trained radiologists and technologists proficient in cardiac imaging, limited physician awareness of CMR’s diagnostic value, and financial constraints that make the procedure inaccessible for many patients23. Furthermore, referring physicians may default to more familiar and widely available modalities, such as echocardiography, even when MRI could provide superior diagnostic accuracy24.
High costs and out-of-pocket payments
MRI procedures in sub-Saharan Africa often cost more than the monthly income of many households, rendering CMR financially inaccessible for the majority25. Most patients must pay out-of-pocket for MRI, as insurance coverage for advanced imaging is minimal2. This financial barrier contributes to low scan volumes and underutilization of existing MRI machines. For instance, a study reported that only 8% of MRI facilities in the region perform 15 or more clinical scans daily per scanner10.
Equipment maintenance and obsolescence
Many African MRI units are older or low-field models that are prone to breakdown and difficult to service2. MRI maintenance is hindered by a lack of trained biomedical engineers, infrequent vendor servicing, and bureaucratic procurement delays26. Many machines face frequent downtime or premature obsolescence due to limited local technical support and inadequate training resources26. The absence of maintenance manuals in commonly spoken local or regional languages poses a practical challenge, especially for mid-level technicians or equipment caretakers who may not have advanced proficiency in international technical English27,28. Although formal biomedical engineers are typically proficient in English or French, basic operational and maintenance tasks are often delegated to locally trained staff or on-site technicians with varied language and technical backgrounds. These operational barriers limit the effective use of even the few MRI scanners currently available.
High costs of AI integration
Although AI holds promise for improving cardiovascular care, its deployment is hindered by the requirement for sophisticated computing hardware, proprietary software, and significant IT infrastructure upgrades, which pose considerable challenges for resource-limited hospitals in Africa29,30. The integration of AI into CMR necessitates investments in advanced data management and high-performance computing that elevate overall imaging costs31. Furthermore, the limited availability of financing, due to financial institutions’ reluctance to support technologies with extended payback periods, further restricts the procurement of essential imaging equipment such as MRI scanners32.
Competing healthcare priorities
Health budgets in Sub-Saharan Africa are constrained and usually directed toward urgent priorities such as infectious diseases, maternal-child health, and basic care33. Expensive modalities such as CMR and AI analytics compete with these priorities and often fall low on the list.
Bias in AI models from limited data
Most medical AI models are trained on datasets from North America, Europe, or Asia, with African populations grossly underrepresented34. This raises concerns that AI algorithms for CMR may not generalize well to African patients. Differences in genetics, disease patterns, scanner protocols, or image quality can lead to skewed results if the AI’s training data lacked diversity34.
A well-documented concern is domain shift, wherein algorithms trained on datasets from high-resource settings underperform when applied to data from LMICs due to differences in patient demographics, scanner models, or imaging protocols35. For instance, segmentation algorithms trained predominantly on Caucasian pediatric cohorts may show reduced accuracy in African children due to differences in body size, composition, or disease presentation. A study on retinal imaging AI models demonstrated nearly 20% lower sensitivity in underrepresented populations36. Similar performance drop-offs have been observed in chest radiography classification tasks when applied to African datasets37. These findings suggest that CMR algorithms require retraining or adaptation using local imaging data to maintain diagnostic fidelity and avoid algorithmic bias38.
However, there is a severe shortage of large, curated imaging datasets from African countries39. For CMR specifically, locally sourced data remains scant, as advanced cardiovascular imaging modalities, including CMR, are often unavailable or severely limited in many African regions33. The lack of African CMR data means researchers and vendors must rely on foreign datasets, which might not capture important local disease variants23.
Systemic and contextual variability in SSA
An additional challenge lies in the considerable heterogeneity among health systems across sub-Saharan Africa, introducing unmeasured confounding when interpreting implementation studies40. For example, the presence of informal or parallel healthcare systems, reliance on donor-driven equipment donations, and inconsistent training pathways all influence CMR deployment and AI adoption. Urban-rural disparities in power stability and internet connectivity further limit the generalizability of findings41. Additionally, inter-study variability in AI performance metrics due to differences in imaging protocols, equipment quality, and reader expertise complicates meta-analysis and benchmarking42. These unmeasured factors highlight the critical need to contextualize pilot results before wider implementation43.
Shortage of radiologists and other healthcare professionals
Sub-Saharan Africa has one of the lowest ratios of radiologists to the population in the world. For example, in Nigeria, which is the most populous country in Africa, there is approximately one radiologist for every 566,000 people44. In Malawi, the situation is even more challenging, with about one radiologist serving 4.4 million people45. In contrast, countries with more robust healthcare systems, such as Germany and the United States, have significantly higher radiologist-to-population ratios. Germany has approximately 12 radiologists per 100,000 people, while the United States has between 10 and 12 radiologists per 100,000 people46.
This shortage means there may be no expert able to run a CMR scan or read the images, even if the equipment is present. Overburdened general radiologists must cover all imaging modalities, leaving little time or incentive to refer patients for CMR. The “brain drain” of skilled professionals to higher-income countries further exacerbates this gap2. This shortage of healthcare professionals is not limited to radiologists alone, as Sub-Saharan Africa also faces significant deficits in other healthcare roles, including nurses, medical physicists, and radiographers, further straining the healthcare system and compromising the quality and safety of imaging services47,48.
Limited AI training programs
There remains a significant shortage of locally trained experts in medical AI in Africa49. Recent studies indicate that radiology training programs in Africa are beginning to incorporate modules on AI and advanced image analysis, though these subjects are often minimally addressed50. For instance, a study evaluating the perceptions and attitudes of trainee and qualified radiologists in South Africa found that while there is an awareness of AI’s role in radiology, formal training and integration into curricula remain limited50. Similarly, discussions on AI integration in cardiovascular healthcare in Africa show that its adoption in medical training programs is still at an early stage51.
Data privacy issues
The absence of strong data policies and regulations in Africa raises serious privacy concerns for AI imaging23. Public data repositories risk unauthorized access and breaches, while poor data quality and unreliable connectivity make safe implementation even harder23. Comprehensive data protection measures are essential to address these challenges49. At present, such controls are often absent or not uniformly applied52. The result is a hesitancy to adopt AI solutions due to fear of breaching patient privacy or ethical standards53. Until national policies catch up (e.g., establishing guidelines for health data usage and ownership), large-scale CMR data sharing for AI remains difficult54.
Medicolegal challenges
The introduction of AI into clinical imaging raises questions about legal responsibility and standards of care55. If an AI system used for CMR interpretation makes an error, such as missing a diagnosis or causing a delay, it is unclear who should be held accountable: the radiologist, the hospital, or the software developer56. Currently, most African countries have no specific regulations or case law addressing AI in healthcare57. This creates medicolegal risk. Healthcare providers worry about being held responsible for AI errors, while at the same time, there is no formal recourse if an AI fails.
To address these ethical and medicolegal challenges, regionally tailored regulatory frameworks are essential58. The African Union’s Digital Transformation Strategy for Africa (2020–2030) and the Africa CDC Health Information Exchange (HIE) Guidelines and Standards offer a practical blueprint for developing ethical AI governance in healthcare59,60. These frameworks emphasize data sovereignty, consent-based data sharing, and regional standardization. Similarly, WHO’s 2021 Guidance on Ethics and Governance of Artificial Intelligence for Health recommends human oversight, transparency, and accountability in AI deployment, principles that could guide national regulation across SSA58. Policymakers in the region should adapt these international guidelines to local realities to facilitate safe and equitable AI adoption in CMR61.
Device suitability for resource-limited settings
Up to 70% of medical equipment in sub-Saharan Africa is donated, yet only 10-30% of donated devices are operational, indicating major issues with suitability and upkeep of such technology62,63. Most health technologies are designed for high-income settings (with reliable power and technical support) and thus often perform poorly in African hospitals that lack stable electricity and maintenance resources64.
Lack of AI integration with existing infrastructure
A fundamental barrier to AI in African healthcare is the paucity of well-structured, digitized health data, as many healthcare facilities do not systematically capture or organize data in formats usable by AI algorithms65. In addition, computational and network infrastructure is limited; only about 28% of the sub-Saharan African population has regular internet access, reflecting connectivity gaps that undermine AI-driven healthcare solutions66,67. Existing healthcare information systems are often fragmented and outdated, making it difficult to incorporate new AI tools into clinical workflows or to interface them with hospital record systems67.
Interoperability challenges
Attaining true interoperability among healthcare systems remains a difficult challenge in Africa, as many digital health initiatives still run as isolated silos with incompatible data standards68. Notably, numerous pilot health information exchange projects across African countries were not guided by common data-sharing standards or policies, resulting in fragmented systems that cannot communicate with each other69. This fragmentation poses a serious barrier for AI-enhanced diagnostics such as CMR, since lack of interoperability means imaging data and AI outputs cannot be easily shared or integrated across different devices and hospital systems49.
Role of CMR for CHD diagnosis
Approximately 500,000 children are born with CHD every year in SSA70. While only 137 children per million individuals are estimated to be born with CHD in the United States, there are nearly 463 children per million born with CHD in SSA. This significantly higher burden can be attributed to the high fertility rates in the region, as well as the incidence of maternal infectious diseases such as rubella and syphilis, amongst other complex environmental and genetic factors6. While there have been no known efforts to determine the breakdown of CHDs across the entire region, several country-specific studies have established hospital-specific prevalence of various CHDs. For instance, analysis of a registry of 3982 patients from a pediatric cardiac clinic of Bugando Medical Center (Tanzania) found ventricular septal defects were the most common, followed by patent ductus arteriosus and atrial septal defects71. A study of 4621 pediatric patients at Uganda’s Heart Institute found a high incidence of truncus arteriosus, but the distribution of acyanotic conditions mirrored the findings in Tanzania72.
Additionally, it has been noted that while CHD mortality has decreased globally over the past decade, it has increased in Africa. A contributing factor could possibly be the lack of timely diagnosis. While children from developed countries receive a diagnosis before their first year of life, their SSA counterparts receive theirs much later, with one study reporting that in certain countries, such as Mozambique, the average age of diagnosis is 4 years73. Typically, pediatric patients are initially suspected of having CHD upon caregivers reporting feeding difficulties or frequent infections, as well as discovery of a heart murmur or cyanosis by healthcare providers74. Pulse oximetry is also becoming more widely employed to screen cyanotic conditions across LMICs, including those in SSA74. However, there is a paucity of facilities that provide echocardiography or other diagnostic imaging modalities to appropriately confirm diagnoses for both cyanotic and acyanotic CHDs, particularly in resource-poor and rural areas75.
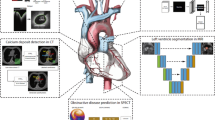
Cardiovascular magnetic resonance (CMR) imaging is a non-invasive modality that offers a comprehensive evaluation of cardiac anatomy, function, and tissue properties, making it indispensable in the assessment of complex congenital heart disease cases76. CMR enables quantitative assessment of cardiac volumes, ventricular function, and flow, and it offers excellent spatial resolution. Unlike echocardiography, it can acquire images in any orientation and provides complementary tissue characterization capabilities77 (Fig. 1).
a Overview of structural barriers to CMR imaging in SSA, highlighting infrastructure limitations, maintenance challenges, and limited human resources. b Conceptual overview illustrating how limited access to CMR imaging modalities contributes to the high burden of CHD in SSA, while also highlighting the potential of AI to reduce this burden and expand accessibility to care. c Overview of challenges associated with AI integration in CMR imaging, including data scarcity, infrastructure limitations, and competing health priorities2,9,34,39.
Although echocardiography remains the first-line diagnostic tool, CMR serves as a crucial adjunct, especially when echocardiographic findings are inconclusive. It provides more detailed anatomical and functional insights with high diagnostic accuracy. The reported sensitivity and specificity of postnatal echocardiography are 89–100% and 100%, respectively78, whereas CMR demonstrates sensitivity and specificity ranging from 93% to 100% and 87% to 100%, respectively76.
CMR utilizes a range of pulse sequences to visualize both anatomy and physiology. Flow sequences, such as two-dimensional (2D) phase-contrast and four-dimensional (4D) flow imaging, quantify blood flow through vessels and cardiac chambers79. Balanced steady-state free precession (bSSFP), a rapid gradient-echo sequence, provides high-contrast images of cardiac chambers and is commonly used to assess ventricular anatomy, size, and function. T1 and T2 mapping techniques offer quantitative data on tissue properties, helping detect myocardial edema or fibrosis. Late gadolinium enhancement (LGE) imaging is used to identify myocardial necrosis and replacement fibrosis, and in certain contexts, it can suggest active inflammation80. In atrial septal defects (ASDs), phase-contrast flow sequences are useful for evaluating shunt severity and associated complications such as pulmonary hypertension79. 4D Flow sequences can further aid in assessing pulmonary regurgitation, particularly in patients with repaired TOF79. bSSP-based cine imaging is also essential in accurately measuring and visualizing the dimensions of VSDs and PDAs, as well as hypertrophy secondary to aortic coarctation-related hypertension77.
However, there are several limitations to CMR, particularly in pediatric patients. Imaging for the pediatric population necessitates greater spatial and temporal resolution. Furthermore, breath-holding can be challenging in this population, with sedation or general anesthesia required79. The integration of AI and compressed sensing, and other accelerating techniques, can be useful in addressing these shortcomings81,82. Compressed sensing is a mathematical technique that enables high-quality image reconstruction from undersampled data, thereby reducing scan time without compromising diagnostic accuracy83.
Despite its advantages, CMR services are available in only eight African countries, with the majority of centers located in South Africa and primarily confined to private and academic institutions79. Of note, a systematic review revealed only two CMR research studies had been published from SSA as of 20229. Although echocardiography remains the first-line diagnostic tool, CMR provides essential detailed anatomical and functional insights with much more diagnostic accuracy when echocardiographic findings are inconclusive. The scarcity of CMR in resource-limited settings contributes to a diagnostic gap that affects both pediatric and adult CHD patients, thereby delaying early intervention and optimal care81,82,84. There are several obstacles that hinder CMR and MRI implementation in SSA. These include lack of funding for the research and training of MRI scientists in Africa, difficulties in procuring sequences and replicating standardized imaging protocols, unreliable electricity in various parts of the region, and expensive service/maintenance costs84.
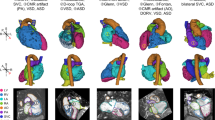
The figures below show how CMR plays a key role in the diagnosis and management of complex CHD. Figure 2 demonstrates balanced double-outlet right ventricle in an adolescent patient, where CMR delineated the relationship of the ventricular septal defect and great arteries and guided surgical planning. Complementary to this, Fig. 3 illustrates three-dimensional virtual segmentation of the same patient, providing enhanced spatial understanding that enabled a successful biventricular repair. Figure 4 presents a case of heterotaxy syndrome with left atrial isomerism, in which CMR identified thrombotic occlusion of a surgically placed graft and residual pulmonary artery stenosis. Complementary to this, Fig. 5 shows time-resolved MR angiography in the same patient, demonstrating hypoperfusion of the right lung with flow quantification and collaterals. Finally, Fig. 6 depicts an adult with repaired tetralogy of Fallot and a residual muscular ventricular septal defect complicated by pulmonary hypertension and Eisenmenger physiology, highlighting the importance of CMR in long-term follow-up. They present real patient cases where CMR provided clear anatomical and functional detail that helped guide decisions before surgery, assess complications after surgery, and evaluate long-term outcomes in adults with repaired or undiagnosed CHD. These examples show how CMR can be essential when other imaging methods are limited, especially in patients with complex or unclear heart anatomy (Fig. 7).
Associated findings include a large secundum ASD and previous pulmonary artery banding. The aorta is anterior and mildly leftward; the PA is posterior and distant from the septum. Several surgical teams previously judged the patient unsuitable for biventricular repair due to unfavorable VSD–PA alignment and concerns regarding the feasibility of intracardiac rerouting. Serial slices of black blood MRI sequences offered detailed morphological delineation of intracardiac anatomy, aiding in precise spatial understanding. The green line represents the hypothetical baffle pathway from the VSD toward the pulmonary artery, illustrating the unfavorable alignment that initially discouraged surgical repair. CMR enabled quantitative assessment of biventricular volumes, systolic function, and Qp/Qs ratio (~1.5), confirming the physiological suitability for biventricular circulation. AA ascending aorta, ASD atrial septal defect, CMR cardiac magnetic resonance imaging, CHD congenital heart disease, LV left ventricle, PA pulmonary artery, PV pulmonary valve, Qp/Qs pulmonary-to-systemic flow ratio, RV right ventricle, VSD ventricular septal defect.
The left panel shows the STL-based 3D anatomical model, while the right panel illustrates blood-pool segmentations of the ventricles and great vessels, with the red and green regions representing intracavitary blood pools for clear visualization of chamber spaces. The 3D colored models are presented as sequential views, collectively demonstrating the interventricular septal defect and its relationship with the great arteries. These imaging-based insights, building upon the prior detailed CMR findings, facilitated surgical planning and allowed the team to proceed with a successful Rastelli-type biventricular repair. This reversed the earlier consensus by multiple teams that the patient was unsuitable for biventricular correction. CMR cardiac magnetic resonance imaging.
The clinical course was complicated by right pleural effusions and ascites. Cardiac catheter angiography failed to cannulate the previously placed Dacron graft, raising suspicion of graft thrombosis. CMR revealed thrombotic occlusion of the Dacron graft (asterisks; a, c), a large AVSD (arrow; a, b) and pleural effusion (E; a, c), together with significant narrowing of the right pulmonary artery and restricted flow toward the right lung. AVSD atrioventricular septal defect, CMR cardiac magnetic resonance imaging, E effusion, IVC inferior vena cava, L-SVC left superior vena cava, RPA right pulmonary artery, R-SVC right superior vena cava.
Time-resolved MRA demonstrated decreased perfusion in the right lung parenchyma (asterisks; a–d), consistent with hypoperfusion, veno-venous collaterals (arrows; a–c), and aortopulmonary collaterals (arrow; d). Flow quantification was performed using phase-contrast CMR and demonstrated restricted flow towards the right pulmonary artery (RPA-to-LPA flow ratio is calculated as 27/73%). Manually drawn planes, illustrated in the planning image, were used to target key sites of systemic and pulmonary venous return and to provide a rapid visual explanation for the clinician. This complex anatomy and suspected vascular complication highlight the challenges in both diagnosis and management, particularly in patients with complex congenital heart disease. CHD congenital heart disease, CMR cardiac magnetic resonance imaging, LPA left pulmonary artery, MRA magnetic resonance angiography, RPA right pulmonary artery.
The patient had not been followed in regular cardiology care for years, and the residual lesion had remained unrecognized until adulthood. Peripheral oxygen saturation was 80%, raising concern for Eisenmenger physiology. Late presentation with hypoxemia and residual lesions highlights the importance of lifelong surveillance in congenital heart disease, which is challenging in LMIC settings. CMR revealed marked right ventricular dilation and dysfunction, with RV EDVI of 331 mL/m², ESVI of 228 mL/m², and an ejection fraction of 31%. The left ventricle showed preserved function (LV EF: 53%), but was also dilated (LV EDVI: 175 mL/m²). Pulmonary regurgitation fraction was significantly elevated at 45%. Importantly, phase-contrast imaging revealed early and mid-diastolic right-to-left shunting across the VSD, a finding highly suggestive of bidirectional or Eisenmenger-level physiology. Flow quantification demonstrated Qp:Qs = 1.8, consistent with significant left-to-right shunting. Pulmonary flow distribution was relatively symmetric (RPA-to-LPA flow ratio is calculated as 48:52%). The RVOT was aneurysmal, classified as Type IV RVOT morphology. This case illustrates the long-term consequences of incomplete follow-up after congenital heart defect repair and emphasizes the role of CMR in accurately characterizing residual lesions, ventricular function, regurgitation severity, and shunt physiology in adult congenital heart disease. CMR cardiac magnetic resonance imaging, EDVI end-diastolic volume index, EF ejection fraction, ESVI end-systolic volume index, LMIC low- and middle-income countries, LV left ventricle, LPA left pulmonary artery, Qp:Qs pulmonary-to-systemic flow ratio, RPA right pulmonary artery, RV right ventricle, RVOT right ventricular outflow tract, VSD ventricular septal defect.
a Highlights clinical enhancements enabled by AI, including improved segmentation accuracy, optimized image acquisition, standardized diagnostics, and reduced inter-observer variability, which together improve the consistency and accuracy of CMR imaging in CHD diagnosis. b Lists key limitations associated with implementing AI-enhanced CMR imaging in SSA. c Illustrates the central role of AI in transforming CHD diagnosis through its integration with CMR imaging. d Lists future potential applications of incorporating AI in CMR systems. e Outlines strategies for scalable implementation of AI-enhanced CMR imaging in low- and middle-income countries87.
While echocardiography remains the first-line modality for CHD assessment in sub-Saharan Africa, CMR and CT provide complementary insights, particularly for complex cases. Table 1 summarizes the comparative advantages and limitations of echocardiography, CMR, and CT for evaluating CHD in SSA85.
Role of artificial intelligence in CMR imaging for CHD
AI is transforming CMR imaging by improving various aspects of the diagnostic process, including image acquisition, processing, reporting, follow-up planning, and data storage84. Deep learning algorithms help with image acquisition by shortening scan times and reducing motion-related artifacts, particularly benefiting pediatric patients by potentially minimizing the need for anesthesia9. A study evaluating 1178 patients for a variety of cardiovascular conditions illustrated a 30% reduction in scan time upon implementation of AI guidance86. AI-driven computer vision can streamline image analysis, while specialized CHD algorithms can enhance efficiency by enabling more precise diagnoses86. Additionally, AI models can help with cardiovascular risk stratification by processing large, temporally diverse datasets without increasing the burden on healthcare professionals86. Advances in telemedicine, including federated and swarm learning, offer enhanced remote data integration and expanded opportunities for translational research87. Federated learning refers to a decentralized machine learning approach in which local data remain on-site while only model updates are shared with a central server, preserving privacy88.
AI has shown promise in optimizing CHD imaging, particularly in automated segmentation and disease classification, potentially reducing dependency on highly specialized expertise87. Due to the lack of large annotated datasets available for pediatric CHD patients, a generative adversarial network was developed and trained on a fully convolutional network to automatically segment the left and right ventricles in patients with complex CHDs. The automatic process demonstrated strong agreement with the results obtained manually87. These capabilities suggest the possibility of shifting the role of technologists to that of quality control89. For instance, the prescription of MRI imaging planes is handled by qualified MRI technologists and physicians. Utilizing deep-learning-based localizations (U-Net and cascaded system-based) yielded similar imaging planes as those manually determined by radiologists and technologists90.
Moreover, an AI-based automatic CMR planning software resulted in fewer errors compared to manual planning91. Another study compared measurements of ventricular volumes that were determined manually by a group of eight observers and automatically. While there was no significant difference between automatic versus manual, there was a significant difference in measurements between observers, suggesting that the performance of automated analysis is comparable to human experts and offers greater precision92. AI diagnostic models, via CMR, have also previously outperformed cardiologists in diagnosing pulmonary hypertension93. More studies are still needed to compare the performance of humans versus AI models in diagnosing and evaluating CHDs. AI tools, including natural language processing, can also compile decades of imaging and other EMR data together94 and trigger automatic follow-up notifications for incidental findings95. However, the integration of AI into routine clinical practice remains limited due to data heterogeneity, lack of standardized CHD imaging datasets, and infrastructural challenges in low-resource settings9. There has also been a paucity of research highlighting the cost-effectiveness of AI implementation in CMR, though its economic benefits have been widely alluded to96.
Strategies for expanding CMR and AI access for CHD in LMICs
Public-private partnerships (PPP)
Innovative financing models such as PPPs can help overcome budgetary barriers to acquiring and operating CMR equipment. In West Africa, experts have called for greater public-private collaboration to improve MRI availability, noting that government partnerships with private investors could bridge funding and infrastructure gaps2. Nigeria’s experience has shown that radiology departments can acquire nearly all major imaging equipment, including MRI, through PPP arrangements97. This approach has led to improvements in service delivery and enhanced residency training for specialists97. While challenges like unclear definitions of the partnership’s scope, staff responsibilities, and lines of authority must be managed, PPPs remain a viable option to bolster imaging capacity when public funding alone is insufficient98.
Low-field MRI adoption
Low-field MRI refers to MRI systems with a field strength typically between 0.25 and 1.0 Tesla, which offer reduced image resolution and signal to noise (SNR) compared to high-field systems but at significantly lower cost99,100. Low-field MRI is particularly useful in limited-resource settings due to its significantly lower cost, reduced infrastructure and power requirements, and portability compared to conventional high-field systems80. Their smaller size and low power consumption make them deployable in rural or mobile settings, expanding access in areas lacking advanced imaging facilities81. Furthermore, simplified hardware, machine learning-enhanced image reconstruction, and safer imaging for patients with implants or devices increase their utility where resources and trained personnel are scarce80. In the context of CMR, adopting lower-field MRI technology presents a practical solution to expand access. This cost-efficient approach is already evident in West Africa, where 77.6% of MRI units are permanent magnet low-field systems with field strengths below 0.3 Tesla, highlighting a model for sustainable imaging expansion in low- and middle-income countries2.
Recent advances in low-field MRI have significantly enhanced clinical potential, particularly for cardiac applications. Balanced steady-state free precession (bSSFP) sequences at 0.35 Tesla have been shown to produce diagnostically useful cardiac images, with only minor compromises in image quality compared to 1.5 Tesla systems101. Assessments of cardiac function, blood flow, and myocardial relaxation parameters at 0.35 Tesla further support the feasibility of low-field systems in capturing essential hemodynamic and tissue characteristics102. Comparisons between 0.55 Tesla and 1.5 Tesla systems have demonstrated that low-field cardiovascular MRI is capable of detecting myocardial infarctions with comparable diagnostic confidence103. Moreover, the development of efficient spiral in-out and echo-planar imaging (EPI) bSSFP cine sequences has improved temporal resolution and reduced artifacts at 0.55 Tesla, enhancing image quality for cine CMR104. In addition to cost savings, low-field MRI offers several other advantages. It is particularly suitable for cardiac imaging in patients with implants, for MRI-guided interventional procedures, and for assessing cardiopulmonary interactions. Furthermore, low-field systems are better tolerated by obese patients and can be more readily deployed within patient care environments such as cardiology units, intensive care, emergency departments, and community-based centers, expanding the accessibility and integration of cardiovascular MRI into routine clinical workflows105. Embracing these low-field MRI technologies could enable many centers in sub-Saharan Africa to perform basic CMR for evaluating ventricular function, blood flow, and congenital heart disease anatomy at a fraction of the cost of a conventional high-field scanner (Fig. 8).
a Description of the key advantages of low-field CMR imaging, including its affordability, portability, and sustainability for pediatric patients and low-resource settings. b Findings from clinical studies have demonstrated that low-field CMR can produce diagnostic-quality cardiac images, with only minor compromises to high-field systems. c Summary of the potential impact of low-field CMR on CHD care, including earlier diagnosis, decentralized imaging access, and the establishment of task-sharing models. d Proposed implementation strategies to expand low-field CMR in SSA, including regional training programs, global partnerships, and the integration of AI to support image acquisition and interpretation80,101,102,104.
Dedicated CHD CMR units in tertiary centers
Creating specialized CMR units for CHD within major hospitals is a practical strategy for low-resource settings. One way to start such a center/unit can be starting with a workshop. A notable example of early adoption occurred in Ethiopia, where a multi-day CMR workshop was conducted in August 2019 at Ayder University Hospital in Mekelle. This initiative introduced local MRI technologists to cardiac imaging techniques and trained an Ethiopian cardiologist in the foundational interpretation of CMR images. This effort marked a milestone in expanding access to advanced imaging in a resource-constrained setting and reflects the growing feasibility of low-field CMR across SSA106.
In South India, one such unit was set up using an existing 1.5 Tesla MRI machine and a small team that included a pediatric cardiologist and a cardiac radiologist107. Imaging protocols were adjusted to meet each patient’s needs, allowing for detailed evaluations of both heart anatomy and function. This approach led to important new findings in some cases and helped guide treatment decisions.
Imaging protocols were adjusted based on each patient’s clinical needs, allowing for detailed evaluations of intracardiac and extracardiac anatomy, ventricular function, and blood flow dynamics. In 10% of patients, CMR identified new anatomic details that were not detected by other imaging modalities. These findings contributed to clinical decision-making, and 23% of patients subsequently underwent cardiac surgery based on information obtained or augmented by CMR. The modality was particularly helpful in surgical planning for complex congenital heart lesions, including double-outlet right ventricle, L-TGA, heterotaxy syndromes, and single-ventricle physiology107.
This experience shows that with focused staff, tailored protocols, and proper planning, high-quality CHD CMR can be done effectively even when resources are limited. It offers a useful model for other countries looking to improve imaging services for children and adults with CHD.
This model of innovation in a resource-limited setting was further strengthened by complementary technologies designed to improve preoperative planning. In a related initiative from the same center in South India, 3D printing was integrated into CHD care to support surgical decision-making for complex cases108. Using CMR and CT data, patient-specific, life-sized cardiac models were printed to visualize intricate anatomy and anticipate surgical challenges. In five difficult cases where surgery had previously been deferred due to anatomical complexity, the 3D prototypes significantly improved spatial understanding and enabled precise planning. All patients subsequently underwent successful surgery, validating the use of 3D-printed models as a powerful tool to enhance clinical outcomes in limited-resource environments108. A recent study reported that, although the average cost of producing a 3D-printed anatomic model in the hospital ranged from $2,180 to $2,737, specialists found the models highly valuable in clinical practice, improving pre-procedural planning and, on average, reducing surgery time by about 30 min. When considering hospital operating room costs, the time savings could theoretically cover the cost of the model itself, potentially saving up to $2,900 per patient109.
Despite all the developments and results, the high initial investment cost and hardware dependency of 3D printing remain significant barriers, particularly in low-resource settings. In this context, patient-specific 3D visual models, generated from CMR or CT datasets using open-source platforms, offer a feasible and cost-effective alternative110,111. These virtual reconstructions preserve the anatomical fidelity necessary for surgical planning and can be manipulated interactively on standard computer systems or viewed in immersive virtual reality environments. By eliminating the need for physical printing while still providing detailed spatial orientation, such models extend the benefits of personalized preoperative planning to a broader range of clinical settings where access to advanced fabrication technologies may be limited.
AI integration across the CMR workflow
Integrating AI into the CMR workflow offers transformative potential for LMICs by addressing key barriers such as limited expertise, infrastructure, and access. AI-enabled automated cardiac image planning can streamline complex, skill-dependent imaging processes, making it possible to perform CMR outside highly specialized centers112. In LMICs, where low-field MRI systems are more accessible but often limited by poor image quality, AI-enhanced reconstruction techniques can improve clarity and enable accurate visualization of cardiac structures113. Additionally, AI can accelerate scan acquisition and automate post-processing, reducing the burden on limited personnel and improving overall diagnostic efficiency114. This is especially valuable in the context of CHD, where detailed anatomical and functional assessment is critical, as AI can aid in tissue characterization, structure identification, and diagnostic support115. By minimizing reliance on high-end infrastructure and shortening traditionally long scan times, AI also supports more scalable imaging solutions suitable for constrained environments116. Portable AI-powered MRI systems, though currently focused on neuroimaging, demonstrate the feasibility of fast, point-of-care imaging that could guide the development of similar cardiac applications for use in low-resource settings117.
Current efforts to improve MRI accessibility, AI, and CHD diagnostics
Several initiatives are underway to address the current challenges in expanding CMR access and integrating AI into CHD diagnostics in LMICs. The Consortium for Advancement of MRI Education and Research in Africa (CAMERA) has launched programs such as the Scan-With-Me (SWiM) initiative, which enhances the skills of MRI technologists through hands-on training and standardized imaging protocols105. AI-driven screening tools are enhancing cardiovascular diagnostics. There also exist governmental partnerships, such as the one between the Kenyan government and General Electric, which have made MRI scanners more affordable118. Via the Medical Credit Fund, this collaboration allows for small private health providers to borrow $100,000 and enhance imaging services119. To address power outages, Crestview Radiology in Nigeria coupled their MRIs with generators and other alternative forms of electricity, so that the MRIs can run even when power from the National Grid is down119.
The integration of AI into cardiology is progressing through strategic partnerships, such as the collaboration between Point G Hospital in Bamako, Mali, and HealthTech Mali, where AI systems are being incorporated to support diagnostic interpretations and clinical decision-making120. CAMERA has also launched the SPARK academy, which teaches individuals from a variety of disciplines, ranging from radiology to computer science, how artificial intelligence can be leveraged in medical imaging121. Another initiative is RAD-AID’s Friendship Data Trust, which addresses the lack of infrastructure, which hinders AI implementation in LMICs122. By providing donated on-site servers, PACS software, and a cloud-based system to run AI applications, resource-limited hospitals are able to readily take advantage of the greater feasibility and efficiency afforded by AI122.
Efforts to develop adaptable imaging technologies for CHD are exemplified by the University of Manchester’s PROTEA project, which addresses CHD diagnostic challenges in Africa by implementing a patient-specific computational fluid dynamics (CFD) pipeline. Given the limited availability of high-resolution MRI in low-resource settings, this initiative adapts CFD analysis using CT scans and Doppler echocardiography to improve diagnostic insights of hemodynamic parameters such as outlet volumetric flow rates and pressure measures in the context of coarctation of aorta123,124. Similarly, the CHD AI project is leveraging AI to assist non-expert sonographers in acquiring optimal echocardiographic images for neonates suspected of having CHD, enabling remote expert interpretation and reducing the need for long-distance travel for diagnosis confirmation125. Another key advance is the development of low-field MRI systems, designed to be more affordable and suitable for regions with unstable power supplies. Researchers are constructing portable, low-field MRI scanners for on-site assembly in African settings, aiming to make imaging technology more accessible and sustainable104. Finally, the feasibility of CMR can be improved by adopting an abbreviated protocol. One such effort in Peru that limited the protocol to left ventricular function reduced the scan time to 18 min (typically 45 min) and reduced the scan cost to $150 USD121.
Future directions should prioritize the sustainable integration of CMR and AI into CHD care in low-resource settings through structured, institution-based models. Central to this strategy is the development of regional satellite centers that provide dedicated CMR units integrated with congenital heart surgery, neonatal care, and specialized nursing, ensuring comprehensive and multidisciplinary management. Previous initiatives led by pediatric cardiovascular teams have demonstrated the value of establishing a cardiovascular surgery program for CHD126,127. Building on these efforts, the incorporation of CMR and AI into cardiologist-led care pathways could enhance CHD services by fostering clinical integration and interdisciplinary collaboration.
To strengthen local capacity, short-term observerships or fellowships at high-capacity centers can provide essential hands-on experience. Although remote support, including a virtual consultation, reporting assistance, or online education, offers high-impact value, these programs should be supported by in-person opportunities in high-capacity centers to strengthen local capacity. Sustainable CMR applications should be longitudinally encouraged with hands-on CMR training, improvement of image acquisition quality, and protocol development embedded within on-site clinical environments.
These initiatives represent promising steps toward bridging the diagnostic gap for CHD in Africa by leveraging both technological innovation and collaborative frameworks.
Several additional limitations constrain the conclusions of this review and should be addressed in parallel. First, there is a lack of large, curated CMR datasets derived from SSA populations, which limits the development and validation of locally relevant AI models. Second, the distribution of imaging infrastructure is uneven, with a concentration in private urban centers, reducing generalizability to rural or public settings. Third, few economic evaluations exist that quantify the cost-effectiveness or return on investment for AI-enabled CMR in low-resource environments. Fourth, substantial regulatory uncertainty persists regarding AI integration into clinical care, including questions around liability, data privacy, and software approval pathways in SSA countries. These issues need to be addressed so that CMR can provide optimal diagnostics.
Whilst considering our findings, it must also be considered that as a narrative review, our findings may be influenced by literature availability and publication bias.
Conclusions
The integration of CMR and AI has the potential to revolutionize the diagnosis and management of CHD in SSA and other LMIC settings. CMR provides detailed anatomic and functional information critical for accurate diagnosis and intervention planning, especially in complex cases inadequately resolved by echocardiography alone. AI further enhances this capability by significantly reducing scan time, automating complex image interpretation, and improving diagnostic precision through machine learning-driven analysis. By enabling detailed cardiovascular assessments with lower reliance on specialized personnel and infrastructure, AI-integrated CMR can help address the scarcity of trained radiologists and imaging experts in sub-Saharan Africa, making advanced cardiac care accessible to underserved communities.
Nonetheless, realizing the full transformative potential of AI-driven CMR requires overcoming substantial infrastructure, technological, and economic barriers pervasive across SSA. Strategic investments in low-field MRI technology, targeted training initiatives to address skill shortages, and ethical frameworks governing AI use in healthcare are essential. Collaborative approaches, including PPP and locally adapted AI solutions, are particularly promising for expanding sustainable access. By leveraging innovative technologies in conjunction with context-specific solutions, AI-enhanced CMR can significantly reduce the clinical burden of CHD in SSA, improving patient outcomes through timely diagnosis, informed intervention, and enhanced healthcare delivery in the region.
References
Nakamura, T. & Sasano, T. Artificial intelligence and cardiology: current status and perspective. J. Cardiol. 79, 326–333 (2022).
Ogbole, G. I., Adeyomoye, A. O., Badu-Peprah, A., Mensah, Y. & Nzeh, D. A. Survey of magnetic resonance imaging availability in West Africa. Pan Afr. Med. J. 30, 240 (2018).
van Schalkwyk, C., van Zyl, B. C., Herbst, P. G. & Ackermann, C. An audit of the establishment of a cardiac magnetic resonance imaging service in a public tertiary hospital setting in the Western Cape Province of South Africa. Pan Afr. Med. J. 49, 15 (2024).
Kwan, G. F. et al. Endemic cardiovascular diseases of the poorest billion. Circulation 133, 2561–2575 (2016).
Bruneau, B. G. The developmental genetics of congenital heart disease. Nature 451, 943–948 (2008).
Zimmerman, M. & Sable, C. Congenital heart disease in low-and-middle-income countries: focus on sub-Saharan Africa. Am. J. Med. Genet. C Semin. Med. Genet. 184, 36–46 (2020).
Murala, J. S. K., Karl, T. R. & Pezzella, A. T. Pediatric cardiac surgery in low-and middle-income countries: present status and need for a paradigm shift. Front. Pediatr. 7, 214 (2019).
Stout, K. K. et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139, e698–e800 (2018).
Hasford, F. et al. A review of MRI studies in Africa with special focus on quantitative MRI: Historical development, current status, and the role of medical physicists. Phys. Med. 103, 46–58 (2022).
Anazodo, U. C. et al. A framework for advancing sustainable MRI access in Africa. NMR Biomed. https://doi.org/10.1002/nbm.4846 (2022).
International Atomic Energy Agency (IAEA). IMAGINE—IAEA Medical Imaging and Nuclear Medicine Global Resources Database. Human Health Campus. https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINE.html. Accessed 23 February 2025.
Hricak, H. et al. Medical imaging and nuclear medicine: a Lancet Oncology Commission. Lancet Oncol. 22, e136–e172 (2021).
Kabongo, J. M., Nel, S. & Pitcher, R. D. Analysis of licensed South African diagnostic imaging equipment. Pan Afr. Med. J. 22, 57 (2015).
Ofori, E. K. et al. An audit of MRI machines and services in Ghana. Radiography 27, 127–131 (2021).
Einstein, A. J. et al. Cardiac procedures remain low in low-income countries. Radiological Society of North America. Available at: https://www.rsna.org/news/2023/september/cardiac-procedures-remain-low. Accessed 23 February 2025 (2023).
Majeed, A. et al. Screening and diagnostic imaging at centres performing congenital heart surgery in middle-income countries. Cardiol. Young 33, 780–786 (2023).
Ayanore, M. A. et al. Towards resilient health systems in Sub-Saharan Africa: a systematic review of the English language literature on health workforce, surveillance, and health governance issues for health systems strengthening. Ann. Glob. Health 85, 113 (2019).
Annan, N. Violent conflicts and civil strife in West Africa: causes, challenges and prospects. Stability 3, 3 (2014). Art.
Vines, A. & Dideberg, R. Working Paper: policy response options for West Africa’s security and democracy crisis. https://www.imvf.org/wp-content/uploads/2025/01/policy-paper-working-responses-observatorio-en.pdf. Accessed 9 July 2025 (Instituto Marquês de Valle Flôr, 2024).
Nyangara, F. & Ngatia, P. Health systems in Africa: community perceptions and perspectives. UNICEF Eastern and Southern Africa Regional Office. https://www.unicef.org/esa/media/551/file/Health-Systems-in-Africa.pdf. Accessed 9 July 2025 (2012).
Osuchukwu, C. The Alliance of Sahel States and the Future of West African Regional Integration. SSRN Electronic Journal. https://doi.org/10.2139/ssrn.5283877 (2024).
Acevedo, E. AI network in Africa seeks to solve resource disparities by uniting imaging stakeholders. RSNA News. February 16. https://www.rsna.org/news/2023/february/solving-ai-disparities-in-africa. Accessed 12 March 2025 (2023).
Kawooya, M. G. Training for rural radiology and imaging in sub-Saharan Africa: addressing the mismatch between services and population. J. Clin. Imaging Sci. 2, 37 (2012).
Bertrand, T., Bartlett-Esquilant, G., Fischer, K. & Friedrich, M. G. Patient and physician preferences for non-invasive diagnostic cardiovascular imaging technologies: a discrete choice experiment. J. Patient Rep. Outcomes 6, 15 (2022).
HEC Stories. Democratizing healthcare in sub-Saharan Africa. https://hecstories.fr/en/democratizing-healthcare-in-sub-saharan-africa/. Accessed 12 March 2025 (2023).
Radiological Society of North America. How radiologists overcome barriers to provide imaging in low to middle-income countries. https://www.rsna.org/news/2024/july/imaging-in-lmics. Accessed 12 March 2025 (2024).
Murali, S. et al. Bringing MRI to low- and middle-income countries: directions, challenges and potential solutions. NMR Biomed. 37, e4992 (2024).
Mollura, D. J., Culp, M. P. & Lungren, M. P. Radiology in Global Health Strategies, Implementation, and Applications 2nd edn (Springer International Publishing, 2019).
Davenport, T. & Kalakota, R. The potential for artificial intelligence in healthcare. Future Health. J. 6, 94–98 (2019).
Milic, M. K. The role of artificial intelligence in strengthening healthcare delivery in Sub-Saharan Africa: challenges and opportunities. Zenodo, https://doi.org/10.5281/zenodo.14714238 (2025).
Tolu-Akinnawo, O. Z., Ezekwueme, F., Omolayo, O., Batheja, S. & Awoyemi, T. Advancements in artificial intelligence in noninvasive cardiac imaging: a comprehensive review. Clin. Cardiol. 48, e70087 (2025).
Lobig, F. et al. To pay or not to pay for artificial intelligence applications in radiology. npj Digit. Med. 6, 117 (2023).
Minja, N. W. et al. Cardiovascular diseases in Africa in the twenty-first century: gaps and priorities going forward. Front. Cardiovasc. Med. 9, 1008335 (2022).
Tripathi, S. et al. Understanding biases and disparities in radiology AI datasets: a review. J. Am. Coll. Radiol. 20, 836–841 (2023).
Guan, H. & Liu, M. Domain adaptation for medical image analysis: a survey. IEEE Trans. Biomed. Eng. 69, 1173–1185 (2022).
Nakayama, L. F. et al. Artificial intelligence, data sharing, and privacy for retinal imaging under Brazilian Data Protection Law. Int. J. Retina Vitr. 11, 41 (2025).
Musa, A., Prasad, R. & Hernandez, M. Addressing cross-population domain shift in chest X-ray classification through supervised adversarial domain adaptation. Sci. Rep. 15, 11383 (2025).
KeyaMedical. The evolving role of AI: generalizability, domain shift, and localization. Keya Medical Blog. March 14. https://www.keyamedical.com/generalizability-domain-shift-localization-ai/. Accessed 11 July 2025 (2020).
Lekadir, K. et al. From MICCAI to AFRICAI: African Network for Artificial Intelligence in Biomedical Imaging. In Presented at: 3rd Workshop on Practical Machine Learning for Developing Countries: Learning Under Limited/Low Resource Scenarios (International Conference on Learning Representations (ICLR), 2022).
Nabyonga-Orem, J. et al. The state and significant drivers of health systems efficiency in Africa: a systematic review and meta-analysis. J. Glob. Health 13, 04131 (2023).
Alliance for Affordable Internet (A4AI). Community networks: a real opportunity for Africa and the Global South. https://a4ai.org/research/community-networks-a-real-opportunity-for-africa-and-the-global-south. Accessed 11 July 2025 (World Wide Web Foundation, 2019).
Aggarwal, R. et al. Diagnostic accuracy of deep learning in medical imaging: a systematic review and meta-analysis. npj Digit. Med. 4, 65 (2021).
Gage, A. D. Health System Performance in the Delivery of Interventions for Reducing Child Mortality in Sub-Saharan Africa. Dissertation, Harvard T.H. Chan School of Public Health (2018).
Soroosh, G., Ninalowo, H., Hutchens, A. & Khan, S. Nigeria Country Report: For Use in Radiology Outreach Initiatives Accessed 16 March 2025 (2015).
RAD-AID International. Malawi Country Report. RAD-AID International. https://rad-aid.org/wp-content/uploads/Malawi-CR.pdf. Accessed 23 March 2025.
GE Health Partners. Workforce review: radiology. Aligning Demand and Capacity in a Changing Health Care Environment. https://emea.gehealthcarepartners.com/images/pdfs/Rapid-Review--Radiology-Workforce-Review-FINAL.pdf. Accessed 16 March 2025 (2018).
World Health Organization. State of the world’s nursing 2020: Investing in education, jobs and leadership. https://www.who.int/publications/i/item/9789240003279. Accessed 21 March 2025 (WHO, 2020).
Kawooya, M. G. et al. An Africa point of view on quality and safety in imaging. Insights Imaging 13, 58 (2022).
Alaran, M. A. et al. Challenges and opportunities of artificial intelligence in African health space. Digit. Health 11, 20552076241305915 (2025).
Nciki, A. I. & Hlabangana, L. T. Perceptions and attitudes towards AI among trainee and qualified radiologists at selected South African training hospitals. SA J. Radiol. 29, 3026 (2025).
Ashinze, P. et al. Artificial intelligence: transforming cardiovascular healthcare in Africa. Egypt Heart J. 76, 120 (2024).
Price, W. N. 2nd & Cohen, I. G. Privacy in the age of medical big data. Nat. Med. 25, 37–43 (2019).
Char, D. S., Shah, N. H. & Magnus, D. Implementing machine learning in health care - addressing ethical challenges. N. Engl. J. Med. 378, 981–983 (2018).
Morley, J. et al. The ethics of AI in health care: a mapping review. Soc. Sci. Med. 260, 113172 (2020).
McKee, M. & Wouters, O. J. The challenges of regulating artificial intelligence in healthcare comment on “Clinical Decision Support and New Regulatory Frameworks for Medical Devices: Are We Ready for It?—A Viewpoint Paper”. Int. J. Health Policy Manag. 12, 7261 (2023).
Ranschaert, E. R., Morozov, S. & Algra, P. R. (eds) Artificial Intelligence in Medical Imaging: Opportunities, Applications and Risks https://doi.org/10.1007/978-3-319-94878-2 (Springer Nature Switzerland AG, 2019).
World Health Organization. Ethics and Governance of Artificial Intelligence for Health https://www.who.int/publications/i/item/9789240029200. Accessed 21 March 2025 (World Health Organization, 2021).
World Health Organization. Ethics & Governance of Artificial Intelligence for Health: WHO Guidance https://www.who.int/publications/i/item/9789240029200. Accessed 12 July 2025 (World Health Organization, 2021).
African Union Commission. Digital Transformation Strategy for Africa (2020–2030). https://au.int/sites/default/files/documents/38507-doc-dts-english.pdf. Accessed 12 July 2025 (African Union Commission, 2020).
Africa Centres for Disease Control and Prevention. African Union Health Information Exchange Guidelines and Standards. https://africacdc.org/download/african-union-health-information-exchange-guidelines-and-standards/. Accessed 12 July 2025 (Africa CDC, 2024).
Muralidharan, V. et al. Global Initiative on AI for Health (GI-AI4H): strategic priorities advancing governance across the United Nations. npj Digit. Med. 8, 219 (2025).
Gatrad, A. R., Gatrad, S. & Gatrad, A. Equipment donation to developing countries. Anaesthesia 62, 90–95 (2007).
World Health Organization. Barriers to innovation in the field of medical Devices: Background Paper 6. (World Health Organization, 2010).
Chawla, S. et al. Electricity and generator availability in LMIC hospitals: improving access to safe surgery. J. Surg. Res. 223, 136–141 (2018).
Musa, S. M. et al. Paucity of health data in Africa: an obstacle to digital health implementation and evidence-based practice. Public Health Rev. 44, 1605821 (2023).
Vurayai, S. COVID-19 pandemic and the narrative of the digital divide gap in universities in sub-Saharan Africa. Afr. Identities 22, 760–771 (2024).
Victor, A. Artificial intelligence in global health: an unfair future for health in Sub-Saharan Africa? Health Aff. Sch. 3, qxaf023 (2025).
eHealth Lab Ethiopia. AU HIE Policy and Standards. Available from https://ehealthlab.org/africa-cdc/. Accessed 23 March 2025 (eHealth Lab Ethiopia, 2022).
Mamuye, A. L. et al. Health information exchange policy and standards for digital health systems in Africa: a systematic review. PLOS Digit. Health 1, e0000118 (2022).
GBD 2017 Congenital Heart Disease Collaborators. Global, regional, and national burden of congenital heart disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet Child Adolesc Health. 2020 Mar;4(3):e6. doi: 10.1016/S2352-4642(20)30031-6.]. Lancet Child Adolesc. Health 4, 185–200 (2020).
Zuechner, A. et al. Spectrum of heart diseases in children presenting to a paediatric cardiac echocardiography clinic in the Lake Zone of Tanzania: a 7 years overview. BMC Cardiovasc. Disord. 19, 291 (2019).
Namuyonga, J. et al. High prevalence of truncus arteriosus in pediatric congenital heart disease in Uganda. Ann. Pediatr. Cardiol. 12, 186 (2019).
Manuel, V., Morais, H., Manuel, A., David, B. & Gamboa, S. Ventricular septal defect in children and adolescents in Angola: experience of a tertiary center. Rev. Port. Cardiol. 33, 637–640 (2014).
Rossouw, B. Congenital heart disease in Africa threatens Sustainable Development Goals. South Afr J. Crit. Care. 37, https://doi.org/10.7196/SAJCC.2021.v37i1.486 (2021).
Awori, M. N., Ojuka, D., Marangu, D., & Bannon, P. Current status of the pediatric congenital heart disease management pathway in low and low-middle income countries: a review. Afr. Ann. Thorac. Cardiovasc. Surg. 15, 42–49 (2023).
Mohrs, O. K. et al. Time-resolved contrast-enhanced MR angiography of the thorax in adults with congenital heart disease. Am. J. Roentgenol. 187, 1107–1114 (2006).
Moscatelli, S. et al. Importance of cardiovascular magnetic resonance applied to congenital heart diseases in pediatric age: a narrative review. Children 11, 878 (2024).
Mamalis, M. et al. Comparison of the results of prenatal and postnatal echocardiography and postnatal cardiac MRI in children with a congenital heart defect. J. Clin. Med. 12, 3508 (2023).
Simonetti, O. P. & Cook, S. Technical aspects of pediatric CMR. J. Cardiovasc. Magn. Reson. 8, 581–593 (2006).
Arnold, T. C., Freeman, C. W., Litt, B. & Stein, J. M. Low-field MRI: clinical promise and challenges. J. Magn. Reson. Imaging 57, 25–44 (2023).
Fotaki, A. et al. Artificial intelligence in cardiac MRI: Is clinical adoption forthcoming? Front. Cardiovasc. Med. 8, 818765 (2022).
Jaspan, O. N., Fleysher, R. & Lipton, M. L. Compressed sensing MRI: a review of the clinical literature. Br. J. Radiol. 88, 20150487 (2015).
Lustig, M., Donoho, D., Santos, J. M. & Pauly, J. M. Compressed sensing MRI. IEEE Signal Process. Mag. 25, 72–82 (2008).
Gulati, A. et al. CMR Guide: Congenital Heart Disease. https://www.escardio.org/static-file/Escardio/Subspecialty/EACVI/CMR-guide-CHD-2014.pdf Accessed 1 April 2025 (European Society of Cardiology, 2014).
Lakshmanan, S. & Mbanze, I. A comparison of cardiovascular imaging practices in Africa, North America, and Europe: two faces of the same coin. Eur. Heart J. Imaging Methods Pract. 1, qyad005 (2023).
Kwong, R. Y. et al. Artificial intelligence-guided cardiac magnetic resonance imaging as a clinical routine procedure leads to substantial reduction of scan time and improvement of imaging quality. Comparative results of 1,147 patient studies from a single US center. J. Am. Coll. Cardiol. 81, 1363–1363 (2023).
Karimi-Bidhendi, S. et al. Fully‑automated deep‑learning segmentation of pediatric cardiovascular magnetic resonance of patients with complex congenital heart diseases. J. Cardiovasc. Magn. Reson. 22, 80 (2020).
Kairouz, P. et al. Advances and open problems in federated learning. Found. Trends Mach. Learn. 14, 1–210 (2021).
Morales, M. A., Manning, W. J. & Nezafat, R. Present and future innovations in AI and cardiac MRI. Radiology https://doi.org/10.1148/radiol.231269 (2024).
Blansit, K., Retson, T., Masutani, E., Bahrami, N. & Hsiao, A. Deep learning–based prescription of cardiac MRI planes. Radiol. Artif. Intell. 1, e180069 (2019).
Glessgen, C. et al. Automated vs manual Cardiac MRI planning: a single-center prospective evaluation of reliability and scan times. Eur. Radiol. https://doi.org/10.1007/s00330-025-11364-z (2025).
Bai, W. et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. J. Cardiovasc. Magn. Reson. 20, 65 (2018).
Wang, Y. R. et al. Screening and diagnosis of cardiovascular disease using artificial intelligence-enabled cardiac magnetic resonance imaging. Nat. Med. 30, 1471–1480 (2024).
Miller, D. D. & Brown, E. W. Artificial intelligence in medical practice: the question to the answer? Am. J. Med. 131, 129–133 (2018).
Domingo, J. et al. Preventing delayed and missed care by applying artificial intelligence to trigger radiology imaging follow-up. NEJM Catal. Innov. Care Deliv. 3, CAT.21 (2022).
Siddiqui, T. A. et al. The merits, limitations, and future directions of cost-effectiveness analysis in CMR with a focus on coronary artery disease: a literature review. J. Cardiovasc. Dev. Dis. 9, 357 (2022).
Jinadu, F. O., Agunloye, A. M., Adeyomoye, A. A., Adekoya, A. O. & Obajimi, G. O. Public–private partnerships in Nigerian teaching hospitals: potential and challenges. West Afr. J. Radiol. 27, 143–149 (2020).
World Bank. Public-private partnership and contracting out projects for imaging and laboratory diagnostics and their potential contribution to universal health coverage in low- and middle-income countries: lessons learned. https://documents1.worldbank.org/curated/en/099228212222328226/pdf/IDU13028bee71430614487187b41f19dfa71b870.pdf. Accessed 31 March 2025 (World Bank, 2022).
Marques, J. P., Simonis, F. F. J. & Webb, A. G. Low-field MRI: An MR physics perspective. J. Magn. Reson Imaging 49, 1528–1542 (2019).
Klein, H.-M. Clinical Low Field Strength Magnetic Resonance Imaging (Springer, 2016).
Rashid, S. et al. Cardiac balanced steady-state free precession MRI at 0.35 T: a comparison study with 1.5 T. Quant. Imaging Med. Surg. 8, 627–636 (2018).
Varghese, J. et al. Assessment of cardiac function, blood flow and myocardial tissue relaxation parameters at 0.35 T. NMR Biomed. 33, e4317 (2020).
Bandettini, W. P. et al. Evaluation of myocardial infarction by cardiovascular magnetic resonance at 0.55-T compared to 1.5-T. JACC Cardiovasc. Imaging 14, 1866–1868 (2021).
Restivo, M. C., Ramasawmy, R., Bandettini, W. P., Herzka, D. A. & Campbell-Washburn, A. E. Efficient spiral in-out and EPI balanced steady-state free precession cine imaging using a high-performance 0.55T MRI. Magn. Reson. Med. 84, 2364–2375 (2020).
Consortium for Advancement of MRI Education and Research in Africa (CAMERA). Scan With Me (SWiM) Program. Available at: https://event.fourwaves.com/swim/pages. Accessed 10 February 2025.
Leuner, C. & Hamza, S. First cardiac MRI workshop in Ethiopian Ayder University Hospital performed. https://etiopia-witten.de/details-62/first-cardiac-mri-workshop-in-ethiopian-ayder-university-hospital-performed.html. Accessed 31 March 2025 (Etiopia-Witten e.V., 2020).
Kappanayil, M., Rajeshkannan, R., Sapre, A. & Kumar, K. Initial experience with a dedicated cardiac MRI program for congenital heart disease in a limited resource environment. J. Cardiovasc. Magn. Reson. 15, P290 (2013).
Kappanayil, M., Koneti, N. R., Kannan, R. R., Kottayil, B. P. & Kumar, K. Three-dimensional-printed cardiac prototypes aid surgical decision-making and preoperative planning in selected cases of complex congenital heart diseases: early experience and proof of concept in a resource-limited environment. Ann. Pediatr. Cardiol. 10, 117–125 (2017).
Ravi, P. et al. University of Cincinnati 3D Printing Clinical Service Participants. Utility and costs during the initial year of 3D printing in an academic hospital. J. Am. Coll. Radiol. 20, 193–204 (2023).
Böttcher, B. et al. 3D cinematic reconstructions of cardiovascular CT presented in augmented reality: subjective assessment of clinical feasibility and potential use cases. Eur. Radiol. Exp. 9, 27 (2025).
Ells, Z. et al. A free method for patient-specific 3D-VR anatomical modeling for presurgical planning using DICOM images and open-source software. Methods 236, 10–16 (2025).
Mastrodicasa, D. et al. Use of AI in cardiac CT and MRI: a scientific statement from the ESCR, EuSoMII, NASCI, SCCT, SCMR, SIIM, and RSNA. Radiology 314, https://doi.org/10.1148/radiol.240516 (2025).
National Institute of Standards and Technology (NIST). AI for low-field MRI. https://www.nist.gov/programs-projects/ai-low-field-mri. Accessed 31 March 2025 (2023).
Lanzafame, L. R. M. et al. Artificial intelligence in cardiovascular CT and MR imaging. Life 13, 507 (2023).
Castellaccio, A. et al. Artificial intelligence in cardiovascular magnetic resonance imaging. Radiologia https://doi.org/10.1016/j.rxeng.2025.03.001 (2025).
Alsharqi, M. & Edelman, E. R. Artificial intelligence in cardiovascular imaging and interventional cardiology: emerging trends and clinical implications. J. Soc. Cardiovasc. Angiogr. Inter. 4, 102558 (2025).
Hyperfine, Inc. AI-powered portable MRI. https://hyperfine.io/. Accessed 31 March 2025 (2025).
Anazodo, U., Obungoloch, J. & Kwikima, U. Looking towards the future of MRI in Africa. Nat. Commun. 15, 2260 (2024).
Anazodo, U. dunnaC. et al. A framework for advancing sustainable magnetic resonance imaging access in Africa. NMR Biomed. 36, e4846 (2023).
Resilient Digital Africa. E-health: AI changing the game in Mali. Available at: https://resilient.digital-africa.co/en/blog/2024/02/28/e-health-ai-changing-the-game-in-mali/. Accessed 14 February 2025.
Menacho, K. et al. INCA (Peru) Study: impact of non-invasive cardiac magnetic resonance assessment in the developing world. J. Am. Heart Assoc. 7, e008981 (2018).
Mollura, D. J. et al. Artificial intelligence in low- and middle-income countries: innovating global health radiology. Radiology 297, 513–520 (2020).
Swanson, L. et al. A patient-specific CFD pipeline using doppler echocardiography for application in coarctation of the aorta in a limited resource clinical context. Front. Bioeng. Biotechnol. 8, 409 (2020).
Understanding children’s heart disease in Africa. The University of Manchester. Available at: https://www.manchester.ac.uk/collaborate/global-influence/collaborations/regional/manchesters-work-in-africa/childrens-heart-disease/. Accessed 14 February 2025.
CHD AI Project. Data Science for Health Discovery and Innovation in Africa. Available at: https://dsi-africa.org/project/32. Accessed 14 February 2025.
Youssef, T. et al. Establishing a high-quality pediatric cardiac surgery program in post-conflict regions: a model for limited resource countries. Pediatr. Cardiol. 46, 279–286 (2025).
Shidhika, F. F. et al. The Namibian Children’s Heart Project: a South-South partnership to provide cardiac care. Cardiol. Young 29, 206–213 (2019).
Author information
Authors and Affiliations
Contributions
Michael Negussie: writing—original draft, writing—review and editing. Nicole Sanchez: figure preparation, writing—original draft. Sherin Aboobucker Sidiq: writing—original draft. Arcadia Trvalik: writing—original draft, writing—review and editing. Eduardo Baettig: writing—review and editing. Sercin Ozkok: conceptualization, writing—review and editing, supervision. Muhammad Umair: conceptualization, writing—review and editing, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Negussie, M., Sanchez, N., Sidiq, S.A. et al. Applying artificial intelligence to cardiac MRI to diagnose congenital heart disease in low-resource settings such as Sub-Saharan Africa. Commun Med 5, 473 (2025). https://doi.org/10.1038/s43856-025-01179-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-01179-w