Abstract
Background
Pancreatic ductal adenocarcinoma is projected to become the second leading cause of cancer-related deaths by 2040, with the highest disease burden expected amongst Non-Hispanic Black patients. One of the most significant predictors of poor outcomes is the presence of cancer-associated cachexia (CCa). Yet, race- and ethnicity-specific biomarkers for early CCa diagnosis are lacking.
Methods
We evaluated a panel of candidate biomarkers of CCa in a diverse cohort of patients with pre-treatment serum using multiplex ELISA-based methods.
Results
We find that growth/differentiation factor-15 (GDF-15) is associated with cachexia severity, is superior to standard biomarkers at classifying cachexia, and differentiates between non-cachexia and pre-cachexia status, but only among Hispanic/Latinx and non-Hispanic White participants. Furthermore, high GDF-15 levels at diagnosis are associated with a greater weight loss from 3.3% (95%CI = −0.14–6.7) to 8.0% (CI = 5.9–10.1) over the 6 months post-diagnosis. Finally, both ENA-78/CXCL5 and GRO-α/CXCL1 are elevated in non-Hispanic Black individuals in a disease-independent manner (P < 0.001 for both analytes).
Conclusions
GDF-15 may be a potential biomarker for “pre-cachexia” in the non-Hispanic White and the Hispanic population, but not non-Hispanic Black individuals. These findings underscore the unmet need to enroll non-Hispanic Black participants in clinical trials for CCa.
Plain language summary
Pancreatic cancer is projected to become the second leading cause of cancer-related deaths by 2040, with the highest burden expected amongst non-Hispanic Black patients. Cancer-associated cachexia, a multifactorial wasting condition, is the most significant predictor of poor treatment response and survival, but race-specific biomarkers for diagnosis of cachexia are lacking. In the current study, we evaluated blood-based biomarkers of cachexia while accounting for race and ethnicity. We found that growth/differentiation factor (GDF)−15 was better than standard biomarkers at classifying cachexia and cachexia severity in non-Hispanic White and Hispanic/Latinx patients, but not non-Hispanic Black patients. However, inflammatory markers ENA-78/CXCL5 and GRO-α/CXCL1 were specifically elevated amongst non-Hispanic Black participants. Our findings highlight the need to target non-Hispanic Black patients for enrollment in clinical trials studying cancer-associated cachexia.
Similar content being viewed by others
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is increasing in incidence in the United States (US) and globally and is projected to be the second leading cause of cancer-related deaths in the US by 20401. Up to 80% of PDAC cases develop cancer-associated cachexia (CCa), defined as a multifactorial wasting disorder characterized by loss of appetite, body weight, and/or lean body mass (i.e., skeletal muscle) leading to fatigue, functional impairment, treatment-related toxicities, poor quality of life, and reduced survival2,3,4,5. Properly detecting CCa in its earliest stage and intervening to maintain muscle mass remain key goals in the care of patients with PDAC. Unfortunately, due to the lack of a well-established definition of CCa and heterogeneity of presentation, diagnosis of CCa typically does not occur until late in the disease course6,7,8,9.
Blood-based biomarkers of CCa have the potential to aid in earlier diagnosis and to monitor the effects of therapy on CCa. Laboratory values obtained as part of standard of care shown to have prognostic value for patients with CCa include serum C-reactive protein (CRP), albumin, hemoglobin (HgB), white blood cell (WBC) count, CRP:albumin ratio or the Glasgow Prognostic Score, neutrophil to lymphocyte ratio, platelet count, and bilirubin10,11,12,13,14,15,16,17,18. Human studies conducted among patients with PDAC have also identified candidate circulating biomarkers of CCa which include: pro-inflammatory cytokines (e.g. interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor (TNF)-α, monocyte chemoattractant protein-1 (MCP-1)); transforming growth factor (TGF)-beta family members (e.g. activin A, growth/differentiation factor (GDF)−15); growth factors (e.g. Insulin-like growth factor binding protein 2 (IGFBP2)); adipokines (e.g. Leptin, Adiponectin); glycoproteins (Zinc-α2-glycoprotein (ZAG)); glucagon-like-peptide-1 (GLP-1)) and branched chain amino acids19,20,21,22,23,24. Unfortunately, sample sizes in these studies were limited ranging from 60-99 PDAC cases, with most studies evaluating patients with metastatic disease at the time of blood collection.
Since prior associations with blood-based biomarkers were generally made in patients with advanced disease (who oftentimes were undergoing treatment), these associations may be linked to disease progression rather than CCa. Further, several of these studies were conducted in Asia and may not be racially diverse or generalizable to other racial/ethnic populations18,25,26. Of those conducted in the US21,22,23,27, all primarily were comprised of self-reported Non-Hispanic White (NHW) individuals, with only two of these studies23,27 including self-reported Non-Hispanic Black (NHB) individuals (n = 723 and n = 1127), and only one study27 including self-reported Hispanic/Latinx (H/L) individuals (n = 5).
Collectively, it is apparent that investigations of serum biomarkers of CCa in larger and more racially and ethnically diverse cohorts of newly-diagnosed PDAC cases are lacking. Given that PDAC incidence and mortality rates are highest in NHB participants followed by NHW and H/L participants28, it is prudent to focus research efforts on including historically underserved populations in studies of CCa. Thus, the goal of the current study is to investigate levels of candidate serum biomarkers in a diverse cohort of treatment-naïve (i.e., prior to receipt of cancer-related therapy) PDAC cases and determine their association with CCa status and survival by race and ethnicity. Importantly, we assess the added value of a race- and ethnicity-specific panel of biomarkers in predicting clinical outcomes above and beyond standard criteria obtained at the point of care. Our findings suggest that GDF-15 is a useful marker for early (prior to weight loss) cachexia but is racially-influenced.
Methods
Study participants
The Florida Pancreas Collaborative (FPC) is a multi-institutional prospective cohort study and biobanking initiative established to advance PDAC research in racially/ethnically diverse populations29. Participants in the FPC include 500 individuals with a suspected pancreatic mass presenting to one of the participating study sites. FPC participants meeting the following inclusion criteria were included in this analysis: 1) diagnosed with PDAC; 2) had available pre-treatment blood collection; and 3) were able to have their cachexia status ascertained. A total of 202 participants met these criteria (Supplementary Fig. 1). A subset of 69 participants who had alternative pancreas-associated diagnoses and who had baseline blood available are included in a supplemental analysis as a non-PDAC comparison group (Supplementary Fig. 1, see also supplemental methods). This study was approved by the Moffitt Cancer Center Scientific Review Committee (MCC19717, Pro00029598), and Advarra IRB (IRB00000971). All patients provided informed consent for participation29.
Data collection and clinical outcomes
Demographic, clinical, and epidemiologic data were collected from participants at diagnosis and 6-month follow-up timepoints through: self-administered online- or teleform-based questionnaires; questions administered by the research coordinator at each participating study site; and abstraction from the electronic medical record29. Using available data, participant cachexia status was classified using two published categorization schemas. First, we used criteria by Vigano et al.30, which classifies the cachexia continuum into 4 stages (non-cachectic (NCa), pre-cachectic (PCa, i.e. those with abnormal labs (within 3 months of diagnosis) or appetite but who have not lost a significant amount of weight in the last 6 months), cachectic (Ca), and refractory cachectic (RCa)) based on clinical, laboratory, functional, and nutritional criteria (used in Fig. 1B). We also used a simplified method for CCa classification described by Fearon et al.31 based on self-reported weight loss (WL) over the 6 months before PDAC diagnosis, with those having >5% WL or an underweight body mass index (BMI) plus WL > 2% categorized as cachectic, and those with <=5% WL as non-cachectic).
Boxplots showing a log-scale value of all significantly different serum biomarkers between cachectic and non-cachectic PDAC patients (A) and when stratified into 4 stages as in Vigano et al. B NCa = non cachectic, PCa = pre cachectic, Ca = cachectic, RCa = refractory cachectic. All plots in (A, B) are significant based on a Wilcoxon rank sum or Kruskal-Wallis test and BH-adjusted p-value (two-sided hypothesis). Significant differences in serum biomarkers between groups (BH-adjusted pairwise Dunn’s test, two-sided hypothesis) are denoted with a red line in (B). Outliers are denoted by a red dot. C ROC curves for percent self-reported weight loss (Pct Weightloss), GDF-15, TNF-alpha, white blood cell count (WBC), Hemoglobin and Albumin and a combined TNF-alpha * GDF-15 model (Combined Model) using cachexia status at baseline as the “ground truth”. Youden’s optimal thresholds are denoted with a black dot. D Boxplot of percent weight loss (negative values indicate weight gain) from baseline to 6-month follow-up time point. Patients were dichotomized based on levels of GDF-15 (Low vs High based on the Youden’s threshold values calculated by the ROC in Fig. 2C or by the values used in Groarke et al. (37). P-values represent a Wilcoxon rank sum test (two-sided hypothesis).
Blood collection and processing
Blood was collected at diagnosis (pre-treatment) and follow-up timepoints via phlebotomy and processed into serum using previously described methods29 and stored at −80 °C. Supplementary Table 1 lists all candidate biomarkers, the assay performance, and the percent of samples falling within the detectable range. Detailed methods for assays performed are found in Supplemental Materials. Mean percent coefficient of variation values (CV) (intra-assay variability) for all successful assays are found in Supplementary Table 2, ranging from 1.16% (ENA-78/CXCL5) to 40.02% (Angiotensin II).
Statistics and reproducibility
First, we tested for differences in clinicodemographic characteristics according to cachexia status31, race and ethnicity, and availability of serum at diagnosis (inclusion criterion) using Pearson’s chi-squared test or Fisher’s exact test for categorical variables and Wilcoxon rank sum test, analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables. Tukey’s Honest Standard Differences or Dunn’s test were used to test pairwise comparisons when ANOVA or Kruskal-Wallis tests were performed, respectively.
To test for differences in serum biomarkers according to dichotomous cachexia status31, we performed Wilcoxon rank sum tests or Kruskal Wallis tests in the overall study population (n = 202) and in subgroups by tumor stage and gender. To understand differences in serum biomarkers across the CCa continuum, we used Kruskal Wallis tests, Cochrane-Armitage trend tests and ordinal regression to test for differences across the four-stage and five-stage cachexia status classification systems defined by Vigano et al.30 and Martin et al.32. We then dichotomized biomarkers that significantly differed by CCa status31 at their medians and used multivariable logistic regression to assess the association of these biomarkers with CCa status31. To further test the ability of these biomarkers to identify cachectic patients, we examined their discriminatory capability by generating receiver operating characteristic (ROC) curves to assess the area under the curve (AUC), Youden’s Index to identify optimal cut-points, sensitivity, and specificity using the ‘pROC’ R package33. We also performed this ROC analysis using common blood-based biomarkers associated with cachexia status (WBC count, albumin, hemoglobin)30 and body composition metrics (BMI, waist circumference and waist to hip ratio) as comparisons, and percent WL was used as a positive control.
Considering that unintentional WL is a hallmark of CCa, we identified a subset of patients with body weight data collected at diagnosis and follow-up (n = 121) to test for differences in percent WL across the 4-stage cachexia continuum34 using a Kruskal-Wallis test with pairwise comparisons. Further, we explored differences in weight loss over time for select analytes that significantly differed by CCa status31 using median splits and published cut-points35.
Next, to understand the mediating role of race and ethnicity on biomarkers of CCa, we tested for differences in serum biomarkers by CCa status31 in subgroups by race and ethnicity using Kruskal Wallis tests. Further, to aid in our understanding of race-based differences in biomarkers of cachexia, we tested for differences in biomarkers by race and ethnicity, irrespective of cachexia status, using Kruskal Wallis tests and multivariable linear regression.
Finally, we examined the association of biomarkers that significantly differed by CCa status31 with overall survival using Kaplan-Meier curves and log-rank tests. We also used Cox proportional hazards regression (‘survival’ R package) to examine the association of several factors36 with all-cause mortality. Overall survival time was calculated in months from the date of diagnosis to the date of last contact or death.
Benjamini-Hochberg (BH) method was used to adjust for multiple comparisons where appropriate. All analyses were performed in R version 4.4.0 (RRID:SCR_000432). All tests were two-sided, and all p-values were calculated based on non-missing values and considered statistically significant at P < 0.05.
Inclusion and ethics statement
This manuscript conforms to the Nature Journal reporting guidelines for reporting on Gender, Race and Ethnicity and other social constructs. Race and Ethnicity were self-reported for these studies due to genetic ancestry not being available for these participants. This research was conducted in the state of Florida and most participants were residents of this US state.
Results
Characteristics of the study population by cachexia status
Of 500 patients with a suspected pancreatic mass that were recruited to the FPC study, 318 were confirmed to have a diagnosis of PDAC29. Select sociodemographic and clinical characteristics of PDAC cases who donated blood pre-treatment (n = 206) are shown in Supplementary Table 3 (stratified by race and ethnicity). The final analytic dataset includes 202 of the 206 PDAC cases with blood for whom cachexia status could be determined using established criteria30,31, and comprised 132 NHW, 30 NHB, and 40 H/L cases (see Table 1 for a high-level overview of patient characteristics, Supplementary Fig. 1 for a flowchart of enrolled patients). At diagnosis, males were significantly more likely than females to be cachectic (P = 0.025). Other predictors of CCa included lower BMI (P = 0.006), diabetes (P = 0.027), and a worse ECOG performance status (P < 0.001) (Table 1). A higher proportion of patients with late-stage PDAC (tumor stage III/IV) were classified as cachectic (36.4%) than non-cachectic (32.1%). Cases classified as Ca (n = 121) had significantly worse survival than those classified as NCa (n = 81) (log-rank P = 0.009, Supplementary Fig. 2b, Table 1, see Supplementary Fig. 2c for survival stratified by cachectic stage as described by Vigano et al.30).
Characteristics of the study population by race and ethnicity
The prevalence of cachexia was highest in H/L cases (67.5%) followed by NHB (63.3%) and NHW (55.2%) individuals. H/L patients had lower BMI (P = 0.018) than other racial and ethnic groups (Supplementary Table 3). NHB patients presented with more late-stage tumors (P = 0.039) than their NHW and H/L counterparts (Table S3). Although NHB individuals presented more frequently with late-stage tumors (50.0%) than NHW and H/L patients (37.5% and 29.4%, respectively), NHB patients did not have significantly poorer overall survival (OS) when compared to the NHW patients (P = 0.192, HR (95%CI):1.37(0.85–2.21) based on a pairwise comparison between group levels with adjustments for multiple testing). H/L PDAC patients in our cohort demonstrated a significantly longer OS time than other groups (P = 0.021, HR (95%CI): 0.58(0.35–0.97) when compared to NHW patients as a reference, Supplementary Table 3, Supplementary Fig. 2d). Finally, NHW patients were more likely to have a smoking history (P = 0.026), and higher age-adjusted Charlson comorbidity index (ACCI) scores (P < 0.0001) (Supplementary Table 3).
Importantly, we did not observe any unexpected systematic differences between characteristics of PDAC cases who did and did not have available pre-treatment blood (Supplementary Table 429,37). Unsurprisingly, due to the observational nature of this study and the reliance upon blood collected at the time of standard of care, survival was shorter and ECOG performance score was worse in the group without pre-treatment blood available for research. All participants self-reported gender, race and ethnicity.
Significant differences were observed between cachectic and non-cachectic PDAC cases overall for multiple serum biomarkers
Univariate analysis: Among PDAC cases with versus without CCa based on weight loss (Fearon et al.)31, serum levels of 16 biomarkers were significantly altered (14 increased, 2 decreased) after adjustment for multiple comparisons (Table 2, Fig. 1A). The two most significantly increased markers in cachectic patients were TNF-α and GDF-15 (both ~1.4-fold increase; Padj < 0.001).
When stratified into subgroups according to tumor stage, three biomarkers emerged that were significantly elevated in cachectic patients with both early-stage (stage I/II) and late-stage (stage III/IV) tumors: TNF-α (P = 0.014 (early) and P = 0.001 (late)), GDF-15 (P = 0.003 (early) and P = 0.018 (late)), and IL-22 (P = 0.004 (early) and P = 0.005 (late)), see Supplementary Table 5 and Supplementary Fig. 3a). Additionally, we found that IL-6 (P = 0.001), MIP3-α (P = 0.002), and IL-8 (P = 0.009) were significantly higher in cachectic versus non-cachectic PDAC patients with early-stage tumors, but not late-stage (Supplementary Table 5 and Supplementary Fig. 3b).
Further, when we stratified the PDAC cohort by gender, both males and females with Ca demonstrated significant increases in TNF-α, IL-10, IL-22, and GDF-15 compared to NCa. A decrease in leptin was observed in Ca males compared to their non-cachectic counterparts (Supplementary Table 6, Supplementary Fig. 4a). Intriguingly, for several biomarkers (IL-1β, IL-6, IL-8, TIMP-1, and IFN-γ) only females demonstrated significantly increased levels in Ca vs. NCa cases. In contrast, MIP-3α was significantly increased in males with Ca but not females (Supplementary Table 6, Supplementary Fig. 4b).
Exploratory analysis: In an exploratory analysis using hierarchical clustering, biomarker clustering was not observed by CCa status (cachectic versus non-cachectic based on Fearon et al.31; Supplementary Fig. 5). Analytes with >80% assay success were assessed due to the need for no missingness in the heatmap.
Pre-cachexia stage can be differentiated by GDF-15 and TIMP-1 levels
Analytes which differentiate “Pre-cachexia”: When cachexia status was classified using a 4-stage system30, we found that patients with PCa can be differentiated from NCa patients by CRP, TIMP-1, and GDF-15 (Fig. 1B, Supplementary Table 7). CRP was omitted from further analysis since it was used to stage cachexia30. When TIMP-1 and GDF-15 levels were dichotomized at the median, both TIMP-1 (P < 0.001) and GDF-15 (P < 0.001) were significantly increased over worsening cachexia stage, demonstrating a dose-dependent relationship between these biomarkers and the CCa continuum. When ordinal regression was performed with TIMP-1 and GDF-15 as dichotomized predictor variables in the same model, GDF-15 remained a significant predictor of cachexia (P = 0.021), but TIMP-1 did not. Indeed, GDF-15 remained significantly increased over all four cachexia stages even when adjusting for race and ethnicity, tumor stage, diabetes status, gender, age at diagnosis, and BMI (P < 0.001, Table 3).
We also assessed GDF-15 levels across the five grades of cachexia as described in Martin et al.32 to assess the rigor and reproducibility of our findings using other cachexia classification systems. We found that GDF-15 increased over all 5 grades: stage 0 median (LCL-UCL): 1337.8 (1376.9–1972.6); stage 1 median (LCL-UCL): 1466.1 (1252.1–2537.3); stage 2 median (LCL-UCL): 1648.7 (1535.4–2638.8); stage 3 median (LCL-UCL): 2373.1 (2378.9–3646.7); stage 4 median (LCL-UCL): 2496.5 (1988.5–4332.6), though the increases were not statistically significant.
GDF-15 and TNF-α can be used to predict cachexia status and perform better at cachexia classification than other commonly used classifiers of cachexia
Regression models for cachexia status: When dichotomized at the median, both GDF-15 (P < 0.001, OR = 4.81, 95%CI = 2.3–10.5) and TNF-α (P < 0.001, OR = 4.10, 95%CI = 1.89–9.34) were predictive of cachexia status when controlling for age at diagnosis, gender, tumor stage, BMI, R&E, and diabetes. Even when including all significant analytes as continuous variables in our model, GDF-15 remained significantly associated with cachexia status (P = 0.002)
AUC results: Both TNF-α and GDF-15 had AUC values of >0.7 and these analytes were used to generate a combined model by multiplying the two concentrations together (Fig. 1C). The combined model did have significantly higher AUC than GDF-15 alone (P = 0.009) but not better than TNF-α alone (P = 0.76); AUCs for TNF-α and GDF-15 were not significantly different (P = 0.730). Other markers such as white blood cell count, hemoglobin, and albumin did not perform better at classification (Fig. 1C, Supplementary Table 8)
GDF-15 levels predict future weight loss
Weight loss over time stratified by GDF-15 levels at baseline: Patients with high GDF-15 levels at diagnosis (based on the optimal ROC threshold of 2044.7 pg/mL) lost a mean of 8.0% body weight and patients with low GDF-15 lost a mean of 4.4% body weight (P = 0.05, Fig. 1D) in the 6 months after GDF-15 assessment. Patients with high GDF-15 levels at diagnosis (defined by Groarke et al.35, 1500 pg/mL) lost a mean 8.0% body weight compared to patients with a low GDF-15 levels (3.3% body weight, P = 0.01, Fig. 1D) in the 6 months after GDF-15 assessment. TNF-α levels and combined levels of GDF-15 and TNF-α did not significantly predict weight loss over the same time period (P = 0.17 and P = 0.06 for TNF-α and combined model, respectively).
Verification of weight loss for individuals classified as PCa: As verification of the Vigano et al. cachexia classification, we compared weight loss in the 6 months post-diagnosis for participants classified as NCa or PCa. Individuals classified as PCa lost more weight than individuals classified as NCa (median/SD = 2.2%/12.7 for non-cachectic individuals and 7.7%/9.7 for pre-cachectic individuals) in the 6 months post-diagnosis, though the difference is not significant (P = 0.10). Furthermore, a greater proportion (70.8%, or 17 out of 24) of PCa individuals lost >5% of their body weight over the 6 months post-diagnosis as compared to the NCa group (40.0% or 8 out of 20) but this also did not achieve significance (P = 0.22 via Fisher’s Exact test).
Stratification by race and ethnicity (R&E) reveals novel biomarkers of CCa for NHW and H/L participants, but not NHB participants
Of the serum biomarkers significantly deregulated in cachectic31 PDAC cases overall (Table 2), several continued to show differences by R&E. For instance, GDF-15, IL-22, MIP-1α, TIMP-1, and TNF-α levels were significantly higher in H/L and NHW PDAC cases with CCa than those without CCa. Although statistically significant differences were not observed for any of these biomarkers by CCa status among NHB cases (Fig. 2, Supplementary Table 9), TNF-α and IL-22 levels did trend upwards for NHB cachectic patients, similar to the other R&E groups (Fig. 2). In contrast to H/L and NHW cases, GDF-15 did not show any difference by cachexia status in NHB cases (Padj=0.95). Intriguingly, we observed that NHW and H/L patients with cachexia had median GDF-15 levels that far exceeded 1500 pg/mL, but NHB patients with cachexia had a median GDF-15 level of 1488 pg/mL35 (Supplementary Table 9).
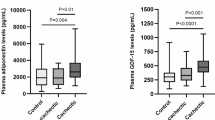
Boxplots showing a log-scale value of serum biomarkers which are significantly different between cachectic and non-cachectic PDAC patients for at least two R/Es stratified into Non-Hispanic Black, Hispanic/Latinx and Non-Hispanic White patients. Significance was determined by Kruskal-Wallis test (BH-adjusted) followed by a BH-adjusted Dunn’s pairwise test (two-sided hypothesis). NHB Non-Hispanic Black, H/L Hispanic/Latinx, NHW Non-Hispanic White, NCa non cachectic, Ca cachectic. Significant differences in serum biomarkers between groups are denoted with a red line. Outliers are denoted by a red dot.
Racial and ethnic variation is apparent for neutrophil- and stress-linked cytokine biomarkers ENA-78/CXCL5 and GRO-α/CXCL1, independent of CCa or pancreas tumor type
A total of 15 biomarkers had significantly different expression levels when comparing NHB, H/L, and NHW PDAC cases with baseline blood (n = 206) (Supplementary Table 10). 2 of the 13 biomarkers were significantly elevated in NHB participants compared to both NHW and H/L cases and included GRO-α/CXCL1 (P < 0.001) and ENA-78/CXCL5 (P < 0.001) (Supplementary Table 10, Fig. 3A).
A Boxplots showing a log-scale value of select significant biomarkers when PDAC R&E groups are compared (ENA-78/CXCL5 and GRO-α/CXCL1) and B when stratified into cachexia groups (NHB Non-Hispanic Black, HL Hispanic/Latino, NHW Non-Hispanic White, NCa non cachexia, Ca Cachexia). C Boxplots showing ENA-78 and GRO-alpha levels for non-PDAC diagnoses. D Boxplot of neutrophil to lymphocyte ratio (NLR) for PDAC patients in the study. Significant differences between relevant groups are denoted with a red line. Significance between NHBCa/NHBNCa and all other groups is denoted with a *. Significance was determined by Kruskal-Wallis test (BH-adjusted) followed by a BH-adjusted Dunn’s pairwise test (two-sided hypothesis). N/S not significant. Outliers are denoted by a red dot.
In response to finding higher ENA-78/CXCL5 and GRO-α /CXCL1 in NHB patients, we performed an ad hoc analysis further stratifying by cachexia status for ENA-78/CXCL5 and GRO-α /CXCL1. These analyses revealed that ENA-78/CXCL5 and GRO-α/CXCL1 levels were unchanged for any R&E group regardless of cachexia status31 (Fig. 3B). When using linear regression with either ENA-78/CXCL5 or GRO-α/CXCL1 as response variables and R&E, cachexia status31, tumor stage, gender, and BMI as predictor variables, NHB patients demonstrated significantly increased ENA-78/CXCL5 and GRO-α/CXCL1 compared to other R&E groups (Supplementary Table 11). Furthermore, when ENA-78/CXCL5 and GRO-α/CXCL1 levels were compared by R&E in patients with other pancreatic conditions from the FPC cohort, NHB individuals continued to demonstrate significantly elevated baseline levels of these biomarkers compared to other R&E groups (Fig. 3C).
Since both GRO-α/CXCL1 and ENA-78/CXCL5 are linked to neutrophil function38,39, the serum neutrophil to lymphocyte ratio (NLR) was calculated using abstracted clinical data and tested for differences by R&E using a Kruskal-Wallis test. We found that NHB PDAC patients had significantly lower NLR than H/L and NHW patients (P < 0.001 for both pairwise comparisons; Fig. 3D). Our data indicate that both higher levels in total lymphocyte count and lower levels in neutrophil count in NHB participants are responsible for this measure.
Survival is predicted by inflammatory and proliferative cytokine levels
Among all biomarkers that were significantly different in Ca vs NCa PDAC patients (based on the Fearon crtieria31), GRO-α, CRP, TNF-α, GDF-15, HGF, IL-10, IL-6, IL-8, and TIMP-1 were independent predictors of all-cause mortality in the PDAC cohort as continuous variables in univariate analyses (Supplementary Table 12). However, after dichotomizing these analytes via a median split and controlling for BMI, R&E, stage, cachexia status, gender, diabetes and age at diagnosis (Fig. 4A), GDF-15, IL-6 and tumor stage remained significant predictors of all-cause mortality. Furthermore, when dichotomized at the median, high GDF-15 and high IL-6 are associated with shorter overall survival (Log-rank P = 0.003 and P < 0.001 for GDF-15 and IL-6, respectively; Fig. 4B, C).
A Forest plot of dichotomized analytes found to be associated with survival as continuous variables in a univariate analysis when controlled for demographic and patho-clinical variables. NHB Non-Hispanic Black, H/L Hispanic/Latinx, NHW Non-Hispanic White, NCa non cachectic, Ca Cachectic. P-values represent a pairwise comparison of the adjusted CoxPH model relative to the reference group (two-sided hypothesis). Median levels of GDF-15 (B) or IL-6 (C) were calculated and participants were classed into either low (Low) or high (High) groups based on a median split. B, C represent Kaplan-Meier survival curves (Time = time to event in months) for GDF-15 (P = 0.003) and IL-6 (P < 0.0001), respectively. P-values represent global p-values of the survival analysis (two-sided hypothesis).
Discussion
The FPC cohort represents one of the most racially and ethnically diverse group of PDAC and patients with other pancreatic-related conditions, with CCa status and available serum biomarker data analyzed to date18,19,20,21,22,23,24,25,40,41,42,43,44.
In the current investigation, GDF-15, also known as macrophage inhibitory cytokine 1 (MIC1), was identified as an important marker of pre-cachexia which continues to increase through the cachexia continuum, and which predicts survival among male and female H/L and NHW participants. GDF-15 is a pleiotropic molecule involved in the pathophysiology of cancers and other conditions and is tightly linked to stress pathways45,46,47. It is known as a key biomarker of stress, dysfunctional metabolism and energy production and has emerged as an attractive therapeutic target for obesity/metabolic disorders, cachexia, and immunotherapy35,48,49. Anti-GDF-15 monoclonal antibody treatment with ponsegromab is both well tolerated and may reverse anti-PD-L1 resistance in clinical trials50,51, thus this treatment modality may be useful in contexts other than CCa. In contrast to others52, we did not observe sexual dimorphism in cachexia-related GDF-15 upregulation53. Importantly, this molecule is found to be upregulated in the blood of patients with both early (I/II) and late (III/IV) stage tumors, suggesting that increases in GDF-15 are not secondary to cancer progression. Furthermore, our findings suggest that GDF-15 levels should be interpreted with caution in the NHB population as this molecule may not be useful as a marker for cachexia due to the possibility of false negatives and given that GDF-15 did not significantly differ by cachexia status in NHB patients. Importantly, GDF-15 was one of only two markers which was predictive of “pre-cachexia” status.
Early identification and intervention for cancer-related cachexia is key to alleviating the negative outcomes associated with this condition5,21,30,34,52,54. Ponsegromab has shown great promise in clinical trials35,50 but, notably, this trial did not include any NHB individuals. The Phase II trial of Ponsegromab used elevated GDF-15 ( > 1500 pg/mL) as an inclusion criteria. The observed difference in GDF-15 levels by R&E in cachectic patients may therefore explain the lack of inclusion of NHB individuals in this trial. Furthermore, our ROC results suggest that the Youden threshold for classification of cachexia with GDF-15 (2045 pg/mL) is somewhat higher than the inclusion cutoffs used in this trial. Importantly, in our PDAC cohort, patients with levels >1500 pg/mL at baseline are at greater risk for losing weight in the upcoming months. Thus, these findings not only underscore the importance of minority inclusion in clinical trials but are valuable from an interventional standpoint to identify H/L and NHW populations with pre-cachexia.
For non-NHB PDAC patients, our findings suggest that GDF-15 can be used to differentiate early cachexia from non-cachexia. Further cementing our findings, high levels of GDF-15 were found to predict greater weight loss in the 6 months after assessment. Collectively, our findings suggest that GDF-15, while less predictive of survival outcomes (than e.g. IL-6), has utility as an early marker of metabolic stress and can be useful to predict weight loss. Thus, there may be a clinical benefit to evaluating GDF-15 levels as soon as possible after a probable PDAC diagnosis is made to alert clinicians of potential future weight loss and supportive care needs necessitating intervention (Fig. 5, created in biorender.com). However, this benefit would only be for NHW and H/L patients until a more reliable biomarker of cachexia can be discerned for NHB patients.
The role of GDF-15 and race is understudied. However, some studies, including a prostate cancer risk study by Rybicki et al.55, observed lower GDF-15 expression in male NHB participants. Contrary to these findings, a 2012 study identified Black race as being predictive of higher plasma GDF-15 levels in a study of atherosclerosis56. Hence, this work represents the first time that GDF-15 has been studied in the context of a response to metabolic stress (cachexia) and race. Collectively, these findings suggest that monitoring of weight/weight loss is paramount in the diagnosis of CCa in NHB patients, as biomarkers of CCa for this population remain elusive.
One intriguing finding that emerged from this study is that, although TNF-α and IL-22 did not reach significance for the smaller NHB group in our PDAC cohort, the means did trend upwards for these two markers in cachectic NHB patients, in stark contrast to GDF-15 and TIMP-1. Thus, these molecules may warrant further investigation as racially agnostic indicators of metabolic stress. TNF-α may be of special interest, as this molecule has long been studied as a metabolic stress and inflammatory cytokine57,58. Our data demonstrate that NHB and H/L participants have slightly higher circulating TNF-α levels than NHW participants, concordant with published literature and secondary to increased chronic or acute social stressors experienced by minority groups that increase inflammation59,60. Higher baseline levels of TNF-α in minorities may thus serve to blunt increases in this cytokine related to cachectic stress.
Another molecule we found to be predictive of PCa is the tissue inhibitor of metalloproteinases, TIMP-161. TIMP-1 is a pro-inflammatory cytokine associated with proliferative processes61. Although TIMP-1 is not known to be associated with GDF-15 activity in cancer, TIMP-1 and GDF-15 were weakly correlated in a study of cardiovascular disease in aging patients, suggesting a prognostic use for these markers in a fingerprint62 to better predict cachexia status.
Other main findings are the distinct racial differences in the aging/inflammatory biomarkers ENA-78/CXCL5 and GRO-α/CXCL120,40,63. ENA-78/CXCL5 and GRO-α/CXCL1 are of particular interest, as they are upregulated in NHB patients independent of CCa status and pancreas-related diagnosis. ENA-78/CXCL5 is a member of the CXC chemokine family and is involved in neutrophil signaling and recruitment64. Similarly, the chemokine CXCL1 is an angiogenic inflammatory marker which has been linked to neutrophil recruitment and migration of adipose stromal cells into the tumor microenvironment65. ENA-78/CXCL5 has been shown to promote pancreatic cancer cell growth, migration, and invasion, and to predict poor prognosis66,67,68. Other findings indicate that GRO-α/CXCL1 and ENA-78/CXCL5 are associated with stress due to social disruption, adult adversity and/or psychological comorbidity in chronic pain disorders69,70. Moreover, these signaling molecules have been associated with PDAC immune evasion67,71, lending credence to a racially unbalanced CXCL1/5-signaling “tumor-friendly” environment. We find that a higher circulating CXCL1/ENA-78/CXCL5 signature is associated with the NHB race, but not with higher circulating neutrophils in NHB patients in agreement with published literature72, suggesting that this minority group may be desensitized to cytokine-mediated neutrophil mobilization or signaling.
Interestingly, the cytokine in our panel with the strongest association with overall survival was IL-6. Consistent with other reports, IL-6 was strongly associated with worse survival in PDAC patients in our study73, suggesting a renewed interest in this prognostic marker may be warranted.
Our cohort of patients was recruited not only from academic centers, but also from community hospitals in the area29,74 representing a more “real-world” scenario regarding choice of therapy, outcomes, and racial and ethnic diversity than many previous studies. Nevertheless, a limitation of the study is that none of the participants were “normal” healthy controls because all participants presented with a suspicion of a pancreatic mass. In addition, we note large variability in analyte levels (including GDF-15 levels)35, suggesting that GDF-15 is not universally upregulated during the cachectic state. Nevertheless, all reliably dysregulated biomarkers chosen for further evaluation from our panel, including GDF-15, had an intra assay variability of <20%. Furthermore, we note that decreased survival for patients with high IL-6 or high GDF-15 could plausibly be due to other tumor-related characteristics. Finally, although our PDAC cohort was more diverse than others, including clinical trials of cachexia therapies, there were only 30 NHB PDAC patients with baseline blood available. Thus, sample size is a limitation for this study, especially considering multiple stratification strategies, and larger follow-up studies are needed to validate these findings. We argue that trials aimed at specifically recruiting minority populations are crucial to fully understanding the nature of racial disparities in CCa and PDAC and that eligibility criteria may perpetuate these disparities due to lack of minority enrollment and participation75. Future studies by this group will investigate a possible link between adipose tissue deposition, cytokine signaling, race and PDAC progression.
Conclusions
GDF-15 has emerged as a promising cachexia biomarker and is specifically increased prior to weight loss for non-NHB PDAC patients. Furthermore, although GDF-15 did not associate with cachexia status/weight loss in NHB patients, it was associated with survival even when controlled for R&E. Future work should endeavor to ensure that minority participants are well represented in PDAC cohorts so that the true biomarker landscape is evaluated from diverse populations. Furthermore, these studies suggest that gender should be included in any cachexia biomarker evaluation.
In addition to our GDF-15 findings, we report that the serum cytokine landscape for NHB PDAC patients is decidedly neutrophil-linked and pro-inflammatory compared to either other minorities (H/L participants) or NHW participants, but that circulating neutrophils are lower in the NHB individuals. Caution should be taken in interpreting these findings, however, to avoid over-simplifying the complex interplay between genetic background, social stressors, R&E, and the metabolic stress response resulting from cancer-associated WL.
Data availability
A source file of all analyte levels, neutrophil and lymphocyte counts, longitudinal weight loss, cachexia status, diagnosis and survival data for all patients with baseline blood available for analysis (including non-PDAC diagnoses) can be found in Supplementary Dataset 1. Source data for Figs. 1–4 can be accessed in Supplementary Dataset 1.
References
Rahib, L., Wehner, M. R., Matrisian, L. M. & Nead, K. T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 4, e214708 (2021).
Poulia, K. A. et al. Pancreatic cancer and cachexia-metabolic mechanisms and novel insights. Nutrients 12 (2020).
Kordes, M., Larsson, L., Engstrand, L. & Lohr, J. M. Pancreatic cancer cachexia: three dimensions of a complex syndrome. Br. J. Cancer 124, 1623–1636 (2021).
Takeda, T. et al. The impact of cachexia and sarcopenia in elderly pancreatic cancer patients receiving palliative chemotherapy. Int J. Clin. Oncol. 26, 1293–1303 (2021).
Fearon, K., Arends, J. & Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 10, 90–99 (2013).
O’Connell, T. M. et al. Metabolic biomarkers for the early detection of cancer cachexia. Front Cell Dev. Biol. 9, 720096 (2021).
Chen, X. Y. et al. A comparison of four common malnutrition risk screening tools for detecting cachexia in patients with curable gastric cancer. Nutrition 70, 110498 (2020).
Penna, F. et al. Autophagy exacerbates muscle wasting in cancer cachexia and impairs mitochondrial function. J. Mol. Biol. 431, 2674–2686 (2019).
Han, J., Meng, Q., Shen, L. & Wu, G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 17, 14 (2018).
McMillan, D. C. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat. Rev. 39, 534–540 (2013).
Pointer, D. T. Jr. et al. Neutrophil to lymphocyte ratio, not platelet to lymphocyte or lymphocyte to monocyte ratio, is predictive of patient survival after resection of early-stage pancreatic ductal adenocarcinoma. BMC Cancer 20, 750 (2020).
Nakamura, Y. et al. Neutrophil/lymphocyte ratio has a prognostic value for patients with terminal cancer. World J. Surg. Oncol. 14, 148 (2016).
Menter, D. G. et al. Platelet “first responders” in wound response, cancer, and metastasis. Cancer Metastasis Rev. 36, 199–213 (2017).
Liu, Y. et al. Association between platelet count with 1-year survival in patients with cancer cachexia. J. Cancer 12, 7436–7444 (2021).
Klocker, E. V. et al. Decreased activity of circulating butyrylcholinesterase in blood is an independent prognostic marker in pancreatic cancer patients. Cancers 12, 1154 (2020).
Koh, H. H. et al. Association of albumin-bilirubin grade and myosteatosis with its prognostic significance for patients with colorectal cancer. Ann. Surg. Oncol. 29, 3868–3876 (2022).
Li, X. R. et al. Association of serum total bilirubin with survival outcomes in patients with cancer cachexia: a prospective, multicenter cohort study. Nutrition 102, 111711 (2022).
Sato, K. et al. Pentraxin 3 is an adipose tissue-related serum marker for pancreatic cancer cachexia predicting subsequent muscle mass and visceral fat loss. Cancer Sci. 113, 4311–4326 (2022).
Tsai, V. W., Brown, D. A. & Breit, S. N. Targeting the divergent TGFbeta superfamily cytokine MIC-1/GDF15 for therapy of anorexia/cachexia syndromes. Curr. Opin. Support Palliat. Care 12, 404–409 (2018).
Paval, D. R. et al. A systematic review examining the relationship between cytokines and cachexia in incurable cancer. J. Cachexia Sarcopenia Muscle 13, 824–838 (2022).
Talbert, E. E. et al. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naive pancreatic cancer patients. J. Cachexia Sarcopenia Muscle 9, 358–368 (2018).
Lewis, H. L. et al. Perioperative cytokine levels portend early death after pancreatectomy for ductal adenocarcinoma. J. Surg. Oncol. 117, 1260–1266 (2018).
Babic, A. et al. Postdiagnosis loss of skeletal muscle, but not adipose tissue, is associated with shorter survival of patients with advanced pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 28, 2062–2069 (2019).
Cao, Z., Zhao, K., Jose, I., Hoogenraad, N. J. & Osellame, L. D. Biomarkers for cancer cachexia: a mini review. Int. J. Mol. Sci. 22, 4501 (2021).
Dong, J. et al. Serum insulin-like growth factor binding protein 2 levels as biomarker for pancreatic ductal adenocarcinoma-associated malnutrition and muscle wasting. J. Cachexia Sarcopenia Muscle 12, 704–716 (2021).
Suzuki, R. et al. Diagnostic yield of endoscopic retrograde cholangiopancreatography-based cytology for distinguishing malignant and benign intraductal papillary mucinous neoplasm: systematic review and meta-analysis. Dig. Endosc. 26, 586–593 (2014).
Fogelman, D. R. et al. A predictive model of inflammatory markers and patient-reported symptoms for cachexia in newly diagnosed pancreatic cancer patients. Support Care Cancer 25, 1809–1817 (2017).
Siegel, R. L., Kratzer, T. B., Giaquinto, A. N., Sung, H. & Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 75, 10–45 (2025).
Permuth, J. B. et al. The florida pancreas collaborative next-generation biobank: infrastructure to reduce disparities and improve survival for a diverse cohort of patients with pancreatic cancer. Cancers 13, 809 (2021).
Vigano, A. A. L. et al. Use of routinely available clinical, nutritional, and functional criteria to classify cachexia in advanced cancer patients. Clin. Nutr. 36, 1378–1390 (2017).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Martin, L. et al. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 33, 90–99 (2015).
Robin, X. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinforma. 12, 77 (2011).
Vigano, A. L. et al. The abridged patient-generated subjective global assessment is a useful tool for early detection and characterization of cancer cachexia. J. Acad. Nutr. Diet. 114, 1088–1098 (2014).
Groarke, J. D. et al. Ponsegromab for the treatment of cancer cachexia. N. Engl. J. Med. (2024).
Li, J. C. A. Modeling survival data: Extending the Cox model. Sociol. Methods Res. 32, 117–120 (2003).
Permuth, J. B. et al. Leveraging real-world data to predict cancer cachexia stage, quality of life, and survival in a racially and ethnically diverse multi-institutional cohort of treatment-naïve patients with pancreatic ductal adenocarcinoma. Front. Oncol. 14 (2024).
De Filippo, K. et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121, 4930–4937 (2013).
Li, H., Li, J., Bai, Z., Yan, S. & Li, J. Collagen-induced DDR1 upregulates CXCL5 to promote neutrophil extracellular traps formation and Treg infiltration in breast cancer. Int Immunopharmacol. 120, 110235 (2023).
Fong, Y. et al. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am. J. Physiol. 256, R659–R665 (1989).
Felix, K. et al. Identification of serum proteins involved in pancreatic cancer cachexia. Life Sci. 88, 218–225 (2011).
Sutandyo, N. The role of microRNA in cancer cachexia and muscle wasting: a review article. Casp. J. Intern Med 12, 124–128 (2021).
Loumaye, A. & Thissen, J. P. Biomarkers of cancer cachexia. Clin. Biochem 50, 1281–1288 (2017).
Li, X., Du, L., Liu, Q. & Lu, Z. MicroRNAs: novel players in the diagnosis and treatment of cancer cachexia (Review). Exp. Ther. Med. 24, 446 (2022).
Molfino, A. et al. Association between growth differentiation factor-15 (GDF-15) serum levels, anorexia and low muscle mass among cancer patients. Cancers 13, 99 (2020).
Lerner, L. et al. Growth differentiating factor-15 (GDF-15): A potential biomarker and therapeutic target for cancer-associated weight loss. Oncol. Lett. 12, 4219–4223 (2016).
Alserawan, L. et al. Growth differentiation factor 15 (GDF-15): a novel biomarker associated with poorer respiratory function in COVID-19. Diagnostics 11 (2021).
Varhaug, K. N., Hikmat, O., Nakkestad, H. L., Vedeler, C. A. & Bindoff, L. A. Serum biomarkers in primary mitochondrial disorders. Brain Commun. 3, fcaa222 (2021).
Haake, M. et al. Tumor-derived GDF-15 blocks LFA-1 dependent T cell recruitment and suppresses responses to anti-PD-1 treatment. Nat. Commun. 14, 4253 (2023).
Groarke, J. D. et al. Phase 2 study of the efficacy and safety of ponsegromab in patients with cancer cachexia: PROACC-1 study design. J. Cachexia Sarcopenia Muscle 15, 1054–1061 (2024).
Crawford, J. et al. A Phase Ib First-In-Patient Study Assessing the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Ponsegromab in Participants with Cancer and Cachexia. Clin. Cancer Res. 30, 489–497 (2024).
Chakedis, J. M. et al. Identification of circulating plasma ceramides as a potential sexually dimorphic biomarker of pancreatic cancer-induced cachexia. JCSM Rapid Commun. 5, 254–265 (2022).
Zhong, X. et al. Sex specificity of pancreatic cancer cachexia phenotypes, mechanisms, and treatment in mice and humans: role of Activin. J. Cachexia Sarcopenia Muscle 13, 2146–2161 (2022).
Park, M. A. et al. Defining and addressing research priorities in cancer cachexia through transdisciplinary collaboration. Cancers 16 (2024).
Rybicki, B. A. et al. Growth and differentiation factor 15 and NF-kappaB expression in benign prostatic biopsies and risk of subsequent prostate cancer detection. Cancer Med 10, 3013–3025 (2021).
Rohatgi, A. et al. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: observations from the Dallas Heart Study. Clin. Chem. 58, 172–182 (2012).
Silva, C. L. & Faccioli, L. H. Tumor necrosis factor (cachectin) mediates induction of cachexia by cord factor from mycobacteria. Infect. Immun. 56, 3067–3071 (1988).
Yehia, R. et al. Impact of TNF-alpha gene polymorphisms on pancreatic and non-small cell lung cancer-induced cachexia in adult Egyptian patients: a focus on pathogenic trajectories. Front Oncol. 11, 783231 (2021).
Gillespie, S. L. et al. Lifetime stressor exposure, systemic inflammation during pregnancy, and preterm birth among Black American women. Brain Behav. Immun. 101, 266–274 (2022).
Strahler, J., Rohleder, N. & Wolf, J. M. Acute psychosocial stress induces differential short-term changes in catecholamine sensitivity of stimulated inflammatory cytokine production. Brain Behav. Immun. 43, 139–148 (2015).
Ma, B. et al. TIMP1 promotes cell proliferation and invasion capability of right-sided colon cancers via the FAK/Akt signaling pathway. Cancer Sci. 113, 4244–4257 (2022).
Eggers, K. M. et al. Relations of growth-differentiation factor-15 to biomarkers reflecting vascular pathologies in a population-based sample of elderly subjects. Scand. J. Clin. Lab Invest 72, 45–51 (2012).
Zhu, Z., Tang, C., Xu, T. & Zhao, Z. Molecular Analysis of Prognosis and Immune Pathways of Pancreatic Cancer Based on TNF Family Members. J. Oncol. 2021, 2676996 (2021).
Deng, J. et al. DDR1-induced neutrophil extracellular traps drive pancreatic cancer metastasis. JCI Insight 6 (2021).
Zhang, T. et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat. Commun. 7, 11674 (2016).
Ando, Y. et al. Necroptosis in pancreatic cancer promotes cancer cell migration and invasion by release of CXCL5. PLoS One 15, e0228015 (2020).
Walsh, R. M. et al. Adipose-tumor crosstalk contributes to CXCL5 mediated immune evasion in PDAC. bioRxiv (2023).
Li, A. et al. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am. J. Pathol. 178, 1340–1349 (2011).
Lin, J. et al. Proinflammatory phenotype in major depressive disorder with adulthood adversity: In line with social signal transduction theory of depression. J. Affect Disord. 341, 275–282 (2023).
Karshikoff, B. et al. Inflammatory blood signature related to common psychological comorbidity in chronic pain. Biomedicines 11 (2023).
Kemp, S. B. et al. Apolipoprotein E promotes immune suppression in pancreatic cancer through NF-kappaB-mediated production of CXCL1. Cancer Res 81, 4305–4318 (2021).
Hsieh, M. M. et al. Neutrophil count in African Americans: lowering the target cutoff to initiate or resume chemotherapy?. J. Clin. Oncol. 28, 1633–1637 (2010).
Fan, M. et al. Atractylenolide I ameliorates cancer cachexia through inhibiting biogenesis of IL-6 and tumour-derived extracellular vesicles. J. Cachexia Sarcopenia Muscle 13, 2724–2739 (2022).
Permuth, J. B. et al. Racial and ethnic disparities in a state-wide registry of patients with pancreatic cancer and an exploratory investigation of cancer cachexia as a contributor to observed inequities. Cancer Med 8, 3314–3324 (2019).
Riner, A. N. et al. Eligibility criteria perpetuate disparities in enrollment and participation of black patients in pancreatic cancer clinical trials. J. Clin. Oncol. 40, 2193–2202 (2022).
Acknowledgements
This work was supported in part by the James and Esther King Biomedical Research Program, Florida Department of Health (Grants #8JK02 awarded to J.B.P. and J.G.T.; #24K03 awarded to J.B.P. and A.R.J.), the Department of Defense Health Program Congressionally Directed Medical Research Program (W81XWH-22-1-1021 LOG#PA210192; awarded to J.B.P., J.B.F., and M.M.), the Tissue Core Facility, the Cancer Pharmacokinetics and Pharmacodynamics Core (PK/PD) Core Facility, and the Biostatistics and Bioinformatics Shared Resource at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). Research reported in this publication was performed in part at the Biospecimen Shared Resource (BSSR) of the Sylvester Comprehensive Cancer Center at the University of Miami, RRID: SCR_022889, which is also supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award number P30CA240139. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors or the participating institutions.
Author information
Authors and Affiliations
Contributions
J.B.P. conceived the study jointly with A.R.J. M.A.P. and E.W.D. designed and implemented the analyses and prepared the manuscript; Q.M. provided statistical support, A.B.M. and S.S. performed the cytokine assays, K.J. provided pathology analyses. M.A.P., E.W.D., S.A., J.P.A., T.L.B., M.B., T.L.C, T.B.deC., D.T.C., E.M.C., S.L.C., M.G.D. W.G.D., J.B.F., P.H., K.L.H., K.J., D.W.K., J.K., A.K.L., M.M., A.A.M., R.M., N.B.M., K.M., Q.M., M.A.M-V., L.M-U., K.D.O., N.P., J.M.P., G.R., K.A.R., L.M.S., P.S., A.F.T., J.K.T., D.V.T., J.G.T., V.V., C.J.W., D.J., S.M.J., A.R.J., and J.B.P. edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
J.M.P. is a consultant for ASTELLAS, J&J Worldwide, Ferranova, and ADVOCARE. All other authors noted that they have no conflicts or perceived conflicts of interest related to this work.
Peer review
Peer review information
Communications Medicine thanks Jonathan Blackburn, Sidsel Christy Lindgaard and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, M.A., Davis, E.W., Alhassan, S. et al. Race-based differences in serum biomarkers for cancer-associated cachexia in a diverse cohort of patients with pancreatic ductal adenocarcinoma. Commun Med 6, 19 (2026). https://doi.org/10.1038/s43856-025-01277-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-01277-9