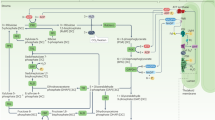
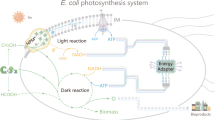
Abstract
Achieving high yields of chemicals via biosynthesis is desirable but challenging because only a small amount of energy molecules (such as adenosine triphosphate (ATP), reduced nicotinamide adenine dinucleotide (NADH) and reduced nicotinamide adenine dinucleotide phosphate (NADPH)) within microorganisms are utilized for target chemical production. Drawing inspiration from the ability of plant-derived thylakoid to convert solar energy into energy molecules, an Escherichia coli–thylakoid hybrid with a dual-channel energy pathway combining energy molecule supply and electron transfer was created by implanting thylakoid in E. coli. Under light, photoelectrons produced in thylakoid were directly utilized to synthesize ATP and NADPH, which were then supplied to E. coli. Photoelectrons from thylakoid can transport and be captured by redox mediators, elevating the level of ATP and NADPH by facilitating the electron transport chain of E. coli. This dual-channel energy pathway boosted the level of energy molecules, enabling the E. coli–thylakoid hybrid to achieve an impressive H2 production rate of \(15.1\,{\mathrm{mmol}}\,{\mathrm{h}}^{-1}\,{\mathrm{g}}_{\mathrm{dcw}}^{-1}\) (dcw, dry cell weight), comparable to top-performing E. coli-based systems. Biogenic thylakoid presented excellent biocompatibility and the E. coli–thylakoid hybrid did not exhibit oxidative stress.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated in this study are provided in the Supplementary Information and Source Data files. Source data are provided with this paper.
References
Sakimoto, K. K., Wong, A. B. & Yang, P. D. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 351, 74–77 (2016).
Kalathil, S. et al. Solar-driven methanogenesis through microbial ecosystem engineering on carbon nitride. Angew. Chem. Int. Ed. 63, e202409192 (2024).
Wang, Q., Kalathil, S., Pornrungroj, C., Sahm, C. D. & Reisner, E. Bacteria–photocatalyst sheet for sustainable carbon dioxide utilization. Nat. Catal. 5, 633–641 (2022).
Xu, Z. et al. Algal cell bionics as a step towards photosynthesis-independent hydrogen production. Nat. Commun. 14, 1872 (2023).
Koh, S. et al. Light-driven ammonia production by Azotobacter vinelandii cultured in medium containing colloidal quantum dots. J. Am. Chem. Soc. 144, 10798–10808 (2022).
Niu, Y. et al. Sustainable power generation from sewage with engineered microorganisms as electrocatalysts. Nat. Sustain. 7, 1182 (2024).
Guo, J. et al. Light-driven fine chemical production in yeast biohybrids. Science 362, 813–816 (2018).
Reimer, C. et al. Engineering the amoeba Dictyostelium discoideum for biosynthesis of a cannabinoid precursor and other polyketides. Nat. Biotechnol. 40, 751–758 (2022).
Zhang, Z. et al. Systems engineering of Escherichia coli for high-level glutarate production from glucose. Nat. Commun. 15, 1032 (2024).
Ren, M. & Schada von Borzyskowski, L. Two modules for biosynthesis from CO2. Nat. Synth. 2, 906–908 (2023).
Li, C., Yin, L., Wang, J., Zheng, H. & Ni, J. Light-driven biosynthesis of volatile, unstable and photosensitive chemicals from CO2. Nat. Synth. 2, 960–971 (2023).
Giger, G. H. et al. Inducing novel endosymbioses by implanting bacteria in fungi. Nature 635, 415–422 (2024).
Reiter, M. A. et al. A synthetic methylotrophic Escherichia coli as a chassis for bioproduction from methanol. Nat. Catal. 7, 560–573 (2024).
Yang, Y., Liu, L.-N., Tian, H., Cooper, A. I. & Sprick, R. S. Making the connections: physical and electric interactions in biohybrid photosynthetic systems. Energy Environ. Sci. 16, 4305–4319 (2023).
Walker, K. T. et al. Self-pigmenting textiles grown from cellulose-producing bacteria with engineered tyrosinase expression. Nat. Biotechnol. 43, 345–354 (2024).
Tang, H., Lin, S., Deng, J., Keasling, J. D. & Luo, X. Engineering yeast for the de novo synthesis of jasmonates. Nat. Synth. 3, 224–235 (2024).
Guan, X., Xie, Y. & Liu, C. Performance evaluation and multidisciplinary analysis of catalytic fixation reactions by material–microbe hybrids. Nat. Catal. 7, 475–482 (2024).
Reis, A. C. et al. Simultaneous repression of multiple bacterial genes using nonrepetitive extra-long sgRNA arrays. Nat. Biotechnol. 37, 1294–1301 (2019).
Liang, J. et al. Revisiting solar energy flow in nanomaterial–microorganism hybrid systems. Chem. Rev. 124, 9081 (2024).
Guan, X. et al. Maximizing light-driven CO2 and N2 fixation efficiency in quantum dot–bacteria hybrids. Nat. Catal. 5, 1019–1029 (2022).
Hu, G. et al. Light-driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals. Nat. Catal. 4, 395–406 (2021).
Wu, D., Zhang, W., Fu, B. & Zhang, Z. Living intracellular inorganic-microorganism biohybrid system for efficient solar hydrogen generation. Joule 6, 2293–2303 (2022).
Zhang, Y., Feng, T., Zhou, X. & Zhang, Z. Photoelectrocatalytic‐microbial biohybrid for nitrogen reduction. Adv. Mater. 63, 2407239 (2024).
Ye, J. et al. Sustainable conversion of microplastics to methane with ultrahigh selectivity by a biotic–abiotic hybrid photocatalytic system. Angew. Chem. Int. Ed. 61, e202213244 (2022).
Li, W. et al. Enhanced light-harvesting and energy transfer in carbon dots embedded thylakoids for photonic hybrid capacitor applications. Angew. Chem. Int. Ed. 136, e202308951 (2024).
Chen, P. et al. A plant-derived natural photosynthetic system for improving cell anabolism. Nature 612, 546–554 (2022).
Zhang, J. Z. & Reisner, E. Advancing photosystem II photoelectrochemistry for semi-artificial photosynthesis. Nat. Rev. Chem. 4, 6–21 (2019).
Zan, J. et al. A microbial factory for defensive kahalalides in a tripartite marine symbiosis. Science 364, 1056–1066 (2019).
Zhao, H. et al. Biomimetic 4D-printed breathing hydrogel actuators by nanothylakoid and thermoresponsive polymer networks. Adv. Funct. Mater. 31, 2105544 (2021).
Gao, F. et al. Artificial photosynthetic cells with biotic–abiotic hybrid energy modules for customized CO2 conversion. Nat. Commun. 14, 6783 (2023).
Su, Z., Zhang, X., Wang, W., Dong, M. & Han, X. Light‐harvesting artificial cells for the generation of ATP and NADPH. Chin. J. Chem. 41, 57–63 (2022).
Wang, W. et al. Light‐driven carbon fixation using photosynthetic organelles in artificial photosynthetic cells. Angew. Chem. Int. Ed. 64, e202421827 (2025).
Gwon, H. J., Park, G., Yun, J., Ryu, W. & Ahn, H. S. Prolonged hydrogen production by engineered green algae photovoltaic power stations. Nat. Commun. 14, 6768 (2023).
Zhou, W. et al. External electrons directly stimulate Escherichia coli for enhancing biological hydrogen production. ACS Nano 18, 10840–10849 (2024).
Yan, J. et al. Rapidly inhibiting the inflammatory cytokine storms and restoring cellular homeostasis to alleviate sepsis by blocking pyroptosis and mitochondrial apoptosis pathways. Adv. Sci. 10, 2207448 (2023).
Zhu, X. et al. Photosynthesis-mediated intracellular biomineralization of gold nanoparticles inside chlorella cells towards hydrogen boosting under green light. Angew. Chem. Int. Ed. 62, e202308437 (2023).
Tong, L. et al. Atomically precise regulation of the N-heterocyclic microenvironment in triazine covalent organic frameworks for coenzyme photocatalytic regeneration. J. Am. Chem. Soc. 146, 21025–21033 (2024).
Tian, S. et al. A coupled system of Ni3S2 and Rh complex with biomimetic function for electrocatalytic 1,4-NAD(P)H regeneration. J. Am. Chem. Soc. 146, 15730–15739 (2024).
Han, H.-X. et al. Reversing electron transfer chain for light-driven hydrogen production in biotic–abiotic hybrid systems. J. Am. Chem. Soc. 144, 6434–6441 (2022).
Ma, J.-Y. et al. A hybrid photocatalytic system enables direct glucose utilization for methanogenesis. Proc. Natl Acad. Sci. USA 121, e2317058121 (2024).
Chen, Y. et al. Electric-field-driven redistribution of carriers on catalyst particles to improve photocatalytic performance. J. Am. Chem. Soc. 146, 31456–31463 (2024).
Li, J. et al. A self-assembled MOF–Escherichia coli hybrid system for light-driven fuels and valuable chemicals synthesis. Adv. Sci. 11, 2308597 (2024).
Chen, N. et al. A photosynthesis-derived bionic system for sustainable biosynthesis. Angew. Chem. Int. Ed. 64, e202414981 (2025).
Tan, H. et al. Photocatalysis of water into hydrogen peroxide over an atomic Ga-N5 site. Nat. Synth. 2, 557–563 (2023).
Liu, Y. et al. Enhanced hydrogen peroxide photosynthesis in covalent organic frameworks through induced asymmetric electron distribution. Nat. Synth. 4, 134–141 (2024).
Chen, Y. et al. Hierarchical assembly of donor–acceptor covalent organic frameworks for photosynthesis of hydrogen peroxide from water and air. Nat. Synth. 3, 998–1010 (2024).
Li, Z. et al. Multi‐enzyme mimetic MoCu dual‐atom nanozyme triggering oxidative stress cascade amplification for high‐efficiency synergistic cancer therapy. Angew. Chem. Int. Ed. 64, e202413661 (2024).
Koo, S. et al. Ceria–vesicle nanohybrid therapeutic for modulation of innate and adaptive immunity in a collagen-induced arthritis model. Nat. Nanotechnol. 18, 1502–1514 (2023).
Yuan, Y. et al. Earth-abundant photocatalyst for H2 generation from NH3 with light-emitting diode illumination. Science 378, 889–893 (2022).
Zhou, P. et al. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 613, 66–70 (2023).
Li, Z. et al. Blocking the reverse reactions of overall water splitting on a Rh/GaN–ZnO photocatalyst modified with Al2O3. Nat. Catal. 6, 80–88 (2023).
Fang, X., Kalathil, S. & Reisner, E. Semi-biological approaches to solar-to-chemical conversion. Chem. Soc. Rev. 49, 4926–4952 (2020).
Asantewaa, G. et al. Glutathione synthesis in the mouse liver supports lipid abundance through NRF2 repression. Nat. Commun. 15, 6152 (2024).
Zhang, L., Morello, G., Carr, S. B. & Armstrong, F. A. Aerobic photocatalytic H2 production by a [NiFe] hydrogenase engineered to place a silver nanocluster in the electron relay. J. Am. Chem. Soc. 142, 12699–12707 (2020).
Miller, T. E. et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts. Science 368, 649–654 (2020).
Ye, J. et al. Methanogenesis in the presence of oxygenic photosynthetic bacteria may contribute to global methane cycle. Nat. Commun. 15, 5682 (2024).
Fang, G. et al. Ultrafine sulfur-doped carbon nanoparticles enhanced the transmembrane bioelectricity of Clostridium butyricum for biohydrogen production. Nano Energy 110, 108382 (2023).
Wang, X.-M. et al. Anaerobic self-assembly of a regenerable bacteria–quantum dot hybrid for solar hydrogen production. Nanoscale 14, 8409–8417 (2022).
Lin, S. et al. Enhancing photocatalytic hydrogen production from engineered Escherichia coli–biohybrid system via intracellular electron redirection. Chem. Eng. J. 499, 156488 (2024).
Ramprakash, B. & Incharoensakdi, A. Encapsulated titanium dioxide nanoparticle–Escherichia coli hybrid system improves light driven hydrogen production under aerobic condition. Bioresour. Technol. 318, 124057 (2020).
Hou, T. et al. Cd1−xZnxS biomineralized by engineered bacterium for efficient photocatalytic hydrogen production. Mater. Today Energy 22, 100869 (2021).
Martins, M., Toste, C. & Pereira, I. A. C. Enhanced light-driven hydrogen production by self-photosensitized biohybrid systems. Angew. Chem. Int. Ed. 60, 9055–9062 (2021).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22134004, B.T.; 2247041268, L.L.), the Taishan Scholars Program of Shandong Province (tstp20250528, L.L.), the Natural Science Foundation of Shandong Province of China (ZR2024QB013, J.A.), the Key R&D Plan of Shandong Province (2021ZDPT01, L.L.) and the Project of Shandong Provincial Center for Fundamental Science Research (YDZX2024150, L.L. and J.A.).
Author information
Authors and Affiliations
Contributions
The project was conceptually designed by B.T., L.L. and J.A. The majority of the experiments were conducted by T.C. and J.A, assisted by F.G and W.Z. Data analysis and interpretation were done by J.A and T.C. The paper was prepared by J.A, L.L and B.T. All authors have given approval to the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Bo Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of thylakoid.
(a) The SDS-PAGE analysis of thylakoid. (b) Calibration curve for DCPIP. Data points are reported as mean ± standard deviation derived from 3 independent experiments (n = 3). The UV-vis absorption intensity of DCPIP in the thylakoid solutions against time under dark (c), with light irradiation (d), with light irradiation and DCMU (e). (f) UV-vis absorption spectroscopy of thylakoid after being placed for five days.
Extended Data Fig. 2 Characterization of E. coli–thylakoid hybrid.
(a) Zeta potential value of E. coli and thylakoid. Data points are reported as mean ± standard deviation derived from 3 independent experiments (n = 3). (b) The UV-vis absorption spectra of E. coli, PDA, and E. coli@PDA. (c) The UV-vis absorption spectra of the E. coli–thylakoid hybrid and E. coli. (d) The CLSM image of E. coli. (e) Bio-TEM image of thin-sectioned E. coli. (f) Bio-TEM image of thin-sectioned the E. coli–thylakoid hybrid.
Extended Data Fig. 3 Light-driven NADPH production in thylakoid.
(a) The UV-vis absorption spectra of NADPH with different concentrations. (b) Calibration curve for NADPH. Data points are reported as mean ± standard deviation derived from 3 independent experiments (n = 3). The UV-Vis absorption spectra of NADPH generated on thylakoid (40 µg Chl·mL−1) against time under dark (c) and light radiation (d) condition. (e) The UV-vis absorption spectra of NADPH generated on thylakoid (80 µg Chl·mL−1) against time under light condition. (f) The UV-vis absorption spectra of NADPH generated on thylakoid (160 µg Chl·mL−1) under light condition.
Extended Data Fig. 4 Electron transfer characteristics between thylakoid and redox mediator.
(a) Photocurrent responses of thylakoid, FAD, and physically mixed group of thylakoid and FAD. (b) Photocurrent responses of thylakoid, coenzymes Q, and mixed group of thylakoid and coenzymes Q. (c) Photocurrent responses of thylakoid and thylakoid@PDA.
Extended Data Fig. 5 H2 production rate of E. coli, E. coli–thylakoid hybrid, and E. coli–thylakoid with DCMU.
Data points are reported as mean ± standard deviation derived from 3 independent experiments (n = 3).
Extended Data Fig. 6 Stability analysis of E. coli–thylakoid.
(a) Fluorescence microscopy image of E. coli and E. coli–thylakoid hybrid after 6 h reaction under real light. Live/dead stained cells via SYTO 9 (green) and PI (red). (b) UV-vis absorption spectroscopy of thylakoid in the E. coli–thylakoid hybrid after 6 h reaction. (c) The 3-dimensional confocal laser scanning microscopy image of the E. coli–thylakoid hybrid after 6 h reaction.
Extended Data Fig. 7 Glucose consumption of E. coli and the E. coli–thylakoid hybrid at different reaction times under light.
Data points are reported as mean ± standard deviation derived from 3 independent experiments.
Supplementary information
Supplementary Information
Supplementary Methods and Tables 1–5.
Source data
Source Data Fig. 2
Unprocessed TEM, SEM and CLSM; UV–vis and flow cytometry.
Source Data Fig. 3
UV–vis, I-T EIS, time-resolved spectroscopy, energy cofactor generation test and Single-molecule fluorescence imaging.
Source Data Fig. 4
Unprocessed SEM and CLSM; UV–vis, ROS test and biocompatibility test.
Source Data Fig. 5
Hydrogen production rate under xenon lamp and sunlight.
Source Data Fig. 6
Transcriptome analysis.
Source Data Extended Data Fig. 1
Unprocessed gel; DCPIP reduction and thylakoid stability.
Source Data Extended Data Fig. 2
Unprocessed CLSM and bio-TEM; zeta potential and UV–vis.
Source Data Extended Data Fig. 3c
UV–vis of NADPH reduction.
Source Data Extended Data Fig. 4
Photocurrent test.
Source Data Extended Data Fig. 5
DCMU inhibits hydrogen production.
Source Data Extended Data Fig. 6
Unprocessed CLSM; UV–vis.
Source Data Extended Data Fig. 7
Glucose consumption result.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
An, J., Chen, T., Ge, F. et al. Dual-channel energy pathway combining energy molecule supply and electron transfer to support solar-to-chemical production in an E. coli–thylakoid hybrid. Nat. Synth 4, 1408–1421 (2025). https://doi.org/10.1038/s44160-025-00853-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00853-0