Abstract
Abdominal aortic aneurysms (AAA), with approximately 200,000 new diagnoses each year, represent a prevalent clinical concern. Current treatment includes monitoring and surgical procedures once the aneurysm reaches a certain size. However, the lack of effective, timely therapies leads to a high mortality rate due to rupture. With recent advancements and innovations in biomedical science, stem cell therapy has moved closer to widespread clinical use, with the field experiencing rapid growth since its inception in the late 20th century. Given the pathophysiology of AAA, stem cell therapies have high potential impact in the treatment for both early and late-stage disease, targeting underlying mechanisms such as inflammation, vascular degeneration, and extracellular matrix degradation. There are many considerations and innovative potential approaches being explored in this type of treatment, such as strategically leveraging cell properties and their associated secretome and incorporating biomaterials-based strategies. This review article summarizes and critically assesses the efficacy of cell-based therapies in AAA preclinical models, current clinical trials in this area, and other emerging bioengineering approaches for the treatment of AAA.
Similar content being viewed by others
Introduction
Abdominal aortic aneurysm (AAA) is a vascular disease consisting of the abnormal dilation in the abdominal aorta diameter and simultaneous weakening of the aortic wall until rupture. AAAs have a prevalence of 150,000–200,000 new diagnoses each year1, with over 35 million active cases world-wide in 20192. Often termed as the “silent killers”, the majority of AAAs are asymptomatic. As imaging has become broadly more common in healthcare check-ups, asymptomatic AAA have been increasingly identified earlier. Otherwise, AAAs tend to be discovered upon rupture, with a rupture mortality rate of approximately 80%3. AAAs are unique and vary in their morphology as well as progression among individuals. The heterogeneity of AAA and the ongoing challenges in diagnosis and understanding pathophysiology contribute to the challenge in both diagnostics and therapeutics of AAA.
Stem cell therapies, whereby stem cells are applied (systemically or locally) to repair tissues, have been an increasingly investigated possibility for AAA. With the term “stem cell” first coined in 1888, stem cell therapy has emerged as a field with major developments in the 20th century4. The first role of stem cells in regenerative medicine was for the first bone marrow transplantation in 19565. Important recent developments include the first isolation of mesenchymal stromal cells (MSCs) in 1991, especially relevant in the cardiovascular field, followed by the discovery of human pluripotent stem cells4. The therapeutic use of these stem cells enables and promises novel advancements in regenerative medicine, addressing the need for precision approaches to complex diseases. For AAA, stem cells can potentially address limitations of conventional treatments by targeting the underlying cellular and molecular mechanisms contributing to wall degradation, inflammation, and the other pathways occurring in the AAA cascade. Stem cell and other cell-based therapies aim to repair or regenerate damaged aortic tissue through various mechanisms, including processes improving regenerative, anti-inflammatory, and matrix-stabilizing effects. Cell-based therapies broadly present a unique advantage over current surgical interventions, which can only stabilize aneurysmal dilation at the late stage without reversing tissue degeneration. The long-term goal and potential of this therapy is to enable effective biological solutions to prevent further growth of AAA. With novel earlier-stage treatment options (currently non-existent in humans), this would dramatically improve both short and long-term outcomes for AAA care.
There are many key considerations in the investigation and development of stem cell-based therapeutics (Fig. 1). The choice of stem cells (e.g., MSCs, adipose-derived stem cells (ADSCs), induced pluripotent stem cells, etc.) directly impacts the efficacy, sourcing, usability, and scalability, which are critical in developing effective technologies. The type of model for in vivo investigation, used for exploring the intricate effects of the different cells as well as possible delivery mechanisms, are also important to understand and establish the underlying mechanisms of the cells and their effects, as well as their limitations and implications for translation to humans. These, in addition to current pre-clinical and clinical studies, other approaches and developments, as well as future perspectives, will be discussed in this review to better understand the current state and promise of cell-based therapies for AAA treatment.
A Therapeutic cell types, their respective sources, and other considerations. B Cell delivery modalities. C Status of relevant clinical trials. D Common animal models and their general techniques. E The aortic wall layers and relevant cells. VSMCs vascular smooth muscle cells, MSCs mesenchymal stromal cells, ADSCs adipose-derived stem cells, iPSCs induced pluripotent stem cells, ECM extracellular matrix, EMT epithelial-to-mesenchymal transition, AAA abdominal aortic aneurysm, IV intravenous, PPE porcine pancreatic elastase, Ang II angiotensin II, ApoE−/− apolipoprotein E−/− Created in BioRender. Badawy, S. (2025) https://BioRender.com/zf2ju2q.
AAA pathology
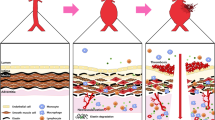
AAA pathophysiology is complex and consists of a cascade of multiple mechanisms with no known individual cause and reasonable contention surrounding the exact etiology (Fig. 2). The abdominal aorta contains VSMCs as a primary component of the medial layer that secrete extracellular matrix (ECM) proteins, such as elastin and collagen, as well as matrix metalloproteinases (MMPs)6,7. This allows for VSMCs to modulate the ECM for repair and degradation. There is evidence that VSMCs in AAA exhibit abnormal behaviour; a study by Airhart et al. shows that AAA VSMCs have greater elastolytic activity and MMP gene expression6. In AAA, inflammatory cells induce VSMC apoptosis, further reducing the structural integrity of the vessel and progressing the disease state7. High numbers of inflammatory macrophages, neutrophils, and helper T cells can be found in AAA lesions8. Studies have shown increased expression of proinflammatory transcription factors and cytokines produced by lymphocytes such as nuclear factor-κB (NF-κB), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α), some of which, when inhibited, have prevented aneurysm progression in animal models8,9,10. VSMCs also exhibit phenotypic switching from a quiescent, contractile phenotype (healthy) to a synthetic phenotype. In this synthetic state, the VSMCs release calcifying signals in the form of extracellular vesicles (EVs). They also move toward the site of injury and show increased proliferation11,12. This phenotypic switch occurs early in AAA development in both humans and mouse models12,13. Additionally, this synthetic/proliferative state has shown decreased SMC marker expression and increased phagocytic marker expression, in addition to upregulated MMP expression and downregulated contractile proteins, leading to vascular dysfunction and contributing to AAA pathogenesis11,13,14.
A The healthy aorta cell environment, with surrounding perivascular adipose tissue (PVAT), compared to (B) the aortic aneurysm environment, characterized by thrombi, infiltration of inflammatory cells, ECM degradation, vascular smooth muscle cell (VSMC) phenotypic switching, apoptosis, excessive cytokine production, reactive oxygen species, and matrix metalloproteinases (MMPs). Reproduced with permission from Lu et al.11.
Moreover, many of the secreted factors by macrophage-like VSMCs form chronic inflammation contributing to the destruction of the aortic wall14. MMPs, which break down the ECM, have been shown to be present at high levels in AAA, specifically in their active forms and with MMP-2 and MMP-9 being particularly relevant in literature. These MMPs are likely contributed by and in response to local inflammatory cytokine production as well as VSMCs6,8,15. Tissue inhibitors of MMPs (TIMPs) are relevant as the negative modulator of MMP activity, both being key factors in AAA pathophysiology, especially in arterial wall integrity16. Oxidative stress and senescence are also relevant to the pathological conditions in AAA11. Due to vascular endothelial growth factor (VEGF) and other marker infiltration in the wall, angiogenesis has been observed at higher rates and contributes to the AAA environment, with neovascularization also being a critical contributor to AAA progression17,18. Ongoing research efforts focus on further understanding the exact mechanical and biological mechanisms and cascade that cause AAAs. Moreover, therapeutic and pharmacological approaches to AAA leverage or manipulate this complex disease environment to remediate AAA growth and progression.
Aortic anatomy and possible targets for therapy
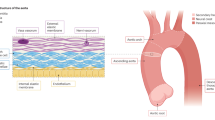
The aortic wall is composed of three tissue layers, the tunica intima, tunica media, and tunica adventitia, respectively from the lumen outwards. These are relevant to understanding the different cell source options and how they may interact with the aortic wall. The innermost intima layer is a thin monolayer of endothelial cells lining the vessel in direct contact with the blood, supported by a basement membrane19. The basement membrane is a highly specialized ECM structure that provides anchoring of the endothelial cells20. This intima layer is most susceptible to injury from atherosclerosis and aortic dissection. The middle “tunica media” layer is the thickest component of the aortic wall, particularly in arteries as opposed to veins21. It is comprised of mainly VSMCs, surrounded by a basal lamina and ECM with proteins including elastin and collagen, among connective tissue components like proteoglycans22. This layer is critical to structural integrity, withstanding the high pressure of the vessel, while retaining elasticity21; it is also responsible for maintaining intravascular pressure and tissue perfusion23. The VSMCs composing this layer are considered the most important cell type in AAA pathogenesis, with other cell types playing relatively minor roles24. The aortic VSMCs are able to contract, proliferate, and regulate extracellular matrix synthesis and degradation23. Given that VSMC dysfunction is prevalent to AAA, creating a stimulating/regenerative therapy for these cells could be a promising target. The outer adventitia layer is a thin layer composed mainly of fibroblasts and collagen fibers. It contains the vasa vasorum, microvessels that supply nutrients and oxygen to the outer layers of the larger vessel walls, and nerves25. This layer has the greatest tensile strength of the three layers due to its collagen-rich composition25.
Initial VSMC therapies for AAA treatment
Since AAA is characterized by the loss of VSMCs, the therapeutic benefit of transplanted VSMCs has been tested in preclinical settings. Implementation of VSMCs for treatment of AAA (Table 1) utilized common experimental models to induce AAA including porcine pancreatic elastase (PPE), Angiotensin II (Ang II) and CaCl2 (Fig. 1D). Allaire et al. examined the effect of VSMC loss in the abdominal aorta on inflammation and formation of AAA. Using guinea pig xenografts seeded with rat VSMCs transplanted into male recipient rats, the authors showed macrophage infiltration into the autograft which is a hallmark of clinical AAA26. The implantation of xenografts seeded with VSMCs group were associated with a significant decrease in aneurysmal growth over time, with only a 35.3 ± 17.8% increase in mean aortic diameter 8 weeks after implantation, compared to xenografts with no cell seeding having a 198.2 ± 106.6% increase26. Animals treated with VSMC-seeded xenografts have also shown reduced elastin degradation, decreased monocyte-macrophage infiltration, and increased production of tissue inhibitor of matrix metalloproteinases (TIMPs), which contributed to reduced dilation26. This study found that MMPs may have been decreased by TIMPs, but was suggested to be decreased at the transcriptional level by VSMCs as well26. However, this study also pointed out that VSMCs alone could not completely prevent dilation26.
In another study, the timing of VSMC delivery was tested in which the cells were injected into xenografts at two weeks post-surgery, during which the aneurysm had already started to form27. Eight weeks post-surgery, the VSMC group aneurysms remained relatively stable with limited growth in aneurysmal diameter and limited decrease in medial elastin content compared to the control group, without cells, and had a marked increase in aneurysmal diameter and decrease in elastin content27. Additionally, VSMC seeding showed a subsequent decrease in mononuclear infiltration as well as key MMP marker mRNA content in the diseased wall, while MMP-inhibitors increased27. Furthermore, VSMCs are sufficient to prevent further expansion in a pre-formed AAA model through prevention of further ECM degradation, notably in terms of elastin content, mainly via regulation of the MMP-dependent proteolytic balance in the aortic wall. Together, these animal studies provide evidence of VSMC therapy on significantly slowing AAA growth and restoring the healing capabilities of proteolytically injured ECM in vivo.
Bridging the path from VSMCs to stem cell-based therapies, the promise of iPSC-derived vascular smooth muscle progenitors, compared to primary human VSMCs, was shown by Mulorz et al. They investigated the localized delivery of both primary human-derived VSMCs and human iPSC-derived smooth muscle progenitors using porous collagen scaffolds to mice AAA with 5×105 cells/scaffold28. Both the iPSC smooth muscle progenitors and VSMCs were validated as phenotypically similar, penetrated approximately 80% into the scaffold, and had no statistical difference in cell viability28. After 28 days from the AAA induction in the mice, the results indicate that the primary VSMC-seeded scaffold showed significantly decreased AAA growth compared to an acellular control and to the iPSC group (at 21 days)28. Moreover, 87% of the mice treated with the primary VSMC-seeded scaffolds were free from AAA compared to 33% in the iPSC-seeded group and no significant difference in AAA expansion in the acellular group28. This evidence supports primary VSMCs as a better candidate for AAA treatment than iPSCs and further demonstrates their promise in slowing AAA growth. VSMC-seeded scaffolds were also more immunologically active, with higher fold change increases across pro and anti-inflammatory cytokines compared to iPSCs, though both groups seemed to promote native SMC retention in the aortic tunica media layer28. Another study by Park et al. differentiated murine skeletal muscle-derived stem cells into VSMC-like progenitors and implanted these in the lumen of elastase-induced AAAs in rats (following enzymatic wall degradation)29. This cell therapy group again had a decreased rate of aneurysm formation compared to control groups, with significantly decreased MMP gene and protein expressions, especially MMP-9 and MMP-229. Immunofluorescence imaging suggested that the VSMC-like progenitor cells inhibited the MMPs, thereby attenuating AAA formation29. These studies highlight the emerging area of smooth muscle progenitor or smooth muscle-like cells for treatment of AAA, giving rise to eventual stem cell-based therapies.
Therapeutic stem cell types for AAA treatment
For the purpose of this review, we will focus on the most promising cell-based therapies and provide an update on the current state of this field. Stem cells identified for therapeutic use in AAA include MSCs, adipose derived stem cells, and induced pluripotent stem cell derivatives (Fig. 1A). Yamawaki-Ogata et al.30 provides a more comprehensive discussion of various cell-based therapies for AAA.
Mesenchymal stromal cells (MSCs)
MSCs are multipotent cells known for their ability to differentiate into various cell types from the mesoderm, such as cartilage, bone, and adipose cells31. MSC populations can be extracted from multiple sources within the body including bone marrow, adipose tissue, and amniotic fluid32. Bone marrow-derived MSCs (BM-MSCs) are a popular source for cell therapy due to their availability, expandability in culture, and overall safety in clinical trials. Other types of MSCs include those derived from umbilical cords (UC-MSCs)33 and placental tissue (PL-MSCs)34. While no single phenotypic marker can characterize MSCs, these cells are generally thought to express phenotypic markers such as CD44, CD90, CD105, and CD73, as defined by the International Society for Cellular Therapy35.
In areas of tissue damage, MSCs are shown to release various cytokines and paracrine factors of potential therapeutic value36. In particular, MSC conditioned media was also shown to stimulate the proliferation of SMCs in vitro, likely through the expression of various cytokines and growth factors such as hepatocyte growth factor (HGF) or fibroblast growth factor-2 (FGF-2)37. Due to these immunosuppressive properties, MSC treatment may be beneficial to AAA as inflammation significantly contributes to aneurysm dilation and progression. MSCs also have a crucial role in AAA pathogenesis through dysregulation of their functional activities in the pathological environment, namely impaired immunomodulatory behavior, and increased levels of MMP-938. MSCs have also been found to upregulate elastin and downregulate collagen expression in fibroblasts, participating in vascular injury remodeling39. These immunomodulatory pathways and regenerative properties of MSCs establish a strong molecular basis for the potential of MSCs to combat AAA progression at a cellular level.
Despite the potential of MSCs, some limitations of their use include: 1) MSC harvesting can be invasive and painful40; 2) although they have a high expansion ratio, MSCs cannot be expanded long-term33; and 3) their potential differentiation into osteogenic lineages is not desirable at the site of AAA. With respect to delivery, systemic delivery is less invasive but is prone to non-targeted effects to other organs (e.g. kidneys, spleen, etc.)34,41. Conversely, direct cell injection or implantation of a cell sheet is highly invasive despite its high targeting ability39,42. Endovascular delivery via catheter is less invasive with a high targeting ability43,44.
Adipose-derived stem cells (ADSCs)
The potential of MSCs extends to ADSCs, proven to be an abundant, accessible and rich source of adult multipotent stem cells45. Although MSCs are commonly sourced from the bone marrow, particularly the iliac crest which produces a higher percentage of MSCs46, they can also be derived from adipose tissue using minimally invasive procedures like lipoaspiration47. ADSCs can be extracted from adipose tissue by collagenase digestion and then expanded in vitro48. Being isolated from more easily available subcutaneous fat, these cells can be rapidly acquired with high cellular activity40. Notably, ADSCs can be expanded in vitro for long periods of time while maintaining their differentiation capacity40, a stark difference from MSCs that favorably facilitates their practical use. Moreover, for both allogeneic and autologous applications, ADSCs have demonstrated optimal efficacy and efficiency40.
ADSCs show similar differentiation ability to MSCs, including immune regulatory properties. In the context of AAA, these cells demonstrate immunomodulatory and anti-inflammatory effects mediated by paracrine factors49. ADSCs were found to also increase select tissue-reparative markers49. They have also been used successfully in other illnesses including Parry-Romberg syndrome, Crohn’s disease, inflammatory and autoimmune disorders, and more50. Owing to the minimally invasive, less painful techniques to harvest these cells40, availability, and regenerative properties, ADSCs are an appealing cell source for therapeutic use, especially given their MSC-like properties. Other studies, however, have found that ADSCs can stimulate epithelial-mesenchymal transition and other challenges in cancer therapy uses and have shown previous negative results in cardiac disease treatment50, necessitating further clinical investigation of ADSCs for AAA.
Induced pluripotent stem cells (iPSCs)
Induced pluripotent stem cells (iPSCs) hold significant promise as a novel cell source. iPSCs, derived from reprogrammed somatic cells, using only four transcription factors, have the capacity to differentiate into various cell types, including VSMCs or endothelial cells51. In AAA therapy, iPSC-derived cells can be used to repair or replace damaged aortic tissue, counteracting the inflammatory, apoptotic, and extracellular matrix-degrading processes characteristic of the disease. iPSCs have also been used as an effective in vitro cell model towards the development of aortic disease treatments51. Moreover, iPSCs provide a platform for patient-specific modeling of AAA pathophysiology and precision medicine options, generally serving as an unlimited cell source for lineage and patient-specific cells without many ethical concerns52. Though their large-scale production depends on the culture and differentiation, they are relatively more promising in this respect to other cell sources both for scalability and personalized medicine, with the capacity for almost unlimited expansion53.
The use of iPSC derivatives for treating abdominal aortic aneurysms offers several advantages, including their ability to generate patient-specific cells, reducing the risk of immune rejection and enabling personalized therapies with autologous cells. Their pluripotency allows differentiation into multiple cell types essential for vascular repair, and they serve as a valuable tool for studying AAA mechanisms and testing therapeutic interventions51. The ease of use and potential benefits of iPSCs does depend on the stage and type of differentiation, delivery method, and other therapeutic design components.
The challenges with iPSC-based therapies include their tumorigenicity (i.e. teratoma formation), for which some mitigation strategies include ensuring full removal of undifferentiated iPSCs and stem cell-like intermediates; the genetic heterogeneity and potential for genetic instability and mutations; and the technical complexity of reprogramming and differentiation protocols53. One particular major hurdle to efficacious use in therapeutics is incomplete maturation of iPSC-derived cells, for which many efforts are underway to improve53. iPSC-derived cells also have shown poor transplant engraftment and limited therapeutic response, making cell delivery and integration in the body another challenge53. Additionally, as with other cell therapies, scalability and quality control remain important challenges to consider.
Preclinical trials of stem cell therapies
MSC localized delivery
Compared to systemic delivery, MSCs have also been locally delivered in preclinical models of AAA (Fig. 1B). As an example of local delivery to the site of the aneurysm, BM-MSCs were generated into cell sheets and wrapped around the adventitial region of Ang II-infused AAs of ApoE−/− mice39. The authors found that infrarenal diameter of the BM-MSC group was significantly smaller than the control aneurysm group at 4 weeks, and not significantly different from the sham group of ApoE-/- mice that did not have induced aneurysm, while phrenic and ascending diameters had similar diameters of both aneurysmal groups, significantly increased compared to the sham group39. There was also significantly more elastin deposition in the aneurysms of mice that were treated with BM-MSC sheets, than in the AAA control group, and the degree of elastin deposition was not different when compared to the sham group39. MMPs, including MMP-2 and 9, IL-6, MCP-1, and TNF-α were significantly decreased in the MSC group, while the expression of IGF-1 and TIMP-1 were up-regulated, further evidencing the positive effects of BM-MSCs on AAA at the molecular level similar to other studies39. In another example, BM-MSC-seeded guinea pig xenografts were implanted in a rat model, in which BM-MSCs (106) decreased AAA diameter growth more effectively than (5 million) VSMCs or AAA controls (infused only with culture media)43. Additionally, BM-MSCs decreased MMP-9 expression and macrophage infiltration and increased TIMP-1, compared to controls43. Interestingly, while neither BM-MSCs or VSMCs initiated repair of ECM in the medial wall layer, the BM-MSCs seemed to induce the accumulation of α-smooth muscle actin expressing cells surrounded by a collagen and elastin ECM43. This differs from the effects of more mature MSCs43, and shows the regenerative potential in effectively healing AAA.
Towards scaling to larger animal models, Turnbull et al. tested the therapeutic benefit of autologous implantation of BM-MSCs in a porcine model of AAA that was induced with an angioplasty balloon and collagenase type 142. Following injection of 107 BM-MSCs directly into the vessel wall, it was observed that the levels of VEGF increased at 72 hours after cell implantation, but returned to control levels at one week42. There was also increased capillary density in AAA tissues, but therapeutic benefits on aortic expansion was not described42. The results of these studies showed that induced aortic dilation was attenuated by BM-MSCs potentially through inflammatory and MMP modulation42. These results show that BM-MSC therapy abrogates dilation of the aorta, preserves elastin content and reduces MMP-2 and MMP-9 activity. These results indicate the potential of localized BM-MSCs as a treatment for halting aneurysm progression as evidenced in small-animal models but yet to be shown in larger models. These findings are supported by Li et al.54., who reported that MSC intervention across 18 studies is associated with reduced aortic diameter enlargement, reduced elastin degradation, and inflammatory cytokines. However, a drawback of localized cell treatment is the involvement of direct injection or placement of cells to the vessel itself, which may require invasive surgical implantation and thus may not be optimal for clinical translation.
MSC systemic delivery
There have been studies of MSCs through intravenous administration, as it this can be less invasive, compared to localized delivery, while allowing the possibility for MSC homing to the site of the aneurysm41. Intravenous injections containing one million to three million cells each have been reported, ranging from single to multiple injections over the course of the study. The result of these experiments showed the attenuation of aneurysm dilation and formation even after one injection of one million cells after 14 days41. Sharma et al. found that treatment with human PL-MSCs (106 injected on day 1) on mice showed suppression of mononuclear cell proliferation and proinflammatory cytokine IL-17 production (which promotes vascular inflammation and atherosclerosis, with proven effects in AAA also in this study), attenuating AAA formation34. There is evidence that MMP-2 and MMP-9 is decreased due to MSC treatment41. In a related work, Yamawaki-Ogata et al.55 reported that the pro- and active forms of MMP-2 and MMP-9 were significantly reduced after two weeks of delivery, but not significantly reduced at later time points, suggesting limited early-term benefits.
To examine the basic signaling mechanism of UC-MSC therapy, another study evaluated the role of NADPH oxidase, which is upregulated in human AAA, through HMGB1 (high mobility group box 1)56, suggesting a pro-inflammatory role57. HMGB1 is a damage associated molecular pattern molecule secreted rapidly by activated macrophages that stimulates IL-1756. HMGB1-blockade has been shown to suppress the development of AAA formation in animal models, reducing infiltration of macrophages and reducing MMP activity57. UC-MSCs showed inhibition of NADPH oxidase activation in macrophages, which attenuates HMGB1 production56. The authors showed a significant increase in HMGB1 production with the formation of AAA. NADPH oxidase 2 (Nox2) knockout mice with induced AAA had reduced HMGB1 expression, MMP-2 and MMP-9 activity, pro-inflammatory cytokine activity, and a decreased aortic diameter compared to wildtype mice with induced AAA56. This work demonstrates the intricate signaling pathways that mediate the effect of MSCs on AAA progression.
Similar to the localized treatments, IV delivered MSCs appeared to attenuate aneurysm expansion, and protect against elastin degradation. Wen et al. investigated intravenously injected (106) umbilical-cord MSCs (UC-MSCs) into a AAA rat model58. UC-MSCs showed attenuation of aneurysmal expansion, reduction of elastin degradation and fragmentation, inhibition of MMPs (pro and active MMP2 and MMP9) and TNF-α expression58. The VSMC healthy contractile phenotype was preserved and/or restored in AAA as well, showing positive effects on the existing VSMCs and phenotype plasticity in the environment, further evidencing benefits of MSC therapies58.
Together, these studies, summarized in Table 2, demonstrate that systemic delivery of MSCs can attenuate aneurysm expansion, reduce inflammation, inhibit MMP activity, and promote elastin preservation, contributing to overall AAA stabilization. Compared to localized delivery, MSCs can be delivered systemically using less invasive means to deliver cells for treatment. However, some of the limitations of systemic delivery include dilution of cells, non-specific distribution, and potential sequestration of MSCs in off-target tissues, which may reduce their effectiveness at the aneurysm site. Additionally, there is evidence that a substantial number of cells are trapped in other tissues before reaching the aneurysm, potentially reducing the effects55,59.
ADSC therapy
As an alternative to BM-MSCs, studies have been conducted using ADSCs to look at their effect on AAA. Blose et al. delivered ADSCs (105 cells) using a port targeting the periadventitial aneurysm in a mouse model60. After two weeks, the aortic diameter and elastin content of the treated group was similar to that at 5 days, when the treatment was administered, suggesting that localized ADSC therapy abrogated the progression of the disease60. In another study, 4×106 ADSCs were injected systemically through the common carotid artery in a rat model61. Assessment of the elastin fibers showed no significant difference between ADSC or BM-MSC groups61. However, protein expression of elastin was significantly higher in the ADSCs than the BM-MSCs and control at 21 days61. Gene expression of elastin demonstrated a significant increase over time with the ADSCs group61. Active and pro forms of MMP-2 and 9 in the ADSCs group were lower than in the sham or control groups61. Gene expression of MMP-2 and MMP-9 in SMCs increased then plateaued after 48 hours with a subsequent decrease, illustrating ADSCs’ inhibition of MMP secretion61. This suggests a beneficial role in the reconstruction of elastic fiber and modulating the MMP pathway, both of which are shown to slow or prevent AAA growth.
However, the beneficial effects of ADSCs appear to be mixed and may be dependent on the frequency or timing of cell delivery. When comparing the efficacy of ADSCs to BM-MSCs on AAA physiology, the authors reported that ADSCs supported higher in vitro protein expression for elastin, but in vivo there was no significant difference between the two cell therapies61. In another study62, 106 “adipose-derived mesenchymal regenerative cells” from male Sprague Dawley rats were intravenously injected in a rat model. The authors demonstrated that these autologous rat ADSCs were not able to attenuate AAA progression, with no significant difference between the saline control and ADSC group in neutrophil or macrophage infiltration, elastin content, or aortic diameter at day 2862. The authors speculated that additional dosages could be needed to have an effect62. In contrast, another study having weekly MSC IV administration reported positive results and effective attenuation of AAA in a mouse model41. These reports of mixed results highlight the need to optimize and then standardize cell dosing for each cell type. Together, these studies (Table 3) illustrate the therapeutic benefit of ADSCs for treatment of AAA.
iPSC-based therapy
Although iPSCs have been investigated for different use cases, such as examining their differentiation into aortic SMCs for modelling Marfan syndrome in mice63, their preclinical investigation as a therapeutic is limited. An early study from Mulorz et al., described above, that compares iPSC-derived vascular smooth muscle progenitors with VSMCs in mice showed that these iPSC-derived cells can be delivered using scaffold-based approaches, localize to the aneurysm wall, and support native SMC retention28. However, the primary VSMCs were more effective therapeutically, causing more significantly decreased AAA growth28. Given the many potential advantages of iPSCs however, both biologically and in terms of clinical translation and scalability, further preclinical investigation is necessary to fully explore this potentially promising cell source.
Clinical trials of stem cell therapies
Planned clinical trials for cell therapies for treatment of AAA have been initiated to explore the utility of MSC treatments for AAA (Fig. 1C). The STOP-AAA trial studied autologous BM-MSCs in attenuating the expansion of small aneurysms64. Three doses of 2 × 106 MSC/Kg or placebo were delivered at baseline, 24, and 53 weeks with the experiment planned to end at 18 months64. No update on this trial has been reported. As of December 2024, there were two relevant cell-therapy related clinical trials with activity since 2020 on ClinicalTrials.gov to the knowledge of the authors. First is the “VIVAAA/ARREST” trial (ClinicalTrials.gov ID NCT02846883) from Indianapolis, U.S.A., which sought to inject allogeneic MSCs to stimulate T-regulatory cells via IV injection in patients with small AAA and evaluate therapeutic safety and efficacy65. This was with the goal of determining if MSCs can decrease inflammation and slow aneurysm growth. Interventions included intravenous infusion of 1 million MSCs/kg, 3 million MSCs/kg, and a placebo65. First posted in 2016, this study was terminated in September 2021 due to slow enrollment after being put on hold during the COVID-19 pandemic65. The second trial from Madrid, Spain first posted in July 2024 (ClinicalTrials.gov ID NCT06488898) evaluates AAA stabilization using allogeneic adipose-derived MSCs (ADSCs) injected locally in the aneurysmal sac, with methods of MSC administration described previously44,66. Currently recruiting and with a target completion date for data collection of December 2025, if completed successfully, this trial should demonstrate the outcomes in humans of cell therapy products in AAA, particularly MSCs, providing the first major clinical outcomes in this area44,66. Results from these ongoing trials will reveal the plausibility of these therapies in humans as well as investigate and translate the findings from the many preclinical studies.
Cell-derived products for AAA treatment
As cell therapies can carry regulatory hurdles, recent studies have pursued cell free methods as an alternative. Included within these cell free methods are EVs (Fig. 3)67,68,69,70. Spinosa et al. utilized human umbilical cord from Wharton’s Jelly MSC derived EVs in a mouse model of AAA71. EVs are nano-sized, membranous structures containing cellular components and surface markers (e.g. proteins, mRNAs, miRNAs, DNA, and lipids) that are secreted into the extracellular space under healthy and pathological condition72. The importance of EVs in cellular communication and other processes makes them relevant and promising as biomarkers and in therapeutics72. In this study, both MSCs and EVs had decreased AAA expansion compared to untreated controls71. They also had decreased inflammatory cytokines and better preservation of elastin compared to untreated controls71. Their study also investigated miR-147 as a prominent microRNA for reducing inflammation. In another study, Chen et al. intravenously injected umbilical cord MSC-EVs into a mouse model of AAA and saw decreased aortic diameter expansion and increased elastin preservation compared to controls73. Kozakai et al. used mouse derived bone marrow MSC-EVs for intravenous injection into a mouse model of AAA74. The bone marrow MSC-EV treated group had decreased diameter expansion, decreased pro and active MMP2 and MMP9 activity, increased elastin preservation, decreased F4/80+ macrophages, and increased CD206+ macrophages compared to the controls74. Additionally, analysis of the MSC-EVs revealed they contained inhibitory microRNAs of aneurysm formation74, implicating that these EVs would cause the downstream effects observed. Hu et al. used mouse adipose MSC-EVs as a delivery system of miR-17-5p via an intravenous injection into a AAA mouse model75. With these EVs, there was increased elastin preservation, decreased inflammatory cytokines, decreased aneurysm expansion all of which was further enhanced by miR-17-5p75. They also tracked their EVs and saw lots of accumulation in the liver with some accumulation in heart, spleen, kidney, and lung75.
VSMCs, immune cells (T cells, macrophages, and monocytes), and endothelial cells are depicted. Created in BioRender. Marini, A. (2025) https://BioRender.com/h8r5hjb.
There have also been some in vitro studies performed with EVs76,77,78. In one study by Cunnane et al., adipose MSC-EVs stimulated elastin and collagen production in 3D SMC seeded fibrin gel constructs76. Sajeesh et al. performed two different EV studies. In the first study, human bone marrow MSC-EVs were used for treatment of elastase damaged SMCs77. The EVs decreased MMP2 and increased TIMP1 and 2 gene expression, decreased active MMP2 and overall MMP protein level, increased TIMP2 level, decreased MMP2/TIMP2 ratio, decreased MMP2 activity, increased elastin (ELN), fibulin 5 (FBLN5), lysyl oxidase (LOX) gene expression, increased LOX protein, and increased elastin content77. In another study, human BM-MSC-EVs were functionalized with cathepsin targeting molecule, as an eventual means for targeting AAA78. These EVs decreased MMP2 expression and increased LOX expression in elastase damaged SMCs78. The functionalization also improved EV uptake78. These studies indicate the regenerative potential of MSC EVs for treatment of AAA. Circulating miRNAs have also been investigated as markers particularly for early-stage diagnosis79. While more is being investigated, 9 key miRNAs in related pathways (e.g., vascular inflammation, VSMC phenotype switching, ECM degradation, etc.) are implicated in known AAA mechanisms79.
Biomaterials-based approaches for AAA treatment
The development of a stem-cell based therapeutic targeted at both the early and late-stage AAA treatment requires a combination of novel and continuously emerging bioengineering strategies and techniques. The integration of biomaterials and scaffolds to enhance stem cell delivery for to create aortic grafts is a major area of interest. Depending on the biomaterial and its associated or designed properties (e.g., ceramics, natural polymers, and synthetic polymers), different characteristics can be exploited for more optimal delivery and cell survivability80.
ECM patches have been tested for the delivery of therapeutic cells to the peri-adventitial space of the aneurysm. For example, Parvizi et al. performed an intraluminal infusion of elastase into rats. A recombinant collagen peptide patch was seeded with 2×106 ADSCs81. After two weeks the ADSC group diameter didn’t differ significantly from the sham group and also had better looking elastin than the aneurysm controls81. The cell group had a significantly higher number of SMCs in the vessel as well as lower levels of macrophages81. In larger animals, Zilberman et al. delivered 106 human ADSCs suspended in GelFoam (an absorbable gelatin sponge82) placed peri-adventitially on a pig model AAA, induced via elastase and collagenase I82. In the stem cell-treated group, there was reduced aortic dilation, higher preservation of elastin, preserved SMCs, and increased VEGF (vascular endothelial growth factor), TIMP1, and TIMP3 in the aorta82. The untreated group saw significantly decreased collagen content, α-smooth muscle actin, and elastin perturbation82. Kooragayala et al. expanded upon this work with the same porcine model, cell type, and GelFoam delivery and saw increased Young’s elastic modulus (ability to withstand stress) as well as preservation of elastin in the treated group, showing efficacy and promise of ADSCs in larger pig models83.
Besides the use of biomaterials for therapeutic cell therapy, there is also a rise of biomaterials to be used for generating vascular grafts. To address the concerns of EVAR procedures leading to due to endoleaks and limited long-term device durability, several bioengineered stent grafts have been investigated focusing on improved tissue integration post-EVAR84. Takeuchi et al. reported that polyethylene terephthalate/polyglycolic acid-based grafts in mongrel dogs promoted both histologic and mechanical integration by recruiting the host tissue into the graft scaffold, thereby preventing potential postoperative migration and endoleaks85. Furthermore, through two consecutive studies, Kawajiri et al. demonstrated the application of in-body tissue architecture technology to fabricate self-expandable aortic stent allografts86,87. An acryl/nitinol mold was subcutaneously embedded into beagles for four weeks to produce an autologous implantable collagenous tissue with the desired shape86. Subsequently, the structure was harvested and implanted as allograft into the infrarenal abdominal aorta of the animal. The implanted stent grafts were observed to be integrated with the native aorta within a month while exhibiting excellent neo-endothelialization87. These studies highlight the emerging area of biomaterials for co-delivery of therapeutic cells or for the generation of vascular grafts to treat AAA.
Clinical translation barriers and considerations
Clinical translation of cell-based products is challenging, from the complex biological environment in the human body, to real-world barriers in manufacturing and scalability, and the importance of quality control and regulatory compliance. Translation not only requires resolving technical challenges and current limitations, as described below, but also building and fitting into robust translational frameworks (e.g. regulatory, manufacturing) in a cost-effective way.
Biologically, clinical integration is complicated by factors like variability in cell quality, survival, engraftment, and functional maturation. For stem cell and cell-based technologies, risks such as tumorigenicity, off-target differentiation, and unexpected immune responses remain major safety considerations88. Stem cells also face specific challenges with differentiation needing to yield mature and stable cell populations at scale89. Long-term functional integration and effects in humans are critical to elucidate as technologies move to clinical trials and beyond. Moreover, working closely alongside and in adherence to regulatory bodies is critical to safely and effectively bringing these technologies to patients with regulatory approval. Consideration of validated clinical trial endpoints and evaluation criteria for therapeutics, like in earlier-stage AAA, is also important to ensure outcomes reflect the true biological effects being investigated.
Stem cell-based products face unique quality control and manufacturing considerations, including the need for reproducible large-scale production under good manufacturing practice (GMP) standards and authorization, validated potency assays, and strict release criteria to ensure batch-to-batch consistency90,91. Moving from smaller-scale academic studies to clinical-grade manufacturing requires addressing hurdles in cryopreservation, storage stability, and distribution, while maintaining phenotype and function. As this and other supporting technology has improved, the translation of stem cell technologies has become increasingly possible and scalable.
There are significant costs and strategic trade-offs throughout the translation of stem-cell technologies that shape product design choices and implementation. Decisions such as autologous vs. allogeneic sources, selection of cell type and harvesting, and complexity of the delivery mechanism must be balanced with scalability, manufacturing, cost-effectiveness, and the realities of adoption into healthcare systems, all critical determinants of successful clinical translation. This necessitates comprehensive and early consideration of the trade-offs and eventual clinical implementation to enable these therapies to successfully reach patient populations in the face of so many barriers. Clinical success becomes more likely with rigorous quality controls, effective scalability and manufacturing, demonstrated durability of benefit, real-world economic viability, and alignment with regulatory pathways, in addition to strong scientific development and testing. Iterative feedback between preclinical studies and early clinical trials will be essential to first identifying and improving upon approaches with true therapeutic and clinical promise for AAAs.
Current limitations and future perspectives
As illustrated throughout this review, the AAA field is witnessing significant innovation across diagnostics, therapeutics, monitoring, and understanding its pathophysiology. Key areas requiring further exploration include the identification of optimal cell sources. While MSCs, ADSCs, and VSMCs have been widely studied, iPSC-derived SMCs present a novel, patient-specific option with reduced immunogenicity and enhanced differentiation potential. Comparative studies are needed to assess their relative efficacy while using consistent models. Furthermore, additional factors such as ease of harvesting, cell stability, and regulatory approvals must also be considered while evaluating their translational ability.
Another critical issue is the limited control over the differentiation pathways and long-term fate of administered stem cells, particularly with respect to off-target differentiation that could compromise therapeutic efficacy or induce adverse effects. For example, MSCs have demonstrated a tendency toward osteogenic differentiation, which is undesirable in the vascular environment and could exacerbate vascular calcification. Similarly, iPSC-derived cells, while offering patient-specific advantages, carry risks of incomplete or aberrant differentiation, potentially leading to tumorigenicity or fibrosis. Moreover, most existing studies on AAA treatment have reported limited data on the biodistribution and long-term response of the transplanted cells, leaving significant gaps in understanding their mechanisms of action and safety profile. Therefore, future studies should address these shortcomings through rigorous assessments of cell engraftment, fate, and genetic stability using standardized in vivo tracking methods, along with stringent quality control, to enable targeted repair and regeneration of the aortic tissue.
Another key consideration is the optimization of dosing, timing, and delivery methods. The therapeutic efficacy of cell-based treatments is influenced not only by the number of cells administered, but also by when and how they are delivered. Preclinical studies highlight the critical importance of timing, with administration after aneurysm formation better reflecting the clinical context and yielding more translatable outcomes compared to interventions initiated at model induction. Furthermore, while systemic administration is less invasive and potentially more scalable, it is limited by poor cell localization and significant off-target effects. In contrast, targeted delivery methods, such as direct injection into the aortic wall using hydrogel-based systems, offer improved therapeutic performance by ensuring higher cell retention and localized effects. Future work should integrate optimal dosing with post-aneurysm timing and localized delivery strategies to validate the clinical potential of stem cell-driven AAA treatment.
Additionally, the lack of studies that investigate sex-based differences in AAA models and stem cell therapies limits critical insight into how the treatments would behave in male and female patients. The majority of studies discussed in this review only used male animals or cells from male animals or human male patients. While AAA mostly affects men, women have worse outcomes related to rupture and surgical repair92. These worse outcomes underscore the need to investigate the effects of stem cell treatment on both male and female animals, as there has been evidence that the sex of the animal affects AAA progression in vivo93,94,95. Also, there could be sex-based differences in the cell source for treatment96,97. Thus, it is necessary to further investigate the effects of sex of the animals and sex of the cell source on the treatment of AAA.
In the future, omics and high throughput techniques (e.g. proteomics, single-cell RNA sequencing) will provide deeper insights into cell therapy-induced changes. These approaches have enabled the discovery of relevant biomarkers and will help validate the therapeutic efficacy of these approaches. Single-cell RNA sequencing provides granular insights into how different cell types in the aortic wall will respond to the applied treatments98. Furthermore, integration with spatial transcriptomics enables an understanding of gene expression within tissue sections, thereby revealing the underlying cellular interactions99. Proteomic analyses can identify the interactions, function, composition, and structure of proteins and their activities, responsible for ECM remodeling and inflammation reduction, as described by Al-Amrani et al.100. Combining these methods with advanced computational tools and machine learning, provides a more intricate understanding of cellular and tissue mechanisms in AAA for development of advanced therapeutic approaches.
With the rapid advancement of artificial intelligence (AI), it is increasingly applied to improve AAA outcomes as well. Besides ‘omics, AI has proven especially valuable in boosting the current imaging and monitoring technologies. Ongoing clinical trials are evaluating its usage for better aneurysm modelling for predictive rupture and enhanced monitoring. These include: the VASCUL-AID-RETRO trial, using AI for vascular disease risk and progression prediction (NCT06206369)101; the ART in EVAR trial, predicting AAA shrinkage following stent-placement with AI (NCT06250998)102; the ViTAA registry pre- and post-operative monitoring for EVAR using aortic mapping technology (NCT05004051)103; and the IAVASC trial, implementing AI into automatic analysis of vascular network segmentation to predict risk for AAA (NCT06451315)104, with the ViTAA and IAVASC trials using AI technology developed previously103,104. These efforts aim to enhance predictive modeling, monitoring, and decision-making, enabling more proactive care for surgical treatment.
In the immediate term, more efforts should be focused on working towards the clinical stage by validating current findings and hypotheses in human studies. Additionally, future research should explore the potential for combinatorial approaches. Integrating cell therapies with biomaterials has shown evident benefits in the delivery of the regenerative effects. How these cells may interact and be enhanced by pharmacological agents for example would be beneficial to investigate for how they could enhance therapeutic efficacy and durability. For example, combining MSCs with matrix-modifying enzymes or anti-inflammatory drugs may synergistically enhance ECM regeneration and reduce aortic wall stress. Additionally, cell-derived products, such as exosomes, will be interesting to further investigate and ascertain as potential therapeutic candidates.
Overall, stem-cell-based therapies hold significant promise for the treatment of AAA, with numerous studies evidencing the therapeutic effects and benefits of cell-based therapies. However, their full clinical potential has yet to be realized. As research in this area expands, clinical trials are necessary to understand the effects of these cells in humans and eagerly awaited. Addressing current limitations through rigorous investigation of cell sources, dosing, timing, and delivery methods, alongside leveraging advanced technologies like omics, will be crucial for translating these therapies into more effective treatments for AAA.
Data availability
All data described here are cited. No new data was generated.
References
Kessler, V., Klopf, J., Eilenberg, W., Neumayer, C. & Brostjan, C. AAA revisited: a comprehensive review of risk factors, management, and hallmarks of pathogenesis. Biomedicines 10, 94 (2022).
Song, P. et al. The global and regional prevalence of abdominal aortic aneurysms: a systematic review and modeling Analysis. Ann. Surg. 277, 912–919 (2023).
Reimerink, J. J., van der Laan, M. J., Koelemay, M. J., Balm, R. & Legemate, D. A. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br. J. Surg. 100, 1405–1413 (2013).
Hoang, D. M. et al. Stem cell-based therapy for human diseases. Signal Transduct. Target Ther. 7, 272 (2022).
Poliwoda, S. et al. Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthop. Rev. ((Pavia)) 14, 37498 (2022).
Airhart, N. et al. Smooth muscle cells from abdominal aortic aneurysms are unique and can independently and synergistically degrade insoluble elastin. J. Vasc. Surg. 60, 1033–1042.e1035 (2014).
Riches, K. et al. Exploring smooth muscle phenotype and function in a bioreactor model of abdominal aortic aneurysm. J. Transl. Med. 11, 208 (2013).
Abdul-Hussien, H. et al. The pathophysiology of abdominal aortic aneurysm growth: corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J. Vasc. Surg. 51, 1479–1487 (2010).
Xiong, W. et al. Blocking TNF-α attenuates aneurysm formation in a murine model. J. Immunol. 183, 2741–2746 (2009).
Dawson, J. et al. Aortic aneurysms secrete interleukin-6 into the circulation. J. Vasc. Surg. 45, 350–356 (2007).
Lu, H. et al. Vascular smooth muscle cells in aortic aneurysm: from genetics to mechanisms. J. Am. Heart Assoc. 10, e023601 (2021).
Petsophonsakul, P. et al. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arterioscler Thromb. Vasc. Biol. 39, 1351–1368 (2019).
Ailawadi, G. et al. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc Surg. 138, 1392–1399 (2009).
Cao, G. et al. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 20, 180 (2022).
Ailawadi, G., Eliason, J. L. & Upchurch, G. R. Jr. Current concepts in the pathogenesis of abdominal aortic aneurysm. J. Vasc. Surg. 38, 584–588 (2003).
Vijungco, J. et al. Enhanced abdominal aortic aneurysm (AAA) formation in timp-1-deficient (timp-1-/-) mice after local elastase perfusion. J. Surgical Res. 114, 248 (2003).
Vijaynagar, B., Bown, M. J., Sayers, R. D. & Choke, E. Potential role for anti-angiogenic therapy in abdominal aortic aneurysms. Eur. J. Clin. Investig. 43, 758–765 (2013).
Sano, M. et al. Lymphangiogenesis and angiogenesis in abdominal aortic aneurysm. PLOS ONE 9, e89830 (2014).
Cheung, A. T. & Weiss, S. J. in Intraoperative Echocardiography (ed Donald C. Oxorn) 161-182 (W.B. Saunders, 2012).
Boland, E., Quondamatteo, F. & Van Agtmael, T. The role of basement membranes in cardiac biology and disease. Biosci. Rep. 41, BSR20204185 (2021).
van Thiel, B. S., van der Pluijm, I., Kanaar, R., Danser, A. H. J. & Essers, J. in The ESC Textbook of Vascular Biology (eds Robert Krams & Magnus Bäck) 5-16 (Oxford University Press, 2017).
Halper, J. in Advances in Pharmacology Vol. 81 95-127 (Academic Press, 2018).
Wang, G., Jacquet, L., Karamariti, E. & Xu, Q. Origin and differentiation of vascular smooth muscle cells. J. Physiol. 593, 3013–3030 (2015).
Mackay, C. D. A., Jadli, A. S., Fedak, P. W. M. & Patel, V. B. Adventitial fibroblasts in aortic aneurysm: unraveling pathogenic contributions to vascular disease. Diagnostics 12, 871 (2022).
Augoustides, J. G. & Cheung, A. T. in Perioperative Transesophageal Echocardiography (eds David L. Reich & Gregory W. Fischer) 191-217 (W.B. Saunders, 2014).
Allaire, E. et al. Paracrine effect of vascular smooth muscle cells in the prevention of aortic aneurysm formation. J. Vasc. Surg. 36, 1018–1026 (2002).
Allaire, E. et al. Vascular smooth muscle cell endovascular therapy stabilizes already developed aneurysms in a model of aortic injury elicited by inflammation and proteolysis. Ann. Surg. 239, 417–427 (2004).
Mulorz, J. et al. peri-Adventitial delivery of smooth muscle cells in porous collagen scaffolds for treatment of experimental abdominal aortic aneurysm. Biomater. Sci. 9, 6903–6914 (2021).
Park, H. S. et al. Potential role of vascular smooth muscle cell-like progenitor cell therapy in the suppression of experimental abdominal aortic aneurysms. Biochem Biophys. Res Commun. 431, 326–331 (2013).
Yamawaki-Ogata, A., Mutsuga, M. & Narita, Y. A review of current status of cell-based therapies for aortic aneurysms. Inflamm. Regen. 43, 40 (2023).
Chamberlain, G., Fox, J., Ashton, B. & Middleton, J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25, 2739–2749 (2007).
Wagner, W. et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 33, 1402–1416 (2005).
De Witte, S. F. et al. Aging of bone marrow–and umbilical cord–derived mesenchymal stromal cells during expansion. Cytotherapy 19, 798–807 (2017).
Sharma, A. K. et al. Experimental Abdominal Aortic Aneurysm Formation Is Mediated by IL-17 and Attenuated by Mesenchymal Stem Cell Treatment. Circulation 126, S38–S45 (2012).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
Ren, G. et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. cell stem cell 2, 141–150 (2008).
Kinnaird, T. et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 94, 678–685 (2004).
Ciavarella, C. et al. Human vascular wall mesenchymal stromal cells contribute to abdominal aortic aneurysm pathogenesis through an impaired immunomodulatory activity and increased levels of matrix metalloproteinase-9. Circulation J. 79, 1460–1469 (2015).
Hashizume, R., Yamawaki-Ogata, A., Ueda, Y., Wagner, W. R. & Narita, Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J. Vasc. Surg. 54, 1743–1752 (2011).
Mazini, L., Rochette, L., Amine, M. & Malka, G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int. J. Mol. Sci. 20, 2523 (2019).
Fu, X. -m. et al. Intravenous administration of mesenchymal stem cells prevents angiotensin II-induced aortic aneurysm formation in apolipoprotein E-deficient mouse. J. Transl. Med. 11, 175 (2013).
Turnbull, I. C. et al. Aortic implantation of mesenchymal stem cells after aneurysm injury in a porcine model. J. Surgical Res. 170, e179–e188 (2011).
Schneider, F. et al. Bone marrow mesenchymal stem cells stabilize already-formed aortic aneurysms more efficiently than vascular smooth muscle cells in a rat model. Eur. J. Vasc. Endovasc. Surg. 45, 666–672 (2013).
del Moral, L. R. et al. Experimental model for coadjuvant treatment with mesenchymal stem cells for aortic aneurysm. Am. J. stem cells 1, 174 (2012).
Bunnell, B. A., Flaat, M., Gagliardi, C., Patel, B. & Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 45, 115–120 (2008).
Narbona-Carceles, J., Vaquero, J., Suárez-Sancho, S., Forriol, F. & Fernández-Santos, M. E. Bone marrow mesenchymal stem cell aspirates from alternative sources is the knee as good as the iliac crest?. Injury 45, S42–S47 (2014).
Zhu, M., Heydarkhan-Hagvall, S., Hedrick, M., Benhaim, P. & Zuk, P. Manual isolation of adipose-derived stem cells from human lipoaspirates. JoVE (J. Visualized Exp.) 79, e50585 (2013).
Frese, L., Dijkman, P. E. & Hoerstrup, S. P. Adipose tissue-derived stem cells in regenerative medicine. Transfus. Med Hemother 43, 268–274 (2016).
Xie, J. et al. Human adipose-derived stem cells suppress elastase-induced murine abdominal aortic inflammation and aneurysm expansion through paracrine factors. Cell Transplant. 26, 173–189 (2017).
Bacakova, L. et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells – a review. Biotechnol. Adv. 36, 1111–1126 (2018).
Zhu, K. et al. Modeling aortic diseases using induced pluripotent stem cells. Stem Cells Transl. Med. 10, 190–197 (2021).
Klein, D. iPSCs-based generation of vascular cells: reprogramming approaches and applications. Cell Mol. Life Sci. 75, 1411–1433 (2018).
Cerneckis, J., Cai, H. & Shi, Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 9, 112 (2024).
Li, X. et al. Therapeutic efficacy of mesenchymal stem cells for abdominal aortic aneurysm: a meta-analysis of preclinical studies. Stem Cell Res Ther. 13, 81 (2022).
Yamawaki-Ogata, A. et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells in formed aortic aneurysms of a mouse model. Eur. J. cardio-Thorac. Surg. 45, e156–e165 (2014).
Sharma, A. K. et al. Mesenchymal stem cells attenuate NADPH oxidase-dependent high mobility group box 1 production and inhibit abdominal aortic aneurysms. Arteriosclerosis, thrombosis, Vasc. Biol. 36, 908–918 (2016).
Kohno, T. et al. High-mobility group box 1 protein blockade suppresses development of abdominal aortic aneurysm. J. Cardiol. 59, 299–306 (2012).
Wen, H. et al. Human umbilical cord mesenchymal stem cells attenuate abdominal aortic aneurysm progression in Sprague-Dawley rats: implication of vascular smooth muscle cell phenotypic modulation. Stem Cells Dev. 29, 981–993 (2020).
Hosoyama, K. et al. Intravenously injected human multilineage-differentiating stress-enduring cells selectively engraft into mouse aortic aneurysms and attenuate dilatation by differentiating into multiple cell types. J. Thorac. cardiovascular Surg. 155, 2301–2313.e2304 (2018).
Blose, K. J. et al. Periadventitial adipose-derived stem cell treatment halts elastase-induced abdominal aortic aneurysm progression. Regenerative Med. 9, 733–741 (2014).
Tian, X. et al. Adipose stem cells promote smooth muscle cells to secrete elastin in rat abdominal aortic aneurysm. PLoS One 9, e108105 (2014).
Kavaliunaite, E. et al. A Single Injection of ADRCs Does Not Prevent AAA Formation in Rats in a Randomized Blinded Design. Int. J. Mol. Sci. 25, 7591 (2024).
Nakamura, K. et al. Lineage-specific induced pluripotent stem cell-derived smooth muscle cell modeling predicts integrin alpha-v antagonism reduces aortic root aneurysm formation in marfan syndrome mice. Arterioscler Thromb. Vasc. Biol. 43, 1134–1153 (2023).
Murphy & Network, M. P. I. o. t. C. C. T. R. The stem cell therapy to prevent expansion of abdominal aortic aneurysm (STOP-AAA) Trial: rationale and design. Arteriosclerosis, Thrombosis, Vasc. Biol. 34, A295 (2014).
Wang, S. K. et al. Rationale and design of the ARREST trial investigating mesenchymal stem cells in the treatment of small abdominal aortic aneurysm. Ann. Vasc. Surg. 47, 230–237 (2018).
Riera del Moral, L. et al. Potential of mesenchymal stem cell in stabilization of abdominal aortic aneurysm sac. J. Surgical Res. 195, 325–333 (2015).
Hong, P., Yang, H., Wu, Y., Li, K. & Tang, Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 10, 242 (2019).
Gallina, C., Turinetto, V. & Giachino, C. A New paradigm in cardiac regeneration: the mesenchymal stem cell secretome. Stem Cells Int. 2015, 1–10 (2015).
Foo, J. B. et al. Comparing the therapeutic potential of stem cells and their secretory products in regenerative medicine. Stem Cells Int. 2021, 1–30 (2021).
Műzes, G. & Sipos, F. Mesenchymal stem cell-derived secretome: a potential therapeutic option for autoimmune and immune-mediated inflammatory diseases. Cells 11, 2300 (2022).
Spinosa, M. et al. Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. Faseb j. 32, fj201701138RR (2018).
Kumar, M. A. et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 9, 27 (2024).
Chen, L. et al. Mesenchymal stem cell-derived extracellular vesicles protect against abdominal aortic aneurysm formation by inhibiting NET-induced ferroptosis. Exp. Mol. Med. 55, 939–951 (2023).
Kozakai, M. et al. Alternative therapeutic strategy for existing aortic aneurysms using mesenchymal stem cell–derived exosomes. Expert Opin. Biol. Ther. 22, 95–104 (2022).
Hu, J. et al. Exosomal miR-17-5p from adipose-derived mesenchymal stem cells inhibits abdominal aortic aneurysm by suppressing TXNIP-NLRP3 inflammasome. Stem Cell Res Ther. 13, 349 (2022).
Cunnane, E. M., Ramaswamy, A. K., Lorentz, K. L., Vorp, D. A. & Weinbaum, J. S. Extracellular vesicles derived from primary adipose stromal cells induce elastin and collagen deposition by smooth muscle cells within 3D fibrin gel culture. Bioeng. ((Basel)) 8, 51 (2021).
Sajeesh, S., Broekelman, T., Mecham, R. P. & Ramamurthi, A. Stem cell derived extracellular vesicles for vascular elastic matrix regenerative repair. Acta Biomaterialia 113, 267–278 (2020).
Sajeesh, S., Camardo, A., Dahal, S. & Ramamurthi, A. Surface-functionalized stem cell-derived extracellular vesicles for vascular elastic matrix regenerative repair. Mol. Pharmaceutics 20, 2801–2813 (2023).
Tasopoulou, K. M., Argiriou, C., Tsaroucha, A. K. & Georgiadis, G. S. Circulating miRNAs as Biomarkers for Diagnosis, Surveillance, and Postoperative Follow-Up of Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 93, 387–404 (2023).
Koyyada, A. & Orsu, P. Recent advancements and associated challenges of scaffold fabrication techniques in tissue engineering applications. Regenerative Eng. Transl. Med. 7, 147–159 (2021).
Parvizi, M., Petersen, A. H., van Spreuwel-Goossens, C. A. F. M., Kluijtmans, S. G. J. M. & Harmsen, M. C. Perivascular scaffolds loaded with adipose tissue-derived stromal cells attenuate development and progression of abdominal aortic aneurysm in rats. J. Biomed. Mater. Res. Part A 106, 2494–2506 (2018).
Zilberman, B. et al. Treatment of abdominal aortic aneurysm utilizing adipose-derived mesenchymal stem cells in a porcine model. J. Surgical Res. 278, 247–256 (2022).
Kooragayala, K. et al. Impact of adipose-derived stem cells on aortic tensile strength in a model of abdominal aortic aneurysm. Am. Heart J. : Cardiol. Res. Pract. 27, 100279 (2023).
Vahabli, E. et al. The technological advancement to engineer next-generation stent-grafts: design, material, and fabrication techniques. Adv. Healthc. Mater. 11, 2200271 (2022).
Takeuchi, M. et al. Tissue-engineered stent-graft integrates with aortic wall by recruiting host tissue into graft scaffold. J. Thorac. Cardiovasc Surg. 148, 1719–1725 (2014).
Kawajiri, H. et al. Development of tissue-engineered self-expandable aortic stent grafts (Bio stent grafts) using in-body tissue architecture technology in beagles. J. Biomed. Mater. Res B Appl Biomater. 103, 381–386 (2015).
Kawajiri, H. et al. Implantation study of a tissue-engineered self-expanding aortic stent graft (bio stent graft) in a beagle model. J. Artif. Organs 18, 48–54 (2015).
Heslop, J. A. et al. Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl. Med 4, 389–400 (2015).
Francis, N. et al. Scaling up pluripotent stem cell-based therapies - considerations, current challenges and emerging technologies: perspectives from the ISCT Emerging Regenerative Medicine Working Group. Cytotherapy 27, 1031–1042 (2025).
Lowdell, M. W. Considerations for manufacturing of cell and gene medicines for clinical development. Cytotherapy 27, 874–883 (2025).
Capelli, C. et al. Potency assays and biomarkers for cell-based advanced therapy medicinal products. Front Immunol. 14, 1186224 (2023).
Villard, C. & Hultgren, R. Abdominal aortic aneurysm: Sex differences. Maturitas 109, 63–69 (2018).
Cullen, J. M. et al. Sex-based differences among experimental swine abdominal aortic aneurysms. J. Surgical Res. 260, 488–498 (2021).
Cho, B. S. et al. Differential regulation of aortic growth in male and female rodents is associated with AAA development. J. Surgical Res. 155, 330–338 (2009).
Laser, A. et al. Differential gender- and species-specific formation of aneurysms using a novel method of inducing abdominal aortic aneurysms. J. Surg. Res 178, 1038–1045 (2012).
Bianconi, E. et al. Sex-specific transcriptome differences in human adipose mesenchymal stem cells. Genes 11, 909 (2020).
Maged, G., Abdelsamed, M. A., Wang, H. & Lotfy, A. The potency of mesenchymal stem/stromal cells: does donor sex matter? Stem Cell Res. Ther. 15, https://doi.org/10.1186/s13287-024-03722-3 (2024).
Jovic, D. et al. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med 12, e694 (2022).
Miranda, A. M. A. et al. Single-cell transcriptomics for the assessment of cardiac disease. Nat. Rev. Cardiol. 20, 289–308 (2023).
Al-Amrani, S., Al-Jabri, Z., Al-Zaabi, A., Alshekaili, J. & Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J. Biol. Chem. 12, 57–69 (2021).
Rijken, L. et al. Developing Trustworthy Artificial Intelligence Models to Predict Vascular Disease Progression: the VASCUL-AID-RETRO Study Protocol. Journal of Endovascular Therapy, https://doi.org/10.1177/15266028251313963 (2025).
van Rijswijk, R. E. et al. Multimodal artificial intelligence model for prediction of abdominal aortic aneurysm shrinkage after endovascular repair (the ART in EVAR study). J Endovasc Ther 0, https://doi.org/10.1177/15266028251314359 (2025).
Forneris, A. et al. AI-powered assessment of biomarkers for growth prediction of abdominal aortic aneurysms. JVS Vasc. Sci. 4, 100119 (2023).
Caradu, C. et al. Fully automatic volume segmentation using deep learning approaches to assess aneurysmal sac evolution after infrarenal endovascular aortic repair. J. Vasc. Surg. 76, 620–630.e623 (2022).
Davis, J. P. et al. Attenuation of aortic aneurysms with stem cells from different genders. J. Surg. Res. 199, 249–258 (2015).
Acknowledgements
This work was supported in part by grants to NFH from the US National Institutes of Health (R01CA285372, R41HL170875, and R21 HL172096), the US Department of Veterans Affairs (1I01BX004259, RX004898, and 1I01BX006882), the National Science Foundation (1829534 and 2227614), and the Tobacco Related Disease Research Program (T33IP6580). NFH is a recipient of a Research Career Scientist award (IK6 BX006309) from the Department of Veterans Affairs. This work was partially funded by a Mitacs Globalink research project award (IT40957) to SB, a Rubicon fellowship from the Dutch Research Council to SA (doi.org/10.61686/PQIVV59136), and a postdoctoral fellowship from the US National Institutes of Health to AM (2T32EY027816). Where noted, figures were prepared using Biorender.com.
Author information
Authors and Affiliations
Contributions
Conceptual design and methodology: J.M., N.F.H. Data searching, data collection, and data curation: S.B., S.A., A.X.M., J.M. Data visualization: S.B., S.A., A.X.M., J.M. Writing the original draft and editing: S.B., S.A., A.X.M., J.M., N.F.H. Review and editing: S.B., S.A., A.X.M., J.M., P.S.T., N.F.H.
Corresponding author
Ethics declarations
Competing interests
Author N.H. is a Guest Editor at npj Biomedical Innovations. N.H. was not involved in the journal’s review of, or decisions related to, this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Badawy, S., Anand, S., Marini, A.X. et al. Stem cell-based therapies for treatment of abdominal aortic aneurysm: development, application, and future potential. npj Biomed. Innov. 2, 41 (2025). https://doi.org/10.1038/s44385-025-00044-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44385-025-00044-8