Abstract
Nuclear receptors (NRs) are a large family of ligand-dependent transcription factors that regulate the expression of a wide range of target genes in response to endogenous and exogenous ligands, including steroid hormones, thyroid hormone, vitamin D, retinoic acid, fatty acids, and oxidative steroids. Upon ligand binding, nuclear receptors form dimer complexes with transcriptional cofactors, which interact with specific DNA sequences in the promoter or enhancer regions of target genes to modulate gene expression. This process plays a crucial role in many physiological processes such as reproduction, development, immune responses, metabolism, and homeostasis. Dysregulation of nuclear receptor signaling is implicated in the pathogenesis of numerous diseases, including cancers, metabolic disorders, cardiovascular diseases, and autoimmune conditions. Therefore, understanding the molecular mechanisms underlying nuclear receptor functions is essential for the development of novel therapeutic strategies. This review summarizes the current understanding of nuclear receptors in both physiological and pathological contexts, providing insights into the signaling pathways they regulate. Additionally, we discuss recent advances in drug development targeting nuclear receptors, with a focus on preclinical and clinical studies aimed at improving therapeutic efficacy. By exploring these therapeutic avenues, this article highlights the potential of nuclear receptors as promising targets for future treatments of a variety of human diseases, paving the way for more personalized and effective therapies in clinical medicine.
Similar content being viewed by others
Introduction
A cell’s ability to detect various extracellular signals depends on the chemical nature of the signaling ligands and the permeability of the plasma membrane to polar compounds, such as polypeptides.1,2 This necessitates the production of two main types of signaling ligands and receptors. The first type, represented by growth factors, consists of polypeptides that generally cannot penetrate the cell membrane3,4,5; therefore, their receptors must present sophisticated extracellular binding domains. The second type of nuclear receptor ligand includes small, relatively hydrophobic molecules such as steroid hormones, vitamins, fatty acid derivatives, dexamethasone and enzalutamide.6 The hydrophobicity of these ligands allows them to pass through the cell membrane and reach the nucleus to bind to and activate nuclear DNA-binding proteins that then act as transcription factors.7
Nuclear receptors (NRs) are a class of ligand-activated transcription factors that sense certain molecules, such as steroids, thyroid hormones and vitamins, to directly bind DNA and modulate gene expression, bypassing the need for cytoplasmic signal cascades that mediate surface receptor and nuclear transcription mechanisms.8,9,10 They are essential for numerous physiological processes, including development, metabolism, reproduction, and homeostasis. To date, 48 NRs have been identified in the human genome, and 49 have been identified in mice. The typical structure of a nuclear receptor consists of a hinge region, a conserved ligand-binding domain and a DNA-binding domain, which are surrounded by various N- and C-terminal domains.11 While some nuclear receptors are physically connected to chromatin, others remain in the cytoplasm until ligand interactions allow nuclear entry. These receptors bind to a DNA recognition sequence in or close to the promoters of the genes they control once they are attached to chromatin, either as homodimers or heterodimers.12,13 These recognition sequences are commonly called hormone response elements (HREs) and are composed of two hexanucleotide sequences separated by a variable number of spacer sequences.14 In addition, NRs can be indirectly recruited to the genome by tethering mechanisms by other DNA-bound transcription factors.15 Several posttranslational modifications, such as phosphorylation, ubiquitination and SUMOylation, have been reported to modulate the activities of NRs.16,17,18,19
The regulation of gene expression by nuclear receptors influences the proliferation,20 differentiation21 and death22,23 of many different cell types, thereby playing important roles in the reproduction, development and physiology of metazoans.24,25,26,27 The characterization of the NR superfamily marked a significant turning point in the field of endocrine physiology by establishing common molecular pathways governing the development and physiology of various animal species. The dysregulation of these genes can lead to cardiovascular disease,28 cancer29 and diabetes.30 Research on NRs began in the mid-1980s when molecular cloning of several hormone receptors revealed their common structural framework.31 As a consequence of their central role in governing cell fate, NRs constitute targets for 15–20% of all pharmacologic drugs.32,33
Drugs that target specific NRs are frequently employed to treat a wide range of illnesses. Tamoxifen and raloxifene, for example, target the estrogen receptor (ER) and are used to treat osteoporosis and breast cancer.34,35 Enzalutamide is used to treat prostate cancer by targeting the androgen receptor (AR),36,37 whereas thiazolidinediones are used to treat type 2 diabetes by targeting peroxisome proliferator-activated receptor-gamma (PPARγ).38,39 Compounds with stronger binding affinities and better specificity are currently under development as new NR-targeted drugs because the available drugs often lack specificity and exhibit significant side effects, including severe heart failure.40,41 Thus, understanding the mechanism of NR gene selection and the development of specific inhibitors targeting NRs has become a pressing scientific issue in the field of biomedicine.
In this review, we systematically introduce NRs and their members, structure, functions and regulatory signals. We summarize recent findings regarding the function of NRs in regulating physiology and pathology and the relevance of these receptors to clinical drug development. Emphasis is given to research on the targeting of these receptors for disease management, the preclinical and clinical testing and development of novel drugs and the techniques and strategies that facilitate targeting and therapeutic efficacy.
Overview of nuclear receptors
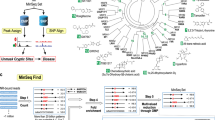
The discovery of hormones dates back to the early 1900s42,43,44,45 (Fig. 1). In 1905, Ernest Starling coined the term ‘hormone’.44,45 In 1926, Tadeus Reichstein and Edward Calvin Kendall elucidated the structures of cortisone and thyroxine,46 while Adolf Butenandt and Edward Adelbert Doisy first isolated estrogen in 1929.47,48 became clear only after Elwood Jensen conducted a series of experiments in the late 1950s to determine how estrogen regulates reproductive organ maturation.49 The rise of molecular biology in the 1980s led to groundbreaking advances, including the cloning of the human glucocorticoid receptor (GR) and the identification of the first estrogen receptor, ERα, from the ESR1 gene.50,51,52 In 1985, Ronald Evans successfully cloned the human glucocorticoid receptor (GR),50,51 while Pierre Chambon’s lab identified the first estrogen receptor, ERα, from the ESR1gene.52 They found that these receptors shared similarities with v-erbA, a viral oncogene recognized as a thyroid hormone receptor (TR) transcribed from the THRA gene. This discovery resulted in the placement of steroid and thyroid hormone receptors into a single grouping. A sequence comparison revealed a conserved evolutionary framework, highlighting structural and functional features that predicted the development of the nuclear receptor superfamily. Each receptor contains DNA-binding, ligand-binding, and transactivation domains. Soon after, receptors for other binding factors, such as retinoic acid and vitamin D, were cloned, confirming the existence of a receptor superfamily unified by structure. The formal establishment of the NR superfamily represented a major milestone, bolstering the hypothesis that the development and physiology of all animal species might be regulated by related molecular processes.
Historical timeline of nuclear receptor expression. Schematic timeline illustrating key milestones in the nuclear receptor research field. The timeline begins with the cloning of the first steroid hormone receptor cDNA and highlights significant advancements, culminating in recent discoveries enabled by “omics” technologies. Major breakthroughs and pivotal discoveries are annotated along the timeline
The therapeutic potential of NRs was recognized in the 1970s when it was shown that the estrogen blocker tamoxifen could inhibit ER-dependent breast cancer cells.53,54 In prostate cancer, multiple nuclear receptors have been shown to inhibit tumor growth, proliferation, and metastasis, leading to significant interest in targeting these receptors as therapeutic strategies.55,56,57 Dysfunction of NRs has been associated with specific diseases, such as infertility, obesity, and diabetes.58 As key drug targets, NRs are crucial in studying the pathological mechanisms of metabolic diseases and related drug development. Today, they represent a huge family of pharmaceutically targetable proteins. This review discusses the current knowledge on NR-based therapeutic intervention in different types of disease.
Family members
The known sequence of the human genome revealed that forty-eight human NRs detect ligands, such as hormones, and translocate them into the nucleus to function as transcription factors regulating gene expression.59 These receptors are classified into three main types on the basis of their ligand types or into eight subgroups on the basis of sequence homology.11,60 Type I receptors are steroid receptors, including the ER, AR, progesterone receptor (PR), mineralocorticoid receptor (MR) and GR.61,62 Their ligands are steroid hormones such as sex hormones, glucocorticoids and mineralocorticoids.63 Type II receptors are nonsteroid receptors, such as thyroid hormones (TRα and TRβ), retinoic acid receptors (RARα, β), vitamin D receptors (VDRs) and peroxisome proliferator-activated receptors (PPARα, β and γ).64 Type III receptors include orphan receptors whose endogenous ligands are unknown or that do not bind to any ligands, such as the testicular receptor and germ cell nuclear factor.65 Alternatively, NRs can be divided into eight subgroups that are similar to classical receptors but differ enough individually, named thyroid hormone receptor-like family, retinoid X receptor-like family, estrogen receptor like family, nerve growth factor IB-like family, steroidogenic factor-like family, germ cell nuclear factor-like family, and those with two DNA-binding domains, as well as recently identified typically structured NR (CgNR8A1) in metazoans such as mollusks, annelids, cnidarians, echinoderms and hemichordates.66,67
Structure
Except for the dose-sensitive sex reversal-adrenal hypoplasia congenital critical region on the X chromosome, gene 1 (DAX1) and the small heterodimer partner (SHP), all NRs are typically single-chain polypeptides that share four or five functional domains,68 including the N-terminal transcription activation domain (NTD), the DNA-binding domain (DBD), the ligand-binding domain (LBD), the C-terminal transcription activation domain (CTD) and a hinge domain (H) that connects the DBD and LBD (Fig. 2).
Diagram of the structural model of nuclear receptors. Nuclear receptors share four functional domains: the N-terminal transcription activation domain (NTD), the DNA binding domain (DBD), the ligand binding domain (LBD), and a hinge domain (H) that connects the DBD and LBD. Some NRs also contain a highly variable C-terminal transcription activation domain (CTD)
The NTD, which is located at the N-terminus and comprises 25–603 amino acids (AAs), contains the first of two transactivation regions (AF-1) and possesses transcriptional activator functions.69,70 The A/B domain is intrinsically disordered, which makes obtaining full-length 3D representations of the receptor difficult.71,72 The second identified subunit is the DBD, with 66--68 AAs, including two zinc fingers, which are typically obscured because of lower DNA affinity when the hormone is absent.73,74,75 Once a hormone is bound, the zinc fingers dock the hormone–receptor complex to hexanucleotide response elements located within NR-regulated promoters that determine the receptor’s regulatory specificity. The DBD also serves as an intermolecular interaction site for receptor dimerization.76 The LBD is located at the receptor’s C-terminus and contains 220--250 AAs.77 The LBD binds to the cognate hormone or ligand through an interior binding pocket.78,79 It also has an AF-2 site for recruiting various coactivating proteins, such as heat shock proteins (HSPs), to facilitate interactions with chromatin-remodeling proteins and the transactivation domain.80,81,82 The LBD plays a crucial role in mediating self-assembly reactions, such as dimerization or tetramerization, which are essential for high-affinity binding to DNA response elements.83 The DBD is connected to the LBD via a flexible domain, the ‘hinge’, which is related primarily to the nuclear localization signal of NRs and may influence the intracellular trafficking and subcellular distribution of the hormone‒receptor complex.84,85 The hinge region also contains a short NLS, and its phosphorylation is coupled to elevated transactivation.86,87
However, not all NRs have all four typical domains. Members of the NR0 subfamily, for example, frequently have neither a DBD nor an LBD in their structures.88 Among these, NRs with only the LBD and no DBD, such as Dax-1 and SHP, are nonetheless capable of binding to other transcription factors (TFs) and suppressing the expression of genes downstream. Finally, some NRs also possess a short, highly variable C-terminal domain (CTD or F domain) that often has unknown functions.89,90 However, current research has revealed a critical function of the F domain in the ERα reaction to select ER modulators (SERMs), particularly in SERM-mediated LBD dimerization and AF-1 activity.91
Origins and evolution
NRs were thought to have evolved approximately 600 million years ago in the common ancestor of bilaterian animals, enabling them to sense and respond to internal and external signals.92 This evolution likely involved genetic amplification and mutation, contributing to the functional diversity of the NR superfamily.93 Most NR ligands are small, hydrophobic molecules that pass through cell membranes, although some, such as thyroid hormones, are actively transported into cells.94 While NRs were initially termed ligand-binding proteins, it remains debated whether their common ancestor bound ligands or functioned as constitutively active receptors. Over 50% of NRs are orphan receptors lacking known ligands, although some have been shown to bind metabolites, suggesting a role in sensing metabolic changes.95,96 The evolution of the LBD enabled interactions with small molecules and coregulators, increasing NR versatility.97 Gene duplication during early metazoan evolution expanded and specialized the NR superfamily into subfamilies such as steroid hormone receptors, thyroid hormone receptors, and orphan receptors. The coevolution of NRs and their ligands increases their physiological complexity. For example, vertebrate NRs, such as glucocorticoid and estrogen receptors, have evolved high specificity for steroid hormones, which are crucial for the stress response and reproduction.98
In 1992, a comparison of the DNA-binding domains of known NRs led to the construction of a phylogenetic tree, suggesting a common ancestor.99 By 1997, an alternative hypothesis emerged, suggesting that the original NR was an orphan receptor that gradually gained ligand-binding ability.100 The earliest NRs are believed to have evolved as orphan receptors that lack defined ligands and are involved in regulating fundamental processes such as metabolism and cellular signaling.101 Over time, orphan receptors adapt to regulate broader metabolic pathways, including lipid homeostasis and detoxification. A recent discovery of the NR7 subfamily has filled a critical gap in the understanding of the evolution of NR dimerization capabilities. Phylogenetically situated between class I NRs (monomers or homodimers) and class II NRs (heterodimers), the NR7 subfamily acts as a “missing link” in the transition from homodimerizing to heterodimerizing nuclear receptors.102
Mechanisms of action
Nuclear receptors can modulate gene expression through direct binding to target genes as well as through interactions with other NRs.103,104 The interplay between NR actions can be described at progressively more detailed levels, with various regulatory modes involved in upstream events that influence downstream gene transcription and crosstalk events at the DNA level that directly influence gene transcription.105 The existence or lack of physical connections between the relevant NRs could further alter the crosstalk mechanisms at the DNA level.
Crosstalk between nuclear signalings
Nuclear receptors can share overlapping arrays of target genes or regulate distinct genes that are part of the same downstream activity or pathway (Fig. 3). There is a wealth of experimental evidence to support endpoint modifications in gene expression that occur after NR crosstalk. The relationship between ERα and the PR was among the first observed examples of crosstalk between nuclear receptors.106 Estradiol-stimulated ERα activity was found to be suppressed by liganded PR isoforms, and the ligand, promoter and cell type were demonstrated to influence this suppressive crosstalk.106,107 Interactions between GRα and PPARα are critical in regulating shared subsets of target genes and different stages of signal transduction cascades involved in inflammatory and metabolic pathways.108,109 For example, both GRα and PPARγ have been shown to repress genes associated with TLR4- and TLR9-induced innate immune responses in primary peritoneal macrophages.110,111 However, there are significant differences in how these NRs achieve gene repression, which involves distinct transcriptional corepressor complexes. These variations in regulatory mechanisms highlight the complexity and specificity of gene regulation mediated by GRα and PPARγ in immune and metabolic contexts.
Crosstalk modes of NR signaling. Various regulatory modes involved in NR signaling influence downstream gene transcription. For example, different types of nuclear receptor signaling can simultaneously regulate the expression of the same gene (Modes 1, 3 and 4) or regulate different genes belonging to the same pathway (Mode 2). Different nuclear receptors may share the same target genes (Mode 5). In addition, crosstalk between two NRs can influence each other’s gene or protein expression (Mode 6)
More complex types of crosstalk, such as reciprocal or unidirectional regulation of the expression of NR genes or proteins and ligand availability, can sometimes occur.112,113 For example, glucocorticoid-activated GRα was reported to upregulate PPARα mRNA expression.109,114 Conversely, fenofibrate-activated PPARα was shown to downregulate GRα expression at the posttranscriptional level in a time- and dose-dependent manner.115,116 In rodent adipocytes, antidiabetic PPARγ ligands increase the expression of PEPCK-C mRNA, which encodes the key glyceroneogenic enzyme EC4.1.1.32.117 In contrast, dexamethasone-activated GRα counteracted this induction at the transcriptional level. These results show that the processes involved in NR crosstalk are not unique; different pairs of nuclear receptors can interact through distinct crosstalk mechanisms according to the stimulus, expression level, cell type, pathway and genes involved. It is probable that many other modes of crosstalk will be uncovered as our knowledge expands to include other intracellular organelles and the shuttling of NRs.
Crosstalk between nuclear receptors at the DNA level
At the DNA level, NR crosstalk can take two primary forms13,118 (Fig. 4). In one scenario, NRs physically interact to regulate specific target genes (direct crosstalk). In another context, NRs do not actually connect but still engage in unidirectional or bidirectional interactions (indirect crosstalk). When two nuclear receptors interact directly, they can bind to DNA sequences that are either closely positioned or farther apart. Alternatively, they can bind DNA sequences through the action of other transcription factors. A well-known example of this is the peroxisome proliferator-activated receptor PPARs, which are of three types: PPARα, PPARβ/δ and PPARγ. Most of these proteins are heterodimers connected to retinoid X receptors (RXRs) that regulate gene transcription,119,120 but they can also be fine-tuned by pairing with a different ligand. Multiple coactivators can interact with heterodimer complexes to supplement and stabilize their activity in regulating lipid metabolism, adipogenesis and inflammation.121,122 Further instances of direct NR connections have been reported for steroid receptors. GRα and AR have many target genes in common due to the recognition of partial palindromic repeats of the sequence 5′-TGTTCT-3′.123 GRα–AR heterodimerization has been proposed as the mechanism underlying the mutual inhibition of the transcriptional activities of these two receptors, allowing for differential regulation of specific genes.124,125 Furthermore, AR and GR share significant similarities in the dimerization structure of their LBDs, which sheds new light on the previously reported structure of the GR LBD.126 The mechanisms of direct crosstalk may allow for dual NR ligand control, but the exact conditions for this process have not been determined. Context-specific influences, such as tissue or cell type and differentiation status, are likely to lead to different interaction patterns, resulting in varied biological responses.127,128
Crosstalk modes at the DNA level. At the DNA level, nuclear receptors engage in crosstalk by indirectly or directly interacting with DNA. Part a outlines indirect regulation modes, where no physical interaction occurs, whereas Part b describes regulatory mechanisms involving direct physical interactions among nuclear receptors (NRs). Indirect regulation modes include: NR signaling pathways may compete for overlapping DNA-binding sites; NR heterodimer partners may cause redistribution or squelching; Components of the transcriptional machinery, such as RNA polymerase II (Pol II) and transcriptional coregulators, may cause redistribution or squelching; The alteration of shared coregulators can influence the activity of other NRs. One NR family member can act as a pioneering factor to facilitate chromatin loosening and enable the subsequent binding of other NRs. Direct physical interactions includes: Paired receptors may cooperate for direct DNA binding or through DNA looping; Direct crosstalk can occur with just one partner contacting the DNA; Two nuclear receptors engage in crosstalk through protein-protein interactions with other transcription factors (TFs); The sequestration of one or both NRs (originating from other modes, (f–i) away from the DNA due to heterodimer interactions
In the absence of an actual connection, NR signaling pathways can indirectly influence crosstalk through several mechanisms.129,130,131 They may compete for overlapping DNA-binding sites by redistributing shared proteins or parts of the transcriptional machinery, such as RNA polymerase II or transcriptional coregulators; by regulating the expression of shared coregulators; and by acting as pioneering factors that loosen chromatin to facilitate the binding of additional NRs. For example, peroxisome proliferator-activated receptor alpha (PPARα) binds to estrogen-related receptor (ERR) target genes in heart tissue through ERR response element motifs via a histone deacetylase SIRT1-dependent mechanism.132 RXR, the PPAR partner, can trigger PPAR-independent activities on PPAR response element (RE)-controlled genes via RXR homodimerization, adding another layer of control to an intricate metabolic pathway.133 Members of the PPAR and ERR subfamilies can be further connected through shared coregulators, including PGC1α and PGC1β, as well as through common regulators such as low-energy-sensing AMP kinase.134,135 For example, during adipocyte differentiation and osteoclastogenesis, PGC1β, a coregulator that is upregulated by ERRα, can be recruited by PPARγ and other transcriptional regulators to enhance their adipogenic properties.136 Reduced PGC1β expression levels due to downregulation of ERRα resulted in decreased transcriptional activity of PPARγ. These reports provide evidence for the existence of complex combinations of indirect regulatory modes.
Genomic and nongenomic mechanisms
The function of nuclear receptors can be achieved via genomic or nongenomic pathways (Fig. 5). Genomic mechanisms involve the direct regulation of gene expression through binding to DNA. This mechanism usually plays a critical role in long-term physiological processes, such as development, metabolism, and homeostasis. Nongenomic mechanisms involve rapid, transcription-independent actions of nuclear receptors. These processes occur within minutes and are crucial for swift cellular responses, such as ion channel modulation, cell migration, and acute stress responses.
Nuclear receptors precisely regulate cell fate and metabolic processes. Nuclear receptors play crucial roles in regulating cell fate by influencing various cellular processes, including proliferation, differentiation, senescence and apoptosis, by acting as transcription factors. Additionally, members of the nuclear receptor family control the synthesis rates of different metabolic enzymes across various tissues, affecting processes such as glucose, lipid and bile acid metabolism, as well as adipocyte differentiation and plasma lipoprotein modulation. By regulating the balance between the storage, utilization and production of these macromolecules, nuclear receptors ensure metabolic flexibility, enabling the organism to adapt to fluctuations in energy availability and demand
Genomic mechanisms
Prior to binding to the specific DNA sequences of target genes known as hormone HREs to control transcription, NRs are typically in an inactive state and require activation through the binding of a cognate ligand.15,137 With many receptors, in the absence of ligands, molecular chaperone proteins such as heat shock protein 90 (HSP90) and HSP70 anchor them in the cytoplasm in an inactive state.138 Upon ligand binding, the NR undergoes a conformational shift that causes it to separate from the heat shock protein and become active.139 This allows the hormone–receptor complex to translocate into the cell nucleus after its nuclear localization sequence (NLS) has been revealed.140,141 Once inside, the complex binds to a specific sequence in the target gene known as an HRE to regulate its transcription. In addition to detaching the HSPs, ligand binding can promote receptor phosphorylation, which further enhances the ability of the receptor to bind to HREs.142 In general, NRs undergo a change from a non-DNA-binding form to a DNA-binding form upon activation, but some types, such as thyroid hormone receptors, retinoic acid receptors and retinoid X receptors, are retained in the nucleus regardless of their ligand binding status.143 They are already bound to HREs but complex with corepressor proteins, which makes them transcriptionally inactive.144,145 However, upon receiving ligand activation signals, the corepressor dissociates, and coactivator proteins are recruited. The NR/DNA complex then enlists more proteins, such as RNA polymerase, to aid in the transcription of DNA into mRNA.146,147,148
Nuclear receptors can directly bind DNA via three different modes, namely, homodimers, heterodimers, or monomers, depending on the type of NR.13 For example, glucocorticoid receptors (GRs) bind to HREs after forming homodimers.149,150 In contrast, retinoic acid receptor-related orphan receptor gamma (RORγ) binds as a monomer to the DNA response region to control gene transcription and expression.151,152 The retinoid X receptor (RXR) can either bind to itself as a homodimer or pair with other nuclear receptors as a heterodimer to activate downstream gene transcription.153,154,155 To make this process even more complicated, some NRs can form nonclassical heterodimers, which are only activated by outside ligands. Thus, unanticipated adverse effects could appear when developing therapeutics because NRs can operate through nonclassical routes.156,157,158,159
The identification of coregulatory proteins that interact with and control the transcriptional action of NRs in the mid-1990s was another pivotal development in our knowledge of the mechanism of action of NRs.160,161 These coregulators have a variety of functions, such as chromatin remodeling, which modifies the target gene’s transcriptional accessibility, or bridging, which maintains the binding of other coregulatory proteins.162 The intrinsic histone acetyltransferase (HAT) activity of coactivator proteins can promote gene transcription by reducing the affinity of histones for DNA.163 On the other hand, the recruitment of histone deacetylases (HDACs) by corepressor proteins increases the number of bonds between histones and DNA and suppresses gene transcription.164 These coactivators or corepressors enable nuclear receptors to function as inducible scaffolds that coordinate large transcriptional complexes, allowing different ligands to induce distinct receptor conformations.163,165 These changes could expose specific protein–protein interaction surfaces, making them available for coregulator binding. This hypothesis provides an explanation for how structurally different ligands, such as selective estrogen receptor modulators, could influence multiple genes in the same cell via the same receptor or how they could jointly regulate the expression of a single gene. Depending on the tissue-specific location of coregulators, the same ligand can also control specific genes in different tissues or at different developmental stages.166,167 Nuclear receptor/coregulator complexes that alter chromatin shape and DNA accessibility are produced by receptor-bound coregulators, which can also directly interact with the core RNA polymerase through their enzyme action. In addition, there is no set link between two distinct nuclear receptors, as they can change depending on the target genes and tissues that are engaged. Transcriptional regulation by NRs can be affected by epigenetic variables such as chromatin remodeling, DNA methylation and histone modification.168,169
Nongenomic mechanisms
In addition to acting as transcription factors in the nucleus to regulate target gene expression (genomic function), NRs can also operate through a molecular mechanism independent of transcriptional activity (nongenomic function)170,171,172 (Fig. 5, Fig. 6). This nongenomic role requires NRs to be localized to specific subcellular structures,173,174,175 where they directly interact with various intracellular proteins to rapidly regulate cellular stress responses and signal transduction pathways. Their subcellular translocation can occur either by alternative splicing or posttranslational modifications through free shuttling or with the assistance of other proteins.176,177 As an example, the plasma membrane is home to a truncated variant of the thyroid hormone receptor. This localization enables them to participate in several biological processes, including the endoplasmic reticulum stress response, apoptosis, autophagy, fatty acid oxidation within mitochondria and the regulation of centrosome homeostasis and cell mitosis. Nongenomic NR signaling frequently involves phosphorylation cascades mediated through pathways associated with AMPK, cAMP/PKA, PI3K/AKT, and Hippo/Yap and signaling via Wnt/β-catenin and MAPK.178,179,180,181,182,183
Platelets are good models for investigating the possible nongenomic impacts of nuclear receptors. Because of their anucleate origin, well-characterized mechanisms, and quick reactions, platelets offer a model system for examining any non-genomic impacts of the NRs. Non-genomic roles of NRs include controlling intracellular calcium levels, ion channel function, kinase and phosphatase activity, and second messenger synthesis. It has been discovered that human platelets contain a number of NRs, and that certain NR agonists have anti-platelet actions through a range of mechanisms
Several NRs have been found to propagate rapid nongenomic effects for cellular protection in addition to their defined genomic effects. Their nongenomic functions operate through the control of kinase/phosphatase activity,184,185 intracellular calcium levels,186,187 ion channel function188,189 and the production of second messengers.190 Accumulating data indicate that RXRα also possesses extranuclear nongenomic functions. Previous studies have demonstrated that RXRα engages in some nongenomic activities through the creation of heterodimers with Nur77.191 In response to specific apoptotic stimuli, RXRα-Nur77 heterodimers are translocated from the nucleus to the mitochondria, where they regulate apoptosis.192 Recent research has shown that truncated RXRα (tRXRα) has an important function in the PI3K/AKT signaling pathway, where it interacts with the p85α subunit to increase AKT activation and promote cell proliferation both in vitro and in vivo.193,194 Notably, glucocorticoids (GCs) manifested nearly instantaneous nongenomic effects through nonspecific interactions with cell membranes and targeted interactions with cytosolic GRs (cGRs) or membrane-bound GRs (mGRs).124,195,196 This rapid nongenomic action of GCs could also contribute to their anti-inflammatory effects through prolonged genomic processes, particularly in managing inflammatory diseases (Fig. 6).197,198 For example, recent studies revealed that GCs quickly enhanced the effects of bronchodilators used to treat allergic asthma.199 Other NRs, including PPARα, LXR and FXR, have also been reported to perform nongenomic functions.189,191,200 The possibility of turning NR ligands into effective medicines should be explored through the characterization of these extragenomic processes and the discovery of new binding partners and pathways in addition to their known genomic effects.
Platelets are useful models for investigating the potential nongenomic effects of NRs because they lack nuclei and possess well-characterized reactions.189,201 ERβ, but not ERα, is known to be expressed on human platelets and to affect their function.202 In addition, some studies have shown that platelets isolated from male rats exhibit a stronger aggregation response than those from female rats do, likely due to an increase in androgenic steroids.203,204 This conclusion was supported by findings that castration reduced platelet aggregation in male rats,205 an effect that was reversed with testosterone treatment.206 LXR and FXR ligands negatively regulate platelet function by inhibiting platelet signaling and the formation of procoagulant-coated platelets.207,208,209,210 For example, LXR ligands induce a procoagulant state in platelets, which is marked by the exposure of phosphatidylserine and α-granule contents on the surface of the platelet, coupled with mitochondrial membrane depolarization, reduced calcium mobilization and decreased affinity of integrin αIIbβ3, which ultimately inhibits platelet aggregation.209,211 Similarly, FXR ligands can cause platelet swelling and transformation into procoagulant-coated platelets, a process dependent on cyclophilin D activity.212 FXR ligands also increased cGMP levels, which increased PKG activity and VASP S239 phosphorylation, further suppressing platelet activation.189 Other studies have identified PPARα as a key mediator of the antiplatelet effects of statins and fenofibrate.213 Treating platelets with PPARα ligands, such as fenofibrate or statins (e.g., simvastatin), inhibited ADP-stimulated platelet activation by increasing intracellular cAMP levels through a PPARα-dependent mechanism.214 This role of PPARα was further confirmed by experiments showing that fenofibrate-induced inhibition of platelet activation and prolonged bleeding time were absent in PPARα-deficient mice215; the reversion of this inhibitory effect by the PPARα antagonist GW6471 further highlighted its dependence on PPARα.
Subsequent studies revealed the unexpected presence of retinoic acid receptors (RARs) in the cytosol of Sertoli cells, hepatic stellate cells and neurons.216,217,218 In Sertoli cells, RARα was found to be a substrate for small ubiquitin-like modifier-2 (SUMO-2), and its cytosolic or nuclear localization was influenced by a dynamic process of sumoylation and desumoylation.219,220 Additionally, in Sertoli and hepatic stellate cells, RARα is retained in the cytosol through interactions with the cytoplasmic adaptor for RAR and TR (CART1) protein or with cytoskeletal proteins that sequester the receptor outside the nucleus.144 Furthermore, in hippocampal neurons, independent studies have shown that RARα is exported to dendritic RNA granules, where it is associated with a specific subset of mRNAs and RNA-binding proteins.221,222,223 Interestingly, in response to retinoic acid (RA), this extranuclear pool of RARα rapidly initiates the local translation of the postsynaptic glutamate receptor GluR1, leading to an increase in synaptic strength.224,225 In light of this growing body of knowledge, understanding these nongenomic mechanisms can lead to the development of new therapeutic strategies that exploit the rapid and diverse roles of nuclear receptors outside of their traditional genomic functions. By targeting the nongenomic pathways of nuclear receptors, it may be possible to create drugs with faster onset times and more specific effects, thereby increasing the efficacy of treatments for various diseases.
Functions in physiology and pathology
Nuclear receptors are of key importance in physiology and pathology because they regulate multiple cellular functions (Fig. 5). For example, NHR-6, the only NR4A-type nuclear receptor in the roundworm Caenorhabditis elegans, has been implicated in the development of the nervous system.226 During development, the nervous system produces highly specialized neurons with distinct functional characteristics. A prime example is the C. elegans BAG chemosensory neurons.227 Under certain circumstances, BAG neurons sense the concentration of carbon dioxide in respiratory gas and translate it into reactions of attraction or repulsion; ETS-5 and its target gene NHR-6 are essential for this process.228 In contrast to ETS-5, which is engaged in both the attraction and avoidance of carbon dioxide, NHR-6 is an NR that is exclusively involved in the attraction process.229 These findings imply that gene regulatory mechanisms supported by NR subtypes have undergone evolutionary conservation. NHR-6 is necessary for BAGs to exhibit exceptional capacity to adaptively assign positive or negative valences to chemosensory stimuli, and it is a key modulator of the functional plasticity of the neural system.226 It is clear that NRs are of the greatest importance for the proper functioning of numerous processes in cellular lipid metabolism,230,231 energy homeostasis232 and the inflammatory response233; cell proliferation and differentiation are critically dependent on nuclear receptors.
Pathological changes in nuclear receptors, such as mutations, altered expression levels, or dysregulated activity, are linked to a wide range of diseases, including cancer, metabolic disorders, inflammation, and cardiovascular conditions. For example, the overexpression of ER in breast cancer drives tumor growth,234 whereas aberrant AR can cause therapy resistance in prostate cancer.235 Impaired PPAR function is associated with metabolic disorders such as obesity and type 2 diabetes,236 whereas dysregulated liver X receptors (LXRs) disrupt cholesterol homeostasis, exacerbating cardiovascular disease.237 Aberrant GR activity exacerbates inflammatory conditions and may induce corticosteroid resistance. Altered retinoid X receptor (RXR) function has been linked to neurodegenerative diseases such as Alzheimer’s disease. These pathological changes not only hold diagnostic and prognostic value but also serve as therapeutic targets. For example, ER, PR, and HER2 expression levels guide breast cancer classification and treatment, with SERMs such as tamoxifen proving effective in ER-positive patients. AR inhibitors are crucial in prostate cancer, where AR expression levels and mutations influence therapeutic outcomes. Additionally, radiolabeled ligands such as [F-18]-estradiol enable imaging of ER-positive tumors via PET scans,238 aiding in diagnosis and treatment monitoring. In summary, nuclear receptor alterations play a dual role in clinical practice as both therapeutic targets and biomarkers, underscoring their critical contribution to precision medicine and improved patient outcomes.
Cell fate
Nuclear receptors are essential for regulating cell fate through their function as transcription factors with effects on proliferation,239 differentiation,240 senescence241 and apoptosis.242 Through nongenomic mechanisms, NRs modulate signaling pathways that determine whether a cell will continue to divide, differentiate into a specific cell type, or undergo programmed cell death.243
The ER has been the most studied ER, mainly because of the estrogen dependence of the growth of certain breast cancer cells. The function of estrogen receptor alpha (ERα/ER) defines the most common type of breast cancer.244,245 Numerous associated elements that function as regulators of estrogen-driven transcriptional pathways have been discovered, indicating that the ER cannot function solely by itself.246 Enhancer sequences are cis-regulatory ER binding sites that are located mainly away from transcriptional start sites, according to genome-wide profiling of chromatin binding.247 There is growing evidence that global enhancer activation is involved in tumor aneuploidy and carcinogenesis.248 Short DNA enhancer elements are crucial for maintaining a coordinated gene expression program specific to cell types during development and differentiation.249 Other nuclear receptor ligands, such as all-trans retinoic acid (atRA) and 1,25(OH)2D3, induce cell differentiation.250,251,252 RA, the primary bioactive metabolite of retinol (vit A), has a range of pleiotropic effects on cell growth and differentiation, which are important in adult physiology and embryonic development.253 Members of the NR class of transactivators, specifically the retinoic acid receptors RARα, RARβ and RARγ, are the main mediators of activity.254 For example, prostate cancer-induced bone formation is decreased, and the endothelial-to-osteoblast transition (EC-to-OSB) is inhibited when the retinoic acid receptor (RAR) is activated.255,256 Treatment with the RARγ agonist palovarotene, which has been tested for heterotopic ossification in fibrodysplasia ossificans progressiva, inhibited osteoblast mineralization and the EC-to-OSB transition in vitro. It also reduces the growth and formation of tumor-induced bone in several models of osteogenic prostate cancer.255 Compared with mature hepatocytes, hepatocytes induced in a 3D environment exhibited lower activity of the nuclear receptor THRB. The addition of THRB ligands during differentiation upregulated not only the expression of CYP3A4 but also the expression of multiple hepatocyte-related genes and increased histone acetylation; the induced differentiated hepatocytes were more similar to mature hepatocytes.21 In addition, the nuclear receptor Nur77 regulates the expression of genes involved in fatty acid absorption and transport, thereby blocking the uptake of exogenous fatty acids by cells and inhibiting the signaling pathways that promote breast cancer cell proliferation.27 These findings highlight the significant role of NRs in controlling cell growth and differentiation.
NRs have also been shown to be involved in cellular senescence and cell death processes. Three members of the NR4A1/Nur77/NGFIB orphan nuclear hormone receptor subfamily (NR4A1, NR4A2 and NR4A3) have been the most studied. NR4A2 was reported to maintain anchorage-independent growth, thereby reducing cell death, known as anoikis, in HeLa cells.257 Following acute kidney injury (AKI), Nur77 and its family members Nurr1 and Nor-1 stimulate epithelial apoptosis. A conformational shift in Bcl2 and an increase in proapoptotic Bcl-xS protein levels are involved in Nur77-mediated kidney damage.258 Furthermore, YAP controls the transcription, phosphorylation and mitochondrial localization of NR4A1 to mediate the proapoptotic and antitumor actions of the Hippo pathway. In turn, NR4A1 acts as a feedback inhibitor of YAP, leading to its destruction, which prevents YAP from participating in carcinogenesis and liver regeneration.259 In addition, Nur77 interacts with DNMT3b, increasing GLS1 promoter methylation and decreasing GLS1 expression and glutaminolysis, which leads to the induction of HSC senescence.260 Hepatocyte nuclear factor 4α (HNF4α, NR2A1), another strongly conserved NR superfamily member, suppresses prostate tumorigenesis by arresting the cell cycle at the G2/M phase and p21-driven cell senescence.261 NR subfamily 1 group I member 2 (NR1I2), also called PXR (pregnane-X receptor) or PAR (pregnane-activated receptor), has been implicated in blunting the expression of the proapoptotic genes TP53 and BAK1 (BCL2 antagonist/killer 1) to prevent apoptosis from toxic bile acids.262 The retinoid RXRα has been shown to suppress the production of ITPR2 and control calcium signaling via ITPR2 and the mitochondrial calcium uniporter (MCU). After reducing the degree of DNA damage, the overexpression of RXRα delays replicative senescence.263 ZBTB17, a zinc finger protein, was shown in another study to interact with RXRα. Notably, ZBTB17 knockdown initiates a series of events, including DNA damage, decreased mitochondrial membrane potential (MMP), RXRα-dependent intracellular calcium signaling and cellular senescence.264 Under metabolic and genotoxic stresses, LXR/CD38 activation promoted lysosomal cholesterol efflux and nicotinamide adenine dinucleotide (NAD+) depletion in macrophages, which caused cholesterol-induced macrophage senescence and neurodegeneration.265
Cellular metabolism
Members of the NR family control the pace at which different metabolic enzymes are synthesized in different tissues,266 which affects processes including the metabolism of glucose, lipids and bile acids; adipocyte differentiation; and plasma lipoprotein modulation.267,268 By influencing the balance between the storage, utilization and production of these macromolecules, NRs ensure metabolic flexibility, allowing the organism to adapt to fluctuations in energy availability and demand (Fig. 7). Their regulatory functions are particularly critical during periods of fasting, stress, or increased physical activity, where their precise control is essential for maintaining overall metabolic health.
Examples of nuclear receptors involved in regulating metabolic pathways. Cellular metabolic pathways provide the energy and materials essential for cell survival. Nuclear receptors transcriptionally regulate genes that encode nutrient transporters and metabolic enzymes involved in these processes. The pathways include the following: 1. Glycolysis, 2. Pentose phosphate pathway (PPP), 3. Gluconeogenesis, 4. Lactate oxidation, 5. Autophagy, 6. Amino acid catabolism, 7. Redox homeostasis, 8. Mitochondrial biogenesis/dynamics, 9. Oxidative phosphorylation (OXPHOS), 10. Tricarboxylic acid (TCA) cycle, 11. Ketogenesis/ketolysis, 12. Glutaminolysis, 13. Ca2+ homeostasis, 14. Fatty acid oxidation (FAO), 15. Lipolysis
Glucose metabolism and gluconeogenesis
Glucose is the primary metabolic fuel for mammals and plays a crucial role in maintaining energy homeostasis.269,270 During the fed state, circulating glucose originates primarily from the absorption of nutrients in the intestines.271 However, under fasting or energy-demanding conditions, the body relies on two additional sources to maintain glucose levels: the breakdown of stored glycogen through glycogenolysis and the production of new glucose molecules via gluconeogenesis.272,273,274 These complex metabolic processes are intricately regulated, and NRs play a key role in coordinating them. As molecular sensors, NRs detect changes in the body’s energy status and modulate the expression of genes involved in glucose metabolism.275,276 The activation of PXR by its ligand, pregnenolone-16α-carbonitrile (PCN), impaired glucose tolerance by dysregulating glucose transporter 2 (GLUT2) function through two distinct mechanisms.277 First, it reduced the expression of GLUT2 in both mouse liver and wild-type hepatocytes. Second, it triggers the transport of GLUT2 from the plasma membrane to the cytosol in the liver, thereby suppressing glucose uptake in primary hepatocytes. Additionally, data mining of published chromatin immunoprecipitation/sequencing results revealed that the Glut2 gene is directly targeted by PXR. These findings may explain the diabetogenic effects of certain medications and environmental contaminants, positioning PXR in a potential novel diabetogenic pathway. At physiological concentrations found in the liver, glucose binds to and activates the transcriptional activity of LXRs, inducing the expression of LXR target genes with similar efficacy to that of oxysterols, which are well-known LXR ligands. Cholesterol homeostasis genes, which depend on LXR for their expression, were upregulated in the liver and intestines of fasted mice after refeeding with a glucose-rich diet,278 suggesting that glucose acted as an endogenous ligand for LXR.
Nuclear orphan receptor subfamily 4 group A member 1 (NR4A1), an important regulator of hepatic glucose homeostasis, was found to interact with the nuclear glycerol kinase Gyk during hepatic gluconeogenesis in the unfed state and in diabetes. Gyk acts as a corepressor of NR4A1, which attenuates the expression of target genes involved in hepatic gluconeogenesis and obstructs blood glucose regulation.279 Nuclear receptor subfamily 5 group A member 2 (NR5A2 or LRH1) and steroidogenic factor 1 (SF1 or NR5A1) work synergistically as key regulators of glucose-sensing systems in hepatic and steroidogenic tissues.280 These receptors are essential for the normal processing of glucose after eating through direct control of GCK and HK1 transcription.281 FXR combines signals from PKA and FOXA2 for hepatic glucose metabolism and production through two regulatory arms.282 The first is the phosphorylation of FXR by protein kinase A, which induces glucagon, agonist-activated FXR and CREB to combine and activate gluconeogenic genes. The physical interaction between FXR and the glucagon-activated FOXA2 transcription factor constitutes the second arm, which prevents FXR from stimulating the anti-gluconeogenic nuclear receptor SHP. Foxa2 knockdown did not influence the expression of glucagon-induced or FXR agonist-boosted gluconeogenic genes, suggesting that distinct subsets of FXR-sensitive genes are controlled by the PKA and FOXA2 pathways.282
NR coregulators play crucial roles in the regulation of glucose metabolism. For example, NR corepressors (NCoRs) operate within multiprotein complexes containing histone deacetylase 3 (HDAC3) to inhibit transcription, primarily through repressive chromatin remodeling at target loci. This process ultimately drives glucocorticoid receptor-dependent activation of hepatic gluconeogenesis. In hepatocytes, the double knockout of both NCoR1 and NCoR2 mimicked the hepatomegaly and fatty liver phenotype observed in HDAC3 knockouts by preventing glucocorticoid receptor binding.283 Similarly, nuclear receptor coactivator 3 (NCOA3) regulates the transcription of tyrosine-protein kinase (Fyn) in a PPARγ-dependent manner. NCOA3 deficiency was shown to worsen diabetic kidney disease by exacerbating albuminuria, glomerular sclerosis, and podocyte injury and impairing autophagy.284
Bile acid, sterol and lipid metabolism
Increasing evidence suggests that NRs play critical roles not only in glucose metabolism but also in the regulation of sterol, bile acid and lipid metabolism.285,286,287 The three ERR isoforms, as well as PPARα and PPARδ, are the main transactivators of genes involved in mitochondrial bioenergetics and fatty acid oxidation (FAO) and are viewed as primary controllers of mitochondrial FAO.288,289 For example, in addition to being a regulator of mitochondrial function, ERRα was established as a major transcriptional regulator of lipid biosynthesis and a promoter of nonalcoholic fatty liver disease (NAFLD/NASH).290 Medium-chain specific acyl-CoA dehydrogenase (ACADM), the first-discovered target gene of ERRα, encodes the initial rate-limiting enzyme in mitochondrial FAO; the promoter is also targeted by PPARα.291 In addition, a muscle-specific protein induced by PGC-1 and ERRs, PERM1 (PGC-1/ERR-induced regulator in muscle-1), promoted mitochondrial biogenesis and metabolism in cardiomyocytes. In this study, PERM1 was shown to interact with the proximal regions of PPAR response elements (PPREs) in the endogenous promoters of genes involved in fatty acid oxidation, and further results revealed that PERM1 interacted with PPRE to promote transcription, partly in a PPARα- and PGC-1α-dependent manner.292
PPARα is also a key regulator of fatty acid oxidation and can induce spontaneous fatty liver and hyperlipidemia in mice fed a standard diet. For example, during the early night, fasting mice exhibited acute PPARα-dependent hepatocyte activity, accompanied by increased circulating free fatty acids that could be further stimulated by adipocyte lipolysis. However, fasting resulted in mild hypoglycemia and hypothermia in PPARαhep−/− mice, suggesting a role of PPARα activity in nonhepatic tissues.293 Liver-specific inactivation of Vps15, the essential regulatory subunit of class 3 PI3K, elicited mitochondrial depletion and failure to oxidize fatty acids through blunting of the transcriptional activity of PPARα. Vps15 deficiency led to the accumulation of the PPARα repressors HDAC3 and nuclear receptor corepressor 1 (NCoR1) in the liver due to disrupted autophagy. Activation of PPARα or inhibition of HDAC3 restored mitochondrial biogenesis and FAO in Vps15-deficient hepatocytes, revealing functions for class 3 PI3K and autophagy in the transcriptional coordination of mitochondrial metabolism.294 In addition, hepatic Ncor1 deletion in mice retarded atherosclerosis development by reprogramming bile acid metabolism and enhancing fecal cholesterol excretion, resulting in reduced plasma cholesterol levels and decreased hepatic cholesterol content in liver-specific Ncor1 knockout mice compared with those in controls fed an atherogenic diet for twelve weeks.295
NR4A1 was identified as a previously unrecognized constitutive regulator of adipocyte progenitor (AP) quiescence.296 In ex vivo experiments, NR4A1 gain-of-function reduced adipogenesis, whereas its loss-of-function increased adipogenesis.296 Compared with control mice, NR4A1 knockout mice fed a high-fat diet were more prone to obesity, with increased gene expression of PPARγ and FAS. Conversely, NR4A1 overexpression in 3T3-L1 preadipocytes inhibited adipogenesis. NR4A1 upregulated GATA binding protein 2 (GATA2), which in turn inhibited PPARγ. NR4A1 also suppressed sterol regulatory element-binding protein 1 (SREBP1) and its downstream target fatty acid synthase (FAS) by upregulating p53. Overall, NR4A1 inhibits adipocyte differentiation and lipid accumulation by increasing the expression of GATA2 and p53.297 Nuclear receptor subfamily 0 group B member 2 (NR0B2, also called SHP) is expressed at high levels in the liver and intestine, and its transcriptional activity can be increased by fibroblast growth factor 19 (human FGF19, mouse FGF15) signaling. FGF19 and SHP were observed to inhibit SREBF2 (sterol regulatory element binding transcription factor 2), which led to a reduction in intestinal NPC1L1 expression, cholesterol absorption and hypercholesterolemia.298 Another member of the nuclear receptor family, NR1A1, was reported to influence hepatic autophagy, lipid metabolism and adipocyte homeostasis by interacting with MED1 through a bridge formed by PGC1α.299,300
The expression of RORα/β/γ and REV-ERBα/β in the liver follows circadian rhythms. The liver genome contains high-order transcriptionally repressive hubs where REV-ERBα condensates are found, and these hubs are strongly associated with circadian gene repression.301,302,303 In mouse models, RORs bind to ROREs at night, whereas REV-ERBα/β bind to ROREs during the day to decrease lipogenesis. In the liver, REV-ERBα and REV-ERBβ work in concert, increasing the ability of HDAC3 and NCoR to control SREBP activity and maintain lipid homeostasis. They also affect cytochrome P4507A1 expression levels, which helps to maintain the equilibrium of bile acid metabolism.304 RORγ serves as a crucial activator of the entire cholesterol biosynthesis program, controlling SREBP2 by binding to genes involved in cholesterol biosynthesis and facilitating SREBP2 recruitment. At the loci of genes involved in cholesterol production, RORγ inhibition decreases chromatin acetylation and disrupts its connection with SREBP2. In immunocompetent animals and patient-derived xenografts, RORγ antagonists induce tumor regression.305
The regulation of bile acids, sterols and lipids is also associated with many other types of nuclear receptors. Through the regulation of Akr1b7 transcription, the nuclear receptor PXR effectively reduces AKI by increasing reactive oxygen species (ROS) generation, mitochondrial autophagy and mitochondrial dysfunction in lipid metabolism.306 It was recently demonstrated that the function of FXR in BA homeostasis depends on the phosphorylation of Tyr-67 by the FGF15/19 signaling-activated nonreceptor tyrosine kinase Src. According to Byun and colleagues, hepatic FXR phosphorylation by FGF15/19-induced Src preserves cholesterol homeostasis and protects against atherosclerosis.307 PXR activation upregulated intermediates in the Kandutsch–Russell cholesterol synthesis pathway in the liver and induced a number of cholesterol synthesis genes, including the rate-limiting HMRCR. In both mice and humans, PXR activation increased the level of plasma proprotein convertase subtilisin/kexin type 9 (PCSK9), a negative regulator of hepatic LDL absorption. A new regulator of PCSK9 and cholesterol synthesis, the PXR-SREBP2 pathway, also serves as a molecular mechanism for drug- and chemical-induced hypercholesterolemia.308
Mitochondria, OxPhos and the TCA cycle
Mitochondria serve as vital energetic and biosynthetic signaling hubs, taking in substrates from the cytoplasm to generate bioenergetic and biosynthetic building blocks.309 This process is achieved through the coordinated activity of several key metabolic pathways, including the electron transport chain (ETC), oxidative phosphorylation (OxPhos), the tricarboxylic acid (TCA) cycle, glycolysis, glutaminolysis and fatty acid oxidation (FAO). These pathways not only produce ATP but also provide intermediates essential for biosynthesis. Crucially, the function of mitochondria is tightly regulated by various nuclear receptors, which modulate gene expression to ensure proper mitochondrial activity and metabolic balance.
Several nuclear receptors are involved in regulating genes associated with the TCA cycle and OxPhos, including estrogen-related receptor alpha (ERRα), NUR77 (NR6A1) and PPARγ.310,311,312 One of the first major datasets used to highlight the role of ERRα in OxPhos regulation was a study that integrated PGC1α-induced GW transcriptional profiling with a software approach to identify cis-regulatory sequences.313,314 Mice lacking both ERRα and ERRγ presented the most extensive and profound disruption in skeletal muscle gene expression, with the ‘mitochondrial function’ pathway genes involved in OxPhos and the TCA cycle being the most significantly affected. Moreover, mice deficient in ERRβ and ERRγ exhibit impairments in lipid metabolism and branched-chain amino acid metabolism, specifically in the soleus muscle.315 Similarly, muscle-specific deletion of Esrrg (the gene encoding ERRγ) in mice reduces exercise capacity because of deficient mitochondrial activity.316 adipocytes lacking all ERR isoforms exhibited significant downregulation of genes involved in oxidative metabolic pathways, including those controlling the TCA cycle and OxPhos, leading to marked reductions in mitochondrial content and oxidative capacity.317 These results suggest that many mitochondrial actions are mediated through the stimulation of ERR expression and activity, underscoring the critical role of ERR in mitochondrial energy metabolism across various tissues.
The nuclear receptor Nur77 (also known as TR3 or NGFI-B), encoded by Nr4a1, belongs to the steroid/thyroid/retinoid superfamily and serves as a key regulator of energy metabolism. Nur77 is phosphorylated by ERK2 and translocated to the mitochondria upon glucose deprivation. Mitochondrial Nur77 binds to TPβ, a rate-limiting enzyme in FAO, to protect it from oxidation. This promoted the metabolic adaptation of melanoma cells by evading ROS-induced cell death.318 Ubiquitinated mitochondrial Nur77 interacts with the ubiquitin-binding domain of p62/SQSTM1 to form condensates capable of sequestering damaged mitochondria. An additional interaction between the N-terminal intrinsically disordered region (IDR) of Nur77 and the N-terminal PB1 domain of p62/SQSTM1 allows tethering of clustered mitochondria to the autophagy machinery, which endows Nur77-p62/SQSTM1 condensates with the magnitude and liquidity to act on the mitochondria.319 In response to the pathological accumulation of α-synuclein (α-syn) fibrils in Parkinson’s disease (PD), Nur77 is translocated from the cytoplasm to the mitochondria to improve PHB-mediated mitophagy by regulating c-Abl phosphorylation. Nur77 overexpression alleviated the expression of pS129-α-syn and protected dopamine (DA) neurons from the loss of α-syn in PD.320
NR1D1 (nuclear receptor subfamily 1 group D member 1) is the most highly upregulated nuclear receptor in abdominal aortic aneurysm (AAA) tissues. Knockout of Nr1d1 in vascular smooth muscle cells (VSMCs), but not in endothelial or myeloid cells, led to significant inhibition of AAA formation. Mechanistic investigations revealed that ACO2 (aconitase-2), a key enzyme in the TCA cycle, is a direct transcriptional target of NR1D1. By repressing ACO2, NR1D1 modulates mitochondrial metabolism. In the absence of NR1D1, ACO2 expression is restored, which subsequently corrects mitochondrial dysfunction during the early stages of Ang II infusion, even prior to the onset of AAA development.321
Catabolism of amino acids
Amino acid catabolism is vital during starvation to sustain glucose levels and provide alternative carbon sources, primarily in the liver but also in the kidneys, muscles and adipose tissue.322 This process involves transamination and oxidative deamination in the cytoplasm, producing metabolites such as α-ketoglutarate, pyruvate and acetyl-CoA, which feed into the TCA cycle.323 An essential NR in AA catabolism is ERRα, which is active in regulating genes involved in amino acid uptake and branched-chain amino acid metabolism.324 ERRα is also linked to reduced leucine oxidation under energy-demanding conditions.325 PPARα and HNF4α also regulate amino acid utilization. PPARα, in the presence of RXRα, inhibited HNF4α activity, suppressing serine dehydratase expression by promoting HNF4α degradation via the proteasome pathway. In PPARα-knockout mice, HNF4α levels and amino acid catabolizing enzyme (AACE) expression are elevated.326 In response to amino acid deficiency (AAD), MEF2D (myocyte enhancer factor 2D) and NR4A1 induce FAM134B2 expression and promote FAM134B2-mediated reticulophagy to maintain intracellular amino acid levels.327
Inflammation and immunity
Compelling evidence suggests that NRs can regulate inflammation and immunity, contributing to the pathogenesis of human diseases. For example, Nur77 binds to LPS, acting as an LPS receptor protein and playing a role in macrophage pyroptosis. Further research indicated that Nur77-LPS interactions activated NLRP3, resulting in the formation of an atypical inflammasome. These findings demonstrated that Nur77 promotes NLRP3 inflammasome activation, enhancing the host immune response to endotoxins.174 Similarly, Nur77 was identified as a key transcriptional regulator of the proinflammatory metabolic switch in macrophages. IDH expression was not downregulated in Nur77-deficient macrophages, which led to increased levels of succinate and other TCA cycle metabolites, independent of glutamine, resulting in increased nitric oxide and proinflammatory cytokine production in an SDH-dependent manner. Thus, Nur77 promotes an anti-inflammatory metabolic state in macrophages, offering protection against chronic inflammatory diseases such as atherosclerosis.328 Another nuclear receptor, RORα, regulates hepatic macrophage polarization and inflammation by inducing kruppel-like factor 4 (KLF4) expression. In myeloid-specific RORα-deficient animals, Kupffer cells (KCs) and bone marrow-derived macrophages fail to undergo M2 polarization, rendering them more susceptible to high-fat-diet-induced nonalcoholic steatohepatitis (NASH).329 Additionally, the orphan nuclear receptor TLX directly bound to the CD274 (PD-L1) gene promoter, showing a strong positive correlation with PD-L1 overexpression. Suppressing TLX significantly reduced the in vivo growth of glioma allografts and xenografts, preserved the antitumor immune response and markedly decreased the number of PD-L1-positive cells and glioma-associated macrophages.330 Nuclear receptor subfamily 1 group D member 1 (NR1D1) acts as a transcriptional repressor and plays a key role in regulating inflammation. The activation of NR1D1 decreased the expression of proinflammatory cytokines and matrix metalloproteinases (MMPs), whereas NR1D1 silencing had the opposite effect. Additionally, NR1D1 activation reduces ROS production and increases the generation of Nrf2-associated antioxidant enzymes.331
NRs also play crucial roles in modulating immune responses by directly regulating the function and activity of immune cells, such as influencing their development, differentiation and activation. For example, overexpression of NR4A1 inhibited the development of effector T cells through binding to the transcription factor AP-1 and inhibited its promotion of the expression of effector genes. NR4A1 binding encouraged the acetylation of histone 3 at lysine 27 (H3K27ac), which activated genes linked to tolerance. Deletion of NR4A1 increases immunity against tumors and chronic viruses, overcomes T-cell tolerance and enhances effector function.332 Similarly, another study revealed that NR4A triple knockout CAR-T lymphocytes presented traits and gene expression profiles typical of CD8+ effector T cells. The chromatin regions in these cells were enriched in binding motifs for transcription factors involved in T-cell activation, such as NF-κB and AP-1.333 These studies suggest that NR4A is a major regulator involved in the induction of T-cell dysfunction and that NR4A inhibition is a promising approach for cancer immunotherapy.
RARs and RA signaling play crucial roles in immune responses, influencing immune tolerance, tissue homing, lymph node formation and protective immunity.334 RARs include RARα, RARβ and RARγ, which are encoded by different genes with unique transcriptional variants and isoforms. In the resting state, RARα binds to transcriptional repressors to inhibit gene expression. However, upon binding to RA, RARα tends to bind to transcriptional activators, promoting gene expression334 RAR signaling has been shown to regulate immunity. For example, following TCR stimulation, extranuclear RARα is rapidly phosphorylated and recruited to the TCR signalosome. RA disrupted extranuclear RARα signaling, leading to reduced TCR activation and increased conversion of FOXP3+ regulatory T cells. RA is translocated to the nucleus by CRABP2, which is upregulated by TCR activation. Deletion of Crabp2 resulted in increased cytoplasmic RA, disrupting signalosome-associated RARα and consequently weakening autoimmune responses while diminishing antipathogen immunity.335
Temporal expression changes
The expression of nuclear receptors is not only related to tissue, sex, and disease but also varies across different age groups within the same individual.336 These variations reflect developmental needs, metabolic changes, hormonal shifts, and adaptations to aging-related physiological demands. Generally, developmental NRs such as RARs, PPARs and RXRs are highly expressed during embryogenesis and early development to support rapid growth, differentiation, and development.337 During adolescence, ARs, ERα and ERβ are elevated to correlate with puberty and the onset of secondary sexual characteristics, whereas GRs act to moderate stress responses and energy metabolism during periods of rapid physical and emotional development. PPARs, LXRs, and FXRs live in adult individuals to maintain lipid and glucose homeostasis, supporting metabolic stability during peak years of physical activity and reproductive capability. Diet, exercise, and stress significantly modulate NR expression, affecting metabolic and endocrine balance during this period. The expression of these NRs decreases with increasing age, which causes impaired lipid metabolism and insulin sensitivity, cholesterol and bile acid metabolism, and calcium homeostasis and immune function, leading to an increased risk of metabolic syndrome, compromised organ function and chronic inflammation. For example, Nr4a1 is reduced in peripheral blood mononuclear cells and CA1 pyramidal neurons with aging, which impairs cognition and excitatory synaptic function.338 Monitoring age-specific NR expression patterns could guide personalized therapies to optimize metabolic health and prevent age-related diseases.
Degradation and metabolic cycling
The metabolic cycling process of NRs, including their synthesis, activation, degradation, and recycling, is integral to maintaining homeostasis and ensuring that NRs can adapt to changing physiological demands. Since the activation and transcriptional regulation of nuclear receptors have been discussed above, we mainly emphasize their degradation and recycling. The degradation of nuclear receptors is a highly regulated process that involves proteasomal and lysosomal pathways, ensuring the dynamic control of receptor levels in response to physiological and environmental cues. Ligand binding can either stabilize or destabilize NRs by inducing conformational changes that influence ubiquitination.339,340 In addition, posttranslational modifications (PTMs), such as phosphorylation,341 SUMOylation342 and acetylation,343 modify NR degradation rates by altering their interaction with ubiquitin ligases or stabilizing proteins. Moreover, many NRs, such as REV-ERBs and PPARs, exhibit circadian cycling, with their expression and activity oscillating in a 24-hour rhythm to align with metabolic needs.344,345 These rhythms are coregulated by core circadian clock genes (e.g., CLOCK and BMAL1), which synchronize metabolic functions with environmental cycles such as light and feeding. Altogether, cycling of NRs ensures appropriate receptor levels, preventing excessive or prolonged signaling that could lead to cellular dysfunction.
Alterations cause by aging
With aging, the efficiency of key regulatory pathways declines, leading to altered NR turnover, disrupted signaling, and increased susceptibility to age-related diseases.346 Proteasomal dysfunction, a hallmark of aging, impairs the degradation of damaged or misfolded proteins, including NRs. This results in the accumulation of inactive or malfunctioning receptors, which disrupt signaling and contribute to metabolic and endocrine dysregulation, key features of aging. These disruptions play a central role in the development of diseases such as obesity, diabetes, cardiovascular conditions, and neurodegenerative disorders.347,348 Additionally, aging reduces autophagic flux, hindering the clearance of damaged proteins, including aggregated NRs. As autophagy becomes less efficient with age, NR aggregates persist, disrupting receptor signaling and promoting chronic inflammation, mitochondrial dysfunction, and cellular stress.349,350 These factors are implicated in the progression of age-related diseases like Alzheimer’s, diabetes, and frailty. Oxidative stress also contributes to aging-related cellular damage by directly modifying NRs. ROS cause conformational changes in NRs, preventing their proper ubiquitination and degradation. This oxidation makes NRs resistant to degradation, leading to the accumulation of damaged receptors and further dysfunction in signaling pathways.351 Understanding these age-related changes offers valuable insights for developing targeted therapies to restore NR homeostasis and mitigate aging-associated disorders.
Targeting nuclear receptors for disease management
Given the critical role of NRs in physiological and pathological processes, they constitute a highly significant and privileged class of intracellular druggable targets for treating various diseases.52,352,353,354 By modulating these receptors, it is possible to influence a wide range of conditions, from metabolic disorders such as diabetes and obesity to immune-related diseases such as autoimmune disorders and inflammatory conditions (Tables 1, 2). NRs are central to cancer progression and hormone regulation, making them prime targets for oncology and hormone therapies. The ability to design drugs that specifically interact with nuclear receptors enhances their potential for precision medicine, offering more targeted treatments with fewer side effects across a broad spectrum of diseases.
Targeting nuclear receptors in cancer
NRs, especially those that bind to three steroid hormones, namely, estrogen, progesterone and androgen, play a significant role in the development of human malignancies, particularly breast cancer,355,356 ovarian cancer357 and prostate cancer.358 In recent years, NR-targeted drugs have attracted widespread attention as novel therapeutic strategies359,360,361 that play a significant role in inhibiting the growth and progression of many types of cancer.
Within the nuclear receptor superfamily, ERs were the first nuclear receptors to be described. Breast cancer is the most common cancer in women, with ER+ breast cancer accounting for approximately 75% of all cases. Numerous studies have confirmed that ERα plays a causative role in carcinogenesis. As a result, an increasing number of ERα-based targeted medicines have entered preclinical and clinical trials (Table 1), and some have even received FDA approval. This includes directly antagonizing the estrogen receptor via SERMs and selective estrogen receptor degraders (SERDs). These drugs include ER antagonists, selective ER modulators and aromatase inhibitors.362 ER antagonists competitively inhibit the ER, whereas selective ER modulators function as partial agonists or antagonists on the basis of their tissue affinity. Aromatase inhibitors decrease estrogen production by blocking aromatase in tissues such as the ovaries and adipose tissue.363,364 Tamoxifen is the most widely used antiestrogen for hormone-dependent breast cancer and is indicated for various treatment settings. Evidence shows that patients with estrogen receptor-positive tumors are more likely to benefit from tamoxifen. FDA-approved treatments include treating breast cancer in both females and males, providing adjuvant therapy after surgery and radiation, treating female patients with ductal carcinoma in situ (DCIS) postsurgery and radiation to lower the risk of invasive breast cancer and reduce the risk of breast cancer in certain high-risk patients.365,366 In addition to tamoxifen, many other SERMs, including raloxifene, lasofoxifene, bazedoxifene and fulvestrant, have been developed and used for breast cancer treatment.363 These drugs have also been used to treat other tumors with high estrogen expression. In addition to hormone-dependent breast cancer, antiestrogen medications such as tamoxifen and aromatase inhibitors have shown efficacy in managing conditions such as endometrial cancer and certain types of ovarian cancer.367,368 Although estrogen levels are low in prostate tissue, ER expression has been detected in some prostate cancer patients.369 ER activation is believed to be associated with the proliferation and metastasis of prostate cancer cells.370 Thus, antiestrogen therapy targeting the ER has also been proposed as a new treatment strategy for prostate cancer. For example, in males with advanced prostate cancer, an ERα agonist, GTx-758, has been shown to lower testosterone with fewer side effects associated with androgen-deprivation therapy.371
Bidedoxifene is the most recent generation of SERMs. Fanning and colleagues demonstrated that bazedoxifene works in concert with palbociclib, a CDK4/6b inhibitor, and is more effective than tamoxifen against specific ERα mutants, such as Y537S and D538G. A phase II clinical trial in patients with ductal carcinoma in situ [NCT02694809] and a phase I/II clinical trial on bazedoxifene in combination with palbociclib in hormone receptor-positive breast cancer [NCT02448771] are two examples of the numerous clinical trials that have examined bazedoxifene across a variety of cancer types. Degradation of this receptor by SERDs is another tactic to target ERs. Fulvestrant is the most researched SERD, and its clinical application has been growing. Clinical trials have combined fulvestrant with CDK4/6 inhibitors, such as ribociclib in men and postmenopausal women with advanced breast cancer [NCT02422615] and palbociclib in hormone receptor+HER2-metastasized breast cancer after endocrine failure [NCT01942135], as well as phosphatidylinositol 3-kinasitol kinase (PI3K)/AKT/mTOR pathway inhibitors, such as pictilisib, in advanced or metastatic breast cancer in participants resistant to aromatase inhibitor therapy [NCT01437566].
The abnormal expression of AR in prostate cancer cells makes anti-AR drugs a logical treatment strategy.372 These drugs inhibit AR function through various mechanisms, reducing androgen stimulation of tumor cells. Among the most common anti-AR agents are AR antagonists such as abiraterone and enzalutamide. These drugs competitively bind to the AR, preventing androgen attachment and inhibiting receptor activity. Abiraterone has been shown to prolong survival in patients with metastatic castration-resistant prostate cancer (mCRPC).373 In the context of metastatic castration-sensitive prostate cancer (mCSPC), a combination treatment involving abiraterone, prednisone and docetaxel has favorable effects.374 Furthermore, for metastasis-free survival, combining enzalutamide with leuprolide has shown superior outcomes compared with leuprolide alone in high-risk patients with biochemical recurrence. Additionally, enzalutamide monotherapy has outperformed leuprolide monotherapy.36 The prostate cancer community has recently focused much attention on a number of newly created AR proteolysis-targeting chimeras (PROTACs), which were created as SARDs. ARV-110 [NCT03888612], the first AR PROTAC to reach clinical trials, is now being used in patients with metastatic CRPC and is undergoing a phase I/II clinical trial. In contrast to clinically licensed AR antagonists, ARD-61, a novel AR PROTAC, has been shown to efficiently promote on-target AR degradation, elicit more potent antiproliferative and proapoptotic effects, and reduce downstream AR target gene expression in prostate cancer cells. The most effective AR PROTACs may eventually be utilized extensively in cancer patients, and we expect more advancements to the clinical trial stage.
Additionally, RARs and their heterodimer partners, such as PPARs and RXR, which are involved in vitamin A metabolism, cell differentiation and immune regulation, also have therapeutic potential.375,376 Aberrant RXR signaling can promote cancer development by disrupting normal cellular homeostasis and enhancing oncogenic processes. RXR agonists have shown potential in treating various advanced cancers in clinical trials. IRX4204, in combination with erlotinib, is under investigation for previously treated advanced non-small cell lung cancer (NSCLC) [NCT02991651]. NRX194204 is being studied for its efficacy in castration- and taxane-resistant prostate cancer [NCT01540071] and advanced NSCLC [NCT00964132]. Additionally, bexarotene is being evaluated for the treatment of metastatic breast cancer and relapsed or refractory cutaneous T-cell lymphoma in patients with stage III or IV disease [NCT00003752, NCT00514293, NCT01134341, NCT00316030]. These studies underscore the therapeutic potential of RXR agonists in managing resistant and advanced malignancies. PPAR agonists have shown promising antitumor effects across a range of cancers, either as standalone treatments or in combination therapies. Troglitazone has shown efficacy in prostate cancer and liposarcoma [NCT00003058], whereas pioglitazone is being studied for advanced solid tumors [NCT02133625], pancreatic cancer [NCT01838317], and thyroid cancer [NCT01655719], often with chemotherapy. Inolitazone combined with paclitaxel is under investigation for advanced anaplastic thyroid cancer [NCT02152137]. Rosiglitazone is being explored for the treatment of liposarcoma [NCT00004180], metastatic thyroid cancer [NCT00098852], and androgen-dependent prostate cancer [NCT00182052]. These trials demonstrate the therapeutic potential of PPAR agonists in treating challenging malignancies. Retinoic acid, a natural RAR agonist, regulates the expression of genes related to cell growth. Studies suggest that RAR/RXR agonists may have antitumor effects on prostate cancer. For example, the RAR agonist adapalene has been shown to induce a tumor-suppressive senescence-associated secretory phenotype (SASP) in prostate cancer cells. In combination with docetaxel, adapalene enhanced tumor suppression and improved natural killer (NK) cell-mediated clearance in preclinical models. This approach also increased the efficacy of NK cell infusion in mice with human prostate cancer, leading to greater tumor suppression.377
REV-ERBs, including REV-ERBα (NR1D1) and REV-ERBβ (NR1D2), are key regulators of the circadian clock. Notably, many tumor types exhibit elevated expression of REV-ERBβ, regardless of ERBB2 or ER status, whereas nonmalignant mammary epithelial cells primarily express REV-ERBα. High levels of REV-ERBβ in cancer cells have been linked to reduced sensitivity to chloroquine via the modulation of autophagy, suggesting a role in therapy resistance. REV-ERB agonists, such as SR9009 and SR9011, selectively target cancer cells and oncogene-induced senescent cells, such as melanocytic naevi, without impacting normal cell viability. These agonists have shown promising anticancer effects, including reduced glioblastoma growth and improved survival in mice, without causing significant toxicity.378
Targeting nuclear receptors in metabolic-related diseases
Metabolic diseases such as nonalcoholic fatty liver disease (NAFLD), obesity, type 2 diabetes and atherosclerosis represent a growing global health burden.379,380 These conditions are frequently associated with dysregulated lipid or glucose metabolism.381,382 NRs play crucial roles in maintaining metabolic homeostasis by controlling the expression of genes involved in lipid, glucose and energy metabolism. Among NRs, PPARs, farnesoid X receptor (FXR), liver X receptor (LXR) and pregnane X receptor (PXR) have been identified as promising therapeutic targets. Drugs that modulate these receptors, such as PPAR agonists and FXR agonists, have demonstrated promising activity in clinical trials for the treatment of metabolic disorders, particularly NAFLD. Here, we explore current and emerging therapies that target these receptors.
One of the most well-known nuclear receptors is the PPAR family. PPAR agonists, such as pioglitazone and rosiglitazone, can increase insulin sensitivity in individuals with T2D. By activating PPARγ, these drugs enhance glucose uptake and lipid storage, which helps to lower blood sugar levels and improve metabolic control. However, these drugs are associated with certain risks, such as weight gain and fluid retention, necessitating careful patient management. PPARα agonists, such as fibrates, have been used clinically for 35 years to regulate blood lipids and prevent atherosclerosis.383 Pemafibrate was introduced in Japan in June 2018 and has since been demonstrated to be effective in inhibiting VLDL secretion and enhancing triglyceride (TG) clearance by activating LPL.384 In mice fed a high-fat diet, it suppressed postprandial hyperlipidemia by downregulating the mRNA expression of the intestinal cholesterol transporter NPC1L1 and upregulating genes involved in β-oxidation to inhibit VLDL secretion from the liver. In a 4-week trial with dyslipidemic patients (0.4 mg/day), pemafibrate significantly reduced fasting and nonfasting serum TG, RemL-C, and apo B-48 levels, with a marked reduction in the postprandial TG AUC, indicating improved postprandial hypertriglyceridemia.385 Similar results were observed in diabetic patients treated with pemafibrate.386 PPARγ and PPARδ regulate energy utilization and insulin sensitivity in skeletal muscle and adipose tissue. High insulin levels accelerate the development of NAFLD through a PPARγ-dependent pathway.387 PPARγ agonists, thiazolidinediones, have been used as insulin sensitizers in diabetic patients.388 PPARδ agonists improved the pathological manifestations of nonalcoholic steatohepatitis (NASH) without affecting body weight. The dual PPARα/δ agonist elafibranor (GFT505) improved glycemic and lipid metabolism in NASH patients in a phase II clinical trial, reduced liver inflammation and did not exacerbate fibrosis, demonstrating good tolerability. Phase III clinical trials targeting NASH patients [NCT02704403] are ongoing. A phase II clinical trial of the dual PPARα/γ agonist Saroglitazar [NCT03061721] in NASH patients was completed in April 2020. Midterm reports revealed not only regression of NASH tissue lesions but also decreased serum ALT levels, indicating improved liver function. The Indian pharmaceutical company Zydus Cadila has submitted a marketing application for this drug.
Another good NR drug target is FXR, which regulates bile acid metabolism and lipid homeostasis. FXR activation improves lipid metabolism and reduces liver inflammation, offering a new avenue for managing metabolic liver disorders.389,390,391 Obeticholic acid, an FXR agonist also known as INT-747, is approved for treating NAFLD and NASH392 [NCT02548351]. Owing to its ability to lower ALP levels and combat cholestasis and fibrosis, obeticholic acid (Ocaliva) was initially approved as a treatment for primary biliary cholangitis. In a phase III clinical trial for NASH [NCT02548351], obeticholic acid improved liver function and reduced tissue pathology, although it exhibited some side effects, such as pruritus and increased low-density lipoprotein levels. Given that pruritus can be managed symptomatically and that dyslipidemia can be addressed by the concomitant use of statins, the United States Food and Drug Administration has approved obeticholic acid as the primary treatment for NASH. Several other FXR agonists with diverse physicochemical characteristics, including bile acid derivatives, nonbile acid-derived steroidal FXR agonists, nonsteroidal FXR agonists and partial FXR agonists, are also progressing through advanced stages of clinical development. The noncarboxylic acid steroidal FXR agonist EDP-305 has been shown to modulate many pathways that trigger antilipogenic, anti-inflammatory and antifibrotic gene signatures. This resulted in hepatoprotective and antisteatotic effects in a mouse model of NASH.393 Patients with intestinal failure require more complicated care when they have parenteral nutrition (PN)-associated cholestasis (PNAC). By restoring hepatic FXR signaling, GW4064 protected animals against PNAC by increasing the expression of sterol and phospholipid transporters and canalicular bile and suppressing the recruitment and activation of macrophages. These findings suggest that increasing FXR activity could be used as a therapeutic approach to treat or prevent PNAC.394
In addition to PPAR and FXR, LXR is involved in cholesterol metabolism and can be targeted for atherosclerosis treatment. LXR agonists have been shown to increase cholesterol efflux and reduce the development of atherosclerotic plaques in preclinical models.395 However, treating patients with medications that increase cholesterol is not a practical option for metabolic illnesses; instead, methods for separating the advantageous effects of LXR ligands from unfavorable side effects must be investigated. Taken together, these examples highlight the growing potential of NR-targeted drugs in addressing the underlying causes of metabolic disorders, offering more precise and effective treatments than traditional approaches do.
Targeting nuclear receptors in cardiovascular diseases
The targeting of NRs for the treatment of cardiovascular diseases has gained significant attention because of the pivotal role these receptors play in regulating the expression of genes involved in metabolic, inflammatory and lipid processes.361,396,397,398 Nuclear receptors, such as PPARs, LXR and FXR, are key regulators of lipid metabolism, glucose homeostasis and inflammation, all of which are critical factors in the development of cardiovascular diseases. By modulating these receptors, it is possible to control pathological processes such as atherosclerosis, hypertension and other cardiovascular disorders.
The NRs RORα and REV-ERBα are key components of the circadian system and regulate biological rhythms, glucose metabolism, lipid metabolism, vascular inflammation and fibrinolysis.399 RORα is instrumental in endothelial progenitor cell (EPC) differentiation and promotes angiogenesis, tissue repair and vascular stability by inducing erythropoietin production and activating AMPK.400 RORα mediates the protective effects of melatonin, including its anti-inflammatory, antioxidant and antiapoptotic effects, making it a potential therapeutic target for atherosclerosis and endothelial dysfunction.401,402 In hypertensive rats, melatonin altered clock gene expression patterns, maintaining high levels of REV-ERBα mRNA, which regulated blood pressure rhythms. This effect is linked to melatonin receptor activity and may involve posttranslational mechanisms.403 Increased RORα expression, which is stimulated by melatonin and the sympathetic nervous system, also protects against programmed hypertension. A number of small-molecule agonists for RORα and REV-ERBα, such as the pharmacological agents SR9009 and GSK4112 for REV-ERBα, have been developed404 and are available for use in cellular and animal experiments, but prospective studies on the impact of stable circadian rhythms on CHD and heart failure are still limited.405,406 When considered collectively, the bulk of current research points to considerable clinical value and therapeutic potential for medications that target RORα and REV-ERBα to treat hypertension and atherosclerosis.
Several studies have highlighted the function of RXRs in cardiovascular disease. Treatment with 9cRA or the synthetic RXR agonist LGD1069 (bexarotene) has been shown to reduce oxidative stress-induced cell damage and preserve the mitochondrial membrane potential.407 In heart failure, oxidative stress activates angiotensin-II (AngII), leading to harmful cardiac remodeling and dysfunction. Bexarotene treatment of cultured rat aortic smooth muscle cells inhibited AngII-driven inflammation and p38 MAPK activation, suggesting that RXR activation has protective effects on cardiac disease.408 ERRα and ERRγ are essential for normal postnatal cardiovascular function, whereas ERRβ supports maximal ATP generation in contracting cardiomyocytes. ERR agonists help maintain oxidative metabolism, which protects the heart against pressure overload-induced heart failure (HF) in vivo. Using a structure-based design, Xu and colleagues synthesized two pan-ERR agonists, SLU-PP-332 and SLU-PP-915, each of which significantly improved the ejection fraction, reduced fibrosis and enhanced survival in models of pressure overload-induced HF without affecting cardiac hypertrophy.409 Targeting other nuclear receptors, such as RARs, PPARs, and LXRs, has also shown promise for ameliorating cardiovascular disease.410,411 These findings provide strong pharmacological evidence supporting the potential of targeting NRs for cardiovascular disease management.
Targeting nuclear receptors in central nervous system disorders
Alzheimer’s disease (AD), PD, Huntington’s disease (HD), multiple sclerosis (MS), cerebral ischemia, stroke and other conditions are common central nervous system disorders that currently affect approximately one hundred million people worldwide. Compelling evidence suggests that nuclear receptors are important in these disorders and that targeting these receptors has significant therapeutic potential.353
Liver X receptor LXRs are key regulators of cholesterol trafficking in the central nervous system (CNS), working with ApoE to facilitate cholesterol recycling and efflux, which are essential for maintaining CNS homeostasis. Dysregulation of LXR signaling is linked to neurodegenerative diseases such as AD, PD and Niemann‒Pick type C disease. LXR activation offers therapeutic potential by modulating neuroinflammation in CNS disorders. In AD models, LXR agonists such as T0901317 reduce inflammation associated with amyloid-beta (Aβ) deposition and tau tangles by suppressing microglial activation and the expression of inflammatory markers such as iNOS, COX-2, and NF-κB. Similar effects are observed in PD models, where T0901317 decreases iNOS and COX-2 levels. In Niemann–Pick type C disease, LXR activation promotes cholesterol clearance, reduces cerebellar inflammation, and extends lifespan. LXRs also enhance blood‒brain barrier (BBB) integrity by increasing the expression of ABC transporters such as ABCB1, which prevents toxin entry into the brain. Natural LXR agonists (e.g., oxysterols) and RXR activation (e.g., with bexarotene) improve BBB function in coculture models, whereas LXR agonists support tissue regeneration in ischemic stroke by stabilizing the BBB and reducing inflammatory cytokines. Although preclinical results are promising, the clinical translation of LXR agonists is hindered by side effects, including elevated triglycerides and LDL cholesterol.284 Efforts to mitigate these issues include the development of selective LXRβ agonists, regional drug delivery, and the targeting of specific downstream pathways, making LXRs promising therapeutic targets for CNS diseases.
PPARs, particularly PPARγ, play pivotal roles in neuroprotection by regulating neuroinflammation, mitochondrial function, and antioxidant defenses. It is expressed in various CNS cell types, including neurons, astrocytes, oligodendrocytes, and microglia. Dysregulation of PPARs is associated with neurodegenerative diseases characterized by inflammation, such as AD and amyotrophic lateral sclerosis (ALS). PPARγ agonists, including endogenous ligands such as OA and synthetic compounds such as rosiglitazone and pioglitazone, have shown promise in preclinical models because they reduce inflammatory cytokines, increase IL-10 levels, and enhance mitochondrial function in conditions such as stroke, ischemia, and traumatic brain injury. Moreover, PPARγ activation has exhibited potential benefits in neuropsychiatric disorders (e.g., depression, anxiety, and mood disorders) and neurodegenerative diseases, with ongoing clinical trials exploring its applications in AD, PD, MS, and bipolar disorder412 [NCT00982202, NCT00736996, NCT00265148, NCT00733785, NCT00688207, NCT01280123]. However, mixed clinical outcomes highlight gaps in understanding the mechanisms of PPARγ within the CNS.413 Further research aimed at optimizing PPARγ activity may unlock its full therapeutic potential for treating neurodegenerative and neuropsychiatric conditions.
The GR also plays a critical role in neuroinflammation, neuroprotection, and brain homeostasis. Its anti-inflammatory effects in the brain are mediated through interactions with AP-1 and NF-κB, limiting their transcriptional activity.414 GR also regulates brain-derived neurotrophic factor (BDNF), promotes neurogenesis, neuronal survival, and synaptic plasticity, and contributes to memory, aging, and behavior. Additionally, GR supports BBB integrity by upregulating tight junction proteins and establishing metabolic barriers to xenobiotics.415,416 GR dysfunction has been implicated in various neuropathologies, including AD, HD and epilepsy. Abnormal GR expression in AD is correlated with hippocampal overexpression, impaired cortisol feedback, and memory deficits.417 Similarly, GR overexpression in epilepsy has been associated with reactive gliosis and coregulation with PXR in epileptic brain endothelial cells. GR dysregulation also affects HD-related changes, such as increased cortisol secretion and muscular atrophy, alongside reduced GR expression in the hypothalamic and pituitary regions. GR abnormalities also underlie mood disorders such as depression and anxiety, as dysfunction in GR and MR signaling leads to maladaptive stress responses, dendritic retraction, and impaired serotonin activity.418,419 GR dysregulation in the prefrontal cortex and hippocampus has been linked to anxiety and depression-like behaviors in animal models. Pharmacological modulation of the GR provides therapeutic opportunities. GR agonists, such as dexamethasone and methylprednisolone, are widely used to treat neurological conditions such as gliomas, MS, and traumatic brain injuries. Dexamethasone reduces edema, prevents cerebrovascular leakage, and upregulates tight junction proteins at the BBB.420,421 Methylprednisolone improves memory and long-term potentiation at low doses but has dose-dependent effects, with high doses alleviating motor deficits in MS models but not cognition.422,423 GR antagonists, such as mifepristone and relacorilant, have shown efficacy in treating mood disorders, neurodegenerative conditions, and PTSD. Mifepristone restores serotonin signaling and alleviates depression-like behaviors, whereas relacorilant is undergoing phase II and III trials for conditions such as Cushing syndrome and prostate cancer424,425 [NCT036748142019, NCT036971092019]. These findings underscore the potential of targeting GRs in a range of CNS disorders.
Estrogens and their receptors play critical roles in neuroprotection and the regulation of inflammatory and neurodegenerative processes in the CNS. Owing to their antiapoptotic, antioxidative, and anti-inflammatory properties, estrogens provide early protection against ischemic injury. ERα and ERβ agonists, such as diarylpropionitrile (DPN), have demonstrated neuroprotective effects by mitigating damage in ischemic models, including the caudate nucleus and CA1 region of ovariectomized mice and the striatum of male mice. The membrane receptor GPER-1 also contributes to ischemic protection, with its agonist G1 offering estrogen-like neuroprotection, particularly in males, as suggested by sex-dependent differences in GPER-1 expression. In addition to estrogens, NRs such as Nr4a2 and bile acid receptors play vital roles in alleviating neuroinflammation and neuropathic pain. Nr4a2 helps regulate depressive-like behaviors by preventing structural and functional damage to anterior cingulate cortex (ACC) microglia and excitatory neurons during chronic neuroinflammation.426 Its upregulation through the dopaminergic agonist pramipexole has demonstrated neuroprotective effects on dopaminergic neurons in Parkinson’s disease.427 Similarly, bile acids activate TGR5 and FXR, alleviating mechanical hyperalgesia by reducing glial activation and neuronal sensitization in the spinal dorsal horn. The presence of bile acid synthesis enzymes in spinal astrocytes suggests an endogenous mechanism for decreasing nociceptive neuron transmission and mechanical hypersensitivity, emphasizing bile acid receptors as promising targets for neuropathic pain relief.428 In MS, Th17-driven inflammation is aggravated by RORγt overexpression, which promotes Th17 cell differentiation. By suppressing RORγt, vitamin A has shown therapeutic potential for MS429[NCT01225289]. Collectively, these findings highlight the diverse and pivotal roles of estrogens and nuclear receptors in neuroprotection, inflammation regulation, and the development of innovative therapeutic strategies.
Targeting nuclear receptors in infectious diseases
In recent decades, global spending on infectious diseases has increased significantly, with treatment costs rising faster than those for other diseases. Infectious diseases caused by bacteria, viruses, fungi, and parasites include common conditions such as pneumonia, influenza, gastroenteritis, hepatitis, malaria, and dengue fever, as well as diseases such as HIV/AIDS, tuberculosis, and COVID-19. NRs play a key role in regulating immune responses by modulating genes involved in inflammation, pathogen defense, and immune cell function, thus influencing the outcome of both bacterial and viral infections. Given the increasingly appreciated roles of NRs in the context of infections, targeting NRs may provide a new, largely unexplored area for infectious disease treatment.430
The role of FXR extends to hepatitis C virus (HCV) infection, where it, along with other NRs, such as HNF4α, PPARα, and PXR, reprograms hepatocyte metabolism, influencing glycolysis, ketogenesis, and drug metabolism.431 The targeting of FXR or PPARα in HCV infection has shown complex antiviral effects, with receptor inhibition reducing viral replication by reversing HCV-induced metabolic shifts. Furthermore, FXR agonists such as GW4064 have demonstrated potential in alleviating inflammation and preserving intestinal barrier function in experimental colitis, primarily through the modulation of NF-κB signaling.432,433 In addition, GW4064 has been reported to downregulate scavenger receptors via FXR and indirectly inhibits HCV entry into cells.434
In addition to their roles in viral infections, NRs are essential in bacterial infection. For example, PPARγ signaling is crucial for resolving Staphylococcus aureus (MRSA) infections and improving outcomes in sepsis models by modulating inflammation.435 PPARγ agonists such as pioglitazone have been shown to restore mitochondrial function and reduce oxidative stress in macrophages, highlighting their potential in treating inflammatory and infectious diseases.436 LXRs are involved in macrophage phagocytic responses, particularly during Salmonella Typhimurium infections.437 In a mouse model, LXR agonist treatment ameliorated clinical signs of Salmonella infection in a CD38-dependent manner.438 Recent transcriptomic studies investigating susceptibility to dengue virus in a Cuban cohort of African ancestry revealed increased gene expression of RXR in individuals who were more resistant to infection and suggested a protective role for LXR/RXR against dengue virus.439 In the case of tuberculosis (TB), NRs such as Nur77 and Nor1 are upregulated in response to Mycobacterium tuberculosis infection. Interestingly, the first-line TB drug rifampicin activates PXR, which can impair its effectiveness in infected macrophages, underscoring the complex interactions between NRs and drug efficacy.440,441 Rifampicin, a first-line anti-tuberculosis (TB) drug, is a potent activator of PXRs, which in turn compromises the effect of rifampicin in M. tuberculosis-infected human macrophages.442
NRs also influence fungal infections, with PPARγ playing a central role in defending against Candida albicans in the gastrointestinal tract. PPARγ ligands reduce fungal colonization through a dectin-1-dependent mechanism. Sepsis models have revealed a critical role for PPARγ in controlling inflammation and improving overall disease outcomes. PPARγ gene expression was downregulated in freshly isolated peripheral blood mononuclear cells (PBMCs) from human sepsis patients as well as in the lungs in a murine sepsis model.443 Treatment with PPARγ agonists improved sepsis outcomes, decreased proinflammatory cytokine levels, increased IL-10 levels, and improved mouse survival.444 Other PPAR isoforms have also been linked to decreased severity of sepsis through the downregulation of inflammation. In a cecal ligation and puncture rat sepsis model, treatment with a PPARβ/δ agonist inhibited signal transducer and activator of transcription 3 (STAT3) activation in AMs, reduced the inflammatory response, and prolonged rat survival.445,446
Parasitic worms are among the global health threats to billions of humans worldwide.447,448 Although several anthelmintic drugs, including benzimidazole, levamisole/morantel and ivermectin, have been used to treat these parasites, resistance frequently occurs. Therefore, identifying novel therapeutic targets in parasitic diseases is urgently needed. Nuclear receptors, which are well-known therapeutic targets in humans, are currently being investigated in parasitic worms because of their widespread distribution and important functions in controlling transcriptional networks involved in metabolism and development.449 Among them, DAF-12 is a nuclear receptor that is necessary for the typical development of nematodes, including the crucial infectious stage. In free-living nematodes such as C. elegans, a lack of DAF-12 ligands (such as dafachronic acid (DA)) under stress conditions promotes entry into the L3d (dauer diapause) state, whereas supplementation with DAF-12 ligands in prosperous environments leads to their exit from L3d and the beginning of reproductive development.450 In contrast, in parasitic nematodes such as S. stercoralis, the absence of a DAF-12 ligand leads to an L3d ortholog stage, termed the infectious L3 (iL3) stage, during preinfection, whereas the presence of a DAF-12 ligand in the definitive host can resume reproductive development.451
The mechanism underlying DAF-12-mediated cell fate decisions is similar to that of other nuclear receptors. Briefly, under favorable conditions or in a host-specific environment, DAF-12 binds with its ligand and is thus activated, which induces the transcription of target genes that facilitate metabolism and development. In contrast, under stress conditions, DAF-12 is inactive without ligand binding, resulting in transcriptional repression and subsequent development arrest and infection. Thus, strategies targeting DAF-12 or its ligands have potential for the treatment of parasitic diseases. In addition to exogenous DA, endogenous Δ7-DA was recently reported as a novel ligand of DAF-12 in S. stercoralis. The abundance of Δ7-DA is regulated by several enzymes, including DAF-36, DHS-16, and DAF-9, which govern the on/off switch of the reproduction, infection and development processes in this parasitic nematode.452 In addition to receptors and ligands, other components, such as cofactors, can also be potential targets for the manipulation of the life cycle of parasitic nematodes. For example, DAF-12 interacting protein-1 (DIP-1) was recently identified as a coactivator of DAF-12, which is indispensable for the ligand-activated transcriptional activity of DAF-12 in Strongyloides spp.453 Together, this evidence suggests that the nuclear receptor DAF-12 is a promising target for the treatment of parasitic diseases.
Targeting nuclear receptors in reproductive diseases
Polycystic ovary syndrome (PCOS) is a type of reproductive disease that affects 5–18% of women worldwide who are of reproductive age.454 Interestingly, patients with PCOS are frequently associated with metabolic syndrome, such as obesity, dyslipidemia, impaired glucose tolerance or increased fasting insulin,455 which is closely linked to an abnormal gut microbiome. A recent study revealed that PCOS can be induced by alterations in the gut microbiome via the activation of the nuclear receptor FXR.456 Mechanistically, agmatine produced by B. vulgatus can activate FXR, which in turn prevents L cells from secreting glucagon-like peptide-1 (GLP-1), suggesting that FXR is a possible therapeutic target for the treatment of PCOS.456 Moreover, PPAR is a large nuclear receptor superfamily related to energy metabolism and is thus involved in the progression of PCOS. In fact, all three PPAR family members, including PPARα, PPARβ/δ and PPARγ, play critical roles in PCOS via multiple mechanisms, such as the regulation of lipogenesis; glucose homeostasis; and fatty acid oxidation, synthesis and storage.457 Given the important function of PPARs in PCOS pathogenesis, modulating the activity of PPARs profoundly affects the development of PCOS. For example, the activation of PPARγ by a polyphenolic compound, sinapic acid, was shown to ameliorate metabolic dysfunction and attenuate PCOS phenotypes in rats.458 Another study focusing on the downstream mechanism underlying PPAR-mediated alleviation of PCOS indicated that PPARα can bind to the promoter of FADS2, a ferroptosis-related gene, thus increasing FADS2 transcription. This event inhibits ferroptosis of granulosa cells in a dehydroepiandrosterone (DHEA)-induced PCOS mouse model, suggesting that PPARα alleviates PCOS by protecting granulosa cells from ferroptosis.459
Uterine leiomyomas, another name for uterine fibroids, are benign tumors of the fibrotic smooth muscle in the reproductive system. The ovarian steroid hormone estrogen, as well as its receptor ER, are involved in this disease.460 In addition, other nuclear receptors, such as the NR4A protein family, may also participate in the progression of uterine fibroids.461 NR4A restricts the proliferation of primary leiomyoma smooth muscle cells by modeling the extracellular matrix, whereas low expression of NR4A contributes to the pathogenesis of uterine fibroids. Moreover, leuprolide acetate, a GnRH analog used to treat uterine fibroids, can restore the expression of NR4A1 and NR4A3 in clinical samples, suggesting that these two NR4A family members might be additional targets of leuprolide acetate in addition to sex steroid receptors.
Targeting nuclear receptors in renal diseases
While NRs are intimately involved in proper kidney function, they are also implicated in a variety of renal diseases, such as diabetes, acute kidney injury, and other conditions, such as aging. In the last 10 years, our understanding of renal disease etiology and progression has been greatly shaped by knowledge regarding how NRs are dysregulated in these conditions. Importantly, NRs have also become attractive therapeutic targets for the attenuation of renal diseases, and their modulation for this purpose has been the subject of intense investigation. Here, we review the roles of six key renal NRs, including PPAR, ERR, FXR, ER, LXR, and VDR, in health and disease, with an emphasis on recent findings over the last decade.
PPARs are involved in various renal diseases, both acute and chronic. PPARα-null mice treated with streptozotocin (STZ) exhibit accelerated diabetic nephropathy, including worsened albuminuria, glomerular sclerosis, mesangial matrix expansion, collagen deposition, and macrophage infiltration. PPARα agonists have been studied as potential treatments for diabetic nephropathy (DN) in rodents, with evidence supporting their renoprotective effects. Recent research by Chung et al.demonstrated that impairment of PPARα and fatty acid oxidation exacerbates kidney fibrosis during aging, with reduced PPARα expression and lipid accumulation in tubular cells.462 PPARα-null mice also exhibit more severe renal aging and fibrosis. Jao et al.reported that ATF6 downregulates PPARα transcription, leading to decreased fatty acid oxidation and increased lipotoxicity in proximal tubule cells, whereas ATF6-deficient mice exhibit increased PPARα expression and decreased lipid accumulation.463 In addition to PPARα, PPARγ has also been studied in DN, with evidence suggesting that its activity is suppressed in this disease. Unlike other PPAR family members, PPARβ/δ is ubiquitously expressed in both cortical and medullary cells. Recent studies have highlighted a potential role for PPARβ/δ in renal homeostasis. For example, Kirkby et al. demonstrated that activation of renal PPARβ/δ by endogenous prostacyclin induces vasodilation and improves blood flow, a process regulated by cyclooxygenase-2 (COX-2).464 This COX-2/prostacyclin/PPARβ/δ axis offers new insights into kidney vascular physiology and therapeutic possibilities. Additionally, activation of PPARβ/δ has been shown to improve renal dysfunction in a lupus mouse model by lowering blood pressure, reducing renal hypertrophy, decreasing albuminuria, and preventing renal injury.465
Additionally, other nuclear receptors play key roles in kidney health. ERRs are crucial regulators in the kidneys, where they help maintain metabolic balance, regulate monovalent cation homeostasis, and control blood pressure.466 The importance of ERRs is further highlighted by the fact that the deletion of ERRγ leads to significant mitochondrial dysfunction and progressive renal failure.467 For example, ERRα has been shown to protect against AKI in tubular epithelial cells, particularly in cases of cisplatin-induced AKI in mice.468 Compared with those in wild-type mice, in ERRα-null mice subjected to cisplatin treatment, tubular injury, a reduced mitochondrial content, abnormal mitochondrial morphology, and increased oxidative stress were observed. A recent study demonstrated that Nr4a1 was upregulated in the kidneys of mice with unilateral ureteral obstruction (UUO), and its expression was positively correlated with the degree of interstitial kidney injury and the level of fibrotic proteins. Nr4a1 may contribute to renal fibrosis by activating the p38 MAPK pathway, as treatment with the p38 MAPK inhibitor SB203580 successfully inhibited fibrotic protein expression and p38 MAPK phosphorylation.184 Furthermore, VDR has been shown to exert a renoprotective effect by inhibiting fibrosis. VDR translocates into the nucleus to form the VDR:RXR heterodimer, which inhibits fibrogenic gene expression in HK-2 cells exposed to high glucose and reduces fibrogenic gene expression in proximal renal tubules.469 In the aging kidney, the expression of FXR and TGR5 is decreased. However, a dual agonist of FXR and TGR5, INT-767, has been found to reverse age-related kidney disease in mice, demonstrating the therapeutic potential of targeting these receptors in renal aging.470 In conclusion, nuclear receptors play vital roles in regulating kidney function and protecting against kidney injury and fibrosis. Understanding their mechanisms and interactions could open new avenues for therapeutic strategies to treat kidney diseases, particularly those related to aging and fibrosis.
Application of emerging technologies in NR research
Several challenges remain in targeting NRs therapeutically due to the complexity of NR signaling, tissue specificity, and the need for precise drug design. However, recent advancements in genomics, proteomics, imaging, and computational modeling have enabled more precise exploration of NR function, regulation, and their involvement in various physiological and pathological processes.
Technologies such as ChIP-chip, ChIP-Seq, and GRO-Seq have enabled unbiased, genome-wide profiling of NR binding sites, revealing new insights into NR mechanisms and their regulatory networks in breast cancer. These genomic methods have highlighted the dynamic interactions between NRs and the chromatin landscape. It is increasingly clear that chromatin, in its varying states of condensation, both regulates and is regulated by NR activity, with NR-chromatin interactions playing a major role in cell type-specific transcriptional regulation.471 Single-cell RNA sequencing (scRNA-seq) has further advanced our understanding of NR function by enabling gene expression analysis at the single-cell level. This technology is invaluable for investigating tissue-specific NR roles and identifying distinct signaling pathways in various cell populations, which is crucial for understanding their contributions to tissue homeostasis, development, and diseases like cancer and metabolic disorders.472 For instance, single-cell transcriptomics revealed the involvement of a previously uncharacterized retinoic acid receptor family member, SmRAR, in reproductive development in Schistosoma mansoni.473 CRISPR-Cas9 technology has revolutionized NR research by allowing precise manipulation of genes in vitro and in vivo. This has facilitated studies of NR roles in diseases such as type 2 diabetes, neurodegeneration, and cancer, and provide reversible and tunable control of NR expression, enabling dynamic investigation of NR function.474,475
Recent advances in proteomics, particularly mass spectrometry (MS), have enabled the identification and quantification of NR interactomes, providing insights into the molecular mechanisms regulating NR activity. MS also helps identify post-translational modifications like phosphorylation, acetylation, and SUMOylation, which are crucial for NR activation and signaling.306 Cryo-EM has emerged as a powerful tool for visualizing the 3D structure of proteins, including NRs, at near-atomic resolution. This technique has provided key insights into the conformational changes NRs undergo upon ligand binding and co-factor recruitment, essential for drug discovery, as many NRs are targeted by small molecules in diseases like cancer, diabetes, and inflammation.476 For example, cryo-EM has elucidated the assembly of the AR:coactivator complex and its role in coactivator assembly and transcriptional regulation.70 Additionally, the cryo-EM structure of the NCOA4-FTH1 interface revealed key amino acids crucial for interaction, paving the way for developing ferritinophagy modulators.477 These technologies, including cryo-EM and omics, have advanced our understanding of NR activation, NR-chromatin interactions, transcriptional regulation, and non-genomic effects, offering new avenues for therapeutic development.
Artificial intelligence (AI) and machine learning (ML) are increasingly applied in NR research to predict receptor-ligand interactions, identify novel signaling pathways, and design drugs targeting specific NR subtypes. These technologies analyze large datasets to uncover hidden patterns within complex biological systems, enhancing our understanding of NRs in health and disease. For example, chemical language models combined with beam search algorithms have been used for automated molecule design, leading to the development of novel inverse agonists for retinoic acid receptor-related orphan receptors (RORs) with low micromolar to nanomolar potency.478 AI and deep docking methodologies have also facilitated the development of VPC-260724, a novel ER-AF2 binder that inhibits ER activity and exhibits antiproliferative effects in ER-positive breast cancer models.479 Additionally, AI has been applied to create an adverse outcome pathway (AOP) network for chemical-induced cholestasis, facilitating automated data collection and the identification of key molecular events.480 Moreover, Bhimsaria and colleagues identified previously hidden binding sites of human NRs via high-throughput SELEX and a novel MinSeq Find algorithm, revealing new biological roles of NRs and their involvement in disease-associated genetic variations while providing a valuable resource for drug repurposing and precision medicine.481 With AI and ML, high-throughput screening (HTS) has become more efficient, enabling better prediction of drug candidates and optimization of lead compounds for clinical applications.
In vivo imaging techniques like positron emission tomography (PET) and magnetic resonance imaging (MRI) are increasingly used to study nuclear receptor (NR) activity in living organisms. These methods allow real-time tracking of NR signaling and the effects of ligands or drugs on receptor function in tissues. Live-cell imaging with fluorescent biosensors also reveals dynamic interactions between NRs and their co-regulatory proteins at the cellular level.482 This approach offers a quantitative, in vivo equivalent to classic radioligand binding assays.483 In vivo imaging helps identify when and where NRs are active during development, aging, and disease, providing insights into how ligands or receptor modulators influence these processes.484 Other technologies, such as organoids, 3D cell cultures, nanotechnology, and systems biology, have also advanced NR research. Notably, PROTACs (proteolysis-targeting chimeras), which degrade target proteins via the 26S proteasome, offer new possibilities for targeting challenging NRs, including orphan receptors or those without enzymatic activity. In cases of drug resistance due to NR mutations (e.g., in hormone-dependent cancers), PROTACs can bypass resistance by degrading the mutated receptors instead of inhibiting their activity.
Emerging technologies are significantly enhancing the field of nuclear receptor research by providing deeper insights into their structure, function, and regulation. The combination of high-resolution imaging, gene editing tools, and advanced computational techniques has opened new avenues for understanding how NRs control physiological processes and contribute to disease. As these technologies continue to evolve, they hold promise for the development of more precise and effective therapeutic strategies targeting nuclear receptors in a variety of disorders.
Concluding remarks
Nuclear receptors are a crucial class of transcription factors that regulate gene expression in response to various ligands, such as hormones, vitamins and metabolic products. They play pivotal roles in processes such as metabolism, immune responses and development, making them key targets for therapeutic intervention in cardiovascular disease, cancer, metabolic disorders and inflammatory conditions. In recent years, targeted therapies aimed at NRs, such as ERs, ARs and VDRs, have shown significant promise in clinical applications, particularly in oncology and metabolic regulation. Newer nuclear receptors, such as LXR and PXR, are being investigated for their therapeutic potential, revealing novel pathways for drug development.
Despite recent advances, several challenges remain in targeting NRs for therapeutic purposes. One key difficulty is the extensive crosstalk between NRs and their signaling pathways, which can lead to synergistic or antagonistic effects, limiting the effectiveness of targeting a single receptor and causing off-target effects. Additionally, NRs exhibit tissue-specific expression and functional outcomes, making designing drugs that selectively modulate receptor activity in specific tissues or cell types challenging. The specificity of ligand-receptor interactions is another hurdle, as NRs can be activated by various small molecules, and ligand promiscuity or cross-reactivity can lead to unwanted side effects. Moreover, long-term NR modulation may result in side effects or even toxicity, such as liver damage or increased cardiovascular risk. Finally, many NRs are regulated by complex feedback mechanisms, complicating predictions about how receptor activity changes in response to treatment. These dynamic regulatory loops require careful consideration to avoid unintended consequences and therapeutic resistance.
Future research will likely focus on understanding the complex interactions of nuclear receptors in various physiological contexts, developing more selective modulators, and exploring novel strategies for more precise and selective targeting of NRs to minimize off-target effects and enhance therapeutic efficacy. Integration of data from transcriptomics, proteomics, and metabolomics will help model NR signaling networks and predict the effects of targeting a specific NR in the context of broader cellular networks. Structure-based drug design employing leverage X-ray crystallography, cryo-EM, and computational modeling will facilitate the identification or design of small molecules that specifically interact with a target NR’s ligand-binding domain, minimizing cross-reactivity. Cutting-edge technologies such as gene editing CRISPR/Cas9 technology and advanced drug delivery systems achieve tissue- or cell-specific targeting of NRs to minimize off-target effects and enhance therapeutic efficacy. Noninvasive techniques to monitor the activity of NRs via specifically binding imaging agents to activated nuclear receptors or linking reporter genes (e.g., luciferase, GFP) to NR-responsive promoters will allow real-time visualization and quantification of receptor activity in preclinical models. In addition, the fact that NRs are key host sensors of microbial signals offers a promising avenue for gut microbiota-targeted drug discovery by leveraging NR-mediated environmental adaptations.485,486,487 For example, FXR plays a pivotal role in modulating the gut microbiota and is thereby involved in various biological processes, including the metformin response,488 bile acid homeostasis,489 and core metabolic functions.490 Overall, with the advancement of these technologies, new breakthroughs will be made in the discovery of novel drugs that target NRs.
Data availability
Not applicable.
References
Cheng, X. & Smith, J. C. Biological membrane organization and cellular signaling. Chem. Rev. 119, 5849–5880 (2019).
Ammendolia, D. A., Bement, W. M. & Brumell, J. H. Plasma membrane integrity: implications for health and disease. BMC Biol. 19, 71 (2021).
Wang, Z. Regulation of cell cycle progression by growth factor-induced cell signaling. Cells. 10, 3327 (2021).
Bartlett, C. S., Jeansson, M. & Quaggin, S. E. Vascular growth factors and glomerular disease. Annu Rev. Physiol. 78, 437–461 (2016).
Brown, G. S., Jang, J. & Li, D. Growth factors and their roles in cardiac development and regeneration: a narrative review. Pediatr. Med. 6, (2022).
Shindo, S. et al. Phosphorylation of nuclear receptors: Novelty and therapeutic implications. Pharmacol. Ther. 248, 108477 (2023).
Fernandez, E. J. Allosteric pathways in nuclear receptors - Potential targets for drug design. Pharmacol. Ther. 183, 152–159 (2018).
De Bosscher, K. et al. Nuclear receptor crosstalk - defining the mechanisms for therapeutic innovation. Nat. Rev. Endocrinol. 16, 363–377 (2020).
Pavek, P. Pregnane X receptor (PXR)-mediated gene repression and cross-talk of PXR with other nuclear receptors via coactivator interactions. Front. Pharmacol. 7, 456 (2016).
Poreba, E. & Durzynska, J. Nuclear localization and actions of the insulin-like growth factor 1 (IGF-1) system components: Transcriptional regulation and DNA damage response. Mutat. Res. Rev. Mutat. Res. 784, 108307 (2020).
Porter, B. A., Ortiz, M. A., Bratslavsky, G. & Kotula, L. Structure and Function of the Nuclear Receptor Superfamily and Current Targeted Therapies of Prostate Cancer. Cancers (Basel). 11, (2019).
Butt, T. R. & Walfish, P. G. Human nuclear receptor heterodimers: opportunities for detecting targets of transcriptional regulation using yeast. Gene Expr. 5, 255–268 (1996).
Penvose, A. et al. Comprehensive study of nuclear receptor DNA binding provides a revised framework for understanding receptor specificity. Nat. Commun. 10, 2514 (2019).
Zhang, D. & Heaney, A. P. Nuclear receptors as regulators of pituitary corticotroph pro-opiomelanocortin transcription. Cells 9, 900 (2020).
Wilkenfeld, S. R., Lin, C. & Frigo, D. E. Communication between genomic and non-genomic signaling events coordinate steroid hormone actions. Steroids 133, 2–7 (2018).
Alegre-Martí, A. et al. A hotspot for posttranslational modifications on the androgen receptor dimer interface drives pathology and anti-androgen resistance. Sci. Adv. 9, eade2175 (2023).
Saito, K. & Cui, H. Estrogen receptor alpha splice variants, post-translational modifications, and their physiological functions. Cells. 12, (2023).
Malbeteau, L. et al. HoW Protein Methylation Regulates Steroid Receptor Function. Endocr. Rev. 43, 160–197 (2022).
Brunmeir, R. & Xu, F. Functional regulation of ppars through post-translational modifications. Int. J. Mol. Sci. 19, (2018).
Fu, T. et al. FXR regulates intestinal cancer stem cell proliferation. Cell 176, 1098–1112.e1018 (2019).
Ma, H. et al. The nuclear receptor THRB facilitates differentiation of human PSCs into more mature hepatocytes. Cell Stem Cell 29, 795–809.e711 (2022).
Tschuck, J. et al. Farnesoid X receptor activation by bile acids suppresses lipid peroxidation and ferroptosis. Nat. Commun. 14, 6908 (2023).
Lambrecht, R. et al. Liver receptor homolog-1 (NR5A2) orchestrates hepatic inflammation and TNF-induced cell death. Cell Rep. 42, 113513 (2023).
Meinsohn, M. C., Smith, O. E., Bertolin, K. & Murphy, B. D. The orphan nuclear receptors steroidogenic factor-1 and liver receptor homolog-1: StrUcture, Regulation, And Essential Roles In Mammalian Reproduction. Physiol. Rev. 99, 1249–1279 (2019).
Font-Díaz, J. et al. Nuclear receptors: Lipid and hormone sensors with essential roles in the control of cancer development. Semin. Cancer Biol. 73, 58–75 (2021).
Zhao, L., Hu, H., Gustafsson, J. & Zhou, S. Nuclear receptors in cancer inflammation and immunity. Trends Immunol. 41, 172–185 (2020).
Yang, P. B. et al. Blocking PPARγ interaction facilitates Nur77 interdiction of fatty acid uptake and suppresses breast cancer progression. Proc. Natl. Acad. Sci. USA 117, 27412–27422 (2020).
Wu, Q. et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Invest. 131, (2021).
Jiang, J. et al. Systematic pan-cancer characterization of nuclear receptors identifies potential cancer biomarkers and therapeutic targets. Cancer Res 82, 46–59 (2022).
Doyon, G. & Bruemmer, D. Vascular smooth muscle cell dysfunction in diabetes: nuclear receptors channel to relaxation. Clin. Sci. (Lond.) 130, 1837–1839 (2016).
Pritchard, K. I. et al. Randomized trial of cyclophosphamide, methotrexate, and fluorouracil chemotherapy added to tamoxifen as adjuvant therapy in postmenopausal women with node-positive estrogen and/or progesterone receptor-positive breast cancer: a report of the National Cancer Institute of Canada Clinical Trials Group. Breast Cancer Site Group. J. Clin. Oncol. 15, 2302–2311 (1997).
Kenda, M. & Sollner Dolenc, M. Computational study of drugs targeting nuclear receptors. Molecules 25, 1616 (2020).
van de Wiel, S. M. W., Bijsmans, I. T. G. W., van Mil, S. W. C. & van de Graaf, S. F. J. Identification of FDA-approved drugs targeting the Farnesoid X Receptor. Sci. Rep. 9, 2193 (2019).
Kim, H. et al. Tamoxifen response at single-cell resolution in estrogen receptor-positive primary human breast tumors. Clin. Cancer Res. 29, 4894–4907 (2023).
Zhang, N. et al. Identifying actionable druggable targets for breast cancer: Mendelian randomization and population-based analyses. EBioMedicine 98, 104859 (2023).
Freedland, S. J. et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N. Engl. J. Med. 389, 1453–1465 (2023).
Armstrong, A. J. et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 40, 1616–1622 (2022).
Lazar, M. A. Reversing the curse on PPARγ. J. Clin. Invest. 128, 2202–2204 (2018).
Dormandy, J. A. et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366, 1279–1289 (2005).
Huss, J. M. & Kelly, D. P. Nuclear receptor signaling and cardiac energetics. Circ. Res. 95, 568–578 (2004).
Vega, R. B. & Kelly, D. P. Cardiac nuclear receptors: architects of mitochondrial structure and function. J. Clin. Invest. 127, 1155–1164 (2017).
Vance, M. L. Growth-hormone-releasing hormone. Clin. Chem. 36, 415–420 (1990).
Xu, H. E. Family reunion of nuclear hormone receptors: Structures, diseases, and drug discovery. Acta Pharm. Sin. 36, 1–2 (2015).
Henderson, J. Ernest Starling and ‘Hormones’: An historical commentary. J. Endocrinol. 184, 5–10 (2005).
Bussmann, B. et al. Bayliss Starling Prize Lecture 2023: Neuropeptide-Y being ‘unsympathetic’ to the broken hearted. J. Physiol. 603, 1841–1864 (2024).
Raju, T. N. The Nobel chronicles. 1950: Edward Calvin Kendall (1886-1972); Philip Showalter Hench (1896-1965); and Tadeus Reichstein (1897-1996). Lancet 353, 1370 (1999).
Shampo, M. A., Kyle, R. A. & Steensma, D. P. Adolf Butenandt-Nobel Prize for chemistry. Mayo Clin. Proc. 87, e27 (2012).
Simoni, R. D., Hill, R. L. & Vaughan, M. The discovery of estrone, estriol, and estradiol and the biochemical study of reproduction. The work of Edward Adelbert Doisy. J. Biol. Chem. 277, e1–e2 (2002).
O’Malley, B. W., Khan, S. & Elwood, V. Jensen (1920-2012): father of the nuclear receptors. Proc. Natl. Acad. Sci. USA 110, 3707–3708 (2013).
Hollenberg, S. M., Giguere, V., Segui, P. & Evans, R. M. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell 49, 39–46 (1987).
Weinberger, C. et al. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Sci 228, 740–742 (1985).
Frigo, D. E., Bondesson, M. & Williams, C. Nuclear receptors: From molecular mechanisms to therapeutics. Essays Biochem 65, 847–856 (2021).
Obiorah, I. E., Fan, P. & Jordan, V. C. Breast cancer cell apoptosis with phytoestrogens is dependent on an estrogen-deprived state. Cancer Prev. Res. 7, 939–949 (2014).
Mohd Idris, R. A. et al. The effects of tamoxifen on tolerogenic cells in cancer. Biology (Basel). 11, (2022).
Shiota, M., Fujimoto, N., Kashiwagi, E. & Eto, M. The role of nuclear receptors in prostate cancer. Cells. 8, (2019).
Ramesh, S. et al. State-of-the-art therapeutic strategies for targeting cancer stem cells in prostate cancer. Front. Oncol. 13, 1059441 (2023).
Hsu, E.-C. et al. Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype via PARP1. Proc. Natl. Acad. Sci. USA 117, 2032–2042 (2020).
Ghosh, A., Tripathy, A. & Ghosh, D. Impact of endocrine disrupting chemicals (EDCs) on reproductive health of human. Proc. Zool. Soc. 75, 16–30 (2022).
Mazaira, G. I. et al. The nuclear receptor field: A historical overview and future challenges. Nucl. Receptor Res. 5, (2018).
Ellmann, S. et al. Estrogen and progesterone receptors: From molecular structures to clinical targets. Cell. Mol. Life Sci. 66, 2405–2426 (2009).
Paakinaho, V. & Palvimo, J. J. Genome-wide crosstalk between steroid receptors in breast and prostate cancers. Endocr. Relat. Cancer 28, R231–R250 (2021).
Bridgham, J. T., Carroll, S. M. & Thornton, J. W. Evolution of hormone-receptor complexity by molecular exploitation. Sci 312, 97–101 (2006).
Dube, N., Khan, S. H., Sasse, R. & Okafor, C. D. Identification of an evolutionarily conserved allosteric network in steroid receptors. J. Chem. Inf. Model. 63, 571–582 (2023).
Hantusch, B. et al. Targeting androgen, thyroid hormone, and vitamin A and D receptors to treat prostate cancer. Int. J. Mol. Sci. 25, 9245 (2024).
Sever, R. & Glass, C. K. Signaling by nuclear receptors. Cold Spring Harb. Perspect. Biol. 5, a016709 (2013).
Xu, P. Nuclear receptors in health and diseases. Int. J. Mol. Sci. 24, (2023).
Gissendanner, C. R. et al. Expression and function of conserved nuclear receptor genes in Caenorhabditis elegans. Dev. Biol. 266, 399–416 (2004).
D’Arrigo, G. et al. Exploring ligand binding domain dynamics in the NRs superfamily. Int. J. Mol. Sci. 23, (2022).
Lavery, D. N. & McEwan, I. J. Structure and function of steroid receptor AF1 transactivation domains: Induction of active conformations. Biochem J. 391, 449–464 (2005).
Yu, X. et al. Structural insights of transcriptionally active, full-length androgen receptor coactivator complexes. Mol. Cell 79, 812–823.e814 (2020).
Fernandez, E. J., Gahlot, V., Rodriguez, C. & Amburn, J. DNA-induced unfolding of the thyroid hormone receptor α A/B domain through allostery. FEBS Open Bio 7, 854–864 (2017).
Xie, G. et al. Centrosomal localization of RXRα promotes PLK1 activation and mitotic progression and constitutes a tumor vulnerability. Dev. Cell. 55, 707–722.e709 (2020).
Ma, B. et al. De novo design of an androgen receptor DNA binding domain-targeted peptide PROTAC for prostate cancer therapy. Adv. Sci. (Weinh.) 9, e2201859 (2022).
Choi, W. J., Haratipour, Z. & Blind, R. D. Full-length nuclear receptor allosteric regulation. J. Lipid Res. 64, 100406 (2023).
Liu, Y. et al. Structures of human TR4LBD-JAZF1 and TR4DBD-DNA complexes reveal the molecular basis of transcriptional regulation. Nucleic Acids Res. 51, 1443–1457 (2023).
Wasmuth, E. V. et al. Allosteric interactions prime androgen receptor dimerization and activation. Mol. Cell 82, 2021–2031.e2025 (2022).
Kolonko, M. et al. The intrinsically disordered region of GCE protein adopts a more fixed structure by interacting with the LBD of the nuclear receptor FTZ-F1. Cell Commun. Signal 18, 180 (2020).
Fang, N. et al. Identification of a novel melatonin-binding nuclear receptor: Vitamin D receptor. J. Pineal Res 68, e12618 (2020).
Erratum: Recognition of fold- and function-specific sites in the ligand-binding domain of the thyroid hormone receptor-like family. Front. Endocrinol. (Lausanne). 14, 1175369, (2023).
Webb, P. et al. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol. Endocrinol. 12, 1605–1618 (1998).
Nakadai, T. et al. Two target gene activation pathways for orphan ERR nuclear receptors. Cell Res. 33, 165–183 (2023).
Useini, A., Engelberger, F., Künze, G. & Sträter, N. Structural basis of the activation of PPARγ by the plasticizer metabolites MEHP and MINCH. Environ. Int. 173, 107822 (2023).
Louw, A. GR dimerization and the impact of GR dimerization on GR protein stability and half-life. Front. Immunol. 10, 1693 (2019).
Huang, W. et al. Multidomain architecture of estrogen receptor reveals interfacial cross-talk between its DNA-binding and ligand-binding domains. Nat. Commun. 9, 3520 (2018).
da Silva, M. A. et al. Case report: Two novel PPARG pathogenic variants associated with type 3 familial partial lipodystrophy in Brazil. Diabetol. Metab. Syndr. 16, 145 (2024).
Lu, J. et al. Types of nuclear localization signals and mechanisms of protein import into the nucleus. Cell Commun. Signal. 19, 60 (2021).
Nagandla, H. & Thomas, C. Estrogen signals through ERβ in breast cancer; what we have learned since the discovery of the receptor. Receptors 3, 182–200 (2024).
Huang, W. et al. Evolution of a novel nuclear receptor subfamily with emphasis on the member from the Pacific oyster Crassostrea gigas. Gene 567, 164–172 (2015).
Więch, A., Tarczewska, A., Ożyhar, A. & Orłowski, M. Metal ions induce liquid condensate formation by the f domain of aedes aegypti ecdysteroid receptor. New perspectives of nuclear receptor studies. Cells. 10, (2021).
Jakób, M. et al. Novel DNA-binding element within the C-terminal extension of the nuclear receptor DNA-binding domain. Nucleic Acids Res. 35, 2705–2718 (2007).
Arao, Y. & Korach, K. ennethS. The physiological role of estrogen receptor functional domains. Essays Biochem 65, 867–875 (2021).
Baker, M. E. Origin and diversification of steroids: co-evolution of enzymes and nuclear receptors. Mol. Cell Endocrinol. 334, 14–20 (2011).
Magner, D. B. & Antebi, A. Caenorhabditis elegans nuclear receptors: insights into life traits. Trends Endocrinol. Metab. 19, 153–160 (2008).
Schulman, I. G. & Heyman, R. A. The flip side: Identifying small molecule regulators of nuclear receptors. Chem. Biol. 11, 639–646 (2004).
Jobe, A. & Vijayan, R. Orphan G protein-coupled receptors: the ongoing search for a home. Front. Pharmacol. 15, 1349097 (2024).
Siepe, D. H. et al. Identification of orphan ligand-receptor relationships using a cell-based CRISPRa enrichment screening platform. eLife 11, e81398 (2022).
Thornton, J. W. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc. Natl. Acad. Sci. USA 98, 5671–5676 (2001).
Escriva, H., Delaunay, F. & Laudet, V. Ligand binding and nuclear receptor evolution. Bioessays 22, 717–727 (2000).
Laudet, V. et al. Evolution of the nuclear receptor gene superfamily. EMBO J. 11, 1003–1013 (1992).
Laudet, V. Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J. Mol. Endocrinol. 19, 207–226 (1997).
Markov, G. V. & Laudet, V. Origin and evolution of the ligand-binding ability of nuclear receptors. Mol. Cell Endocrinol. 334, 21–30 (2011).
Beinsteiner, B. et al. A novel nuclear receptor subfamily enlightens the origin of heterodimerization. BMC Biol. 20, 217 (2022).
Bwayi, M. N. et al. Molecular basis of crosstalk in nuclear receptors: Heterodimerization between PXR and CAR and the implication in gene regulation. Nucleic Acids Res. 50, 3254–3275 (2022).
De Bosscher, K. et al. Nuclear receptor crosstalk — defining the mechanisms for therapeutic innovation. Nat. Rev. Endocrinol. 16, 363–377 (2020).
Ogawa, S. et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122, 707–721 (2005).
Huggins, R. J. & Greene, G. L. ERα/PR crosstalk is altered in the context of the ERα Y537S mutation and contributes to endocrine therapy-resistant tumor proliferation. npj Breast Cancer 9, 96 (2023).
Katzenellenbogen, B. & Katzenellenbogen, B. S. Mechanisms of action and cross-talk between estrogen receptor and progesterone receptor pathways. J Soc Gynecol Investig 71(Suppl): S33-S37. J. Soc. Gynecol. Investig. 7, S33–S37 (2000).
Ratman, D. et al. Chromatin recruitment of activated AMPK drives fasting response genes co-controlled by GR and PPARα. Nucleic Acids Res. 44, 10539–10553 (2016).
Bougarne, N. et al. Mechanisms underlying the functional cooperation between PPARα and GRα to attenuate inflammatory responses. Front. Immunol. 10, 1769 (2019).
Dana, N., Vaseghi, G. & Haghjooy Javanmard, S. Activation of PPARγ inhibits TLR4 signal transduction pathway in melanoma cancer in vitro. Adv. Pharm. Bull. 10, 458–463 (2020).
Toobian, D., Ghosh, P. & Katkar, G. D. Parsing the role of PPARs in macrophage processes. Front. Immunol. 12, (2021).
Cantile, M. et al. Endocrine nuclear receptors and long non‑coding RNAs reciprocal regulation in cancer (Review). Int. J. Oncol. 64, (2024).
Freire, P. R. & Conneely, O. M. NR4A1 and NR4A3 restrict HSC proliferation via reciprocal regulation of C/EBPα and inflammatory signaling. Blood 131, 1081–1093 (2018).
Gao, H., Li, Y. & Chen, X. Interactions between nuclear receptors glucocorticoid receptor α and peroxisome proliferator–activated receptor α form a negative feedback loop. Rev. Endocr. Metab. Disord. 23, (2022).
Bougarne, N. et al. PPARα blocks glucocorticoid receptor α-mediated transactivation but cooperates with the activated glucocorticoid receptor α for transrepression on NF-κB. Proc. Natl. Acad. Sci. USA 106, 7397–7402 (2009).
Chen, X. et al. Peroxisome proliferator-activated receptor alpha agonist-induced down-regulation of hepatic glucocorticoid receptor expression in SD rats. Biochem. Biophys. Res Commun. 368, 865–870 (2008).
Cadoudal, T. et al. Proposed involvement of adipocyte glyceroneogenesis and phosphoenolpyruvate carboxykinase in the metabolic syndrome. Biochimie 87, 27–32 (2005).
Antwi, M. B. et al. Unlocking therapeutic potential: Exploring cross-talk among emerging nuclear receptors to combat metabolic dysfunction in steatotic liver disease. npj Metab. Health Dis. 2, 13 (2024).
Wang, J. et al. Forsythiaside A alleviates acute lung injury by inhibiting inflammation and epithelial barrier damages in lung and colon through PPAR-γ/RXR-α complex. J. Adv. Res. 60, 183–200 (2024).
Capitão, A. M. F. et al. An ancestral nuclear receptor couple, PPAR-RXR, is exploited by organotins. Sci. Total Environ. 797, 149044 (2021).
Chen, L. et al. Modulation of nongenomic activation of PI3K signalling by tetramerization of N-terminally-cleaved RXRα. Nat. Commun. 8, 16066 (2017).
Kilu, W. et al. Heterodimer formation with retinoic acid receptor RXRα modulates coactivator recruitment by peroxisome proliferator-activated receptor PPARγ. J. Biol. Chem. 297, 100814 (2021).
Moehren, U. et al. Identification of androgen-selective androgen-response elements in the human aquaporin-5 and Rad9 genes. Biochem J. 411, 679–686 (2008).
Petta, I. et al. The interactome of the glucocorticoid receptor and its influence on the actions of glucocorticoids in combatting inflammatory and infectious diseases. Microbiol Mol. Biol. Rev. 80, 495–522 (2016).
Hirayama, Y. & Sadar, M. D. Does increased expression of glucocorticoid receptor support application of antagonists to this receptor for the treatment of castration resistant prostate cancer? AME Med. J. 3, (2018).
Bianchetti, L. et al. Alternative dimerization interfaces in the glucocorticoid receptor-α ligand binding domain. Biochim. Biophys. Acta Gen. Subj. 1862, (2018).
Dubey, N. et al. The ESC/E(Z) complex, an effector of response to ovarian steroids, manifests an intrinsic difference in cells from women with premenstrual dysphoric disorder. Mol. Psychiatry 22, 1172–1184 (2017).
Pihlajamaa, P., Sahu, B. & Jänne, O. A. Determinants of receptor- and tissue-specific actions in androgen signaling. Endocr. Rev. 36, 357–384 (2015).
Clarisse, D. et al. Glucocorticoid receptor signaling: intricacies and therapeutic opportunities. Trends Biochem. Sci. 49, 431–444 (2024).
Præstholm, S. M., Correia, C. M. & Grøntved, L. Multifaceted control of GR signaling and its impact on hepatic transcriptional networks and metabolism. Front. Endocrinol. (Lausanne) 11, 572981 (2020).
Huss, J. M., Garbacz, W. G. & Xie, W. Constitutive activities of estrogen-related receptors: Transcriptional regulation of metabolism by the ERR pathways in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 1852, 1912–1927 (2015).
Oka, S. et al. PPARα-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab. 14, 598–611 (2011).
Tzeng, J. et al. An ideal PPAR response element bound to and activated by PPARα. PLoS One 10, e0134996 (2015).
Fan, W. et al. ERRγ promotes angiogenesis, mitochondrial biogenesis, and oxidative remodeling in PGC1α/β-deficient muscle. Cell Rep. 22, 2521–2529 (2018).
Wang, X. X. et al. Estrogen-related receptor agonism reverses mitochondrial dysfunction and inflammation in the aging kidney. Am. J. Pathol. 193, 1969–1987 (2023).
Abe, Y. et al. A TLR4/TRAF6-dependent signaling pathway mediates NCoR coactivator complex formation for inflammatory gene activation. Proc. Natl. Acad. Sci. USA 121, e2316104121 (2024).
Mays, S. G., Hercules, D., Ortlund, E. A. & Okafor, C. D. The nuclear receptor LRH-1 discriminates between ligands using distinct allosteric signaling circuits. Protein Sci. 32, e4754 (2023).
Hu, C. et al. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm (2020) 3, e161 (2022).
Messner, E. A. et al. The androgen receptor in prostate cancer: Effect of structure, ligands and spliced variants on therapy. Biomedicines. 8, (2020).
Ni, L. et al. Androgen induces a switch from cytoplasmic retention to nuclear import of the androgen receptor. Mol. Cell Biol. 33, 4766–4778 (2013).
Mohammad Nezhady, M. A., Rivera, J. C. & Chemtob, S. Location bias as emerging paradigm in GPCR biology and drug discovery. iScience 23, 101643 (2020).
Avilla, M. N. et al. The Ah receptor: Adaptive metabolism, ligand diversity, and the Xenokine Model. Chem. Res. Toxicol. 33, 860–879 (2020).
Liu, K., Zou, C. & Qin, B. The association between nuclear receptors and ocular diseases. Oncotarget 8, 27603–27615 (2017).
Park, U. H., Kim, E. J. & Um, S. J. A novel cytoplasmic adaptor for retinoic acid receptor (RAR) and thyroid receptor functions as a Derepressor of RAR in the absence of retinoic acid. J. Biol. Chem. 285, 34269–34278 (2010).
Yu, T., Dube, N. & Okafor, C. D. Simulations reveal the flexible domain architecture of full-length nuclear receptor complexes. bioRxiv, (2024).
Fili, N. et al. NDP52 activates nuclear myosin VI to enhance RNA polymerase II transcription. Nat. Commun. 8, 1871 (2017).
Dos Santos, Á et al. Autophagy receptor NDP52 alters DNA conformation to modulate RNA polymerase II transcription. Nat. Commun. 14, 2855 (2023).
Furuya, N. et al. NDP52 interacts with mitochondrial RNA poly(A) polymerase to promote mitophagy. EMBO Rep. 19, (2018).
Paakinaho, V., Johnson, T. A., Presman, D. M. & Hager, G. L. Glucocorticoid receptor quaternary structure drives chromatin occupancy and transcriptional outcome. Genome Res 29, 1223–1234 (2019).
Schöne, S. et al. Sequences flanking the core-binding site modulate glucocorticoid receptor structure and activity. Nat. Commun. 7, 12621 (2016).
Jiang, L. et al. Structural characterization of the DNA binding mechanism of retinoic acid-related orphan receptor gamma. Structure 32, 467–475.e463 (2024).
Johnson, D. R. et al. NuRD complex component Mi-2beta binds to and represses RORgamma-mediated transcriptional activation. Biochem. Biophys. Res Commun. 318, 714–718 (2004).
Jenniskens, M. et al. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med. 42, 16–27 (2016).
Zheng, Y. et al. COX-2 mediates tumor-stromal prolactin signaling to initiate tumorigenesis. Proc. Natl. Acad. Sci. USA 116, 5223–5232 (2019).
Dauwe, Y. et al. Steatosis and metabolic disorders associated with synergistic activation of the CAR/RXR heterodimer by pesticides. Cells. 12, (2023).
Huber, A. D. & Chen, T. A new mode of inhibition. eLife 13, e101446 (2024).
Jaladanki, C. K. et al. Virtual screening of potentially endocrine-disrupting chemicals against nuclear receptors and its application to identify PPARγ-bound fatty acids. Arch. Toxicol. 95, 355–374 (2021).
Martens, N. et al. Activation of liver X receptors and peroxisome proliferator-activated receptors by lipid extracts of Brown Seaweeds: A potential application in Alzheimer’s disease? Nutrients. 15, (2023).
Tan, H. et al. Structures of endocrine-disrupting chemicals correlate with the activation of 12 classic nuclear receptors. Environ. Sci. Technol. 55, 16552–16562 (2021).
Blackburn, C. M. R. et al. Myeloid-associated lipin-1 transcriptional co-regulatory activity is atheroprotective. Atherosclerosis 330, 76–84 (2021).
Kumar, R., Lee, J. C., Bolen, D. W. & Thompson, E. B. The conformation of the glucocorticoid receptor af1/tau1 domain induced by osmolyte binds co-regulatory proteins. J. Biol. Chem. 276, 18146–18152 (2001).
Hosseinzadeh, L. et al. The androgen receptor interacts with GATA3 to transcriptionally regulate a luminal epithelial cell phenotype in breast cancer. Genome Biol. 25, 44 (2024).
Talukdar, P. D. & Chatterji, U. Transcriptional co-activators: emerging roles in signaling pathways and potential therapeutic targets for diseases. Signal Transduct. Target Ther. 8, 427 (2023).
Bahl, S. & Seto, E. Regulation of histone deacetylase activities and functions by phosphorylation and its physiological relevance. Cell. Mol. Life Sci. 78, 427–445 (2021).
Corso-Díaz, X. et al. Co-activator candidate interactions for orphan nuclear receptor NR2E1. BMC Genomics 17, 832 (2016).
Perez-Mockus, G. et al. The Drosophila ecdysone receptor promotes or suppresses proliferation according to ligand level. Dev. Cell. 58, 2128–2139.e2124 (2023).
Akiyama, T., Raftery, L. A. & Wharton, K. A. Bone morphogenetic protein signaling: the pathway and its regulation. Genetics. 226, (2023).
Bure, I. V., Nemtsova, M. V. & Kuznetsova, E. B. Histone Modifications and Non-Coding RNAs: Mutual Epigenetic Regulation and Role in Pathogenesis. Int. J. Mol. Sci. 23, 5801 (2022).
Kumar, S. & Mohapatra, T. Epigenetic modifications in genome help remembering the stress tolerance strategy adopted by the plant. FBL. 29, (2024).
Flora, G. D. et al. Non-genomic effects of the Pregnane X receptor negatively regulate platelet functions, thrombosis and haemostasis. Sci. Rep. 9, 17210 (2019).
Burgermeister, E. Mitogen-activated protein kinase and nuclear hormone receptor crosstalk in cancer immunotherapy. Int. J. Mol. Sci. 24, 13661 (2023).
Großkopf, H. et al. Non-genomic AhR-signaling modulates the immune response in endotoxin-activated macrophages after activation by the environmental stressor BaP. Front. Immunol. 12, 620270 (2021).
Gourdy, P. et al. Estrogen receptor subcellular localization and cardiometabolism. Mol. Metab. 15, 56–69 (2018).
Zhu, F. et al. The orphan receptor Nur77 binds cytoplasmic LPS to activate the non-canonical NLRP3 inflammasome. Immunity 56, 753–767.e758 (2023).
Ma, Y. L. et al. Semaglutide ameliorates cardiac remodeling in male mice by optimizing energy substrate utilization through the Creb5/NR4a1 axis. Nat. Commun. 15, 4757 (2024).
Rehman, S. U. et al. Identification of two novel isoforms of mouse NUR77 lacking N-terminal domains. IUBMB Life 69, 106–114 (2017).
Nyati, K. K. et al. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. NAR 45, 2687–2703 (2017).
Santos, R. S. et al. Activation of estrogen receptor alpha induces beiging of adipocytes. Mol. Metab. 18, 51–59 (2018).
Bennett, L. et al. Glucocorticoid receptor (GR) activation is associated with increased cAMP/PKA signaling in castration-resistant prostate cancer. Mol. Cancer Ther. 23, 552–563 (2024).
Jung, K. et al. Farnesoid X receptor activation impairs liver progenitor cell-mediated liver regeneration via the PTEN-PI3K-AKT-mTOR axis in zebrafish. Hepatology 74, 397–410 (2021).
Li, X. et al. YAP inhibits ERα and ER(+) breast cancer growth by disrupting a TEAD-ERα signaling axis. Nat. Commun. 13, 3075 (2022).
Leung, Y. K. et al. The loss of an orphan nuclear receptor NR2E3 augments Wnt/β-catenin signaling via epigenetic dysregulation that enhances Sp1-β catenin-p300 interactions in hepatocellular carcinoma. Adv. Sci. (Weinh.) 11, e2308539 (2024).
Hori, T. et al. Nuclear receptor CAR suppresses GADD45B-p38 MAPK signaling to promote phenobarbital-induced proliferation in mouse liver. Mol. Cancer Res. 16, 1309–1318 (2018).
Tao, Y. et al. Nr4a1 promotes renal interstitial fibrosis by regulating the p38 MAPK phosphorylation. Mol. Med. 29, 63 (2023).
Ashraf, S., Hegazy, Y. K. & Harmancey, R. Nuclear receptor subfamily 4 group A member 2 inhibits activation of ERK signaling and cell growth in response to β-adrenergic stimulation in adult rat cardiomyocytes. Am. J. Physiol. Cell Physiol. 317, C513–c524 (2019).
Romero-Martínez, B. S. et al. Estrogenic modulation of ionic channels, pumps and exchangers in airway smooth muscle. Int. J. Mol. Sci. 24, (2023).
de Hoog, E., Lukewich, M. K. & Spencer, G. E. Retinoic acid inhibits neuronal voltage-gated calcium channels. Cell Calcium 72, 51–61 (2018).
Sahoo, N. et al. Intracellular hemin is a potent inhibitor of the voltage-gated potassium channel Kv10.1. Sci. Rep. 12, 14645 (2022).
Unsworth, A. J., Flora, G. D. & Gibbins, J. M. Non-genomic effects of nuclear receptors: insights from the anucleate platelet. Cardiovasc. Res. 114, 645–655 (2018).
Nuñez, F. J. et al. Glucocorticoids rapidly activate cAMP production via G(αs) to initiate non-genomic signaling that contributes to one-third of their canonical genomic effects. FASEB J. 34, 2882–2895 (2020).
Chen, L., Wu, L., Zhu, L. & Zhao, Y. Overview of the structure-based non-genomic effects of the nuclear receptor RXRα. Cell. Mol. Biol. Lett. 23, 36 (2018).
Liu, Y. et al. Effect of retinoid X receptor-α nuclear export inhibition on apoptosis of neurons in vivo and in vitro. Mol. Med. Rep. 16, 2037–2044 (2017).
Xie, C. K. et al. Sulindac (K-80003) with nab-paclitaxel and gemcitabine overcomes drug-resistant pancreatic cancer. Mol. Cancer 23, 215 (2024).
Li, H. et al. Oroxylin A, a natural compound, mitigates the negative effects of TNFα-treated acute myelogenous leukemia cells. Carcinogenesis 39, 1292–1303 (2018).
Abdoul-Azize, S. et al. Glucocorticoids paradoxically promote steroid resistance in B cell acute lymphoblastic leukemia through CXCR4/PLC signaling. Nat. Commun. 15, 4557 (2024).
Zhou, F. et al. A narrative review of the role of glucocorticoid receptors in prostate cancer: developments in last 5 years. Transl. Androl. Urol. 11, 1189–1199 (2022).
Ehrchen, J. M., Roth, J. & Barczyk-Kahlert, K. More than suppression: Glucocorticoid action on monocytes and macrophages. Front. Immunol. 10, 2028 (2019).
Ma, Y., Huang, Y. & Xu, G. New insights into the mucosal immune pathogenesis of IgA nephropathy from the perspective of COVID-19 vaccination. QJM 116, 181–195 (2023).
Panettieri, R. A. et al. Non-genomic effects of glucocorticoids: An updated view. Trends Pharmacol. Sci. 40, 38–49 (2019).
Maczewsky, J., Kaiser, J., Krippeit-Drews, P. & Drews, G. Approved LXR agonists exert unspecific effects on pancreatic β-cell function. Endocrine 68, 526–535 (2020).
Kierans, S. J. et al. Hypoxia induces a glycolytic complex in intestinal epithelial cells independent of HIF-1-driven glycolytic gene expression. Proc. Natl. Acad. Sci. USA 120, e2208117120 (2023).
Ambhore, N. S. et al. Differential estrogen-receptor activation regulates extracellular matrix deposition in human airway smooth muscle remodeling via NF-κB pathway. FASEB J. 33, 13935–13950 (2019).
Roşca, A. E. et al. Effects of exogenous androgens on platelet activity and their thrombogenic potential in supraphysiological administration: A literature review. J Clin Med. 10, (2021).
Sesti, F. et al. Late-onset hypogonadism: Reductio ad absurdum of the cardiovascular risk-benefit of testosterone replacement therapy. Andrology 8, 1614–1627 (2020).
Li, S. et al. Experimental arterial thrombosis regulated by androgen and its receptor via modulation of platelet activation. Thromb. Res. 121, 127–134 (2007).
Chang, S. et al. Platelet aggregation in Klinefelter syndrome is not aggravated by testosterone replacement therapy: A longitudinal follow-up study. Andrology 11, 456–463 (2023).
Unsworth, A. J. et al. RXR ligands negatively regulate thrombosis and hemostasis. Atertio. Thromb. Vasc. Biol. 37, 812–822 (2017).
Grover, S. P., Bergmeier, W. & Mackman, N. Platelet signaling pathways and new inhibitors. Atertio. Thromb. Vasc. Biol. 38, e28–e35 (2018).
Moraes, L. A. et al. Farnesoid X receptor and its ligands inhibit the function of platelets. Arterioscler. Thromb. Vasc. Biol. 36, 2324–2333 (2016).
Zhou, X. et al. Mechanism of bile acid in regulating platelet function and thrombotic diseases. Adv. Sci. (Weinh.) 11, e2401683 (2024).
Unsworth, A. J. et al. Farnesoid X receptor and liver X receptor ligands initiate formation of coated platelets. Arterioscler. Thromb. Vasc. Biol. 37, 1482–1493 (2017).
Topalov, N. N. et al. Two types of procoagulant platelets are formed upon physiological activation and are controlled by integrin α(IIb)β(3). Arterioscler. Thromb. Vasc. Biol. 32, 2475–2483 (2012).
Ferreira, J. P. et al. Fenofibrate and heart failure outcomes in patients with type 2 diabetes: Analysis from ACCORD. Diab. Care 45, 1584–1591 (2022).
Bye, A. P., Unsworth, A. J. & Gibbins, J. M. Platelet signaling: A complex interplay between inhibitory and activatory networks. J. Thromb. Haemost. 14, 918–930 (2016).
Ali, F. Y. et al. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler. Thromb. Vasc. Biol. 29, 706–711 (2009).
Al Tanoury, Z., Piskunov, A. & Rochette-Egly, C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J. Lipid Res 54, 1761–1775 (2013).
Yu, Z. et al. ATRA-mediated-crosstalk between stellate cells and Kupffer cells inhibits autophagy and promotes NLRP3 activation in acute liver injury. Cell. Signal. 93, 110304 (2022).
Harding, E. K., Ferron, L. & Zamponi, G. W. RAR-alpha: Creator, protector, and tormentor. Neuron 110, 4033–4035 (2022).
Sengör, B. et al. Effects of acitretin on spermatogenesis of rats. J. Eur. Acad. Dermatol. Venereol. 20, 689–692 (2006).
Zhu, L., Doyle, T. J. & Kim, K. H. Retinoic acid modulates the subcellular localization of small ubiquitin-related modifier-2/3 (SUMO-2/3) in the testis. J. Androl. 31, 406–418 (2010).
Walker, S. E. et al. Retinoid X receptor α downregulation is required for tail and caudal spinal cord regeneration in the adult newt. Neural Regen. Res. 13, 1036–1045 (2018).
Chen, L., Lau, A. G. & Sarti, F. Synaptic retinoic acid signaling and homeostatic synaptic plasticity. Neuropharmacology 78, 3–12 (2014).
Park, E., Lau, A. G., Arendt, K. L. & Chen, L. FMRP interacts with RARα in synaptic retinoic acid signaling and homeostatic synaptic plasticity. Int. J. Mol. Sci. 22, 6579 (2021).
Khatib, T., Muller, B. & McCaffery, P. Vol. 2524 197-207 (2022).
Zhong, L. R. et al. Retinoic acid receptor RARα-dependent synaptic signaling mediates homeostatic synaptic plasticity at the inhibitory synapses of mouse visual cortex. J. Neurosci. 38, 10454–10466 (2018).
Rossillo, M. & Ringstad, N. Development of specialized sensory neurons engages a nuclear receptor required for functional plasticity. Genes Dev. 34, 1666–1679 (2020).
Riedl, J., Fieseler, C. & Zimmer, M. Tyraminergic corollary discharge filters reafferent perception in a chemosensory neuron. Curr. Biol. 32, 3048–3058.e3046 (2022).
Brandt, J. et al. A single gene target of an ETS-family transcription factor determines neuronal CO2-chemosensitivity. PLoS One 7, e34014 (2012).
Smith, E., Martinez-Velazquez, L. & Ringstad, N. A chemoreceptor that detects molecular carbon dioxide. J. Biol. Chem. 288, (2013).
Lee, Y. H. et al. Hepatic MIR20B promotes nonalcoholic fatty liver disease by suppressing PPARA. Elife. 10, (2021).
Lee, M. C. et al. Tributyltin affects retinoid X receptor-mediated lipid metabolism in the marine Rotifer Brachionus koreanus. Environ. Sci. Technol. 53, 7830–7839 (2019).
Xu, Y., O’Malley, B. W. & Elmquist, J. K. Brain nuclear receptors and body weight regulation. J. Clin. Invest. 127, 1172–1180 (2017).
Ding, L., Yang, L., Wang, Z. & Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 5, 135–144 (2015).
Nicolini, A., Ferrari, P. & Duffy, M. J. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin. Cancer Biol. 52, 56–73 (2018).
Xu, Y. et al. ZNF397 deficiency triggers TET2-driven lineage plasticity and AR-targeted therapy resistance in prostate cancer. Cancer Discov. 14, 1496–1521 (2024).
Alfarhan, M. W., Al-Hussaini, H. & Kilarkaje, N. Role of PPAR-γ in diabetes-induced testicular dysfunction, oxidative DNA damage and repair in leptin receptor-deficient obese type 2 diabetic mice. Chem. Biol. Interact. 361, 109958 (2022).
Dragoljevic, D. et al. Administration of an LXR agonist promotes atherosclerotic lesion remodelling in murine inflammatory arthritis. Clin. Transl. Immunol. 12, e1446 (2023).
Kumar, M., Salem, K., Jeffery, J. J. & Fowler, A. M. PET imaging of estrogen receptors using (18)F-based radioligands. Methods Mol. Biol. 2418, 129–151 (2022).
Yang, J. et al. Mecp2 fine-tunes quiescence exit by targeting nuclear receptors. Elife. 12, (2024).
Zhao, Y. et al. Nr5a2 ensures inner cell mass formation in mouse blastocyst. Cell Rep. 43, 113840 (2024).
Wan, R. et al. NR2F2 alleviates pulmonary fibrosis by inhibition of epithelial cell senescence. Respir. Res. 25, 154 (2024).
Wang, C. et al. Z-ligustilide selectively targets AML by restoring nuclear receptors Nur77 and NOR-1-mediated apoptosis and differentiation. Phytomedicine 82, 153448 (2021).
Clarisse, D., Offner, F. & De Bosscher, K. Latest perspectives on glucocorticoid-induced apoptosis and resistance in lymphoid malignancies. Biochim. Biophys. Acta 1874, 188430 (2020).
Arnesen, S., et al. Estrogen receptor alpha mutations in breast cancer cells cause gene expression changes through constant activity and secondary effects. Cancer Res. 81, 539–551 (2021).
Pakdel, F. The role of estrogen receptors in health and disease. Int. J. Mol. Sci. 24, (2023).
Farcas, A. M., Nagarajan, S., Cosulich, S. & Carroll, J. S. Genome-wide estrogen receptor activity in breast cancer. Endocrinology. 162, (2021).
Ross-Innes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Chen, H. et al. A pan-cancer analysis of enhancer expression in nearly 9000 patient samples. Cell 173, 386–399.e312 (2018).
Maurya, S. S. Role of enhancers in development and diseases. Epigenomes. 5, (2021).
Rashid, A. et al. Roscovitine enhances All-trans retinoic acid (ATRA)-induced leukemia cell differentiation: Novel effects on signaling molecules for a putative Cdk2 inhibitor. Cell. Signal. 71, 109555 (2020).
Rashid, A. et al. Roscovitine enhances all- trans retinoic acid (ATRA)-induced nuclear enrichment of an ensemble of activated signaling molecules and augments ATRA-induced myeloid cell differentiation. Oncotarget. 11, (2020).
Salehpour, A. et al. 1,25-Dihydroxyvitamin D3 modulates adipogenesis of human adipose-derived mesenchymal stem cells dose-dependently. Nutr. Metab. (Lond.). 18, 29 (2021).
Caccavale, F. et al. Crosstalk between nitric oxide and retinoic acid pathways is essential for amphioxus pharynx development. Elife. 10, (2021).
di Masi, A. et al. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol. Asp. Med. 41, 1–115 (2015).
Yu, G. et al. Retinoic acid receptor activation reduces metastatic prostate cancer bone lesions by blocking the endothelial-to-osteoblast transition. Cancer Res 82, 3158–3171 (2022).
Yu, G. et al. Multiple pathways coordinating reprogramming of endothelial cells into osteoblasts by BMP4. iScience 24, 102388 (2021).
Ke, N. et al. Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res 64, 8208–8212 (2004).
Balasubramanian, S. et al. Orphan nuclear receptor Nur77 promotes acute kidney injury and renal epithelial apoptosis. J. Am. Soc. Nephrol. 23, 674–686 (2012).
He, L. et al. A Regulation Loop Between YAP and NR4A1 balances cell proliferation and apoptosis. Cell Rep. 33, 108284 (2020).
Chen, L. et al. Emodin promotes hepatic stellate cell senescence and alleviates liver fibrosis via a nuclear receptor (Nur77)-mediated epigenetic regulation of glutaminase 1. Br. J. Pharm. 180, 2577–2598 (2023).
Wang, Z. et al. Nuclear receptor HNF4α performs a tumor suppressor function in prostate cancer via its induction of p21-driven cellular senescence. Oncogene 39, 1572–1589 (2020).
Xing, Y., Yan, J. & Niu, Y. PXR: A center of transcriptional regulation in cancer. Acta Pharm. Sin. B 10, 197–206 (2020).
Ma, X. et al. The nuclear receptor RXRA controls cellular senescence by regulating calcium signaling. Aging Cell 17, e12831 (2018).
Wang, C. et al. Zinc finger protein ZBTB17 controls cellular senescence via interacting with nuclear receptor RXRA and its downstream calcium signaling. FASEB J. 37, e23193 (2023).
Terao, R. et al. LXR/CD38 activation drives cholesterol-induced macrophage senescence and neurodegeneration via NAD(+) depletion. Cell Rep. 43, 114102 (2024).
Kumar, R. et al. Metabolic pathways, enzymes, and metabolites: Opportunities in cancer therapy. Cancers (Basel). 14, (2022).
Marino, J. S. et al. Glucocorticoid Receptor β induces hepatic steatosis by augmenting inflammation and inhibition of the peroxisome proliferator-activated receptor (PPAR) α. J. Biol. Chem. 291, 25776–25788 (2016).
Shimizu, M. & Sato, R. Endocrine fibroblast growth factors in relation to stress signaling. Cells. 11, (2022).
Chatterjee, N. & Perrimon, N. What fuels the fly: Energy metabolism in Drosophila and its application to the study of obesity and diabetes. Sci. Adv. 7, eabg4336 (2021).
Gonzalez-Rellan, M. J., Fondevila, M. F., Dieguez, C. & Nogueiras, R. O-GlcNAcylation: A sweet hub in the regulation of glucose metabolism in health and disease. Front. Endocrinol. (Lausanne) 13, 873513 (2022).
Aronoff, S. L., Berkowitz, K., Shreiner, B. & Want, L. Glucose metabolism and regulation: Beyond insulin and glucagon. Diab. Spectr. 17, 183–190 (2004).
Hatano, R. et al. Hepatic ketone body regulation of renal gluconeogenesis. Mol. Metab. 84, 101934 (2024).
Zhao, Y. et al. Histone phosphorylation integrates the hepatic glucagon-PKA-CREB gluconeogenesis program in response to fasting. Mol. Cell 83, 1093–1108.e1098 (2023).
Yook, J. S. et al. The SLC25A47 locus controls gluconeogenesis and energy expenditure. Proc. Natl. Acad. Sci. USA 120, e2216810120 (2023).
Hassan, F. U. et al. Role of peroxisome proliferator-activated receptors (PPARs) in energy homeostasis of dairy animals: ExplOiting Their Modulation Through Nutrigenomic Interventions. Int. J. Mol. Sci. 22, (2021).
Ramatchandirin, B., Pearah, A. & He, L. Regulation of liver glucose and lipid metabolism by transcriptional factors and coactivators. Life (Basel). 13, (2023).
Hassani-Nezhad-Gashti, F. et al. Activation of nuclear receptor PXR impairs glucose tolerance and dysregulates GLUT2 expression and subcellular localization in liver. Biochem Pharm. 148, 253–264 (2018).
Mitro, N. et al. The nuclear receptor LXR is a glucose sensor. Nature 445, 219–223 (2007).
Miao, L. et al. Glycerol kinase interacts with nuclear receptor NR4A1 and regulates glucose metabolism in the liver. FASEB J. 33, 6736–6747 (2019).
Martin Vázquez, E. et al. NR5A2/LRH-1 regulates the PTGS2-PGE(2)-PTGER1 pathway contributing to pancreatic islet survival and function. iScience 25, 104345 (2022).
Sun, Y., Demagny, H. & Schoonjans, K. Emerging functions of the nuclear receptor LRH-1 in liver physiology and pathology. Biochim Biophys. Acta Mol. Basis Dis. 1867, 166145 (2021).
Ploton, M. et al. The nuclear bile acid receptor FXR is a PKA- and FOXA2-sensitive activator of fasting hepatic gluconeogenesis. J. Hepatol. 69, 1099–1109 (2018).
Hauck, A. K. et al. Nuclear receptor corepressors non-canonically drive glucocorticoid receptor-dependent activation of hepatic gluconeogenesis. Nat. Metab. 6, 825–836 (2024).
Xie, Y. et al. Deficiency of nuclear receptor coactivator 3 aggravates diabetic kidney disease by impairing podocyte autophagy. Adv. Sci. (Weinh.) 11, e2308378 (2024).
Wang, F. et al. Essential role of nuclear receptors for the evaluation of the benefits of bioactive herbal extracts on liver function. Biomed. Pharmacother. 99, 798–809 (2018).
Chiang, J. Y. L. & Ferrell, J. M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell Endocrinol. 548, 111618 (2022).
Bougarne, N. et al. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 39, 760–802 (2018).
Lamichane, S., Dahal Lamichane, B. & Kwon, S. M. Pivotal roles of peroxisome proliferator-activated receptors (PPARs) and their signal cascade for cellular and whole-body energy homeostasis. Int. J. Mol. Sci. 19, (2018).
Guajardo-Correa, E. et al. Estrogen signaling as a bridge between the nucleus and mitochondria in cardiovascular diseases. Front. Cell Dev. Biol. 10, (2022).
Chen, C. Y. et al. Inhibition of estrogen-related receptor α blocks liver steatosis and steatohepatitis and attenuates triglyceride biosynthesis. Am. J. Pathol. 191, 1240–1254 (2021).
Gulick, T. et al. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc. Natl. Acad. Sci. USA 91, 11012–11016 (1994).
Huang, C. Y. et al. PERM1 regulates genes involved in fatty acid metabolism in the heart by interacting with PPARα and PGC-1α. Sci. Rep. 12, 14576 (2022).
Montagner, A. et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 65, 1202–1214 (2016).
Iershov, A. et al. The class 3 PI3K coordinates autophagy and mitochondrial lipid catabolism by controlling nuclear receptor PPARα. Nat. Commun. 10, 1566 (2019).
Geiger, M. et al. Genetic deletion of hepatic NCOR1 protects from atherosclerosis by promoting alternative bile acid-metabolism and sterol excretion. Cardiovasc. Diabetol. 22, 144 (2023).
Zhang, Y. et al. Targeting nuclear receptor NR4A1-dependent adipocyte progenitor quiescence promotes metabolic adaptation to obesity. J. Clin. Invest. 128, 4898–4911 (2018).
Qin, D. D. et al. NR4A1 retards adipocyte differentiation or maturation via enhancing GATA2 and p53 expression. J. Cell. Mol. Med. 22, 4709–4720 (2018).
Kim, Y. C. et al. Small heterodimer partner and fibroblast growth factor 19 inhibit expression of NPC1L1 in mouse intestine and cholesterol absorption. Gastroenterology 156, 1052–1065 (2019).
Ito, K. et al. Critical roles of transcriptional coactivator MED1 in the formation and function of mouse adipose tissues. Genes Dev. 35, 729–748 (2021).
Zhou, J. et al. MED1 mediator subunit is a key regulator of hepatic autophagy and lipid metabolism. Autophagy 17, 4043–4061 (2021).
Cai, C., Cai, P. & Chu, G. Selection of suitable reference genes for core clock gene expression analysis by real-time qPCR in rat ovary granulosa cells. Mol. Biol. Rep. 46, 2941–2946 (2019).
Zhu, K. et al. An intrinsically disordered region controlling condensation of a circadian clock component and rhythmic transcription in the liver. Mol. Cell 83, 3457–3469.e3457 (2023).
Schibler, U. How the circadian nuclear orphan receptor REV-ERBα represses transcription: Temporal and spatial phase separation combined. Mol. Cell 83, 3399–3401 (2023).
Zhao, X. et al. Nuclear receptors rock around the clock. EMBO Rep. 15, 518–528 (2014).
Cai, D. et al. RORγ is a targetable master regulator of cholesterol biosynthesis in a cancer subtype. Nat. Commun. 10, 4621 (2019).
Yu, X. et al. Nuclear receptor PXR targets AKR1B7 to protect mitochondrial metabolism and renal function in AKI. Sci. Transl. Med. 12, (2020).
Byun, S. et al. Phosphorylation of hepatic farnesoid X receptor by FGF19 signaling-activated Src maintains cholesterol levels and protects from atherosclerosis. J. Biol. Chem. 294, 8732–8744 (2019).
Karpale, M. et al. Activation of pregnane X receptor induces atherogenic lipids and PCSK9 by a SREBP2-mediated mechanism. Br. J. Pharm. 178, 2461–2481 (2021).
Jin, P. et al. Mitochondrial adaptation in cancer drug resistance: prevalence, mechanisms, and management. J. Hematol. Oncol. 15, 97 (2022).
Scholtes, C. et al. Identification of a chromatin-bound ERRα interactome network in mouse liver. Mol. Metab. 83, 101925 (2024).
Wu, Z. et al. HDAC1 disrupts the tricarboxylic acid (TCA) cycle through the deacetylation of Nur77 and promotes inflammation in ischemia-reperfusion mice. Cell Death Discov. 9, 10 (2023).
Sacco, S. A. et al. Overexpression of peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α) in Chinese hamster ovary cells increases oxidative metabolism and IgG productivity. Metab. Eng. 79, 108–117 (2023).
Kida, Y. S. et al. ERRs mediate a metabolic switch required for somatic cell reprogramming to pluripotency. Cell Stem Cell 16, 547–555 (2015).
Singh, B. K. et al. Thyroid hormone receptor and ERRα coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci Signal. 11, (2018).
Wattez, J. S. et al. Loss of skeletal muscle estrogen-related receptors leads to severe exercise intolerance. Mol. Metab. 68, 101670 (2023).
Sopariwala, D. H. et al. Innately expressed estrogen-related receptors in the skeletal muscle are indispensable for exercise fitness. FASEB J. 37, e22727 (2023).
Gantner, M. L. et al. Complementary roles of estrogen-related receptors in brown adipocyte thermogenic function. Endocrinology 157, 4770–4781 (2016).
Li, X. X. et al. Nuclear receptor Nur77 facilitates melanoma cell survival under metabolic stress by protecting fatty acid oxidation. Mol. Cell 69, 480–492.e487 (2018).
Peng, S. Z. et al. Phase separation of Nur77 mediates celastrol-induced mitophagy by promoting the liquidity of p62/SQSTM1 condensates. Nat. Commun. 12, 5989 (2021).
Yin, S. et al. Nur77 increases mitophagy and decreases aggregation of α-synuclein by modulating the p-c-Abl/p-PHB2 Y121 in α-synuclein PFF SH-SY5Y cells and mice. Eur. J. Med. Chem. 268, 116251 (2024).
Sun, L. Y. et al. Nuclear receptor NR1D1 regulates abdominal aortic aneurysm development by targeting the mitochondrial tricarboxylic acid cycle enzyme aconitase-2. Circulation 146, 1591–1609 (2022).
Ling, Z.-N. et al. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 8, 345 (2023).
Torres, N. et al. Amino acid catabolism: An overlooked area of metabolism. Nutrients. 15, (2023).
Dhillon, P. et al. The nuclear receptor ESRRA protects from kidney disease by coupling metabolism and differentiation. Cell Metab. 33, 379–394.e378 (2021).
Sjögren, R. J. O. et al. Branched-chain amino acid metabolism is regulated by ERRα in primary human myotubes and is further impaired by glucose loading in type 2 diabetes. Diabetologia 64, 2077–2091 (2021).
Tobón-Cornejo, S. et al. PPARα/RXRα downregulates amino acid catabolism in the liver via interaction with HNF4α promoting its proteasomal degradation. Metabolism 116, 154705 (2021).
Shiozaki, Y., Miyazaki-Anzai, S., Keenan, A. L. & Miyazaki, M. MEF2D-NR4A1-FAM134B2-mediated reticulophagy contributes to amino acid homeostasis. Autophagy 18, 1049–1061 (2022).
Koenis, D. S. et al. Nuclear receptor Nur77 limits the macrophage inflammatory response through transcriptional reprogramming of mitochondrial metabolism. Cell Rep. 24, 2127–2140.e2127 (2018).
Han, Y. H. et al. RORα induces KLF4-mediated M2 polarization in the liver macrophages that protect against nonalcoholic steatohepatitis. Cell Rep. 20, 124–135 (2017).
Zhou, J. et al. Orphan nuclear receptor TLX promotes immunosuppression via its transcriptional activation of PD-L1 in glioma. J. Immunother. Cancer. 9, (2021).
Liu, H. et al. NR1D1 modulates synovial inflammation and bone destruction in rheumatoid arthritis. Cell Death Dis. 11, 129 (2020).
Liu, X. et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature 567, 525–529 (2019).
Chen, J. et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 567, 530–534 (2019).
Ghyselinck, N. B. & Duester, G. Retinoic acid signaling pathways. Development. 146, (2019).
Larange, A. et al. A regulatory circuit controlled by extranuclear and nuclear retinoic acid receptor α determines T cell activation and function. Immunity 56, 2054–2069.e2010 (2023).
Sarubin, N. et al. The sex-dependent role of the glucocorticoid receptor in depression: variations in the NR3C1 gene are associated with major depressive disorder in women but not in men. Eur. Arch. Psychiatry Clin. Neurosci. 267, 123–133 (2017).
Mark, M., Ghyselinck, N. B. & Chambon, P. Function of retinoic acid receptors during embryonic development. Nucl. Recept Signal 7, e002 (2009).
Chen, J. et al. Progressive reduction of nuclear receptor Nr4a1 mediates age-dependent cognitive decline. Alzheimers Dement 20, 3504–3524 (2024).
Ekimoto, T., Kudo, T., Yamane, T. & Ikeguchi, M. Mechanism of vitamin D receptor ligand-binding domain regulation studied by gREST simulations. J. Chem. Inf. Model. 61, 3625–3637 (2021).
Wang, N. et al. Ligand binding and heterodimerization with retinoid X receptor α (RXRα) induce farnesoid X receptor (FXR) conformational changes affecting coactivator binding. J. Biol. Chem. 293, 18180–18191 (2018).
Hashiguchi, T. et al. Phosphorylation of farnesoid X receptor at serine 154 links ligand activation with degradation. Mol. Endocrinol. 30, 1070–1080 (2016).
Stein, S. et al. Impaired SUMOylation of nuclear receptor LRH-1 promotes nonalcoholic fatty liver disease. J. Clin. Invest. 127, 583–592 (2017).
He, Y. et al. PPARγ acetylation orchestrates adipose plasticity and metabolic rhythms. Adv. Sci. (Weinh.) 10, e2204190 (2023).
Ikegami, K., Refetoff, S., Van Cauter, E. & Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 15, 590–600 (2019).
Kim, Y. H. & Lazar, M. A. TranscRiptional Control Of Circadian Rhythms And Metabolism: A matter of time and space. Endocr. Rev. 41, bnaa014 (2020).
Guo, J. et al. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 7, 391 (2022).
Frankowska, N., Lisowska, K. & Witkowski, J. M. Proteolysis dysfunction in the process of aging and age-related diseases. Front Aging 3, 927630 (2022).
Reich, N. & Hölscher, C. The neuroprotective effects of glucagon-like peptide 1 in Alzheimer’s and Parkinson’s disease: An in-depth review. Front. Neurosci. 16, 970925 (2022).
Brown, M. R. et al. The nuclear receptor REV-ERBα is implicated in the alteration of β-cell autophagy and survival under diabetogenic conditions. Cell Death Dis. 13, 353 (2022).
Li, P. et al. Autophagy and aging: Roles in skeletal muscle, eye, brain and hepatic tissue. Front Cell Dev. Biol. 9, 752962 (2021).
Doering, K. R. S., Ermakova, G. & Taubert, S. Nuclear hormone receptor NHR-49 is an essential regulator of stress resilience and healthy aging in Caenorhabditis elegans. Front. Physiol. 14, 1241591 (2023).
Yang, Z. et al. Targeting nuclear receptors for cancer therapy: Premises, promises, and challenges. Trends Cancer 7, 541–556 (2021).
Moutinho, M., Codocedo, J. F., Puntambekar, S. S. & Landreth, G. E. Nuclear receptors as therapeutic targets for neurodegenerative diseases: Lost in translation. Annu. Rev. Pharmacol. Toxicol. 59, 237–261 (2019).
Dubois, V., Lefebvre, P., Staels, B. & Eeckhoute, J. Nuclear receptors: pathophysiological mechanisms and drug targets in liver disease. Gut 73, 1562–1569 (2024).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 38, 1346–1366 (2020).
Xu, G. et al. ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer. Nat. Genet. 52, 198–207 (2020).
González, E. R. Estrogen, progesterone receptors in ovarian cancer. JAMA 245, 1626 (1981).
Williams, A. et al. SOX2 expression in prostate cancer drives resistance to nuclear hormone receptor signaling inhibition through the WEE1/CDK1 signaling axis. Cancer Lett. 565, 216209 (2023).
Zhang, J. et al. Naringenin in Si-Ni-San formula inhibits chronic psychological stress-induced breast cancer growth and metastasis by modulating estrogen metabolism through FXR/EST pathway. J. Adv. Res. 47, 189–207 (2023).
Zhao, L., Zhou, S. & Gustafsson, J. Nuclear receptors: Recent drug discovery for cancer therapies. Endocr. Rev. 40, 1207–1249 (2019).
Dierickx, P. et al. Circadian REV-ERBs repress E4bp4 to activate NAMPT-dependent NAD(+) biosynthesis and sustain cardiac function. Nat. Cardiovasc Res 1, 45–58 (2022).
van Weelden, W. J., Massuger, L., Pijnenborg, J. M. A. & Romano, A. Anti-estrogen treatment in endometrial cancer: A systematic review. Front. Oncol. 9, 359 (2019).
Patel, H. K. & Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 186, 1–24 (2018).
Miki, Y., Suzuki, T. & Sasano, H. Aromatase inhibitor and bone. Biomed. Pharmacother. 61, 540–542 (2007).
Tian, Y. et al. MIR497HG-Derived miR-195 and miR-497 mediate tamoxifen resistance via PI3K/AKT signaling in breast cancer. Adv. Sci. (Weinh.) 10, e2204819 (2023).
Gao, Y. et al. CD63(+) cancer-associated fibroblasts confer tamoxifen resistance to breast cancer cells through exosomal miR-22. Adv. Sci. (Weinh.) 7, 2002518 (2020).
Woolas, J., Davis, M. & Rahimi, S. Recurrence of endometrial cancer in a hysterectomised patient treated with tamoxifen for breast cancer: A case report. J. R. Soc. Med. 113, 454–456 (2020).
Trédan, O. et al. Regorafenib or Tamoxifen for platinum-sensitive recurrent ovarian cancer with rising CA125 and no evidence of clinical or RECIST progression: A GINECO randomized phase II trial (REGOVAR). Gynecol. Oncol. 164, 18–26 (2022).
Bonkhoff, H. Estrogen receptor signaling in prostate cancer: Implications for carcinogenesis and tumor progression. Prostate 78, 2–10 (2018).
Qu, L. G. et al. Effects of estrogen receptor signaling on prostate cancer carcinogenesis. Transl. Res. 222, 56–66 (2020).
Yu, E. Y. et al. Selective estrogen receptor alpha agonist GTx-758 decreases testosterone with reduced side effects of androgen deprivation therapy in men with advanced prostate cancer. Eur. Urol. 67, 334–341 (2015).
Huang, J., Lin, B. & Li, B. Anti-androgen receptor therapies in prostate cancer: A brief update and perspective. Front. Oncol. 12, 865350 (2022).
Waltering, K. K. et al. Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res. 69, 8141–8149 (2009).
Fizazi, K. et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 399, 1695–1707 (2022).
Hong, F. et al. PPARs as nuclear receptors for nutrient and energy metabolism. Molecules. 24, (2019).
Hu, P. et al. Nuclear receptor PPARα as a therapeutic target in diseases associated with lipid metabolism disorders. Nutrients 15, 4772 (2023).
Colucci, M. et al. Retinoic acid receptor activation reprograms senescence response and enhances anti-tumor activity of natural killer cells. Cancer Cell 42, 646–661.e649 (2024).
Sulli, G. et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018).
Lonardo, A., Leoni, S., Alswat, K. A. & Fouad, Y. History of nonalcoholic fatty liver disease. Int. J. Mol. Sci. 21, (2020).
Schulman, I. G. Nuclear receptors as drug targets for metabolic disease. Adv. Drug Deliv. Rev. 62, 1307–1315 (2010).
Mitrovic, B. et al. Non-alcoholic fatty liver disease, metabolic syndrome, and type 2 diabetes mellitus: where do we stand today?. Arch. Med. Sci. 19, 884–894 (2023).
Genua, I. & Cusi, K. Pharmacological approaches to nonalcoholic fatty liver disease: Current and future therapies. Diab. Spectr. 37, 48–58 (2024).
Ali, A. H. et al. Lipid-lowering therapies for atherosclerosis: Statins, fibrates, ezetimibe and PCSK9 monoclonal antibodies. Curr. Med. Chem. 28, 7427–7445 (2021).
Yamashita, S., Masuda, D. & Matsuzawa, Y. Pemafibrate, a new selective PPARα modulator: Drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr. Atheroscler. Rep. 22, 5 (2020).
Yamashita, S. et al. Effects of pemafibrate (K-877) on cholesterol efflux capacity and postprandial hyperlipidemia in patients with atherogenic dyslipidemia. J. Clin. Lipidol. 12, 1267–1279.e1264 (2018).
Araki, E. et al. Effects of pemafibrate, a novel selective PPARα modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: A randomized, double-blind, placebo-controlled, phase 3 trial. Diab. Care 41, 538–546 (2018).
Shannon, C. E. et al. Insulin resistance is mechanistically linked to hepatic mitochondrial remodeling in non-alcoholic fatty liver disease. Mol. Metab. 45, 101154 (2021).
Della-Morte, D. et al. Pharmacogenomics and pharmacogenetics of thiazolidinediones: role in diabetes and cardiovascular risk factors. Pharmacogenomics 15, 2063–2082 (2014).
Adorini, L. & Trauner, M. FXR agonists in NASH treatment. J. Hepatol. 79, 1317–1331 (2023).
Stofan, M. & Guo, G. L. Bile acids and FXR: Novel targets for liver diseases. Front. Med. 7, (2020).
Yang, S. et al. Ginsenoside Rh4 improves hepatic lipid metabolism and inflammation in a model of NAFLD by targeting the gut liver axis and modulating the FXR signaling pathway. Foods 12, 2492 (2023).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
Ratziu, V. et al. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J. Hepatol. 76, 506–517 (2022).
El Kasmi, K. C. et al. Pharmacologic activation of hepatic farnesoid X receptor prevents parenteral nutrition-associated cholestasis in mice. Hepatology 75, 252–265 (2022).
Hernández-Hernández, I. et al. Endogenous LXR signaling controls pulmonary surfactant homeostasis and prevents lung inflammation. Cell. Mol. Life Sci. 81, 287 (2024).
Zhao, Y. et al. Disruption of circadian rhythms by shift work exacerbates reperfusion injury in myocardial infarction. J. Am. Coll. Cardiol. 79, 2097–2115 (2022).
Pourcet, B. & Duez, H. Nuclear receptors and clock components in cardiovascular diseases. Int. J. Mol. Sci. 22, (2021).
Griffett, K. et al. Antihyperlipidemic activity of gut-restricted LXR inverse agonists. ACS Chem. Biol. 17, 1143–1154 (2022).
Kim, E. J. et al. RORα suppresses proliferation of vascular smooth muscle cells through activation of AMP-activated protein kinase. Int. J. Cardiol. 175, 515–521 (2014).
Ling, S. et al. Natural compound bavachalcone promotes the differentiation of endothelial progenitor cells and neovascularization through the RORα-erythropoietin-AMPK axis. Oncotarget 8, 86188–86205 (2017).
Huang, H. et al. Melatonin prevents endothelial dysfunction in SLE by activating the nuclear receptor retinoic acid-related orphan receptor-α. Int. Immunopharmacol. 83, 106365 (2020).
Ding, S. et al. Melatonin stabilizes rupture-prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor RORα-dependent manner. J. Pineal Res. 67, e12581 (2019).
Peliciari-Garcia, R. A. et al. Expression of circadian clock and melatonin receptors within cultured rat cardiomyocytes. Chronobiol. Int 28, 21–30 (2011).
Chang, H. Y. & Tain, Y. L. Postnatal dexamethasone-induced programmed hypertension is related to the regulation of melatonin and its receptors. Steroids 108, 1–6 (2016).
Hong, H. et al. REV-ERBα agonist SR9009 suppresses IL-1β production in macrophages through BMAL1-dependent inhibition of inflammasome. Biochem Pharm. 192, 114701 (2021).
Grant, D. et al. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbα. ACS Chem. Biol. 5, 925–932 (2010).
Shan, P. et al. RXR agonists inhibit oxidative stress-induced apoptosis in H9c2 rat ventricular cells. Biochem Biophys. Res Commun. 375, 628–633 (2008).
Lehman, A. M. et al. Activation of the retinoid X receptor modulates angiotensin II-induced smooth muscle gene expression and inflammation in vascular smooth muscle cells. Mol. Pharm. 86, 570–579 (2014).
Xu, W. et al. Novel Pan-ERR agonists ameliorate heart failure through enhancing cardiac fatty acid metabolism and mitochondrial function. Circulation 149, 227–250 (2024).
Nakajima, Y. Retinoic acid signaling in heart development. Genesis 57, e23300 (2019).
Wang, S. et al. Retinoic acid signaling promotes the cytoskeletal rearrangement of embryonic epicardial cells. FASEB J. 32, 3765–3781 (2018).
Jamwal, S., Blackburn, J. K. & Elsworth, J. D. PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther. 219, 107705 (2021).
Tufano, M. & Pinna, G. Is there a future for PPARs in the treatment of neuropsychiatric disorders? Molecules. 25, (2020).
Ghosh, C. et al. Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood-brain barrier model. Epilepsia 59, 2049–2060 (2018).
Ghosh, C. et al. Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells. Epilepsia 58, 576–585 (2017).
Tertil, M. et al. Glucocorticoid receptor signaling in astrocytes is required for aversive memory formation. Transl. Psychiatry 8, 255 (2018).
Hartmann, J. et al. Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol. Psychiatry 22, 466–475 (2017).
McKlveen, J. M. et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol. Psychiatry 74, 672–679 (2013).
Baes, C., Martins, C. M., Tofoli, S. M. & Juruena, M. F. Early life stress in depressive patients: HPA axis response to GR and MR agonist. Front Psychiatry 5, 2 (2014).
Hue, C. D. et al. Dexamethasone potentiates in vitro blood-brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J. Cereb. Blood Flow. Metab. 35, 1191–1198 (2015).
Tsai, H. C. et al. Dexamethasone inhibits brain apoptosis in mice with eosinophilic meningitis caused by Angiostrongylus cantonensis infection. Parasit. Vectors 8, 200 (2015).
de Vargas, L. D. S. et al. Methylprednisolone as a memory enhancer in rats: Effects on aversive memory, long-term potentiation and calcium influx. Brain Res. 1670, 44–51 (2017).
Dos Santos, N. et al. High dose of dexamethasone protects against EAE-induced motor deficits but impairs learning/memory in C57BL/6 mice. Sci. Rep. 9, 6673 (2019).
Baglietto-Vargas, D. et al. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol. Psychiatry 74, 357–366 (2013).
Nguyen, E. T. et al. A mixed glucocorticoid/mineralocorticoid receptor modulator dampens endocrine and hippocampal stress responsivity in male rats. Physiol. Behav. 178, 82–92 (2017).
He, Y. et al. Protective effect of Nr4a2 (Nurr1) against LPS-induced depressive-like behaviors via regulating activity of microglia and CamkII neurons in anterior cingulate cortex. Pharmacol. Res. 191, 106717 (2023).
Zhang, L. M. et al. Dopamine agonists Exert Nurr1-inducing effect in peripheral blood mononuclear cells of patients with Parkinson’s disease. Chin. Med. J. (Engl.). 128, 1755–1760 (2015).
Wu, Y. et al. Activation of the bile acid receptors TGR5 and FXR in the spinal dorsal horn alleviates neuropathic pain. CNS Neurosci. Ther. 29, 1981–1998 (2023).
Mohammadzadeh Honarvar, N. et al. The effect of vitamin A supplementation on retinoic acid-related orphan receptor γt (RORγt) and interleukin-17 (IL-17) gene expression in Avonex-treated multiple sclerotic patients. J. Mol. Neurosci. 51, 749–753 (2013).
Leopold Wager, C. M., Arnett, E. & Schlesinger, L. S. Macrophage nuclear receptors: Emerging key players in infectious diseases. PLoS Pathog. 15, e1007585 (2019).
Levy, G. et al. Nuclear receptors control pro-viral and antiviral metabolic responses to hepatitis C virus infection. Nat. Chem. Biol. 12, 1037–1045 (2016).
Liu, H. M., Lee, T. Y. & Liao, J. F. GW4064 attenuates lipopolysaccharide‑induced hepatic inflammation and apoptosis through inhibition of the Toll‑like receptor 4‑mediated p38 mitogen‑activated protein kinase signaling pathway in mice. Int. J. Mol. Med. 41, 1455–1462 (2018).
Hu, Y. B., Liu, X. Y. & Zhan, W. Farnesoid X receptor agonist INT-767 attenuates liver steatosis and inflammation in rat model of nonalcoholic steatohepatitis. Drug Des. Devel. Ther. 12, 2213–2221 (2018).
Wu, Z. Y. et al. Farnesoid X receptor agonist GW4064 indirectly inhibits HCV entry into cells via down-regulating scavenger receptor class B type I. Eur. J. Pharm. 853, 111–120 (2019).
Thurlow, L. R., Joshi, G. S. & Richardson, A. R. Peroxisome proliferator-activated receptor γ is essential for the resolution of staphylococcus aureus skin infections. Cell Host Microbe 24, 261–270.e264 (2018).
Fernandez-Boyanapalli, R. F. et al. Pioglitazone restores phagocyte mitochondrial oxidants and bactericidal capacity in chronic granulomatous disease. J. Allergy Clin. Immunol. 135, 517–527.e512 (2015).
Han, S. et al. Liver X receptor agonist therapy prevents diffuse alveolar hemorrhage in murine lupus by repolarizing macrophages. Front. Immunol. 9, 135 (2018).
Matalonga, J. et al. The nuclear receptor LXR limits bacterial infection of host macrophages through a mechanism that impacts cellular NAD metabolism. Cell Rep. 18, 1241–1255 (2017).
Sierra, B. et al. OSBPL10, RXRA and lipid metabolism confer African-ancestry protection against dengue haemorrhagic fever in admixed Cubans. PLoS Pathog. 13, e1006220 (2017).
Lith, S. C. et al. Nuclear receptor Nur77 regulates immunomechanics of macrophages. Eur. J. Cell Biol. 103, 151419 (2024).
Saini, A. et al. An accord of nuclear receptor expression in M. tuberculosis infected macrophages and dendritic cells. Sci. Rep. 8, 2296 (2018).
Bhagyaraj, E. et al. A human xenobiotic nuclear receptor contributes to nonresponsiveness of Mycobacterium tuberculosis to the antituberculosis drug rifampicin. J. Biol. Chem. 293, 3747–3757 (2018).
Wang, D. et al. Activation of PPARγ inhibits pro-inflammatory cytokines production by upregulation of miR-124 in vitro and in vivo. Biochem Biophys. Res Commun. 486, 726–731 (2017).
Ferreira, A. E. et al. PPAR-γ/IL-10 axis inhibits MyD88 expression and ameliorates murine polymicrobial sepsis. J. Immunol. 192, 2357–2365 (2014).
Wang, C., Zhou, G. & Zeng, Z. Effects of peroxisome proliferator-activated receptor-β/δ on sepsis induced acute lung injury. Chin. Med. J. (Engl.). 127, 2129–2137 (2014).
Kim, I. S., Silwal, P. & Jo, E. K. Peroxisome proliferator-activated receptor-targeted therapies: Challenges upon infectious diseases. Cells. 12, (2023).
Leon Rodriguez, D. A., Carmona, F. D., González, C. I. & Martin, J. Evaluation of VDR gene polymorphisms in Trypanosoma cruzi infection and chronic Chagasic cardiomyopathy. Sci. Rep. 6, 31263 (2016).
Mubaraki, M. A. et al. Vitamin D receptor regulates intestinal inflammatory response in mice infected with blood stage malaria. Microb. Pathog. 117, 299–303 (2018).
Wang, Z., Schaffer, N. E., Kliewer, S. A. & Mangelsdorf, D. J. Nuclear receptors: emerging drug targets for parasitic diseases. J. Clin. Invest. 127, 1165–1171 (2017).
Wang, Z. et al. The nuclear receptor DAF-12 regulates nutrient metabolism and reproductive growth in nematodes. PLoS Genet 11, e1005027 (2015).
Albarqi, M. M. et al. Regulation of life cycle checkpoints and developmental activation of infective larvae in strongyloides stercoralis by dafachronic acid. PLoS Pathog. 12, e1005358 (2016).
Wang, Z. et al. Characterization of the endogenous DAF-12 ligand and its use as an anthelmintic agent in Strongyloides stercoralis. Elife. 10, (2021).
Cheong, M. C. et al. Identification of a nuclear receptor/coactivator developmental signaling pathway in the nematode parasite Strongyloides stercoralis. Proc Natl Acad Sci USA. 118, (2021).
Joham, A. E. et al. Polycystic ovary syndrome. Lancet Diab. Endocrinol. 10, 668–680 (2022).
Livadas, S. et al. Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine 47, 631–638 (2014).
Yun, C. et al. The microbial metabolite agmatine acts as an FXR agonist to promote polycystic ovary syndrome in female mice. Nat. Metab. 6, 947–962 (2024).
Przybycień, P., Gąsior-Perczak, D. & Placha, W. Cannabinoids and PPAR ligands: The future in treatment of polycystic ovary syndrome women with obesity and reduced fertility. Cells. 11, (2022).
Lan, H. et al. Sinapic acid modulates oxidative stress and metabolic disturbances to attenuate ovarian fibrosis in letrozole-induced polycystic ovary syndrome SD rats. Food Sci. Nutr. 12, 2917–2931 (2024).
Liu, Y. et al. PPAR-α inhibits DHEA-induced ferroptosis in granulosa cells through upregulation of FADS2. Biochem Biophys. Res Commun. 715, 150005 (2024).
Borahay, M. A. et al. Estrogen receptors and signaling in fibroids: Role in pathobiology and therapeutic implications. Reprod. Sci. 24, 1235–1244 (2017).
Yin, H. et al. Expression profiling of nuclear receptors identifies key roles of NR4A subfamily in uterine fibroids. Mol. Endocrinol. 27, 726–740 (2013).
Chung, K. W. et al. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J. Am. Soc. Nephrol. 29, 1223–1237 (2018).
Jao, T. M. et al. ATF6α downregulation of PPARα promotes lipotoxicity-induced tubulointerstitial fibrosis. Kidney Int 95, 577–589 (2019).
Kirkby, N. S. et al. Cyclooxygenase-2 selectively controls renal blood flow through a novel PPARβ/δ-dependent vasodilator pathway. Hypertension 71, 297–305 (2018).
Romero, M. et al. Activation of peroxisome proliferator activator receptor β/δ improves endothelial dysfunction and protects kidney in murine lupus. Hypertension 69, 641–650 (2017).
Lee, G. et al. PGC-1α, a potential therapeutic target against kidney aging. Aging Cell 18, e12994 (2019).
Zhao, J. et al. Genomic integration of ERRγ-HNF1β regulates renal bioenergetics and prevents chronic kidney disease. Proc. Natl. Acad. Sci. USA 115, E4910–e4919 (2018).
Tsushida, K. et al. Estrogen-related receptor α is essential for maintaining mitochondrial integrity in cisplatin-induced acute kidney injury. Biochem Biophys. Res Commun. 498, 918–924 (2018).
Li, A. et al. LC3 promotes the nuclear translocation of the vitamin D receptor and decreases fibrogenic gene expression in proximal renal tubules. Metabolism 98, 95–103 (2019).
Wang, X. X. et al. A dual agonist of farnesoid X receptor (FXR) and the G protein-coupled receptor TGR5, INT-767, reverses age-related kidney disease in mice. J. Biol. Chem. 292, 12018–12024 (2017).
Doan, T. B., Graham, J. D. & Clarke, C. L. Emerging functional roles of nuclear receptors in breast cancer. J. Mol. Endocrinol. 58, R169–r190 (2017).
Xu, S. et al. Nr4a1 marks a distinctive ILC2 activation subset in the mouse inflammatory lung. BMC Biol. 21, 218 (2023).
Moescheid, M. F. et al. The retinoic acid family-like nuclear receptor SmRAR identified by single-cell transcriptomics of ovarian cells controls oocyte differentiation in Schistosoma mansoni. Nucleic Acids Res. 53, gkae1228 (2024).
Li, Q. S. & Zheng, P. S. ESRRB Inhibits the TGFβ signaling pathway to drive cell proliferation in cervical cancer. Cancer Res 83, 3095–3114 (2023).
Farr, J. N. et al. Osteoprotection through the deletion of the transcription factor Rorβ in mice. J. Bone Miner. Res. 33, 720–731 (2018).
Li, W. et al. Molecular basis of nucleosomal H3K36 methylation by NSD methyltransferases. Nature 590, 498–503 (2021).
Hoelzgen, F. et al. Structural basis for the intracellular regulation of ferritin degradation. Nat. Commun. 15, 3802 (2024).
Moret, M. et al. Beam search for automated design and scoring of novel ROR ligands with machine intelligence*. Angew. Chem. Int. Ed. Engl. 60, 19477–19482 (2021).
Foo, J. et al. Characterization of novel small molecule inhibitors of estrogen receptor-activation function 2 (ER-AF2). Breast Cancer Res. 26, 168 (2024).
van Ertvelde, J. et al. Optimization of an adverse outcome pathway network on chemical-induced cholestasis using an artificial intelligence-assisted data collection and confidence level quantification approach. J. Biomed. Inf. 145, 104465 (2023).
Bhimsaria, D. et al. Hidden modes of DNA binding by human nuclear receptors. Nat. Commun. 14, 4179 (2023).
Ryan, C. et al. Nobiletin stimulates adrenal hormones and modulates the circadian clock in mice. Nutrients. 16, (2024).
Parent, E. E. & Fowler, A. M. Nuclear receptor imaging in vivo-clinical and research advances. J. Endocr. Soc. 7, bvac197 (2023).
Dart, D. A. & Bevan, C. L. In vivo imaging of nuclear receptor transcriptional activity. Methods Mol. Biol. 1443, 203–217 (2016).
Guan, X. J., Zhang, Y. Y., Zheng, X. & Hao, H. P. Drug discovery inspired from nuclear receptor sensing of microbial signals. Trends Mol. Med. 27, 624–626 (2021).
Dvořák, Z. et al. Targeting the pregnane X receptor using microbial metabolite mimicry. EMBO Mol. Med. 12, e11621 (2020).
Dvořák, Z., Sokol, H. & Mani, S. Drug mimicry: Promiscuous receptors PXR and AhR, and microbial metabolite interactions in the intestine. Trends Pharmacol. Sci. 41, 900–908 (2020).
Sun, L. et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 24, 1919–1929 (2018).
Friedman, E. S. et al. FXR-dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid. Gastroenterology 155, 1741–1752.e1745 (2018).
Pathak, P. et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588 (2018).
Pignolo, R. J. et al. Palovarotene for fibrodysplasia ossificans progressiva (FOP): Results of a randomized, placebo-controlled, double-blind phase 2 trial. J. Bone Miner. Res. 37, 1891–1902 (2022).
Shimono, K. et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat. Med. 17, 454–460 (2011).
Kowdley, K. V. et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J. Hepatol. 73, 94–101 (2020).
Camilleri, M. et al. Randomised clinical trial: Significant biochemical and colonic transit effects of the farnesoid X receptor agonist tropifexor in patients with primary bile acid diarrhoea. Aliment. Pharmacol. Ther. 52, 808–820 (2020).
Mudaliar, S. et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582.e571 (2013).
Bullock, T. A. et al. Significant improvement of facial actinic keratoses after blue light photodynamic therapy with oral vitamin D pretreatment: An interventional cohort-controlled trial. J. Am. Acad. Dermatol. 87, 80–86 (2022).
Kumar, S. et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 119, 4375–4382 (2012).
Schroten, N. F. et al. Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: an open-label, blinded end point, randomized prospective trial (VitD-CHF trial). Am. Heart J. 166, 357–364.e352 (2013).
Alshahawey, M. et al. The impact of cholecalciferol on markers of vascular calcification in hemodialysis patients: A randomized placebo controlled study. Nutr. Metab. Cardiovasc. Dis. 31, 626–633 (2021).
Mily, A. et al. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: A randomized controlled trial. PLoS One 10, e0138340 (2015).
Ceglia, L. et al. Effect of vitamin D(3) vs. calcifediol on VDR concentration and fiber size in skeletal muscle. J. Bone Miner. Metab. 41, 41–51 (2023).
Borzutzky, A. et al. Effect of weekly vitamin D supplementation on the severity of atopic dermatitis and type 2 immunity biomarkers in children: A randomized controlled trial. J. Eur. Acad. Dermatol. Venereol. 38, 1760–1768 (2024).
Holt, R. et al. Low serum anti-Müllerian hormone is associated with semen quality in infertile men and not influenced by vitamin D supplementation. BMC Med 21, 79 (2023).
Lundwall, K., Jörneskog, G., Jacobson, S. H. & Spaak, J. Paricalcitol, microvascular and endothelial function in non-diabetic chronic kidney disease: A randomized trial. Am. J. Nephrol. 42, 265–273 (2015).
Ganmaa, D. et al. Vitamin D supplements for prevention of tuberculosis infection and disease. N. Engl. J. Med. 383, 359–368 (2020).
Oblak, M. et al. Paricalcitol versus placebo for reduction of proteinuria in kidney transplant recipients: a double-blind, randomized controlled trial. Transpl. Int. 31, 1391–1404 (2018).
Wang, A. Y. et al. Effect of paricalcitol on left ventricular mass and function in CKD-the OPERA trial. J. Am. Soc. Nephrol. 25, 175–186 (2014).
Parvanova, A. et al. Moderate salt restriction with or without paricalcitol in type 2 diabetes and losartan-resistant macroalbuminuria (PROCEED): a randomised, double-blind, placebo-controlled, crossover trial. Lancet Diab. Endocrinol. 6, 27–40 (2018).
de Zeeuw, D. et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376, 1543–1551 (2010).
Ussif, A. et al. Paricalcitol supplementation during the first year after kidney transplantation does not affect calcification propensity score. BMC Nephrol. 19, 212 (2018).
Tu, H. et al. Effects of calcium and vitamin D3 on transforming growth factors in rectal mucosa of sporadic colorectal adenoma patients: A randomized controlled trial. Mol. Carcinog. 54, 270–280 (2015).
Rorie, A., Goldner, W. S., Lyden, E. & Poole, J. A. Beneficial role for supplemental vitamin D3 treatment in chronic urticaria: a randomized study. Ann. Allergy Asthma Immunol. 112, 376–382 (2014).
Meireles, M. S. et al. Effect of cholecalciferol on vitamin D-regulatory proteins in monocytes and on inflammatory markers in dialysis patients: A randomized controlled trial. Clin. Nutr. 35, 1251–1258 (2016).
Mendes, M. M. et al. Vitamin D supplementation and sunlight exposure on serum vitamin D concentrations in 2 parallel, double-blind, randomized, placebo-controlled trials. J. Nutr. 151, 3137–3150 (2021).
Noskova, P. et al. Neonatal effect of remifentanil in general anaesthesia for caesarean section: a randomized trial. BMC Anesthesiol. 15, 38 (2015).
Mueller, M. et al. Ursodeoxycholic acid: Effects on hepatic unfolded protein response, apoptosis and oxidative stress in morbidly obese patients. Liver Int 38, 523–531 (2018).
Debono, M. et al. Mifepristone reduces insulin resistance in patient volunteers with adrenal incidentalomas that secrete low levels of cortisol: a pilot study. PLoS One 8, e60984 (2013).
Pivonello, R. et al. Glucocorticoid receptor antagonism upregulates somatostatin receptor subtype 2 expression in ACTH-producing neuroendocrine tumors: New insight based on the selective glucocorticoid receptor modulator relacorilant. Front. Endocrinol. (Lausanne) 12, 793262 (2021).
Golier, J. A. et al. A randomized, double-blind, placebo-controlled, crossover trial of mifepristone in Gulf War veterans with chronic multisymptom illness. Psychoneuroendocrinology 64, 22–30 (2016).
Serritella, A. V. et al. Phase I/II trial of enzalutamide and mifepristone, a glucocorticoid receptor antagonist, for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 28, 1549–1559 (2022).
Acknowledgements
This work was supported by the following research grants: the Department of Science and Technology of Sichuan Province (2023YFS0037), the National Natural Science Foundation of China (32170759, 82303506, 82273126), the Sichuan Administration of Traditional Chinese Medicine (2023MS267), Sichuan Science and Technology Department (2025ZNSFSC1893) the Technological Innovation Research and Development Project from the Chengdu Science and Technology Bureau (2022-YF05-01950-SN) and the Basic Research Program of Yunnan Province (202401CF070184). Figures were created by BioRender (BioRender. com). Data of clinical trials presented in Tables 1, 2 were obtained from https://clinicaltrials.gov/.
Author information
Authors and Affiliations
Contributions
P.J., X.D. and Z.H wrote the manuscript and made the figures, J.Z and H.T revised the Figure, Y.D. and H.G. organized the tables, C.Z. and K.X. conceived, designed, and revised the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, P., Duan, X., Huang, Z. et al. Nuclear receptors in health and disease: signaling pathways, biological functions and pharmaceutical interventions. Sig Transduct Target Ther 10, 228 (2025). https://doi.org/10.1038/s41392-025-02270-3
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41392-025-02270-3
This article is cited by
-
The assumption of a threshold is false in risk assessments for endocrine disrupting chemicals (EDCs) when endogenous hormones being disrupted are already above any possible threshold: a policy failure by the US FDA
Environmental Health (2026)
-
Exploring the metaphysical foundations for the ontological unity of biochemical complexes
Foundations of Chemistry (2026)