Abstract
Current clinical strategies for the delivery of pulmonary therapeutics to the lung are primarily targeted to the upper portions of the airways, such as treatment with nebulized instillation and inhalation. However, targeted delivery to the lower regions of the lung is necessary for the treatment of parenchymal lung injury and disease. Here, we show the development of an mRNA therapeutic for the lower lung in mice using one-component Ionizable Amphiphilic Janus Dendrimers as a delivery vehicle. We deliver an anti-inflammatory cytokine mRNA, transforming growth factor-beta, to produce transient protein expression in the lower regions of the lung. This study highlights a method for precise, effective, and safe delivery of TGF-β mRNA to the lung in mice. This delivery system offers a promising approach for targeting therapeutics to the specific tissues, a strategy necessary to fill the current clinical gap in treating parenchymal lung injury and disease.
Similar content being viewed by others
Introduction
Acute lung injury (ALI) is a prevalent condition in the United States, with 200,000 new diagnoses each year, resulting in high morbidity and mortality1,2,3,4. Injury progression is a result of epithelial barrier dysfunction, alveolar damage, pulmonary edema, and surfactant dysfunction brought on by changes in innate immune, epithelial, and endothelial cell dysfunction1,5,6,7,8. There are 23 generations of division in the lung, where Generations 0–16 are the conductive airways and 16–23 are the respiratory airways. Within the pulmonary circuit, blood flow is matched to gas exchange in the respiratory portion of the lung9. Many of the effects of ALI, a result of the liquid leak, are observed in the lung parenchyma beyond the 16th generation. However, drug delivery to this area is challenging often leading to poor specificity and an uneven distribution10,11,12,13,14. Therefore, there is a therapeutic gap in the treatment of ALI requiring the development of methods to deliver pharmacological agents to the lower lung. This is a particular challenge when administering a complex biologic, such as anti-inflammatory cytokine mRNA, to the site of injury.
Since the beginning of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV2) pandemic in 2019, there have been a series of major breakthroughs in the development of nucleoside-modified mRNA vaccines by both major research institutions and the pharmaceutical industry15,16,17,18. Currently, the leading delivery system for mRNA vaccines are four-component lipid nanoparticle (LNP) synthetic delivery systems, which consist of ionizable lipids, phospholipids, cholesterol, and polyethylene glycol (PEG)-conjugated lipids, which can be assembled by microfluidic or T-tube technology19,20. Despite their wide adoption of COVID-19 vaccinations, the targeted delivery of mRNA to the lung with four-component LNPs has not proven successful21,22,23,24,25,26,27.
Recently, we reported a novel self-assembling one-component multifunctional sequence-defined ionizable amphiphilic Janus dendrimer (IAJD) synthetic delivery system for mRNA, consisting of functional hydrophilic dendrons conjugated to hydrophobic dendrons28,29. IAJDs and mRNA can be co-assembled into unilamellar and multilamellar onion-like dendrimersome nanoparticles (DNPs) by simple mixing in acetate buffer rather than microfluidic and T-tube devices. Previously we have shown that certain of these IAJDs display organ specificity29. In addition to eliminating structural tissues that can arise from mixtures of four-component LNPs, this technology may allow for programmed delivery to specific organs, such as the lung.
In this study, we explore the potential of one-component IAJDs to deliver therapeutic mRNA encoding the anti-inflammatory cytokine, transforming growth factor-beta (TGF-β) to the lung parenchyma. TGF-β is essential for the resolution of inflammatory events through the regulation of immune cell migration, division, and pro-resolution signal transduction30,31,32,33. If introduced early in the inflammatory response, TGF-β has the capacity to accelerate resolution, ultimately reducing the incidence of complex lung injury and disease. In addition, we test the nontoxic and transient nature of its delivery, as persistent TGF-β delivery may result in excessive pro-resolution signaling, leading to the development of lung fibrosis over time32,34,35,36. Here, we confirm that IAJDs can be utilized for the successful and targeted delivery of cytokine mRNA diffusely throughout the lung and that the effects of this delivery are transient, reducing the risk of fibrotic development. Furthermore, we evaluate the potential for IAJD targeted delivery of TGF-β mRNA to limit ALI using an intratracheal bleomycin (ITB) model3,36,37. These studies contribute to the progress of genetic nanomedicine and raise the possibility of lung-based nanotherapeutics.
Results and discussion
Characterization of lung-specific IAJD34 for targeted mRNA delivery to the lung
Many IAJDs identified are capable of targeting the lung38. With a relatively large chemical structure and a pKa of 6.74 lending to a strongly lung-specific targeting, IAJD34 was selected for further characterization (Fig. 1A). To evaluate lung-specific delivery, the Luc mRNA-IAJD34 formulation was injected into mice at an initial dose of 10 µg per mouse. Four hours post-injection, whole-body, and organ luminescence were quantified via IVIS. Luciferin intensity in the lungs was strong and several magnitudes higher compared to other organs (Fig. 1B). To evaluate the kinetics of luciferin protein production over time, mice were injected with the Luc mRNA-IAJD34 formulation at a dose of 10 µg per mouse. Live mice were imaged at various time points (4, 24, 48, and 72 h) post-injection, and the luciferase intensity was quantified as a whole-body flux (p/s) (Fig. 1C). Luciferin intensity peaked at 4 h post-injection (3.12 × 107 ± 9.15 × 106), by 24 h, the flux intensity decreased but remained elevated by 37%. At 48 and 72-h post-injection, there was a more significant drop to 3% and 1.9%, respectively (Fig. 1D and Supplementary Table 2).
IAJD34 formulated with luciferase mRNA (Luc mRNA-IAJD34) was i.v. injected into BALB/c mice and analyzed for efficient pulmonary mRNA using IVIS. A The chemical structure of one-component IAJD3440. B Specificity of Luc mRNA-IAJD34 delivery to the lung. Representative IVIS images of whole-body and organs of mice at 4 h post-injection of 10 μg Luc mRNA-IAJD34 show luminescent biodistribution of luciferase expression predominantly in the lungs. IVIS images were analyzed, and the photon radiance (s−1sr−1m−2) of each image was quantified. The quantitative bioluminescence imaging (BLI) data are shown as total flux (p/s). C Time course luciferase expression in vivo. Representative IVIS images of whole-body mice following 10 μg Luc mRNA-IAJD34, imaged at 4, 24, 48, or 72 h post-injection. D Whole-body radiant flux (p/s) at various time points following the injection of 10 μg Luc mRNA-IAJD34. Data presented as mean ± SE, with n = 12 for 4 h, n = 15 for 24 h, n = 18 for 48 h, and n = 6 for 72 h. E Dose-dependent pulmonary delivery of Luc mRNA-IAJD34. Representative IVIS images of whole-body and organs of mice injected with Luc mRNA-IAJD34 at doses of 10, 20, or 30 μg, imaged at 24 h post-injection. Particle size (nm) and polydispersity index (PDI) for Luc mRNA-IAJD34 at each dose were measured prior to injection and are shown above the corresponding mouse IVIS image. F Delivery of Luc mRNA-IAJD34 to the lung parenchyma. At 24 h post-injection with 30 μg Luc mRNA-IAJD34, lung tissue was harvested and immunohistochemistry (IHC) for luciferase or IgG was performed and compared to naïve animals. Experiment was repeated 2 independent times. Scale bar = 100 µm. Black = Naïve, Red = 10 μg, Blue = 20 μg, Green = 30 μg. Source data are provided as a Source Data file.
The stability of the Luc mRNA-IAJD34 formulation was studied over time at +4 °C. The formulation was stored at +4 °C for 5 days and then injected into mice. Whole-body luciferase intensity did not change significantly compared to the freshly prepared formulation (Supplementary Fig. 2A). Thus, the one-component IAJD34 formulated with Luc mRNA maintains stability and activity at 4 °C and can be stored for at least 5 days prior to use. The stability of particle size and PDI of IAJD34 formulated with TGF-β mRNA was also evaluated and was found to be stable for at least 5 weeks at +4 °C (Supplementary Fig. 2B).
To evaluate the impact of dose on luciferase expression, Luc mRNA-IAJD34 was injected at 10, 20, and 30 µg doses per mouse and imaged at 24 h post-injection. There was a dose-dependent increase in lung-specific luciferase expression, reaching 9.3 × 108 at 30 µg per mouse (Fig. 1E, Supplementary Table 3 and Supplementary Fig. 3). Immunohistochemical staining of lung tissue with the 30 µg dose revealed that expression of luciferase protein was well distributed throughout the alveolar epithelium, with minimal staining in the upper airway compared to the control (Fig. 1F). The mRNA delivery to the alveolar level (i.e., below the 16th generation of the airway tree) had previously been unsuccessful, the expression of luciferase in these lower respiratory zones suggests our approach may serve as a promising therapeutic.
Notably, our evaluation of IAJD34 toxicity and efficacy was limited using this formulation because luciferase is encoded by a large mRNA and has no biological function within the cell. Thus, delivery of clinically relevant mRNA was essential to fully characterize the therapeutic nature of IAJD34.
Evaluation the pulmonary delivery of therapeutic mRNA
Following confirmation of IAJD34 lung specificity, we focused on designing a therapeutic mRNA for lung-targeted delivery. TGF-β, a prominent anti-inflammatory cytokine associated with the resolution of ALI, was selected as our mRNA of interest31,32. The production and quality of TGF-β mRNA were validated (Supplementary Fig. 4A). Protein expression of TGF-β was confirmed in Human epithelial kidney (HEK293) cells following transfection with TGF-β mRNA (Supplementary Fig. 4B).
To evaluate inflammatory effects in the lung, IAJD34 formulated with TGF-β mRNA (TGF-β mRNA-IAJD34) was injected at 10, 20, and 30 µg per mouse and bronchoalveolar lavage fluid (BAL) markers were measured 24 h post-injection. Increasing doses (10 µg and 20 µg) had no effect on BAL phospholipids or total cell count in the BAL fluid (Fig. 2A). However, a significant increase in BAL fluid protein and neutrophils was observed at the 30 µg dose, indicating epithelial barrier dysfunction (Fig. 2A, B). Although BAL fluid protein was increased, there was no significant increase in BAL IL-6 levels (Fig. 2D), suggesting this may have been a downstream effect of abundant TGF-β delivery rather than a side effect of IAJD34 formulation.
Various doses of TGFβ mRNA-IAJD34 (10, 20, and 30 μg per mouse) were delivered to BALB/c mice and compared to naïve animals. At 24 h post-injection, BAL fluid and cells, whole lung tissue, and serum were collected. A BAL cells were fixed and stained using a Hema 3 Stat kit. Total BAL protein, differential BAL cell count, and BAL phospholipid levels were quantified as markers of lung injury: MP, Macrophage, NP, neutrophil, LP, Lymphocyte. B Representative cell differentials are shown. C Formaldehyde-fixed lung tissue was processed, stained with H&E, and scored by a board-certified pathologist. Representative H&E images are shown. D IL-6 was measured in BAL fluid via ELISA according to manufacturer’s guidelines (n/group = 10/Naïve, 5/10 μg, 7/20 μg, 3/30ug). E ALT and AST were measured in serum via ELSIA according to the manufacturer’s guidelines (n/group = 5/Naïve, 5/20 μg, 5/30 μg). Data are presented as mean ± SE, n = 3-10/group from 2 independent experiments. For (A, D, and E), samples were compared using the Shapiro-Wilk test for normality followed by an ordinary one-way ANOVA with Tukey’s Multiple comparisons where *indicates a significant difference from the naïve control, p < 0.05. ((A) BAL protein: Naïve vs 30 μg p = 0.0001; NP: Naïve vs 30 μg p = 0.0134; (E) ALT: Naïve vs 30 μg p = 0.0164). Scale bar = 50 µm. Source data are provided as a Source Data file.
Histological sections of organs were stained with H&E and evaluated by a board-certified pathologist. No pathological alterations were observed in the liver, spleen, or kidney at any of the tested doses (Supplementary Fig. 5). In the lung histology, a dose-dependent increase in fibrin deposition and lymphocyte infiltration was observed, as was expected at higher doses of TGF-β (Fig. 2C). Liver function was also evaluated by measuring serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), known markers of liver function39. Increased ALT was observed at the 30 µg dose, with no significant change in AST (Fig. 2E). Thus, systemic pathological and toxicological markers are limited in the 10 µg and 20 µg doses of TGF-β mRNA-IAJD34. Even at the highest dose, 30 µg, increases in systemic toxicity markers were minimal. Established four-component LNPs are typically dosed between 1 and 10 μg for vaccinations for SARS-COV2 in mice, indicating that 10 µg would likely be sufficient40. In addition, most studies using IAJDs report that 10 µg or less of mRNA to be effective for organ-specific detection38.
To determine alveolar macrophage (AM) activation, BAL cells were evaluated by flow cytometry as previously described3. AMs were defined as viable CD45+F4/80+SiglecF+ cells (Supplementary Fig. 6A). Inflammatory activation was characterized by the expression of Ly6c and CD11c. No dose-dependent difference was observed in the AM phenotype, with a majority of cells being resident AMs (Ly6c−/CD11c+) under all conditions. This was consistent with the lack of observed acute inflammatory activation (Fig. 3A). There were also no differences in Ly6C/CD206 AM populations at any dose of TGF-β mRNA-IAJD34 compared to the control (Supplemental Table 4). Interstitial macrophages (IMs) were identified in CD45+ lung digest cells. IMs were defined as viable being CD45+, CD11b+, and SiglecF- cells as previously described (Supplementary Fig. 6B)3. Ly6c+ IMs were increased in all doses of TGF-β mRNA-IAJD34 with no increases in either CD11c+ or CD206+ expression at any dose (Fig. 3B and Supplementary Fig. 7). Ultimately, no acute inflammatory activation was observed in IMs as a result of TGF-β mRNA-IAJD34 treatment when compared to control. It is unclear why treatment with TGF-β mRNA-IAJD34 increased Ly6c in IMs, but a previous study evaluating renal interstitial macrophages in Tgfbr2fl/fl mice showed a decrease in Ly6c+ macrophages indicating that there may be a relationship between Ly6c and TGF-β signaling41. Further analysis will be necessary to fully understand TGF-β mRNA-IAJD34’s effects on Ly6c+ IMs.
Various doses of TGFβ mRNA-IAJD34 (10, 20, and 30 μg per mouse) were delivered to BALB/c mice and compared to naïve animals at 24 h post-injection. A Cells from the BAL were isolated, immunostained, and analyzed by flow cytometry. Cells that were positively stained for both Siglec F and F4/80 were determined to be alveolar macrophages (AMs) and were evaluated for Ly6c and CD11c expression. B Cells from digested lung tissue were immunomagnetically separated based upon CD45 expression. CD45+ cells were isolated, immunostained, and analyzed. Cells that expressed F4/80 and CD11b in the absence of Siglec F were categorized as interstitial macrophages (IMs) and were analyzed for Ly6c and CD206 expression. Data are presented as mean ± SE for n = 4–10 per group. Samples were compared using the Shapiro-Wilk test for normality followed by an ordinary one-way ANOVA with Šídák’s multiple comparisons test where * indicates a significant difference from the naïve control, p < 0.05. B Ly6c-/CD11c- IMs: Naïve vs 10 μg p = 0.0135, Naïve vs 30 μg p = 0.0427; Ly6c + /CD11c- IMs: Naïve vs 20 μg p < 0.0001, Naïve vs 30 μg p = 0.0003; Ly6c-/CD11c+ IMs: Naïve vs 10 μg p = 0.0069, Naïve vs 20 μg p < 0.0001, Naïve vs 30 μg p < 0.0001; Ly6c + /CD11c+ IMs: Naïve vs 10 μg p = 0.0026. Source data are provided as a Source Data file.
TGF-β protein exists in an unprocessed form with a latency-associated peptide that is cleaved to form an active protein, which can then initiate signal transduction42,43. To confirm that TGF-β mRNA-IAJD34 was reaching the lung and being processed, TGF-β protein expression was confirmed in lung tissue digest using western blotting. Cleaved TGF-β was significantly increased at all doses compared to the control, whereas unprocessed and total TGF-β were only significantly increased in the 20 µg and 30 µg groups (Fig. 4).
Various doses of TGF-β mRNA-IAJD34 (10, 20, and 30 μg per mouse) were delivered to BALB/c mice, and lung tissue was collected 24 h post-injection and analyzed for TGF-β protein expression. A Western blots for TGF-β were performed on homogenized whole lung tissue at indicated time points. Representative images are shown. B Western blots results were quantified using densitometry. TGF-β protein expression was normalized to β-actin for each band, and the density was expressed relative to the naïve control. Data are presented as mean ± SE for n = 4–9 per group. Statistical comparisons were made using a two-tailed Mann-Whitney U test where * indicates a significant difference from the control, p < 0.05. ((B) Unprocessed TGF-β: Naïve vs 20 μg p = 0.0012, Naïve vs 30 μg p = 0.0046; Cleaved TGF-β: Naïve vs 10 μg p = 0.0557, Naïve vs 20 μg p = 0.0029, Naïve vs 30 μg p = 0.0026; Total TGF-β: Naïve vs 20 μg p = 0.0003, Naïve vs 30 μg p = 0.0444). Source data are provided as a Source Data file.
We next evaluated the presence of other cytokines and chemokines in the BAL fluid that can be upregulated or downregulated by TGF-β signaling (Supplementary Table 5). Granulocyte-colony stimulating factor (G-CSF), identified as an anti-inflammatory pro-neutrophilic growth factor, was elevated at the 30 µg dose of TGF-β mRNA-IAJD34 compared to the control (Fig. 5A)44. By contrast, pro-inflammatory cytokines interleukin-1 alpha (IL-1a), interleukin-9 (IL-9), and tumor necrosis factor (TNFα) were decreased in a dose-dependent manner (Fig. 5B–D)45,46. Thus, at 24 h post-treatment, TGF-β protein expression alters the downstream signaling of pro- and anti-inflammatory cytokines. These observations are likely directly associated with increased TGF-β signaling and not an off-target effect of IAJD34.
Various doses of TGF-β mRNA-IAJD34 (10, 20, and 30 μg per mouse) were delivered to BALB/c mice, and BAL fluid was collected 24 h post-injection. A–D Cell- free BAL was evaluated for cytokines using a Milliplex Max Mouse Cytokine/Chemokine Magnetic Bead Panel - Premixed 32 Plex - Immunology Multiplex Assay. The concentration of (A) G-SCF, (B) IL-1α, (C) IL-9, and (D) TNFα are presented as pg/mL. E, F Cells from digested lung tissue were immunomagnetically separated based upon CD45 expression. CD45 + cells were isolated and analyzed metabolically using an Agilent Seahorse according to manufacturer’s guidelines; (E) ECAR was measured following injections of glucose, oligomycin (Oligo), and 2-DG, and glycolysis was determined following injection of glucose; (F) OCR was measured with injections of oligo, FCCP, and rotenone/antimycin (R/A) and normalized to non-mitochondrial energy production. Maximal respiration was determined following FCCP injection. Data are shown as mean ± SE, for all experiments n/group = 10/Naïve, 5/10 μg, 8/20 μg, 4/30ug. Data were evaluated for normality using a Shapiro- Wilk’s test and compared using a one-way ANOVA followed by Tukey’s multiple comparisons test. * indicates a significant difference from the control, p < 0.05. ((A): Naïve vs 30 μg p = 0.0034; (B): Naïve vs 20 μg p = 0.0322, Naïve vs 30 μg p = 0.0003; (C): Naïve vs 20 μg p = 0.0129, Naïve vs 30 μg p = 0.0233; (E) Glycolysis: Naïve vs 20 μg p = 0.0001, Naïve vs 30 μg p < 0.0001). Black = Naïve, Red = 10 μg, Blue = 20 μg, Green = 30 μg. Source data are provided as a Source Data file.
Finally, we studied metabolic upregulation, which is predicted to occur with increased protein synthesis and cell signaling stimulated by TGF-β mRNA. CD45+ lung-digest cells were evaluated by employing the glycolysis stress test and the mito stress test. For the glycolysis stress test, the extracellular acidification rate (ECAR) was recorded. Glycolysis was determined by the ECAR measurement prior to glucose injection subtracted from the maximum ECAR before oligomycin injection. Injection with TGF-β mRNA-IAJD34 caused a dose-dependent increase in glycolysis, with a significant increase observed in the 20 µg and 30 µg groups compared to the control (Fig. 5E). This increase was associated with increased cytokine production, likely a direct impact of TGF-β signaling cascades47,48. For the mito stress test, oxygen consumption rate (OCR) was used to determine oxidative phosphorylation. OCR data were first normalized to the post rotenone/antimycin (R/A) injections to control for non-mitochondrial oxygen consumption. Maximal respiration was determined from OCR data as the maximum OCR following the injection of FCCP (Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone), a potent mitochondrial uncoupler. A TGF-β mRNA-IAJD34 induced dose-dependent increase was observed in maximal respiration, though this was not significant (Fig. 5F). Although increased metabolic demand is likely a part of increased translational processes, it has also been hypothesized to be a direct effect of increased TGF-β expression and macrophage activation48. Thus, treatment with TGF-β mRNA-IAJD34 successfully alters macrophage cell phenotype and cytokine expression as expected with the production of TGF-β protein.
Evaluating pulmonary delivery of TGF-β mRNA-IAJD34 over time
Due to the lack of toxicity, limited alterations in cell phenotype, and efficient expression of TGF-β protein and downstream signals, the 10 µg dose of TGF-β mRNA-IAJD34 was selected for further development.
The 10 µg dose of empty-IAJD34 or TGF-β mRNA-IAJD34 was further evaluated at 4, 24, and 48 h post-injection. Treatment with TGF-β mRNA-IAJD34 did not increase BAL protein or phospholipids at any time point (Supplementary Fig. 8).
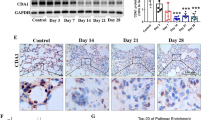
Immunohistochemistry (IHC) staining of lung tissue confirmed that TGF-β protein expression was transiently expressed in lung tissue, peaking at 4 h post-injection, and persisting up to 48 h (Fig. 6). Unprocessed and cleaved TGF-β were also measured in whole lung tissue, showing significant increases in all forms of TGF-β at 4 h post-injection, an increase in cleaved TGF-β at 24 h, and no significant increases in any TGF-β isoforms at 48 h post-injection compared to the control (Fig. 7). Consistent with TGF-β dose-response findings, there were no changes in AM or IM inflammatory activation at any time point compared to control, though slight increases in Ly6c+ IMs were observed (Supplementary Fig. 9 and Supplementary Table 6). Thus, a 10 µg dose of TGF-β mRNA-IAJD34 can deliver robust and transient TGF-β to the lung without significant signs of inflammation or toxicity. Transient expression is therapeutically beneficial as prolonged TGF-β expression can lead to fibrin deposition and fibrosis34. However, in the future, it may be necessary to deliver repeat doses of TGF-β mRNA-IAJD34 until resolution is achieved.
Empty IAJD34 or 10 μg TGF-β mRNA-IAJD34 were delivered to BALB/c mice, and lung tissues were evaluated for TGF-β protein expression at 4, 24, and 48 h post-injection. Lung tissues was stained for TGF-β or IgG control via immunohistochemistry. Representative IHC images are shown. The slides are at 100x magnification, and the scale bars presented 100 µm. Increases in TGF-β protein expression were observed in the TGF-β mRNA-IAJD34 group through immunohistochemical staining from 4 to 48 h. The experiment was repeated 2 independent times. Scale bar = 100 µm.
Empty IAJD34 or 10 ug TGF-β mRNA-IAJD34 were delivered to BALB/c mice, and lung tissue homogenates were evaluated for TGF-β protein expression at 4, 24, and 48 h post-injection. A Western blots for TGF-β were performed on homogenized whole lung tissue. Representative images are shown. B Western blots bands were quantified by densitometry and TGF-β protein expression was normalized to β-actin for each band, and the density was expressed relative to the naïve control. Data are presented as mean ± SE, n = 3–5 per group. Statistical comparisons were made using a two-tailed Mann-Whitney U test, where * indicates a significant difference from the matched empty control, p < 0.05. ((B) Unprocessed TGF-β: Control vs 4 hr p = 0.0357; Cleaved TGF-β: Control vs 4 hr p = 0.0357, Control vs 24 hr p = 0.0159; Total TGF-β: Control vs 4 hr p = 0.0357). Source data are provided as a Source Data file.
TGF-β mRNA-IAJD34 can limit pulmonary injury following exposure to bleomycin
The ultimate goal of lung-targeted delivery of therapeutic mRNA is to generate treatment strategies for pulmonary injury and disease. Intratracheal bleomycin (ITB) is a laboratory model of ALI, characterized by acute pulmonary inflammation occurring over the first 7 days, transitioning to fibrotic development around 14 days, and resolving at 21–28 days post initial exposure3,4,49. It should be noted that not all models of ITB result in the same level of fibrosis50,51,52. To have interventive therapeutic potential, treatment with TGF-β is predicted to be most effective during the development of pro-inflammatory cellular activation and pathology.
For initial studies, animals were treated with a 10 µg dose of TGF-β mRNA-IAJD34 or empty-IAJD34 concurrently to instillation of bleomycin or PBS control. To align with initial pulmonary inflammation development and the expression of TGF-β mRNA-IAJD34, animals were euthanized 3 days post ITB or PBS exposure. ITB causes weight loss in mice over the course of the first 7 days51. Substantial weight loss was observed in groups exposed to ITB, where treatment with TGF-β mRNA-IAJD34 had no impact on ITB-induced decreases in percent body weight (Supplementary Fig. 10).
ITB is also associated with increases in alveolar thickness, immune cell infiltration, and tissue consolidation that variably develop over the first 7 days post-exposure3,4,49,53. An increase in alveolar epithelial thickness was observed in ITB + empty-IAJD34 animals when compared to PBS control, with no change in percent white space and nuclei count (Fig. 8). Treatment with TGF-β mRNA-IAJD34 alone did not significantly increase epithelial thickness compared to PBS control. However, in the presence of TGF-β mRNA-IAJD34, there was no significant increase in epithelial thickness in response to ITB (Fig. 8). The limited ITB-induced histological alterations are likely a consequence of evaluating injury at such an early time point and a limitation of using BALB/c mice as a model. Notably, BALB/c mice are slightly more resistant to ITB exposure as compared to C57BL6/J mice50,51,52,54. BALB/c mice were chosen for this study as IAJD targeting and development has primarily been characterized in this strain of mice29,38,55. The 3-day timepoint was chosen as the peak of inflammatory signaling post ITB injury. Successful delivery of TGF-β is predicted to have its greatest effect on inflammatory cellular phenotype at this time point.
BALB/c mice were exposed to PBS or bleomycin (ITB) and subsequently received either an empty IAJD34 or 10 µg TGF-β mRNA-IAJD34. Lung tissue was collected 3 days post-exposure and injection. Fixed lung tissue was processed and stained with H&E. Representative H&E images are shown. The slides are shown at 400x magnification, with scale bars indicating 50 µm. An inset image shows the data of evaluating alveolar thickness, where 10 fields per slide were randomly selected and analyzed using ImageJ. Data are presented as Median ± SE, n = 6–10 per group. Statistical comparisons were made using a two-tailed Wilcoxon ranked sum test, where * indicates a significant difference from the PBS/empty-IAJD34 group, p < 0.05. (Alveolar Thickness: PBS/Empty-IAJD34 vs ITB/Empty-AJD34 p = 0.0273, PBS/Empty-IAJD34 vs PBS/TGF-β mRNA-IAJD34 p = 0.0625, ITB/Empty-IAJD34 vs ITB/TGF-β mRNA-IAJD34 p = 0.2188). Scale bar = 50 µm. Source data are provided as a Source Data file.
Beyond histopathological alterations, ITB-induced ALI is also associated with increases in pulmonary epithelial injury, marked by increased BAL fluid protein and disrupted epithelial lipid barriers56. Exposure to ITB caused a significant increase in BAL fluid protein content as compared to control. TGF-β mRNA-IAJD34 treatment reduced the protein level within the BAL but not significantly (Fig. 9A). Neither ITB nor TGF-β mRNA-IAJD34 had an effect on BAL fluid phospholipid levels, hypothesized to be a limitation of the 3-day time-point (Fig. 9B). As shown previously, ITB induces a loss of BAL cells, a consequence of early pulmonary inflammation and cell death. However, in the presence of TGF-β mRNA-IAJD34, this ITB-induced loss was not observed (Fig. 9C)3.
BALB/c mice were exposed to PBS or bleomycin (ITB) and subsequently received either an empty IAJD34 or 10 µg TGF-β mRNA-IAJD34. Cell-free BAL fluid, BAL cells, and large aggregate surfactant fractions were collected 3 days post-exposure and injection. A Cell-free BAL fluid was evaluated for total protein content using a BCA assay (n/group = 7/PBS + Empty-IAJD34, 6/PBS + TGF-β mRNA+IAJD34, 10/ITB+Empty-IAJD34, 8/ITB + TGF-β mRNA+IAJD34). Data were evaluated for normality using a Shaprio-Wilks normality test. PBS and ITB were compared using a 2-way ANOVA. B Total phospholipids were determined from the large aggregate surfactant fraction (n/group = 4/PBS + Empty-IAJD34, 3/PBS + TGF-β mRNA + IAJD34, 5/ITB + Empty-IAJD34, 5/ITB + TGF-β mRNA + IAJD34). Data were compared using a 2-way ANOVA. C Total BAL cells were counted using a colter counter (n/group = 8/PBS + Empty-IAJD34, 6/PBS + TGF-β mRNA + IAJD34, 10/ITB + Empty-IAJD34, 10/ITB + TGF-β mRNA+IAJD34). Data were compared using a 2-way ANOVA with Šídák’s multiple comparisons test. D, E BAL cells were immunostained for flow cytometric analysis. Cells that were positively stained for both Siglec F and F4/80 were determined to be alveolar macrophages (AMs). Resident macrophages (CD11c+ /CD11b−), can be differentiated from recruited (CD11c−/CD11b+) or migratory macrophages (CD11c+/CD11b+) (n/group = 7/PBS + Empty-IAJD34, 6/PBS + TGF-β mRNA + IAJD34, 10/ITB+Empty-IAJD34, 8/ITB + TGF-β mRNA+IAJD34). Resident AMs exposed to PBS and ITB were compared using a 2-way ANOVA. Recruited AMs were compared using a two-tailed Wilcoxon Signed Rank test. F Cells from digested lung tissue were immunomagnetically separated based upon CD45 expression. CD45+ cells were isolated, immunostained, and analyzed. Cells that expressed F4/80 and CD11b in the absence of Siglec F were categorized as interstitial macrophages (IMs) and were analyzed for Ly6c expression (n/group = 7/PBS + Empty-IAJD34, 6/PBS + TGF-β mRNA + IAJD34, 10/ITB + Empty-IAJD34, 8/ITB + TGF-β mRNA + IAJD34). Ly6c+ IMs exposed to PBS and ITB were compared using a 2-way ANOVA. All data were evaluated for normality using a Shaprio-Wilks normality test and are presented as Mean ± SE, n = 3–10/group. * indicates a significant difference from the control, p < 0.05. ((A): PBS vs ITB p = 0.0486; (C): PBS/Empty-IAJD34 vs ITB/Empty-IAJD34 p = 0.0087; (D): PBS vs ITB p = 0.0360; (E): PBS/Empty-IAJD34 vs ITB/Empty-IAJD34 p = 0.0156; (F): PBS vs ITB p = 0.0089). Source data are provided as a Source Data file.
To characterize the loss of BAL cells, AMs (viable CD45+Siglec F+F4/80+ BAL cells) were characterized further as being resident (CD11c+CD11b−), recruited (CD11c−CD11b+), or migratory macrophages (CD11c+CD11b+). In line with previous 7-day studies, exposure to bleomycin reduced resident AMs and increased recruited AMs compared to PBS control (Fig. 9D, E)3,4. Although treatment with TGF-β mRNA-IAJD34 did not prevent ITB-induced decreases in resident AMs, treatment did mitigate ITB-induced increases in recruited AMs (Fig. 9D, E). In contrast to TGF-β mRNA-IAJD34 dose-response studies, Ly6c+ IMs (viable CD45+/CD11b+SiglecF− lung-digest cells) were decreased in ITB exposed animals irrespective of TGF-β mRNA-IAJD34 treatment (Fig. 9F). ITB-induced changes in AM and IM populations were variable between animals due to the early inflammatory time point and the less sensitive BALB/c model. Treatment with TGF-β mRNA-IAJD34 helped mitigate some AM alterations but had no effect on IMs at this time point. Innate TGF-β expression is expressed more highly in human AMs when compared to other lung cell types33. There is some evidence that TGF-β plays a less significant role in IM cell maturation and activity, but this remains largely speculative33. Further model development will be necessary to understand the role that TGF-β plays in modulating ITB-induced AM and IM cell characteristics.
We next evaluated the downstream effects of TGF-β mRNA-IAJD34 on cytokine signaling following exposure to ITB beyond pathological and inflammatory indicators (Fig. 10 and Supplementary Table 7). As anticipated, exposure to ITB elevated levels of G-CSF, IL-6, and CXCL-10 compared to control. These neutrophilic or pro-inflammatory cytokines are elevated following ITB exposure (Fig. 10A–C)57,58,59,60. Treatment with TGF-β mRNA-IAJD34 prevented ITB-induced increases in all three of these pro-inflammatory cytokines, consistent with the anti-inflammatory signaling of TGF-β (Fig. 10A–C). Exposure to ITB caused a reduction in IL-1α and IL-2 compared to PBS control, irrespective of treatment with TGF-β mRNA-IAJD34 (Fig. 10D, E). Both IL-1α and IL-2 are also pro-inflammatory cytokines, and it is unclear why ITB exposure decreased these cytokines, though it may be a result of AM resident cell loss. Thus, treatment with TGF-β mRNA-IAJD34 can prevent components of ITB-induced pulmonary inflammatory signaling.
BALB/c mice were exposed to PBS or bleomycin (ITB) and subsequently received either an empty IAJD34 or 10 µg TGF-β mRNA-IAJD34. Cell-free BAL fluid was collected 3 days post-exposure and injection. A–E Cell-free BAL fluid was evaluated for cytokines using a Milliplex Max Mouse Cytokine/Chemokine Magnetic Bead Panel - Premixed 32 Plex - Immunology Multiplex Assay. The concentrations of (A) G-SCF, (B) IL-6, (C) CXCL10, (D) IL-1α, and (E) IL-2 are presented as pg/mL. Date shown a mean ± SE, n/group = 7/PBS + Empty-IAJD34, 6/PBS + TGF-β mRNA + IAJD34, 10/ITB + Empty-IAJD34, 8/ITB + TGF-β mRNA+IAJD34. Data were evaluated for normality using a Shapiro Wilk’s test. If data were normally distributed, they were compared using a 2-way ANOVA followed by Šídák’s multiple comparisons test. Data points that were identified as 0 were set to the limit of detection according to manufacture values for statistical analysis. If data were not normally distributed, they were evaluated using a Kruskal Wallis test with Dunn’s multiple comparison test. * indicates a significant difference, p < 0.05. ((A): PBS/Empty-IAJD34 vs ITB/Empty-IAJD34 p = 0.0231, ITB/Empty-IAJD34 vs ITB/TGF-β mRNA-IAJD34 p = 0.0300; (B): PBS/Empty-IAJD34 vs ITB/Empty-IAJD34 p = 0.0057, ITB/Empty-IAJD34 vs ITB/TGF-β mRNA-IAJD34 p = 0.0285; (C): PBS/Empty-IAJD34 vs ITB/Empty-IAJD34 p = 0.0168; (D): PBS/Empty-IAJD34 vs ITB/Empty-IAJD34 p = 0.0296; (E) PBS vs ITB p = 0.0107). Source data are provided as a Source Data file.
IAJD34 is a single-component lipid particle designed for the targeted delivery of mRNA to the lung38. The current studies have shown that IAJD34 can successfully deliver luciferase mRNA to the lung, specifically targeting lung parenchyma. This is of particular therapeutic advantage as targeting the smaller airways, especially the respiratory regions beyond the 16th generation of the respiratory tree, is necessary to treat most lung injury and consequent disease1,5,7. While necessary, targeting these areas of the lung is challenging and has proven difficult in clinical settings1.
It is not clear why IAJD34 particles localize to the lung. However, we hypothesize that IAJD34 is able to target these areas due to its lipophilic and charged nature. When injected intravenously, we propose that these characteristics lead to pulmonary accumulation as it is the first pass organ for i.v. administration. TGF-β mRNA-IAJD34 was able to deliver dose-dependent levels of TGF-β mRNA to the lung with limited inflammation and toxicity observed. Once delivered, TGF-β protein was produced, processed, and mediated downstream cytokine signaling. Delivery of TGF-β was transiently expressed over the course of 48 h, which is important as long-term delivery of TGF-β can lead to significant fibrosis.
TGF-β mRNA-IAJD34 was used to treat ITB effects on the lung 3 days post-injury showed only mild improvements in lung histology and barrier function. Although the use of the day 3 timepoint in BALB/c mice meant that these factors were only mildly affected by ITB, our intention was to demonstrate that delivery of TGF-β mRNA-IAJD34 to the lung would reduce inflammatory signaling. Indeed, there was a significant TGF-β mRNA-IAJD34 effect that correlated with TGF-β expression. These results indicate that we could successfully deliver TGF-β to the lower lung and observe a significant signaling effect. Furthermore, this effect appears to be transitory in nature as would be required in a therapeutic setting.
To establish TGF-β mRNA-IAJD34 as a therapeutic in this ALI model, we will need to characterize the effect of TGF-β at 7 days or longer post-injury, which may require multiple dosing regimens. This study establishes the potential use of IAJD34 to treat ALI and other pulmonary ailments that currently require targeted clinical interventions and demonstrates the potential of mRNA delivery for therapeutic use in the lung.
Methods
mRNA production and characterization
Codon-optimized sequences of firefly luciferase (Luc-mRNA, GenBank: MK484108.1) or tumor growth factor-β (TGFβ-mRNA, GenBank: NM_011577.2) were synthesized and cloned into an mRNA production plasmid25,61. Briefly, nucleoside-modified mRNAs were transcribed to contain 101 nucleotide-long poly(A) tails. mRNAs were modified with m1Ψ-5′-triphosphate (TriLink, # N-1081) instead of UTP and capped cotranscriptionally using the trinucleotide cap1 analog, CleanCap (TriLink, # N-7413). mRNA was purified by cellulose (Sigma-Aldrich, # 11363-250 G) purification25. All mRNA were analyzed for quality control through agarose gel electrophoresis, dsRNA, endotoxin, and IFN-α assays and in vitro transfection. mRNA was stored at −20 °C until ready for use.
TGF-β mRNA transfection in human embryonic kidney 293 cell line
Human Embryonic Kidney (HEK) 293 cells (ATCC CRL-1573) were seeded into a 24-well cell culture plate at a density of 150,000 per well in Dulbecco’s Modified Enriched Medium (DMEM) containing fetal bovine serum (10%), L-glutamine, and penicillin-streptomycin. Cells were allowed to grow for 24 h, and then TGF-β mRNA (500 n per/well) was transfected into cells in triplicate using lipofectamine MessengerMax (Life Technologies, Carlsbad, CA) and OptiMEM serum-free medium (ThermoFisher Scientific, # 31985-062, Rockford, IL) according to manufacturer guidelines. Cells were allowed to grow for an additional 24 h, and then samples collected in RIPA buffer (Sigma Aldrich, # R0278-500mL) containing cOmpleteMini protease cocktail inhibitors (Roche Diagnostics, # 11836153001). Samples were centrifuged (300 × g, 5 min, 4 °C) to remove cellular debris, and supernatant was then collected. Protein concentrations were determined using a Pierce™ BCA Protein Assay (Peirce, # 23227).
Immunoblots
Whole cell lysates samples (10 µg per well) were analyzed for TGF- β by denaturing SDS-PAGE 4–12% Bis-Tris followed by western blot analysis. Samples were transferred to PVDF membrane using an iBlot 2 dry apparatus (ThermoFisher Scientific, Rockford, IL), The membranes were blocked in non-fat dried milk (10% with 5% TTBS), and TGF- β was detected using primary anti TGF-β antibody (1:5000, Abcam ab215715; Waltham, MA) overnight at 4 °C, followed by a secondary goat anti-rabbit IgG HRP at 1:10,000 for 1 h at room temperature. Membranes were stripped and re-probed with anti-GAPDH (Cell Signaling Technology, # 2118S, Danvers, MA) for 1 h at room temperature at a 1:1000 dilution, and a secondary goat anti-rabbit IgG HRP (Bio-Rad, # 170-6515 Bio-Rad, Hercules, CA) at a 1:10,000 for 1 h at room temperature. Membranes were washed in ECL Prime Western Blotting Detection Reagent (Amersham Biosciences, Amersham, UK, # RPN2232) prior to visualization using an Amersham Cytiva imager (Amersham Biosciences, Amersham, UK).
To analyze TGF-β protein expression in lung tissue, immunoblots were performed on collected lung tissue. Tissue from the accessory lobe was mechanically homogenized on dry ice and digested in lysis buffer with protease inhibitors (100 µL/0.33 g tissue). The tissues were sonicated, centrifuged (2 min, 1000 × g), and supernatants were assessed for protein concentration using a Pierce™ BCA Protein Assay. Equal amounts of protein from lung tissue samples (45 µg per well) were analyzed for TGF-β protein expression as described above. Antibodies for TGF-β were used at 1:5000, and goat anti-rabbit HRP at 1:5000. Membranes were washed in ECL Prime Western Blotting Detection Reagent prior to visualization on x-ray film. Uncropped gel images are available in the Source Data file.
Co-assembly of IAJD34 and mRNA
IAJD34 was synthesized as previously described29, and the purity and structural identity of final products and intermediates were determined using various techniques, including thin-layer chromatography (TLC), high-pressure liquid chromatography (HPLC), 1H and 13C NMR, and Electrospray Ionization Time-of-Flight (ESI-TOF) mass spectrometry29. Co-assembly of IAJD34 and mRNA was performed29,55. Nucleoside-modified mRNA encoding Luc mRNA or TGF-β mRNA was dissolved at a concentration of 4 mg/mL in UltraPure DNase/RNase-free PCR-certified water (Teknova, # W3440). IAJD34 was dissolved in ethanol at a concentration of 80 mg/mL. For the co-assembly of IAJD34 with mRNA, the mRNA was mixed with 10 mM acetate buffer (pH 4.0), and this solution was rapidly mixed with IAJD34 in ethanol at an IAJD34/mRNA weight-to-weight ratio of 40 and vortexed for 5 s. The prepared formulation was analyzed for size (nm), polydispersity index (PDI), and zeta potential using dynamic light scattering (DLS) prior to injection of empty-IAJD34, Luc mRNA-IAJD34, and TGF-β mRNA-IAJD34. Prepared formulations were also evaluated for mRNA encapsulation efficiency, defined as the amount of mRNA encapsulated versus free, was determined using a Quant-iT™ RiboGreen™ RNA Assay Kit (Invitrogen) according to manufacturer instructions. Briefly, to measure non-encapsulated mRNA (free mRNA), mRNA-IAJD formulations were diluted with Tris-EDTA buffer (TE) and treated with the RiboGreen™ reagent. To measure total mRNA, mRNA-IAJD formulations were disrupted by treatment with 2% Triton X-100 in TE buffer and treated with RiboGreen™ reagent. Both conditions were performed in triplicate to ensure proper release of encapsulated mRNA. Fluorescence changes were measured using a Qubit 4 Fluorometer (Invitrogen) at 480 nm excitation and 520 nm emission. mRNA encapsulation efficiency was calculated as: (total mRNA – free mRNA)/total mRNA × 100% (EE, %). (Supplementary Fig. 1A).
Cryogenic Transmission Electron Microscopy (Cryo-TEM) imaging was done to characterize the morphologies of mRNA-IAJD34 nanoparticles (Supplementary Fig. 1B). Samples were prepared by deposition of 5 μL sample solution onto a 300-mesh copper TEM grid, and samples were immediately flash-frozen in liquid ethane using a Vitrobot Mark IV system (Thermo Fisher) and maintained at liquid nitrogen temperature. Images were recorded at − 170 °C with an acceleration voltage of 200 kV using a Talos F200C G2 microscope.
Purity of IAJD34 was also confirmed using carbon 13 nuclear magnetic imaging (13C NMR), hydrogen nuclear magnetic imaging (1H NMR), High-performance liquid chromatography (HPLC), and Electrospray Ionization Time-of-Flight (ESI-TOF) (Supplementary Fig. 1C–F). 1H NMR and 13C NMR spectra were recorded on a Bruker Avance Neo (400 MHz) spectrometer at 298 K. Deuterated chloroform (CDCl3) was used as the solvent. NMR chemical shifts were calculated with the residual protic solvent of CDCl3 (1H, δ 7.26 ppm; 13 C, δ 77.16 ppm) as an internal reference. The purity of the products was determined by HPLC. HPLC was performed using a Thermo Fisher Vanquish Core at 298 K on a Chiralpak AD column. Methanol was used as an elute at a flow rate of 1.0 mL/min; λ = 210 nm; tR = 3.122 min. ESI-TOF mass spectrometry was performed on a Thermo Fisher Scientific ExactiveTM. IAJD 34 was dissolved in dichloromethane (3 mg/mL). The report contains the ionization method (ESI in positive mode), chemical formula, [ion]charge, mass to charge ratio (m/z): calculated value, found value.
Animal studies
Male and female wild-type BALB/c (8–10 weeks) mice obtained from Charles River (Luc mRNA study) or Jackson Laboratories (TGF-β studies) were used for all experiments. Studies used male and/or female mice and are indicated in the source data file. Studies were not powered to compare sex. Mice were housed under standard conditions (12 hr light/12 hr dark, 65–75 °F, 40–60% humidity) with food (Lab diet 5053, Irradiated Rodent Diet) and water provided ad libitum. All experiments were conducted in accordance with the University of Pennsylvania and Rutgers University IACUC-approved protocols adhering to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were housed and cared for in the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facilities. These studies abide by the ARRIVE guidelines. During experimentation, animal welfare was monitored daily, and animals that displayed extensive pain or discomfort beyond the allowance of the study were humanely euthanized via CO2 in accordance with the IACUC protocols.
Bioluminescence characterization for Luc mRNA delivery to mouse lungs
Female or male 6-8-week-old BALB/c mice were administered 10 μg of Luc mRNA-IAJD34 in a100 μL volume via retro-orbital intravenous injection. Four hours post-injection, mice were injected intraperitoneally (i.p.) with D-luciferin (Regis Technologies) at a dose of 150 mg/kg of body weight. The mice were anesthetized in a ventilated anesthesia chamber with 3% isoflurane (Piramal Healthcare Limited) in oxygen and imaged 10 min post D-luciferin injection using an in vivo imaging system (IVIS, PerkinElmer, Waltham, MA). For organs imaging, mice were sacrificed, organs were collected immediately, and bioluminescence imaging was performed. Bioluminescence was quantified as proton flux (photons/seconds) in each region of interest using Living Image software v3.2 (PerkinElmer)55.
TGF-β mRNA-IAJD34 formulation toxicity studies
TGF-β mRNA was co-assembled with IAJD34 as described above. The formulation was then concentrated using a Vivaspin ultrafiltration spin column MWCO 10,000 (Cytiva # 28-9322-47) according to manufacture guidelines. Mice were randomly assigned to control or treated. Mice were anesthetized with isoflurane and received a single retro-orbital intravenous injection of 10 µg, 20 µg, or 30 µg of TGF-β mRNA co-assembled with IAJD34 or empty IAJD34. The following criteria to assess the toxicity of formulated TGF-β mRNA-IAJDs was utilized: mice behavior, serum and BAL fluid cytokine analysis, liver enzyme activities, and main organs’ histological score. Mice were sacrificed 24 h post-injections, and their serum samples and organs were collected. Organs were fixed in 4% paraformaldehyde and further stained with H&E as described below. The liver, spleen, kidney, and lungs were inspected for signs of inflammation and deviations from normal histology.
Enzyme-linked immunosorbent assay (ELISA)
ELISAs for aspartate aminotransferase (AST; Abcam ab263882) and alanine aminotransferase (ALT; Abcam ab282882) were performed in serum in a 1:80 dilution according to manufacture guidelines. Interleukin-6 (IL-6; Abcam ab222503) was used to measure serum and first wash BAL fluid levels in accordance with manufacturer guidelines.
Multiplex cytokine analysis
Cytokine in the first 1 mL of BAL fluid was performed by the University of Pennsylvania Human Immunology Core (RRID: SCR_022380) using a Milliplex Max Mouse Cytokine/Chemokine Magnetic Bead Panel - Premixed 32 Plex - Immunology Multiplex Assay (MCYTMAG-70K-PX32; MilliporeSigma, Burlington, MA). Samples were run in duplicate, and cytokine and chemokine concentrations were determined from a matched standard curve.
Bleomycin mRNA-IAJD34 studies
Mice were randomly assigned to treatment groups. Mice were anesthetized with isoflurane and received a single retro-orbital intravenous injection of 10 µg of TGF-β mRNA formulated with IAJD34 or empty IAJD34. while still under anesthesia, mice immediately received a single intratracheal instillation of either 50 µL PBS or 50 µL of bleomycin (3U/kg of body weight) (Santa Cruz Biotechnology, Inc., Dallas, TX; # sc-200134B) diluted in PBS3. Following injections and treatment, mice were observed to ensure full recovery from anesthesia, and that dose was successfully administered. Animals were weighed daily and provided supportive care when necessary. Mice were sacrificed 3 days post intratracheal administration of bleomycin via a single intraperitoneal injection of ketamine (135 mg/kg of body weight) and xylazine (30 mg/kg of body weight) (Fort Dodge Animal Health, Fort Dodge, IA).
Bronchoalveolar lavage
Lungs were lavaged with 1 mL of ice-cold PBS, followed by five 1 mL washes of ice-cold PBS through a 20-gauge canula inserted into the trachea. Collected BAL fluid was centrifuged at 300 × g for 8 mins. The cell-free supernatant from the first wash was collected for protein and cytokine analysis, and the cell-free supernatant from the five subsequent washes (5 mL) was collected for phospholipid analysis. Cell pellets from both washes were combined and resuspended in 1 mL of staining buffer (5% FBS in PBS, 0.2% sodium azide) and assessed for viability using Trypan Blue Solution (0.4%, ThermoFisher Scientific, Rockford, IL). The total cell count was determined using a Z1 Counter particle counter (Beckman Colter). Approximately 10,000 cells were centrifuged on a Thermo Shandon Cytospin-3 at 750 rpm for 3 min onto a microscope slide, followed by Giemsa staining using a Hema 3™ Stat Pack (Fisherbrand, ThermoFisher Scientific, Rockford, IL). Total and differential cell counts were obtained. Cells were identified as macrophages, eosinophils, neutrophils, and lymphocytes by standard morphology.
Phospholipids
Collected cell-free BAL fluid (5 mL) was centrifuged (20,000 × g, 4 °C, 1 hour) and separated into large and small aggregate surfactant fractions3,62. Phospholipids were extracted from the large aggregate fraction and resuspended in 30 µL 0.9% sodium chloride. Total phospholipids were measured by light absorbance at 830 nm using a standard curve ranging from 0 to 3.1 µg phosphate3,63,64.
Lung tissue digest
Lung tissue from the right lobes was incubated at 37 °C for 30 min with intermittent shaking in 5 mL of collagenase buffer (2 mg/mL collagenase type IV (Sigma Aldrich, St. Louis, MO) in RPMI 1640 (ThermoFisher Scientific, Rockford, IL) with 5% FBS (ThermoFisher Scientific, Rockford, IL)). The digested tissue was filtered through a 70 µm strainer, washed with RPMI with 5% FBS, and centrifuged (6 min, 400 × g). The cell pellet was lysed with Red Blood Cell Lysis Buffer (Sigma Aldrich, St. Louis, MO) for 5 min. The purified cell pellet was resuspended at a concentration of 1 × 108 cells/mL PBS with 2% FBS and 1 mM EDTA. CD45 + leukocytes were isolated using the EasySep™ Mouse CD45 Positive Selection Kit (Stemcell Technologies, Cambridge, MA) and prepared for flow cytometry.
Flow cytometry
Cells from the BAL fluid or lung tissue digest were resuspended in 100 µL of staining buffer (PBS with 5% FBS, with 0.2% w/v sodium azide). Cells were incubated with TruStain FcX anti-mouse CD16/32 (Fc Block, 1:100) for 10 min at 4 °C to prevent non-specific antibody binding. Cells were then incubated with antibodies against CD11b, CD206, CD11c, CD45, F4/80, Ly6c, MHC II, and Siglec F at a 1:100 dilution in staining buffer (Supplementary Table 1). After centrifugation and washing, cells were stained with eFluor 780-conjugated fixable viability dye for 30 min at 4 °C, washed with staining buffer, and fixed in paraformaldehyde (3%). Fluorescence was analyzed using a Gallios 10-color flow cytometer (Beckman Colter, Brea, CA). Cells were analyzed after sorting based upon forward and side scatter, doublet discrimination, and viability using Kaluza software v 2.2.1 (Beckman Colter, Brea, CA). Discrete alveolar and interstitial macrophage phenotypes were determined3.
Seahorse real-time cellular metabolic analysis
Positively selected CD45 lung digest cells were plated at 200,000 cells per well in a poly-D-lysine coated Seahorse XF96 Cell Culture Microplate (Agilent, Santa Clara, CA) and incubated at 37̊ °C, 5% CO2 for 1 h to allow for cell adhesion. The cells were fed with ECAR medium (DMEM media, pH 7.4 (Agilent # 103575-100) with 2 mM L-glutamine) or OCR medium (DMEM media, pH 7.4 with 25 mM glucose, 1 mM pyruvate, and 2 mM L-glutamine). The extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) were measured using a Seahorse XF96 Analyzer (Agilent Technologies, Santa Clara, CA). For ECAR analysis, CD45 + cells were sequentially treated with 25 mM glucose, 4 μM oligomycin, and 50 mM 2-deoxy-d-glucose (2-DG). For OCR measurement, the cells were sequentially treated with 4 μM oligomycin, 1 μM carbonyl cyanide ptrifluoromethoxyphenylhydrazone (FCCP), 0.5 μM rotenone, and 0.5 μM antimycin A. Data were normalized to µg of protein per well and analyzed using Agilent Wave software.
Histology and immunohistochemistry (IHC)
After BAL fluid collection, the left lung lobe was inflation fixed in 3% paraformaldehyde and embedded in paraffin. Liver, spleen, and kidney tissue were also fixed in 3% paraformaldehyde and embedded in paraffin. Four-micrometer sections were cut, slide-mounted, and left unstained for IHC or stained with hematoxylin and eosin (H&E) to observe histological changes. For the TGF-β dose-response toxicity study, tissues were blindly scored by a board-certified pathologist to determine overt toxicological pathology. For bleomycin studies, scans were blindly scored and quantified via ImageJ (NIH). For ImageJ quantification, samples were analyzed as previously described3,53. Briefly, randomly selected histological areas (n = 10, 400X) from each sample were captured and used to determine tissue consolidation (% white space), alveolar wall thickness, and cell infiltration (number of nuclei).
Mounted unstained tissue sections were deparaffinized in xylenes followed by decreasing concentrations of ethanol (100–50%) and water. Antigen retrieval was performed in heated citrate buffer (10 mM sodium citrate, pH 6.0) for 30 min followed by quenching of endogenous peroxidase (3% H2O2 in methanol). Tissue sections were incubated in blocking buffer (10% normal goat serum in PBS) for 1 h at ambient temperature to prevent non-specific binding. Tissues were incubated at 4 °C for 18 h with anti-firefly luciferase (Abcam ab238448; Waltham, MA 1:100) or TGF-β (Abcam ab215715; Waltham, MA 1:100) antibody in a blocking buffer along with IgG controls (Pro-Sci 3703; Fort Collins, CO, matched concentrations). Sections were washed in decreasing concentrations of Tween-PBS (1%-0.5%) and incubated with biotin-conjugated secondary antibody (Vector Laboratories Vectastain Rabbit Kit; Newark, CA) for 1 h at ambient temperature. Antibody binding was visualized beneath a microscope using a DAB Peroxidase Substrate Kit (Vector Laboratories, Newark, CA). Slides were scanned at 40X magnification using a VS120 Virtual Slide Microscope (Olympus, Waltham, MA) and viewed with OlyVIA software (Olympus, Waltham, MA) at 400X magnification.
Statistical analysis
Statistical analyses were completed using GraphPad Prism version 9 or 10. Results are reported as means ± SE unless otherwise indicated. Data were tested for normal distribution using a Shapiro-Wilks test. If normally distributed, statistical significance for multiple group comparisons was determined using a one-way ANOVA with Tukey’s post-hoc test or Šídák’s multiple comparison test. If not normally distributed, statistical significance for multiple comparisons was determined using a Kruskal-Walli’s test with a Dunn’s multiple comparison test if needed. For parametric single comparisons, statistical significance was determined using an unpaired t-test with Welch’s correction compared to control groups as indicated in figure legends. If single comparisons were nonparametric, statistical significance was determined using a Mann-Whitney U test. All P-values < 0.05 were considered statistically significant. For data presented as Median ± SE, statistical comparisons were made using a Wilcoxon ranked sum test. Statistical tests were conducted using a 5% significance level. n = 3–18 animals/group and is further indicated in figure legends. Studies are represented as at least 2 independent studies.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are included in the manuscript. Source data generated in this study are provided in the Supplementary and Source Data files. Additional data is deposited on figshare [https://doi.org/10.6084/m9.figshare.26128828]. Codon-optimized sequences are available under the following ascension codes for firefly luciferase MK484108.1 [https://www.ncbi.nlm.nih.gov/nuccore/MK484108.1] (Luc) or tumor growth factor-β NM_011577.2 [https://www.ncbi.nlm.nih.gov/nuccore/930697458] (TGFβ), All materials used in this manuscript are available from the corresponding author upon reasonable request. Source data are provided in this paper.
References
Johnson, E. R. & Matthay, M. A. Acute lung injury: epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 23, 243–252 (2010).
Dushianthan, A., Grocott, M., Postle, A. & Cusack, R. Acute respiratory distress syndrome and acute lung injury. Postgrad. Med. J. 87, 612–622 (2011).
Stevenson, E. R., Wilkinson, M. L., Abramova, E., Guo, C. & Gow, A. J. Intratracheal administration of Acyl Coenzyme A Acyltransferase-1 inhibitor K-604 reduces pulmonary inflammation following bleomycin-induced lung injury. J. Pharmacol. Exp. Ther. 382, 356–365 (2022).
Wilkinson, M. L. et al. Fatty acid nitroalkenes inhibit the inflammatory response to bleomycin-mediated lung injury. Toxicol. Appl. Pharmacol. 407, 115236 (2020).
Matthay, M. A. & Zimmerman, G. A. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am. J. Respir. Cell Mol. Biol. 33, 319–327 (2005).
Tam, A., Wadsworth, S., Dorscheid, D., Man, S. P. & Sin, D. D. The airway epithelium: more than just a structural barrier. Thera. Adv. Respir. Dis. 5, 255–273 (2011).
Butt, Y., Kurdowska, A. & Allen, T. C. Acute lung injury: a clinical and molecular review. Arch. Pathol. Lab. Med. 140, 345–350 (2016).
Cross, L. M. & Matthay, M. A. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit. Care Clin. 27, 355–377 (2011).
Bates, J. H. Lung Mechanics: An Inverse Modeling Approach. (Cambridge University Press, 2009).
Schwaiblmair, M. et al. Drug induced interstitial lung disease. Open Respir. Med. J. 6, 63 (2012).
He, S. et al. A roadmap to pulmonary delivery strategies for the treatment of infectious lung diseases. J. Nanobiotechnol. 20, 101 (2022).
Labiris, N. R. & Dolovich, M. B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 56, 588–599 (2003).
Newman, S. P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 8, 647–661 (2017).
O’Callaghan, C. Targeting drug delivery to the lungs by inhalation. Mediators of Inflamm. 3, S31–S33 (1994).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Pilkington, E. H. et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 131, 16–40 (2021).
Rele, S. COVID-19 vaccine development during pandemic: gap analysis, opportunities, and impact on future emerging infectious disease development strategies. Hum. Vaccines Immunother. 17, 1122–1127 (2021).
Parhiz, H., Atochina-Vasserman, E. N. & Weissman, D. mRNA-based therapeutics: looking beyond COVID-19 vaccines. Lancet 403, 1192–1204 (2024).
Sabnis, S. et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26, 1509–1519 (2018).
Ansell, S. M. & Du, X. (Google Patents, 2017).
Hibbitts, A. J., Ramsey, J. M., Barlow, J., MacLoughlin, R. & Cryan, S.-A. In vitro and in vivo assessment of PEGylated PEI for anti-IL-8/CxCL-1 siRNA delivery to the lungs. Nanomaterials 10, 1248 (2020).
Kaczmarek, J. C. et al. Optimization of a degradable polymer–lipid nanoparticle for potent systemic delivery of mRNA to the lung endothelium and immune cells. Nano Lett. 18, 6449–6454 (2018).
Kaczmarek, J. C. et al. Polymer–lipid nanoparticles for systemic delivery of mRNA to the lungs. Angew. Chem. 128, 14012–14016 (2016).
Kaczmarek, J. C. et al. Systemic delivery of mRNA and DNA to the lung using polymer-lipid nanoparticles. Biomaterials 275, 120966 (2021).
Pardi, N. et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 217, 345–351 (2015).
Patel, A. K. et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 31, 1805116 (2019).
Sahu, I., Haque, A. A., Weidensee, B., Weinmann, P. & Kormann, M. S. Recent developments in mRNA-based protein supplementation therapy to target lung diseases. Mol. Ther. 27, 803–823 (2019).
Percec, V. et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science 328, 1009–1014 (2010).
Zhang, D. et al. One-component multifunctional sequence-defined ionizable amphiphilic Janus dendrimer delivery systems for mRNA. J. Am. Chem. Soc. 143, 12315–12327 (2021).
Yoshimura, A., Wakabayashi, Y. & Mori, T. Cellular and molecular basis for the regulation of inflammation by TGF. J. Biochem. 147, 781–792 (2010).
Pittet, J.-F. et al. TGF-β is a critical mediator of acute lung injury. J. Clin. Invest. 107, 1537–1544 (2001).
Saito, A., Horie, M. & Nagase, T. TGF-β signaling in lung health and disease. Int. J. Mol. Sci. 19, 2460 (2018).
Yu, X. et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity 47, 903–912 (2017).
Meng, X. Nikolic‑Paterson dJ and Lan HY: TGF‑β: The master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325 (2016).
Yue, X., Shan, B. & Lasky, J. A. TGF-β: titan of lung fibrogenesis. Curr. Enzyme Inhib. 6, 67–77 (2010).
Frangogiannis, N. G. Transforming growth factor–β in tissue fibrosis. J. Exp. Med. 217, https://doi.org/10.1084/jem.20190103 (2020).
Casey, J. et al. Alveolar surfactant protein D content modulates bleomycin-induced lung injury. Am. J. Respir. Crit. Care Med. 172, 869–877 (2005).
Zhang, D. et al. Targeted delivery of mRNA with one-component ionizable amphiphilic Janus dendrimers. J. Am. Chem. Soc. 143, 17975–17982 (2021).
Gowda, S. et al. A review on laboratory liver function tests. Pan Afr. Med. J. 3, 17 (2009).
Naderi Sohi, A. et al. Development of an mrna-lnp vaccine against sars-Cov-2: Evaluation of immune response in mouse and rhesus macaque. Vaccines 9, 1007 (2021).
Chung, S. et al. TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight 3, https://doi.org/10.1172/jci.insight.123563 (2018).
Lyons, R. M., Keski-Oja, J. & Moses, H. L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J. Cell Biol. 106, 1659–1665 (1988).
Böttinger, E. P. et al. The recombinant proregion of transforming growth factor beta1 (latency-associated peptide) inhibits active transforming growth factor beta1 in transgenic mice. Proc. Natl. Acad. Sci. USA 93, 5877–5882 (1996).
Visani, G. & Manfroi, S. G-CSF in the biology and treatment of acute myeloid leukemias. Leukemia lymphoma 18, 423–428 (1995).
Noelle, R. J. & Nowak, E. C. Cellular sources and immune functions of interleukin-9. Nat. Rev. Immunol. 10, 683–687 (2010).
Mukhopadhyay, S., Hoidal, J. R. & Mukherjee, T. K. Role of TNFα in pulmonary pathophysiology. Respir. Res. 7, 1–9 (2006).
Ogger, P. P. & Byrne, A. J. Macrophage metabolic reprogramming during chronic lung disease. Mucosal Immunol. 14, 282–295 (2021).
Drzewicka, K. & Zasłona, Z. Metabolism-driven glycosylation represents therapeutic opportunities in interstitial lung diseases. Front. Immunol. 15, 1328781 (2024).
Izbicki, G., Segel, M., Christensen, T., Conner, M. & Breuer, R. Time course of bleomycin‐induced lung fibrosis. Int. J. Exp. Pathol. 83, 111–119 (2002).
Pottier, N. et al. Relationships between early inflammatory response to bleomycin and sensitivity to lung fibrosis: a role for dipeptidyl-peptidase I and tissue inhibitor of metalloproteinase-3? Am. J. Respir. Crit. Care Med. 176, 1098–1107 (2007).
Manali, E. D. et al. Static and dynamic mechanics of the murine lung after intratracheal bleomycin. BMC Pulm. Med. 11, 1–13 (2011).
Schrier, D. J., Kunkel, R. G. & Phan, S. H. The role of strain variation in murine bleomycin-lnduced pulmonary fibrosis. Am. Rev. Respir. Dis. 127, 63–66 (1983).
Golden, T., Murray, A., Venosa, A. & Gow, A. J. Comprehensive dataset to assess morphological changes subsequent to bleomycin exposure. Data Brief 37, 107270 (2021).
Gur, I. et al. Lymphokines in bleomycin-induced lung injury in bleomycin-sensitive C57BL/6 and-resistant BALB/c mice. Exp. Lung Res. 26, 521–534 (2000).
Zhang, D. et al. The unexpected importance of the primary structure of the hydrophobic part of one-component ionizable amphiphilic Janus dendrimers in targeted mRNA delivery activity. J. Am. Chem. Soc. 144, 4746–4753 (2022).
Allawzi, A., Elajaili, H., Redente, E. F. & Nozik-Grayck, E. Oxidative toxicology of bleomycin: role of the extracellular redox environment. Curr. Opin. Toxicol. 13, 68–73 (2019).
Tager, A. M. et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am. J. Respir. Cell Mol. Biol. 31, 395–404 (2004).
Karmiol, S., Remick, D. G., Kunkel, S. L., & Pahn, S. H. Regulation of rat pulmonary endothelial cell interleukin-6 production by bleomycin: effects of cellular fatty acid composition. Am. J. Respir. Cell Mol. Biol. 9, 628–636 (1993).
Saito, F. et al. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am. J. Respir. Cell Mol. Biol. 38, 566–571 (2008).
Takamizawa, A. et al. Bleomycin stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activity. J. Immunol. 162, 6200–6208 (1999).
Martinez, D. R. et al. Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice. Science 373, 991–998 (2021).
Botelho, D. et al. Exposure to silver nanospheres leads to altered respiratory mechanics and delayed immune response in an in Vivo murine model. Front. Pharmacol. 9, 213 (2018).
Atochina-Vasserman, E. N. et al. Immune reconstitution during Pneumocystis lung infection: disruption of surfactant component expression and function by S-nitrosylation. J. Immunol. 182, 2277–2287 (2009).
Bartlett, G. R. Phosphorus assay in column chromatography. J. Biol. Chem. 234, 466–468 (1959).
Acknowledgements
The authors thank Drs. Yangzhu Du, Honghong Sun, and Eline Luning Prak of the Human Immunology Core (supported in part by NIH P30 AI045008 and P30 CA016520. HIC RRID: SCR_022380) at the Perelman School of Medicine at the University of Pennsylvania for their assistance with Luminex assays. They also would also like to thank Drs. Michael Goedken and Marianne Polunas of the Rutgers University Histology Core for their assistance with histological preparation and pathology services; Jessica Cervelli and Rita Hahn of the Rutgers Flow Cytometry Core for assistance with flow cytometry. In addition, they thank Sungjae Lee, Tony Hu, and Houping Ni for their technical assistance. They also express gratitude to Dr. Yongfeng Zhou at Shanghai Jiao Tong University for his assistance with cryo-TEM imaging of formulated IAJD34. Special thanks to Dr. Wook-Jin Park and Jessica A. Vasserman for their help with the characterization of formulated particles (size, PDI, Zeta potential, and encapsulation efficiency). Financial support was provided by the National Institutes of Health National Institute of Environmental Health Sciences [Grant T32-ES01984] and [Grant P30-ES005022] for A.J.G., E.R.S, R.S., M.W., E.A., and C.J.G. NSF-DMR-1807127, 1720530, and 2104554 financially supported V.P. This work was partially supported by the Wellcome Leap R3 program.
Author information
Authors and Affiliations
Contributions
A.J.G., D.W., and E.N.A.-V. conceived the concept. V.P., D.S.M. conceived and designed the IAJD used in this study. D.Z., Y.Y., L.P., and Y.L. synthesized the IAJD used in this study. J.A.M., E.R.S., D.Z., R.S., N.A.O., E.K.R., E.A., C-J.G., M.W., I.B., and E.N.A-V. performed the experiments. J.A.M., E.R.S., D.Z., R.S., N.A.O., E.K.R., E.A., C-J.G., M.W., I.B., A.J.G., and E.N.A.-V. performed the data analysis. J.A.M., E.R.S., A.J.G., and E.N.A.-V. wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, we report that D.W., V.P., D.Z., E.N.A.-V, and A.J.G. are named on patents and patent applications relevant to the work done in this publication. We have disclosed those interests fully to the University of Pennsylvania, and we have in place an approved plan for managing any potential conflicts arising from the licensing of our patents. All other authors declare no competing interests.
Inclusion and Diversity
One or more of the authors of this paper self-identifies as a gender minority or ethnicity minority in their field of research. We support the inclusive, diverse, and equitable conduct of scientific research.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meshanni, J.A., Stevenson, E.R., Zhang, D. et al. Targeted delivery of TGF-β mRNA to murine lung parenchyma using one-component ionizable amphiphilic Janus Dendrimers. Nat Commun 16, 1806 (2025). https://doi.org/10.1038/s41467-025-56448-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56448-y
This article is cited by
-
Azobenzene-bridged ionizable amphiphilic Janus glycosides for light-controlled, single-component and organ-modulable pDNA delivery
Communications Chemistry (2026)
-
Microrobots for pulmonary drug delivery
Nature Reviews Bioengineering (2026)
-
Dendrimer engineering to overcome delivery challenges of nucleic acids
Nature Reviews Bioengineering (2025)