Abstract
Development of an efficacious universal influenza vaccines remains a long-sought goal. Current vaccines have shortfalls such as mid/low efficacy and needing yearly strain revisions to account for viral drift/shift. Horses undergo bi-annual vaccines for the H3N8 equine influenza virus, and surveillance of sera from vaccinees demonstrated very broad reactivity and neutralization to many influenza strains. Subsequently, vaccinating mice using the equine A/Kentucky/1/1991 strain or recombinant hemagglutinin (HA) induced similar broadly reactive and neutralizing antibodies to seasonal and high pathogenicity avian influenza strains. Challenge of vaccinated mice protected from lethal virus challenges across H1N1 and H3N2 strains. This protection correlated with neutralizing antibodies to the HA head, esterase, and stem regions. Vaccinated ferrets were also protected after challenge with H1N1 influenza A/07/2009 virus using whole viral or HA. These data suggest that equine H3N8 induces broad protection against multiple influenzas using a unique antigen that diverges from other universal vaccine approaches.
Similar content being viewed by others
Introduction
Seasonal influenza, especially pandemic influenza viruses, continues to be one of the most significant public health concerns of the 21st century1,2. Seasonal influenza strains cause three to five million severe infections and 500,000 deaths yearly (WHO)3. Circulating influenza strains can be very diverse because they can undergo antigenic shift and drift4, thus making vaccine design difficult. Occasional highly lethal zoonotic infections, like the current circulating H5N15, demonstrate that humans remain in constant threat of these viruses crossing the species divide and causing pandemics6,7. Moreover, strains from subtypes with pandemic potential, such as H5N1, H5N8, H7N2, H10N8, and H9H2, are in circulation in permissive species with the possibility for mutations or reassortment to allow crossing of the species barrier8,9. The number of influenza strains that have crossed from birds into swine has increased dramatically in the last few years, further increasing the likelihood of crossing into humans10,11,12,13,14. Moreover, H5N1 has recently been found infecting swine. Thus, discovering a vaccine that can elicit protective immune responses against antigenically diverse strains is paramount for human health. Seasonal vaccination remains the best defense against influenza available. However, current vaccines have limited efficacy and provide a narrow breadth of protection15.
Current FDA. licensed influenza virus vaccines for humans contain H1N1 (phylogenetic group 1 hemagglutinin), H3N2 (phylogenetic group 2 hemagglutinin), and 1-2 influenza B virus components16. These vaccines are moderately efficacious for closely matched strains predominantly by eliciting antibodies recognizing type-specific epitopes in the globular head domain of HA17. These licensed vaccines are comprised of cold-adapted “attenuated” virus (LAIV), inactivated virus (IV), recombinant baculovirus-expressed HA, or virosome delivery of influenza peptides18,19 with mRNA-based ones currently in clinical testing phases20. IV still remains the most commonly administered vaccine, but efficacy is generally quite moderate21,22,23, with LAIV often giving, but not always, comparable protection24. Moreover, LAIV has failed to protect from infection, as demonstrated by the low vaccine efficacy of the vaccine’s 2013-14 H1N1 pnd09 component (CDC)25. As typified by the emergence of the H1N1 pnd09 viruses in the 2008–09 influenza season, some vaccines based on early season prediction strategies have failed to prevent strains that emerged late in the influenza season.
Furthermore, mutations in the predicted strains can further limit the protection afforded by current influenza vaccines, as typified by the H3N2v2 that emerged in 2012-1326. Due to the limited efficacy of current influenza vaccine technology, predicting the next year’s relevant influenza strain or variance in antigenicity is not precise. Vaccine mismatches, antigenic variants, and viral evolution of novel highly pathogenic strains such as avian H5N1, H7, and swine H1 and H3 variants complicate vaccine design. Vaccine failures due to antigenic mismatch and the pandemic threat from emergent viruses from reservoir hosts infected with avian or swine viruses warrant the development of influenza A vaccine candidates that induce broader protection. A universal influenza vaccine capable of broad protection against group 1 and 2 influenza viruses would be ideal. Still, a broadly protective vaccine that extends coverage to most seasonal and likely pandemic strains is desirable. Several potential universal influenza strains are in development or testing phases27,28,29, but nothing is yet licensed for use in humans.
Neutralizing antibodies (nAbs) generally bind to epitopes on the HA’s highly variable globular head domain, inhibiting receptor binding and mediating strain-specific hemagglutination inhibition (HAI) activity30. Thus, nAbs to H1N1 pnd09 often only neutralize that same virus strain and are not broadly neutralizing Abs (bnAbs). Conversely, the stem domain of the HA (sHA2)31 is relatively conserved but less immunogenic32. sHA2 binding Abs can mediate HAI activity by inducing conformational changes within the head region. These mAbs have broadly neutralizing activity identified33,34. Humans also recognize antigenic sites in the trimer interface domain that are broadly protective35,36,37. These observations suggest that a vaccine that can induce such Abs would be more efficacious against divergent HA subtypes. However, vaccine approaches that seek to generate HA stem binding Abs may need to carefully ensure that the right Ab profiles are generated, as certain profiles of sHA2 binding Abs are associated with increased vaccine-enhanced respiratory disease in pigs38. Whether or not enhanced disease can be induced in other species is uncertain. In addition, there is evidence that influenza may be able to modify its HA stem region to avoid stem-binding Abs39. However, this is unusual and may occur at a fitness cost for virus replication40.
An optimal future universal vaccine may target conserved antigenic sites within the head and the stem regions. A collection of nAbs that bind to a broad range of influenza virus strains and subtypes by binding to the sHA2 domain have been characterized41,42. However, a strategy of eliciting cross-phylogenetic group HAI Abs via strain-specific vaccination has been complicated. Despite significant sHA2 conservation between similar groups of influenza strains43, research to discover nAbs with activity against group 1 and group 2 viruses shows that B cells specifying these nAbs are rare. However, bnAbs can occur in humans that cross-react with H5N1 viruses44 and generally are elicited by H1N1 pnd09 infections. Despite many common epitopes, infections with H3N2 viruses do not usually elicit broad protection against other antigenically divergent H3N2 strains, nor does H1N1 infection provide protection from similar subtypes, suggesting that conserved epitopes are not a major component of the typical immune response in healthy subjects with prior influenza exposure.
We recently discovered an alternative immunogen based on the equine H3N8 virus, which can elicit bnAbs by directing antibodies toward multiple HAs. We discovered that the equine HA3 antigen elicited HAI Abs toward many human groups 1 and 2 viruses. We did not find equine H1N1 pnd09 infection, but we did find it in cats, prompting further equine sera HAI and neuraminidase ELISA testing. Neutralization activity in equine serum with no evidence of infection by other strains prompted us to test vaccinations in mice and ferrets. This new immunogen elicited a favorable protective immune profile targeting multiple HA sites and cross-protecting from heterosubtypic virus challenges in multiple species.
Results
Serum neutralizing antibody activity measured by HAI and microneutralization assays suggested the immunogenicity of the HA head domain
HAI screening assays were then used with sera from vaccinated horses. These samples possessed neutralizing activity toward multiple human seasonal influenza strains (Fig. 1A). We then tested the responses of foals that had never been vaccinated with H3N8. We found similar neutralizing titers as the other horses with unknown vaccination histories (Fig. 1B) but no responses before their first H3N8 vaccine.
Sera from vaccinated horses were heat inactivated and RDE treated. A HAI titers were assessed across multiple strains of influenza (n = 15). Line represents a protective reciprocal titer of 40. We do not show baseline pre-vaccination titers here since the horses are a mixed age population. B However, in a limited number of foals that we vaccinated for the first time, we did not find antibodies to any influenza antigen prior to vaccination and found equivalent HAI titers as their peers after vaccination (n = 7, as finding vaccine-free horses is difficult). p < 0.001 against unvaccinated foal sera. Mice received two doses of C H3N8 or D H3N2(A/Texas/50/12) in alum, and HAI serum titers were tested across many strains of influenza. Line indicates a minimum protective reciprocal titer of 40.Similar influenza strains were at least p < 0.001 between H3N8 and H3N2 vaccinees (n = 5 for two reps).
In further agreement with the equine data, serum from vaccinated mice also had neutralizing activity across multiple human seasonal influenza strains (Fig. 1C). Vaccination with an H3N2 virus (to match the H3 used in both vaccines) did not induce as broad of an HAI response as H3N8 vaccination since most responses were below an HAI titer of 40, with many having no response whatsoever (Fig. 1D). While we also determined that we could detect cross-reactive antibodies after one vaccine dose in mice, we could not detect cross-neutralizing antibodies until after the second vaccination (data not shown). We also tested sera from mice for neutralization activity and saw similar responses versus controls, thus validating our HAI results (Supplementary Fig. 1). Further testing of the serum by adoptive transfers and subsequent lethal challenge demonstrated protection in mice that received antibodies from vaccinees that received the equine H3N8 or rHA. While not an exhaustive determination, these data do support that the antibodies elicited from equine HA may be protective (Supplementary Fig. 2).
Vaccination with H3N8 induced cross-reactive antibodies to group 1 and 2 viruses
To further examine whether our vaccine induced cross-reactive antibodies and further bolster the HAI data in Fig. 1, we performed ELISAs using several different rHAs including H5N1.
Horse sera with limited known prior vaccination histories were screened initially for serum antibody reactivity by ELISA across multiple rHAs. They contained broadly reactive antibodies of both the IgM and pan IgG (Fig. 2A, B). This reactivity pattern included both group 1 and 2 viruses and multiple potential pandemic strains. To assess the effect of prior influenza exposure on the broad reactivity pattern observed, we also tested for antibodies directed toward NA from H1N1 A/California/07/2009. We did not detect reactivity (data not shown).
A, B Antibodies from horses were tested against a panel of rHAs (IRR Resources) from seasonal and avian flu strains by ELISA (n = 15). C We vaccinated mice with two doses of our LAIV H3N8 without adjuvant and tested reactivity by ELISA again for IgM and IgG. D, E We then tested for IgM and IgG again after inclusion of alum in the immunization (C–E: n = 5 for two reps each).
Next, we vaccinated mice with H3N8 virus by intramuscular injection so that the same viral vaccine used in horses was also used in mice. We found their serum antibodies exhibited a similar cross-reactive pattern by ELISA after two vaccinations as that of the horses. We intramuscularly injected the virus to simulate a normal route for humans, but we also found that equine H3N8 viruses would not infect mice at all doses tested. However, unlike the horses, the murine reactivity was predominantly of the IgM isotype (Fig. 2C) with lower amounts of IgG detected (1:10–1:20 titers, data not shown). Next, we tested whether including an alum adjuvant in each intramuscular vaccination altered the isotype profile. We chose alum, knowing it’s a weaker adjuvant since it is an approved and common adjuvant in humans. We found that alum altered the antibody profile toward a mixed IgM/IgG response (Figs. 2D, E). All further mouse experiments used alum as an adjuvant in vaccine immunizations. Vaccination with rHA induced similar levels of IgM/IgG that were also improved with Alum adjuvant in mice (data not shown).
After vaccination, mice and horses develop antibodies to the head, stem, and esterase regions
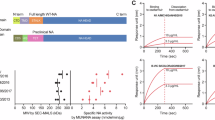
We also compared the antibody reactivity of mouse vaccinee sera with that of multiple broadly neutralizing antibodies by ELISA. We found a pattern like that of CR9114, although our antibody pools did not react to influenza B strains (Table 1). We then tested mouse vaccinee sera against the head (HA1) or stem (HA2) domains by ELISA and found that serum antibodies cross-reacted to both domains (Fig. 3A). Finally, we used competition-binding assays to determine the approximate location of antibody binding against three HA antibodies, H3v-95, H3v-47, and CR9114, specific to head, esterase-domain, or stem, respectively45. We found that sera from horses or vaccinated mice could at least partially inhibit the binding of antibodies directed to the head, esterase, or stem regions (Fig. 3B). Mouse sera was not tested for head domain reactivity due to insufficient serum sample volume. However, prior figures’ HAI data clearly demonstrated that mice also target the head region broadly. We also used the HAI and ELISA data against the various strains (Fig1/2) to examine potential differences between neutralized and non-neutralized strains to map potential broadly neutralizing sites (Supplementary Fig. 3). Future studies will be directed toward isolating and characterizing such broadly reactive antibodies.
A We performed ELISA for endpoint titers against headless HAs (sHA2) or recombinant HA1 portions of various rHAs (n = 6 mice, 2 reps). HA-reactive antibodies were not detected in sham-vaccinated mice (not shown). p < 0.05 or less for all comparisons to controls. B We performed ELISA competition-binding assays for antigens with pooled vaccinated mice (2x) or horse (1x) sera against panels of known bnAbs. Our sera competed for binding of antibodies specific for the esterase region (mAb H3v-47), head region (mAb H3v-95), or stem region (a recombinant mAb-based on the sequence of CR9114) for binding to HA from H3N2 A/Texas/50/2012. Pools of n = 5 mice or pools of n = 10 horses were used. Antibodies used for this assay are published prior67,68,69.
Vaccination protected mice from heterosubtypic challenge
Mice were vaccinated either with attenuated H3N8, rHA, control viruses, or PBS alone. To confirm that our rHA vaccine elicited similar HAI toward many seasonal strains like the whole H3N8, we first tested for neutralization after two vaccinations of our H3N8 rHA or PR8 H1 rHAs (Fig. 4A, B). In agreement with data in Fig. 2C, we found neutralization of many seasonal strains for which PR8 rHA did not elicit neutralization (Fig. 4B). We then challenged vaccinated mice with lethal doses of murine-adapted H1N1 or H3N2 viruses. We found that animals vaccinated with H3N8 or rHA were protected from lethality (Fig. 4C–F). We next vaccinated mice similarly as before and used a 2 × LD50 dose of A/PR/8/34 virus and found that H3N8- and rHA-vaccinated animals had what appeared to be less lung histopathology (Fig. 5A–D) and had lower viral burdens in the lungs after challenge (Fig. 5E).
A We vaccinated mice as stated prior in the results twice using rHAs from either H3N8 and tested for HAI responses across many seasonal strains of influenza (n = 5 for two reps). B Next, we tested for survival after H1N1 A/California/07/2009 challenge (4 × LD50). PR8 HA was used here instead of our H1/H3 controls since they contained the H1N1 A/California/07/2009 virus (n = 5 for two reps). C–F Next, we tested for survival after the challenge with 2 × LD50 of viruses stated in the figures. Here, we compared our H3N8 whole vaccine against our H3N8 rHA and a sham or control vaccine containing H1 and H3 viruses (n = 5 for two reps for all animals). Per our animal protocol, we needed to use a 25% weight cutoff before euthanizing animals after the challenge. * p < 0.01 for each survival curve between equine vaccinees (LAIV or rHA) and both controls. The xPR8 in each challenge virus indicates these were viral crosses with A/PR/8/34 to facilitate infection in mice given its mouse adaptation.
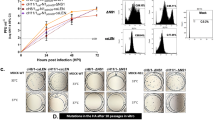
A Again, we vaccinated mice with either H3N8 whole virus, rHA from H3N8, sham, or our mix of H1/H3 Cali viruses. We assessed the collected lungs after 5 DP. and stained them with hematoxylin and eosin. (n = 5 with two reps, but representative photos are shown. Shown are A sham control, B H1/H3 Cali, C equine rHA, and D H3N8 LAIV. E Viral lung burdens were determined in 1 mg of the upper right lobe after 6 DPIs from the same mice as (A) by TCID50 assay. 20× magnification was used. The inset for 5C is also 20× magnification but the image size was reduced to show more of the lung histology.
Immunogenicity and protective efficacy in ferrets suggest that H3N8 protects from challenges in other species
Next, ferrets were vaccinated with rHA, LAIV, H3N2 A/Texas/50/12, or PBS sham by intramuscular vaccination as done in mice. HAI titers were then examined after one, two, or three vaccinations and found to have increasing levels of HAI to H3N8 virus (Fig. 6A) and cross-reactive HAI against H1N1 A/California/07/2009 after each vaccination (Fig. 6B). We then challenged the ferrets 4 weeks after the third vaccination with 107 TCID50 of the H1N1 A/California/07/09 virus. Ferrets vaccinated with sham or H3N2 A/Texas/50/12 had significant lung consolidation similar to mice from Fig. 5 (not shown). Ferrets vaccinated with rHA from H3N8 had mixed histopathology at 6 days post infection (DPI), with most exhibiting areas of clearance but some areas with lung infiltration and consolidation that still appeared better than that in controls, like Fig. 5. Ferrets vaccinated with LAIV H3N8 had lung histology that looked similar to uninfected controls. Sham and A/Texas/50/12 inoculated ferrets also had higher sneezing and lethargy scores than equine vaccine recipients (data not shown). Next, we stained those same lungs with pan anti-influenza A antibody (Millipore). We found that sham-immunized ferrets had widespread virus distribution in the lungs (Fig. 7A), while H3N2 A/Texas/50/12 immunized animals had less virus but still substantial virus antigen staining.
Ferrets received 3 doses of immunogens with alum and were then challenged 4 weeks after the third vaccination. A HAI responses to the H3N8 virus after each vaccination. * p < 0.001 between equine and control groups. B HAI responses to H1N1 A/California/07/2009 strain after each vaccination. Notice the third vaccine axis is different from subsequent vaccinations. p < 0.001 between equine and control groups. Lines represent HAI titer of 40. A/Texas/50/12 is from H3N2 A/2012.
Ferrets rested 3 weeks post their third vaccine and were then challenged with 107 TCID50 of H1N1/Cali/09. A Ferrets were euthanized after 6 days, and lungs were stained with anti-influenza A antibody. A representative stain is shown for all groups. Brightfield images were merged with FITC images by the camera software (4× magnification). Green indicates the presence of the influenza virus. B We assessed the viral burdens in nasal swabs 3 days post-infection from the same ferrets using one-step qRT-PCR for a flu-conserved M2 region. C 1 mg of lung tissue was homogenized and filtered, and then virus titer was determined by TCID50 assay. 10× magnification was used in the images.
In contrast, rHA-immunized ferrets had significantly less but still detectable virus by staining, while it was more difficult to find areas of viral staining in LAIV-vaccinated ferrets. Quantitative RT-PCR was also determined for viral burdens using nasal samples, and we found that H3N8-vaccinated ferrets had the least viral burdens, followed by rHA-vaccinated animals and then the controls in nasal swabs at 3 DPI (Fig. 7B). Alternatively, viral titers were determined as TCID50 of 1 mg sections of upper right lobe lungs at 5 DPI (Fig. 7C).
Discussion
There is an urgent need for a broadly protective influenza vaccine given the yearly circulation of strains that infect humans, the constant antigenic drift and possibility for an antigenic shift, and the threat of potentially highly pathogenic strains crossing into humans. In the present study, we demonstrated the efficacy of an equine influenza H3N8 virus to protect from a heterosubtypic influenza virus challenge in multiple animal species, suggesting further development of this novel antigen as a vaccine candidate is warranted.
Interestingly, dogs infected by the H3N8 virus from cross-species exposure to infected horses were susceptible to infection by H3N2 in subsequent cross-species transfers, but only H3N8 viruses infect horses46. While experimental infections with high doses of other viruses are possible in horses, those infections are generally attenuated compared to H3N847. While these observations could be due to differences in viral tropism, another possibility is that H3N8 exposure in horses makes them immune to other influenza viruses. Further evidence for this possibility is that before H3N8 adaption into horses, H7N7 viruses were the predominant infecting viral species, but that viral strain disappeared from horses after H3N8 crossed into horse populations48.
It has been previously reported that mice or ferrets vaccinated with a cold-adapted virus incorporating equine HA induced protection from equine influenza challenge49, suggesting that vaccination might prevent that particular zoonosis due to equine influenza virus. Here, we extend those findings to show that equine H3N8 antigens or epitopes might provide a promising component for a more broadly protective vaccine against human influenza. The previous studies used equine H3N8 Georgia/81 virus, and key differences exist with the influenza strain used here, H3N8 A/Kentucky/1/91, as shown in Supplementary Fig. 4. Specifically, there are key amino acid substitutions in areas that we predict to induce bnAbs (e.g., site S70P has no glycosylation site in the Georgia/81 vaccine, meaning a key immunodominant and non-protective epitope is exposed that might decoy the immune response near a site we hypothesize contributes to protection). As we continue to examine this vaccine candidate, testing other equine influenza viruses against heterosubtypic protection could be an interesting avenue of exploration.
The need for high concentrations of alum adjuvant to push the antibody profile from IgM- towards IgG-dominant suggests that a lack of CD4 T cell help may occur with this antigen, at least in mice. To help with this shift, we chose to study alum here since it is an approved adjuvant in human vaccines. Other adjuvants might likely increase HAI titers or induce a better IgG dominance than we observed with the alum. In our preliminary studies, including cyclic dinucleotide adjuvants can improve the HAI titers observed at least 4-fold in mice (not shown). Still, we do not yet know about the effect of such adjuvants on the switch from IgM to IgG. Our goal in inducing class switching is to induce an antibody response of higher affinity and longer duration. IgM antibodies are known to be produced in the lungs after the challenge of mice and can have significant reactivity toward influenza, but their duration may be short-lived50. In humans, neutralizing IgM antibodies may exhibit a longer half-life after influenza exposure, and thus, IgM might contribute to clinical protection in humans51.
The difference in protection between our baculovirus-expressed HA and the live attenuated virus likely is due to two factors: (1) the whole virus contains more antigenic proteins such as NP and neuraminidase (NA) and (2) baculovirus-expressed HA protein generally needs much higher concentrations in the injection to confer similar efficacy as split vaccines. We saw at least some serum antibody cross-reactivity in NA-based ELISAs in our whole virus H3N8 vaccinees that we did not see in the serum of H3N2 vaccinees. Work to qualify and quantify any contributions from antibodies toward NA is of interest (Supplementary Fig. 5, not exhaustive testing across strains yet). We also did not examine cell-mediated responses toward the vaccine, and likely CD8+ T cells directed toward the NP contribute to heterosubtypic protection observed in animals. Moreover, there is evidence in humans that CD8+ T cells directed toward NP may impart some protection.
The need to use three vaccine doses in ferrets stems from the fact that we did not see robust HAI titers toward H1N1 A/California/07/09 after two doses. This limited response could stem from a lack of prior exposure to influenza vaccines. Some H3N2 viruses elicit low immunity by intramuscular injection after the first dose versus intraperitoneal routes in mice. Alternatively, we may not have included a high enough dose to optimally boost the HAI titers after the prime and boosting doses, as we have evidence in our murine vaccinations that a third and fourth vaccination increased the total sera concentration of H3N8 reactive antibodies. This observation suggests that maximal immunogenicity is not achieved after two doses with the current regimen and formulation. In fact, we found in subsequent experiments that further boosting mice with another 2 doses of H3N8 or rHA increased antibody titers, suggesting that stronger adjuvants or higher doses might increase the level of response. We also believe a similar trend might occur in our vaccinated ferrets, as even three doses of immunization likely did not maximize the full level of HAI we could have obtained.
We demonstrated that equine H3N8 can induce broadly reactive and neutralizing antibodies to multiple human season influenza strains. Follow-on studies will compare rHA in higher concentrations or with other viral proteins (i.e., NP or NA) to determine whether we can bolster the protection afforded similar to that induced by the whole virus. We are also evaluating other antigen delivery methods, including prolonged polyanhydride nanoparticle release. We do not yet know if prior exposure to other influenza strains for vaccines changes the immunity imparted by this vaccine candidate. We also do not know how long the induced immunity lasts, and thus do not know whether an annual booster would be necessary to maintain immunity over time. We are exploring the antigenic sites recognized by the induced neutralizing antibodies and are actively generating hybridomas from vaccinees (equine and murine). While the H3N8 virus is not very infectious for humans, including the vaccine as a killed or rHA vaccine could foster protective responses. The best format for this vaccine such as a detergent killed vaccine or a rHA using baculovirus or even an mRNA vaccine will need to be compared to the rHA or whole viruses used here to select the one that elicits a similar response in humans as we round in the multiple animals we have used in this study. We demonstrated that the H3N8 virus or its HA can broadly protect influenza A strains across multiple animal species and seasonal influenza strains. We consider this vaccine candidate worthy of further development. Future studies will explore the use of other equine strains or HAs and test higher doses or alternative adjuvants to bolster and enhance immunogenicity. Of course, cellular immunity from the vaccine might also contribute to protection and will need to be studied. However, these studies establish proof of principle for the potential of this immunogen to induce broadly protective responses.
Methods
Animals
The Iowa State University Institutional Animal Care and Use Committee and Institutional Biosafety Committee approved all procedures. Euthanasia of mice and ferrets was consistent with the American Association of Veterinarians guidelines. Moreover, mice were euthanized by using CO2 with cervical dissociation as a secondary mechanism. Ferrets were euthanized by intravenous injection of a fatal dose of Fatal-Plus (Vortech Pharmaceuticals, Dearborn, MI) administered by animal resources veterinarians. No horses were euthanized in this study.
Mice
Female BALB/c mice, 6–10 weeks of age, were purchased from the Jackson Laboratory and housed under ABSL2 conditions at Iowa State University. Mice were vaccinated twice with 25 μg of HA containing LAIV H3N8 or a mix of H1N1 A/07/2009 and California H3N2 A/4230/2014 or recombinant HA (rHA) by intramuscular injections (caudal muscle) containing alum in 50 μl doses. Three weeks after the second vaccination, mice were challenged with influenza strains (2–4 × LD50 dose) H1N1s A/PR/8/1934 or A/New Jersey/1976 or A/California/07/2009, or H3N2 A/Victoria/361/2011in 40 µL of PBS under isofluorane gas for anesthesia to unconsciousness. Mice were euthanized after >25% body weight loss or when exhibiting signs of respiratory distress.
Horses
Horse sera from horses aged 2–10 years old were obtained retrospectively from the Iowa State University Diagnostic Laboratory. In addition, male and female horses aged 25 weeks (receiving their first dose of FluAvert (Merck), were used from the Iowa State University herd before influenza vaccination. Doses were consistent with the manufacturer’s guidelines, using intranasal administration of one ml of the reconstituted vaccine containing the manufacturer’s saline diluent. Horse peripheral blood samples were obtained by phlebotomy of the jugular vein 3 weeks post-H3N8 vaccine that was administered by the intranasal route according to the manufacturer’s instructions (Merck). All vaccinations and blood withdrawals were performed by veterinarians at ISU. Horses did not need anesthesia for vaccinations or blood withdrawals, which is standard practice at ISU.
Ferrets
Male ferrets, aged 4 weeks, castrated and vaccinated for rabies and canine distemper virus by Marshall Bioresources (Utica, NY) were purchased and housed under ABSL2 conditions at Iowa State University. Ferrets were vaccinated thrice, 3 weeks. Apart, with 50 μL of H3N8, rH3 from H3N8, or H3N2 Hong Kong/2014 containing 25 μg HA and 0.2 μg of alum in PBS per intramuscular injection in hind leg muscles. Ferrets were anesthetized by isoflurane gas when vaccinated to unconsciousness. Another group was vaccinated with alum alone in PBS. Ferrets were then challenged intranasally with human influenza strain H1N1 A/California/7/2009 (107 TCID50) in 1 mL of PBS under isofluorane gas 3 weeks post-final vaccination. Weight loss was measured daily, and clinical signs of disease were noted blindly by care staff. Ferrets were set to be euthanized after >25% body weight loss or when exhibiting signs of respiratory distress, but no ferrets needed this intervention. For nasal swabs and qRT-PCR viral burden determinations, we used a floxed cotton swab soaked in viral transport media (PBS, 1% glucose, and 1% Pen/Strep antibiotic) and swabbed the nasal flares twice before placing the tip of the swab into viral transport media on ice.
Challenge viruses
Challenge viruses were obtained from B.E.I. Resources or the International Reagent Resource (IRR) as reassortants with H1N1 A/PR/8/34 virus. BALB/c mice were inoculated with the virus by intranasal delivery to deliver a virus to the lower respiratory tract (50 μl), and lungs were harvested 3 days post-inoculation. The virus was passed in mouse lungs in 5 serial passages. The recovered strains were expanded in 10-day-old embryonated eggs (Charles River). Two additional H1N1 A/California/07/2009 (Cali/09) human isolates and an H3N2 A/Texas/50/12 isolate also were obtained from IRR and expanded similarly in eggs (Charles River). All strains were sequenced after RT-PCR for HA and NP (nucleoprotein) nucleotides, and the original parent strains from IRR were ensured to match.
Vaccine antigen
LAIV, based on the equine H3N8 A/Kentucky/1/91 strain, was obtained from Merck & Co. and expanded at 28 °C in 10-day-old embryonated chicken eggs for 3 days. H1N1 A/California/07/2009 and H3N2 A/California/4230/2014 and H3N2 A/Texas/50/12 were passed serially in Madin-Darby canine kidney (MDCK) cell monolayer cultures at 37, 35, 30, and 28 °C before expansion in embryonated eggs, as described above for H3N8. These viruses were confirmed to grow in MDCKs at 28 °C to match the H3N8 LAIV vaccine. rHA was generated from H3N8 A/Kentucky/1/91 or H1N1 A/PR/8/34 viruses by overlapping synthesis of oligonucleotides (Genscript) that removed the transmembrane domain of the protein but inserted a flexible glycine/serine peptide spacer, followed by a T4 foldon domain, and a 6 × His tag. The sequence was subcloned into a baculovirus shuttle vector (Mirus Biotech) and used to produce infectious baculovirus according to the manufacturer’s instructions using the FlashBac Ultra system (Mirus Biotech) and transfection into Sf21 cells (Clontech). After 5 days, the virus was titrated using a commercial titration kit (Clontech) by qPCR. HiFive cells (Invitrogen) were then inoculated with an M.O.I. of 5 for 2 days before cleavage with N-tosyl-L-phenylalanine chloromethyl ketone (TPCK) treated trypsin. rHA was purified on Ni-NTA columns and dialyzed for 2 days with PBS using 4 buffer exchanges. rHA was routinely run on a native gel and verified to contain a ~230 kDa protein that reacted with LAIV-immunized or PR8-immunized animal sera. Alum adjuvant was purchased from Invivogen and mixed with immunogen in PBS buffer 30 min before injection. Furthermore, vaccine stocks were tested for other viruses by H1 or H3 RT-PCR detection primers, and we only detected H3 amplicons or H1 amplicons in the matched vaccines (data not shown). In some experiments, we directly compared H3 vaccines (i.e., H3N2 versus H3N8) to determine whether the responses were just from H3 immunizations. In others, we compared H3N8 to a mix of H1/H3 to simulate a human vaccine without the B strains. H3N8 was not infectious for mice or ferrets, and we believe that LAIV injected into the muscles would not behave as a live attenuated virus but as a killed one. The LAIV strain used was confirmed not to grow in infected mice as no evidence of virus was found in the lungs 3 days post-challenge by TCID50 or qRT-PCR which is not surprising given this strain’s 28° cold-adaptation (data not shown).
ELISA
Recombinant HAs were obtained from B.E.I. Resources or the IRR. Antigen was added to PBS at 0.1 μg/mL and plated into 384-well Maxsorb ELISA plates (Thermo Fisher) overnight at 4 °C. Plates then were blocked with 3% nonfat dry milk (NDM) in PBS for 1 h. at 37 °C. Plates then were washed 4 times with PBS with 0.5% Tween-20 (Thermo Fisher). Sera were diluted in duplicates with two-fold dilutions down the short sides of the plates in NDM and incubated for 1 h. Plates then were washed, and anti-horse or anti-mouse IgM (Southern Biotech) or anti-horse (Southern Biotech) or anti-mouse pan IgG HRP. (Biolegend) was added (1:10,000) in NDM and incubated for 1 h at 37 °C. Plates were washed, and 3,3′,5,5′-tetramethylbenzidine (TMB) (Thermo Fisher) was added before reading optical densities. To calculate endpoint titers, a cutoff was determined as 3×, the standard deviation of non-specific murine sera.
Microneutralization assays
Murine, ferret, or equine sera were incubated 1:3 with receptor-destroying enzyme (Sigma) overnight at 37 °C, followed by heat inactivation at 56 °C for 30 min. Influenza virus (100 TCID50/well) was incubated for 1 h at 37 °C with sera diluted in DMEM (Thermo Fisher) medium with penicillin/streptomycin and 1% B.S.A. The virus was then added to plates containing MDCK cells at 85% confluency and allowed to absorb for 2 h at 37 °C, followed by washing with DMEM. Cells were then incubated with culture media in the presence of TPCK-treated trypsin at 1 μg/mL (Sigma) for 3 days. Viral neutralization titers were determined by incubation with 0.5% turkey blood (Lampire Biologicals) and calculated as reciprocal titers.
Hemagglutination inhibition
HAI titers were determined similar to other studies52 using serially diluted serum with 8 HAU. per 50 μL of H1N1 or H3N2 viruses in PBS containing 0.5% Bovine Serum Albumin (Sigma) in V-bottom assay plates (Thermo Fisher). The virus/ antibody was incubated for 0.5 h at room temperature, and then 0.5% turkey or chicken blood cells containing 0.5% B.S.A. were added, and plates were read within 45 min. Data were calculated as the reciprocal titer above agglutination.
Antibody competition assays
Competition with serum antibodies was performed by ELISA as described previously53. Briefly, 96-well plates coated with 1 µg/ml of H3 A/Texas/50/2012 HA were blocked with 5% NDM, 2% goat serum, and 0.1% Tween-20 in PBS. The plates were washed with PBS containing 0.1% Tween-20 and incubated with serum diluted (1:10) with PBS, followed by wash and incubation with 5 µg/ml of either mAb H3v-47 or mAb H3v-95 or mAb CR9114. Anti-human IgG alkaline phosphatase conjugate antibody (Meridian Life Science, W99008A) was used for detection with phosphatase substrate. The corresponding optical density values were measured at 405 nm wavelength on a BioTek plate reader.
qPCR for viral burdens
50 mg tissue slices were extracted using the Total RNA extraction kit (Qiagen) following the manufacturer’s directions. From that, 50 ng of RNA was then subjected to one-step qRT-PCR (NEB) with a universal M1 probe conjugated to Fluorescein amidites (F.A.M.) and Iowa Black quencher (IDT) for 40 cycles.
Immunohistochemistry
5 μM sections were cut from paraffin blocks and embedded. Slides were washed through xylene and rehydrated in a series of ethanol from 100% to 0% before blocking with bovine F.B.S. (5%) in PBS for 1 h at room temperature. Slides were then stained overnight at 4 °C with mouse anti-influenza A1 amd A3 (Millipore: MAB9251-KC) in PBS with 1% B.S.A. at 1:100 dilution followed by three washes in PBS containing 0.05% Tween 20. Slides were then stained with anti-mouse FITC (BioLegend) at 1:100 in PBS with B.S.A. at room temperature for 1 h. Slides were washed again and stained further with anti-FITC Alexa488 (Jackson Immuno) in PBS with bovine serum albumin at 1:100 for 1 h at room temperature. Slides were then rewashed, and VECTASHIELD with DAPI (Vector) was added before imaging. Microscope gain and contrast were set using the sham vaccinees and uninfected ferrets, and then all slides were imaged with identical settings. Slides were photographed in random spots on a fluorescent ZOE. microscope (Bio-Rad), and images were merged with no further manipulations except for automatic contrast, which was done in PowerPoint for figure generation (Microsoft).
Data graphing and statistical analysis
Prism software (GraphPad) was used for making all graphs and for all parametric analysis using Student T-tests or One-Way ANOVA tests. A p value less than 0.05 was considered significant.
Data availability
Data will be uploaded to a searchable Iowa State University repository upon acceptance and link provided to journal.
References
Harrington, W. N., Kackos, C. M. & Webby, R. J. The evolution and future of influenza pandemic preparedness. Exp. Mol. Med. 53, 737–749 (2021).
Javanian, M. et al. A brief review of influenza virus infection. J. Med. Virol. 93, 4638–4646 (2021).
Krammer, F. et al. Influenza. Nat. Rev. Dis. Prim. 4, 3 (2018).
Liang, Y. Pathogenicity and virulence of influenza. Virulence 14, 2223057 (2023).
Charostad, J. et al. A comprehensive review of highly pathogenic avian influenza (HPAI) H5N1: an imminent threat at doorstep. Travel Med. Infect. Dis. 55, 102638 (2023).
Hui, D. S. & Zumla, A. Emerging respiratory tract viral infections. Curr. Opin. Pulm. Med. 21, 284–292 (2015).
Shi, J., Zeng, X., Cui, P., Yan, C. & Chen, H. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg. Microbes Infect. 12, 2155072 (2023).
Trombetta, C., Piccirella, S., Perini, D., Kistner, O. & Montomoli, E. Emerging influenza strains in the last two decades: a threat of a new pandemic? Vaccines 3, 172–185 (2015).
Graziosi, G., Lupini, C., Catelli, E. & Carnaccini, S. Highly pathogenic avian influenza (HPAI) H5 clade 2.3.4.4b virus infection in birds and mammals. Animals 14, https://doi.org/10.3390/ani14091372 (2024).
Henningson, J. N. et al. Comparative virulence of wild-type H1N1pdm09 influenza A isolates in swine. Vet. Microbiol. 176, 40–49 (2015).
Lewis, N. S. et al. Substitutions near the hemagglutinin receptor-binding site determine the antigenic evolution of influenza A H3N2 viruses in U.S. swine. J. Virol. 88, 4752–4763 (2014).
Nfon, C. K. et al. Characterization of H1N1 swine influenza viruses circulating in Canadian pigs in 2009. J. Virol. 85, 8667–8679 (2011).
Rajao, D. S. et al. Novel reassortant human-like H3N2 and H3N1 influenza A viruses detected in pigs are virulent and antigenically distinct from endemic viruses. J. Virol. https://doi.org/10.1128/JVI.01675-15 (2015).
Kang, M. et al. Zoonotic infections by avian influenza virus: changing global epidemiology, investigation, and control. Lancet Infect. Dis. 24, e522–e531 (2024).
Soema, P. C., Kompier, R., Amorij, J. P. & Kersten, G. F. Current and next generation influenza vaccines: formulation and production strategies. Eur. J. Pharm. Biopharm. 94, 251–263 (2015).
McLean, H. Q. & Belongia, E. A. Influenza vaccine effectiveness: new insights and challenges. Cold Spring Harb. Perspect. Med. 11, https://doi.org/10.1101/cshperspect.a038315 (2021).
Ellebedy, A. H. et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc. Natl. Acad. Sci. USA 111, 13133–13138 (2014).
Moser, C., Muller, M., Kaeser, M. D., Weydemann, U. & Amacker, M. Influenza virosomes as vaccine adjuvant and carrier system. Expert Rev. Vaccines 12, 779–791 (2013).
Wong, S. S. & Webby, R. J. Traditional and new influenza vaccines. Clin. Microbiol Rev. 26, 476–492 (2013).
Lee, I. T. et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: interim analysis. Nat. Commun. 14, 3631 (2023).
Joshi, A. Y., Iyer, V. N., Hartz, M. F., Patel, A. M. & Li, J. T. Effectiveness of trivalent inactivated influenza vaccine in influenza-related hospitalization in children: a case-control study. Allergy Asthma Proc. 33, e23–e27 (2012).
Ng, S. et al. The effect of age and recent influenza vaccination history on the immunogenicity and efficacy of 2009-10 seasonal trivalent inactivated influenza vaccination in children. PLoS ONE 8, e59077 (2013).
Kissling, E. et al. Interim 2022/23 influenza vaccine effectiveness: six European studies, October 2022 to January 2023. Euro. Surveill. 28, https://doi.org/10.2807/1560-7917.ES.2023.28.21.2300116 (2023).
Dibben, O. et al. Defining the root cause of reduced H1N1 live attenuated influenza vaccine effectiveness: low viral fitness leads to inter-strain competition. NPJ Vaccines 6, 35 (2021).
Ilyushina, N. A. et al. Live attenuated and inactivated influenza vaccines in children. J. Infect. Dis. 211, 352–360 (2015).
Epperson, S. et al. Human infections with influenza A(H3N2) variant virus in the United States, 2011-2012. Clin. Infect. Dis. 57(Suppl 1), S4–S11 (2013).
Nachbagauer, R. & Palese, P. Is a universal influenza virus vaccine possible? Annu Rev. Med 71, 315–327 (2020).
Wang, W. C., Sayedahmed, E. E., Sambhara, S. & Mittal, S. K. Progress towards the development of a universal influenza vaccine. Viruses 14, https://doi.org/10.3390/v14081684 (2022).
Lim, C. M. L., Komarasamy, T. V., Adnan, N., Radhakrishnan, A. K. & Balasubramaniam, V. Recent advances, approaches and challenges in the development of universal influenza vaccines. Influenza Other Respir. Viruses 18, e13276 (2024).
Gomez Lorenzo, M. M. & Fenton, M. J. Immunobiology of influenza vaccines. Chest 143, 502–510 (2013).
Cotter, C. R., Jin, H. & Chen, Z. A single amino acid in the stalk region of the H1N1pdm influenza virus HA protein affects viral fusion, stability and infectivity. PLoS Pathog. 10, e1003831 (2014).
Krammer, F. & Palese, P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr. Opin. Virol. 3, 521–530 (2013).
Impagliazzo, A. et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 349, 1301–1306 (2015).
Yassine, H. M. et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 21, 1065–1070 (2015).
Bangaru, S. et al. A site of vulnerability on the influenza virus hemagglutinin head domain trimer interface. Cell 177, 1136–1152. e1118 (2019).
Zost, S. J. et al. Canonical features of human antibodies recognizing the influenza hemagglutinin trimer interface. J. Clin. Investig. 131, https://doi.org/10.1172/JCI146791 (2021).
Dong, J. et al. Anti-influenza H7 human antibody targets antigenic site in hemagglutinin head domain interface. J. Clin. Investig. 130, 4734–4739 (2020).
Khurana, S. et al. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci. Transl. Med. 5, 200ra114 (2013).
Magadan, J. G. et al. Biogenesis of influenza a virus hemagglutinin cross-protective stem epitopes. PLoS Pathog. 10, e1004204 (2014).
Lee, C. Y. et al. Epistasis reduces fitness costs of influenza A virus escape from stem-binding antibodies. Proc. Natl. Acad. Sci. USA 120, e2208718120 (2023).
Hashem, A. M. Prospects of HA-based universal influenza vaccine. Biomed. Res. Int. 2015, 414637 (2015).
Stropkovska, A. et al. Broadly cross-reactive monoclonal antibodies against HA2 glycopeptide of Influenza A virus hemagglutinin of H3 subtype reduce replication of influenza A viruses of human and avian origin. Acta Virol. 53, 15–20 (2009).
Fan, X. et al. Targeting the HA2 subunit of influenza A virus hemagglutinin via CD40L provides universal protection against diverse subtypes. Mucosal Immunol. 8, 211–220 (2015).
Sui, J. et al. Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies. Clin. Infect. Dis. 52, 1003–1009 (2011).
Dreyfus, C. et al. Highly conserved protective epitopes on influenza B viruses. Science 337, 1343–1348 (2012).
Parrish, C. R., Murcia, P. R. & Holmes, E. C. Influenza virus reservoirs and intermediate hosts: dogs, horses, and new possibilities for influenza virus exposure of humans. J. Virol. 89, 2990–2994 (2015).
Zhu, H. et al. Absence of adaptive evolution is the main barrier against influenza emergence in horses in Asia despite frequent virus interspecies transmission from wild birds. PLoS Pathog. 15, e1007531 (2019).
Murcia, P. R., Wood, J. L. & Holmes, E. C. Genome-scale evolution and phylodynamics of equine H3N8 influenza A virus. J. Virol. 85, 5312–5322 (2011).
Baz, M. et al. A live attenuated equine H3N8 influenza vaccine is highly immunogenic and efficacious in mice and ferrets. J. Virol. 89, 1652–1659 (2015).
Wolf, A. I. et al. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. J. Clin. Investig. 121, 3954–3964 (2011).
Skountzou, I. et al. Influenza virus-specific neutralizing IgM antibodies persist for a lifetime. Clin. Vaccin. Immunol. 21, 1481–1489 (2014).
Rafiq, S. et al. Serological response to influenza vaccination among children vaccinated for multiple influenza seasons. PloS ONE 7, e51498 (2012).
Bangaru, S. et al. A multifunctional human monoclonal neutralizing antibody that targets a unique conserved epitope on influenza HA. Nat. Commun. 9, 2669 (2018).
Ghafoori, S. M. et al. Structural characterisation of hemagglutinin from seven Influenza A H1N1 strains reveal diversity in the C05 antibody recognition site. Sci. Rep. 13, 6940 (2023).
Krause, J. C. et al. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J. Virol. 85, 10905–10908 (2011).
Lee, P. S. et al. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc. Natl. Acad. Sci. USA 109, 17040–17045 (2012).
Whittle, J. R. et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA 108, 14216–14221 (2011).
Han, A. et al. Safety and efficacy of CR6261 in an influenza A H1N1 healthy human challenge model. Clin. Infect. Dis. 73, e4260–e4268 (2021).
Tharakaraman, K., Subramanian, V., Cain, D., Sasisekharan, V. & Sasisekharan, R. Broadly neutralizing influenza hemagglutinin stem-specific antibody CR8020 targets residues that are prone to escape due to host selection pressure. Cell Host Microbe 15, 644–651 (2014).
Sui, J. et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16, 265–273 (2009).
Wu, Y. et al. A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat. Commun. 6, 7708 (2015).
Sauter, N. K. et al. Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: analysis by proton nuclear magnetic resonance spectroscopy and X-ray crystallography. Biochemistry 31, 9609–9621 (1992).
Song, Y. et al. High-resolution comparative modeling with RosettaCM. Structure 21, 1735–1742 (2013).
Collins, P. J. et al. Recent evolution of equine influenza and the origin of canine influenza. Proc. Natl. Acad. Sci. USA 111, 11175–11180 (2014).
Yang, H. et al. Structural and functional analysis of surface proteins from an A(H3N8) influenza virus isolated from New England harbor seals. J. Virol. 89, 2801–2812 (2015).
Corti, D. et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 (2011).
Qiu, Y. et al. Mapping of a novel H3-specific broadly neutralizing monoclonal antibody targeting the hemagglutinin globular head isolated from an elite influenza virus-immunized donor exhibiting serological breadth. J. Virol. 94, https://doi.org/10.1128/JVI.01035-19 (2020).
Wu, N. C. & Wilson, I. A. Influenza hemagglutinin structures and antibody recognition. Cold Spring Harb. Perspect. Med. 10, https://doi.org/10.1101/cshperspect.a038778 (2020).
Wu, N. C. et al. Different genetic barriers for resistance to HA stem antibodies in influenza H3 and H1 viruses. Science 368, 1335–1340 (2020).
Acknowledgements
The work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC. We further acknowledge the NIH Biodefense and Emerging Infections Research Resources Repository NIAID, NIH and Influenza Reagent Resource, Influenza Division, WHO Collaborating Center for Surveillance, Centers for Disease Control and Prevention for providing rHAs and viruses used in this study.
Author information
Authors and Affiliations
Contributions
D.V. conceptualized the study, performed most experiments, analyzed the data, wrote the manuscript, obtained the funding. B.S. helped conceptualize the study and gave input on experiments and obtained equine samples. J.C. helped edit the manuscript and oversaw the antibody competition assays. S.B. ran the antibody competition assays with J.C. R.W. performed validating HAI assays on equine serum. B.L. performed protein modeling and determination of antibody binding sites.
Corresponding author
Ethics declarations
Competing interests
The vaccine used in this study is based on a veterinary vaccine (FluAvert) manufactured by Merck Sharp & Dohme LLC. However, Merck was not involved in the planning or execution of this study. D.V. and B.S. have a patent granted for the use of A/equine/Kentucky/91 for use as a universal influenza vaccine and are developing it for use for our company Syntherna. J.E.C. has served as a consultant for Luna Labs USA, Merck Sharp & Dohme Corporation, Emergent Biosolutions, a former member of the Scientific Advisory Boards of Gigagen (Grifols), of Meissa Vaccines, and BTG International, is founder of IDBiologics and receives royalties from UpToDate. The laboratory of J.E.C. received unrelated sponsored research agreements from AstraZeneca, Takeda Vaccines, and IDBiologics during the conduct of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Verhoeven, D., Sponseller, B.A., Crowe, J.E. et al. Use of equine H3N8 hemagglutinin as a broadly protective influenza vaccine immunogen. npj Vaccines 9, 247 (2024). https://doi.org/10.1038/s41541-024-01037-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-024-01037-1