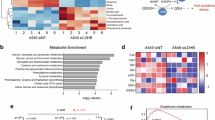
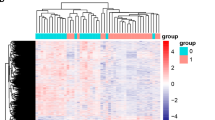
Abstract
The aberrant accumulation of intracellular disulfides promotes cancer cell disulfidptosis; however, how disulfide stress influences tumour-infiltrating CD8+ T cell function remains unknown. Here we demonstrate that lactate dehydrogenase B (LDHB) facilitates intratumoural CD8+ T cell disulfidptosis and exhaustion, leading to impaired antitumour immunity. SLC7A11-mediated cystine uptake by CD8+ T cells induces disulfidptosis, which plays critical roles in the development of exhausted CD8+ T cells. LDHB restricts glucose-6-phosphate dehydrogenase (G6PD) activity in exhausted CD8+ T cells by interacting with G6PD, causing NADPH depletion and consequently triggering disulfidptosis. Accordingly, the loss of LDHB in T cells prevents disulfidptosis-dependent CD8+ T cell exhaustion and improves antitumour immunity. Mechanistically, STAT3 directs LDHB expression to limit G6PD activity and mediate disulfidptosis in exhausted CD8+ T cells. Our results highlight the distinct roles of disulfidptosis and ferroptosis in driving CD8+ T cell exhaustion and suggest a potential therapeutic strategy to target LDHB in cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq data that support the findings of this study have been deposited in the Genome Sequence Archive in National Genomics Data Center under accession codes CRA018798 and CRA022687. ATAC-seq data that support the findings of this study have been deposited in the Genome Sequence Archive in National Genomics Data Center under accession codes CRA018765 and CRA022688. The processed CRC public scRNA-seq datasets were collected from different databases, including Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/, GSE178341, GSE164522 and GSE146771), GSA (https://ngdc.cncb.ac.cn/gsa-human/, HRA000979) and synapse (https://www.synapse.org/, syn26844071), which includes count matrices from five cohorts (SMC, CRC-SG1, CRC-SG2, KUL3 and KUL5). The processed LC public scRNA-seq dataset was downloaded from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE179994). The RCC public scRNA-seq dataset was downloaded from European Genome-phenome Archive (EGA, https://ega-archive.org/studies/EGAS00001002325). The pan-cancer scRNA-seq CD8+ T cells dataset with three cancer types (CRC, LC and RCC) was downloaded from an online data browser (http://cancer-pku.cn:3838/PanC_T). Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding authors on reasonable request.
References
Hayes, J. D., Dinkova-Kostova, A. T. & Tew, K. D. Oxidative stress in cancer. Cancer Cell 38, 167–197 (2020).
Cheung, E. C. & Vousden, K. H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 22, 280–297 (2022).
Harris, I. S. & DeNicola, G. M. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 30, 440–451 (2020).
Liu, X., Zhuang, L. & Gan, B. Disulfidptosis: disulfide stress-induced cell death. Trends Cell Biol. 34, 327–337 (2024).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Koppula, P., Zhuang, L. & Gan, B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12, 599–620 (2021).
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Liu, X. et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat. Cell Biol. 25, 404–414 (2023).
Hu, Z. et al. Acylglycerol kinase maintains metabolic state and immune responses of CD8+ T cells. Cell Metab. 30, 290–302 (2019).
Zhang, Y. et al. Dietary fructose-mediated adipocyte metabolism drives antitumor CD8+ T cell responses. Cell Metab. 35, 2107–2118 (2023).
Philip, M. & Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 22, 209–223 (2022).
Cao, T. et al. Cancer SLC6A6-mediated taurine uptake transactivates immune checkpoint genes and induces exhaustion in CD8+ T cells. Cell 187, 2288–2304 (2024).
Park, J., Hsueh, P. C., Li, Z. & Ho, P. C. Microenvironment-driven metabolic adaptations guiding CD8+ T cell anti-tumor immunity. Immunity 56, 32–42 (2023).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Quinn, W. J. 3rd et al. Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep. 33, 108500 (2020).
Ma, X. et al. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 33, 1001–1012 (2021).
Xu, S. et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity 54, 1561–1577 (2021).
Zheng, L. et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 374, abe6474 (2021).
Liao, P. et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 40, 365–378 (2022).
Zhang, H. L. et al. PKCbetaII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat. Cell Biol. 24, 88–98 (2022).
Hwang, S. et al. Correcting glucose-6-phosphate dehydrogenase deficiency with a small-molecule activator. Nat. Commun. 9, 4045 (2018).
Li, M. et al. Aldolase B suppresses hepatocellular carcinogenesis by inhibiting G6PD and pentose phosphate pathways. Nat. Cancer 1, 735–747 (2020).
TeSlaa, T., Ralser, M., Fan, J. & Rabinowitz, J. D. The pentose phosphate pathway in health and disease. Nat. Metab. 5, 1275–1289 (2023).
Kristensen, A. R., Gsponer, J. & Foster, L. J. A high-throughput approach for measuring temporal changes in the interactome. Nat. Methods 9, 907–909 (2012).
Young, M. D. et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 361, 594–599 (2018).
Zhang, L. et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181, 442–459 (2020).
Pelka, K. et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184, 4734–4752 (2021).
Joanito, I. et al. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat. Genet. 54, 963–975 (2022).
Liu, B. et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat. Cancer 3, 108–121 (2022).
Liu, Y. et al. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell 40, 424–437 e425 (2022).
Qi, J. et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer. Nat. Commun. 13, 1742 (2022).
Wei, X., Kixmoeller, K., Baltrusaitis, E., Yang, X. & Marmorstein, R. Allosteric role of a structural NADP+ molecule in glucose-6-phosphate dehydrogenase activity. Proc. Natl Acad. Sci. USA 119, e2119695119 (2022).
Peng, M. et al. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354, 481–484 (2016).
Hu, Z. et al. Glycolysis drives STING signaling to facilitate dendritic cell antitumor function. J. Clin. Invest. 133, e166031 (2023).
Xu, K. et al. Glycolytic ATP fuels phosphoinositide 3-kinase signaling to support effector T helper 17 cell responses. Immunity 54, 976–987 e977 (2021).
Xu, K. et al. Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science 371, 405–410 (2021).
Ma, J. et al. Lithium carbonate revitalizes tumor-reactive CD8+ T cells by shunting lactic acid into mitochondria. Nat. Immunol. 25, 552–561 (2024).
McLane, L. M., Abdel-Hakeem, M. S. & Wherry, E. J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev. Immunol. 37, 457–495 (2019).
Sun, Q. et al. STAT3 regulates CD8+ T cell differentiation and functions in cancer and acute infection. J. Exp. Med. 220, e20220686 (2023).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (grant no. 82225020, Q.Z.; grant no. 82430054, Q.Z.), the National Key Research and Development Program of China (grant no. 2020YFA0803603, Q.Z.; grant no. 2021YFA1301402, Q.Z.) and the Baoding Innovation Capability Enhancement Project (grant no. 2494F019, J.-H.L.).
Author information
Authors and Affiliations
Contributions
J.W., J.-H.S., M.S. and H.H. designed and performed the experiments, prepared the figures and wrote the paper; Z.Z., W.L., C.G., R.B., X.Y., Q.H., X.D., S.L. and Y.Y. contributed to the performance of the experiments, X.C., X.L., J.-H.L. and Q.Z. supervised the work and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Boyi Gan, Hazem Ghoneim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 T cell development of Slc7a11fl/+Cd8-Cre and Slc7a11fl/+Cd4-Cre mice.
a, Flow cytometric analysis of the percentage of PD-1+Tim-3+ and IFN-γ+ CD8+ T cells in the exhaustion-induced condition in vitro in the presence of 5 mM disulfidptosis inhibitor (TCEP) at day 2, 4, 6 and 8 (n = 4). b, Flow cytometric analysis of the percentage of CD4+ and CD8+ T cells in the thymus, spleen and lymph node of Slc7a11+/+Cd8-Cre, Slc7a11fl/+Cd8-Cre and Slc7a11fl/flCd8-Cre mice (n = 3). c, Flow cytometric analysis of the percentage of CD4+ and CD8+ T cells in the thymus, spleen and lymph node of Slc7a11+/+Cd4-Cre and Slc7a11fl/+Cd4-Cre mice (n = 3). d, Flow cytometric analysis of the expressions of CD44 and CD62L in CD4+ and CD8+ T cells in the spleen of Slc7a11+/+Cd4-Cre and Slc7a11fl/+Cd4-Cre mice (n = 3). Data were presented as mean ± SEM. P values were calculated by two-tailed Student’s t test (a, c, d) or one-way ANOVA with Dunnett’s multiple comparison (b); ns, no statistically significance. In a-d, n indicates the number of biological replicates. For a-d, three independent experiments were performed.
Extended Data Fig. 2 T cell activity of Slc7a11fl/+Cd8-Cre and Slc7a11fl/+Cd4-Cre T cells.
a, The percentage of PD-1+Tim-3+ and IFN-γ+ cells of CD8+ T cells in exhaustion-induced condition in vitro at day 2, 4, 6 and 8 (n = 4). b, Actin cytoskeleton protein in exhausted Slc7a11+/+Cd8-Cre (labelled as 1), Slc7a11fl/+Cd8-Cre (labelled as 2) and Slc7a11fl/flCd8-Cre (labelled as 3) CD8+ T cells at day 2, 4, 6 and 8. c, FLNA in exhausted Slc7a11+/+Cd8-Cre (WT), Slc7a11fl/+Cd8-Cre (Her) and Slc7a11fl/flCd8-Cre (KO) CD8+ T cells. d, Actin cytoskeleton protein in exhausted Slc7a11+/+Cd4-Cre (WT) and Slc7a11fl/+Cd4-Cre (Her) CD8+ T cells. e, Intracellular cystine level (n = 4) and PD-1+Tim-3+ cell percentage (n = 3) of Slc7a11+/+Cd4-Cre (WT) and Slc7a11fl/+Cd4-Cre (Her) CD8+ T cells in exhaustion induced condition in vitro. f, The percentage of TNF-ɑ+ and IFN-γ+ in Slc7a11+/+Cd4-Cre and Slc7a11fl/+Cd4-Cre splenic CD4+ and CD8+ T cells stimulated with anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) for 48 hours (n = 4). g, Tumor size of Slc7a11+/+Cd4-Cre (WT) and Slc7a11fl/+Cd4-Cre (Her) mice injected s.c. with 5 × 105 MC38 colon cancer cells (n = 6). h, The percentage of tumor-infiltrating PD-1+Tim-3+, TNF-ɑ+, IFN-γ+ CD8+ T cells (n = 6) and IFN-γ+ (n = 6), Foxp3+ (n = 7), CXCR5+PD-1+ (n = 6) CD4+ T cells in tumors of Slc7a11+/+Cd4-Cre and Slc7a11fl/+Cd4-Cre mice injected with MC38 cells (day 16). i, Tumor growth of Slc7a11+/+Cd4-Cre (WT) and Slc7a11fl/+Cd4-Cre (Her) mice injected with MC38 cells. These mice were treated (i.p.) with 200 mg anti-CD4 (ɑ-CD4) or control antibody (IgG) every three days for five times (n = 6). Data were presented as mean ± SEM. P values were calculated by two-tailed Student’s t test (e, f, h), one-way ANOVA with Dunnett’s multiple comparison (a) or two-way ANOVA with Geisser-Greenhouse correction (g, i); ns, no statistically significance. In a, e-f, n indicates the number of biological replicates. In g-i, n indicates the number of mice. For a-i, three independent experiments were performed.
Extended Data Fig. 3 T cell development of G6pdfl/flCd4-Cre mice.
a, Flow cytometric analysis of the percentage of CD4+ and CD8+ T cells in the thymus, spleen and lymph node of G6pd+/+Cd4-Cre (WT) and G6pdfl/flCd4-Cre (KO) mice (n = 3). b, Flow cytometric analysis of the cell survival of G6pd+/+Cd4-Cre (WT) and G6pdfl/flCd4-Cre (KO) CD8+ T cells stimulated in vitro for 48 hours. c, Flow cytometric analysis of the percentage of PD-1+Tim-3+ cells of Slc7a11+/+Cd8-Cre (WT), Slc7a11fl/+Cd8-Cre (Her), TCEP-treated WT and Her CD8+ T cells in exhaustion-induced condition in vitro (n = 4). Data were presented as mean ± SEM. P values were calculated by two-tailed Student’s t test (a) or one-way ANOVA with Tukey’s multiple comparisons (c). ns, no statistically significance. In a,c, n indicates the number of biological replicates. For a-c, three independent experiments were performed.
Extended Data Fig. 4 T cell development of Ldhbfl/flCd4-Cre mice.
a, IP and IB analysis of the interaction of G6PD protein with LDHB protein in WT exhausted CD8+ T cells. b, Signature score of T cell exhaustion-associated gene set in CRC and RCC-infiltrating LDHBhigh (CRC, n = 27262; RCC n = 6529) and LDHBlow (CRC, n = 22246; RCC n = 5396) CD8+ T cells. The boxes in a show the median ±1 quartile, with the whiskers extending from the hinge to the smallest or largest value within 1.5× the interquartile range from the box boundaries. c, RT-qPCR analysis of the expression levels of CD8+ T cell exhaustion signature genes in RCC-infiltrating CD8+ T cells (n = 7). The lowest expression level of LDHB in RCC-infiltrating CD8+ T cells was normalized to 1. LDHBhigh group, higher than 1.2; LDHBlow group, less than 1.2. d, IB analysis of LDHB protein level in Ldhb+/+Cd4-Cre and Ldhbfl/flCd4-Cre CD8+ T cells. e, Flow cytometric analysis of the percentage of CD4+ and CD8+ T cells in the thymus, spleen and lymph node of Ldhb+/+Cd4-Cre and Ldhbfl/flCd4-Cre mice (n = 3). f, Flow cytometric analysis of the percentage of tumor-infiltrating CD4+ T cells in tumors of Ldhb+/+Cd4-Cre and Ldhbfl/flCd4-Cre mice injected s.c. with MC38 colon cancer cells (day 16; IFN-γ+, n = 5; Foxp3+, n = 6; CXCR5+PD-1+, n = 7). Data were presented as mean ± SEM. P values were calculated by two-sided Wilcoxon rank-sum test (b) or two-tailed Student’s t test (a,c,e,f). ns, no statistically significance. In b, n indicates the number of CD8+ T cells. In c, n indicates the number of human samples. In e, n indicates the number of biological replicates. In f, n indicates the number of mice. For a, d-f, three independent experiments were performed.
Extended Data Fig. 5 LDHB-G6PD axis in exhausted CD8+ T cells.
a, Native-PAGE analysis of the G6PD dimmer and G6PD monomer proteins in exhausted Ldhb+/+Cd4-Cre (WT) and Ldhbfl/flCd4-Cre (KO) CD8+ T cells. b, Heatmap of glycolysis-related metabolites in exhausted Ldhb+/+Cd4-Cre (WT) and Ldhbfl/flCd4-Cre (KO) CD8+ T cells (n = 3). c, IB analysis of LDHB protein levels in the cytosol and membrance of exhausted CD8+ T cells. Data were independently repeated 3 times (a,c). In b, n indicates the number of biological replicates. For a, c, three independent experiments were performed.
Extended Data Fig. 6 RNA-seq and ATAC-seq of exhausted Ldhbfl/flCd4-Cre CD8+ T cells.
a, Principal component analysis (PCA) of RNA-seq (upper panel) and ATAC-seq (lower panel) of exhausted Ldhb+/+Cd4-Cre and Ldhbfl/flCd4-Cre CD8+ T cells induced in vitro. b,c, Heatmap showing the differentially regulated gene expression (b) and peaks (c) among exhausted Ldhb+/+Cd4-Cre (WT) and Ldhbfl/flCd4-Cre (KO) CD8+ T cells (n = 3). d, qPCR analysis of the expression level of Ldhb in naïve (Tn) and exhausted (Tex) CD8+ T cells (n = 4). e, IB analysis of the protein level of LDHB in naïve (Tn) and exhausted (Tex) CD8+ T cells. Data were independently repeated 3 times (d,e). Data were presented as mean ± SEM and P values were calculated by two-tailed Student’s t test (d). In b-d, n indicates the number of biological replicates. For d, e, three independent experiments were performed.
Supplementary information
Supplementary Table 1
Primer sequences used in this Article.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data
Unprocessed western blots and gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, J., Shi, JH., Shi, M. et al. Lactate dehydrogenase B facilitates disulfidptosis and exhaustion of tumour-infiltrating CD8+ T cells. Nat Cell Biol 27, 972–982 (2025). https://doi.org/10.1038/s41556-025-01673-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41556-025-01673-2
This article is cited by
-
Exploiting metabolic cell death for cancer therapy
Nature Reviews Cancer (2026)
-
Disulfidptosis in cancer: from redox stress to therapeutic strategy
Cancer Gene Therapy (2026)
-
Comprehensive analysis of disulfidptosis-related genes identifies clinically actionable prognostic biomarkers in cholangiocarcinoma
Discover Oncology (2026)
-
Therapeutic targeting of cell death-immune crosstalk in cancer to rewire the tumor immune microenvironment
Molecular Cancer (2025)
-
Engineered iron oxide nanoplatforms: reprogramming immunosuppressive niches for precision cancer theranostics
Molecular Cancer (2025)