Abstract
Hepatocellular carcinoma (HCC) is accompanied by an altered gut microbiota but whether the latter contributes to carcinogenesis is unclear. Here we show that faecal microbiota transplantation (FMT) using stool samples from patients with HCC spontaneously initiate liver inflammation, fibrosis and dysplasia in wild-type mice, and accelerate disease progression in a mouse model of HCC. We find that HCC-FMT results in gut barrier injury and translocation of live bacteria to the liver. Metagenomic analyses and bacterial culture of liver tissues reveal enrichment of the gut pathogen Klebsiella pneumoniae in patients with HCC and mice transplanted with the HCC microbiota. Moreover, K. pneumoniae monocolonization recapitulates the effect of HCC-FMT in promoting liver inflammation and hepatocarcinogenesis. Mechanistically, K. pneumoniae surface protein PBP1B interacts with and activates TLR4 on HCC cells, leading to increased cell proliferation and activation of oncogenic signalling. Targeting gut colonization using K. oxytoca or TLR4 inhibition represses K. pneumoniae-induced HCC progression. These findings indicate a role for an altered gut microbiota in hepatocarcinogenesis.
Similar content being viewed by others
Main
Hepatocellular carcinoma (HCC) is a leading cause of cancer-associated deaths1. HCC proceeds via a multistep cascade involving liver inflammation, fibrosis, cirrhosis and HCC2. Beyond host factors, gut dysbiosis plays important roles in HCC pathogenesis3. Recent studies have also unravelled the existence of an intratumoural microbiome4 that promotes tumorigenesis and modifies the immune microenvironment5,6,7,8. However, the function of gut and intrahepatic microbes in HCC pathogenesis remains largely unclear.
The gut microbiome regulates liver homoeostasis via the gut–liver axis9,10. The gut barrier is the first line of defence against deleterious gut pathogens and their products, and a leaky gut might enable transfer of gut microbes to the liver in disease states11. Intriguingly, germ-free mice displayed delayed HCC progression due to reduced activation of Toll-like receptor 4 (TLR4)12, a sensor of bacterial infection. This highlights the possibility that gut dysbiosis may contribute to hepatocarcinogenesis.
Here we sought to establish the causality of HCC-associated gut dysbiosis in promoting HCC. We performed faecal microbiota transplantation (FMT) of microbial consortia from patients with HCC and healthy donors to mice, revealing that HCC-FMT promotes inflammation, fibrosis, dysplasia and HCC. HCC-associated gut microbiota disrupts gut barrier function and promotes translocation of live bacteria to the liver, especially Klebsiella pneumoniae (K. pneumoniae). K. pneumoniae monocolonization studies in mice validated its causative role in HCC. Mechanistically, HCC-FMT and K. pneumoniae activate hepatic inflammatory and oncogenic signalling. Corroborating these results, live K. pneumoniae is enriched in liver tissues of individuals with HCC. Altogether, we revealed a causative role of gut dysbiosis in hepatocarcinogenesis.
Results
HCC stool accelerates DEN-induced liver tumorigenesis
We initially investigated the effect of polymicrobial consortia transplantation from stool of patients with HCC or liver cirrhosis (LC) and healthy donors on liver pathologies in germ-free and specific pathogen-free (SPF) mice. 16S ribosomal (r)RNA sequencing revealed a progressive decrease in microbial diversity in stool samples from healthy donors, LC to HCC, as determined by Shannon, Chao1 and Ace indices (Supplementary Fig. 1a). Klebsiella was highly enriched in stool samples from individuals with HCC (Supplementary Fig. 1a). We observed accurate recapitulation of gut microbiome differences of donor consortia in germ-free mice stool after FMT treatment (Supplementary Fig. 1b,c), as evidenced by reduced microbial diversity and enrichment of Klebsiella in mice receiving HCC-FMT compared with other groups.
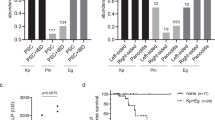
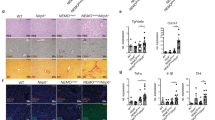
Faecal microbiota consortia were transplanted from 15 HCC (GFD/HCC-FMT), 17 LC (GFD/LC-FMT) and 16 healthy donors (GFD/HD-FMT) to diethylnitrosamine (DEN)-injected germ-free (GFD) mice once per week (Fig. 1a), with PBS as blank control. Compared with the other groups, HCC-FMT increased maximum tumour diameter (P = 0.007 vs LC-FMT; P < 0.0001 vs HD-FMT), tumour number (P = 0.0001 vs HD-FMT) and tumour burden (P = 0.0012 vs LC-FMT; P < 0.0001 vs HD-FMT) (Fig. 1b and Supplementary Fig. 2a), indicating that HCC-associated microbes promote tumorigenesis. Immunohistochemistry (IHC) showed that HCC-FMT induced hepatocyte proliferation (PCNA, Ki-67) (Fig. 1c and Supplementary Fig. 2b) compared with LC-FMT and HD-FMT. Meanwhile, HCC-FMT and LC-FMT similarly induced liver inflammation, with increased pro-inflammatory T helper 1 (TH1) and TH17 cells, together with reduced anti-inflammatory TH2 compared with HD-FMT (Supplementary Fig. 2c). Fibrosis markers desmin, α-smooth muscle actin (α-SMA) and Sirius red staining were also increased by HCC-FMT and LC-FMT (Supplementary Fig. 2d). Cancer pathway PCR array revealed that HCC-FMT upregulated genes involved in cell proliferation (G6pdx, Skp2, Pgf, and Sox10), angiogenesis and embryonic development (Angpt2 and Gsc), epithelial–mesenchymal transition (Foxc2 and Snai1) and anti-apoptosis (Epo) compared with LC-FMT and HD-FMT (Fig. 1d). Inflammatory response and autoimmunity PCR array showed that pro-inflammatory response (Tlr4, Tnfsf14, Il6ra, Ccl4, andTlr2) and lymphocyte chemotaxis (Ccr2 and Cxcr2) were increased by HCC-FMT compared with LC-FMT and HD-FMT groups (Fig. 1e).
a, Design of FMT experiment for DEN-treated germ-free mice (GFD/PBS group n = 8; GFD/HD-FMT group n = 16; GFD/LC-FMT group n = 17; GFD/HCC-FMT group n = 15) and relevant results (b–e). b, Representative images of liver gross morphology (dashed yellow circles indicate tumour) with tumour incidence, tumour number and tumour burden. n = 8 (GFD/PBS), 16 (GFD/HD-FMT), 17 (GFD/LC-FMT) and 15 (GFD/HCC-FMT) mice. c, Immunohistochemistry (IHC) staining for PCNA and Ki-67. n = 6 biologically independent samples. d, Mouse cancer pathway finder array of liver tissues. e, Mouse inflammatory response and autoimmunity PCR array of liver tissues. In d and e, n = 3 independent experiments with similar results. f, Design of FMT experiment for DEN-treated SPF mice (SPFD/PBS group n = 20; SPFD/HD-FMT group n = 16; SPFD/LC-FMT group n = 16; SPFD/HCC-FMT group n = 16) and relevant results (g–j). g, Representative images of liver gross morphology (yellow circles indicate tumour) with tumour incidence, tumour number and tumour burden. n = 20 (SPFD/PBS), 16 (SPFD/HD-FMT), 16 (SPFD/LC-FMT), 16 (SPFD/HCC-FMT). h, IHC staining for PCNA and Ki-67. n = 6 biologically independent samples. i, Mouse cancer pathway finder array of liver tissues. n = 3 biologically independent samples of each group were mixed for assay. j, Mouse inflammatory response and autoimmunity PCR array of liver tissues. In i and j, n = 3 independent experiments with similar results. In b,c,g,h, data are presented as mean ± s.e.m. Each data point in bar plots represents one mouse. Statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
The protumorigenic effect of HCC-FMT compared with LC-FMT and HD-FMT were recapitulated in DEN-injected SPF mice (Fig. 1f–j and Supplementary Fig. 3a–d) and validated by another mixed stool consortium from HCC (N = 5) and healthy donors (N = 5) in DEN-injected germ-free mice (Extended Data Fig. 1a–d). Furthermore, individual FMT from healthy donors (N = 2) and patients with HCC (N = 2) to DEN-injected germ-free mice again showed that HCC-FMT accelerated HCC progression (Extended Data Fig. 1e–h). Together, faecal transplantation of HCC microbiota to mice accelerates DEN-induced HCC progression.
HCC stool promotes inflammation, fibrosis and dysplasia
Next, we performed FMT of polymicrobial consortia from 16 patients with HCC (GF/HCC-FMT) and 12 healthy donors (GF/HD-FMT) to germ-free mice without DEN injection (Extended Data Fig. 2a). Upon killing, mice receiving HCC-FMT displayed liver nodules (in 9 out of 16 mice) that were absent from HD-FMT and PBS groups (P < 0.0001) (Extended Data Fig. 2b). Haematoxylin and eosin (H&E) staining confirmed diagnosis of dysplasia in the HCC-FMT group (Extended Data Fig. 2b). Compared with HD-FMT, HCC-FMT increased hepatocyte proliferation (PCNA and Ki-67) (Extended Data Fig. 2c), hepatic inflammation (TH1, TH17 and TH2) (Extended Data Fig. 2d) and liver fibrosis markers (desmin, α-SMA and Sirius red) (Extended Data Fig. 2e). Cancer pathway PCR array revealed that HCC-FMT upregulated genes involved in cell proliferation (Cdc20 and Dkc1), angiogenesis (Angpt2), epithelial–mesenchymal transition (Foxc2 and Snai2) and anti-apoptosis (Nol3) (Extended Data Fig. 2f). Inflammatory response and autoimmunity PCR array also showed that pro-inflammatory response (Tlr4, Myd88, Nfkb1 and Il1a) and lymphocyte chemotaxis (Ccl12, Ccl11 and Ccl7) were increased by HCC-FMT (Extended Data Fig. 2g). Concordant results were obtained by FMT of HCC stool to SPF mice (Extended Data Fig. 3a–f) and of independent stool consortia from individuals with HCC (N = 5) and healthy donors (N = 5) to germ-free mice (Extended Data Fig. 4a–f). Collectively, HCC-FMT spontaneously induces liver inflammation, fibrosis and dysplasia in mice.
HCC stool promotes gut leakage and pathogen translocation
Gut dysbiosis modulates liver pathologies via the gut–liver axis13. We examined the effect of FMT treatment on gut barrier integrity and demonstrated that HCC-FMT increased gut permeability to 500 kDa FITC-dextran (without DEN: P < 0.0001; with DEN: P = 0.0024) compared with HD-FMT and PBS in germ-free mice (Extended Data Fig. 5a,b). Transmission electron microscopy (TEM) confirmed abnormalities of colon intercellular junctions in HCC-FMT, with disappearance of tight junctions and widening of adherens junctions (Fig. 2a and Supplementary Fig. 4a). HCC-FMT reduced the thickness of colonic mucus and downregulated the expression of tight junction markers (claudin 3 (CLDN3), CLDN1 and E-cadherin (E-cad)), leading to increased bacterial infiltration into mucosal lamina propria and muscularis mucosa (Extended Data Fig. 5a,b, Fig. 2a and Supplementary Fig. 4a). As a result, HCC-FMT induced inflammation in colon, as evidenced by PCR array and flow cytometry (Extended Data Fig. 5c,d). These suggest that HCC-FMT disrupts the gut barrier and promotes bacterial infiltration into colonic mucosa.
a, Quantitative analysis of TEM, Alcian blue staining, E-cad IHC staining and Cy3-conjugated EUB338 probe FISH of colon tissues. n = 6 biologically independent samples. b, Quantitative analysis of bacterial culture of liver tissues under anaerobic and aerobic conditions with quantitative analysis. n = 8 (GFD/PBS), 16 (GFD/HD-FMT), 17 (GFD/LC-FMT), 15 (GFD/HCC-FMT), 6 (GF/PBS), 12 (GF/HD-FMT), 16 (GF/HCC-FMT) mice. c, Representative TEM images of colon tissues and liver tissues in GFD/HCC-FMT mice and representative TEM image of liver tissues in GF/HCC-FMT mice. n = 3 independent experiments with similar results. d, Stacked bar plot of relative abundance of culturable bacterial strains as determined by microbial mass spectrometry identification. e, Absolute number of K. pneumoniae (KP) in human faeces (left), mice faeces (middle) and mice liver tissues (right) as detected by qPCR. n = 10 biologically independent samples. f, Representative images (left) and quantification (right) of Cy3-conjugated K. pneumoniae probe FISH detection in mice liver tissues. n = 6 biologically independent samples. In a (excluding tight junction disappear rate), b (excluding liver with living bacteria) and f, data are presented as mean ± s.e.m. Each data point in bar plots represents one mouse. Tight junction disappearance rate and liver with living bacteria were analysed using Fisher’s exact test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
We next asked whether disrupted gut barrier function might enable translocation of gut microbes to the liver. Anaerobic and aerobic culture of fresh liver homogenates from germ-free mice demonstrated that almost all liver samples from HCC-FMT displayed positive culture, while those from the HD-FMT group were largely negative (P < 0.0001) (Fig. 2b, Extended Data Fig. 5e and Supplementary Fig. 4b). Cultures of the PBS group and contamination controls all tested negative (Fig. 2b, Extended Data Fig. 5f and Supplementary Fig. 4b). Fluorescence in situ hybridization (FISH) (universal 16S rRNA) confirmed that bacteria were readily detected in livers of the HCC-FMT group but were absent in the HD-FMT (close to none) and PBS groups (Extended Data Fig. 5g). TEM of colon and hepatic tissues confirmed the presence of bacteria in HCC-FMT-treated germ-free mice (Fig. 2c). Corroborating these results, gut barrier disruption and translocation of live bacteria to the liver was validated in SPF mice with HCC-FMT (Extended Data Fig. 6a–c), germ-free mice transplanted with another mixed stool consortium from individuals with HCC (N = 5) and healthy donors (N = 5) (Extended Data Fig. 7a–h), and individual HCC-FMT (N = 2) and HD-FMT (N = 2) (Extended Data Fig. 7i–k). Notably, LC-FMT evidently caused gut barrier dysfunction and bacterial translocation to the liver, consistent with the involvement of gut dysbiosis in liver pathologies.
To decipher the live bacterial composition in the liver, colonies were randomly selected from liver tissues for identification (Fig. 2d). K. pneumoniae was the top bacterium enriched in the HCC-FMT group compared with HD-FMT, followed by Enterococcus faecalis, Proteus mirabilis, Enterobacter hormaechei and Bacteroides fragilis (Fig. 2d). Absolute quantification by qPCR in human donors and recipient mouse samples demonstrated that faecal K. pneumoniae was increased in individuals with HCC compared with those with LC and healthy donors, and a consistent pattern was reproduced in the stool of FMT recipient mice (Fig. 2e). Correspondingly, liver K. pneumoniae levels were higher in the HCC-FMT group compared with LC-FMT and HD-FMT groups (Fig. 2e), which was confirmed by FISH using a K. pneumoniae-specific probe (Fig. 2f). Together, HCC-associated gut dysbiosis disrupts gut barrier function, thereby promoting translocation of bacteria to the liver and enrichment of intrahepatic K. pneumoniae.
Live K. pneumoniae is enriched in liver tissue of patients with HCC
We next sought to validate our discoveries in human patients. Universal 16S rRNA FISH of tissue arrays revealed the enrichment of bacteria in tumorous compared with the paracancerous region in patients with HCC (P < 0.0001) (N = 45) (Supplementary Fig. 5a). Anaerobic and aerobic culture of fresh liver homogenates from 54 individuals with HCC and 15 with LC demonstrated a living microbiome in HCC and LC tissues, which were confirmed by FISH, lipopolysaccharide (LPS) and lipoteichoic acid (LTA) staining (Fig. 3a,b). Mass spectrometry identification of randomly picked live bacterial colonies and 16S rRNA sequencing (Fig. 3c,d) revealed that Klebsiella (K. pneumoniae) dominates the liver microbiome of individuals with HCC compared with LC, which was supported by absolute quantification (P = 0.0002) and K. pneumoniae-specific FISH probe (P < 0.0001) (Fig. 3e,f). Finally, we demonstrated that upregulation of faecal K. pneumoniae in individuals with HCC negatively correlated with overall survival and progression-free survival of HCC patients (Supplementary Fig. 5b). These results implicate a living hepatic microbiome in individuals with HCC with preferential enrichment of K. pneumoniae.
a, Top: representative images of bacterial culture of liver tissues from patient with LC under anaerobic and aerobic conditions. N = 15 biologically independent samples. From left to right and top to bottom, the culture plates are blood agar plates, chocolate blood agar plates, MacConkey agar plates and Columbia blood agar plates, respectively. Bottom: Cy3-conjugated EUB338 probe FISH detection, LPS and LTA IHC staining of liver tissues from patient with LC (n = 3 independent experiments with similar results). b, Top: representative images of bacterial culture of tumour tissues from patient with HCC under anaerobic and aerobic conditions. N = 54 biologically independent samples. From left to right and top to bottom, the culture plates are blood agar plates, chocolate blood agar plates, MacConkey agar plates and Columbia blood agar plates, respectively. Bottom: Cy3-conjugated EUB338 probe FISH detection, LPS and LTA IHC staining of HCC patient liver tissues (n = 3 independent experiments with similar results). c, Stacked bar plot of relative abundance of culturable bacterial strains from liver tissues from individuals with LC or with HCC as determined by microbial mass spectrometry identification. d, Stacked bar plot of relative abundance at genus level of bacteria from liver tissues from patients with LC or HCC as detected by 16S rRNA sequencing. N = 11 (LC), 35 (HCC). e, Absolute number of K. pneumoniae in liver tissues from patient with LC and HCC as detected by qPCR. n = 10 biologically independent samples. f, Representative images of Cy3-conjugated K. pneumoniae probe FISH detection in liver tissues from patient with LC (top) and HCC (bottom). n = 10 biologically independent samples. In f, data are presented as mean ± s.e.m. Each data point in bar plots represents one participant. Statistical significance was calculated using Student’s t-test.
K. pneumoniae initiates gut barrier disruption by gelatinase
To identify the driver bacterium in promoting HCC, we next selected 5 top enriched Gram-negative bacteria (K. pneumoniae, Bacteroides thetaiotaomicron (BT), Enterobacter hormaechei (EH), Escherichia coli (EC) and P. mirabilis (PM)) from liver tissues of HCC-FMT-treated mice and gavaged them to germ-free mice for 10 weeks (Fig. 4a). Compared with PBS control, only K. pneumoniae elevated gut permeability (P < 0.0001) and translocated to the liver as live bacteria (Fig. 4b). Moreover, K. pneumoniae disrupted colonic intercellular junctions, reduced thickness of colonic mucus, downregulated expression of tight junction proteins (E-cad, CLDN3 and CLDN1) and increased bacterial infiltration into mucosal lamina propria and muscularis mucosa (Fig. 4c and Extended Data Fig. 8a), while other candidates had no effect. This suggests that K. pneumoniae as a driver bacterium that disrupts the gut barrier to enable its translocation to the liver.
a, Design of 5 Gram-negative bacteria gavage in germ-free mice without DEN (PBS group n = 6; BT group n = 6; PM group n = 6; EC group n = 6; EH group n = 6; KP group n = 6) and related results (b,c). b, Gut permeability assays using 500 kDa FITC-dextran (top), live bacteria translocated into liver cultured in blood agar plates (middle) and Cy3-conjugated EUB338 probe FISH assay of liver tissues (bottom). n = 6 biologically independent samples. c, Quantitative analysis of TEM, Alcian blue staining, E-cad, CLDN3, CLDN1 IHC staining, and Cy3-conjugated EUB338 probe FISH of colon tissues. n = 10 biologically independent samples. d, THP-1-induced macrophage using phorbol ester (top row) and MMP-2/-9 activity in K. pneumoniae and THP-induced macrophage co-culture conditional medium using gelatin hydrolysis assay and gelatin zymography (bottom row). n = 3 independent experiments with similar results. Ctrl: BHI and macrophage co-culture conditional medium; EC: E. coli and macrophage co-culture conditional medium; KP: K. pneumoniae and macrophage co-culture conditional medium. e, Design of colon macrophage depletion using Clodrosome and its effect on K. pneumoniae-mediated gut barrier dysfunction in germ-free mice without DEN treatment (PBS group n = 6; KP group n = 6; KPC group n = 6) and related results (f–h). f, In vivo imaging of body and liver after Cy5.5-d-Lys-labelled K. pneumoniae gavage (top), and live bacterial culture of liver tissues on blood agar plates and quantification (bottom). n = 6 biologically independent samples. g, Gut permeability assays using 500 kDa FITC-dextran (top left) (n = 6 biologically independent samples) and MMP-2/-9 activity in mouse faecal supernatant detected using gelatin hydrolysis assay (top middle), gelatin zymography (top right) (n = 3 independent experiments with similar results) and dynamic activity assay (bottom) (n = 6 biologically independent samples). h, Quantitative analysis of TEM, Alcian blue staining, E-cad and CLDN1 IHC staining, bacteria number per slide using Cy3-conjugated EUB338 probe (PBS group) and K. pneumoniae probe (KP and KPC groups) FISH of colon tissues. n = 10 biologically independent samples. In b, c (excluding tight junction disappear rate) and f–h (excluding liver with live bacteria and tight junction disappear rate) data are presented as mean ± s.e.m. Each data point in bar plots represents one mouse. Liver with living bacteria and tight junction disappearance rate were analysed using Fisher’s exact test. MMP activity was analysed using two-way ANOVA. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Breakdown of extracellular matrix by gelatinase is the initial step in the invasion of pathogens into gut mucosa14. We thus assessed faecal gelatinase activities in germ-free mice treated with individual bacteria. Only faecal supernatants from the K. pneumoniae group liquefied gelatin, suggesting gelatinase activity (Supplementary Fig. 6a). Gelatin zymography indicated Collagenase IV activity, as evidenced by clear bands at 72 kDa and 92 kDa (Supplementary Fig. 6b). Matrix metallopeptidase (MMP) activity assays validated the elevated MMP-2/-9 (type IV collagenases) activities in faecal supernatant from mice treated with K. pneumoniae (Supplementary Fig. 6c). Consistent with in vitro data, Masson’s trichrome staining revealed collagen depletion in colonic mucosa of K. pneumoniae-gavaged germ-free mice compared with other bacteria or PBS, which was confirmed by IHC of collagen type IV (COLIV) and fibronectin (Supplementary Fig. 6d). In this connection, faecal supernatants from individuals with HCC and LC exhibited increased gelatinase activity (Supplementary Fig. 7a), as did faecal supernatants from HCC-FMT- and LC-FMT-treated mice (Supplementary Fig. 7b). The direct effect of MMP-2/-9 in attenuating gut barrier markers (COLIV, fibronectin, E-cad and ZO-1) was demonstrated in normal NCM460 cells (Supplementary Fig. 7c). Hence, K. pneumoniae and HCC-FMT disrupt colonic barrier function by promoting gelatinase activity.
Interestingly, K. pneumoniae-conditioned medium lacks gelatinase activity (Supplementary Fig. 8a–c), implying an indirect effect of this bacterium. Macrophages are known to secrete MMP-2/-9 (refs. 15,16), which could damage gut barrier integrity17. We thus asked whether K. pneumoniae might boost macrophage-derived MMP-2/-9. Supporting our notion, in vitro co-culture of THP-1-induced macrophages with K. pneumoniae-conditioned medium induced MMP-2/-9 activity, as assessed by gelatin hydrolysis assay and gelatin zymography assays (Fig. 4d). To test this hypothesis in vivo, we depleted macrophages in germ-free mice using Clodrosome, followed by gavage of K. pneumoniae (Fig. 4e–h and Extended Data Fig. 8b–d). Before collection, we gavaged these mice with Cy5.5-d-Lys-labelled K. pneumoniae (Supplementary Fig. 9a) to trace its fate in vivo. While the K. pneumoniae-only group showed positive fluorescence and culture in liver tissues with 100% incidence, Clodrosome pretreatment reduced intrahepatic K. pneumoniae (2 out of 6) (Fig. 4f). K. pneumoniae-specific FISH confirmed that K. pneumoniae levels were reduced in livers of macrophage-depleted mice (Extended Data Fig. 8c). Macrophage depletion also alleviated K. pneumoniae-promoted gut barrier dysfunction, gelatinase activity (Fig. 4g), collagen degradation (Supplementary Fig. 9b), disruption of colonic intercellular junctions and bacterial infiltration into gut mucosa (Fig. 4h, Extended Data Fig. 8d and Supplementary Fig. 9c). Consequently, Clodrosome relieved K. pneumoniae-induced liver pathologies (Supplementary Fig. 9d,e) and oncogenic and pro-inflammatory signalling (Supplementary Fig. 9f,g). Overall, K. pneumoniae initiates gut barrier disruption by elevating macrophage-mediated gelatinase activity, which facilitates its eventual translocation to the liver.
K. pneumoniae promotes precancerous lesions and HCC in mice
To validate the function of K. pneumoniae in HCC, we gavaged K. pneumoniae to germ-free mice (GF/KP) for 36 weeks (Fig. 5a), with E. coli MG1655 (GF/EC) and PBS as controls. Fluorescence tracing of Cy5.5-d-Lys-labelled K. pneumoniae and E. coli identified K. pneumoniae-derived signals in both the gut and liver, whereas E. coli signals were restricted to the gut (Supplementary Fig. 10a and Fig. 5b). Bacterial culture and FISH showed the presence of live K. pneumoniae in GF/KP mouse liver (8 out of 10), whereas E. coli and PBS were both negative (Fig. 5b). These results indicate that monocolonization with K. pneumoniae is sufficient for its translocation to the liver. We confirmed previous observations that K. pneumoniae compromised gut barrier function by inducing gelatinase activity (Fig. 5c–e, Extended Data Fig. 8e and Supplementary Fig. 10b,c). Critically, K. pneumoniae-gavaged germ-free mice developed dysplasia that were absent in E. coli or PBS-gavaged mice (P < 0.0001) (Fig. 5f). K. pneumoniae also induced hepatocyte proliferation (Fig. 5g and Extended Data Fig. 8f), fibrotic injury (Fig. 5h and Extended Data Fig. 8g) and oncogenic and pro-inflammatory signalling (Extended Data Fig. 8h). In addition, the protumorigenic effect of K. pneumoniae was further reproduced in SPF mice (Fig. 5i–n and Supplementary Fig. 11a–d) and DEN-injected SPF mice (Fig. 5o,p and Supplementary Fig. 12a–g). In these models, K. pneumoniae disrupted gut barrier integrity and translocated to the liver. Our results implicate K. pneumoniae as a gut pathogen that promotes precancerous lesions and HCC in mice.
a, Design of K. pneumoniae gavage for promoting precancerous lesions in germ-free mice without DEN treatment (GF/PBS group n = 10; GF/EC group n = 8; GF/KP group n = 10) and related results (b–h). b, In vivo imaging of body and liver after Cy5.5-d-Lys-labelled K. pneumoniae gavage (left), live bacteria culture of liver tissues on blood agar plate (top right) and Cy3-conjugated EUB338 probe (GF/PBS group), E. coli probe (GF/EC group) or K. pneumoniae probe (GF/KP group) FISH detection in liver tissues (bottom right). c, Gut permeability assays using 500 kDa FITC-dextran (left) (n = 10 (GF/PBS), 8 (GF/EC), 10 (GF/KP)) and quantitative analysis of TEM (middle) (n = 6 biologically independent samples) and E-cad IHC staining (right) (n = 6 biologically independent samples). d, MMP-2/-9 activity in mouse faecal supernatant using gelatin hydrolysis assay (bottom left), gelatin zymography (top left) and dynamic activity assay (right). n = 3 biologically independent samples. e, Masson’s trichrome staining (top left) and COLIV IHC staining (top right) of colon tissues and COLIV protein level by western blot (bottom). n = 6 biologically independent samples. f, Representative images of liver gross morphology (top left) (dashed yellow circles indicate nodules), H&E staining of mice liver sections (bottom left), and nodule number and incidence of dysplasia (right). n = 10 (GF/PBS), 8 (GF/EC), 10 (GF/KP). g, IHC staining for PCNA and Ki-67 (left) (n = 6 biologically independent samples), and western blot assay of PCNA (right) (n = 3 biologically independent samples). h, Desmin (left) and α-SMA (middle) IHC staining, and Sirius red staining (right) of liver sections. n = 6 biologically independent samples. i, Design of K. pneumoniae gavage on precancerous lesions in SPF mice without DEN treatment (PBS group n = 6; EC group n = 6; KP group n = 6) and relevant results (j–n). j, Gut permeability assays using 500 kDa FITC-dextran. n = 6 biologically independent samples. k, Live bacterial culture of liver tissues on blood agar plate (top), and Cy3-conjugated EUB338 probe (PBS group), E. coli probe (EC group) or K. pneumoniae probe (KP group) FISH detection in liver tissues (bottom). n = 6 biologically independent samples. l, TEM (left) and quantitative analysis (right) of colon tissues. n = 6 biologically independent samples. m, Representative images of liver gross morphology (top) (dashed yellow circles indicate nodules), H&E staining of mice liver sections (middle), and nodule number and incidence of dysplasia (bottom). n = 6 biologically independent samples. n, Representative images of IHC staining for PCNA and Sirius red staining of liver sections (left) with quantitative analysis (right). n = 6 biologically independent samples. o, Design of K. pneumoniae gavage on HCC in SPF mice with DEN treatment (DPBS group n = 7; DEC group n = 6; DKP group n = 7) and related results (p). p, Representative images of liver gross morphology (left) (dashed yellow circles indicate tumour) and H&E staining of mice liver sections (middle) with tumour incidence and tumour number quantitative analysis (right). n = 7 (DPBS), 6 (DEC), 7 (DKP). In c (excluding tight junction disappear rate), d, e–h (excluding incidence of dysplasia), j,k,m (excluding incidence of dysplasia), n and p (excluding tumour incidence), data are presented as mean ± s.e.m. Each data point in bar plots represents one mouse. Tight junction disappearance rate, incidence of dysplasia and tumour incidence were analysed using Fisher’s exact test. MMP activity was analysed using two-way ANOVA. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
K. pneumoniae cell wall protein binds to and activates TLR4
We next investigated the molecular mechanism of K. pneumoniae in HCC. Because K. pneumoniae-conditioned medium (KPCM) had no effect on HCC cell viability (Supplementary Fig. 13a,b), we hypothesized that bacteria–HCC cells interaction is necessary. In line with this, scanning electron microscopy (SEM), TEM and cell attachment assay demonstrated attachment of K. pneumoniae to Hep3B and Huh7 cells (Fig. 6a). Co-culture of pasteurized K. pneumoniae promoted growth of HCC cells and HCC patient-derived organoids by inducing proliferation (Fig. 6b,c), suggesting that surface proteins of K. pneumoniae might be involved. To unravel K. pneumoniae proteins that interact with HCC receptors, we performed biotin pulldown and identified putative candidates by mass spectrometry (Fig. 6d). One candidate protein, penicillin-binding protein 1B (PBP1B), is reported to localize to the peptidoglycan-based cell wall of K. pneumonia. We next constructed glutathione S-transferase (GST)-tagged PBP1B (Supplementary Fig. 13c), followed by GST pulldown to identify its corresponding HCC receptor (Fig. 6e and Supplementary Fig. 13d). Overlap of common candidate proteins unravelled TLR4 as a receptor for PBP1B (Fig. 6e and Supplementary Fig. 13d). Surface plasmon resonance (SPR) using purified proteins confirmed direct PBP1B–TLR4 interaction with an affinity of 0.269 nM (Fig. 6f and Supplementary Fig. 13e). In silico modelling showed that PBP1B docks into the TLR4 catalytic site with binding energy of −318 kcal mol−1 (Fig. 6g). Co-immunoprecipitation (Co-IP) assays confirmed the interplay between GST–PBP1B and TLR4 in Hep3B and Huh7 cells (Fig. 6h,i).
a, Representative SEM (top) and TEM (bottom) images of Hep3B and Huh7 HCC cell lines after co-culture with K. pneumoniae (MOI = 10). After co-culture of HCC cells with K. pneumoniae, cell lysate was spread on BHI agar for bacterial culture. n = 3 independent experiments with similar results. b, Effect of pasteurized K. pneumoniae (MOI = 10) on HCC cell proliferation (top) (n = 3 biologically independent samples), colony formation (middle) (n = 3 biologically independent samples) and growth of HCC patient-derived organoid (bottom) (n = 20 biologically independent samples). c, Effect of pasteurized K. pneumoniae (MOI = 10) on PCNA expression in HCC cells. n = 3 (Ctrl), 6 (pasteurized KP). d, Screening of K. pneumoniae adhesins by biotin pull-down assay. e, Hep3B membrane protein was incubated with GST or GST–PBP1B together with GST magnetic beads for GST pull-down assay. Corresponding bands in GST–PBP1B and Hep3B cell membrane protein groups were subjected to mass spectrometry analysis. In d and e, n = 3 independent experiments with similar results. f, Binding affinity between PBP1B and TLR4 was detected using SPR. Kd, dissociation constant. g, Representative structure of PBP1B and TLR4 after molecular docking (left) and the residues involved in the interaction between PBP1B with TLR4 (right). The structure of TLR4 is coloured pink while the PBP1B is coloured yellow. The light green dash represents hydrogen bond. h, TLR4 from Hep3B and Huh7 cell lysates was pulled down by GST–PBP1B according to the GST pull-down assay. i, Immunoprecipitation of TLR4 from Hep3B or Huh7 cells lysates validated the binding between TLR4 and GST–PBP1B. In h and i, n = 3 independent experiments with similar results. j, Effect of PBP1B (0.05 μM) on HCC cell proliferation (top) (n = 3 biologically independent samples), colony formation (middle) (n = 3 biologically independent samples) and growth of HCC patient-derived organoid (bottom) (n = 20 biologically independent samples). n = 3 independent experiments with similar results. k, Effect of PBP1B (0.05 μM) on TLR4, MyD88, p-P65, P65 and PCNA protein expression in HCC cells, as determined by western blot. n = 3 biologically independent samples. l,m, TLR4i (30 μM) abolished the effect of PBP1B on HCC cell proliferation (l) and colony formation (m). n = 3 biologically independent samples. n, Effect of K. pneumoniae on TLR4 expression in liver tissues of both germ-free and SPF mice with or without DEN treatment by IHC staining. In b,c, j–m, data are presented as mean ± s.e.m. Each data point in bar plots represents one mouse. Cell proliferation was analysed using two-way ANOVA. In b,c,j,k,m, comparisons between two groups were analysed using Student’s t-test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
To investigate whether PBP1B promotes malignant behaviour of HCC cells, we incubated recombinant PBP1B with HCC cells in vitro. PBP1B promoted growth of HCC cells and HCC patient-derived organoids (Fig. 6j). PBP1B robustly increased TLR4, MyD88, p-P65 and PCNA protein levels (Fig. 6k), suggesting TLR4 signalling activation. TLR4 inhibitor (TLR4i; TLR4-IN-C34) abolished PBP1B-induced HCC cell growth (Fig. 6l,m), suggesting TLR4 as its functional target. Furthermore, K. pneumoniae gavage to germ-free and SPF mice elevated TLR4 expression (Fig. 6n). Our data suggest that K. pneumoniae PBP1B binds to TLR4 in HCC cells, leading to TLR4-mediated tumorigenesis.
Anticolonization or TLR4i reverses K. pneumoniae-induced HCC
Given that colonic infiltration of K. pneumoniae is obligatory for translocation to the liver, suppressing its colonization in the gut represents a potential therapeutic strategy. Klebsiella oxytoca has been reported to promote colonization resistance against pathogens18,19. We thus gavaged DEN-treated germ-free mice with K. oxytoca, K. pneumoniae and K. oxytoca plus K. pneumoniae (Extended Data Fig. 9a). Remarkably, K. oxytoca co-treatment reduced tumour incidence (P = 0.0027), tumour number (P < 0.0001) and tumour burden (P = 0.0001) compared with K. pneumoniae alone (Extended Data Fig. 9b). K. oxytoca co-treatment also restored gut barrier integrity (Extended Data Fig. 9c,d), thereby preventing infiltration of K. pneumoniae into mucosal lamina propria and muscularis mucosa (Extended Data Fig. 9c) and translocation to the liver (Extended Data Fig. 9e and Supplementary Fig. 14a). K. oxytoca also abrogated the effect of K. pneumoniae on TLR4 activation, hepatocyte proliferation (PCNA, Ki-67) and oncogenic and pro-inflammatory signalling (Supplementary Fig. 14a,b). K. oxytoca itself had no effect on HCC development. These results indicate that K. oxytoca antagonizes K. pneumoniae-induced HCC by arresting its colonic intrusion and translocation to the liver.
Next, we asked whether TLR4 blockade might overcome HCC-promoting K. pneumoniae via the PBP1B–TLR4 axis. Supporting our hypothesis, the TLR4i TLR4-IN-C34 reversed the effect of K. pneumoniae on tumour incidence (P = 0.0011), tumour number (P < 0.0001) and tumour burden (P = 0.0004) in DEN-injected germ-free mice (Extended Data Fig. 9f,g). Although TLR4-IN-C34 failed to restore the gut barrier or prevent translocation of K. pneumoniae to the liver (Extended Data Fig. 9h–j and Supplementary Fig. 15a), it effectively inhibited TLR4 activation, hepatocyte proliferation (PCNA, Ki-67), fibrosis marker expression, and oncogenic and pro-inflammatory signalling (Extended Data Fig. 9k and Supplementary Fig. 15b–d). TLR4-IN-C34 thus acts by blocking local effects of K. pneumoniae in the liver, without impacting gut barrier dysfunction or its translocation to the liver. We also confirmed that TLR4-IN-C34 blocked the HCC-promoting function of HCC-FMT in DEN-treated germ-free mice (Extended Data Fig. 10a,b), an effect independent of gut barrier dysfunction (Extended Data Fig. 10c,d) but is driven by TLR4 downregulation (Extended Data Fig. 10e–g). Collectively, both impairment of gut permeability and local activation of the PBP1B–TLR4 axis in the liver are essential for the HCC-promoting effect of K. pneumoniae, which can be targeted to overcome K. pneumoniae-induced hepatocarcinogenesis.
Discussion
Here we provide evidence of the causality of HCC-associated gut dysbiosis in contributing to hepatocarcinogenesis. Reconstitution of HCC microbiota in both germ-free mice and SPF mice led to spontaneous liver inflammation, fibrosis and dysplasia, and exacerbated carcinogen-induced HCC. Intriguingly, HCC microbiota was found to impair gut barrier integrity, thereby enabling the transfer of live pathogenic bacteria to the liver and consequential activation of pro-inflammatory and oncogenic signalling to perpetuate tumorigenesis. Characterization of the liver microbiota identified enrichment of pathogenic K. pneumoniae in mice given HCC-FMT and in human patients with HCC. Finally, K. pneumoniae monocolonization in mice phenocopied the effect of HCC-FMT, corroborating K. pneumoniae as an oncogenic pathogen in HCC.
Emerging evidence suggests that gut dysbiosis impacts liver diseases via the gut–liver axis13. We investigated this proposition in HCC by transplantation of HCC patient stool to germ-free mice or SPF mice, revealing spontaneous development of liver inflammation and fibrosis, characterized by induction of pro-inflammatory TH1/TH17 cells and suppression of anti-inflammatory TH2 cells. Chronic inflammation and fibrosis are contributors to hepatocarcinogenesis20. Indeed, half of HCC-FMT mice developed dysplasia. Furthermore, HCC-FMT accelerated DEN-induced HCC, thus establishing a causative role of HCC-associated dysbiosis in hepatocarcinogenesis.
Gut barrier integrity is fundamental to a functional gut–liver axis, as it prevents indiscriminate access of bacteria and their products to portal circulation21. Here we observed that HCC-FMT increased colonic gelatinase activities. Excess gelatinase activities can degrade extracellular matrix and impair epithelial integrity in the gut14,22,23,24. Gelatinase activity thus has a crucial role in induction of mucosal damage, loss of barrier function and disruption of intestinal tight junctions in HCC-FMT-treated mice. This is manifested by a marked increase in gut permeability after HCC-FMT, exposing the liver to gut pathogens, pathogen-associated molecular patterns and metabolites25.
Consequently, impaired gut barrier function led to colonization of the liver by live and culturable gut microbes in HCC-FMT-treated mice that were absent in mice given HD-FMT. Although recent studies have described intratumoural microbiota in many cancers4, the origin of these microbes remains enigmatic. Our results implicate the gut as the origin of intrahepatic pathogens in HCC. Cross-validation in patients with HCC and HCC-FMT-treated mice demonstrated increased K. pneumoniae in both stool and liver microbiomes. This is consistent with a previous study indicating that Klebsiella was increased in patients with early HCC26. K. pneumoniae has been reported to disrupt the intestinal barrier27,28,29; however, its molecular mechanism remains unclear. Here we showed that K. pneumoniae promoted macrophage-derived gelatinase activity in colon to induce gut barrier dysfunction. Together, K. pneumoniae is a driver pathogen that impairs gut barrier function and enables its own colonization in the liver.
K. pneumoniae is part of the human gut flora (~5% prevalence)30 and an opportunistic pathogen involved in hepatobiliary infections31, pyogenic liver abscess32 and non-alcoholic fatty liver disease28; however, its role in tumorigenesis is unknown. We revealed that K. pneumoniae exerts protumorigenic function by stimulating the growth of HCC cells and HCC patient-derived organoids. In mice, intrahepatic colonization of K. pneumoniae robustly promotes inflammation, fibrosis and oncogenic/inflammation signalling. This indicates K. pneumoniae as a gut pathogen promoting hepatocarcinogenesis. In line with our data, tumour-resident microbiota have been shown to have contributing roles in promoting cancer progression and immunosuppression7,33.
Mechanistically, K. pneumoniae promotes HCC via its surface protein PBP1B, which interacts with and activates TLR4 signalling in HCC cells. Indeed, TLR4 blockade reversed the protumorigenic effect of K. pneumoniae and HCC-FMT in mice. Importantly, the abilities of K. pneumoniae to disrupt gut barrier integrity and locally activate TLR4 in the liver are both essential for the HCC-promoting effect of this bacterium. Hence, targeting its gut colonization or TLR4 represent viable strategies to repress K. pneumoniae-induced HCC progression. Alternatively, recent work showed that K. pneumoniae could also be targeted by bacteriophages34.
In summary, in this study we established a causal link between HCC-associated gut dysbiosis and hepatocarcinogenesis. HCC dysbiosis disrupts gut barrier function and promotes translocation of live, pathogenic bacteria via the gut–liver axis. We identified K. pneumoniae as a gut pathogen that initiates gut barrier dysfunction and translocates to the liver, where it exacerbates HCC development via the PBP1B–TLR4 axis (Supplementary Fig. 17). Targeting K. pneumoniae represents a strategy for HCC prevention and treatment. The DEN-induced HCC mouse model used in this study is not a perfect tumour model and probably does not reflect human disease very well.
Methods
Ethics oversight
Written informed consent was obtained for all human samples and this study was approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (ID No. [2024]141 and [2020]361). Corresponding compensation was provided to participants.
All experiments were approved by the Animal Experimentation Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (ID No. [2021]506, [2023]361, [2024]141) or Shenzhen Jingtuo Biotechnology (ID No. [2023]51), which required a maximum tumour volume not exceeding 2,000 mm3, maximum diameter not exceeding 15 mm and tumour weight not exceeding 5% of body weight. The maximum tumour size and tumour burden in this study did not exceed this limit.
Human samples collection
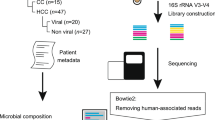
As shown in Supplementary Table 1, we collected stool from healthy donors (N = 20), individuals with LC (N = 20) and individuals with HCC (N = 20). In addition, we also collected stool from another batch of healthy donors (N = 5) and individuals with HCC (N = 5) (Supplementary Table 2) at The First Affiliated Hospital of Sun Yat-sen University. The exclusion criteria were as follows: (1) use of antibiotics within the past 3 months, (2) had received previous cancer treatment, (3) had a history of other cancers and (4) presence of intrahepatic cholangiocarcinoma and a history of gastrointestinal surgeries. Fresh faecal samples were collected using sterile stool collection tubes, placed in anaerobic conditions created by the AnaeroPack-Anaero (C-35, Mitsubishi Gas Chemical) and transported to the laboratory on ice. Stool samples were suspended in sterile PBS in an anaerobic glove box (COY-8300400, Coy Laboratory) and the suspension was filtered using a 40-μm cell strainer and centrifuged at 100 g for 1 min to remove debris. Supernatant was then centrifuged at 10,000 g for 8 min, and the bacterial pellet was suspended with 25% sterile glycerol and stored at −80 °C. We also collected stool samples from individuals with HCC (N = 24) with overall survival and progression-free survival data (Supplementary Table 3).
Fresh LC tissues (N = 15) and HCC tumours tissues (N = 54) were collected for live bacterial culture, 16S rRNA sequencing and wax block embedding (Supplementary Table 4) from patients who underwent surgery at The First Affiliated Hospital of Sun Yat-sen University.
Mice
Animals used in experiments were gender and age matched. SPF BALB/c and C57BL/6J mice were purchased from GemPharmatech and raised at Jingtuo Biotechnology. Germ-free male BALB/c mice were bred and housed at the Germ-Free Mouse Research Facility, The First Affiliated Hospital of Sun Yat-sen University or Shenzhen Jingtuo Biotechnology. Mice were raised in SPF or sterile facilities, following a cycle of 12 h light and 12 h dark conditions. Food (for SPF mice: 1010058, Jiangsu Xietong; for germ-free mice: 1019018, Jiangsu Xietong) and sterile water were provided ad libitum. Mice were randomly assigned to various experimental groups and experimental treatments. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications6. All mice were euthanized by pentobarbital sodium and cervical dislocation.
Bacteria
K. pneumoniae (isolated from tumour tissues of patients with HCC in this study), E. coli (used in Fig. 4a, isolated from tumour tissues of patients with HCC in this study), B. thetaiotaomicron (isolated from tumour tissues of HCC-FMT-treated mice in this study), P. mirabilis (isolated from tumour tissues of HCC-FMT-treated mice in this study), Enterobacter hormaechei (isolated from tumour tissues of HCC-FMT-treated mice in this study), E. coli MG1655 (700926, American Type Culture Collection (ATCC)) and K. oxytoca (DSM5175, DSMZ) were all cultured in brain heart infusion (BHI) broth (024053, Huanan Microbial) at 37 °C under aerobic/anaerobic conditions.
Cell lines
Both human HCC cell lines Hep3B (HB-8064, ATCC), Huh7 (01042712, Sigma-Aldrich) and normal colonic epithelial cells NCM460 (NCM460D, Incell) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (11965092, Thermo Fisher) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere of 5% CO2.
Germ-free and SPF mouse models
For germ-free mice without DEN treatment, 8-week-old male germ-free BALB/c mice were selected for FMT or bacterial gavage. For SPF mice without DEN treatment, 8-week-old male SPF BALB/c or C57BL/6J mice were selected for FMT or bacterial gavage. The number of mice in each group is shown in the figure legends.
For germ-free mice with DEN treatment, 14-day-old male germ-free BALB/c mice were selected and given 25 mg kg−1 DEN (N0756, Sigma-Aldrich) intraperitoneal injection. For SPF mice with DEN treatment, 14-day-old male SPF BALB/c or C57BL/6J mice were selected and given 25 mg kg−1 DEN intraperitoneal injection. The number of mice in each group is shown in the figure legends. FMT or other bacterial (as shown in figure legends) gavage only started when mice reached adulthood at 8 weeks of age.
At the end point, mice were anaesthetized with sodium pentobarbital intraperitoneally. Tumour number and size in the liver were recorded. Tumour volume was measured by length (a) and width (b) using a digital caliper and calculated as tumour volume = ab2/2. Tumour burden was the summation of tumour volumes of each liver. Liver and colon tissues were collected in 4% paraformaldehyde, 2% glutaraldehyde or snap-frozen. For bacterial culture and 16S rRNA sequencing, fresh liver tissues were collected in the biosafety cabinet. Liver histology was assessed by H&E staining (BL735B, Biosharp) of paraffin-embedded sections. Fresh liver and colon tissues were collected for flow cytometry. Liver histology was evaluated by a pathologist, B.L., blinded to the treatment conditions.
Bacterial culture from liver tissue and strain identification
Fresh liver tissues from mice, and patients with LC or HCC were collected in sterile tubes with BHI broth under aerobic and anaerobic conditions. Tissues were washed six times with sterile phosphate-buffered saline (PBS) and chopped into pieces. Tissue pieces were homogenized using TissueLyser II (85300, QIAGEN), and 100 μl tissue homogenate was directly spread onto different agar plates including blood agar plates, chocolate agar plates, MacConkey agar plates and Columbia blood agar plates in both anaerobic and aerobic conditions at 37 °C. Colony-forming units (c.f.u.) per mg tissue was counted. Full-length 16S rRNA sequencing or microbial mass spectrometry (MALDI Biotyper smart, Bruker) was used for strain identification.
Whole-genome sequencing and assemblies of K. pneumoniae
Genomic DNA was extracted from the isolated K. pneumoniae using the Tris-EDTA-NaCl buffer extraction method. The collected DNA was detected using agarose gel electrophoresis and quantified using Qubit. After quality control, the high-quality genomic DNA was subjected to sequencing library building and the genome of K. pneumoniae was sequenced using single-molecule, real-time technology (Novogene Bioinformatics). Low-quality reads were filtered using SMRT Link v.8.0 and the filtered reads were assembled using the software Canu to generate one contig without gaps. PacBio long-read sequencing of our K. pneumoniae isolate from HCC tissues revealed the absence of virulence factors associated with liver abscess (for example, peg-344, iroB, iucA, rmpA and rmpA2 encoding proteins) (Supplementary Table 6), which might explain why it colonizes in HCC without causing abscesses.
16S rRNA sequencing of liver tissues and stool samples
Liver tissues from mice and humans were collected in a biosafety cabinet for 16S rRNA sequencing. Liver tissues were disrupted by bead beating after digestion with mutanolysin and lysozyme. DNA extraction was performed with the QIAamp DNA mini kit (51304, QIAGEN). DNA of mice and human faecal bacteria were extracted using the Quick-DNA Faecal/Soil Microbe Miniprep kit (D6010, Zymo). DNA library preparation and 16S rRNA gene sequencing were performed on an Illumina NovaSeq 6000 platform by Gene Denovo. The V3–V4 region of bacterial 16S rRNA was PCR amplified using 16S primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′), with no template DNA reaction as negative control. Sequencing libraries were generated using the TruSeq DNA PCR-Free Sample Preparation kit (FC-121-3001, Illumina) and sequenced on an Illumina NovaSeq platform. After quality control, paired-end reads were overlapped to assemble the final sequences (overlap region >10 bp, mismatch ratio <0.2). Chimaera tags were filtered out using the Gold database by UCHIME (v.4.2.40). Operational taxonomic unit (OTU) analysis was performed using the Uparse package (v.7.0.1001) with 97% sequence identity. Each OTU was taxonomically assigned to the silva database using the RDP classifier. OTUs with relative abundance values >0.001% in at least one sample were retained.
Shotgun metagenomic sequencing
DNA of mouse faecal bacteria was extracted using the Quick-DNA Faecal/Soil Microbe Miniprep kit (D6010, Zymo). Shotgun metagenomic sequencing of faecal DNA was performed on an Illumina NovaSeq 6000 platform by Gene Denovo. Taxonomy was assigned to metagenomic reads using k-mer-based algorithms implemented in the Kraken taxonomic annotation pipeline. A standard database comprising 13,844 bacterial genomes from NCBI was built using the Jellyfish programme by counting distinct 31-mers. Each k-mer in a read was mapped to the lowest common ancestor of all reference genomes with exact k-mer matches. Each query was thereafter classified to a taxon, with the highest total hits of k-mers matched by pruning the general taxonomic trees affiliated with mapped genomes. Final metagenomic read counts were normalized with the cumulative sum scaling method using metagenome Seq R/Bioconductor.
K. pneumoniae correlates with prognosis of patients with HCC
K. pneumoniae relative abundance in stool samples from individuals with HCC (N = 24) was detected using qPCR assay (Forward: 5′-CGATGCTACTTATCCCGACA-3′; Reverse: 5′-AGCCGGTTGAGACGTAAAC-3′), and correlation analysis between K. pneumoniae relative abundance and overall survival or progression-free survival was performed.
IHC
IHC on paraffin-embedded tissues was used for the detection of PCNA (1:5,000), Ki-67 (1:400), TLR4 (1:5,000), E-cad (1:200), CLDN1 (1:200), CLDN3 (1:100), α-SMA (1:1,000), desmin (1:2,000), fibronectin (1:2,000), COLIV (1:500), LPS (1:200) and LTA (1:400) (antibodies listed in Supplementary Table 5). Quantitative analysis was performed using ImageJ (v.1.51, National Institutes of Health (NIH)).
Sirius red staining
Sirius red staining was conducted using the commercial kit (BP-DL030, Nanjing SenBeiJia) according to manufacturer instructions to evaluate collagen fibre content in liver tissues.Quantitative analysis was performed using ImageJ (v.1.51, NIH).
Masson’s trichrome staining
Masson’s trichrome staining was conducted using the commercial kit (BP-DL022, Nanjing SenBeiJia) according to manufacturer instructions to evaluate collagen fibre content in colon tissues. Quantitative analysis was performed using ImageJ (v.1.51, NIH).
Western blot
Total protein was extracted using Minute Total Protein Extraction Kit for Animal Cultured Cells/Tissues (SD-001, Invent Biotechnologies). Protein levels were measured using the BCA Protein Assay kit (ZJ101, Shanghai Epizyme). Proteins (30 μg) were separated using 12% gel and transferred onto PVDF membranes (SEQ85R, Merck Millipore). Membranes were incubated with primary antibody (1:1,000) overnight at 4 °C and then secondary antibody (1:5,000) (HRP-linked anti-mouse IgG 7076, HRP-linked anti-rabbit IgG 7074, Cell Signaling Technology) for 1 h. Proteins were visualized using a chemiluminescence reagent (SQ201, Shanghai Epizyme). β-actin was used as the total protein loading control. Antibodies used are listed in Supplementary Table 5.
FITC-dextran permeability assay
Mice were gavaged with 500 kDa FITC-dextran (FD500S, Sigma-Aldrich) after overnight fasting. Blood samples were collected from the tail vein at 4 h post administration. FITC-dextran in the plasma was measured in a spectrophotometer (Varioskan LUX, Thermo Scientific).
Gelatinase activity assay
The gelatin hydrolysis activity of faecal supernatant with various treatments or KPCM were detected using a gelatin biochemical identification tube (075310, HuanKai Microbial) and a gelatin zymography assay kit (RTD6143, Real-Times Biotechnology). MMP-2 and MMP-9 activities were detected using the MMP Activity Assay kit (ab112146, Abcam).
Effects of MMP-2/9 protein on gut barrier markers in vitro
MMP-2 (HY-P70268, MCE) and MMP-9 (HY-P70145, MCE) were used at 0.01 μg ml−1 and co-cultured with NCM460 cells for 3 days. Then, western blot assay was conducted to evaluate the effects of MMP-2/9 protein on gut barrier markers COLIV, fibronectin, E-cad and ZO-1. Antibodies used are listed in Supplementary Table 5.
FISH assay
Paraffin-embedded liver and colon sections were used for FISH assay using the Bacterial DNA FISH Assay kit (D-0015, FOCO Biology). Hybridization was performed at 37 °C for 2 days with a universal 16S rRNA probe EUB338 (5′-GCTGCCTCCCGTAGGAGT-3′, with a Cy3 label), E. coli probe (5′-GCATAAGCGTCGCTGCCG-3′, with a Cy3 label) or K. pneumoniae probe (5′-CCTACACACCAGCGTGCC-3′, with a Cy3 label) at 0.25 μM in hybridization buffer. After washing three times in wash buffer, slides were rinsed with sterile double-distilled water, air-dried in the dark and mounted in mounting medium with DAPI (ab104139, Abcam). Microscopic observations were performed using a fluorescence microscope (BX63F, Olympus).
RT2 profiler qPCR array
Total RNA was isolated from liver and colon tissues using TRIzol reagent (15596026, Thermo Fisher) and reverse transcribed using RT2 First Strand kit (330401, QIAGEN). RT2 Profiler PCR Array Mouse Cancer Pathway Finder (PAMM-033ZA-6, QIAGEN) or RT2 Profiler PCR Array Mouse Inflammatory Response and Autoimmunity (PAMM-077Z, QIAGEN) was used for qPCR analysis.
HCC patient-derived organoid culture and experiment
Organoids from patients with HCC (668T) were provided by N. Wong (The Chinese University of Hong Kong), embedded into Matrigel (356231, Corning) and cultured in AIM-V medium (12055083, Thermo Fisher) supplemented with 10% FBS, 1% penicillin/streptomycin, 1% l-glutamine (25030081, Thermo Fisher), MEM non-essential amino acids solution (11140050, Thermo Fisher) and insulin-transferrin-selenium (41400045, Thermo Fisher). Culture medium was changed every 3 days. After 10 days, HCC organoids were digested using TrypLE Express (12604013, Thermo Fisher) and collected for experiments. To investigate the effects of candidate bacteria or proteins on organoid growth, images were captured and the diameter of organoids in each random field was measured using ImageJ (v.1.51, NIH) after treatment for 5 days. The effect of PBP1B (0.05 μM) on the growth of HCC patient-derived organoids was also investigated as stated above.
Flow cytometry analysis of immune cells
Multicolour flow cytometry was performed to study the immune cell types in liver and colon tissues. Single-cell suspensions of liver and colon tissues were prepared with the Liver Dissociation kit (130-105-807, Miltenyi Biotec) or the Lamina Propria Dissociation kit (130-097-410, Miltenyi Biotec), respectively. For surface staining, cells were incubated with Fc block antibody purified rat anti-mouse CD16/CD32 (1:100) (553142, BD Biosciences) for 30 min. Then, cells were stained with the following antibodies: CD45 (1:100; 157213, Biolegend), FVS510 (1:100; 564406, BD Biosciences), CD3 (1:100; 100330, Biolegend), CD4 (1:100; 100434, Biolegend), CD11b (1:100; 101216, Biolegend), MHC II (1:100; 107622, Biolegend) or Ly-6C (1:100; 560525, BD Biosciences). For intracellular markers, cells were stimulated for 8 h in DMEM with leucocyte activation cocktail. Then, cells were fixed and permeabilized with BD GolgiPlug kit (550583, BD Bioscience) and then stained with intracellular cytokine IFNγ (1:100; 557649, BD Bioscience), IL-4 (1:100; 564005, BD Bioscience) and IL-17 (1:100; 563354, BD Bioscience) antibodies. Flow cytometry was performed on an Attune NxT Acoustic Focusing cytometer (ThermoFisher). Gating strategy for flow cytometry is shown in Supplementary Fig. 16a. Antibodies used are listed in Supplementary Table 5.
THP-1-induced macrophage in vitro and co-culture experiment
THP-1 monocyte cells (TIB-202, ATCC) were cultured in RPMI 1640 medium (11875093, Gibco). THP-1 cells were differentiated into macrophages by 3 days incubation with 150 nM phorbol ester (P8139, Sigma), followed by morphological observation and flow cytometry to evaluate CD14 (1:100; 367140, Biolegend) expression. THP-1-induced macrophages were then co-cultured with K. pneumoniae (multiplicity of infection (MOI) = 10) for 2 h and subsequently cultured for another 3 days. After that, the cell suspension was centrifuged at 10,000g for 10 min at 4 °C to collect the supernatant for gelatinase activity assay. The gating strategy for flow cytometry is shown in Supplementary Fig. 16b. Antibodies used are listed in Supplementary Table 5.
Colon macrophage depletion mouse model
We used the macrophage depletion reagent Clodrosome (CLD-8909, Encapsula NanoSciences) according to manufacturer instructions to deplete colon macrophages. After killing, macrophages in colon were detected by flow cytometry. Macrophages in colon were marked as CD45+ MHC II+ CD11b+ Ly6C−. The gating strategy for flow cytometry is shown in Supplementary Fig. 16c. Antibodies used are listed in Supplementary Table 5.
Live K. pneumoniae attachment to HCC cells assay
Live K. pneumoniae (MOI = 10) was suspended in DMEM medium supplemented with 10% FBS and co-cultured with Hep3B or Huh7 cells for 2 h in 6-well plates. Then, cells were washed with warm PBS six times and scraped off from plates. The cells were collected and stripped in BHI agar for culture.
SEM and TEM assay of K. pneumoniae and HCC cell attachment
Hep3B or Huh7 cells (1 × 106) were seeded in sterile coverslips and co-cultured with K. pneumoniae (MOI = 10) for 2 h. Next, cells were washed with PBS three times and fixed with 2.5% glutaraldehyde fixative at 4 °C overnight. The fixed coverslips were washed with Sorensen’s phosphate buffer three times and fixed in 1% osmium tetroxide for 2 h, followed by rinsing with double-distilled water. After dehydration and coating with gold-palladium, specimens were examined using a Cold Field Scanning Electron Microscope (SU8010, Hitachi). Hep3B or Huh7 cells (1 × 106) were seeded in 6-well plates and co-cultured with K. pneumoniae for 4 h (MOI = 10), followed by fixation in 2.5% glutaraldehyde. Fixed samples were further fixed in 2% osmium tetroxide and dehydrated with a gradient of ethanol and propylene oxide. Samples were then embedded, sectioned and stained with 3% uranyl acetate and lead citrate. Pictures were taken using an HT7700 TEM (Hitachi).
Cell membrane protein extraction
Hep3B or Huh7 cells were extracted with 0.5 ml ice-cold lysis buffer (150 mM NaCl, 10 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA (pH 8.0), 0.2 mM sodium orthovanadate, 0.2 mM phenylmethylsulfonylfluoride, 1% Triton X-100, 0.5% NP-40) for 1 h at 4 °C, followed by sonication on ice for 15 min. The cell suspension was centrifuged at 10,000 g for 10 min at 4 °C to collect the supernatant, which was then used as the cell membrane protein lysate.
Biotin pull-down assay and silver staining
Biotin pulldown was performed as previously described35. In brief, Hep3B or Huh7 cells were labelled with 1 mM EZ-Link Sulfo-NHS-LC-Biotin (21338, Thermo Scientific) at 4 °C for 2 h, and then incubated with K. pneumoniae surface membrane proteins overnight at 4 °C, followed by incubation with streptavidin magnetic beads for 6 h at 4 °C. As a negative control, streptavidin agarose resin was incubated with non-biotinylated Hep3B or Huh7 surface proteins. Finally, the beads were washed with PBST (PBS with 0.1% Tween 20) three times, boiled with SDS–PAGE gel loading buffer and centrifuged. Eluted proteins were subjected to SDS–PAGE and visualized by silver staining using the Pierce Silver Stain for Mass Spectrometry kit (24600, Thermo Scientific). Corresponding bands of gels were identified by mass spectrometry.
Expression and purification of PBP1B protein with or without GST tag
To generate recombinant GST–PBP1B protein, the non-transmembrane domain of PBP1B (accession: A0A086I8Y5) was cloned into the pGEX-6P-1 vector and transformed into E. coli BL21 (DE3). Transformed E. coli was grown in ampicillin-supplemented LB broth at 37 °C. When optical density reached 1.0, 0.5 mM IPTG (HY-15921, MCE) was added to induce protein synthesis at 20 °C overnight. Bacterial pellets were collected and suspended in PBS-basic lysis buffer (50 mM NaH2PO4, 150 mM NaCl, pH 7.2, 10 mg ml−1 lysozyme and proteinase inhibitors). After centrifugation at 10,000 g for 30 min, GST–PBP1B (113 kDa) was purified from the supernatant using GST-tag Protein Purification kit (P2262, Beyotime). GST–PBP1B was incubated with PreScission protease (27084301, Cytiva) to obtain PBP1B without the GST tag (87 kDa).
GST pull-down assay
To perform the GST pulldown, Hep3B or Huh7 membrane proteins were incubated with or without recombinant GST–PBP1B protein overnight at 4 °C, followed by addition of glutathione magnetic agarose beads (HY-K0234, MCE) for another 4 h at 4 °C. The beads were then washed and boiled with SDS–PAGE gel loading buffer. Eluted proteins were separated by SDS–PAGE. Then, gel was analysed by silver staining, followed by mass spectrometry analysis of target bands, or western blot assay. Antibodies used are listed in Supplementary Table 5.
Co-immunoprecipitation
For co-immunoprecipitation of TLR4, anti-TLR4 antibody (1:100) and Hep3B or Huh7 cell membrane protein were incubated with or without recombinant GST-PBP1B overnight at 4 °C. Mouse IgG (1:100) served as the negative control. Dynabeads Protein G magnetic beads (10004D, Invitrogen) were then added and incubated for another 4 h. The beads were washed and boiled with loading buffer. Boiled samples were subjected to SDS–PAGE and immunoblotted with anti-TLR4 (1:1,000) or anti-GST (1:5,000) (2624, Cell Signaling Technology). Antibodies used are listed in Supplementary Table 5.
SPR
Binding affinity between PBP1B and TLR4 was evaluated by SPR using Biacore T200 (GE Healthcare). TLR4 human recombinant protein (HY-P73586, MCE) was immobilized on a Series S Sensor Chip CM5 (29149603, GE Healthcare) using an amine coupling kit (BR-1000-50, GE Healthcare) with a flow rate of 10 μl min−1, resulting in a final ligand coupling of ~180 response units. Different concentrations of PBP1B (100, 50, 25, 12.5, 6.25, 3.125 and 0 nM) diluted using an analyte buffer were injected at a flow rate of 30 μl min−1 for an association phase of 120 s, followed by a 300-s dissociation. The association and dissociation processes were all handled in the analyte buffer. We repeated 7 cycles of analysis according to analyte concentration in ascending order. After each cycle of interaction analysis, the sensor chip surface was regenerated completely with 10 mM glycine-HCl as injection buffer at a flow rate of 30 μl min−1 for 30 s to remove the analyte, then the injection and regeneration steps were repeated for the next concentration cycle of the analyte PBP1B. The Biacore T200 Evaluation Software v.2.0 was used to determine the binding affinity.
Molecular docking analysis
Molecular docking analysis was performed to determine receptor–ligand interactions. The three-dimensional structures of PBP1B (accession: A0A086I8Y5) and TLR4 (O00206) were predicted using AlphaFold. Protein was pretreated using Discovery Studio software to remove water molecules, hydrogenation and ions. The primary ligand was extracted from the structure and the treated protein was visualized using Pymol. The 2D ligand–protein interaction diagrams and hydrophobic interactions were constructed using the software LigPlus.
Alcian blue staining
Paraffin-embedded colonic sections were stained using the Alcian Blue Stain kit (S0134, Bioss). Alcian blue staining-positive areas were analysed using ImageJ (v.1.51, NIH).
TEM assay of colon and liver tissues
Ultra-thin sections of colon and liver tissues were prepared and examined using an HT7700 TEM (Hitachi), then photographed for analysis.
K. pneumoniae quantification by qPCR
Stool DNA was prepared using the Quick-DNA Faecal/Soil Microbe kit (D6110, Zymo), while bacterial DNA from liver tissues was prepared using the QIAamp DNA Mini kit (51306, QIAGEN) combined with RNase A (R6513, Sigma-Aldrich) and lysozyme (L6876, Sigma-Aldrich). Quantification of K. pneumoniae was performed using the K. pneumoniae-EASY Genesig kit (Z-Path-K. pneumoniae-standard, Primer Design) and Oasig lyophilised 2× qPCR Master Mix (Z-oasig-standard-150, Primer Design) in an Applied Biosystems Real-Time PCR System (QuantStudio 5, Thermo Fisher).
Colony formation and cell proliferation
KPCM was prepared by culture in BHI broth for 2 days, followed by bacterial removal by centrifugation and filtration with a 0.22-µm filter. Pasteurized K. pneumoniae was prepared by incubating live K. pneumoniae at 70 °C for 30 min. For colony formation, cells were seeded on 6-well plates (1,000 cells per well). KPCM (5% or 10%) or pasteurized K. pneumoniae (MOI = 10) treatment was performed for 3 h day−1, then the medium was replaced with MEM or DMEM containing 10% FBS, 1% penicillin/streptomycin and 20 mg ml−1 gentamycin. The procedure was repeated for 14 days and colonies were stained with 0.5% crystal violet. For the cell proliferation assay, cells were seeded into 96-well plates (1,000 cells per well), followed by live K. pneumoniae or culture supernatant treatment. At different time intervals, cell viability was determined using the MTT (475989, Sigma-Aldrich) assay and measured under an optical density of 570 nm using a spectrophotometer. In addition, PBP1B (0.05 μM) effects on HCC cell proliferation and colony formation were also assessed as stated above.
Bacterial fluorescence tracing experiment
To label K. pneumoniae and E. coli MG1655, bacteria were cultured in BHI containing 100 μmol l−1 Cy5.5-d-Lys at 37 °C for 48 h. Bacterial suspension was collected by centrifugation and washed with PBS six times. Labelling was determined using a fluorescence microscope (BX63F, Olympus). To trace bacteria in vivo, labelled bacteria were gavaged to germ-free mice and fluorescence detection was performed before bacterial gavage and 8 h after gavage using the in vivo imaging system (IVIS Lumina III, Perkin Elmer). Hepatic fluorescence detection was also conducted after collecting the liver.
TLR4i treatment in vitro and in vivo
For in vitro treatment, the TLR4i TLR4-IN-C34 (HY-107575, MCE) was used at 30 μM, while mice received TLR4-IN-C34 (1 mg kg−1) gavaged daily in vivo.
Colonization resistance challenge of K. pneumoniae in vivo
K. oxytoca was used to cause colonization resistance to K. pneumoniae in the gut of germ-free mice. Mice were gavaged with 1 × 108 c.f.u. of K. oxytoca diluted in 200 µl PBS. After 3 days of precolonization, mice were gavaged with 1 × 108 c.f.u. of K. pneumoniae. This process was conducted once a week. For mice receiving only K. oxytoca treatment, mice were given 1 × 108 c.f.u. of K. oxytoca gavaged once a week.
Statistical analysis
No animals or data points were excluded from the analyses. Data collection and analysis were not performed blind to the conditions of the experiments. Data distribution was assumed to be normal but this was not formally tested. Measurements were taken from distinct samples. All in vitro experiments were repeated three times as three independent experiments unless otherwise stated. For in vitro experiments, all attempts at replication were successful. For in vivo experiments, all experiments were successful without replication. Data are expressed as mean ± s.e.m. for both in vivo and in vitro experiments. Comparison between two groups was performed using Student’s t-test. One-way ANOVA was used to compare differences among multiple groups. Differences in cell proliferation and MMP activity were determined using two-way ANOVA. Fisher’s exact test was used to evaluate the proportional difference in categorical variables between groups. Simple linear regression analysis was conducted to characterize the correlation between two variables. Correlation coefficient, r, was calculated using Goodness of Fit test. All statistical analyses were conducted using GraphPad Prism v.10.3.1. Flow cytometry data were analysed with the FlowJo v.10.9.0 software. P value < 0.05 indicates statistical significance.
Reagent and resource sharing
Further information and requests for resources, biological materials and reagents should be directed to and will be fulfilled by the lead contact, J.Y.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All datasets generated and/or analysed during the study are available. All related raw data have been deposited to public datasets with accession numbers. Whole-genome sequencing data of Klebsiella pneumoniae reported in this study have been deposited at the Genome Sequence Archive (GSA) of the China National Center for Bioinformation (CNCB) under accession number CRA019172. 16S rRNA sequencing data of liver tissues and stools from patients with LC and HCC have been deposited at the GSA of CNCB under accession numbers CRA019234 and CRA019232, respectively. 16S rRNA sequencing data and metagenomic sequencing sequencing data of mice faeces have been deposited at the GSA of CNCB under accession numbers CRA019231 and CRA019388, respectively. Source data are provided with this paper.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Fujiwara, N., Friedman, S. L., Goossens, N. & Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 68, 526–549 (2018).
Behary, J. et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 12, 187 (2021).
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020).
Dejea, C. M. et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597 (2018).
Iida, N. et al. Chronic liver disease enables gut Enterococcus faecalis colonization to promote liver carcinogenesis. Nat. Cancer 2, 1039–1054 (2021).
Fu, A. et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 185, 1356–1372 (2022).
Dohlman, A. B. et al. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 185, 3807–3822 (2022).
Pabst, O. et al. Gut–liver axis: barriers and functional circuits. Nat. Rev. Gastroenterol. Hepatol. 20, 447–461 (2023).
Schnabl, B. & Brenner, D. A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524 (2014).
Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018).
Dapito, D. H. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516 (2012).
Albillos, A., de Gottardi, A. & Rescigno, M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72, 558–577 (2020).
McCawley, L. J. & Matrisian, L. M. Matrix metalloproteinases: they’re not just for matrix anymore! Curr. Opin. Cell Biol. 13, 534–540 (2001).
Kirkegaard, T., Hansen, A., Bruun, E. & Brynskov, J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn’s disease. Gut 53, 701–709 (2004).
Tan, T. K. et al. Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am. J. Pathol. 176, 1256–1270 (2010).
Bai, X. et al. Changes in MMP‑2, MMP‑9, inflammation, blood coagulation and intestinal mucosal permeability in patients with active ulcerative colitis. Exp. Ther. Med. 20, 269–274 (2020).
Osbelt, L. et al. Klebsiella oxytoca causes colonization resistance against multidrug-resistant K. pneumoniae in the gut via cooperative carbohydrate competition. Cell Host Microbe 29, 1663–1679 (2021).
Osbelt, L. et al. Klebsiella oxytoca inhibits Salmonella infection through multiple microbiota-context-dependent mechanisms. Nat. Microbiol. 9, 1792–1811 (2024).
Okuda, K. Hepatocellular carcinoma: recent progress. Hepatology 15, 948–963 (1992).
Brescia, P. & Rescigno, M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol. Med. 27, 844–855 (2021).
Zitta, K. et al. Serum from patients undergoing remote ischemic preconditioning protects cultured human intestinal cells from hypoxia-induced damage: involvement of matrixmetalloproteinase-2 and-9. Mol. Med. 18, 29–37 (2012).
Jakubowska, K. et al. Expressions of matrix metalloproteinases (MMP-2, MMP-7, and MMP-9) and their inhibitors (TIMP-1, TIMP-2) in inflammatory bowel diseases. Gastroenterol. Res. Pract. 2016, 2456179 (2016).
Rodrigues, D. M. et al. Matrix metalloproteinase 9 contributes to gut microbe homeostasis in a model of infectious colitis. BMC Microbiol. 12, 105 (2012).
Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015).
Ren, Z. et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 68, 1014–1023 (2019).
Nakamoto, N. et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat. Microbiol. 4, 492–503 (2019).
Yuan, J. et al. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. 30, 675–688 (2019).
Atarashi, K. et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358, 359–365 (2017).
Ganaway, J. R. in The Biology of the Guinea Pig (eds Wagner. J. E. & Manning, P. J.) 121–135 (Elsevier, 1976).
Wagner, K. A., Hartmann, F. A. & Trepanier, L. A. Bacterial culture results from liver, gallbladder, or bile in 248 dogs and cats evaluated for hepatobiliary disease: 1998–2003. J. Vet. Intern. Med. 21, 417–424 (2007).
Lederman, E. R. & Crum, N. F. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am. J. Gastroenterol. 100, 322–331 (2005).
Niño, J. L. G. et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611, 810–817 (2022).
Federici, S. et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185, 2879–2898 (2022).
Long, X. et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 4, 2319–2330 (2019).
Acknowledgements
This project was funded by the National Science and Technology Major Project of China (2023ZD0500200, J.Y.), the GuangDong Basic and Applied Basic Research Foundation (2022B1515120031, J.Y.), RGC-CRF Hong Kong (C4039-19GF and C4008-23W, J.Y.), the Strategic Seed Funding Collaboration Research Scheme CUHK (3133344, J.Y.), the Strategic Impact Enhancement Fund CUHK (3135509, J.Y.), the Impact case for RAE CUHK (3134277, J.Y.), the National Natural Science Foundation of China (82173191, L.X.), and the Guangdong Basic and Applied Basic Research Foundation (2019B151502009, L.X.).
Author information
Authors and Affiliations
Contributions
X.W. and J.Y. conceptualized the project. X.W., H.S., H.W., X. Li and J.J.Y.S. developed the methodology. X.W., Y.F., W. Liang, Y.C., J.W., N.W., L.S., H.C.-H.L., Y.J., X.Z., L.Y., M.M., T.Y., M.F., H.Z., E.S.-H.C., M.L., W. Liu, X. Liu, S.Z. and X. Li conducted investigations. Q.Z., L.X., H.S., H.W., X. Li and M.K. acquired resources. X.W., L.S., M.F., J.W., N.W., T.Y., M.M. and B.L. performed visualization. L.X. and J.Y. acquired funding. J.Y. administered and supervised the project. X.W., C.C.W. and J.Y. wrote the original draft. X.W., C.C.W. and J.Y. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Tim Greten and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mixed HCC fecal microbiota transplantation and fecal microbiota from two individual HCC patients promote hepatocarcinogenesis in DEN-treated germ-free mice.
(a) Design of mixed FMT experiment to DEN-treated germ-free mice (DEN+PBS group n=6; DEN+HD group n=12; DEN+HCC group n=11) and related results (b-d). (b) Representative images of liver gross morphology (yellow circles indicate tumors) and H&E staining of mouse liver sections (yellow circles indicate tumor areas). Tumor incidence, tumor number and the maximum tumor diameter per mice. n=6 (DEN+PBS), 12 (DEN+HD), 11 (DEN+HCC). (c) IHC staining of Ki-67 and PCNA. n=6 biologically independent samples. (d) Desmin and α-SMA IHC staining, and Sirius red staining of liver sections. n=6 biologically independent samples. (e) Design of FMT experiment to germ-free mice with DEN treatment (PBS group n=9; DEN+PBS group n=10; DEN+IHD group n=12; DEN+IHCC group n=9) and related results (f-h). (f) Representative images of liver gross morphology (yellow circles indicate tumors) and H&E staining. Tumor incidence, tumor number, and maximum tumor diameter per mouse in liver. n=9 (PBS), 10 (DEN+PBS), 12 (DEN+IHD), 9 (DEN+IHCC). (g) Ki-67 and PCNA IHC staining. n=6 biologically independent samples. (h) α-SMA and Desmin IHC staining, and Sirius red staining of mouse liver sections. n=6 biologically independent samples. Data (excluding tumor incidence) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Tumor incidence was calculated using Fisher’s exact test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Extended Data Fig. 2 Individual HCC patient fecal microbiota initiates liver precancerous lesions in germ-free mice without DEN treatment.
(a) Design of FMT experiment to germ-free mice without DEN treatment (GF/PBS group n=6; GF/HD-FMT group n=12; GF/HCC-FMT group n=16). (b) Representative images of liver gross morphology (yellow circles indicate nodules), and H&E staining of mice liver sections with incidence of dysplasia and nodules number statistical results. n=6 (GF/PBS), 12 (GF/HD-FMT), 16 (GF/HCC-FMT). (c) IHC staining for PCNA and Ki-67. n=6 biologically independent samples. (d) Hepatic infiltration of Th1, Th17, and Th2 cells was evaluated by flow cytometry. n=3 biologically independent samples. (e) Desmin and α-SMA IHC staining, and Sirius red staining of liver sections. n=6 biologically independent samples. (f) Mouse Cancer Pathway Finder Array and (g) Inflammatory Response and Autoimmunity PCR Array of liver tissues. f and g, n=3 independent experiments with similar results. Data (excluding incidence of dysplasia and PCR array results) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Incidence of dysplasia was calculated using Fisher’s exact test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Extended Data Fig. 3 Individual HCC patient fecal microbiota initiates liver precancerous lesions in SPF mice without DEN treatment.
(a) Design of FMT experiment to SPF mice without DEN treatment (SPF/PBS group n=19; SPF/HD-FMT group n=16; SPF/HCC-FMT group n=16). (b) Representative images of liver gross morphology (yellow circles indicate nodules), and H&E staining of mice liver sections. Incidence of dysplasia and number of liver nodules. n=19 (SPF/PBS), 16 (SPF/HD-FMT), 16 (SPF/HCC-FMT). (c) IHC staining for PCNA and Ki-67. n=6 biologically independent samples. (d) Desmin and α-SMA IHC staining, and Sirius red staining of liver sections. n=6 biologically independent samples. (e) Hepatic infiltration of Th1 (n=3 biologically independent samples), Th17 (n=4 biologically independent samples), and Th2 (n=3 biologically independent samples) cells was evaluated by flow cytometry. (f) Mouse Cancer Pathway Finder Array and Inflammatory Response and Autoimmunity PCR Array of liver tissues. n=3 independent experiments with similar results. Data (excluding incidence of dysplasia and PCR array results) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Incidence of dysplasia was calculated using Fisher’s exact test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Extended Data Fig. 4 Five mixed HCC fecal microbiota transplantation initiates hepatocarcinogenesis in germ-free mice without DEN treatment.
(a) Design of FMT experiment to germ-free mice (PBS group n=10; HD-FMT group n=12; HCC-FMT group n=9). (b) Representative images of liver gross morphology (yellow circles indicate nodules) and H&E staining of mice liver sections. n=10 (PBS), 12 (HD-FMT), 9 (HCC-FMT). (c) IHC staining for Ki-67 and PCNA. n=6 biologically independent samples. (d) Mouse Cancer Pathway Finder PCR Array and Inflammatory Response and Autoimmunity PCR Array of liver tissues. n=3 independent experiments with similar results. (e) Th1, Th17, and Th2 cells infiltration in liver was determined by flow cytometry. n=3 biologically independent samples. (f) α-SMA and Desmin IHC staining, and Sirius red staining of liver sections. n=6 biologically independent samples. Data (excluding incidence of dysplasia and PCR array results) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Incidence of dysplasia was calculated using Fisher’s exact test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Extended Data Fig. 5 HCC patient fecal microbiota impairs gut barrier function, induces intestinal inflammation, and promotes bacteria translocation into liver in germ-free mice with or without DEN treatment.
(a) Gut permeability assays using 500 kDa FITC-dextran, CLDN 3 and CLDN 1 IHC staining of germ-free mice without DEN. n=6 biologically independent samples. (b) Gut permeability assays using 500 kDa FITC-dextran, CLDN 3 and CLDN 1 IHC staining of germ-free mice with DEN. n=6 biologically independent samples. (c) Mouse Inflammatory Response and Autoimmunity PCR Array of colon tissues. n=3 independent experiments with similar results. (d) Colon Th1, Th17, and Th2 cells in germ-free mice without DEN was evaluated by flow cytometry. n=3 biologically independent samples. (e) Workflow of fresh liver homogenate preparation for bacteria culture. (f) PBS contamination control used in tissues bacteria culture experiment. (g) Cy3-conjugated EUB338 probe FISH detection of liver tissues. n=6 biologically independent samples. Data (excluding PCR array result) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Extended Data Fig. 6 HCC patient fecal microbiota impairs gut barrier function and promotes bacteria translocation into liver in SPF mice with or without DEN treatment as shown in Fig. 1f and Extended Data Fig. 3a.
(a) Gut permeability assay using 500 kDa FITC-dextran. n=6 biologically independent samples. (b) Representative pictures of Alcian blue staining, E-Cad, CLDN 3 IHC staining, and Cy3-conjugated EUB338 probe FISH of colon tissues with quantitative analysis. n=3 biologically independent samples. (c) Representative bacterial culture of liver tissues under anaerobic and aerobic conditions with quantitative analysis. n= 19 (SPF/PBS), 16 (SPF/HD-FMT), 16 (SPF/HCC-FMT), 20 (SPFD/PBS), 16 (SPFD/HD-FMT), 16 (SPFD/LC-FMT), 16 (SPFD/HCC-FMT). From left to right and top to bottom, the culture plates are blood agar plate, chocolate blood agar plate, MacConkey agar plate, and Columbia blood agar plate, respectively. And, representative images of Cy3-conjugated EUB338 probe FISH detection in mice liver tissues. n=6 biologically independent samples. Data (excluding liver with live bacteria) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Liver with live bacteria was calculated using Fisher’s exact test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Extended Data Fig. 7 Mixed HCC fecal microbiota transplantation and fecal microbiota from two individual HCC patients impair gut barrier function and promotes bacteria translocation into liver in germ-free mice with or without DEN treatment.
(a) Gut permeability assay using FITC-dextran. n=6 (DEN+PBS), 12 (DEN+HD), 11 (DEN+HCC). (b) Representative pictures of Alcian blue staining, CLDN 3 and E-Cad IHC staining of colon tissues with quantitative analysis. n=6 biologically independent samples. (c) Representative bacterial culture of liver tissues under anaerobic and aerobic conditions with quantitative analysis. n=6 (DEN+PBS), 12 (DEN+HD), 11 (DEN+HCC). From left to right and top to bottom, the culture plates are blood agar plate, chocolate blood agar plate, MacConkey agar plate, and Columbia blood agar plate, respectively. (d) Images of Cy3-conjugated EUB338 probe detection. n=6 biologically independent samples. a–d are related to Extended Data Fig. 1a. (e) Pictures of CLDN 3 and E-Cad IHC staining, and Alcian blue staining of colon tissues with quantitative analysis. n=6 biologically independent samples. (f) Bacterial culture of liver tissues under anaerobic and aerobic conditions with quantitative analysis. n=10 (PBS), 12 (HD-FMT), 9 (HCC-FMT). From left to right and top to bottom, the culture plates are blood agar plate, chocolate blood agar plate, MacConkey agar plate, and Columbia blood agar plate, respectively. (g) Gut permeability assay using FITC-dextran and (h) Cy3-conjugated EUB338 probe detection of liver tissue. n=6 biologically independent samples. e-h are related to Extended Data Fig. 4a. (i) Pictures of Alcian blue staining, CLDN 3 and E-Cad IHC staining of colon tissues with quantitative analysis. n=6 biologically independent samples. (j) Gut permeability assay using FITC-dextran. n=9 (PBS), 10 (DEN+PBS), 12 (DEN+IHD), 9 (DEN+IHCC). (k) Bacterial culture of liver tissues under anaerobic and aerobic conditions with quantitative analysis. n=9 (PBS), 10 (DEN+PBS), 12 (DEN+IHD), 9 (DEN+IHCC). i-k are related to Extended Data Fig. 1e. Data (excluding liver with live bacteria) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Liver with living bacteria was calculated using Fisher’s exact test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Extended Data Fig. 8 K. pneumoniae initiates gut barrier dysfunction through elevating colon macrophage mediated MMP-2/-9 activities and promotes precancerous lesions in germ-free mice.
(a) Representative pictures of TEM, Alcian blue staining, E-Cad, CLDN 3, CLDN 1 IHC staining, and Cy3-conjugated EUB338 probe FISH of colon tissues related to Fig. 4c. (b) Macrophage abundance in colon was evaluated by flow cytometry related to Fig. 4e. n = 6 biologically independent samples. (c) Cy3-conjugated conjugated EUB338 probe (PBS group), K. pneumoniae probe (KP and KPC groups) FISH detection in liver tissue related to Fig. 4f. n = 6 biologically independent samples. (d) Representative pictures of TEM, Alcian blue staining, E-Cad, CLDN 1 IHC staining, and Cy3-conjugated conjugated EUB338 probe (PBS group), K. pneumoniae probe (KP and KPC groups) FISH detection of colon tissues with quantitative analysis related to Fig. 4h. (e) Representative pictures of TEM and E-Cad IHC staining of liver tissues related to Fig. 5c. Masson’s trichrome staining and COL IV IHC staining of colon tissues related to Fig. 5e. (f) Representative pictures of PCNA and Ki-67 IHC staining related to Fig. 5g. (g) Representative pictures of Desmin and α-SMA IHC staining, and Sirius red staining of liver sections related to Fig. 5h. (h) Mouse Cancer Pathway Finder Array and Mouse Inflammatory Response and Autoimmunity PCR Array of liver tissues related to Fig. 5a. n = 3 independent experiments with similar results. Data (excluding PCR array results) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Extended Data Fig. 9 Antagonizing K. pneumoniae colonic colonization or TLR4 inhibition reverse K. pneumoniae induced-HCC development.
(a) Design of K. oxytoca on K. pneumoniae induced HCC promotion in germ-free mice with DEN treatment (GFD/PBS group n=6; GFD/EC group n=6; GFD/KO group n=6; GFD/KP group n=7; GFD/KOP group n=8) and relevant results (b–e). (b) Images of liver gross morphology (yellow circles indicate tumor area), and H&E with tumor incidence, tumor number and tumor burden. n=6 (GFD/PBS), 6 (GFD/EC), 6 (GFD/KO) 7 (GFD/KP), 8 (GFD/KOP). (c) Alcian blue staining, E-Cad, CLDN 3, CLDN 1 IHC, and Cy3-conjugated EUB338 probe detection with quantitative analysis. n=6 biologically independent samples. (d) Gut permeability assay using FITC-dextran. n=6 (GFD/PBS), 6 (GFD/EC), 6 (GFD/KO) 7 (GFD/KP), 8 (GFD/KOP). (e) Live bacteria culture of liver tissues on blood agar plates with quantitative analysis. n=6 (GFD/PBS), 6 (GFD/EC), 6 (GFD/KO) 7 (GFD/KP), 8 (GFD/KOP). (f) Design of TLR4i on K. pneumoniae induced HCC promotion in germ-free mice with DEN treatment (GFD/PBS group n=6; GFD/EC group n=6; GFD/KP group n=7; GFD/KPi group n=7) and relevant results (g-k). (g) Images of liver gross morphology (yellow circles indicate tumor area), and H&E with tumor incidence, tumor number and tumor burden. n=6 (GFD/PBS), 6 (GFD/EC), 7 (GFD/KP), 7 (GFD/KPi). (h) E-Cad IHC and Cy3-conjugated EUB338 probe (GFD/PBS group), E. coli probe (GFD/EC), K. pneumoniae probe (GFD/KP) and GFD/KPi groups) detection of colon tissues with quantitative analysis. n=6 biologically independent samples. (i) Gut permeability assay using FITC-dextran. n=6 biologically independent samples. (j) Live bacteria culture of liver tissues on blood agar plates with quantitative analysis. n=6 (GFD/PBS), 6 (GFD/EC), 7 (GFD/KP), 7 (GFD/KPi). (k) Cy3-conjugated conjugated EUB338 probe (GFD/PBS group), E. coli probe (GFD/EC), K. pneumoniae probe (GFD/KP) and GFD/KPi groups) FISH, TLR4, PCNA, Ki-67 IHC, and Sirius red staining of liver sections. n=6 biologically independent samples. Data (excluding tumor incidence and liver with live bacteria) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Tumor incidence and Liver with live bacteria were calculated using Fisher’s exact test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Extended Data Fig. 10 TLR4 inhibition reverses HCC-FMT induced-HCC development.
(a) Design of TLR4i on HCC-FMT induced HCC promotion in germ-free mice with DEN treatment (GFD/PBS group n=8; GFD/HCC-FMT group n=15; GFD/HCC-FMTi group n=10) and relevant results (b–g). TLR4i was daily gavaged in this experiment. (b) Representative images of liver gross morphology (yellow circles indicate tumor area), and H&E staining of mice liver sections with tumor incidence, tumor number, and tumor burden. n=8 (GFD/PBS), 15 (GFD/HCC-FMT), 10 (GFD/HCC-FMTi). (c) Representative bacterial culture of liver tissues under anaerobic and aerobic conditions with quantitative analysis. n=8 (GFD/PBS), 15 (GFD/HCC-FMT), 10 (GFD/HCC-FMTi). From left to right and top to bottom, the culture plates are blood agar plate, chocolate blood agar plate, MacConkey agar plate, and Columbia blood agar plate, respectively. Gut permeability assay using 500 kDa FITC-dextran. n=6 biologically independent samples. (d) Representative pictures of Alcian blue staining, E-Cad IHC staining, and Cy3-conjugated EUB338 probe FISH detection of colon tissues with quantitative analysis. n=6 biologically independent samples. (e) Cy3-conjugated EUB338 probe FISH, Cy3-conjugated K. pneumoniae probe FISH, TLR4, PCNA, Ki-67 IHC of liver sections. n=6 biologically independent samples. (f) α-SMA, Desmin IHC staining, and Sirius red staining of liver sections. n=6 biologically independent samples. (g) Mouse Cancer Pathway Finder Array and Inflammatory Response and Autoimmunity PCR Array of liver tissues. n=3 independent experiments with similar results. Data (excluding tumor incidence, liver with live bacteria and PCR array results) are presented as mean ± SEM. Each data point in bar plots represents one mouse. Tumor incidence and Liver with live bacteria were calculated using Fisher’s exact test. Unless otherwise stated, statistical significance was calculated using one-way ANOVA. Adjustments were made for multiple comparisons.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16, and unprocessed scans of blots and gels.
Supplementary Tables
Supplementary Tables 1–6.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., Fang, Y., Liang, W. et al. Gut–liver translocation of pathogen Klebsiella pneumoniae promotes hepatocellular carcinoma in mice. Nat Microbiol 10, 169–184 (2025). https://doi.org/10.1038/s41564-024-01890-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-024-01890-9
This article is cited by
-
Microbiome modulation of tumorigenesis and immune responses
Journal of Biomedical Science (2026)
-
Genetic evidence for causal links between diet, gut microbiota, and hepatobiliary cancer: a Mendelian randomization study
AMB Express (2025)
-
Gut microbiome in metabolic dysfunction-associated steatotic liver disease and associated hepatocellular carcinoma
Nature Reviews Gastroenterology & Hepatology (2025)
-
Lactobacillus acidophilus potentiates oncolytic virotherapy through modulating gut microbiota homeostasis in hepatocellular carcinoma
Nature Communications (2025)
-
Microbial collagenase activity is linked to oral–gut translocation in advanced chronic liver disease
Nature Microbiology (2025)