Abstract
Monitoring the emergence and spread of drug-resistant parasites is essential for effective malaria control. Here, we describe the prevalence of genetic markers of Plasmodium falciparum antimalarial drug resistance and parasite population structure in Mozambique. Drug resistance loci and microhaplotypes were genotyped by multiplex targeted amplicon sequencing of 1146 P. falciparum samples collected in 2021 (n = 321) and 2022 (n = 825 rainy season, and n = 155 dry season). pfpm2 gene copy number (associated to piperaquine resistance) was assessed using real-time quantitative PCR. No pfk13 markers of partial artemisinin resistance nor pfpm2 duplications were observed. Prevalence of pfdhfr/pfdhps quintuple mutants associated with sulfadoxine-pyrimethamine (SP) resistance was high across all regions (> 92.5% in 2021 and > 87.8% in 2022), but pfdhps-A581G mutation was rare (1.6% in 2021 and 0.8% 2022). Both prevalence of mutations in pfdhps-436 (p < 0.001) and genetic complexity of infections increased from South to North. These results support the continued use of artemisinin-based combination therapies in Mozambique, call for a close monitoring of chemopreventive efficacy based on SP, and confirm the spatial genetic distinction in P. falciparum population observed across the country.
Similar content being viewed by others
Introduction
Mozambique is one of the four African countries that accounts for over half of all malaria deaths worldwide, the vast majority attributable to Plasmodium falciparum1. Malaria transmission is highly heterogeneous across the country2,3, requiring different strategies to both reduce the burden of malaria where the transmission is high (northern and central Mozambique), and to eliminate malaria where the transmission is low (southern Mozambique). Pharmacological-based interventions are one of the main pillars of National Malaria Control Program (NMCP) approaches to malaria control and elimination across transmission strata4. These include the effective treatment of malaria clinical cases using artemisinin-based combination therapy (ACT; first line treatments: artemether-lumefantrine [AL] and artesunate-amodiaquine [AS-AQ])5, chemoprevention in children (i.e., perennial malaria chemoprevention [PMC] with sulfadoxine-pyrimethamine [SP] and seasonal malaria chemoprevention [SMC] with SP-AQ), chemoprevention in pregnant women (i.e., intermittent preventive treatment [IPTp] with SP), and treatment of the whole population in near elimination or emergency settings (mass drug administration [MDA] with dihydroartemisinin-piperaquine [DP])5.
P. falciparum has shown a remarkable ability to develop resistance to multiple antimalarial drugs, and the emergence of resistance to artemisinin and partner drugs within ACTs in Africa poses a significant threat for their potential impact on antimalarial interventions6,7,8,9,10,11. Monitoring the emergence, evolution and spread of drug-resistant parasites is crucial for implementing effective malaria control strategies. The gold standard for drug efficacy, the in vivo therapeutic efficacy studies (TES), are logistically challenging and costly, thus conducted intermittently and at a limited number of sites, potentially missing geographical heterogeneities. Genomic surveillance constitutes a powerful approach for high-throughput and routine data generation that can complement and/or inform TES, as it has the potential to provide information not only on a wide range of resistance genotypes but also on other genotypes of programmatic interest, such as vaccine targets or markers of genetic diversity for TES case classification12, parasite importation or transmission intensity stratification.
Several genetic polymorphisms have been validated as markers of antimalarial resistance and associated with failure to ACT treatment13. Point mutations in kelch 13 gene (pfkelch13) confer partial resistance to artemisinin derivatives14, and multiple gene copies in plasmepsin-2 (pfpm2) have been associated with resistance to the piperaquine, the partner drug in DP formulation15. However, there is no well-validated marker of resistance for lumefantrine in AL, which is currently the most common treatment in both public and private sector markets in Africa13. In the case of SP, parasitological resistance can be conferred by accumulation of mutations in the genes dihydrofolate reductase (pfdhfr; codons 16, 51, 59, 108, 164) and dihydropteroate synthase (pfdhps; codons 431, 436, 437, 540, 581, 613). The quintuple combination pfdhfr 51-59-108 + pfdhps 437–540 and, most notably, the sextuple combination that includes pfdhps-581 have been associated to clinical and parasitological failures to SP treatment16,17. Resistance to chloroquine is conferred by mutation 76 in chloroquine transporter gene (pfcrt), whereas point mutations in multidrug resistance gene 1 (pfmdr1) in combination with pfcrt mutants have been associated to amodiaquine resistance18. To date, only one study has reported the spatial and temporal distribution of antimalarial resistance in Mozambique with data from 201819. This study indicated that parasite population was characterized by a south-to-north decreasing gradient in resistance to SP (i.e., prevalence of quintuple mutants), accompanied by a concentration of pfdhps-436 mutations (A/C/F/H) in the North, and south-to-north increases in genetic complexity, pointing to the importance of understanding geographical heterogeneity of P. falciparum population, its relation with transmission intensity, and its potential impact on policy.
Since 2021, Mozambique’s NMCP has been relying on the genomic surveillance for guiding programmatic decisions20. Among priority use cases are (1) the routine monitoring of drug and diagnostic resistance markers and (2) characterization of genetic diversity metrics across different malaria transmission intensity strata to inform malaria burden (i.e. as complementary indicators of epidemiological metrics of transmission intensity)20,21. For this purpose, a novel multiplex amplicon sequencing assay with capacity to generate data for 15 drug-resistance genes and 165 highly diverse microhaplotypes was established22. Here we present the first report of this surveillance effort with high throughput sequencing data generated in-country and investigate parasite population structure at different regions as well as the effect of seasonality on genetic metrics.
Results
Sample selection and geographical coverage during 2021 and 2022 rainy seasons
Patients with a confirmed diagnosis of uncomplicated P. falciparum malaria by rapid diagnostic test (RDT) were recruited at different cross-sectional surveys at health facility level during two consecutive rainy seasons (January to May) in 2021 and 2022. One hundred dried blood spot (DBS) samples were randomly selected for sequencing per province and year, unless the sample size was less than 100, resulting in a total of 1285 DBS (387 from 2021 to 898 from 2022). The locations where the samples were collected are presented in Fig. 1. Provinces with initial number of DBS < 100 were Inhambane (80), Manica (33) and Niassa (67) in 2021, and Sofala (86) and Cabo Delgado (91) in 2022. Positive qPCR confirmation followed by successful sequencing (i.e., with allele calls passing negative controls and allele frequency filters, see Methods) was obtained from a total of 321 DBS for 2021 (83%) and 825 (92%) from 2022, as detailed in Table 1. With sample size computed at the regional level (South, Center and North), the study had a > 80% power for the detection of genetic variants at a frequency of 2% for 2021, and 1% for 202223.
Demographic characteristics of patients and parasite densities determined by P. falciparum 18S qPCR are shown in Table 1. Age differed between regions, with a higher proportion of older than five-year patients in the South compared to Centre and North. Note that children were the targeted population in all surveys, except in Maputo Province (in the South) in 2022, which also included adults. Patients did not differ in gender (p > 0.116). Overall parasite densities did not differ between 2021 and 2022 (p = 0.305). Between regions, densities were significantly higher in the South only in 2021 (p < 0.001; Table 1).
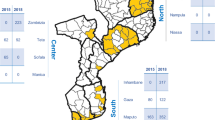
Location of health facilities included in the study. Map of Mozambique with province (thick black lines) and district (thin grey lines) administrative divisions. Yellow dots indicate the location of health facilities in the 2021 (A) and 2022 (B) surveys. Geographical regions used in primary analysis are colored (South in blue, Centre in green, North in red). Map generated from OpenStreetMap data available under the Open Database License and modified in Python 3.
Genetic markers of drug resistance
Extracted DNA was subject to amplicon-based sequencing using the MAD4HatTeR modular assay22. The following drug resistance genes were analyzed for genetic polymorphisms, based on current, past or planned uses of antimalarials in Mozambique: pfk13, pfdhps, pfdhfr, pfcrt, pfmdr1 and pfpm2. In addition, pfexonuclease, pfarps10, pfferredoxin, pfmdr2 were screened for their potential role in artemisinin and DP resistance. The sequencing depth for the amplicons covering selected loci was 1155 median reads (interquartile range, IQR: 592, 2086). By study year, the median was 653 reads/sample/amplicon (IQR 352, 1025) in 2021 samples, and 1499 reads/sample/amplicon (IQR 807, 2512) in 2022 samples (Supplementary Fig. 1).
Polymorphisms in pfk13 gene and artemisinin-resistance background
The prevalence of infections carrying mutant parasites by year and geographical region is presented in Table 2 (detail by province in Supplementary Tables 1 and 2). None of the samples sequenced from the 2021 or 2022 rainy seasons carried pfk13 validated or candidate markers of partial artemisinin resistance. However, up to 17 different non-synonymous mutations were found in 20 different samples, accounting for 2% (6/297) of infections in 2021 and 1.8% (14/772) in 2022 (Tables 2 and 3). Seventeen out of the 20 non-synonymous mutants were part of mixed infections with the wild-type allele. A higher prevalence of non-synonymous mutations was detected in the North (3.1–3.2%) as compared to Centre (0.9-2%) and South (0-1.1%), but the trend was not statistically significant. Mutations G449S (South, Maputo province, 2022) and R561C (North, Nampula province, 2022) occurred at the same codon as other validated/associated mutations13. Mutations associated with a parasite genetic background favorable for the emergence of artemisinin resistance in South-East Asia, namely, pfarps10 (V127M), pfferredoxin (D193Y) and pfmdr2 (T484I)24, were not present in Mozambique.
Polymorphisms in pfpm2 and other piperaquine-resistance associated markers
Read counts were used to screen for candidate samples carrying putative pfpm2 copy number variations (CNV, defined as > 1.5-fold deviations from the expected read counts for single-copy pfpm2), a genotype associated with resistance to the partner drug piperaquine in DP formulation25. The screening was conducted in samples with parasitemia > 1000 parasites/µl to enhance robustness (74,6%, corresponding to 855 out of 1146; see Methods). Twenty-three (2.6%) samples had an observed fold-change > 1.5 (median 1.6 [IQR 1.5, 1.8]), but none was confirmed to carry CNV in subsequent qPCR analysis (see Methods). Mutation E415G in pfexonuclease, previously linked to reduced piperaquine IC50 in genome-wide association analysis26, and mutations T93S, H97Y, F145I, I218F and C350R in pfcrt, associated to minor-to-moderate decreases in in vivo or in vitro piperaquine resistance outside Africa27, were not detected in any sample.
Polymorphisms in Pfdhfr and Pfdhps genes
SP resistance markers were evaluated based on the combination of mutations in pfdhfr and pfdhps. Overall, 822/897 (91.6%) samples carried pfdhfr and pfdhps quintuple mutant (IRNGE; 94.1% in 2021 and 90.7% in 2022, p = 0.103; Table 2). Comparisons by region indicated a higher prevalence of quintuple mutants in the South of the country (174/182 [97%]) as compared to Centre (237/270 [88%]) and North (186/206 [90%]; p = 0.018) in 2022, which was independent of age group (older/younger than 5 years), gender and parasite density category (below or above 500 parasites/µL; Table 2 and Supplementary Table 4). No statistically significant differences were observed in the prevalence of quintuple mutants by age groups, gender or parasite density (Supplementary Tables 3 and 4). Eleven samples carried pfdhps variant A581G (five in 2021 [1.6%] and six in 2022 [0.8%]). Ten of the A581G samples were detected in the North or Centre of the country and one in the South (Table 2; detail by province in Supplementary Tables 1 and 2). All A581G mutants with available data at other dhps loci (n = 10) were accompanied by K540E mutation.
Following previous reports from 201819, we looked for variation at codon 436 of pfdhps. Four variants were identified (A/C/F/H), with alleles A and F as the most common substitutions (93% of mutants in 2021 and 92% in 2022). South to North prevalence of pfdhps S436ACFH ranged from 1.4 to 23.7% in 2021 and from 2.1 to 24.4% in 2022 (Table 2 and Supplementary Tables 3 and 4). Prevalence of pfdhps 436 mutants was higher in children below 5 years of age (71/485 [15%]) compared to older children (7/309 [2%]; odds ratio for > 5 years in multivariate analysis = 0.24 (95% CI 0.1, 0.6); p < 0.001) (Supplementary Table 4). No mutations at pfdhps codon 431 were detected.
Polymorphisms in Pfcrt and Pfmdr1
All but one sample were wild type at codons 72–76 of pfcrt (CVMNK haplotype). A single CVIET haplotype sample was detected in Nampula in 2022 (Supplementary Table 2). pfmdr1 mutation Y184F prevalence also presented a geographical South-North gradient, being lowest in the South (< 60%) and higher in the North of the country both in 2021 and 2022 (> 76.3%; p = 0.001; Table 2) independently of age, gender or parasite density (Supplementary Tables 3 and 4). Mutations in codons pfmdr1-86 and pfmdr1-1246 were rare (< 1%; Supplementary Tables 1 and 2), and no variations were detected at pfmdr1-1034 and pfmdr1-1042 positions in any sample.
Genetic diversity and population structure in 2022 rainy season
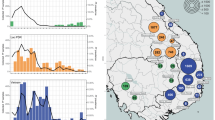
Genetic diversity was estimated using data from 165 microhaplotypes. The analysis focused on 2022 samples due to its wider geographical coverage and larger sample size per region (Supplementary Fig. 2). Median naive COI of all 2022 infections was 2 (IQR 1, 4). Median eCOI, which incorporates intra-host relatedness between clones (see Methods), was 1.6 (IQR 1, 2.6). eCOI was higher in the North (1.8, IQR 1.1, 2.9) and Centre (1.7, IQR 1, 2.7) as compared to the South (1.3, IQR 1, 1.9; p < 0.05; Fig. 2A) Similarly, the proportion of polyclonal infections was highest for the North (193/264, 73%), followed by the Centre (216/336, 64%) and lowest in the South (112/206, 54%; p < 0.001; Fig. 2B). Intra-individual heterozygosity measured by 1-Fws showed a similar north-south gradient, suggesting inbreeding in the parasite population in southern provinces (Fig. 2C). Statistically significant differences in eCOI, proportion of polyclonal infections and 1-Fws were maintained in the North and Center as compared to South after correcting for the confounding effect of age, gender or parasite density in multivariate regression models (Supplementary Table 5). The intra-host diversity metrics results stratified at province level is provided in the Supplementary material (Supplementary Fig. 3). Population mean expected heterozygosity (HE) at 165 microhaplotype loci was 0.54 (95% CI 0.53, 0.55), ranging from < 0.01 to 0.92. Comparisons between the three areas indicated highest HE in Centre provinces (Fig. 2D). The exploratory analysis of genetic diversity metrics for 2021 (n = 321) showed no differences in intra-individual metrics as compared to 2022, but lower HE, which can be attributable to low sample size and lower number of provinces included within each region (Supplementary Fig. 4).
Population structure was determined by clustering analysis of Principal Coordinates (PCoA). PCoA coordinates 1 and 2 allowed to differentiate South from North populations, whereas samples from central provinces showed more proximity to those in the North. As expected, based on prevalence data, most samples with pfdhps-436 clustered in the North, but there was no further particular sub-clustering of this group (Fig. 3A). Correlation of Bray-Curtis distance from population allele frequencies with the geographical distance between samples was significant (Mantel’s rho = 0.73 p = 0.001; Fig. 3B), further supporting geographical patterns based on latitude.
Population structure in samples from 2022 rainy season. A) Principal Coordinates Analysis with allele frequencies. Colors indicate administrative regions (red tones, North; green tones, Centre; blue tones, South). Samples with mutations in pfdhp-s436 and pfdhps-581 codons are indicated with hollow shapes. Bray-Curtis was used as the dissimilarity metric. B) Permutational Mantel test of Bray-Curtis distance calculated from population allele frequencies vs. geographic distance (Mantel’s rho = 0.73 p = 0.001; Spearman’s test with 10000 permutations).
Population structure of Pfdhps allelic haplotypes
To further investigate the evolutionary history of pfdhps mutations, we analyzed 607 monoallelic infections for 6 loci covering or flanking the pfdhps gene (total concatenated sequences 1143 nucleotides). The set included 12 pfdhps-436 mutants: 11 from the North (9 S436A, 1 S436C, 1 S436F), and 1 from the South (S436A). Phylogenetic reconstruction focusing on pfdhps-436/437/540 haplotypes indicated a strong differentiation between the most common wild-type/mutant/mutant (wt/mut/mut) haplotype and the mut/wt/wt, with the latter more closely related to wt/wt/wt (Fig. 4A). Infections with a mutant pfdhps-436 were more likely to be wild type for pfdhps-437/540 (p < 0.001; Cramér’s V = 0.543; Fig. 4B), while wild type pfdhps-436 infections were more likely to be mutant for both pfdhps-437/540. Triple mutants for the pfdhps-437/540 codons were rare. Overall, haplotypes wt/wt/wt, wt/mut/mut, and mut/wt/wt were significantly associated with populations at regional level, although the association was weak (p < 0.001; Cramér’s V = 0.108) (Fig. 4). The distribution of all three haplotypes across regions was significantly different from what would be expected by chance (wt/wt/wt: p = 0.017, Cramér’s V = 0.063; wt/mut/mut: p < 0.001, Cramér’s V = 0.101; mut/wt/wt: p < 0.001, Cramér’s V = 0.082). At the province level, statistically significant associations were moderate for wt/mut/mut (p < 0.001, Cramér’s V = 0.26) and weak for mut/wt/wt (p < 0.001, Cramér’s V = 0.132; Supplementary Fig. 5).
Population structure of pfdhps-436/437/540 haplotypes for 2022 rainy season in Mozambique. A) Maximum likelihood phylogeny. Branch support (> 50%) from 1000 bootstraps is shown. Tips are colored by genotype and labeled by region. B) Heatmap of genotype occurrences. C) Relative frequency of haplotypes for each region with error bars.
Effect of seasonality on drug resistance and genetic diversity
The provinces of Maputo and Manica were used to study the effect of interannual malaria seasonality on drug resistance markers in 2022. According to the DHIS2 data, the RDT positivity rate among less than 5 year old children (who sought care at either a heath facility or community healthcare worker level differed significantly in Manica province between rainy (January to May; median 59.5% [IQR 41.1–72.2]) and dry season (June to September; 47.8% [31.0-64.2]; p < 0.001), but was similar throughout the year in Maputo (7.2% [3.3–10.6] in rainy vs. 5.7% [3.0-8.1] in dry; p = 0.230; Supplementary Fig. 6). One-hundred samples were randomly selected per province, out of which 70 and 85 were confirmed qPCR positive and produced valid sequences for Maputo and Manica, respectively. No statistically significant differences in main demographic or parasitological characteristics were found between seasons (Tables 4). Prevalence of pfmdr1-Y184F in Maputo province was higher in the dry season, and both pfmdr1-Y184F and pfk13 non-synonymous mutations were more common in the dry season when adjusting for age, gender and parasite levels (Table 4 and Supplementary Table 6). No differences were found in the percentage of polyclonal infections, eCOI and 1-Fws (in rainy compared to dry seasons (Table 4), neither after adjusting by age gender or parasite density. HE was higher in rainy as compared to dry season in Manica (p = 0.017) but not in Maputo.
Discussion
In this study, we applied a sensitive, high-throughput genomic assay to detect signals of drug-resistance and genetic diversity in malaria parasites from nine provinces and three regions in Mozambique, representative of the different malaria transmission strata in the country. We found that (1) markers of partial resistance to artemisinin derivatives and resistance to piperaquine partner drug were residual or absent; (2) there was a high prevalence of pfdhfr/pfdhps quintuple mutants in all three regions, but the prevalence of sextuple mutants remains very low; (3) the mutations were not influenced by gender and parasite density; (4) there was a south-to-north gradient in genetic complexity and prevalence of mutations at pfdhps-436.
The continued monitoring and identification of drug-resistant P. falciparum parasites is crucial in the fight against malaria. Out results show the absence of validated or candidate mutations associated with partial artemisinin resistance in 2022, in line with previous studies conducted in the country with samples from before 202128,29,30,31,32. However, 17 non-synonymous mutations were detected in the pfk13 gene, two of which had already been reported in Mozambique (G436S19 and A578S19,29). To date no mutants detected in Mozambique have been associated with a resistant phenotype, although a recent case of imported malaria from Mozambique to Portugal reported elevated ex vivo half-maximal inhibitory concentrations values to dihydroartemisinin in the presence of pfk13-I416V mutation33. We did not find that mutation in this study. The absence of pfpm2 mutants (and any other candidate markers of piperaquine resistance)27 is reassuring for an adequate efficacy of DP, currently being used as the ACT of choice in mass drug administration campaigns led by NMCP. However, the risk of emergence of ACT resistance in Mozambique remains due to continuous drug pressure and the recent emergence of artemisinin partial resistance in neighboring Tanzania9,34 and other East African countries6,8,10,11.
The overall rate of pfdhfr-pfdhps quintuple was high across the three regions and, despite South still had the highest rates, there was a notable increase in the North (90.3% in 2022) compared to levels observed in 2015 (40%) and 2018 (72%)19, leading to an homogenization of pfdhfr-pfdhps prevalences across the country. The fact that sextuple mutants were uncommon, together with evidence generated from clinical studies35, suggests that chemopreventive strategies based on the use of SP still retain some chemopreventive effect in the current parasite molecular resistance background35.
A clear North-South divide was also found in the distribution of pfdhps-S436A, pfmdr1-Y184F and in the genetic complexity of P. falciparum, with higher prevalence of mutations and diversity found in the high burden provinces of the North. PCoA and a Mantel test support a notable regional structuring of parasite populations in line with previous observations in years 2015 and 2018 derived from WGS data19. The large geographical distance between regions, local heterogeneity in intervention coverage and difficult inter-regional population movement, likely contribute to ecological barriers with restricted parasite migration and limited gene flow. Interestingly, mutations in pfdhps-S436A clustered with those wild-type at 437 and 540 codons, suggesting a common origin. In the case of mdr1-Y184F, not only levels in the North were relatively higher, but prevalence in the region increased from 50 to 56% in 2015/18 to 77% in 202219. Drivers of differences in these resistant markers are unclear. There are no differences in treatment policy in the North compared to the South of the country, but coverage of antimalarial interventions may vary between regions due to the unequal distribution of resources and security issues, what could exert differential drug pressures. In addition, we cannot exclude that a pilot implementation of SMC with amodiaquine-SP ongoing in Nampula province since 2020 could contribute to expansion of pfmdr1-Y184F, as previously observed in other countries36.
Parasite genetic diversity has been proposed as an indicator to characterize the key drivers of ongoing transmission, to identify foci and to discriminate between indigenous and imported cases in areas approaching elimination37. It has been suggested that reductions in P. falciparum genetic diversity and increased similarity due to inbreeding and recent common ancestry reflect bottlenecks in parasite population driven by control and elimination efforts37,38. Our data indicates that intra-host metrics of genetic diversity align with a South-to-North increase in regional transmission intensity measured with traditional epidemiological indicators. Although the study was not designed to capture fine scale/local changes, these results add-up to the reports supporting the use of P. falciparum genetic diversity measures to supplement traditional surveillance for improving stratification, monitoring and impact evaluations in different epidemiological contexts, especially where epidemiologic surveillance data is sparse39. This use case still requires development of analytical and interpretative tools to infer malaria burden and effectiveness of interventions, as well as validation of sampling frameworks38,40.
One of the concerns in the design of sampling schemes for malaria molecular surveillance is the time of collection, especially in areas where malaria transmission is highly seasonal23. We addressed this question by sequencing samples from the dry season in Manica (a province with marked seasonality in malaria positivity rates) and Maputo (a province with no seasonality, selected as control). Differences in both pfk13 and pfmdr1 Y184F were observed in the Maputo control province when adjusting for demographic factors, whereas no impact was seen in Manica (although comparisons were limited by sample size and lack of variation). Overall data suggests that time of sampling may have some impact in the genetic metrics measured especially in dry season for low transmission areas, where outbreaks, cluster effects or importation events, among other situations, could impact in both increasing or decreasing point prevalence estimates.
The study presents some limitations. First, different sampling schemes were followed in 2021 and 2022. Surveys in 2021 were conducted at multiple randomized health facilities but in a limited number of provinces, some of which had very low sample sizes. Surveys in 2022 covered a wider, more balanced geographical area, but health facilities were selected by convenience. Although most results showed consistency across years, we cannot exclude biases due to this heterogeneity. Second, we acknowledge some technical limitations that should be considered in data interpretation, such as the exclusion of polyclonal infections in haplotype reconstruction, or low densities samples in the estimation of pfpm2 CNV. Finally, although genetic markers of resistance provide a baseline and early warning signal for antimalarial resistance, drug efficacy should ultimately be confirmed using therapeutic and/or chemoprevention efficacy studies.
Conclusions
P. falciparum parasites circulating in Mozambique in 2021 and 2022 were characterized by the absence of molecular markers of partial resistance to artemisinin and of resistance to ACT partner drugs, suggesting an appropriate efficacy of artemisinin-based combination therapy for P. falciparum treatment. Infections presented high rates of pfdhfr/pfdhps quintuple haplotype, which increased in the North compared to data from 2018, but were mostly wild type for dhps-581 mutation that would confer additional SP resistance. Parasite populations in Northern provinces also present higher genetic diversity and a cluster of pfdhps-436 mutants, pointing towards the importance of continuous surveillance of these mutations in Mozambique. The results of this study are informative for public health decision makers on discussions about malaria treatment and control.
Methods
Study design and sample collection
In 2021, malaria patients aged 2–10 years old were recruited as part of the National Health Facility Survey (NMCP, Ministry of Health) that evaluated quality of malaria case management at public outpatient clinics in six provinces of Mozambique (Maputo, Inhambane, Manica, Zambézia, Niassa and Nampula). 40 health facilities were randomized out of all eligible centers to maximize representativity (Fig. 1). In 2022, targeted sampling was conducted at selected health facilities in Maputo province (all ages > 6 months) and Inhambane, Manica, Zambézia, Sofala and Manica (children 2–10 years old). Samples from Nampula were obtained from children aged 3 months to 5 years old at health facilities in areas where seasonal malaria chemoprevention was implemented. Samples from Tete and Cabo Delgado were obtained from the 2022 therapeutic efficacy survey (children aged 6 months to 5 years old), with an additional inclusion criterion of parasite density > 2500 parasites per microliter by microscopy20. Additional surveys were conducted in 2022 during dry season (June to October) for Maputo and Manica provinces to study the effect of malaria seasonality on molecular markers of antimalarial resistance and parasite diversity.
In all surveys, patients presenting with a confirmed diagnosis of uncomplicated P. falciparum malaria by RDT were invited to participate20. After they provided informed consent, two to four 50 µL DBS were prepared onto one or two filter papers through finger prick. DBS were identified with anonymous barcodes, air-dried during 72 h and stored at 4 °C in sealed bags with silica gel until laboratory processing.
Genomic DNA extraction and quantification
Genomic DNA was extracted from DBS samples using a Tween-Chelex based protocol as described by Brokhattingen et al.30, with some modifications. Five mm (~ 12,5 µL blood) discs were cut from each DBS into 96-well deep well plates with a manual puncher. One mL of freshly made 0.5% Tween 20® detergent diluted in PBS was added to each well plate containing a DBS punch and incubated overnight in a thermomixer at 15ºC and 300 rpm. The next morning, the supernatant was removed, 1 mL of fresh PBS was added per well and the plate was briefly vortexed and then incubated at 4ºC for 30 min. After incubation, the liquid was aspirated, and 150 µL of a solution of 10% Chelex (C7901, Merck) in molecular grade water was added. The samples were incubated at 95ºC in a water bath for 15 min with gentle vortexing every 5 min. The plate was then centrifuged for 5 min at 1500 rpm to pellet the Chelex® beads. Supernatant (approximately 130 µL) containing the eluted DNA was transferred to a new PCR 96 well plate, centrifuged again and finally 100 uL transferred to barcoded tubes placed on 96 well plate (Wilmut).
P. falciparum infection was confirmed in all DNA samples by qPCR targeting the 18 S rRNA gene on an ABI PRISM 7500 HT Real-Time System (Applied Biosystems), as previously described41. Parasite density was quantified by extrapolation to an external standard curve composed of six 1:10 dilutions of 3D7 (MRA-151, MR4, Bei Resources) cultured parasites in whole blood spotted onto filter paper (range 100.000 to 1 parasites/µl). DNA was stored at -20ºC until sequencing.
Amplicon-based sequencing
Sequencing was performed using the MAD4HatTeR multiplex amplicon sequencing panel using CleanPlex reagents and CleanMag Magnetic Beads (Paragon Genomic Inc, California, USA) as previously described22. Briefly, we used two multiplexed PCR reactions with primer pools D1.1, R1.2 (reaction 1) and R2.1 (reaction2). Together, these primer pools target 241 P. falciparum loci of 225–300 bp22. Multiplex PCR was performed with 10 cycles if parasite density ≥ 500 parasites/µL, or 20 cycles for those samples with < 500 parasites/µL. Following multiplex PCR, reactions proceeded in a single tube for each sample, where products were bead-cleaned, digested, and indexed via PCR to generate Illumina-compatible libraries. A randomly selected subset of 10 libraries from each full plate was assessed using automated capillary electrophoresis in a TapeStation 4150 (Agilent technologies, California, USA) to confirm library quality (size and concentration). Finally, libraries from each sample were pooled adjusting volumes based on parasitemia, and the pool was bead-cleaned using a 1X bead ratio to remove primer dimers. Pooled libraries were run on an agarose gel, from which the amplicon-sized band was excised. DNA was extracted using Monarch® DNA Gel Extraction Kit (New England Biolabs Inc., Massachusetts, USA), and products were quantified using a TapeStation and a Qubit fluorometer. For 2021 samples, pools contained 96 samples and were 150 paired-end sequenced in a MiSeq System with v2-300 cycles reagents (Illumina, USA) at CISM laboratory; for 2022 samples, pools of 288 samples were also sequenced with 150 paired-end reads in a NextSeq 2000 System using P1 reagents at ISGlobal laboratory.
Positive (n = 2, matching the parasitemia category of the samples in the plate) and negative (n = 2) controls were included in every library preparation plate to control for run quality and contaminations. Positive controls were prepared from P. falciparum laboratory strains 3D7 (MRA-151), HB3 (MRA-155), Dd2 (MRA-156 and MRA-1255). Cultures were synchronized in the ring stage, mixed with uninfected human whole blood to obtain a range of parasite densities (1, 10, 100, 1,000, 10,000 and 100.000 parasites/µL) and spotted onto filter paper. Negative controls were prepared from P. falciparum negative DBS.
Sequence data analysis
FASTQ files were subjected to filtering, demultiplexing and allele inference using a Nextflow-based pipeline version 0.1.8 (https://github.com/EPPIcenter/mad4hatter)22. The 3D7 genome sequence was used as reference for alternative allele calling (https://github.com/EPPIcenter/mad4hatter/blob/main/resources/v4/ALL_refseq.fa). The resulting allele tables were subsequently filtered based on read counts and coverage across loci within a sample and across samples. Alleles with fewer reads than the maximum observed reads in any locus for negative controls were removed, along with alleles with < 1% within-sample frequency.
Prevalence of antimalarial drug resistance markers was defined as the number of P. falciparum infections carrying mutant alleles (including mixed infections) out of total infections with valid allele calls per each locus. Reconstruction of pfdhps double, pfdhfr triple and pfdhfr/pfdhps quintuple haplotypes was done for samples with no mixed genotypes at selected loci and for those with allele frequency > 95%. Within-host and population level metrics of diversity were calculated from diversity locus (n = 165) as recently described30. Samples with < 50 diversity loci covered at a read depth of at least 100, and diversity loci with < 100 samples covering them with at least 100 reads were filtered out. Intra-host complexity of infection (COI) and effective COI (eCOI) were estimated from polyallelic genomics data using a Markov Chain Monte Carlo (MCMC)-based approach implemented in MOIRE v3.4.0 (https://github.com/EPPIcenter/moire). eCOI considers within-host relatedness, and can be interpreted as the expected COI if population diversity was infinite (heterozygosity = 1). Polyclonal infections were defined as having eCOI > 1.130. Wright’s inbreeding co-efficient (Fws) was calculated as 1-Fws across all diversity loci as the allele heterozygosity of the individual (HW) relative to the population42. Genetic diversity of the parasite population was measured using expected heterozygosity (HE), i.e., the probability that two randomly selected parasites carry distinct alleles at each diversity locus30. Principal Coordinates Analyses (PCoA) of in-sample allele frequencies and presence/absence of alleles were done to visualize the differences between provinces and regions. Bray-Curtis dissimilarity matrices were computed using the R package vegan (https://vegandevs.github.io/vegan/)43. A permutational Mantel test (10000 permutations) utilizing Spearman’s correlation method was conducted to evaluate the correlation between genetic (Bray-Curtis dissimilarity) and geographic distances using vegan. Geographic coordinates for each province were used to calculate a Haversine matrix.
To infer the evolutionary history of the mutant alleles in pfdhps, we focused on six specific amplicons surrounding the pfdhps gene covering positions 549,583 to 596,266 on chromosome 8 (549583–549807, 549960–550215, 550057–550318, 585331–585590, 585703–585949, and 585993–586266). Each amplicon was aligned separately with the R package msa44 and concatenated into a 1143-nucleotide sequence. Pairwise distances between infections were calculated, and an initial unrooted tree was constructed using the minimum evolution algorithm45. The best evolutionary model was identified using the Bayesian Information Criterion (BIC), and 1000 bootstrap replicates were performed for statistical support. Phylogenetic reconstruction was conducted with the Phangorn package in R46. Chi-squared tests assessed associations between pfdhps-436 codon (mutant or wild type) and pfdhps-437/540 codons, between haplotypes (wt/wt/wt, wt/mut/mut, and mut/wt/wt) and populations (region or province) and between frequency of each haplotype across regions and provinces. Cramér’s V was used to measure effect sizes. Haplotypes mix/wt/wt and mix/mix/wt were considered mut/wt/wt, and the rest of mixed haplotypes were excluded from the analysis. Single mutants for dhps437/540 were grouped into one category for the codon association analysis. The population association analysis only included haplotypes wt/wt/wt, wt/mut/mut, and mut/wt/wt.
Copy number of pfpm2
MAD2HatTeR data was screened for CNV at pfpm2 locus using read counts. A generalized additive model was constructed based on amplicon read counts for each sequencing run under the assumption that most amplicons correspond to single-copy genes. Fold change values were calculated from the difference between observed and expected read counts, and further normalized using the resulting fold change of single-copy laboratory 3D7 controls sequenced in each run to account for technical variation. Additionally, the 5% of amplicons with the highest read count variability across sequencing runs were excluded from the fold change calculation to reduce noise. Samples with normalized fold changes greater than 1.5 were flagged as potential CNV. The analysis was restricted to samples with a parasite density > 1000 parasites/µL, as lower parasitaemia samples are more prone to produce inconsistent read counts across runs22.
Samples with read fold-change > 1.5 were tested for pfpm2 CNV adapting a previously published qPCR protocol15 by using ubiquitin conjugated enzyme (pfuce) as reference gene47. Reactions were set-up in an ABIPrism 7500 Thermocycler with Power SYBR Green master mix (ThermoFisher). Amplification efficiencies calculated from standard curve build with 3D7 genomic DNA were 0.90% for pfpm2 and 0.81% for pfuce. Copy numbers were calculated using ddCt method and 3D7 (one pfpm2 copy) as calibrator sample. Samples with CNV > 1.5 by qPCR were considered pfpm2 duplications. DBS from a field isolate with confirmed four pfpm2 copies were included as positive control (kindly donated by Prof. Didier Ménard, Institute Pasteur).
Definitions and statistical analysis
Regions for geographical analysis were South (Maputo, Inhambane), Centre (Manica, Sofala, Zambézia, Tete) and North (Nampula, Niassa and Cabo Delgado). Maputo Province and Maputo City, which are administratively independent provinces, were considered as a single unit for sampling purposes and in secondary analysis at the province level. Maps were created using Python 3.9 from the public OpenStreetMap data.
The prevalence of infections carrying parasites with markers of antimalarial resistance were estimated per region, year and transmission season. Validated and associated markers of antimalarial drug resistance were considered as those included in WHO’s 2020 review report on antimalarial drug-resistance13. Rainy season was defined as the period between January 1st and May 30th, and samples collected after this date and up to October 31st were considered dry season. Malaria case data per each province and season was extracted from the Health Information System for Monitoring and Evaluation (SIS-MA, Ministry of Health, Mozambique). Health facilities reporting a minimum of 96 malaria rapid diagnostic tests per study month were included to avoid biases for health facility with sporadic testing patters (95% confidence interval and 80% power). Positivity rate was defined as the percentage of positive malaria RDTs out of the total tests conducted in each of the health facilities.
Statistical analyses were performed in Stata version 15.0 or R version 4.3.1. Chi-square test, Fisher’s exact were used to compare frequencies of resistance markers and differences in percentage of polyclonal infections by location and/or year. Kruskal–Wallis rank sum test was used for the comparison of the distribution of parasite densities, read counts, eMOI or 1-Fws between populations. Multivariate regression models for genetic metrics were build adjusting for the age (older/younger than 5 years), gender and parasite density (below or above 500 parasites/µL. Genome-wide He estimate and 95% confidence intervals were calculated by a linear mixed model fitting locus as a random effect. To compare the malaria positivity rates between the rainy and dry seasons within each province, the Wilcoxon rank sum test was employed. P-values lower than 0.05 was considered to indicate statistical significance.
Ethical considerations
Clinical-demographic data and blood samples were collected only after written informed consent was obtained from participants and/or their accompanying adults. All RDT positive individuals were treated according to national treatment guidelines. Study protocols were approved by the Mozambican National Committee for Bioethics in Health (CNBS, references number 354/CNBS/2021 and 604/CNBS/21) and Hospital Clinic de Barcelona Ethics Review Committee (ref. HCB/2022/0097).
Data availability
Sequencing data is available at NCBI Sequence Read Archive (SRA) under BioProject PRJNA1107381 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1107381): accession numbers SAMN41182041-SAMN41182361 (2021 samples), and SAMN41181143-SAMN41182039 and SAMN41180171-SAMN41180265 (2022 samples). The raw epidemiological data includes personal information and is protected by data privacy laws. Anonymized databases can be made available for research purposes from the corresponding author upon reasonable request by email. Requests for data will be reviewed in a three-month timeframe by Manhiça Health Research Center to verify that data sharing is not subject to any intellectual property or confidentiality obligations. If data can be shared, it will be released via a Data Transfer Agreement.
References
WHO. Strategy to Respond to Antimalarial Drug Resistance in Africa. (2022).
Arroz, J. A. H. Increase in cases of malaria in Mozambique, 2014: epidemic or new endemic pattern? Rev Saude Publica 50, (2016).
Ejigu, B. A. Geostatistical analysis and mapping of malaria risk in children of Mozambique. PLoS One 15, (2020).
Oladipo, H. J. et al. Increasing challenges of malaria control in sub-Saharan Africa: Priorities for public health research and policymakers. Annals of Medicine and Surgery vol. 81 Preprint at (2022). https://doi.org/10.1016/j.amsu.2022.104366
WHO. Global Technical Strategy for Malaria 2016–2030, 2021 Update. (2021).
Fola, A. A. et al. Plasmodium falciparum resistant to Artemisinin and diagnostics have emerged in Ethiopia. Nat. Microbiol. 8, 1911–1919 (2023).
Rosenthal, P. J. et al. The emergence of artemisinin partial resistance in Africa: how do we respond? The Lancet Infectious Diseases Preprint at (2024). https://doi.org/10.1016/S1473-3099(24)00141-5
Assefa, A., Fola, A. A. & Tasew, G. Emergence of plasmodium falciparum strains with Artemisinin partial resistance in East Africa and the Horn of Africa: is there a need to panic? Malar J 23, (2024).
Ishengoma, D. S. et al. Evidence of Artemisinin partial resistance in Northwestern Tanzania: clinical and molecular markers of resistance. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(24)00362-1 (2024).
Uwimana, A. et al. Association of plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect. Dis. 21, 1120–1128 (2021).
Balikagala, B. et al. Evidence of Artemisinin-Resistant malaria in Africa. N. Engl. J. Med. 385, 1163–1171 (2021).
June Technical Consultation on the Role of Parasite and Anopheline Genetics in Malaria Surveillance. (2019).
WHO. Report on Antimalarial Drug Efficacy, Resistance and Response: 10 Years of Surveillance (2010–2019). (2020).
Ariey, F. et al. A molecular marker of artemisinin-resistant plasmodium falciparum malaria. Nature 505, 50–55 (2014).
Witkowski, B. et al. A surrogate marker of piperaquine-resistant plasmodium falciparum malaria: a phenotype–genotype association study. Lancet Infect. Dis. 17, 174–183 (2017).
Picot, S. et al. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J 8, (2009).
Naidoo, I. & Roper, C. Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitology 29, (2013).
Hodoameda, P. P. falciparum and Its Molecular Markers of Resistance to Antimalarial Drugs. in (Intechopen, 2021). https://doi.org/10.5772/intechopen.98372
da Silva, C. et al. Targeted and whole-genome sequencing reveal a north-south divide in P. falciparum drug resistance markers and genetic structure in Mozambique. Commun Biol 6, (2023).
Mayor, A. et al. Prospective surveillance study to detect antimalarial drug resistance, gene deletions of diagnostic relevance and genetic diversity of plasmodium falciparum in Mozambique: protocol. BMJ Open 12, (2022).
Schaffner, S. F. et al. Malaria surveillance reveals parasite relatedness, signatures of selection, and correlates of transmission across Senegal. Nat Commun 14, (2023).
Aranda-Díaz, A. et al. Sensitive and modular amplicon sequencing of plasmodium falciparum diversity and resistance for research and public health. Sci. Rep. 15, 10737 (2025).
Mayor, A., Ishengoma, D. S., Proctor, J. L. & Verity, R. Sampling for malaria molecular surveillance. Trends in Parasitology vol. 39 954–968 Preprint at (2023). https://doi.org/10.1016/j.pt.2023.08.007
Miotto, O. et al. Genetic architecture of artemisinin-resistant plasmodium falciparum. Nat. Genet. 47, 226–234 (2015).
Rahmasari, F. V. et al. Evolution of genetic markers for drug resistance after the introduction of dihydroartemisinin–piperaquine as first-line anti-malarial treatment for uncomplicated falciparum malaria in Indonesia. Malar J 22, (2023).
Amato, R. et al. Genetic markers associated with dihydroartemisinin–piperaquine failure in plasmodium falciparum malaria in Cambodia: a genotype–phenotype association study. Lancet Infect. Dis. 17, 164–173 (2017).
Hagenah, L. M. et al. Plasmodium falciparum African PfCRT mutant isoforms conducive to Piperaquine resistance are infrequent and impart a major fitness cost. J. Infect. Dis. https://doi.org/10.1093/infdis/jiae617 (2024).
Escobar, C. et al. Polymorphisms in plasmodium falciparum K13-propeller in Angola and Mozambique after the introduction of the acts. PLoS One 10, (2015).
da Silva, C. et al. Anti-malarial resistance in Mozambique: absence of plasmodium falciparum Kelch 13 (K13) propeller domain polymorphisms associated with resistance to artemisinins. Malar J 22, (2023).
Brokhattingen, N. et al. Genomic malaria surveillance of antenatal care users detects reduced transmission following elimination interventions in Mozambique. Nat. Commun. 15, 2402 (2024).
Coonahan, E. et al. Whole-genome surveillance identifies markers of plasmodium falciparum drug resistance and novel genomic regions under selection in Mozambique. mBio https://doi.org/10.1128/mbio.01768-23 (2023).
Gupta, H. et al. Drug-resistant polymorphisms and copy numbers in plasmodium falciparum, Mozambique, 2015. Emerg. Infect. Dis. 24, 40–48 (2018).
Casanova, D. et al. Artemisinin resistance-associated gene mutations in plasmodium falciparum: A case study of severe malaria from Mozambique. Travel Med. Infect. Dis 57, (2024).
Bakari, C. et al. Trends of plasmodium falciparum molecular markers associated with resistance to artemisinins and reduced susceptibility to lumefantrine in Mainland Tanzania from 2016 to 2021. Malar J 23, (2024).
Matambisso, G. et al. Sustained clinical benefit of malaria chemoprevention with sulfadoxine-pyrimethamine (SP) in pregnant women in a region with high SP resistance markers. Journal Infection 88, (2024).
Millogo, K. S. et al. Trend of N86Y and Y184F mutations in Pfmdr1 gene in children under seasonal malaria chemoprevention coverage in Nanoro, Burkina Faso. Acta Parasitol. https://doi.org/10.1007/s11686-024-00923-x (2024).
Koepfli, C. & Mueller, I. Malaria Epidemiology at the Clone Level. Trends in Parasitology vol. 33 974–985 Preprint at (2017). https://doi.org/10.1016/j.pt.2017.08.013
Rice, B. L. et al. Genetic evidence that the Makira region in Northeastern Madagascar is a hotspot of malaria transmission. Malar J 15, (2016).
Ralinoro, F. et al. Genetic diversity of plasmodium falciparum populations in three malaria transmission settings in Madagascar. Malar J 20, (2021).
Watson, O. J. et al. Evaluating the performance of malaria genetics for inferring changes in transmission intensity using transmission modeling. Mol. Biol. Evol. 38, 274–289 (2021).
Mayor, A. et al. How hidden can malaria be in pregnant women? Diagnosis by microscopy, placental histology, polymerase chain reaction and detection of histidine-rich protein 2 in plasma. Clin. Infect. Dis. 54, 1561–1568 (2012).
Manske, M. et al. Analysis of plasmodium falciparum diversity in natural infections by deep sequencing. Nature 487, 375–379 (2012).
Oksanen, J. et al. Package ‘vegan’: Community Ecology Package. Preprint at (2024). https://github.com/vegandevs/vegan
Bodenhofer, U., Bonatesta, E., Horejš-Kainrath, C. & Hochreiter, S. Msa: an R package for multiple sequence alignment. Bioinformatics 31, 3997–3999 (2015).
Desper, R. & Gascuel, O. Fast and accurate phylogeny reconstruction algorithms based on the Minimum-Evolution principle. J. Comput. Biol. 9, 687–705 (2002).
Schliep, K. P. Phangorn: phylogenetic analysis in R. Bioinformatics 27, 592–593 (2011).
Joice, R. et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med 6, (2014).
Acknowledgements
We are very grateful to all individuals and their corresponding parents/guardians who agreed to participate in the study, clinical officers, field supervisors, data managers and lab teams from all participating institutions. We thank Dr Nanna Brokhattingen for data analysis support and comments to the manuscript. We also thank Antoni Sanchez for his support on the manuscript-related follow-ups.
Funding
This work was supported financially by the Bill and Melinda Gates Foundation (INV-019032 and INV-067310, A.M.), the Departament d’Universitats i Recerca de la Generalitat de Catalunya (AGAUR; grant 2021 SGR 01517, A.M. and grant 2022 FI_B 00148, S.B.), Ministerio de Ciencia e Innovación (PID2020-118328RB-I00/AEI/10.13039/501100011033, A.M.), the European Union’s Horizon 2020 research and innovation programme (Marie Skłodowska-Curie grant agreement 890477, A.P.), and ”la Caixa” Foundation (ID 100010434; fellowship code LCF/BQ/PR24/12050009,A.P.). The Centro de Investigaçâo em Saude de Manhica (CISM) is supported by the Government of Mozambique and the Spanish Agency for International Development Cooperation (AECID). This research is part of ISGlobal’s Program on the Molecular Mechanisms of Malaria which is partially supported by the Fundación Ramón Areces. We acknowledge support from the grant CEX2023-0001290-S funded by MCIN/AEI/10.13039/501100011033, and support from the Government of Catalonia through the CERCA Program. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
A.M., B.C. designed the study.S.B., C.S., K.C., S.E., P.A., M.D., E.C., coordinated field work and participant data collection.S.B., C.S., N.C., N.D., A.C., G.M., A.S., J.I., A.R.C., M.S., M.P., A.N. L.N., and Gui. M. participated in sample collection and/or managed samples and databases.S.B., C.S., D.T., E.R.V., P.C. and C.G.F. conducted the laboratory analysis. M.G.U., A.A.D., B.P. processed the raw sequencing files and/or developed bioinformatic tools. S.B., E.R.V., M.G.U., A.M. analyzed and interpreted the data. A.A.D., A.P., B.A.G., B.G., contributed to data analysis and interpretation. S.B., E.R.V., A.M. wrote the main manuscript text. All authors reviewed the manuscript and agreed to submit it for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boene, S., Rovira-Vallbona, E., da Silva, C. et al. Antimalarial drug resistance and population structure of Plasmodium falciparum in Mozambique using genomic surveillance at health facilities in 2021 and 2022. Sci Rep 15, 29335 (2025). https://doi.org/10.1038/s41598-025-02166-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02166-w