Abstract
Sepsis, a severe infectious disease causing multiple organ dysfunction, requires further exploration of its pathomechanisms and therapeutic options. Hydroxysafflor Yellow A (HYSA), extracted from Carthamus tinctorius L, has shown anti-inflammatory and antioxidant properties effective in treating sepsis, though its precise mechanism remains unclear. In this study, a cecum ligation puncture (CLP) model was used to evaluate HYSA’s effects on sepsis-induced organ injury. HYSA (300 mg/kg) was administered intraperitoneally 4 h post-CLP. Results showed that HYSA inhibited the inflammatory response and improved pathological scores in the liver, lungs, and kidneys. Metabolomics analysis identified key metabolites and pathways influenced by HYSA, including nicotinate and nicotinamide metabolism and glycine/serine metabolism. Network pharmacology and molecular docking identified JAK2/STAT1 as potential targets, with Western blotting confirmed that HYSA inhibits JAK2/STAT1 phosphorylation. These findings suggest that HYSA protects against sepsis-induced organ injury by regulating metabolic networks and modulating the JAK2/STAT1 pathway.
Similar content being viewed by others
Introduction
Sepsis is a complex disease characterized by a dysregulated host response and multi-organ dysfunction resulting from infection1,2. It affects approximately 50 million individuals globally and is responsible for 11 million deaths annually, presenting a significant global health challenge3. Therefore, reducing its high mortality rate is essential. Research indicates that the pathological mechanisms of sepsis are primarily related to uncontrolled inflammatory responses, tissue metabolism, and energy imbalances, which lead to tissue damage, organ failure, and death1. Consequently, controlling the inflammatory response is a primary treatment strategy for sepsis4. However, the current mainstream use of anti-inflammatory drugs faces challenges such as drug resistance5. This underscores the urgent need for developing effective therapeutic strategies with minimal side effects.
Hydroxysafflor Yellow A (HYSA), the main component extracted from Carthamus tinctorius L6. possesses anti-inflammatory, antioxidant, and anticoagulant properties7. In clinical practice, XueBiJing is a potent agent commonly used for the treatment of sepsis, with HYSA being one of its main active compounds. HYSA has been shown to regulate immune responses, suppress excessive inflammation, modulate coagulation, and improve organ dysfunction. Animal studies have demonstrated that HYSA can inhibit inflammation and ameliorate sepsis-induced acute injuries to the lungs and kidneys8,9. In addition, our previous studies have confirmed that HYSA can alleviate liver injury in septic mice and reduce their mortality rate10. Despite these findings, the specific targets and overall molecular mechanisms of HYSA in treating sepsis remain poorly understood and require further elucidation and validation.
The advent of omics technologies provides a valuable solution. Metabolomics is an approach that involves a comprehensive analysis of all endogenous metabolites in biological systems11. It is instrumental in studying changes in metabolic networks during disease and in examining the absorption and distribution of drugs both in vivo and in vitro12. For example, metabolomics has been used to reveal amino acid metabolism disorders in septic patients13. Network pharmacology is a new discipline based on systems biology theory, biological system network analysis, and the selection of specific signal nodes for multi-target drug molecule design14. It is particularly effective in identifying potential drug targets for treating diseases15. Therefore, combining these two techniques can facilitate an in-depth exploration of the molecular mechanisms of HYSA in treating sepsis.
In this study, the cecum ligation puncture (CLP) method was employed to establish an animal model of sepsis to observe the protective effects of HYSA on sepsis-induced lung, liver, and kidney injuries. Untargeted metabolomics was subsequently utilized to identify metabolic pathways and compounds influenced by HYSA in different organs affected by sepsis. Network pharmacology and molecular docking were used to uncover the underlying molecular mechanisms. Finally, Western blotting analysis was conducted to validate that HYSA’s amelioration of multi-organ damage is particularly related to the janus kinase 2 (JAK2)/signal transducer and activator of transcription 1 (STAT1) signaling pathway.
Materials and methods
Materials
HYSA (purity > 98%) was procured from Yuan Ye Biotechnology Co., Ltd. (batch number 20240112, Shanghai, China). Methanol (HPLC, 99.90%), acetonitrile (HPLC, 99.95%), and formic acid (HPLC, 99.00%) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α (batch number A28231233), MPO (batch number A213340115), and IL-6 (batch number A20631141) were sourced from MULTI SCIENCES (Shanghai, China). ALT reagent kit (batch number 20230901), AST reagent kit (batch number 20230725), Crea reagent kit (batch number 20231107), and BUN reagent kit (batch number 20231008) were purchased from Yichuan Biotechnology Co. (Guangzhou, China). Primary antibodies for phospho-JAK2 (Tyr1007/1008) (batch number 11) was obtained from CST (Shanghai, China), JAK2 (batch number M06DE5P), phospho-STAT1 (Ser727) (batch number N29JA8P), and STAT1 (batch number N23JA7P) were obtained from Zenbio (Chengdu, China).
CLP model preparation
All experimental protocols and procedures complied with the NIH Guide for the Care and Use of Laboratory Animals, and were reviewed and approved by the Animal Ethics Committee of Hunan Provincial People’s Hospital (No.2023-10). All procedures were performed in accordance with the ARRIVE guidelines.
C57BL/6 male mice (18 ± 1 g) were procured from Henan Sibecks Biotechnology Co., Ltd (SCXK [Yu] 2020-0005) and housed in a specific pathogen-free (SPF) animal facility. All mice were acclimatized for 7 days. The CLP model was established by anesthetizing the mice with 2.5% isoflurane via inhalation and performing a midline abdominal incision to expose, ligate (approximately 75% length), and puncture the cecum with a sterile 21G needle. Post-surgery, 1 ml of saline was injected subcutaneously to facilitate recovery16.
Animal grouping and drug administration
Based on previous literature results, including studies on HYSA dosage and toxicity experiments17,18we found that HYSA has high safety in animals. Furthermore, according to our preliminary experimental results, we used the dose of 300 mg/kg for subsequent animal experiments. Following a one-week acclimation period, the mice were divided into three groups using the random number table method: Sham, CLP, and HYSA300. The Sham group underwent a procedure where the cecum was exposed without ligation or puncture, mirroring the rest of the CLP group’s procedure. The CLP group adhered to the previously described CLP surgical procedure. The HYSA300 group followed the same CLP procedure, with an additional intraperitoneal injection of 300 mg/kg HYSA administered 4 h post-surgery. At 24 h post-CLP surgery, the mice were anesthetized with 2.5% isoflurane via inhalation, and blood was collected from their eyeballs. After standing for 2 h, the blood was centrifuged at 3000 rpm for 15 min to obtain serum. The bronchoalveolar lavage fluid (BALF) was centrifuged at 1500 rpm at 4℃ for 10 min. Tissue samples from the lungs, liver, and kidneys were collected. Both serum and tissue samples were frozen and stored at −80 °C for future experiments.
Histopathological analysis
Lung, liver, and kidney tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned for pathological analysis using hematoxylin-eosin (HE) staining. The prepared slides were observed for pathological changes and photographed using an LED LEICA DM3000 microscope. The results were presented in a PPT format. The personnel responsible for the histopathological analysis were unaware of the group assignments.An observer evaluated five randomly selected fields of view for each sample, with scores assigned as follows: 0 for none, 1 for mild, 2 for moderate, and 3 for severe. Evaluation criteria included the following: (1) Lung: Alveolar structural disturbance, inflammatory cell infiltration, vascular congestion, and interstitial edema; (2) Liver: Dilation and vacuolization of hepatocytes, vascular congestion, dilation of sinusoids, and Kupffer cell infiltration; (3) Kidney: Degeneration of Bowman space and glomeruli, degeneration of proximal and distal tubules, vascular congestion, interstitial edema, and inflammatory cell infiltration19.
Biochemical and inflammatory marker analyses
Serum levels of ALT, AST, Crea, and BUN were measured with an automatic biochemical analyzer (Dongzhi Toshiba, TBA-40FR, Japan). ALT, AST, and BUN were analyzed using kinetic assays, whereas Crea was measured using an endpoint assay. TNF-α and IL-6 in serum, as well as MPO levels in lung tissue, were determined according to the ELISA kit instructions. BALF protein content was assessed using the QUAWEIIQ5000 machine.
Metabolomics analysis
A 30 mg sample was mixed with 400 µL methanol-water (4:1 v/v) containing a 4 µg/mL internal standard. After cooling at −40 °C for 2 min, the mixture was ground for 2 min, followed by ultrasonic extraction in an ice-water bath for 10 min and overnight storage at −40℃. Centrifugation was performed at 12,000 rpm for 10 min at 4℃. The supernatant was filtered and stored at −80℃ for LC-MS analysis. The LC system used was a Waters ACQUITY UPLC I-Class Plus with a Thermo QE Plus mass spectrometer. An ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm) was used, with a mobile phase of water (0.1% formic acid) and acetonitrile, at 0.35 mL/min flow rate. A linear gradient from 5 to 100% B over 16 min was applied. MS settings: spray voltage 3800 V (+) and 3000 V (−), sheath gas flow rate 35 arbitrary units, capillary temperature 320℃, full MS resolution 70,000, and MS/MS resolution 17,500 with MS/MS range 70-1050. Compounds were identified based on multiple dimensions such as retention time, exact mass number, secondary fragmentation, and isotopic distribution. They were analyzed using the Human Metabolome Database, Lipidmaps (v2.3), METLIN databases, and a local database. The extracted data underwent missing value processing, zero-value replacement, score screening, and data merging. Orthogonal partial least squares analysis (OPLS-DA) was used to differentiate the overall differences in metabolic profiles between the groups and identify differential metabolites. Variable importance of projection (VIP) values obtained from the OPLS-DA model ranked the overall contribution of each variable to group discrimination. A two-tailed Student’s t-test was used to verify whether the differences in metabolites between the two groups were significant. Differential metabolites were selected with VIP values > 1.0 and P < 0.05.
Network pharmacological analysis
Potential target genes of HYSA were identified using Swiss Target Prediction, PharmMapper, and SEA Search Server. Disease-related targets were searched in the GeneCards database with keywords “Sepsis,” “Acute liver injury,” “Acute lung injury,” and “Acute kidney injury.” Intersection targets were visualized in a Venn diagram and analyzed with the STRING database. Visualization was done using Cytoscape 3.8.2 software. GO and KEGG pathway enrichment analyses were performed using the DAVID 6.8.0 database20.
Molecular docking
The 2D structure of the active ingredient was downloaded from the PubChemCID database, while the 2D structure of gene-related proteins was obtained from the PDB database. PyMOL software was used to remove ligands and non-protein molecules (such as water) from the target proteins. The crystal structures were hydrogenated using AutoDock Tools 1.5.6, and protein charge calculations were performed. Molecular docking was conducted with AutoDock Vina, and the lowest binding energies were calculated and visualized using PyMOL software.
Western blotting analysis
Liver, lung, and kidney tissues were prepared separately, and a mixture of RIPA, phosphatase inhibitor A, phosphatase inhibitor B, and a general-purpose protease inhibitor (in a ratio of 100:2:2:2) was used to extract total protein from each organ. Protein concentration was determined using proteinA280 software. An appropriate amount of SDS loading buffer was added to the mixtures, which were then boiled for 10 min. Proteins were electrophoresed and transferred to PVDF membranes. The membranes were blocked for 1 h, washed three times with TBST/PBST solution, and incubated with primary antibodies overnight at 4℃. After washing the membranes three times with TBST/PBST solution, homologous secondary antibodies were added, and incubation continued at room temperature for 1 h. The membranes were washed three more times with TBST/PBST solution. Specific bands were visualized using Enhanced Chemiluminescence and detected by the Omega Lum C gel imaging system (Bio-Rad). Protein levels were quantified and analyzed using ImageJ software. Antibody dilution information for different organs is provided in Table 1.
Statistical analysis
Data were presented as mean ± SD and analyzed using GraphPad Prism software 9.5 and IBM SPSS Statistics 25, statistical comparisons were performed using one-way ANOVA, with significance defined as P < 0.05.
Results
HYSA improved survival rate in CLP mice
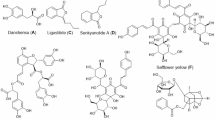
To evaluate the efficacy of HYSA (Fig. 1A) on survival in CLP-induced septic mice, the drug was administered four hours post-CLP surgery. Given that the inflammatory response peaks at 24 h, the mice were sacrificed by cervical dislocation 24 h later to collect tissues for subsequent testing (Fig. 1B). For survival analysis, mice were observed every 24 h for seven days. Notably, the 300 mg/kg HYSA group showed no deaths on the first day and had a significantly lower mortality rate over seven days compared to the CLP and 150 mg/kg groups (Fig. 1C). Therefore, a dose of 300 mg/kg was selected as the dose for subsequent experiments.
HYSA protects multiple organs from CLP-induced damage
High-dose HYSA reduced protein content in BALF and MPO activity in the lungs (Fig. 2A). It also suppressed serum levels of liver function markers (ALT, AST) (Fig. 2B), kidney function markers (Crea, BUN) (Fig. 2C), and inflammatory cytokines (IL-6, TNF-α) (Fig. 2D). CLP surgery induced interstitial edema, alveolar wall thickening, and inflammatory cell infiltration in the lungs; hepatocellular necrosis, inflammatory cell infiltration, and hepatic sinusoidal stasis in the liver; and glomerular structural degeneration, reduction of Bowman’s space, and inflammatory cell infiltration in the kidneys. Notably, HYSA partially reversed these pathological changes in the lungs, liver, and kidneys (Fig. 2E, F).
HYSA protects multiple organs of mice from CLP-induced damage. (A) Protein content in BALF and MPO content in lung tissue. (B) ALT and AST expression levels in serum determined by biochemical analysis. (C) BUN and Crea expression levels in serum determined by biochemical analysis. (D) TNF-α and IL-6 expression levels in serum measured by ELISA. (E) Histopathology of lung, liver, and kidney using HE staining, scale bar = 200 μm. (F) Histopathological scores of lung, liver, and kidney. Data are presented as mean ± SD. ****P < 0.0001, ***P < 0.001, **P < 0.01 and *P < 0.05 vs. the CLP group. n = 3–4 mice per group.
Metabolomics analysis of lung tissue
Using the OPLS-DA model, 247 differentially expressed metabolites (DEMs) were identified between the CLP and Sham groups, with 124 up-regulated and 123 down-regulated metabolites. Additionally, 102 DEMs were identified between the CLP and HYSA groups, including 43 up-regulated and 59 down-regulated metabolites (Fig. 3A-C). The screening criteria were VIP > 1 and P < 0.05. Venn diagram analysis revealed 75 common DEMs among these groups (Fig. 3D). These DEMs were analyzed using the MetaboAnalyst 6.0 online platform, focusing on pathways with an impact > 0.02. The analysis indicated that these metabolites were mainly involved in phenylalanine metabolism (L-phenylalanine) and glycine, serine, and threonine metabolism (L-serine) (Fig. 3E). Further analysis of the 75 DEMs showed that HYSA partially restored 62 metabolites. Detailed information on these 62 metabolites is provided in Table 2. Six key metabolites associated with the identified metabolic pathways were identified, including L-serine, L-phenylalanine, L-allothreonine, L-arginine, L-proline, and 5’-methylthioadenosine (Fig. 3F). Heat maps of these six metabolites were generated using an online platform (https://www.bioinformatics.com.cn/). Compared to the Sham group, these six metabolites are elevated in the CLP group, while HYSA can partially suppress the levels of these metabolites (Fig. 3G).
Metabolomics analysis of metabolic profiling changes in lung tissue. (A) DEMs between CLP vs. Sham and between CLP vs. HYSA300 groups using OPLS-DA, with screening criteria of VIP > 1 and P < 0.05. (B) Volcano plots of DEMs between CLP and Sham groups. (C) Volcano plot of DEMs between CLP and HYSA300 groups. (D) Venn diagram of common DEMs among the three groups. (E) Pathway analysis of common DEMs. (F) Common DEMs that can be reverted after HYSA treatment. (G) Heat map of common DEMs that can be reverted after HYSA treatment. Data are presented as mean ± SD. ***P < 0.001, **P < 0.01 and *P < 0.05 vs. the CLP group. n = 8–10 mice per group.
Metabolomics analysis of liver tissue
Using the OPLS-DA model, 237 DEMs were identified between the CLP and Sham groups, including 79 up-regulated and 158 down-regulated metabolites. Additionally, 125 DEMs were identified between the CLP and HYSA groups, with 51 up-regulated and 74 down-regulated metabolites (Fig. 4A-C). Venn diagram analysis revealed 24 common DEMs (Fig. 4D). These metabolites were primarily involved in nicotinate and nicotinamide metabolism (Nad) and glutathione metabolism (Glutathione) (Fig. 4E). Further analysis of these 24 common DEMs among groups showed that HYSA partially restored the levels of 10 metabolites (Fig. 4F), including Glutathionate(1-), Creatine, Glutathione, Tazobactam, N,N’-Diethylthiourea, 2 S-Hydroxybutanoic Acid, Nad, Epiafzelechin 3-O-Gallate, Dehydroascorbate (Bicyclic Form), and S-Adenosylhomocysteine. Detailed information on these metabolites is provided in Table 3. Heat maps of the 10 metabolites were plotted. Compared to the Sham group, the levels of Glutathionate(1-), Glutathione, Tazobactam, NAD, Epiafzelechin 3-O-Gallate, and S-Adenosylhomocysteine were elevated in the CLP group. In contrast, the levels of Creatine, N,N’-Diethylthiourea, 2 S-Hydroxybutanoic Acid, and Dehydroascorbate (Bicyclic Form) were decreased. HYSA partially restored or suppressed the levels of these metabolites (Fig. 4G).
Metabolomics analysis of metabolic profiling changes in liver tissue. (A) DEMs between CLP vs. Sham and between CLP vs. HYSA300 groups using OPLS-DA, with screening criteria of VIP > 1 and P < 0.05. (B) Volcano plots of DEMs between CLP and Sham groups. (C) Volcano plot of DEMs between CLP and HYSA300 groups. (D) Venn diagram of common DEMs among the three groups. (E) Pathway analysis of common DEMs. (F) Common DEMs that can be reverted after HYSA treatment. (G) Heat map of common DEMs that can be reverted after HYSA treatment. Data are presented as mean ± SD. ***P < 0.001, **P < 0.01 and *P < 0.05 vs. the CLP group. n = 8–10 mice per group.
Metabolomics analysis of metabolic profiling changes in kidney tissue
Using the OPLS-DA model, 239 DEMs were identified between the CLP and Sham groups, including 111 up-regulated and 128 down-regulated metabolites. Additionally, 128 DEMs were identified between the CLP and HYSA groups, with 27 up-regulated and 101 down-regulated metabolites (Fig. 5A-C). Venn diagram analysis revealed 75 common DEMs (Fig. 5D). These metabolites were primarily involved in ketone body metabolism (Acetoacetic acid), fructose and mannose degradation (D-fructose), and glycine and serine metabolism (Glycine) (Fig. 5E). Further analysis of these 75 common DEMs among groups showed that HYSA partially restored 49 metabolites (Fig. 5F). Detailed information on these metabolites is provided in Table 4. Five key metabolites were selected for visualization: Methylimidazole Acetaldehyde, 1-methylhistamine, D-fructose, Acetoacetic Acid and Glycine. Compared to the Sham group, the levels of Acetoacetic Acid are elevated in the CLP group, while the levels of 1-methylhistamine, Methylimidazole Acetaldehyde, D-fructose, and Glycine are decreased in the CLP group. HYSA can partially restore or suppress the levels of these metabolites (Fig. 5F, G).
Metabolomics analysis of metabolic profiling changes in kidney tissue. (A) DEMs between CLP vs. Sham and between CLP vs. HYSA300 groups using OPLS-DA, with screening criteria of VIP > 1 and P < 0.05. (B) Volcano plots of DEMs between CLP and Sham group. (C) Volcano plot of DEMs between CLP and HYSA300 group. (D) Venn diagram of common DEMs among the three groups. (E) Pathway analysis of common DEMs. (F) Five Common DEMs that can be reverted after HYSA treatment. (G) Heat map of five common DEMs that can be reverted after HYSA treatment. Data are presented as the mean ± SD. ***P < 0.001, **P < 0.01 and *P < 0.05 vs. the CLP group. n = 8–10 mice per group.
Network pharmacology analysis
HYSA was analyzed across three databases, identifying 88 target genes in Swiss Target Prediction, 289 in PharmMapper, and 17 in SEA Search Server. After eliminating duplicates, 365 unique target genes were obtained. Searching the GeneCards database with “sepsis” yielded 3196 target genes, while “Acute lung injury,” “Acute liver injury,” and “Acute kidney injury” resulted in 8701, 8315, and 8619 target genes, respectively. Intersection analysis revealed 176 common target genes for treating these acute organ injuries (Fig. 6A). The STRING database was used to study the interactions among these 176 target genes. After removing isolated genes, the data were visualized using Cytoscape 3.8.2, creating a protein-protein interaction (PPI) network with 175 nodes and 2182 edges (Fig. 6B). The MCODE plugin identified the highest-scoring subnetwork, consisting of 28 nodes and 332 edges (Fig. 6C). Further analysis of these 28 target genes using the DAVID database revealed that, in biological processes, HYSA is mainly involved in positive regulation of transcription, regulation of apoptotic processes, and positive regulation of cell migration. For cellular components, HYSA is associated with the cytosol, cytoplasm, and nucleus. In terms of molecular functions, HYSA primarily involves protein binding, ATP binding, and enzyme binding (Fig. 6D). KEGG pathway analysis indicated that HYSA potentially regulates the PI3K-Akt signaling pathway, Th17 cell differentiation, NOD-like receptor signaling pathway, IL-17 signaling pathway, and TNF signaling pathway (Fig. 6E). These findings suggest HYSA can modulate inflammatory responses and oxidative stress in sepsis, thereby improving multi-organ function.
Network pharmacologic analysis of HYSA for the treatment of multiple organ injuries in sepsis. (A) Target genes of HYSA for the treatment of acute lung injury, acute liver injury, and acute kidney injury in sepsis. (B) PPI of HYSA treatment targets for sepsis-induced multi-organ injuries. (C) Highest scoring subnetwork analyzed by the Mcode plugin. (D) GO analysis of subnetwork target genes. (E) KEGG analysis of subnetwork target genes.
Molecular docking
Molecular docking was conducted to verify whether HYSA interacts with the core targets in the subnetwork. The top 9 core target genes in terms of score were selected for docking. Binding energy was used to evaluate the docking results. Typically, a binding energy below − 5.0 kcal/mol indicates moderate affinity, while a value below − 7.0 kcal/mol suggests high affinity21. As shown in Table 5, all binding energies were less than − 7 kcal/mol, demonstrating that HYSA has a strong affinity for the target genes. In addition, HYSA forms hydrogen bonds with most proteins. The results show that the binding energy between ALB protein and HYSA is −8.3 kcal/mol. ALB interacts with HYSA through the amino acid residues GLU-542, LYS-394, and ASN-405. The binding energy between ANX5 and HYSA is −7.1 kcal/mol, and this interaction depends on ARG-245, ARG-207, LEU-5, and GLN-3. ASP-15 and SER-18 residues are essential for the interaction between BCL2L1 and HYSA. IKBKB binds to HYSA using residues such as LEU-307 and PRO-71. The binding energy between IL1B and HYSA is −7.1 kcal/mol, and it relies on interactions between LYS-74 and LEU-80. JAK2 binds to HYSA via hydrogen bonds involving residues like ASP-1092 and LYS-1055. MMP2 interacts with HYSA through ASP-618 and ASP-476. GLU-416 and PHE-425 residues are necessary for the interaction between MMP9 and HYSA. The binding energy between STAT1 and HYSA is −7.9 kcal/mol, and this interaction is influenced by ARG-245, ARG-207, LEU-5, and GLN-3 (Fig. 7).
HYSA downregulates the JAK2/STAT1 pathway in sepsis mice
To validate the network pharmacology results, the focus was placed on the JAK2/STAT1 pathway. In the CLP group, compared to the sham group, JAK2 phosphorylation at site 1007/1008 was enhanced in lung, liver, and kidney tissues, promoting downstream STAT1 phosphorylation and initiating inflammatory responses. Interestingly, the phosphorylation sites of STAT1 differed: Ser727 in the lungs and kidneys, and Ser701 in the liver. HYSA treatment inhibited the phosphorylation of p-JAK2 and p-STAT1, thereby reducing multi-organ inflammatory responses (Fig. 8).
HYSA inhibits JAK2/STAT1 phosphorylation in multiple organs. (A) HYSA inhibits p-JAK2 and p-STAT1(727) in sepsis-induced lung injury. (B) HYSA inhibits p-JAK2 and p-STAT1(701) in sepsis-induced liver injury. (C) HYSA inhibits p-JAK2 and p-STAT1(727) in sepsis-induced kidney injury. Data are represented as the mean ± SD. **P < 0.001 and *P < 0.05 vs. the CLP group. n = 3 mice per group.
Discussion
Current research indicates that HYSA, a promising anti-inflammatory compound, has potential in treating acute diseases such as acute lung injury8. However, its impact on the metabolic network involved in sepsis-induced multi-organ damage and its underlying molecular mechanisms remain unclear. This study demonstrates that HYSA ameliorates multi-organ injury induced by CLP in mice, possibly by regulating the metabolic network and inhibiting the JAK2/STAT1 pro-inflammatory pathway (Fig. 9).
Sepsis-induced acute lung injury (ALI) is consistently accompanied by elevated levels of MPO and protein22. Our findings align with these observations, showing increased protein content, high MPO levels, and morphological alterations, indicating that CLP exacerbates lung damage. Notably, HYSA treatment significantly ameliorated ALI. Metabolomics studies indicated that phenylalanine metabolism and glycine, serine, and threonine metabolism are key pathways affected by HYSA in ALI. Phenylalanine metabolism is associated with the release of phenylalanine during ALI, which increases the expression of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) and promotes macrophage death23. A prospective clinical study linked elevated phenylalanine levels to higher mortality in patients with sepsis24. Additionally, phenylalanine enhances the inflammatory response by activating the NLRP3 pro-inflammatory pathway and inducing pyroptosis in lung macrophages25. Notably, high levels of phenylalanine can impair mitochondrial bioenergetics, leading to changes in oxidative, inflammatory states, and inducing cell apoptosis26. Glycine, serine, and threonine metabolism also play crucial roles in sepsis-induced ALI by increasing serine levels, which exacerbate lung cell apoptosis and inflammatory cell infiltration27. Other studies have shown that L-serine activates the IGF1/JAK1/STAT1 pro-inflammatory pathway and promotes LPS-induced macrophage polarization towards the M1-type28. There is more direct evidence showing that metabolic enzymes involved in de- novo serine synthesis support the maturation of macrophage IL-1β through activation of the NLRP3 inflammasome29. As depicted in Fig. 9, L-phenylalanine and L-serine levels were elevated in CLP-induced septic mice but reduced following HYSA treatment. This indicates that HYSA may alleviate lung inflammation and apoptosis by inhibiting L-serine and L-phenylalanine.
The liver, a crucial immune organ, is frequently targeted by lipopolysaccharide (LPS) during sepsis, leading to acute liver injury characterized by elevated serum levels of ALT and AST30. Our results demonstrated that CLP significantly increased serum levels of ALT, AST, TNF-α, and IL-6, along with histomorphological changes in the liver. HYSA treatment partially reversed these effects, indicating its potential therapeutic efficacy in sepsis-induced acute liver injury. Metabolite dysregulation significantly impacts the pathogenesis and recovery from acute liver injury in sepsis31. Metabolomics analysis revealed alterations in nicotinate and nicotinamide metabolism and glutathione metabolism in the CLP group. Nicotinamide adenine dinucleotide (Nad), a critical component of nicotinate and nicotinamide metabolism, reduces oxidative stress in liver cells, lowers reactive oxygen species (ROS) levels, enhances bacterial phagocytosis in macrophages, and inhibits bacterial dissemination and liver damage32. In addition, NAD+ mediates the function of the cellular energy center—mitochondria. Studies have shown that increased NAD + metabolism enhances mitochondrial function, reduces mitochondrial DNA damage and leakage, with some mechanisms related to the activation of the cGAS-STING pathway33. Glutathione, composed of glutamate, cysteine, and glycine, acts as a vital antioxidant preventing oxidative stress in liver cells34. Additionally, glutathione protects the immune system and mitigates liver injury in septic rats by inhibiting nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) inflammatory and oxidative stress pathways35. As shown in Fig. 9, HYSA treatment increased Nad and glutathione levels in the liver compared to the CLP group, indicating that HYSA may protect the liver by modulating these metabolic pathways, thereby reducing oxidative stress and inhibiting inflammatory responses.
Sepsis is a primary cause of acute kidney injury (AKI), characterized by excessive inflammatory responses and elevated levels of inflammatory cytokines such as TNF-α and IL-6. These factors induce endothelial cell apoptosis and dysfunction in the kidneys36. Elevated serum levels of BUN and Crea serve as markers of AKI37. HYSA significantly reduced serum Crea and BUN levels in septic mice, suggesting its potential to ameliorate sepsis-induced AKI. Metabolomics results revealed that, compared to the sham group, the CLP group exhibited altered metabolic pathways primarily involving fructose and mannose degradation, glycine and serine metabolism, and ketone body metabolism. Physiologically, fructose serves as a substrate for gluconeogenesis and is reabsorbed by the proximal tubules of the kidney to maintain systemic blood glucose concentration and energy balance30. During AKI, fructose is not reabsorbed, leading to reduced levels in renal tissue. Glycine, synthesized mainly from serine and threonine, possesses anti-inflammatory, immune-regulatory, and cell-protective functions and is used to treat AKI38,39,40. Previous studies have indicated reduced glycine levels in patients with AKI41. A clinical trial result shows that glycine can partially improve oxidative stress, mitochondrial dysfunction, inflammation, and aging42. Additionally, Acetoacetic acid, a key metabolic product of ketone body metabolism, is primarily formed in the kidneys through the deacylation of acetoacetyl-CoA43. Acetoacetic acid increases the sensitivity of kidney proximal tubule cells (LLC-PK1) to toxins, exacerbating kidney damage44. As shown in Fig. 9, HYSA treatment elevated D-fructose and glycine levels while reducing acetoacetic acid levels in the kidney. This suggests that HYSA may serve as a potential therapeutic drug for AKI by reducing inflammation, maintaining energy balance, and lowering the toxic sensitivity of renal proximal tubules.
Network pharmacology analyses indicate that HYSA inhibits pro-inflammatory pathways, such as the JAK2/STAT1 pathway, to mitigate inflammatory responses and alleviate sepsis-induced injuries in the lungs, liver, and kidneys. Following activation by cell surface receptor signaling, JAK2 undergoes phosphorylation, facilitating the dimerization and nuclear translocation of the STAT1 protein45. Overexpression of these proteins triggers inflammatory responses and signifies immune system disorders46,47. In septic lung injury, inhibiting JAK2/STAT1 phosphorylation reduces protein leakage and macrophage apoptosis48. Further studies have shown that JAK2 can activate STAT1, leading to reduced phagocytic function in alveolar macrophages. This activation also increases the levels of inflammatory factors such as IL-6, which exacerbates acute lung injury in mice49. Similarly, pathway inhibition improves hepatocyte apoptosis in acute liver injury50. In addition, the activation of hepatic stellate cells can induce excessive extracellular matrix deposition, promoting pathological liver damage. Studies have shown that activation of the JAK2/STAT1 pathway induces macrophage polarization towards the M1 phenotype (increased CD80 expression), which in turn promotes the upregulation of Stard13 expression in hepatic stellate cells, leading to their activation51. JAK2/STAT1 activation correlates with elevated creatinine levels and apoptosis in HK-2 cells, aggravating AKI52,53. As shown in Fig. 8, our study demonstrated that HYSA inhibits JAK2 phosphorylation at the 1007/1008 site in the lungs, liver, and kidneys. However, the sites of STAT1 phosphorylation inhibition vary, with site Ser727 in the lungs and kidneys and site Ser701 in the liver. These findings lay the groundwork for future studies on the specific mechanisms through which HYSA affects the JAK2/STAT1 pathway.
Our study results indicate that HYSA improves the molecular mechanism network of sepsis-induced multi-organ injury, including its effect on organ metabolite levels and inhibition of the JAK2/STAT1 pathway. A literature review suggests that these metabolites may have direct or indirect links to JAK2/STAT1. For example, endogenous glutathione can regulate STAT1 expression to mediate innate immune responses54. NAD+ can stimulate the phosphorylation of STAT1 in RAW264.7 cells55. Defects in serine metabolism increase the expression of insulin-like growth factor 1, which enhances the JAK-STAT1 axis and promotes M1 macrophage polarization28. Therefore, these findings suggest that future studies could investigate how HYSA regulates these metabolites and inhibits JAK2/STAT1 activation to influence macrophage function and improve sepsis-induced multi-organ injury.
Of course, this study has some limitations. During the study, although we strictly controlled the experimental environment and animal housing conditions, there may still be differences in metabolic responses between individual mice. These differences could be influenced by factors such as genetic background and gut microbiota. Additionally, there are differences in metabolism and immunity between mice and humans, which may affect the efficacy and safety of HYSA. Therefore, future clinical trials are needed to explore the specific mechanisms of HYSA. Furthermore, we have not yet conducted a systematic study on the off-target effects of HYSA, which requires further attention in future research. This study only tested a dose of 300 mg/kg of HYSA, and the optimal therapeutic dose remains to be determined. Finally, future studies could integrate techniques such as transcriptomics, proteomics, and single-cell genomics to gain a better understanding of the mechanisms underlying the action of HYSA.
Conclusions
This study analyzed metabolite changes and molecular mechanisms in sepsis, along with the therapeutic effects of HYSA, using multi-organ metabolomics, network pharmacology, and experimental validation. Our findings indicated that HYSA administration ameliorated tissue damage and reduced inflammation in the lungs, liver, and kidneys of septic mice. Multi-organ metabolomics studies revealed that HYSA regulates levels of L-phenylalanine and L-serine in the lungs, Nad and glutathione in the liver, and D-fructose, glycine, and acetoacetic acid in the kidneys, influencing multiple metabolic pathways to exert organ-protective effects. Additionally, network pharmacology and molecular docking results suggest a potential mechanism for HYSA against multi-organ damage. Specifically, HYSA inhibits JAK2/STAT1 phosphorylation, thereby attenuating the inflammatory response and immune activation in the lungs, liver, and kidneys. This study suggests that HYSA could serve as a novel therapeutic agent for sepsis, paving the way for further research and development in this field.
Data availability
Data is provided within the manuscript or supplementary information files. For more detailed information or any other inquiries, please contact the corresponding author via email.
References
Sygitowicz, G. & Sitkiewicz, D. Molecular mechanisms of organ damage in sepsis: an overview. Braz J. Infect. Dis. 24, 552–560. https://doi.org/10.1016/j.bjid.2020.09.004 (2020).
Font, M. D., Thyagarajan, B. & Khanna, A. K. Sepsis and septic Shock - Basics of diagnosis, pathophysiology and clinical decision making. Med. Clin. North. Am. 104, 573–585. https://doi.org/10.1016/j.mcna.2020.02.011 (2020).
Rudd, K. E. et al. Global, regional, and National sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet 395, 200–211. https://doi.org/10.1016/S0140-6736(19)32989-7 (2020).
Vincent, J. L. Current sepsis therapeutics. EBioMedicine 86, 104318. https://doi.org/10.1016/j.ebiom.2022.104318 (2022).
Iroh Tam, P. Y. et al. Neonatal sepsis and antimicrobial resistance in Africa. Lancet Child. Adolesc. Health. 7, 677–679. https://doi.org/10.1016/S2352-4642(23)00167-0 (2023).
Wang, Y., Li, X., Deng, F. & Yin, R. Hydroxy-Safflower yellow A alleviates osteoporosis in ovariectomized rat model by inhibiting carbonic anhydrase 2 activity. Front. Pharmacol. 12, 734539. https://doi.org/10.3389/fphar.2021.734539 (2021).
Zhao, F. et al. Hydroxysafflor yellow A: A systematical review on botanical resources, physicochemical properties, drug delivery system, pharmacokinetics, and Pharmacological effects. Front. Pharmacol. 11, 579332. https://doi.org/10.3389/fphar.2020.579332 (2020).
Wang, Y. P. et al. Three ingredients of safflower alleviate acute lung injury and inhibit NET release induced by lipopolysaccharide. Mediators Inflamm. 2020 (2720369). https://doi.org/10.1155/2020/2720369 (2020).
Bai, J. et al. Protective effect of hydroxysafflor yellow A against acute kidney injury via the TLR4/NF-kappaB signaling pathway. Sci. Rep. 8, 9173. https://doi.org/10.1038/s41598-018-27217-3 (2018).
Pan, B. et al. Potential roles of HSYA in attenuating sepsis-induced liver injury through multi-omics analysis. J. Pharm. Biomed. Anal. 238, 115801. https://doi.org/10.1016/j.jpba.2023.115801 (2024).
Ni, Y., Xie, G. & Jia, W. Metabonomics of human colorectal cancer: new approaches for early diagnosis and biomarker discovery. J. Proteome Res. 13, 3857–3870. https://doi.org/10.1021/pr500443c (2014).
Lee, J. & Banerjee, D. Metabolomics and the Microbiome as biomarkers in Sepsis. Crit. Care Clin. 36, 105–113. https://doi.org/10.1016/j.ccc.2019.08.008 (2020).
Chen, Q. et al. Integrative analysis of metabolomics and proteomics reveals amino acid metabolism disorder in sepsis. J. Transl Med. 20 https://doi.org/10.1186/s12967-022-03320-y (2022).
Zhao, L. et al. Network Pharmacology, a promising approach to reveal the Pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 309, 116306. https://doi.org/10.1016/j.jep.2023.116306 (2023).
Jiashuo, W. U., Fangqing, Z., Zhuangzhuang, L. I., Weiyi, J. & Yue, S. Integration strategy of network Pharmacology in traditional Chinese medicine: a narrative review. J. Tradit Chin. Med. 42, 479–486. https://doi.org/10.19852/j.cnki.jtcm.20220408.003 (2022).
Rittirsch, D., Huber-Lang, M. S., Flierl, M. A. & Ward, P. A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36. https://doi.org/10.1038/nprot.2008.214 (2009).
Liu, Y. et al. Hydroxysafflor yellow A, a natural food pigment, ameliorates atherosclerosis in ApoE(-/-) mice by inhibiting the SphK1/S1P/S1PR3 pathway. Food Sci. Nutr. 12, 8939–8955. https://doi.org/10.1002/fsn3.4466 (2024).
Liu, Z., Li, C., Li, M., Li, D. & Liu, K. The subchronic toxicity of hydroxysafflor yellow A of 90 days repeatedly intraperitoneal injections in rats. Toxicology 203, 139–143. https://doi.org/10.1016/j.tox.2004.06.007 (2004).
Atici, A. E. et al. Neuropeptide W attenuates oxidative Multi-Organ injury in rats induced with Intra-Abdominal Sepsis. Inflammation 45, 279–296. https://doi.org/10.1007/s10753-021-01545-5 (2022).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Jin, L. et al. Combining network pharmacology, molecular Docking and experimental validation to explore the effects and mechanisms of indirubin on acute lymphoblastic leukemia. Drug Des. Devel Ther. 19, 1083–1103. https://doi.org/10.2147/DDDT.S500249 (2025).
Wang, Y. C. et al. Dihydromyricetin alleviates Sepsis-Induced acute lung injury through inhibiting NLRP3 Inflammasome-Dependent pyroptosis in mice model. Inflammation 42, 1301–1310. https://doi.org/10.1007/s10753-019-00990-7 (2019).
Wang, J., Ishfaq, M. & Li, J. Baicalin ameliorates Mycoplasma gallisepticum-induced inflammatory injury in the chicken lung through regulating the intestinal microbiota and phenylalanine metabolism. Food Funct. 12, 4092–4104. https://doi.org/10.1039/d1fo00055a (2021).
Huang, S. S. et al. Phenylalanine- and leucine-defined metabolic types identify high mortality risk in patients with severe infection. Int. J. Infect. Dis. 85, 143–149. https://doi.org/10.1016/j.ijid.2019.05.030 (2019).
Tang, Y. et al. Phenylalanine promotes alveolar macrophage pyroptosis via the activation of CaSR in ARDS. Front. Immunol. 14, 1114129. https://doi.org/10.3389/fimmu.2023.1114129 (2023).
Wyse, A. T. S., Santos, D., Seminotti, T. M., Leipnitz, G. & B. & Insights from animal models on the pathophysiology of hyperphenylalaninemia: role of mitochondrial dysfunction, oxidative stress and inflammation. Mol. Neurobiol. 58, 2897–2909. https://doi.org/10.1007/s12035-021-02304-1 (2021).
He, W. et al. Liang-Ge Decoction ameliorates acute lung injury in septic model rats through reducing inflammatory response, oxidative stress, apoptosis, and modulating host metabolism. Front. Pharmacol. 13, 926134. https://doi.org/10.3389/fphar.2022.926134 (2022).
Shan, X. et al. Serine metabolism orchestrates macrophage polarization by regulating the IGF1-p38 axis. Cell. Mol. Immunol. 19, 1263–1278. https://doi.org/10.1038/s41423-022-00925-7 (2022).
Wang, C. et al. Serine synthesis sustains macrophage IL-1beta production via NAD(+)-dependent protein acetylation. Mol Cell 84, 744–759 e746, (2024). https://doi.org/10.1016/j.molcel.2024.01.002
Kenig, A. et al. Low-Dose Colchicine attenuates Sepsis-Induced liver injury: A novel method for alleviating systemic inflammation. Inflammation 46, 963–974. https://doi.org/10.1007/s10753-023-01783-9 (2023).
Zhang, J. et al. Combined metabolomic and proteomic analysis of sepsis related acute liver injury and its pathogenesis research. Int. Immunopharmacol. 130, 111666. https://doi.org/10.1016/j.intimp.2024.111666 (2024).
Cao, T. et al. Nicotinamide mononucleotide as a therapeutic agent to alleviate multi-organ failure in sepsis. J. Transl Med. 21, 883. https://doi.org/10.1186/s12967-023-04767-3 (2023).
Myakala, K. et al. NAD metabolism modulates inflammation and mitochondria function in diabetic kidney disease. J. Biol. Chem. 299, 104975. https://doi.org/10.1016/j.jbc.2023.104975 (2023).
Biolo, G., Antonione, R. & De Cicco, M. Glutathione metabolism in sepsis. Crit. Care Med. 35, 591–595. https://doi.org/10.1097/01.CCM.0000278913.19123.13 (2007).
Xu, D., Liao, S., Lv, Y., Wang, J. & Kong, L. NMR-based metabolomics approach reveals effects of antioxidant nutrients in sepsis-induced changes in rat liver injury. J. Nutr. Biochem. 85, 108440. https://doi.org/10.1016/j.jnutbio.2020.108440 (2020).
Chang, Y. M., Chou, Y. T., Kan, W. C. & Shiao, C. C. Sepsis and acute kidney injury: A review focusing on the bidirectional interplay. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23169159 (2022).
Yu, Y. Y. et al. Self-developed NF-kappaB inhibitor 270 protects against LPS-induced acute kidney injury and lung injury through improving inflammation. Biomed. Pharmacother. 147, 112615. https://doi.org/10.1016/j.biopha.2022.112615 (2022).
Vallon, V. & Nakagawa, T. Renal tubular handling of glucose and Fructose in health and disease. Compr. Physiol. 12, 2995–3044. https://doi.org/10.1002/cphy.c210030 (2021).
Razak, M. A., Begum, P. S., Viswanath, B. & Rajagopal, S. Multifarious beneficial effect of nonessential amino acid, glycine: A review. Oxid. Med. Cell. Longev. 2017, 1716701. https://doi.org/10.1155/2017/1716701 (2017).
Zhong, Z. et al. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care. 6, 229–240. https://doi.org/10.1097/00075197-200303000-00013 (2003).
Zhang, C. et al. Supramolecular nanofibers containing Arginine-Glycine-Aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano. 14, 12133–12147. https://doi.org/10.1021/acsnano.0c05681 (2020).
Gisewhite, S., Stewart, I. J., Beilman, G. & Lusczek, E. Urinary metabolites predict mortality or need for renal replacement therapy after combat injury. Crit. Care. 25, 119. https://doi.org/10.1186/s13054-021-03544-2 (2021).
Kumar, P. et al. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, physical function, and aging hallmarks: A randomized clinical trial. J. Gerontol. Biol. Sci. Med. Sci. 78, 75–89. https://doi.org/10.1093/gerona/glac135 (2023).
Brady, P. S. et al. Pathways of acetoacetate’s formation in liver and kidney. J. Biol. Chem. 257, 9290–9293 (1982).
Denoon, T., Sunilkumar, S. & Ford, S. M. Acetoacetate enhances oxidative metabolism and response to toxicants of cultured kidney cells. Toxicol. Lett. 323, 19–24. https://doi.org/10.1016/j.toxlet.2020.01.015 (2020).
Agashe, R. P., Lippman, S. M. & Kurzrock, R. J. A. K. Not just another kinase. Mol. Cancer Ther. 21, 1757–1764. https://doi.org/10.1158/1535-7163.MCT-22-0323 (2022).
Perner, F., Perner, C., Ernst, T. & Heidel, F. H. Roles of JAK2 in aging, inflammation, hematopoiesis and malignant transformation. Cells 8 https://doi.org/10.3390/cells8080854 (2019).
Roy, A. et al. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharmacol. 40, 79–89. https://doi.org/10.1016/j.intimp.2016.08.026 (2016).
Liu, Q. et al. JAK2/STAT1-mediated HMGB1 translocation increases inflammation and cell death in a ventilator-induced lung injury model. Lab. Invest. 99, 1810–1821. https://doi.org/10.1038/s41374-019-0308-8 (2019).
Wang, Y. H., Li, A. G., Wang, H. Y. & Tu, Y. S. Repression of JAK2-STAT1 and PD-L1 by CEP-33779 ameliorates the LPS-induced decline in phagocytic activity of alveolar macrophages and mitigates lung injury in mice. Front. Immunol. 15, 1472425. https://doi.org/10.3389/fimmu.2024.1472425 (2024).
Li, J. et al. Pretreatment with fucoidan from Fucus vesiculosus protected against ConA-Induced acute liver injury by inhibiting both intrinsic and extrinsic apoptosis. PLoS One. 11, e0152570. https://doi.org/10.1371/journal.pone.0152570 (2016).
Ma, C. et al. Phillygenin inhibited M1 macrophage polarization and reduced hepatic stellate cell activation by inhibiting macrophage Exosomal miR-125b-5p. Biomed. Pharmacother. 159, 114264. https://doi.org/10.1016/j.biopha.2023.114264 (2023).
Xie, L. B. et al. LINC00963 targeting miR-128-3p promotes acute kidney injury process by activating JAK2/STAT1 pathway. J. Cell. Mol. Med. 24, 5555–5564. https://doi.org/10.1111/jcmm.15211 (2020).
Diotallevi, M. et al. Glutathione Fine-Tunes the innate immune response toward antiviral pathways in a macrophage cell line independently of its antioxidant properties. Front. Immunol. 8, 1239. https://doi.org/10.3389/fimmu.2017.01239 (2017).
Peng, Y. C. et al. NAD activates olfactory receptor 1386 to regulate type I interferon responses in plasmodium yoelii YM infection. Proc. Natl. Acad. Sci. U S A. 121, e2403796121. https://doi.org/10.1073/pnas.2403796121 (2024).
Acknowledgements
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Funding
This work was supported by Natural Science Foundation of Hunan Province (No. 2023JJ60102, 2022JJ80067, 2024JJ8203), Research Project of Hunan Provincial Department of Education (No. 22B0049), Project of Changsha Natural Science Foundation (No. kq2208124), Hunan Province Traditional Chinese Medicine Research Program Project (No. A2024040) and Ren-Shu Key Cultivation Project of Hunan Provincial People’s Hospital (No. RS2022A08).
Author information
Authors and Affiliations
Contributions
BB Pan: Conceptualization, Visualization, Writing original draft; FT Chen, SY Shen and Y Jiang: Methodology; Y Yang, J Wang, YJ Liu, L Wei and JT Liu: Editing; GY Kong and X He: Validation; B Guo: Conceptualization, Supervision and Writing & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pan, B., Chen, F., Shen, S. et al. Hydroxysafflor Yellow A modulation of metabolite networks and inhibition of JAK2/STAT1 pathway in sepsis. Sci Rep 15, 25861 (2025). https://doi.org/10.1038/s41598-025-11545-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-11545-2