Abstract
The chemical properties of extra virgin olive oil (EVOO) from Arbequina variety grown in Türkiye were evaluated, and its major phenolic compounds (PC) (oleocanthal, oleacein, luteolin and tyrosol) were compared with drugs (bempedoic acid and ezetimibe) involved in LDL metabolism through in silico analyses. The fatty acids composition (FA), PC and volatile organic compound (VC) profiles of EVOO obtained from Arbequina olive were evaluated via chromatographic methods (GC-FID, HPLC). The quality parameters, including total phenolic content (TPC), pigment content, peroxide value (PV), free fatty acid levels (FFA) and absorption coefficients, were determined via spectrophotometric methods. ADMET profiles, density functional theory (DFT), molecular docking, and the biological targets and activities of oleocanthal, oleacein, luteolin, tyrosol, bempedoic acid and ezetimibe were calculated and compared. Oleocanthal, oleacein, and luteolin completely passed the rules of Lipinski, Ghose, Veber, Egan, and Muegge, whereas only luteolin met the optimum ranges of all the criteria on the radar map. All 4 PC strongly inhibited OATP1B1 and OATP1B3, whereas oleocanthal, oleacein, and luteolin inhibited CYP3A4. Additionally, luteolin, oleocanthal and ezetimibe had individual inhibitory effects on CYP1A2, CYP2C9 and CYP2D6, respectively. Oleacein had the best binding affinity for LDLR, whereas luteolin had the best binding affinity for PCSK9 and ACLY. Oleacein was biologically effective against pathogens such as Leishmania species, but showed high reactivity with a low energy gap and high malleability. In conclusion, oleacein, oleocanthal and luteolin have potential therapeutic functions in LDL metabolism, which plays a role in atherosclerosis. However, experimental and clinical studies are needed for more evidence.
Similar content being viewed by others
Introduction
Evidence from both clinical and epidemiological studies has revealed a close relationship between dietary patterns and the incidence of chronic diseases, such as cardiovascular disease, cancer, diabetes, and obesity1. For this reason, in recent years, interest in healthy dietary patterns has increased among both the scientific community and the public. At this point, the Mediterranean diet has emerged as a prominent model of healthy nutrition, leading to its growing popularity worldwide. Numerous studies have demonstrated a significant association between adherence to the Mediterranean diet and a reduced incidence of major diet-related chronic diseases. The health benefits of this diet are attributed predominantly to its plant-based food content, with olive oil (OO) as its main lipid component2. EVOO is an element that has contributed to the popularity of this diet. The therapeutic effects of EVOOs are due to their high oleic acid content as a monounsaturated fatty acid (MUFA) and their rich and diverse phytochemical constituents, including PCs, phytosterols, tocopherols, squalenes, and pigments3. The chemical qualities of EVOOs are generally evaluated by their FFA content, PV, FA composition; and tocopherol, sterol, pigment, and PC contents. On the other hand, its VC composition has also been frequently investigated in recent years because it plays a critical role in sensory quality4. The amounts of all these constituents in EVOOs vary depending on the olive variety used and the conditions of agricultural growth5,6, as do both the EVOOs obtained from the olive and the subsequent storage conditions7. Oleic acid is the major MUFA in OOs and offers a protective effect against cardiovascular risks by preventing oxidative modifications of low-density lipoproteins (LDLs), lowering blood cholesterol8, and positively affecting the management of metabolic syndrome9. However, PCs in EVOOs have attracted increasing attention in studies focused on their bioactive effects on various diseases. PCs in EVOOs include phenolic acids, lignans, flavonoids, phenolic alcohols (hydroxytyrosol and tyrosol) and secoiridoids derived from oleuropein and ligstroside isomers10. The secoiridoids oleocanthal and oleacein are the quantitative main PCs of EVOOs and have a notable effect on their positive sensory properties11.
The studies conducted to date on the biological properties of EVOOs have focused mostly on the activities of oleuropein, hydroxytyrosol, and tyrosol. However, previous studies have shown that the biological roles of these individual compounds are less potent than those of the phenolic extracts from EVOOs. This is largely attributed to secoiridoids, as well as other components such as tocopherols, carotenoids, VC, and squalenes12. Oleocanthal and oleacein, which are PCs found in olive oil, have garnered significant attention in the scientific community in recent years because of their biological properties, including anti-inflammatory and antioxidant effects, as well as their ability to regulate cell proliferation13. In a clinical study, the consumption of EVOO with a high concentration of oleocanthal was associated with beneficial effects on metabolic parameters, inflammatory cytokines, and abdominal fat distribution in individuals with hepatic steatosis who are part of the metabolic syndrome category, a group of patients at high cardiometabolic risk14. Tyrosol is a phytochemical mostly found in olives, olive oil, wine, and various herbal preparations. It possesses antioxidant, anti-inflammatory, anticancer, antistress, antiosteoporotic, cardioprotective, and neuroprotective properties15,16. Luteolin, a flavonoid, is believed to play a significant role in the prevention of atherosclerotic diseases and to exhibit anti-inflammatory and anticancer properties17,18,19. Atherosclerotic cardiovascular disease (ASCVD) remains one of the leading causes of death worldwide, encompassing conditions such as myocardial infarction and ischemic stroke. Consistent data from clinical and genetic studies indicate that low-density lipoprotein cholesterol (LDL-C) levels contribute to the development of ASCVD; therefore, lowering LDL-C levels is a primary goal in both the primary and secondary prevention of ASCVD20,21. Interventions aimed at reducing LDL-C levels have led to significant advancements in addressing this global health challenge. In recent years, therapeutic innovations have emerged that effectively lower LDL-C levels, resulting in continuous improvements in cardiovascular outcomes. Notably, the discovery of the role of proprotein convertase subtilisin/kexin type 9 (PCSK9) in relation to autosomal dominant hypercholesterolemia has spurred the rapid development of novel therapeutic agents capable of reducing LDL-C concentrations beyond previous methods and mitigating cardiovascular risks22.
PCSK9 proteins bind to LDL receptors (LDLRs) in hepatocytes and accelerate their degradation, thereby increasing the level of circulating LDL-C. PCSK9 inhibitory agents are monoclonal antibodies that irreversibly bind to blood-borne PCSK9 proteins, preventing them from binding to and degrading hepatic LDLR23. Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9is) lower LDL-C by increasing both the quantity and durability of hepatic LDLR. Bempedoic acid reduces the synthesis of hepatic cholesterol. Ezetimibe, PCSK9i, and bempedoic acid are evidence-based, nonstatin therapies that work synergistically to lower LDL levels and reduce the risk of major adverse cardiovascular events. They also have favorable side effect profiles and are generally well tolerated23. ATP citrate lyase (ACLY) is a crucial enzyme in cellular metabolism that links carbohydrate and lipid metabolism, serving as the primary source of acetyl-coenzyme A, an important precursor for the biosynthesis of FAs, cholesterol, and isoprenoids. Additionally, ACLY plays a role in protein acetylation. Alterations in ACLY expression are associated with hyperlipidemia and cardiovascular disease24.
Bempedoic acid is a drug that inhibits cholesterol synthesis by targeting ACLY, which acts upstream of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This action, in turn, increases LDL receptor expression25,26,27. Recent evidence suggests that bempedoic acid and ezetimibe significantly lower lipid levels and have an acceptable safety profile in the treatment of hypercholesterolemia and atherosclerotic cardiovascular disease (ASCVD)28. Research aimed at reducing ASCVD has focused specifically on the effects of unsaturated FAs. In our previous study, we discussed the PCs hydroxytyrosol, oleuropein, pinoresinol, and apigenin, presenting important data from the literature29. However, compounds such as oleocanthal, oleacein, luteolin, and tyrosol, which are also derived from phenolic sources, have not been adequately explored in the context of lipid metabolism and ASCVD. This study aimed to minimize the number of biological target analyses for in vitro or in vivo studies and focus on the most promising compounds, such as oleocanthal, oleacein, luteolin, and tyrosol, which are thought to play a role in LDL metabolism. To design and analyze new potential candidates that exhibit superior biological activity compared to existing LDL-lowering agents such as ezetimibe and bempedoic acid, we aimed to achieve this goal through computational studies that included molecular docking of known targets in ASCVDs, including LDLR, PCSK9, and ATP-citrate lyase, as well as the ligands oleocanthal, oleacein, luteolin, and tyrosol. To further support these analyses, DFT calculations were used to elucidate the electronic and structural properties of the investigated molecules, providing valuable information on their reactivity, stability, and potential interaction mechanisms with biological targets. Given the importance of this study, it has the potential to contribute to further research in this field and, ultimately, benefit humanity. We hypothesized that PC of EVOO, such as oleacein and oleocanthal, exhibit LDL-lowering activity comparable to that of current lipid-lowering agents.
Materials and methods
Chemicals and reagents
Cyclohexane, hexane, Folin–Ciocalteu reagent, potassium hydroxide, sodium carbonate, methanol, and acetonitrile (both used for liquid chromatography), phosphoric acid, isobutyl acetate, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and PC standards (hydroxytyrosol, oleuropein pinoresinol, apigenin, luteolin, tyrosol, vanillic acid, p-coumaric acid, t-ferulic acid, catechin, and caffeic acid), a mixture of fatty acid methyl esters (FAMEs), and an alkane standard solution (C8-C20) were purchased from Sigma‒Aldrich (Darmstadt, Germany).
Extra Virgin Olive oil (EVOO)
The EVOO used in this study was obtained from Arequipa olives, a Spanish cultivar harvested during the 2021 season. The Arequipa olive variety is among the most widely cultivated and marketed varieties because of its adaptability to high-density cultivation systems and varying environmental conditions. Its small fruit size, early ripening, high oil yield, and excellent oil quality further contribute to its popularity30,31. Olives used for the EVOO production were collected from a grove located in the Aegean Region of Türkiye (Salihli, Manisa) in western Turkey. The olives were harvested during the second week of October, reaching the ripeness level classified by the International Olive Oil Council (IOC, 2011)32 as category 2 (green to red transition stage). The olives were transported to an industrial-scale oil press for processing on the same day. An Oliomio Cultivar 1000-2GV (MORI-TEM srl, Florence, Italy) oil press mill was utilized to extract the EVOO. The malaxation temperature was maintained at 27 ± 1 °C, and the extraction time was set to 25 min. A sufficient quantity of the obtained EVOO was stored in dark glass bottles and kept at 4 °C until analysis.
Quality index analysis
PV, FFA, and absorption coefficients (K232 and K270) were analyzed according to the Turkish Official Methods33. Sensory properties were assessed by a trained panel of 10 members, whose positive and negative perceptions of EVOO were measured4,34. The chlorophyll and carotenoid contents were determined following the methods of Isabel Minguez-Mosquera et al.35.
Extraction of phenolic compounds
PCs were extracted via a slightly modified version of the method described by Rodrigues et al.36. Three grams of EVOO was mixed with 3 mL of a methanol: water (80:20, v v− 1) mixture, 1.5 mL of hexane, and 0.5 mL of syringic acid (60 mg mL− 1) as an internal standard (IS). After incubation for 2 min, the mixture was centrifuged at 5000 rpm and 10 °C for 5 min. The methanolic phase was recovered, and the extraction process was repeated twice. The supernatants were collected and treated twice with 3 mL of hexane. The resulting the extract of PCs was utilized for total phenolic content (TPC) and individual PC analyses.
Total phenolic content (TPC) analysis
TPC was assayed using the Folin–Ciocalteu method as described by Capanoglu et al.37. Briefly, 100 µL of the extract PCs was placed into a tube, followed by the addition of 900 µL of distilled water and 5 mL of Folin-Ciocalteu reagent. This mixture was shaken vigorously and allowed to stand for 8 min. Subsequently, 5 mL of 7.5% sodium carbonate was added, and mixed in a vortex for 20 s. The mixture was then kept in the dark at room temperature for 2 h, after which its absorbance was measured at 765 nm using a UV spectrophotometer (Biochrom Libra S70). The results were calculated using a calibration curve of solutions prepared at various concentrations of gallic acid and expressed as gallic acid equivalents (GAE) per kg sample.
Fatty acid (FA) composition
The FA composition was determined by converting the FAs to methyl esters (FAMEs) according to the European Official Methods38. The analysis was conducted using a gas chromatography‒flame ionization detector (GC‒FID) system (Shimadzu QP2020, Shimadzu) equipped with a Restek Rtx‒2330 capillary column (60 m × 0.25 mm, 0.20 μm). A 0.1 g sample of EVOO was mixed with 10 mL of n-hexane in a tube and agitated vigorously. Next, 0.5 mL of 2 N potassium hydroxide in methanol was added to this mixture, which was shaken vigorously again and allowed to sit in the dark for 2 h. Subsequently, 1 µL of the upper phase was injected into the GC system in split mode (1:100). The injection temperature was set to 250 °C. The oven temperature was initially maintained at 140 °C for 5 min, then increased to 240 °C at a rate of 4 °C/min and held at this temperature for 12 min. Helium was used as the carrier gas at a flow rate of 1 mL/min. The identification and quantification of each FA were performed using FAME standards.
Analysis of phenolic compounds
The analysis of individual PCs was performed using high-performance liquid chromatography (HPLC) (Waters, e2695) coupled with a photodiode array detector (DAD) (Waters, 2996). The separation was achieved using an inerstsustain C18 (4.6 × 250 mm, 5 μm) (GL Sciences). The extrct of PCs was filtered through a polyvinylidene fluoride (PVDF) filter and injected into the HPLC system. The oven temperature, injection volume, and flow rate were set at 35 °C, 10 µL, and 1 mL/min, respectively. The mobile phases consisted of a phosphoric acid solution adjusted to pH 2.10 (A) and a mixture of ethanol: acetonitrile (9:1) (B). The gradient flow program was applied as described by Veneziani et al.39. The detection and quantification of PCs were performed using their respective standards at 280 nm (hydroxytyrosol, tyrosol, catechin, caffeic acid, vanillic acid, syringic acid (IS), p-coumaric acid, transferrulic acid, and pinoresinol), 335 nm (apigenin and luteolin), and 230 nm (oleuropein), with the exception of oleocanthal acid and oleacein. Owing to the unavailability of commercial standards for these secoiridoids, their peaks were identified on the basis of retention times according to the IOC method40. The quantities of these two PCs were calculated relative to the IS using the response factor of tyrosol and expressed as mg tyrosol per kg of EVOO40,41.
Volatile compound (VC) analysis
VCs were extracted by solid-phase microextraction (SPME) and then analyzed via gas chromatography‒mass spectrometry (GC‒MS) (Shimadzu QP2020). All procedures were conducted according to Korkmaz4.
Physicochemical and molecular descriptor analyses
The canonical smile formats of oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid were downloaded from PubChem and used as inputs for evaluating drug-like properties on the SwissADME online server (http://www.swissadme.ch/, access date, January 20, 2025)42. These compounds were subjected to in silico calculations of their physicochemical, molecular, and pharmacokinetic properties using ADMETlab 3.0 software (https://admetlab3.scbdd.com, access date, January 21, 2025)43.
Identification of molecular biological targets
Potential biological targets of oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid were predicted using the web tool MolPredictX (https://www.molpredictx.ufpb.br/, access date, January 22, 2025). Potential targets, outcomes, probabilities of activity or inactivity, and reliability are reported in the same Tables44,45.
Biological activity site of metabolism
The biological activity of the metabolic zone was assessed using a prediction service known as PASS Online (https://www.way2drug.com/passonline/, access date, January 23, 2025), and http://www.swisstargetprediction.ch/access date, July 11, 2025). These websites provide a set of bioinformatics tools that facilitate the analysis of medicinal properties based on the structure information (SMILE) of compounds45,46,47.
Molecular docking method
Ligand screening
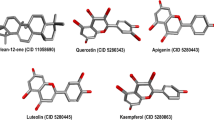
3D chemical structures and simplified molecular line entry system (SMILES) representations of oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) (Fig. 1).
Protein targets
LDLR was retrieved in PDB format from AlphaFold (ID: AF-H0YMD1-F1-v4) (https://alphafold.ebi.ac.uk/entry/H0YMD1). PCSK9, known as Proprotein Convertase Subtilisin/Kexin Type 9 (PDB ID: 2P4E), and the crystal structure of human ATP-Citrate Lyase with citrate bound (PDB ID: 3MWD) were obtained from the Protein Data Bank in 3D structures in PDB format (https://www.rcsb.org/).
Molecular Docking study
Molecular docking was performed using AutoDock Vina, while Discovery Studio Visualizer and PyMOL Molecular Graphics System, Version 3.1 (https://www.pymol.org/), were used for visualisation48,49,50,51. The crystal structures of the proteins were retrieved from the Protein Data Bank (PDB) in the PDB format. Co-crystallized ligands, ions, and water molecules belonging to the crystal structure were removed from the protein structure, as these molecules can cause non-specific interactions at the binding site. The binding pocket coordinates with the LDLR, PCSK9 and ACLY proteins center x, y, z: 0.75 Å, -1.34 Å, 3.34 Å/Size x, y, z: 80.00, 80.00, 80.00, center x, y, z: 29.49, 31.58, 38.84/size x, y, z: 80.00, 80.00, 80.00, center x, y, z: -0.68, 46.82, 9.36/size x, y, z: 80.00, 80.00, 80.00.
Redocking method
In the protein preparation step, water molecules and non-crystalline ligands were removed from the structure, and polar hydrazenes and Collman charges were added. Redocking procedures were applied for each protein and the ligands in the crystal structure were re-docked to the active site48.
Gene enrichment analysis
The ShinyGO 0.81 database was utilized for gene enrichment analysis. ShinyGO offers graphical visualizations of enrichment, pathways, gene features, and protein interactions, facilitating an in-depth analysis of gene lists. Gene Ontology (GO) encompasses not only functional categorizations but also gene identity mappings and other quantitative gene features. Gene queries are mapped to all gene IDs in the database, enabling both identity transformation and the identification of potential organisms. Gene lists are routinely generated from various omics studies. Enrichment analysis can link these gene lists to underlying molecular pathways and functional categories, such as GO and other relevant databases52.
DFT
Quantum chemistry simulations based on density functional theory (B3LYP with a 6-311 + + G(d, p) basis set) were employed to optimize the geometry via the Gaussian 09 software package. One of the calculations aimed to determine the energy gap (ΔE) between the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO). Additionally, the electronegativity (χ), hardness (η), nucleophilicity (Nu), softness (s), and electrophilicity (ω) properties were evaluated. Through these quantum chemistry techniques, the dipole moment (µ) and Mulliken charges on the backbone atoms were also calculated53,54,55,56,57,58,59,60,61,62.
The energy gap (ΔE) was calculated via the following equation:
The softness (s), hardness (η), and electronegativity (χ) were derived as follows:
The electrical characteristics of the molecules under investigation are well understood, which is essential for predicting their stability and chemical reactivity. By elucidating these characteristics, this information offers valuable insights into the behavior of molecules and their potential chemical and biological applications. This knowledge will aid in the design and synthesis of novel compounds with desirable stability and reactivity profiles. From a biological perspective, understanding these properties is vital for anticipating how these compounds interact with biological targets, maximizing their efficacy as medications, and assessing their safety and metabolic stability.
Results and discussion
Quality indices
The quality parameters of the EVOO samples are listed in Table 1. The values of FFAs, PV, K232, and K270 were 0.05 ± 0.00% oleic acid, 2.35 ± 0.01 mEq O2 kg− 1, 1.81 ± 0.02 and 0.17 ± 0.00, respectively. All of these values comply with the standards of the European Commission Regulation (EU)63. According to this regulation, the limits of FFAs and PV for the EVOO category are ≤ 0.8% oleic acid and ≤ 20, respectively. The FFA content is negatively correlated with positive sensory properties64. FFAs in OO are released from triglycerides via the hydrolytic activity of lipase enzymes on ester bonds. The FFA content in OO increases because of damage to olive fruits and storage for a long time before processing. In addition, inappropriate processing and storage conditions also increase this content65. Similarly, the PV is a main parameter used to assess the flavor defects of EVOO and is a measure of the concentration of hydroperoxide radicals formed as a result of FA oxidation. These reactions form several VCs in OO that cause an unpleasant aroma and rancid odor66.
The values of the coefficients K270 and K232 are also used to determine the degree of oxidation. K232 provides information about compounds belonging to the primary stage of oxidation in oil, whereas K270 is related to the level of compounds such as aldehydes and ketones formed in the second stage of oxidation67. The total chlorophyll and total carotenoid contents were 4.09 ± 0.09 and 6.05 ± 0.30 mg kg− 1, respectively (Table 1). The pigment content of OOs is significantly affected by the cultivar used, olive cultivation conditions, degree of maturity, OOs processing technique, storage conditions, and selected analysis methods68. In a previous study69, the total chlorophyll and total carotenoid levels in an EVOO from a cultivar of Arbequina olives grown in Tunisia were 2.53 and 0.42 mg kg− 1, respectively, whereas these two contents in another EVVO of Arbequina grown in Morocco were 9.16 and 4.06 mg kg− 1, respectively70. Chlorophylls are responsible for the characteristic greenish color of OOs, whereas carotenoids are responsible for their yellowish color. These pigments not only increase the oxidative stability of OOs but can also be used as indicators to detect other seed oils added to oils for adulteration71. The median fruity, bitterness, and pungency attributes of the EVOO were 3.2 (medium green fruitiness), 3.4 (medium bitterness), and 3.1 (medium pungency), respectively (Table 1). The median intensity of the defects was 0. In this regard, the EVOO samples were classified under the EVOO category according to the IOC34.
Total phenolic compounds (TPCs) and individual phenolic compounds (PCs)
PCs in EVOOs have been associated with several health benefits, including reduced cardiovascular disease incidence and antidiabetic and anticancer effects72. Additionally, both the content and diversity of PCs have decisive importance for the organoleptic quality of EVOOs. Furthermore, PCs play a key role in ensuring the oxidative stability of EVOO during storage and thermal processing68. Hence, these ingredients have become widely used for evaluating EVOO quality73. The TPC and PC contents of the EVOO samples are presented in Table 2. The most abundant PCs in the EVOO of Arbequina studied were the secoiridoids oleacein (45.51 ± 1.54 mg kg− 1) and oleocanthal (23.79 ± 0.69 mg kg− 1), as found in many previous studies on EVOOs from Arbequina and some other olive genotypes74,75.
Oleacein and oleocanthal are derived from oleuropein and its precursor ligstroside glycoside, respectively. Oleacein is responsible for the perception of the bitterness of EVOOs, whereas oleocanthal is responsible for their pungency74. Hydroxytyrosol (16.98 ± 0.85 mg kg− 1) and pinoresinol (12.32 ± 0.62 mg kg− 1) were the next most abundant compounds. The amounts of these compounds, including the tyrosol content (1.52 ± 0.05 mg kg− 1), agreed with those of a previous study on two EVOOs from Arbequina olives grown in different regions of Türkiye73. The phenolic alcohols hydroxytyrosol and tyrosol are also formed from oleuropein and ligstroside by chemical (acidic) or/and enzymatic hydrolysis, respectively74.
The most abundant flavonoids in EVOO are luteolin (1.98 ± 0.02 mg kg− 1) and apigenin (6.49 ± 0.33 mg kg− 1), which have been reported to have anticancer and other bioactive properties76. The TPC in the EVOO was 442.8 ± 7.64 mg GAE kg− 1. This content was consistent with the level (454.68 mg GAE kg− 1) reported by Uluata et al.73 for an EVOO of Arbequina cultivated in Izmir but clearly higher than that (50.86 mg GAE kg− 1) reported in Adana; both locations are in Türkiye. Both the total and individual PC amounts found in this study exceeded the minimum amount (200 mg kg− 1) required by the European Food Safety Authority (EFSA)77 for health claims on OOs. PC levels in OOs are influenced by cultivar, ripening period, pre- and postharvest practices, and conditions78 as well as the selected extraction method and instrument systems used during their analysis6.
Fatty acid (FA) composition
A recent study revealed that a diet containing OO resulted in lower levels of triglycerides, total cholesterol, malondialdehyde, LDL-C, and its oxidized form in the serum of rats than did soybean oil or a mixture of oils79. The FA values in the EVOO sample are summarized in Table 3. The predominant FA fractions in the EVOO were oleic (68.11 ± 0.76%), palmitic (15.32 ± 0.98%), linoleic (10.56 ± 0.11%), stearic (2.16 ± 0.34%), and palmitoleic acids (1.12 ± 0.15%). The percentages of FAs in the EVOO from Arbequina olive used in this study were within the range reported by Rey-Giménez and Sánchez-Gimeno80 for FAs in 97 different OOs obtained from the Arbequina cultivar. The FA composition of OOs is strongly influenced by cultivar. Therefore, it is used as a distinguishing feature for olive varieties72. The oleic/linoleic and linoleic/linolenic ratios (ω-6/ω-3) of EVOO were 6.45 ± 0.14 and 11.93 ± 0.01, respectively. The oleic/linoleic ratio is characteristic of the olive variety, and a higher ratio in the diet, which means more oleic acid, is desirable for cardiovascular health80. In contrast, a lower ω-6/ω-3 ratio is recommended to reduce the risk of some chronic diseases, including type 2 diabetes79. The value of ω-6/ω-3 (6.45 ± 0.14) found in this study was slightly greater than the recommended ratio (1–5) for a healthier individual. Kmiecik et al.68 demonstrated that the ω-6/ω-3 ratios of OOs from an Arbequina (15.48 ± 0.92) and an Armonia (14.14 ± 0.52) olive were also markedly greater than the suggested range, whereas the ratios reported for OOs from Picual (3.76 ± 0.13) and Cornicabra (5.31 ± 0.6) OOs were lower.
Volatile compounds (VCs)
Both the typical pleasant and possible defective odors of EVOOs are associated with their VC profiles66. Therefore, VC composition has recently been suggested to be used to determine the authenticity of OOs and to characterize monovarietal EVOOs81,82. The typical desirable aroma of EVOOs comes mostly from compounds with fruity and grassy odors formed during extraction, while their off-flavors are due to microbial activities and the oxidation of FA83. The relative concentrations of VC identified in the EVOO of Arbequina olive are presented in Table 4. A total of 31 VCs were identified in EVOO, and the most abundant chemical classes of VC in terms of diversity were aldehydes (7), alcohols (10) and terpenoids (5). The C6 compounds hexanal (2.076 ± 0.108), (Z)-3-hexenal (3.890 ± 0.163), (E)-2-hexenal (50.119 ± 0.140), 2,4-hexanedienal (1.684 ± 0.587), 1-hexanol (0.954 ± 0.054), (Z)-3-hexen-1-ol (2.150 ± 0.126) and (E)-2-hexen-1-ol (0.721 ± 0.062 mg kg− 1) were the most abundant VC in the EVOO sample.
VCs with C6 and C5 are generated via the lipoxygenase pathway and are primarily responsible for the fruity and grassy notes of EVOOs83. Most of these properties are related to fruitiness, with hexanal, (Z)-3-hexenal, and (Z)-3-hexen-1-ol providing green apple, tomato leaf, and green-grassy odors to EVOOs, respectively74,84. C6 aldehydes and alcohols have also previously been found in the EVOOs of Arbequina74, and other olive varieties are the major VC in EVOO4,85. The temperature of malaxation during extraction has a significant effect on the concentrations of these compounds86. (E)-2-Hexenal (herbaceous) alone accounted for 60.92% of the total amount of VC in the sample, agreeing with previous findings of EVOO from the same variety74. This volatile aroma is related to a pleasant and herbaceous aroma and is considered a freshness indicator for OOs84.
β-Sesquiphellandrene (herbal-fruity) (5.504 ± 0.154 mg kg− 1) had the highest content among terpenoids in EVOO, whereas 1-penten-3-one (1.910 ± 0.012 mg kg− 1) was the main ketone compound. Although Lozano-Castellón et al.86 mentioned 1-penten-3-one and (E)-2-pentenal (0.213 ± 0.011 mg kg− 1) as contributing positive aromas, similar to other enzymatically formed C5 compounds, they were associated with the positive aroma of EVOOs. Calatayud et al.83 recently proposed them as indicators for rancid defects of OOs. This contradiction may be related to their contents, perception thresholds, and other VC content in OOs. The ester compounds hexyl acetate (0.861 ± 0.044) and (E)-3-hexen-1-ol-acetate (3.557 ± 0.145 mg kg− 1) found in EVOO also contribute to fruitiness, whereas hexanoic (0.752 ± 0.073) and decanoic acid (0.209 ± 0.020 mg kg− 1) are associated with a musty odor and are attributed to microbial activity83 or oxidation87.
Radar maps of the compounds
To evaluate and compare these compounds, a radar map was created using six physicochemical properties: size, polarity, lipophilicity, solubility, flexibility, and saturation. The region indicated in the diagram corresponds to the optimal range of values for each parameter. The results revealed an optimal range (highlighted by the pink area) for all criteria except luteolin. Oleacanthal exhibited an optimal range for all criteria except for the flexibility of oleacein and bempedoic acid. Luteolin and ezetimibe, on the other hand, showed an optimal range for all other criteria except saturation (Fig. 2). The number rotatable values of oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid were 10, 10, 1, 2, 6, and 14, respectively, while the numbers of hydrogen bond acceptors were 15, 6, 5, 2, 5, and 5, respectively. The Csp3 values for oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid were 0.35, 0.35, 0.00, 0.25, 0.21, and 0.89, respectively. Notably, the Csp3 values for luteolin, tyrosol, and ezetimibe were less than 0.25. The Mw values for oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid were 304.34, 320.34, 286.24, 138.16, 409.43, and 344.49, respectively. These values fall within the acceptable range of 180–480. The expected total polar surface area (TPSA) values, which should be between 20 Ų and 130 Ų, were 80.67, 100.90, 111.13, 138.16, 60.77, and 94.83 for oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid, respectively. However, tyrosol did not meet these criteria (Table 5).
The compounds oleocanthal, oleacein, and luteolin were able to pass the rules of Lipinski, Ghose, Veber, Egan, and Muegge without violation. Tyrosol was able to pass the Ghose rule with 2 violations (No. 2 violations: MW < 160, MR < 40) and the Muegge rule with 1 violation. While the Ezetimibe compound passed the rules of Ghose, Veber, Egan, and Muegge without violations, it passed the Lipinski rule with 1 violation (Yes; 1 violation: MLOGP > 4.15). Bempedoic acid, on the other hand, was able to pass the rules of Lipinski, Ghose, and Egan Muegge without violation but could not completely pass the Veber rule with a violation (Table 5). The bioavailabilities of these compounds as oral drugs were 55%, 55%, 55%, 55%, 56%, respectively. The synthetic accessibility score (SA) evaluates how easy a medical molecule is to synthesize. The SA is calculated by dividing the sum of the contributions of molecular components by the number of components and is used to determine the ease of molecule synthesis in drug development processes. This evaluation was performed on many potential drug candidates, supported by software models, combinatorial libraries, and de novo molecular design methods88,89,90. The synthetic accessibility values of the compounds were 3.08, 3.16, 3.02, 1.00, 3.37, and 2.69, respectively. The highest value was for ezetimibe, while the lowest was for tyrososol. In general, all the compounds are easy to chemically synthesize. However, the lower synthetic accessibility value of tyrosol than others means that it can be produced more easily in the pharmaceutical industry (Table 5).
Absorption properties of the compounds
Caco-2 permeability, MDCK permeability, PAMPA, substrate or inhibitor (P-gpinh/P-gpsub), and human intestinal absorption (HIA) were estimated to evaluate the absorption properties of oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid. In addition, 20% bioavailability (F20), 30% bioavailability (F30), and 50% bioavailability (F50) were evaluated (Table 6). Transwell experiments with human colorectal adenocarcinoma (Caco-2) or Madin‒Darby canine kidney (MDCK) cells, which carry some morphological and functional characteristics, are the gold standard for measuring in vitro permeability, which governs the absorption of chemicals from the gut91,92. When the Caco-2 values of the compounds were examined, oleocanthal: -4.789, oleacein: -4.865, luteolin: -5.192, tyrosol: -4.856, ezetimibe: -5.019 and bempedoic acid: -5.154 were determined. Oleocanthal and oleacein had fairly good values (Table 6). A high negative Caco-2 permeability value indicates that these compounds can increase intestinal permeability and therefore provide better absorption than other compounds. When the MDCK permeability values were examined, oleocanthal: -4.691, oleacein: -4.659, luteolin: -4.799, tyrosol: -4.689, ezetimibe: -4.722 and bempedoic acid: -4.764 were determined. Oleocanthal, oleacein, luteolin, tyrosole, ezetimibe, and bempedoic acid do not have high pass rates. All the compounds exhibited low permeability in the MDCK cell membrane (Table 6). When the PAMPA values used to measure the membrane permeation rates, i.e., permeability properties, of the compounds were examined, oleocanthal: 0.753, oleacein: 0.723, luteolin: 0.345, tyrosol: 0.773, ezetimibe: 0.042 and bempedoic acid: 0.999 were determined. If the logPeff values of all these compounds are less than 2.0, then all the compounds have low permeabilities (Table 6).
Since the logPeff values of all compounds were less than 2.0, they exhibited low permeability characteristics. These findings suggest that the compounds have limited membrane permeability and may show low absorption in terms of bioavailability. Oleocanthal and oleacein exhibited optimal permeability, as they had the highest Caco-2 values. Tirosol and ezetimibe met the optimal permeability criteria but showed slightly lower permeability characteristics than oleocanthal and oleacein. This suggests that these compounds have good potential for intestinal absorption in humans. However, the low permeability of bempedoic acid and luteolin may require distribution strategies or additional formulations in terms of bioavailability.
When the Pgp inhibitor and Pgp substrate values were examined, oleocanthal: 0.205; 0.768, oleacein: 0.04; 0.329, luteolin: 0.001; 0.209, tyrosol: 0.018; 0.775, ezetimibe: 0.974; 0.031 and bempedoic acid: 0.0; 0.297 were determined, respectively. P-gp is a transport protein found in cell membranes that transports many drugs and compounds out of the cell. P-gp inhibitors inhibit the function of this protein, whereas P-gp substrates are substances that this protein is responsible for carrying (Table 6). Compounds such as oleocanthal, tyrosol, and bempedoic acid were observed with high probabilities as P-gp substrates. Among the P-gp inhibitors, ezetimibe has the highest probability, whereas compounds such as oleocanthal and tyrosol tend to be low-probability inhibitors. These data show that P-gp studies and substrates have different potentials.
When HIA values, which are important absorption parameters and constitute a significant obstacle in the formulation of new drug substances, were examined, oleocanthal: 0.01, oleacein: 0.097, luteolin: 0.015, tyrosol: 0.093, ezetimibe: 0.0 and bempedoic acid: 0.898 were determined. Bempedoic acid had a high HIA value, whereas ezetimibe, oleocanthal, oleacein, luteolin, and tyrosol had low absorption values (Table 6). This, in turn, can limit the effectiveness of these compounds and may require different formulation strategies. In addition, compounds with lower absorption values can increase the difficulty of drug development.
The HIA values of oleocanthal, oleacein, luteolin, tyrosol, and ezetimibe were very low, indicating a high intestinal absorption potential, which is advantageous in terms of bioavailability when administered orally. Although the low HIA value of ezetimibe theoretically indicates that it is well absorbed, its bioavailability is low. This suggests that the effects of ezetimibe may be limited by metabolic pathways. Conversely, the high HIA + of bempedoic acid likely indicates low absorption. The low permeability of all compounds in the PAMPA data partially contradicts HIA results. However, this may be explained by the fact that PAMPA is an artificial membrane model and better represents in vivo HIA conditions.
Distribution properties of the compounds
When the PPB and VDss values used to analyze the pharmacokinetic properties and potential therapeutic effects of the compounds were examined, oleocanthal: 90.175; 0.113, oleacein: 71.226; -0.223, luteolin: 97.642; -0.614, tyrosol: 34.6; 0.454, ezetimibe: 90.458; 0.082 and bempedoic acid: 77.687; -0.326 were determined, respectively (Table 7). When the F20%, F30% and F50% values of the compounds were examined, oleocanthal: 0.691; 0.697; 0.998, oleacein: 0.987; 0.99; 1.0, luteolin: 0.925; 0.992; 0.998, tyrosol: 0.226; 0.183; 0.975, ezetimibe: 0.007; 0.073; 0.007 and bempedoic acid: 0.231; 0.281; 0.441 were determined, respectively. Oleocanthal, oleacein, and luteolin had high bioavailability probabilities, especially at F50% values (Table 7). Because the F20% and F30% values of ezetimibe and tyrosol are quite low, the bioavailability of these compounds may be limited. The optimal value for PPB should be 90%, whereas the values for oleocanthal, luteolin, and ezetimibe are high. These results suggest that oleocanthal, luteolin, and ezetimibe may remain in circulation for a longer period of time, but caution is required in terms of the treatment index because high protein binding can lead to decreased efficacy or increased side effects in some cases. Tyrosol has a low value, indicating that it is found in more free forms and therefore could exhibit greater pharmacological activity.
The VDss values of oleocanthal and tyrosol indicate that these compounds can be well distributed in the body. Ezetimibe, on the other hand, is in the optimal range, but its lower value may indicate that it has a limited volume of distribution. Because the VDss values of oleacein, luteolin, and bempedoic acid were negative, no comment could be made on this issue.
To aid in candidate selection in the early stages of drug discovery, Fu values, an estimate of the fraction released in plasma from the pharmacokinetic properties of a drug, were examined, and oleocanthal: 11.572, oleacein: 26.918, luteolin: 2.286, tyrosol: 59.526, ezetimibe: 9.367, and bempedoic acid: 16.98 were identified (Table 7). Ingredients with high Fu values, such as oleacein, tyrosol, and bempedoic acid, are potentially more effective, meaning that they more easily reach the treated tissues. Luteolin, on the other hand, has a low Fu value, which may lead to activity restrictions due to its high protein binding rate. Therefore, the dosage of these drugs should be carefully adjusted93. Organic anion transporter polypeptides, which play important roles in the excretion of drugs in the human body, are OATP1B1 and OATP1B3 membrane proteins that allow drugs to enter the liver and then be excreted through the biliary tract by conjugation94. When the OATP1B1 and OATP1B3 inhibitor values were examined, oleocanthal: 0.944; 0.805; oleacein: 0.925; 0.84; luteolin: 0.871; 0.984; tyrosol: 0.922; 0.882; ezetimibe: 0.096; 0.076; and bempedoic acid: 0.506; 0.106 were determined (Table 7). Compounds such as oleocanthal, oleacein, luteolin, and tyrosol showed high inhibitory effects on OATP1B1 and OATP1B3, whereas ezetimibe and bempedoic acid had very low inhibitory effects on both transporters. While possible drug interactions of drugs with high inhibitory effects may pose a risk, the risk of interaction is low since ezetimibe and bempedoic acid are drugs with low inhibitory effects. When the BCRP inhibitor and MRP1 inhibitor values were examined, oleocanthal: 0.372; 0.164, oleacein: 0.552; 0.229, luteolin: 0.955; 0.804, tyrosol: 0.493; 0.404, ezetimibe: 0.002; 0.74 and bempedoic acid: 0.008; 0.998 were determined (Table 7). Owing to its high concentration, luteolin can be considered a powerful inhibitor of both BCRP and MRP1. Although the inhibitory effects of oleocanthal and oleacein remain weak, ezetimibe and bempedoic acid can be considered important inhibitors of MRP1.
Metabolic properties of the compounds
Cytochrome P450 is a membrane-bound hemoprotein isozyme superfamily with several classifications. These enzymes are found in the liver, intestines, and kidneys; their highest density is observed in the liver. Among the 57 isozymes discovered thus far, six important isozymes involved in the oxidative metabolism of xenobiotics and endogenous compounds are as follows: Cytochrome P450 1A2 (CYP1A2), Cytochrome P450 2C9 (CYP2C9), Cytochrome P450 2C19 (CYP2C19), Cytochrome P450 2D6 (CYP2D6), Cytochrome P450 2E1 (CYP2E1), and Cytochrome P450 3A4 (CYP3A4). These enzymes are responsible for approximately 90% of drug metabolism. When the inhibition values of oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid on CYP3A4, CYP2D6, CYP2C19, CYP2C9 and CYP1A2 enzymes were examined, respectively, the following values of CYP1A2 inhibition were determined: 0.007, 0.003, 1.0, 0.023, 0.003, 0.0; the values of CYP2C19 inhibition were 0.498, 0.372, 0.01, 0.013, 0.017, 0.0; the values of CYP2C9 inhibition were 0.972, 0.139, 0.001, 0.016, 0.321, 0.015; the values of CYP2D6 inhibition were 0.005, 0.001, 0.578, 0.02, 0.64, 0.0; and the values of CYP3A4 inhibition were 0.999, 0.999, 0.998, 0.036, 0.006, and 0.001 (Table 8). Luteolin has a strong inhibitory effect on CYP1A2, oleocanthal on CYP2C9, oleocanthal on oleacein, luteolin on CYP3A4, and ezetimibe on CYP2D6. These compounds can strongly affect the function of these enzymes. Bempedoic acid does not act on any enzyme. Oleocanthal, oleacein, and ezetimibe exhibit very low inhibitory effects on CYP1A2. In particular, molecules that strongly inhibit CYP enzymes can interact with other drugs to have side effects or alter drug metabolism. These molecules strongly influence the functions of these enzymes.
Excretion properties of the compounds
When the plasma clearance (CLplasma) and T1/2 values of the compounds were examined, oleocanthal: 8.796; 0.57, oleacein: 12.738; 1.031, luteolin: 8.482; 1.373, tyrosol: 12.254; 1.483, ezetimibe: 5.128; 0.731 and bempedoic acid: 3.214; 1.024 were determined (Table 9). Although there were no compounds characterized by high clearance (CLplasma > 15 ml/min/kg), oleacein, Tyrosol, oleocanthal, luteolin, and ezetimibe were characterized by medium-high clearance (15 ml/min/kg: moderate clearance). The half-lives of all the compounds were short (1.5 h). This suggests that the effect of a compound with high clearance and a short half-life quickly disappears, as the effects of these substances quickly subside. Inhibition of the human ether-a-go-go-related gene (hERG) channel is an important task for medicinal chemists in the development of various classes of drugs, and determining the potential of this inhibition at an early stage has become a rapidly evolving standard in drug design and development to reduce the risk of arrhythmias95,96. Drug-induced liver injury (DILI) is a predictable or unpredictable form of liver injury that can develop as a result of the toxic effects of certain drugs in certain individuals due to genetic and environmental factors97.
Toxicity properties of the compounds
When the hERG blockers and DILI values of the compounds were examined, oleocanthal: 0.073; 0.089, oleacein: 0.049; 0.209, luteolin: 0.069; 0.796, tyrosol: 0.152; 0.039, ezetimibe: 0.624; 0.187 and bempedoic acid: 0.013; 0.104 were determined (Table 10). hERG blockade is typically associated with low IC50 values and a strong blockade effect, indicating the potential for severe side effects, such as heart rhythm disturbances. Oleocanthal, oleacein, luteolin, and tyrosol were in the hERG + class in this regard, whereas ezetimibe and bempedoic acid were exempt from this risk. In addition, oleocanthal, oleacein, tyrosol, ezetimibe, and bempedoic acid pose a low risk of DILI. However, the use of compounds with a high risk of DILI, such as luteolin, requires careful monitoring.
When the AMES mutagenicity values used to determine whether a compound is mutagenic or not and the Rat Oral Acute Toxicity values used to determine whether a compound has toxic effects in rats, oleocanthal: 0.304; 0.147, oleacein: 0.39; 0.177, luteolin: 0.65, 0.51, tyrosol: 0.317; 0.086, ezetimibe: 0.415; 0.67, bempedoic acid: 0.03; 0.032 were determined (Table 10)98. We found that all the compounds have a certain mutagenic potential for the AMES test, i.e., Ames-positive, except for bempedoic acid, i.e., Ames negative. Higher values may indicate stronger mutagenic effects. Therefore, luteolin appears to carry the highest risk of mutation. When acute oral toxicity was examined in rats, oleocanthal, oleacein, luteolin, and ezetimibe showed high toxicity, whereas tyrosol and bempedoic acid showed low toxicity. Oleocanthal, oleacein, luteolin, and ezetimibe have both mutagenic potential and high toxicity in rats. These findings suggest that these compounds may carry health risks and should therefore be carefully evaluated. In particular, since luteolin exhibits both high mutagenicity and toxicity, further research on this compound is recommended.
The maximum daily dose recommended by the FDA (FDA MDD) estimates the toxic dose threshold of chemicals in humans99. The FDAMDD and carcinogenicity values were as follows: oleocanthal: 0.624; 0.359; oleacein: 0.656; 0.118; luteolin: 0.88; 0.689; tyrosol: 0.062; 0.456; ezetimibe: 0.901; 0.646; and bempedoic acid: 0.128; 0.115 (Table 10). Although luteolin and ezetimibe are safe for daily use, they should be used with caution because they carry carcinogenic risks. The FDAMDD values are quite high for both compounds, making them safe for daily use. However, the risk of carcinogenicity is greater in oleochantal than in the oleacein. Bempedoic acid has a very low risk for FDAMDD and carcinogenicity, making it a low risk for daily use. Although luteolin has a high FDAMDD value, its risk of carcinogenicity is high. Even if this makes luteolin safe for use in low amounts, caution should be exercised in long-term or high-dose use. Ezetimibe, on the other hand, has a high FDAMDD value and a moderate risk of carcinogenicity, making this ingredient safer for daily use. In summary, the use of compounds with a high risk of FDAMDD and low carcinogenicity is safer.
When the hematotoxicity and genotoxicity values of the compounds were examined, oleocanthal: 0.136; 0.927, oleacein: 0.962; 0.024, luteolin: 0.029; 0.986, tyrosol: 0.116; 0.019, ezetimibe: 0.577; 0.998 and bempedoic acid: 0.272; 0.003 were determined, respectively (Table 10). In terms of hematotoxicity, oleacein had the highest risk, whereas luteolin and tyrosol had the lowest risk. However, oleacein and bempedoic acid appear to be safe in terms of genotoxicity. Tyrosol and bempedoic acid are generally noted for their low levels of risk, which suggests their safety. Oleocanthal and luteolin have a high risk of genotoxicity but a low risk of hematotoxicity. Ezetimibe and luteolin have a remarkably high risk of genotoxicity; these compounds can have negative effects on DNA. When the RPMI-8226 immunotoxicity values of the compounds were examined, oleocanthal: 0.045, oleacein: 0.024, luteolin: 0.025, tyrosol: 0.042, ezetimibe: 0.029 and bempedoic acid: 0.036 were determined. Oleocanthal, oleacein, luteolin, tyrosol, ezetimibe, and bempedoic acid are noncytotoxic (Table 10). In other words, the compounds did not have a pronounced toxic effect on the RPMI-8226 cell line. Hence, these compounds can offer positive usability and reliability profiles. When the A549 cytotoxicity values and Hek293 cytotoxicity values of the compounds were examined, oleocanthal: 0.026; 0.205, oleacein: 0.147; 0.107, luteolin: 0.096; 0.242, tyrosol: 0.477; 0.473, ezetimibe: 0.619; 0.942 and bempedoic acid: 0.003; 0.003 were determined (Table 10). Although ezetimibe and tyrosol had higher cytotoxicity values in the A549 cell line, ezetimibe and tyrosol seemed to be effective in the Hek293 cell line. Bempedoic acid is not cytotoxic to either cell line. When the neurotoxicity values of the compounds were examined, oleocanthal: 0.729, oleacein: 0.255, luteolin: 0.212, tyrosol: 0.204, ezetimibe: 0.969, and bempedoic acid: 0.006 were determined. Among these compounds, ezetimibe and oleocanthal appear to have a high probability of being neurotoxic (Table 10). Oleacein, luteolin, and tyrosol are less likely to be neurotoxic. In contrast, bempedoic acid has almost no neurotoxic effect. These data may require caution in clinical practice, particularly for ezetimibe- and oleocanthal-treated patients.
The targets identified for pathogens from the investigated compounds were as follows: ezetimibe for Sars-Cov; oleocanthal, oleacein, tyrosol ve bempedoic acid for C. albicans; oleocanthal, oleacein, tirosol and ezetimibe for Dang larvicida; oleacein, luteolin and ezetimibe for Leishmania infantum-Promastigota; oleacein and ezetimibe for Leishmania braziliensis; and oleocanthal and oleacein for the epimastigote Chagas. In particular, oleacein, oleocanthal, and ezetimibe have shown activity against multiple biological targets. The development of some treatment strategies is important for revealing the potential activities of these compounds against different pathogens or diseases. In particular, the biological activity of oleacein against pathogens such as Leishmania species suggests that it may be a potential treatment option (Table 11)43.
When the biological activities of the compounds were examined, oleocanthal (PASS server) (Ubiquinol-cytochrome-c reductase inhibitor, protein-disulfide reductase (glutathione) inhibitor, aldose reductase substrate, polyporopopsin inhibitor, GST A substrate) oleacein (chemopreventive, ubiquinol-cytochrome-c reductase inhibitor, protein-disulfide reductase (glutathione) inhibitor, feruloyl esterase inhibitor, preneoplastic conditions treatment), luteolin (chorcone reductase inhibitor, membrane integrity agonist, HIF1A expression inhibitor, membrane permeability inhibitor, 2-dehydropantoate 2-reductase inhibitor), Tyrosol (Aspulvinone dimethylallyltransferase inhibitor, linoleate diol synthase inhibitor, membrane integrity agonist, CYP2C12 substrate, chlordecone reductase inhibitor), ezetimibe (atherosclerosis treatment, lipoprotein disorders treatment, Niemann-Pick C1-like 1 protein antagonist, Muramoyltetrapeptide carboxypeptidase inhibitor, hypolipemic), bempedoic acid (APOA1 expression enhancer, sphinganine kinase inhibitor, lipid metabolism regulator, alkylacetylglycerophosphatase inhibitor, acylcarnitine hydrolase inhibitor) were used (Table 12).
According to SwissTargetPrediction analysis of the compounds’ biological activities, oleocanthal (Hepatocyte growth factor receptor, Arachidonate 5-lipoxygenase, Histone deacetylase 6, Histone deacetylase 2, Histone deacetylase 8) oleacein (Arachidonate 5-lipoxygenase, Hepatocyte growth factor receptor, Histone deacetylase 6, Histone deacetylase 8, Histone deacetylase 1), luteolin (NADPH oxidase 4, Aldose reductase, Cyclin-dependent kinase 5/CDK5 activator 1, Xanthine dehydrogenase, Monoamine oxidase A), Tyrosol (Carbonic anhydrase II, Estrogen-related receptor gamma, GABA-A receptor; alpha-1/beta-2/gamma-2, Cyclooxygenase-1, Androgen Receptor), ezetimibe (Cannabinoid receptor 1, Cannabinoid receptor 2, 11-beta-hydroxysteroid dehydrogenase 1, Estrogen receptor alpha, Estrogen receptor beta), bempedoic acid (Peroxisome proliferator-activated receptor delta, Peroxisome proliferator-activated receptor alpha, 11-beta-hydroxysteroid dehydrogenase 1, Free fatty acid receptor 1, Solute carrier family 22 member 6 (by homology)) were evaluated (Table 12)47.
The binding energies of LDLR and compounds resulting from ligand‒protein interactions, H bonds, and hydrophobic interactions are shown in Table 13. The negative value of the ΔG results indicates that the reaction occurred spontaneously. The tested compounds presented binding energies in the range of -8.3–4.6 kcal/mol. The H-bond interaction, hydrophobic interaction, electrostatic interaction and binding energies of LDLR with the compounds are as follows: oleocanthal (-4.6 kcal/mol, H-bond interaction: LEU414, ASP415, hydrophobic interaction: LEU414, electrostatic interaction: LYS617), oleacein (-8.3 kcal/mol, H-bond interaction: LEU479, ILE522, VAL524, THR567, LEU568, GLN660, hydrophobic interaction: TRP483, VAL523, LEU570, VAL615, electrostatic interaction: ASP651), luteolin (-7.6 kcal/mol, H-bond interaction: CYS173, ASN428, ARG520, hydrophobic interaction: ILE623, electrostatic interaction: GLU174, GLU650), tyrosol (-5.8 kcal/mol, H-bond interaction: LEU568, GLU615, hydrophobic interaction: LEU571, electrostatic interaction: ASP569), ezetimibe (-7.5 kcal/mol, H-bond interaction: ASP172, LYS518, SER648, hydrophobic interaction: ALA475, electrostatic interaction: ARGstatic Interation: ARGstatic Interation: ASP569), ezetimibe (Figs. 3 and 4 and, Table 13).
As a result of ligand‒protein interactions, H bonding, and hydrophobic interactions, PCSK9 and the binding energies of the compounds are shown in Table 14. The tested compounds showed a binding energy ranging from − 8.6 to -5.6 kcal/mol. The H-bond interaction, hydrophobic interaction, electrostatic interaction and binding energies of PCSK9 with the compounds are as follows: oleocanthal (-6.9 kcal/mol, H-bond interaction: ARG495, hydrophobic interaction: TRP566, LEU571, electrostatic interaction: No), oleacein (-7.6 kcal/mol, H-bond interaction: ARG495, SER564, LEU571, HIS591) hydrophobic interaction: PRO639 electrostatic interaction: No), luteolin (-8.6 kcal/mol), H-bond interaction: THR437, TRP461, hydrophobic interaction: PRO438, VAL650, electrostatic interaction: No), tyrosol (-5.6 kcal/mol, H-bond interaction: LYS69, hydrophobic interaction: No, electrostatic interaction: No), ezetimibe (-7.4 kcal/mol, H-bond interaction: ALA478, PRO479, hydrophobic interaction: No, electrostatic interaction: GLU332, ARG357, CYS358, ASP360, ARG458) and bempedoic acid (-6.8 kcal/mol, H bond Interaction: ASP360, ARG521, ASP651), Hydrophobic Interaction: ILE416, PRO438, ARG458, VAL460, Electrostatic Interation: No) (Figs. 5 and 6, and Table 14).
The binding energies of ACLY and its compounds as a result of ligand‒protein interactions, H bonding, and hydrophobic interactions are shown in Table 15. The tested compounds showed a binding energies in the range of -8.6–5.3 kcal/mol. The H-bond interaction, hydrophobic interaction, electrostatic interaction and binding energies of ACLY with the compounds are as follows: oleocanthal (-6.1 kcal/mol, H-bond interaction: GLY281, SER343, ASN346, GLY664, GLY665), hydrophobic interaction: ALA280, ALA345, electrostatic interaction: no), oleacein (-6.0 kcal/mol, H-bond interaction: GLY309, ASN346, VAL626, GLY664, GLY665), hydrophobic interaction: ALA345, PHE347 electrostatic interaction: no), luteolin (-8.6 kcal/mol), H-bond interaction: SER7, ARG33, ASP60, GLN61, PHE242, GLY243, GLU245, ALA246, hydrophobic interaction: PRO239, PRO240, electrostatic interaction: no), Tyrosol (-5.3 kcal/mol, H-bond interaction: ASN346, PHE347, THR348, hydrophobic interaction: no, electrostatic interaction: no), ezetimibe (− 6.0 kcal/mol, H-bond interaction: GLU250, hydrophobic interaction: PRO241 (Figs. 7 and 8 and Table 15).
The results of the molecular docking study revealed that the order of compounds with the best binding affinity to the LDLR protein structure was oleacein > luteolin > ezetimibe > tyrosol > bempedoic acid > oleocanthal. The order of the compounds showing the best binding affinity for the PCSK9 protein structure was luteolin > oleacein > ezetimibe > oleocanthal > bempedoic acid > tyrosol. According to these data, luteolin, oleacein and ezetimibe had the best binding scores. For the ACLY protein structure, the order was luteolin > oleacanthal > oleacein = ezetimibe = bempedoic acid > tyrosol.
According to these results, luteolin, oleacein, and ezetimibe had the best binding scores. Tyrosol had the lowest binding score. The gene enrichment analysis results revealed that therapeutic compounds were associated with diseases such as arteriosclerosis, corneal artery disease, and hypertension with known atherosclerosis activity according to gene targets (Table 16 and Figs. 9 and 10).
Chemical reactivity and stability can be predicted via DFT calculations, which offer valuable insights into the electronic characteristics of molecules. To determine a molecule’s electronic stability and reactivity, the energy gap (ΔE) between the HOMO and LUMO was computed. Electrophilicity (ω), nucleophilicity (Nu), hardness (), softness (s), and electronegativity (χ) were evaluated. The measured dipole moment (µ) reflects how the charge is distributed within a molecule.
According to the results of the analysis evaluated together with Fig. 11, oleocanthal (A), with a binding energy of -4.6 kcal/mol, formed hydrogen bonds with LEU414 and ASP415, as indicated by blue dashed lines, hydrophobic interaction with LEU414 marked with yellow regions, and electrostatic interaction with LYS617 indicated by red/blue color scale (2.3–2.8 Å RMSD). In particular, the green hydrophobic pocket on the protein surface is observed to interact strongly with the aromatic ring of oleocanthal. Oleacein (B) with − 8.3 kcal/mol is prominently embedded in the active site of the protein and interacts with numerous blue dashed lines (hydrogen bonds with LEU479, ILE522, VAL524) and yellow surfaces (hydrophobic interactions with TRP483, VAL523) (0.9–1.4 Å RMSD). The electrostatic attraction of the red coloured ASP651 in the image is particularly striking. Luteolin (C) with − 7.6 kcal/mol is seen tightly bound to the protein surface in the image and its hydrogen bonds with CYS173 and ARG520 can be clearly distinguished by the blue dashed lines and the electrostatic interaction with GLU174 by the red/blue colour transitions (1.2–1.7 Å RMSD). The π-π stacking interactions of the aromatic rings in the image are particularly prominent. Tyrosol (E) with − 5.8 kcal/mol appears more loosely bound on the protein surface in the image, showing only a few blue dashed lines (hydrogen bonds with LEU568 and GLU615) and limited interaction with pale yellow regions (LEU571 hydrophobic interaction) (3.0-3.8 Å RMSD). The solvent-exposed position in the image explains the binding instability. Ezetimibe (D) with − 7.5 kcal/mol was located in the electrostatic region of the protein in the image, hydrogen bonds with ASP172 and LYS518, and strong electrostatic interaction (red/blue regions) with ARG520 (1.5-2.0 Å RMSD). Its position adjacent to the alpha helix structure in the image stands out as an important feature. Bempedoic acid (F) with − 5.5 kcal/mol forms hydrogen bonds with PRO171 and ASP477 in the image, while showing hydrophobic interaction (yellow region) with ILE623 (~ 2.0-2.5 Å RMSD). The conformation of the carboxylate group in the image can be considered as a factor limiting the binding stability.
Analyzing the molecular interactions in Fig. 12, oleocanthal (A) (-6.8 kcal/mol) showed moderate stability (2. 1 Å RMSD), while oleacein (B) (-8.5 kcal/mol) exhibits the most stable binding (~ 1.2 Å RMSD) with multiple hydrogen bonds with ASP289, ARG292 and GLN295 and hydrophobic interactions with PHE286/ILE290, completely buried in the active site. Luteolin (C) (-9.1 kcal/mol) shows excellent compatibility with hydrogen bonds with THR279, ASN282 and ARG292 and electrostatic interaction with GLU278 (< 1.0 Å RMSD), with a compact structure in the image that fits perfectly into the protein fold. Tyrosol (E) (-5.9 kcal/mol) only forms a single hydrogen bond with SER283, while its solvent-exposed position and lack of interaction in the image explain the high RMSD value (~ 3.5 Å). Ezetimibe (D) (-7.2 kcal/mol) formed hydrogen bonds with GLU277 and LYS280 and hydrophobic interactions with PRO275 (1.8 Å RMSD), whereas its restricted interaction with side chains was noticeable in the image. Bempedoic acid (F) (-6.3 kcal/mol) formed hydrogen bonds with ASP289 and GLY293 and hydrophobic interactions with ILE290 (2.0 Å RMSD), and the rotational flexibility of the carboxyl group is evident in the image. When the image is evaluated as a whole, the structures of the multi-interacting compounds (B, C) completely embedded in the protein surface and the hydrogen bonds shown by the intense blue dashed lines show the most stable binding in accordance with the low RMSD values, while the looser binding profiles of the single interacting compounds (A, E) can be clearly distinguished in the image. In particular, the critical role of electrostatic interactions (red/blue regions) and hydrophobic pockets (yellow surfaces) in binding stability is clearly seen by the colour coding in the image.
Considering the molecular interactions and binding conformations shown in Fig. 13, oleocanthal (A) (-6.1 kcal/mol) exhibits moderately stable bonding (1.8–2.3 Å RMSD) due to hydrogen bonds with GLY281 and ASN346 (blue dashed lines) and hydrophobic interactions with ALA280/ALA345 (yellow surfaces), as shown in the image. The solvent-exposed position in the image explains the limited stability of the single hydrogen bond. Oleacein (B) (-6.0 kcal/mol) appeared to be tightly bound to the protein surface (1.5-2.0 Å RMSD), forming multiple hydrogen bonds (GLY309, VAL626, blue dashed lines) and hydrophobic packing (yellow regions) with PHE347, as evident from the visual analysis. The compact structure in the image favours the low RMSD value. Luteolin (C) (-8.6 kcal/mol) is observed to form the most stable complex in the image, showing excellent compatibility (< 1.0 Å RMSD) due to numerous hydrogen bonds with SER7 and ARG33 (dense blue dashed network), hydrophobic interactions with PRO239/240 (prominent yellow regions) and electrostatic attraction with LYS103 (red/blue colour transitions). In the image, its structure completely embedded in the active site is striking. Tyrosol (E) (-5.3 kcal/mol) only forms a single hydrogen bond (sparse blue line) with ASN346 in the image, while it appears loosely positioned on the protein surface due to the lack of hydrophobic interaction (absence of yellow region) (2.8–3.5 Å RMSD). The completely solvent-exposed position in the image explains the high RMSD value. Ezetimibe (D) (-6.0 kcal/mol) formed a single hydrogen bond with GLU250 and hydrophobic interactions with ALA254/LEU264 (light yellow regions) in the image but appeared partially unstable due to the lack of electrostatic interaction (2.0-2.5 Å RMSD). Its peripheral position in the image reflects its binding instability. Bempedoic acid (F) (-6.0 kcal/mol) exhibits a moderately stable conformation (1.5-2.0 Å RMSD), forming hydrogen bonds with GLY281 and GLY665 and hydrophobic interactions with VAL626. The position of the carboxyl group in the image shows that it is open to rotamer changes.
With an energy gap of 0.77 eV, oleacein exhibited significant electronic instability (Table 17; Fig. 14) compared with the other compounds investigated. This low ΔE indicates greater chemical reactivity. While its dipole moment of 3.6989 Debye shows significant charge separation, which may affect its interactions with biological targets, its high softness (2.57 eV− 1) and low hardness (0.38 eV) further confirm its reactive nature. Ezetimibe exhibited a significantly larger energy gap of 4.56 eV, suggesting reduced reactivity and increased electronic stability. Its softness (0.43 eV− 1) and hardness (2.82 eV) both indicate a stable molecular structure. Its pharmacological interactions may be affected by the comparatively lower dipole moment of 5.3366 Debye, which indicates less noticeable charge separation within the molecule. At 3.38 eV, luteolin exhibits an intermediate energy gap, indicating moderate stability and reactivity. According to its electrical properties, the hardness was 1.69 eV, and the softness was 0.59 eV− 1. Moderate charge separation was indicated by a dipole moment of 3.7055 Debye, which may have affected the solubility and binding affinity. With an energy gap of 0.78 eV, the oleocanthal exhibited a balance between stability and reactivity. Higher chemical reactivity is indicated by a smaller ΔE value. These qualities are compatible with its softness (2.54 eV− 1) and hardness (0.39 eV). Minimal charge separation is indicated by a dipole moment of 2.9436 Debye, which may affect the bioavailability and target contact. The largest energy gap, 6.95 eV, was observed for bempedoic acid, indicating strong electronic stability and low reactivity. The low softness (0.28 eV− 1) and strong hardness (3.47 eV) further support this stability. Significant charge separation is indicated by a dipole moment of 3.4369 Debye, which could impact the material’s solubility and interaction dynamics. Among the compounds under study, tyrosol exhibited the lowest reactivity and maximum electronic stability, with an energy gap of 6.13 eV. Its low softness (0.32 eV− 1) and high hardness (3.06 eV) reinforce its stability. Its interaction with biological systems may be affected by the moderate degree of charge separation, as shown by its dipole moment of 0.1693 Debye (Fig. 14 and Table 17).
The electrostatic potential (ESP) maps of the compounds are provided in Fig. 14. Since the ESP concept captures the intricate electron‒electron interactions and electron density distribution in a system, it is crucial for density functional theory (DFT) calculations. Theoretically, ESPs dramatically advance our knowledge of material properties, electrical structure, and molecular energetics. The ESP maps generated from the DFT computations display positive zones in green or blue and negative regions in orange or red. These colors can be used to better visualize the distribution of electron density in molecules and materials. This study examined the stability and reactivity of chemicals via molecular orbital theory. The quantum reactivity parameters were also computed from the HOMO and LUMO energies. The negative regions show regions with comparatively greater electron concentrations, suggesting an electron-rich or abundant environment. Electron-rich functional groups, such as lone pairs of electrons or π-electron systems in organic compounds, are typically represented by these regions. In chemistry, negative regions are associated with possible sites of electron donation or nucleophilic activity. The global electrophilicity component demonstrates how well electron acceptors can absorb extra electronic charges from the system100,101,102,103. Conversely, positive zones on the ESP maps suggest lower electron concentrations and, consequently, electron-poor or electron-deficient conditions. These areas are typically observed near electrophilic functional groups or atoms with much higher electronegativity than their neighboring atoms. Positive regions tend to be connected with electrophilic activity, where molecules or atoms try stealing electrons from other species. ESP (electrostatic potential) examination of chemical structures has shown that areas with oxygen atoms are negatively charged104,105,106,107. This finding is true for the following compounds: ezetimibe, bempedoic acid, oleocanthal, oleacein, luteolin, and tyrosol. The oxygen atoms in these compounds usually take part in electron-rich functional groups such as carbonyl (C=O) and hydroxyl (-OH) moieties. The capacity of these oxygen-containing groups to draw electrons contributes to their total negative electrostatic potential (Fig. 14). Understanding the chemical reactivity and intermolecular interactions of these compounds, especially in situations such as hydrogen bonding or interactions with positively charged species, requires an understanding of this property. Moreover, the presence of negative ESP regions around oxygen atoms could affect the solubility, stability, or biological activity of compounds in various settings108,109,110. The energy difference between the HOMO and LUMO is a useful descriptor of the chemical and biological activities of molecules. A smaller energy difference makes the compound more easily polarizable, and thus more chemically and biologically active111. Because oleocanthal and oleacein have different chemical compositions and functional groups, they can have various reactivity properties, which could indicate differences in their electrostatic profiles and reactivity patterns. Oleacein also exhibits high binding affinity (ΔG = − 7.6 and − 8.3 kcal/mol) to PCSK9 and LDLR proteins due to its low ΔE = 0.77 eV, supporting the relationship between reactivity markers and biological activity. Ultimately, DFT calculations reveal significant variations in the electronic properties and reactivities of the molecules being studied, which are critical for understanding their chemical behavior and potential applications in a range of fields.
Conclusion
The findings obtained in our study show that among the PCs of EVOO, oleacein, oleocanthal, and luteolin are notable compounds. Oleocanthal, oleacein, and luteolin have successfully passed the Lipinski, Ghose, Veber, Egan, and Muegge rules regarding their drug-like properties. Luteolin, on the other hand, stands out as a potential component because it has reached optimum values for all the criteria on the radar map. A strong inhibitory effect was observed on OATP1B1 and OATP1B3 of oleocanthal, oleacein, luteolin, and tyrosol on CYP1A2, oleocanthal on CYP2C9, oleocanthal, oleacein, and luteolin on CYP3A4, and ezetimibe on CYP2D6. In particular, oleacein’s high electronic instability made this compound more reactive, whereas the interactions of oleocanthal and luteolin with different enzymes offered important insights into their potential therapeutic properties. Molecular docking studies revealed that luteolin and oleacein had the best binding scores. The compound with the best binding affinity for the LDLR protein structure was oleacein, whereas the compound with the best binding affinity for PCSK9 and ACLY was luteolin. These findings suggest that these compounds play a role in ASCVD and LDL metabolism in particular. In conclusion, oleacein, oleocanthal, and luteolin offer important opportunities for improving the lipid profile in ASCVD management. Further examination of the health effects of these compounds may contribute to the identification of potential pharmacological applications. However, experimental and clinical studies on dose amount and dose frequency are needed.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ASCVD:
-
Atherosclerotic cardiovascular disease
- ACLY:
-
ATP citrate lyase
- APOA1:
-
Apolipoprotein A-I
- CDK5 activator:
-
Cyclin-dependent kinase 5 activator
- CYP:
-
Cytochrome P450
- DFT:
-
Density functional theory
- DILI:
-
Drug-induced liver injury
- ESP:
-
Electrostatic potential
- EVOO:
-
Extra virgin olive oil
- FA:
-
Fatty acids
- GC-FID:
-
Gas chromatography flame ionization detector
- hERG:
-
Human ether-a-go-go-related gene
- HIA:
-
Human intestinal absorption
- HIF1A:
-
Hypoxia-inducible factor 1-alpha
- HOMO:
-
Highest occupied molecular orbital
- LDL-C:
-
Low-density lipoprotein cholesterol
- LDLR:
-
LDL receptor
- LUMO:
-
lowest unoccupied molecular orbital
- MUFA:
-
Monounsaturated fatty acid
- PASS:
-
Prediction of activity spectra for substances
- PC:
-
Phenolic compounds
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- SPME:
-
Solid-phase microextraction
- SMILES:
-
Simplified molecular line entry system
- TPC:
-
Total phenolic content
- VC:
-
Volatile organic compounds
References
Galan, P., & Hercberg, S. [SU.VI.MAX and NutriNet-Santé: lessons from large cohorts]. La Revue Du Praticien. 2018;68(1).
Mentella, M. C., Scaldaferri, F., Ricci, C., Gasbarrini, A. & Miggiano, G. A. D. Cancer and mediterranean diet: A review. Nutrient. 19(1), 2059. https://doi.org/10.3390/nu11092059 (2019).
Farhan, N., Al-Maleki, A. R., Sarih, N. M., Yahya, R. & Shebl, M. Therapeutic importance of chemical compounds in extra virgin olive oil and their relationship to biological indicators: A narrative review and literature update. Food Biosci. 52, 102372. https://doi.org/10.1016/j.fbio.2023.102372 (2023).
Korkmaz, A. Characterization and comparison of extra virgin olive oils of Turkish olive cultivars. Molecules https://doi.org/10.3390/molecules28031483 (2023).
Benlloch-González, M., Martos-García, I., Benlloch, M. & Fernández-Escobar, R. High temperatures have a different effect on vegetative and reproductive processes of ‘Picual’ and ‘Arbequina’ olive in Spain. Sci. Hortic. 337, 113560. https://doi.org/10.1016/j.scienta.2024.113560 (2024).
Blasi, F., Ianni, F. & Cossignani, L. Phenolic profiling for geographical and varietal authentication of extra virgin olive oil. Trends Food Sci. Technol. 147, 104444. https://doi.org/10.1016/j.tifs.2024.104444 (2024).
Olmo-Cunillera, A. et al. High hydrostatic pressure enhances the formation of oleocanthal and oleacein in ‘Arbequina’ olive fruit. Food Chem. 437(Part 1), 137902. https://doi.org/10.1016/j.foodchem.2023.137902 (2024).
Moreno, J. A. et al. A monounsaturated fatty acid-rich diet reduces macrophage uptake of plasma oxidised low-density lipoprotein in healthy young men. Br. J. Nutr. 100(3), 569–575. https://doi.org/10.1017/S0007114508911508 (2008).
Pastor, R., Bouzas, C. & Tur, J. A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free Radic. Biol. Med. 172, 372–385. https://doi.org/10.1016/j.freeradbiomed.2021.06.017 (2021).
Nikou, T., Sakavitsi, M. E., Kalampokis, E. & Halabalaki, M. Metabolism and bioavailability of olive bioactive constituents based on in vitro, in vivo and human studies. Nutrients 14(18), 3773. https://doi.org/10.3390/nu14183773 (2022).
Lozano-Castellón, J. et al. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 60(15), 2532–2548. https://doi.org/10.1080/10408398.2019.1650715 (2020).
Filardo, S. et al. Olea europaea L-derived secoiridoids: Beneficial health effects and potential therapeutic approaches. Pharmacol. Ther. 254, 108595. https://doi.org/10.1016/j.pharmthera.2024.108595 (2024).
Ruiz-García, I. et al. Rich oleocanthal and oleacein extra virgin olive oil and inflammatory and antioxidant status in people with obesity and prediabetes. The APRIL study: A randomised, controlled crossover study. Clin. Nutr. 42(8), 1389–1398. https://doi.org/10.1016/j.clnu.2023.06.027 (2023).
Patti, A. M. et al. Daily use of extra virgin olive oil with high oleocanthal concentration reduced body weight, waist circumference, alanine transaminase, inflammatory cytokines and hepatic steatosis in subjects with the metabolic syndrome: A 2-month intervention study. Metabolites 10(10), 392. https://doi.org/10.3390/metabo10100392 (2020).
Ali, F. E. M., Badran, K. S. A., Baraka, M. A., Althagafy, H. S. & Hassanein, E. H. M. Mechanism and impact of heavy metal-aluminum (Al) toxicity on male reproduction: Therapeutic approaches with some phytochemicals. Life Sci. 340, 122461. https://doi.org/10.1016/j.lfs.2024.122461 (2024).
Güvenç, M. et al. Protective effects of tyrosol against dss-induced ulcerative colitis in rats. Inflammation 42(5), 1680–1689. https://doi.org/10.1007/s10753-019-01028-8 (2019).
Ding, X., Zheng, L., Yang, B., Wang, X. & Ying, Y. Luteolin attenuates atherosclerosis via modulating signal transducer and activator of transcription 3-mediated inflammatory response. Drug Des. Devel. Ther. 13, 3899–3911. https://doi.org/10.2147/DDDT.S207185 (2019).
Fan, X. et al. Evaluation of anti-nociceptive and anti-inflammatory effect of luteolin in mice. J. Environ. Pathol. Toxicol. Oncol. 37(4), 351–364. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2018027666 (2018).
Imran, M. et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 112, 108612. https://doi.org/10.1016/j.biopha.2019.108612 (2019).
Sandesara, P. B., Virani, S. S., Fazio, S. & Shapiro, M. D. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr. Rev. 40(2), 537–557. https://doi.org/10.1210/er.2018-00184 (2019).
Ference, B. A. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement fromthe European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38(32), 1. https://doi.org/10.1093/eurheartj/ehx144 (2017).
Libby, P. & Tokgözoğlu, L. Chasing LDL cholesterol to the bottom—PCSK9 in perspective. Nat. Cardiovasc. Res. 1(6), 554–561. https://doi.org/10.1038/s44161-022-00085-x (2022).
Gunta, S. P., O’Keefe, J. H., O’Keefe, E. L. & Lavie, C. J. PCSK9 inhibitor, ezetimibe, and bempedoic acid: Evidence-based therapies for statin-intolerant patients. Prog. Cardiovasc. Dis. 79, 12–18. https://doi.org/10.1016/j.pcad.2023.02.007 (2023).
Granchi, C. ATP-citrate lyase (ACLY) inhibitors as therapeutic agents: A patenting perspective. Expert Opin Ther. Pat. https://doi.org/10.1080/13543776.2022.2067478 (2022).
Nikolic, D., Mikhailidis, D. P., Davidson, M. H., Rizzo, M. & Banach, M. TC-1002: A future option for lipid disorders?. Atherosclerosis 237(2), 705–710. https://doi.org/10.1016/j.atherosclerosis.2014.10.099 (2014).
Pinkosky, S. L. et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat. Commun. 7(1), 13457. https://doi.org/10.1038/ncomms13457 (2016).
Penson, P., McGowan, M. & Banach, M. E. valuating bempedoic acid for the treatment of hyperlipidaemia. Expert Opin Investig. Drugs. 26(2), 251–269. https://doi.org/10.1080/13543784.2017.1280458 (2017).
Bhagavathula, A. S., Al Matrooshi, N. O., Clark, C. C. T. & Rahmani, J. Bempedoic acid and ezetimibe for the treatment of hypercholesterolemia: A systematic review and meta-analysis of randomized phase II/III trials. Clin. Drug Investig. 41(1), 19–28. https://doi.org/10.1007/s40261-020-00989-1 (2021).
Unsal, V. et al. Evaluation of extra virgin olive oil compounds using computational methods: In vitro, ADMET, DFT, molecular docking and human gene network analysis study. BMC Chem. 19(1), 3. https://doi.org/10.1186/s13065-024-01369-y (2025).
Torres, M. M. & Maestri, D. M. Chemical composition of Arbequina virgin olive oil in relation to extraction and storage conditions. J. Sci. Food Agric. 86(14), 2311–2317. https://doi.org/10.1002/jsfa.2614 (2006).
Borges, T. H. et al. Characterization of Arbequina virgin olive oils produced in different regions of Brazil and Spain: Physicochemical properties, oxidative stability and fatty acid profile. Food Chem. 215, 454–462. https://doi.org/10.1016/j.foodchem.2016.07.162 (2017).
International Olive Council (IOC). COI/OH/Doc. No 1 November 2011, Guide for The Determination of The Characteristics of Oil-Olives. https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-OH-Doc.-1-2011-Eng.pdf (2011). Accessed 10 October 2021
TR Ministry of Agriculture and Forestry, Turkish Food Codex on Olive Oil and Pomace Oil Analysis Methods Notification. Turkish Official Gazette, 29181. https://www.resmigazete.gov.tr/eskiler/2014/11/20141120-21.htm (2014). Accessed 10 December 2022.
International Olive Council (IOC), COI/T.20/Doc. No 15/Rev. 10, Method for the Organoleptic Assessment of Virgin Olive Oil. https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T20-Doc.-15-REV-10-2018-Eng.pdf (2018). Accessed 10 December 2022.
Isabel Minguez-Mosquera, M., Rejano-Navarro, L., Gandul-Rojas, B., Sanchez Gomez, A. H. & Garrido-Fernandez, J. Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 68(5), 332–336. https://doi.org/10.1007/BF02657688 (1991).
Rodrigues, N. et al. Ancient olive trees as a source of olive oils rich in phenolic compounds. Food Chem. 276, 231–239. https://doi.org/10.1016/j.foodchem.2018.09.106 (2019).
Capanoglu, E., De Vos, R. C. H., Hall, R. D., Boyacioglu, D. & Beekwilder, J. Changes in polyphenol content during production of grape juice concentrate. Food Chem. 139(1–4), 521–526. https://doi.org/10.1016/j.foodchem.2013.01.023 (2013).
EEC Commission Regulation (EEC). No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Official Journal of the European Communities, L 248. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:1991:248:FULL (1991). Accessed 15 December 2022.
Veneziani, G. et al. Characterization of phenolic and volatile composition of extra virgin olive oil extracted from six Italian cultivars using a cooling treatment of olive paste. LWT. 87, 523–528. https://doi.org/10.1016/j.lwt.2017.09.034 (2018).
International Olive Council (IOC), Method 1: COI/T.20/Doc. No 29/Rev.1 2017. Determination of Biophenols in Olive Oils by HPLC. https://www.internationaloliveoil.org/wp-content/uploads/2022/06/Doc.-No-29-REV-2_ENK.pdf (2022). Accessed 7 November 2022.
Pierguidi, L. et al. Markers of sensory dynamics in phenols-rich virgin olive oils under optimal storage conditions. Int. Food Res. 187, 14438. https://doi.org/10.1016/j.foodres.2024.114438 (2024).
Daina, A., Michielin, O. & Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7, 42717. https://doi.org/10.1038/srep42717 (2017).
Fu, L. et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucl. Acids Res. 52(11), 422–431. https://doi.org/10.1093/nar/gkae236 (2024).
Tullius Scotti, M. et al. MolPredictX: Online biological activity predictions by machine learning models. Mol. Inform. 41(12), 2200133. https://doi.org/10.1002/minf.202200133 (2022).
Unsal, V., Oner, E., Yıldız, R. & Mert, B. D. Comparison of new secondgeneration H1 receptor blockers with some molecules: A study involving DFT, molecular docking, ADMET, biological target and activity. BMC Chem. 19(1), 4. https://doi.org/10.1186/s13065-024-01371-4 (2025).
Filimonov, D. A. et al. Prediction of the biological activity spectra of organic compounds using the pass online web resource. Chem. Heterocycl. Compd. 50(3), 444–457. https://doi.org/10.1007/s10593-014-1496-1 (2014).
Daina, A., Michielin, O. & Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucl. Acids Res. 47(W1), 357–364. https://doi.org/10.1093/nar/gkz382 (2019).
Trott, O. & Olson, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chemy. 31(2), 455–461. https://doi.org/10.1002/jcc.21334 (2010).
Hanwell, M. D. et al. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 4, 17. https://doi.org/10.1186/1758-2946-4-17 (2012).
Biovia, D.S. 2021. Discovery studio visualiser. San Diego, CA, USA, 936.
DeLano W.L., PyMOL Molecular Graphics System, Version 2.3. Schrödinger LLC (2020).
Ge, S. X., Jung, D. & Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 36(8), 2628–2629. https://doi.org/10.1093/bioinformatics/btz931 (2020).
Keleşoğlu, A. et al. Inhibition efficiency of pyrazinecarboxylic acid on mild steel in acidic environment. JAST 35(13), 1426–1446. https://doi.org/10.1080/01694243.2020.1850055 (2021).
Kartal, A., Yıldız, R., Döner, A. & Baran, M. F. Thorough examination of Polygonum aviculare L. plant leaf extract as a green corrosion inhibitor for mild steel in 1M HCl: Synthesis, characterization, observations from surface analysis, experimental and DFT studies. Inorg. Chem. Commun. 176, 11424. https://doi.org/10.1016/j.inoche.2025.114241 (2025).
Bitew, M. et al. Pharmacokinetics and drug-likeness of antidiabetic flavonoids: Molecular docking and DFT study. PLoS ONE 16(12), e0260853. https://doi.org/10.1371/journal.pone.0260853 (2021).
Döner, A., Yıldız, R., Arslanhan, S. & Baran, M. F. A comprehensive analysis of Arum dioscoridis plant leaf extract as a corrosion inhibitor for mild steel in 1 M HCl: Synthesis, characterization, surface analysis observations, experimental and DFT studies. J. Taiwan Inst. Chem. Eng. 169, 105955. https://doi.org/10.1016/j.jtice.2025.105955 (2025).
Primas, H. & Shimony, A. Chemistry, quantum mechanics and reductionism: Perspectives in theoretical chemistry. Am. J. Phys. https://doi.org/10.1119/1.13329 (1983).
Karelson, M., Lobanov, V. S. & Katritzky, A. R. Quantum-chemical descriptors in QSAR/QSPR studies. Chem. Rev. https://doi.org/10.1021/cr950202r (1996).
Abd-El-Aziz, A. S. et al. A new family of benzo[h]chromene based Azo dye: Synthesis, in-silico and DFT studies with in vitro antimicrobial and antiproliferative assessment. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22062807 (2021).
Ismael, M., Abdel-Mawgoud, A. M. M., Rabia, M. K. & Abdou, A. Design and synthesis of three Fe(III) mixed-ligand complexes: Exploration of their biological and phenoxazinone synthase-like activities. Inorgan. Chim. Acta. 505, 119443. https://doi.org/10.1016/j.ica.2020.119443 (2020).
Unsal, V., Yıldız, R., Cicek, M., Gungor, M. & Kurutas, E. B. Trans-chalcone attenuate arsenic-induced toxicity in 3T3 embryonic fibroblast cells: An in vitro and in silico study. J. Mol. Struct. 1318(Part 2), 139338. https://doi.org/10.1016/j.molstruc.2024.139338 (2024).
Arslanhan, S., Sığırcık, G., Yıldız, R. & Baran, M. F. Lavandula angustifolia extract as a green corrosion inhibitor for protection of mild steel in HCl acid solution. Prot. Met. Phys. Chem. Surf. 60(3), 554–570. https://doi.org/10.1134/S2070205124701739 (2024).
Commission Implementing Regulation (EU). 2105 of 29 July 2022 laying down rules on conformity checks of marketing standards for olive oil and methods of analysis of the characteristics of olive oil. Official Journal of the European Communities, L 284, 23–47. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R2105 (2022). Accessed 19 December 2022.
Di Serio, M. G. et al. Ethyl esters versus fermentative organoleptic defects in virgin olive oil. Food Chem. 219, 33–39. https://doi.org/10.1016/j.foodchem.2016.09.109 (2017).
Ariza-Ortega, J. A. et al. Fatty acids and squalene and quality parameters of extra virgin olive oil (Olea europaea L.) produced in Mexico. Food Hum. 3, 100367. https://doi.org/10.1016/j.foohum.2024.100367 (2024).
Lozano-Castellón, J. et al. Extra virgin olive oil: A comprehensive review of efforts to ensure its authenticity, traceability, and safety. Compr. Rev. Food Sci. Food Saf. 21(3), 2639–2664. https://doi.org/10.1111/1541-4337.12949 (2022).
Mousavi, S. A., Nateghi, L. & Hosseini, E. Exploring the physicochemical, antioxidant and sensory properties of the flavoured virgin olive oil with chavir extract during storage. Flavour. Fragr. J. 40(1), 53–62. https://doi.org/10.1002/ffj.3814 (2025).
Kmiecik, D., Fedko, M., Małecka, J., Siger, A. & Kowalczewski, P. Ł. Effect of heating temperature of high-quality arbequina, picual, manzanilla and cornicabra olive oils on changes in nutritional indices of lipid, tocopherol content and triacylglycerol polymerization process. Molecules 8(10), 4247. https://doi.org/10.3390/molecules28104247 (2023).
Chtourou, M., Gargouri, B., Jaber, H., Abdelhedi, R. & Bouaziz, M. Comparative study of olive oil quality from Chemlali Sfax versus Arbequina cultivated in Tunisia. Eur. J. Lipid Sci. 115(6), 631–640. https://doi.org/10.1002/ejlt.201200234 (2013).
Belbachir, Y. et al. Exploring accelerated oxidative and physicochemical properties of Arbequina and Moroccan Picholine olive oils: A preliminary study on molecular interactions. Biocatal. Agric. Biotechnol. 56, 103037. https://doi.org/10.1016/j.bcab.2024.103037 (2024).
Ferreiro-González, M. et al. Authentication of virgin olive oil by a novel curve resolution approach combined with visible spectroscopy. Food Chem. 220, 331–336. https://doi.org/10.1016/j.foodchem.2016.10.015 (2017).
Reboredo-Rodríguez, P. et al. Effect of olive ripening degree on the antidiabetic potential of biophenols-rich extracts of Brava Gallega virgin olive oils. Int. Food Res. 137, 109427. https://doi.org/10.1016/j.foodres.2020.109427 (2020).
Uluata, S., Altuntaş, Ü. & Özçelik, B. biochemical characterization of arbequina extra virgin olive oil produced in Turkey. JAOCS J. Am. Oil Chem. Soc. 93(5), 617–626. https://doi.org/10.1007/s11746-016-2811-z (2016).
Marx, Í. M. G. et al. Impact of fresh olive leaves addition during the extraction of Arbequina virgin olive oils on the phenolic and volatile profiles. Food Chem. 393, 133327. https://doi.org/10.1016/j.foodchem.2022.133327 (2022).
Criado-Navarro, I. et al. Monitoring the partition of bioactive compounds in the extraction of extra virgin olive oil. LWT. 162, 113433. https://doi.org/10.1016/j.lwt.2022.113433 (2022).
Schmidt, L., Prestes, O. D., Augusti, P. R. & Fonseca Moreira, J. C. Phenolic compounds and contaminants in olive oil and pomace–A narrative review of their biological and toxic effects. Food Biosci. 53, 102626. https://doi.org/10.1016/j.fbio.2023.102626 (2023).
European Food Safety Authority (EFSA), Panel on Dietetic Products, & Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to polyphenols in olive oil and protection of LDL particles from oxidative damage. EFSA J., 9(4), 2033. https://doi.org/10.2903/j.efsa.2011.2033 (2011). Accessed 25 December 2022.
Ghreishi Rad, S. A., Jalili, M., Ansari, F., Rashidi Nodeh, H. & Rashidi, L. Maturity impact on physicochemical composition and polyphenol properties of extra virgin olive oils obtained from Manzanilla, Arbequina, and Koroneiki varieties in Iran. Food Sci Nutr. https://doi.org/10.1002/fsn3.3497 (2023).
Zhou, Y. et al. Normal fat intake with high MUFA content and an appropriate SFA/MUFA/PUFA ratio improved the health of rats. Eur. J. Lipid. Sci. Technol. 126(6), 2300214. https://doi.org/10.1002/ejlt.202300214 (2024).
Rey-Giménez, R. Sánchez-Gimeno ACEffect of cultivar and environment on chemical composition and geographical traceability of Spanish olive oils. JAOCS J. Am. Oil Chem. Soc. 101(4), 371–382. https://doi.org/10.1002/aocs.12774 (2024).
Quintanilla-Casas, B. et al. The volatile metabolome—gas chromatography–mass spectrometry approaches in the context of food fraud. Curr. Opin Food Sci. 61, 101235. https://doi.org/10.1016/j.cofs.2024.101235 (2025).
Freitas, F., Cabrita, M. J. & da Silva, M. G. Early identification of olive oil defects throughout shelf life. Separations. 11(6), 167. https://doi.org/10.3390/separations11060167 (2024).
Calatayud, M. V. et al. Relationships between chemical compounds and sensory properties of virgin olive oil in the us and israel: Development of a prediction model for defects. J. Agric. Food Chem. 72(45), 25391–25402. https://doi.org/10.1021/acs.jafc.4c06012 (2024).
Genovese, A., Caporaso, N. & Sacchi, R. Flavor chemistry of virgin olive oil: An overview. Appl Sci. 11(4), 1639. https://doi.org/10.3390/app11041639 (2021).
Nucciarelli, D. et al. Extra virgin olive oil from stoned olives with oxygen supply during processing: Impact on volatile and phenolic fraction and sensory characteristics. Foods. 13(19), 3073. https://doi.org/10.3390/foods13193073 (2024).
Lozano-Castellón, J. et al. A targeted foodomic approach to assess differences in extra virgin olive oils: Effects of storage, agronomic and technological factors. Food Chem. 435, 137539. https://doi.org/10.1016/j.foodchem.2023.137539 (2024).
Chiaia, V. et al. Study of oxidation products in aged olive oils by GC and HPLC techniques coupled to mass spectrometry to discriminate olive oil lipid substances in archaeological artifacts from ancient Taormina (Italy). J. Chromatogr. A. 1731, 465154. https://doi.org/10.1016/j.chroma.2024.465154 (2024).
Ertl, P. & Schuffenhauer, A. Estimation of synthetic accessibility score of drug-like molecules based on molecular complexity and fragment contributions. J. Cheminform. https://doi.org/10.1186/1758-2946-1-8 (2009).
Thakkar, A., Chadimová, V., Bjerrum, E. J., Engkvist, O. & Reymond, J. L. Retrosynthetic accessibility score (RAscore)-rapid machine learned synthesizability classification from AI driven retrosynthetic planning. Chem. Sci. https://doi.org/10.1039/d0sc05401a (2021).
Warr, W. A. A short review of chemical reaction database systems, computer-aided synthesis design, reaction prediction and synthetic feasibility. Mol. Inform. 33(6–7), 469–476. https://doi.org/10.1002/minf.201400052 (2014).
Hubatsch, I., Ragnarsson, E. G. E. & Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2(9), 2111–2119. https://doi.org/10.1038/nprot.2007.303 (2007).
Tian, H., Ketkar, R. & Tao, P. ADMETboost: A web server for accurate ADMET prediction. J. Mol. Model. 28(12), 408. https://doi.org/10.1007/s00894-022-05373-8 (2022).
Watanabe, R. et al. Predicting fraction unbound in human plasma from chemical structure: Improved accuracy in the low value ranges. Mol. Pharm. 15(11), 5302–5311. https://doi.org/10.1021/acs.molpharmaceut.8b00785 (2018).
Ciută, A.-D. et al. Structure of human drug transporters OATP1B1 and OATP1B3. Nat. Commun. 14(1), 5774. https://doi.org/10.1038/s41467-023-41552-8 (2023).
Kalyaanamoorthy, S. & Barakat, K. H. Development of safe drugs: The hERG Challenge. Med. Res. Rev. 38(2), 525–555. https://doi.org/10.1002/med.21445 (2018).
Garrido, A., Lepailleur, A., Mignani, S. M., Dallemagne, P. & Rochais, C. hERG toxicity assessment: Useful guidelines for drug design. Eur. J. Med. Chem. 195, 112290. https://doi.org/10.1016/j.ejmech.2020.112290 (2020).
Andrade, R. J. et al. Drug-induced liver injury. Nat. Rev. Dis. Primers. 5(1), 58. https://doi.org/10.1038/s41572-019-0105-0 (2019).
Chu, C. S. M., Simpson, J. D., O’Neill, P. M. & Berry, N. G. Machine learning—Predicting Ames mutagenicity of small molecules. J. Mol. Graph. Model. 109, 10811. https://doi.org/10.1016/j.jmgm.2021.108011 (2021).
Babalola, S., Igie, N. & Odeyemi, I. Molecular docking, drug-likeness analysis, in silico pharmacokinetics, and toxicity studies of p-nitrophenyl hydrazones as anti-inflammatory compounds against COX-2, 5-LOX, and H+/K+ ATPase. Pharm. Fronts. https://doi.org/10.1055/s-0042-1759688 (2022).
Panahande, Z., Mohammadi Ziarani, G., Feizi-Dehnayebi, M., Badiei, A. & Abu-Dief, A. M. Synthesis and DFT Calculation of Hg2+ Fluorescence Chemosensor Based on a New Hybrid Organic-Inorganic Nanoporous Material of SBA-Pr-Is-Hy. Appl. Organomet. Chem. 39(2), e7818. https://doi.org/10.1002/aoc.7818 (2025).
Lanez, T., Feizi-Dehnayebi, M. & Lanez, E. Assessment of the electrostatic binding of ferrocenylmethyl-nitroaniline derivatives to DNA: A combined experimental and theoretical study. J. Mol. Struct. 1308, 138386. https://doi.org/10.1016/j.molstruc.2024.138386 (2024).
Andonova, V. et al. Spectral characteristics, in silico perspectives, density functional theory (DFT), and therapeutic potential of green-extracted phycocyanin from spirulina. Int. J. Mol. Sci. 25(17), 9170. https://doi.org/10.3390/ijms25179170 (2024).
Mohammadi Ziarani, G., Rezakhani, M., Feizi-Dehnayebi, M. & Nikolova, S. Fumed-Si-Pr-Ald-Barb as a fluorescent chemosensor for the Hg2+ detection and Cr2O72− ions: A combined experimental and computational perspective. Molecules 29(20), 4825. https://doi.org/10.3390/molecules29204825 (2024).
Chai, Y.-M., Zhang, H.-B., Zhang, X.-Y. & Chai, L.-Q. X-ray structures, spectroscopic, antimicrobial activity, ESP/HSA and TD/DFT calculations of Bi(III) complex containing imidazole ring. J. Mol. Struct. 1256, 132517. https://doi.org/10.1016/j.molstruc.2022.132517 (2022).
An, H.-L., Duan, Y., Chen, T.-T. & Chai, L.-Q. Experimental and theoretical studies of trinuclear cadmium(II) complex containing pyridine ring: Synthesis, crystallographic, spectroscopic, TD/DFT calculations and Hirshfeld surface analysis. J. Mol. Struct. 1310, 138320. https://doi.org/10.1016/j.molstruc.2024.138320 (2024).
Majumdar, D., Roy, S., Elizabeth Philip, J., Tüzün, B. & Hazra, S. In-situ Salen-type ligand formation-driven of a heterometallic Cu(II)-Hg(II) complex: Synthetic update, crystallographic features, DFT calculations, and unveil antimicrobial profiles. Inorg. Chem. Commun. 160, 111933. https://doi.org/10.1016/j.inoche.2023.111933 (2024).
Wu, C. et al. Investigating haloacetic acids - human serum albumin interactions: A comprehensive approach using multi-spectroscopy, DFT calculations, and molecular docking. J. Mol. Struct. 1299, 137143. https://doi.org/10.1016/j.molstruc.2023.137143 (2024).
Abu-Dief, A. M. et al. Design, preparation, physicochemical characterization, structural conformational, biological evaluation, and DNA interaction for some new benzimidazole complexes. Appl. Organomet. Chem. https://doi.org/10.1002/aoc.7358 (2024).
Sahin, D. et al. Biological activities, DFT calculations, and molecular docking simulation of thymol-based compounds. ChemistrySelect 9(23), e202304572. https://doi.org/10.1002/slct.202304572 (2024).
Zahirović, A. et al. Dual Antimicrobial-Anticancer Potential, Hydrolysis, and DNA/BSA Binding Affinity of a Novel Water-Soluble Ruthenium-Arene Ethylenediamine Schiff base (RAES) Organometallic. Spectrochim. Acta A Mol. Biomol. Spectrosc. 318, 124528. https://doi.org/10.1016/j.saa.2024.124528 (2024).
Mohamed, G. G., Mahmoud, W. H. & Refaat, A. M. Nano-azo ligand and its superhydrophobic complexes: Synthesis, characterization, DFT, contact angle, molecular docking, and antimicrobial studies. J. Chem. 1, 6382037. https://doi.org/10.1155/2020/6382037 (2020).
Acknowledgements
We would like to thank Birsen Can Pehlivan, a certified olive oil taster, for her support in providing the EVOO used in this study. Her name was added to the acknowledgements section with his permission and knowledge.
Funding
This research was funded by the Ministry of Industry and Technology of the Republic of Turkey and the Southeast Anatolia Project (GAP) Regional Development Administration, and Derik (Mardin) Municipality (Grant number MAÜ-GAP-20-SBF-005).
Author information
Authors and Affiliations
Contributions
Aziz Korkmaz: Project administration, formal analysis, conceptualization, investigation, methodology, supervision, visualization, writing-original draft, writing-review and editing. Velid Unsal: Supervision, formal analysis, conceptualization, investigation, methodology, writing-original draft, writing-review & editing Reşit Yıldız: Methodology, formal analysis, Writing-review & editing; Erkan Oner: Formal analysis, conceptualization, investigation, methodology, writing-review & editing; Ahmet Ferit Atasoy: Conceptualization, formal analysis, visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to publish the study
Permission to use the olive samples for this study was obtained from the orchard owner.
Institutional review board
Not applicable.
Informed consent
Not applicable.
Clinical trial number
Not applicable.
Limitations
This study did not include in vitro validation experiments (e.g., enzyme inhibition or LDL uptake assays) due to lack of funding.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Korkmaz, A., Unsal, V., Yıldız, R. et al. Chemical evaluation of arbequina extra virgin olive oil with in silico analysis of its key phenolic compounds targeting LDL metabolism. Sci Rep 15, 33536 (2025). https://doi.org/10.1038/s41598-025-18144-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18144-1