Abstract
The advent of immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway has revolutionized cancer treatment, resulting in improved clinical outcomes. However, resistance remains a critical challenge. This study aimed to comparatively elucidate immunophenotypic changes in syngeneic mouse models sensitive (MC-38) or resistant (LLC1) to anti-PD-1 monoclonal antibody (mAb) treatment. In the sensitive MC-38 model, anti-PD-1 therapy increased dendritic cells (DCs) and macrophages, while decreasing myeloid-derived suppressor cells (MDSCs) within the tumor microenvironment. Enhanced expression of antigen presentation molecules (MHC I/II) and costimulatory molecules (CD80/CD86) was observed on tumor-associated DCs and macrophages. Tumor-infiltrating CD4+T, CD8+T, regulatory T, NK, and NKT cells also significantly increased. Importantly, treatment boosted lymphocyte cytotoxic potential, with perforin identified as a key marker of efficacy. Notably, perforin expression in CD4+T and NKT cells strongly negatively correlated with tumor volume. In contrast, the resistant LLC1 model exhibited minimal immunophenotypic changes upon treatment. These findings highlight critical immune modifications induced by anti-PD-1 therapy, particularly the role of perforin, and the DC/MDSC ratio in predicting therapeutic outcomes. This research offers valuable insights into potential predictive biomarkers and informs strategies to overcome resistance, emphasizing the complex interplay between anti-PD-1 treatment and the tumor microenvironment, ultimately aiming to improve immunotherapy response rates.
Similar content being viewed by others
Introduction
The advent of immune checkpoint inhibitors, particularly anti-PD-1 mAbs, has revolutionized cancer therapy by harnessing the body’s immune system to target and eliminate tumor cells. Despite the success of these therapies, resistance remains a significant challenge, necessitating a deeper understanding of the underlying immunological mechanisms. This study aims to elucidate the immunophenotypic changes in syngeneic tumor model mice that are either sensitive or resistant to anti-PD-1 mAb treatment.
Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor expressed on T cells, which, upon binding to its ligands PD-L1 and PD-L2, inhibits T cell activation and proliferation, thereby allowing tumors to evade immune surveillance1,2. Anti-PD-1 mAbs block this interaction, reinvigorating T cell activity against tumor cells. However, the response to anti-PD-1 mAb therapy is highly variable among patients, with some showing remarkable tumor regression while others exhibit resistance3.
The tumor microenvironment (TME) plays a crucial role in the efficacy of immune checkpoint inhibitors. The TME is composed of various immune cells, including T cells, natural killer (NK) cells, dendritic cells (DCs), and myeloid-derived suppressor cells (MDSCs), which can either promote or inhibit tumor growth2,4. Understanding the dynamic changes in these cell populations in response to anti-PD-1 mAb therapy can provide insights into resistance mechanisms and identify potential biomarkers for therapeutic response5,6.
Previous studies have shown that the presence of tumor-infiltrating lymphocytes (TILs), particularly CD8+ cytotoxic T cells, is associated with a favorable response to anti-PD-1 mAb therapy5,7. However, the role of other immune cells, such as NK cells and myeloid cells, in mediating the response to anti-PD-1 mAb therapy is less well understood4,8. Additionally, the expression of activation markers such as CD107a, granzyme B, and perforin on these cells can provide further insights into their functional status and contribution to anti-tumor immunity9,10.
In this study, we used two syngeneic mouse tumor models, MC-38 and LLC1, which exhibit differential responses to anti-PD-1 mAb treatment, to investigate the effects of anti-PD-1 mAb on tumor growth and immune cell populations in tumor draining lymph nodes (TdLNs) and tumors. Flow cytometry was employed to analyze the expression of activation markers on various immune cells, providing a comprehensive immunophenotypic profile of the TME. By comparing the immune profiles of mice sensitive and resistant to anti-PD-1 mAb therapy, we aimed to identify key factors that contribute to therapeutic resistance and potential strategies to overcome it.
Materials and methods
Animal models and tumor inoculation
All animal experiments were conducted in accordance with institutional guidelines and approved by the Institutional Animal Care and Use Committee of Shin Nippon Biomedical Laboratories (Approval No. IACUC999-897), which is a fully AAALAC International-certified facility. Female C57BL/6J mice (4 weeks old) were obtained from Jackson Laboratory Japan (Shiga, Japan). The animals were housed under specific-pathogen-free conditions and provided with food and water ad libitum. The mouse Lewis lung carcinoma cell line, LLC1, was purchased from the American Type Culture Collection (Manassas, VA, USA). The mouse colon adenocarcinoma cell line, MC-38, was purchased from Kerafast, Inc. (Boston, MA, USA). All cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. LLC1 cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 units/mL penicillin and 100 μg/mL streptomycin. MC-38 cells were cultured in DMEM supplemented with 10% heat-inactivated FBS, 100 units/mL penicillin, 100 μg/mL streptomycin and 50 μg/mL gentamicin. Mice were inoculated subcutaneously with 1 × 106 cells/body of MC-38 or LLC1 cells suspended in phosphate-buffered saline (PBS). DMEM, penicillin, streptomycin, gentamycin, and PBS were purchased from Nacalai Tesque, Inc. (Kyoto, Japan), and FBS was purchased from Cytiva (Marlborough, MA, USA).
Treatment protocol
Six days after tumor inoculation, C57BL/6 mice were randomized into treatment groups and intraperitoneally administered either anti-PD-1 mAb (clone: RMP1-14, 100 μg/body) or isotype control antibody (clone: 2A3, 100 μg/body) every 3 days for a total of 3 doses (Fig. 1A). Tumor growth was monitored by caliper measurements of tumor length and width every 3 days from 5 days after the first dosing of anti-PD-1 mAb or isotype control antibody. Tumor volume was calculated using the formula (length × width2)/2. Anti-PD-1 mAb and isotype control antibody were purchased from BioXcell (Lebanon, NH, USA). All experiments in this manuscript were planned and conducted in accordance with the ARRIVE guidelines.
Comparison of syngeneic tumor model mice intraperitoneally treated with anti-PD-1 antibodies. Schedule for inoculation of tumor cells (MC-38 or LLC1) and administration of anti-PD-1 mAb or isotype control to mice (A). Tumor growth in mice administered anti-PD-1 mAb or isotype control after the inoculation with LLC1 cells or MC-38 cells (each 1 × 106 cells/body, n = 5) is shown as the average values in panel (B) and as individual values in panel (C). The average body weight of mice administered anti-PD-1 mAb or isotype control after inoculation with LLC1 cells or MC-38 cells (each 1 × 106 cells/body) are shown in panel (D). Line graphs depict the average values with SEM (B) and SD (D).
Tissue collection and preparation
Seven days after the final dosing, blood was collected for flow cytometry under isoflurane inhalation anesthesia. Then, mice were exsanguinated from the abdominal aorta and euthanized. The tissues were digested into single-cell suspensions using 1 mg/mL collagenase D and 0.2 mg/mL DNase I. The cell suspensions were then filtered through a 70 μm cell strainer and washed with PBS. Collagenase D and DNase I were purchased from Roche Diagnostics (Basel, Switzerland).
Flow cytometry
After treatment with Zombie UV Fixable Viability Kit (BioLegend, San Diego, CA, USA) following treatment with purified rat anti-mouse CD16/CD32 (clone: 2.4G2, BD Biosciences, Franklin Lakes, NJ, USA), cell suspensions of tumors and TdLNs were used for staining. The following fluorescence-conjugated antibodies were used for staining the surface markers of the lymphocytes: CD45 (clone: 30-F11), CD3 (clone: 145-2C11), CD4 (clone: RM4–5), CD8 (clone: 53–6.7), CD25 (clone: PC61), CD44 (clone: IM7), CD62L (clone: MEL-14), CD45R (clone: RA3-6B2), CD49b (clone: DX5), and CD107a (clone: 1D4B), and for staining the intracellular markers of the lymphocytes: FoxP3 (clone: MF-14), granzyme B (clone: GB11), and perforin (clone: S16009A). The following fluorescence-conjugated antibodies were used for staining the surface markers of monocytes/granulocytes: CD45 (clone: 30-F11), CD11b (clone: M1/70), I-A/I-E (clone: M5/114.15.2), F4/80 (clone: BM8), CD80 (clone: 16-10A1), CD86 (clone: GL-1), Gr-1 (clone: RB6-8C5), CD11c (clone: N418), CD206 (clone: C068C2), PD-L1 (clone: 10F.9G2), CD197 (clone: 4B12), and H-2 Kb/H-2Db (clone: 28–8–6). All fluorescence-conjugated antibodies were obtained from Biolegend. Flow cytometry was performed using a BD LSRFortessa X-20 flow cytometer, and data were analyzed using FlowJo software. For lymphocytes, live cells (Zombie UV−) were classified into CD3+T cells (CD45+CD3+CD49b−), CD4+T cells (CD45+CD3+CD49b−CD4+FoxP3−CD25−), CD8+T cells (CD45+CD3+CD49b−CD8+), Tregs (CD45+CD3+CD49b−CD4+FoxP3+CD25+), B cells (CD45+CD3−CD49b−CD45R+), NK cells (CD45+CD3−CD49b+), and NKT cells (CD45+CD3+CD49b+). For monocytes/granulocytes, live cells (Zombie UV−) were classified into DCs (CD45+CD11c+), MΦ (CD45+F4/80+CD11b+), M1 phenotype MΦ (CD45+F4/80+CD11b+CD11c+CD206−), M2 phenotype MΦ (CD45+F4/80+CD11b+CD11c−CD206+), and MDSCs (CD45+Gr-1+CD11b+).
Statistical analysis
Data were analyzed using Microsoft Excel for Microsoft 365. Differences between groups were assessed using appropriate statistical tests (e.g., Student’s t-test, one-way ANOVA) based on the data distribution and number of groups. Correlation analyses were performed using Pearson’s correlation coefficients. P-values < 0.05 were considered statistically significant.
Results
Anti-PD-1 mAb treatment inhibited tumor growth in the MC-38 model but not in the LLC1 model.
To evaluate the therapeutic efficacy of anti-PD-1 mAb treatment, tumor growth was monitored in the MC-38 and LLC1 syngeneic mouse models (Fig. 1A). In the MC-38 model, anti-PD-1 mAb treatment remarkably inhibited tumor growth compared to the isotype control group (Fig. 1B,C)). However, in the LLC1 model, the difference in tumor growth between the anti-PD-1 mAb and isotype control groups (Fig. 1B,C) was minimal. Body weight measurements did not reveal any significant treatment-related toxicity, suggesting no severe side effects in mice (Fig. 1D).
Differential effects of anti-PD-1 mAb treatment on monocytes/granulocytes populations in TdLNs and tumors.
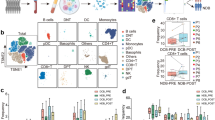
We conducted flow cytometry analysis to characterize distinct immune cell profiles of monocytes/granulocytes among all CD45+ leukocytes harvested from TdLNs and tumors in the MC-38 and LLC1 models upon anti-PD-1 mAb treatment. In the TdLNs, both mouse models treated with anti-PD-1 mAb showed a subtle increase in the ratios of dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs) and macrophages (MΦ) compared to the isotype control (Fig. 2A). In the tumor microenvironment, the MC-38 model sensitive to anti-PD-1 mAb exhibited a significant increase in the ratios of DCs and MΦ compared to the isotype control (Fig. 2B). The ratios of DCs and MΦ were 18.8% and 39.6%, respectively, in the isotype control group and increased to 25.7% and 41.0%, respectively, in the anti-PD-1 mAb treated group. In contrast, the ratio of MDSCs decreased from 18.6 to 27.2% upon anti-PD-1 treatment (Fig. 2B). Both rate of increase in DCs and rate of decrease in MDSCs in tumors were greatest in MC38-derived tumors treated with PD-1 (Fig. 2C). Conversely, in the LLC1 model resistant to anti-PD-1 mAb treatment, anti-PD-1 mAb treatment had minimal effect on the ratios of these monocyte/granulocyte populations.
Immunophenotyping analysis of monocytes/granulocytes in TdLNs and tumors from mice intraperitoneally administered anti-PD-1 antibody after inoculation with LLC1 cells or MC-38 cells. Anti-PD-1 mAb or isotype control (each 100 μg/body, n = 5) was administered a total of 3 times to mice every 3 days from 6 days after the inoculation with LLC1 cells and MC-38 cells (each 1 × 106 cells/body). TdLNs and tumors were harvested 7 days after the final dosing, digested into single cell suspensions and analyzed by flow cytometry using fluorescence-conjugated antibodies. The average ratios of several monocytes/granulocytes, including DCs, MΦ and MDSCs, and non-monocytes/granulocytes in CD45+ cells from TdLNs (A) and tumors (B) are shown in pie charts with the respective ratios in parentheses. The percent differences between anti-PD-1 mAb treatment and isotype control-treatment for each monocyte/granulocyte in TdLNs and tumors were obtained by subtracting the average ratios of isotype control-treatment from those of anti-PD-1 mAb treatment in panel (C). The average ratios of CD80, CD86, MHC class I (MHC I), MHC class II (MHC II), CD197, and PD-L1 of several monocytes/granulocytes, including DCs, MΦ, and MDSCs, in CD45+ cells from TdLNs (D) and tumors (E) are shown in bar graphs. The average ratios of M1 phenotype MΦ (CD11c+CD206–), M2 phenotype MΦ (CD11c–CD206+), and MΦ in CD45+ cells from TdLNs (F) and tumors (G) are shown in bar graphs. Bar graphs depict the average values with SD (D–G). Significant differences are denoted with asterisks (*P < 0.05) (E, G).
We next investigated the functional status and activation status of monocyte/granulocyte populations. The expression levels of antigen presentation molecules (MHC class I and MHC class II) and immune checkpoint molecules (CD80, CD86, CD197, and PD-L1) on tumor-associated DCs, MΦ, and MDSCs were analyzed. In the MC-38 model, anti-PD-1 mAb treatment significantly upregulated the expression levels (MFI, mean fluorescence intensity) of MHC class I and PD-L1 on DCs and MΦ within the tumor microenvironment but not in TdLNs (Supplementary Fig. 1). However, in the LLC1 model, no significant changes in the expression of these molecules were observed upon anti-PD-1 mAb treatment (Supplementary Fig. 1). Similarly, anti-PD-1 mAb treatment in the MC-38 model significantly increased the ratios of the aforementioned activation-related markers such as CD80, MHC class I, MHC class II, CD197 (CCR7), and CD274 (PD-L1) in DCs among CD45+ cells in tumors, whereas anti-PD-1 mAb treatment in the LLC1 model had minimal effect (Fig. 2E). Similarly, increases in these activation markers on monocytes/granulocytes were also observed in TdLNs from the MC-38 model, although they were not significant (Fig. 2D). The anti-PD-1 mAb treatment in the MC-38 model significantly also increased the ratios of MΦ expressing MHC class II and CD197 and those of MDSCs expressing MHC class II in tumors compared to the isotype control (Fig. 2E). For tumor-associated MΦ (TAM) in TdLNs, anti-PD-1 mAb treatment in the MC-38 model increased M1 phenotype macrophages (CD11c+CD206−), which represent a pro-inflammatory phenotype that plays a crucial role in the host defense against tumor cells, and the same treatment in the LLC1 model showed a significant increase in M2 phenotype macrophages (CD11c−CD206+), which represented an inhibitory phenotype in anti-tumor immunity (Fig. 2F). Similarly, only anti-PD-1 mAb treatment in the MC-38 model significantly increased the M1 phenotype macrophages, but no change was seen in M2 phenotype macrophages (Fig. 2G).
Differential effects of anti-PD-1 mAb treatment on lymphocyte populations in TdLNs and tumors.
We characterized distinct immune cell profiles of lymphocytes among all CD45+ leukocytes harvested from TdLNs and tumors in the MC-38 and LLC1 models upon anti-PD-1 mAb treatment. In the TdLNs, both mouse models treated with anti-PD-1 mAb showed an approximately 3% to 6% decrease in the ratios of CD4+T cells and CD8+T cells and an approximately 8% increase in the ratio of B cells compared to the isotype control, although there was no statistically significant difference (Fig. 3A,C). In NK cells, there was a slight increase in the ratio upon anti-PD-1 mAb treatment (Fig. 3A,C). Notably, in the tumor microenvironment, the MC-38 model sensitive to anti-PD-1 mAb treatment revealed a statistically significant increase in the ratios of CD4+T cells, CD8+T cells, Tregs, NK cells, and NKT cells but not B cells compared to the isotype control (Fig. 3B,D), demonstrating a vigorous influx of various immune cell fractions into the tumor bed. Conversely, in the LLC1 model resistant to anti-PD-1 mAb treatment, this intervention had minimal effect on the ratios of these immune cell populations (Fig. 3B,D).
Immunophenotyping analysis of lymphocytes in TdLNs and tumors from mice intraperitoneally administered anti-PD-1 antibodies after inoculation with LLC1 cells or MC-38 cells. Anti-PD-1 mAb or isotype control (100 μg/body, n = 5) was administered a total of 3 times to mice every 3 days from 6 days after inoculation with LLC1 cells or MC-38 cells (1 × 106 cells/body). TdLNs and tumors were harvested 7 days after the final dosing, digested into cell suspensions, and analyzed by flow cytometry using fluorescence-conjugated antibodies. The average ratios of several lymphocytes, including CD4+T cells, CD8+T cells, regulatory T cells (Tregs), B cells, NK cells, and NKT cells, and non-lymphocytes in CD45+ cells from TdLNs (A) and tumors (B) are shown in pie charts with the respective ratios in parentheses. The average ratios of CD3+T cells, CD4+T cells, CD8+T cells, Tregs, B cells, NK cells, and NKT cells in CD45+ cells from TdLNs (C) and tumors (D) are shown in bar graphs. Bar graphs depict the average values with SD. Significant differences are denoted with asterisks (*P < 0.05, **P < 0.005).
Subsequently, we assessed the profiles of memory phenotypes of lymphocytes among all CD45+ leukocytes harvested from TdLNs and tumors in the MC-38 and LLC1 models upon anti-PD-1 mAb treatment. In TdLNs, there was a robust decrease in the ratio of naïve CD4+T cells and CD8+T cells but minimal effects on the ratios of CD62L+CD44+ (central memory: TCM) and CD62L–CD44+ (effector memory: TEM) in mice treated with anti-PD-1 mAb compared to the isotype control in both LLC1 and MC-38 models (Fig. 4A). Conversely, in the tumor microenvironment, the MC-38 model sensitive to anti-PD-1 mAb treatment showed statistically significant increases in the ratios of both CD8+TCM cells and CD8+TEM cells but not in the ratios of CD4+TCM cells and CD4+TEM cells compared to the isotype control, whereas minimal changes of these immune cell subsets were observed in the LLC1 model (Fig. 4B). These results illustrated that anti-PD-1 mAb treatment robustly promoted the infiltration of both memory phenotypes of effector CTLs into tumors to exert their cytocidal effects.
Analysis of naïve and memory CD4+T cells and CD8+T cells in TdLNs and tumors from mice intraperitoneally administered anti-PD-1 antibodies after inoculation with LLC1 cells or MC-38 cells. Anti-PD-1 mAb or isotype control (100 μg/body, n = 5) was administered a total of 3 times to mice every 3 days from 6 days after the inoculation with LLC1 cells or MC-38 cells (1 × 106 cells/body). TdLNs and tumors were harvested 7 days after the final dosing, digested into cell suspensions and analyzed by flow cytometry using fluorescence-conjugated antibodies. The average ratios of CD62L+CD44– (naïve), CD62L+CD44+ (central memory: TCM), and CD62L–CD44+ (effector memory: TEM) in CD4+T cells or CD8+T cells in CD45+ cells from TdLNs (A, left: CD4+T cells, right: CD8+T cells) and tumors (B, left: CD4+T cells, right: CD8+T cells) are shown in bar graphs. Bar graphs depict the average values with SD. Significant differences are denoted with asterisks (*P < 0.05).
Anti-PD-1 mAb treatment enhanced T cell, B cell, and NK cell activation in the MC-38 model.
While tumor killing is ascribed to CD8+T cell (CTL) function, pre-clinical and clinical studies have identified intratumoral cytotoxic CD4+T cells that can directly kill cancer cells by secreting granzyme B and perforin11,12,13. To assess the cytotoxic functional status of immune cells, the expression of 3 different cytotoxic markers (CD107a, granzyme B, and perforin) on CD4+T cells, CD8+T cells, Tregs, B cells, NK cells, and NKT cells in tumors were comparatively analyzed. In cytotoxic CD4+T cells, among 3 cytotoxic makers, only a subset of perforin-expressing CD4+T cells was significantly higher in the anti-PD-1 mAb treatment compared to the isotype control (Fig. 5A). In CD8+CTL, all subpopulations of CD107-, granzyme B-, and perforin-expressing CD8+T cells were significantly increased in the MC-38 model, with perforin-positive cells showing the highest absolute number and change of rate (Fig. 5B). Recent studies demonstrated new roles of Tregs and B cells that possess cytotoxic activities14,15,16,17. Similar to CD8+T cells, CD107-, granzyme B-, and perforin-expressing Tregs and B cells were significantly increased in the MC-38 model, with perforin-positive cells showing the highest absolute number and change of rate (Fig. 5C,D). Interestingly, in regard to other innate cytotoxic cells such as NK and NKT cells, only perforin-expressing NK cells were significantly increased in the MC-38 model (Fig. 5E,F). In order to evaluate the most reliable cytolytic activation markers among these 3 molecules, we next performed correlation analyses to investigate the relationships between tumor volumes and average ratios of a range of lymphocytes expressing CD107a, granzyme B, or perforin in tumors treated with anti-PD-1 mAb in both LLC1 cells and MC-38 cells. In the LLC1 model resistant to anti-PD-1 mAb treatment, no data showed a significant negative correlation upon anti-PD-1 mAb treatment (Fig. 6A and Table 1). In contrast, in the MC-38 model sensitive to anti-PD-1 mAb treatment, granzyme B- and perforin-expressing cytotoxic CD4+T cells and perforin-expressing NKT cells exhibited a significant negative correlation upon anti-PD-1 mAb treatment (r = − 0.975, − 0.995, − 0.934; P < 0.01, P < 0.01, P < 0.05; respectively, Fig. 6B and Table 1). Regarding CD8+CTLs as immunologically representative cytotoxic immune cells, perforin-expressing CD8+T cells showed the strongest negative correlation among the 3 markers in the MC-38 model treated with anti-PD-1 mAb, although there was no statistically significant difference (r = − 0.497; Fig. 6B and Table 1). Only perforin-expressing cells showed negative correlation coefficients upon anti-PD-1 mAb treatment in all immune fractions, although there were marked differences in the values (r = − 0.995, − 0.497, − 0.464, − 0.405, − 0.003, − 0.934; Fig. 6B and Table 1). These results collectively implicated that perforin may be the most reliable marker associated with the magnitude of efficacy of anti-PD-1 mAb treatment, and furthermore suggested anti-PD-1 mAb treatment enhances the cytotoxic potential of various lymphocytes, including not only CD8+T cells, NK cells and NKT cells, but also CD4+T cells, Tregs, and B cells within the tumor microenvironment.
Analysis for average ratios of lymphocytes expressing CD107a, granzyme B or perforin in tumors from mice intraperitoneally administered anti-PD-1 antibodies after inoculation with LLC1 cells or MC-38 cells. Anti-PD-1 mAb or isotype control (100 μg/body, n = 5) was administered a total of 3 times to mice every 3 days from 6 days after the inoculation with LLC1 cells or MC-38 cells (1 × 106 cells/body). Tumors were harvested 7 days after the final dosing, digested into cell suspensions, and analyzed by flow cytometry using fluorescence-conjugated antibodies. The average ratios of CD107a+, granzyme B+ or perforin+ in several lymphocytes, including CD4+T cells (A), CD8+T cells (B), regulatory T cells (Tregs; C), B cells (D), NK cells (E), and NKT cells (F) in CD45+ cells from tumors are shown in bar graphs. Bar graphs depict the average values with SD. Significant differences in bar graphs are denoted with asterisks (*P < 0.05, **P < 0.005).
Correlation analysis between tumor volume and average ratios of lymphocytes expressing CD107a, granzyme B or perforin in tumors from mice intraperitoneally administered anti-PD-1 antibodies after inoculation with LLC1 cells or MC-38 cells. Anti-PD-1 mAb or isotype control (100 μg/body, n = 5) was administered a total of 3 times to mice every 3 days from 6 days after the inoculation with LLC1 cells or MC-38 cells (1 × 106 cells/body). Tumors were harvested 7 days after the final dosing, digested into cell suspensions and analyzed by flow cytometry using fluorescence-conjugated antibodies. The relationships between tumor volumes and the average ratios of CD107a+, granzyme B+ or perforin+ of several lymphocytes including CD4+T cells, CD8+T cells, regulatory T cells (Tregs), B cells, NK cells, and NKT cells in CD45+ cells in tumors from LLC1 tumor-bearing mice (A) and MC-38 tumor-bearing mice (B) are shown in scatter plots with approximation lines.
When looking at TdLNs that foster the antigen-specific effector adaptive immune cells primed by antigen presenting cells, granzyme B- and perforin-expressing CD8+T cells were significantly decreased in the MC-38 model treated with anti-PD-1 mAb, with perforin-positive cells showing the highest reduction (P < 0.05) (Supplementary Fig. 2B). Similarly, in Tregs and NKT cells, there were some reductions of granzyme B-, perforin-expressing Tregs, and NKT cells (Supplementary Fig. 2C,F).
Discussion
Immune checkpoint inhibitors targeting the PD-1/PD-L1 axis have revolutionized the treatment landscape for various cancers, including colorectal and lung cancer18,19,20. However, a significant proportion of patients does not respond to these therapies, highlighting the need for a better understanding of the tumor microenvironment and the mechanisms underlying resistance. In this study, through comprehensive immunophenotyping analyses of tumor-infiltrating immune cells harvested from two different syngeneic mouse models subcutaneously implanted with either MC-38 or LLC1 cells, we uncovered distinct immunological changes associated with anti-PD-1 mAb responsiveness, providing insights into potential biomarkers and combination strategies to enhance immunotherapy outcomes.
The MC-38 model, which is sensitive to anti-PD-1 mAb therapy, exhibited a significant inhibition of tumor growth upon treatment with anti-PD-1 mAb. Conversely, the LLC1 model, which is resistant to anti-PD-1 mAb therapy, did not show any significant tumor growth inhibition. These findings were consistent with previous reports21,22,23, and the contrasting responses between these two models provided an excellent opportunity to elucidate the immunological determinants of anti-PD-1 mAb efficacy.
Our immunophenotypic analyses revealed significant alterations in tumor-infiltrating myeloid cell populations following anti-PD-1 treatment in the responsive MC-38 model. Notably, we observed increases in DCs and MΦ, coupled with a decrease in MDSCs. These changes were not apparent in the resistant LLC1 model. The expansion of intratumoral DCs is particularly noteworthy, in line with previous reports24,25. as DCs play a crucial role in priming and sustaining anti-tumor memory T cell responses26. The increased DC infiltration may enhance tumor antigen presentation and T cell activation, contributing to the efficacy of anti-PD-1 therapy. Concurrently, the reduction in MDSCs is significant, as these cells are known to suppress T cell function and promote tumor progression27. The shift from an MDSC-rich to a DC-rich myeloid landscape likely creates a more favorable immune microenvironment for anti-tumor responses. The increase in tumor-associated macrophages (TAMs) in the MC-38 model was accompanied by a shift towards the M1 pro-inflammatory phenotype. This polarization from immunosuppressive M2 to anti-tumor M1 macrophages has been associated with improved outcomes in various cancer immunotherapies28,29,30.The ability of anti-PD-1 treatment to induce this phenotypic switch in macrophages may also be a key mechanism underlying its efficacy in the MC-38 model. In addition to the quantitative changes in myeloid cell populations, we observed qualitative alterations indicative of enhanced antigen presentation and T cell stimulation. Upregulation of MHC class I and II molecules, as well as costimulatory markers CD80 and CD86 on DCs and macrophages, was evident in the MC-38 model following anti-PD-1 treatment. These findings further support an increased capacity for antigen presentation and T cell priming within the tumor microenvironment. Interestingly, we also noted an increase in PD-L1 expression on these myeloid cells. While seemingly counterintuitive, this may reflect an adaptive response to interferon-γ production by activated T cells, creating a feed-forward loop that sustains anti-PD-1 efficacy31. The concurrent upregulation of antigen presentation machinery and PD-L1 on myeloid cells may highlight the complex dynamics of immune regulation within the tumor microenvironment.
A hallmark of effective anti-PD-1 therapy is the enhancement of tumor-infiltrating lymphocytes (TILs). Our results elicited a significant increase in CD4+T cells, CD8+T cells, regulatory T cells (Tregs), NK cells, and NKT cells within MC-38 tumors following anti-PD-1 treatment. This broad lymphocyte infiltration was not observed in the LLC1 model, underscoring its importance in mediating anti-PD-1 mAb-mediated anti-tumor responses. The expansion of effector T cell populations, particularly CD8+cytotoxic T lymphocytes (CTLs), is widely recognized as a key mechanism of anti-PD-1 efficacy32. Our findings of increased both CD8+TCM cells and CD8+TEM cells in MC-38 tumors, which are consistent with results shown in melanoma patients in a clinical setting33, suggest the robust development of a sustained anti-tumor immune response. The generation of these memory populations may contribute to long-lasting tumor control and potentially explain the durable responses seen with checkpoint inhibitors in some patients.
To further characterize the functional status of TILs, we assessed the expression of cytotoxic markers CD107a, granzyme B and perforin. Notably, we for the first time observed significant increases in perforin-expressing CD4+T cells, CD8+T cells, Tregs, B cells, and NK cells within MC-38 tumors following anti-PD-1 treatment. The broad enhancement of cytotoxic potential across multiple lymphocyte subsets suggests a coordinated anti-tumor immune response. The increase in perforin-expressing CD4+T cells is particularly intriguing, as it points to the potential contribution of cytotoxic CD4+T cells in tumor control. Recent studies have highlighted the importance of these cells in anti-tumor immunity, and our findings suggest that anti-PD-1 therapy may harness this often-overlooked CD4+T cell function12,34,35. In contrast, in the MC-38 model sensitive to anti-PD-1 mAb treatment, granzyme B- and perforin-expressing cytotoxic CD4+T cells and perforin-expressing NKT cells exhibited a significantly negative correlation upon anti-PD-1 mAb treatment. Unexpectedly, we observed that anti-PD-1 treatment increased subpopulations of Treg and B cells with cytotoxic markers CD107a, granzyme B, and perforin, implicating their direct cytotoxicity against cancer cells driven by PD-1/PD-L1 blockade. Our correlation analyses revealed that perforin expression in various lymphocyte subsets, especially CD4+T cells and NKT cells, showed the strongest negative correlation with tumor volume in the MC-38 model. This suggests that perforin may be a reliable marker of anti-PD-1 efficacy and highlights the potential importance of non-conventional cytotoxic cells like CD4+T cells and NKT cells, perhaps even Tregs and B cells, which are not originally envisioned to be directly cytotoxic, in mediating tumor regression. These unforeseen events may imply the depth of the PD-1/PD-L1 blockade-induced anti-tumor immune system.
Our comprehensive immunophenotyping approach identified several potential biomarkers that may predict or indicate response to anti-PD-1 therapy in the future. We speculate that the ratio of DCs to MDSCs, the polarization state of TAMs and the frequency of perforin-expressing lymphocytes may emerge as promising candidates. These markers could be explored in clinical samples to stratify patients and guide treatment decisions. For example, perforins or perforin-related proteins in serum from cancer patients could be detected before and after ICI-based therapies in liquid biopsies. Additionally, the observed increase in PD-L1 expression on myeloid cells within responding tumors suggests that PD-L1 upregulation may be a marker of ongoing anti-tumor immune responses rather than just a mechanism of immune evasion. This nuanced understanding of PD-L1 dynamics could refine its use as a biomarker for immunotherapy response31.
The lack of anti-tumor efficacy and immunological changes in the LLC1 model provides insights into potential mechanisms of resistance to anti-PD-1 therapy. The failure to induce significant alterations in myeloid cell populations or enhance lymphocyte infiltration suggests that LLC1 tumors may have intrinsic or adaptive mechanisms that prevent the development of an inflamed tumor microenvironment. Recent studies have identified various tumor-intrinsic factors that can contribute to immunotherapy resistance, including defects in antigen presentation, activation of oncogenic pathways that suppress T cell function and epigenetic alterations that create an immunosuppressive microenvironment36,37,38. Further investigation of these mechanisms in the LLC1 model could reveal new targets for combination therapies to overcome resistance.
The distinct immunological changes observed in the responsive MC-38 model raise the following potential combination strategies to enhance anti-PD-1 efficacy or overcome resistance. First, combining anti-PD-1 mAb with agents that deplete MDSCs or repolarize TAMs towards an M1 phenotype could enhance efficacy. For example, CSF1R, expressed in a wide range of myeloid cells, and PI3Kγ inhibitors have shown promise in preclinical models and clinical settings by modulating myeloid cell function39,40,41. Second, strategies to boost DC function and antigen presentation, such as Flt3L administration or STING agonists, could synergize with anti-PD-1 therapy by promoting T cell priming and activation42,43. Third, given the observed increases in NK and NKT cells, combining anti-PD-1 with approaches to activate these innate lymphocytes, such as IL-15 super-agonists or invariant NKT cell agonists44,45, may broaden the anti-tumor immune response. Fourth, for primarily resistant tumors like LLC1, combining anti-PD-1 with blockade of other inhibitory receptors (e.g., TIM-3, LAG-3) or agonism of costimulatory molecules (e.g., OX40, 4-1BB) could help overcome stubborn immunosuppression46,47.
While our study provides valuable insights into the immunological changes associated with anti-PD-1 efficacy, several limitations should be addressed in future investigations.
Expanding the analysis to genetically engineered humanized mouse models would provide a more comprehensive understanding of the spectrum of anti-PD-1 responses. Assessing immune changes at multiple time points during treatment could reveal the kinetics of the anti-tumor immune response. Employing spatial transcriptomics or multiplex immunohistochemistry techniques would provide more insights into the geographical relationships between immune cell subsets and their functional states within the tumor microenvironment.
In conclusion, our study provides a comprehensive immunological portrait of anti-PD-1 responsiveness in syngeneic mouse tumor models. The contrasting responses between MC-38 and LLC1 tumors highlight the complex interplay between myeloid cells, lymphocytes, and the tumor microenvironment in determining immunotherapy outcomes. The identified changes in immune cell composition, phenotype, and functional status offer potential biomarkers and therapeutic targets to enhance the efficacy of anti-PD-1 therapy. The broad remodeling of both innate and adaptive immune compartments in responding tumors also underscores the systemic nature of effective anti-tumor immunity. Our findings emphasize the importance of considering multiple immune cell types and their functional states when developing strategies to improve cancer immunotherapy. As the field continues to evolve, unraveling and integrating insights from preclinical models with emerging clinical data will be crucial in realizing the full potential of cancer immunotherapy, harnessing the power of our immune system to combat and cure cancer.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CTL:
-
Cytotoxic T lymphocyte
- DCs:
-
Dendritic cells
- mAb:
-
Monoclonal antibody
- MDSC:
-
Myeloid-derived suppressor cell
- PD-1:
-
Programmed cell death protein 1
- TAM:
-
Tumor-associated macrophage
- TCM :
-
Central memory T cell
- TdLN:
-
Tumor-draining lymph node
- TEM :
-
Effector memory T cell
- TIL:
-
Tumor-infiltrating lymphocyte
- Treg:
-
Regulatory T cell
References
Martín-Ruiz, A. et al. Effects of anti-PD-1 immunotherapy on tumor regression: Insights from a patient-derived xenograft model. Sci. Rep. 10, 7078 (2020).
Wang, Q. et al. What happens to the immune microenvironment after PD-1 inhibitor therapy?. Front. Immunol. 12, 773168 (2021).
Nagasaki, J., Ishino, T. & Togashi, Y. Mechanisms of resistance to immune checkpoint inhibitors. Cancer Sci. 113, 3303–3312 (2022).
Laba, S., Mallett, G. & Amarnath, S. The depths of PD-1 function within the tumor microenvironment beyond CD8+ T cells. Semin. Cancer Biol. 86, 1045–1055 (2022).
Jin, Y. et al. Different syngeneic tumors show distinctive intrinsic tumor-immunity and mechanisms of actions (MOA) of anti-PD-1 treatment. Sci. Rep. 12, 3278 (2022).
Shi, G., Scott, M., Mangiamele, C. G. & Heller, R. Modification of the tumor microenvironment enhances anti-PD-1 immunotherapy in metastatic melanoma. Pharmaceutics 14, 2429 (2022).
Hammerl, D. et al. Spatial immunophenotypes predict response to anti-PD1 treatment and capture distinct paths of T cell evasion in triple negative breast cancer. Nat. Commun. 12, 5668 (2021).
Ma, Y. et al. Remodeling the tumor-immune microenvironment by anti-CTLA4 blockade enhanced subsequent anti-PD-1 efficacy in advanced nasopharyngeal carcinoma. NPJ Precis. Oncol. 8, 65 (2024).
Ricciuti, B. et al. Genomic and immunophenotypic landscape of acquired resistance to PD-(L)1 blockade in non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 42, 1311–1321 (2024).
Li, K. et al. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy. Cancer Cell 40, 1374-1391.e7 (2022).
Takeuchi, A. & Saito, T. CD4 CTL, a cytotoxic subset of CD4+ T cells, their differentiation and function. Front. Immunol. 8, 194 (2017).
Lin, W. et al. Human CD4 cytotoxic T lymphocytes mediate potent tumor control in humanized immune system mice. Commun. Biol. 6, 447 (2023).
Oh, D. Y. & Fong, L. Cytotoxic CD4+ T cells in cancer: Expanding the immune effector toolbox. Immunity 54, 2701–2711 (2021).
Arabpour, M., Rasolmali, R., Talei, A.-R., Mehdipour, F. & Ghaderi, A. Granzyme B production by activated B cells derived from breast cancer-draining lymph nodes. Mol. Immunol. 114, 172–178 (2019).
Zhu, J.-Q. et al. Granzyme B+ B cells detected by single-cell sequencing are associated with prognosis in patients with intrahepatic cholangiocarcinoma following liver transplantation. Cancer Immunol. Immunother. CII 73, 58 (2024).
Choi, B. D. et al. Human regulatory T cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody. Cancer Immunol. Res. 1, 163–167 (2013).
Cao, X. et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 27, 635–646 (2007).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Ganesh, K. et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 16, 361–375 (2019).
Sun, L. et al. Clinical efficacy and safety of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or metastatic cancer: a systematic review and meta-analysis. Sci. Rep. 10, 2083 (2020).
Bernardo, M. et al. An experimental model of anti-PD-1 resistance exhibits activation of TGFß and Notch pathways and is sensitive to local mRNA immunotherapy. Oncoimmunology 10, 1881268 (2021).
Fellermeyer, M. et al. Combination CD200R/PD-1 blockade in a humanised mouse model. Immunother. Adv. 3, 006 (2023).
Ajona, D. et al. Short-term starvation reduces IGF-1 levels to sensitize lung tumors to PD-1 immune checkpoint blockade. Nat. Cancer 1, 75–85 (2020).
Oba, T. et al. Overcoming primary and acquired resistance to anti-PD-L1 therapy by induction and activation of tumor-residing cDC1s. Nat. Commun. 11, 5415 (2020).
Fernandez-Rodriguez, L. et al. Dual TLR9 and PD-L1 targeting unleashes dendritic cells to induce durable antitumor immunity. J. Immunother. Cancer 11, e006714 (2023).
Gardner, A. & Ruffell, B. Dendritic cells and cancer immunity. Trends Immunol. 37, 855–865 (2016).
Parker, K. H., Beury, D. W. & Ostrand-Rosenberg, S. Myeloid-derived suppressor cells. Adv. Cancer Res. 128, 95–139 (2015).
Gao, J., Liang, Y. & Wang, L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front. Immunol. 13, 888713 (2022).
Khan, S. U. et al. Reprogramming tumor-associated macrophages as a unique approach to target tumor immunotherapy. Front. Immunol. 14, 1166487 (2023).
Chen, S. et al. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 8, 207 (2023).
Taube, J. M. et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 20, 5064–5074 (2014).
Zuazo, M. et al. Systemic CD4 immunity as a key contributor to PD-L1/PD-1 blockade immunotherapy efficacy. Front. Immunol. 11, 586907 (2020).
Yamaguchi, K. et al. Activation of central/effector memory T cells and T-helper 1 polarization in malignant melanoma patients treated with anti-programmed death-1 antibody. Cancer Sci. 109, 3032–3042 (2018).
Zuazo, M. et al. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol. Med. 11, e10293 (2019).
Spitzer, M. H. et al. Systemic immunity is required for effective cancer immunotherapy. Cell 168, 487-502.e15 (2017).
Chen, M., Bie, L. & Ying, J. Cancer cell-intrinsic PD-1: Its role in malignant progression and immunotherapy. Biomed. Pharmacother. Biomedecine Pharmacother. 167, 115514 (2023).
Rieth, J. & Subramanian, S. Mechanisms of intrinsic tumor resistance to immunotherapy. Int. J. Mol. Sci. 19, 1340 (2018).
Kalbasi, A. & Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 20, 25–39 (2020).
Saung, M. T. & Zheng, L. Adding combination immunotherapy consisting of cancer vaccine, anti-PD-1 and anti-CSF1R antibodies to gemcitabine improves anti-tumor efficacy in murine model of pancreatic ductal adenocarcinoma. Ann. Pancreat. Cancer 2, 21 (2019).
Peng, X. et al. A novel pan-PI3K inhibitor KTC1101 synergizes with anti-PD-1 therapy by targeting tumor suppression and immune activation. Mol. Cancer 23, 54 (2024).
Hong, D. S. et al. Eganelisib, a first-in-class PI3Kγ inhibitor, in patients with advanced solid tumors: Results of the phase 1/1b MARIO-1 trial. Clin. Cancer Res. 29, 2210–2219 (2023).
Gajewski, T. F. & Cron, K. R. cDC1 dysregulation in cancer: An opportunity for intervention. J. Exp. Med. 217, e20200816 (2020).
Murgaski, A., Bardet, P. M. R., Arnouk, S. M., Clappaert, E. J. & Laoui, D. Unleashing tumour-dendritic cells to fight cancer by tackling their three A’s: Abundance, activation and antigen-delivery. Cancers 11, 670 (2019).
Knudson, K. M., Hicks, K. C., Alter, S., Schlom, J. & Gameiro, S. R. Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. J. Immunother. Cancer 7, 82 (2019).
Nelson, A., Lukacs, J. D. & Johnston, B. The current landscape of NKT cell immunotherapy and the hills ahead. Cancers 13, 5174 (2021).
Messenheimer, D. J. et al. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin. Cancer Res. 23, 6165–6177 (2017).
Cai, L., Li, Y., Tan, J., Xu, L. & Li, Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J. Hematol. Oncol. 16, 101 (2023).
Acknowledgements
The authors would like to thank the technical staff at the Animal Care Facility of Shin Nippon Biomedical Laboratories for their assistance with animal husbandry and care.
Author information
Authors and Affiliations
Contributions
Conceptualization, H.I., and N.H.; data curation, T.H., K.I., and H.M.; investigation, H.I., T.H., K.I., and H.M.; visualization, H.I., T.H., K.I., and H.M.; methodology, H.I.,T.H., K.I., and H.M.; software, T.H., K.I., and H.M.; data analysis, T.H., K.I., and H.M.; formal analysis, T.H., K.I., and H.M.; validation, T.H., K.I., and H.M.; writing—original draft, H.I.; writing—review & editing, H.I., T.H., K.I., H.M., A.N., N.E., I.N., M.F., and N.H.; resources, T.H., K.I., H.M., I.N. and N.H.; supervision, H.I.; project administration, H.I. and N.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Inoue, H., Hamasaki, T., Inoue, K. et al. Comprehensive immunophenotyping reveals distinct tumor microenvironment alterations in anti-PD-1 sensitive and resistant syngeneic mouse model. Sci Rep 15, 8311 (2025). https://doi.org/10.1038/s41598-025-91979-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91979-w