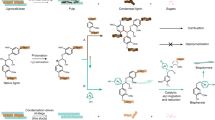
Abstract
Lignocellulosic biomass, mainly composed of cellulose, hemicellulose, and lignin, is an abundant renewable resource in agricultural and forestry residues. In contrast to cellulose and hemicellulose, lignin remains underutilized due to its complex structure. In this study, a halophilic bacterium, Virgibacillus salarius was isolated, and its laccase production was optimized for delignification. Using response surface methodology (RSM), a maximum laccase activity of 392.0 U L⁻¹ was achieved. This enzyme demonstrated high efficiency, reducing lignin content in garlic peel from 32.4 to 3.6% and increasing cellulose content from 42.1 to 44.1%. Additionally, the delignified biomass exhibited increased crystallinity and a porous surface, enhancing its suitability for further processing. The delignification process yielded valuable by-products, including 3-hydroxybenzoic acid (a food preservative and flavor enhancer) and aromatic compounds such as 2-methoxyphenol (guaiacol) and ethyl-2-methoxyphenol (homovanillin), which are widely used as flavoring agents. These findings emphasize the potential of V. salarius laccase in sustainable biomass conversion for industrial applications.
Similar content being viewed by others
Introduction
Lignocellulosic biomass, commonly consisting of agricultural residues, lumber, fruit and vegetable waste, and forest leftovers, makes Earth’s most plentiful source of renewable organic material1. Much lignocellulosic biomass is utilized in the paper and textile industries, heat and electricity generation, and bioethanol production2. Moreover, easy availability and high sustainability make it a vital component of the green biorefinery strategy2,3. Hemicellulose, cellulose, and lignin constitute the main building blocks of lignocellulosic biomass with several chemical and physical properties4. Lignin, unlike cellulose and hemicellulose, is relatively underutilized due to its chemical stability and inherent complexity5. It is traditionally used as a low-tech heat and power generation or a by-product in wood and pulp processing. This natural polymer, the second-most abundant carbon source in the environment, comprises a mass fraction of 19–28% in hardwoods, 25–30% in softwoods, and 11–27% in nonwoody sources6. However, over the past decade, lignin has demonstrated considerable potential as a valuable resource for the synthesis of hydrocarbons (e.g., toluene, benzyl, and xylene), simple phenols (e.g., vanillin, eugenol, catechol, and quinones), polymeric compounds (e.g., carbon fiber and thermosets), and for the production of nutraceuticals, pharmaceuticals, and cosmetics3. Currently, mechanical methods (such as milling), ultrasonic disruption, and chemical treatments (pyrolysis, hydrogenolysis, hydrolysis, etc.) are the predominant approaches for delignification and densification3. However, mechanical processing is typically prevalent; it makes the fiber less intense and requires high energy consumption. The chemical process has also produced numerous significant pollutants, posing a serious environmental threat7. As an alternative method, biological treatments developed by microorganisms producing ligninolytic enzymes offer a more environmentally friendly approach owing to their non-toxic nature7. Ligninolytic enzymes are broadly classified into two major groups: peroxidases (including lignin peroxidase, manganese peroxidase, versatile peroxidase, and dye-decolorizing peroxidase) and oxidases (primarily encompassing laccases)7. Although peroxidases appear more potent for delignification due to their high redox potential, laccase is the preferred industrial choice8. Laccases are preferred to peroxidases since they require only molecular oxygen (O2) as a co-substrate, while peroxidases need hydrogen peroxide (H2O2), which is known to be destabilizing to enzymes and further leads to higher costs of the processes9. Bacterial laccases often possess a wide range of activity, thermal stability, and chloride resistance, making them more attractive than fungal enzymes10. In this context, extremophiles have received significant attention due to their ability to produce stable laccases as extremozymes, which can exhibit activity on substrates even under extreme reaction conditions11. Employing extremophilic enzymes for treating lignocellulose can increase solubility, enhance bioavailability, reduce viscosity, improve reaction rate, and decrease the potential for mesophilic microbial contamination3. Therefore, applications of halophiles in bio-delignification processes present a promising avenue for enhancing the feasibility and sustainability of this industrial sector. This study was undertaken to isolate and characterize a halophilic, laccase-producing bacterium from hypersaline environments in Iran and evaluate its potential for the sustainable delignification of garlic peel biowaste. Indeed, biowaste refers to the discarded peels of garlic. These peels result from garlic processing and consumption, often regarded as waste and discarded. Garlic is a globally significant crop, ranking among the top 10 vegetables produced worldwide12. Global garlic production has increased from 27.6 million tons in 2017 to approximately 30.1 million tons today12. China, the world’s largest garlic producer, harvested 21,197,131 tons in 2016, followed by India (1,400,000 tons) and Bangladesh (381,851 tons)13. Garlic processing generates significant by-products, including skins, outer leaves, tops, and bottoms, often discarded or used as soil amendments, leading to environmental concerns. To address this issue, these by-products can be valued through various applications. Delignification of garlic peels, for instance, can produce cellulose, tannins, oligosaccharides, allicin, and inulin14. Additionally, they can serve as a substrate for mushroom cultivation and are used as a dietary fiber source in animal feed. Extracts from skins and outer leaves have the potential as corrosion inhibitors, emulsifiers, and antioxidants15. Furthermore, garlic by-products can be employed as sorbents to remove pollutants (metal ions, dyes, and other organic compounds) from water16. Some studies have also explored the potential of utilizing treated garlic waste as silage additives to enhance fermentation quality and improve feed efficiency17. The novelty of this study lay in isolating a halophilic, laccase-producing bacterium from hypersaline environments, which offered an innovative, eco-friendly approach for the sustainable delignification of garlic peel biowaste. This creative method reduced lignin and generated valuable by-products, enabling efficient agricultural waste valorization and supporting green biorefinery strategies. The study aimed to minimize environmental impact and maximize by-product recovery from a significant waste stream by harnessing this unique bacterium.
Materials and methods
Chemicals and reagents
Vanillin, o-toluidine, xylidine, 2,6-dimethoxyphenol (2,6-DMP), 4-hydroxybenzoic acid (HBA), 1-hydroxy benzotriazole (HBT), guaiacol, and gallic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). Yeast extract, peptone, casein, inulin, pectin, and soluble starch were supplied from Merck (Darmstadt, Germany). All other chemicals were of analytical grade and purchased from commercial sources.
Isolation of halophilic and laccase-producing bacterium
Soil samples were collected from the hypersaline environments of four provinces (Golestan (37°13’4"N, 54°7’16"E), Isfahan (33°41’28"N, 55°41’24"E), Qom (34°59’19’’N, 50°53’31’’E), and Mazandaran (36°05’15"N, 53°02’45"E)) in Iran to isolate a halophilic and laccase-producing bacterium. The samples were cultivated on Luria-Bertani (LB) agar plates containing 0.5% yeast extract, 1% peptone, and 3 M sodium chloride at pH 7 and 37 °C under aerobic conditions. This process was repeated until pure colonies were obtained. The isolated bacteria were then transferred onto LB agar supplemented with 0.1% w/v 2,6-DMP, a laccase indicator, and incubated at 37 °C for 6 days. The strain that exhibited a colored halo around the colonies, resulting from the enzyme’s oxidative activity on the substrate, was selected for laccase activity assessment in an LB liquid medium. The bacterium was stored at − 80 °C in LB broth containing 15% v/v glycerol for long-term storage. Short-term storage involved monthly cultivation of the LB agar plate, which was then kept at 4 °C in a refrigerator.
Enzyme activity and protein assay
The laccase activity was measured by centrifuging 1 mL of the bacterial broth culture for 10 min at 8000g. The supernatant was mixed with an equal volume of 10 mM 2,6-DMP dissolved in Britton-Robinson buffer (BRB; 100 mM, pH 7) containing 3 M sodium chloride. After incubating the mixture with agitation at 40 °C for 15 min, the density of red brick color due to the oxidation of the substrate was measured using a UV-vis spectrophotometer (Synergy HTX, BioTek, Germany) at 468 nm (ε468 = 35,640 M–1 cm–1). Laccase activity was reported in international units (IU), representing the amount of enzyme that converts 1 µmol of substrate to product per min18. Protein concentration was determined using the Bradford method, with UV-vis absorbance measured at 595 nm and a standard curve prepared using bovine serum albumin18.
Biochemical characterizations and phylogenetic study on the isolate
The pigmentation of the grown colonies was observed on an LB agar plate after incubating the medium at 37 °C for 48 h under aerobic conditions. Microscopic assessment was conducted by Gram staining the microbial isolate. Biochemical characterizations were evaluated through experiments that included measuring the activity of catalase, oxidase, and urease; fermenting arabinose, fructose, galactose, glucose, lactose, mannitol, sucrose, and trehalose; and hydrolyzing aesculin and gelatin. To amplify the 16 S rRNA gene, the primers 27 F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1525R (5’-AAGGAGGTGATCCAGCC-3’) were employed19. The thermal cycler was programmed with an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 60 s, 55 °C for 60 s, and 72 °C for 90 s, with a final extension at 72 °C for 10 min. Subsequently, the PCR product (ca. 1.5 kbp) was sequenced and analyzed using the Basic Local Alignment Search Tool (BLAST) in GenBank (http://www.ncbi.nlm.nih.gov/BLAST). A phylogenetic tree was constructed using the neighbor-joining (NJ) algorithm in MEGA 7 software.
Enhancing laccase production by the halophilic isolate bacterium
One-Factor-at-a-Time (OFAT) study on enzyme production
An initial OFAT study was conducted to determine the nutritional and environmental factors influencing laccase production. This method involved systematically varying one parameter at a time while keeping all other variables constant, allowing for the identification of factors that significantly impact laccase production. These parameters included the concentration of sodium chloride (0–5 M, 1 M intervals), temperature (10–45°C, 10°C intervals), pH level (4–9, 1 unit intervals), the medium-to-air ratio (1:10–5:10, 1:10 intervals), and inoculum size (1–5%, 1% intervals). Furthermore, various substrates, including glucose, fructose, galactose, sucrose, maltose, lactose, inulin, pectin, and soluble starch, were tested to evaluate the effect of different carbon sources on laccase production. Each carbon source was assessed at concentrations of 0.5%, 1%, and 2% w/v. In addition, nitrogen sources, including ammonium chloride, ammonium oxalate, sodium nitrate, peptone, yeast extract, and casein, were investigated at concentrations of 0.5%, 1%, and 2% w/v. The influence of trace elements, specifically cobalt (II) chloride, calcium chloride, zinc sulfate, magnesium sulfate, manganese (II) sulfate, and nickel (II) chloride, was investigated at concentrations of 0.5 mM, 1 mM, and 2 mM. Inducers for enzyme production (i.e., vanillin, xylidine, tannic acid, gallic acid, caffeine, o-toluidine, and copper sulfate) were also evaluated at 1 mM, 5 mM, and 10 mM. The production of the enzyme was studied in the presence of common salts, lithium chloride, sodium chloride, and potassium chloride, at concentrations ranging from 1 M to 5 M, with 1 M intervals. The studies on enzyme production were conducted in a modified M9 medium consisting of 6 g of sodium hydrogen phosphate dibasic, 3 g of potassium dihydrogen phosphate, 116 g of sodium chloride, 1 g of ammonium chloride, 25 mg of magnesium sulfate, 1 mg of calcium chloride, and 100 mg of glucose per liter.
Experimental design for screening nutritional and conditional factors in laccase production
Following the preliminary study, fractional factorial design was utilized to examine the impact of key variables on laccase production. This method was selected because it efficiently identified significant factors while minimizing the number of experimental trials, offering a more practical alternative to other approaches.
Optimization of laccase production
After identifying the variables with significant effects on laccase production, Response Surface Methodology (RSM) was employed as a statistical tool to optimize laccase production. The optimization was performed using the Box-Behnken design (BBD) and analyzed with Design-Expert software. Laccase production was considered a response variable, whereas the concentration of sodium chloride (M), pH, the content of peptone, and the amount of copper sulfate were four independent variables. The BBD used all the factors at three levels assigned as − 1, 0, and + 1 for the lowest, central, and highest values.
Delignification of Garlic Peel waste by laccase from the halophilic bacterial isolate
OFAT study on laccase-mediated delignification of garlic peel
Following the optimization of laccase production by the halophilic bacterial isolate, the enzyme’s ability to degrade garlic peel biowaste was evaluated. Therefore, garlic cloves (Allium sativum) were purchased from a local market. The peels were removed, dried at room temperature, ground in a knife mill, sieved to a particle size of 50 mesh, and stored in a refrigerator for subsequent experiments. Using a conventional one-factor-at-a-time (OFAT) approach, the effect of a wide range of factors, including temperature (25–55°C, 5°C intervals), pH (5.0–9.5, 0.5 intervals), laccase concentration (50 U L–1–300 U L–1, 50 U L–1 intervals), and enzyme mediator type and concentration (HBT, HBA, gallic acid, guaiacol, and vanillin; 5–20 mM), on delignification efficiency and polyphenol reduction was measured20,21.
Optimizing the bio-delignification process
A user-defined design (UDD) under response surface methodology (RSM) was used to optimize delignification by systematically varying independent factors over a specific range. Following OFAT experiments, HBA concentration (1–10 mM, 4.5 mM intervals), enzyme content (50 U L−1–200 U L−1, 75 U L−1 intervals), pH (5–9, 2 intervals), and temperature (30–50 °C, 10 °C intervals) were selected as independent variables for further optimization. For each experiment, the delignification efficiency (%) was measured as the response variable, and the control group, lacking the enzyme, was processed simultaneously with the test sample. Before delignification tests, laccase was partially purified by centrifuging a 4-day-old bacterial culture at 5500g for 20 min and separating the bacterial cells from the supernatant. Then, the enzyme was precipitated from the supernatant by adding 80% v/v cold ethanol, incubating at 4 °C, and centrifuging at 12000g. Subsequently, the obtained precipitate was redissolved in 100 mM BRB buffer (pH 8) containing 1 M sodium chloride and 0.25 mM copper sulfate. The laccase was dialyzed overnight against the same buffer to remove residual impurities. The DMP assay was subsequently conducted to confirm the presence and activity of the halophilic laccase.
Assessment of polyphenol reduction percentage in laccase-treated Garlic Peel
Following bio-delignification of garlic peel, the solid residue was separated by filtration and analyzed for polyphenol content. Polyphenol content was evaluated using the Folin-Ciocalteu method, where phenolic groups interact with the reagent, resulting in a blue color at 765 nm21. For accurate measurements, a calibration curve was generated using gallic acid. The results were expressed as milligrams of gallic acid equivalents per gram for polyphenols.
Evaluation of laccase-mediated delignification efficiency (%) in treated Garlic Peel
The iodometric method was used to measure the lignin content of garlic peels before and after the enzymatic modification22. This analytical technique was based on the oxidation of the lignocellulosic material using 7.5 mL of a 0.1 N potassium permanganate solution in a sulfuric acid medium (4 N). The subsequent addition of 1 N potassium iodide (1.5 mL) resulted in the liberation of iodine in direct proportion to the lignin content. The released iodine was then titrated with a 0.1 N sodium thiosulfate solution, with the endpoint visually indicated by the appearance of the blue starch-iodine complex22. Finally, the Kappa number representing lignin content in a sample was calculated using Eqs. (1) & (2). In these calculations, V1 shows the volume (mL) of potassium permanganate consumed, V2 refers to the volume (mL) of sodium thiosulfate used for the blank, and V3 represents the volume (mL) of sodium thiosulfate used during the test sample. The concentration of the sodium thiosulfate solution is indicated by C. In the calculations, m presents the weight of the moisture-free pulp in the sample. In Eq. 2, the correction factor (d), accounting for a 50% (w/w) pulp consistency, is derived based on the volume of potassium permanganate consumed, indicated by (V1).
Delignification efficiency (%) was subsequently determined using a specific equation (Eq. 3). In this equation, K0 denotes the initial Kappa number, reflecting the lignin content before delignification, while K1, the final Kappa number, indicates the remaining lignin content following the treatment.
Compositional analysis of the laccase-delignified Garlic Peel Biowaste
The compositional content of the biowaste, including lignin, hemicellulose, and cellulose, was analyzed before and after the enzymatic treatment using standardized methods outlined in the Agricultural Chemical Association guideline23. The biomass was characterized using acid detergent fiber (ADF), neutral detergent fiber (NDF), and acid detergent lignin (ADL) methods. The ADF method was used to determine the content of lignin and cellulose. This method involved treating the biomass with a hot solution of 1 N sulfuric acid and 20 g L−1 cetyltrimethylammonium bromide (CTAB), which solubilizes non-fibrous components like hemicellulose and proteins. The remaining material was then dried at 105 °C, cooled in a desiccator, and weighed. Subsequently, the ADF fraction underwent further treatment with 72% w/v sulfuric acid to dissolve the cellulose, leaving predominantly lignin, which was quantified through gravimetric analysis and identified as ADL. The NDF method used a neutral detergent solution containing 0.5% w/v sodium dodecyl sulfate (SDS), 1 g L− 1 disodium ethylenediaminetetraacetic acid (EDTA), and 0.5 g L− 1 sodium phosphate monobasic (NaH2PO4) at pH 7.0 to solubilize all components except for cellulose, hemicellulose, and lignin. The remaining material, termed NDF, represented the total cell wall content of the biomass. This NDF fraction was also dried at 105 °C for at least 3 h, cooled in a desiccator, and weighed23.
Structural characterization of the laccase-treated Garlic peels
Scanning electron microscopy (SEM; TESCAN, MIRA II, Czech Republic) was employed to observe the morphological changes in the substrate before and after the enzymatic treatment. Fourier-transform infrared spectroscopy (FTIR; Equino × 55, Shimadzu, Japan) was conducted to investigate changes in the functional groups of untreated and treated garlic peels. FTIR spectra were acquired over a wavenumber range of 400 cm− 1–4000 cm− 1 at a resolution of 0.5 cm− 1. X-ray diffraction (XRD; PW1730, Philips, Netherlands) analysis was conducted to determine the crystallinity of both raw and enzyme-treated garlic peels. The XRD scans were performed over a 2θ range of 15°–75° at a scanning rate of 3° per min using CoKα radiation (λ = 1.79 Å) at a current of 20 mA and voltage of 40 kV.
Predicting bio-delignification by-products
Gas chromatography-mass spectrometry (GC-MS) analysis was conducted to identify the products generated during enzymatic delignification. For this purpose, a Shimadzu gas chromatograph (Agilent Technologies-7890 A) equipped with a mass detector (MS Agilent 5975) and a Porapak-Q column (12 ft × 2 mm; 60/80 mesh) was utilized.
Statistical analysis
Experiments were conducted in triplicate, and results are expressed as mean ± standard deviation (SD). Statistical analyses were performed using Sigmaplot version 14.0 (Systat Software, Inc. UK). Significant differences between two or more factors were determined using Tukey’s post-hoc test following analysis of variance (ANOVA). A p-value less than 0.05 was considered statistically significant.
Results and discussion
Isolation of identification of a halophilic and laccase-producing bacterium
To screen for halophilic and laccase-producing bacterial isolates, soil samples were collected from four different provinces of Iran and cultivated on LB agar plates containing the laccase indicator 2,6-DMP. The culture media were incubated at 37 °C for 6 days until an orange halo appeared around the colonies, indicating substrate oxidation. During an initial screening of 26 soil samples, 14 bacterial colonies were identified as producing a colored halo on the plates (Table S1). Among these isolates, strain TA-16 exhibited the largest colored zone surrounding its colony after six days of incubation. This isolate was subsequently subjected to further molecular, biochemical, and morphological analysis. After sequencing the 16 S rRNA gene and performing phylogenetic analysis, the PCR product (1500 bp) showed 99% homology to Virgibacillus salarius (Fig. S1 and Fig. 1a). The phylogenetic analysis confirmed that the isolate belongs to the genus Virgibacillus and was identified as V. salarius MAF01 (GenBank accession no. PP494041, http://www.ncbi.nlm.nih.gov/).
(a) A phylogenetic tree depicting the evolutionary relationships among Virgibacillus strains derived from their 16 S rRNA gene sequences. (b) Oxidation of 2,6-DMP by V. salarius. (c) The microscopic image of the microorganism. Scanning electron micrograph (SEM) of V. salarius at (d) 5 kx and (e) 50 kx magnification.
The isolate exhibited a Gram-positive reaction (Fig. 1c) with a rod-shaped morphology (Fig. 1d and e) under microscopic examination. It formed pale white-yellow colonies with a ridged perimeter on agar plates after a 3-day incubation at 37 °C (Fig. 1b). The isolate could not ferment trehalose and galactose or reduce nitrate. However, the bacterium demonstrated catalase and oxidase activities and the capacity to hydrolyze gelatin and aesculin. Carbohydrate fermentation analysis indicated that the bacterial isolate could metabolize arabinose, fructose, glucose, lactose, mannitol, and sucrose (Table S2).
Enhancement of laccase production in V. salarius
OFAT analysis of laccase production
Enzyme production significantly increased during the exponential phase, reaching a peak of 102 U L− 1 in the presence of 2 M salt on day 3, marking the onset of the stationary phase (Fig. S2). The growth pattern of the isolated strain correlated well with the enzyme production. After 3 days, laccase production showed a slight decline. The isolate was cultured in M9 basal medium across a temperature range of 15–45 °C and pH values of 5–11 to evaluate the effects of these parameters. The highest enzyme activity (98 U L− 1) was achieved at 35 °C and pH 7 on 3rd day (Fig. S3). The results indicate that enzyme production remained stable within pH 6–9 at 25–45 °C temperatures. The study on the medium-to-air ratio revealed that maximum enzyme production (98 U L− 1) was obtained at a ratio of 2:10, with a subsequent decline to 50 U L− 1 as the ratio increased to 5:10 (Fig. S4). The findings also showed that enzyme production was higher with a 2% inoculum size (102 U L− 1) than more enormous proportions, likely due to reduced metabolic activity and nutrient depletion (Fig. S5). The impact of carbon sources on growth and enzyme production was investigated, revealing that 1% w/v glucose resulted in 145 U L− 1 of laccase activity (Fig. S6). Maximum laccase activity (183 U L− 1) was observed with 1% w/v peptone as the nitrogen source, followed by yeast extract, ammonium chloride, sodium nitrate, ammonium oxalate, and casein, highlighting the significant effect of organic over inorganic sources (Fig. S7). The influence of trace elements was assessed by adding metal ions (iron(II) sulfate, nickel chloride, zinc chloride, cobalt chloride, calcium chloride, magnesium sulfate, and manganese sulfate) at concentrations of 0.5 mM, 1 mM, and 2 mM. The highest laccase activity was recorded in the presence of iron(II) sulfate, magnesium sulfate, and zinc chloride, achieving 124 U L− 1, 122 U L− 1, and 112 U L− 1, respectively, while nickel chloride showed a moderate positive effect. Conversely, cobalt chloride and manganese sulfate exhibited inhibitory effects on enzyme production (Fig. S8). This study demonstrated that the highest enzyme production (144 U L− 1) was achieved with 5 mM CuSO4, followed by vanillin, gallic acid, tannic acid, and caffeine, due to enhanced transcription of the relevant gene. However, xylidine and o-toluidine had an inhibitory effect on enzyme production (Fig. S9). The impact of different salt types on enzyme production was also evaluated by supplementing the culture medium with sodium chloride, lithium chloride, and potassium chloride at concentrations of 1 M–5 M. Maximum laccase activity (97 U L− 1) was achieved with 2 M sodium chloride, while lithium chloride and potassium chloride inhibited production, reducing it to 52 U L− 1 and 62 U L− 1, respectively (Fig. S10). Overall, the OFAT studies indicated that optimal laccase production is achieved under conditions of pH 7, 2% inoculum size, a medium-to-air ratio of 2:10, at 35 °C, with glucose, peptone, iron(II) sulfate, copper sulfate, and sodium chloride, which were selected for further optimization.
Experimental screening of factors for laccase production
Table 1 shows the factors selected for a fractional factorial design to assess their influence on laccase production(U L–1). Table 2 also shows the experimental design matrix generated using Design-Expert software (version 10.0.0, Stat-Ease, Inc., Minneapolis, USA). The experimental design evaluated 9 factors at two levels, low (–1) and high (+ 1), in 20 experiments. Three additional experiments were conducted at the center point to assess the curvature of the response. All experiments were independently performed three times, and the results were presented as the average values.
Analysis of variance for screening variables (ANOVA) was displayed in Table 3. Pareto chart revealed that four variables (i.e., pH level, sodium chloride concentration, peptone amount, and copper sulfate concentration, significantly affected laccase production (Fig. S11). Consistent with previous studies, pH was a crucial factor influencing laccase production. This effect could be explained by two distinct factors: pH-mediated changes in medium properties, such as nutrient transportation and solubility, and pH-dependent alterations in enzyme ionizable groups, affecting the laccase stability24. Cu2+ also proved to be an effective inducer of laccase production in bacteria, an effect likely attributed to its ability to upregulate the transcription level of the laccase gene. Nitrogen sources significantly influence bacterial physiology and metabolism, thereby affecting enzyme production. However, effective nutritional sources vary across microbial species. Numerous studies have examined the impact of single or mixed cations on laccase production in basal or defined media. For instance, yeast extract supplemented with CuSO4 significantly increased laccase production in Pleurotus ostreatus25.
However, the inductive effect of Cu2+ on the enzyme activity was time and dose-dependent. Arockiasamy et al. employed the Plackett-Burman design to screen critical parameters affecting laccase production by a Coriolus versicolor strain26. Their findings indicated that MgSO4∙7 H2O noticeably enhanced enzymatic activity relative to CuSO4∙5 H2O. Conversely, Mn2+ was the most potent laccase inducer for Trametes orientalis compared to other cations such as Cu2+, Fe2+, and Mg2 + 27. The combination of Cu2+ and Mn2+ enhanced Pleurotus ostreatus laccase production, while Cu2+ alone improved Flammulina velutipes yield28.
Optimization of laccase production by V. salarius
After the OFAT studies (Figs. S2–S10) and screening analysis (Fig. S11, Tables 1, 2 and 3), four variables, including concentration of sodium chloride (X1), pH (X2), content of peptone (X7), and amount of copper sulfate (X9) were found to have significant effect on laccase production by V. salarius (Table 4). Therefore, the D-optimal RSM was employed to optimize the culture conditions to maximize laccase production by the isolated bacterium. The results obtained from performing 29 runs are summarized in Table 5. ANOVA for the quadratic model is shown in Table 6. The p-value of less than 0.05 (p < 0.05) indicates the significance of the model. Additionally, the lack of fit was insignificant (p > 0.05) in the response, suggesting that the model was adequate. All independent variables (X1, X2, X7, and X9) and their interactions; X1 ×2, X1×7, X1 × 9, X2×9, X2 × 7, X7 ×9, X12, X22, and X92 exhibited a significant effect on laccase production (p-value < 0.05). The quadratic model was the most suitable for describing the statistical relationship between the selected variables and the response, summarized as follows:
This model’s coefficient of determination (R2 and adjusted R2 were 0.994 and 0.996, respectively. The three-dimensional (3D) response surfaces illustrating the impact of the independent variables on laccase production are shown in Fig. 2. In each plot, the interaction of two variables was assessed simultaneously while the others were kept at their middle-level value. Surface plots indicate that elevated pH levels and increased peptone content improved enzyme production. At the same time, changes in copper sulfate concentration from 3 to 7 mM had only a minor impact on the response. By analyzing Eqs. 4 and 3D response surface graphs, the maximum laccase production (396 U L− 1) was achieved under the following conditions: 2 M sodium chloride, 1.3% peptone, 3.2 mM copper sulfate, and pH 7. Five verification experiments were performed under statistically optimized conditions to validate the model’s accuracy. The results demonstrated a maximum laccase production of 392 U L− 1, closely matched (99%) with the predicted value. It indicated a high level of agreement between the experimental and expected outcomes, confirming the accuracy and reliability of the model.
Response surface analysis of laccase production (U L− 1) by V. salarius as influenced by sodium chloride concentration (1–3 M), pH level (6–8), peptone content (0.5–1.5%), and amount of copper sulfate (3–7 mM). The graph visualizes the interactive effects of these variables on enzyme production (U L− 1).
In contrast to Bacillus ligniniphilus and Halomonas sp., which exhibited tolerance to 1 M NaCl, V. salarius maintained robust laccase production under higher salinity29,30. Additionally, while Alkalibacillus salilacus demonstrated optimal laccase activity at pH 8 and up to 1 M sodium chloride, V. salarius performed well at neutral pH and higher sodium chloride concentrations (2 M), indicating greater adaptability to a broader range of environmental conditions31. The obtained results highlight the potential of V. salarius for use in high-salt environments, particularly in biotechnological applications such as bioremediation and organic compound degradation.
Bio-delignification of garlic peel biowaste
The full-factorial design approach to bio-delignification
Some experiments were carried out using the OFAT method to establish a baseline for the RSM design, focusing on the critical factors (temperature, pH, laccase content, type, and concentration of mediator) affecting delignification efficiency and polyphenols reduction. The initial phase involved conducting full-factorial experiments across a range of temperatures, with 40 °C identified as the most effective baseline for subsequent RSM analysis due to its superior performance in delignification efficiency (32%) and polyphenolic reduction (25%). These experiments were conducted under various pH levels, from acidic to alkaline, with a neutral condition (pH 7), yielding the highest delignification efficiency (34%). Moreover, the enzyme content was progressively increased from 5 U L− 1 to 200 U L− 1, resulting in a significant enhancement in delignification efficiency, which rose from 18 to 70% and decreased the polyphenolic content by approximately 78%. Beyond a laccase content of 200 U L− 1, delignification efficiency and the reduction of polyphenols reached a plateau, indicating a saturation point in the enzymatic reaction under the specified conditions (Fig. S12). The study also evaluated the effects of inducers on the bio-delignification efficiency and polyphenol reduction in garlic peels at pH 7 and 40 °C. Based on the findings, the highest delignification efficiency (90.0%) was achieved at a concentration of 20 mM HBA. Following this, HBT demonstrated significant delignification ability, with a maximum efficiency of 70.3%. Vanillin, gallic acid, and guaiacol showed moderate delignification effects, with efficiencies ranging from 33.7 to 55.2%. Although all inducers moderately increased polyphenol reduction (%), HBA and HBT generally produced slightly higher levels than the others. HBA was particularly effective in lowering polyphenols, reducing 82.8% (Table S3). One of the primary objectives in enhancing laccase catalytic activity is the selection of a suitable mediator to elevate its redox potential. Previous studies have also shown that HBA is a highly effective laccase mediator for degrading lignin phenolic and non-phenolic aromatic structures. This natural molecule efficiently mediates the oxidation of environmentally significant polycyclic aromatic hydrocarbons like benzo[a]pyrene and perylene. Its oxidation pattern is comparable to that of HBT as a synthetic mediator. These findings underscore HBA’s potential as a natural and environmentally friendly alternative to synthetic mediators in lignin biodegradation and environmental remediation32.
Optimization, model development, and validation for delignification
After identifying the most critical factors affecting bio-delignification, an experimental design approach was used to minimize experiments and eliminate trial-and-error. The UDD under RSM was utilized to predict the optimal conditions for the delignification process. The selected variables and their corresponding ranges are specified in Table 7.
Additionally, ANOVA was applied to study the impact of independent variables on the resulting response (Tables 8 and 9). Based on the funding, a quadratic second-order polynomial equation could appropriately fit the data. The insignificance of the lack of fit compared to pure error indicated that the model adequately conformed to the experimental design, demonstrating sufficient accuracy. Moreover, the similarity between the R2 (0.998) and adjusted R2 (0.997) values confirmed the adequacy of the model in predicting the bio-delignification efficiency (%) during the optimization process. The plot of residual values versus predicted ones shows no discernible trends, indicating homogeneous variance in the data and no outliers in the experimental runs (Fig. S13). A strong correlation between the predicted and observed lignin removal values also indicated that the RSM model accurately predicted the delignification efficiency of garlic peel using laccase from V. salarius (Fig. 3). A low coefficient of variation further supported the model’s precision and reliability. Based on the multiple regression analysis, the linear and interaction coefficients of all variables and the squared coefficient of pH were statistically significant (p < 0.05) in predicting delignification efficiency (%). The resulting equation was as follows:
3D response surface plots were generated using Design-Expert software to investigate the interaction between two variables. As shown in Fig. 3, the response surface exhibits a curvature along the pH axis, suggesting a significant interaction with the concentration of HBA, temperature, and laccase content. This curvature can be attributed to the statistical significance of the quadratic pH term in the model. Additionally, increasing laccase content and HBA concentration as the reaction temperature was elevated from 30 to 50 °C enhanced the delignification efficiency. The model was validated using the optimal conditions recommended by the software. The results confirmed that the maximum lignin removal of 99.3% was achieved with 9.4 mM HBA and 198.5 U L− 1 of the enzyme at a pH of 7.1 and a temperature of 31.1 °C. The validation tests were performed five times following the predicted experiments, yielding a delignification efficiency of 95.4%, nearly matching the expected value. The results from this study were noteworthy when compared to previous studies on lignin delignification using various laccases. For instance, studies on laccase activity from the halophilic bacterium Aquisalibacillus elongatus demonstrated significantly lower delignification efficiency with rates of 32.1% and 39.3% at laccase concentrations of 10 and 25 U mL−1, respectively33. Furthermore, a bacterial laccase from Bacillus tequilensis achieved 28% delignification without a mediator and 47% delignification in the presence of HBT34. Similarly, the high redox potential laccase from Pycnoporus cinnabarinus resulted in a slight increase in lignin content when used on wheat straw34. Another study on A. elongatus reported a 45% reduction in the lignin content of peanut shells after 24 h of treatment34. This suggests that the efficiency of A. elongatus laccase was relatively modest compared to V. salarius. Table 10 compares recent research on laccase-mediated delignification of diverse biomass substrates with the delignification of garlic peel obtained in this study under specific conditions. It is important to note that the extent of lignin removal by laccase depends on the biochemical composition of the biomass and the redox potential of the biocatalyst35. For example, peanut shells contain approximately 30-40% lignin, while garlic peels typically contain 15-20% lignin36,37. The lower lignin content in garlic peel waste than in peanut shell bio-waste suggests that garlic peels may be more amenable to enzymatic delignification. In this regard, laccase can play a significant role as a leading biocatalyst and a potent substitute for chemical-based deconstruction of lignocellulosic materials. Extremophiles and extremozymes are indeed very promising for the efficient degradation and utilization of lignin. Their unique properties make them particularly effective in breaking down recalcitrant lignin structures, such as garlic peel waste3.
Surface characterization of laccase-associated Garlic peels
The SEM analysis revealed significant changes in the surface morphology of laccase-treated garlic peels, showing a rough, porous, and distorted structure. The enzymatic treatment also led to visible holes and cracks in the biomass, as illustrated in the SEM images. This starkly contrasted with the intact, smooth surface observed in the untreated control group (Fig. 4a and b).
SEM imaging (500x magnification) of garlic peel (a) before and (b) after treatment with laccase (198.5 U L− 1) from V. salarius and with 9.4 mM HBA at a pH of 7.1 and a temperature of 31.1 °C. (b) FTIR spectroscopy analysis and (c) XRD analysis of garlic peel before and after treatment with laccase (198.5 U L− 1) from V. salarius. The XRD plot shows X-ray intensity on the y-axis versus the diffraction angle (2θ) on the x-axis.
FTIR spectroscopy was also employed qualitatively to characterize the effects of the enzymatic treatments on garlic peel biowaste (Fig. 4c). After treatment, the intensity at 1612–1634 cm−1, 1420–1425 cm−1(CH₂ deformation), 1504 cm−1 (aromatic stretch), and 1053–1056 cm−1 (mainly polysaccharides) decreased, which may indicate a reduction in cellulose crystallinity. A reduction in the intensity at 620 cm−1 could also confirm a decrease in the cellulose crystallinity of the biowaste after treatment. FTIR analysis of the laccase-treated garlic peels revealed a significant reduction in peak intensities at 3417–3427 cm−1 (OH vibration), 2919 cm−1 (C–H methyl and methylene groups), 1670–1731 cm−1 (C = C stretching), 1515 cm−1 (C = C aromatic), 1249–1256 cm−1 (O–H phenolic)47,48. These findings suggest that the enzymatic treatment effectively disrupted the lignin structure, potentially facilitating subsequent deconstruction processes. Additionally, the XRD pattern revealed a substantial increase in the diffractive signal intensity of cellulose I at 2θ = 15.5° and 22 and cellulose II at 2θ = 20.3° due to the breakdown of linkages within the lignocellulose matrix, which exposed more accessible regions of cellulose (Fig. 4d). These findings demonstrate the efficacy of the treatment in enhancing the accessibility of cellulose in the biowaste. This enhances cellulose accessibility to enzymes, creating a more favorable environment for enzymatic hydrolysis. Thus, laccase treatment could significantly improve the biofuel potential of garlic peels49,50.
Composition profiling of the laccase-delignified garlic peel biowaste
Garlic peels initially contained about 47.1% cellulose, 30.4% hemicellulose, and 22.4% lignin, with an estimated kappa number 36.8. During delignification, the weight decreased from 5 g to 3.7 g. Following enzymatic treatment, the biowaste composition changed to 49.1% cellulose, 31.4% hemicellulose, and 1.1% lignin, all measured on a dry weight basis (Table S4). Based on the results, the enzymatic reaction significantly altered the lignin content, while no significant changes were observed in the hemicellulose and cellulose percentage compared to the untreated samples. Lignin is difficult to break down with enzymes or chemicals, which is the main challenge in making cellulose and hemicellulose more susceptible to hydrolysis in industrial processes. The results were comparable to those from the pretreatment of feedstock with a commercial laccase, which removed up to 50% of lignin and significantly increased glucose and xylose content after the subsequent hydrolysis51. While bacterial laccases typically have low redox potential, they show high ligninolytic activity during biomass degradation. Conversely, fungal pretreatment often results in significant losses of cellulose and hemicellulose, as these components are used as carbon sources to support fungal growth and metabolism. Bacterial laccases, however, can effectively degrade lignin with minimal loss of these valuable components, making it more efficient in processes where preserving cellulose and hemicellulose is essential, such as in industrial applications aimed at maximizing the yield of fermentable sugars52,53.
GC-MS analysis of residual compounds in laccase-delignified garlic peel biowaste
Laccase-treated garlic peels were analyzed using GC-MS to understand better the chemical modifications in the lignin structure (Fig. S14). The identities and relative abundances of the lignin-derived compounds released are listed in Table 11. These compounds were subsequently grouped into four categories according to their aromatic structure: phenol-type compounds (phenol, 3-hydroxybenzoic acid), guaiacyl-type compounds (2-methoxyphenol, 4-ethyl-2-methoxyphenol), syringol-type compounds (2-methoxybenzene-1,4-diol), and catechol-type compounds (3,4-dihydroxybenzoic acid, hydroxybenzaldehyde). It is possible to appreciate that the syringyl-derived compounds were more abundant than the guaiacyl type. Among these compounds, 3-hydroxybenzoic acid has applications in the food industry as a preservative and flavor enhancer. 2-Methoxyphenol (Guaiacol) and Ethyl-2-methoxyphenol (Homovanillin) also have flavoring properties. Previous research has also demonstrated the lignin-degrading potential of several extremophilic bacteria, such as Thermobifida fusca54, Clostridium thermocellum55, Caldicellulosiruptor kronotskyensis54, and Arthrobacter sp. C2 54. A common delignification mechanism of extremophilic laccases involves the cleavage of Cα—Cβ and aryl—Cα bonds, coupled with the oxidation of Cα—OH to Cα = O. Demethylation and increased carbonyl group formation, indicative of Cα-oxidation, are frequently observed during laccase-mediated lignin modification. However, the product distribution resulting from laccase-catalyzed reactions of lignin is notably sensitive to reaction conditions. Specifically, pH and enzyme dosage, which are influenced by thermodynamic and kinetic factors, play a key role in determining the final product distribution4.
Conclusion
This study highlights the potential of V. salarius, a halophilic, laccase-producing bacterium, for effectively delignification of garlic peel biomass. Optimizing enzyme production resulted in efficient lignin removal, significantly reducing its content and substantially altering the biomass’s structural composition. Microscopic and analytical techniques confirmed these changes, revealing visible holes and cracks within the biomass. This environmentally friendly method offers a promising alternative to traditional chemical and mechanical processes, suggesting that V. salarius could be a valuable asset in sustainable biomass conversion for industrial applications such as biofuel production and the synthesis of high-value chemicals. The findings highlight the potential of extremophiles in advancing biorefinery processes, paving the way for further exploration of their biotechnological applications.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files.
References
Tanis, M. H., Wallberg, O., Galbe, M., Al-Rudainy, B. & & Lignin extraction by using two-step fractionation: A review. Molecules 29 (1), 98 (2023).
Vohra, M., Manwar, J., Manmode, R., Padgilwar, S. & Patil, S. Bioethanol production: feedstock and current technologies. J. Environ. Chem. Eng. 2 (1), 573–584 (2014).
Zhu, D., Qaria, M. A., Zhu, B., Sun, J. & Yang, B. Extremophiles and extremozymes in lignin bioprocessing. Renew. Sustainable Energy Rev. 157, 112069 (2022).
Agustin, M. B. et al. Laccase as a tool in Building advanced lignin-based materials. ChemSusChem 14, 4615–4635 (2021).
Wang, Z. & &Deuss, P. J. The isolation of lignin with native-like structure. Biotechnol. Adv. 68, 108230 (2023).
Tarasov, D., Leitch, M. & Fatehi, P. Lignin–carbohydrate complexes: properties, applications, analyses, and methods of extraction: a review. Biotechnol. Biofuels. 11, 1–28 (2018).
Ishak, N., Kassim, A. S., Aripin, A. M., Sharifah, S. M. & Oluwatosin, A. F. A review on lignin and biodelignification. J. Eng. Health Sci. 3 (1), 41–72 (2022).
Xu, N. et al. Biomethane production from lignocellulose: biomass recalcitrance and its impacts on anaerobic digestion. Front. Bioeng. Biotechnol. 7, 191 (2019).
Zhou, N., Thilakarathna, W. W., He, Q. S. & Rupasinghe, H. V. A review: depolymerization of lignin to generate high-value bio-products: opportunities, challenges, and prospects. Front. Energy Res. 9, 758744 (2022).
Albulaihed, Y. et al. Optimization of laccase from Stenotrophomonas maltophilia E1 by submerge fermentation using coconut husk with its detoxification and biodecolorization ability of synthetic dyes. Bioresour Bioprocess. 10 (1), 80 (2023).
Liu, Y., Luo, G., Ngo, H. H., Guo, W. & Zhang, S. Advances in thermostable laccase and its current application in lignin-first biorefinery: a review. Bioresour Technol. 298, 122511 (2020).
Pathania, D. et al. Garlic Peel based mesoporous carbon nanospheres for an effective removal of malachite green dye from aqueous solutions: detailed isotherms and kinetics. Spectrochim. Acta Part. A. 276, 121197 (2022).
Shaikhiev, I. G., Kraysman, N. V. & Sverguzova, S. V. Use of Garlic processing by-products to remove pollutants from aqueous media. Biointerface Res. Appl. Chem. 12 (4), 4518 (2022).
Lara-Fiallos, M. V. et al. Optimization of inulin extraction from Garlic (Allium sativum L.) waste using the response surface methodology. Rev. Educ. Madrid. 392 (54), 2–31 (2021).
Carreón-Delgado, D. F. et al. Evaluation of pretreatments and extraction conditions on the antifungal and antioxidant effects of Garlic (Allium sativum) Peel extracts. Plants 12 (1), 217 (2023).
Roy, P. et al. Mesoporous carbon nanospheres derived from agro-waste as novel antimicrobial agents against gram-negative bacteria. Environ. Sci. Pollut Res. 28, 13552–13561 (2021).
Chen, J. et al. Effects of mixing Garlic skin on fermentation quality, microbial community of high-moisture Pennisetum hydridum silage. Front. Microbiol. 12, 770591 (2021).
Najafabadipour, N., Mojtabavi, S., Jafari-Nodoushan, H., Samadi, N. & Faramarzi, M. A. High efficiency of osmotically stable laccase for biotransformation and micro-detoxification of Levofloxacin in the urea-containing solution: catalytic performance and mechanism. Colloids Surf. B. 207, 112022 (2021).
Tatar, D. Isolation, phylogenetic analysis and antimicrobial activity of halophilic actinomycetes from different saline environments located near çorum Province. Biologia 76 (2), 773–780 (2021).
Malhotra, M. & Suman, S. K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: removing Obstacles in energy generation. Environ. Sci. Pollut Res. 28, 58929–58944 (2021).
Sadeghian-Abadi, S., Rezaei, S., Yousefi-Mokri, M. & Faramarzi, M. A. Enhanced production, one-step affinity purification, and characterization of laccase from solid-state culture of Lentinus Tigrinus and delignification of pistachio shell by free and immobilized enzyme. J. Environ. Manage. 244, 235–246 (2019).
Rajeswari, G. & Jacob, S. Deciphering the aloe vera leaf rind as potent feedstock for bioethanol through enzymatic delignification and its enhanced saccharification. Ind. Crops Prod. 143, 111876 (2020).
Agricultural Chemical Association. Official guideline for agricultural chemical usage (3rd ed.). Agricultural Chem. Association ISSN 2166–983X (2023).
Mohsen, L. Y., Lilo, R. A. & AL-Maamori, A. M. Optimization of laccase production from Marasimus palmivorus. AIP Conf. Proc. 2776: 020012 (2023).
Zhou, C. et al. Effect of common metal ions and anions on laccase catalysis of Guaiacol and lignocellulosic fiber. BioResources 12 (3), 5102–5511 (2017).
Arockiasamy, S. et al. Enhanced production of laccase from Coriolus versicolor NCIM 996 by nutrient optimization using response surface methodology. Appl. Biochem. Biotechnol. 151, 371–379 (2008).
Zheng, F. et al. A novel laccase from white rot fungus Trametes orientalis: purification, characterization, and application. Int. J. Biol. Macromol. 102, 758–770 (2017).
An, Q. et al. Enhanced laccase activity of white rot fungi induced by different metal ions under submerged fermentation. BioResources 15 (4), 8369–8383 (2017).
Rahimi, E. et al. Bioremoval and detoxification of anthracene by a halophilic laccase from Alkalibacillus salilacus. Iran. J. Biotechnol. 20 (2), e3058 (2022).
Zhu, D. et al. Insight into depolymerization mechanism of bacterial laccase for lignin. ACS Sustainable Chem. Eng. 8 (34), 12920–12933 (2020).
Bisaccia, M. et al. A novel promising laccase from the psychrotolerant and halotolerant Antarctic marine Halomonas Sp. M68 strain. Front. Microbiol. 14, 1078382 (2023).
Christopher, L. P., Yao, B. & Ji, Y. Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2, 12 (2014).
Rezaie, R. et al. Delignification and detoxification of peanut shell bio-waste using an extremely halophilic laccase from an Aquisalibacillus elongatus isolate. Extremophiles 21, 993–1004 (2017).
Rezaei, S., Shahverdi, A. R., Faramarzi, M. A. & Isolation one-step affinity purification, and characterization of a polyextremotolerant laccase from the halophilic bacterium Aquisalibacillus elongatus and its application in the delignification of sugar beet pulp. Bioresource Technol. 230, 67–75 (2017).
Singh, G. et al. Laccase mediated delignification of wasted and non-food agricultural biomass: recent developments and challenges. Int. J. Biol. Macromol. 235:, 123840 (2023).
Mandala, R., Hegde, G., Kodali, D. & Kode, V. R. From waste to strength: unveiling the mechanical properties of peanut-shell-based polymer composites. J. Compos. Sci. 7 (8), 307 (2023).
Qiu, Z., Zheng, Z. & Xiao, H. Sustainable valorization of Garlic by-products: from waste to resource in the pursuit of carbon neutrality. Compr. Rev. Food Sci. Food Saf. 24 (2), e70151 (2025).
Suresh, G. & Johney, J. Optimization of laccase production by Pleurotus pulmonarius through solid substrate fermentation of tender coconut fiber: enhanced laccase production and biomass delignification. Biomass Convers. Biorefin 1–13 (2024).
da Silva, G. R., Pratto, B. & Morandim-Giannetti A.D.A. Optimization of sugarcane Bagasse pretreatment with Xylaria Sp. laccase for bioethanol production. Waste Biomass Valorization 1–11 (2025).
Wang, C., Jia, Y., Luo, J., Chen, B. & Pan, C. Characterization of thermostable Recombinant laccase F from Trametes hirsuta and its application in delignification of rice straw. Bioresour Technol. 395, 130382 (2024).
Xiang, Y. et al. Preparation of a novel laccase-modified Fe3O4/TiO2 catalyst for simultaneous delignification and saccharification of Spartina alterniflora Loisel. Process. Biochem. 146, 387–400 (2024).
Ying, W. et al. Efficient crop straws biotreatment using the fungus Cerrena unicolor GC. u01. AMB Express. 14 (1), 28 (2024).
Sharma, L. et al. Combined NaOH-laccase mediator system assisted pretreatment and additive mediated saccharification enhances bioethanol production from Kenaf (Hibiscus cannabinus L.) biomass. Available SSRN 4921060 (2024).
Subramaniam, S., Karunanandham, K., Raja, A. S. M., Shukla, S. K. & Uthandi, S. EnZolv delignification of cotton spinning mill waste and optimization of process parameters using response surface methodology (RSM). Biotechnol. Biofuels Bioprod. 17 (1), 37 (2024).
Maulita, F. et al. Enzymatic degradation of oil palm empty fruit bunch fiber using laccase from Trametes hirsuta EDN 082. In American Institute of Physics Conference Series (Vol. 3186, No. 1, p. 020048). (2025). https://doi.org/10.1063/5.0234710
Adelpour, T., Shahverdi, A. R., Amini, M., Faramarzi, M. A. & Mojtabavi, S. In the pursuit of an efficient delignification from pumpkin seed shells by the polyextremophilic and laccase-producing bacterial isolate Halomonas elongata. Biomass Convers. Biorefin 1–22 (2025).
Moshfegh, M., Shahverdi, A. R., Zarrini, G. & Faramarzi, M. A. Biochemical characterization of an extracellular polyextremophilic α-amylase from the halophilic archaeon Halorubrum xinjiangense. Extremophiles 17, 677–687 (2013).
Vojdanitalab, K. et al. Instantaneous synthesis and full characterization of organic–inorganic laccase-cobalt phosphate hybrid nanoflowers. Sci. Rep. 12 (1), 9297 (2022).
Deng, Z. et al. Laccase pretreatment of wheat straw: effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnol. Biofuels. 12, 1–2 (2019).
Pula, B., Ramesh, S., Pamidipati, S. & Doddipatla, P. A comparative study of greener alternatives for nanocellulose production from sugarcane Bagasse. Bioresour Bioprocess. 8, 1–8 (2021).
Rencoret, J., Pereira, A., Del Río, J. C., Martínez, A. T. & Gutiérrez, A. Laccase-mediator pretreatment of wheat straw degrades lignin and improves saccharification. Bioenergy Res. 9, 917–930 (2016).
De La Torre et al. Comparison of the efficiency of bacterial and fungal laccases in delignification and detoxification of steam-pretreated lignocellulosic biomass for bioethanol production. J. Ind. Microbiol. Biotechnol. 44 (11), 1561–1573 (2017).
Guo, H., Zhao, Y., Chen, X., Shao, Q. & Qin, W. Pretreatment of miscanthus with biomass-degrading bacteria for increasing delignification and enzymatic hydrolysability. Microb. Biotechnol. 12 (4), 787–798 (2019).
Rahmanpour, R., Rea, D., Jamshidi, S., Fülöp, V. & Bugg, T. D. Structure of Thermobifida fusca DyP-type peroxidase and activity towards kraft lignin and lignin model compounds. Arch. Biochem. Biophys. 594, 54–60 (2016).
Akinosho, H. O. et al. Elucidating the structural changes to Ppopulus lignin during consolidated bioprocessing with Clostridium thermocellum. ACS Sustainable Chem. Eng. 5 (9), 7486–7491 (2017).
Funding
The Elite Researcher Grant Committee supported research reported in this publication under award number 4021494 from the National Institute for Medical Research Development (NIMAD), Tehran, Iran, to M.A.F.
Author information
Authors and Affiliations
Contributions
Tina Adelpour: Methodology, Writing Original Draft, and Conceptualization. Somayeh Mojtabavi: Methodology, Data Curation, Writing-Review & Editing. Zahra Mahmoudabadi-Arani: Data Curation, Visualization, and Software. Maryam Bozorgi-Koshalshahi: Methodology, Formal Analysis, and Conceptualization. Mohammad Ali Faramarzi: Supervision, Conceptualization, Methodology, Resources, Data Curation, Writing-Review & Editing, and Project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Adelpour, T., Mojtabavi, S., Mahmoudabadi-Arani, Z. et al. Green valorization of garlic peel waste using halophilic laccase for efficient biomass delignification and biorefinery applications. Sci Rep 15, 15885 (2025). https://doi.org/10.1038/s41598-025-99715-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99715-0