Abstract
Snakebite envenoming, a neglected tropical disease, affects millions globally, causing significant morbidity and mortality. Developing broadly neutralising monoclonal antibodies offers a promising approach to address the antigenic variation present in snake venoms. In this study, we designed a long-chain consensus α-neurotoxin, LCC, to serve as an antigen in a phage display–based antibody discovery campaign. Utilising a yeast expression system, we expressed LCC and identified 21 variable domains of heavy-chain-only antibodies (VHHs) from immune libraries. These VHHs were assessed for their binding affinity to various long-chain α-neurotoxins and their neutralising capability in vitro. The VHH with the broadest cross-reactivity and highest affinity, TPL1158_01_C09, was co-crystallised with α-cobratoxin to elucidate its binding mechanism. In vivo rodent studies demonstrated the neutralisation potential of TPL1158_01_C09. Our findings highlight that the use of a consensus toxin as an antigen coincided with the discovery of broadly neutralising VHHs against snake venom toxins.

Similar content being viewed by others
Introduction
Snakebite envenoming is a non-communicable disease that affects millions of individuals and results in approximately 100,000 deaths annually1. When a snakebite occurs, complex mixtures of protein-based toxins are injected, leading to severe symptoms such as neurotoxicity, coagulopathy, or local tissue damage2. The significant antigenic variation within and across venoms from different snake species poses a substantial challenge in developing effective treatments3. To address the challenge of antigenic variation, researchers have explored strategies similar to those used for the development of therapies against various infectious diseases. In this regard, the application of broadly neutralising monoclonal antibodies (bnAbs), defined here as antibodies capable of functionally neutralising multiple related toxins despite sequence or structural differences, has emerged as a promising solution for developing therapeutics with broad efficacy against these complex targets4,5,6,7. Beyond their ability to cross-neutralise toxins across various snake species, bnAbs can be produced using standardised cell cultivation processes commonly employed in the pharmaceutical industry. Moreover, these bnAbs can be combined into oligoclonal mixtures, resulting in exceptionally broadly neutralising recombinant antivenoms with enhanced safety, efficacy, and cost-effectiveness8,9. Consequently, bnAbs offer significant potential for the development of improved envenoming therapies.
In recent years, increased efforts to discover bnAbs against snake venom toxins have led to the identification of the first broadly neutralising human monoclonal immunoglobulin G antibodies (IgGs)5,10. These discoveries were made possible using in vitro display technologies, cross-panning strategies6,10,11,12, and extensive screening efforts5. A major disadvantage of the early discovery approaches was their reliance on (native) snake toxins isolated directly from venoms8. As a potential alternative, recent studies have explored the use of consensus toxins—recombinant proteins representing the average sequence of a toxin group13—as antigens for bnAb discovery. When combined with phage display or animal immunisation, this strategy has shown promise for identifying antibodies with broad cross-reactivity4,7,8,14, although it has not been directly compared with other selection strategies.
In this work, we focused on the same medically important subfamily of toxins studied by Khalek et al. and Ledsgaard et al., namely the long-chain α-neurotoxins found in elapid snakes, such as cobras and mambas5,10. We developed a rational design approach to strategically engineer consensus toxins, representing this toxin subfamily, which yielded a consensus toxin that maintained structural similarities with the natural toxins. We subsequently expressed these toxins in yeast and then employed them as antigens in a phage display–based antibody discovery campaign, utilising immune VHH libraries previously reported to contain high-affinity, cross-binding VHHs against other snake toxins8. Through rigorous selection and screening efforts, we identified 21 binders. These were further evaluated for their affinity to different long-chain α-neurotoxins, and the top five VHHs were assessed for their ability to neutralise α-neurotoxins in vitro. The VHH with the broadest cross-reactivity and highest binding affinity to most targets was co-crystallised with α-cobratoxin from the venom of the monocled cobra (Naja kaouthia), which revealed the structural mechanisms behind its mode of action. Finally, to assess its potential clinical utility, this VHH was evaluated in rodent studies, where it successfully neutralised α-cobratoxin.
Results
LCC represents an average of the long-chain α-neurotoxins included in the design
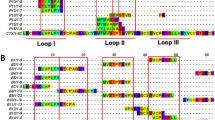
For the design of a long-chain consensus α-neurotoxin sequence, the sequences of 14 long-chain α-neurotoxins produced by venomous sub-Saharan African snakes of the family Elapidae were retrieved from the NCBI. These sequences were aligned, and a consensus sequence was designed based on the most commonly present physicochemical properties at each position (acidic, basic, polar, or hydrophobic amino acid side chains). The signal peptides were manually trimmed from the final sequence to obtain the mature protein. The resulting consensus toxin, LCC, did not mirror any known natural sequence; its sequence identity ranged from 64.8% to 87.5% compared to the sequences used in its formation (Supplementary Fig. 1). Specifically, the lowest sequence identity observed was with P01383 from Naja melanoleuca, while the highest was with P01397 from Dendroaspis polylepis polylepis (Fig. 1A, Supplementary Fig. S1). The structure of LCC, as predicted by AlphaFold2 (overall pLDDT of 0.86), aligns with the characteristic fold observed in long-chain α-neurotoxins. This fold is defined by a compact core composed of an antiparallel β-sheet structure consisting of three β-strands and one or two additional shorter β-strands. Additionally, there is a long loop that bridges the third and the fourth β-strands, which assumes a short α-helical configuration (Fig. 1B). The sequence conservation within the three-dimensional structure suggests that most of the differences between LCC and the original sequences are concentrated within the loops, while the β-sheet core shows notable conservation, as illustrated in Fig. 1C. When comparing the structural alignment of LCC with the natural sequences used in its design, the root mean square deviation (RMSD) values highlight minimal spatial differences in residue positions between the designed protein and its natural counterparts (Fig. 1D). This emphasises the structural resemblance, despite variations in their primary sequences.
A Alignment of the long-chain α-neurotoxins included in the consensus sequence design. The alignment was performed with ClustalW using a BLOSUM62 substitution matrix, and a percentage identity matrix is detailed in Supplementary Fig. S1. The chemical characteristics of the amino acids are colour coded as shown in the legend. The final consensus sequence (LCC) is displayed. B AlphaFold2 structure prediction of LCC coloured by pLDDT scores (overall pLDDT 0.86). Numbering of loops is labelled. C Sequence conservation of the LCC based on sequence alignment to the native toxins (PDB IDs: 4FLT35 and 8D9Z17). Remaining structures are AlphaFold2 predictions obtained from UniProt. D Structure conservation based on the structural alignment of native structures (grey; same structures as in C) to LCC’s structure (cyan) and corresponding RMSD values (mean RMSD = 1.8 Å).
LCC’s structure resembles that of a native α-neurotoxin
Successful expression of the consensus toxin was confirmed in Komagataella phaffii (previously known as Pichia pastoris), checking for a close replication of the structural attributes of native long-chain α-neurotoxins purified from snake venoms. The genes responsible for encoding LCC were cloned into the pPICZα A expression vector (Fig. 2A). A secretion signal sequence was utilised to secrete the toxin into the culture media. To assess the success of the expression and purification process, SDS-PAGE (Fig. 2B), confirming the expected size of the toxin and successful purification. The secondary structure of LCC was evaluated using Far-UV circular dichroism (CD) spectroscopy (Fig. 2C). This analysis showed that the secondary structure of LCC resembled that of the native α-cobratoxin purified from snake venom. The variations observed between the recombinant consensus toxin LCC and native α-cobratoxin have been observed before in the expression of recombinant α-cobratoxin in K. phaffii, so the disparities might stem from minor structural disparities and changes in the surrounding environment of pH-sensitive residues or possibly the arrangement of disulfide bonds in the toxin’s hydrophobic core, as previously described15,16.
A Schematic representation of the vector employed for electroporation of LCC into K. phaffii. LCC, in phase with the AOX1 promoter, is preceded by the α-mating factor secretion signal (α-MF) and an 6xHis-tag (His). B SDS-PAGE analysis of the supernatant samples collected at 24 h, 48 h, 72 h, and 96 h during the expression as well as the flow-through (FT), wash (W), and elution (E) fractions collected during the affinity purification. C Circular dichroism spectra comparing the secondary structure of LCC (pink) to a native long-chain α-neurotoxin: α-cobratoxin from N. kaouthia (black).
Effective VHH selection from immunised camelid libraries
Before selection, the successful biotinylation of LCC was confirmed, showing one biotin molecule per toxin (data not shown). Phage display selection was performed using mixed VHH libraries derived from two immunised llamas. A selection campaign was carried out with two rounds using LCC as the antigen (the campaign is named TPL1158). The enrichment of the rounds was evaluated using a polyclonal phage ELISA.
Following the selection rounds, the VHH-encoding genes were isolated and subcloned into a bacterial expression vector for further characterisation. A total of 186 clones were randomly chosen to evaluate the binding of the encoded VHHs to biotinylated LCC using a dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA) (Supplementary Fig. S2). The VHHs that demonstrated a positive signal in the primary screening were selected for further analysis. A total of 88 clones exhibiting a TRF signal 10 times over background underwent a second DELFIA screening and were assessed for their binding affinity to various biotinylated native toxins.
VHHs selected with LCC can bind to multiple native long-chain α-neurotoxins
Clones from TPL1158 were tested against α-cobratoxin (a long-chain α-neurotoxin from N. kaouthia) and venom fractions containing primary long-chain α-neurotoxins [fraction 8 from N. nivea (Nn8) and fraction 4 from D. viridis (Dv4)] (Fig. 3). Additionally, the binding of the VHHs to Eu-labelled streptavidin alone was evaluated in samples without added toxins. Surprisingly, the majority of the tested VHHs showed the capability to bind to a diverse range of native long-chain α-neurotoxins purified from venom produced by different snake species, confirming their cross-binding ability. Signals comparable to the background were detected in the negative control, confirming that the observed signals are due to VHH binding to the toxins, not to non-specific binding to streptavidin. A subset of 61 clones was chosen for DNA sequencing based on signal intensity and cross-reactivity capacity. As a result, 26 unique clones were identified, and the antibody framework and complementarity-determining regions (CDRs) of the clones were annotated and analysed (Fig. 3B and C). Five of these clones were selected based on CDR diversity for a kinetics screen using BLI (Fig. 3C).
A The binding of 88 VHH clones from TPL1158 was assessed against LCC, α-cobratoxin, and two venom fractions enriched in long-chain α-neurotoxins (fractions Dv4 and Nn8). The negative control included no toxin to ensure the Eu-labelled streptavidin did not bind to the VHH clones. B DELFIA results depicting the binding of the selected 21 VHHs from TPL1158 to the various toxins. C Sequence alignment of the complementarity-determining regions (CDRs) from the selected 21 clones. The clones chosen for kinetics experiments are highlighted in bold, and their full sequences can be found in Supplementary Data 1.
Binding affinity of selected clones
To investigate the binding capability of the top five VHH clones (highlighted in Fig. 3C), BLI analysis was employed using a panel of native biotinylated long-chain α-neurotoxins (Dv4, α-cobratoxin, and Nn8) as immobilised antigens. The selected VHHs were assayed in a concentration range between 9.4 and 300 nM (Supplementary Fig. S3). Some VHH-toxin combinations showed a non-canonical dissociation profile, and therefore the KD could not be calculated using the 1:1 standard binding model (Table 1). The clones showed high binding affinity towards the tested toxins, whose dissociation constant (KD) values ranged from 200 nM (TPL1158_01_A11 with α-cobratoxin) to 0.6 nM (TPL1158_01_C09 with Nn8). Supplementary Table S1 contains the kinetic constants and error values. The observed low nanomolar KD values for most of the clones highlight the strong and stable interaction between the VHHs and the respective toxins.
The selected VHHs bind to a range of long-chain α-neurotoxins from different snake species
To evaluate the binding capabilities of the VHHs and their recognition of various α-neurotoxins, a comprehensive DELFIA was conducted, covering multiple toxin fractions to assess the extent of cross-reactivity (Fig. 4). The VHHs were tested against five different long-chain α-neurotoxins (Dv4, Dv6, Dp7, α-cobratoxin, and α-bungarotoxin). Additionally, binding to a cytotoxic three-finger toxin (3FTx) (Nan13) that shares 40% sequence similarity with LCC was tested (Supplementary Fig. S4).
The binding of five different VHHs to five different native α-neurotoxins purified from venom (fractions Dv4 and Dv6 from D. viridis, fraction Dp7 from D. polylepis polylepis, α-cobratoxin from N. kaouthia, and α-bungarotoxin from Bungarus multicinctus) was analysed. The toxins were tested at concentrations ranging from 250 nM to 16 pM.
Supplementary Table S2 provides information about the toxins and venom fractions used in this study. A sequence alignment and percentage identity of the tested toxins can be found in Supplementary Tables S3 and S4, providing insights into the similarities and differences between LCC and the toxins used. Differences in sequence conservation and structural comparison are shown in Supplementary Fig. S5.
The results indicated that the selected VHHs exhibited binding to most of the tested long-chain α-neurotoxins, with TPL1158_01_C04, TPL1158_01_C09, and TPL1158_02_C06 also showing binding towards α-bungarotoxin when the VHH concentration was higher (above 10 nM). This is notable because α-bungarotoxin is the long-chain α-neurotoxin with the least sequence similarity to LCC among those tested, displaying only 62% sequence similarity on average. Notably, signal intensity for binding Dp7 was consistently low across all clones tested, despite its high sequence identity to Dv4 and Dv6 (>95%). This discrepancy may reflect variation in the biotinylation of this particular toxin batch, which could have affected capture efficiency and, consequently, the DELFIA signal. Nonetheless, the data remain qualitatively informative and support the overall binding trends observed across the panel. Lastly, the tested VHHs showed weak to no binding to the cytotoxic 3FTx (Nan13).
Following the same tendency observed in the BLI experiments, TPL1158_01_A11 bound only toxins purified from D. viridis and D. polylepis polylepis venom, though no signals were detected for any of the other long-chain α-neurotoxins from other elapid species. In this context, it is important to recognise that DELFIA measures total binding at equilibrium and does not provide kinetic parameters, such as association and dissociation rates, which are essential for accurately determining KD values. While DELFIA is a useful tool for gaining an overall understanding of binding strength and cross-reactivity, it cannot substitute for kinetic affinity measurements. These measurements are influenced by critical factors such as molecular orientation, biotinylation efficiency, and specific assay conditions, which DELFIA does not account for.
Selected VHHs neutralise toxicity in vitro
The neutralisation of toxicity by the selected VHHs was assessed using two distinct methods. First, a DELFIA assay was utilised to evaluate the inhibition of toxin binding to the nicotinic acetylcholine receptor (nAChR), which is the natural target of long-chain α-neurotoxins (Fig. 5). Second, the ability to protect nAChR function was measured using patch-clamp electrophysiology (Fig. 6).
The ability of VHH clones to prevent long-chain α-neurotoxins from binding to the human acetylcholine receptor was assessed with a DELFIA. An isotype control VHH that was specific to cytotoxins was included as a negative control. The vertical red dotted line indicates a 1:1 molar ratio of VHH:toxin.
The VHHs were preincubated with different long-chain α-neurotoxins at molar ratios ranging from 1:9 to 9:1 (3:1 for αBgtx) VHH:toxin. The resulting normalised peak current is shown. Toxins were used at concentrations ranging between IC10 and IC90, as indicated by the peak current obtained in the absence of VHH.
In the DELFIA assay, clones TPL1158_01_C04, TPL1158_01_C09, and TPL1158_02_C06 showed disruption of the interaction between all the tested long-chain α-neurotoxins and the nAChR. TPL1158_01_A11 and TPL1158_01_B05, however, showed significant signs of disruption only for fraction Dv4 and fractions Dv4 and Dp7, respectively. At a 1:1 molar ratio, the binding of both Dv4 and Dp7 to the nAChR was completely disrupted. A higher molar excess was required to achieve complete disruption for Dv6, α-cobratoxin, and α-bungarotoxin.
In the electrophysiology experiment, all the clones exhibited broad neutralisation capabilities, with TPL1158_01_C04 and TPL1158_01_C09 showing the ability to neutralise all tested long-chain α-neurotoxins. The molar ratios necessary to inhibit the toxic activity for each of the toxins were comparable in both VHHs, suggesting a similar mechanism for binding and neutralisation. Dv4 toxicity was drastically reduced even in a molar deficit of VHH (1:3 VHH:toxin); Dv6 and Dp7 were neutralised in a 1:1 ratio, while the complete neutralisation of α-cobratoxin and α-bungarotoxin necessitated a molar excess of VHH (>3:1VHH:toxin). TPL1158_01_A11 neutralised only toxins originating from mambas, consistent with the data obtained from BLI and DELFIA. Finally, TPL1158_02_C06 showed the lowest neutralisation capacity among the tested VHHs, and it was not capable of neutralising α-bungarotoxin in any of the tested conditions.
Selected VHHs neutralise toxicity in vivo
Neutralisation experiments were performed to evaluate the ability of the two selected VHHs to prevent death of mice when preincubated with three median lethal doses (LD50s) of α-cobratoxin (Fig. 7a). After injection with the toxin preincubated with TPL1158_01_C09, no evident signs of envenomation appeared over the 24 h observation period, demonstrating complete in vivo neutralisation. Conversely, mice injected with the toxin preincubated with TPL1158_01_C04 showed severe signs of envenomation and died approximately 2 h after injection. These signs of envenomation included weakness, paralysis of limbs, and eventual respiratory distress and death. Nonetheless, this represents an extension of time of death compared with the control (toxin preincubated with PBS only), where mice died within the first 10 min after injection.
A 3 × LD50s of α-cobratoxin was preincubated with either PBS or one of the VHHs and injected intravenously in mice. B Three LD50s of α-cobratoxin were injected subcutaneously, and 5 min later, VHH was injected intravenously. n = 3; * indicates a significant difference to PBS control (P < 0.05) in a Mantel-Cox log-rank test.
Given that complete prevention of lethality was observed with TPL1158_01_C09, this VHH was also tested in a rescue format, mimicking a real-life envenomation scenario. Here, three LD50s of α-cobratoxin were injected subcutaneously, followed 5 min later by intravenous injection of the VHH at a 1:3 VHH to toxin molar ratio. All the mice in this experiment survived (Fig. 7b) and showed no evident signs of envenomation.
Broad neutralisation capacity of selected VHH is explained by receptor mimicry
A structural and mechanistic explanation for the cross-reactivity of TPL1158_01_C09 was provided from X-ray crystallography and simulations. We determined the crystal structure of TPL1158_01_C09 in complex with α-cobratoxin at a 3 Å resolution, refined to R/Rfree value of 0.243/0.301. A structural analysis of the binding interface reveals that most direct atomic interactions, including hydrophobic or hydrogen bonds and salt bridges, are formed through the CDR-H3 loop of the VHH. The longest loop of the α-cobratoxin, which connects the second and third β-strand of the β-sheet core, primarily governs antigen recognition. This interaction is further stabilised by residues in the CDR-H1 and CDR-H2 loops of the VHH, as well as P7 from the third β-strand of α-cobratoxin (Fig. 8A). Key interactions include the formation of a salt bridge between VHH-D27 and α-cobratoxin-R36, as well as a hydrogen bond between VHH-D100G and α-cobratoxin-R33. Additionally, a hydrogen bond network stabilises the interface through VHH-W99 interacting with α-cobratoxin-P7, through VHH-T100A interacting with α-cobratoxin-K35, and through VHH-S100B interacting with α-cobratoxin-R33. This network is further reinforced by VHH-Y100, which interacts with α-cobratoxin-D27, forming a highly stable binding interface. The binding pose of TPL1158_01_C09 in complex with α-cobratoxin directly mimics the binding mode of α-cobratoxin to the nAChR, as both the VHH and the receptor engage similar toxin residues, particularly R33, R36, and K35. These residues are critical for the neurotoxin’s interaction with the receptor, and the VHH effectively blocks toxin binding to the nAChR by occupying this shared binding interface. This type of mimicry has been described before for Fab fragments co-crystallised with either an α-neurotoxin from D. polylepis polylepis17 or α-bungarotoxin from B. multicinctus5. Here, we show that TPL1158_01_C09 neutralises the toxin via the same mechanism (Fig. 9).
A Hydrogen bond interactions in the binding interface of the complex crystal structure of TPL1158_01_C09 (light blue) with α-cobratoxin (purple) mapped onto the respective paratope (bottom) and the epitope (top). The left close-up shows the predicted hydrogen bond network obtained from molecular dynamics simulations. The right close-up shows the local electron density map around the paratope/epitope region. B Hydrogen bond network obtained from molecular dynamics simulations mapped onto the respective epitope (top) and the paratope (bottom). The interactions for the α-cobratoxin complex are coloured in orange, for the α-neurotoxin (D. polylepis) complex in green, for the LCC complex in blue, and for the α-bungarotoxin complex in pink. The colour gradient corresponds to the probability of the respective interactions. C Sequence alignment with epitope residues highlighted in the colours corresponding with the structures in (B). A detailed paratope–epitope mapping per residue can be found in Supplementary Table S5.
Overlay of the TPL1158_01_C09 with α-cobratoxin crystal structure (this study) with the nAChRα1-subunit in complex with α-bungarotoxin (PDB accession code: 2QC1), highlighting the similar binding modes. The toxin structures are coloured in purple; the structure of TPL1158_01_C09 with α-cobratoxin (this study) is shown in cyan, and the nAChRα1-subunit is depicted in grey. A close-up of three aromatic residues in the nAChR α1-loop C (F189, Y190, and Y198), along with similar structural features in the VHH’s CDR3 loop (Y100, Y102, and W99) mimicking the structural features of the acetylcholine receptor.
To characterise the binding interface of TPL1158_01_C09 with additional toxins, we used the available X-ray structure (this study) as a template to model the VHH–toxin complexes (α-cobratoxin, α-neurotoxin from D. polylepis polylepis, LCC, and α-bungarotoxin). To assess critical contacts, we initially calculated the interactions in the interface found in the X-ray structure (Fig. 8A). Based on the molecular dynamics (MD) simulations of the modelled complexes, we calculated hydrogen bond and salt bridge interactions, revealing similar binding poses and interactions of TPL1158_01_C09 with the four different toxins. While in general the epitope is very conserved, the biggest difference in the paratope footprint can be found for α-bungarotoxin. In particular, one key arginine residue at position 39, which is involved in both salt bridge and hydrogen bond interactions in all other complexes, is missing in α-bungarotoxin. This results in an increased variability of the TPL1158_01_C09–α-bungarotoxin complex, which is also reflected in a slightly shifted paratope interaction footprint (Fig. 8B and 8C). Additionally, we find a higher number of aromatic and hydrophobic contacts in the consensus toxins complex compared to complexes with α-neurotoxin, α-cobratoxin, and α-bungarotoxin, as it has a higher number of tryptophan residues in the antigen-binding site. The key residues involved in the epitopes identified through co-crystallisation and molecular dynamics simulations—such as D27, R33, K35, R36, and V37 in α-cobratoxin—are highly conserved or exhibit equivalent physicochemical properties in the same or nearby positions (within a 1–2 amino acid window) across the toxins used for the consensus toxin design (Fig. 1 and 8C). The interactions in which the conserved residues participate also occur for more than 80% of the simulation time, highlighting their critical role in stabilising the complex and contributing to the unique cross-reactive profile of TPL1158_01_C09 (Supplementary Table S5). Supporting residues, such as P7 and D8, show a lower degree of conservation but still contribute to stabilising interactions (Figs. 8C, Supplementary Table S5).
Discussion
In this study, we designed and utilised LCC, a consensus long-chain α-neurotoxin, as an antigen in a phage display–based discovery campaign to identify VHHs capable of broadly neutralising (in vitro) long-chain α-neurotoxins from Asian and African elapids, including the monocled cobra (N. kaouthia) and the western green mamba (D. viridis). We further demonstrated that one of these VHHs (TPL1158_01_C09) could also neutralise α-cobratoxin in vivo, both in preincubation and rescue settings. Our findings underscore several potential advantages of using consensus toxins as antigens in antibody discovery and development, particularly with highly variable and complex targets like snake venom neurotoxins. Notably, our results suggest that the antigenic properties of an entire subfamily of toxins can be represented in a single, rationally designed antigen, which may support the selection of binders with broad neutralising capacity. The success of this approach was not entirely anticipated, given the challenges inherent in designing a single antigen capable of addressing the diversity within this toxin subfamily. These results therefore, highlight the feasibility of the consensus design strategy and its potential utility in addressing complex antigenic targets in future therapeutic efforts. While the consensus toxin approach has not yet been directly compared with cross-panning or other selection strategies, including panning on selected native or recombinant toxins, another theoretical advantage is that it reduces the number of selection rounds to be performed6. Nevertheless, it is important to emphasise that while the use of consensus toxins as antigens may not be inherently superior to other approaches for discovering broadly neutralising VHHs, it represents a complementary strategy that might be useful in some cases.
The structural mechanism of antigen recognition and broad neutralisation by TPL1158_01_C09 was elucidated through the determined X-ray structure (this study) in complex with α-cobratoxin. The binding pose of TPL1158_01_C09 to α-cobratoxin mimics the binding interface of neurotoxins with the nAChR and reveals highly similar (mainly aromatic) interacting residues of loop C in the nAChR (F189, Y190, and Y198) and the CDR3 loop of the nanobody (Y100, Y102, and W99) (Figs. 8 and 9)17. Glanville et al.17 reported the discovery of a Fab fragment capable of binding α-neurotoxins from D. polylepis polylepis, N. nivea, and Oxyuranus scutellatus, with binding affinities ranging from 37 to 490 pM, providing full protection in vivo for all toxins tested. Khalek et al. and Ledsgaard et al. have also described the discovery of cross-reactive IgGs that can bind to multiple long-chain α-neurotoxins with binding affinities between 1 and 100 nM. In comparison, TPL1158_01_C09 demonstrates binding affinities between 63 nM and 120 nM for α-neurotoxins from N. kaouthia, D. viridis, and N. nivea5,10. No direct comparison regarding binding breadth and binding affinity between the IgGs from these studies and TPL1158_01_C09 was made, as the VHH was tested on a more limited panel of toxins. Although the absolute binding affinities are lower for the discovered VHH, this antibody format has the benefit of being exceptionally stable and consisting only of a single polypeptide chain, which encompasses the entire paratope and is simple to manufacture. In contrast, Fabs (and IgGs) require both a heavy and a light chain, making these molecules both less stable and more complex to manufacture. This highlights the unique ability of the presented VHHs to efficiently target this conserved neutralising epitope while mimicking the binding interface of neurotoxins with the nAChR using only a single antibody domain. Due to the high level of sequence and structural conservation, the overall binding interface and respective paratope/epitope footprints are highly similar. The major difference noted was with α-bungarotoxin, which lacks one of the critical arginine residues that form a highly conserved salt bridge to stabilise the binding interface. Conversely, the consensus toxin contains additional tryptophan residues that form extra hydrophobic and hydrogen bond interactions with TPL1158_01_C09.
Our results demonstrate the broad in vitro neutralisation capacities of the selected VHHs against a variety of long-chain α-neurotoxins. Notably, clones TPL1158_01_C04 and TPL1158_01_C09 showed the ability to neutralise all tested toxins in vitro, including those from snake species beyond the African elapids used in the design of LCC. Despite the high similarity of the CDR3 regions between TPL1158_01_C04 and TPL1158_01_C09, the former failed to neutralise venom toxicity in vivo, possibly due to negative influences of other CDR2 regions that need further characterisation. This discrepancy between in vitro and in vivo results underscores the critical role of pharmacokinetics, including factors such as stability, biodistribution, and clearance rates, which can differ significantly even between closely related VHHs. Such differences highlight the importance of conducting in vivo studies to fully validate the therapeutic potential of neutralising antibodies, as in vitro assays alone cannot account for these complex biological factors. The VHH TPL1158_01_C09 was even able to rescue envenomated mice 5 min after toxin injection, showing its potential in an experimental setting mimicking real-life envenomation.
The BLI experiments were challenging to perform and analyse due to buffer-dependent artifacts, e.g., pH changes and baseline drift. These limitations affected some interactions but did not impact the overall conclusions regarding specificity and affinity. The discovered VHHs displayed KD values between 0.6 nM and 200 nM, comparable to previously discovered affinity-matured IgGs with therapeutic promise against the same toxin subfamily5,10. We speculate that some of our discovered VHHs could be useful in developing recombinant antivenoms based on oligoclonal mixtures of such VHHs. VHHs have demonstrated a generally stable, monomeric, and aggregation-resistant behaviour18,19. This therapeutic concept was previously demonstrated with VHHs against short-chain α-neurotoxins and phospholipases A2s from North American coral snake venoms, highlighting that broad neutralisation of several toxin subfamilies could be achieved with a surprisingly low number of VHHs8. This finding is crucial, as it is essential to keep the number of molecules in a therapeutic product low to ensure low-cost manufacturing and simple quality control measures20,21. Broadly neutralising antibodies, such as the VHHs discovered here, are thus highly beneficial for ensuring wide coverage of snake venoms, potentially enabling the development of polyvalent recombinant antivenoms3,5,8. In this light, it is therefore particularly noteworthy that the combined consensus design and phage display selection approach presented here was able to identify VHHs that can bind and neutralise the toxicity of two medically relevant elapid long-chain α-neurotoxins, namely α-bungarotoxin and α-cobratoxin, whereas most antibodies discovered so far can neutralise only one or the other toxin10,17,22. Nevertheless, while long-chain α-neurotoxins are particularly dangerous components of elapid venoms, this group of toxins represents just one of several medically important toxin families. To fully address the diversity of venom components, this approach would need to be extended to other toxin families, such as phospholipases A2 and additional neurotoxins, by designing consensus antigens for these targets and using these for discovering additional VHHs or antibodies23. It would also be necessary to understand the stoichiometry of neutralisation and the compositions of the venoms that were to be covered by any new recombinant antivenom product24.
In addition to their broad and improved neutralisation capacity, recombinant antivenoms offer several advantages over traditional animal plasma–derived antivenoms. They can be designed and manufactured as standardised biopharmaceutical products with minimal batch-to-batch variation and improved safety9. This aspect is important not only for treatment costs but also for clinical trials, where safer products than current antivenoms could potentially be tested more thoroughly, including trials involving healthy volunteers, in which current plasma-derived antivenoms are not tested due to their potential for adverse reactions21,25,26. Additionally, the use of VHHs as active ingredients in new types of antivenoms could improve shelf-life, as VHHs are known for their stability at elevated temperatures27. This feature is particularly desirable in tropical regions with recurring electrical brownouts, potentially eliminating the need for cold-chain storage. However, it is emphasised that safety, efficacy, and low-cost manufacturing remain the primary priorities for novel snakebite therapeutics.
Beyond snakebite envenoming, the use of consensus antigens could find wide utility in the discovery of bnAbs for other indications requiring recognition of multiple similar targets, such as hypermutable targets in cancer, bacterial serotypes, parasitic infections with high antigenic variation, and escape mutations in viruses7,8,28,29,30. Moreover, the data obtained from antibody–antigen interactions in campaigns using consensus antigens can be used for further antibody engineering and optimisation. Combined with MD, machine learning, and artificial intelligence, these data can inform the design of binding proteins with desired levels of cross-reactivity in silico31,32,33, enabling a more rational design of advanced biotherapeutics. Comprehensive approaches involving these disciplines may ultimately enhance our ability to tackle challenges involving complex and variable disease targets, potentially aiding the development of effective therapeutic and diagnostic tools for snakebite envenoming and other conditions.
Methods
Consensus toxin design
Amino acid sequences of long-chain α-neurotoxins from African snakes were obtained from UniProt (http://www.uniprot.org). SnapGene® software (from Insightful Science; https://www.snapgene.com) was used to perform multiple alignments of 14 sequences of long-chain α-neurotoxins, and from the alignments, a consensus amino acid sequence was determined: LCC (Supplementary Table S2). The consensus sequence was built by making decisions for each position depending on the most abundant amino acid property. For each position, the most repeated physicochemical property was identified, and then the most abundant amino acid within that group was chosen. In case of a tie, the amino acid with the bulkiest side chain was chosen. Amino acids were grouped as acidic (E, D), basic (K, R), polar (S, T, Y, N, N), and non-polar (G, A, V, L, I, M, W, F). Proline (P) and cysteine (C) formed a group themselves.
Structure prediction of LCC and comparison to native toxins
The structure of LCC was predicted using the local Colab version of AlphaFold234. Structure predictions were conducted using the X-ray crystallography resolved structures of two native long-chain α-neurotoxins (PDB IDs: 4LFT, 8D9Z) as templates. For comparison of LCC’s structure to the native toxins, all structures were obtained from UniProt (12 AlphaFold2 predicted structures and the two resolved structures 4LFT35 and 8D9Z17. Pymol (version 2.5.5) was used to align all native structures to LCC and colour by sequence conservation using a previously described script (https://pymolwiki.org/index.php/Color_by_conservation). RMSD values were obtained using the matchmaker function from ChimeraX (version 1.6.1), aligning all native structures to LCC with default settings.
Plasmid construction for toxin expression
The gene for LCC was purchased from Eurofins and cloned into a plasmid as described elsewhere15. Briefly, the gene encoding the 6xHis-LCC was inserted into a pPICZα A vector (Invitrogen) using the NEBuilder HiFi DNA Assembly method. The resulting plasmid was verified by DNA sequencing to confirm the correct insertion (Eurofins Genomics Sanger Sequencing, Ebersberg, Germany).
Expression and purification of LCC
The expression and purification of LCC followed a previously described protocol15. Briefly, K. phaffii KM71H were electroporated with the Sanger Sequenced–confirmed plasmid, and positive transformants were identified by plating on YPDS plates (20 g/L peptone, 10 g/L yeast extract, 100 mL/L dextrose 20% (w/v), 182.2 g/L sorbitol, 20 g/L agar) containing 1,000 µg/mL of Zeocin. Following a preliminary assessment of small-scale expression, clones exhibiting higher LCC production, as determined by SDS-PAGE analysis of culture supernatants, were selected. Subsequently, the selected clones were cultured in YPD medium overnight at 30 °C. To ensure long-term preservation, 15% (v/v) glycerol was added to the cultures, which were then aliquoted and stored at -80 °C.
A 5 mL overnight culture of the selected clone in YPD medium (20 g/L peptone, 10 g/L yeast extract, 20% (w/v) dextrose) was inoculated with a single colony of transformed K. phaffii and incubated overnight at 30 °C. The culture was then scaled up to 1 L of BMGY medium (10 g/L yeast extract, 20 g/L peptone, 0.1 M potassium phosphate pH 6.0, 1.34% (w/v) YNB, 0.04 µg/mL biotin, 1% (v/v) glycerol) and grown for 24 h at 30 °C. The cells were harvested, resuspended in BMMY medium (10 g/L yeast extract, 20 g/L peptone, 0.1 M potassium phosphate pH 6.0, 1.34% (w/v) YNB, 0.04 µg/mL biotin, 0.5% (v/v) methanol), and cultured at 25 °C for 4 d with methanol induction. After 96 h, the cells were harvested, and the supernatant was collected and sterilised by filtration. The filtered supernatant was dialysed against wash buffer (50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 20 mM imidazole) and purified using HIS-Select® Nickel Affinity Gel resin. LCC was eluted with elution buffer (50 mM Tris-HCl, pH 8.0, 300 mM NaCl, 400 mM imidazole), dialysed against a dialysis buffer (50 mM Tris-HCl, pH 8.0, 300 mM NaCl), and concentrated using Amicon® Ultra-15 Centrifugal Filters (Millipore, Burlington, USA).
The supernatant and purification steps samples were run on an SDS-PAGE gel and stained with colloidal Coomassie blue.
Native toxin preparation
Lyophilised forms of α-cobratoxin (L8114), α-bungarotoxin (L8115), short neurotoxin 1 (L8101), and whole venom derived from D. polylepis polylepis (L1309), D. viridis (L1310), and N. nivea (L1328) were obtained from Latoxan SAS (Portes-lès-Valence, France) and prepared following established procedures36. The whole venom underwent fractionation using RP-HPLC, as detailed in a separate publication37, to isolate fractions enriched with long-chain α-neurotoxins.
ChromaLINK biotinylation of LCC
LCC was biotinylated using the ChromaLINK Biotin (DMF-soluble) reagent (Vector Laboratories, B-1012-010) according to the manufacturer’s instructions. Briefly, LCC was dissolved in PBS and incubated with a 10-fold molar excess of ChromaLINK biotin reagent, freshly prepared in dimethylformamide (DMF), for 2 h at room temperature with gentle agitation. Unreacted biotin was removed by buffer exchange using a Zeba Spin Desalting Column (Thermo Fisher Scientific).
The degree of biotinylation was determined spectrophotometrically by measuring absorbance at 280 nm and 354 nm. The protein concentration was estimated from A280 using the extinction coefficient E0.1% = 1.75 for LCC, while A354 was used to quantify the incorporated biotin. For the biotinylated LCC sample, A280 = 0.30 and A354 = 0.35, resulting in a calculated biotinylation ratio of 1.09 mol of biotin per mol of LCC.
Phage display
Phage display selection was conducted using an immune VHH library. To generate the VHH-displaying phage libraries, two camelids were immunised with a mixture of 18 elapid venoms (Dendroaspis angusticeps, Dendroaspis jamesoni kaimosae, D. polylepis polylepis, D. viridis, Naja anchietae, Naja annulifera, Naja ashei, N. haje haje, Naja katiensis, N. melanoleuca, Naja mossambica, Naja nigricincta, Naja nigricollis, Naja nubiae, Naja pallida, Naja senegalensis, and Hemachatus haemachatus) over a 16-week period, followed by three booster injections within a 6-week period after 1 year to enhance the immune response8. The libraries were constructed as previously described38. The resulting libraries exhibited a diversity of 5.108 and 1.6.108 individual clones, respectively. The two libraries were mixed before usage.
The selection process followed a previously described methodology39, with a modification in the technique for capturing biotinylated toxins. Instead of direct coating onto a 96-well microtiter plate, the biotinylated toxins were captured on streptavidin-coated beads (Dynabeads M-280, Invitrogen, Waltham, MA, USA).
Two consecutive rounds of selections were performed using 50 nM LCC as the antigen. The phage display campaign using LCC was designated as TPL1158.
Screening and sequencing VHHs
One hundred eighty-six cherry-picked clones were screened in a DELFIA against LCC. Briefly, the clones were expressed in 150 µl autoinduction media36 and incubated overnight at 30 °C. The following day the cultures were centrifuged at 3000 × g for 15 min at 4 °C. The supernatants were diluted 1:1 in 6% milk-PBS (MPBS) and then added to black MaxiSorp plates (Nunc A/S, Roskilde, Denmark) coated with anti-FLAG M2 at a concentration of 2.5 µg/mL. The plates were blocked with 3% MPBS and thoroughly washed with PBS with 0.1% Tween-20 (PBS-T) and PBS before 25 nM biotinylated LCC was added and detected using Eu-labelled streptavidin at a concentration of 0.2 µg/mL using a VICTOR Nivo Multimode Microplate Reader.
A subset of clones having a signal greater than 10 times the background was then subjected to a DELFIA assay to determine their capability to recognise native toxins. In total, 88 clones were selected. The clones were screened against α-cobratoxin (N. kaouthia), venom fractions containing primary long-chain α-neurotoxins [fraction 8 from N. nivea (Nn8) and fraction 4 from D. viridis (Dv4)], short neurotoxin 1 (N. pallida), and streptavidin.
The chosen clones were subjected to expression in a 96-deep well plate using a 1 mL autoinduction media11, allowing for overnight incubation at 30 °C. The following day, the plates were centrifuged at 4000 × g for 15 min at 4 °C, and subsequently frozen for 24 h. After thawing on ice, the cell pellets were reconstituted in 110 µL of PBS supplemented with complete EDTA-free Protease Inhibitor (Roche). Another centrifugation step at 4000 × g at 4 °C for 30 min was performed to collect the supernatant, which contained the periplasmic fraction along with the VHHs. Sixty µL of the periplasmic fraction containing VHHs was diluted 1/100 in 3% (w/v) MPBS. The diluted periplasmic fraction was then added to black MaxiSorp plates (Nunc A/S, Roskilde, Denmark) coated with anti-FLAG M2 at a concentration of 2.5 µg/mL. The plates were blocked with 3% MPBS and thoroughly washed with PBS-T and PBS before 25 nM of the indicated biotinylated toxins was added and detected using Eu-labelled streptavidin at a concentration of 0.2 µg/mL using a VICTOR Nivo Multimode Microplate Reader.
The selected clones were subsequently sent for sequencing (Eurofins Genomics Sanger Sequencing, Ebersberg, Germany).
Expression and purification of VHHs
Twenty-one VHHs showing binding to at least three different toxins and having unique CDR sequences were selected and expressed in 5 mL autoinduction media at 30 °C overnight. After centrifugation at 4000 × g for 10 min at 4 °C, the cell pellets were frozen at −20 °C overnight. The pellets were thawed on ice and resuspended in 1 mL ice-cold PBS supplemented with complete EDTA-free Protease Inhibitor (Roche) and 10 mM imidazole. Following a centrifugation step at 20,000 × g for 45 min at 4 °C, the supernatant containing VHH was collected.
Nickel affinity chromatography was performed by incubating the supernatant with HIS-Select® Nickel Affinity Gel resin (Millipore) pre-equilibrated with wash buffer (PBS, 20 mM imidazole, pH 8.0). After 1 h incubation at 4 °C with end-over-end rotation, the resin was transferred to chromatography columns, and the flow-through fractions were collected. The column was washed with 6 column volumes of wash buffer, and the VHHs were eluted using 500 µL elution buffer (PBS, 250 mM imidazole, pH 8.0).
Elution fractions containing the VHHs were dialysed twice against PBS at 4 °C to remove the imidazole. The purified VHHs were then ready for downstream applications and further characterisation.
The purified VHHs were run on an SDS-PAGE gel and stained with colloidal Coomassie blue to confirm their purity.
Binding affinity determination by biolayer interferometry
The binding affinities of the selected VHHs were determined using the Octet K2 system (FortéBio, Fremont, CA, USA). The measurements were conducted in 96-well black microplates (655209, Greiner Bio-One, Kremsmünster) at 25 °C with agitation at 1000 rpm. Kinetics buffer (KB, 18-1105, Sartorius) was prepared in PBS and used as the loading buffer in the experiment. Prior to the experiment, streptavidin Biosensors (18-5019, Sartorius, Göttingen, Germany) were dipped in KB for at least 10 min, followed by loading of the biosensors with the biotinylated toxins (ligand) at a concentration of 1 µg/mL in KB. A brief acid conditioning step with glycine buffer (10 nM, pH 2.0) followed by neutralisation in KB was carried out for 5 s for five cycles.
For the interactions between α-cobratoxin and TPL1158_01_A11, Nn8 and TPL1158_01_A11 and TPL1158_02_C06, and Dv4 and TPL1158_01_B05 and TPL1158_02_C06, a baseline was established in running buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 50 mM MES hydrate, and 0.05% P20 (MES-HEPES), pH 7.2) for 120 s, and the biosensors were immersed in wells containing 9.4–300 nM VHHs in a 2-fold dilution in MES-HEPES for 600 s, followed by a dissociation step of 900 s in MES_HEPES. For all other interactions, the same protocol was followed using KB instead of MES-HEPES.
Biosensors were regenerated using glycine buffer pH 2.0 for 10 s for seven cycles between rounds. FortéBio’s data analysis software was used to fit the curves to a 1:1 binding model to derive the kinetic constants (global fitting model). Curves exhibiting baseline drifts during dissociation or sudden signal shifts between association and dissociation were exempted from analysis. In cases where reliable KD values could not be determined, results were reported as “non calculable” (Supplementary Table S1).
Cross-binding assessment of VHHs using DELFIA
To assess the cross-binding capabilities of the top five VHHs, we conducted binding experiments against a range of α-neurotoxins. The VHHs were evaluated for binding to six different long-chain α-neurotoxins and venom fractions enriched for long-chain α-neurotoxins. Additionally, one cytotoxin with a three-finger fold was included in the testing panel. A detailed compilation of the toxins employed in this analysis can be found in Supplementary Table S1. The evaluation of VHH binding was conducted using a DELFIA assay, as described above.
In the DELFIAs assays, 30 nM of each VHH was captured using anti-FLAG antibodies. To evaluate the binding of the VHHs to different toxins, varying concentrations of the toxins, ranging from 16 pM to 250 nM, were tested.
Each VHH was tested in triplicate for each toxin concentration. The results were analysed and plotted using the following equation in GraphPad Prism 9 (GraphPad Software): log(agonist) vs. response – variable slope.
nAChR blocking DELFIA
A blocking DELFIA was performed to assess the ability of VHHs to prevent long-chain α-neurotoxins from binding to the human α7-acetylcholine receptor chimera, following a previously described protocol40,41. Briefly, black MaxiSorp plates (Nunc A/S, Roskilde, Denmark) were coated with 5 μg/mL α7-AChR in PBS (500 ng per well). The plates were washed thrice with PBS-T and blocked using 3% MPBS. Mixtures of 0.1 μg/mL of biotinylated toxin with various concentrations of VHHs or a negative control VHH specific to cytotoxins were prepared in 3% MPBS and preincubated for 30 min at room temperature. Plates were thoroughly washed before 50 μL of the toxin:VHH mixture was added to the wells. The signal was generated with europium-labelled streptavidin at a concentration of 0.2 μg/mL and detected using a VICTOR Nivo Multimode Microplate Reader. Measurements were performed in duplicates. The data were analysed in Graphpad Prism 10 (GraphPad Software) with a nonlinear fit using ‘ECanything’, a variable slope model, while constraining the F-value to 50.
In vitro neutralisation by whole-cell patch-clamp
To evaluate the potential of the VHHs to neutralise the blocking effects of different α-neurotoxins of the muscle-type nAChR function, we employed planar whole-cell patch-clamp (Qube 384, Sophion Bioscience), following a previously established method36. Human-derived rhabdomyosarcoma cells, endogenously expressing muscle-type nAChRs (α1, β1, δ, and γ-subunit), were patch-clamped for the experiments.
To determine the concentration of the toxins inhibiting 80% of a nAChR-mediated current (IC80) we elicited a current using 70 µM acetylcholine (ACh) followed by toxin administration and measurement of their inhibitory effects. To ensure full toxin effect, we preincubated with toxin for at least 5 min before the addition of ACh. For testing VHH neutralisation, various concentrations of the VHHs were preincubated with toxins. The chosen toxin concentrations ranged between IC10 and IC90, allowing for a toxin:VHH molar ratio between 1:3 and 3:1. The inhibitory effect was quantified by normalising the elicited current to that of the initial ACh addition.
Data analysis was performed using the Sophion Analyzer (Sophion Bioscience) and GraphPad Prism 10 (GraphPad Software).
In vivo neutralisation of α-cobratoxin toxicity
Groups of three CD1 mice between 18 and 20 g of total body weight and indistinct sex were used for in vivo LD50 determination and lethality neutralisation experiments. The mice were kept under 12 h light and dark cycles with food and water ad libitum. All animals and in vivo methodologies used were approved by the bioethics committee of the IBt-UNAM under project #345.
The LD50 of α-cobratoxin was determined through intravenous and subcutaneous routes of injecting groups of mice with varying doses of the toxin in a final volume of 200 µL and 100 µL PBS, respectively. The percentage of dead mice was determined 24 h after injection. Data were analysed using a non-linear regression (semi-logarithmic dose-response curve) using the software GraphPad Prism 10 (GraphPad Software), as previously described42.
For preincubation experiments, three LD50s of α-cobratoxin (equivalent to 4.5 µg per mouse when injected intravenously) were combined with each of the VHHs at a 1:1 toxin to VHH molar ratio (equivalent to 9.9 µg per mouse for the VHH TPL1158_01_C04 and 10.1 µg per mouse for the VHH TPL1158_01_C09) in a total volume of 200 µL of PBS. The mixture was preincubated at 37 °C for 30 min before intravenous injection into groups of three mice. For rescue experiments, three LD50s of α-cobratoxin (equivalent to 6.6 µg per mouse when injected subcutaneously) in 100 µL PBS, were injected using the subcutaneous route to groups of three mice. Five min later, the nanobody TPL1158_01_C04 (equivalent to 44.2 µg per mouse) was injected intravenously in a total of 200 µL of PBS. The percentage of survival in both cases was determined 24 h after injection and graphed in Kaplan-Meier survival curves using GraphPad Prism 10 (GraphPad Software). All mice were observed during the first 5 h and then approximately every 6 h for appearance of any signs of envenomation.
To estimate the significance of the results obtained in the in vivo experiments, a Mantel-Cox log-rank test was performed43. Data were compared to the negative control (PBS only), and the significance value was set to α = 0.05.
All experimental protocols were approved by the relevant institutional and licensing committees. All animals and in vivo methodologies used were approved by the bioethics committee of the IBt-UNAM under project #345. Efforts were made to minimise animal suffering and the number of animals used
Co-crystallisation of VHH and α-cobratoxin
Lyophilised α-cobratoxin was dissolved in a buffer of 5 mM Tris and 20 mM NaCl at pH 8.0 to a concentration of 10 mg/mL. The VHH TPL1158_01_C09 was then added at a 1:3 molar ratio to α-cobratoxin and allowed to bind overnight at 4 °C. For purification, the mixture was processed through an NGC QuestTM 10 Plus Chromatography System (Biorad) using a Superdex 75 10/300GL column (Cytiva) at 4 °C with the same buffer as the mobile phase. Before analysis, the concentration of the VHH:α-cobratoxin complex was increased to 15.2 mg/mL using 3.0 kDa MWCO Ultracentrifugation Units (UFC500324, Merck).
Crystallisation was performed using the sitting drop vapour diffusion method at 21 °C. Drops were set up either at a 2:1, 1:1, or 1:2 molar ratio of reservoir to protein in a total volume of 0.3 µL in a 96-well drop format on SWISSCI MRC 2 Well Crystallisation Plates (JENA) using Index screening solutions (Hampton Research). The wells were sealed with crystal clear tape and equilibrated against 50 µL of reservoir solution. Small crystals appeared in the 1:2 condition (25% (w/v) PEG1500) within 48 h. Crystals developed were harvested using mounted CryoLoops (Hampton Research) with cryoprotection performed by adding glycerol to a neighbour drop with no crystals to a final concentration of 20%. A 300 µm loop was used to fish several crystals. The loop edge was kept in contact with the cryo solution for approximately 5 s to equilibrate before flash freezing the crystal in liquid nitrogen and shipping these to the beamline for remote data collection. Model and structure factors are deposited to the Protein Data Bank (PDB) under accession code 9GCN.
Data collection and structure determination
Diffraction data were obtained at the Biomax beamline (MAX IV synchrotron facility, Lund, Sweden). A complete dataset was collected over a 360° rotation, of which 190° were processed (1,900 images). The data were processed with XDSAPP344,45,46 are summarised in Supplementary Table S6. The structure of TPL1158_01_C09 in complex with α-cobratoxin was determined by molecular replacement with Phaser-MR46 using an AlphaFold model of VHH and PDB ID 7ULG as a search model. Model building and refinement were performed with Phenix.refine47 and Coot48.
The structure was evaluated using MolProbity with final statistics presented in Supplementary Table S6. Molecular graphics were presented with the PyMOL molecular visualisation system (Version 2.2r7pre, Schrödinger, LLC). Coordinates and structure factors have been submitted to the PDB database under the accession code 9GCN.
MD simulations
As starting structures for MD simulations, we used the available X-ray structure of TPL1158_01_C09 in complex with α-cobratoxin (this study). In addition, we modelled the complexes of TPL1158_01_C09 in complex with α-bungarotoxin, α-neurotoxin, and LCC (presented in this study), using the X-ray structure as a template. To characterise and quantify the interactions and dynamics of the VHH:toxin complexes, we performed five repetitions of 500 ns of MD simulations (10,000 frames each).
MD simulations were performed in an NpT ensemble using pmemd.cuda49. Bonds involving hydrogen atoms were restrained by applying the SHAKE algorithm50, allowing a time step of 2 fs. The complexes were placed into cubic water boxes of TIP3P water molecules51 with a minimum wall distance to the protein of 12 Å52,53. Parameters for all simulations were derived from the AMBER force field 14SB54. To neutralise the charges, we used uniform background charges55,56,57. Each system was carefully equilibrated using a multistep equilibration protocol58. The multistep equilibration protocol allowed us to use the full length of the respective production runs for further analysis. Atmospheric pressure of the system was preserved by weak coupling to an external bath using the Berendsen algorithm59. The Langevin thermostat was used to maintain the temperature during simulations at 300 K60,61.
We calculated the interactions using the GetContacts tool, which calculates the interface contacts in a time-resolved way and allows monitoring of the evolution of contacts during the simulation. The hydrogen bonds from the X-ray structure were calculated using a heavy-atom distance criterion of 3.5 Å. Molecular graphics were presented with the PyMOL molecular visualisation system (Version 2.5.2, Schrödinger, LLC).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data that support the findings of this study are included in the manuscript and/or Supplementary Information files. The crystal structure has been deposited to the Protein Data Bank (PDB) under accession code 9GCN. These data are also available from the corresponding authors upon reasonable request.
References
Gutiérrez, J. M. et al. Snakebite envenoming. Nat. Rev. Dis. Prim. 3, 17063 (2017).
Casewell, N. R. et al. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl Acad. Sci. 111, 9205–9210 (2014).
Casewell, N. R., Jackson, T. N. W., Laustsen, A. H. & Sunagar, K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 41, 570–581 (2020).
de la Rosa, G. et al. Horse immunization with short-chain consensus α-neurotoxin generates antibodies against broad spectrum of elapid venomous species. Nat. Commun. 10, 3642 (2019).
Khalek, I. S. et al. Synthetic development of a broadly neutralizing antibody against snake venom long-chain α-neurotoxins. Sci. Transl. Med. 16, eadk1867 (2024).
Ahmadi, S. et al. An in vitro methodology for discovering broadly-neutralizing monoclonal antibodies. Sci. Rep. 10, 10765 (2020).
Rivera-de-Torre, E. et al. Discovery of broadly-neutralizing antibodies against brown recluse spider and Gadim scorpion sphingomyelinases using consensus toxins as antigens. Protein Sci. 33, e4901 (2024).
Benard-Valle, M. et al. In vivo neutralization of coral snake venoms with an oligoclonal nanobody mixture in a murine challenge model. Nat. Commun. 15, 4310 (2024).
Jenkins, T. P. & Laustsen, A. H. Cost of manufacturing for recombinant snakebite antivenoms. Front. Bioeng. Biotechnol. 8, 703 (2020).
Ledsgaard, L. et al. Discovery and optimization of a broadly-neutralizing human monoclonal antibody against long-chain α-neurotoxins from snakes. Nat. Commun. 14, 682 (2023).
Sørensen, C. V. et al. Cross-reactivity trends when selecting scFv antibodies against snake toxins using a phage display-based cross-panning strategy. Sci. Rep. 13, 10181 (2023).
Sørensen, C. V. et al. Antibody-dependent enhancement of toxicity of myotoxin II from Bothrops asper. Nat. Commun. 15, 173 (2024).
Rivera-de-Torre, E. et al. Strategies for heterologous expression, synthesis, and purification of animal venom toxins. Front. Bioeng. Biotechnol. 9, 811905 (2022).
de la Rosa, G., Corrales-García, L. L., Rodriguez-Ruiz, X., López-Vera, E. & Corzo, G. Short-chain consensus alpha-neurotoxin: a synthetic 60-mer peptide with generic traits and enhanced immunogenic properties. Amino Acids 50, 885–895 (2018).
Damsbo, A. et al. A comparative study of the performance of E. coli and K. phaffii for expressing α-cobratoxin. Toxicon 239, 107613 (2024).
Hider, R. C. et al. Molecular conformation of α-cobratoxin as studied by nuclear magnetic resonance and circular dichroism. J. Mol. Biol. 158, 275–291 (1982).
Glanville, J. et al. Snake venom protection by a cocktail of varespladib and broadly neutralizing human antibodies. Cell 188, 3117–3134 (2025).
Harmsen, M. M. & De Haard, H. J. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol. Biotechnol. 77, 13–22 (2007).
Steeland, S., Vandenbroucke, R. E. & Libert, C. Nanobodies as therapeutics: big opportunities for small antibodies. Drug Discov. Today 21, 1076–1113 (2016).
Knudsen, C. et al. Engineering and design considerations for next-generation snakebite antivenoms. Toxicon 167, 67–75 (2019).
Thumtecho, S., Burlet, N. J., Ljungars, A. & Laustsen, A. H. Towards better antivenoms: navigating the road to new types of snakebite envenoming therapies. J. Venom Anim. Toxins Incl. Trop. Dis. 29, e20230057 (2023).
Li, Q. et al. Generation of nanobodies acting as silent and positive allosteric modulators of the α7 nicotinic acetylcholine receptor. Cell Mol. Life Sci. 80, 164 (2023).
Gutiérrez, J. M., Casewell, N. R. & Laustsen, A. H. Progress and challenges in the field of snakebite envenoming therapeutics. Annu. Rev. Pharmacol. Toxicol. 65, 465–485 (2024).
Laustsen, A. H. Guiding recombinant antivenom development by omics technologies. N. Biotechnol. 45, 19–27 (2018).
Williams, D. J., Habib, A. G. & Warrell, D. A. Clinical studies of the effectiveness and safety of antivenoms. Toxicon 150, 1–10 (2018).
Hamza, M. et al. Clinical management of snakebite envenoming: Future perspectives. Toxicon X. 11, 100079 (2021).
Muyldermans, S. Applications of nanobodies. Annu. Rev. Anim. Biosci. 9, 401–421 (2021).
Laustsen, A. How can monoclonal antibodies be harnessed against neglected tropical diseases and other infectious diseases?. Expert Opin. Drug Discov. 14, 1–10 (2019).
Wang, R. et al. Vaccination with a single consensus envelope protein ectodomain sequence administered in a heterologous regimen induces tetravalent immune responses and protection against dengue viruses in mice. Front. Microbiol. 10, 1113 (2019).
Duperret, E. K., Yan, J. & Weiner, D. B. Designing consensus immunogens to break tolerance to self-antigens for cancer therapy. Oncotarget 9, 35513–35514 (2018).
Cao, L. et al. Design of protein-binding proteins from the target structure alone. Nature 605, 551–560 (2022).
Bennett, N. R. et al. Atomically accurate de novo design of single-domain antibodies. bioRxiv 2024.03.14.585103, https://doi.org/10.1101/2024.03.14.585103 (2024).
Bennett, N. R. et al. Improving de novo protein binder design with deep learning. Nat. Commun. 14, 2625 (2023).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Wang, C.-I. A. et al. Isolation and structural and pharmacological characterization of α-Elapitoxin-Dpp2d, an amidated three finger toxin from black mamba venom. Biochemistry 53, 3758–3766 (2014).
Ledsgaard, L. et al. In vitro discovery of a human monoclonal antibody that neutralizes lethality of cobra snake venom. mAbs 14, e2085536 (2022).
Lomonte, B. et al. Venomics of New World pit vipers: Genus-wide comparisons of venom proteomes across Agkistrodon. J. Proteom. 96, 103–116 (2014).
Pardon, E. et al. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 9, 674–693 (2014).
Schofield, D. J. et al. Application of phage display to high throughput antibody generation and characterization. Genome Biol. 8, R254 (2007).
Ledsgaard, L. et al. Advances in antibody phage display technology. Drug Discov. Today 27, 2151–2169 (2022).
Ratanabanangkoon, K. et al. An in vitro potency assay using nicotinic acetylcholine receptor binding works well with antivenoms against Bungarus candidus and Naja naja. Sci. Rep. 8, 9716 (2018).
Laustsen, A. H. Toxin-centric development approach for next-generation antivenoms. Toxicon 150, 195–197 (2018).
Bewick, V., Cheek, L. & Ball, J. Statistics review 12: Survival analysis. Crit. Care 8, 389 (2004).
Krug, M., Weiss, M. S., Heinemann, U. & Mueller, U. XDSAPP: a graphical user interface for the convenient processing of diffraction data using XDS. J. Appl. Cryst. 45, 568–572 (2012).
Kabsch, W. XDS. Acta Cryst. D. 66, 125–132 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. Biol. Crystallogr. 66, 213–221 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Cryst. D. 66, 486–501 (2010).
Salomon-Ferrer, R. et al. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput. 9, 3878–3888 (2013).
Miyamoto, S. & Kollman, P. A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 13, 952–962 (1992).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
El Hage, K., Hédin, F., Gupta, P. K., Meuwly, M. & Karplus, M. Valid molecular dynamics simulations of human hemoglobin require a surprisingly large box size. eLife 7, e35560 (2018).
Gapsys, V. & de Groot, B. L. Comment on ‘Valid molecular dynamics simulations of human hemoglobin require a surprisingly large box size’. eLife 8, e44718 (2019).
Maier, J. A. et al. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Salomon-Ferrer, R., Case, D. A. & Walker, R. C. An overview of the Amber biomolecular simulation package. WIREs Comput. Mol. Sci. 3, 198–210 (2013).
Hub, J. S., de Groot, B. L., Grubmüller, H. & Groenhof, G. Quantifying artifacts in ewald simulations of inhomogeneous systems with a net charge. J. Chem. Theory Comput. 10, 381–390 (2014).
Wallnoefer, H. G., Handschuh, S., Liedl, K. R. & Fox, T. Stabilizing of a globular protein by a highly complex water network: a molecular dynamics simulation study on Factor Xa. J. Phys. Chem. B. 114, 7405–7412 (2010).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Doll, J. D., Myers, L. E. & Adelman, S. A. Generalized Langevin equation approach for atom/solid-surface scattering: Inelastic studies. J. Chem. Phys. 63, 4908–4914 (1975).
Adelman, S. A. & Doll, J. D. Generalized Langevin equation approach for atom/solid-surface scattering: General formulation for classical scattering off harmonic solids. J. Chem. Phys. 64, 2375–2388 (1976).
Acknowledgements
AHL is supported by a grant from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (850974), by a grant from the Villum Foundation (00025302), and by a grant from Wellcome (221702/Z/20/Z). MBV is supported by a Eurotech postdoctoral fellowship from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement (899987). AA has received funding from the Mexican Consejo Nacional de Ciencia y Tecnología, FORDECyT-PRONAII (303045). We acknowledge the EuroHPC Joint Undertaking for awarding us access to MeluXina, Luxembourg. We acknowledge the MAX IV Laboratory for beamtime on the Beamline Biomax under proposal 20220405. Research conducted at MAX IV, a Swedish national user facility, is supported by Vetenskapsrådet (Swedish Research Council, VR) under contract 2018-07152, Vinnova (Swedish Governmental Agency for Innovation Systems) under contract 2018-04969 and Formas under contract 2019-02496. We thank Dr. Ana Gonzalez for her assistance during the data collection. We thank Suzana Siebenhaar for assistance with crystallisation and shipping from the DTU Catalyse4x platform.
Author information
Authors and Affiliations
Contributions
A.D., E.R.T., and A.H.L. conceptualised the idea. A.D., E.R.T., M.B.V., N.J.B., A.H.L., J.P.M., A.V., I.B., T.T., and K.B. designed the methodology. A.D., E.R.T., N.J.B., M.B.V., A.G.M., J.R.L., M.L.F.Q., M.D.O., C.V., and J.P.M. were in charge of the investigation. Visualisation of the results was handled by A.D., E.R.T., N.J.B., J.R.L., M.L.F.Q., and M.D.O. Funding acquisition was managed by M.B.V., A.A., and A.H.L. Project administration was overseen by E.R.T. and A.H.L. Resources were provided by A.A., A.B.W., and A.H.L. Supervision was conducted by E.R.T., A.B.W., and A.H.L. The original draft was written by A.D., A.H.L., and E.R.T. All authors contributed to the review and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent application (WO2025078529) has been submitted based on the work presented in this article with AD, ERT, and AHL named as inventors. All other authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Damsbo, A., Burlet, N.J., Fernández-Quintero, M.L. et al. Structural mechanisms behind the neutralisation of long-chain α-neurotoxins by broadly neutralising VHHs discovered using a consensus antigen. Commun Chem 8, 209 (2025). https://doi.org/10.1038/s42004-025-01600-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-025-01600-4