Abstract
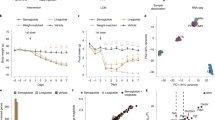
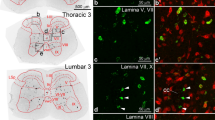
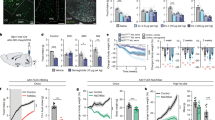
Central glucagon-like peptide-1 (GLP-1), secreted by a distinct population of nucleus tractus solitarius neurons, suppresses feeding but the exact mechanisms of action in the brain remain unclear. Here, we investigate a descending circuit formed by GLP-1 receptor (GLP-1R) neurons in the paraventricular hypothalamic nucleus (PVNGLP-1R) projecting to the dorsal vagal complex (DVC) of the brain stem in mice. PVNGLP-1R→DVC synapses release glutamate and are augmented by GLP-1. Chemogenetic activation of PVNGLP-1R→DVC suppresses feeding. Under an energy deficit (that is, hunger) state, synaptic strength is weaker but is more profoundly augmented by GLP-1R activation than under energy-replete state. In an obese condition, the dynamic synaptic changes in this circuit are disrupted. Optogenetic activation of PVNGLP-1R→DVC projections suppresses food intake energy state dependently, and blocking its synaptic release or ablating GLP-1Rs in the presynaptic neurons impairs metabolic health. These findings indicate that the state-dependent synaptic regulation by GLP-1 in PVNGLP-1R→DVC descending circuit is important for energy homeostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data and datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request. Original histological images are available via figshare at https://doi.org/10.6084/m9.figshare.28602683 (ref. 67).
Code availability
Code used to analyse fibre photometry and open-field data is available via GitHub at https://github.com/RohanSavani/PVN_GLP1R.
References
Sandoval, D. CNS GLP-1 regulation of peripheral glucose homeostasis. Physiol. Behav. 94, 670–674 (2008).
Drucker, D. J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 57, 101351 (2022).
Zhang, C. et al. Area postrema cell types that mediate nausea-associated behaviors. Neuron 109, 461–472 e465 (2021).
He, L. et al. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med. 182, 513–519 (2022).
Brierley, D. I. et al. Central and peripheral GLP-1 systems independently suppress eating. Nat. Metab. 3, 258–273 (2021).
Huang, K. P. et al. Dissociable hindbrain GLP1R circuits for satiety and aversion. Nature 632, 585–593 (2024).
Kim, K. S. et al. GLP-1 increases preingestive satiation via hypothalamic circuits in mice and humans. Science 385, 438–446 (2024).
Acuna-Goycolea, C. & van den Pol, A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J. Neurosci. 24, 8141–8152 (2004).
Liu, J. & Pang, Z. P. Glucagon-like peptide-1 drives energy metabolism on the synaptic highway. FEBS J. 283, 4413–4423 (2016).
Alhadeff, A. L. et al. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology 42, 1471–1479 (2017).
Xu, W. & Sudhof, T. C. A neural circuit for memory specificity and generalization. Science 339, 1290–1295 (2013).
Singh, U. et al. Neuroanatomical organization and functional roles of PVN MC4R pathways in physiological and behavioral regulations. Mol. Metab. 55, 101401 (2022).
Li, M. M. et al. The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653–667 e656 (2019).
Ludwig, M. Q. et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat. Metab. 3, 530–545 (2021).
Grill, H. J. & Hayes, M. R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 16, 296–309 (2012).
Alhadeff, A. L. & Grill, H. J. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R465–R470 (2014).
Thorens, B. et al. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes 42, 1678–1682 (1993).
Xu-Friedman, M. A. & Regehr, W. G. Probing fundamental aspects of synaptic transmission with strontium. J. Neurosci. 20, 4414–4422 (2000).
Grzelka, K. et al. A synaptic amplifier of hunger for regaining body weight in the hypothalamus. Cell Metab. 35, 770–785 e775 (2023).
Liu, J. et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron 96, 897–909 e895 (2017).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Li, C. et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 29, 681–694 e685 (2019).
Cui, G. et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242 (2013).
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Holt, M. K. et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes 68, 21–33 (2019).
Campbell, J. E. & Drucker, D. J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 17, 819–837 (2013).
Holst, J. J. The physiology of glucagon-like peptide 1. Physiol. Rev. 87, 1409–1439 (2007).
Kreymann, B., Williams, G., Ghatei, M. A. & Bloom, S. R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 2, 1300–1304 (1987).
Pannacciulli, N. et al. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage 35, 511–517 (2007).
Sisley, S. et al. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J. Clin. Invest. 124, 2456–2463 (2014).
Wilson-Perez, H. E. et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes 62, 2380–2385 (2013).
Wang, C. Y., Liu, Z., Ng, Y. H. & Sudhof, T. C. A synaptic circuit required for acquisition but not recall of social transmission of food preference. Neuron 107, 144–157 e144 (2020).
Enriquez-Traba, J. et al. Dissociable control of motivation and reinforcement by distinct ventral striatal dopamine receptors. Nat. Neurosci. 28, 105–121 (2025).
Xu, Y. et al. Lateral septum as a melanocortin downstream site in obesity development. Cell Rep. 42, 112626 (2023).
Xu, W. et al. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron 73, 990–1001 (2012).
Kirchgessner, A. L. & Sclafani, A. PVN-hindbrain pathway involved in the hypothalamic hyperphagia-obesity syndrome. Physiol. Behav. 42, 517–528 (1988).
Meeran, K. et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology 140, 244–250 (1999).
Plamboeck, A. et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G1117–G1127 (2013).
Burmeister, M. A. et al. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes 66, 372–384 (2017).
Ludwig, M. & Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 7, 126–136 (2006).
Ludwig, M. et al. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature 418, 85–89 (2002).
Cheng, W. et al. Hindbrain circuits in the control of eating behaviour and energy balance. Nat. Metab. 4, 826–835 (2022).
Martinez de Morentin, P. B. et al. A brainstem to hypothalamic arcuate nucleus GABAergic circuit drives feeding. Curr. Biol. 34, 1646–1656 e1644 (2024).
NamKoong, C. et al. Chemogenetic manipulation of parasympathetic neurons (DMV) regulates feeding behavior and energy metabolism. Neurosci. Lett. 712, 134356 (2019).
Qiu, W. et al. Multiple NTS neuron populations cumulatively suppress food intake. eLife https://doi.org/10.7554/eLife.85640 (2023).
Zhan, C. et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 33, 3624–3632 (2013).
Travagli, R. A. & Anselmi, L. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 13, 389–401 (2016).
Lyu, Q. et al. A brain-to-gut signal controls intestinal fat absorption. Nature 634, 936–943 (2024).
Chang, H. et al. Stress-sensitive neural circuits change the gut microbiome via duodenal glands. Cell https://doi.org/10.1016/j.cell.2024.07.019 (2024).
Orskov, C., Wettergren, A. & Holst, J. J. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand. J. Gastroenterol. 31, 665–670 (1996).
Vahl, T. P., Drazen, D. L., Seeley, R. J., D’Alessio, D. A. & Woods, S. C. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology 151, 569–575 (2010).
Hayes, M. R., Bradley, L. & Grill, H. J. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150, 2654–2659 (2009).
Ly, T. et al. Sequential appetite suppression by oral and visceral feedback to the brainstem. Nature 624, 130–137 (2023).
Pinto, S. et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304, 110–115 (2004).
Horvath, T. L. et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc. Natl Acad. Sci. USA 107, 14875–14880 (2010).
Rossi, M. A. et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science 364, 1271–1274 (2019).
Stinson, S. E. et al. Fasting plasma GLP-1 is associated with overweight/obesity and cardiometabolic risk factors in children and adolescents. J. Clin. Endocrinol. Metab. 106, 1718–1727 (2021).
Faerch, K. et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: The ADDITION-PRO Study. Diabetes 64, 2513–2525 (2015).
Chen, Z. et al. GLP-1R-positive neurons in the lateral septum mediate the anorectic and weight-lowering effects of liraglutide in mice. J. Clin. Invest. https://doi.org/10.1172/JCI178239 (2024).
Erika et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Liu, J. J., Tsien, R. W. & Pang, Z. P. Hypothalamic melanin-concentrating hormone regulates hippocampus-dorsolateral septum activity. Nat. Neurosci. 25, 61–71 (2022).
Dirice, E. et al. Increased β-cell proliferation before immune cell invasion prevents progression of type 1 diabetes. Nat. Metab. 1, 509–518 (2019).
Zhang, R. et al. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol. Psychiatry 22, 733–744 (2017).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Rossi, M. A. et al. Transcriptional and functional divergence in lateral hypothalamic glutamate neurons projecting to the lateral habenula and ventral tegmental area. Neuron 109, 3823–3837 e3826 (2021).
Luis-Islas, J., Luna, M., Floran, B. & Gutierrez, R. Optoception: perception of optogenetic brain perturbat. eNeuro https://doi.org/10.1523/ENEURO.0216-22.2022 (2022).
Wang, L. Histology data related to Wang et al. Nature Metabolism 2025. figshare https://doi.org/10.6084/m9.figshare.28602683 (2025).
Acknowledgements
We thank H. Kwon for help with the CLAMS assay; A. Tahiri and N. Hammed for help on the ITT and GTT experiments; M. Yang for help with part of the histology and J. Wu for the helpful discussions. This study was supported by grants from the Robert Wood Johnson Foundation to the Child Health Institute of New Jersey (RWJF grant no. 74260, Z.P.P.), NIH grant no. NIMH RF1MH120144 (Z.P.P.), NIH grant no. NIDDK R01DK131452 (Z.P.P.), NIH grant no. NIDDK R01DK122167 (A.E.), startup funding from New York Medical College (A.E.), grant no. CIHR PJT 180576 (M.B.W.), grant no. NSERC 72067156 (M.B.W.) NIH grant no. NIDDK R01DK136641 (M.A.R.) and the Whitehall Foundation grant (no. 2022-12-051, M.A.R.). L.W. was supported by the New Jersey Governor’s Council for Medical Research and Treatment of Autism Postdoctoral Fellowship (grant no. CAUT24DFP) and the NExT-Metabolism Pilot Award (grant no. 500301). I.S. was supported by an NSERC/CREATE fellowship.
Author information
Authors and Affiliations
Contributions
L.W. conducted animal surgery, electrophysiology and animal behaviour experiments. R.H.S. conducted fibre photometry recording, data analysis and animal behaviour analysis. Y.L. conducted part of the animal surgery. M.B. conducted part of the electrophysiology recording. J.L.-I. conducted part of the animal behaviour experiments. E.P. conducted part of the histology and analysis. I.S. conducted part of the analysis. W.X. provided the AAV for expressing AAV-CMV-fDIO-TeNT-EYFP and AAV-DIO-SynaptoTag2. A.E., M.B.W., H.J.G. and M.A.R. provided conceptual input on experimental design. Z.P.P. and L.W. conceived the project, designed the experiments, and wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Revati Dewal, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 PVNGLP-1R neurons project to different downstream targets.
a. Experimental paradigm for tracing PVNGLP-1R neuronal outputs using AAV-mediated Cre-dependent expression of SynaptoTag2. The bicistronic expression cassette expresses cell-filling mCherry and a synapse-specific EGFP-synaptobrevin-2 fusion protein (EGFP-Syb2). b. Representative images of the PVN and downstream targets of PVNGLP-1R neurons in the brain of GLP-1R-ires-Cre mice with AAV-SynaptoTag2 injected into the PVN. c. Quantifications of downstream targets of PVNGLP-1R neurons in the brain. EGFP-Syb2 signal is normalized to the fluorescence intensity of the PVH (n = 4 mice). d. Experimental paradigm for retrograde tracing of DVC inputs from the GLP-1R expressing neurons. AAVrg-DIO-EYFP was injected into the DVC in GLP-1R-ires-Cre mice. e. Representative image of retrogradely labeled PVNGLP-1R →DVC neurons. f. Quantification of PVNGLP-1R→DVC neurons along the anterior to posterior axis (n = 3 mice). Data are presented as mean ± SEM. 3 V: the third ventricle; cc: central canal; scp: superior cerebellar peduncle.
Extended Data Fig. 2 PVNGLP-1R→DVC neurons do not have apparent collateral projections.
a. Experimental paradigm using AAVrg-DIO-Flpo and AAV-fDIO-EYFP to trace PVNGLP-1R →DVC neuronal projections. b. Representative images of PVNGLP-1R →DVC neurons using the strategy depicted in (a).(n = 3 mice) c. Experimental paradigm for dual-color retrograde tracing using AAVrg-DIO-EYFP injected into the DVC and AAVrg-DIO-tdTomato into the LPBN of GLP-1R-ires-Cre mouse. d. Images showing retrogradely labeled PVNGLP-1R neurons using the strategy depicted in (c) (n = 1 mouse). e. Experimental paradigm for dual-color retrograde tracing using AAVrg-DIO-EYFP into the DVC region and AAVrg-DIO-tdTomato into the LC region in GLP-1R-ires-Cre mouse. f. Images showing retrogradely labeled PVNGLP-1R neurons via the strategy depicted in (e) (n = 1 mouse).
Extended Data Fig. 3 GLP-1R mediated signaling enhances DVC neuron synaptic inputs.
a. Representative image of a recorded DVC neuron labeled with neurobiotin. DMV neurons were visualized with ChAT immunostaining (n > 3 mice). b. Representative traces of sEPSCs with or without Exn-4 (100 nM). c. Pooled data of frequency and amplitude of sEPSCs recorded in DVC neurons (frequency: two-tailed Wilcoxon matched-pairs signed rank test, p < 0.0001; amplitude: two-tailed Wilcoxon matched-pairs signed rank test, p = 0.5876; n = 35 cells/12 mice). d. The vast majority of DMV preganglionic motor neurons (ChAT-positive) do not express GLP-1R. GLP-1R-expressing neurons are visualized via ChR2-EYFP expression. e. Enlarged view of the area shown in panel d. n = 3 mice. f. Quantification of AMPAR-mediated synchronous oEPSC (that is, first peak) amplitudes before and after Exn-4 application with 5 mM Sr2+in external ACSF (two-tailed paired t-test, t(10) = 2.935, p = 0.0166, n = 10 cells/3 mice). Note that these traces are the same from Fig. 1n, but to emphasize the synchronous release phase for quantifications. Data are presented as mean ± SEM. ∗p < 0.05; ∗∗∗∗p < 0.0001.
Extended Data Fig. 4 Chemogenetic activation of PVNGLP-1R→DVC neurons via hM3Dq suppresses food intake.
a. Food intake consumption after i.p. saline injection (control) in GLP1R-ires-Cre mice expressing hM3Dq- or control-virus in PVNGLP-1R→DVC neurons during the dark cycle (related to Fig. 2e) (two-way ANOVA, Group effect: F(1, 14) = 2.492, p = 0.1368; Time effect: F(3, 42) = 89.98, p < 0.0001; interaction: F(3, 42) = 0.6374, p = 0.5952, control n = 9 mice, hM3Dq n = 7 mice). b. Food intake upon activation of PVNGLP-1R→DVC neurons in a fasted-refeeding paradigm during the light cycle (two-way ANOVA, main effect of Group: F(1, 14) = 10.83, p = 0.0054; main effect of Time: F(1.570, 21.98) = 60.54, p < 0.0001; interaction between Group and Time: F(3, 42) = 1.921, p = 0.1408; Sidak’s multiple comparisons test vs. control: 30 min p = 0.0056; 60 min p = 0.0188; 120 min p = 0.0296; 180 min p = 0.0346; control n = 9 mice, hM3Dq n = 7 mice). c. Food intake consumption upon activation of PVNGLP-1R→DVC neurons in the fed light cycle (two-way ANOVA, Group effect: F(1, 14) = 6.781, p = 0.0208; Time effect: F(1.903, 26.64) = 21, p < 0.0001; interaction: F(3, 42) = 3.899, p = 0.0152; Sidak’s multiple comparisons test vs. control: 30 min p = 0.063; 60 min p = 0.2495; 120 min p = 0.0623; 180 min p = 0.0256; control n = 9 mice, hM3Dq n = 7 mice). d. Glucose tolerance test with or without chemogenetic activation (two-way ANOVA, Group effect: F(1, 14) = 1.663, p = 0.2181; Time effect: F(2.740, 38.36) = 355.1, p < 0.0001; interaction: F(4, 56) = 1.342, p = 0.2659; control n = 9 mice, hM3Dq n = 7 mice). e. Representative traces of animal exploration in the open field with or without chemogenetic activation of PVNGLP-1R→DVC neurons. f. Time spent in the center of the open field (two-tailed t-test, t (16) = 1.617, p = 0.1282; control n = 9 mice, hM3Dq n = 7 mice). g. Total traveled distance in the open field (two-tailed t-test, t(16) = 1.283, p = 0.2205; control n = 9 mice, hM3Dq n = 7 mice). h. Quantification of light-dark box assay for anxiety-like behaviors: time spent in the dark zone (two-tailed t-test, t(16) = 0.1509, p = 0.8822; control n = 9 mice, hM3Dq n = 7 mice). i. Dark zone entries (two-tailed t-test, t(16) = 0.0516, p = 0.9596; control n = 9 mice, hM3Dq n = 7 mice). Data are presented as mean ± standard error of the mean (SEM). ∗p < 0.05; ∗∗p < 0.01.
Extended Data Fig. 5 Fiber Photometry measurement of PVNGLP-1R→DVC neuronal calcium activity in responding to various sensory inputs.
a. Average response of fiber photometry data showing calcium dynamics of PVNGLP-1R→DVC neurons during self-grooming behavior. b. Pooled data (two-tailed paired t-test, t(5) = 2.822, p = 0.0477; n = 5 mice). c. Average response of fiber photometry calcium dynamics of PVNGLP-1R→DVC neurons during tail picking-induced stress d. Pooled data (two-tailed paired t-test, t(5) = 4.109, p = 0.0147; n = 5 mice). e. Heatmap showing calcium dynamics of PVNGLP-1R→DVC neurons during different food/object tea ball drop stimuli across different energy states. f. Quantitation of the areas under the curve (AUC) of calcium response (one-way ANOVA, F(3,16) = 4.383, p = 0.0197, n = 5 mice). Data are presented as mean ± SEM. ∗p < 0.05.
Extended Data Fig. 6 Electrophysiological characterization of PVNGLP-1R→DVC neurons under different energy states.
a. Experimental paradigm. b. Intrinsic electrophysiological characterization of PVNGLP-1R→DVC neurons. Summary of capacitance (two-tailed t-test, t(52) = 0.6357, p = 0.5279; Fed n = 24 cells/3 mice, Fasted n = 28 cells/3 mice). c. Input resistance (two-tailed Mann-Whitney test, p = 0.7134; Fed n = 24 cells/3 mice, Fasted n = 28 cells/3 mice). d. Resting membrane potential (two-tailed Mann-Whitney test, p = 0.0049; Fed n = 24 cells/3 mice, Fasted n = 28 cells/3 mice). e. Representative traces of spontaneous action potentials (sAPs). f. Quantification of sAP frequency (two-tailed Mann-Whitney test, p = 0.3148; Fed n = 24 cells/3 mice, Fasted n = 28 cells/3 mice). g. Representative traces of neurons responding to ramping current injection. Insert show the current injection protocol. h. Quantification of ramping current injection action potential firing number (two-tailed Mann-Whitney test, p = 0.3461; Fed n = 25 cells/3 mice, Fasted n = 27 cells/3 mice). i, Representative traces of neurons in response to stepped current injection. Insert shows the current injection protocol. j. Plot of the number of APs as a function of injected current (two-way ANOVA, Group effect: F(1, 48) = 0.7961, p = 0.3767; Time effect: F(1.340, 64.33) = 98.47, p < 0.0001; interaction: F(5, 240) = 0.3641, p = 0.8728; Fed n = 24 cells/3 mice, Fasted n = 26 cells/3 mice). Data are presented as mean ± SEM. ∗∗p < 0.01.
Extended Data Fig. 7 Electrophysiological characterization of PVNGLP-1R→DVC neurons in HFD-induced obesity animals.
a. Experimental paradigm. b. Body weight of control and HFD-induced obese animals (two-tailed t-test, t (11) = 4.671, p = 0.0012; control n = 5 mice, HFD n = 6 mice). c. Intrinsic electrophysiological characterization of PVNGLP-1R→DVC neurons. Summary of capacitance (c) (two-tailed Mann-Whitney test, p = 0.2638; control n = 20 cells/3 mice, HFD n = 32 cells/3 mice). d. Resting membrane potential (RMP) (two-tailed t-test, t (51) = 3.212, p = 0.0023; control n = 20 cells/3 mice, HFD n = 31 cells/3 mice). e. Representative traces of spontaneous action potentials (sAPs). f. Pooled data of sAP frequency (two-tailed t-test, t (20) = 2.042, p = 0.0561; control n = 6 cells/3 mice, HFD n = 14 cells/3 mice). g. Representative traces of neurons in response to ramp current injection. Inset shows the current injection protocol. h. Quantification plot of ramping current injection-induced AP firing number (two-tailed Mann-Whitney test, p = 0.5784; control n = 20 cells/3 mice, HFD n = 30 cells/3 mice). i. Representative traces of neurons in response to stepped current injection. Inset shows the current injection protocol. j. Plot of the number of APs as a function of injected current (two-way ANOVA, Group effect: F(1, 48) = 0.1073, p = 0.7447; Time effect: F(1.362, 64.97) = 157.7, p < 0.0001; interaction: F(10, 477) = 0.7476, p = 0.6795; control n = 20 cells/3 mice, HFD n = 30 cells/3 mice). Data are presented as mean ± SEM. ∗∗p < 0.01.
Extended Data Fig. 8 Liraglutide augments PVNGLP-1R→DVC synaptic release in HFD-fed mice.
a. Representative traces of AMPAR-oEPSCs and NMDAR oEPSCs in HFD-fed mice with or without i.p. injection of 400 µg/kg liraglutide. b. Pooled data of NMDAR-oEPSCs (two-tailed t-test, t (43) = 2.561, p = 0.0153; HFD n = 18 cells/3 mice, Liraglutide n = 25 cells/3 mice). c. AMPAR/NMDAR oEPSCs ratio (two-tailed t-test, t(43) = 1.391, p = 0.1718; HFD n = 18 cells/3 mice, Liraglutide n = 18 cells/3 mice). Data are presented as mean ± SEM. ∗p < 0.05.
Extended Data Fig. 9 Impact of optogenetic activation of PVNGLP-1R projections in the DVC on food intake and aversion.
a. Quantification of fasted-refeeding normalized chow intake for ChR2/EYFP mice (normalized to EYFP/ChR2 off average chow intake, 470 nm 20 Hz, 1 s on, 0.5 s off; two-tailed Mann-Whitney test, p = 0.0146, EYFP n = 6 mice, ChR2 n = 7 mice). b. Experimental paradigm for testing sucrose licking in mouse in a head-fixed format. Animals were injected with AAV-ChR2 or EYFP into the PVN, and fiber optics were implanted in the DVC. c. Quantification of body weight changes after 12 weeks of HFD-DIO (two-tailed paired t-test, t(13) = 4.257, p = 0.0011; EYFP n = 6 mice, ChR2 n = 7 mice). d. Raster plots of licks (consumption of 10% sucrose) of energy-replete (fed) ChR2 and EYFP mice (post 12 weeks HFD) after pre (5 min), during blue light photostimulation (470 nm 20 Hz, 1 s on, 0.5 s off, 5 min), and 5 min post optogenetic stimulation. e. Normalized sucrose licking rates before, during and after optogenetic activation (two-way ANOVA, Group effect: F (3, 22) = 1.335e + 017, p < 0.0001; Time effect: F (1.294, 28.47) = 13.27, p = 0.0005; interaction between Group and Time: F (6, 44) = 0.5878, p = 0.7381; Sidak’s multiple comparisons test vs. EYFP Light On ChR2 p = 0.0003; EYFP n = 6 mice, ChR2 n = 7 mice). f. Representative heatmaps depicting the time spent in the real-time place preference task. The colorbar denotes time spent in an area normalized to the maximum time spent. g. Preference of EYFP and ChR2 mice for the photostimulation chamber (470 nm, 20 Hz) (two-tailed Mann-Whitney test, p = 0.9452; EYFP n = 6 mice, ChR2 n = 7 mice). Data are presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Illustration in b adapted from SciDraw under a Creative Commons license CC BY 4.0.
Extended Data Fig. 10 Comprehensive metabolic analyses of animals after inactivation of PVNGLP-1R→DVC synaptic release and daily food consumption.
a. Energy expenditure (EE) (two-way ANOVA, the main effect of Group: F(1, 14) = 0.01004, p = 0.9216; main effect of Time: F(6.449, 90.29) = 4.545, p = 0.0003; interaction between Group and Time: F(159, 2226) = 1.054, p = 0.3132; control n = 7 mice, TeNT n = 9 mice). b. The volume of carbon dioxide produced (VCO2) (two-way ANOVA, the main effect of Group: F(1, 14) = 0.1053, p = 0.7503; main effect of Time: F (6.291, 88.08) = 4.765, p = 0.0002; interaction between Group and Time: F(159, 2226) = 0.9634, p = 0.6126; control n = 7 mice, TeNT n = 9 mice). c. Respiratory exchange ratio (RER) (two-way ANOVA, Group effect: F (1, 14) = 4.844, p = 0.0450; Time effect: F(3.463, 48.49) = 2.22, p = 0.0893; interaction: F (159, 2226) = 0.442, p > 0.9999; control n = 7 mice, TeNT n = 9 mice). d. Oxygen consumed (VO2) (two-way ANOVA, Group effect: F(1, 14) = 0.003172, p = 0.9559; Time effect: F(6.303, 88.25) = 3.659, p = 0.0023; interaction: F(119, 1666) = 1.157, p = 0.1256; control n = 7 mice, TeNT n = 9 mice). e. Average locomotor activity of TeNT and control (GFP) animals (two-way ANOVA, Group effect: F(1, 12) = 1.862, p = 0.1974; Time effect: F(8.698, 104.4) = 2.654, p = 0.0089; interaction between Group and Time: F(159, 1908) = 1.101, p = 0.1919; control n = 6 mice, TeNT n = 8 mice). f. Experimental paradigm for inactivating PVNGLP-1R→DVC synaptic release. Cre-dependent expression of Flpo and Flpo-dependent expression of TeNT in PVNGLP-1R →DVC neurons. g. Average daily food intake of EYPF HFD-fed obese animals’ baseline for 2 days and after i.p. injection of 200 μg/kg liraglutide for 5 days (two-tailed paired t-test, t(7) = 4.658, p = 0.0035; EYFP n = 7 mice). h. Average daily food intake consumption of TeNT HFD-fed obese animals’ baseline for 2 days and after i.p. injection of 200 μg/kg liraglutide for 5 days (two-tailed paired t-test, t(7) = 5.595, p = 0.0014; TeNT n = 7 mice). Data are presented as mean ± SEM. ∗∗p < 0.01.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Savani, R.H., Lu, Y. et al. State-dependent central synaptic regulation by GLP-1 is essential for energy homeostasis. Nat Metab 7, 1443–1458 (2025). https://doi.org/10.1038/s42255-025-01305-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s42255-025-01305-x