Abstract
Chronic diseases affecting the cardiovascular system, diabetes mellitus, neurodegenerative diseases, and various other organ-specific conditions, involve different underlying pathological processes. However, they share common risk factors that contribute to the development and progression of these diseases, including air pollution, hypertension, obesity, high cholesterol levels, smoking and alcoholism. In this review, we aim to explore the connection between four types of diseases with different etiologies and various risk factors. We highlight that the presence of risk factors induces regulated necrotic cell death, leading to the release of damage-associated molecular patterns (DAMPs), ultimately resulting in sterile inflammation. Therefore, DAMP-mediated inflammation may be the link explaining how risk factors can lead to the development and maintenance of chronic diseases. To explore these processes, we summarize the main cell death pathways activated by the most common life-threatening risk factors, the types of released DAMPs and how these events are associated with the pathophysiology of diseases with the highest mortality.

Various risk factors, such as smoking, air pollution, alcoholism, hypertension, obesity, and high cholesterol levels induce regulated necrosis. Subsequently, the release of DAMPs leads to chronic inflammation, which increases the risk of many diseases, including those with the highest mortality rates.
Similar content being viewed by others
Facts
-
Environmental, physiological or behavioral risk factors can induce regulated necrotic cell death and DAMP production.
-
DAMP-related sterile inflammation plays a role in the development and progression of cardiovascular diseases, neurodegenerative diseases, diabetes or alcoholic and non-alcoholic liver diseases.
-
Current anti-inflammatory treatments do not target the root cause of cell death processes and the release of DAMPs.
Open questions
-
To what extent can the harmful effects of risk factors be mitigated by regulating necrotic cell death?
-
To what extent do the DAMP patterns of pathologies associated with sterile inflammation overlap?
-
In which diseases can drugs targeting the pathomechanism of sterile inflammation be used, such as drugs that inhibit the effects of regulated cell death or DAMPs?
Introduction
Over 50% of global deaths are associated with a fairly limited spectrum of conditions, including cardiovascular and neurodegenerative diseases, diabetes mellitus, autoimmune disorders, as well as various organ-specific diseases and certain types of cancers. Extensive research conducted over the past few decades has demonstrated that the pathogenesis of each of these disorders is tightly associated with sterile inflammation [1].
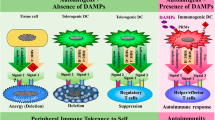
The inflammatory response is primarily triggered by cells of innate immunity upon activation of pattern recognition receptors (PRRs). PRRs detect both pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Accordingly, sterile inflammation, in the absence of pathogens, is predominantly triggered by DAMPs released by cell or tissue damage. Sterile inflammation is a common phenomenon that occurs in various contexts, including autoimmune and autoinflammatory disorders, as a hallmark feature of tumors, in response to sports injuries, and as a consequence of certain genetic deficiencies. During the resulting inflammation, the activation of effector cells of the immune system leads to tissue degeneration, which can be compensated by the subsequent tissue regeneration steps [2].
The release of DAMPs typically occurs when cells undergo necrosis, and consequent membrane rupture leads to the leakage of intracellular components, generating danger signals [3]. In addition to the primary necrosis, several regulated necrotic cell death modalities have been identified, such as necroptosis, pyroptosis, ferroptosis and parthanathos. Although different forms of cell death can be triggered by a wide spectrum of stimuli, all are characterized by a loss of membrane integrity and the consequent necroinflammation [4].
Risk factors, including environmental (air pollution), physiological (high blood pressure, obesity, high blood cholesterol), and even behavioral risk factors (smoking, alcoholism), are all closely related to life-threatening disorders. It is not clear how each of the different lifestyle risk factors affects diseases with such different pathomechanisms, but a common characteristic of all these factors is that they initiate sterile inflammation. The effects of different risk factors can converge in necrotic cell death, which leads to DAMP release and then inflammation. Since inflammation is not only the consequence of cell death, but can also its cause through tissue destruction, the process can turn into a chronic reaction.
While the impact of health hazards on the most lethal diseases is well documented, how these factors influence necrotic cell death and DAMP production is poorly understood. We first summarize our knowledge of how different life-threatening risk factors influence the necrotic cell death pathways and then review how regulated necrotic cell death and DAMP-related chronic inflammation can lead to the development of high-mortality diseases.
Although cancer is also one of the leading causes of death, and about 25% of cancers are related to chronic inflammation [5], we do not cover this disease in our article. Specific processes characteristic of the pathomechanism of tumors, such as mutagenesis, abnormal proliferation, and metastasis, make it difficult to compare cancer with the other diseases mentioned. Comprehensive reviews of the complex roles of chronic inflammation, cell death and DAMPs in cancer processes are available to readers [6,7,8,9].
Cell death pathways and DAMP release in chronic inflammation
In recent decades, several regulated necrotic cell death modalities have been described (Fig. 1). During the immune response, activation of cytotoxic cells through death receptors, detection of pathogens via pattern recognition receptors, integrin signaling, immune complexes, or various cytokines can all stimulate necroptosis, pyroptosis, and netosis [4]. In addition to immune system-related stimuli, many extra- or intracellular disturbances can trigger regulated necrosis (Fig. 2), such as ion imbalance (pyroptosis), pH dysregulation (alkaliptosis), ATP depletion (parthanathos), generation of reactive oxygen species (parthanathos, pyroptosis, mitochondrial permeability transition), redox imbalance (ferroptosis), metals overload (ferroptosis, cuproptosis), lipid peroxidation (ferroptosis), nitrogen species formation (parthanatos), hypoxia (parthanatos) and exposure to various drugs, chemical compounds or radiation [4, 10].
Apoptosis is carried out by regulated mechanisms triggered by various stress signals through the intrinsic pathway, and death receptors via the extrinsic pathway. In the intrinsic pathway, cytochrome-C is released from the mitochondrial intermembrane space, leading to the activation of caspase-9. In the extrinsic pathway, ligand binding to death receptors triggers the activation of caspase-8 and -10. Initiator caspases (caspases-9, -8, and -10) activate effector caspases, such as caspases-3 and -7, which carry out the controlled, energy-dependent dismantling of the cell. Apoptosis is characterized by cell shrinkage and membrane blebbing, followed by the formation of apoptotic bodies. These apoptotic bodies are typically engulfed by efferocytes or neighboring cells, preventing the release of damage-associated molecular patterns (DAMPs) and minimizing inflammation. Necroptosis is a form of regulated necrosis, which is activated by the necrosome containing Receptor-interacting protein kinase 1 (RIPK1) and RIPK3 (after stimulation of death receptors). Necrosome activates mixed lineage kinase domain-like protein (MLKL) by phosphorylation, which results in its oligomerization and translocation to the plasma membrane. MLKL pores compromise membrane integrity, allowing an increased influx of cations, leading to osmotic imbalance. This imbalance causes cell swelling, membrane rupture, and the subsequent release of DAMPs into the extracellular space. During pyroptosis, caspases—primarily caspase-1, which is activated by NOD-like receptors (NLR) organized inflammasomes, or by caspases-4/5 (homologous to caspase-11 in mice), which are activated by lipopolysaccharide (LPS)—cleave gasdermin proteins to initiate cell death. The ASC adapter protein also promotes the activation of caspase-1 upon various stimuli. After the cleavage of gasdermin proteins their N-terminal domains oligomerize and create pores in the plasma membrane leading to cell death. These pores are not ion-selective, thus do not lead to osmotic shock. Additionally, activated caspase-1 cleaves the pro-forms of the inflammatory cytokines IL-1β and IL-18 into their mature forms, facilitating their release. Ferroptosis is a regulated cell death induced by iron-dependent lipid peroxidation. It can occur due to increased ROS levels (which may be a consequence of iron-mediated Fenton reactions) or a reduced capacity of the cell’s antioxidant system. The oxidation of phospholipids containing polyunsaturated fatty acids increases lipid peroxide levels, leading to enhanced membrane permeability and potential rupture. The oxidative balance of the cells is maintained by the cooperation of the xCT transporter and glutathione peroxidase 4 (GPx4). xCT ensures the transport of cysteine, a glutathione precursor, into the cell, while GPx4 protects cells from oxidative stress by catalyzing the reduction of lipids and other organic hydroperoxides to their corresponding alcohol forms, using glutathione as a substrate. Parthanatos is initiated by hyperactivation of PARP1, a key enzyme in the DNA damage response, leading to depletion of the PARP substrates NAD+ and ATP. As a result, the cells’ energy source is exhausted. In addition to cellular energy deficiency, cell death is accompanied by the release of apoptosis-inducing factor (AIF) from the mitochondria, which is translocated to the nucleus after parylation by the PARP enzyme and induces Macrophage migration inhibitory factor (MIF)-dependent DNA fragmentation. Activation of mitochondrial permeability transition (MPT) is a consequence of intracellular stress signals that lead to dysregulation of the inner mitochondrial membrane. This can cause osmotic rupture of both mitochondrial membranes, resulting in cyclophilin D (CYPD)-dependent necrosis. CYPD in conjunction with a voltage dependent anion channel (VDAC) and adenine-nucleotide translocase (ANT) forms the permeability transition pore complex. Under oxidative stress, low ATP levels, or high Ca2+ concentrations, this complex forms open pores between the inner and outer mitochondrial membranes. This results in mitochondrial swelling and allows unregulated diffusion of molecules, ions and apoptogenic proteins. For a deeper understanding of signaling pathways, we recommend the review of the Cell Death Nomenclature Committee [4]. Apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC), Apoptosis-inducing factor mitochondria associated 1 (AIF), Cyclophilin D (CyPD), cystine/glutamate antiporter (xCT), damage–associated molecular pattern (DAMP), Gasdermin (GSDM), glutathione GSH, glutathione peroxidase (GPx4), inner mitochondrial membrane (IMM), Macrophage migration inhibitory factor (MIF), mitochondrial permeability transition (MPT), Mixed lineage kinase domain-like protein (MLKL), NOD-like receptor (NLR), oxidized glutathione (GSSG), pattern recognition receptor (PRR), Poly(ADP-ribose) polymerase 1 (PARP-1), Receptor-interacting serine/threonine-protein kinase 1 (RIPK1), reactive oxygen species (ROS).
Under sterile conditions, tissue or cellular damage leads to various forms of necrotic cell death, which trigger DAMP-mediated inflammation. DAMPs are endogenous molecules that are not recognized by the immune system under normal physiological conditions. For example, DAMPs characteristic of disrupted extracellular matrix include versican, biglycan, decorin, while intracellular components released from necrotic cells can include ATP, HMGB1, histons, nuclear DNA, mitochondrial factors (such as N-formyl peptides, mtDNA), lysosomal proteins (e.g., Cold-inducible RNA-binding protein), and endoplasmic reticulum stress signals (such as PKR-like endoplasmic reticulum kinase or calreticulin), all of which can act as alarm signals [11].
Particular regulated necrotic cell death processes may differ in their molecular background, pore-forming proteins, and the pattern of released molecules [10]. NINJ1-mediated membrane disruption is characteristic of the late stages of many necrotic processes [12]. Therefore, DAMP release may differ in individual cell death processes, and possibly even in their phases.
In addition, DAMPs can act synergistically with PAMPs, but they are also responsible for the immune response independent of infection. As a result of tissue or cell damage, DAMPs activate the immune response, especially the components of innate immunity, alerting it to unwanted pathological reactions [13]. They can stimulate the production of chemokines to attract the cells of the immune system to the site of danger and facilitate the production of inflammatory cytokines [14]. The inflammatory response mediated by DAMPs ultimately translates into subsequent tissue regeneration [11, 15].
The role of individual DAMPs in sterile inflammation has been investigated in various in vivo models. Administration of recombinant DAMPs leads to sterile inflammation and some human pathologies are directly linked to elevated DAMP levels. Recombinant IL-1α, HMGB1 and S100A9 exhibited immune adjuvant activity, increased the overall incidence of arthritis and induced an inflammatory response in the lung in mouse models, respectively [16,17,18]. Overproduction of IL-1β results in systemic inflammatory diseases, and elevated serum uric acid leads to gout and nephrolithiasis in humans [19, 20].
Neutralization or inactivation of specific DAMPs may be beneficial in several inflammatory diseases. Anti-HMGB1 was shown to be protective in arthritis models [21], and in high-fat diet-induced atherosclerosis in ApoE-/- mice [22]. Canakinumab, a monoclonal antibody that targets IL-1β, reduced inflammatory markers and cardiovascular events in patients with prior myocardial infarction [23]. Neutralization of extracellular ATP with apyrase reduced systemic injury in an experimental acute pancreatitis model [24]. Administration of RNase1 significantly reduced the incidence of myocardial infarction [25], while DNase-1 treatment reduced the inflammatory response in a rat ischemia-reperfusion model [26].
Release of DAMPs exacerbates various inflammatory processes, and persistent activation of cell death pathways can lead to chronic inflammation caused by DAMP production unless the resolution mediators become dominant [11, 27]. This process is also involved in the development of diseases such as cardiovascular diseases, neurodegenerative disorders, diabetes or alcoholic and non-alcoholic liver diseases (Table 1).
Risk factor-induced cell death processes and DAMP secretion
It is well known that environmental, physiological and behavioral risk factors such as air pollution, high blood pressure, obesity, cholesterol levels, smoking or intensive alcohol consumption significantly contribute to the increased risk of cardiovascular and neurodegenerative diseases, diabetes and various organ-specific disorders. These life hazards are known to directly contribute to the exacerbation of disorders by inducing cell death and DAMP secretion leading to chronic inflammation, as detailed in the subsequent sections and Table 1.
Air pollution-related cell death processes and DAMP secretion
Particulate matter (PM) and/or adsorbed chemicals are risk factors for the development of pulmonary diseases and have become a major concern worldwide. Air pollution results in endoplasmic reticulum stress and mitochondrial dysfunction, promoting pancreatic β-cell apoptosis, which impairs insulin synthesis over the long-term [28]. Necroptosis can also be induced by heavy metals, as CdCl2 exposure has been shown to induce necroptosis and hepatic injury both in vivo and in vitro [29]. Exposure of A549 epithelial or RAW264.7 macrophage cell lines to PM with an aerodynamic diameter of less than 2.5 μm (PM2.5) or black carbon, a key component of PM2.5, caused necroptosis and consequently enhanced inflammation [30, 31]. Necroptosis also contributes to PM exposure-related inflammation during ocular surface injuries [32].
Various factors of environmental pollution, like water-soluble inorganic ions, carbonaceous materials, and heavy metals in PM2.5, activate the NLRP3 inflammasome and pyroptosis. Inflammatory responses associated with elevated levels of IL-1β in bronchoalveolar lavage fluid (BALF) were shown in mice exposed to PM2.5. Wood smoke particulate matter activates the NLRP3 inflammasome by regulating extracellular ATP levels and mediates pyroptosis in human bronchial epithelial 16-HBE cells [33]. PM2.5 significantly increased mitochondrial dysfunction, causing oxidative damage and GPX-dependent ferroptotic cell death in neurons [34]. Additionally, PM2.5 enhances the ferroptosis sensitivity of HUVEC cells, inducing iron overload, lipid peroxidation, GSH depletion, and redox imbalance [35]. Heavy metal components of air pollution, such as As, Pb, and Cd can also induce redox imbalance causing ferroptosis in the liver, central nervous system, and the kidneys [36].
Inhaling air pollutants disrupts the epithelial barrier, causing oxidative stress [37]. PM2.5 directly activates NLRP3 by various substances, such as elevated extracellular ATP [38]. Similarly, PM2.5-induced ROS production and systemic oxidative stress increase DAMP production, further activating the NLRP3 inflammasome and IL-1 family cytokine production [37]. Consequently, elevated IL-1β and IL-33 expression regulate airway barrier function [39]. PM2.5-induced cell death causes the secretion of HMGB1, mtDNA, and N-formyl peptides triggering inflammation in the airways of mice [39, 40]. In motor vehicle extract-treated rats, a larger ratio of HMGB1 and RAGE-positive bronchial epithelial cells were detected in the lungs and in vitro in the supernatant of a human bronchial epithelial cell line [41]. Furthermore, organic dust was found to cause sustained neuroinflammation in in vivo mice study involving HMGB1 and RAGE signaling [42].
Blood pressure-related cell death processes and DAMP secretion
Hypertensive stimuli such as altered mechanical forces, increased velocity of blood flow and friction lead to endothelial dysfunction and induce multiple modes of vascular cell death [43,44,45]. An increase in blood pressure leads to ER stress resulting in apoptosis of cardiomyocytes with increased caspase-3 expression in a rat model [46]. RIPK3-mediated necroptosis and its role in the progression of inflammation have also been identified in the pathogenesis of pulmonary arterial hypertension (PAH) [47, 48].
Both canonical and non-canonical pyroptosis pathways have been implicated in hypertension. Caspase-11 deficiency in vivo and caspase-4 silencing in the human pulmonary arterial endothelial cells in vitro alleviated the development of PAH [49], and inhibition of Caspase-1 also attenuated the pathogenesis of PAH [50].
The role of ferroptosis in hypertension is controversial. PAH is characterized by decreased serum iron levels. However, whether iron deficiency contributes to the disease or is merely a consequence of it remains debated [51]. Solute carrier family member 7, 11 (SLC7A11), the Xc subunit of the cystine/glutamate antiporter system which inhibits ferroptosis, is upregulated in PAH patients and hypoxia-induced PAH rat models [52]. On the contrary, ferroptosis was observed in pulmonary artery endothelial cells from a monocrotaline-induced PH in a rat model and was characterized by the upregulation of the labile iron pool and lipid peroxidation, and the downregulation of GPX4 expression.
It’s suggested that, high blood pressure and pro-hypertensive factors induce vascular injury. In patients with idiopathic or congenital heart disease-related PAH, HMGB1 release is elevated [53, 54]. Mitochondrial dysfunction also plays a role in PAH, activating inflammasomes [53, 55]. Inflammasome activation results in the production of interleukin IL-1β and IL-18, both of which are key biomarkers of PAH. Accordingly, clinical trials using IL-1β blockade have shown reductions in recurrent cardiac events and inflammation [53]. Moreover, increased serum levels of S100B are detected in hypertension [56], and S100a8/a9 produced by neutrophils was identified as an initial proinflammatory factor during acute hypertension [57]. Hypertension and mechanical stress contribute to endothelial injury which further accelerates the development of atherosclerosis via the activation of oxidative stress, leading to lipid oxidation and tissue damage [58,59,60]. Markedly, one of the most common risk factors for brain aging is arterial hypertension, causing endothelial damage that disrupts the blood-brain barrier, and is associated with the production of ROS and β-amyloid, which causes neuroinflammation [61].
Obesity-related cell death processes and DAMP secretion
Obesity causes low-grade chronic inflammation due to the release of adipokines and cytokines from fat cells. Also, the destruction of fat cells is a major factor triggering the release of inflammatory substances, attracting macrophages around the necrotizing cells [62].
Both the intensity of the death receptor- and mitochondria-mediated apoptotic pathways increased in adipocytes from humans and mice with obesity and insulin resistance [63], but the role of necrotic cell death in obesity has also been intensively investigated. Functionally, increased RIPK1 expression in adipose tissue has been proven, however, the causative role of necroptosis in obesity remains unclear [64, 65].
The NLRP3 inflammasome contributes to obesity-induced inflammation, and ablation of NLRP3 in mice prevents obesity-induced inflammasome activation in fat depots [66]. Caspase-1 protein levels are more abundantly expressed in the adipose tissue of hyperglycemic ob/ob animals and high glucose levels activate Caspase-1 in human and murine adipose tissue [67]. Nonetheless, GSDMD deficiency does not protect mice against HFD-induced adipose tissue inflammation [68]. AGEs contribute to the acceleration of the development of atherosclerosis through activation of the NLRP3-ASC inflammasome pathway in a mouse monocyte cell line, and by increasing the level of HMOX1 (a member of the ferroptotic pathway) in primary human aortic endothelial cells [69,70,71]. Ferroptosis is intricately linked to obesity [72], and it is characterized by iron buildup, lipid peroxide hyperplasia, GPX4 inhibition, and systemic Xc inhibition in the adipose tissue under obesity conditions [73]. Several studies have shown that overexpression of GPX4 is protective against adipocyte inflammation, while iron chelators reduce the generation of inflammatory agents and the infiltration of adipose tissue macrophages [74].
In obesity, apoptotic, necroptotic, and pyroptotic adipocytes are the major sources of DAMPs in visceral fat tissue [75]. The mechanically stressed adipocytes secrete factors, like HMGB1 that attract immune cells, and favor M1-like macrophage differentiation. HMGB1 levels are increased in adipose tissue in obese individuals compared to those of normal-weight [76]. Moreover, it was shown that the deficiency of its receptor, TLR4, decreases inflammation and insulin resistance in adipose tissue. Furthermore, adipocyte death raises free fatty acid levels, causing mitochondrial dysfunction, mtDNA release, ROS accumulation, and inflammasome activation in endothelial cells [77, 78]. Additionally, DNA released by white adipose tissue can enhance inflammation together with secreted HMGB1 [77]. As well as, hyperglycemia causes ROS production, which promotes the development and progression of diabetes through the induction of M1-like pro-inflammatory macrophages [79].
High cholesterol level-related cell death processes and DAMP secretion
Cholesterol accumulation in macrophages and other immune cells mediates cell death, both apoptotic and necrotic processes, and promotes inflammatory responses as well [80, 81]. In addition to cholesterol-induced intrinsic [82], and extrinsic apoptosis [81], necroptotic death also plays a role in the development of atherosclerosis. While elevated levels of phosphorylated RIPK3 were detected in atherosclerotic plaques, knockdown of RIPK3 showed delayed mortality compared to apolipoprotein E (ApoE) single knockout mice [83]. Moreover, ox-LDL and cholesterol crystals induce NLRP3 inflammasome activation, upregulate the expression of various necroptotic molecules (RIPK3, MLKL), and activate ferroptotic (HMOX-1) protein [84,85,86,87].
The cholesterol accumulation disrupts lysosomal membrane structure leading to cathepsin B release, which activates NLRP3 and induces pyroptosis [88]. Furthermore, cholesterol crystals, together with the complement system, also induce NLRP3 activation [89]. However, cholesterol directly enhances ferroptosis resistance in hematopoietic stem cells [90].
Additionally, extracellular free cholesterol was demonstrated to enhance ROS production [91], and oxDNA levels are significantly increased in the plasma of obese individuals with NASH [78]. In HFD-fed obese mice, obesity induced blood-brain barrier damage, microglial activation, elevated proinflammatory cytokines, and increased oxidative stress [92]. Another risk factor for vascular endothelium damage is ox-LDL which upon its accumulation results in the secretion of DAMPs. Accordingly, ox-LDL upregulates HMGB1 expression in human umbilical vein endothelial cells [93].
Smoking-related cell death processes and DAMP secretion
Smoking is one of the most significant factors that increases the risk of many diseases and contributes to life-threatening pathological processes. Cigarette smoke (CS) contains thousands of chemicals, several of which can have cytotoxic effects on human tissues and organs.
It has long been acknowledged that CS induces apoptosis of bronchial epithelial cells, but it has now been proven that it can also induce necrotic cell death in these cells [94]. RIPK3 or MLKL-deficient mice showed reduced airway inflammation in a chronic CS-induced COPD model [95] and expression of necroptosis-associated markers (RIPK1, RIPK3, and MLKL) were elevated in cigarette tar treated vascular smooth muscle cells [96].
Furthermore, CS stimulates NLRP3 inflammasome-induced pyroptosis and subsequent increase in IL-1β and IL-18 levels in human and mouse models [97, 98]. CS-induced acute pulmonary inflammation was not affected by NLRP3 deficiency but was attenuated upon IL-1α or IL-1β neutralization [99]. It was established that, compared to non-smokers, heavy smokers with coronary artery disease display activation of the NLRP3 inflammasome which facilitates the progression of atherosclerosis [100].
Ferroptosis has also been shown to play a role in cigarette smoke-induced atherosclerosis [101]. In addition, CS induces ferroptosis in human bronchial epithelial cells in culture, a process that is attenuated by the treatment with an iron chelator or ferroptosis inhibitors. Consistent with its role in ferroptosis, mice overexpressing GPX4 have a milder response to CS [102]. Additionally, the disruption of Nrf2 in mice, a transcription factor that is involved in the regulation of many antioxidant genes, leads to a widespread CS-induced emphysema than in wild-type littermates [103].
Cigarette smoking also causes DNA damage, which leads to the activation of enzymes, and PARP-1-mediated DNA repair to reduce CS-induced cell death [104]. However, overactivation of PARP-1 can lead to parthanatos cell death. CS induces the mitochondrial translocation of AIF and Endonuclease G, characteristics of parthanatos. Accordingly, the application of a specific PARP-1 inhibitor was shown to abolish the smoke-induced parthanatos pathway [105].
CS containing a complex array of toxic chemicals induces different forms of necrotic cell death in bronchial epithelial cells, consequently leading to a significant increase in DAMP levels (dsDNA, HMGB1, HSP70, mtDNA, and IL-1β) [106]. Elevated levels of HMGB1 and its receptors, RAGE and TLR4 proteins, were found in the lung tissue of smoking COPD patients [107], and CS-induced HMGB1 secretion may lead to insulin resistance [108]. Supporting this, RAGE KO mice are protected from smoke-induced lung pathologies due to reduced macrophage infiltration and chemokine release [109]. Elevated HMGB1 expression may synergize with the function of additional DAMPs, as HMGB1 forms a complex with mtDNA, leading to the co-signaling of RAGE and TLR9 receptors [110]. Moreover, RAGE activation may cause sustained ER stress and consequently the release of an ER-related DAMP, calreticulin (CRT). During the CS-induced ER stress, CRT acts as an “eat me” signal for surrounding phagocyte [110]. Furthermore, the upregulation of HMGB1 was positively correlated with IL-1β [110]. Significantly increased IL-1α levels were also detected in smokers [99]. CS triggers HSP70 production [106] activating TLR2 and TLR4 signaling and the release of proinflammatory cytokines [110]. Additionally, higher ATP levels [111], and upregulation of ATP receptors (P2X7 and P2Y2) are also observed in neutrophils, macrophages, and lung tissue of smokers. Knockout mice lacking P2Y2 receptors showed reduced lung inflammation after acute CS exposure compared to wild-type mice [112].
Alcohol consumption-related cell death processes and DAMP secretion
Chronic alcohol exposure induces multiple forms of cellular stress, including oxidative stress, hypoxia, failed protein folding, and ER stress, resulting in the activation of both intrinsic and extrinsic pathways of apoptosis [113,114,115]. However, inhibition of apoptosis is not a protective mechanism in mouse models of ALD, suggesting that necroptosis may play an important role in ethanol-induced liver injury. Indeed, RIPK3 expression in the liver is elevated in both mouse and human ALD models. Additionally, RIPK3 KO mice were protected from ethanol-induced hepatocyte injury and inflammation [116].
ER stress stimulates NLRP3 activation in human steatohepatitis liver [117], and NLRP3 deficiency leads to attenuation of alcoholic steatosis [118]. Caspase-1-dependent upregulation of IL-1β also plays an important role in ALD pathogenesis according to the KO mouse model [119]. Accumulating evidence suggests the role of ferroptosis in liver diseases, including ALD. Approximately 50% of patients with alcoholic liver disease are characterized by iron overload in the liver, which contributes to liver inflammation [120]. Long-term ethanol feeding in mice was found to enhance polyunsaturated lipid peroxidation and indirectly deactivate GPX4 through severe GSH exhaustion [121].
In late-stage alcohol metabolism, diverse DAMPs (HMGB1, mtDNA, miRNA, and ATP) are secreted not only in the liver but also in adipose tissue, the gut, and bone marrow, resulting in a heightened production of proinflammatory cytokines and chemokines [122]. DAMPs amplify liver injury through the stimulation of the inflammasome [123], consequently, pro-IL-1β mRNA levels were shown to be increased in the peripheral monocytes as well as in the cerebellum of alcoholics [124]. Alcohol-associated neurodegenerative diseases have been linked to increased apoptosis, excitotoxicity, lipid peroxidation, and elevated DAMP secretion such as IL1β, ROS, mtDNA, and HMGB1 [125]. Interestingly, HMGB1 and IL-1β heterocomplexes could be detected in the postmortem human alcoholic hippocampus. Ethanol intake promotes ROS generation and raises serum uric acid levels by boosting urate production and excretion, as well as its release from dying cells [126, 127]. Additionally, serum uric acid triggers the production of inflammasome-related molecules in bone marrow-derived macrophages [128].
The role of cell death and related DAMP release in cardiovascular diseases
Ischemic heart disease (IHD) is the leading cause of death worldwide, representing 32% of all global deaths. Of these deaths, 45% are due to myocardial infarction (MI). The main cause of IHD is the formation of atherosclerotic plaques in coronary arteries. As the disease progresses, destabilization and rupture of atherosclerotic plaques followed by thrombotic events result in complete occlusion of the coronary arteries, leading to MI. Interestingly, the outcomes of MI worsen upon restoration of the tissue perfusion, known as ischemia-reperfusion injury (IRI).
Recent studies have demonstrated the significant contribution of various cell death pathways in the progression of atherosclerosis and IHD (Table 2). Pyroptosis of endothelial cells, macrophages, and smooth muscle cells has been established to be intimately connected to the development and destabilization of atherosclerotic lesions. A significant decrease in the atherosclerotic lesion size was observed in high-fat diet mice reconstituted with NLRP3-, ASC-, IL-1α-knockout bone marrow cells [8, 88, 129, 130].
Mitochondrial permeability transition pore (MPT)-mediated necrosis has also been demonstrated to have a role in the progression of atherosclerosis since the deletion of CyPD decreased the necrotic core size in advanced atherosclerotic lesions [131].
Ferroptosis may also contribute to plaque instability. GPX4 counteracts ferroptosis and inhibits the development of atherosclerosis by reducing lipid peroxidation and vascular cell sensitivity to oxidized lipids. Accordingly, GPX4 is downregulated in advanced atherosclerotic plaques in human arteries [132].
RIPK3 knockout attenuated cardiac dysfunction 30 days after MI injury [133], and necroptosis has also been demonstrated to play a significant role in cardiac IRI, since inhibition of necroptosis or global deletion of RIPK3 was shown to reduce infarct size following IRI in mice [133].
Additional studies have confirmed a strong association of NLRP3 inflammasome and pyroptosis with the pathological processes of MI and myocardial ischemia‒reperfusion injury (MIRI). Cardiac dysfunctions were significantly alleviated in ASC- or caspase-1-deficient mice, or following silencing of either NLRP3 or P2X7, a pyroptosis stimulating ATP receptor [88, 129, 130].
Furthermore, iron excess contributed to ferroptosis in cardiac cells in the early and middle stages of MI [132], and downregulation of GPX4 with subsequent ferroptosis was revealed in an MI mouse model. Following an MI, mice lacking CyPD were shown to have a smaller infarct size, as well as less adverse cardiac dysfunction and remodeling [131]. Calcium overload and oxidative stress, observed in reperfusion injury, caused MPT-induced necrosis, and inhibitors or genetic ablation of CyPD provide a strong protection from reperfusion injury [131].
Several DAMPs have been linked to the inflammatory processes underlying the progression of atherosclerosis, MI, and IRI. These include oxidized LDL (ox-LDL), cholesterol crystals and calcium phosphate crystals in the former case, as well as HSPs, HMGB1, ATP, nuclear and mtDNA, and RNA in the latter one [134]. Cholesterol crystals, ox-LDL, and calcium phosphate crystals have been shown to exert their proinflammatory effects via NLRP3 inflammasome-mediated pathways and elevated pro-IL-1 expression. In addition, excessive cholesterol crystals can promote lysosomal destabilization and rupture, resulting in the leakage of cathepsin B, further activating the NLRP3 inflammasome [135]. HSP70 is a biomarker of the clinical outcome of MIRI; however, the causal role of HSP70 in the inflammatory response after MI has not yet been proven [134]. Upon IRI, both HMGB-1 and mtDNA are released into the bloodstream. Treatment of either HMGB-1 or mtDNA infusion separately did not result in increasing infarct size, yet the combined treatment of HMGB-1 and mtDNA did have clear harmful effects [134]. It was further demonstrated that the treatment with RNase attenuates necrosis-induced cytokine production in cardiomyocytes and protects mice against IRI, marked by smaller infarct size [134].
The role of cell death and related DAMP release in neurodegeneration
Neurodegenerative diseases are a group of disorders characterized by progressive dysfunction and loss of neurons in the central nervous system (CNS). According to the World Health Organization (WHO), currently more than 55 million people worldwide have dementia, and it ranks as the seventh leading cause of death and one of the major reasons for disability and dependency among older people globally. Sterile inflammation in neurodegenerative diseases, like Alzheimer’s (AD) and Parkinson’s (PD), is fueled by endogenous danger signals such as misfolded proteins, oxidative stress, and neuronal cell death [136]. This chronic inflammatory response involves the activation of the microglia and astrocytes and the release of pro-inflammatory cytokines and chemokines in the CNS [137, 138]. Several types of cell death mechanisms have been identified in the pathogenesis of neurodegenerative diseases including apoptosis, necroptosis, pyroptosis, ferroptosis and a special form of neuronal cell death known as excitotoxicity [139] (Table 3). Excitotoxicity is triggered by excessive stimulation of the N-methyl-D-aspartate receptor (NMDA) subtype of glutamate receptors leading to the loss of ATP, causing the general depolarization of neurons, thus the cytosolic Ca2+ is sequestered resulting in ER stress and/or mitochondrial permeability transition [140].
In addition to apoptosis which was demonstrated to be a contributing cell death pathway in neurodegeneration [141], elevated levels of various markers of necroptosis were also detected in the postmortem brain tissues of AD patients. Mechanistically, TNFα production by activated microglia induces necroptosis in astrocytes [142]. Studies on knockout mice mechanistically confirm the role of various cell death processes in neurodegenerative diseases. Data observed in neuron-specific RIPK1- or RIPK3-deficient mice confirmed that necroptosis contributed to delayed neuronal cell death in traumatic brain injury [143].
Furthermore, the levels of key components of pyroptosis, NLRP3, caspase-1, GSDMD, IL-1β and IL-18 were also increased in PBMCs of AD patients [144], and GSDME is found to be highly expressed in the brain tissue and neurons of patients with neurodegenerative diseases executing its role in the pyroptotic pathway [145]. Cortical iron has also been shown to be elevated in AD possibly inducing a ferroptosis-related inflammatory response [146].
Knockdown of NLRP3 or caspase-1 reduced neuronal death and reversed cognitive impairments [147], whereas PARP-1 -/- mice were observed to prevent synaptic damage, cognitive dysfunction, and microglial activation in an AD mouse model referring to the significant role of pyroptosis and parthanathos in the pathogenesis of AD [148, 149]. Accordingly, GSDMD depletion inhibited the expression and release of IL-1β and TNF-α while promoting those of anti-inflammatory cytokines (IL-10 and TGF-β1) in neurodegenerative pathologies [149]. MPT-related cell death mechanisms are also implicated in various neurological diseases based on CyPD knockout studies [150].
Various danger-associated molecules, including HMGB1, ATP, and S100B, participate in neuroinflammation and activate cerebral myeloid cells and other brain cells to produce inflammatory factors. Soluble amyloid β (Aβ) is considered an inducible DAMP molecule, the secretion of which leads to inflammatory cytokine production [151]. HMGB1 seems to colocalize with Aβ in senile plaque that is associated with activated microglia, enhancing Aβ neurotoxicity and inhibiting microglial clearance. Moreover, various cells have been identified as sources of DAMP secretion in neurodegeneration, such as astrocytes (ATP, HSP70, uric acid and S100B), injured neurons (ATP, HSP70, uric acid, mtDNA, formylated peptides, HMGB1, PRXs [152], oligodendrocytes (HSP70) and glia cells (HSP70, mtDNA, formylated peptides, HMGB1 [152]. In addition, Chromogranin A released by neuronal stress or demise stimulates the further release of cytotoxins and neurotoxins by microglia acting as DAMP in AD [153]. Several of these DAMPs (mtDNA, ATP, uric acid) have been implicated in inducing pyroptosis, contributing to the exacerbation of the inflammatory process [154, 155].
The role of cell death and related DAMP release in diabetes mellitus
Type 2 diabetes (T2D) mellitus is a multifactorial disease with increasing incidence worldwide. The β-cell apoptosis reduces the β-cell mass [156], but different kinds of regulated non-apoptotic cell deaths also contribute to its pathogenesis (Table 4) [157].
Necroptosis plays a role in a form of diabetes named maturity-onset diabetes of the young (MODY) which is an autosomal dominant disease with incomplete penetrance. Interestingly, a very rare heterozygous damaging mutation in MLKL was found exclusively in patients with diabetes [158].
Recent studies have demonstrated that activation of NLRP3 inflammasome contributes to insulin resistance and β-cell death in T2D. Activation of NLRP3 inflammasome has also been observed in macrophages under inflammatory conditions. For instance, in conditions of hypoxia and high glucose levels, the saturated fatty acid palmitate can trigger NLRP3 inflammasome/caspase-1-mediated pyroptosis and consequently IL-1β, and IL-18 production. The loss of β-cells is not the only factor that causes complications; IL-1β production also plays a critical role in chronic inflammation in T2D as it interferes with insulin signaling, resulting in impaired glucose tolerance and reduced insulin sensitivity [159].
Several studies have shown that pancreatic β-cells are predisposed to ferroptosis due to low levels of antioxidant enzymes, such as superoxide dismutase, GPx4, and catalase. Furthermore, Fe2+ in the labile iron pool promotes ROS synthesis via the Fenton reaction in β-cells. In addition, ferroptosis in the liver, fat, and muscles also causes insulin resistance [160].
The role of different cell death pathways in T2D has also been intensively investigated in knock-out mouse models, mice lacking Caspase-8 in β-cells were protected from in vivo models of type 1 and type 2 diabetes, but with aging, these mice gradually developed hyperglycemia and a concomitant decline in β-cells mass, confirming that several different cell death pathways may play a role in T2D [161]. Whole-body deficiency of MLKL [65], NLRP3 [67, 162], or CyPD [163] also prevented obesity-induced, or high-fat diet (HFD)-induced insulin resistance and glucose intolerance.
Increased levels of various DAMPs, including RAGE ligands such as advanced glycation end products (AGE), HMGB1, S100 proteins, mtDNA, uric acid, and ROS, have been reported in both diabetic patients and animal models. Either AGE, HMGB1, S100, or mtDNA induce AIM2 or NLRP3 inflammasome activation which plays a critical role in the pathogenesis of type 2 diabetes [164, 165]. Elevated levels of these DAMPs regulate the secretion of the proinflammatory cytokines IL-1β and IL-18, leading to chronic inflammation. Accordingly, a reduction in glucose levels and increased glucose tolerance has been observed in HMGB1 knockout mice [166]. Elevated uric acid levels also increase the secretion of IL-6 and TNFα, in addition to the production of IL-1β. High blood glucose, glycemic variability, hypoglycemia, and uric acid are all related to increased ROS production, which leads to the development of insulin resistance and impaired insulin secretion [164, 167].
The role of cell death and related DAMP release in liver diseases
Hepatocyte cell death is a critical event in the progression of liver disease due to resultant inflammation leading to fibrosis. Apoptosis, necroptosis, pyroptosis, and ferroptosis have all been investigated in the pathogenesis of various liver diseases, such as alcoholic liver disease (ALD), non-alcoholic fatty liver disease, steatohepatitis (NAFLD/NASH), acetaminophen-induced hepatotoxicity, autoimmune hepatitis and cholestatic liver disease [168]. In this chapter, we review the two most prevalent types of liver conditions, ALD and NASH/NAFLD (Table 5).
ALD is associated with a complex pathogenesis involving sterile inflammation and is the second most common cause of annual human mortality [169]. Alcohol metabolism via alcohol dehydrogenase, aldehyde dehydrogenase, cytochrome P450 2E1, and catalase increases ROS production, which triggers mitochondrial damage and hepatocyte cell death. However, alcohol-induced hepatocyte apoptosis does not account for the inflammatory cytokine production observed in mice after chronic alcohol feeding [170]. In contrast to apoptosis, inhibition of necroptosis or pyroptosis was shown to reduce alcohol-induced inflammation. RIPK3 knockout mice had attenuated levels of proinflammatory cytokines and chemokines, and decreased plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities during ethanol-induced liver damage or Gao-binge alcohol treatment [116, 171]. However, the role of MLKL in ALD remains questionable [172]. Biopsy samples from ALD showed increased RIPK3 expression, but not RIPK1 expression [116]. Also, the deficiency of NLRP3, Caspase-1, ASC, IL-1R1, and Caspase-11 prevents alcohol-induced liver inflammation and liver damage [119, 173].
It is currently unknown how ferroptosis plays a role in the pathogenic mechanism of alcoholic steatohepatitis. Nevertheless, long-term ethanol feeding increased the expression of ferroptosis-related genes, lipid peroxidation, and the amount of labile iron. Ethanol can also sensitize the liver to ferroptosis by depleting cysteine and suppressing the xCT glutamate antiporter [121, 174].
NAFLD is the most common cause of liver disease in the U.S. NASH occurs in 20% of NAFLD, in which hepatocyte cell death causes inflammation and fibrosis [168]. While apoptosis is undoubtedly important in the development of NASH [168], the role of necroptosis in this disease is highly controversial depending on the model used and the molecules knocked down [172]. While MLKL KO mice were protected from steatohepatitis in various models, symptoms could be exacerbated in RIPK3-/- mice. Presumably, RIPK3 deficiency exacerbated apoptosis in various models [172].
Furthermore, pyroptosis in hepatocytes and macrophages is also involved in the development of liver fibrosis in NAFLD, as the pro-inflammatory cytokines released during pyroptosis are key molecules for NAFLD development [168]. In GSDMD KO mice fed with HFD, liver histology was significantly improved, steatohepatitis and the level of inflammatory cytokines were reduced, and elevated levels of the cleaved GSDMD-N fragment were detected in liver biopsy samples from patients with NASH [175].
The liver plays a significant role in iron metabolism and hepatic lipogenesis, and abnormal lipid accumulation promotes hepatic steatosis in NASH/NAFLD, accordingly, ferroptosis also takes on an important part in the disease’s pathogenesis [176]. Iron overload is a key characteristic of NAFLD, and elevated serum ferritin levels have been observed in patients with this disease [177]. Steatosis and inflammation were significantly improved using ferroptosis inhibitors in mice exposed to a methionine- and choline-deficient diet, a classical dietary model of NASH [178].
Hepatocytes release HMGB1 in a dose- and time-dependent manner in response to ethanol treatment in vitro, and an increase in HMGB1 expression was detected in liver biopsies from patients with ALD. HMGB1 seems to be a dominant DAMP in ALD, since HMGB1 ablation in hepatocytes protected mice from alcohol-induced liver injury [179]. Ethanol-damaged hepatocytes release uric acid and ATP, activating the inflammasome and IL-1β-related inflammation. This creates a pyroptosis-mediated cycle that maintains inflammation in ALD. Alcohol metabolism in liver cells further increases the production of ROS, which results in mitochondrial dysfunction [180, 181].
Clinical opportunities for regulating cell death in fatal diseases
Understanding the interplay between sterile inflammation, cell death and DAMP release offers potential targets for therapeutic intervention to mitigate disease progression. Several diseases are linked to risk factors, regulated necrosis, or DAMP production, but only a small number of approved drugs have been developed to inhibit cell death or DAMP action.
Emricasan (approved for the treatment of non-alcoholic steatohepatitis cirrhosis) as an apoptosis inhibitor, Rilonacept (used for the treatment of cryopyrin-associated periodic syndromes), Canakinumab (for the treatment of systemic juvenile idiopathic arthritis, active Still’s disease) as inhibitors or antibodies neutralizing IL-1β, or Anakinra (used to treat rheumatoid arthritis, cryopyrin-associated periodic syndromes, familial Mediterranean fever, and Still’s disease) as an IL-1R antagonist are currently used. These drugs are also being studied in clinical trials focusing on cardiovascular diseases [182], neurodegenerative diseases [183], diabetes [184] and ALD [185]. Four PARP inhibitors [186] niraparib, olaparib, rucaparib, and talazoparib, have been approved for the treatment of various cancers by inhibiting DNA damage repair. Since these drugs could potentially be used to block cell death as well, strategies to block parthanatos offer promising therapeutic approaches for the treatment of parthanatos-related disorders such as neurodegenerative diseases. For example, veliparib, rucaparib, and talazoparib were shown to prevent the α-synuclein preformed fibrils-mediated cell death in mice PD models. In ALS veliparib and olaparib can inhibit TAR DNA-binding protein 43-associated neuronal death. A PARP-1 inhibitor, PJ34, inhibits Aβ-induced neuronal death in AD models, and another PARP-1 inhibitor, INO-1001, has shown neuroprotective effects in a mouse model of human Huntington’s disease [187].
Efforts to inhibit necroptosis have so far focused on RIPK1, but the development of probes targeting RIPK3 and MLKL would also be essential. Off-target activity, species specificity, and interference with non-necroptosis functions of RIPK1 and RIPK3 pose challenges to the development of a successful necroptosis inhibitor [188].
In recent years, research into small-molecule drugs that inhibit pyroptosis has received more and more attention. For example, INF4E, a newly synthesized small-molecule inhibitor of NLRP3, significantly inhibits the release of LDH characteristic of necrosis, moderates the development of NLRP3-dependent inflammation, and reduces infarct size. Disulfiram, a drug used to treat alcohol dependence, has been shown to effectively inhibit GSDMD pore formation in human and mouse cells, thereby inhibiting pyroptosis [189].
Considerable progress has been made in developing pharmacological agonists and antagonists for the treatment of these ferroptosis-related conditions. As an antioxidant, (N-acetylcysteine NAC) has been shown to inhibit ferroptosis by targeting cysteine metabolism. It is clinically approved to treat acetaminophen toxicity overdose and NAC has also been clinically shown to improve neurodegeneration-related symptoms by increasing cysteine levels and facilitating the synthesis of γ-glutamyl-cysteine and GSH. A modified form of NAC with increased membrane permeability, NACA, is being tested to mitigate the side effects of NAC. To date, NACA has been shown to have antioxidant effects in several preclinical models but is still awaiting approval for clinical use. Both the ALOX15-specific inhibitor ML351 and Baicalein, a specific ALOX12 antagonist, protect against I/R-induced cardiac injury in mice. Polyunsaturated fatty acids deuterated at the bis-allyl position have also been shown to inhibit ferroptosis in animal models of PD and Friedreich’s ataxia [190]. In addition, copper-diacetyl-bis(N4-methylthiosemicarbazone), a candidate drug for treating ALS and PD, suppresses ferroptosis via its RTA activity [191].
Regulation of NINJ1, a common point of various necrotic cell death processes, is a new therapeutic approach to downregulate inflammation. The anti-NINJ1 neutralizing monoclonal antibody attenuated inflammation induced by liver damage or ischemia-reperfusion injury and also reduced gout flare [192, 193].
Prophylactic anti-HMGB1 therapy constitutes a promising new tool to reduce HMGB1-dependent inflammation/damage and improve patient outcomes. For example, pretreatment with HMGB1 antibody protects against secondary liver damage and ameliorates renal dysfunction due to ischemia-reperfusion in vivo. Intraperitoneal injection of a monoclonal anti-HMGB1 has been shown to attenuate serum IL-17 elevation and reduce the demyelination associated with experimental autoimmune encephalomyelitis [194].
For deeper understanding, current improvements targeting necroptosis [188], pyroptosis [189], ferroptosis [191], parthanatos [187], or HMGB1 [194] are included in the indicated review articles.
Similar cell death or DAMP secretion processes are behind various diseases, which suggests that drugs that are effective in certain diseases should also be tested in other diseases with intensive cross-examination. This is confirmed by the fact that several drugs currently preferred in the treatment of human cardiovascular diseases, neurodegenerative disorders and diabetes can also regulate cell death in other indications according to mouse models (Table 6). A more precise exploration of the regulatory functions of cell death would expand the applicability in humans of these agents to other diseases as well.
Several other approved drugs have been found to influence cell death processes by either activating or inhibiting cell death mechanisms, suggesting exploring these drugs for use in treating sterile inflammation-related diseases. Here we refer to the studies related to necroptosis [195], pyroptosis [196, 197] and ferroptosis [191].
Conclusions
Sterile inflammation takes place in several steps, different stimuli induce regulated necrosis. Innate immune cells, mainly macrophages and DCs, respond to the resultant DAMPs release by producing inflammatory mediators. Consequently, some mediators like TNFα and interferons, certain DAMPs can trigger further cell death, establishing a feedback loop leading to chronic reactions, unless pro-resolving pathways are dominante. The tissue destruction characteristic of various diseases can be caused by several mechanisms, even simultaneously. Cell death can be induced [1] directly by risk factors, [2] due to inflammatory effector mechanisms triggered by DAMP release during necrotic cell death and [3] by exacerbation of inflammation through necrotic cell death induced by secreted DAMPs. It is not entirely known to what extent these effects are involved in the development or progression of each disease, and which effects cause inflammation to become a chronic process. Current anti-inflammatory treatments do not address the root cause of either the cell death process or the dysregulated release of DAMPs. A deeper understanding of cell death and DAMPs’ role in the disease pathogenesis could enable causal, rather than merely symptomatic therapy.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32.
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621.
Huang Y, Jiang W, Zhou R. DAMP sensing and sterile inflammation: intracellular, intercellular and inter-organ pathways. Nat Rev Immunol. 2024;24:703–719.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541.
Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Seminars Cancer Biol. 2012;22:33–40.
Wen Y, Zhu Y, Zhang C, Yang X, Gao Y, Li M, et al. Chronic inflammation, cancer development and immunotherapy. Front Pharm. 2022;13:1040163.
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6:263.
Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L, et al. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther. 2022;7:286.
Fucikova J, Moserova I, Urbanova L, Bezu L, Kepp O, Cremer I, et al. Prognostic and predictive value of DAMPs and DAMP-associated processes in cancer. Front Immunol. 2015;6:402.
Mazlo A, Jenei V, Burai S, Molnar T, Bacsi A, Koncz G. Types of necroinflammation, the effect of cell death modalities on sterile inflammation. Cell Death Dis. 2022;13:423.
Mazlo A, Tang Y, Jenei V, Brauman J, Yousef H, Bacsi A, et al. Resolution potential of necrotic cell death pathways. Int J Mol Sci. 2022;24:16.
Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O’Rourke K, Li Q, et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591:131–6.
Land WG. The role of damage-associated molecular patterns in human. I. Promoting inflammation and immunity. Sultan Qaboos Univ Med J. 2015;15:e9–e21.
Martin SJ. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. The. FEBS J. 2016;283:2599–615.
Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28.
Afonina IS, Tynan GA, Logue SE, Cullen SP, Bots M, Luthi AU, et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1alpha. Mol Cell. 2011;44:265–78.
Pullerits R, Jonsson IM, Verdrengh M, Bokarewa M, Andersson U, Erlandsson-Harris H, et al. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis Rheum. 2003;48:1693–700.
Chen B, Miller AL, Rebelatto M, Brewah Y, Rowe DC, Clarke L, et al. S100A9 induced inflammatory responses are mediated by distinct damage associated molecular patterns (DAMP) receptors in vitro and in vivo. PLoS ONE. 2015;10:e0115828.
Broderick L, Hoffman HM. IL-1 and autoinflammatory disease: biology, pathogenesis and therapeutic targeting. Nat Rev Rheumatol. 2022;18:448–63.
Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:j2376.
Schierbeck H, Lundback P, Palmblad K, Klevenvall L, Erlandsson-Harris H, Andersson U, et al. Monoclonal anti-HMGB1 (high mobility group box chromosomal protein 1) antibody protection in two experimental arthritis models. Mol Med. 2011;17:1039–44.
Kanellakis P, Agrotis A, Kyaw TS, Koulis C, Ahrens I, Mori S, et al. High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:313–9.
Truong R, Thankam FG, Agrawal DK. Immunological mechanisms underlying sterile inflammation in the pathogenesis of atherosclerosis: potential sites for intervention. Expert Rev Clin Immunol. 2021;17:37–50.
Dixit A, Cheema H, George J, Iyer S, Dudeja V, Dawra R, et al. Extracellular release of ATP promotes systemic inflammation during acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2019;317:G463–G75.
Cabrera-Fuentes HA, Ruiz-Meana M, Simsekyilmaz S, Kostin S, Inserte J, Saffarzadeh M, et al. RNase1 prevents the damaging interplay between extracellular RNA and tumour necrosis factor-alpha in cardiac ischaemia/reperfusion injury. Thromb Haemost. 2014;112:1110–9.
Wang S, Xie T, Sun S, Wang K, Liu B, Wu X, et al. DNase-1 treatment exerts protective effects in a rat model of intestinal ischemia-reperfusion injury. Sci Rep. 2018;8:17788.
Koncz G, Jenei V, Toth M, Varadi E, Kardos B, Bacsi A, et al. Damage-mediated macrophage polarization in sterile inflammation. Front Immunol. 2023;14:1169560.
Li Y, Xu L, Shan Z, Teng W, Han C. Association between air pollution and type 2 diabetes: an updated review of the literature. Ther Adv Endocrinol Metab. 2019;10:2042018819897046.
Zhang S, Che L, He C, Huang J, Guo N, Shi J, et al. Drp1 and RB interaction to mediate mitochondria-dependent necroptosis induced by cadmium in hepatocytes. Cell Death Dis. 2019;10:523.
Liu Q, Weng J, Li C, Feng Y, Xie M, Wang X, et al. Attenuation of PM(2.5)-induced alveolar epithelial cells and lung injury through regulation of mitochondrial fission and fusion. Part Fibre Toxicol. 2023;20:28.
Cui Y, Xiao Q, Zhang Q, Liu Y, Hao W, Jiang J, et al. Black carbon nanoparticles activate the crosstalk mechanism between necroptosis and macrophage extracellular traps to change macrophages fate. Environ Res. 2023;232:116321.
Shi K, Yin Q, Tang X, Yu X, Zheng S, Shentu X. Necroptosis contributes to airborne particulate matter-induced ocular surface injury. Toxicology. 2022;470:153140.
Mou Y, Liao W, Liang Y, Li Y, Zhao M, Guo Y, et al. Environmental pollutants induce NLRP3 inflammasome activation and pyroptosis: roles and mechanisms in various diseases. Sci Total Environ. 2023;900:165851.
Xiong Q, Tian X, Xu C, Ma B, Liu W, Sun B, et al. PM(2) (.5) exposure-induced ferroptosis in neuronal cells via inhibiting ERK/CREB pathway. Environ Toxicol. 2022;37:2201–13.
Wang Y, Tang M. PM2.5 induces ferroptosis in human endothelial cells through iron overload and redox imbalance. Environ Pollut. 2019;254:112937.
Sharma B, Singh S, Siddiqi NJ. Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int. 2014;2014:640754.
Zeng X, Liu D, Wu W, Huo X. PM(2.5) exposure inducing ATP alteration links with NLRP3 inflammasome activation. Environ Sci Pollut Res Int. 2022;29:24445–56.
Jia H, Liu Y, Guo D, He W, Zhao L, Xia S. PM2.5-induced pulmonary inflammation via activating of the NLRP3/caspase-1 signaling pathway. Environ Toxicol. 2021;36:298–307.
Aghapour M, Ubags ND, Bruder D, Hiemstra PS, Sidhaye V, Rezaee F, et al. Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur Respir Rev. 2022;31:210112.
Basith S, Manavalan B, Shin TH, Park CB, Lee WS, Kim J, et al. The impact of fine particulate matter 2.5 on the cardiovascular system: a review of the invisible killer. Nanomaterials (Basel). 2022;12:2656.
Zou W, He F, Liu S, Pu J, Hu J, Sheng Q, et al. PM2.5 Induced the expression of fibrogenic mediators via HMGB1-RAGE signaling in human airway epithelial cells. Can Respir J. 2018;2018:1817398.
Massey N, Shrestha D, Bhat SM, Padhi P, Wang C, Karriker LA, et al. Mitoapocynin attenuates organic dust exposure-induced neuroinflammation and sensory-motor deficits in a mouse model. Front Cell Neurosci. 2022;16:817046.
Cho A, Mitchell L, Koopmans D, Langille BL. Effects of changes in blood flow rate on cell death and cell proliferation in carotid arteries of immature rabbits. Circ Res. 1997;81:328–37.
Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler Thromb Vasc Biol. 2005;25:957–62.
Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. 2020;36:635–47.
Wang Q, Cui Y, Lin N, Pang S. Correlation of cardiomyocyte apoptosis with duration of hypertension, severity of hypertension and caspase-3 expression in hypertensive rats. Exp Ther Med. 2019;17:2741–5.
Xiao G, Zhuang W, Wang T, Lian G, Luo L, Ye C, et al. Transcriptomic analysis identifies Toll-like and Nod-like pathways and necroptosis in pulmonary arterial hypertension. J Cell Mol Med. 2020;24:11409–21.
Sun Y, Liu S, Chen C, Yang S, Pei G, Lin M, et al. The mechanism of programmed death and endoplasmic reticulum stress in pulmonary hypertension. Cell Death Discov. 2023;9:78.
Wu Y, Pan B, Zhang Z, Li X, Leng Y, Ji Y, et al. Caspase-4/11-mediated pulmonary artery endothelial cell pyroptosis contributes to pulmonary arterial hypertension. Hypertension. 2022;79:536–48.
Zhang M, Xin W, Yu Y, Yang X, Ma C, Zhang H, et al. Programmed death-ligand 1 triggers PASMCs pyroptosis and pulmonary vascular fibrosis in pulmonary hypertension. J Mol Cell Cardiol. 2020;138:23–33.
Quatredeniers M, Mendes-Ferreira P, Santos-Ribeiro D, Nakhleh MK, Ghigna MR, Cohen-Kaminsky S, et al. Iron deficiency in pulmonary arterial hypertension: a deep dive into the mechanisms. Cells. 2021;10:477.
Hu P, Xu Y, Jiang Y, Huang J, Liu Y, Wang D, et al. The mechanism of the imbalance between proliferation and ferroptosis in pulmonary artery smooth muscle cells based on the activation of SLC7A11. Eur J Pharm. 2022;928:175093.
Foley A, Steinberg BE, Goldenberg NM. Inflammasome activation in pulmonary arterial hypertension. Front Med (Lausanne). 2021;8:826557.
Li Y, Li Y, Li L, Yin M, Wang J, Li X. PKR deficiency alleviates pulmonary hypertension via inducing inflammasome adaptor ASC inactivation. Pulm Circ. 2021;11:20458940211046156.
Dasgupta A, Wu D, Tian L, Xiong PY, Dunham-Snary KJ, Chen KH, et al. Mitochondria in the pulmonary vasculature in health and disease: oxygen-sensing, metabolism, and dynamics. Compr Physiol. 2020;10:713–65.
Gonzalez-Quevedo A, Garcia SG, Concepcion OF, Freixas RS, Sotolongo LQ, Menendez MC, et al. Increased serum S-100B and neuron specific enolase—potential markers of early nervous system involvement in essential hypertension. Clin Biochem. 2011;44:154–9.
Wu Y, Li Y, Zhang C, Wang AX, Cui Y. W, et al. S100a8/a9 released by CD11b+Gr1+ neutrophils activates cardiac fibroblasts to initiate angiotensin II-Induced cardiac inflammation and injury. Hypertension. 2014;63:1241–50.
Dzau VJ. Atherosclerosis and hypertension: mechanisms and interrelationships. J Cardiovasc Pharm. 1990;15:S59–64.
Li JJ, Chen JL. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med Hypotheses. 2005;64:925–9.
Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25:155–61.
Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17:639–54.
Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55.
Luk CT, Chan CK, Chiu F, Shi SY, Misra PS, Li YZ, et al. Dual role of caspase 8 in adipocyte apoptosis and metabolic inflammation. Diabetes. 2023;72:1751–65.
Karunakaran D, Turner AW, Duchez AC, Soubeyrand S, Rasheed A, Smyth D, et al. RIPK1 gene variants associate with obesity in humans and can be therapeutically silenced to reduce obesity in mice. Nat Metab. 2020;2:1113–25.
Xu H, Du X, Liu G, Huang S, Du W, Zou S, et al. The pseudokinase MLKL regulates hepatic insulin sensitivity independently of inflammation. Mol Metab. 2019;23:14–23.
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88.
Koenen TB, Stienstra R, van Tits LJ, de Graaf J, Stalenhoef AF, Joosten LA, et al. Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1beta transcription in human adipose tissue. Diabetes. 2011;60:517–24.
Ma EB, Javaid HMA, Jung DH, Park JH, Huh JY, Gasdermin D. Deficiency does not protect mice from high-fat diet-induced glucose intolerance and adipose tissue inflammation. Mediators Inflamm. 2022;2022:7853482.
Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc Diabetol. 2002;1:1.
Meng Z, Liang H, Zhao J, Gao J, Liu C, Ma X, et al. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sci. 2021;284:119935.
Ananthakrishnan R, Kaneko M, Hwang YC, Quadri N, Gomez T, Li Q, et al. Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2009;296:H333–41.
Zhou D, Lu P, Mo X, Yang B, Chen T, Yao Y, et al. Ferroptosis and metabolic syndrome and complications: association, mechanism, and translational applications. Front Endocrinol (Lausanne). 2023;14:1248934.
He LP, Zhou ZX, Li CP. Narrative review of ferroptosis in obesity. J Cell Mol Med. 2023;27:920–6.
Tajima S, Ikeda Y, Sawada K, Yamano N, Horinouchi Y, Kihira Y, et al. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. Am J Physiol Endocrinol Metab. 2012;302:E77–86.
Ferriere A, Santa P, Garreau A, Bandopadhyay P, Blanco P, Ganguly D, et al. Self-nucleic acid sensing: a novel crucial pathway involved in obesity-mediated metaflammation and metabolic syndrome. Front Immunol. 2020;11:624256.
Gunasekaran MK, Viranaicken W, Girard AC, Festy F, Cesari M, Roche R, et al. Inflammation triggers high mobility group box 1 (HMGB1) secretion in adipose tissue, a potential link to obesity. Cytokine. 2013;64:103–11.
Zhang J, Zhang L, Zhang S, Yu Q, Xiong F, Huang K, et al. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol Cell Endocrinol. 2017;454:103–11.
Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859–64.
Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224:242–53.
Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16.
Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–11.
Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–92.
Meng L, Jin W, Wang X. RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc Natl Acad Sci USA. 2015;112:11007–12.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61.
Song D, Li M, Yu X, Wang Y, Fan J, Yang W, et al. The molecular pathways of pyroptosis in atherosclerosis. Front Cell Dev Biol. 2022;10:824165.
Uyy E, Suica VI, Boteanu RM, Cerveanu-Hogas A, Ivan L, Hansen R, et al. Regulated cell death joins in atherosclerotic plaque silent progression. Sci Rep. 2022;12. 2814.
Puylaert P, Zurek M, Rayner KJ, De Meyer GRY, Martinet W. Regulated necrosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2022;42:1283–306.
Ji N, Qi Z, Wang Y, Yang X, Yan Z, Li M, et al. Pyroptosis: a new regulating mechanism in cardiovascular disease. J Inflamm Res. 2021;14:2647–66.
Niyonzima N, Bakke SS, Gregersen I, Holm S, Sandanger O, Orrem HL, et al. Cholesterol crystals use complement to increase NLRP3 signaling pathways in coronary and carotid atherosclerosis. EBioMedicine. 2020;60:102985.
Liu C, Liao W, Chen J, Yu K, Wu Y, Zhang S, et al. Cholesterol confers ferroptosis resistance onto myeloid-biased hematopoietic stem cells and prevents irradiation-induced myelosuppression. Redox Biol. 2023;62:102661.
Amiya E. Interaction of hyperlipidemia and reactive oxygen species: Insights from the lipid-raft platform. World J Cardiol. 2016;8:689–94.
Davanzo GG, Castro G, Monteiro LB, Castelucci BG, Jaccomo VH, da Silva FC, et al. Obesity increases blood-brain barrier permeability and aggravates the mouse model of multiple sclerosis. Mult Scler Relat Disord. 2023;72:104605.
Huo X, Su B, Qin G, Zhao L. HMGB1 promotes Ox-LDL-induced endothelial cell damage by inhibiting PI3K/Akt signaling pathway. BMC Cardiovasc Disord. 2022;22:555.
Chen D, Gregory AD, Li X, Wei J, Burton CL, Gibson G, et al. RIP3-dependent necroptosis contributes to the pathogenesis of chronic obstructive pulmonary disease. JCI Insight. 2021;6:e144689.
Lu Z, Van Eeckhoutte HP, Liu G, Nair PM, Jones B, Gillis CM, et al. Necroptosis signaling promotes inflammation, airway remodeling, and emphysema in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;204:667–81.
Bai X, Wang Y, Luo X, Bao X, Weng X, Chen Y, et al. Cigarette tar accelerates atherosclerosis progression via RIPK3-dependent necroptosis mediated by endoplasmic reticulum stress in vascular smooth muscle cells. Cell Commun Signal. 2024;22:41.
Mehta S, Srivastava N, Bhatia A, Dhawan V. Exposure of cigarette smoke condensate activates NLRP3 inflammasome in vitro and in vivo: a connotation of innate immunity and atherosclerosis. Int Immunopharmacol. 2020;84:106561.
Rumora L, Hlapcic I, Hulina-Tomaskovic A, Somborac-Bacura A, Bosnar M, Rajkovic MG. Pathogen-associated molecular patterns and extracellular Hsp70 interplay in NLRP3 inflammasome activation in monocytic and bronchial epithelial cellular models of COPD exacerbations. APMIS. 2021;129:80–90.
Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Vanden Berghe T, et al. Role of IL-1alpha and the Nlrp3/caspase-1/IL-1beta axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J. 2011;38:1019–28.
Mehta S, Vijayvergiya R, Dhawan V. Activation of NLRP3 inflammasome assembly is associated with smoking status of patients with coronary artery disease. Int Immunopharmacol. 2020;87:106820.
Bao X, Luo X, Bai X, Lv Y, Weng X, Zhang S, et al. Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic Biol Med. 2023;201:76–88.
Yoshida M, Minagawa S, Araya J, Sakamoto T, Hara H, Tsubouchi K, et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun. 2019;10:3145.
Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–59.
Kovacs K, Erdelyi K, Hegedus C, Lakatos P, Regdon Z, Bai P, et al. Poly(ADP-ribosyl)ation is a survival mechanism in cigarette smoke-induced and hydrogen peroxide-mediated cell death. Free Radic Biol Med. 2012;53:1680–8.
Kunzi L, Holt GE. Cigarette smoke activates the parthanatos pathway of cell death in human bronchial epithelial cells. Cell Death Discov. 2019;5:127.
Pouwels SD, Zijlstra GJ, van der Toorn M, Hesse L, Gras R, Ten Hacken NH, et al. Cigarette smoke-induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2016;310:L377–86.
Lin L, Li J, Song Q, Cheng W, Chen P. The role of HMGB1/RAGE/TLR4 signaling pathways in cigarette smoke-induced inflammation in chronic obstructive pulmonary disease. Immun Inflamm Dis. 2022;10:e711.
Taylor OJ, Thatcher MO, Carr ST, Gibbs JL, Trumbull AM, Harrison ME, et al. High-mobility group box 1 disrupts metabolic function with cigarette smoke exposure in a ceramide-dependent manner. Int J Mol Sci. 2017;18:1099.
Wolf L, Herr C, Niederstrasser J, Beisswenger C, Bals R. Receptor for advanced glycation endproducts (RAGE) maintains pulmonary structure and regulates the response to cigarette smoke. PLoS ONE. 2017;12:e0180092.
Pouwels SD, Heijink IH, ten Hacken NH, Vandenabeele P, Krysko DV, Nawijn MC, et al. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014;7:215–26.
Lommatzsch M, Cicko S, Muller T, Lucattelli M, Bratke K, Stoll P, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–34.
Cicko S, Lucattelli M, Muller T, Lommatzsch M, De Cunto G, Cardini S, et al. Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J Immunol. 2010;185:688–97.
Miyata T, Nagy LE. Programmed cell death in alcohol-associated liver disease. Clin Mol Hepatol. 2020;26:618–25.
Shin SK, Kaiser EE, West FD. Alcohol induced brain and liver damage: advantages of a porcine alcohol use disorder model. Front Physiol. 2020;11:592950.
Fernandez-Sola J The effects of ethanol on the heart: alcoholic cardiomyopathy. Nutrients. 2020;12:572.
Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–83.
Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6:e1879.
Cui K, Yan G, Xu C, Chen Y, Wang J, Zhou R, et al. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1beta in mice. J Hepatol. 2015;62:1311–8.
Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–89.
Mueller S, Rausch V. The role of iron in alcohol-mediated hepatocarcinogenesis. Adv Exp Med Biol. 2015;815:89–112.
Luo J, Song G, Chen N, Xie M, Niu X, Zhou S, et al. Ferroptosis contributes to ethanol-induced hepatic cell death via labile iron accumulation and GPx4 inactivation. Cell Death Discov. 2023;9:311.
Shim YR, Jeong WI. Recent advances of sterile inflammation and inter-organ cross-talk in alcoholic liver disease. Exp Mol Med. 2020;52:772–80.
Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–48.
Coleman LG Jr., Zou J, Qin L, Crews FT. HMGB1/IL-1beta complexes regulate neuroimmune responses in alcoholism. Brain Behav Immun. 2018;72:61–77.
Kamal H, Tan GC, Ibrahim SF, Shaikh MF, Mohamed IN, Mohamed RMP, et al. Alcohol use disorder, neurodegeneration, Alzheimer’s and parkinson’s disease: interplay between oxidative stress, neuroimmune response and excitotoxicity. Front Cell Neurosci. 2020;14:282.
Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–84.
Choi HK, Curhan GBeer. liquor, and wine consumption and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2004;51:1023–9.
Braga TT, Forni MF, Correa-Costa M, Ramos RN, Barbuto JA, Branco P, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep. 2017;7. 39884.
Chen X, Tian PC, Wang K, Wang M, Wang K. Pyroptosis: role and mechanisms in cardiovascular disease. Front Cardiovasc Med. 2022;9:897815.
Zeng C, Wang R, Tan H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int J Biol Sci. 2019;15:1345–57.
Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009;1787:1402–15.
Fratta Pasini AM, Stranieri C, Busti F, Di Leo EG, Girelli D, Cominacini L. New Insights into the Role of Ferroptosis in Cardiovascular Diseases. Cells. 2023;12:867.
Guo X, Chen Y, Liu Q. Necroptosis in heart disease: molecular mechanisms and therapeutic implications. J Mol Cell Cardiol. 2022;169:74–83.
Silvis MJM, Kaffka Genaamd Dengler SE, Odille CA, Mishra M, van der Kaaij NP, Doevendans PA, et al. Damage-associated molecular patterns in myocardial infarction and heart transplantation: the road to translational success. Front Immunol. 2020;11:599511.
Cho S, Ying F, Sweeney G. Sterile inflammation and the NLRP3 inflammasome in cardiometabolic disease. Biomed J. 2023;46:100624.
McGinnis MM, Parrish BC, McCool BA. Withdrawal from chronic ethanol exposure increases postsynaptic glutamate function of insular cortex projections to the rat basolateral amygdala. Neuropharmacology. 2020;172:108129.
Halliday M, Mallucci GR. Targeting the unfolded protein response in neurodegeneration: A new approach to therapy. Neuropharmacology. 2014;76:169–74.
Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68:930–41.
Goel P, Chakrabarti S, Goel K, Bhutani K, Chopra T, Bali S. Neuronal cell death mechanisms in Alzheimer’s disease: An insight. Front Mol Neurosci. 2022;15:937133.
Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57:1041–8.
Erekat NS. Apoptosis and its therapeutic implications in neurodegenerative diseases. Clin Anat. 2022;35:65–78.
Mitroshina EV, Saviuk M, Vedunova MV. Necroptosis in CNS diseases: focus on astrocytes. Front Aging Neurosci. 2022;14:1016053.
Wehn AC, Khalin I, Duering M, Hellal F, Culmsee C, Vandenabeele P, et al. RIPK1 or RIPK3 deletion prevents progressive neuronal cell death and improves memory function after traumatic brain injury. Acta Neuropathol Commun. 2021;9:138.
Rui W, Xiao H, Fan Y, Ma Z, Xiao M, Li S, et al. Systemic inflammasome activation and pyroptosis associate with the progression of amnestic mild cognitive impairment and Alzheimer’s disease. J Neuroinflamm. 2021;18. 280.
Wu KJ, Wang WR, Cheng QH, Li H, Yan WZ, Zhou FR, et al. Pyroptosis in neurodegenerative diseases: from bench to bedside. Cell Biol Toxicol. 2023;39:2467–99.
Ayton S, Wang Y, Diouf I, Schneider JA, Brockman J, Morris MC, et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry. 2020;25:2932–41.
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8.
Kauppinen TM, Suh SW, Higashi Y, Berman AE, Escartin C, Won SJ, et al. Poly(ADP-ribose)polymerase-1 modulates microglial responses to amyloid beta. J Neuroinflamm. 2011;8. 152.
Du H, Li CH, Gao RB, Cen XQ, Li P. Ablation of GSDMD attenuates neurological deficits and neuropathological alterations after traumatic brain injury. Front Cell Neurosci. 2022;16:915969.
Du H, Guo L, Wu X, Sosunov AA, McKhann GM, Chen JX, et al. Cyclophilin D deficiency rescues Abeta-impaired PKA/CREB signaling and alleviates synaptic degeneration. Biochim Biophys Acta. 2014;1842:2517–27.
Clark IA, Vissel B. Amyloid beta: one of three danger-associated molecules that are secondary inducers of the proinflammatory cytokines that mediate Alzheimer’s disease. Br J Pharm. 2015;172:3714–27.
Otani K, Shichita T. Cerebral sterile inflammation in neurodegenerative diseases. Inflamm Regen. 2020;40:28.
Venegas C, Heneka MT. Danger-associated molecular patterns in Alzheimer’s disease. J Leukoc Biol. 2017;101:87–98.
Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1037–45.
Zheng X, Wan J, Tan G. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in diabetic retinopathy. Front Immunol. 2023;14:1151185.
Costes S, Bertrand G, Ravier MA Mechanisms of beta-cell apoptosis in type 2 diabetes-prone situations and potential protection by GLP-1-based therapies. Int J Mol Sci. 2021;22:5303.
Pang H, Huang G, Xie Z, Zhou Z. The role of regulated necrosis in diabetes and its complications. J Mol Med (Berl). 2024;102:495–505.
Hildebrand JM, Lo B, Tomei S, Mattei V, Young SN, Fitzgibbon C, et al. A family harboring an MLKL loss of function variant implicates impaired necroptosis in diabetes. Cell Death Dis. 2021;12. 345.
Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–15.
Miao R, Fang X, Zhang Y, Wei J, Zhang Y, Tian J. Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: mechanisms and therapeutic opportunities. Cell Death Dis. 2023;14. 186.
Liadis N, Salmena L, Kwan E, Tajmir P, Schroer SA, Radziszewska A, et al. Distinct in vivo roles of caspase-8 in beta-cells in physiological and diabetes models. Diabetes. 2007;56:2302–11.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–40.
Taddeo EP, Laker RC, Breen DS, Akhtar YN, Kenwood BM, Liao JA, et al. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol Metab. 2014;3:124–34.
Shin JJ, Lee EK, Park TJ, Kim W. Damage-associated molecular patterns and their pathological relevance in diabetes mellitus. Ageing Res Rev. 2015;24:66–76.
Bae JH, Jo SI, Kim SJ, Lee JM, Jeong JH, Kang JS, et al. Circulating cell-free mtDNA contributes to AIM2 inflammasome-mediated chronic inflammation in patients with type 2 diabetes. Cells. 2019;8:328.
Liu Z, Annarapu G, Yazdani HO, Wang Q, Liu S, Luo JH, et al. Restoring glucose balance: Conditional HMGB1 knockdown mitigates hyperglycemia in a Streptozotocin induced mouse model. Heliyon. 2024;10:e23561.
Xiong Q, Liu J, Xu Y. Effects of uric acid on diabetes mellitus and its chronic complications. Int J Endocrinol. 2019;2019:9691345.
Shojaie L, Iorga A, Dara L. Cell death in liver diseases: a review. Int J Mol Sci. 2020;21:9682.
Kong LZ, Chandimali N, Han YH, Lee DH, Kim JS, Kim SU, et al. Pathogenesis, early diagnosis, and therapeutic management of alcoholic liver disease. Int J Mol Sci. 2019;20:2712.
Roychowdhury S, Chiang DJ, Mandal P, McMullen MR, Liu X, Cohen JI, et al. Inhibition of apoptosis protects mice from ethanol-mediated acceleration of early markers of CCl4 -induced fibrosis but not steatosis or inflammation. Alcohol Clin Exp Res. 2012;36:1139–47.
Wang S, Ni HM, Dorko K, Kumer SC, Schmitt TM, Nawabi A, et al. Increased hepatic receptor interacting protein kinase 3 expression due to impaired proteasomal functions contributes to alcohol-induced steatosis and liver injury. Oncotarget. 2016;7:17681–98.
Zhou Y, Wu R, Wang X, Bao X, Lu C. Roles of necroptosis in alcoholic liver disease and hepatic pathogenesis. Cell Prolif. 2022;55:e13193.
Khanova E, Wu R, Wang W, Yan R, Chen Y, French SW, et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology. 2018;67:1737–53.
Zhou Z, Ye TJ, Bonavita G, Daniels M, Kainrad N, Jogasuria A, et al. Adipose-specific Lipin-1 overexpression renders hepatic ferroptosis and exacerbates alcoholic steatohepatitis in mice. Hepatol Commun. 2019;3:656–69.
Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68:773–82.
Cheng Z, Chu H, Zhu Q, Yang L. Ferroptosis in non-alcoholic liver disease: molecular mechanisms and therapeutic implications. Front Nutr. 2023;10:1090338.
Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85.
Qi J, Kim JW, Zhou Z, Lim CW, Kim B. Ferroptosis affects the progression of nonalcoholic steatohepatitis via the modulation of lipid peroxidation-mediated cell death in mice. Am J Pathol. 2020;190:68–81.
Ge X, Antoine DJ, Lu Y, Arriazu E, Leung TM, Klepper AL, et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J Biol Chem. 2014;289:22672–91.
Petrasek J, Iracheta-Vellve A, Saha B, Satishchandran A, Kodys K, Fitzgerald KA, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol. 2015;98:249–56.
Wu J, Lin S, Wan B, Velani B, Zhu Y. Pyroptosis in liver disease: new insights into disease mechanisms. Aging Dis. 2019;10:1094–108.
Delbaere Q, Chapet N, Huet F, Delmas C, Mewton N, Prunier F, et al. Anti-inflammatory drug candidates for prevention and treatment of cardiovascular diseases. Pharmaceuticals (Basel). 2023;16:78.
Cavalli G, Dinarello CA. Anakinra therapy for non-cancer inflammatory diseases. Front Pharm. 2018;9:1157.
Hensen J, Howard CP, Walter V, Thuren T. Impact of interleukin-1beta antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: results of secondary endpoints from a randomized, placebo-controlled trial. Diabetes Metab. 2013;39:524–31.
Iracheta-Vellve A, Petrasek J, Gyogyosi B, Bala S, Csak T, Kodys K, et al. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int. 2017;37:968–73.
Li K, Deng Y, Deng G, Chen P, Wang Y, Wu H, et al. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res Ther. 2020;11:131.
Liu L, Li J, Ke Y, Zeng X, Gao J, Ba X, et al. The key players of parthanatos: opportunities for targeting multiple levels in the therapy of parthanatos-based pathogenesis. Cell Mol Life Sci. 2022;79:60.
Gardner CR, Davies KA, Zhang Y, Brzozowski M, Czabotar PE, Murphy JM, et al. From (Tool)Bench to bedside: the potential of necroptosis inhibitors. J Med Chem. 2023;66:2361–85.
Wei S, Feng M, Zhang S. Molecular characteristics of cell pyroptosis and its inhibitors: a review of activation, regulation, and inhibitors. Int J Mol Sci. 2022;23:16115.
Yanpiset P, Maneechote C, Sriwichaiin S, Siri-Angkul N, Chattipakorn SC, Chattipakorn N. Gasdermin D-mediated pyroptosis in myocardial ischemia and reperfusion injury: Cumulative evidence for future cardioprotective strategies. Acta Pharm Sin B. 2023;13:29–53.
Sun S, Shen J, Jiang J, Wang F, Min J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct Target Ther. 2023;8:372.
Kayagaki N, Stowe IB, Alegre K, Deshpande I, Wu S, Lin Z, et al. Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury. Nature. 2023;618:1072–7.
Zhang H, Gao J, Fang W, Tang Y, Fang X, Jin T, et al. Role of NINJ1 in gout flare and potential as a drug target. J Inflamm Res. 2022;15:5611–20.
Xue J, Suarez JS, Minaai M, Li S, Gaudino G, Pass HI, et al. HMGB1 as a therapeutic target in disease. J Cell Physiol. 2021;236:3406–19.
Molnar T, Mazlo A, Tslaf V, Szollosi AG, Emri G, Koncz G. Current translational potential and underlying molecular mechanisms of necroptosis. Cell Death Dis. 2019;10. 860.
Jin X, Ma Y, Liu D, Huang Y. Role of pyroptosis in the pathogenesis and treatment of diseases. MedComm. 2023;4:e249.
Yang K, Bao T, Zeng J, Wang S, Yuan X, Xiang W, et al. Research progress on pyroptosis-mediated immune-inflammatory response in ischemic stroke and the role of natural plant components as regulator of pyroptosis: a review. Biomed Pharmacother. 2023;157:113999.
Dagher Z, Garcon G, Billet S, Gosset P, Ledoux F, Courcot D, et al. Activation of different pathways of apoptosis by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. Toxicology. 2006;225:12–24.
Li A, Zhang Q, Zhou L, Luo H, Yu K, Meng X, et al. Long-term exposure to ambient air pollution and incident gout: a prospective cohort study in the UK Biobank. Environ Pollut. 2024;345:123540.
Vogel CFA, Van Winkle LS, Esser C, Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34:101530.
Costa-Beber LC, Heck TG, Fiorin PBG, Ludwig MS. HSP70 as a biomarker of the thin threshold between benefit and injury due to physical exercise when exposed to air pollution. Cell Stress Chaperones. 2021;26:889–915.
Byun HM, Colicino E, Trevisi L, Fan T, Christiani DC, Baccarelli AA. Effects of air pollution and blood mitochondrial DNA methylation on markers of heart rate variability. J Am Heart Assoc. 2016;5:e003218.
Gangwar RS, Bevan GH, Palanivel R, Das L, Rajagopalan S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020;34:101545.
Bauer RN, Diaz-Sanchez D, Jaspers I. Effects of air pollutants on innate immunity: the role of Toll-like receptors and nucleotide-binding oligomerization domain-like receptors. J Allergy Clin Immunol. 2012;129:14–24. quiz 5-6.
Sepand MR, Aliomrani M, Hasani-Nourian Y, Khalhori MR, Farzaei MH, Sanadgol N. Mechanisms and pathogenesis underlying environmental chemical-induced necroptosis. Environ Sci Pollut Res Int. 2020;27:37488–501.
Li X, Zhang Y, Li B, Yang H, Cui J, Li X, et al. Activation of NLRP3 in microglia exacerbates diesel exhaust particles-induced impairment in learning and memory in mice. Environ Int. 2020;136:105487.
Jiang M, Li D, Piao J, Li J, Sun H, Chen L, et al. Real-ambient exposure to air pollution exaggerates excessive growth of adipose tissue modulated by Nrf2 signal. Sci Total Environ. 2020;730:138652.
Janko C, Filipovic M, Munoz LE, Schorn C, Schett G, Ivanovic-Burmazovic I, et al. Redox modulation of HMGB1-related signaling. Antioxid Redox Signal. 2014;20:1075–85.
Nakayama H, Otsu K, Mitochondrial DNA. as an inflammatory mediator in cardiovascular diseases. Biochem J. 2018;475:839–52.
Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am. 2017;101:169–93.
Sanchez-Lozada LG, Rodriguez-Iturbe B, Kelley EE, Nakagawa T, Madero M, Feig DI, et al. Uric acid and hypertension: an update with recommendations. Am J Hypertens. 2020;33:583–94.
Zhuang L, Ge Y, Zong X, Yang Q, Zhang R, Fan Q, et al. High proteoglycan decorin levels are associated with acute coronary syndrome and provoke an imbalanced inflammatory response. Front Physiol. 2021;12:746377.
Yin X, Cao H, Wei Y, Li HH. Alteration of the IL-33-sST2 pathway in hypertensive patients and a mouse model. Hypertens Res. 2019;42:1664–71.
De Miguel C, Pelegrin P, Baroja-Mazo A, Cuevas S. Emerging role of the inflammasome and pyroptosis in hypertension. Int J Mol Sci. 2021;22:1064.
Zhang WH, Wang X, Narayanan M, Zhang Y, Huo C, Reed JC, et al. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci USA. 2003;100:16012–7.
Fang Z, Wu G, Sheng J, Ye B, Huang Z, Xu J, et al. Gasdermin D affects aortic vascular smooth muscle cell pyroptosis and Ang II-induced vascular remodeling. Heliyon. 2023;9:e16619.
Cero FT, Hillestad V, Sjaastad I, Yndestad A, Aukrust P, Ranheim T, et al. Absence of the inflammasome adaptor ASC reduces hypoxia-induced pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol. 2015;309:L378–87.
Yang J, Wang M, Wang S, Li G, Gao Y. Study on ferroptosis pathway that operates in hypertensive brain damage. Clin Exp Hypertens. 2020;42:748–52.
Erkens R, Kramer CM, Luckstadt W, Panknin C, Krause L, Weidenbach M, et al. Left ventricular diastolic dysfunction in Nrf2 knock out mice is associated with cardiac hypertrophy, decreased expression of SERCA2a, and preserved endothelial function. Free Radic Biol Med. 2015;89:906–17.
Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428–38.
Almog T, Kandel Kfir M, Levkovich H, Shlomai G, Barshack I, Stienstra R, et al. Interleukin-1alpha deficiency reduces adiposity, glucose intolerance and hepatic de-novo lipogenesis in diet-induced obese mice. BMJ Open Diabetes Res Care. 2019;7:e000650.
Yuzefovych LV, Pastukh VM, Ruchko MV, Simmons JD, Richards WO, Rachek LI. Plasma mitochondrial DNA is elevated in obese type 2 diabetes mellitus patients and correlates positively with insulin resistance. PLoS One. 2019;14:e0222278.
Kolb H. Obese visceral fat tissue inflammation: from protective to detrimental?. BMC Med. 2022;20. 494.
Salem ESB, Vonberg AD, Borra VJ, Gill RK. Nakamura T. RNAs and RNA-binding proteins in immuno-metabolic homeostasis and diseases. Front Cardiovasc Med. 2019;6:106.
Antoniotti V, Bellone S, Goncalves Correia FP, Peri C, Tini S, Ricotti R, et al. Calreticulin and PDIA3, two markers of endoplasmic reticulum stress, are associated with metabolic alterations and insulin resistance in pediatric obesity: a pilot study. Front Endocrinol (Lausanne). 2022;13:1003919.
Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13:423–44.
Buckman LB, Anderson-Baucum EK, Hasty AH, Ellacott K. Regulation of S100B in white adipose tissue by obesity in mice. Adipocyte. 2014;3:215–20.
Kim J, Lee SK, Shin JM, Jeoun UW, Jang YJ, Park HS, et al. Enhanced biglycan gene expression in the adipose tissues of obese women and its association with obesity-related genes and metabolic parameters. Sci Rep. 2016;6. 30609.
Bolton K, Segal D, McMillan J, Jowett J, Heilbronn L, Abberton K, et al. Decorin is a secreted protein associated with obesity and type 2 diabetes. Int J Obes (Lond). 2008;32:1113–21.
Han CY, Kang I, Harten IA, Gebe JA, Chan CK, Omer M, et al. Adipocyte-derived versican and macrophage-derived biglycan control adipose tissue inflammation in obesity. Cell Rep. 2020;31:107818.
Romo M, Lopez-Vicario C, Perez-Romero N, Casulleras M, Martinez-Puchol AI, Sanchez B, et al. Small fragments of hyaluronan are increased in individuals with obesity and contribute to low-grade inflammation through TLR-mediated activation of innate immune cells. Int J Obes (Lond). 2022;46:1960–9.
Tang H, Liu N, Feng X, Yang Y, Fang Y, Zhuang S, et al. Circulating levels of IL-33 are elevated by obesity and positively correlated with metabolic disorders in Chinese adults. J Transl Med. 2021;19. 52.
Hasan A, Al-Ghimlas F, Warsame S, Al-Hubail A, Ahmad R, Bennakhi A, et al. IL-33 is negatively associated with the BMI and confers a protective lipid/metabolic profile in non-diabetic but not diabetic subjects. BMC Immunol. 2014;15:19.
Hildebrandt X, Ibrahim M, Peltzer N. Cell death and inflammation during obesity: “Know my methods, WAT(son)”. Cell Death Differ. 2023;30:279–92.
Zhang S, Sun Z, Jiang X, Lu Z, Ding L, Li C, et al. Ferroptosis increases obesity: Crosstalk between adipocytes and the neuroimmune system. Front Immunol. 2022;13:1049936.
Xia Y, Zhai X, Qiu Y, Lu X, Jiao Y The Nrf2 in obesity: a friend or foe? Antioxidants (Basel). 2022;11:2067.
Kovanen PT, Pentikainen MO. Decorin links low-density lipoproteins (LDL) to collagen: a novel mechanism for retention of LDL in the atherosclerotic plaque. Trends Cardiovasc Med. 1999;9:86–91.
Tovey Crutchfield EC, Garnish SE, Hildebrand JM The Role of the Key Effector of Necroptotic Cell Death, MLKL, in Mouse Models of Disease. Biomolecules. 2021;11:803.
Yan J, Li S, Zhang Y, Deng Z, Wu J, Huang Z, et al. Cholesterol induces pyroptosis and matrix degradation via msrebp1-driven endoplasmic reticulum stress in intervertebral disc degeneration. Front Cell Dev Biol. 2021;9:803132.
Ioannou GN, Horn CL, Kothari V, Yeh MM, Shyu I, Lee SP, et al. Genetic deletion or pharmacologic inhibition of the Nlrp3 inflammasome did not ameliorate experimental NASH. J Lipid Res. 2023;64:100330.
Kamisako T, Tanaka Y, Kishino Y, Ikeda T, Yamamoto K, Masuda S, et al. Role of Nrf2 in the alteration of cholesterol and bile acid metabolism-related gene expression by dietary cholesterol in high fat-fed mice. J Clin Biochem Nutr. 2014;54:90–4.
Ramage L, Jones AC, Whelan CJ. Induction of apoptosis with tobacco smoke and related products in A549 lung epithelial cells in vitro. J Inflamm (Lond). 2006;3:3.
Le Y, Wang Y, Zhou L, Xiong J, Tian J, Yang X, et al. Cigarette smoke-induced HMGB1 translocation and release contribute to migration and NF-kappaB activation through inducing autophagy in lung macrophages. J Cell Mol Med. 2020;24:1319–31.
Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health. 2009;6:445–62.
Railwah C, Lora A, Zahid K, Goldenberg H, Campos M, Wyman A, et al. Cigarette smoke induction of S100A9 contributes to chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2020;319:L1021–L35.
Mandraffino G, Imbalzano E, Mamone F, Aragona CO, Lo Gullo A, D’Ascola A, et al. Biglycan expression in current cigarette smokers: a possible link between active smoking and atherogenesis. Atherosclerosis. 2014;237:471–9.
Bracke KR, Dentener MA, Papakonstantinou E, Vernooy JH, Demoor T, Pauwels NS, et al. Enhanced deposition of low-molecular-weight hyaluronan in lungs of cigarette smoke-exposed mice. Am J Respir Cell Mol Biol. 2010;42:753–61.
Pouwels SD, Hesse L, Faiz A, Lubbers J, Bodha PK, Ten Hacken NH, et al. Susceptibility for cigarette smoke-induced DAMP release and DAMP-induced inflammation in COPD. Am J Physiol Lung Cell Mol Physiol. 2016;311:L881–L92.
Faiz A, Mahbub RM, Boedijono FS, Tomassen MI, Kooistra W, Timens W, et al. IL-33 Expression is lower in current smokers at both transcriptomic and protein levels. Am J Respir Crit Care Med. 2023;208:1075–87.
Zhang MY, Jiang YX, Yang YC, Liu JY, Huo C, Ji XL, et al. Cigarette smoke extract induces pyroptosis in human bronchial epithelial cells through the ROS/NLRP3/caspase-1 pathway. Life Sci. 2021;269:119090.
Fatokun AA, Dawson VL, Dawson TM. Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharm. 2014;171:2000–16.
Rodriguez A, Chawla K, Umoh NA, Cousins VM, Ketegou A, Reddy MG, et al. Alcohol and Apoptosis: Friends or Foes?. Biomolecules. 2015;5:3193–203.
Hao F, Cubero FJ, Ramadori P, Liao L, Haas U, Lambertz D, et al. Inhibition of Caspase-8 does not protect from alcohol-induced liver apoptosis but alleviates alcoholic hepatic steatosis in mice. Cell Death Dis. 2017;8. e3152.
Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharm. 2010;5:83–91.
Chen Y, Liu M, Wei H, Guo J, Zhang S, Bu X, et al. Alcohol induces hepatocytes necroptosis through the LC3/RIPK1/RIPK3 pathway. Food Chem Toxicol. 2023;182:114124.
Miyata T, Wu X, Fan X, Huang E, Sanz-Garcia C, Ross CKC, et al. Differential role of MLKL in alcohol-associated and non-alcohol-associated fatty liver diseases in mice and humans. JCI Insight. 2021;6:e140180.
Hou S, Wang D, Yuan X, Yuan X, Yuan Q. Identification of biomarkers co-associated with M1 macrophages, ferroptosis and cuproptosis in alcoholic hepatitis by bioinformatics and experimental verification. Front Immunol. 2023;14:1146693.
Li Y, Gao Y, Li G. Preclinical multi-target strategies for myocardial ischemia-reperfusion injury. Front Cardiovasc Med. 2022;9:967115.
Webster I, Salie R, Marais E, Fan WJ, Maarman G, Huisamen B, et al. Myocardial susceptibility to ischaemia/reperfusion in obesity: a re-evaluation of the effects of age. BMC Physiol. 2017;17. 3.
Makkos A, Szantai A, Paloczi J, Pipis J, Kiss B, Poggi P, et al. A comorbidity model of myocardial ischemia/reperfusion injury and hypercholesterolemia in rat cardiac myocyte cultures. Front Physiol. 2019;10:1564.
Miyamae M, Diamond I, Weiner MW, Camacho SA, Figueredo VM. Regular alcohol consumption mimics cardiac preconditioning by protecting against ischemia-reperfusion injury. Proc Natl Acad Sci USA. 1997;94:3235–9.
Montone RA, Rinaldi R, Bonanni A, Severino A, Pedicino D, Crea F, et al. Impact of air pollution on ischemic heart disease: evidence, mechanisms, clinical perspectives. Atherosclerosis. 2023;366:22–31.
Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, et al. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–26.
Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–9.
Zhu P, Hu S, Jin Q, Li D, Tian F, Toan S, et al. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol. 2018;16:157–68.
Xu B, Zhang J, Strom J, Lee S, Chen QM. Myocardial ischemic reperfusion induces de novo Nrf2 protein translation. Biochim Biophys Acta. 2014;1842:1638–47.
Son YJ, Lee HJ. Association between persistent smoking after a diagnosis of heart failure and adverse health outcomes: a systematic review and meta-analysis. Tob Induc Dis. 2020;18. 05.
Triposkiadis F, Sarafidis P, Briasoulis A, Magouliotis DE, Athanasiou T, Skoularigis J, et al. Hypertensive heart failure. J Clin Med. 2023;12:5090.
Aryee EK, Ozkan B, Ndumele CE. Heart failure and obesity: the latest pandemic. Prog Cardiovasc Dis. 2023;78:43–8.
Tada H, Kaneko H, Suzuki Y, Okada A, Takeda N, Fujiu K, et al. Familial hypercholesterolemia is related to cardiovascular disease, heart failure and atrial fibrillation. Results from a population-based study. Eur J Clin Invest. 2024;54:e14119.
Rasoul D, Ajay A, Abdullah A, Mathew J, Lee Wei En B, Mashida K, et al. Alcohol and heart failure. Eur Cardiol. 2023;18:e65.
Jia Y, Lin Z, He Z, Li C, Zhang Y, Wang J, et al. Effect of air pollution on heart failure: systematic review and meta-analysis. Environ Health Perspect. 2023;131:76001.
Kim NH, Kang PM. Apoptosis in cardiovascular diseases: mechanism and clinical implications. Korean Circ J. 2010;40:299–305.
Wang Y, Liu X, Shi H, Yu Y, Yu Y, Li M, et al. NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin Transl Med. 2020;10:91–106.
Li Q, Zhao Z, Zhou X, Yan Y, Shi L, Chen J, et al. Ferroptosis: the potential target in heart failure with preserved ejection fraction. Cells;11. 2022.
Amgalan D, Garner TP, Pekson R, Jia XF, Yanamandala M, Paulino V, et al. A small-molecule allosteric inhibitor of BAX protects against doxorubicin-induced cardiomyopathy. Nat Cancer. 2020;1:315–28.
Cao T, Ni R, Ding W, Ji X, Li L, Liao G, et al. MLKL-mediated necroptosis is a target for cardiac protection in mouse models of type-1 diabetes. Cardiovasc Diabetol. 2022;21:165.
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604.
Jiang K, Tu Z, Chen K, Xu Y, Chen F, Xu S, et al. Gasdermin D inhibition confers antineutrophil-mediated cardioprotection in acute myocardial infarction. J Clin Invest. 2022;132:e151268.
Liu Y, Li H, Wang J, Xue Q, Yang X, Kang Y, et al. Association of cigarette smoking with cerebrospinal fluid biomarkers of neurodegeneration, neuroinflammation, and oxidation. JAMA Netw Open. 2020;3:e2018777.
Turana Y, Shen R, Nathaniel M, Chia YC, Li Y, Kario K. Neurodegenerative diseases and blood pressure variability: a comprehensive review from HOPE Asia. J Clin Hypertens (Greenwich). 2022;24:1204–17.
Neto A, Fernandes A, Barateiro A. The complex relationship between obesity and neurodegenerative diseases: an updated review. Front Cell Neurosci. 2023;17:1294420.
Perna L, Mons U, Stocker H, Beyer L, Beyreuther K, Trares K, et al. High cholesterol levels change the association of biomarkers of neurodegenerative diseases with dementia risk: Findings from a population-based cohort. Alzheimers Dement. 2023;19:2913–22.
Araujo I, Henriksen A, Gamsby J, Gulick D. Impact of alcohol abuse on susceptibility to rare neurodegenerative diseases. Front Mol Biosci. 2021;8:643273.
Roy R, D’Angiulli A. Air pollution and neurological diseases, current state highlights. Front Neurosci. 2024;18:1351721.
Tehranian R, Rose ME, Vagni V, Pickrell AM, Griffith RP, Liu H, et al. Disruption of Bax protein prevents neuronal cell death but produces cognitive impairment in mice following traumatic brain injury. J Neurotrauma. 2008;25:755–67.
Roltsch E, Holcomb L, Young KA, Marks A, Zimmer DB. PSAPP mice exhibit regionally selective reductions in gliosis and plaque deposition in response to S100B ablation. J Neuroinflamm. 2010;7. 78.
Campagna D, Alamo A, Di Pino A, Russo C, Calogero AE, Purrello F, et al. Smoking and diabetes: dangerous liaisons and confusing relationships. Diabetol Metab Syndr. 2019;11:85.
Przezak A, Bielka W, Pawlik A. Hypertension and type 2 diabetes-the novel treatment possibilities. Int J Mol Sci. 2022;23:6500.
Chandrasekaran P, Weiskirchen R. The role of obesity in type 2 diabetes mellitus—an overview. Int J Mol Sci. 2024;25:1882.
Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313:1029–36.
Knott C, Bell S, Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care. 2015;38:1804–12.
Tomita T. Apoptosis in pancreatic beta-islet cells in Type 2 diabetes. Bosn J Basic Med Sci. 2016;16:162–79.
Prasad MK, Mohandas S, Ramkumar KM. Dysfunctions, molecular mechanisms, and therapeutic strategies of pancreatic beta-cells in diabetes. Apoptosis. 2023;28:958–76.
Wang M, Wu H, Wu R, Tan Y, Chang Q. Application of multiple machine learning approaches to determine key pyroptosis molecules in type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2023;14:1112507.
Cheng Q, Pan J, Zhou ZL, Yin F, Xie HY, Chen PP, et al. Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharm Sin. 2021;42:954–63.
Li S, Zheng L, Zhang J, Liu X, Wu Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic Biol Med. 2021;162:435–49.
Wang Y, Zhong J, Zhang X, Liu Z, Yang Y, Gong Q, et al. The role of HMGB1 in the pathogenesis of type 2 diabetes. J Diabetes Res. 2016;2016:2543268.
Kohnert KD, Freyse EJ, Salzsieder E. Glycaemic variability and pancreatic beta-cell dysfunction. Curr Diabetes Rev. 2012;8:345–54.
Hagstrom H. Alcohol, smoking and the liver disease patient. Best Pr Res Clin Gastroenterol. 2017;31:537–43.
Marsano LS, Mendez C, Hill D, Barve S, McClain CJ. Diagnosis and treatment of alcoholic liver disease and its complications. Alcohol Res Health. 2003;27:247–56.
Chiang DJ, McCullough AJ. The impact of obesity and metabolic syndrome on alcoholic liver disease. Clin Liver Dis. 2014;18:157–63.
Jia L. Dietary cholesterol in alcohol-associated liver disease. Immunometabolism (Cobham). 2023;5:e00026.
Mackowiak B, Fu Y, Maccioni L, Gao B. Alcohol-associated liver disease. J Clin Invest. 2024;134:e176345.
Lopez-Bueno JA, Padron-Monedero A, Diaz J, Navas-Martin MA, Linares C. Short-term impact of air pollution, noise and temperature on emergency hospital admissions in Madrid (Spain) due to liver and gallbladder diseases. Environ Res. 2024;249:118439.
Jang YS, Joo HJ, Park YS, Park EC, Jang SI. Association between smoking cessation and non-alcoholic fatty liver disease using NAFLD liver fat score. Front Public Health. 2023;11:1015919.
Ng CH, Wong ZY, Chew NWS, Chan KE, Xiao J, Sayed N, et al. Hypertension is prevalent in non-alcoholic fatty liver disease and increases all-cause and cardiovascular mortality. Front Cardiovasc Med. 2022;9:942753.
Chen Y, Wang W, Morgan MP, Robson T. Annett S. Obesity, non-alcoholic fatty liver disease and hepatocellular carcinoma: current status and therapeutic targets. Front Endocrinol (Lausanne). 2023;14:1148934.
Sinha RA, Bruinstroop E, Singh BK, Yen PM. Nonalcoholic fatty liver disease and hypercholesterolemia: roles of thyroid hormones, metabolites, and agonists. Thyroid. 2019;29:1173–91.
Choi JH, Sohn W, Cho YK. The effect of moderate alcohol drinking in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26:662–9.
Cheng WC, Wong PY, Wu CD, Cheng PN, Lee PC, Li CY. Non-linear association between long-term air pollution exposure and risk of metabolic dysfunction-associated steatotic liver disease. Environ Health Prev Med. 2024;29:7.
Thapaliya S, Wree A, Povero D, Inzaugarat ME, Berk M, Dixon L, et al. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig Dis Sci. 2014;59:1197–206.
Saeed WK, Jun DW, Jang K, Oh JH, Chae YJ, Lee JS, et al. Decrease in fat de novo synthesis and chemokine ligand expression in non-alcoholic fatty liver disease caused by inhibition of mixed lineage kinase domain-like pseudokinase. J Gastroenterol Hepatol. 2019;34:2206–18.
Wang X, Du H, Shao S, Bo T, Yu C, Chen W, et al. Cyclophilin D deficiency attenuates mitochondrial perturbation and ameliorates hepatic steatosis. Hepatology. 2018;68:62–77.
Hatting M, Zhao G, Schumacher F, Sellge G, Al Masaoudi M, Gabetaler N, et al. Hepatocyte caspase-8 is an essential modulator of steatohepatitis in rodents. Hepatology. 2013;57:2189–201.
Shaker ME. The contribution of sterile inflammation to the fatty liver disease and the potential therapies. Biomed Pharmacother. 2022;148:112789.
Peserico D, Stranieri C, Garbin U, Mozzini CC, Danese E, Cominacini L, et al. Ezetimibe prevents ischemia/reperfusion-induced oxidative stress and up-regulates Nrf2/ARE and UPR signaling pathways. Antioxidants (Basel). 2020;9:349.
Xia X, Li J, Liang X, Zhang S, Liu T, Liu J, et al. Ticagrelor suppresses oxidized low‑density lipoprotein‑induced endothelial cell apoptosis and alleviates atherosclerosis in ApoE‑/‑ mice via downregulation of PCSK9. Mol Med Rep. 2019;19:1453–62.
Kong N, Xu Q, Cui W, Feng X, Gao H. PCSK9 inhibitor inclisiran for treating atherosclerosis via regulation of endothelial cell pyroptosis. Ann Transl Med. 2022;10:1205.
Li H, Yang H, Qin Z, Wang Q, Li L. Colchicine ameliorates myocardial injury induced by coronary microembolization through suppressing pyroptosis via the AMPK/SIRT1/NLRP3 signaling pathway. BMC Cardiovasc Disord. 2024;24:23.
Lu N, Cheng W, Liu D, Liu G, Cui C, Feng C, et al. NLRP3-mediated inflammation in atherosclerosis and associated therapeutics. Front Cell Dev Biol. 2022;10:823387.
Hassan MQ, Akhtar MS, Akhtar M, Ali J, Haque SE, Najmi AK. Edaravone protects rats against oxidative stress and apoptosis in experimentally induced myocardial infarction: Biochemical and ultrastructural evidence. Redox Rep. 2015;20:275–81.
Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, et al. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci. 2013;14:13909–30.
Hassan MQ, Akhtar MS, Akhtar M, Ali J, Haque SE, Najmi AKEdaravone. a potent free radical scavenger and a calcium channel blocker attenuate isoproterenol induced myocardial infarction by suppressing oxidative stress, apoptotic signaling and ultrastructural damage. Ther Adv Cardiovasc Dis. 2016;10:214–23.
Li C, Mu N, Gu C, Liu M, Yang Z, Yin Y, et al. Metformin mediates cardioprotection against aging-induced ischemic necroptosis. Aging Cell. 2020;19:e13096.
Zhang J, Huang L, Shi X, Yang L, Hua F, Ma J, et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging (Albany NY). 2020;12:24270–87.
Zhang H, Huang C, Zhang D, Zhu Y. Pioglitazone protects against hypoxia-induced cardiomyocyte apoptosis through inhibiting NLRP3/Caspase-1 pathway in vivo and in vitro. Int Heart J. 2022;63:893–903.
Chen W, Zhang Y, Wang Z, Tan M, Lin J, Qian X, et al. Dapagliflozin alleviates myocardial ischemia/reperfusion injury by reducing ferroptosis via MAPK signaling inhibition. Front Pharm. 2023;14:1078205.
Naoi M, Maruyama W, Shamoto-Nagai M. Neuroprotective function of rasagiline and selegiline, inhibitors of type b monoamine oxidase, and role of monoamine oxidases in synucleinopathies. Int J Mol Sci. 2022;23:11059.
Jami MS, Salehi-Najafabadi Z, Ahmadinejad F, Hoedt E, Chaleshtori MH, Ghatrehsamani M, et al. Edaravone leads to proteome changes indicative of neuronal cell protection in response to oxidative stress. Neurochem Int. 2015;90:134–41.
Song G, Li Y, Lin L, Cao Y. Anti-autophagic and anti-apoptotic effects of memantine in a SH-SY5Y cell model of Alzheimer’s disease via mammalian target of rapamycin-dependent and -independent pathways. Mol Med Rep. 2015;12:7615–22.
Wang H, Wang X, Li Y, Yu H, Wang C, Feng C, et al. Chronic ethanol exposure induces SK-N-SH cell apoptosis by increasing N-methyl-D-aspartic acid receptor expression and intracellular calcium. Exp Ther Med. 2018;15:3791–800.
Xu C, Mei Y, Yang R, Luo Q, Zhang J, Kou X, et al. Edaravone dexborneol mitigates pathology in animal and cell culture models of Alzheimer’s disease by inhibiting neuroinflammation and neuronal necroptosis. Cell Biosci. 2024;14:55.
Chen X, Wang Z, Yang W, Fu Y. Levodopa improves behavioral deficits of mice with parkinson’s disease symptoms via curbing NLRP3 inflammasome activation and enhancing tyrosine hydroxylase levels in the striatum and substantia nigra. J Integr Neurosci. 2024;23:2.
Arab HH, Eid AH, Alsufyani SE, Ashour AM, El-Sheikh AAK, Darwish HW, et al. Targeting autophagy, apoptosis, and oxidative perturbations with dapagliflozin mitigates cadmium-induced cognitive dysfunction in rats. Biomedicines. 2023;11:3000.
Wicinski M, Wodkiewicz E, Slupski M, Walczak M, Socha M, Malinowski B, et al. Neuroprotective activity of sitagliptin via reduction of neuroinflammation beyond the incretin effect: focus on Alzheimer’s disease. Biomed Res Int. 2018;2018:6091014.
Xia P, Pan Y, Zhang F, Wang N, Wang E, Guo Q, et al. Pioglitazone confers neuroprotection against ischemia-induced pyroptosis due to its inhibitory effects on HMGB-1/RAGE and Rac1/ROS pathway by activating PPAR-ɤ. Cell Physiol Biochem. 2018;45:2351–68.
Yang J, Shi X, Wang Y, Ma M, Liu H, Wang J, et al. Multi-target neuroprotection of thiazolidinediones on Alzheimer’s disease via neuroinflammation and ferroptosis. J Alzheimers Dis. 2023;96:927–45.
Ge Q, Zhao L, Ren XM, Ye P, Hu ZY. LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis. Exp Biol Med (Maywood). 2019;244:1028–39.
Kothari V, Galdo JA, Mathews ST. Hypoglycemic agents and potential anti-inflammatory activity. J Inflamm Res. 2016;9:27–38.
Adhikari J, Hirai T, Kawakita E, Iwai K, Koya D, Kanasaki K. Linagliptin ameliorated cardiac fibrosis and restored cardiomyocyte structure in diabetic mice associated with the suppression of necroptosis. J Diabetes Investig. 2023;14:844–55.
Liu P, Zhang Z, Wang J, Zhang X, Yu X, Li Y. Empagliflozin protects diabetic pancreatic tissue from damage by inhibiting the activation of the NLRP3/caspase-1/GSDMD pathway in pancreatic beta cells: in vitro and in vivo studies. Bioengineered. 2021;12:9356–66.
Hamouda AO, Abdel-Hamed AR, Abo-Elmatty DM, Khedr NF, Ghattas MH. Pentoxifylline and its association with kaempferol improve NASH-associated manifestation in mice through anti-apoptotic, anti-necroptotic, antioxidant, and anti-inflammatory mechanisms. Eur Rev Med Pharm Sci. 2022;26:8644–59.
Yang L, Bi L, Jin L, Wang Y, Li Y, Li Z, et al. Geniposide ameliorates liver fibrosis through reducing oxidative stress and inflammatory respose, inhibiting apoptosis and modulating overall metabolism. Front Pharm. 2021;12:772635.
Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, et al. Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in high fat diet fed ApoE((-/-)) mice by activating autophagy and reducing er stress and apoptosis. Int J Mol Sci. 2021;22:818.
Fontanella RA, Ghosh P, Pesapane A, Taktaz F, Puocci A, Franzese M, et al. Tirzepatide prevents neurodegeneration through multiple molecular pathways. J Transl Med. 2024;22. 114.
Acknowledgements
GK was supported by The National Research, Development and Innovation Office NKFIH, 146330, JV was supported by the PhD Excellence Scholarship from the Count István Tisza Foundation for the University of Debrecen and Scholarship for New National Excellence Program of the Ministry for Innovation and Technology., (ÚNKP-23-3-I-DE-338) A.M. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences BO/00952/23/5 and by the ÚNKP-23-5-DE-500 New National Excellence Program of the Ministry for Innovation and Technology. Figures were created using BioRender.
Funding
Open access funding provided by University of Debrecen.
Author information
Authors and Affiliations
Contributions
Conceptualization, ZsM, VJ, AM, and GK; Writing—Original Draft Preparation, MZs, VJ, RM, EM, SD, RCs, AM, and GK—Review and Editing, AB, AM, and GK; Visualization, AM and ZsM; Supervision, GK. Funding Acquisition, JV, AM, and GK. All authors have read and agreed to the published final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited By Massimiliano Agostini
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muszka, Z., Jenei, V., Mácsik, R. et al. Life-threatening risk factors contribute to the development of diseases with the highest mortality through the induction of regulated necrotic cell death. Cell Death Dis 16, 273 (2025). https://doi.org/10.1038/s41419-025-07563-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41419-025-07563-7
This article is cited by
-
Mitochondria as Inducers of Neutrophil Extracellular Traps
Inflammation (2026)
-
Diabetes and periodontitis: the role of a high-glucose microenvironment in periodontal tissue cells and corresponding therapeutic strategies
Stem Cell Research & Therapy (2025)