Abstract
Secretome-based therapies that target angiogenesis are promising for the treatment of ischemic heart disease (IHD). The effects of Chemokine C-C motif ligand (CCL) 28 on IHD remain unclear. In this study, we investigated the role of CCL28 in angiogenesis during IHD in male mice using the myocardial infarction (MI) and hindlimb ischemia (HI) models. The upregulated CCL28/ C-C motif receptor (CCR)10 axis has been observed in HI and MI. Additionally, CCR10 is highly expressed in endothelial cells (ECs). Compared to CCR10- ECs, CCR10+ ECs exhibited robust proangiogenic capacity, which was induced by CCL28 through CCR10/ERK/SOX5 positive feedback signaling. The deletion of CCL28 results in impaired angiogenesis, whereas the use of recombinant CCL28 protein has therapeutic potential for myocardial and hindlimb ischemia, including that in diabetes. Endothelial-specific CCR10 deficiency impairs angiogenesis and blocks the therapeutic effects of rCCL28 in ischemic models. Serum CCL28 levels have a predictive effect on coronary collateral vessels (CCV) in patients with chronic total occlusions. This study highlights the angiogenic role of CCL28 and suggests that recombinant CCL28 protein may be a potential therapeutic option to attenuate IHD.
Similar content being viewed by others
Introduction
Ischemic heart disease (IHD) is the principal cause of the global health burden. Although therapeutic strategies for IHD have progressed greatly, patients experience adverse clinical outcomes, including heart failure and even death1. Angiogenesis is the formation of new vasculature from pre-existing vessels to restore the blood supply to the ischemic area2. Angiogenesis facilitates the establishment of coronary collateralization and reperfusion in the ischemic myocardium, which are essential for prolonging the survival of dying cardiomyocyte3. Dysfunctional angiogenesis is a major problem in advanced heart failure4. Thus, significant efforts have been made to find potential strategies to improve angiogenesis in IHD and peripheral artery diseases.
The endothelial cell (EC) seems to be more sensitive to ischemia than cardiomyocytes. Prolonged ischemia results in irreversible EC dysfunction and apoptosis after myocardial infarction (MI), with impaired angiogenesis and loss of vascular integrity5. Therapeutic stimulation of EC is associated with improved angiogenesis and heart function in the MI model of mice1,6. The endogenous cardiac angiogenesis is insufficient to sustain the demand of the myocardium. Secretome-based therapies have been demonstrated to enhance EC function and angiogenesis following MI7,8,9. For example, myeloid-derived growth factor (MYDGF) secreted by macrophages can promote cardiac angiogenesis and myocyte survival10. The bone marrow-derived endoplasmic reticulum membrane protein complex subunit 10 (EMC10) enhances capillarization in the infarct border-zone and exerts a sustained beneficial effect on left ventricular remodeling11.
Chemokine C-C motif ligand (CCL) 28, also known as mucosa-associated epithelial chemokine, is a highly conserved chemokine that plays a vital role in mucosal immunity, in addition to CCL2712. Hypoxia promotes the recruitment of regulatory T cells by inducing the expression of CCL28, which in turn promotes tumor angiogenesis13. CCL28 exerts chemotactic and immunomodulatory functions by interacting with its chemokine receptors CCR3 and CCR1012. Our previous study reported a protective role for CCL28 in ischemia reperfusion-induced renal injury14. However, the involvement of CCL28 in angiogenesis during IHD remains unclear. In this study, we investigated the role of CCL28 in angiogenesis and its possible underlying mechanisms using MI and hindlimb ischemia (HI) models. In total, 416 chronic total occlusions (CTO) patients were enrolled to evaluate the predictive value of CCL28 for coronary collateral vessels (CCV).
Results
CCL28 is upregulated in myocardial and hindlimb ischemia
The mRNA levels of the homologs chemokine Ccl27/Ccl28 were analyzed using the Single Cell Portal database (https://singlecell.broadinstitute.org) from 99,684 nuclei of cells from the non-infarcted region of the left ventricle of human patients with ischemic cardiomyopathy (n = 8) and non-failing controls (n = 8) (accession number phs001539.v4.p1)15. The snRNA-seq data revealed that Ccl28 was significantly more abundant than Ccl27 in human ischemic cardiomyopathy samples and that Ccl28 was expressed in non-myocardial cells of the heart (Fig. S1A, 1B). In hindlimb ischemia (HI) and myocardial ischemia (MI), the mRNA levels of Ccl27 and Ccl28 were upregulated, and the increase in Ccl28 was more significant than that in Ccl27 (Fig. 1A, 1B). The protein and mRNA levels of CCL28 peaked on the 7 days post HI and 3 days post MI (Fig. 1A–1D). Enzyme-linked immunosorbent assay (ELISA) data also confirmed an increase in serum CCL28 expression in both HI and MI (Fig. S1C). Immunofluorescence staining for CCL28 (red) showed that CCL28 expression was elevated in both HI and MI, with a peak at 7 days post HI and 3 days post MI (Fig. 1E). The gating strategy for CCL28+ cells and their cell subsets were presented in Figure S1D, including immunocytes (CCL28+CD45+ cells), fibroblasts (CCL28+CD45-CD140a+ cells) and ECs (CCL28+CD45-CD31+ cells). CCL28+ cells were most abundant on 7 days post HI and 3 days post MI (Fig. 1F). Furthermore, the largest proportion of CCL28+CD45+ immunocytes was detected on 7 days post HI and 3 days post MI, respectively, within the CCL28+ cell subsets (Fig. S1E–1G). RT-qPCR results showed a significant increase of Ccl28 in the MI and HI groups compared to the sham group in immunocytes and fibroblasts, but not in other cell types (Fig. S1H). The subgroups of CCL28+ immunocytes were subsequently analyzed, and the gating strategy for CCL28+CD45+ immunocytes and their cell subsets was presented in Fig. S1I, including neutrophils (CCL28+CD45+CD11b+Ly6G+ cells), monocytes (CCL28+CD45+CD11b+Ly6G-Ly6C+F4/80- cells), and macrophages (CCL28+CD45+CD11b+Ly6G-Ly6C-F4/80+ cells). Of the CCL28+CD45+ cells, the highest proportion of CCL28+CD45+CD11b+Ly6G-Ly6C-F4/80+ macrophages was observed on the 7 days post HI and 3 days post MI (Fig. 1G, H). Immunofluorescence staining using tyramide signal amplification showed that CCL28 was mainly located in macrophages (CD45+F4/80+ cells) (Fig. S1J). These results suggest that CCL28 is expressed by various cells, primarily macrophages, in both HI and MI.
The hindlimb ischemia (HI) and myocardial ischemia (MI) were constructed on wild-type mice at specific time points (biological replicates, n = 4 per group). A The mRNA levels of Ccl27 and Ccl28 in the gastrocnemius of mice subjected to sham or HI surgery were determined by RT-qPCR at specific time points (biological replicates, n = 4 per group, One-Way ANOVA using Tukey multiple comparisons test). B The mRNA levels of Ccl27 and Ccl28 in the hearts of mice subjected to sham or MI surgery were determined by RT-qPCR at specific time points (biological replicates, n = 4 per group, One-Way ANOVA using Tukey multiple comparisons test). C Representative bands of CCL28 and ACTB protein in the gastrocnemius of mice subjected to sham or HI surgery measured by Western blot assay. Representative bands of CCL28 and ACTB protein in the hearts of mice subjected to sham or MI surgery measured by Western blot assay. D Quantification of CCL28 protein (biological replicates, n = 3 per group, One-Way ANOVA using Tukey multiple comparisons test). E Representative immunofluorescence images and quantification of CCL28 in gastrocnemius slice 7 days post HI and heart slice 3 days post MI with staining for CCL28 (red) and nuclei (blue). Scale bar, 200 μm. (biological replicates, n = 4 per group, One-Way ANOVA using Tukey multiple comparisons test). F Flow cytometry-based representative histogram (isotype control, red; CCL28 staining, blue) and quantification of CCL28+ cells isolated from the gastrocnemius of mice subjected to sham or HI surgery, and the hearts of mice subjected to sham or MI surgery at specific time points (biological replicates, n = 4 per group, One-Way ANOVA using Tukey multiple comparisons test). G Flow cytometry-based representative dot plots of CD11b+Ly6G-Ly6C-F4/80+ cells among the CCL28+CD45+ cells 7 days post HI and 3 days post MI. H The percentage of multiple cells, including neutrophils (black), monocytes (yellow), macrophages (blue), and other cells (red), among the CCL28+ cells 7 days post HI and 3 days post MI (biological replicates, n = 4 per group). Quantitative real-time polymerase chain reaction, RT-qPCR; chemokine C-C motif ligand (CCL). Data are expressed as mean ± SD. Source data are provided as a Source Data file.
Next, we isolated bone marrow-derived macrophages (BMDMs), cardiomyocytes, fibroblasts, and ECs were isolated from normal hearts. IL-4 stimulation promoted the expression and secretion of CCL28 in BMDMs (Fig. S2A, 2B). Hypoxia mediated the expression and secretion of CCL28 in fibroblasts, but not in cardiomyocytes or ECs (Figure S2C–2F).
CCR10+ endothelial cells response to myocardial and hindlimb ischemia
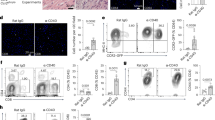
We evaluated the expression of the CCL28 receptor, chemokine C-C motif receptor (CCR) 3, and CCR10 in ischemic diseases. The mRNA levels of Ccr3 and Ccr10 were upregulated in the ischemic myocardium and gastrocnemius muscle, and the increase in Ccr10 expression was more significant than that in Ccr3 (Fig. 2A, 2B). The protein and mRNA expression levels of CCR10 peaked on the 7 days post-HI and 3 days post-MI (Fig. 2A–2D). The gating strategy for CCR10+ cells and their cell subsets were presented in Figure S2G, including immunocytes (CCR10+CD45+ cells), fibroblasts (CCR10+CD45-CD140a+ cells), and ECs (CCR10+CD45-CD31+ cells). CCR10+ cells were the most abundant on 7 days post HI and 3 days post MI (Fig. 2E). Additionally, the largest percentage of CCR10+CD45-CD31+ ECs was observed on 7 days post HI among the CCR10+ cell subsets. Meanwhile, CCR10+CD45+ immunocytes were the most prevalent on 3 days post MI, followed by CCR10+CD45-CD31+ ECs (Fig. 2F). Immunofluorescence staining displayed that CCR10 was highly expressed in ECs on 7 days post HI and 3 days post MI (Fig. 2G). Immunofluorescence staining for isolectin b4 (IB4, red) and CCR10 (green) showed that CCR10 was also located in the capillaries (Fig. S2H). These findings suggest that the CCL28-CCR10 axis is involved in EC function in HI and MI, and that CCR10+ ECs may play an important role in ischemic diseases.
The hindlimb ischemia (HI) and myocardial ischemia (MI) were constructed on wild-type mice at specific time points (biological replicates, n = 4 per group). A The mRNA levels of Ccr3 and Ccr10 in the gastrocnemius of mice subjected to sham or HI surgery were determined by RT-qPCR at specific time points (biological replicates, n = 4 per group, One-Way ANOVA using Tukey multiple comparisons test). B The mRNA levels of Ccr3 and Ccr10 in the hearts of mice subjected to sham or MI surgery were determined by RT- qPCR at specific time points (biological replicates, n = 4 per group, One-Way ANOVA using Tukey multiple comparisons test). C Representative bands of CCR10 and ACTB protein in the gastrocnemius of mice subjected to sham or HI surgery measured by Western blot assay. Representative bands of CCR10 and ACTB protein in the hearts of mice subjected to sham or MI surgery measured by Western blot assay. D Quantification of CCR10 protein (biological replicates, n = 3 per group, One-Way ANOVA using Tukey multiple comparisons test). E Flow cytometry-based representative histogram (isotype control, red; CCR10 staining, blue) and quantification of CCR10+ cells isolated from the gastrocnemius of mice subjected to sham or HI surgery, and the hearts of mice subjected to sham or MI surgery at specific time points (biological replicates, n = 4 per group, One-Way ANOVA using Tukey multiple comparisons test). F Flow cytometry-based representative dot plots of CCR10+CD45-CD31+CD140a- cells 7 days post HI and 3 days post MI. The percentage of multiple cells, including endothelial cells (black), immunocytes (yellow), fibroblasts (blue), and others (red), among the CCR10+ cells 7 days post HI and 3 days post MI (biological replicates, n = 4 per group). G Immunofluorescence images of gastrocnemius slice 7 days post HI and heart slice 3 days post MI with staining for CCR10 (green), CD31 (red) and nuclei (blue) (n = 1). Scale bar, 100μm. The colocalization of CCR10 (green) and CD31 (red) was analyzed by Pearson’s correlation. quantitative real-time polymerase chain reaction, RT-qPCR; chemokine C-C motif receptor, CCR. Data are expressed as mean ± SD. Source data are provided as a Source Data file.
CCR10+ endothelial cells are characterized by an enhanced proangiogenic capacity
Flow cytometry was performed to evaluate Ki67 expression levels in CCR10- and CCR10+ ECs on 7 days post HI and 3 days post MI (Fig. S3A). The mean fluorescence intensity (MFI) of KI67 was higher in CCR10+ ECs than that in CCR10- ECs, indicating that CCR10+ ECs have a strong proliferate ability (Fig. 3A, 3B). Next, we used flow cytometry to sort CCR10- and CCR10+ ECs and then examined their endothelial function (Fig. 3C). Annexin V/propidium iodide (PI) staining was utilized to evaluate the anti-apoptotic ability of CCR10- and CCR10+ ECs after 24 h of hypoxia treatment. The results of flow cytometry demonstrated that the apoptosis level of CCR10+ ECs was significantly lower than that of CCR10- ECs (Fig. 3D). Wound healing assays showed that CCR10+ ECs had a stronger migration ability than CCR10- ECs (Fig. 3E). We performed a spheroid-based sprouting assay to study angiogenesis. Interestingly, a significant increase in both sprout number and length was observed in CCR10+ ECs compared with that in CCR10- ECs (Fig. 3F, 3G). Tube formation assay showed that more tube numbers were observed in CCR10+ ECs to CCR10- ECs (Fig. 3H). Finally, we constructed an EC aging model using hydrogen peroxide and found that CCR10+ ECs could resist the aging stimuli (Fig. 3I). In addition, RT-qPCR analysis revealed that the expression of angiogenic programs in CCR10+ ECs was higher than that in CCR10- ECs, including IGF-1, VEGF-A and FGF-2 (Fig. 3J). In addition, we detected the response of CCR10+ ECs to CCL28 and found that CCL28 promoted angiogenesis in CCR10+ endothelial cells (Fig. S3B). These in vivo and in vitro experiments showed that CCR10+ ECs were characterized by an enhanced proangiogenic capacity.
A, B Flow cytometry-based representative histogram and quantification of fluorescence intensity of KI67 for CD45-CD140a-CD31+CCR10- cells (blue) and CD45-CD140a-CD31+CCR10+ cells (red) 7 days post hindlimb ischemia (HI) and 3 days post myocardial ischemia (MI) (biological replicates, n = 6 per group, Unpaired two-tailed Student’s t-test). C Gating strategy for flow cytometric sorting of CD45-CD140a-CD31+CCR10- cells and CD45-CD140a-CD31+CCR10+ cells from the hearts of mice subjected to MI surgery at 3 days. D The apoptotic proportion (biological replicates, n = 5 per group, Unpaired two-tailed Student’s t-test), E migration ability (biological replicates, n = 4 per group, Two-Way ANOVA using Tukey multiple comparisons test. scale bar, 200 μm), F, G sprouting ability (biological replicates, n = 4 per group, Unpaired two-tailed Student’s t-test. scale bar, 100 μm), H tube formation ability (biological replicates, n = 4 per group, Unpaired two-tailed Student’s t-test. scale bar, 500μm), I aging proportion (biological replicates, n = 6 per group, Unpaired two-tailed Student’s t-test. scale bar, 250μm), and J mRNA levels of angiogenic genes IGF-1, VEGF-A and FGF-2 in CCR10- and CCR10+ endothelial cell (biological replicates, n = 4 per group, Unpaired two-tailed Student’s t-test). Data are expressed as mean ± SD. Source data are provided as a Source Data file.
CCL28 activates the CCR10/ERK/SOX5 positive feedback signaling in endothelial cells
Given the consistent correlation between the number of CCR10+ ECs and CCL28 expression in myocardial and hind limb ischemia, CCL28 was considered to be promoting the expression of CCR10 in ECs. The recombinant CCL28 protein (rCCL28) upregulated the levels of CCR10 mRNA and protein in a concentration-dependent manner (Fig. 4A, Fig. S3C). We also found that 10 ng/mL recombinant CCL28 protein (rCCL28) increased CCR10 protein levels in BEND.3 cells (Fig. S3C). The flow cytometry results also confirmed that rCCL28 increased the proportion of CCR10+ ECs in a concentration-dependent manner (Fig. 4B, S3D). Ccl28 global knockout mice (Ccl28-KO) were generated (Fig. S3E) and Ccl28 was successfully deleted on 3 days post MI, as confirmed by RT-qPCR and Western blot assay (Fig. S3F, 3G). H&E staining and echocardiography revealed no differences between the WT and Ccl28-KO mice under physiological conditions (Fig. S3H). RNA-seq was performed using CD45-CD31+ ECs magnetic bead sorted from WT and Ccl28-KO mice (Fig. S4A). Gene set enrichment analysis (GSEA) showed that Ccl28 deletion inhibited angiogenesis and blood vessel remodeling, and promoted oxidative stress and redox pathways (Fig. 4C). A volcano plot of the differential RNA is shown in Fig. S4B, from which the three most significantly downregulated transcription factors were the transcription factor CP2-like 1 (Tfcp2l1), double homeobox B-like 1 (Duxbl1), and sex determining region Y-box protein 5 (Sox5) (Fig. S4C). RT-qPCR data revealed that si-Sox5, rather than si-Tfcp2l1 and si-Duxbl1, decreased the mRNA level of Ccr10 (Fig. S4D). These genes were involved in mitogen-activated protein kinase (MAPK) signaling, chemokine signaling, and inflammatory response (Fig. S4E–S4G).
Endothelial cells (ECs) are sorted by anti-CD31 magnetic beads and used for subsequent detection. A The mRNA levels of Ccr10 in ECs treated with mouse recombinant CCL28 protein (rCCL28) at different concentrations for 24 h were determined by RT-qPCR (biological replicates, n = 4 per group, One-Way ANOVA using Tukey multiple comparisons test). B Flow cytometry-based representative dot plots and quantification of CCR10+ ECs treated with rCCL28 at different concentrations (biological replicates, n = 4 per group, One-Way ANOVA using Tukey multiple comparisons test). C Gene set enrichment analysis (GSEA) for endothelial cells isolated from hearts of the Ccl28-KO (KO) and littermate control mice (WT) mice subjected to MI surgery at 3 days, as tested by RNA-sequencing. D Representative bands of p-ERK1/2, ERK1/2, VEGFA, SOX5, CCR10 and ACTB protein measured by Western blot assay (biological replicates, n = 3 per group). E Representative bands of SOX5, CCR10 and ACTB protein measured by Western blot assay (biological replicates, n = 3 per group). F Western blot analysis of input proteins and proteins immunoprecipitated with either IgG or SOX5 antibody. G ChIP analysis of the Ccr10 promoter site 3 (−400 to +200) in ECs by RT-qPCR (biological replicates, n = 3 per group, Unpaired two-tailed Student’s t-test). H Dual-luciferase reporter assay was performed after co-transfection with a reporter vector in NIH/3T3 (biological replicates, n = 4 per group, Two-Way ANOVA using Tukey multiple comparisons test). I, J The sprouting ability (biological replicates, n = 4 per group, Unpaired two-tailed Student’s t-test. scale bar, 100 μm) and K aging proportion (biological replicates, n = 4 per group, Unpaired two-tailed Student’s t-test. scale bar, 250 μm) of ECs were measured. L Mechanism diagram of CCL28-induced CCR10/ERK/SOX5 positive feedback signaling. Data are expressed as mean ± SD. Source data are provided as a Source Data file.
SOX5 involves in angiogenesis16 and EC function17,18. MAPK/extracellular-signal-regulated kinase (ERK) signaling is the main regulator of angiogenesis19. It is assumed that the CCL28-CCR10 axis mediates angiogenesis through MAPK signaling and SOX5. We found that rCCL28 upregulated the protein expression of p-ERK1/2, VEGFA, SOX5, and CCR10, whereas the ERK1/2 inhibitor Ravoxertinib and CCR10 inhibitor BI-6901 blocked CCL28-mediated increases in these proteins (Fig. 4D and S4H). SOX5 knockdown resulted in a reduction of CCR10 expression during both control and rCCL28 stimulation (Fig. 4E and S4I). The JARSPAR database predicted the presence of SOX5 binding sites in the Ccr10 promoter region (site1: −2000 to −1400; site 2: −1000 to −400; site 3: −400 to +200) (Fig. S5A). To confirm this, a chromatin immunoprecipitation (ChIP)-qPCR assay was performed on ECs using either an anti-SOX5 antibody or an immunoglobulin G (IgG) control (Fig. 4F). Ccr10 promoter region 3 (−400 to +200) was preferentially enriched after immunoprecipitation with the anti-SOX5 antibody compared to the IgG control (Fig. 4G and S5B). Dual-luciferase assays confirmed that SOX5 knockdown or Ravoxertinib inhibited the transcriptional activity of pGL3-promoter containing the Ccr10 promoter region (−400 to +200). Additionally, rCCL28 stimulation promoted its transcriptional activity and this increase was reversed by SOX5 knockdown or Ravoxertinib (Fig. 4H and S5C). Finally, the role of SOX5 in EC function was evaluated. The ability of ECs to sprout and resist aging was suppressed by SOX5 knockdown. Furthermore, SOX5 knockdown blocked the enhancement of sprouting and anti-aging abilities induced by rCCL28 (Figs. 4I–K and S5D). In addition, we explored the crosstalk between macrophages and endothelial cells using in vitro co-cultures. The results showed that M2 macrophages promoted the expression of CCR10 and tube formation ability, while the CCL28 antibody inhibited these effects (Fig. S5E, F). We investigated the effect of CCL28 on human endothelial cells (HMEC-1). The results showed that rCCL28 promote the expression of CCR10 and the ability of tube formation in HMEC-1 (Fig. S5G, H). Taken together, CCL28 enhances endothelial ability by activating CCR10/ERK/SOX5 positive feedback signaling (Fig. 4L).
CCL28 is required for post-ischemic revascularization
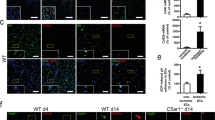
WT and Ccl28-KO mice were subjected to femoral artery ligation in one lower limb. RT-qPCR and Western bolt assays showed reduced CCR10 expression in Ccl28-KO mice on 7 days post HI (Fig. S6A–C). A decrease in the proportion of CCR10+CD31+ ECs was observed in Ccl28-KO mice compared with WT mice (Fig. S6D). We found that Ccl28 knockout significantly attenuated blood flow recovery after femoral artery ligation, as examined by Laser Doppler imaging (Fig. 5A). Immunofluorescence analysis of ECs by CD31 and IB4 staining revealed a decrease in vascular density in the ischemic gastrocnemius muscle of Ccl28-KO mice compared to that in WT mice (Fig. 5B and S6E). Furthermore, Ccl28 knockout exacerbated gastrocnemius fibrosis, as assessed by Masson’s trichrome staining (Fig. 5C).
The hindlimb ischemia (HI) and myocardial ischemia (MI) were constructed on Ccl28-KO (KO) and littermate control mice (WT). A Representative images and quantification of blood flow in mice examined by laser Doppler imaging at specific time points (biological replicates, n = 8 per group, Two-Way ANOVA using Tukey multiple comparisons test). B Representative immunofluorescence images and quantification of vessel numbers in the WT and Ccl28-KO mice of gastrocnemius 7 days post HI with staining for CD31 (red) and nuclei (blue) (biological replicates, n = 5 per group, Unpaired two-tailed Student’s t-test). Scale bar, 200 μm. C The percentage of collagen volume fraction in the WT and Ccl28-KO mice of gastrocnemius 14 days post HI was assessed by Masson’s trichrome staining (biological replicates, n = 5 per group, Unpaired two-tailed Student’s t-test). Scale bar, 100 μm. D Representative immunofluorescence images and quantification of vessel numbers in the WT and Ccl28-KO of hearts 7 days post MI with staining for CD31 (red) and nuclei (blue) (biological replicates, n = 5 per group, Unpaired two-tailed Student’s t-test). Scale bar, 200 μm. E, F The percentage of infarct size and collagen volume fraction in the WT and Ccl28-KO of hearts 7 days post MI were assessed by Masson’s trichrome staining (biological replicates, n = 4 per group, Unpaired two-tailed Student’s t-test). Scale bar, 1 mm. G Echocardiography was performed to assess cardiac function of the left ventricle in the WT and Ccl28-KO mice subjected to sham or MI surgery at specific time points (biological replicates, n = 6 per group, Two-Way ANOVA using Tukey multiple comparisons test). H The FITC-Dextran (green) penetrance was used to detect vascular leakage in the WT and Ccl28-KO of hearts 3 days post MI (biological replicates, n = 6 per group, Unpaired two-tailed Student’s t-test). Scale bar, 100 μm. Data are expressed as mean ± SD. Source data are provided as a Source Data file.
Then, we assessed the role of CCL28 in myocardial ischemia. It was observed that CCR10 expression decreased (Fig. S6A–C) as well as the proportion of CCR10+CD31+ ECs (Fig. S6D) in Ccl28-KO mice in contrasted to WT mice on 3 days post MI. Additionally, there was a notable decrease in vascular numbers in Ccl28-KO hearts compared to WT controls (Fig. 5D and S6E). Furthermore, Ccl28-KO mice exhibited increased infarct size and cardiac fibrosis compared with WT controls (Fig. 5E, 5F). Echocardiography was performed on WT and Ccl28-KO mice that underwent sham or MI surgery on the 3 days and 7 days post MI. The results showed that Ccl28 deficiency markedly impaired cardiac function and worsened heart enlargement after MI (Figs. 5G, S6F). Furthermore, Ccl28-KO hearts demonstrated increased coronary permeability following tail vein injection of FITC-dextran (Fig. 5H).
Recombinant CCL28 protein promotes angiogenesis in myocardial and hindlimb ischemia
The hindlimb ischemia model mice were administered with an intraperitoneal injection of rCCL28 (50 μg/kg, every four days) or vehicle. We found the increased CCR10 expression (Fig. S6G–I) and the proportion of CCR10+CD31+ ECs (Fig. S6J) in the rCCL28 group compared to the vehicle group on 7 days post HI. Laser Doppler imaging showed the improved blood flow recovery after femoral artery ligation following treatment with rCCL28 (Fig. 6A). Further staining results demonstrated that rCCL28 increased vascular numbers (Fig. 6B and S6E) and reduced gastrocnemius fibrosis (Fig. 6C). Further, we evaluate the therapeutic potential of rCCL28 (50 μg/kg, every four days) in MI. An increase in CCR10 expression (Fig. S6G–I) and the proportion of CCR10+CD31+ ECs (Fig. S6J) was observed under the rCCL28 treatment on 3 days post MI. The intervention of rCCL28 promoted cardiac angiogenesis (Fig. 6D and S6K) and improved cardiac function, remodeling (Fig. 6E–G and S6L), and coronary permeability (Fig. 6H).
The hindlimb ischemia (HI) and myocardial ischemia (MI) were constructed on wild-type mice treated with mouse recombinant CCL28 protein (rCCL28, 50 μg/kg, every four days) or vehicle. A Representative images and quantification of blood flow in mice examined by laser Doppler imaging at specific time points (biological replicates, biological replicates, n = 8 per group, Two-Way ANOVA using Tukey multiple comparisons test). B Representative immunofluorescence images and quantification of vessel numbers in the Vehicle and rCCL28 groups of gastrocnemius 7 days post HI with staining for CD31 (red) and nuclei (blue) (biological replicates, n = 5 per group, Unpaired two-tailed Student’s t-test). Scale bar, 200 μm. C The percentage of collagen volume fraction in the Vehicle and rCCL28 groups mice of gastrocnemius 14 days post HI was assessed by Masson’s trichrome staining (biological replicates, n = 5 per group, Unpaired two-tailed Student’s t-test). Scale bar, 100 μm. D Representative immunofluorescence images and quantification of vessel numbers in the Vehicle and rCCL28 groups of hearts 3 days post MI with staining for CD31 (red) and nuclei (blue) (biological replicates, n = 5 per group, Unpaired two-tailed Student’s t-test). Scale bar, 200 μm. E, F The percentage of infarct size and collagen volume fraction in the Vehicle and rCCL28 groups of hearts 7 days post MI were assessed by Masson’s trichrome staining (biological replicates, n = 4 per group, Unpaired two-tailed Student’s t-test). Scale bar, 1 mm. G Echocardiography was performed to assess cardiac function of the left ventricle in the Vehicle and rCCL28 groups subjected to sham or MI surgery at specific time points (biological replicates, n = 6 per group, Two-Way ANOVA using Tukey multiple comparisons test). H The FITC-Dextran (green) penetrance was used to detect vascular leakage in the Vehicle and rCCL28 groups of hearts 3 days post MI (biological replicates, n = 6 per group, Unpaired two-tailed Student’s t-test). Scale bar, 100 μm. Data are expressed as mean ± SD. Source data are provided as a Source Data file.
We then investigated whether rCCL28 could be used to treat Ccl28-knockout mice. Treatment with rCCL28 improved blood flow recovery (Fig. S7A), increased vascular numbers (Fig. S7B), and reduced gastrocnemius fibrosis (Fig. S7D) following hind limb ischemia in KO mice. Similar therapeutic effects of rCCL28 were also demonstrated in KO mice with myocardial infarction (Fig. S7C and S7E, F).
The proangiogenic effect of CCL28 depends on CCR10+ ECs
Considering the CCL28-CCR10 regulatory axis, we speculated that CCL28 promotes angiogenesis in CCR10+ endothelial cells. AAV9-Tie1-sh-CCR10 (2 × 1011 vg/mouse) was transfected into endothelial cells via tail vein injection20. After 4 weeks, we examined the expression of CCR10 and found that the mRNA level of CCR10 in ECs from the hearts and gastrocnemius (Fig. S8A) and the proportion of CCR10+CD45-CD31+ ECs were significantly reduced by AAV9-Tie1-sh-CCR10 compared to AAV9-Tie1-sh-Control (Fig. S8B, C).
Mice subjected to tail vein injections of AAV9-Tie1-sh-CCR10 or AAV9-Tie1-sh-Control for 4 weeks underwent femoral artery ligation in one lower limb. Hindlimb ischemia model mice were administered with an intraperitoneal injection of rCCL28 (50 μg/kg, every four days) or vehicle. Laser Doppler imaging showed that the downregulation of CCR10 resulted in impaired blood flow recovery and blocked the therapeutic effect of rCCL28 (Fig. S8D). Further staining that demonstrated AAV9-Tie1-sh-CCR10 decreased vascular numbers (Fig. S8E) and increased gastrocnemius fibrosis (Fig. S8F) compared to AAV9-Tie1-sh-Control. The effect of rCCL28 on angiogenesis and fibrosis is blocked by AAV9-Tie1-sh-CCR10 (Fig. S8E, F).
We determined the effect of AAV9-Tie1-sh-CCR10 on MI. Intervention with AAV9-Tie1-sh-CCR10 inhibited cardiac angiogenesis (Fig. S9A) and impaired remodeling (Fig. S9B), cardiac function (Fig. S9C), and coronary permeability (Fig. S9D). In addition, tail vein injection of AAV9-Tie1-sh-CCR10 blocked the therapeutic effect of rCCL28 in MI (Fig. S9A–D). Taken together, these results indicate that CCR10 is crucial for endothelial angiogenesis, and the pro-angiogenic effect of CCL28 depends on CCR10+ ECs.
Recombinant CCL28 protein ameliorates revascularization disorder in diabetic mice
Diabetes impairs endothelial function and angiogenesis21. Here, we investigated the role of the CCL28-CCR10 axis in myocardial and hindlimb ischemia in diabetes. Western blot analysis showed that CCL28 and CCR10 expression was reduced in HI and MI of db/db mice compared to that in WT mice on 7 days post HI and 3 days post MI (Figs. 7A–D and S10A). The proportion of CCR10+CD31+ ECs decreased in diabetic mice (Fig. 7E–H). Further, we evaluate the therapeutic potential of rCCL28 (50 μg/kg, every four days) in HI and MI of db/db mice. Immunofluorescence analysis indicated increased vascular density in the diabetic ischemic gastrocnemius and heart treated with rCCL28 (Fig. 7I–L and Fig. S10B). Treatment with rCCL28 significantly improved blood flow recovery after femoral artery ligation, as shown by Laser Doppler imaging (Fig. 7M). And gastrocnemius fibrosis, and cardiac infarct size and fibrosis were mitigated by treatment of rCCL28 (Fig. S10C–D). Angiogenesis is related to wound healing and we observed that rCCL28 accelerated wound healing caused by lower limb femoral artery ligation in db/db mice (Fig. S10E).
A–H the hindlimb ischemia (HI) and myocardial ischemia (MI) were constructed on wild-type and db/db mice. A Representative bands of CCL28 and ACTB protein in the gastrocnemius of mice subjected to sham or HI surgery at 7 days was measured by Western blot assay (biological replicates, n = 3 per group). B Representative bands of CCL28 and ACTB protein in the hearts of mice subjected to sham or MI surgery at 3 days measured by Western blot assay (biological replicates, n = 3 per group). C Representative bands of CCR10 and ACTB protein in the gastrocnemius of mice subjected to sham or HI surgery at 7 days measured by Western blot assay (biological replicates, n = 3 per group). D Representative bands of CCR10 and ACTB protein in the hearts of mice subjected to sham or MI surgery at 3 days measured by Western blot assay (biological replicates, n = 3 per group). E–H Flow cytometry-based representative dot plots and quantification of CCR10+CD31+ endothelial cells isolated from the gastrocnemius of mice subjected to sham or HI surgery at 7 days, and the hearts of mice subjected to sham or MI surgery at 3 days (biological replicates, n = 4 per group, Unpaired two-tailed Student’s t-test). I–M the hindlimb ischemia (HI) and myocardial ischemia (MI) were constructed on db/db mice treated with mouse recombinant CCL28 protein (rCCL28, 50 μg/kg, every four days) or vehicle. I–L Representative immunofluorescence images and quantification of vessel numbers in the Vehicle and rCCL28 groups of gastrocnemius 7 days post HI, and hearts 3 days post MI with staining for CD31 (red) and nuclei (blue) (biological replicates, n = 5 per group, Unpaired two-tailed Student’s t-test). Scale bar, 200 μm. M Representative images and quantification of blood flow in mice examined by laser Doppler imaging at specific time points (biological replicates, n = 8 per group, Two-Way ANOVA using Tukey multiple comparisons test). Data are expressed as mean ± SD. Source data are provided as a Source Data file.
Low serum levels of CCL28 are associated with poor coronary collateralization in patients with chronic total occlusion
This nested case-control study was conducted in a cohort of 493 consecutive patients with stable angina and CTO between February 2019 and September, 2023. Seventy-seven patients were excluded for the following reasons: pregnancy, lactation or malignant tumor with<1-year life expectancy (9), chronic heart failure (22), pulmonary heart disease (6), immune system disorders (2), history of coronary artery bypass grafting (3), percutaneous coronary intervention within the prior 3 months (31), and intermittent claudication (4). Finally, 416 patients were enrolled in the study (Fig. 8A). Rentrop scores of 0, 1, 2, and 3 were observed in 24, 133, 145, and 114 patients, respectively. Baseline characteristics according to Rentrop scores are detailed in Table S1. Serum levels of CCL28 gradually increased across the Rentrop scores of groups 0, 1, 2, and 3 (p < 0.05) (Fig. 8B). The levels of CCL28 were significantly lower in patients with a poorer CCV cluster (Rentrop score 0 and 1 groups) than in those with a better CCV cluster (Rentrop score 2 and 3 groups) [582 (521-666) vs. 629 (571–792) pg/mL, p < 0.001]. CCL28 levels were significantly correlated with the Rentrop score (Spearman’s r = 0.2967, p < 0.0001). The association between CCL28 and Rentrop scores was investigated using univariate and multivariate logistic regression analyses. In Model 1, after adjusting for age and sex, CCL28 was correlated with Rentrop score (OR, 2.373; 95% CI, 1.582–3.586; p < 0.0001). In Model 2, after adjusting for sex, age, body mass index, smoking, hypertension, diabetes, prior myocardial infarction, and serum creatinine (sCr), the association between CCL28 and Rentrop score remained significant (OR, 2.133; 95% CI, 1.392–3.289; p = 0.0005) (Table 1). In the receiver operating characteristic (ROC) curve analysis, the area under the curve (AUC) was 0.650 (95% CI 0.595–0.705, p < 0.0001) for serum levels of CCL28 in predicting poor CCV (Rentrop scores 0 and 1), with an optimal cutoff point of 605 pg/mL (sensitivity, 59.1%; specificity, 63.1%) (Fig. 8C). CCL28 significantly improved the prediction efficacy for the Rentrop score in addition to traditional CTO risk factors including sex, age, body mass index, smoking, hypertension, diabetes, prior myocardial infarction, and sCr. ROC analysis showed AUC of 0.657 (95%CI 0.604–0.711) and 0.695 (95%CI 0.642–0.746) for traditional CTO risk factors and traditional CTO risk factors+CCL28, respectively. The CTO risk model+CCL28 had higher AUC values than the CTO risk model alone (0.695 vs.0.657, p = 0.0115) (Fig. 8D). Moreover, the addition of CCL28 to the conventional model improved risk reclassification (0.388, 95%CI: 0.205–0.572, p < 0.0001) and integrated discrimination improvement (0.035, 95%CI: 0.020–0.049, p < 0.0001) for the Rentrop score (Table 2).
A Flowchart of study participants. B The serum CCL28 levels were assessed by ELISA assay (biological replicates, the numbers of participants with Rentrop score 0, 1, 2, 3 were 24, 133, 145, and 114, respectively. Kruskal-Wallis test using Dunn multiple comparisons test). In detail, the boxplots depict the distribution of CCL28 levels across four Rentrop classifications (Rentrop 0–Rentrop 3). Each box represents the interquartile range (IQR), with the lower boundary corresponding to the 25th percentile (Q1) and the upper boundary to the 75th percentile (Q3); the median is indicated by a central line. The whiskers extend to the 10th and 90th percentiles, respectively, capturing the central 80% of the data, while the minimum and maximum values within each Rentrop group are explicitly marked to illustrate the full data range. Specifically, for Rentrop0, the median is 499.5 (Q1: 493.25, Q3: 561.5) pg/mL, with whiskers spanning from 447.5 (10th percentile) to 738.5 (90th percentile) pg/mL, and the minimum and maximum values are 408 and 807 pg/mL, respectively; for Rentrop1, the median is 592 (Q1: 545, Q3: 686) pg/mL, with whiskers from 507.8 to 833.2 pg/mL, and the minimum and maximum values are 411 and 1832 pg/mL; for Rentrop2, the median is 611 (Q1: 567, Q3: 746) pg/mL, with whiskers from 534.6 to 1010 pg/mL, and the minimum and maximum values are 514 and 2519 pg/mL; and for Rentrop3, the median is 679.5 (Q1: 576.25, Q3: 826.75) pg/mL, with whiskers from 545.5 to 1162.5 pg/mL, and the minimum and maximum values are 408 and 2230 pg/mL. C Receiver operating characteristic curve analysis of CCL28 for predicting CCV (red). D Receiver operating characteristic curve analysis of CTO risk model (red) and its modifications (blue) for predicting CCV. E Graphical abstract of main findings. Source data are provided as a Source Data file.
Discussion
Therapeutic strategies for neovascularization have gradually been approved as valid approaches to improve the prognosis of IHD5,10,22,23. In this study, we identified CCL28 as a notable factor promoting post-ischemic angiogenesis. CCL28 is secreted by various resident cells, mainly macrophages, and its deletion in mice results in impaired angiogenesis during myocardial and hindlimb ischemia. Compared to CCR10- ECs, CCR10+ ECs present robust endothelial functions, including proliferation, migration, sprouting, tube formation, anti-apoptosis, and anti-senescence, which are induced by CCL28 through CCR10/ERK/SOX5 positive feedback signaling. The application of recombinant CCL28 protein has therapeutic potential in myocardial and hindlimb ischemia, including that in diabetes (Fig. 8E).
CCL27 and CCL28 are the homologous chemokines24. CCL28 expression was notably upregulated following myocardial infarction and hindlimb ischemia compared to that of CCL27. Additionally, we confirmed that various cells in the myocardium and gastrocnemius secrete CCL28, primarily macrophages. Previous studies have reported that CCL28 is expressed by resident cells such as mucosal epithelial cells25, tubular epithelial cells14, and tumor cells13. In contrast, in the ischemic myocardium and gastrocnemius, recruited macrophages are the primary source of CCL28. CCR3/CCR10, identified as CCL28 receptors, have been extensively reported to be involved in B-cell and T-cell recruitment and immune response12,26. We constructed a model of myocardial infarction and hindlimb ischemia and found that CCR10 expression was more significantly upregulated than CCR3 expression in the myocardium and gastrocnemius. Further results showed that CCR10 is mainly expressed in ECs, suggesting that the CCL28-CCR10 axis is involved in EC function in myocardial and hindlimb ischemia.
The role of CCR10 in ECs remains unclear. Chen et al. reported that CCR10 inhibits angiogenesis and skin wound healing in both control and diabetic mice27,28 and that ligation of joint CCL28 to CCR10+ ECs promotes rheumatoid arthritis angiogenesis29. These inconsistent findings regarding the role of CCR10 in ECs may be attributed to different mechanisms of pathogenesis mechanisms. In this study, cardiac CCR10+ ECs were characterized by enhanced angiogenesis, including proliferation, migration, sprouting, tube formation, anti-apoptosis, and anti-senescence effects. Functional identification of CCR10+ ECs has been reported for the first time, suggesting that targeting CCR10+ ECs is a potential strategy for improving microcirculatory dysfunction.
In the present study, using RNA-seq analysis, we found that cardiac ischemic ECs lacking stimulation from CCL28 showed a decrease in the ability of angiogenesis and blood vessel remodeling. CCL28 deficiency leads to the inactivation of CCR10/ERK/SOX5 positive feedback signaling in ECs. Previous studies have shown that angiogenesis driven by the ligation of CCL28 to CCR10 is linked to the ERK cascade29. Our results supplement previous findings that the CCR10/ERK cascade triggered by CCL28 is not only crucial for maintaining endothelial function, but also promotes SOX5-dependent Ccr10 transcription and forms a positive feedback loop.
Given that CCL28 is secreted by multiple cell types, Ccl28 global knockout mice were generated to investigate the role of CCL28 in ischemic diseases. Ccl28 deletion decreases blood flow recovery and aggravates gastrocnemius fibrosis in hindlimb ischemia. In MI, CCL28 is essential for maintaining cardiac function and inhibiting adverse remodeling. Our previous studies consistently showed that CCL28 protects the kidney from ischemia-reperfusion injury14. Importantly, we emphasize that the recombinant CCL28 has clinical translational potential. Administration of CCL28 promotes post-ischemic angiogenesis and improves functional recovery in mice with femoral or coronary artery occlusion. Our results identified CCL28 as a wide-spectrum cardiovascular protective factor against ischemic disease. Moreover, we found that endothelial-specific CCR10 deficiency impaired angiogenesis and blocked the therapeutic effect of rCCL28 in myocardial and hindlimb ischemia, confirming that the proangiogenic effect of CCL28 depends on CCR10+ ECs.
The two ischemic models, HI and MI, are commonly used to explore the mechanism of angiogenesis. They share a common mechanism, in which occlusion of the responsible blood vessels leads to ischemia and hypoxia in the corresponding regions, triggering the self-repair process of angiogenesis. When ischemia occurs, macrophages are recruited to the ischemic area and secrete regulatory factors, thus promoting angiogenesis by targeting endothelial cells in both HI and MI30,31. This was further confirmed in our research. Surprisingly, we found that in these two ischemic models, which are similar but have independent characteristics, macrophages are enriched at lesion sites and secrete a large amount of CCL28, which then induces the repair function of endothelial cells through the CCR10/ERK/SOX5 positive feedback signaling pathway, thereby improving the course of the disease. Our research serves as an effective supplement to elucidate the key mechanisms of immune cells in the development of angiogenesis and provides experimental evidence for exploring the effectiveness of immunotherapy in ischemic diseases.
Dysregulation of proangiogenic factors and endothelial cell senescence has been implicated in diabetic ischemic tissues9,32. We found reduced CCL28 expression and a reduced proportion of CCR10+ ECs in the diabetic ischemic myocardium and gastrocnemius. The impaired CCL28-CCR10 axis may be the cause of endothelial dysfunction in diabetes, which leads to impaired angiogenesis and senescence. Administration of CCL28 also promotes post-ischemic angiogenesis and improves functional recovery in diabetic mice. The application of recombinant CCL28 protein may be an effective treatment for myocardial and hindlimb ischemia, including that in diabetes.
Chemokines play an important role in the initiation and progression of IHD. In addition, several studies have demonstrated that the therapeutic strategies targeting chemokines may be promising33,34. In the present study, we examined the predictive value of CCL28 in CTO patients with CCV. The results showed that serum levels of CCL28 in CTO patients with poor CCV were significantly lower than those in patients with good CCV. Moreover, CCL28 levels were independently associated with CCV, could improve the predictive value of the traditional CCV risk model, and could be used as a predictive indicator of CCV in patients with CTO. These findings reveal the clinical significance of CCL28 in patients with CTO.
This study has some limitations. First, AAV9-Tie1-sh-CCR10 was not specific to cardiac endothelial cells. Our research confirmed that CCR10+ endothelial cells are characterized by an enhanced proangiogenic capacity. However, we cannot rule out the possibility that the CCL28-CCR10 axis-mediated proangiogenic effect may occur through cell types other than endothelial cells. Further studies are required to confirm the role of endothelial cell-specific CCR10 knockouts in angiogenesis. Second, this study was conducted with a small sample size at a single center. Third, CCV was defined only by the Rentrop score, although this index has been used in many studies to reflect the presence and degree of coronary collaterals. Rentrop, as an alternative outcome for CTO patients, may have some potential limitations. Future studies are needed to evaluate the association between CCL28 and more robust outcome indexes, such as major adverse cardiac events in these patients.
In conclusion, the present study demonstrated that CCR10+ endothelial cells (ECs) are characterized by an enhanced proangiogenic capacity and are beneficial for angiogenesis. CCL28 promotes angiogenesis in both myocardial and hindlimb ischemia by increasing CCR10+ ECs via the CCR10/ERK/SOX5 positive feedback signaling. The use of recombinant CCL28 protein may be a promising therapeutic approach for myocardial and hindlimb ischemia, including that in diabetes.
Methods
Participants and ethics
We conducted a nested case-control study at Fujian Provincial Hospital between February 2019 and September 2023. This study was approved by the Fujian Provincial Hospital Ethics Committee, and the protocol was carried out in accordance with the Declaration of Helsinki (K2017-12-025). All participants provided written informed consent. Consecutive patients who had stable angina (chronic coronary syndrome) and at least one lesion with angiographic 100% occlusion, were enrolled in our study. Stable angina was diagnosed according to the criteria recommended by the European Society of Cardiology/European Association for Cardiac-Thoracic Surgery35. The exclusion criteria were as follows:(1) pregnancy, lactation, or malignant tumor with <1-year life expectancy; (2) chronic heart failure; (3) pulmonary heart diseases; (4) immune system disorders; (5) had a history of coronary artery bypass grafting; (6) received a percutaneous coronary intervention within the prior 3 months; (7) intermittent claudication. A total of 416 eligible patients were finally enrolled. Blood samples were collected at the time of hospital admission, and they were drawn into an anticoagulant tube and centrifuged for 3000 × g for 15 min.
Coronary angiography
Coronary angiography was performed according to the current guidelines. All angiograms were reviewed by two experienced cardiologists blinded to the study according to the lesion classification scheme of the European Society of Cardiology36. Any difference in interpretation was resolved by a third reviewer. The Rentrop score was used to assess the presence/absence and extent of collateral circulation37. The score is defined as follows: 0 = none, 1 = filling of side branches only, 2 = partial filling of the epicardial segment, and 3 = complete filling of the epicardial segment. Patients were categorized according to the Rentrop score and classified into poor (grade 0 or 1) or good (grade 2 or 3) CCV groups. In patients with more than one total coronary occlusion, the vessel with the highest collateral grade was chosen for the analysis.
Animals
All experimental procedures were approved by the Animal Care Ethics Committee of Fujian Provincial Hospital (IACUC-FPH-SL-20230804[0161]) and were performed in accordance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) for the care and use of laboratory animals. Male C57BL/6J wild-type and db/db mice (6–8 weeks) were purchased from the Beijing Vital River Laboratory Animal Technology Company (Beijing, China) and housed at Fujian Provincial Hospital. The mice were housed at 23 ± 1°C, a humidity of 55 ± 5%, and a 12-hour dark/light cycle. Mice were also given ad libitum access to food and water. Ccl28 heterozygous deficiency mice (Ccl28+/-) were purchased from Cyagen (Suzhou, China). Ccl28+/- mice in C57BL/6JGpt background were crossed to produce Ccl28-/- mice and Ccl28+/+ mice. Male littermate mice (6–8 weeks) were used in subsequent experiments.
Mouse model of hindlimb or myocardial ischemia
Myocardial infarction (MI) was induced in male C57BL/6J mice by ligation of the left coronary artery. Briefly, mice were anesthetized with 1–2% isoflurane, intubated, and mechanically ventilated. The heart was exposed by thoracotomy in the third intercostal space of the left chest, and the left anterior descending coronary artery (LAD) was ligated with 7–0 silk suture. Ischemia and whitening of the area between the ligation site and the apex of the heart indicated successful LAD ligation. We selected the same slide, which contained the largest infarct size, for each group to ensure the comparability of each group.
Unilateral mouse hindlimb ischemia was produced by ligation of the left femoral artery under general anesthesia with 1–2% isoflurane. A skin incision was made over the left femoral artery from the knee towards the medial thigh. The femoral artery was then visualized and ligated proximally at the groin with double knots and again distally near the knee. The incision was then closed with sutures, and the mice were monitored throughout the recovery process. The sham surgery mice underwent the same surgical procedures except for the ligation. The maximal transverse section of each gastrocnemius was obtained and stained.
Application of mouse recombinant CCL28 protein (rCCL28)
The human rCCL28 (300-57, PeproTech) and the mouse rCCL28 (250-30, PeproTech) were administered to the culture medium at a final concentration of 10 ng/mL for 24 h. In addition, mice were given 50 μg/kg of rCCL28 every 4 days after surgery14. The first administration of rCCL28 was conducted on the day of the surgery (mouse model of hindlimb or myocardial ischemia).
Tail vein injection of AAV-tie1-sh-CCR10
We used adeno-associated virus (AAV9) vectors carrying a short hairpin RNA (shRNA) against murine Ccr10 mRNA under control of the tie1 core promoter (AAV-tie1-sh-CCR10, 2 × 1011 vg/mouse, constructed by OBiO Technology) to specifically downregulate CCR10 expression in the endothelial cells by tail vein injection.
Echocardiography analysis
To measure the cardiac function, mice were anesthetized by isoflurane (3% for induction and 1.5% for maintenance), and M-mode images were acquired with a Vevo 2100 high-frequency ultrasound system (VisualSonics, Toronto, ON, Canada).
Laser Doppler imaging
Laser Doppler perfusion imaging measurements were performed using the PeriScan PIM 3 System. Mice were anesthetized with 1–2% isoflurane and restrained in the supine position on a heated platform set at 38 °C. Body hair was removed from the thigh using depilatory cream. A measurement distance of 10 cm, a scanning area of 5 × 3 cm, and a scanning speed of 1 frame/second were chosen and kept constant throughout the measurements. The rate of hind limb reperfusion was measured by analyzing the ratio of blood flow in the ligated and unligated limb using PIMSoft (Perimed, Sweden).
Flow cytometry
Mice were euthanized and perfused intracardially with cold PBS to remove blood cells. The hearts and gastrocnemius were dissected, cut into small pieces, and digested with the enzyme to obtain the single cell suspension. The Zombie Aqua™ Fixable Viability Kit (423102, Biolegend) was used to label dead cells. After incubation with TruStain FcXTM antibody (101320, Biolegend) for 15 min to minimize non-specific binding, cells were then stained with the following antibodies for 30 min. For detection of CCL28+ cells: AF488 anti-CCL28(sc-376654, Santa Cruz), APC/Fire™ 750 anti-CD45 (103154, Biolegend), PE/Cy7 anti-CD31(102524, Biolegend), APC anti-CD140a (135908, Biolegend), PE anti-Ly6C (128008, Biolegend), PerCP/Cyanine5.5 anti-Ly6G (127616, Biolegend), PE/Cy7 anti-CD11b (101216, Biolegend), APC anti-F4/80(123116, Biolegend). For detection of CCR10+ cells: AF405 anti-CCR10 (FAB2815V, R&D), APC/Fire™ 750 anti-CD45 (103154, Biolegend), PE/Cy7 anti-CD31(102524, Biolegend), APC anti-CD140a (135908, Biolegend), AF488 anti-Ki67(NB110-89717AF488, Novus). For detection of endothelial cells treated with rCCL28: APC/Cy7 anti-CD31(102440, Biolegend), PE/Atto594 anti-CCR10(FAB2815PEATT594, Novus). Flow cytometry was performed using a BD FACSAria™ III and BD FACSAria™ II (BD Biosciences, Franklin Lakes, NJ, USA). Data were analyzed using FlowJo 10 software.
For FACS sorting, myocardial tissue was subjected to single-cell suspensions using enzymatic digestion. CCR10+CD45-CD31+ and CCR10-CD45-CD31+cells were then sorted on a BD FACSAria™ III (BD Biosciences, Franklin Lakes, NJ, USA)by labeling with AF405 anti-CCR10 (FAB2815V, R&D), APC-Cy7 anti-CD45 (103154, Biolegend), and PE-Cy7 anti-CD31 (102524, Biolegend). All populations were routinely back-gated to verify gating and purity.
In vivo permeability assessment
To quantify cardiac vascular permeability, mice were injected in the tail vein with 100 μL 50 mg/mL FITC-dextran (70 kDa) (46945, Sigma) 30 min before sacrifice. Hearts were fixed in 4% paraformaldehyde overnight, embedded, and sectioned. Images were taken under a fluorescence microscope (Leica, Germany). The indicators of fluorescence were evaluated in 10 randomly chosen high-powered fields (40×) in each section. These data were measured and calculated using ImageJ software.
Hematoxylin-eosin staining
Hearts were immersed in 4% paraformaldehyde at 4 °C for 24 h, dehydrated through an alcohol gradient, cleared and embedded in paraffin, and serially sectioned at 5-μm thickness using a microtome. Sections were stained with haematoxylin and eosin (abs9217, Absin) according to the manufacturer’s instructions.
Masson’s trichrome staining
Mouse hearts were fixed in 4% paraformaldehyde for 24 h. The fixed tissues were then paraffin-embedded and serially sectioned with a microtome at a 5-μm thickness. Masson’s trichrome staining was used to evaluate the infarct size and the extent of perivascular and interstitial collagen in the heart tissues. By optical microscopy, the ratio of infarct size and collagen volume fraction was verified using ImageJ software. Mouse gastrocnemius were fixed in 4% paraformaldehyde for 24 h. The fixed tissues were then paraffin-embedded and serially sectioned with a microtome at a 5-μm thickness. Masson’s trichrome staining was used to evaluate the extent of perivascular and interstitial collagen in the gastrocnemius tissues. By optical microscopy, the ratio of collagen volume fraction was evaluated in 10 random fields in each section and was verified using ImageJ software.
Isolation and culture of adult primary cardiomyocytes and fibroblasts
Adult primary cardiomyocytes and fibroblasts were isolated from 8-week-old male mice using the Langendorff-free method38. Cardiomyocytes were subsequently resuspended in pre-warmed plating medium and plated into a laminin-precoated culture dish (5 μg/mL). After 1 h, the plating medium was replaced with pre-warmed culture medium. Fibroblasts were plated into a culture dish, then subcultured for subsequent experiments. All cells were maintained in a humidified culture incubator (Thermo Fisher Scientific, Waltham, MA, USA) under 5% CO2 at 37 °C.
Isolation and culture of mouse cardiac endothelial cells
The hearts from 8-week-old male C57BL/6J mice were harvested and cut into pieces, digested with 1 mg/mL collagenase II, 2.5 mg/mL collagenase IV, 0.25 U/mL Dispase, and 7.5 μg/mL DNAse I for 30 min. The cell suspension labeled with CD31 magnetic MicroBeads (130-097-418, Miltenyi Biotec) for heart EC positive selection to obtain cardiac microvascular endothelial cells (CMECs), according to the manual. CMECs isolated from five mice (About 1 million cells) were cultured in 6 cm petri dish with endothelial cell medium (1001, ScienCell) with 5% fetal bovine serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin, and 1% ECGF. The cells were incubated in a 5% CO2 incubator at 37 °C. CMECs are used for subsequent experiments after one passage.
Isolation and culture of macrophages from bone marrow
Bone-marrow-derived macrophages (BMDMs) were isolated from the femur and tibia of 8-week-old male C57BL/6J wild-type mice. Briefly, mouse femurs and tibiae were isolated, the metaphyses were clipped, and bone marrow cells were rinsed from the femurs and tibiae of WT mice with sterile PBS and cultured for 3 days in RPMI 1640 medium supplemented with 10% FBS, 100 units/mL penicillin/streptomycin and 30 ng/mL macrophage colony stimulating factor (M-CSF) (315-02, Peprotech) to allow differentiation into macrophages. 100 ng/mL IL-4 (AF-214-14, PeproTech) was used to induce macrophage M2 polarization.
Cell lines
bEnd.3 cells (SCSP-5267) and HMEC-1(GNHu46) were purchased from National Collection of Authenticated Cell Cultures. bEnd.3 cells were cultured in DMEM medium supplemented with 10% FBS, 100 units/mL penicillin/streptomycin. HMEC-1 were cultured in MCDB131 medium (10372019, Gibco), supplemented with 10% FBS, 100 units/mL penicillin/streptomycin, and 10 ng/mL hEGF (E9644, sigma). The cells were incubated in a 5% CO2 incubator at 37 °C.
Cell intervention
For experiments requiring hypoxic conditions, the cells were maintained in a CO2 incubator (3131, Thermo Scientific) at 37 °C, 5% CO2, 1% O2, and 94% N2 for an indicated amount of time.
To induce senescence, isolated ECs were treated with 50 µM H2O2 (Sigma-Aldrich) for 2 h and then cultured for another 48 h at 37 °C.
siRNA transfection
The si-NC or si-SOX5, si-TFCP2L1 and si-DUXBL1 (RiboBio, Guangzhou, China) was transfected into cardiac endothelial cells using Lipofectamine 3000 reagent (L3000015, Invitrogen) according to the instruction manual.
Annexin V/PI staining
Annexin V-FITC and PI analyses (V13242) were performed to examine the apoptosis rate of endothelial cells. The cells were trypsinized, washed twice with PBS, and centrifuged at 350 g for 5 min. Determine the cell density and dilute in 1X annexin-binding buffer to ∼1 × 106 cells/mL. Add 5 µL of FITC Annexin V and 1 µL of the PI working solution to each 100 µL of cell suspension. Incubate the cells at room temperature for 15 minutes. After the incubation period, add 400 µL of 1X annexin-binding buffer, mix gently, and keep the samples on ice. Apoptotic cells were then measured using the BD FACSAria™ III (Becton Dickinson, Franklin Lakes, NJ), and the results were analyzed using FlowJo 10 software.
Wound healing assay
Confluent CMECs monolayers grown in 6-well plates were scratched with a sterile 200 µL pipette tip. Having been washed with ECM, the culture medium was replaced with ECM supplemented with 0.5% FBS for a further 12 h. At the indicated time points (0 h, 6h and 12 h), scratch closures were observed and photographed under a microscope. The area of the wounds was evaluated in 10 random fields in each biological replication using ImageJ software. Cell migratory ability for wound-healing was assessed by applying the following formula: [(wound area at 0 h) - (wound area at indicated time)]/(wound area at 0 h).
Tube formation assay
A 24-well plate was polymerized with Matrigel (356234, Corning) for 30 min at 37 °C. 2 × 104 CMECs were incubated in 1 mL endothelial cell medium (1001, Sciencell) for 12 h, then stained with calcein (C7600, Solarbio) before imaging. Capillary tubes were quantified under fluorescence microscopy by measuring the total length of the completed tube structure. Three independent experiments were performed for each treatment. Ten random fields were evaluated in each independent experiment.
Spheroid-based sprouting angiogenesis assay
The CMECs spheroid-based sprouting angiogenesis assay was performed as previously described39. Briefly, 8 × 104 CMECs were resuspended in 4 mL of endothelial cell growth medium (1001, ScienCell). 1 mL of methylcellulose was carefully added. The entire mixture was pipetted in 25 μl hanging droplets onto a 10 cm dish and incubated upside down for 24 h to form spheroids. Spheroids were rinsed with PBS and resuspended in 2 mL Methocel containing 20% FBS. Collagen type I (354236, Corning) and 10 × medium 199 (M9163, Sigma) were mixed on ice, and the pH was adjusted to 7.4 with 0.1 M NaOH. Two milliliters of this mixture were added to the spheroid suspension. The final collagen concentration was 2 mg/mL. A 3.5 cm glass-bottom culture dish (D35-20-1-N, Cellvis) was pipetted with 1 mL of the spheroid-collagen solution and incubated at 37 °C for 30 min. VEGF (25 ng/mL) was then added to the dish after polymerization of the collagen gel. Spheroids were incubated for a further 18 h, fixed in 4% paraformaldehyde, and stained with Phalloidin (CA1610, Solarbio). Images were captured by confocal microscopy (Olympus FV3000RS, Japan). The length of each process was measured from the edge of the spheroid to the tip of the process using ImageJ. The sprout number and length were evaluated in 10 random fields in each independent experiment.
Senescence-associated β-galactosidase staining
SA-β-Gal staining was performed using an SA-β-Gal staining kit (C0602; Beyotime Institute of Biotechnology) according to the manufacturer’s instructions. SA-β-Gal+ cells were identified by light microscopy as blue-stained spots. Ten random fields in each independent experiment were analyzed at equal magnification using ImageJ software.
ChIP assay
ChIP was performed using the SimpleChIP® Enzymatic Chromatin IP Kit (9003, CST). Briefly, HCMECs were cross-linked with 1% formaldehyde for 10 min at RT, and the cross-link was quenched with glycine for 5 min at RT. The cells were washed with cold PBS, collected, and nuclei were extracted after cell lysis. To digest the DNA, micrococcal nuclease was added to the nuclear suspension. The nuclei were collected and resuspended in IP dilution buffer. The chromatin was then sonicated to a length of 300-500 base pairs. Chromatin was immunoprecipitated overnight at 4 °C using SOX5 antibodies (ab94396, Abcam) or IgG control (included in the kit). ChIP-grade Protein A/G Magnetic beads were added and incubated at 4 °C. RNase A and Proteinase K were used to digest RNA and protein. The purified DNA was used for qPCR.
Dual luciferase reporter assay
The Ccr10 promoter (−2000– +200 bp, pGL3-Promoter) was amplified by PCR and inserted into the pGL3 vector (Beyotime). The pGL3-Empty and pGL3-Promoter plasmids were individually cotransfected with either the si-NC or si-SOX5 and Renilla luciferase plasmids into NIH/3T3 cells (SCSP-515, National Collection of Authenticated Cell Cultures) using Lipofectamine 3000 reagent (L3000015, Invitrogen). The cells were then treated with vehicle or rCCL28 for 12 h, and then the cells were harvested and lysed. Firefly luciferase activities were detected with a Dual-Luciferase Reporter Assay System E1910 (Promega, USA) and normalized to control Renilla luciferase levels.
ELISA detection
The supernatants of cells culture and the mice serum were used in the assay for CCL28 using the Mouse CCL28 ELISA Kit (ab210578, Abcam). The serum CCL28 levels from CTO patients were detected by Human CCL28 ELISA Kit (ab99988, Abcam). These biochemical features were measured following the manufacturer’s manual.
RNA isolation and RT-qPCR
Total RNA was isolated from murine cardiac tissues and cells using TRIzol reagent (15596-018, Invitrogen), according to the manufacturer’s instructions. The PrimeScript RT Reagent Kit with gDNA Eraser (RR047A, Takara) was used for reverse transcription. Subsequent RT-qPCR was performed using TB Green Premix Ex Taq II (RR820A, Takara) and the primers listed in Table S2. 18S was used as an endogenous control.
RNA-sequence
Total RNA was extracted using TRIzol reagent. Libraries were then constructed using the VAHTS Universal V6 RNA-seq Library Prep Kit. Transcriptome sequencing and analysis were performed by OE Biotech Co., Ltd. (Shanghai, China). Q value < 0.05 and fold change > 2 or fold change <0.5 were set as the threshold for significant differential expression genes (DEGs). Then, hierarchical cluster analysis was performed on the DEGs to prove the expression patterns of genes in different groups and samples. Based on the hypergeometric distribution, GO, KEGG pathway, Reactome, and WikiPathways enrichment analysis of DEGs were performed to screen the significantly enriched terms using R (v 3.2.0). Gene Set Enrichment Analysis (GSEA) was performed using GSEA software. A predefined gene set was used for the analysis, and the genes were ranked according to the degree of differential expression in the two sample types. The raw data of RNA sequencing have been uploaded to the Gene Expression Omnibus (GEO) database (GSE290928).
Western blot
Tissues and cells from the different experimental groups were homogenized in ice-cold lysis buffer and then centrifuged at 12,000 × g for 20 min at 4 °C. Lysates (50 μg protein/lane) were separated by SDS-PAGE on a 12% gel and were then transferred to PVDF membranes (Bio-Rad). The membranes were blocked with 5% BSA for 1 h and were then cut according to the molecular weights of each protein of interest. The cut membranes were incubated with the respective primary antibodies overnight at 4 °C. The following primary antibodies were used: anti-ACTB antibody (ab8226, Abcam), anti-CCL28/MEC antibody (ab231557, Abcam), anti-CCR10 antibody (22071-1-AP, Proteintech), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibody (4370, CST), p44/42 MAPK (Erk1/2) antibody (4695, CST), anti-VEGFA antibody (A0280, Abclonal), anti-SOX5 antibody (ab94396, Abcam), anti-CDKN2A/p16INK4a antibody (ab211542, Abcam), anti-Lamin B1 antibody (ab16048, Abcam). The membranes were then probed with HRP-labeled goat anti-rabbit IgG (H + L) (Beyotime, A0208) or HRP-labeled goat anti-mouse IgG (H + L) (Beyotime, A0216) for 2 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence reagent (Millipore), according to the manufacturer’s instructions. ACTB was used as the loading control, and protein expression was quantified by densitometry.
Immunofluorescence staining
For MI, we selected the same slide that contained the largest infarct size for each group to ensure the comparability of each group. For HI, the maximal transverse section of each gastrocnemius was obtained for staining. Cryosections of mouse tissues were removed from the −20 °C freezer, thawed at room temperature for 30 min, and washed three times with PBS (5 min per wash). A 10% BSA solution was dropped onto the tissue sections, and the sections were then sealed at 37 °C for 1 h and dried with filter paper. Subsequently, anti-CCL28/MEC antibody (ab231557, Abcam), anti-CD31 antibody (ab9498, Abcam), and anti-CCR10 antibody (22071-1-AP, Proteintech) with 0.1% Triton X-100 were prepared and then dropped onto the tissue sections. The sections were then cultured overnight at 4 °C and then washed three times with PBS (5 min per wash). Next, the tissue sections were treated with secondary antibodies Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) (ab150077, Abcam), Goat Anti-Rabbit IgG H&L (Alexa Fluor® 594) (ab150080, Abcam) and Goat Anti-Mouse IgG H&L (Alexa Fluor® 594) (ab150116, Abcam), soaked in water at 37 °C in the dark, and washed three times with PBS (5 min per wash). GS-IB4 Alexa Fluor™ 594 (Thermo Fisher Scientific, I21413) was used to label microvascular endothelial cells according to instructions. Then, the sections were stained with DAPI for 10 min at room temperature in the dark. An anti-fluorescence-quenching agent (Invitrogen, USA) was dropped onto the samples, and the sections were then covered with glass coverslips. Images were obtained using a fluorescence microscope (Leica, Germany). The indicators of fluorescence were evaluated in 10 randomly chosen high-powered fields (20×) in each section. These data were measured and calculated using ImageJ software.
Multiplex immunohistochemical (mlHC) staining using Tyramide Signal Amplification (TSA)
Bas Multi-target Immunohistochemistry Kit (AXB, BXT35010011) was used to perform mIHC according to the instruction manual. The main procedures were as follows: first, after the slides were deparaffinized in xylene, rehydrated in graded alcohol, washed in distilled water, and retrieved in EDTA buffer, the slides were blocked using antibody diluent/block; second, the slides were incubated with primary antibody for 1 h and HRP-labeled goat anti-(Ms+Rb) for 10 min at 37 °C; third, the fluorescent dyes included in the kit were used for visualization. After each cycle of staining, heat-induced epitope retrieval was performed to remove the former antibodies. Finally, the slides were counterstained with DAPI for 5 min and enclosed in Antifade Mounting Medium. The primary antibodies were used: anti-CD31 antibody (ab9498, Abcam), anti-CD45 antibody (ab318154, Abcam), anti-F4/80 antibody (ab300421, Abcam), and anti-CCL28 antibody (ab231557, Abcam). The fluorescence was evaluated in 10 random fields in each section.
Sample size calculation
The sample size calculation was based on the following The incidence of poor CCV in the CTO patients is approximately 40%9; 2. We hypothesize that the incidence of poor CCV in the CTO patients with a lower level of CCL28 is 45% and the incidence of poor CCV in the CTO patients with a higher level of CCL28 is 30%. An estimated sample size of 386 patients would be needed to detect the difference with a statistical power >90% and a probability Type I error of 0.05, considering an attrition rate of 10%. The sample size calculation was performed using PASS 15.0.
Statistical analysis
Experimental data (cell and animal) were expressed as the mean ± standard deviation (SD) of at least three biological replicates. Data normality was estimated using the Shapiro-Wilk test. According to the normality, the Student’s t-test or Wilcoxon rank-sum test was performed to determine differences between two groups, and the One-Way ANOVA test or Kruskal-Wallis test was performed to determine the differences among three or more groups. When studying the effects of two variables, Two-way ANOVA was appropriate. Tukey post hoc multiple comparison test was used for both One- and Two-Way ANOVA tests. Two-sided P < 0.05 indicated significance. The statistical analyses were performed using SPSS 18.0 and GraphPad Prism 9.
Statistical analysis of clinical data was performed using R version 4.0.2 and SPSS 18.0. Normally distributed continuous variables were reported as the mean ± SD, and non-normally distributed continuous variables were expressed as the median and interquartile range. Categorical data were expressed as absolute values and percentages and analyzed using the chi-square or Fisher’s exact test. The Student’s t-test or Wilcoxon rank-sum test was performed to determine differences between groups. Kruskal-Wallis test with Dunn multiple comparisons test was used to evaluate the difference in serum CCL28 levels among patients with Rentrop scores 0, 1, 2, and 3. The association between CCL28 and CCV was evaluated by logistic regression using two multivariate models. Model 1 was adjusted for age and sex. Model 2 was adjusted for variables from Model 1 plus body mass index, smoking, hypertension, diabetes, prior myocardial infarction, and serum creatinine. The predictive values of CCL28 for CCV were analyzed using a receiver operating characteristic (ROC) curve. The area under the curve (AUC), continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were performed to compare the discriminative ability of CCL28 on the traditional CTO risk model. AUC differences were compared using the two-tailed DeLong method. The Spearman’s correlation analysis was used to explore the relationship between CCL28 and CCV. All statistical tests were 2-sided, and P < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this study are available in the article, its Supplementary information, the Source Data file and from the corresponding authors upon request. RNA-sequencing data have been uploaded to the Gene Expression Omnibus database (GSE290928, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE290928). Flow cytometry data has been deposited in Zenodo (https://doi.org/10.5281/zenodo.15774815). Source data are provided with this paper.
References
Pei, J. et al. LPA(2) Contributes to vascular endothelium homeostasis and cardiac remodeling after myocardial infarction. Circ. Res. 131, 388–403 (2022).
Johnson, T., Zhao, L., Manuel, G., Taylor, H. & Liu, D. Approaches to therapeutic angiogenesis for ischemic heart disease. J. Mol. Med. 97, 141–151 (2019).
He, L. et al. Preexisting endothelial cells mediate cardiac neovascularization after injury. J. Clin. Invest 127, 2968–2981 (2017).
Taimeh, Z., Loughran, J., Birks, E. J. & Bolli, R. Vascular endothelial growth factor in heart failure. Nat. Rev. Cardiol. 10, 519–530 (2013).
Wu, X., Reboll, M. R., Korf-Klingebiel, M. & Wollert, K. C. Angiogenesis after acute myocardial infarction. Cardiovasc Res. 117, 1257–1273 (2021).
Li, Z. et al. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur. Heart J. 40, 2507–2520 (2019).
Simons, M. et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 105, 788–793 (2002).
Lu, L. et al. Reduced serum levels of vasostatin-2, an anti-inflammatory peptide derived from chromogranin A, are associated with the presence and severity of coronary artery disease. Eur. Heart J. 33, 2297–2306 (2012).
Bao, X. L. et al. Vasostatin-2 associates with coronary collateral vessel formation in diabetic patients and promotes angiogenesis via angiotensin-converting enzyme 2. Eur. Heart J. 44, 1732–1744 (2023).
Korf-Klingebiel, M. et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat. Med. 21, 140–149 (2015).
Reboll, M. R. et al. EMC10 (Endoplasmic Reticulum Membrane Protein Complex Subunit 10) Is a Bone Marrow-Derived Angiogenic Growth Factor Promoting Tissue Repair After Myocardial Infarction. Circulation 136, 1809–1823 (2017).
Mohan, T., Deng, L. & Wang, B. Z. CCL28 chemokine: An anchoring point bridging innate and adaptive immunity. Int Immunopharmacol. 51, 165–170 (2017).
Facciabene, A. et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 475, 226–230 (2011).
Chen, J. et al. Inhibition of ALKBH5 attenuates I/R-induced renal injury in male mice by promoting Ccl28 m6A modification and increasing Treg recruitment. Nat. Commun. 14, 1161 (2023).
Simonson, B. et al. Single-nucleus RNA sequencing in ischemic cardiomyopathy reveals common transcriptional profile underlying end-stage heart failure. Cell Rep. 42, 112086 (2023).
Chen, X. et al. SOX5 induces lung adenocarcinoma angiogenesis by inducing the expression of VEGF through STAT3 signaling. Onco Targets Ther. 11, 5733–5741 (2018).
Yuan, W. M. et al. SOX5 regulates cell proliferation, apoptosis, migration and invasion in KSHV-infected cells. Virol. Sin. 36, 449–457 (2021).
Jing, Y. et al. Genome-wide CRISPR activation screening in senescent cells reveals SOX5 as a driver and therapeutic target of rejuvenation. Cell Stem Cell 30, 1452–1471.e1410 (2023).
Zhang, Q. et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target Ther. 7, 78 (2022).
Li, H. et al. Lysyl hydroxylase 1 (LH1) deficiency promotes angiotensin II (Ang II)-induced dissecting abdominal aortic aneurysm. Theranostics 11, 9587–9604 (2021).
Shah, M. S. & Brownlee, M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ. Res. 118, 1808–1829 (2016).
Li, Y. et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 7, (2021).
Yu, B. et al. Extracellular vesicles engineering by silicates-activated endothelial progenitor cells for myocardial infarction treatment in male mice. Nat. Commun. 14, 2094 (2023).
Pan, J. et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J. Immunol. 165, 2943–2949 (2000).
Lazarus, N. H. et al. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J. Immunol. 170, 3799–3805 (2003).
Mergia Terefe, E. et al. Roles of CCR10/CCL27-CCL28 axis in tumour development: mechanisms, diagnostic and therapeutic approaches, and perspectives. Expert Rev. Mol. Med. 24, e37 (2022).
Chen, Z. et al. Inhibition of CCL28/CCR10-Mediated eNOS downregulation improves skin wound healing in the obesity-induced mouse Model of Type 2 Diabetes. Diabetes 71, 2166–2180 (2022).
Chen, Z. et al. CCL28-induced CCR10/eNOS interaction in angiogenesis and skin wound healing. FASEB J. 34, 5838–5850 (2020).
Chen, Z. et al. Characterising the expression and function of CCL28 and its corresponding receptor, CCR10, in RA pathogenesis. Ann. Rheum. Dis. 74, 1898–1906 (2015).
Reboll, M. R. et al. Meteorin-like promotes heart repair through endothelial KIT receptor tyrosine kinase. Science 376, 1343–1347 (2022).
Zhang, J. et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 31, 1136–1153.e1137 (2020).
Hwang, H. J., Kim, N., Herman, A. B., Gorospe, M. & Lee, J. S. Factors and pathways modulating endothelial cell senescence in vascular aging. Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms231710135 (2022).
Hanna, A. & Frangogiannis, N. G. Inflammatory Cytokines and Chemokines as therapeutic targets in heart failure. Cardiovasc. Drugs Ther. 34, 849–863 (2020).
Surmi, B. K. & Hasty, A. H. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vasc. Pharm. 52, 27–36 (2010).
Valgimigli, M. et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 39, 213–260 (2018).
Corrigendum to: 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 4242, (2020).
Rentrop, K. P., Cohen, M., Blanke, H. & Phillips, R. A. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J. Am. Coll. Cardiol. 5, 587–592 (1985).
Ackers-Johnson, M. et al. A simplified, Langendorff-free method for concomitant isolation of viable cardiac myocytes and nonmyocytes from the adult mouse heart. Circ. Res. 119, 909–920 (2016).
Tetzlaff, F. & Fischer, A. Human endothelial cell spheroid-based sprouting angiogenesis assay in collagen. Biol. Protoc. 8, e2995 (2018).
Acknowledgements
This study was funded by National Natural Science Foundation of China General Program (Grant number: 82070375, to K.L.); Natural Science Foundation for Distinguished Young Scholars of Fujian Province (Grant number: 2021J06030, to K.L.); The Youth Top Talent Project of Fujian Provincial Foal Eagle Program (no grant number, to K.L.); Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare (no grant number, to K.L.).
Author information
Authors and Affiliations
Contributions
Conceptualization: K.Y., H.C., Y.L., W.W., J.S., R.G. and K.L.; Formal analysis: K.Y., J.S., R.G. and K.L.; Investigation: K.Y., H.C., Y.L., W.W., J.S., R.G. and K.L.; Validation: K.Y., H.C., Y.L., W.W., X.W., Y.Z.L., B.L., G.Z., J.C., J.S., R.G. and K.L.; Methodology: K.Y., H.C., Y.L., W.W., J.S., R.G. and K.L.; Project administration: J.S., R.G. and K.L.; Funding acquisition: K.L.; Data curation: K.Y., H.C., Y.L., W.W., X.W., Y.Z.L., B.L., G.Z. and J.C.; Software and data visualizations: K.Y., H.C., Y.L. and W.W.; Resources: J.S., R.G. and K.L.; Supervision: J.S., R.G. and K.L.; Writing—original draft: K.Y. and K.L.; Writing—review and editing: H.C., Y.L., W.W., J.S. and R.G.; All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, K., Chen, H., Lyu, Y. et al. CCL28 contributes to angiogenesis and cardiac repair through CCR10+ endothelial cells after myocardial infarction in male mice. Nat Commun 16, 9262 (2025). https://doi.org/10.1038/s41467-025-64283-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-64283-4