Abstract
Multiple sclerosis (MS) is a chronic neurodegenerative disease driven by infiltration of activated innate immune cells into the central nervous system (CNS). Current imaging approaches for diagnosing and monitoring disease progression rely on structural lesions and cannot directly assess innate immune activity. Here, we describe a dendrimer positron emission tomography (PET) tracer, 18F-flurimedrimer (18F-FMD), for non-invasive, longitudinal tracking of activated myeloid cells. In an experimental autoimmune encephalomyelitis (EAE) murine model, 18F-FMD specifically detects myeloid activation at presymptomatic and symptomatic stages, with PET signal correlating with disease severity. Moreover, 18F-FMD sensitively captures therapeutic response to fingolimod (FTY720) and a CSF1R dendranib (H74DS3M8), both of which suppress immune cell activation and attenuate disease severity. These findings highlight the potential of 18F-FMD PET for specific, real-time monitoring of innate immune responses, and the applicability of the dendrimer in clinical settings for monitoring therapeutic efficacy, advancing the development of personalized, myeloid-targeted strategies for MS.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is the most prevalent chronic autoimmune disease of the central nervous system (CNS), affecting nearly 2.9 million people worldwide1. Characterized by inflammation, demyelination, and neurodegeneration, MS presents as a heterogeneous disease with clinical manifestations that vary significantly among individuals. Although its exact etiology is unclear, MS is driven by a complex interplay between the adaptive and innate immune systems. Within the adaptive immune response, autoreactive T and B cells are central to MS pathogenesis. Pro-inflammatory T helper 1 (Th1) and Th17 cell subsets secrete cytokines such as interleukin-17 (IL-17) and IL-21, promoting CNS infiltration and amplifying inflammation, while B cells contribute by producing autoantibodies and modulating T cell responses2,3. In parallel, the innate immune system–particularly myeloid cells including microglia, monocytes, macrophages, neutrophils, and dendritic cells–plays a crucial role in both the progression and potential remission of MS4,5. Activated microglia and infiltrating macrophages are the predominant immune cell types found in acute and chronic active MS lesions within the CNS6,7,8,9. These cells drive neuroinflammation through the release of pro-inflammatory cytokines, reactive oxygen species, and other neurotoxic mediators; they also phagocytose myelin and debris, and sustain lymphocyte activation through antigen presentation and cytokine polarization10,11,12. Notably, myeloid cell activation is present early in the disease course and persists throughout various clinical subtypes, including relapsing-remitting MS (RRMS), primary progressive MS (PPMS), and secondary progressive MS (SPMS), which together account for over 85% of MS cases13. Given their central involvement in MS pathology, activated microglia and macrophages can serve as clinically relevant biomarkers of chronic neuroinflammation and disease progression.
Currently, there is no cure for MS, but several disease-modifying therapies (DMT) are approved, primarily for managing RRMS and SPMS, with only one Food and Drug Administration (FDA)-approved option for PPMS14. These treatments mainly work by modulating immune responses or depleting specific immune cell populations to reduce disease activity and slow clinical progression15. While no FDA-approved MS therapies directly target activated myeloid cells, a growing number of investigational treatments aim to address this gap. Moreover, several existing immunomodulatory therapies indirectly affect myeloid cells through changes in cytokine signaling and adaptive immune cell-mediated pathways4. Despite these advancements, PPMS remains particularly challenging to manage. The complexity and heterogeneity of MS–driven by multiple interacting immune cell types–make selecting effective therapies difficult4. Compounding this challenge is the lack of specific in vivo imaging biomarkers for activated myeloid cells, which limits our ability to fully elucidate their role in neurodegeneration, stratify patients for trials of emerging microglia-targeting treatments, or monitor therapeutic responses.
Structural magnetic resonance imaging (MRI) is the current standard of care for diagnosing and staging MS lesions, as well as for monitoring treatment response16. While MRI is effective for visualizing structural changes, it lacks the molecular specificity needed to differentiate different immune cell subsets. As a result, MRI provides limited insight into treatment efficacy at the cellular level and may lead to suboptimal therapeutic choices, increasing the risk of continued CNS damage. Since activated myeloid cells are an early and persistent feature of MS and play a critical role in disease progression and remission, there is a critical need for non-invasive imaging strategies to specifically identify and track maladaptive myeloid populations and their functional phenotypes in the CNS of MS patients.
Positron emission tomography (PET) is a functional molecular imaging modality that has the potential to profoundly enhance our understanding of the in vivo roles of activated myeloid cells in MS. Unfortunately, the current selection of PET tracers for imaging innate immune activation is limited. The most widely evaluated PET tracers for visualizing activated myeloid cells target the translocator protein 18 kDa (TSPO)17,18. Despite providing valuable insights into the spatiotemporal dynamics of neuroinflammation in MS patients in addition to showing potential for predicting disease progression19,20, TSPO-PET imaging has several critical limitations, including its lack of cellular specificity (i.e., it is expressed on microglia, peripheral myeloid cells, astrocytes, and vascular endothelium, thus making interpretation of images challenging), unclear functional role in MS, and a genetic polymorphism in the human TSPO gene resulting in variable binding affinity across patients for all second-generation TSPO-PET tracers21. Moreover, recent post-mortem studies of MS brain tissue revealed that TSPO expression does not increase in activated cells but rather in the number of TSPO-expressing cells, underscoring its limitations as a reliable biomarker for myeloid activation22. While alternative PET imaging targets, such as colony-stimulating factor 1 receptor (CSF1R), cluster of differentiation molecule 11b (CD11b), cyclooxygenase-2 (COX2), triggering receptor expressed on myeloid cells 1 (TREM1), and P2Y purinoceptor 12 (P2Y12R), are being investigated as potentially improved biomarkers for specific in vivo tracking of innate immune activation23, none have yet been validated as effective clinical biomarkers for neuroinflammation.
To address this unmet need for a PET tracer that enables highly specific detection of activated myeloid cells24, we recently developed 18F-flurimedrimer (18F-FMD; previously known as 18F-OP-801), a fluorine-18-labeled synthetic fourth-generation polyamidoamine (PAMAM) hydroxyl dendrimer25,26. Fourth-generation hydroxyl dendrimers have been demonstrated to penetrate regions of a dysfunctional, but not healthy, intact blood-brain barrier (BBB), where they are quickly and selectively taken up by activated myeloid cells via -phase endocytosis and rapidly cleared from tissue that does not contain these cells27,28,29,30,31,32,33,34. In preclinical PET imaging, we have demonstrated that 18F-FMD sensitively detects neuroinflammation induced by systemic sepsis in a lipopolysaccharide mouse model, with signal intensity correlating with the murine sepsis severity score35. Moreover, the synthesis of 18F-FMD has recently been optimized and approved for clinical use26, positioning this PET tracer as a potentially valuable tool for highly specific, functionally relevant, and non-invasive imaging of activated myeloid cells in patients with MS and other inflammatory diseases.
In this study, we evaluate the ability of 18F-FMD to non-invasively detect activated myeloid cells in an experimental autoimmune encephalomyelitis (EAE) mouse model at both presymptomatic and acute disease stages (Fig. 1). We demonstrate that 18F-FMD selectively accumulates in activated myeloid cells and sensitively tracks therapeutic modulation of neuroinflammation by fingolimod (FTY720), an FDA-approved small-molecule immunosuppressant, and H74DS3M8, a recently developed hydroxyl dendrimer-based receptor tyrosine kinase inhibitor (referred to as “dendranib”). These findings establish 18F-FMD as a promising tool for in vivo monitoring of innate immune activation in the CNS and support its translational potential for evaluating therapeutic response and disease progression in neuroinflammatory disorders.
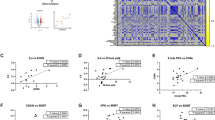
a Multiple sclerosis (MS) is a heterogeneous, multifactorial, immune-mediated disease of the central nervous system (CNS) whereby myelin is attacked by the body’s own immune cells creating areas of sclerosis, also known as lesions. Microglial activation occurs during early stages of MS and is associated with profound dysregulation and breakdown of the blood-brain barrier (BBB), allowing immune cells to infiltrate from the periphery into the CNS, which leads to further microglial activation and demyelination, followed by reactive gliosis and neurodegeneration. Formation of demyelinating lesions in the white and gray matter of the brain and spinal cord is driven by both the adaptive and innate immune system. Here, we are highlighting the role of the innate immune system (involving activated microglia and peripheral myeloid cells) in driving the initiation, progression, and remission of MS. We have identified 18F-FMD positron emission tomography (PET) as a promising approach to detect pathogenic microglia and peripheral CNS-infiltrating myeloid cells in an experimental autoimmune encephalomyelitis (EAE) mouse model. 18F-FMD is known to be taken up specifically by activated myeloid cells via a biophysical mechanism (due to their ultra-fast rates of endocytosis). Created in BioRender. Reyes, S. (2026) https://BioRender.com/m7yvwy3. b 18F-FMD was synthesized in two steps using 18F click chemistry. Briefly, 18F-3-Fluoropropylazide was accessed via nucleophilic fluorination of a tosyl precursor followed by subsequent reaction with the PAMAM-G4-OH alkyne10 dendrimer precursor via CuAAC click chemistry to yield 18F-FMD26.
Results
18F-FMD PET imaging enables early disease detection in EAE mice before symptom onset
We first assessed the ability of 18F-FMD PET imaging to detect and track activated myeloid cells in vivo in an EAE mouse model characterized by a sustained progressive disease course with persistent inflammation, demyelination, and axonal degeneration36,37,38. PET/computed tomography (CT) imaging was performed at presymptomatic (pre-EAE; score 0, no paresis/paralysis, ≥1 g weight loss in 24–48 h) and symptomatic (EAE; score 0.5–3.5, hindlimb paresis/paralysis) stages of disease, compared to naïve controls, 50 min after tracer injection (Fig. 2a). Clinical signs of EAE, which progress rostrocaudally from the tail to hindlimbs and forelimbs, began to manifest by day 12 and peaked by day 15 with an average disease score of 2.3 ± 1.2 (Fig. 2b and Supplementary Table 2).
a Study design and timeline for imaging pre-EAE, EAE, and naïve mice. PET/CT was performed 50-60 min after injection of 18F-FMD, and tissues were subsequently harvested for ex vivo analyses. Parts created in BioRender. Reyes, S. (2026) https://BioRender.com/kkh8mf1. b Disease severity scores for EAE mice (n = 30) as determined by level of paresis and paralysis using a standard scoring metric. c Representative 18F-FMD PET/CT images highlighting elevated signal in the spinal cords (arrow) of pre-EAE and EAE mice versus naïve mice. Quantification of PET images in whole brain, medulla, cerebellum, pons, cervical/thoracic and lumbar regions of spinal cord for d naïve (n = 4) and pre-EAE mice (n = 16) and e naïve (n = 5) and EAE mice (n = 5). C/T cervical/thoracic, L lumbar, SC spinal cord. d, e Statistical analyses were performed using two-tailed unpaired t tests. * denotes direct comparison of groups. d C./T. SC: p = 0.02, L. SC: p = 0.0012; e cerebellum: p = 0.02, pons: p = 0.01, C./T. SC: p = 0.047, L. SC: p = 0.008. Data are mean ± SD.
In naïve mice, 18F-FMD PET signal was predominantly observed in the kidneys and bladder, reflecting the tracer’s urinary excretion pathway. Conversely, pre-EAE and symptomatic EAE mice exhibited visible increases in spinal cord signal (Fig. 2c, white arrow). PET image quantification confirmed elevated 18F-FMD uptake in pre-EAE mice compared to naïve controls in both the cervical/thoracic (0.69 to 1.02 %ID/g [percent injected dose per gram]) and lumbar (2.04–2.71 %ID/g) spinal cord regions (p < 0.05; Fig. 2d). The signal increased further upon symptom manifestation in EAE mice, reaching 2.92 %ID/g in the cervical/thoracic and 4.71 %ID/g in the lumbar spinal cord (p < 0.05; Fig. 2e). While no significant differences in 18F-FMD PET signal were observed in the brain during the pre-EAE stage, symptomatic EAE mice showed elevated tracer uptake in white matter-rich brain regions, particularly the cerebellum (0.29 to 1.05 %ID/g) and pons (0.29 to 1.06 %ID/g; p < 0.05).
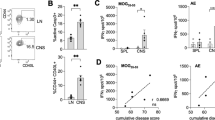
Following PET/CT imaging, mice were perfused with phosphate-buffered saline (PBS) to remove residual tracer in the blood pool. Ex vivo gamma counting was then performed to validate in vivo PET findings in CNS tissues. Notably, in pre-EAE mice with no clinical symptoms, 18F-FMD signal in the lumbar spinal cord was significantly elevated compared to naïve controls (p < 0.01; Fig. 3a). As the disease progressed, tracer accumulation markedly intensified, with symptomatic EAE mice showing a 2- to 4.5-fold increase in PET signal in the cervical/thoracic and lumbar spinal cords compared to pre-EAE mice, and a dramatic 9- to 12-fold increase compared to naïve controls (p < 0.01; Fig. 3a). Additionally, tracer uptake in the blood pool of symptomatic EAE mice was significantly higher than in naïve mice (p < 0.0001), with a trend toward significance in pre-EAE mice (p = 0.29; Supplementary Fig. 3a). However, no significant differences were observed in peripheral organs such as spleen, which is known to exhibit fluctuations in immune cell populations like T cells, B cells, monocytes, and neutrophils during EAE39.
a Ex vivo gamma counting results (%ID/g) in whole brains, cervical/thoracic spinal cords, and lumbar spinal cords of naïve (n = 9), pre-EAE (n = 5), and EAE mice (n = 6). b Representative high-resolution ex vivo digital autoradiography of 60 µm-thick fresh frozen spinal cord sections (representative images based on mean spinal cord uptake) with H&E staining overlay. c Representative images at 40x magnification of H&E and CD68 immunohistochemical (IHC) staining of 12 µm lumbar spinal cord sections from naïve, pre-EAE, and EAE mice. Scale bars, 50 µm. C/T cervical/thoracic, L lumbar, SC spinal cord. a Statistical analyses were performed using one-way ANOVA with Tukey’s multiple comparisons test. +versus naïve mice and *denotes direct comparison of groups. a C./T. SC: p = 0.0001 for naïve vs. EAE, p = 0.0022 for pre-EAE vs. EAE n = 5/group were stained for H&E and CD68 IHC. Data are mean ± SD.
High-resolution autoradiography of lumbar spinal cords validated the in vivo PET and ex vivo gamma counting results. In naïve mice, 18F-FMD signal was minimal (Fig. 3b). In contrast, despite pre-EAE mice showed no clinical symptoms, they exhibited focal tracer uptake in regions corresponding to early immune cell infiltration. Strikingly, symptomatic EAE mice with hindlimb paralysis demonstrated even more pronounced tracer accumulation, forming punctuate hotspots that colocalized with multifocal mononuclear infiltrates and peripheral white matter demyelination in adjacent hematoxylin and eosin (H&E)-stained sections, which are hallmarks of inflammatory lesions in this EAE model40,41 (Fig. 3c). Quantitative analysis of autoradiography supported these findings, revealing a 2.5-fold and 3.5-fold increase in tracer uptake in the spinal cords of pre-EAE and symptomatic EAE mice, respectively, compared to naïve controls (p < 0.05; Supplementary Fig. 3b). Furthermore, CD68 immunohistochemical staining in a subset of mice from the same PET imaging cohort confirmed the presence of activated myeloid cells in the lumbar spinal cords of both pre-EAE and symptomatic EAE mice (Fig. 3c and Supplementary Fig. 4). Together, these results provide compelling evidence for the specificity and sensitivity of 18F-FMD PET in detecting neuroinflammation.
CSF1R dendranib is effective in reducing EAE disease severity
H74DS3M8 is a hydroxyl dendrimer, conjugated with eight dasatinib payloads, engineered to selectively target activated myeloid cells via fluid-phase endocytosis and inhibit CSF1R with high potency42,43. Hydroxyl dendrimers of this class are metabolically stable and are eliminated intact via renal clearance44,45,46. Kinase profiling revealed its sub-nanomolar potency against CSF1R, ABL tyrosine kinase 1 (ABL1), cluster of differentiation 117 (c-Kit), and cellular Src (c-Src), with minimal off-target activity against epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor beta (PDGFRβ; Supplementary Table 3). In cell-based assays, H74DS3M8 inhibited proliferation in bellus verum 2 (BV2) microglial cells in a dose-dependent manner, with an IC50 of 343 nM at 52 h (Supplementary Fig. 1). Importantly, this effect on inhibition of proliferation was seen in the absence of cell death, differentiating the mechanism of action of H74DS3M8 from other CSF1R inhibitors whose primary mechanism is through microglial killing or depletion. Pharmacokinetic studies demonstrated sustained systemic exposure following both intraperitoneal (i.p.) and intravenous administration, supporting its suitability for in vivo applications (Supplementary Table 4). Noncompartmental analysis of sparse-sampling data revealed log-linear elimination kinetics with terminal plasma half-lives (HL_Lambda_z) of 3.44 h (i.p.) and 3.15 h (intravenous, i.v.), respectively (Supplementary Fig. 2), consistent with a clearance profile appropriate for therapeutic targeting of CNS inflammation.
To evaluate its therapeutic potential in a context of neuroinflammation and demyelination, we treated EAE mice daily with H74DS3M8 (60 mg/kg, i.p.). Disease severity was compared to vehicle-treated controls (4.4% [weight per volume] trehalose dihydrate/10 mM histidine via i.p. injection) and FTY720-treated mice (3 mg/kg via oral gavage; Fig. 4a). Treatment began at the presymptomatic stage on day 8 post-EAE induction, the earliest timepoint at which 18F-FMD PET imaging detected molecular changes in myeloid cell activation. By day 15, 100% of vehicle-treated mice developed mild to severe EAE symptoms (Fig. 4b). In contrast, only 52% of H74DS3M8-treated mice and 27% of FTY720-treated mice showed mild symptoms (e.g., tail weakness), with none progressing to severe EAE (e.g., hindlimb paralysis). Both treatments effectively reduced disease severity, with average scores reduced to 0.6 ± 0.9 (H74DS3M8) and 0.4 ± 0.6 (FTY720), compared to 2.7 ± 0.9 in vehicle-treated controls by day 15 (p < 0.0001; Fig. 4b and Supplementary Table 5).
a Timeline for treatment monitoring study. Parts created in BioRender. Reyes, S. (2026) https://BioRender.com/kkh8mf1. b EAE disease severity scores in mice treated daily from the pre-EAE stage with 60 mg/kg H74DS3M8 (n = 30), 3 mg/kg FTY720 (n = 30), or vehicle (n = 40). c Representative 18F-FMD PET/CT images and d quantification of PET images shows reduced signal in the CNS of H74DS3M8- (n = 7) and FTY720-treated EAE mice (n = 6) compared to vehicle-treated EAE mice (n = 5). C/T cervical/thoracic, L lumbar, SC spinal cord. Statistical analyses were performed using b two- or d one-way ANOVA with Tukey’s multiple comparisons tests. # versus H74DS3M8-treated EAE mice, +versus FTY720-treated EAE mice, and *denotes direct comparison of groups. b day 12: p = 0.0016 EAE+veh vs. EAE + H74, p = 0.0002 EAE+veh vs. EAE + FTY720; days 13-15: p < 0.0001 EAE + veh vs. EAE + H74 and EAE+veh vs. EAE + FTY720; day 15: p = 0.0085 EAE + H74 vs. EAE + FTY720. d medulla: p = 0.0026 EAE+veh vs. EAE + H74, p = 0.0039 EAE+veh vs. EAE + FTY720; cerebellum: p = 0.0071 EAE+veh vs. EAE + H74, p = 0.0088 EAE + veh vs. EAE + FTY720; pons: p = 0.008 EAE+veh vs. EAE + H74, p = 0.014 EAE+veh vs. EAE + FTY720; C./T. SC: p = 0.0014 EAE+veh vs. EAE + H74, p = 0.0004 EAE + veh vs. EAE + FTY720; L. SC: p = 0.0117 EAE+veh vs. EAE + H74, p = 0.0043 EAE+veh vs. EAE + FTY720. Data are mean ± SD.
18F-FMD PET imaging sensitively detects therapeutic response of EAE mice to different MS therapies
To assess the ability of 18F-FMD PET to monitor the immunomodulatory effects of H74DS3M8 in vivo, we performed a treatment monitoring study comparing its therapeutic efficacy to FTY720 and vehicle control in EAE mice. On day 15 post-EAE induction, 18F-FMD PET imaging showed a marked reduction in spinal cord signal (white arrow) in both H74DS3M8- and FTY720-treated mice compared to vehicle-treated controls (Fig. 4c). Quantification of in vivo PET images revealed significant decreases in tracer uptake across multiple CNS regions, including the medulla (p < 0.01), cerebellum (p < 0.01), pons (p < 0.05), cervical/thoracic spinal cord (p < 0.01), and lumbar spinal cord (p < 0.05), consistent with a reduction in innate immune activity. These results demonstrate that 18F-FMD PET imaging can sensitively detect the immunomodulatory effects of both treatments (Fig. 4d).
Ex vivo gamma counting and high-resolution autoradiography further confirmed in vivo PET findings. H74DS3M8- and FTY720-treated EAE mice showed significantly lower 18F-FMD signal in the cervical/thoracic and lumbar spinal cords, as well as blood, compared to vehicle controls. The cervical/thoracic spinal cords of treated mice exhibited 5- to 8-fold lower signal, while lumbar spinal cords and blood showed 2- to 3-fold decreases (p < 0.05; Fig. 5a and Supplementary Fig. 3c). Autoradiography confirmed these results, with H74DS3M8 and FTY720 reducing tracer signal to near-naïve levels (Fig. 5b and Supplementary Fig. 3d), and H&E staining revealed significantly less demyelination in treated mice, closely resembling naïve controls (Fig. 5c). Immunohistochemistry staining for CD68+ cells in the same PET imaging cohort confirmed negligible presence of activated myeloid cells in H74DS3M8- and FTY720-treated mice, underscoring their efficacy in modulating neuroinflammation and potentially altering disease severity (Fig. 5c and Supplementary Fig. 4).
a Ex vivo gamma counting of whole brain, cervical/thoracic spinal cord, and lumbar spinal cord of EAE mice treated with vehicle (n = 5), H74DS3M8 (n = 7), and FTY720 (n = 6). b Representative autoradiography images with H&E overlay in EAE mice treated with vehicle, H74DS3M8, and FTY720. c Representative histology images at 40x magnification. Scale bars, 50 µm. C/T cervical/thoracic; L lumbar; SC spinal cord; H74 H74DS3M8. a Statistical analyses were performed using one-way ANOVA with Tukey’s multiple comparisons tests. #versus H74DS3M8-treated EAE mice and *denotes direct comparison of groups. a C./T. SC: p = 0.0005 EAE + veh vs. EAE + H74, p = 0.0004 EAE + veh vs. EAE + FTY720; L. SC: p = 0.018 EAE + veh vs. EAE + H74, p = 0.012 EAE + veh vs. EAE + FTY720. n = 5/group were stained for H&E and CD68 IHC. Data are mean ± SD.
Flow cytometry confirmed therapeutic modulation of CNS and peripheral myeloid responses in EAE
To identify the immune cell subsets driving neuroinflammation and assess their modulation by therapeutic interventions in this EAE mouse model, we performed single-cell flow cytometry on spinal cord and peripheral blood samples from naïve, pre-EAE, vehicle-treated, H74DS3M8-treated, and FTY720-treated EAE mice (Fig. 6a and Supplementary Fig. 5). This approach enabled profiling of immune dynamics across disease stages and treatment responses. Tissues were harvested on day 8 post-induction for pre-EAE mice and on day 15 for EAE mice treated with H74DS3M8, FTY720, and vehicle at various disease stages (score 0.5–3.5, hindlimb paresis/paralysis), as well as from naïve mice.
a EAE was induced in C57BL/6J mice, and a group of them was treated with either H74DS3M8, FTY720, or vehicle. Created in BioRender. Reyes, S. (2026) https://BioRender.com/57g010x. Blood was collected and spinal cords were harvested for single-cell flow cytometry at different disease states. Summarized proportion of infiltrating macrophages, resting microglia, monocytes/dendritic cells, neutrophils, T cells, and B cells in the b spinal cord and c blood of naïve, pre-EAE, EAE with vehicle, EAE with H74DS3M8, and EAE with FTY720 mice. b, c Statistical analyses were performed using one-way ANOVA with Tukey’s multiple comparisons tests. +versus naïve mice, #versus H74DS3M8-treated EAE mice, and *denotes direct comparison of groups. Resting microglia, activated microglia, DCs, neutrophils, monocytes, T and B cells (blood): n = 5/group; infiltrating macrophages: n = 11 naïve, n = 9 pre-EAE, n = 18 EAE+vehicle, n = 6 EAE + H74, n = 5 EAE + FTY720; T cells (spinal cord): n = 11 naïve, n = 9 pre-EAE, n = 16 EAE + vehicle, n = 9 EAE + H74, n = 5 EAE + FTY720; B cells (spinal cord): n = 8 naïve, n = 9 pre-EAE, n = 15 EAE + vehicle, n = 9 EAE + H74, n = 5 EAE + FTY720. b p < 0.0001 for all groups except infiltrating macrophages: p = 0.048 naïve vs. EAE + H74, neutrophils: p = 0.028 naïve vs. EAE + H74, T cells: p = 0.003 naïve vs. EAE + H74, p = 0.017 pre-EAE vs. EAE + H74, p = 0.03 EAE + H74 vs. EAE + FTY720; c p < 0.0001 for all groups except monocytes: p = 0.0007 naïve vs. pre-EAE, p = 0.03 pre-EAE vs. EAE + FTY720; neutrophils: p = 0.02 naïve vs. EAE + H74, T cells: p = 0.0001 naïve vs. pre-EAE, p = 0.0016 pre-EAE vs. EAE+vehicle, p = 0.0055 pre-EAE vs. EAE + H74, p = 0.048 EAE + H74 vs. EAE + FTY720; B cells: p = 0.0001 naïve vs. EAE + H74, p = 0.0004 pre-EAE vs. EAE+vehicle, p = 0.003 pre-EAE vs. EAE + FTY720, p = 0.0014 EAE + vehicle vs. EAE + H74; p = 0.0008 EAE + H74 vs. EAE + FTY720. H74 = H74DS3M8. Data are mean ± SD. Parts of (a) created in BioRender.
Flow cytometric analysis revealed profound immune remodeling in the CNS of EAE mice compared to naïve controls. There was a dramatic reduction in the proportion of resting microglia (CD11b+CD45lowTMEM119+), which declined from 69.9% in naïve mice to 20.28% in EAE mice. In parallel, activated microglia (CD11b+CD45lowTMEM119−) expanded significantly, from 0.13% to 20.84%, representing a >160-fold increase and indicating a marked phenotypic shift. This transformation was accompanied by widespread infiltration of peripheral immune cells, including T cells (CD3+CD45+; 0.49% in naïve vs. 22.39% in EAE), infiltrating macrophages (CD11b+CD45hi; 0.88% vs. 37.53%), monocyte/dendritic cells (DC) (CD11b+CD45hiLy6G−; 1.01% vs. 17.42%), and neutrophils (CD11b+CD45hiLy6G+; 0.99% vs. 13.64%) (all comparisons p < 0.0001; Fig. 6b). Collectively, these data reveal a profound shift from a predominantly homeostatic microglial environment to a highly inflammatory landscape, with infiltrating macrophages emerging as the most abundant immune cell type within the CNS of EAE mice.
Prior to symptom onset, pre-EAE mice showed a substantial reduction in resting microglia (28.24% vs. 69.86% in naïve; p < 0.0001) in the spinal cord, alongside an early expansion of activated microglia (1.32% vs. 0.13% in naïve), suggesting that innate immune activation precedes peripheral immune infiltration and may play a key initiating role in EAE pathogenesis. Notably, B cells (CD19+CD45+) remained absent from the CNS across all groups and stages, indicating a minimal role of B cells in this model. In the periphery, a parallel pattern of immune activation was observed: circulating myeloid cells increased significantly (p < 0.05; Fig. 6c), and T cells underwent a transient expansion during the preclinical phase, suggesting early systemic priming. B cells in blood modestly declined during the pre-EAE stage and returned to baseline levels during acute disease, mirroring levels seen in naïve mice.
Building on this data, we next evaluated the therapeutic potential of H74DS3M8 in modulating immune cell populations and compared its effects to the established immunomodulator FTY720. Both treatments significantly reduced pathogenic myeloid populations in the CNS and peripheral blood of EAE mice, including activated microglia, infiltrating macrophages, and monocytes (p < 0.01; Fig. 6b, c). H74DS3M8 also markedly suppressed CNS-infiltrating T cells, DCs, and neutrophils, and decreased both T and B cell frequencies in the circulation. FTY720 similarly inhibited T cell infiltration into the CNS and significantly reduced circulating monocytes, neutrophils, and B cells (p < 0.001). To further characterize the functional impact of treatment, we analyzed macrophage polarization states. Both therapies significantly decreased the proportion of pro-inflammatory M1-like microglia/macrophages (CD11b+CD45+CD86+; p < 0.0001; Supplementary Fig. 6).
18F-FMD is selectively taken up by activated myeloid cells in EAE mice
To investigate the specific cell populations that endocytose 18F-FMD and thus gain an understanding of what the signal in our 18F-FMD PET scans truly represent, we used Cy5-labeled generation-4 hydroxyl dendrimer (Cy5-HD), a fluorescent analog that closely mirrors the structure, size, and uptake mechanism of 18F-FMD. Cy5-HD was administered intraperitoneally to all experimental groups 24 h prior to tissue collection for flow cytometry analysis. Quantitative analysis revealed a significant increase in Cy5-HD+ myeloid populations, including infiltrating macrophages, activated microglia, neutrophils, and monocyte/DCs, in both the spinal cord and peripheral blood of pre-EAE and symptomatic EAE mice compared to naïve controls (p < 0.001, Fig. 7). Among microglia, Cy5-HD uptake was more than 9-fold higher in cells with a pro-inflammatory, activated phenotype compared to resting microglia, which showed negligible uptake (Supplementary Fig. 7). T cells and B cells lacked Cy5-HD uptake, further supporting the tracer’s specificity for activated myeloid subsets. Following therapeutic intervention, Cy5-HD+ myeloid populations were markedly reduced by up to 19-fold in infiltrating macrophages and 46-fold in activated microglia, highlighting the ability of both treatments to suppress pro-inflammatory innate immune activity (Fig. 7). These findings align with previously published findings demonstrating the selective accumulation of hydroxyl dendrimers in activated myeloid cells under inflammatory conditions27,28,29. Notably, the spatial and temporal distribution of Cy5-HD+ cells observed by flow cytometry closely mirrored the trends captured by in vivo PET imaging, ex vivo gamma counting, and autoradiography. PET signal intensity decreased 2- to 2.5-fold following treatment, paralleling the observed reductions in Cy5-HD+ myeloid populations.
Percentage of Cy5-HD+ cells in a spinal cord and b blood of naïve, pre-EAE, EAE with vehicle, EAE with H74, and EAE with FTY720 mice. a, b Statistical analyses were performed using one-way ANOVA with Tukey’s multiple comparisons tests. +versus naïve mice, #versus H74DS3M8-treated EAE mice, and *denotes direct comparison of groups. Activated microglia, DCs, neutrophils, monocytes: n = 5/group; infiltrating macrophages: n = 12 naïve, n = 8 pre-EAE, n = 15 EAE+vehicle, n = 8 EAE + H74, n = 5 EAE + FTY720. a p < 0.0001 for all groups; b p < 0.0001 for monocytes: naïve vs. pre-EAE, naïve vs. EAE + vehicle, pre-EAE vs. EAE + vehicle, EAE+vehicle vs. EAE + H74, EAE + vehicle vs. EAE + FTY720; monocytes: p = 0.0083 naïve vs. EAE + H74, p = 0.01 naïve vs. EAE + FTY720, p = 0.01 pre-EAE vs. EAE + H74, p = 0.006 pre-EAE vs. EAE + FTY720; p < 0.0001 for neutrophils: naïve vs. pre-EAE, naïve vs. EAE + vehicle, pre-EAE vs. EAE + H74, pre-EAE vs. EAE + FTY720, EAE + vehicle vs. EAE + H74, EAE+vehicle vs. EAE + FTY720; neutrophils: p = 0.003 naïve vs. EAE + H74. H74 = H74DS3M8. Data are mean ± SD.
Discussion
Immune-mediated neurodegeneration is a key pathological hallmark of MS, driven by the activation and infiltration of immune cells, including CNS-resident microglia and peripheral myeloid cells, across a compromised BBB. In both human MS and animal models such as EAE, these activated myeloid populations initiate neuroinflammatory cascades that contribute to demyelination and neuronal damage47,48. While structural MRI remains the clinical standard for MS diagnosis and monitoring, it lacks the sensitivity to distinguish between active and inactive lesions and offers limited insight into real-time specific immune cell activity.
To address this unmet clinical need, we developed and validated a hydroxyl dendrimer PET tracer, 18F-FMD, designed to non-invasively track whole-body innate immune responses25,26. Activation of these innate immune cells is one of the earliest events in EAE pathogenesis, initiating and promoting pro-inflammatory T cell responses while facilitating the recruitment of blood circulating monocytes into the CNS, where they subsequently differentiate into monocyte-derived macrophages and DCs5,49. In an EAE model, we demonstrated that 18F-FMD can detect presymptomatic disease activity before any muscle weakness or paralysis, with PET signal increasing in the spinal cord, brain, and peripheral blood as disease severity worsened. These changes mirror the clinical trajectory of the myelin oligodendrocyte glycoprotein residues 35–55 (MOG35-55) EAE model, in which neurological symptoms ascend rostrocaudally from the tail along the spinal axis50. Ex vivo biodistribution and high-resolution autoradiography of tissues after perfusion further confirmed in vivo findings. Histological analysis of spinal cord sections adjacent to autoradiography images revealed multifocal mononuclear infiltrates and peripheral white matter demyelination in severe EAE, further validating the imaging findings. These patterns are consistent with upregulated expression of pro-inflammatory cytokines, such as tumor necrosis factor (TNF), IL-1β, and interferon gamma (IFN-γ), which sharply increase in the spinal cord around disease onset (day 10 post-induction) and remain elevated throughout the acute phase of EAE39.
To investigate the immune cells that endocytose 18F-FMD, we used Cy5-HD as a fluorescent surrogate for the PET tracer. Cy5-HD mirrors the structure and function of 18F-FMD. Both compounds lack affinity for specific cell surface markers and are internalized via fluid-phase endocytosis, a non-receptor-mediated mechanism that is increased in activated myeloid cells during neuroinflammation. This uptake mechanism ensures that tracer localization reflects active cellular processes rather than passive diffusion or nonspecific retention. Once internalized, endocytosed dendrimers are trafficked to lysosomes and stably retained, enabling prolonged intracellular signal51,52,53. Importantly, the molecular weights of 18F-FMD (14.462 kDa) and Cy5-HD (15.084 kDa) are nearly identical, with the shared dendrimer scaffold (14.277 kDa) comprising the majority of the mass. Each construct carries only a single 18F or Cy5 payload, which does not alter the core structure, surface charge, hydrophilicity, or biodistribution of the dendrimer34,46,54,55. This molecular consistency supports the use of Cy5-HD as a reliable surrogate for 18F-FMD in mechanistic and cell-type validation studies.
Flow cytometry confirmed that hydroxyl dendrimer uptake was selectively restricted to activated myeloid cells, indicating that increases in 18F-FMD PET signal reflect the infiltration and expansion of this population in EAE mice. Additionally, immunostaining with CD68 in the spinal cord of a subset of PET-imaged mice further validated the co-localization of tracer signal with activated myeloid cells. Together, these findings support the conclusion that 18F-FMD detects functionally relevant innate immune activation rather than nonspecific or passive tracer accumulation.
Conventional MRI remains the cornerstone of MS care, serving as the primary tool for both diagnosis and longitudinal monitoring of treatment efficacy. The revised McDonald criteria, introduced at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) in 2024, have further expanded the role of clinical and imaging parameters, reinforcing the central role of structural MRI in facilitating more timely diagnoses56. However, while MRI provides critical structural information, it lacks specificity for innate immune cells–cell populations increasingly recognized as key drivers of the neurodegenerative aspects of MS pathology. As new therapeutic strategies emerge that target these cells directly, there is a growing need for advanced imaging approaches capable of capturing their dynamics and treatment responses in vivo. In this context, 18F-FMD PET has the potential to offer a complementary approach by enabling cell-specific, real-time visualization of innate immune activation. Herein, we demonstrated that 18F-FMD PET sensitively detected reductions in CNS inflammatory activity in EAE mice following treatment with either FTY720, an FDA-approved sphingosine 1-phosphate (S1P) receptor modulator, or H74DS3M8, a CSF1R-targeting dendranib. These reductions were corroborated by flow cytometry and ex vivo analyses, underscoring the tracer’s ability to track immune modulation with high specificity. Together, these results support 18F-FMD PET as a promising biomarker for monitoring innate immune dynamics and therapeutic efficacy, complementing structural MRI and potentially guiding the development and evaluation of emerging MS therapies.
The differential effects observed with H74DS3M8 and FTY720 also illustrate how distinct classes of immunomodulators influence neuroinflammation through complementary mechanisms. H74DS3M8 is a highly potent inhibitor of CSF1R, ABL1, and c-Src, with a kinase inhibition profile similar to that of dasatinib57 but with improved selectivity and reduced off-target activity. By directly modulating the CSF1R signaling pathway, it suppresses pathological microglial proliferation without triggering cell death. Prior studies have shown that CSF1R inhibitors rapidly deplete pro-inflammatory myeloid cells in the spinal cord and significantly ameliorate EAE symptoms43. Moreover, CSF1R-targeted therapies have been associated with slowed disease progression and improved cognitive outcomes in MS patients58,59. In contrast, FTY720 binds to four of the five G protein-coupled S1P receptor subtypes, modulating lymphocyte egress from lymphoid tissues and mitigating neuroinflammation in MS, thereby indirectly suppressing myeloid cell activation and CNS infiltration60,61,62. These mechanistic differences underscore the importance of understanding the functional plasticity of myeloid cells–an area of growing interest in MS research. While macrophages were traditionally categorized into M1 (pro-inflammatory) and M2 (anti-inflammatory) subsets, recent studies suggest that this dichotomy is overly simplistic. Histological analyses of active MS lesions reveal that up to 70% of macrophages co-express both M1 and M2 markers, reflecting intermediate or hybrid activation states shaped by the surrounding cytokine milieu, oxidative stress, and tissue-derived signals63,64,65,66. This context-dependent activation is exemplified by TMEM119, a marker commonly used to identify homeostatic microglia. In the MOG35-55 EAE model, TMEM119 expression is significantly downregulated in the spinal cord, mirroring observations in postmortem MS tissue, where TMEM119 immunoreactivity is lost in white matter lesions but preserved in gray matter67,68,69. These shifts reflect the complex, region-specific immune landscape in MS and emphasize the need for tools like 18F-FMD PET that can capture the dynamic and spatially heterogenous nature of myeloid activation in vivo. Such a tool holds great promise not only for understanding disease biology but also for enhancing clinical trial design.
Building on this translational potential, 18F-FMD could be integrated into trials of emerging therapies such as tolebrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor evaluated in the HERCULES trial, which has shown promise in slowing clinical progression in non-relapsing SPMS70. By enabling quantitative, real-time monitoring of myeloid cell activity, 18F-FMD PET could support pharmacodynamic assessments and facilitate patient stratification. Beyond therapeutic response monitoring, this tracer also offers a unique opportunity to deepen our understanding of the in vivo role of innate immune cells in neuroinflammation, thereby supporting both drug development and biomarker-guided clinical decision making.
This study is not without limitations. Given the renal clearance of 18F-FMD and the small size of EAE mice, there is a possibility of partial volume effects from adjacent kidneys affecting signal quantification in the lumbar spinal cord. Partial volume corrections using post-reconstruction- and reconstruction-based methods could help circumvent this limitation71. However, these correction techniques are challenging to implement effectively in mouse models due to the small size and thin geometry of the spinal cord, which often falls below the spatial resolution of small-animal PET scanners72,73. This leads to significant spillover and underestimation of true activity, even after correction, particularly when adjacent high-uptake organs (like the kidneys) are involved. Instead, we mitigated this issue by dissecting the kidneys and spinal cord out of each mouse for gamma counting and performing autoradiography on thin spinal cord sections. These techniques eliminate contributions from circulating tracer and provide higher sensitivity and spatial resolution, respectively, without any possibility for spillover from adjacent tissues. Using these methods, we observed a dramatic elevation in spinal cord signal in EAE mice compared to naïve controls, corroborating in vivo PET findings. Importantly, this limitation is less relevant in larger species, such as non-human primates or humans, where greater anatomical separation between kidneys and spinal cord reduces the likelihood of signal interference, allowing for more accurate delineation of regions of interest.
While animal models are invaluable for understanding disease mechanisms and evaluating diagnostic and therapeutic approaches, they do not fully recapitulate the clinical progression of human MS. The induction of EAE via MOG35-55 peptide and Complete Freund’s adjuvant (CFA) is an artificial process that does not naturally occur in human MS. CFA triggers strong peripheral innate immune activation to facilitate antigen presentation, which in turn alters CNS-resident immune cell behavior74. This preclinical immune milieu may therefore not adequately represent the pathophysiology of human MS, underscoring the need for clinical studies to evaluate the performance of 18F-FMD.
EAE models involve BBB disruption, which will enable some passive tracer entry into the CNS parenchyma. Nonetheless, the selective uptake and retention of 18F-FMD is mediated by endocytosis of activated myeloid cells in the presence of neuroinflammation; the tracer is not retained in healthy brain tissue or resting immune populations. In the absence of immune activation, hydroxyl dendrimers do not exhibit sustained accumulation. Therefore, even in the context of increased BBB permeability or microglial-driven barrier modulation, nonspecific leakage alone does not account for the tracer signal observed in our study.
Another important limitation is that, due to the biophysical mechanism of uptake of our PET tracer via fluid-phase endocytosis by activated myeloid cells, it is not possible to evaluate 18F-FMD specificity or uptake patterns in vitro using post-mortem tissue sections. Both the Cy5- and 18F-labeled dendrimer conjugates require living, metabolically active cells for internalization, and endocytic activity ceases upon cell death. Consequently, analyses of these agents in fresh-frozen or fixed MS tissues are not possible. Furthermore, BBB disruption in human MS is more heterogenous and typically less pronounced than in EAE, complicating direct extrapolation from animal models. Definitive evaluation of tracer uptake patterns will therefore require clinical validation, where the interplay of BBB integrity, immune activation, and disease heterogeneity can be directly assessed.
Despite these limitations, EAE models remain a powerful and well-characterized preclinical platform for studying MS-like neuroinflammation. In our study, we leveraged an EAE model to evaluate the in vivo performance of 18F-FMD and its ability to selectively monitor activated myeloid cells. While this model does not capture the full spectrum of disease progression seen in clinical MS, it provides a robust system for assessing systemic tracer distribution and uptake by activated myeloid populations within the CNS and throughout the body. These preclinical findings underscore the promise of 18F-FMD and establish a foundation for subsequent clinical evaluation.
Our team has recently translated 18F-FMD for clinical use. It is currently being evaluated in an ongoing phase 1/2 clinical trial (NCT05395624)75 to assess its performance across MS subtypes and other neuroinflammatory diseases26,76. This trial aims to validate the sensitivity, specificity, and uptake patterns of 18F-FMD in patients, and to establish whether they mirror those observed in preclinical models. In addition, the study will evaluate the tracer’s utility for monitoring neuroinflammatory activity, assessing treatment response, and enabling patient stratification in clinical settings.
In conclusion, 18F-FMD PET is a promising, highly sensitive and specific imaging tool that enables detection of aberrant innate immune responses in EAE even before clinical symptoms manifest. The selective endocytosis of 18F-FMD by activated myeloid cells allows precise monitoring of pro-inflammatory activity following treatments, as demonstrated with both the FDA-approved drug FTY720 and the CSF1R dendranib H74DS3M8. This study establishes the utility of a hydroxyl dendrimer-based PET tracer for imaging activated myeloid cells in EAE mice and demonstrates that a hydroxyl dendrimer-based CSF1R dendranib can effectively suppress pathogenic myeloid proliferation with efficacy comparable to fingolimod. By enabling non-invasive visualization of early neuroinflammation and dynamic tracking of disease-modifying therapy effects, 18F-FMD holds promise as a complementary tool to structural MRI, facilitating more precise and personalized management of MS. As 18F-FMD advances through clinical trials, its broader application to other neuroinflammatory and neurodegenerative diseases could establish it as a versatile platform for mapping myeloid cell activation across diverse conditions.
Methods
Pharmacokinetics of H74DS3M8
8–12-week-old male C57BL/6J mice (Jackson Laboratory, strain #: C57BL6J, RRID: 1MSR_JAX:000664) were dosed with 60 mg/kg H74DS3M8 in 6.3% Trehalose/10 mM histidine pH5.5 (at a dose concentration of 12 mg/mL) by i.p. injection into the lower left or right quadrant of the abdomen or by i.v. injection into the lateral tail vein, at Meadowhawk Biolabs (Hayward, CA). Blood was collected from each animal at two timepoints (via submental vein for survival collection and cardiac puncture for terminal collection), of 0.5, 1, 4, 12, 24, 36 h, for a total of n = 3 per timepoint. Samples were collected into K2EDTA tubes and shipped to Alturas Analytics (Moscow, ID) for bioanalytical measurement using a previously established high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) bioanalytical method.
Microglia proliferation assay
BV2 cells (ATCC, Manassas, VA, #CRL-2467) were cultured in the presence of 100 ng/mL macrophage colony-stimulating factor (M-CSF, Thermo Fisher Scientific, Waltham, MA, #PMC2044) in serum-free Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific, #11885084) supplemented with 1% Penicillin-Streptomycin (Thermo Fisher Scientific, #15140122). Cells were treated with various concentrations of H74DS3M8, and confluence was observed every 2 h in the Incucyte (Sartorius, Göttingen, Germany) maintained at 37 °C in 5% CO2. Brightfield images were obtained in one of four field-views per well at 10x magnification at 52 h post-plating and treatment. Concentration data from the 52-h timepoint were used to calculate the IC50.
Animals
Female 8-week-old C57BL/6J mice (Jackson Laboratory, strain #: C57BL6J, RRID: 1MSR_JAX:000664) were housed in a non-barrier, temperature-controlled environment (humidity 40–60 %) under a 12-h light/dark schedule with unrestricted access to food and water. A total of 160 mice were used for this study. Treatment groups were randomly assigned by cage and co-housed in the same room as vehicle groups. All animal care and procedures complied with the Animal Welfare Act and were in accordance with institutional guidelines. Permission to perform all animal experiments was granted by the Stanford Administrative Panel on Laboratory Animal Care (APLAC), which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC International).
EAE induction
EAE was induced in female C57BL/6J WT mice (9 weeks; the Jackson Laboratory, #000664) using MOG35-55 emulsified in CFA (Hooke Laboratories, Lawrence, MA, #EK-0111). All vehicle and therapy-treated mice received two pertussis toxin i.p. injections (110 ng) 2 and 24 h after MOG induction. Naïve littermates were used as additional controls. EAE mice were weighed and scored daily from day 8 onward using a standard scoring protocol for levels of paresis/paralysis (Supplementary Table 1).
Drug treatments
Mice were randomly assigned to the following groups: naïve, vehicle-treated EAE, H74DS3M8-treated EAE, and fingolimod-treated EAE. All therapies were given starting from 8 days post disease-induction for 8 days, till day 15 post-induction. Vehicle-treated EAE mice received 4.4% (w/v) trehalose dihydrate/10 mM histidine via i.p. injection. H74DS3M8 (Ashvattha Therapeutics, Redwood City, CA, 60 mg/kg) was administered via i.p. injection. Fingolimod (Cayman Chemicals, Ann Arbor, MI, 3 mg/kg, #10006292) was administered via oral gavage.
Flow cytometry
Mice were euthanized under anesthesia (with inhaled isoflurane [2–3%]) and blood was collected by cardiac puncture in EDTA-treated tubes and lysed with ACK lysis buffer (Lonza, Walkersville, MD, #BP10-548E) for 10 min at room temperature. All cells were then resuspended in fluorescence-activated cell sorting (FACS) buffer (2% fetal bovine serum [Thermo Fisher Scientific, #A5670701] in PBS [Gibco, #10010023]). Mice were then anesthetized and exsanguinated by cardiac perfusion with PBS. Spinal cords were harvested, mechanically homogenized in ice-cold CNS buffer (2.5% Hepes [pH 7.5, Gibco, #15630080] in Hanks’ balanced salt solution without Ca/Mg/PSP [Thermo Fisher Scientific, #J67681.K2]), and resuspended in FACS buffer. Flow cytometry was performed to delineate immune cell populations using the following antibodies (Supplementary Table 6): AF700 CD45 (BioLegend, San Diego, CA, #147715), PerCP CD19 (BioLegend, #115531), BV421 CD3 (BioLegend, #100228), APC/Fire750 CD11b (BioLegend, #101261), BV570 Ly6G (BioLegend, 127629), PB CD86 (BioLegend, #105021), BUV737 CD80 (BD Biosciences, Franklin Lakes, NJ, #612773), PE CD163 (BioLegend, #156703), PerCP/Cy5.5 CD206 (BioLegend, #141715), PeCy7 TMEM119 (Thermo Fisher Scientific, #25-6119-82), and Cy5-HD (Ashvattha Therapeutics). Samples were washed with FACS buffer and resuspended in 2% paraformaldehyde (PFA, Thermo Fisher Scientific, J19943.K2) for 20 min at room temperature. After fixation, cells were washed twice and resuspended in FACS buffer until being analyzed on the Cytek Aurora (Cytek Biosciences, Fremont, CA). FlowJo software (v.10, BD Biosciences) was used for the analysis and depiction of the gating strategy.
18F-FMD radiosynthesis
All chemicals were acquired from commercial sources except for synthetic precursors 3-azidopropyl-4-methylbenzenesulfonate and PAMAM-G4-OH alkyne10 dendrimer precursor, which were synthesized to order by Snapdragon Chemistry. All chemicals were used without further purification. 18F-FMD was radiolabeled in two steps26: nucleophilic 18F-fluorination of 3-azidopropyl-4-methylbenzenesulfonate yielded 18F-3-fluoropropylazide, which was purified via high-performance liquid chromatography (HPLC). This was subsequently reacted with PAMAM-G4-OH alkyne10 dendrimer precursor (0.5 mg) via copper catalyzed azide-alkyne cycloaddition to produce 18F-FMD, which was purified and reformulated in saline with 10% ethanol for injection. HPLC was used to confirm identity and determine specific activity. Specific activity for these experiments (n = 4 syntheses) was 0.556 mCi/μg and the approximate injected mass of dendrimer per mouse was 0.270 μg for in vivo PET imaging studies and 1.345 µg for ex vivo autoradiography studies.
In vivo PET/CT imaging studies
Mice were anesthetized with isoflurane gas (2.0–3.0% for induction and 1.0–2.0% for maintenance) and intravenously injected with 18F-FMD (150 µCi). 10-min static PET images were acquired 50 min post-injection of 18F-FMD on days 8 and 15 after EAE induction using the GNEXT scanner (Sofie Biosciences, Dulles, VA) in list mode format. CT images were acquired after each PET scan to provide an anatomical reference frame in addition to scatter and attenuation correction for PET data. Isotropic resolution was achieved using OSEM3D/MAP reconstruction algorithms with 24 subsets, 3 iterations, and a matrix size of 240 × 240 × 191.
PET image analysis
PET images were analyzed using VivoQuant 4.0 (Perspective Therapeutics, Seattle, WA). Briefly, CT was overlaid on PET for anatomical reference. A three-dimensional mouse brain atlas was used along with total decay-corrected dose to each mouse to quantify tracer uptake in the whole brain. Cervical/thoracic and lumbar spinal cord quantification was obtained via segmentation and exclusion of the vertebral column using L1-L5 as landmarks77,78. Analysis was not conducted truly blind because the researchers have access to the mouse EAE scoring data. However, all PET/CT imaging data were assigned a scan number, and all mice were allocated unique ID numbers based on mouse positions in the scanner. All data were grouped for graphing data by experimental group after analysis.
Ex vivo gamma counting
Immediately after PET, mice were euthanized under anesthesia (with inhaled isoflurane [2–3%]) and cardiac puncture was performed. Mice were subsequently perfused with 20 mL of PBS and tissues of interest (heart, spleen, kidney, brain, spinal cord, and tail) were individually harvested, weighed and gamma-counted (Hidex automatic gamma counter, Hidex, Turku, Finland). Biodistribution results were calculated as %ID/g using the weight of each dissected organ.
Ex vivo autoradiography studies
A separate cohort of EAE and naïve mice were used to evaluate 18F-FMD distribution in the CNS tissues. Sixty minutes after intravenous injection of a higher dose of 18F-FMD (700 µCi), mice were euthanized under anesthesia (with inhaled isoflurane (2–3%)) and perfused with PBS (20 mL), and CNS tissues were harvested for autoradiography and immunohistochemistry. Spinal cord sections were analyzed via digital autoradiography using a Typhoon phophorimager (Amersham Biosciences, Amersham, United Kingdom) and stained with H&E (Hematoxylin Gills 3, Thermo Fisher Scientific, NC9964763; Eosin-Y Richard-Allan Scientific, Thermo Fisher Scientific, #22-110-637) for anatomical reference and visualization of the distribution of immune cells.
Immunohistochemistry
Dissected spinal cords were fixed in 4% PFA (Thermo Fisher Scientific) in PBS for 24 h, then switched to 30% sucrose (Thermo Fisher Scientific, #A15583.0E) and stored at 4 °C for at least 1 week before being immersed in optimal cutting temperature (OCT) compound (Sakura Finetek USA, Torrance, CA, #4583). 12 μm sections were then cut and mounted onto slides, which were stored at −80 °C for one day before staining. For staining, slides were incubated overnight at 4 °C in rat anti-mouse CD68 primary antibody (1:500) (MCA1957, Bio-Rad Laboratories, Hercules, CA). The next day, slides were incubated with biotinylated anti-rat secondary antibody (1:1000; BA-4001, Vector Laboratories, Newark, CA) for 45 min. Peroxidase substrate solution 3,3’-diaminobenzidine tetrahydrochloride hydrate (DAB; #D5637, Sigma-Aldrich, St. Louis, MO) was used to intensify stain.
Statistics and reproducibility
Statistical analyses of flow cytometry, in vivo PET, ex vivo biodistribution, and autoradiography were performed using Prism (version 10, GraphPad Software, San Diego, CA). All data were assessed for normalization (D’Agostino-Pearson omnibus K2/Shapiro-Wilk tests). Statistical analyses were performed using two-tailed unpaired t tests, Mann-Whitney tests, or one-way analysis of variance (ANOVA) with multiple comparisons tests as indicated in the figure legends for each dataset. Error bars represent standard deviation (SD). PET/CT and autoradiography results are from four experiments. Flow cytometry results are from three experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. The raw numbers for charts and graphs are available in the Source Data file whenever possible. The raw PET imaging and flow cytometry data generated in this study have been deposited in the Figshare database under https://doi.org/10.6084/m9.figshare.30644609. Source data are provided with this paper.
References
National Multiple Sclerosis Society. Number of people with MS | Atlas of MS. https://atlasofms.org/map/global/epidemiology/number-of-people-with-ms.
Loma, I. & Heyman, R. Multiple sclerosis: pathogenesis and treatment. Curr. Neuropharmacol. 9, 409–416 (2011).
Attfield, K. E., Jensen, L. T., Kaufmann, M., Friese, M. A. & Fugger, L. The immunology of multiple sclerosis. Nat. Rev. Immunol. 22, 734–750 (2022).
Mishra, M. K. & Yong, V. W. Myeloid cells—targets of medication in multiple sclerosis. Nat. Rev. Neurol. 12, 539–551 (2016).
Hernández-Pedro, N. Y., Espinosa-Ramirez, G., de la Cruz, V. P., Pineda, B. & Sotelo, J. Initial Immunopathogenesis of Multiple Sclerosis: innate Immune Response. Clin. Dev. Immunol. 2013, 413465 (2013).
Lucchinetti, C. et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707–717 (2000).
Prineas, J. W. et al. Immunopathology of secondary-progressive multiple sclerosis. Ann. Neurol. 50, 646–657 (2001).
Wingerchuk, D. M., Lucchinetti, C. F. & Noseworthy, J. H. Multiple sclerosis: current pathophysiological concepts. Lab. Investig. 81, 263–281 (2001).
Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. V. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Greter, M. et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 11, 328–334 (2005).
Bailey, S. L., Schreiner, B., McMahon, E. J. & Miller, S. D. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ TH-17 cells in relapsing EAE. Nat. Immunol. 8, 172–180 (2007).
Ifergan, I. & Miller, S. D. Potential for targeting myeloid cells in controlling CNS inflammation. Front. Immunol. 11, 571897 (2020).
Confavreux, C. & Vukusic, S. The clinical course of multiple sclerosis. in Handbook of Clinical Neurology Vol. 122, 343–369 (Elsevier, 2014).
Montalban, X. et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 376, 209–220 (2017).
Fischer, S., Proschmann, U., Akgün, K. & Ziemssen, T. Lymphocyte counts and multiple sclerosis therapeutics: between mechanisms of action and treatment-limiting side effects. Cells 10, 3177 (2021).
McDonald, W. I. et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann. Neurol. 50, 121–127 (2001).
Luus, C., Hanani, R., Reynolds, A. & Kassiou, M. The development of PET radioligands for imaging the translocator protein (18 kDa): what have we learned? J. Label. Compd. Radiopharm. 53, 501–510 (2010).
Chen, M.-K. & Guilarte, T. R. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol. Ther. 118, 1–17 (2008).
Airas, L., Rissanen, E. & Rinne, J. O. Imaging neuroinflammation in multiple sclerosis using TSPO-PET. Clin. Transl. Imaging 3, 461–473 (2015).
Polvinen, E., Matilainen, M., Nylund, M., Sucksdorff, M. & Airas, L. M. TSPO-detectable chronic active lesions predict disease progression in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflammation 10, e200133 (2023).
Owen, D. R. et al. An 18-kDa Translocator Protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow. Metab. 32, 1–5 (2012).
Nutma, E. et al. Activated microglia do not increase 18 kDa translocator protein (TSPO) expression in the multiple sclerosis brain. Glia 69, 2447–2458 (2021).
Jain, P. et al. Neuroinflammation PET imaging: current opinion and future directions. J. Nucl. Med. 61, 1107–1112 (2020).
Wu, C., Li, F., Niu, G. & Chen, X. PET imaging of inflammation biomarkers. Theranostics 3, 448–466 (2013).
Carlson, M. L. et al. Development and initial assessment of [18F]OP-801: a novel hydroxyl dendrimer PET tracer for preclinical imaging of innate immune activation in the whole body and brain. Mol. Imaging Biol. 25, 1063–1072 (2023).
Jackson, I. M. et al. Clinical radiosynthesis and translation of [18F]OP-801: a novel radiotracer for imaging reactive microglia and macrophages. ACS Chem. Neurosci. 14, 2416–2424 (2023).
Sharma, A. et al. Dense hydroxyl polyethylene glycol dendrimer targets activated glia in multiple CNS disorders. Sci. Adv. 6, eaay8514 (2020).
Kambhampati, S. P. et al. Systemic and intravitreal delivery of dendrimers to activated microglia/macrophage in ischemia/reperfusion mouse retina. Investig. Ophthalmol. Vis. Sci. 56, 4413–4424 (2015).
Kambhampati, S. P. et al. Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J. Control. Release 335, 527–540 (2021).
Henningfield, C. M. et al. Selective targeting and modulation of plaque associated microglia via systemic hydroxyl dendrimer administration in an Alzheimer’s disease mouse model. Alzheimers Res. Ther. 16, 101 (2024).
Nance, E. et al. Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome. J. Neuroinflammation 14, 252 (2017).
Arteaga Cabeza, O. et al. Neuroprotective effects of a dendrimer-based glutamate carboxypeptidase inhibitor on superoxide dismutase transgenic mice after neonatal hypoxic-ischemic brain injury. Neurobiol. Dis. 148, 105201 (2021).
Niño, D. F. et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci. Transl. Med. 10, eaan0237 (2018).
Mishra, M. K. et al. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS Nano 8, 2134–2147 (2014).
Carlson, M. L. et al. Development and initial assessment of [18F]OP-801: a novel hydroxyl dendrimer PET tracer for imaging maladaptive inflammation in the whole body and brain. Mol. Imaging Biol. 25, 1063–1072 (2023).
Voskuhl, R. R. & MacKenzie-Graham, A. Chronic experimental autoimmune encephalomyelitis is an excellent model to study neuroaxonal degeneration in multiple sclerosis. Front. Mol. Neurosci. 15, 1024058 (2022).
Hamilton, A. M. et al. Central nervous system targeted autoimmunity causes regional atrophy: a 9.4T MRI study of the EAE mouse model of Multiple Sclerosis. Sci. Rep. 9, 8488 (2019).
McCarthy, D. P., Richards, M. H. & Miller, S. D. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler’s virus-induced demyelinating disease. Methods Mol. Biol. Clifton NJ 900, 381–401 (2012).
Barthelmes, J. et al. Induction of experimental autoimmune encephalomyelitis in mice and evaluation of the disease-dependent distribution of immune cells in various tissues. J. Vis. Exp. 53933. https://doi.org/10.3791/53933 (2016).
Dagkonaki, A. et al. Mannan-MOG35-55 reverses experimental autoimmune encephalomyelitis, inducing a peripheral type 2 myeloid response, reducing CNS inflammation, and preserving axons in spinal cord lesions. Front. Immunol. 11, 575451 (2020).
Pyka-Fosciak, G., Stasiolek, M. & Litwin, J. A. Immunohistochemical analysis of spinal cord components in mouse model of experimental autoimmune encephalomyelitis. Folia Histochem. Cytobiol. 56, 151–158 (2018).
Nissen, J. C., Thompson, K. K., West, B. L. & Tsirka, S. E. Csf1R inhibition attenuates experimental autoimmune encephalomyelitis and promotes recovery. Exp. Neurol. 307, 24–36 (2018).
Hagan, N. et al. CSF1R signaling is a regulator of pathogenesis in progressive MS. Cell Death Dis. 11, 904 (2020).
Liaw, K. et al. Dendrimer size effects on the selective brain tumor targeting in orthotopic tumor models upon systemic administration. Bioeng. Transl. Med. 5, e10160 (2020).
Zhang, F. et al. Uniform brain tumor distribution and tumor associated macrophage targeting of systemically administered dendrimers. Biomaterials 52, 507–516 (2015).
Lesniak, W. G. et al. Biodistribution of fluorescently labeled PAMAM dendrimers in neonatal rabbits: effect of neuroinflammation. Mol. Pharm. 10, 4560–4571 (2013).
Wang, X.-S. et al. Idazoxan reduces blood–brain barrier damage during experimental autoimmune encephalomyelitis in mouse. Eur. J. Pharmacol. 736, 70–76 (2014).
Wolburg, H., Wolburg-Buchholz, K. & Engelhardt, B. Involvement of Tight junctions during transendothelial migration of mononuclear cells in experimental autoimmune encephalomyelitis. in Neuroinflammation in Stroke (eds Dirnagl, U. & Elger, B.) 17–38. https://doi.org/10.1007/978-3-662-05426-0_2 (Springer Berlin Heidelberg, 2004).
Gandhi, R., Laroni, A. & Weiner, H. L. Role of the innate immune system in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 221, 7–14 (2010).
Bittner, S., Afzali, A. M., Wiendl, H. & Meuth, S. G. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J. Vis. Exp. 51275. https://doi.org/10.3791/51275 (2014).
Thomas, T. P. et al. Cationic poly(amidoamine) dendrimer induces lysosomal apoptotic pathway at therapeutically relevant concentrations. Biomacromolecules 10, 3207–3214 (2009).
Alnasser, Y. et al. Preferential and increased uptake of hydroxyl-terminated PAMAM dendrimers by activated microglia in rabbit brain mixed glial culture. Mol. J. Synth. Chem. Nat. Prod. Chem. 23, 1025 (2018).
Albertazzi, L., Serresi, M., Albanese, A. & Beltram, F. Dendrimer internalization and intracellular trafficking in living cells. Mol. Pharm. 7, 680–688 (2010).
Sharma, A. et al. Targeting mitochondrial dysfunction and oxidative stress in activated microglia using dendrimer-based therapeutics. Theranostics 8, 5529–5547 (2018).
Zhang, F., Nance, E., Alnasser, Y., Kannan, R. & Kannan, S. Microglial migration and interactions with dendrimer nanoparticles are altered in the presence of neuroinflammation. J. Neuroinflammation 13, 65 (2016).
Goossens, J. Revised McDonald criteria allow earlier and more precise MS diagnosis. Medical Conferences https://conferences.medicom-publishers.com/specialisation/neurology/ectrims-2024/revised-mcdonald-criteria-allow-earlier-and-more-precise-ms-diagnosis-2/ (2024).
Kitagawa, D. et al. Characterization of kinase inhibitors using different phosphorylation states of colony stimulating factor-1 receptor tyrosine kinase. J. Biochem. 151, 47–55 (2012).
Vermersch, P. et al. Masitinib treatment in patients with progressive multiple sclerosis: a randomized pilot study. BMC Neurol. 12, 36 (2012).
Ettcheto, M. et al. Masitinib for the treatment of Alzheimer’s disease. Neurodegener. Dis. Manag. 11, 263–276 (2021).
Webb, M. et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing–remitting experimental autoimmune encephalitis in SJL mice. J. Neuroimmunol. 153, 108–121 (2004).
Di Dario, M. et al. Myeloid cells as target of fingolimod action in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflammation 2, e157 (2015).
Chun, J. & Hartung, H.-P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 33, 91–101 (2010).
Vogel, D. Y. et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflammation 10, 809 (2013).
Leuti, A. et al. Macrophage plasticity and polarization are altered in the experimental model of multiple sclerosis. Biomolecules 11, 837 (2021).
Radandish, M., Khalilian, P. & Esmaeil, N. The role of distinct subsets of macrophages in the pathogenesis of MS and the impact of different therapeutic agents on these populations. Front. Immunol. 12, 667705 (2021).
Boven, L. A. et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129, 517–526 (2006).
Vankriekelsvenne, E. et al. Transmembrane protein 119 is neither a specific nor a reliable marker for microglia. Glia 70, 1170–1190 (2022).
Zrzavy, T. et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017).
van Wageningen, T. A. et al. Regulation of microglial TMEM119 and P2RY12 immunoreactivity in multiple sclerosis white and grey matter lesions is dependent on their inflammatory environment. Acta Neuropathol. Commun. 7, 206 (2019).
Fox, R. J. et al. Tolebrutinib in nonrelapsing secondary progressive multiple sclerosis. N. Engl. J. Med. 392, 1883–1892 (2025).
Rousset, O., Rahmim, A., Alavi, A. & Zaidi, H. Partial volume correction strategies in PET. PET Clin. 2, 235–249 (2007).
Van Der Weijden, C. W. J. et al. Myelin imaging of the spinal cord in animal models and patients with multiple sclerosis using [11C]MeDAS PET: a translational study. J. Nucl. Med. 66, 136–141 (2025).
Kuntner, C. & Stout, D. B. Quantitative preclinical PET imaging: opportunities and challenges. Front. Phys. 2, 12 (2014).
Krishnamoorthy, G. & Wekerle, H. EAE: an immunologist’s magic eye. Eur. J. Immunol. 39, 2031–2035 (2009).
Moradi, F. et al. [18F]Flurimedrimer, a novel nanomedicine radiotracer, selectively targeting activated microglia in human brain. J. Nucl. Med. 66, 251092 (2025).
Ashvattha Therapeutics, Inc. A Phase 1/2 Study to Evaluate Safety, PK and Biodistribution of an Imaging Agent, 18F-OP-801, After Intravenous Administration to Patients With ALS, Alzheimer’s Disease, Multiple Sclerosis, Parkinson’s Disease and Healthy Volunteers. https://clinicaltrials.gov/study/NCT05395624 (2024).
James, M. L. et al. Imaging B cells in a mouse model of multiple sclerosis using 64 Cu-rituximab PET. J. Nucl. Med. 58, 1845–1851 (2017).
Cropper, H. C. et al. Longitudinal translocator protein-18 kDa–positron emission tomography imaging of peripheral and central myeloid cells in a mouse model of complex regional pain syndrome. Pain 160, 2136–2148 (2019).
Acknowledgements
We would like to thank Dr. Emily Becker for her assistance in preparing the manuscript. This work was supported by Ashvattha Therapeutics (to M.L.J.), NIH/NINDS (grant number: 1R21NS135513-01A1, to M.L.J.) and NSF GRFP (grant number: DGE-2146755, to R.C.K.).
Author information
Authors and Affiliations
Contributions
R.C.K. and M.L.C. designed and implemented the study, led data acquisition and analysis, and wrote the manuscript and prepared all figures and tables. S.T.R., S.C.N., M.K., I.S.A., C.J.A., I.N.F., E.C.A., C.B., and M.C. helped with data acquisition. N.M. and I.M.J. synthesized the radiotracer. Y.Z. and L.N. at the Stanford Shared FACS Facility designed the flow cytometry panel and provided guidance on data analysis. N.G.A., S.S.M., and J.L.C. helped conceptualize and design the study and analysis. M.L.J. helped design the study and assisted with developing figures and interpreting data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
N.G.A., M.C., S.S.M., and J.L.C. are employed by Ashvattha Therapeutics during the completion of this work. All other authors have declared no conflicts of interest.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kuo, R.C., Carlson, M.L., Reyes, S.T. et al. A radiolabeled dendrimer non-invasively identifies and tracks innate immune cell activation in a mouse model of experimental autoimmune encephalomyelitis. Nat Commun 17, 1220 (2026). https://doi.org/10.1038/s41467-025-67907-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-67907-x