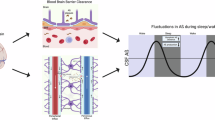
Abstract
Dysfunction of the glymphatic system has been proposed as a mechanistic link between sleep disruption and Alzheimer’s disease (AD). In animal models, glymphatic impairment alone can drive AD pathology. Whether this system clears amyloid beta (Aβ) and tau in humans remains unknown. In a randomized crossover trial with 39 participants, we found that glymphatic clearance during normal sleep increased morning plasma levels of AD biomarkers compared to sleep deprivation. Participants underwent overnight monitoring using an investigational device that measured dynamic changes in synaptic-metabolic release and glymphatic activity. The observed changes mirrored predictions from a multicompartment model based on published data on Aβ and tau release and clearance. Our findings indicate that sleep-active physiological processes, particularly reduced brain parenchymal resistance, enhance overnight glymphatic clearance of AD biomarkers to plasma. These results support a key role for glymphatic function in AD pathophysiology and suggest its enhancement as a potential therapeutic strategy to slow disease progression.
Similar content being viewed by others
Introduction
Sleep disruption is associated with increased risk of Alzheimer’s disease (AD) in epidemiological studies1,2,3, while biomarker studies in cognitively-intact participants suggest that short sleep duration and poor sleep quality are associated with greater AD-related amyloid β (Aβ) and tau pathology4,5,6,7,8,9, even before the onset of clinical symptoms of cognitive and functional decline. Yet the mechanistic link between sleep disruption and AD risk remains undefined. The glymphatic system is a brain-wide network of perivascular pathways along which cerebrospinal fluid (CSF) and interstitial fluid (ISF) exchange, supporting the clearance of interstitial solutes from the brain into the CSF, and thence into the blood. Studies in mice demonstrated that glymphatic function contributes to the clearance of Aβ and tau, is more rapid during sleep, and is impaired by acute sleep deprivation10,11,12,13,14,15. While more rapid glymphatic clearance of solutes during sleep has now been validated in the human brain16,17, whether sleep-active glymphatic activity contributes to the clearance of Aβ and tau in a healthy human population is unknown.
Plasma biomarkers have emerged as a potentially critical tool enabling the non-invasive diagnosis of AD at a large scale within the wider population. Immuno-assay and mass spectrometry-based measures of different Aβ and tau species permit the assessment of AD-related Aβ plaque burden and tau pathology, even during preclinical stages of disease in the years prior to patients’ clinical progression to cognitive impairment and dementia18,19. Although generally regarded as steady-state indicators of brain AD pathological burden, plasma AD biomarker levels are impacted acutely by sleep disruption4,20. While it is possible that these effects reflect reduced rates of cellular Aβ and tau release during sleep, they may also reflect the role of sleep-active glymphatic clearance in the transport of Aβ and tau from the brain to the blood. In a crossover study including 5 healthy participants, plasma Aβ40, Aβ42, non-phosphorylated tau181 (np-tau181), non-phosphorylated tau217 (np-tau217) and phosphorylated tau181 (p-tau181) were each reduced during the overnight period by acute sleep deprivation4. Noting an increased ratio of CSF:plasma Aβ and tau levels, the authors inferred that the processes governing the clearance of Aβ and tau from the brain to the CSF, and thence from the CSF to the plasma must be impaired by acute sleep deprivation. These findings are consistent with the sleep-active glymphatic clearance of Aβ and tau from the brain, yet they do not provide causal support. A second study showed that plasma Aβ and tau levels were reduced in participants that exhibited MRI evidence of impaired glymphatic and CSF-to-plasma clearance21. These findings established a correlation between steady-state AD biomarker plasma levels and impaired CSF circulation, but did not test whether sleep-active glymphatic function directly contributes to the clearance of these peptides during sleep in otherwise healthy individuals.
Here, we designed a study to directly test if sleep-active glymphatic function contributes to the overnight clearance of Aβ and tau from the human brain. We first developed a compartmental pharmacokinetic model to predict the effect that sleep-associated changes in Aβ and tau release and sleep-active glymphatic clearance, reflected by transport between the brain ISF and CSF, would have on morning plasma levels of Aβ and tau species. We then carried out a clinical randomized crossover study where participants underwent overnight normal sleep and sleep deprivation. Prior studies in rodents and humans demonstrate that glymphatic function increases with increasing sleep electroencephalography (EEG) delta and theta power, reduced EEG beta power, reduced heart rate (HR), reduced central noradrenergic tone, and reduced brain parenchymal resistance to fluid flow (RP), and vasomotor oscillations13,17,22,23,24,25. Measuring these key determinants of glymphatic exchange with an investigational device17, we defined the relationship between sleep features measured by EEG, RP measured by dynamic impedance spectroscopy, and HRV and cerebrovascular compliance measured by impedance plethysmography (IPG) and overnight changes in plasma AD biomarker levels. Using this approach, we directly tested the hypothesis that sleep-active glymphatic clearance elevates morning plasma AD biomarker levels, despite a sleep-associated reduction in their production.
Method
All studies were performed between October 2022—June 2023 and were reviewed and approved by University of Florida Institutional Review Board (IRB No. 202201364, Villages study) and Western Institutional Review Board (IRB No. 20225818, UW study). The studies have been registered at ClinicalTrials.gov (https://clinicaltrials.gov/study/NCT06060054 and https://clinicaltrials.gov/study/NCT06222385). Written informed consent was obtained from all study participants during a screening visit, prior to any study activities. Studies were carried out in accordance with the principles of the Belmont Report. The Villages Study enrolled 34 healthy participants 56–66 years of age. The UW Study enrolled 14 healthy participants 49–63 years of age. Participants were excluded if they had cognitive impairment or clinical depression. Cognitive impairment was assessed using the Montreal Cognitive Assessment (MoCA <26) and depression was evaluated using the 15-item Geriatric Depression Scale (GDS > 4). Participants with a self-reported history of diabetes, hypertension, coronary artery disease, pulmonary disease, neurological disease, depression, or anxiety were excluded from the study. Exclusion also applied to participants planning travel to alternate time zones within two weeks of study participation, as well as those with a formal diagnosis of any sleep disorder (e.g., sleep apnea requiring positive airway pressure [PAP] therapy, insomnia, restless leg syndrome, circadian rhythm sleep disorder, or parasomnia). Participants were excluded if they had taken any prescribed or over-the-counter stimulants, sleep aids, or psychiatric medications, including antidepressants, within the past 30 days. During intake, participants were further instructed to abstain from these sleep-altering substances throughout the study period and to avoid caffeine consumption after 12:00 p.m. on the day of the study visit. Compliance with both instructions was confirmed upon arrival at the study visit. Exclusion also applied to individuals who consumed more than 400 mg of caffeine per day; female participants who consumed more than 3 alcoholic drinks on any day or more than 7 drinks per week; and male participants who consumed more than 4 drinks on any day or more than 14 drinks per week. To minimize preparation bias (i.e., altering sleep schedules or taking naps in anticipation of sleep deprivation visits), participants were not informed of their initial visit assignment until 4:00 PM on the day of arrival. Sleep quality prior to and between study visits was subjectively evaluated based on investigator assessment.
Compartmental pharmacokinetic model relating glymphatic exchange to plasma AD biomarker levels
We first developed a compartmental pharmacokinetic model to define the effects that sleep-related changes in interstitial solute release and clearance would have on overnight changes in plasma levels of Aβ and tau. The model (shown in Fig. 1A) is a simplification of prior models26 and includes the minimal number of compartments, transport and elimination pathways needed to define the effect that a change in overnight interstitial solute production/release and glymphatic clearance between the ISF and CSF would have on plasma Aβ and tau levels. The model included a 16-hour period of wake, and either an 8-hour period of sleep or an 8-hour period of sleep deprivation. It includes 6 compartments: Cellular Site of Production/Release (1), Free ISF (2), CSF (3), Plasma22(4), Cellular Site of Uptake/Degradation (5), and Non-Monomeric Pools (6). Solute transport processes and their rate constants for Aβ and tau species based on literature values26,27 are provided in Table 1.
A Amyloid β (Aβ) and tau are produced in neurons (1) and released into the ISF compartment (2) where they are cleared from the ISF via local cellular uptake (2 → 5) and degradation, blood-brain barrier efflux (2 → 4), or glymphatic efflux to the CSF (2 → 3). Aβ42 and phosphorylated tau species are prone to non-monomeric aggregation, unlike Aβ40 and non-phosphorylated tau. Their monomeric forms can also be cleared from the ISF through further aggregation into non-monomeric structures (2 → 6). CSF solutes may recirculate back into the brain interstitium (3 → 2) or be cleared by CSF efflux pathways to the plasma (3 → 4) from whence peripheral degradation occurs. B–C Compartment concentrations of monomeric amyloid β and tau at steady state in the null model and the neuro-glymphatic model following changes in glymphatic efflux/influx or synaptic and metabolic release. Release of cellular Aβ and tau species from neurons (1) into the ISF (2) in the model is controlled by the cellular solute release rate constant \({k}_{{cell\_rel}}\) and was kept constant during the 16 hours of wake and was reduced by 30% during the 8 h of sleep occurring in the shaded window between 16 and 24 h. Solid lines show compartment concentrations under the null model, with time-invariant release rate constant \({k}_{{cell\_rel}}\) and time-invariant exchange between the CSF (3) and ISF (2) compartments. Long dashed lines show change in compartment concentrations when glymphatic efflux/influx rate constants \({k}_{{glymph\_out}}\) and \({k}_{{glymph\_in}}\), were increased by a factor of 1.5 (B) or decreased by a factor 0.5 (C) during the 8-hour sleep window. Dotted lines show change in compartment concentrations when synaptic and metabolic release rate constant \({k}_{{cell\_rel}}\) was increased by a factor of 1.25 (B) or decreased by a factor of 0.5 (C) during the 8-h sleep window. Note that increasing (decreasing) either glymphatic efflux/influx or synaptic and metabolic release during sleep increases (decreases) morning plasma level of Aβ and tau species relative to the null model.
The 24-h cellular synthesis of amyloid precursor protein (APP) and tau is invariant to sleep-wake state and was modeled at a constant rate of 10 arbitrary units (a.u.) per hour28,29. Release of cellular Aβ and tau species into the ISF in the model is controlled by the cellular solute release rate constant \({k}_{{cell\_rel}}\) (Table 1) and was kept constant during the 16 h of wake. During the 8 h of sleep, \({k}_{{cell\_rel}}\) was reduced by 30% consistent with reported 30% sleep reduction in ISF Aβ and tau species28,29, and during the 8 h of sleep deprivation it was reduced by 10% reflecting a circadian time-of-day effect.
This model was evaluated under two conditions. In the null model, sleep-active glymphatic exchange was posited to not occur; therefore solute transport between the Free ISF compartment (2) and the CSF compartment (3), reflected by the rate constants \({k}_{{glymph\_out}}\) and \({k}_{{glymph\_in}}\), were held constant across both sleep and waking (or sleep deprivation) states. In this model, sleep-related decreases in synaptic and metabolic activity, which impact cellular solute release, were not permitted to vary beyond the 30% reduction in the cellular solute release rate constant \({k}_{{cell\_rel}}\) during the overnight 8-hour sleep period and the 10% circadian-related reduction during the overnight 8-hour sleep-deprivation period. In the neuro-glymphatic model, \({k}_{{glymph\_out}}\) and \({k}_{{glymph\_in}}\) increased during sleep, reflecting the sleep-active CSF solute influx and interstitial solute efflux observed in both rodents and humans13,16,17. Additionally, \({k}_{{cell\_rel}}\) was allowed to vary with sleep from its 30% reduced value relative to wake, representing changes in synaptic and metabolic activity. These changes model variations in solute release into the brain’s interstitial space, which are associated with differences in sleep quality—such as the proportion of non-REM sleep relative to wake-after-sleep onset29,30.
The full derivation of the compartmental pharmacokinetic model is provided in Supplementary Methods and Results.
Clinical study design and participant demographic data
As described previously17, we conducted two cross-over clinical studies in which participants underwent one night of normal sleep and one night of sleep deprivation, in randomized order and separated by two or more weeks (Fig. 2A). One study was conducted in The Villages® community in Central Florida where the University of Florida maintains a satellite academic research center, The UF Health Precision Health Research Center (UF Health PHRC). The second study was carried out at the University of Washington (UW) in Seattle. Study participants underwent peripheral blood draws at 1900 hrs and 0700 hrs, prior to and following the overnight sleep and sleep deprivation periods. Plasma AD biomarkers, including Aβ40, Aβ42, np-tau181, np-tau217, p-tau181, and phosphorylated tau217 (p-tau217) were quantified using C2N Diagnostics’ immunoprecipitation liquid chromatography-tandem mass spectrometry platforms31,32,33. Details of specimen collection and processing are provided in Supplementary Methods and Results. During the overnight period, participants were instrumented with an investigational in-ear wearable device from Applied Cognition17 that measured key determinants of glymphatic function, including sleep features (hypnogram and spectral band power) by electroencephalography (EEG), heart rate variability (HRV) by photoplethysmography (PPG), cerebrovascular pulse transit time (PTT) by impedance plethysmography (IPG), and brain parenchymal resistance (RP) by dynamic impedance spectroscopy (Fig. 2B). A detailed description of this device, the validation of its sleep EEG measures against gold-standard overnight polysomnography, and of the measures of RP against contrast-enhanced magnetic resonance imaging (CE-MRI)-based measures of glymphatic function has recently been reported17. The details of EEG processing, as well as hypnogram and spectral band power computations, are provided for completeness in the Supplemental Methods and Results (Signal Processing – Electroencephalography). Cerebrovascular PTT measurements obtained using impedance plethysmography were validated against contrast-enhanced MRI-based measures of cerebrovascular function, as detailed in the Supplemental Methods and Results (Supplementary Tables 2–6).
The Benchmarking Study conducted at The Villages® and the Replication study conducted at the University of Washington were (A) randomized cross-over assignment of overnight sleep opportunity and overnight sleep deprivation designed to define the relationship between parenchymal resistance (RP) and glymphatic function. B Reported here are the overnight investigational device recordings of RP, EEG and HR, and blood analysis of amyloid β and tau levels (Aβ40, Aβ42, np-tau181, np-tau217 and p-tau181). C The Benchmarking Study enrolled 34 participants of which 30 completed both visits. Three were censored due to changes in device data collection and sensor locations. One withdrew following the first MRI scan. Of the 30 that completed the study, 5 overnight sleep visits and 8 overnight wake visits failed the data quality control (QC) criteria to provide sufficient artifact free data to yield results. This resulted in 25 sleep and 22 wake complete data sets. The Replication Study enrolled 14 participants. All 14 completed the study, of which 3 wake visits failed the data QC criteria and one sleep visit failed specific to the analysis of these data because of missing EEG powerband data during REM sleep caused by artifacts. The remaining 13 participants had complete data for analysis.
Testing for Sequence-Related Effects We investigated potential sequence-related effects in the randomized cross-over study by comparing evening-minus-morning plasma biomarker levels following sleep and/or sleep deprivation between the first and second visits. This analysis was conducted separately for amyloid-positive, amyloid-negative, and combined cohorts, resulting in 15 comparisons per group and a total of 45 distinct comparisons.
Development of a multivariate model to relate release and glymphatic clearance features to plasma Aβ and tau concentrations
We developed a series of multivariate mixed models to define the effects that continuous features of glymphatic physiology and synaptic-metabolic activity have on overnight release and clearance of brain interstitial Aβ and tau to the plasma. Within these models, the multiple dependent variable measures for each participant were the morning plasma Aβ and tau (Aβ40, Aβ42, np-tau181, np-tau217, p-tau181) biomarker levels. Plasma p-tau217 levels were measured but excluded from analysis because a large proportion (9 out of 49) of these cognitively-intact individuals exhibited plasma p-tau217 levels below the limit of detection for the assay, consistent with prior study findings34,35. Data from amyloid-negative and amyloid-positive individuals were analyzed separately.
Because of the large number of measured outcomes, we used dimensionality reducing single-index regression36 to combine sleep-related factors into single ‘predictors’. A detailed explanation of this single predictor development is provided in Supplementary Methods and Results. Within these models, two distinct groups of predictors were analyzed: neurophysiological variables (Physio) and hypnographic sleep stages (Hypno). Separating predictors into these groups enabled comparison of both neurophysiological measures and sleep stage durations in explaining observed differences in morning plasma Aβ and tau levels, while mitigating multicollinearity among EEG power bands and sleep stages in the regression models, as previously documented17. The first group of predictors (Physio) included EEG non-rapid eye movement (NREM) delta (0.5–4 Hz) and theta power bands, REM sleep theta and beta power bands, heart rate variability (HRV), and pulse transit time (PTT) during NREM, and parenchymal resistance RP. HRV and PTT values during REM sleep were highly correlated with those during NREM and were excluded. The NREM and REM power bands selected represent the majority of the spectral power during these sleep stages29,30, thus contributing to synaptic–metabolic release, and because EEG delta, beta and theta power bands have established associations with glymphatic clearance13,17,22,23. The second group (Hypno) comprised hypnographic sleep stages, including the durations of REM, N1, NREM (N2 + N3), and wake after sleep onset (WASO). Each group of individual predictors was combined using linear combinations into three single-index predictors for amyloid-negative and amyloid-positive participants separately: the neurophysiological predictor under the sleeping condition (PhysioS) and under the sleep deprivation/wake condition (PhysioW), and the sleep hypnogram predictor under the sleeping condition (HypnoS).
We developed parallel multivariate linear mixed models representing the null model and the neuro-glymphatic model, as described in detail in Supplemental Methods and Results. Under the null model, morning plasma levels of Aβ and tau species were not dependent upon sleep-active glymphatic exchange and synaptic-metabolic release, and thus were not assumed to be influenced by sleep neurophysiological (Physio) and sleep stage (Hypno) predictors. Rather the morning plasma levels of AD biomarkers (ConcAM) were regressed on the evening plasma levels (ConcPM) for Aβ and tau biomarkers, separately for amyloid positive and amyloid negative participants. Potentially confounding variables age, sex, APOE-ε4 status, and study site were included in the model. A circadian confounder was also included in the model, reflecting the interval between the evening AD biomarker sample time and sleep-onset measured by EEG. Multivariate mixed models used participant ID as a random intercept and the categorical biomarker variable as a vector of random slopes. The neuro-glymphatic model shares the features of the null model but included the effects of the single index predictors (PhysioS, PhysioW or HypnoS), and their respective interaction terms with evening levels of plasma AD biomarkers (PhysioS * ConcPM, HypnoS * ConcPM, PhysioW * ConcPM). The likelihood ratio test (LRT) of the neuro-glymphatic model versus the null model was used to determine which model performed better at predicting the morning plasma Aβ and tau biomarker levels. The conditional variances of each model, i.e., the residual variation conditional on a participant’s random effects, were used to define the amount of variance in morning plasma Aβ and tau levels explained by the neuro-glymphatic model over that explained by the null model. This is described in detail in Supplementary Methods and Results.
Results
Statistical significance tests presented in the results have not been adjusted for multiple comparisons.
A Consolidated Standards of Reporting Trials (CONSORT) diagram for the Villages Study and UW Study is provided in Fig. 2C. Within the Villages Study, the first three participants were removed from analysis because of a sensor position change in the investigational device. One participant was unable to complete the first MRI session and withdrew from the study. Of the remaining 30 participants (61.8 ± 2.7 years of age; 14 female, 16 male) that completed the Villages Study, five overnight sleep studies and eight overnight wake studies failed data quality control due to excessive artifacts in the recordings, leaving 25 sleep studies and 22 wake studies with analyzable device and biomarker data in the Villages Study. Of the participants enrolled in the UW Study, one was not compliant with the enforced wake protocol and was removed from analysis. A second participant was also excluded because of missing EEG powerband data during REM sleep caused by artifacts, which was required for the current analysis. The remaining 13 participants (55.9 ± 4.6 years of age; 6 female, 7 male) all completed the protocol. All overnight sleep data were usable, but two overnight wake studies were removed because of excessive artifact in the UW Study. Participant demographics, MoCA and GDS scores are listed for each study site and for the combined dataset in Table 2.
We measured plasma Aβ40, Aβ42, np-tau181, np-tau217, p-tau181, and p-tau217 at evening and morning timepoints in each participant prior to and following overnight sleep or sleep deprivation. Summary plasma AD biomarker levels are provided in Table 3. Of 38 participants, a total of 11 were assessed as ‘amyloid-positive’ by the C2N mass spectrometry with a Aβ42/ Aβ40 cutoff value of 0.08931,33,37 (Table 2). Plasma AD biomarker levels shown in Table 3 are stratified by participant amyloid status. Overall, the measured plasma levels and overnight changes with sleep agree with prior reported values for Aβ40, Aβ42 and p-tau18138. Furthermore, the overnight changes in measured plasma levels following sleep and sleep-deprivation did not differ significantly, consistent with the possibility of competing clearance or production effects during sleep and sleep-deprivation.
Of the 45 comparisons conducted to assess sequence effects on overnight changes in plasma biomarkers, only two showed statistically significant differences between the first and second visits: (i) In the amyloid-negative group under sleep deprivation, np-tau217 differed significantly (P = 0.031); (ii) In the combined group under sleep deprivation, np-tau181 showed a significant difference (P = 0.043).
Predictions of the compartmental pharmacokinetic model relating glymphatic exchange to plasma AD biomarker levels
Figure 1B presents the steady-state compartmental concentrations of Aβ and tau over a 48-hour period under the null model (solid lines), as well as under neuro-glymphatic model scenarios during sleep. These include increased glymphatic clearance relative to its sleep baseline, representing improved sleep-related drivers of clearance (dashed lines); and elevated synaptic-metabolic release relative to its sleep baseline, reflecting poor quality, fragmented sleep (dotted lines). We observe that elevations in Aβ and tau concentrations in plasma or CSF above baseline do not distinguish between an increase in sleep-related clearance (desirable) and an increase in production (undesirable).
Figure 1C depicts alternative neuro-glymphatic model scenarios during sleep involving reduced glymphatic clearance relative to baseline, as would occur with a deterioration in sleep-related clearance drivers (dashed lines) and decreased synaptic-metabolic release relative to baseline, as might result from improved, less fragmentated sleep (dotted lines). Both are compared against the null model (solid lines). Across all scenarios, both Aβ and tau concentrations in the plasma increase or decrease relative to the null model, depending on whether glymphatic clearance or synaptic-metabolic release is enhanced or diminished, respectively. These plots demonstrate that changes in Aβ and tau concentrations within the plasma and CSF compartments cannot independently distinguish between decreased glymphatic clearance and decreased solute release relative to baseline, nor between increased glymphatic clearance and increased solute release. An additional derivation of the model, described in detail in the Supplementary Methods and Results, demonstrates that in the null model, morning plasma levels will be linearly dependent on evening plasma levels only, whereas the neuro-glymphatic model has a contribution from the interaction term between the evening plasma levels and the variable rate constants \({k}_{{glymph\_out}}\), \({k}_{{glymph\_in}}\) and \({k}_{{cell\_rel}}\).
We next compared changes in plasma levels of species that remained primarily monomeric in form (Aβ40, np-tau), and species that are prone to form non-monomeric aggregates (Aβ42, p-tau) following increases and decreases in glymphatic clearance and synaptic-metabolic release. If this tendency to form aggregates is ignored, and rate constants for monomer-to-aggregate conversion is set to zero (\({k}_{{on}-{rate}}=0\)), changes in cellular release \({k}_{{cell\_rel}}\) or clearance \({k}_{{glymph\_out}}\) have no effect on the Aβ42/Aβ40 or p-tau/np-tau ratios (Supplementary Table 1, top row). In contrast, when the tendency for Aβ42 and p-tau to form non-monomeric aggregates is included in the model (\({k}_{{on}-{rate}} > 0\)), increased and decreased solute release and glymphatic clearance shift plasma Aβ42/Aβ40 or p-tau/np-tau ratios (Supplementary Table 1). Both the Aβ42/Aβ40 or p-tau/np-tau ratios increase in response to decreased production or increased glymphatic clearance, and decrease in response to increased production or reduced clearance. This suggests that non-monomeric aggregates act both as a secondary source of monomeric Aβ42 and p-tau species under conditions of reduced production and as an additional sink for these species under conditions of reduced clearance. Under conditions of increased glymphatic clearance, the enhanced removal of Aβ42 and p-tau from the ISF reduces their opportunity to aggregate, resulting in a higher proportion reaching the plasma relative to Aβ40 and np-tau—thus increasing their respective plasma ratios. Thus, unlike Aβ and tau concentrations in plasma and CSF - which cannot independently distinguish between decreased glymphatic clearance and decreased solute release relative to baseline, nor between increased clearance and increased release - changes in plasma Aβ42/Aβ40 or p-tau/np-tau ratios enable us to infer the primary mechanism driving changes in morning plasma AD biomarker levels under sleep or sleep-deprivation conditions.
These pharmacokinetic modeling results provide three concrete predictions that permit us to test the hypothesis that sleep-active glymphatic exchange contributes to overnight changes in plasma AD biomarker levels independent of sleep-dependent changes in solute release:
-
1)
Under the neuro-glymphatic model, compared to normal sleep, decreased clearance during overnight sleep deprivation would reduce morning plasma AD biomarker levels while increased production would increase levels.
-
2)
Under the neuro-glymphatic model, under conditions of sleep deprivation, morning plasma AD biomarker levels would increase by the interaction term between evening plasma levels and features of synaptic-metabolic activity contributing to greater production; under normal sleep conditions morning plasma AD biomarker levels will be increased by the interaction term between evening plasma levels and features of sleep-active glymphatic function contributing to greater clearance.
-
3)
Under the neuro-glymphatic model, under normal sleep conditions morning plasma Aβ42/Aβ40 and p-tau/np-tau ratios will increase with greater sleep-dependent clearance; overnight sleep deprivation will decrease these ratios from greater wake-dependent production.
Effects of sleep-related neurophysiological features on overnight Aβ and tau release and clearance
The predictor PhysioS that led to the best fit of the data based on maximum likelihood for the neuro-glymphatic model in sleep for both amyloid-negative and -positive individuals showed that RP was the overwhelming contributor to PhysioS (Table 4). The output of the null model and neuro-glymphatic model with the predictor PhysioS for both amyloid-positive and amyloid-negative individuals are shown in Table 5. Estimates for single predictor coefficients within the neuro-glymphatic model for each plasma AD biomarker, evaluated at the mean evening biomarker level, are provided in Supplementary Table 7.
Within the null model, evening plasma Aβ and tau levels (ConcPM) were the main predictor of morning plasma levels in both amyloid-positive and -negative participants, reflecting between-participant evening consistency in AD biomarker levels (Table 5). Within the neuro-glymphatic model, in addition to evening plasma biomarker levels, participant age, sex, and circadian alignment each significantly contributed to morning plasma Aβ and tau levels in both amyloid-positive and -negative participants. APOE-e4 status was significantly associated with lower morning AD biomarker levels in amyloid-positive, but not amyloid-negative individuals. Age was significantly associated with higher morning AD biomarker levels in both groups. Across both amyloid-positive and -negative participants, the PhysioS predictor significantly impacted plasma Aβ and tau levels, and the ConcPM*PhysioS interaction terms were consistently significant across Aβ and tau analytes (Table 5).
By including overnight RP, PTT, HRV, and EEG power bands in the single predictor PhysioS, the neuro-glymphatic model led to greater predictive performance over the null model (Table 6, LRT, p < 0.001). When comparing the percent variance of morning plasma AD biomarker levels explained by the neuro-glymphatic model compared to the null model, inclusion of overnight PhysioS in the neuro-glymphatic model explained between 49.1% (Aβ40) to 56.0% (Aβ42) additional variance in amyloid-positive individuals, and between 71.3% (p-tau181) to 97.8% (Aβ40) in amyloid-negative individuals (Table 7).
As detailed above, the PhysioS single-index predictor includes several sleep-related physiological features that could contribute variously to changes in glymphatic clearance to the plasma, or to synaptic-metabolic release of Aβ and tau. We carried out a sensitivity analysis to explore the relative contributions of these individual neurophysiological predictors, and of glymphatic clearance versus synaptic-metabolic release to changes in morning plasma Aβ and tau analyte levels. This sensitivity analysis is provided in detail in Supplementary Methods and Results. The analysis suggests that parenchymal resistance RP, cerebrovascular compliance (measured by PTT) and NREM EEG delta power during sleep, which are each associated with enhanced glymphatic exchange in rodents and humans12,13,17,22,23,24,39,40, facilitated the overnight clearance of brain interstitial Aβ and tau into the plasma made the largest contribution to changes in plasma Aβ and tau levels. Relative to the contributions of these individual predictors, the contribution from increased synaptic-metabolic activity reflected in NREM EEG theta power and REM EEG theta and beta power were comparatively minor (Supplementary Table 3). The combined effects of these predictors which impact both glymphatic clearance and synaptic-metabolic release of Aβ and tau accounted for over 50% of the variance in morning plasma Aβ and tau levels in amyloid-positive individuals, and over 90% of the variance in amyloid-negative individuals, compared to that explained by the null model alone (Table 7). These data suggest that both increased synaptic-metabolic release and glymphatic clearance of Aβ and tau during sleep contribute to increased morning plasma AD biomarker levels, but that increased clearance driven by reduced RP, increased cerebrovascular compliance, and increased EEG delta power are the greatest contributors to these effects.
NREM sleep duration enhanced overnight clearance of Aβ and tau to the plasma
We next tested whether differences in overnight sleep stages influenced the glymphatic clearance or synaptic-metabolic release of Aβ and tau to the plasma. The predictor HypnoS was constructed from regressors chosen from EEG hypnogram stages REM, N1, NREM (N2, N3) and WASO that led to the best fit of the data based on the maximum likelihood optimization for the neuro-glymphatic model for amyloid-positive and amyloid-negative individuals (Table 4). The null model and the neuro-glymphatic model fitted to the morning plasma AD biomarker level data following overnight sleep with the HypnoS predictor are shown in Supplementary Table 10. By including overnight REM, NREM, N1 and WASO in the single predictor HypnoS, the neuro-glymphatic model led to greater predictive performance over the null model (Table 6, LRT, p < 0.001). When comparing the percent variance of morning plasma AD biomarker levels that was explained by overnight sleep stage durations in the neuro-glymphatic model and that was not explained by the null model, the neuro-glymphatic model in amyloid-positive individuals explained between 38% (all tau species) to 62.5% (Aβ42) of the variance (Table 7). In amyloid-negative individuals, sleep stage duration did not perform better than the null model in explaining Aβ40 and Aβ42 levels, but explained between 64% (p-tau181) and 93.1% (np-tau217) of the tau variance (Table 7).
Using a similar approach to that above, we observed that at a mean value of each evening plasma AD biomarker level, a decrease in overnight Hypnos, which corresponds to an increase in NREM sleep duration and a decrease in REM sleep, N1 sleep, and WASO duration (Table 4), increases morning plasma Aβ and tau biomarker levels (Supplementary Table 7). Sensitivity analysis, as described in Supplementary Methods and Results (Supplementary Table 3-4), suggests that increased NREM sleep duration, which is associated with increased glymphatic function in rodents and humans13,16,17, increases the clearance of Aβ and tau to the plasma. A decrease REM sleep, N1 sleep and WASO duration were also associated with an increase in glymphatic clearance (Supplementary Table 3), primarily because of their negative correlation with NREM sleep duration. These effects explain approximately 50% of the unexplained variance in the morning plasma AD biomarker levels from the null model in amyloid-positive individuals but fail to explain additional Aβ40 and Aβ42 variance in amyloid-negative individuals (Table 7). These data demonstrate that increased NREM (N2 + N3) sleep duration contributes to the overnight clearance of Aβ and tau to the plasma.
Combined effects of release and clearance to plasma Aβ and tau levels during sleep deprivation
We next evaluated whether the neurophysiological features used to characterize sleep also influenced Aβ and tau release and clearance under conditions of overnight sleep deprivation (waking). To address this, we constructed the single-index predictor PhysioW from the same regressors, optimizing it to best fit the sleep deprivation data (Table 4). The null model and the neuro-glymphatic model fitted to morning plasma AD biomarker levels following sleep deprivation, using the single predictor PhysioW are shown Supplementary Table 11. By incorporating overnight neurophysiological features related to glymphatic exchange and synaptic-metabolic activity into PhysioW, the neuro-glymphatic model demonstrated superior predictive performance for morning plasma AD biomarker levels compared with the null model (Table 6, LRT, p < 0.001). When assessing the additional variance in morning plasma Aβ and tau levels explained by the neuro-glymphatic model beyond that explained by the null model, we found that in amyloid-positive individuals the model accounted for 72.8% of all tau variance. In amyloid-negative individuals, the model explained 92.5% of Aβ40, 93.3% of Aβ42, and 73.8% of p-tau181 variance (Table 7).
In individuals undergoing overnight sleep deprivation, HRV, PTT, and RP measures contributed to Aβ and tau release and clearance in a manner largely consistent with their effects during sleep, although the contribution of RP was substantially smaller (Supplementary Table 3). In this context, increases in EEG delta and theta power were associated with elevated Aβ and tau release, whereas a reduction in EEG beta power enhanced clearance, an association previously observed in both rodent and human studies17,22. Note that in amyloid-positive individuals, predictor assignment for a decrease in PhysioW was based on all tau effects (Supplementary Table 8), as Aβ40 and Aβ42 effects were not statistically significant (Supplementary Table 11). These data suggest that under conditions of sleep deprivation, both synaptic-metabolic release and glymphatic clearance of Aβ and tau contribute to morning plasma AD biomarker levels.
The predictors Physio S, Hypno S and Physio W in the neuro-glymphatic model replicate the rate constants in the compartmental pharmacokinetic model
The linear interaction terms between the predictors PhysioS, HypnoS and PhysioW and evening plasma AD biomarker levels in the three neuro-glymphatic models for amyloid-positive and amyloid-negative individuals were significant at both the group level (Supplementary Table 12) and individual levels (Table 5, Supplementary Tables 5–6). These interaction terms are the same as described by Equation 3 in the solution of the multi-compartment model (Supplementary Methods and Results). That is, the linear interaction terms between the predictors PhysioS, HypnoS and PhysioW and evening plasma AD biomarker levels from experimental observations are consistent with those derived from multicompartment first-order kinetic modeling under the neuro-glymphatic model assumption with ISF to CSF clearance rate constants and synaptic-metabolic release that are time-varying through PhysioS, HypnoS and PhysioW.
To further demonstrate this correspondence, we compared experimental predictions of morning plasma AD biomarker levels when PhysioS and HypnoS were varied by ±1 standard deviation with predictions generated by the compartmental model under proportional variation of the rate constants \({k}_{{glymph\_out}}\), \({k}_{{glymph\_in}}\) and \({k}_{{cell\_rel}}\). As shown in Fig. 3A–B, varying the magnitude of fitted predictors in the neuro-glymphatic models for sleep, while proportionally varying \({k}_{{glymph\_out}}\) and \({k}_{{glymph\_in}}\) in the compartment model, produced similar changes in predicted morning plasma AD biomarker levels across all conditions. Furthermore, the compartmental model reproduced the experimentally-observed pattern of greater clearance of aggregation-prone species (Aβ42, p-tau181) relative to non-aggregation-prone species (Aβ40, np-tau181), consistent with an increased Aβ42/Aβ40 and p-tau181/np-tau181 ratio over baseline clearance. By contrast, increasing \({k}_{{cell\_rel}}\) in the compartmental model generated similar directional effects on morning plasma biomarker levels but reduced clearance of aggregation-prone species relative to non-aggregation-prone species, a pattern inconsistent with the experimental predictions (Fig. 1, Supplementary Table 1). Taken together, these findings support glymphatic clearance as the predominant physiological mechanism underlying the observed variation in morning plasma AD biomarker levels following overnight sleep.
The percent change prediction in morning plasma level ConcAM of AD biomarkers Aβ40, Aβ42, np-tau181, np-tau217, p-tau181 resulting from a change in units of SD of the single predictors PhysioS (A), HypnoS(B), and PhysioW (C) of the neuro-glymphatic model in amyloid-positive and amyloid-negative participants are compared against the percent change in the morning amyloid β and tau multicompartment model prediction from a corresponding change glymphatic efflux/influx rate constants \({k}_{{glymph\_out}}\) and \({k}_{{glymph\_in}}\) (A) and (B), or synaptic and metabolic release rate constant \({k}_{{cell\_rel}}\) (C). Predicted mean and 95% confidence intervals for Aβ40, Aβ42, np-tau181, np-tau217, p-tau181 at each SD change in the single predictor are shown in order as the first five bars alongside the neuro-glymphatic model predictions for that SD change. Multicompartment model predictions for Aβ40, Aβ42, non-phosphorylated and phosphorylated tau at each corresponding change of the rate constants \({k}_{{glymph\_out}}\) and \({k}_{{glymph\_in}}\) (A) and (B), or \({k}_{{cell\_rel}}\) (C), are shown in order as the last four hatched bars. The change in the negative of the single predictors are shown for comparison with the change in the rate constants. For example, a 1 SD increase in the negative predictor corresponds to a 1 SD decrease in the actual predictor. The plot error bars represent confidence intervals calculated using 95% quantiles of 1000 nonparametric bootstrap replicates. Source data are provided as “Fig. 3 SOURCE DATA.xlsx”.
We next compared experimental predictions of morning plasma AD biomarker levels during sleep deprivation, obtained by varying PhysioW by ±1 standard deviation, with predictions from the compartment model under proportional variation of the rate constants \({k}_{{glymph\_out}}\), \({k}_{{glymph\_in}}\) and \({k}_{{cell\_rel}}\). As shown in Fig. 3C, reproducing the experimental results from the neuro-glymphatic models required varying \({k}_{{cell\_rel}}\) in the compartmental model. This adjustment produced similar changes in predicted morning plasma AD biomarker levels and replicated the experimentally-observed pattern of reduced clearance of aggregation-prone species (Aβ42, p-tau181) relative to non-aggregation-prone species (Aβ40, np-tau181). Thus, under sleep deprivation, increased synaptic-metabolic release emerges as the predominant physiological mechanism underlying the observed morning plasma AD biomarker levels.
It is noteworthy that while the results of the statistical and compartmental models converge, they were derived from independent sources. The glymphatic models were statistically estimated from the experimental data from the Villages and UW clinical studies whereas the compartmental model was based on the elimination kinetics of amyloid β and tau obtained from independent published research26,27.
Discussion
We developed a compartmental transport model based on published kinetic values to predict the effect that sleep-active glymphatic CSF-ISF exchange and synaptic-metabolic activity has on overnight changes in plasma Aβ and tau levels. To rigorously test the glymphatic model of interstitial Aβ and tau clearance from the human brain during sleep, and to define the relative contributions that changes in synaptic-metabolic release and clearance make to plasma Aβ and tau levels, we conducted a multi-site, randomized, crossover clinical study. This study utilized an investigational device to measure parenchymal resistance RP, sleep EEG features, cerebrovascular pulse transit time PTT, heart rate variability HRV, in addition to clinically validated blood-based biomarkers of Aβ and tau provided by C2N31,32,33. Healthy older participants underwent both overnight sleep and sleep deprivation visits. Our findings showed that reduced parenchymal resistance, increased cerebrovascular compliance, and elevated EEG delta power during NREM sleep – each of which are associated with increased glymphatic function in rodents and humans12,13,17,22,23,24,39 – predicted higher morning plasma levels of Aβ and tau. EEG theta power during NREM sleep, and both theta and beta power during REM sleep, also contributed to morning plasma Aβ and tau levels, though to a lesser extent, suggesting a role for synaptic–metabolic activity in Aβ and tau release to the plasma28,29,30. These predictors of sleep-active glymphatic clearance and synaptic–metabolic release explained over 50% of the residual variance in morning plasma AD biomarker levels among amyloid-positive individuals, and over 90% among amyloid-negative individuals, that was not explained by the model that assumed constant overnight CSF–ISF exchange and reduced but uniform synaptic–metabolic activity across individuals.
The burden of Aβ and phosphorylated tau in the brain is diagnostic for AD and prognostic of its progression41,42,43. Recently approved monoclonal antibody therapies targeting Aβ demonstrate marked reduction in brain Aβ burden, but at present provide only modest improvement in cognitive outcomes and quality of life44,45. This apparent shortcoming has led to an increasing interest in extending anti-Aβ immunotherapy into earlier, preclinical stages of AD and into at-risk populations such as APOE-ε4 carriers. It is also possible that if the mechanisms contributing to the development and progression of Aβ and tau pathology can be defined, then it may be possible both to better identify individuals at risk of the development of this pathology and to intervene in at-risk individuals to prevent its development and eventual progression.
In rodent models, sleep-active glymphatic function has been implicated in the clearance of both Aβ and tau. Interstitial Aβ and tau both move through brain tissue along perivascular pathways10,14, while inhibition of glymphatic function by either Aqp4 or Snta1 gene deletion slows the clearance of Aβ and tau and promotes the development of Aβ and tau pathology10,13,14,15,46,47,48,49,50. Glymphatic function is impaired in animal models of aging51, cerebrovascular dysfunction52,53, traumatic brain injury14,54,55,56, and sleep disruption13,57; each of which are non-genetic risk factors for Alzheimer’s disease. This body of results from rodent studies suggests that impairment of glymphatic clearance of Aβ and tau is a key factor in the development of AD, serving as a mechanistic linkage between a wide spectrum non-genetic AD risk factors and the development of AD-related Aβ and tau pathology58,59. If true, then the detection of glymphatic impairment would permit the identification of individuals at risk for the development of Aβ and tau pathology, while targeting glymphatic function would provide a potential approach to the prevention of the development and progression of AD pathology in at-risk individuals.
Despite promising results from rodent studies, key biological features of sleep-active glymphatic function have only recently been confirmed in the human brain. Intrathecal contrast-enhanced MRI studies have demonstrated extensive CSF-ISF exchange in the human brain60, that this exchange is organized along the axis of the cerebral arterial vasculature61, and that ISF solute clearance is more rapid in the sleeping compared to the waking brian16. In a recent study, we demonstrated that glymphatic function is sleep-active, and enhanced during sleep by increasing EEG delta power, reduced EEG beta power, reduced HR, and reduced parenchymal resistance RP17. Yet a role for sleep active glymphatic exchange in the clearance of Aβ and tau from the human brain remains undefined.
One recent study by Lucey and colleagues reported that the CSF-to-blood clearance of Aβ and tau are impaired by acute sleep deprivation4, although this study did not specifically implicate glymphatic clearance in this association. In another important study by Eide et al.21, the authors report that in participants undergoing assessment of glymphatic function and CSF-to-plasma clearance by intrathecal contrast-enhanced MRI, slowed glymphatic clearance was associated with lower plasma total-tau levels, while slowed CSF-to-plasma clearance was correlated with lower Aβ42 levels. While this study supports a role for glymphatic exchange in the clearance of AD-related biomarkers, the interpretation of the findings has key limitations. Because intrathecal administration is an off-label use of gadolinium-based contrast agents such as gadobutrol, the study was carried out in participants being evaluated for disordered CSF circulation and not otherwise ‘healthy’ participants. This introduced a several decade age range and multiple clinical indications in the study group that would be expected to confound the relationship between plasma levels of AD biomarkers and glymphatic function. While the study did evaluate the effect of time of day on plasma biomarker levels, the relationship between measures of glymphatic clearance and overnight dynamic changes in AD biomarker levels, rather than ‘steady state’ levels, was not evaluated. Lastly, while overall self-reported sleep quality was evaluated with the Pittsburgh Sleep Quality Index, objective measures of sleep parameters, sleep stages, or sleep EEG spectral band powers were not evaluated. In the present study, we extend these initial studies by evaluating the effect of overnight parenchymal resistance, sleep stages, sleep EEG powerbands during REM and NREM, cerebrovascular arterial compliance and heart-rate variability, each of which is a key determinate of glymphatic transport12,13,17,22,23,24,39,40 or a key contributor to synaptic-metabolic release28,29,30, on the dynamic clearance of Aβ and tau to the plasma in healthy older individuals.
To test whether sleep-active glymphatic function contributes to the clearance of Aβ and tau from the human brain independent of variations in synaptic-metabolic activity during sleep, we first developed a multicompartment model of Aβ and tau clearance from the brain interstitium. The model was designed to predict the effects of sleep-related changes in glymphatic CSF–ISF exchange and synaptic–metabolic release on evening-to-morning changes plasma Aβ and tau levels. Glymphatic clearance was modeled as changes in the rate constants between ISF and CSF compartments, demonstrating that an overnight increase in exchange would result in higher plasma AD biomarker levels the following morning. Similarly, synaptic–metabolic release was modeled as a change in the rate constant for Aβ and tau release from cells into the ISF, which also predicted elevated morning plasma biomarker levels when release increased.
Monomeric Aβ42 and p-tau181 in the ISF were modeled as being in equilibrium with their non-monomeric forms using a separate compartment and published rate constants between ISF and aggregate pools26,27. This revealed that an increase (or decrease) in the exchange rate between ISF and CSF (glymphatic exchange) preferentially increased (or decreased) the clearance of aggregation-prone species (Aβ42 and p-tau181) relative to non-aggregation-prone species (Aβ40 and np-tau181). In contrast, increasing the release rate from cells into the ISF led to the opposite effect, preferentially elevating non-aggregation-prone over aggregation-prone species. These key differences in model behavior allowed us to directly test the hypothesis that glymphatic clearance of Aβ and tau is the dominant contributor to sleep-related variation in plasma Aβ and tau levels, while synaptic–metabolic release dominates during sleep deprivation (and likely waking).
Analyzing data from two cross-over design clinical studies, we evaluated the validity of two parallel models to explain overnight changes in plasma Aβ and tau levels. Our null hypothesis was that neither glymphatic exchange nor synaptic-metabolic release contributed to the overnight changes in AD plasma biomarkers, and the null model used only the evening AD plasma biomarker levels adjusted by biological confounders to predict the morning AD biomarker levels. The neuro-glymphatic hypothesis was that increased CSF-ISF exchange during sleep increased the overnight clearance of Aβ and tau from brain to the plasma and that individual differences in overnight synaptic-metabolic release further altered morning plasma levels. Because glymphatic function and synaptic-metabolic activity during sleep are regulated by changes in EEG delta, theta and beta power during NREM and REM, and PTT, HRV, and RP12,13,17,22,23,24,28,29,30,39,40, we tested if the inclusion of these features would improve the prediction of plasma Aβ and tau levels consistent with our multicompartment model prediction.
Using a single-index regression that linearly combined these variables into single predictor PhysioS for sleep, the neuro-glymphatic model explained over 50% of the residual variance in morning plasma AD biomarker levels in amyloid-positive individuals and over 90% in amyloid-negative individuals during the sleep condition. The optimal sleep predictor PhysioS which maximized the log-likelihood during sleep, was primarily driven by parenchymal resistance RP, with smaller contributions from cerebrovascular compliance measure by PTT and EEG delta power during NREM sleep, features associated with increased glymphatic clearance. Minor contributions also came from EEG theta power during both NREM and REM sleep, and EEG beta power during REM, features suggestive of increased synaptic-metabolic release.
During the sleep deprivation (awake) condition, the neuro-glymphatic model with the optimal predictor PhysioW accounted for over 70% of np-tau and p-tau variance in amyloid-positive individuals, and between 70% and 90% of Aβ and p-tau variance in amyloid-negative individuals. HRV, PTT, and RP contributed to the predictor PhysioW in a manner largely consistent with their effects during sleep on glymphatic clearance, although RP showed a substantially smaller contribution. In contrast to the optimal sleep predictor, however, EEG delta, theta and beta power that are associated with increased synaptic-metabolic production during wake, contributed strongly to PhysioW.
When we tested the neuro-glymphatic model using the single-index predictor HypnoS for the sleep condition, constructed from sleep stage durations, it did not outperform the null model in explaining Aβ40 and Aβ42 levels in amyloid-negative individuals, although it did explain over 64% of the variance in tau level. In amyloid-positive individuals, HypnoS explained over 30% of the variance. Increased NREM (N2 + N3) sleep duration, associated with enhanced glymphatic clearance, and decreased REM sleep, N1 sleep, and WASO, associated with increased synaptic-metabolic release, contributed to HypnoS. That sleep stage duration alone showed poorer performance in explaining overnight changes in plasma Aβ and tau levels is not surprising. Measuring variations in the underlying neurophysiology occurring in each sleep stage is likely to be more predictive than simply quantifying time spent in each stage.
While it is widely appreciated that glymphatic clearance is more rapid during sleep than during waking, glymphatic clearance does occur during wake13,17. Yet to what extent this clearance is modifiable by sleep-like physiology during waking has remained unknown. Sleep deprivation also increases Aβ and tau release into the ISF28,29,30. The wake prediction model using PhysioW was notable in that it explained a substantial amount of the overnight variance in plasma Aβ and tau levels, with contributions from both increased glymphatic clearance and increased release. The optimal single predictor, PhysioW, showed that changes in cerebrovascular compliance, sympathetic/noradrenergic tone, and parenchymal resistance contributed to increased glymphatic clearance, similar to their effects during sleep in PhysioS. In contrast, wake EEG theta and delta power were associated with increased synaptic-metabolic release. While increased glymphatic clearance during wakefulness contributed to morning Aβ and tau levels, simulated changes in the neuro-glymphatic model’s single-index predictor PhysioW produced predicted shifts in plasma AD biomarker levels that were consistent with release being the primary driver. This interpretation aligns with the multicompartment model, which showed similar results when comparable magnitude changes were made to the cell-to-ISF release rate constant.
We were further able to show from these data that a simulated change in the neuro-glymphatic model PhysioS or HypnoS single-index predictors led to predicted changes in Aβ and tau clearance to plasma that replicated the predictions made by the multicompartment model when similar magnitude changes were made to the CSF-ISF rate constants. Considering that the multicompartment model was derived from external published data26,27, this finding independently corroborates the findings from our experimental data and statistical model. It is also noteworthy that the interaction between the single-index predictor and evening plasma concentration in the experimental neuro-glymphatic model mirrored the interaction between the CSF-ISF and cellular release rate constants and evening plasma concentration in the multicompartment neuro-glymphatic model. This suggests an association between these rate constants and the neurophysiological or hypnogram measures identified by the single-index predictors. Together these results affirm the neuro-glymphatic model, that increased synaptic-metabolic release and glymphatic clearance during sleep supports overnight changes in plasma Aβ and tau levels. These findings further support the predominance of sleep-active glymphatic clearance of Aβ and tau from the brain during sleep, and highlight a key role for synaptic-metabolic production of Aβ and tau during sleep-deprivation.
In addition to supporting the role for glymphatic transport in the clearance of Aβ and tau during sleep, the present results provide additional mechanistic insights into how these processes unfold in the human brain. Consistent with prior studies in rodents and humans, EEG delta power13,17,22,23 during NREM sleep and increased cerebrovascular compliance12,24,39 contributed to increasing clearance of Aβ and tau to the plasma in the overnight period, while higher EEG theta and delta power during REM sleep contributed to increasing synaptic-metabolic release28,29,30. These contributions are in marked contrast to the dominant effect that RP had within these models, with lower overnight RP contributing to greater morning plasma Aβ and tau levels, which may be because RP mediates the downstream effect of these variables. Conceptually, the synchronized low-frequency neural activity reflected in delta band power and increases in cerebrovascular compliance may serve as a key driving force for the process of glymphatic clearance, while astroglial-mediated changes in extracellular volume fraction reflected in the RP parameter13,17 may regulate the effects of this driving force on fluid and solute transport within the brain parenchyma. If true, then RP may represent a promising therapeutic target to increase glymphatic clearance of Aβ and tau.
It is important to point out the distinction between the changes in plasma AD biomarker levels observed through a single overnight period in the present study and changes in plasma AD biomarker levels that reflect steady-state alterations in AD-related pathology. For example, over the timescale of years, declining plasma Aβ42 levels and increasing p-tau levels occur with increasing Aβ plaque and tau pathological burden in AD. Within the setting of the present study, overnight changes in plasma AD biomarker levels reflect the dynamics of both the release of Aβ and tau species into the brain interstitium, and their subsequent clearance to the CSF and the plasma in the overnight period. These dynamics are superimposed upon the existing steady-state Aβ42 and p-tau levels of each participant that were analyzed separately for amyloid-positive and amyloid-negative individuals. Within these two groups, we do not expect individual overnight differences in Cell-ISF release and CSF-ISF exchange to be affected by baseline Aβ42 and p-tau levels. The converse question – whether impaired overnight Aβ and tau clearance leads to increased AD pathological burden and long-term shifts in steady-state plasma AD biomarker levels—is a compelling hypothesis that requires a further investigation.
One limitation of this study was the large number of regressors included in the statistical analysis, particularly given the relatively small number of amyloid-positive participants. To address this concern, we employed a single index regressor, a dimensionality reduction projection that condensed multiple regressors into a single variable. Additionally, by using multivariate linear mixed models for the five dependent variables, the correlated Aβ and tau plasma measures, we improved statistical power. This was evident in the increase in explained variance of the neuro-glymphatic model compared to the null model, as shown by statistically significant likelihood ratio tests. Although most of the potential confounding variables included in each model had no significant effect, we retained them for the sake of clarity and completeness.
Our findings showed that elements of sleep-active physiology, in particular, brain parenchymal resistance, enhanced the clearance of Aβ and tau into plasma during overnight sleep in humans. In contrast, during sleep deprivation, increased synaptic–metabolic release elevated plasma AD biomarker levels overnight. Our null model and neuro-glymphatic model were estimated to predict morning AD biomarker levels from evening AD biomarker levels which reduces the risk of spurious correlations when estimating those models on morning-to-evening differences or ratios of AD biomarker levels62. We further showed that while synaptic-metabolic release was dominant in the awake state, sleep-related physiology also contributed to increasing Aβ and tau clearance to the plasma.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The source data to Fig. 3 is available at the following URL and DOI. All other data supporting this study are also available at the following URL and DOI with Institutional Review Board (IRB) approval and a Data Use Agreement. URL: https://zenodo.org/records/17886744: https://doi.org/10.5281/zenodo.17886744 This ensures compliance with participants’ informed consent and permits non-commercial use for independent validation, publication, and the sharing of new findings. For participants with one or more measures that failed quality control during pipeline processing, we will provide, upon special request, the measures that passed quality control, along with unprocessed sensor data for the others, where possible. Source data are provided with this paper.
Code availability
Code used for the analysis and to produce the figures is available on Zenodo at the URL and DOI below. URL: https://zenodo.org/records/17887324: https://doi.org/10.5281/zenodo.17887324 The code for the compartment model simulations is available on github.com at the URL and DOI below.URL: https://zenodo.org/records/17887407: https://doi.org/10.5281/zenodo.17887407.
References
Robbins, R. et al. Examining sleep deficiency and disturbance and their risk for incident dementia and all-cause mortality in older adults across 5 years in the United States. Aging (Albany NY) 13, 3254–3268 (2021).
Lutsey, P. L. et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: the Atherosclerosis Risk in Communities Study. Alzheimers Dement 14, 157–166 (2018).
Lim, A. S., Kowgier, M., Yu, L., Buchman, A. S. & Bennett, D. A. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 36, 1027–1032 (2013).
Liu, H. et al. Acute sleep loss decreases CSF-to-blood clearance of Alzheimer’s disease biomarkers. Alzheimers Dement https://doi.org/10.1002/alz.12930 (2023).
Sprecher, K. E. et al. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology 89, 445–453 (2017).
Sprecher, K. E. et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol. Aging 36, 2568–2576 (2015).
Spira, A. P. et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 70, 1537–1543 (2013).
Winer, J. R. et al. Association of short and long sleep duration with amyloid-β burden and cognition in aging. JAMA Neurol. 78, 1187–1196 (2021).
Insel, P. S., Mohlenhoff, B. S., Neylan, T. C., Krystal, A. D. & Mackin, R. S. Association of sleep and β-amyloid pathology among older cognitively unimpaired adults. JAMA Netw. Open 4, e2117573 (2021).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012).
Iliff, J. J. et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123, 1299–1309 (2013).
Iliff, J. J. et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Iliff, J. J. et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193 (2014).
Harrison, I. F. et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 143, 2576–2593 (2020).
Eide, P. K., Vinje, V., Pripp, A. H., Mardal, K. A. & Ringstad, G. Sleep deprivation impairs molecular clearance from the human brain. Brain 144, 863–874 (2021).
Dagum, P. et al. A wireless device for continuous measurement of brain parenchymal resistance tracks glymphatic function in humans. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-025-01394-9 (2025).
Blennow, K. et al. The potential clinical value of plasma biomarkers in Alzheimer’s disease. Alzheimers Dement 19, 5805–5816 (2023).
Rissman, R. A. et al. Plasma Aβ42/Aβ40 and phospho-tau217 concentration ratios increase the accuracy of amyloid PET classification in preclinical Alzheimer’s disease. Alzheimers Dement 20, 1214–1224 (2024).
Benedict, C., Blennow, K., Zetterberg, H. & Cedernaes, J. Effects of acute sleep loss on diurnal plasma dynamics of CNS health biomarkers in young men. Neurology 94, e1181–e1189 (2020).
Eide, P. K. et al. Plasma neurodegeneration biomarker concentrations associate with glymphatic and meningeal lymphatic measures in neurological disorders. Nat. Commun. 14, 2084 (2023).
Hablitz, L. M. et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 5, eaav5447 (2019).
Jiang-Xie, L. F. et al. Neuronal dynamics direct cerebrospinal fluid perfusion and brain clearance. Nature 627, 157–164 (2024).
van Veluw, S. J. et al. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105, 549–561.e545 (2020).
Fultz, N. E. et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628–631 (2019).
Elbert, D. L., Patterson, B. W., Lucey, B. P., Benzinger, T. L. S. & Bateman, R. J. Importance of CSF-based Aβ clearance with age in humans increases with declining efficacy of blood-brain barrier/proteolytic pathways. Commun. Biol. 5, 98 (2022).
Michaels, T. C. T. et al. Dynamics of oligomer populations formed during the aggregation of Alzheimer’s Aβ42 peptide. Nat. Chem. 12, 445–451 (2020).
Kang, J. E. et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 (2009).
Holth, J. K. et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884 (2019).
Cirrito, J. R. et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48, 913–922 (2005).
Meyer, M. R. et al. Clinical validation of the PrecivityAD2 blood test: A mass spectrometry-based test with algorithm combining %p-tau217 and Aβ42/40 ratio to identify presence of brain amyloid. Alzheimers Dement 20, 3179–3192 (2024).
Fogelman, I. et al. Independent study demonstrates amyloid probability score accurately indicates amyloid pathology. Ann. Clin. Transl. Neurol. 10, 765–778 (2023).
Hu, Y. et al. Assessment of a plasma amyloid probability score to estimate amyloid positron emission tomography findings among adults with cognitive impairment. JAMA Netw. Open 5, e228392 (2022).
Du, L. et al. Longitudinal plasma phosphorylated-tau217 and other related biomarkers in a non-demented Alzheimer’s risk-enhanced sample. Alzheimers Dement https://doi.org/10.1002/alz.14100 (2024).
Barthélemy, N. R. et al. Highly accurate blood test for Alzheimer’s disease is similar or superior to clinical cerebrospinal fluid tests. Nat. Med. 30, 1085–1095 (2024).
Park, H., Tarpey, T., Petkova, E. & Ogden, R. T. A high-dimensional single-index regression for interactions between treatment and covariates. Stat. Papers https://doi.org/10.1007/s00362-024-01546-0 (2024).
West, T. et al. A blood-based diagnostic test incorporating plasma Aβ42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol. Neurodegener. 16, 30 (2021).
Orduña Dolado, A. et al. Effects of time of the day at sampling on CSF and plasma levels of Alzheimer’ disease biomarkers. Alzheimers Res. Ther. 16, 132 (2024).
Mestre, H. et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 9, 4878 (2018).
Hauglund, N. L. et al. Norepinephrine-mediated slow vasomotion drives glymphatic clearance during sleep. Cell 188, 606–622.e617 (2025).
Jack, C. R. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562 (2018).
Biel, D. et al. Tau-PET and in vivo Braak-staging as prognostic markers of future cognitive decline in cognitively normal to demented individuals. Alzheimers Res. Ther. 13, 137 (2021).
Jack, C. R. et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement https://doi.org/10.1002/alz.13859 (2024).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527 (2023).
Mestre, H. et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7 https://doi.org/10.7554/eLife.40070 (2018).
Pedersen, T. J., Keil, S. A., Han, W., Wang, M. X. & Iliff, J. J. The effect of aquaporin-4 mis-localization on Aβ deposition in mice. Neurobiol. Dis. 181, 106100 (2023).
Simon, M. et al. Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid β plaque formation in mice. Alzheimers Res. Ther. 14, 59 (2022).
Xu, Z. et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 10, 58 (2015).
Ishida, K. et al. Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J. Exp. Med. 219 https://doi.org/10.1084/jem.20211275 (2022).
Kress, B. T. et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861 (2014).
Li, M. et al. Impaired glymphatic function and pulsation alterations in a mouse model of vascular cognitive impairment. Front Aging Neurosci. 13, 788519 (2021).
Wang, M. et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J. Neurosci. 37, 2870–2877 (2017).
Braun, M. et al. Macroscopic changes in aquaporin-4 underlie blast traumatic brain injury-related impairment in glymphatic function. Brain 147, 2214–2229 (2024).
Christensen, J., Wright, D. K., Yamakawa, G. R., Shultz, S. R. & Mychasiuk, R. Repetitive mild traumatic brain injury alters glymphatic clearance rates in limbic structures of adolescent female rats. Sci. Rep. 10, 6254 (2020).
Plog, B. A. et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 35, 518–526 (2015).
Deng, S. et al. Chronic sleep fragmentation impairs brain interstitial clearance in young wildtype mice. J. Cereb Blood Flow Metab. 271678X241230188 https://doi.org/10.1177/0271678X241230188 (2024).
Nedergaard, M. & Goldman, S. A. Glymphatic failure as a final common pathway to dementia. Science 370, 50–56 (2020).
Keil, S. A., Jansson, D., Braun, M. & Iliff, J. J. Glymphatic dysfunction in Alzheimer’s disease: a critical appraisal. Science 389, eadv8269 (2025).
Ringstad, G., Vatnehol, S. A. S. & Eide, P. K. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140, 2691–2705 (2017).
Eide, P. K. & Ringstad, G. Functional analysis of the human perivascular subarachnoid space. Nat. Commun. 15, 2001 (2024).
Kronmal, R. A. Spurious correlation and the fallacy of the ratio standard revisited. J. R. Stat. Soc. A 156, 379–392 (1993).
Acknowledgements
This work was funded by Applied Cognition. V.V.V. and K.Y. at C2N Diagnostics, LLC were supported by funding from the Gerald and Henrietta Rauenhorst Foundation (GHR), Alzheimer’s Drug Discovery Foundation (GC-201711-2013978), BrightFocus (CA2016636), and National Institutes of Health (NIH) (R44 AG059489).
Author information
Authors and Affiliations
Contributions
Conceptualized the study: P.D. and J.J.I. Collected and managed the data: T.S., V.V.V., A.C., S.R.L., C.V. Performed formal analyses: P.D., D.L.E., T.S., A.C., R.M.K., E.L. Developed experimental methods: P.D., D.L.E., J.J.I. Wrote the original draft: P.D. Revised and edited the manuscript: P.D., D.L.E., L.G., T.S., V.V.V., A.C., R.M.K., S.R.L., E.L., K.Y., J.L., C.V., M.M.L., and J.J.I.
Corresponding author
Ethics declarations
Competing interests
The authors P.D., L.G., T.S., A.C., S.R.L., M.M.L., J.J.I. declare the existence of financial and incentive stock options competing interests. The author E.L. declares the existence of financial competing interests. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Bryce Mander, who co-reviewed with Abhishek Dave; Alan Talevi; and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dagum, P., Elbert, D.L., Giovangrandi, L. et al. The glymphatic system clears amyloid beta and tau from brain to plasma in humans. Nat Commun 17, 715 (2026). https://doi.org/10.1038/s41467-026-68374-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-026-68374-8