Abstract
Fabry disease (FD) is a rare X-linked lysosomal storage disorder caused by variants in the alpha-galactosidase A gene (GLA). Cardiac complications are a major cause of mortality, but the large number of variants complicate early identification of at-risk patients. In this study, we assessed the microcirculation using Retinal Vessel Analysis (RVA) in 63 FD patients age- and gender-matched to 60 healthy controls, analyzing associations between RVA parameters, cardiac involvement, and GLA variants. FD patients showed reduced venular flicker-induced dilation, narrower retinal arterioles, and a lower arteriolar-to-venular ratio. Impaired retinal microcirculation was associated with cardiac involvement, and patients with cardiac-associated GLA variants exhibited narrower retinal arterioles. Markers of inflammation and endothelial dysfunction (ED) were significantly higher in FD patients. Higher inflammatory levels correlated with altered retinal microcirculation in patients carrying cardiac-associated GLA variants. RVA detects microvascular ED in FD patients and may serve as a non-invasive biomarker for cardiovascular risk stratification. Registration: https://clinicaltrials.gov/study/NCT06758648; Unique identifier: NCT06758648.

Similar content being viewed by others
Introduction
Fabry disease (FD, OMIM-301500) is a rare, X-linked lysosomal storage disorder with a reduced or completely absent activity of the enzyme α-galactosidase A encoded by the GLA-gene. This malfunction leads to a progressive accumulation of glycosphingolipids, such as globotriaosylceramide (Gb3), in the endothelium of different organs. Primary cellular dysfunction and secondary inflammation result in reduced organ perfusion and fibrosis, which causes progressive, multisystemic organ damage1,2,3. In adult FD patients, cardiovascular manifestations, including left ventricular hypertrophy (LVH), and cerebrovascular complications, including stroke, contribute to a significantly lower life expectancy4,5,6. Heterozygous females with α-galactosidase A deficiency may also develop Fabry-associated complications, although usually with a slower progression7. Diagnostic delay of FD can reduce the efficacy of the treatment, and cardiovascular complications may significantly benefit from early treatment5,8,9.
Although the exact pathophysiological mechanisms underlying endothelial dysfunction (ED) in FD remain unclear, Gb3 has been shown to induce the degradation of endothelial KCa 3.1 channels, which play a crucial role in endothelium-dependent hyperpolarization. Additionally, secondary chronic inflammation may contribute to the degradation of the endothelial glycocalyx, further exacerbating ED in FD patients10,11. Consequently, various markers of endothelial activation and inflammation, including vascular endothelial growth factor (VEGF), intercellular adhesion molecule-1 (ICAM-1), and monocyte chemoattractant protein-1 (MCP1), are elevated in patients and markers of inflammation are associated with cardiac disease progression in FD12,13,14,15,16. After the administration of enzyme replacement therapy (ERT), complete microvascular clearance of Gb3 in the endothelium and podocytes has been observed17,18. However, in advanced FD patients with severe LVH, intracellular clearance of glycosphingolipids in cardiomyocytes appears to be ineffective despite ERT infusion due to fibrosis and inflammation19. Furthermore, Gb3 clearance may not prevent altered molecular signaling, further underscoring the importance of early disease detection17.
Quantifying ED by non-invasive imaging methods has been proposed by the European Society of Cardiology (ESC)20, and studies have shown impaired endothelial function in medium- to large-sized vessels via pulse-wave velocity (PWV) and flow-mediated dilation (FMD)21,22. However, cardiovascular complications in FD are mainly a result of coronary microvascular dysfunction, and monitoring the microcirculation with non-invasive tools could potentially provide a better tool for early diagnosis and disease monitoring18,23,24. To our knowledge to date, there is no functional data on small vessel health in FD.
Dynamic retinal vessel analysis (DVA) and static retinal vessel analysis (SVA) are two quantitative and non-invasive technologies that follow up on changes in retinal microcirculation as a predictive tool for the progression of systemic cardiovascular disease (CVD). DVA quantifies the reaction to flickering light over time, mainly mediated by neuro-vascular coupling followed by shear-stress-induced nitric oxide (NO) release25. This allows direct assessment of the cerebrovascular function, and especially in CVD, DVA and SVA have shown in multiple studies their high potential to monitor endothelial health non-invasively26,27. The Atherosclerosis Risk in Communities (ARIC) study proved the excellent predictive value of retinal arteriolar narrowing and venular widening as a long-term predictor of CV events28. In a large group of dialysis patients, we were able to show that impaired retinal venular dilation (vFID) is an independent predictor of all-cause mortality29.
Early detection of organ damage in FD is crucial, however diagnosis in early stages still presents a considerable challenge. Therefore, the study’s primary aim was to investigate whether the retinal structure and function, measured by DVA and SVA, are altered in FD. The secondary aim was to evaluate how parameters of the retinal microcirculation are associated with clinical disease severity, ERT and/or pharmacological chaperone therapy (PCT), and the cardiac phenotype.
Results
Patients’ characteristics and subgroup analysis
Out of 63 FD patients, 60 (95.2%) had high-quality SVA images (68.3% female, mean age 48.6 ± 16.5 years) and were age- and gender-matched with 60 healthy volunteers (68.3% female, 49.7 ± 16.2 years) from a previously recruited HC (n = 204)30. The occurrence of CV risk factors was comparable between cohorts after matching. There was a tendency towards more nicotine abuse in FD patients; however, not significantly. The most frequent CV risk factors in FD patients were hypercholesterolemia (53.3%) and obesity (36.7%). Fabry-related complications included patients with left ventricular hypertrophy (LVH, 48.3%), patients with central nervous, vascular disease (CNVD, 25.0%), patients with cardiac arrhythmia (21.7%), and patients with chronic kidney disease (CKD, 6.7%). 41 patients (68.3%) were pre-treated with enzyme replacement therapy (ERT) or pharmacological chaperone therapy (PCT; 4/41; 9.8% of treated patients) with a median therapy duration of 1.1 years (0.0–3.4). The average Fabry disease severity scoring system (DS3) was 8.7 (5.2–11.8), and median LysoGb3 levels were 4.3 ng/ml (0.9–8.3) under current ERT/PCT. Laboratory parameters showed significantly higher leukocytes and lower hemoglobin levels in FD patients (Table 1).
Male FD patients (n = 20, mean age 50.4 ± 10.7 years) showed a more severe clinical phenotype than female FD patients (n = 43, mean age 47.4 ± 18.2 years). Frequency of LVH (37.2% vs. 70.0%, p = 0.029), CNVD (13.9% vs. 55.0%, p = 0.0011), and heart valve disease (HVD, 0% vs. 15.0%, p = 0.028) was significantly higher in male patients. Consequently, the ADS3 was significantly higher (7.7 vs. 11.7, p = 0.0052), and male FD patients showed significantly higher levels of LysoGb3 (4.2 vs. 27.0, p = 0.028). CV risk factors and age were not significantly different between male and female FD patients. Ferritin as well as Creatinine levels were significantly elevated in male FD patients (Supplementary Table 1).
Impaired retinal microvascular function in Fabry disease
FD patients demonstrated significantly lower venular flicker-induced dilation (vFID) compared to age- and gender-matched HC (vFID: 3.5% ± 1.6% vs. 4.6% ± 2.4%; p = 0.0058). No significant difference was observed in arteriolar flicker-induced dilation (aFID) between the groups, although FD patients showed a tendency towards higher values (3.9% ± 3.1% vs. 3.0% ± 2.0%; p = 0.079) (Fig. 1a, b). We analyzed retinal fundus pictures to assess retinal vessel diameters in FD patients. FD patients showed significantly narrower retinal arterioles (CRAE) when compared with HC (165.2 µm [153.5–183.2] vs. 183.2 µm [174.7–191.4], p < 0.001). There were no differences in retinal venular diameters (CRVE) between the groups (207.2 µm [196.9–217.2] vs. 212.6 µm [200.2–219.5]; p = 0.35). The arteriolar-venular ratio (AVR), calculated as CRAE/CRVE, was significantly lower in FD patients compared to HC (0.82 [0.74–0.87] vs. 0.86 [0.82–0.91]; p < 0.001) (Fig. 1c–e).
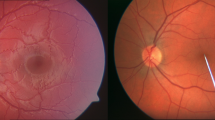
Violin plots of DVA parameters venular flicker-induced dilation (vFID: FD, n = 56; HC, n = 60; a) and arteriolar flicker-induced dilation (aFID: FD, n = 55; HC, n = 60; b) and SVA parameters CRVE; (c), CRAE; (d), and AVR; (e all n = 60) in age- and gender-matched Fabry patients (red) and healthy controls (blue). Boxplots display values as the median (line) and mean (green square). The Wilcoxon rank-sum test was used for non-parametric distributions, and the Student’s t-test was used for parametric distributions. Statistical significance is indicated as follows: *p < 0.05; **p < 0.01; ***p < 0.001. ROC curves for CRAE, AVR and vFID and their AUCs and 95% confidence intervals (CI) are shown (f). g shows representative fundus images of a Fabry disease patient and a healthy control, with semi-automatically labeled retinal arterioles and venules. Vessel calibers were measured one disc diameter away from the optic disc to derive the respective SVA parameters: CRAE, CRVE, and AVR. This figure was created with Affinity Publisher (Version 2.5.5).
After controlling for potential confounders of RVA parameters, lower CRAE (p = 0.0028), lower AVR (p < 0.001), and lower vFID (p = 0.0015) remained significantly associated with FD (Supplementary Table 2). Additionally, vFID, CRAE, and AVR remained significantly lower in FD patients compared to HC after the exclusion of FD patients with a likely benign variant or VUS (Supplementary Fig. 1). CRAE had good accuracy in distinguishing FD patients from HCs, with an area under the curve (AUC) value of 0.71. Both vFID and AVR achieved acceptable discrimination with AUC values of 0.61 and 0.68, respectively (Fig. 1f). Representative fundus images of an FD patient and an HC are shown in Fig. 1g, illustrating the semi-automatic labeling of retinal arterioles and venules used to derive the SVA parameters CRAE, CRVE, and AVR.
FD is an X-linked inherited disease; therefore, we conducted a subgroup analysis. For DVA and SVA parameters, we saw similar trends for both genders. Female FD patients had lower vFID (p = 0.030) as did male patients, which, however, failed to reach significance (p = 0.10). Male patients exhibited a significant increase in aFID compared to HCs (p = 0.027), whereas no significant difference was found in female FD patients (p = 0.72) (Fig. 2a, b). CRVE did not differ significantly between female or male FD patients and HCs. CRAE and AVR were lower in male and female FD patients than HCs, with AVR not reaching significance (p = 0.068) in female patients (Fig. 2c–e). Thus, retinal microvascular changes are evident in male and female FD patients.
Boxplots of DVA parameters, including vFID (a; FD: n = 56, HC: n = 60) and aFID (b; FD: n = 55, HC: n = 60), as well as SVA parameters CRVE, CRAE, and AVR (c–e; all n = 60), are shown for age- and sex-matched FD patients (red) and healthy controls (blue). These groups are subdivided into male (n = 19) and female (n = 41) patients. Boxplots display the mean (rectangle) and median (line). The Wilcoxon rank-sum test was applied for non-parametric distributions, and Student’s t-test was used for parametric distributions. Statistical significance is indicated as follows: *p < 0.05; **p < 0.01; ***p < 0.001. This figure was created with Affinity Publisher (Version 2.5.5).
Retinal vessel diameters and function as markers for clinical outcome in Fabry disease
Microvascular changes and their associated complications drive comorbidities and symptom severity in FD patients. Symptom severity in FD patients was assessed using the average DS3 score. CRAE showed a strong negative correlation with symptom severity (r = −0.40, p = 0.0013) (Fig. 3a). CRVE showed no significant correlation with average DS3 (r = −0.16, p = 0.22). However, lower AVR was significantly correlated with higher average DS3 (r = −0.33, p = 0.0098) (Fig. 3b, c). DVA parameters showed no association with average DS3 (not shown).
Scatterplots show the Pearson correlations between SVA parameters CRAE, CRVE, and AVR (all n=60) with average DS3 (n=63), including the corresponding correlation coefficient (r) and p-value (a–c). Spearman correlations are shown for CRAE, CRVE and AVR with the Cardiac Severity Score, including the corresponding correlation coefficient (ρ) LOESS curves with 95% confidence intervals are displayed to illustrate the negative, potentially non-linear associations (d–f). This figure was created with Affinity Publisher (Version 2.5.5).
Among the four clinical domains of the average DS3, cardiac symptom severity was strongly correlated with narrower retinal arterioles (ρ = −0.45, p < 0.001) (Fig. 3d). Narrower retinal venules were significantly correlated with greater cardiac symptom severity (ρ = −0.31, p = 0.017), while the correlation for lower AVR missed significance (ρ = −0.25, p = 0.054) (Fig. 3e, f). As previously described by others, higher LysoGb3 levels were positively correlated with average DS3 and cardiac symptom (Supplementary Fig. 2a, b)31.
Multivariable linear regression models were fitted to further explore the association between the retinal microcirculation and the cardiac phenotype. Lower CRAE was significantly associated with higher ADS3, cardiac symptom severity and renal symptom severity, which all remained significant after adjusting for CV risk factors. Narrow retinal arterioles were independently linked to specific organ involvement, showing significant associations with LVH, HVD, CNVD, and CKD (Table 2). Similar findings were observed for higher LysoGb3, lower AVR and CRVE, which also showed significant associations with increased cardiac symptom severity and organ involvement (Supplementary Table 3).
Given that SVA parameters and higher LysoGb3 levels were linked to cardiac symptom scores and comorbidities, we further investigated how SVA parameters correlate with cardiac laboratory markers and echocardiographic parameters. Lower CRAE was significantly associated with elevated levels of lactate dehydrogenase (LDH, r = −0.31), high sensitivity Troponin T (hs-Troponin T, r = −0.49) and NT-proBNP (r = −0.54). Lower AVR showed similar results. Regarding echocardiographic changes, both CRAE and AVR were significantly negatively correlated with the left ventricular end-diastolic diameter (LVEDD, r = −0.17 and r = −0.19), intraventricular septum thickness (IVS, r = −0.55 and r = −0.38), and posterior wall thickness (PW, r = −0.46 and r = −0.32) (Fig. 4a, c). Except for the correlation with LVEDD, similar correlations were observed for LysoGb3 (Fig. 4b). Lower CRVE was correlated with higher IVS (r = −0.3), PW (r = −0.28), and LDH (r = −0.3) (Fig. 4d).
Correlation plots show Spearman correlations of static retinal vessel parameters and and LysoGb3 with cardiac laboratory parameters (hsTnT, high-sensitivity Troponin T, n=38; NT-proBNP, N-terminal prohormone of brain natriuretic peptide, n=38; CK, creatine kinase, n=63; LDH, lactate dehydrogenase, n=63) and echocardiographic parameters (LVEDD, left ventricular end-diastolic diameter, n=59; IVS, intraventricular septum thickness, n=60; PW, posterior wall thickness, n=58; EF, ejection fraction, n=46). Correlation coefficients (r) are visualized and displayed on the right. Non-significant correlations are not shown (empty fields)This figure was created with Affinity Publisher (Version 2.5.5).
Fabry genetic variants, cardiac involvement and associations with retinal microvascular health
Sequence variants were classified according to the American College of Medical Genetics and Genomics (ACMG) guidelines, and clinical parameters were compared among four variant categories: pathogenic (n = 33), likely pathogenic (n = 7), variants of uncertain significance (VUS, n = 8), and likely benign (n = 14). There was a trend towards a higher frequency of Fabry-related comorbidities in patients with pathogenic, likely pathogenic, and VUS, though this did not reach statistical significance. High sensitivity (hs)-CRP and hs-TnT levels were significantly different across these clusters, with higher hs-CRP values observed in patients with VUS and pathogenic variants and higher hs-TnT levels in patients with likely pathogenic and pathogenic variants (Supplementary Table 4).
Because left ventricular hypertrophy is one of the most frequent signs of Fabry-related cardiomyopathy, we analyzed the relationship between IVS thickness, variant classification, and specific GLA variants32.
In a multivariable linear regression model, IVS and LysoGb3 were significantly higher in VUS, likely pathogenic and pathogenic variants. Lower CRAE was associated with likely pathogenic and pathogenic variants (Supplementary Table 5).
Next, we assessed the relationship between specific GLA-gene sequence variants and IVS thickness in a multivariable model. Seven gene variants with a potential cardiac phenotype were selected based on their estimates and significant association with higher IVS, suggesting their relevance in FD-related cardiac hypertrophy (Supplementary Table 6).
We plotted a heatmap of scaled estimates for various cardiac outcome variables to further visualize potential cardiovascular involvement in the seven selected gene variants. Except for one variant (c.547 G > A), all selected gene variants showed associations with either levels of higher cardiac laboratory parameters (LDH, CK, NT-proBNP, hsTnT) or higher LVEDD and PW thickness (Supplementary Fig. 3). Based on these associations across multiple cardiac parameters, six variants were finally classified as “potential cardiac variants”. Next, we compared parameters of retinal microcirculation and LysoGb3 between FD patients with the six selected variants with a potential cardiac phenotype (n = 20) and FD patients with other GLA-gene sequence variants (n = 42). FD patients with a potential cardiac phenotype showed a significant increase in LysoGb3 levels (log-transformed, 0.81 vs. 0.02, p = 0.0038). Additionally, they showed significantly narrower retinal arterioles (159.5 µm vs. 171.0 µm, p = 0.039). Both CRVE and AVR tended to lower levels, but not significantly (Fig. 5a–d). Showcasing cardiac involvement, NT-proBNP, and hsTnT were significantly higher in the selected six gene variants (Fig. 5e, f). LysoGb3, hsTnT and NT-proBNP had good accuracy in distinguishing FD patients with a cardiac variant from patients without, with AUC values of 0.72, 0.77 and 0.79. CRAE achieved acceptable discrimination with an AUC value of 0.67 (Fig. 5g). Four of the variants with a potential cardiac phenotype were pathogenic, one likely pathogenic, and interestingly, one was a VUS. The selected gene variants were prominent in female FD patients (female 15/20 vs. male 5/20).
Boxplot of CRAE (a), AVR (b) and CRVE (c; all n = 60), LysoGb3 (d; n = 63), and laboratory parameters NT-proBNP (e; n = 38) and hsTnT (f; n = 38) between FD patients with a potential cardiac variant (red, n = 20) and FD patients with other GLA gene variants (blue, n = 43). LysoGb3, hsTnT and NT-proBNP were log-transformed. Boxplot displays values as median (line) and mean (green square). The Wilcoxon rank-sum test was used for non-parametric distributions, and the Student´s t-test was used for parametric distributions. * p < 0.05; **p < 0.01; ***p < 0.001. ROC curves for distinguishing gene variants are shown for CRAE, LysoGb3, NT-proBNP and hsTnT and their AUCs and 95% confidence intervals (CI) are shown (g).
Inflammatory parameters and markers of endothelial dysfunction in FD patients
Previous studies have reported elevated markers of endothelial activation and chronic inflammation in FD patients. Compared to age- and gender-matched HC, FD patients displayed significantly elevated levels of VEGF (pg/ml, 67.3 [39.5–118.0] vs. 0.7 [0.0–2.1], p < 0.001), ICAM-1 (ng/ml, 159.5 [78.6–333.6] vs. 0.0 [0.0–244.1], p = 0.039) and VCAM-1 (ng/ml, 48.9 [30.9–68.0] vs. 0 [0.0–25.2], p = 0.0011). In addition, levels of CXCL10 (pg/ml, 79.3 [45.9–120.3] vs. 37.5 [29.2–59.6], p < 0.001), MCP1 (pg/ml, 94.9 [63.4– 117.7] vs. 37.0 [26.2– 65.0], p < 0.001) and Rantes (ng/ml, 50.1 [15.0–125.6] vs. 1.4 [0.8–1.6], p < 0.001) were elevated. IL-6 showed a tendency towards higher levels, however failing to reach significance (Supplementary Fig. 4a–f). We fitted regression models with interaction effects to investigate the association of markers of ED and inflammation markers with retinal vessel diameters and function in FD patients with a potential cardiac gene variant (Supplementary Table 7). In FD patients with potential cardiac variants, the association between higher levels of CXCL10 and lower CRAE was stronger, with a significant interaction observed between CXCL10 and cardiac variants (pinteraction = 0.048) (Fig. 6a). Similar trends were found in FD patients with cardiac variants for the association between higher levels of MCP1, Rantes, and VCAM-1 and lower CRAE, although these interactions failed to reach significance (Fig. 6b–d). Consistent with these findings, in FD patients with a potential cardiac variant, we observed a stronger association between higher CXCL10 levels and lower CRVE (pinteraction= 0.011) as well as between higher MCP1 levels and lower AVR (pinteraction= 0.041) (Fig. 6e, f). Interestingly, higher levels of LysoGb3 were associated with higher MCP1 in FD patients with potential cardiac variants (pinteraction= 0.010) (Fig. 6g).
Interaction plots visualize the effect of cardiac gene variants (red line, n = 20) and no cardiac variants (green, dashed line, n = 42) on the correlation between CRAE (a–d), AVR (e), CRVE (f), and LysoGB3 (g) with levels of inflammatory and endothelial dysfunction parameters. MCP1, CXCL10, Rantes and LysoGb3 were log-transformed (all n = 63). Pinteraction displays the interactions between FD patients with a potential cardiac gene variant and laboratory variables calculated in a multivariable linear model. This figure was created with Affinity Publisher (Version 2.5.5).
Discussion
Endothelial dysfunction is a hallmark of Fabry disease, and cardiac impairment such as perfusion defects and slow coronary flow result from luminal narrowing in the microcirculation10,23,33. For the first time we could now show an altered retinal microcirculation in FD patients, as evidenced by significantly reduced flickering-induced venular dilatation, retinal arteriolar narrowing, and smaller AVR. Importantly, these microvascular differences were independent of age, gender, and CV risk factors such as arterial hypertension, BMI, hypercholesterolemia, and smoking.
Smooth muscle cell (SMC) proliferation is one of the consequences of Gb3 deposition, and SMC proliferation is directly induced by LysoGb321,34. As small arteries and arterioles in the retina are known to have relatively large components of SMCs, arteriolar narrowing, as evidenced by smaller CRAE in our FD patients, might be a direct effect of intima thickening25. SMC proliferation may lead to remodeling of the arterioles, increasing shear stress, which ultimately reduces NO synthesis. Studies also postulate a downregulation of the NO signaling pathway in FD35,36. Both could explain our findings of reduced flicker-induced venular dilation. As we have shown that reduced vFID is an independent predictor of mortality in a cohort of patients with end-stage renal disease (ERDS), assessing venular dilation in FD patients could also provide valuable predictive insights, particularly in identifying patients at higher risk of Fabry-associated complications, such as cardiac or cerebrovascular events29. Systemic, ongoing inflammation may serve as the unifying pathophysiological mechanism underlying reduced venular dilation in both conditions3,37.
Interestingly, in male FD patients, flickering-induced arteriolar dilation was significantly higher than in healthy males. This might be explained by a compensatory overregulation in arterioles and, therefore, a stronger reaction to flickering light. Altarescu et al. also showed a stronger arterial vasodilation in FD patients after administration of acetylcholine35. This is in line with findings in Alzheimer’s disease (AD), where the levels of storage products directly correlated with a higher arteriolar pulse amplitude, and the arteriolar pulse amplitude was significantly higher in AD patients38,39.
In female FD patients, CRAE and vFID were significantly reduced compared to HC, indicating that RVA provides a tool to quantify microcirculatory changes in female patients. In patients with residual enzyme activity, such as female patients or those with cardiac variants, SMCs showed deposits even when the endothelium remained clear. Therefore, SMCs may be more susceptible to Gb3 than the endothelium40. Monitoring female FD patients with RVA could provide additional insights into endothelial health beyond measuring often normal LysoGb3 levels. We argue that RVA could offer a cost-effective, non-invasive tool to monitor microvascular endothelial health in FD patients and, therefore, should be incorporated into routine care. Although a retinal phenotype was detectable in both genders, the more pronounced retinal microvascular alterations observed in male Fabry patients likely reflect the X-linked inheritance of the disease, resulting in a higher accumulation of storage products and a more severe vascular phenotype in males compared with females21,41,42. Although a higher cardiovascular burden in males or age-related factors could theoretically contribute to these differences, both cardiovascular risk profiles and age were comparable between genders in our cohort (see Supplementary Table 1). Therefore, the X-linked nature of the disease likely represents the principal explanation for the observed gender-related differences in retinal microvascular health.
Whether ERT effectively clears Gb3 deposits in the heart remains controversial, and microvascular changes may persist well beyond therapy18,24,43. In patients with more advanced disease, ERT’s impact on restoring vascular health is potentially limited or altered, possibly due to longstanding disease-related vascular changes that are less responsive to ERT6,44. One possible explanation could be persistent alterations in endothelial signaling pathways17,45.
Due to the increased availability of genetic testing and disease awareness, there is an evolving number of VUS46. Determining whether to start ERT can be challenging, especially with late-onset or organ-specific variants, such as cardiac ones8,47. Although pathogenic variants tended to be associated with more Fabry-related complications in our cohort, complications were also observed in cases with VUS. After identifying six variants closely associated with primarily cardiac involvement during recruitment and predominantly found in female FD patients, we observed that these variants are strongly linked to arterial narrowing. This finding aligns with our observation that retinal arteriolar narrowing is closely associated with cardiac comorbidities, such as LVH, HVD, and CNVD, independent of traditional cardiovascular risk factors. Barbey et al. showed a proliferative effect of plasma from FD patients on SMC and cardiomyocytes, suggesting the plasma contains factors sustaining proliferative activity48. In our cohort, the FD plasma showed elevated levels of chemokines, such as Rantes, MCP1, and CXCL10.
Rantes and MCP1 are upregulated in human podocytes after Lyso-Gb3 stimulation by the NOTCH-1-dependent pathway, contributing to podocyte injury and kidney fibrosis49. Additionally, in light of evidence linking the CXCL10/CXCR3 axis to proinflammatory and T-cell-mediated responses in cardiac diseases, our finding of elevated CXCL10 in patients may suggest a potential role for CXCL10-mediated inflammation in the pathogenesis of cardiac complications in this cohort50. This, aligns with the findings of Frustaci et al., who reported immune-mediated myocarditis in Fabry cardiomyopathy characterized by CD3 + T-cell infiltration and myocyte necrosis51. Chemokine-driven immune cell recruitment and amplified inflammatory signaling in endothelial cells may upregulate markers of endothelial activation in FD patients. We could show significantly higher levels of VEGF, ICAM-1, and VCAM-1. ICAM-1 and VCAM-1 are upregulated in EC after Gb3 stimulation, and VEGF has been associated with hypertrophic cardiomyopathy in FD patients13,52. In FD patients with potential cardiac variants, we could show that higher levels of MCP1 and CXLC10 are associated with an impaired retinal microcirculation and higher levels of LysoGb3. Cardiac inflammation and endothelial activation are secondary mechanisms sustaining damage to myocardial structures despite ERT2,3. Higher levels of autoantibodies in FD patients further point toward a shared pathophysiology of autoinflammatory diseases and FD53. This further emphasizes how crucial early detection of microvascular changes in FD is and suggests that concomitant anti-inflammatory therapies should be considered in advanced disease.
This study is cross-sectional; therefore, even though we tried to control for confounders, all correlations and associations should be interpreted as exploratory. Longitudinal studies are needed to confirm the predictive value of RVA parameters. While the study includes a relatively large cohort of 63 FD patients, which is significant given the rarity of the disease, subgroup analyses may still lack statistical power to detect certain associations. Furthermore, our cohort consisted primarily of heterozygous patients, and we included FD patients with VUS and likely benign variants. While subgroup analysis was performed, this composition may limit the generalizability of the findings. In addition, all patients were recruited from the Department of Nephrology, which may introduce a selection bias toward individuals with renal involvement. However, each participant underwent comprehensive multidisciplinary evaluations by a nephrologist, ophthalmologist, neurologist, and cardiologist to ensure systematic assessment of multi-organ manifestations.
Regarding the markers analyzed as secondary outcomes, it is essential to note that while the study was sufficiently powered to assess the primary outcome of endothelial dysfunction, the exploratory analyses involving laboratory markers and associations with genetic variants may not have been adequately powered to detect all possible associations. The results of these secondary analyses should be interpreted with caution and viewed as hypothesis-generating, providing a foundation for further studies with larger cohorts to validate these findings. Despite these limitations, this study represents a meaningful contribution to understanding endothelial dysfunction and its potential biomarkers in a rare disease like Fabry. Our study highlights the value of RVA as a quick, inexpensive, and validated tool for early detection of microvascular changes in Fabry disease, independent of traditional cardiovascular risk factors. The significant alterations observed in both static and dynamic retinal vessel parameters in male and female FD patients underline the close link between microvascular changes and systemic disease severity. Notably, the strong correlation between retinal arteriolar narrowing and established echocardiographic and laboratory markers of cardiovascular involvement suggests that RVA may be a reliable, non-invasive approach for monitoring endothelial health in Fabry disease patients.
Given the persistent inflammatory and endothelial activation seen in FD, particularly in patients with a potential cardiac gene variant, early detection and monitoring of microvascular abnormalities through RVA could enable more timely intervention and the possibility of incorporating adjunctive anti-inflammatory therapies to slow disease progression. Longitudinal studies are essential to assess the predictive value of RVA for long-term cardiac- and Fabry-related outcome variables.
Methods
Study design and patients
This study is part of an observational longitudinal investigation examining the retinal microvasculature of 63 FD patients and providing an in-depth clinical characterization of FD patients. All patients were recruited in our outpatient clinic from the department of nephrology (Klinikum Rechts Der Isar, Technical University of Munich, School of Medicine), which all underwent clinical examination, RVA analysis, and blood collection. The local ethics committee approved the study protocol (Ethics Committee of the Klinikum Rechts Der Isar, Technical University of Munich, School of Medicine, No. 432/19S). The study “VASCinFABRY” was registered previously at ClinicalTrials.gov (NCT06758648). All participants of this study gave written informed consent. The study conforms to the principles of the Declaration of Helsinki and was designed following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The sample size was defined previously in the study protocol and was powered for the primary aim of comparing retinal vessel parameters in Fabry patients with a healthy cohort (HC). Assumptions regarding expected differences and variability were informed by comparable patient cohorts, and the calculation was conducted with guidance from a statistical expert. The HC was recruited as described previously30.
Inclusion criteria were age >18, diagnosis of FD by genetic testing of the galactosidase alpha (GLA) gene, α-gal A activity in leukocytes, or elevated LysoGb3 levels. Exclusion criteria were active infection and or malignant disease, operation two weeks before baseline examination, known glaucoma or epilepsy, and lack of written consent.
Genetic analysis was done in 62 patients (Genetic analysis, Molecular Genetics and Metabolism Laboratory, Centogene AG, Rostock, Germany).
Retinal vessel analysis
DVA and SVA were performed by experienced and trained examiners using the Dynamic Retinal Vessel Analyzer (DVAlight; IMEDOS Systems, Jena, Germany) and the Static Vessel Analyzer (IMEDOS Systems GmbH, Jena, Germany). The technique has been extensively described in our studies29,54. SVA was performed before DVA. Before the examination, pupils were dilated using topical tropicamide (0.5% Mydriaticum Stulln; Pharma Stulln, Germany), and patients were seated in a quiet, dark room for a ten-minute rest period.
In the case of DVA, patients were asked to focus on a needle attached inside the camera, and the diameters of one arteriole and venule were automatically and continuously recorded for 350 seconds. Arteriole and venule segments between 0.5 to 1 mm were analyzed approximately one disc diameter away from the optic nerve in the upper-temporal or lower-temporal direction. The baseline recording was 50 seconds, followed by a flickering phase of 20 seconds and then a recovery period of 80 seconds. Three of these cycles were performed, and we calculated the percentage of maximum arteriolar (aFID) and venular dilation (vFID) to baseline as previously described. For data validation, the quality of vessel response curves was compared using a cumulative scoring method (range 0 to 5)29. Retinal measurements with a score <2.5 were excluded after re-evaluation by an experienced observer. In five cases (7.9%), we could not measure vFID due to a lack of information in the measured region, and in six cases (9.5%), we could not obtain high-quality measurements of aFID.
SVA pictures were analyzed using Vesselmap 2® (IMEDOS Systems GmbH, Jena, Germany). One eye was examined, and three images were taken with the focus on the optic disc at an angle of 50°. Roughly one disc diameter away from the optic disc, retinal veins and arterioles segments were semi-automatically labeled. The Paar-Hubbard formula averaged the central retinal arteriolar (CRAE) and central venular (CRVE) equivalents55. SVA parameters CRAE and CRVE were measured in measurement units (MU), where 1 MU corresponds to 1 µm in Gullstrand’s eye model. Inter- and intra-observer inter-class correlation coefficients for CRAE and CRVE range from 0.75 to 0.8755,56. In three patients (4.8%), we could not obtain high-quality static retinal pictures. For both manual and semi-automated image analyses, the examiner was aware that the analyzed cohort consisted of patients with Fabry Disease. However, to minimize potential bias, the examiner was blinded to all individual clinical information, including disease severity, biomarker levels (e.g., LysoGb3), echocardiographic parameters, and genetic data during image evaluation.
Analysis of clinical and laboratory characteristics
We studied the medical records to record baseline characteristics, comorbidities, disease onset, gene variants, FD-specific treatment, and medication. Blood examinations and echocardiography took place within the framework of routine tests on the day of baseline recruitment.
Blood samples were collected following previously described protocols54, and routine parameters were measured in an ISO-certified laboratory. Levels of IL-6, Rantes, VEGF, CXCL10, MCP1, VCAM-1, and ICAM-1 in patient serum were quantified using the Cytometric Bead Array Flex system (BD Biosciences, San Diego, USA), following the manufacturer’s instructions.
The Fabry disease severity scoring system (average DS3) is a validated score that takes four clinical domains (Renal, peripheral nervous system, cardiac, and central nervous system) and one patient-reported outcome (PROM) into account. The average scoring system ranges from “minimal disease severity” (0 p.) to “maximum disease severity” (32 p.)57. Interventricular-septum (IVS) thickness was measured and considered as increased for values > 13 mm. The presence of chronic kidney disease (CKD) was defined as an eGFR of less than 60 ml/min calculated with the CKD-EPI equitation.
In 62 patients, the galactosidase alpha (GLA)-gene sequence variants were analyzed. Variants were classified according to the American College of Medical Genetics and Genomics (ACMG) guidelines, and 33 patients were classified with a pathogenic variant, 7 with a likely pathogenic variant, 8 patients with a variant of uncertain significance (VUS) and 14 with a likely benign variant (Supplementary Table 8)58.
Statistical analysis
All statistical analyses were performed using R (4.2.1) and R Studio (Version: 2024.04.2 + 764). Graphical preparation was done using Affinity Publisher (Version 2.5.5). The Graphical Abstract was Created with BioRender.com. Normally distributed values are shown as mean, ± standard deviation (SD), and non-normally distributed values as mean and their interquartile range (IQR) if not otherwise stated. To compare patients’ characteristics student´s t-test was used for normally distributed values, the Wilcoxon rank sum test for non-normally, and χ2-test for categorical variables. For comparisons of more than two groups, one-way ANOVA was used for normally distributed data, and Kruskal-Wallis was used elsewhere. Normality was assessed with the Shapiro-Wilks test and visually by using histograms. All statistical hypothesis testing was conducted on two-sided 5% significance levels. To match FD patients with the healthy cohort based on age and gender, we used the Matching package; matching success was controlled with the MatchBalance function. Violin- and boxplots were created using the ggplot package. Receiver operating characteristics (ROC) analysis was done with the plotROC and pROC package, and the area under the curve (AUC) was calculated using the trapezoidal rule. The 95% confidence interval (CI) of the AUC was calculated using DeLong´s method. For the correlation plots and the calculation of Spearman´s (for skewed data) and Pearson´s (for normal data) correlation coefficients, we used the ggscatter function, which is part of the ggpubr package. The correlation heatmap was created using the corplot package. In a multivariable linear model, we adjusted for potential confounders (BMI, arterial hypertension, hypercholesterolemia, and nicotine abuse) of retinal vessel analysis59,60,61,62. We used the interaction package to create interaction plots. Two independent researchers typed in all clinical data for double-data verification. Efforts were made to minimize missing data throughout the study, and the extent of missingness was kept to a reasonably achievable minimum. Missing data were not imputed. One author had full access to all the data in the study and took responsibility for its integrity and the data analysis.
Data availability
Data are available from the corresponding author at reasonable request.
References
Zarate, Y. A. & Hopkin, R. J. Fabry’s disease. Lancet 372, 1427–1435 (2008).
Kurdi, H., Lavalle, L., Moon, J. C. C. & Hughes, D. Inflammation in Fabry disease: stages, molecular pathways, and therapeutic implications. Front. Cardiovasc. Med. 11, 1420067 (2024).
Rozenfeld, P. & Feriozzi, S. Contribution of inflammatory pathways to Fabry disease pathogenesis. Mol. Genet. Metab. 122, 19–27 (2017).
Linhart, A. et al. Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur. Heart J. 28, 1228–1235 (2007).
Waldek, S., Patel, M. R., Banikazemi, M., Lemay, R. & Lee, P. Life expectancy and cause of death in males and females with Fabry disease: findings from the Fabry Registry. Genet Med. 11, 790–796 (2009).
Pieroni, M. et al. Cardiac involvement in Fabry disease: JACC review topic of the week. J. Am. Coll. Cardiol. 77, 922–936 (2021).
Wagenhäuser, L. et al. X-chromosomal inactivation patterns in women with Fabry disease. Mol. Genet Genom. Med. 10, e2029 (2022).
Oder, D. et al. α-Galactosidase A genotype N215S induces a specific cardiac variant of fabry disease. Circ. Cardiovasc. Genet. 10, e001918 (2017).
Weidemann, F. et al. Long-term effects of enzyme replacement therapy on fabry cardiomyopathy. Circulation 119, 524–529 (2009).
Pollmann, S., Scharnetzki, D., Manikowski, D., Lenders, M. & Brand, E. Endothelial dysfunction in fabry disease is related to glycocalyx degradation. Front. Immunol. 12, 789142 (2021).
Choi, S. et al. Globotriaosylceramide induces lysosomal degradation of endothelial KCa3.1 in Fabry disease. Arterioscler. Thromb. Vasc. Biol. 34, 81–89 (2014).
Lund, N. et al. Overexpression of VEGFα as a biomarker of endothelial dysfunction in aortic tissue of α-GAL-Tg/KO mice and its upregulation in the serum of patients with Fabry’s disease. Front. Cardiovasc. Med. 11, 1355033 (2024).
Ivanova, M. M., Dao, J., Slayeh, O. A., Friedman, A. & Goker-Alpan, O. Circulated TGF-β1 and VEGF-A as biomarkers for fabry disease-associated cardiomyopathy. Cells 12, 2102 (2023).
Demuth, K. & Germain, D. P. Endothelial markers and homocysteine in patients with classic Fabry disease. Acta Paediatr. Suppl. 91, 57–61 (2002).
Chen, K.-H. et al. Evaluation of proinflammatory prognostic biomarkers for fabry cardiomyopathy with enzyme replacement therapy. Can. J. Cardiol. 32, 1221.e1221–1221.e1229 (2016).
Yogasundaram, H. et al. Elevated inflammatory plasma biomarkers in patients with fabry disease: a critical link to heart failure with preserved ejection fraction. J. Am. Heart Assoc. 7, e009098 (2018).
Braun, F. et al. Enzyme replacement therapy clears Gb3 deposits from a podocyte cell culture model of fabry disease but fails to restore altered cellular signaling. Cell Physiol. Biochem 52, 1139–1150 (2019).
Thurberg, B. L. et al. Cardiac microvascular pathology in Fabry disease: evaluation of endomyocardial biopsies before and after enzyme replacement therapy. Circulation 119, 2561–2567 (2009).
Frustaci, A. et al. Fabry cardiomyopathy: myocardial fibrosis, inflammation, and down-regulation of mannose-6-phosphate receptors cause low accessibility to enzyme replacement therapy. J. Am. Heart Assoc. 14, e036815 (2025).
Alexander, Y. et al. Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on atherosclerosis and vascular biology, aorta and peripheral vascular diseases, coronary pathophysiology and microcirculation, and thrombosis. Cardiovasc. Res. 117, 29–42 (2020).
Rombach, S. M. et al. Vascular aspects of Fabry disease in relation to clinical manifestations and elevations in plasma globotriaosylsphingosine. Hypertension 60, 998–1005 (2012).
Collin, C. et al. Long-term changes in arterial structure and function and left ventricular geometry after enzyme replacement therapy in patients affected with Fabry disease. Eur. J. Prev Cardiol. 19, 43–54 (2012).
Chimenti, C. et al. Angina in Fabry disease reflects coronary small vessel disease. Circulation: Heart Fail. 1, 161–169 (2008).
Elliott, P. M. et al. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart 92, 357–360 (2006).
Hanssen, H., Streese, L. & Vilser, W. Retinal vessel diameters and function in cardiovascular risk and disease. Prog. Retin. Eye Res. 91, 101095 (2022).
Nägele, M. P. et al. Retinal microvascular dysfunction in heart failure. Eur. Heart J. 39, 47–56 (2018).
Li, W. et al. Type 2 diabetes and HbA1c are independently associated with wider retinal arterioles: the Maastricht study. Diabetologia 63, 1408–1417 (2020).
Seidelmann, S. B. et al. Retinal vessel calibers in predicting long-term cardiovascular outcomes: the Atherosclerosis Risk in Communities Study. Circulation 134, 1328–1338 (2016).
Günthner, R. et al. Impaired retinal vessel dilation predicts mortality in end-stage renal disease. Circ Res. 124 (2019).
Günthner, R. et al. Endothelial dysfunction in retinal vessels of hemodialysis patients compared to healthy controls. Sci. Rep. 14, 13948 (2024).
Lavalle, L. et al. Phenotype and biochemical heterogeneity in late onset Fabry disease defined by N215S mutation. PLoS One 13, e0193550 (2018).
Chang, H. C., Kuo, L., Sung, S. H., Niu, D. M. & Yu, W. C. Prognostic implications of left ventricular hypertrophy and mechanical function in Fabry disease: a longitudinal cohort study. J. Am. Soc. Echocardiogr. 37, 787–796 (2024).
Frustaci, A., Chimenti, C., Doheny, D. & Desnick, R. J. Evolution of cardiac pathology in classic Fabry disease: Progressive cardiomyocyte enlargement leads to increased cell death and fibrosis, and correlates with severity of ventricular hypertrophy. Int. J. Cardiol. 248, 257–262 (2017).
Aerts, J. M. et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. 105, 2812–2817 (2008).
Altarescu, G. et al. Enhanced endothelium-dependent vasodilation in Fabry disease. Stroke 32, 1559–1562 (2001).
Shu, L. et al. Decreased nitric oxide bioavailability in a mouse model of Fabry disease. J. Am. Soc. Nephrol. 20, 1975–1985 (2009).
Cobo, G., Lindholm, B. & Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transpl. 33, iii35–iii40 (2018).
Kotliar, K. et al. Altered retinal cerebral vessel oscillation frequencies in Alzheimer’s disease compatible with impaired amyloid clearance. Neurobiol. Aging 120, 117–127 (2022).
Golzan, S. M. et al. Retinal vascular and structural changes are associated with amyloid burden in the elderly: ophthalmic biomarkers of preclinical Alzheimer's disease. Alzheimer’s. Res. Ther. 9, 13 (2017).
Rombach, S. M. et al. Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol. Genet. Metab. 99, 99–108 (2010).
Üçeyler, N. et al. Skin globotriaosylceramide 3 load is increased in men with advanced Fabry disease. PLoS One 11, e0166484 (2016).
Arends, M. et al. Characterization of classical and nonclassical fabry disease: a multicenter study. J. Am. Soc. Nephrol. 28, 1631–1641 (2017).
Frustaci, A. et al. Long-term clinical-pathologic results of enzyme replacement therapy in prehypertrophic Fabry disease cardiomyopathy. J. Am. Heart Assoc. 13, e032734 (2024).
Nordin, S. et al. Myocardial storage, inflammation, and cardiac phenotype in fabry disease after one year of enzyme replacement therapy. Circulation: Cardiovasc.Imaging 12, e009430 (2019).
Jehn, U. et al. α-galactosidase a deficiency in fabry disease leads to extensive dysregulated cellular signaling pathways in human podocytes. Int. J. Mol. Sci. 22, 11339 (2021).
Monda, E. et al. Impact of GLA variant classification on the estimated prevalence of fabry disease: a systematic review and meta-analysis of screening studies. Circulation: Genom. Precis. Med. 16, e004252 (2023).
Lau, K. et al. Gene variants of unknown significance in Fabry disease: clinical characteristics of c.376A>G (p.Ser126Gly). Mol. Genet. Genom. Med. 10, e1912 (2022).
Barbey, F. et al. Cardiac and vascular hypertrophy in Fabry disease. Arteriosclerosis Thrombosis. Vasc. Biol. 26, 839–844 (2006).
Sanchez-Niño, M. D. et al. Lyso-Gb3 activates Notch1 in human podocytes. Hum. Mol. Genet. 24, 5720–5732 (2015).
Altara, R., Mallat, Z., Booz, G. W. & Zouein, F. A. The CXCL10/CXCR3 axis and cardiac inflammation: implications for immunotherapy to treat infectious and noninfectious diseases of the heart. J. Immunol. Res. 2016, 4396368 (2016).
Frustaci, A. et al. Immune-mediated myocarditis in Fabry disease cardiomyopathy. J. Am. Heart Assoc. 7, e009052 (2018).
Shen, J. S. et al. Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Mol. Genet. Metab. 95, 163–168 (2008).
Martinez, P., Aggio, M. & Rozenfeld, P. High incidence of autoantibodies in Fabry disease patients. J. Inherit. Metab. Dis. 30, 365–369 (2007).
Kuchler, T. et al. Persistent endothelial dysfunction in post-COVID-19 syndrome and its associations with symptom severity and chronic inflammation. Angiogenesis 26, 547–563 (2023).
Hubbard, L. D. et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 106, 2269–2280 (1999).
Wong, T. Y. et al. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 111, 1183–1190 (2004).
Giannini, E. H. et al. A validated disease severity scoring system for Fabry disease. Mol. Genet Metab. 99, 283–290 (2010).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Ikram, M. K. et al. Retinal vessel diameters and risk of hypertension. Hypertension 47, 189–194 (2006).
Yanagi, M. et al. Is the association between smoking and the retinal venular diameter reversible following smoking cessation?. Invest. Ophthalmol. Vis. Sci. 55, 405–411 (2014).
Nägele, M. P. et al. Retinal microvascular dysfunction in hypercholesterolemia. J. Clin. Lipido 12, 1523–1531.e1522 (2018).
Köchli et al. Blood pressure, and retinal vessels: a meta-analysis. Pediatrics 141, e20174090 (2018).
Acknowledgements
We want to thank all the patients who participated. Additionally, we want to thank our study nurse, Evi Altmann, and the doctoral students involved in the project: Tarek Assali and Ahed Alachkar.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
C.S., the Chief Investigator, and C.R. conceived the study and led the project. T.W. contributed to the study design, data interpretation and analysis, and drafting of the manuscript. M.L., J.C.L., and A.R. served as lead methodologists for the basic science components and biosample analyses. R.G. contributed to the development of the study and the manuscript. M.W., A.A., and B.H. assisted with figure creation and statistical and data analyses. N.H. supported the analysis of gene variants. H.H., K.K., U.H., and L.S. provided significant expertise in RVA. D.H. played a major role in developing and refining the manuscript. All authors contributed to the manuscript’s development and ensured the accuracy of all parts of the work. All authors read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wallraven, T., Regenbogen, C., Günthner, R. et al. Endothelial dysfunction in Fabry disease: retinal biomarkers link cardiac GLA gene variants with chronic inflammation. npj Genom. Med. 11, 6 (2026). https://doi.org/10.1038/s41525-025-00540-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41525-025-00540-1