Abstract
The escalating global water crisis demands innovative solutions. This review highlights the transformative role of metal–organic frameworks (MOFs) in wastewater treatment, integrating green, scalable synthesis (e.g., mechanochemistry, continuous-flow, supercritical fluid methods) with advanced functionalization. It covers diverse pollutants, performance in real matrices, removal mechanisms, and practical deployment platforms. Key challenges and future directions are discussed, offering a roadmap toward sustainable, industrial-scale MOF applications for water purification.
Similar content being viewed by others
Introduction
Anthropogenic water contamination is a profound threat to global ecosystems and public health, demanding urgent action1. Central to this crisis are emerging organic contaminants (EOCs) that include pollutants from pharmaceuticals (e.g., antibiotics, analgesics), electronic wastes (e-waste), food and beverage additives (e.g., sucralose, synthetic vanillin), personal care products (e.g., triclosan, benzophenone-3), industrial dyes (e.g., methylene blue, malachite green), and pesticides (e.g., atrazine, chlorpyrifos)2,3,4,5,6,7,8,9. These recalcitrant contaminants persist in treated wastewater effluents at concentrations sufficient to trigger severe ecological disruptions and toxicological risks in living systems, owing to their high chemical stability and resistance to conventional treatment processes10,11,12. Conversely, the escalating release of radioactive isotopes such as Cesium-137 (137Cs), Iodine-131 (131I), and Strontium-90 (90Sr), and toxic heavy metals, including cadmium (Cd2+) and lead (Pb2+), from industrial, agricultural, and nuclear activities compounds this peril. Their non-biodegradable nature, prolonged half-lives, and bioaccumulative tendencies pose enduring, systemic threats to biodiversity, water quality, and human well-being13,14,15.
As represented in Fig. 1, traditional wastewater treatment systems (WWTSs), are inadequate to address the complexity of legacy and emerging pollutants, achieving only partial and inconsistent removal efficiencies16,17,18. For instance, activated sludge processes typically remove less than 60% of pharmaceuticals such as carbamazepine, diclofenac, and sulfamethoxazole, owing to their resistance to microbial degradation19,20,21. Filtration and chlorination, while effective against pathogens, fail to mitigate hydrophobic or chemically stable EOCs and are entirely unsuitable for radionuclides like Caesium-137 and Strontium-9019,20,21. Advanced oxidation processes (AOPs), such as ozonation and Fenton reactions, enhances degradation but are energy-intensive and frequently generate toxic transformation products, limiting their practical utility22,23. Similarly, membrane technologies like reverse osmosis (RO) and nanofiltration provide broad-spectrum contaminant removal but are constrained by membrane fouling, high energy costs, and the challenge of managing concentrated brine waste24,25. These critical shortcomings highlight the urgent need for innovative materials that are highly selective, efficient, reusable, scalable, and adaptable to diverse pollutants under variable environmental conditions. Furthermore, a significant portion of domestic (42%) and industrial (~27%) wastewater remains untreated, contributing to ecosystem degradation, greenhouse gas emissions (~1.57% globally), and the persistence of waterborne diseases26,27. Wastewater offers reuse potential of 320 billion m3 annually, exceeding 10 times the current global desalination capacity, and the capacity to generate energy for half a billion people, further highlighting the immense potential of this abundant resource26,27.
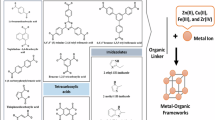
In response to these challenges, metal–organic frameworks (MOFs) have emerged as a transformative platform for advanced wastewater treatment10,28. Composed of metal ions or clusters coordinated with organic linkers, MOFs possess exceptional surface areas (in excess of 6500 m2/g), tunable pore architectures, high crystallinity, and structural modularity29,30,31,32,33. These attributes enable MOFs to capture a broad spectrum of contaminants including EOCs, heavy metals (e.g., Cd2+, Pb2+), and radioactive species (e.g., 137Cs, 90Sr) through mechanisms such as physisorption, chemisorption, ion exchange, and catalytic degradation, capabilities far surpassing traditional WWTs34,35,36,37,38. Recent innovations, including hybrid MOF composites, defect-engineered frameworks, functionalized architectures, and MOF-derived materials (e.g., porous carbons and single-atom catalysts (SACs)) have significantly enhanced their performance and applicability39,40,41,42,43. The integration of MOFs into membranes, electrochemical systems, and modular treatment units further paves the way for practical, real-world deployment39,40,41,42,43.
The seminal review by Rojas et al.12 provided a cornerstone for understanding MOF–EOC interactions, detailing the adsorption and catalytic removal of EOCs using pristine and modified MOFs. However, rapid advancements in MOF chemistry spanning ultrastable frameworks, functionalised architectures, MOF-derived electrocatalysts, and hybrid composites demand a comprehensive reassessment28,44,45,46,47,48. Existing reviews often suffer from limited scope, focusing narrowly on specific applications (e.g., MOF membranes, dye adsorption, or heavy metal removal) while inadequately addressing broader pollutant categories or multifunctional treatment pathways28,44,45,46,47,48. Moreover, critical innovations such as defect engineering, SAC development, and MOF-derived carbonaceous composites are inconsistently integrated, despite their potential to enhance selectivity, reusability, and resilience in practical settings. Equally concerning is the limited emphasis on implementation challenges, including scalability of MOF synthesis, long-term aqueous stability, regeneration strategies, and techno-economic feasibility within existing WWTs infrastructures.
Scope Statement
This review critically explores the convergence of green chemistry, scalable synthesis, and real-world applicability in the development of MOF-based materials for wastewater treatment. The scope encompasses:
-
Eco-friendly and industrially viable MOF synthesis approaches.
-
Advanced material design strategies for improved stability, selectivity, and recyclability.
-
Mechanistic insights into contaminant removal pathways for emerging organic pollutants, heavy metals, and radionuclides.
-
Integration of MOFs into deployable platforms such as membranes, reactors, and composite structures.
-
Evaluation of performance under complex, real-world water matrices.
-
Future directions including techno-economic analysis, interdisciplinary collaboration, and regulatory compliance.
-
By synthesizing current advances and outlining implementation pathways, the review aims to inform the rational development of MOFs as next-generation materials for sustainable water purification systems.
Literature Review
The literature was rigorously curated through searches of academic databases, including ScienceDirect, Google Scholar, Web of Science, Wiley Online Library, and SpringerLink, using keywords such as “metal–organic frameworks,” “wastewater treatment,” “emerging organic contaminants,” “heavy metals,” “radioactive waste,” “MOF composites,” and “electrocatalysis.” Emphasis was placed on peer-reviewed articles and reviews published within the last 5 years (2020 – 2025), prioritizing studies with experimental rigour, mechanistic insights, scalability, or practical relevance. By synthesizing these findings, this work bridges the gap between foundational research and future-oriented strategies, establishing a robust framework for scalable, sustainable MOF-based water purification.
Green Strategies In Synthesis Of Mofs
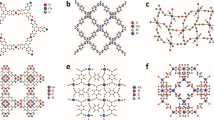
Over the years, a diverse array of solids, collectively known as metal–organic frameworks (MOFs), coordination polymers, hybrid organic–inorganic materials, or organic zeolite analogues, have been developed, characterized by metal ions or clusters interconnected by organic ligands49,50,51,52,53. These terms, while overlapping, carry distinct connotations: MOFs emphasize robust, crystalline frameworks with modifiable organic linkers and well-defined geometries, whereas coordination polymers broadly describe any extended metal–ligand coordination network, regardless of structure or crystallinity49,50,51,52,53. A key distinction lies in composition. For example, MOFs often feature large inorganic clusters linked by short ligands, contrasting with coordination polymers that may involve isolated metal cations and complex organic linkers (exemplified in Fig. 2)49,50,53,54,55.
a Series of MOFs derived from the dicopper paddle-wheel secondary building unit (SBU), Cu2(COO)4, demonstrating systematic pore size modulation achieved by varying the length and topicity of m-benzenedicarboxylate-based organic linkers. The structure labelled HKUST-1 (also known as Cu–BTC or MOF-199) is included as a reference. Atom colour scheme: Cu (blue), O (red), C (grey), H atoms are omitted for clarity. Pore spaces are visualized using semi-transparent green, blue, and pink spheres. Reused with permission from ref. 55. Copyright 2014, Royal Society of Chemistry. b Structural representation of the mmen–Mg2(dobpdc) MOF system: (I) Fundamental building blocks comprising (Ia) Mg2+ metal centres (green), (Ib) dobpdc linker molecules (grey/red), and (Ic) appended mmen ligands (blue/white); (II) Periodic minimum-energy configuration from DFT simulations viewed along the ab-plane; (IIIa,b) Molecular fragments used for cluster model calculations, cut either along the c-axis (IIIa) or across the ab-plane (IIIb). The organic linker dobpdc is 4,4′-dioxidobiphenyl-3,3′-dicarboxylate, and mmen refers to N,N′-dimethylethylenediamine (CH3NHCH2CH2NHCH3). Reproduced from ref. 54. Copyright 2013, American Chemical Society.
The concept of green synthesis for MOFs is grounded in the principles of green chemistry, which aim to reduce environmental impact by minimizing hazardous substances, energy consumption, and waste56. Green MOF synthesis promotes the use of benign solvents (e.g., water, ethanol, supercritical CO2), room temperature or microwave-assisted reactions, solvent-free mechanochemical routes, and recyclable reaction media such as ionic liquids or deep eutectic solvents. These approaches not only mitigate ecological and health risks but also facilitate scale-up by lowering cost, enhancing process safety, and aligning with circular economy goals57,58,59. The following sections examine green synthesis routes with emphasis on their scalability, performance, and applicability in wastewater treatment systems.
To qualify as an MOF, the material must exhibit high crystallinity, structural robustness, and organic linkers amenable to synthetic modification, enabling precise structure–property correlations49,50,51,52,53,54,55. Crystallinity facilitates the systematic analysis of MOF architectures, revealing connectivity trends and enabling reticular synthesis, a strategic approach to designing targeted frameworks with predictable topologies, a paradigm largely unique to MOFs49,51,52,54,55. The rational design capability underscores MOFs’ transformative potential in applications requiring tailored properties. In the context of water treatment, pristine MOFs have garnered significant interest as emerging electrocatalysts, owing to the strategic placement of catalytic sites within either the metal nodes or organic linkers60. Intrinsic modularity enables the precise design of redox-active centres capable of facilitating advanced electrochemical processes, such as contaminant degradation and reactive oxygen species (ROS) generation61,62,63,64. Beyond the native catalytic function, the well-defined porous architecture of MOFs offers an ideal platform for hosting additional active species including metal complexes, polyoxometalates (POMs), and conductive substrates thereby allowing for the fabrication of MOF-based hybrid systems60,62,63. Resultant synergistic interactions between the framework and guest components often lead to enhanced charge transfer, increased catalytic turnover, and improved stability under aqueous conditions, making these hybrid systems particularly promising for electrochemical removal of persistent organic pollutants and emerging contaminants in water60,61,64.
Scalable and green MOF fabrication techniques
Achieving scalable and genuinely eco-friendly synthesis of MOFs is a critical prerequisite for translating their promising laboratory-scale performance into viable, large-scale applications. Traditional solvothermal batch syntheses, which have dominated early MOF research, frequently rely on high-boiling point, toxic organic solvents, with N,N-dimethylformamide (DMF) being a particularly common example49,65,66,67. The use of such solvents raises significant environmental, health, and safety concerns, alongside disposal costs68,69. Besides, these conventional batch methods often suffer from inherent limitations regarding scalability due to reactor size constraints, inefficient heat and mass transfer, and difficulties in achieving consistent batch-to-batch reproducibility, hindering process control and quality assurance70,71. It has been estimated that a vast majority, potentially over 90%, of the MOFs reported in the literature have been synthesized using hazardous solvents, significantly impeding their path towards environmentally compliant and economically feasible commercial adoption70,71. In response to these challenges, innovations have focused intently on developing greener and more scalable synthetic routes. This section will discuss some of the recent strategic innovative methods for the synthesis of MOFs.
Continuous-flow methodologies
Utilizing microreactors or tubular reactors, offer significantly improved control over reaction parameters, enhanced heat and mass transfer rates leading to faster kinetics and potentially higher yields, and lend themselves readily to automation and large-scale production72,73. It has been demonstrated that implementing continuous flow chemistry in MOF synthesis significantly enhances yield and throughput while markedly reducing solvent consumption74. For example, the volumes of N,N-dimethylformamide and formic acid required for producing MOF-808 are lowered by 84% and 67%, respectively, through the use of flow-through reactors. This technique also achieved productivity improvements by nearly two orders of magnitude, with comparable yields to conventional batch processes75. Given that solvent usage heavily influences the cost of MOF production, this strategy offers a more cost-effective and scalable alternative for industrial applications74,75. Moreover, microfluidic flow systems, a subset of flow chemistry, have shown additional potential for reducing costs during scale-up phases74,75. Beyond cost efficiency, scaling up MOF production is feasible through parallelization, an approach where multiple identical reaction channels operate simultaneously, thereby boosting output76,77,78. Additionally, since MOF crystallization is typically the rate-limiting step, this process can be optimized using droplet-based methods. In this technique, each droplet acts as a self-contained microreactor, enabling precise control over crystallization kinetics76,77,78. By restricting reaction volumes to the microliter scale, the system benefits from an elevated surface-area-to-volume ratio, which facilitates faster heat and mass transfer, ultimately improving uniformity and reaction speed77,78. Nevertheless, many MOF syntheses conducted in droplet microfluidic systems rely on organic solvents such as dimethylformamide (DMF), methanol, and others (substances that are not only costly but also environmentally harmful)76,77,78. Consequently, there is growing interest in replacing these with water, a safer, more cost-effective, and sustainable solvent for MOF synthesis.
Wu et al.79 introduce a water-based droplet microfluidic system that enables the continuous and ultrafast synthesis of two representative MOFs, ZIF-8 and MIL-100, under significantly milder and environmentally friendly conditions. The study’s most significant findings include the successful synthesis of highly crystalline ZIF-8 and MIL-100 within 24 to 160 s, considerably shorter than the 200–300 min required by traditional hydrothermal routes79. This was achieved through precise control of microfluidic parameters such as flow rate, residence time, and temperature. The resulting MOFs exhibited superior textural properties, including a higher BET surface area (e.g., 1844 m2/g for microfluidic ZIF-8 vs. 1302 m2/g for hydrothermal ZIF-8 as shown in Fig. 3a) and improved uniformity, as indicated by a significantly lower polydispersity index. Another landmark achievement of this study is the first reported application of de novo synthesis in a continuous microfluidic system to produce gold-encapsulated ZIF-8 (Au@ZIF-8), achieving an unprecedented 80% metal retention as shown in Fig. 3b. This addresses a major drawback of previous post-synthetic metal loading techniques, which typically suffer from low encapsulation efficiency and surface deposition of metal nanoparticles79. The study demonstrates the feasibility of coupling microfluidic nanoarchitectonics with green chemistry principles to enable scalable, high-throughput, and reproducible MOF production. Significantly advancing the process intensification strategies by presenting a flow regime map for droplet generation that prevents common issues such as coalescence and channel clogging. Additionally, it lays foundational insights for process parameter optimization, such as precursor ratios and flow velocities, which are critical for achieving monodispersed products with high crystallinity and surface functionality. The introduction of a water-only solvent system in the microfluidic context further aligns with the global push toward sustainable materials manufacturing. Nonetheless, while the two-phase system reduces reliance on organic solvents, the use of perfluoropolyether (PFPE) oil as a continuous phase introduces a non-trivial processing step that may pose scalability and recyclability challenges. Hence, despite demonstrating successful synthesis for ZIF-8 and MIL-100, the generalizability of this system to a broader range of MOFs, especially those requiring more complex ligands or multi-metal nodes, remains to be validated. Additionally, while the de novo method for Au@ZIF-8 is innovative, the absence of catalytic performance testing, limits the assessment of the material’s real-world functionality and application potential. Therefore, future directions could include expanding the microfluidic approach to a wider library of MOFs and MOF composites, integrating inline monitoring tools such as optical or electrochemical sensors for real-time quality control, and scaling up via multiplexed reactor arrays. Moreover, coupling machine learning algorithms with flow control could enable autonomous synthesis platforms tailored to specific MOF properties.
a Comparative analysis of ZIF-8 synthesized via hydrothermal and microfluidic methods at room temperature, highlighting differences in particle size distribution as determined by dynamic light scattering (DLS). The blue and red curves represent hydrothermal and microfluidic products, respectively, with the microfluidic synthesis yielding a narrower size distribution and enhanced uniformity at a total precursor flow rate of 200 μL min−1. b Characterization of Au@ZIF-8 synthesized via the droplet-based microfluidic de novo approach. a XRD patterns confirm successful gold encapsulation without altering the ZIF-8 crystal structure. b–d SEM-EDX elemental mapping reveals homogeneous dispersion of gold nanoparticles within the ZIF-8 matrix. e TEM imaging shows the morphology and size distribution of embedded gold nanoparticles, demonstrating the efficacy of the microfluidic de novo strategy for high metal content MOF synthesis. Reproduced from ref. 79. Copyright 2023, Royal Society of Chemistry.
Due to the inadequate performance of conventional micromixing strategies, which often suffer from poor mixing efficiency across variable Reynolds numbers (Re), prolonged mixing distances, and long mixing times, which hamper the practical deployment in scalable MOFs80,81, Tan et al.82 propose a strategic innovative approach to overcoming these limitations by developing a 3D homogeneous microreactor (3D-HM) that combines optimized 2D and 3D secondary flow structures, shunt geometries, and zigzag channel designs. They demonstrate that this microreactor achieves superior mixing intensity across a wide Re range (Re = 0–100), with mixing indices exceeding 98% at Re > 10 and over 94% at Re = 0 – 10. This is accompanied by reduced mixing time (τmix ≈ 3.4 ms) and mixing distance (Τmix ≈ 7.2 mm), outperforming traditional T-shaped and split-and-recombine (SAR) microreactors82. In terms of material performance, the ZIF-8 MOFs synthesized using the 3D-HM exhibited comparatively improved properties, including higher yield, smaller and more uniform particle size (most < 100 nm at Re = 500)82. This indicates that the synthesized MOFs are not only structurally robust but functionally enhanced. The method offers multiple merits, for example, by leveraging 3D secondary flow engineering, it introduces controlled, multidirectional Dean vortices that disrupt laminar flow and enhance mixing through both chaotic advection and diffusion, thereby allowing the microreactor to be universally applicable across a wide Re spectrum, addressing one of the core scalability challenges in microfluidics82. The approach significantly contributes to the development of greener and more scalable synthetic routes by enabling efficient synthesis under mild conditions, hence reducing energy input, operational costs, and dependence on high-pressure equipment or harsh solvents82. However, the study presents certain limitations. For example, the synthesis and performance evaluations were conducted primarily on ZIF-8 as a model MOF, so generalizability to other MOF types remains to be demonstrated. Additionally, although the 3D-HM performs well across various Re values, there is a potential trade-off in pressure drop and device fabrication complexity due to the intricate geometry, which we argue may hinder its mass production. Notwithstanding, looking ahead, the prospects for this technique is highly favourable. With the rise of modular, portable, and energy-efficient chemical synthesis platforms, the 3D-HM could be integrated into automated flow synthesis units, facilitating on-demand MOF production. Furthermore, future research could explore multi-MOF or hierarchical MOF synthesis, multi-enzyme cascade reactions, or integration with AI-based flow controllers for real-time optimization.
In furtherance of green chemistry in MOF fabrication, the use of supercritical CO2 (scCO2) offers a strategically innovative contribution to the field of MOF synthesis by addressing critical bottlenecks in sustainability, scalability, and reaction efficiency. For example, the development of a continuous-flow reactor system (setup in Fig. 4) utilizing scCO2 as the mixing and heating medium for the synthesis of UiO-66, a benchmark zirconium-based MOF, achieved a record production rate of 104 g·h−1 with a reaction time under 3 s, surpassing the throughput and efficiency of previously reported batch and flow synthesis strategies83. Moreover, the synthesized UiO-66 exhibited well-defined crystalline structure, high porosity (BET surface area of ~1109 m2·g−1), and favourable particle size distribution (~211 nm), confirming the method’s ability to produce MOFs with comparable or improved material properties relative to conventional synthesis routes83. The use of scCO2 brings key merits in strategic MOF design and green chemistry. For example, compared to supercritical water (scH2O), scCO2 has a lower critical temperature and pressure (304 K, 7.31 MPa), drastically reducing energy consumption and eliminating precursor degradation issues83. This enhances compatibility with a broader range of organic ligands and metal salts. The counter-current mixing (CCM) design employed in the reactor ensures rapid and efficient heat and mass transfer, leading to uniform mixing and fast nucleation83. Importantly, the process allows for natural separation and recycling of CO2 and DMF, which is rare in other systems, significantly minimizing solvent waste and enabling a closed-loop approach to MOF manufacturing83. The method offers a scalable MOF synthesis platform that satisfies key criteria for industrial deployment: speed, yield, safety, and sustainability. It also demonstrates the dual role of scCO2 as a synthesis and activation medium, which eliminates the need for prolonged post-synthetic degassing, commonly a major bottleneck in MOF processing83. This sets the foundation for future research into one-pot MOF synthesis-activation schemes and streamlined downstream processing. However, the study acknowledges several limitations. Firstly, while UiO-66 serves as a useful model system, the applicability of this method to other classes of MOFs, especially those containing sensitive or larger organic linkers, remains unproven83. Secondly, the system requires precise control of flow rates and temperature to maintain scCO2 stability and optimize mixing, which could present operational challenges at larger industrial scales. Lastly, though the study evaluates energy savings and solvent recyclability, it does not conduct a full life-cycle assessment (LCA) or techno-economic analysis (TEA), which are necessary to comprehensively evaluate industrial feasibility83. Nevertheless, as the demand for modular and scalable synthesis increase, this method could be extended to synthesize other MOFs such as ZIFs, MILs, or HKUSTs under low-energy conditions. Future research may focus on coupling this system with machine learning-driven process optimization, in situ reaction monitoring, or multi-material synthesis for composite or hierarchical MOFs. Moreover, given its alignment with circular economy principles and green engineering, this method holds strong potential to serve as a platform technology for sustainable nanomanufacturing.
The diagram identifies key components including the thermocouple (TC), counter-current mixer, heating elements, and back-pressure regulator (BPR), which collectively enable rapid mixing, efficient heat transfer, and stable supercritical conditions necessary for scalable MOF production. Reproduced from ref. 83. Copyright 2020, American Chemical Society.
Mechanochemistry (ball-milling)
Mechanochemical synthesis has gained prominence as a highly promising and environmentally friendly alternative to conventional methods for producing MOFs with exceptional properties suitable for advanced applications such as wastewater treatments, among others57,58. Characterized by its simplicity, excellent reproducibility, and typically solvent-free conditions (ball milling), this approach enables rapid synthesis under mild conditions, aligning well with green chemistry principles (Scheme 1)57,58,59. Recent advances demonstrate that mechanochemistry can facilitate the efficient assembly of MOFs with desirable textural and structural features, often unattainable via traditional solvothermal or hydrothermal routes59,84. For example, to overcome inherent limitations of conventional post-synthetic MOF modification and post-synthetic ligand/metal ex-change (PSLE/PSME), the mechanochemical presents a strategically rational advantage in MOF design by enabling tailored incorporation of functional groups without disrupting the parent framework coupled with the merits of solvent-free, rapid, and scalable, achieving of chiral MOFs in short time, which is important for industrial translation. This method provides flexibility for the construction of multivariate and solvent-sensitive MOFs (e.g., ZIF-L), previously unattainable via classical strategies59,84.
Merits of the mechanochemical technique includes mechanically grinding pre-synthesized MOFs with activated functional ligands, enabling efficient ligand exchange without compromising the original framework structure. This solvent-free approach integrates the benefits of mechanochemistry and post-synthetic modification, allowing rapid and scalable functionalization of MOFs under mild conditions favourable for sustainable development. Reproduced from ref. 59. Copyright 2023, American Chemical Society.
Jin et al.85 present a compelling study that addresses longstanding challenges in the synthesis of chiral metal–organic frameworks (CMOFs) by integrating mechanochemistry, defect engineering, and a truncated mixed-ligand strategy. Driven by the need for a facile, green, and scalable approach to CMOF construction, especially for applications in enantioselective catalysis and chiral separation, the authors devised a method that combines high-energy ball milling with partial replacement of the parent ligand (H3BTC) by structurally similar chiral fragments, specifically varying amounts of (S)-5-(pyrrolidine-2-carboxamido) isophthalic acid (IPA-L-Pro)85. This strategy enables the retention of the parent framework’s topology while introducing chirality and deliberate structural defects. These defects promote the formation of hierarchical micro/mesoporous architectures and enhance catalytic accessibility. In contrast to conventional solvothermal methods, the proposed route preserves crystallinity, shortens reaction time to under 1 h, eliminates solvent waste, and achieves gram-scale yields85. A series of Cu-BTC-derived chiral MOFs (e.g., Cu-BTC-P-6) were synthesized, with PXRD analyses (Fig. 5) confirming structural integrity despite ligand substitution. Among them, Cu-BTC-P-6 demonstrated excellent performance in the asymmetric Aldol reaction, achieving 97.1% conversion and 44.3% enantiomeric excess (ee), values comparable to the homogeneous catalyst IPA-L-Pro, while retaining its activity and crystallinity over five catalytic cycles85. These results underscore the practical viability and catalytic potential of the mechanochemically synthesised CMOFs.
A PXRD patterns of Cu-BTC-P-1 obtained at different milling durations, showing optimal crystallinity at 1 h. B PXRD comparison of Cu-BTC-M and Cu-BTC-P-X samples synthesized mechanochemically, indicating structural retention consistent with simulated Cu-BTC. Reproduced from ref. 85. Copyright 2022, American Chemical Society.
Tegudeer et al.86 provide a pivotal contribution to the field of MOF synthesis by establishing a direct, scalable, and green approach for integrating hydrolysis-sensitive imine-based ligands into MOF architectures through solid-state mechanochemistry. This innovation addresses a longstanding challenge in MOF chemistry: the limited accessibility of imine-based MOFs (IMOFs), whose practical application has been hindered by the susceptibility of imine bonds to hydrolytic degradation under conventional solvothermal conditions. While imine linkages offer desirable features such as modular tunability, dynamic covalent character, and Lewis basicity, their deployment in framework construction has remained constrained due to poor stability during high-temperature synthesis. Conventional strategies, such as solvothermal linker exchange or in situ condensation, have typically yielded low efficiencies ( ≤27%), extended reaction durations, and frequent decomposition of imine functionalities, underscoring the need for a robust, high-yield, and sustainable synthetic platform86. To overcome these limitations, the authors implement a mechanochemical tandem reaction strategy that unifies imine ligand formation and MOF crystallization into a single, solvent-minimized operation. This technique harnesses mechanical force to facilitate both Schiff base condensation and metal–ligand coordination under ambient conditions, eliminating the need for pre-isolated ligands or harsh thermal inputs. Unlike previous approaches, this method achieves exceptional yields (up to 93%) and dramatically reduces synthesis time to 90 min, while operating at room temperature and minimal solvent levels. The result is a sustainable and scalable platform that aligns with industrial green chemistry principles. They successfully synthesized a diverse library of Zr-, Hf-, and Cu-based imine MOFs, including previously inaccessible analogues of PCN-161 and MOF-50586. Structural and textural analyses confirmed high crystallinity, thermal stability, and porosity, with BET surface areas exceeding 2500 m2/g in some Cu-MOFs. Furthermore, CO2 adsorption studies revealed enhanced binding affinity in imine-functionalized MOFs (Qst = 24.5 kJ/mol), surpassing that of analogous azo-linked systems (Qst = 23.2 kJ/mol), indicating superior Lewis basicity. Notably, the imine condensation was shown to benefit from autocatalytic promotion, where protons released from acetate-to-carboxylate substitution catalyse the imine bond formation, further enhancing efficiency in the cascade synthesis process86. However, the report demonstrates some key limitations. For example, the mechanochemically produced MOF particles are typically <300 nm, precluding single-crystal X-ray diffraction and complicating structural resolution. Additionally, not all ligand–metal combinations proved compatible; for instance, the direct mechanochemical synthesis of [Zr6O4(OH)4(L5)6] failed and required post-synthetic ligand exchange, suggesting scope-dependent limitations. Moreover, while tandem synthesis offers operational simplicity, it may introduce competing reaction pathways, especially when utilizing complex or multifunctional precursors, potentially affecting reproducibility86. Hence, future opportunities should focus on scaling up by extending the approach to other labile dynamic bonds (e.g., hydrazones, oximes) and integrating with computational screening and AI-driven design to systematically explore the vast chemical space of imine-based linkers. The concept also opens avenues for tunable gas storage materials, stimuli-responsive systems, and enantioselective catalysts, where imine functionalities offer unique advantages. As such, the study presents a paradigm shift in sustainable framework chemistry, redefining how fragile organic motifs can be integrated into stable, porous architectures through strategic, green design.
Microwave-assisted synthesis
Microwave-assisted synthesis has emerged as a powerful and environmentally friendly technique for the rapid fabrication of MOFs, offering significant advantages over conventional solvothermal methods. Unlike traditional heating, which relies on slow thermal conduction, microwave irradiation delivers rapid, volumetric, and uniform heating, drastically accelerating crystallization kinetics and reducing synthesis times from several hours to just minutes87,88. This approach promotes faster nucleation rates, yielding smaller, more uniform MOF crystals with enhanced textural and functional properties. The high energy efficiency and minimal solvent requirements of microwave synthesis align well with green chemistry principles, making it highly suitable for scalable and sustainable MOF production87,88. A compelling example is the microwave-assisted solvothermal synthesis of bismuth-based MOFs (Bi-MOFs) using various carboxylate ligands89. Among the synthesized materials, Bi-TATB (Bismuth–Triazine-2,4,6-triyl-tribenzoate MOF) demonstrated superior photocatalytic performance, attributed to its exceptionally high surface area (355 m2/g), abundant oxygen vacancies, and enhanced visible-light absorption. These features are directly linked to the π-conjugated structure of the ligand H3TATB (4,4’,4’’-s-Triazine-2,4,6-triyl-tribenzoic acid), which facilitates efficient ligand-to-metal charge transfer (LMCT) and reduces the band gap, thereby improving light harvesting and charge carrier dynamics89. This highlights how the synergistic integration of ligand engineering and microwave synthesis can be strategically leveraged to tailor MOF properties for advanced applications such as photocatalysis. Thus, reinforcing the growing consensus that microwave-assisted methodologies offer a scalable, time-efficient, and greener alternative for producing high-quality MOFs, particularly those incorporating complex or hydrolytically sensitive linkers.
Elsewhere, researchers employed a microwave-assisted hydrothermal method to synthesize Fe–Cu bimetallic MOFs (MW-53(Fe,Cu)-X), marking a notable improvement over conventional solvothermal synthesis90. The microwave irradiation delivered rapid, uniform internal heating, promoting faster nucleation, reduced reaction time, and better particle uniformity. Importantly, it enhanced metal ion substitution dynamics, allowing isomorphous replacement of Fe by Cu and the formation of Fe–O–Cu heteroatomic linkages. The approach contrasts with traditional methods which often yield inhomogeneous doping, lower crystallinity, and reduced catalytic efficiency. Compared to the parent MOF MIL-53(Fe), the microwave-synthesized MOFs exhibit enhanced crystallinity, mesoporosity, and uniform metal dispersion, essential features for efficient catalytic activation90.
In one of the first systematic studies to directly compare the same MOF structure synthesized via solvothermal and microwave-assisted methods, Jung et al.91 provide a compelling demonstration of how microwave-assisted synthesis (MAS) can drive both strategic morphological control and application-specific performance enhancement in MOFs. Through an integrated structure–function analysis and characterizations, the authors reveal how synthetic route selection profoundly influences crystallinity, particle size, surface area, and dispersibility, all of which directly impact sorption kinetics and extraction efficiency91. Most notably, reaction time was reduced by a factor of 504, from 7 days to just 20 min, while maintaining chemical and structural integrity. The resulting Cu–S MOF microrods exhibited a ~ 145-fold reduction in crystal length (from 549 µm to ~3.8 µm), significantly improving dispersibility in aqueous media and enabling faster analyte access to active sites. Additionally, the surface area increased more than threefold, from 3.31 m2/g to 10.5 m2/g, attributed to rapid nucleation and size minimization under microwave conditions91. Importantly, the work transcends conventional MOF applications such as gas adsorption or catalysis, demonstrating for the first time the utility of microwave-synthesized MOFs in the highly relevant and underexplored field of food safety and trace pharmaceutical monitoring; thereby setting a benchmark for functional MOF design tailored for rapid, real-world analytical workflows, highlighting MAS as a transformative tool in advanced material synthesis.
It is increasingly important to explore the integration of multiple green synthesis strategies to advance the sustainable and scalable fabrication of MOFs. A particularly transformative development in this direction is the coupling of continuous-flow (CF) processing with microwave-assisted (MW) synthesis, a method that holds promise to redefine the landscape of MOF production by overcoming critical limitations associated with conventional solvothermal and batch microwave approaches. In a landmark study, Taddei et al.92 demonstrated the industrial feasibility and process intensification potential of this technique by synthesizing three benchmark MOFs (UiO-66, MIL-53(Al), and HKUST-1) using a modular CFMW reactor setup (Scheme 2), achieving exceptional synthesis rates, yields, and material quality. Notably, UiO-66 was synthesized in just 7 min at 280 W, achieving a 94% yield, surface area of 1052 m2/g, and a record-breaking space–time yield (STY) of 7204 kg·m−3·d−1. MIL-53(Al) was obtained with a 65% yield, surface area of 1376 m2/g, and an STY of 3618 kg·−3·d−1, representing the highest productivity reported for microwave synthesis of this framework. Even more impressively, HKUST-1 reached quantitative conversion within just 1 min, with a 96% yield, surface area of 1550 m2/g, and an astonishing STY of 64,800 kg·m−3·d−1, surpassed only by mechanochemical extrusion methods92. The strategic merits of this CFMW approach are multifold: (1.) enabling unprecedented synthesis speeds, facilitating rapid screening and high-throughput optimization of MOF libraries, (2.) delivers superior STY values that far exceed prior methods, particularly for UiO-66 and MIL-53(Al), while maintaining consistent quality across variable reactor volumes (29.6–53 mL)92. The process promotes solvent economy and atom efficiency, operating with stoichiometric reagent ratios and minimal use of modulators such as acetic acid. Furthermore, it exemplifies principles of green chemistry by minimizing energy input, reducing chemical waste, and eliminating the need for hazardous precursors (e.g., halide salts or electrochemical mediators)92. Its successful application to multiple prototypical MOFs highlights its broad applicability, operational safety, and commercial potential, positioning CFMW as a model system for next-generation MOF production in academic, industrial, and environmental domains.
Schematics of the experimental setup for the continuous-flow microwave-assisted synthesis of MOFs. The system comprises a reagent feed mechanism using syringe pumps, a tubular reactor positioned within a microwave cavity for uniform volumetric heating, and a back-pressure regulator to control system pressure. The configuration enables precise adjustment of flow rate, temperature, and residence time, facilitating rapid and scalable MOF crystallization under continuous operation. Reproduced from ref. 92. Copyright 2016, John Wiley & Sons.
Miscellaneous
Over the years, several green synthesis approaches have emerged for the strategic and rational design of MOFs, a few are summarised in Table 1, excluding those already discussed. Additionally, Table 2 provides a side-by-side comparative summary of mentioned MOF synthesis methods, based on key metrics such as energy efficiency, solvent usage, scalability, reaction time, crystallinity control, and alignment with green chemistry principles.
In summary, the development of a diverse and powerful synthetic toolbox is driving a paradigm shift toward greener, more scalable, and application-ready MOF production. Emerging technologies such as continuous-flow reactors, microwave-assisted synthesis, and spray-drying platforms are enabling process intensification while preserving crystallinity, porosity, and functional integrity. Mechanochemical and solvent-free approaches eliminate hazardous solvents entirely, aligning with green chemistry principles and enhancing atom economy. Meanwhile, the adoption of benign media including water, bio-derived solvents, and supercritical CO2 for synthesis and activation underscores a deep commitment to environmental stewardship. Collectively, these innovations are dismantling long-standing barriers related to cost, safety, and industrial viability, transforming MOFs from laboratory curiosities into practically deployable materials. However, key challenges remain, particularly in achieving consistent product quality at scale and broadening the chemical scope of frameworks accessible via green methods. However, the accelerating progress in synthetic strategy and process engineering is closing the gap between laboratory discovery and real-world implementation, especially in critical sectors such as wastewater treatment, CO2 capture, and sustainable catalysis. As the field moves forward, the convergence of materials innovation, green process design, and scale-up readiness is set to define the next generation of MOF-based technologies.
It is deducible from Table 2, that among the green synthesis methods reviewed, mechanochemistry and continuous-flow microwave-assisted synthesis (CFMW) stand out as the most suitable for large-scale production. Mechanochemistry offers high atom economy, minimal solvent use, and excellent scalability via extrusion processes. CFMW synthesis combines rapid reaction times with high space–time yields and consistent material quality, making it industrially attractive. While other methods (e.g., sonochemical, ionothermal) offer niche advantages, they often require specialized setups or face reproducibility and scalability challenges. Hence, mechanochemistry and CFMW provide the best balance of efficiency, environmental compatibility, and industrial feasibility.
Furthermore, to support practical translation of green MOF synthesis strategies from laboratory-scale research to industrial-scale deployment, a general workflow for scaling up MOF production is proposed and illustrated in Fig. 6. This flowchart distils the essential stages involved in developing scalable, eco-friendly MOFs, from the rational selection of green synthesis routes (e.g., mechanochemistry, continuous flow) to integration into functional platforms and technoeconomic validation. It emphasizes modularity, reproducibility, and sustainability, providing a structured overview that complements the detailed methodologies discussed earlier. The inclusion of this workflow offers a visual summary and strategic roadmap for researchers and engineers aiming to bridge the gap between innovation and real-world implementation in water treatment technologies.
The schematic outlines key stages from the selection of eco-friendly synthesis routes through to quality control and technoeconomic assessment. It highlights critical steps for ensuring reproducibility, process efficiency, and environmental sustainability, serving as a roadmap for translating MOFs into practical wastewater treatment solutions. Original image by the authors.
It is noteworthy that, as MOF production scales up, maintaining product quality becomes critical. Key quality parameters include phase purity, crystallinity, surface area, and porosity, which can be affected by rapid heating, inconsistent mixing, or impurities in reagents. Techniques such as real-time process monitoring, in-line particle size analysis, and batch consistency tests (e.g., BET, PXRD, ICP-OES) are essential for quality control. Surface area and porosity, in particular, are influenced by synthesis rate and post-synthetic treatment. Green synthesis platforms must integrate modular reactors, controlled activation steps, and validated purification strategies to ensure reproducibility and performance.
Finally, from the discourse, it is evident that activation and purification are critical yet often overlooked steps in scalable MOF production. Activation typically involves solvent removal or pore opening to expose the MOF’s internal surface area. Green alternatives include supercritical CO2 drying, vacuum-assisted thermal activation at mild temperatures, and microwave drying, which reduce energy use and solvent waste. For purification, replacing DMF washes with ethanol, water, or bio-based solvents minimizes environmental impact. Closed-loop solvent recycling systems and membrane-assisted filtration can further improve sustainability. Developing integrated one-pot synthesis–activation systems is an emerging area that could streamline these steps for scale-up applications.
Mechanisms of water–stable MOFs in contaminant removal
In realistic implementation of MOFs in wastewater treatment, hydrolytic stability and structural integrity is a fundamental requirement. The ability of a MOF to resist degradation in aqueous environments directly influences its operational lifespan, reusability, and reliability in complex water matrices93,94,95. Progress have converged on multiple complementary strategies to address this challenge, with metal–ligand bond strength emerging as a central design criterion96,97. For instance, guided by the Hard and Soft Acids and Bases (HSAB) theory, frameworks have been composed of high-valence “hard” metal clusters such as zirconium (IV) (Zr4+), aluminium (III) (Al3+), and chromium (III) (Cr3+) paired with hard-base carboxylate ligands to form exceptionally robust metal–oxygen (M–O) coordination bonds94,95,98. Similarly, soft–soft pairings, such as zinc (II) (Zn2+) coordinated with imidazolate linkers, as exemplified in zeolitic imidazolate frameworks (ZIFs), also yield chemically stable architectures93,94,95. Beyond intrinsic bond strength, hydrophobicity and steric shielding offer kinetic barriers to hydrolysis; incorporation of long-chain alkyl groups, trifluoromethyl (CF3) substituents, or surface modification with polydimethylsiloxane (PDMS) coatings creates water-repellent pore environments that hinder the diffusion of water molecules and reduce the probability of ligand displacement93,94,95. In parallel, defect engineering, through controlled missing-linker strategies, enhances porosity and exposes coordinatively unsaturated metal sites (CUSs), sites that are not fully saturated by ligands, thus improving accessibility for adsorption and catalytic reactions. While such defects can sometimes lower stability, they are advantageous when carefully optimized93,94,95. Another powerful strategy is post-synthetic functionalization, wherein chemically stable MOFs are modified with polar functional groups such as amine (–NH2), carboxyl (–COOH), or sulfonic acid (–SO3H) groups. These modifications introduce hydrogen-bonding and ionic interaction sites without necessarily compromising the structural integrity of the framework93,94,95,96. For instance, MIL-101(Fe)–NH2, an amine-functionalized derivative of the iron-based Materials of Institut Lavoisier (MIL-101), maintains nearly identical resistance to water, acid, and base as its unmodified counterpart93,94,95.
Taken together, these design principles, have enabled the development of MOFs that remain structurally intact under prolonged aqueous exposure. Hence, subsequently a summary of recent advances of this convergence of chemical robustness and selective physicochemical functionality toward realizing sustainable and high-performance MOF-based technologies for real-world wastewater contaminant remediation are presented.
Ion-exchange and coordination
In addressing the formidable challenge of removing the radioactive and environmentally persistent 99TcO4− anion from legacy defence nuclear waste, Shen et al.99 designed and characterised a two-dimensional cationic metal–organic framework (MOF), SCU-103, with exceptional stability and functional selectivity under extreme chemical and radiological conditions. A pivotal contribution of the study lies in elucidating the precise mechanism by which SCU-103 captures and retains the target pertechnetate species. A combination of experimental validation and computational modelling, establish anion exchange and not coordination or chelation as the dominant mechanism of action governing the uptake of 99TcO4− 99. The SCU-103 framework is constructed from Ni2+ ions and a neutral tridentate nitrogen ligand–tris[4-(1H-imidazol-1-yl)-phenyl]amine (tipa), yielding a positively charged 2D architecture with NO3− counterions residing in the internal cavities (shown in Fig. 7a). These nitrate anions are freely exchangeable and serve as placeholders for the selective uptake of tetrahedral oxoanions. Upon exposure to 99TcO4− (or its nonradioactive surrogate, ReO4−), ion exchange occurs via electrostatic attraction, whereby the incoming target anions displace the framework-bound nitrates99. No direct metal–ligand interaction between TcO4− and the Ni2+ centres was detected, as the metal ions are fully coordinated in a saturated octahedral environment with no accessible coordination sites. This rules out chelation or direct coordination as contributing mechanisms. Remarkably, this exchange proceeds with ultrafast kinetics, achieving over 95% uptake of TcO4− within 5 min, and exhibits robust performance even in the presence of a 6000-fold excess of competing anions, such as SO42− and NO3− (Fig. 7b)99. Crucially, the framework’s sterically protected concave–convex structure prevents hydrolytic degradation by OH− ions, and the π-conjugated tipa ligand backbone confers resistance to radiation-induced radical damage99. These features make SCU-103 an exemplary candidate for deployment in real-world high-level waste (HLW) environments.
a Structural configuration and porosity of the cationic MOF SCU-103: (a) The Ni2+ centre adopts an octahedral coordination, each bonded to six nitrogen atoms from tridentate tipa ligands. b View of the framework’s 2D layers arranged in an ABC stacking pattern, creating periodic concave–convex morphology. c Close-up showing bowl-like cavities formed by the interlocking of adjacent layers, providing confined spaces for ion capture. d Open channels connecting the cavities, enabling diffusion of guest species throughout the porous matrix. (b) Sorption kinetics and selectivity of SCU-103: (a) UV–Vis spectra tracking the rapid decline of 99TcO4− absorbance at 290 nm, indicating swift anion removal. b Kinetic profiles showing > 90% uptake within minutes, confirming the fast anion-exchange process for both 99TcO4 and its surrogate ReO4−. c Langmuir-fitted adsorption isotherm revealing a high ReO4− uptake capacity of 318 mg/g. (d) High selectivity of SCU-103 for ReO4− in the presence of various competing anions. e−f Retention of uptake efficiency even under large excesses of NO3− and SO42−, emphasizing SCU-103’s strong anion preference under competitive conditions. Reproduced from ref. 99. Copyright 2020, Springer Nature.
Zhang et al.100 drawing on insights regarding the high water sensitivity of Co(II)-based coordination environments and the superior hydrolytic resistance of Pd(II), synthesized a novel bimetallic MOF, 1–Th-Co, featuring [Th6O4(OH)4(COO)12] and [CoN4·Cl2] secondary building units, and then implemented a post-synthetic cation exchange process in N,N-diethylformamide to substitute Co(II) centres with Pd(II), to produce a structurally isoreticular yet chemically more stable 1–Th-Pd framework100. This substitution introduced planar PdN4 coordination environments that not only reinforced the structural resilience of the framework in aqueous media but also preserved its cationic nature and permanent porosity, enabling highly efficient and rapid adsorption of 99TcO4− and its nonradioactive analogue ReO4− 100. The authors demonstrated that 1–Th-Pd exhibits superior removal efficiencies, achieving >93% uptake of 99TcO4− in ~5 ppm solutions within minutes, complete (>99%) removal of ReO4− at 1 ppm, and >80% removal even in complex tap water matrices, underscoring its exceptional kinetics, high affinity, and tolerance to competing ions; further highlighting the framework’s remarkable robustness under operationally relevant conditions100. The study provides a compelling demonstration of how selective metal ion modulation, via Co(II)-to-Pd(II) exchange, can simultaneously enhance the chemical durability and anion-removal efficiency of MOFs, establishing a generalizable design strategy for constructing sustainable, high-performance MOF-based sorbents for nuclear waste remediation. Notwithstanding, some limitations remain that temper the immediate translational potential of the work. For example, the post-synthetic ion-exchange approach, although effective for enhancing water stability and preserving framework crystallinity, introduces uncertainties regarding uniformity, long-term structural stability under fluctuating environmental conditions, and potential leaching of palladium during repeated use, which were not systematically addressed beyond the four-cycle regeneration test. Furthermore, while the use of N,N-diethylformamide as the medium for Pd(II) exchange was instrumental for maintaining framework integrity, the toxicity and limited sustainability of this solvent raise environmental and scalability concerns that need to be resolved through greener processing alternatives. In terms of functional evaluation, the adsorption experiments were primarily conducted at relatively low contaminant concentrations ( ~ 1–10 ppm) under controlled laboratory conditions; however, real-world waste streams often contain mixed contaminants, fluctuating pH, and high ionic strength, factors which could interfere with selective anion uptake and were not extensively studied.
In another report, a chemically robust, magnetically separable, and hydrolytically stable metal-organic framework hydrogel (MOFH) was developed to address the persistent challenge of selective uranium (U(VI)) removal from nuclear wastewater characterized by high levels of total dissolved solids, organic matter, and diverse interfering ions101. In this work, ferrocyanide was embedded into a crosslinked polysaccharide-based hydrogel matrix composed of alginate and chitosan, with stabilization via Fe(II)-CN-Fe(III) linkages. This design endowed the material with amphoteric behaviour across a broad pH range, enhanced chemical stability, and convenient magnetic separability for post-use recovery. The resulting MOFH exhibited a synergistic combination of high specific surface area (248.36 m2/g), mesoporous structure, and diverse functional groups (–OH, –COOH, –NH2) critical for effective chelation, ion exchange, and hydrogen bonding with U(VI) ions. The MOFH achieved an impressive maximum adsorption capacity of 486.76 mg/g at pH 6–7, surpassing many benchmark materials such as phosphorylated porous polymers, Prussian blue analogues, and alginate-immobilized MOFs, while retaining over 80% of its efficiency after five adsorption–desorption cycles, underscoring its potential for practical reuse101. Spectroscopic analyses confirmed the binding interactions between U(VI) and functional groups on the MOFH, particularly through complexation with –OH, –COOH, and –NH2 moieties, while the Fe(II)-CN-Fe(III) scaffold further stabilized uranyl capture through coordination101. Adsorption behaviour followed a pseudo-second-order kinetic model and Freundlich isotherm, suggesting multilayer chemisorption on heterogeneous surfaces, an advantageous mechanism for dynamic and complex wastewater environments101. Remarkably, the MOFH maintained high selectivity for U(VI) even in the presence of significant concentrations of Na+, K+, Mg2+, and humic substances, as indicated by high distribution coefficients and sustained removal efficiency in real effluent samples collected from uranium smelting operations101. This resistance to competitive interference and fouling, a common limitation in conventional sorbents, highlights the material’s applicability in realistic wastewater matrices. Its strong magnetic response (saturation magnetization BS = 32 emu/g) further enables rapid and reagent-free recovery, making it suitable for scalable field deployment101. Despite these promising features, several limitations remain. The adsorption performance declined slightly at elevated temperatures and at extreme pH values, indicating the need for future formulations with enhanced thermal and chemical resilience. Moreover, the residual U(VI) concentration in treated real wastewater exceeded regulatory discharge limits (0.05 mg/L), necessitating complementary post-treatment strategies.
The critical limitations of conventional Prussian Blue analogues and MOFs, despite their known ion-exchange capabilities and tunable porosity, lie in their poor mechanical integrity, limited regeneration potential, and insufficient selectivity in complex aqueous systems. To overcome these challenges, a new study introduces a synergistic composite aerogel, termed PBA/ZIF-67/AARH, synthesized via a green, scalable approach rooted in circular economy principles102. This composite integrates cobalt ferrocyanide, ZIF-67, and aged refuse humus (AARH), offering a multifunctional material platform with improved performance and sustainability. From a materials design perspective, AARH not only reduces synthesis costs by over 60% compared to commercial porous carbons but also contributes additional active sites, supporting enhanced adsorption and lifecycle efficiency102. The ZIF-67 framework, known for its high porosity and chemical robustness, is embedded within a sodium alginate/polyvinyl alcohol (SA/PVA) hydrogel, which reinforces structural stability, promotes efficient mass transport, and mitigates pore collapse during handling. Crucially, the in-situ growth of cobalt ferrocyanide nanoparticles introduces high-affinity ion-exchange sites, facilitating selective caesium (Cs+) capture, with cobalt acting as a coordinating bridge to the nitrogen-rich ferrocyanide lattice102. This engineered material exhibits a remarkable maximum adsorption capacity of 51.92 mg/g at pH 5 and a dosage of 0.01 g, outperforming both ZIF-67-only (38.44 mg/g) and AARH-only (32.47 mg/g) analogues. The adsorption mechanism aligns with the Langmuir isotherm model (R2 = 0.97) and pseudo-second-order kinetics (R2 = 0.9985), indicative of monolayer chemisorption governed primarily by ion exchange between Cs+ and K+ within the PBA domain, supplemented by ligand coordination to nitrogen donor atoms. The aerogel maintains 84.5% Cs+ removal efficiency after five adsorption–desorption cycles, highlighting its hydrolytic stability and regenerative potential, significantly mitigating a major drawback of traditional Prussian Blue systems, which often degrade or leach cyanide under alkaline conditions. Importantly, cyanide release remains well within WHO safety thresholds under mildly alkaline conditions, underscoring the material’s environmental compatibility. In real-world matrices, including seawater and solutions enriched with dissolved organic matter (DOM), the aerogel preserves over 83% of its original adsorption capacity, demonstrating strong resilience against ionic competition and organic fouling key for practical application in radioactive wastewater remediation102. Nonetheless, certain limitations remain. For instance, the specific surface area (30.8 m2/g) is moderate compared to high-surface-area MOFs (>500 m2/g), likely due to structural compaction during freeze-drying. Furthermore, the long-term stability of the composite under continuous-flow operation, its resistance to high ionic strength environments, and its selectivity in the presence of multiple radionuclides (for example, Sr2+, UO22+) require further exploration. Elsewhere, a study presents a structurally and mechanistically innovative framework for monovalent anion-selective separations by incorporating MOFs into poly(arylene ether sulfone) (PAES)-based anion exchange membranes (AEMs)103. Specifically, the work exploits the intrinsic advantages of UiO-66-NH2-type MOFs, recognized for their exceptional aqueous stability, tunable pore architecture, and versatile post-synthetic functionalization capacity, to engineer membranes that synergistically combine size-selective transport and chemical affinity-based discrimination. The rational doping of three structurally tailored MOFs (i.e., UiO-66-NH2-Ph, UiO-66-NH2-Py, and UiO-66-NH2-Pyr) into the PAES matrix results in the formation of composite membranes with spatially defined nanochannels and enhanced anion transport control. This dual-functionality mechanism, predicated on precise molecular sieving through rigid, fixed-size MOF apertures and selective coordination interactions enabled by nitrogen-functionalized ligands, substantially elevates the perm-selectivity performance of the membranes. The PAES-UiO-66-Pyr membrane, in particular, achieves unprecedented selectivity coefficients of 54.26 (Cl−/SO42-) and 51.30 (NO3−/SO42-), outperforming the benchmark commercial Neosepta© ACS and all previously reported AEM systems; thereby demonstrating a significant leap forward in monovalent anion separation performance, highlighting the efficacy of hybrid MOF–polymer integration in achieving tailored ionic transport behaviour, in addition to their superior selectivity103. Importantly, the materials maintained structural and functional stability across multiple ED cycles, underscoring their hydrolytic resilience and operational durability, an essential criterion for real-world scalability and long-term deployment in harsh aqueous environments103. Nevertheless, limitations exists. For example, the relatively modest MOF loading ( ~ 7.5 wt. %) potentially underutilizes the full sorptive and selective potential of the MOF domains, while the evaluation under simplified binary ion systems does not fully reflect the complex and heterogeneous nature of real wastewater streams. The influence of fouling, pH variability, competing multivalent ions, and organic contaminants remains unexplored, and osmotic water transport across the membranes, though measured, could exacerbate long-term volume flux imbalances. Hence, future studies could aim to: (1.) optimize MOF loading and dispersion without compromising membrane mechanical integrity, (2.) validate performance under realistic wastewater conditions, (3.) assess antifouling behaviour and long-term cycling under continuous flow, and (4.) quantify the technoeconomic feasibility and regeneration potential of MOF–AEM composites.
A report presents a methodologically rigorous and conceptually innovative strategy to overcome a critical bottleneck in lithium extraction: the selective separation of Li+ from Mg2+ in salt-lake brines with high Mg/Li ratios, a challenge exacerbated by the ions’ comparable radii and similar physicochemical behaviours104. The study introduces AEMs embedded with hydrolytically stable sulfonated UiO-66 (HSO3-UiO-66)–MOFs as high-performance hybrid membranes capable of enhancing ion discrimination through a synergistic combination of electrostatic repulsion and size-exclusion effects104. By incorporating HSO3-UiO-66 into a quaternary ammonium-functionalized poly(2,6-dimethyl-1,4-phenylene oxide) (QPPO) matrix, the authors engineered a scalable and chemically robust hybrid system that simultaneously leverages the MOF’s hydrophilicity and structural integrity and the AEM’s high anion conductivity. The resulting membranes demonstrate significantly improved Li+ permeability (0.238 mol·m–2·h–1) and Li+/Mg2+ selectivity (up to 8.33), representing increases of 114% and 48%, respectively, over the pristine QPPO membrane. These enhancements are attributed to dual transport mechanisms: electrostatic attraction of counter anions coupled with MOF-mediated steric sieving and selective coordination chemistry. Notably, among the MOFs tested, only HSO3-UiO-66 retained its structural integrity in diverse saline environments, highlighting its superior hydrolytic and chemical stability. Molecular dynamics simulations corroborated the experimental findings, revealing higher mean-squared displacements and weaker hydration shells for Li+ relative to Mg2+, as well as weaker interactions with the sulfonate groups, factors that contribute to greater Li+ mobility within the MOF framework. In addition to separation performance, the hybrid membranes exhibited enhanced physicochemical functionality, including increased water uptake, reduced swelling, and sufficient mechanical integrity, thereby enabling operational stability across a range of pH values, temperatures, and salt concentrations104. Nonetheless, performance under extremely acidic conditions (pH < 1) was compromised due to partial MOF degradation, and elevated salt concentrations introduced competitive diffusion effects that diminished separation selectivity. These limitations suggest future efforts could focus on strengthening polymer–MOF interfacial cohesion to minimize non-selective transport pathways and possibly chemical post-modifications to improve acid resistance and long-term resilience under harsh operational conditions.
Adsorptive interactions
As previously discussed, conventional strategies for heavy metal remediation such as chemical precipitation, membrane filtration, and ion exchange while effective, often face challenges related to scalability, cost-effectiveness, and the generation of secondary pollutants. Adsorption has emerged as a promising alternative due to its operational simplicity and energy efficiency; however, traditional adsorbents commonly suffer from limitations such as poor selectivity, limited hydrolytic stability, and inadequate regeneration potential. Addressing these challenges, a study introduced a composite strategy involving the encapsulation of two tailored metal–organic frameworks (MOFs) viz NH2-MIL-101-Fe and EDTA-functionalized MOF-808 within a polyether sulfone (PES) matrix to produce spherical mixed matrix adsorbents105. This hybrid design leverages the high metal-ion affinity and tunable chemistry of MOFs alongside the mechanical resilience and aqueous stability of the PES support. The report argue that both composite systems exhibit appreciable adsorption capacities, with the MOF-808-EDTA spheres significantly outperforming their NH2-MIL-101-Fe counterparts. Specifically, MOF-808-EDTA achieved maximum adsorption capacities of 272.7 mg/g for Hg2+, 151.3 mg/g for As3+, and 125.9 mg/g for Mn2+. Optimal adsorption occurred in the mildly acidic pH range of 5.0 to 5.6, consistent with the deprotonation of functional groups and enhanced electrostatic attraction between the negatively charged sorbent surfaces and metal cations. Adsorption behaviour conformed to the Langmuir isotherm model, suggesting monolayer coverage on homogeneously distributed active sites. Furthermore, dynamic fixed-bed column experiments confirmed the materials’ practical applicability, with removal efficiencies reaching 92% for Hg2+, 84% for As3+, and 75% for Mn2+ under optimized flow and bed height conditions. Notably, the spheres exhibited excellent recyclability, retaining over 95% of their initial performance across five regeneration cycles using dilute HCl, thereby reinforcing their potential for deployment in continuous-flow or modular water treatment systems105. Nevertheless, certain limitations warrant attention. The adsorption studies were conducted using simulated aqueous solutions rather than actual industrial or municipal wastewater, which may contain a complex mixture of competing ions, organic matter, and variable pH conditions that could adversely affect performance. In addition, the relatively low surface areas of the composites (8.5–13.3 m2/g), compared to pristine MOFs, imply partial pore obstruction or reduced accessibility of active sites due to polymer encapsulation. While thermodynamic and surface spectroscopic analyses provided useful insights into adsorption mechanisms, the study lacked direct evidence of changes in the oxidation states and local coordination environments of the adsorbed metal ions.
A notable advancement in the field of multifunctional adsorbent materials is demonstrated in a recent study that presents a sequential and synergistic strategy for addressing two pressing environmental challenges: the recovery of heavy metals specifically copper ions (Cu2+) and the removal of emerging organic micropollutants such as tetracycline hydrochloride (TC)106. The authors developed a novel bifunctional system by first synthesizing ZIF-8@SA hydrogel beads, wherein zeolitic imidazolate framework-8 (ZIF-8) nanocrystals are immobilized within a sodium alginate (SA) matrix. These beads serve as primary adsorbents for Cu2+, achieving a high adsorption capacity of 182.61 mg/g through a combination of chemisorptive mechanisms, most notably ion exchange between Cu2+ and Zn2+ in the ZIF-8 lattice, electrostatic attraction, complexation with –COO− groups, and coordination to imidazole nitrogen sites106. Following Cu2+ capture, the beads undergo in situ transformation into ZIF-8@SA@Cu-MOF composites via post-synthetic coordination with 2-methylimidazole, utilizing the recovered Cu2+ to form copper-based MOF nanostructures on the surface. This transformation repurposes the adsorbed Cu2+ into a new functional entity capable of targeting TC, enabling a second stage of contaminant removal. The resulting Cu-MOF-modified beads exhibit a maximum adsorption capacity of 152.2 mg/g for TC, among the highest reported for similar systems106. The TC removal process is governed predominantly by physical adsorption, as supported by pseudo-first-order kinetic fitting, but also involves hydrogen bonding, π–π stacking interactions, electrostatic attraction, and cationic bridging via Cu–N coordination, underscoring the material’s multifunctional affinity106. Importantly, the structural integrity of the ZIF-8@SA@Cu-MOF beads is maintained across a wide pH range and under ionic interference from Na+, K+, Ca2+, and Mg2+ conditions mimicking real wastewater environments such as tap and snowmelt water. Additionally, negligible leaching of Cu2+ (<2 mg/L) and Zn2+ was observed, validating the composite’s chemical stability and environmental safety during use106. The material retained substantial adsorption efficiency even after five regeneration cycles, highlighting its potential for scalable, cost-effective deployment in industrial water treatment systems. This dual-functionality approach, merging heavy metal recovery with antibiotic removal, illustrates a promising convergence of chemical robustness, material reusability, and mechanistic selectivity, aligning with principles of sustainable materials chemistry and circular economy. Nevertheless, the study has some limitations that warrant future attention. Although laboratory tests with simulated and natural water samples affirm the material’s efficacy, its long-term performance under dynamic flow conditions and high contaminant loads typical of industrial effluents remains untested. Additionally, the degradation behaviour, fouling potential, and structural fatigue under repeated regeneration in complex matrices were not fully investigated. The environmental fate of exchanged Zn2+, while negligible at lab scale, requires closer scrutiny at larger operational scales. Future research should prioritize the scale-up synthesis of ZIF-8@SA@Cu-MOF beads and validate their efficacy in pilot-scale continuous flow systems. Investigations into co-pollutant removal dynamics including simultaneous adsorption of multi-class antibiotics, heavy metals, and endocrine-disrupting compounds would enhance their practical scope. Furthermore, a comprehensive life cycle assessment (LCA) comparing this hybrid MOF-alginate technology to conventional treatment methods is essential to ascertain its true environmental and economic viability.
Driven by the persistent and toxic nature of Congo Red (CR), a widely used anionic diazo dye in the textile and related industries that poses serious ecological and health hazards due to its resistance to biodegradation and conventional chemical treatments, a recent study advances the design of a novel trimetallic MOF, ZnCuCr-TpIm, for efficient dye remediation107. This MOF integrates zinc (Zn), copper (Cu), and chromium (Cr) metal nodes within a single framework, coordinated with terephthalic acid and imidazole (TpIm) linkers. The strategic incorporation of these three metal centres yields synergistic improvements in adsorption performance, structural stability, and contaminant selectivity. Under optimized conditions (40 mg dosage, pH 6–7, 55 °C, 60 min contact time), ZnCuCr-TpIm achieved a CR removal efficiency of 96.5%. Kinetic data fit a pseudo-second-order model (R2 > 0.999), indicating chemisorption as the dominant mechanism, likely governed by electron-sharing or electron-transfer processes between dye molecules and the MOF surface. Complementary isotherm analysis showed excellent agreement with the Langmuir model (R2 = 0.998), with a notably high maximum adsorption capacity of 325 mg/g, placing ZnCuCr-TpIm among the most effective CR adsorbents reported thus far. Mechanistically, dye removal is driven by a combination of electrostatic attractions, hydrogen bonding, and coordination interactions between CR functional groups and unsaturated metal sites or heteroatoms in the framework. Hence, the presence of Zn2+, Cu2+, and Cr3+ enriches the diversity and density of active sites, while TpIm linkers confer structural flexibility that promotes optimal spatial orientation for guest–host interactions. Furthermore, the predominance of subnanometer pores (<1 nm) facilitates size-selective molecular sieving and efficient diffusion of dye molecules into the internal adsorption domains. Beyond its adsorption capacity, ZnCuCr-TpIm demonstrated robust physicochemical stability under environmentally relevant conditions. The MOF retained over 90% of its surface area and adsorption performance after five regeneration cycles and 72 h of aqueous exposure. Even under simulated wastewater containing humic acids and high salinity, the material maintained 82.6% CR removal after 14 days, reflecting excellent operational resilience. Importantly, the MOF exhibited pH-dependent stability: while structural degradation occurred at pH 10 due to ligand hydrolysis, the framework remained largely intact and effective at neutral pH conditions typical of real wastewater, confirming its environmental suitability107. However, several limitations temper the immediate translational outlook. The exclusive use of CR as a model pollutant does not capture the complexity of real wastewater streams, which contain a diverse array of organic and inorganic contaminants. The MOF’s competitive adsorption behaviour and selectivity in multi-component systems remain unexplored. Moreover, the long-term ecotoxicological implications of even trace metal leaching were not evaluated, which is critical for environmental risk assessments. Mechanistic elucidation would also benefit from molecular simulations or operando spectroscopic studies to precisely characterize adsorption pathways and binding energetics. Finally, the solvothermal synthesis route, while effective at the laboratory scale, presents challenges in terms of cost, energy intensity, and scalability, necessitating future work on green, economically viable production methods and full life-cycle assessments.
Catalytic—(Fenton-like and Peroxymonosulfate (PMS)) degradation
The growing presence of pharmaceuticals and personal care products (PPCPs) in aquatic environments poses serious ecological and health concerns, with carbamazepine (CBZ), a widely used antiepileptic, standing out due to its resistance to conventional treatment. Addressing this challenge, a recent study introduces a novel electro-Fenton (EF) system integrated with membrane filtration and a MOF-derived magnetic nanocatalyst (Fe@PC), offering a selective, energy-efficient, and environmentally sustainable approach for PPCP degradation in complex aqueous matrices as shown in Fig. 8108. The Fe@PC catalyst, synthesized via high-temperature carbonization of MIL-101(Fe), features a core–shell architecture of iron and iron carbide nanoparticles embedded in porous carbon. This design preserves MOF morphology while enhancing conductivity, structural stability, and reusability through magnetic recovery. These properties underpin its high catalytic performance (Fig. 7). Under optimal conditions (pH 3, 0.67 mA cm−2, 250 mg L−1 catalyst), the system achieved 98.34% CBZ degradation within 70 min, substantially faster than conventional methods. Mechanistic studies confirmed the in situ generation of hydroxyl (•OH) and superoxide (•O2−) radicals, with •OH predominating during initial stages, facilitating rapid breakdown (Fig. 8)108. The catalyst maintained over 75% efficiency after five reuse cycles, showing negligible iron leaching and retained magnetic integrity. Moreover, the micro-electric field applied across the membrane mitigated fouling, extending membrane life and ensuring consistent performance. The dual functionality of the membrane as filter and cathode exemplifies an integrative strategy for enhancing process efficiency and operational resilience. Despite these advances, the system’s reliance on acidic conditions (pH 3) may hinder real-world application due to the cost and complexity of pH control. Additionally, total organic carbon (TOC) removal reached only ~ 49%, indicating incomplete mineralization and potential accumulation of residual intermediates. Future research could prioritize the development of pH-tolerant catalysts, potentially through heteroatom doping, to broaden the system’s operational range. Integrating EF with photochemical or biological post-treatment may enhance mineralization and eliminate persistent byproducts. Elsewhere, Duan et al.109 report the synthesis of iron-based MOFs, specifically MIL-100(Fe), via a green, hydrothermal route using zero-valent iron as the sole metal source. This approach induces missing ligand defects through heterogeneous nucleation, yielding coordinatively unsaturated Fe sites (Fe-CUS) that function as strong Lewis acid centres, enhancing H2O2 adsorption and activation109. The resulting defective MIL-100(Fe) (Def-MIL-100(Fe)) (Fig. 9) achieves rapid and efficient Fenton-like degradation of bisphenol A (91.26% within 10 min at pH 4, 0.05 g/L catalyst), far outperforming its defect-free counterpart109. The superior activity of Def-MIL-100(Fe) is attributed to three synergistic effects: increased Lewis acidity at Fe-CUS facilitates formation of Fe–H2O2 complexes; the presence of mixed-valent Fe2+/Fe3+ enables fast redox cycling for sustained •OH generation; and defect-induced mesoporosity enhances mass transfer and radical utilization despite a lower overall surface area109. The material demonstrates notable robustness, maintaining 89.9% degradation efficiency over five cycles with minimal Fe leaching (0.11–0.81 mg/L), well below EU limits. It also retains high activity in real water matrices and efficiently degrades diverse contaminants including tetracycline, ciprofloxacin, and 2,4-dichlorophenol109. Nonetheless, limitations remain. Defect density is not precisely controlled or quantified, and long-term durability under variable environmental conditions is unassessed. The focus on batch systems and limited pollutant range restricts insights into scalability and performance against persistent or emerging contaminants such as PFAS and pharmaceuticals. Future work should target controlled defect engineering, continuous-flow applications, and broader contaminant profiles to advance practical implementation.
Each pathway reflects a distinct mechanistic route by which reactive oxygen species, primarily hydroxyl radicals (•OH) and superoxide radicals (•O2−), initiate oxidative transformations of CBZ, leading to its eventual breakdown into smaller, less toxic molecules. Pathway I suggests two sub-routes where free radicals attack CBZ to produce a key intermediate (P9), either directly from CBZ (P1) or from an epoxide intermediate (P8). This is followed by sequential hydroxylation steps forming P11 and ultimately P20. These intermediates suggest gradual functionalization and ring hydroxylation, leading to higher hydrophilicity and facilitating further breakdown. Pathway II involves initial •OH attack on the double bond with the highest electron density in CBZ, forming intermediate P2. This compound undergoes ring-opening to form P3, followed by oxidative transformations that yield P4 or P5. These structures undergo side-chain cleavage to generate P6 or P7, respectively. The transformations in this pathway indicate a rapid deconstruction of the aromatic and heterocyclic rings, which accelerates mineralization. Pathway III describes the formation of an epoxide intermediate (P8) via hydroxyl radical addition, followed by cleavage and oxidation reactions yielding P10, P12, and P13. This pathway is indicative of partial ring retention and slower breakdown steps compared to the more energetically favourable Pathway II. Pathway IV begins with direct •OH addition to the benzene or azepine ring, producing hydroxylated derivatives P17 and P18. These undergo further oxidation and elimination to produce P12 and P13, overlapping with intermediates in Pathway III. The convergence of products suggests multiple routes may lead to common degradation intermediates. Reproduced from ref. 108. Copyright 2023 Elsevier B.V.
It clarifies that using zero-valent iron in a heterogeneous reaction environment, unlike the homogeneous route with soluble Fe2+induces missing ligand defects by promoting uneven nucleation. These defects give rise to coordinatively unsaturated Fe sites essential for enhanced Fenton-like catalytic performance. Reproduced from ref. 109. Copyright 2023 Elsevier B.V.
Li et al.110 report a significant advancement in heterogeneous Fenton-like catalysis for sustainable wastewater treatment by addressing key limitations of traditional systems: metal ion leaching, poor catalyst recovery, and inefficient ROS generation. These persistent challenges have limited the scalability of Fenton-based advanced oxidation processes (AOPs), despite their strong theoretical efficacy. The authors develop a novel iron-based metal-organic framework catalyst (FeNC), featuring dual active sites—Fe–N4 and Fe–Fe clusters anchored in a nitrogen-doped porous carbon matrix derived via a scalable hydrothermal-calcination route110. The FeNC catalyst exhibits a high surface area (229.6 m2/g) and magnetic responsiveness (23.6 emu/g), enabling enhanced pollutant-catalyst contact, efficient electron transfer, and facile magnetic recovery. The catalyst efficiently activates peroxymonosulfate (PMS) through an electro-Fenton-like mechanism, rapidly generating reactive SO4•− and •OH radicals. DFT calculations confirm these radicals as the primary oxidative species, while singlet oxygen (1O2) and superoxide (O2•−) play negligible roles. To realize practical application, the study integrates FeNC into a modular reactor system comprising electrochemical activation, ultrasonic regeneration, and magnetic separation chambers. This system achieves continuous degradation of diverse organic pollutants including dyes, phenols, and antibiotics across a broad pH range (2.5–11.5) and in real water matrices (river, seawater, groundwater), maintaining high efficiency over six cycles with minimal Fe leaching (<70 µg/L), well below the WHO limits110. Nevertheless, challenges remain. The synthesis process, though scalable, is multi-step and may limit industrial deployment in resource-constrained contexts. The system’s dependence on external PMS raises sustainability concerns due to chemical input requirements and potential secondary pollution. Furthermore, the exclusive reliance on radical-driven pathways may constrain efficiency in complex wastewater matrices. Another study introduces a significant advance in sustainable wastewater treatment via the development of a Cu-based MOF, i.e., MAF-wyu2, designed for efficient PMS activation111. The work addresses the urgent need for robust catalysts capable of degrading persistent antibiotics such as sulfamethoxazole (SMX), which resist conventional treatments and pose environmental and health risks. Because conventional AOPs are limited by narrow pH windows, high energy requirements, and catalyst instability, MAF-wyu2 overcomes these limitations through a structurally resilient, chemically stable, and catalytically active framework, effective across a broad pH range with minimal energy input, as illustrated in Fig. 10111. MAF-wyu2 features planar trinuclear [Cu3(μ3-O)]4+ clusters embedded in a porous pyrazolate-based 3D architecture with abundant open metal sites (OMSs) and a high pore volume (69.6%) (Fig. 10). It exhibits exceptional thermal and chemical stability (pH 3–11, diverse solvents) and achieves >95% degradation of multiple antibiotics including SMX, OTC (oxytetracycline), SMZ (sulfamethazine), CIP (ciprofloxacin) and TC (tetracycline) within 30 min. Notably, it enables 100% SMX degradation at pH 11 using only 25 mg L−1 catalyst and 100 mM PMS, outperforming most reported MOFs and representing the first such material to show full PMS activation efficacy under strongly alkaline conditions. Quenching studies confirm the generation of hydroxyl (HO•), sulphate (SO4•–), superoxide (O2•–), and singlet oxygen (1O2) species, with O2•– and 1O2 identified as dominant111. The MAF-wyu2 material demonstrates high reusability, retaining >95% degradation efficiency over five cycles with minimal Cu leaching (0.76 mg L−1), well below regulatory limits. Compared to benchmark MOFs such as MIL-53(Fe), MIL-101(Fe), HKUST-1, and MOF-74, MAF-wyu2 exhibits superior degradation kinetics, attributed to synergistic effects of high OMS density, Cu2+ redox activity, and enhanced porosity111. Turnover frequency and number metrics further affirm its catalytic advantage. Nonetheless, the framework amorphizes upon activation, though reversibly, this presents limitations of long-term structural integrity. Additionally, Humic acid (HA), prevalent in natural waters, significantly impairs performance by scavenging ROS and obstructing OMSs, reducing degradation by over 60%. While Cu leaching is low under lab conditions, extended field use warrants more comprehensive environmental risk assessments.
The figure serves a dual purpose: it provides direct evidence of the MOF’s crystallographic architecture (a) and illustrates the internal pore environment that enables mass transport and access to active sites (b). Specifically, (a) confirms the formation of a three-dimensional coordination network composed of planar trinuclear [Cu3(μ3-O)]4+ clusters interconnected by pyrazolate ligands, resulting in a stable, extended framework rich in open metal sites (OMSs). This structural arrangement is central to PMS activation, as it exposes catalytically active Cu2+ centres throughout the material. b highlights the large, interconnected pores that enhance molecular diffusion of both PMS and antibiotics, thereby facilitating efficient ROS generation and pollutant degradation. The exceptionally high pore ratio (69.6%) visually emphasized directly correlates with the high adsorption capacity and reaction kinetics observed. Reproduced from ref. 111. Copyright 2024, The Royal Society of Chemistry.
Photocatalytic degradation
Perovskite oxides have garnered significant attention for photocatalytic water treatment due to their optical activity and chemical stability, particularly their ability to absorb visible light and generate ROS via electron–hole pair excitation. However, their practical application is hindered by limitations such as low surface area and rapid charge recombination arising from insufficient redox potential. To address these shortcomings, recent strategies have focused on compositional tuning, morphological control, and hybridization with high-surface-area materials such as MOFs, an approach exemplified by Younes et al.112 where they strategically integrated LaFeO3 perovskite and MIL-125-NH2 MOF into a heterostructured photocatalyst for degrading persistent pharmaceuticals such as carbamazepine (CBZ) and caffeine (CAF). The composite exploits LaFeO3’s visible-light responsiveness and redox activity alongside the high surface area and adsorption capacity of MIL-125-NH2, yielding a synergistic photocatalyst with enhanced performance under simulated solar irradiation112. The Z-scheme heterojunction facilitates efficient charge separation, extending carrier lifetimes, as evidenced by photoluminescence quenching and radical scavenger experiments. Superoxide radicals (O2·−) were identified as the dominant reactive species, with contributions from ·OH and h+. Band alignment analysis confirmed directional electron transfer from MIL-125-NH2 to LaFeO3 and reverse hole migration, promoting reactive oxygen species generation. Monte Carlo simulations and NCI analyses revealed stronger adsorption of caffeine via π–π stacking, metal–π, and hydrogen bonding, corroborating the composite’s high affinity for pharmaceutical pollutants. The LaFeO3/MIL-125-NH2 catalyst achieved 87% CAF and 74% CBZ degradation within 60 min, outperforming individual components. Superior activity is linked to its mesoporous structure (pore size ~3.9 nm), increased surface area (187.9 m2/g), and improved interfacial charge dynamics. The kinetics followed pseudo-first-order behaviour, highlighting suitability for low-concentration pollutant removal. Nonetheless, the study is limited by the absence of real wastewater validation, catalyst recyclability data, and by-product toxicity analysis. Additionally, the synthesis relies on prolonged sonication and DMF use, which may challenge scalability unless green alternatives are adopted. In a related development, a comparative evaluation into the use of UiO-66 (a zirconium-based MOF) and La0.7Sr0.3FeO3 (a perovskite oxide) as adsorbents for caffeine (CAF) removal from aqueous media, is reported113. Notably, UiO-66 demonstrated a higher maximum adsorption capacity (62.5 mg/g) relative to the perovskite (35.25 mg/g), attributed to its significantly greater surface area and pore volume, thus enhancing inner-surface-dependent interactions. Perovskite exhibited broader pH tolerance (4–10) and faster initial uptake, showing robustness under neutral and mildly basic conditions. However, materials engaged in multi-faceted interactions with CAF. For the UiO-66, adsorption was governed by inner-pore filling and electrostatic attraction at acidic pH (below its pHzpc of 5.5), as evidenced by the surface area reduction post-adsorption and pH-dependent uptake patterns113. Contrary to photoactive perovskites often used in light-induced radical generation or photocatalytic degradation, the study operates purely on adsorption principles without exploring catalytic or self-photosensitizing properties. As such, while La0.7Sr0.3FeO3 is known for visible-light activation, this aspect remains unutilized, highlighting a missed opportunity to expand the material’s multifunctionality for hybrid adsorption–photocatalysis systems113. Another limitation lies in the poor reusability of the materials as each exhibited significant performance loss after a single adsorption cycle. Additionally, no tests were conducted using real wastewater matrices, and the materials’ long-term chemical and mechanical stability were not evaluated, thus limiting conclusions on real-world applicability and scalability. The high cost of perovskite precursors also challenges its economic feasibility for large-scale deployment.
Xu et al.114 demonstrate an effectual sulphur-modified Cu/Fe Prussian blue analogue (CuFeO@S) integrated into a visible-light-assisted persulfate (PDS) oxidation system, aimed at addressing the persistent issue of sulphonamide antibiotics in water bodies, contaminants known for promoting antimicrobial resistance. The system relies on direct and indirect photocatalytic degradation pathways. Light exposure generates photoinduced holes on CuFeO@S, which either directly oxidize sulphonamides or activate PDS to generate SO4•− and 1O2 radicals. The sulphur-induced Cu+/Cu2+ redox cycle enhances PDS activation, while Fe3+/Fe2+ redox participation contributes to electron–hole separation, reducing recombination losses114. The CuFeO@S catalyst achieves >99% degradation of four sulphonamides within 30 min under visible light and remains robust over 7 reuse cycles with >90% efficiency. It operates effectively across a broad pH range (4–10) and under realistic matrix interference (Cl−, HCO3−, humic acid), showcasing operational flexibility and resilience114. Nevertheless, limitations exists. For example, the moderate total organic carbon (TOC) removal in complex matrices signals incomplete mineralization and potential formation of transformation products. Leaching of Cu or Fe ions, although mitigated by the MOF framework, was not quantified, raising concerns about long-term stability and ecological safety. Moreover, radical suppression by coexisting anions highlights potential performance attenuation in high-ionic-strength waters. The electron–hole recombination rate, though reduced, may further benefit from co-catalyst integration or heterojunction formation. In another development, to overcome the limited light absorption and charge separation efficiency of conventional photocatalysts such as bismuth oxychloride (BiOCl) it was integrated with a two-dimensional nickel-based MOF (Ni-MOF), forming an S-scheme heterojunction to obtain a face-to-face 2D/2D Ni-MOF/BiOCl heterojunction, which significantly boosts photocatalytic efficiency115. The optimal composite (0.1Ni-MOF/BiOCl) achieved a degradation rate constant of 0.00274 min−1 over 7-fold higher than pristine BiOCl under UV irradiation. This is attributed to the improved charge transfer and retention of redox potentials enabled by the S-scheme architecture115. It was confirmed that photoexcited electrons in Ni-MOF recombine with holes in BiOCl at the interface, while energetic electrons in BiOCl and holes in Ni-MOF remain available for redox reactions. The photocatalytic process is governed by efficient light-induced radical generation. Upon UV illumination, electrons from the conduction band (CB) of Ni-MOF migrate across the interface to recombine with holes from the valence band (VB) of BiOCl, forming an internal electric field (IEF) that facilitates spatial charge separation. Consequently, electrons accumulated in BiOCl CB reduce oxygen to generate superoxide radicals (O2•−), while holes retained in Ni-MOF VB oxidize water to form hydroxyl radicals (•OH); with trapping experiments identifying O2•− as the dominant ROS driving TC degradation136. Analysis of intermediates elucidates two major degradation pathways involving hydroxylation, demethylation, and ring cleavage, indicating effective mineralization. However, the catalyst operates under UV light, which is energy-intensive and limits practical applicability in real-world solar-driven systems. Additionally, the synthesis relies on organic solvents and multi-step procedures that may challenge large-scale, cost-effective deployment. Besides, the degradation efficiency under visible or solar light, long-term environmental toxicity of leached Ni species, and performance in complex effluent matrices remain unexplored.
Outlook and conclusions
The transformative potential of MOFs in wastewater treatment hinges on addressing critical gaps between laboratory innovation and industrial deployment, with a focus on sustainable synthesis, enhanced material stability, and practical application in complex water matrices. To achieve scalable and eco-friendly MOF production, future research must prioritize the optimization of green synthesis strategies. Mechanochemical synthesis using continuous-flow reactors can reduce synthesis times to under 30 min for complex MOFs like UiO-66 and ZIF-8 while achieving yields exceeding 90% under solvent-free conditions. Continuous-flow microwave (CFMW) synthesis should be scaled up using multiplexed reactor arrays integrated with real-time monitoring tools, such as in-line spectroscopy, to ensure consistent crystallinity and porosity (e.g., BET surface areas > 1000 m2/g). Additionally, the use of supercritical CO2 as a synthesis and activation medium should be expanded to other MOF classes, such as MIL-101 and NH2-MIL-53, with full life-cycle assessments (LCAs) to quantify energy savings and solvent recyclability, potentially reducing production costs below $ 20 per kg for industrial-grade MOFs.
To bridge the gap between laboratory and real-world applications, MOF performance must be rigorously validated in realistic wastewater matrices. Pilot-scale demonstrations should integrate MOF-based membranes or column reactors into existing wastewater treatment plants to validate removal efficiencies above 90% for emerging organic contaminants (EOCs) like pharmaceuticals (e.g., ibuprofen, diclofenac) and personal care products (e.g., triclosan), as well as heavy metals like lead and cadmium. Specific attention should be given to the removal of persistent organic pollutants (POPs) such as perfluorooctanoic acid (PFOA) and polychlorinated biphenyls (PCBs) from municipal wastewater, where traditional treatment methods are insufficient. For industrial effluents, the selective removal of heavy metals like chromium(VI) and arsenic(V) in the presence of high concentrations of sodium and sulfate ions should be investigated. In nuclear waste treatment, focusing on the sequestration of radionuclides such as technetium-99 and iodine-129 is crucial due to their long half-lives and mobility. Application-specific MOF designs should be tailored: for municipal wastewater, MOFs could serve as high-capacity polishing adsorbents in tertiary treatment stages; for industrial effluents, robust MOF–polymer composites could target specific pollutants like methylene blue or Pb2+; and for nuclear waste, ultra-stable cationic MOFs like SCU-103 could be optimized for selective ion exchange under high-radiation conditions.
Addressing persistent challenges in MOF stability, selectivity, and regeneration is critical for industrial readiness. Water stability can be enhanced by developing hybrid MOF systems, such as MOF–hydrogel composites or MOF–oxide heterostructures. For example, embedding Zr-based MOFs like UiO-66 in polydimethylsiloxane (PDMS) or alginate matrices could extend operational lifespans beyond 6 months in aggressive aqueous environments. Exploring robust linkers, such as perfluorinated biphenyl dicarboxylic acid in UiO-66 analogues, could yield MOFs with enhanced hydrolytic stability, capable of withstanding a wide range of pH. Regeneration strategies should focus on energy-efficient methods, such as pH-triggered desorption or low-temperature thermal cycling (<100 °C), to restore >95% of adsorption capacity over multiple cycles. Investigating alternative desorption agents, such as supercritical CO2 or ionic liquids, could provide more environmentally friendly regeneration methods. To improve selectivity, defect engineering and post-synthetic functionalization should introduce targeted binding sites, such as thiol groups in MIL-101, achieving distribution coefficients (Kd) exceeding 105 mL/g for mercury ions (Hg2+). Machine learning-driven design could optimize MOF pore architectures and ligand chemistries for multi-contaminant removal, enabling simultaneous capture of EOCs, heavy metals, and radionuclides. To combat fouling, innovations like zwitterionic coatings or in situ electrochemical cleaning could maintain >80% performance in the presence of organic foulants such as humic acids and proteins.
Techno-economic analyses (TEAs) and regulatory alignment are essential for industrial viability. Future TEAs should quantify capital and operational costs of MOF-based systems, targeting treatment costs below $0.50/m3 for municipal wastewater and $2/m3 for specialized applications like nuclear waste treatment. Considering value recovery, such as through the extraction of valuable metals or the production of reusable clean water, could enhance economic viability. Collaboration with regulatory bodies, such as the EPA or IAEA, is crucial to establish MOF safety profiles, particularly regarding metal leaching (<0.1 mg/L) and long-term environmental impacts. Standardized protocols for testing MOF stability, toxicity, and performance in water treatment applications will facilitate industry and regulatory acceptance. Pilot projects integrating MOFs into modular treatment units, such as portable MOF–membrane pods or fixed-bed reactors, could provide real-world data to support regulatory approval and market adoption. For example, deploying MOF-based systems in remote or disaster-stricken areas could demonstrate their versatility and effectiveness in providing clean water under challenging conditions.
As summarised in Table 3, the scientific community by synergistically advancing green synthesis, material durability, and application-specific performance, MOFs can transition from promising laboratory materials to robust, scalable solutions, addressing the global water crisis with unprecedented efficiency and sustainability.
Data availability
No datasets were generated or analysed during the current study.
References
Grassly, N. C., Shaw, A. G. & Owusu, M. Global wastewater surveillance for pathogens with pandemic potential: opportunities and challenges. Lancet Microbe. 6, 100939 (2025).
Jurado, A. et al. Emerging organic contaminants in groundwater in Spain: a review of sources, recent occurrence and fate in a European context. Sci. Total Environ. 440, 82–94 (2012).
Farhan Hanafi, M. & Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 31, A141–A150 (2020).
Klančič, V., Gobec, M. & Jakopin, Ž. Environmental contamination status with common ingredients of household and personal care products exhibiting endocrine-disrupting potential. Environ. Sci. Pollut. Res. 29, 73648–73674 (2022).
Lange, F. T., Scheurer, M. & Brauch, H.-J. Artificial sweeteners—a recently recognized class of emerging environmental contaminants: a review. Anal. Bioanal. Chem. 403, 2503–2518 (2012).
Lin, S. et al. Remediation of emerging contaminated sites due to uncontrolled e-waste recycling. Chem. Eng. J. 430, 133169 (2022).
Zhou, Y. et al. Synthetic organic antibiotics residues as emerging contaminants waste-to-resources processing for a circular economy in China: challenges and perspective. Environ. Res 211, 113075 (2022).
Abdel Wahaab, R. & Alseroury, F. A. Wastewater treatment: a case study of electronics manufacturing industry. Int. J. Environ. Sci. Technol. 16, 47–58 (2019).
Meyer, C., Stravs, M. A. & Hollender, J. How wastewater reflects human metabolism─suspect screening of pharmaceutical metabolites in wastewater influent. Environ. Sci. Technol. 58, 9828–9839 (2024).
Badea, S.-L. & Niculescu, V.-C. Recent progress in the removal of legacy and emerging organic contaminants from wastewater using metal–organic frameworks: an overview on adsorption and catalysis processes. Materials 15, 3850 (2022).
Vázquez-Tapia, I. et al. Occurrence of emerging organic contaminants and endocrine disruptors in different water compartments in Mexico – a review. Chemosphere 308, 136285 (2022).
Rojas, S. & Horcajada, P. Metal–organic frameworks for the removal of emerging organic contaminants in water. Chem. Rev. 120, 8378–8415 (2020).
Li, Q. et al. Heavy metal-contained wastewater in China: discharge, management and treatment. Sci. Total Environ. 808, 152091 (2022).
Zhang, X., Gu, P. & Liu, Y. Decontamination of radioactive wastewater: state of the art and challenges forward. Chemosphere 215, 543–553 (2019).
Jin, H. et al. Electrochemical upcycling of uranyl from radioactive organic wastewater with a self-standing covalent-organic framework electrode. Nat. Commun. 16, 3574 (2025).
Geetha Varma, V., Jha, S., Himesh Karthik Raju, L., Lalith Kishore, R. & Ranjith, V. A review on decentralized wastewater treatment systems in India. Chemosphere 300, 134462 (2022).
Abujazar, M. S. S., Karaağaç, S. U., Abu Amr, S. S., Alazaiza, M. Y. D. & Bashir, M. J. K. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: a review. J. Clean. Prod. 345, 131133 (2022).
Korajkic, A., Kelleher, J., Shanks, O. C., Herrmann, M. P. & McMinn, B. R. Effectiveness of two wastewater disinfection strategies for the removal of fecal indicator bacteria, bacteriophage, and enteric viral pathogens concentrated using dead-end hollow fiber ultrafiltration (D-HFUF). Sci. Total Environ. 831, 154861 (2022).
Soares, C. et al. Removal of diclofenac and sulfamethoxazole from aqueous solutions and wastewaters using a three-dimensional electrochemical process. J. Environ. Chem. Eng. 10, 108419 (2022).
Mohapatra, S. et al. Photodegradation of a mixture of five pharmaceuticals commonly found in wastewater: experimental and computational analysis. Environ. Res 216, 114659 (2023).
Srinivasan, N. R., Kamaraj, M. & Prabhu, S. V. Strategies and limitations of water treatment methods for point-of-use application. In Strategies and Tools for Pollutant Mitigation (eds. Aravind, J., Kamaraj, M., Prashanthi Devi, M., Rajakumar, S.) 117–133 (Springer International Publishing, Cham, 2021).
Mahmoodi, M. & Pishbin, E. Ozone-based advanced oxidation processes in water treatment: recent advances, challenges, and perspective. Environ. Sci. Pollut. Res. 32, 3531–3570 (2025).
Bello, M. M., Abdul Raman, A. A. & Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 126, 119–140 (2019).
Ang, W. L., Nordin, D., Mohammad, A. W., Benamor, A. & Hilal, N. Effect of membrane performance including fouling on cost optimization in brackish water desalination process. Chem. Eng. Res. Des. 117, 401–413 (2017).
Jafari, M. et al. Cost of fouling in full-scale reverse osmosis and nanofiltration installations in the Netherlands. Desalination 500, 114865 (2021).
United Nations. Blueprint for Acceleration: Sustainable Development Goal 6 Synthesis Report on Water and Sanitation 2023. https://www.unwater.org/publications/sdg-6-synthesis-report-2023 (2023).
United Nations Human Settlements Programme (UN-Habitat) & World Health Organization (WHO). Progress on the Proportion of Domestic and Industrial Wastewater Flows Safely Treated—Mid-Term Status of SDG Indicator 6.3.1 and Acceleration Needs, with a Special Focus on Climate Change, Wastewater Reuse and Health. https://www.unwater.org/sites/default/files/2024-08/SDG6_Indicator_Report_631_Progress-on-Wastewater-Treatment_2024_EN_0.pdf (2024).
Ahmadijokani, F. et al. COF and MOF Hybrids: advanced materials for wastewater treatment. Adv. Funct. Mater. 34, 2305527 (2024).
Zhai, Q.-G. et al. An ultra-tunable platform for molecular engineering of high-performance crystalline porous materials. Nat. Commun. 7, 13645 (2016).
Qiu, S. & Zhu, G. Molecular engineering for synthesizing novel structures of metal–organic frameworks with multifunctional properties. Coord. Chem. Rev. 253, 2891–2911 (2009).
Zhou, H.-C. “Joe” & Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 43, 5415–5418 (2014).
Li, H., Eddaoudi, M., O’Keeffe, M. & Yaghi, O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402, 276–279 (1999).
Luque, R., Yahya Tahir, M. & Ahmad, A. 9 MOF – a promising material for energy applications. In ZIF-8 Based Materials for Pharmaceutical Waste (eds. Awais Ahmad, A. Pervaiz, M., Saeed, Z., Luque, R., Alsaiari, M., & Harraz, A. F.) 109–136 (De Gruyter, 2023).
Song, K. et al. Tailoring the crystal forms of the Ni-MOF catalysts for enhanced photocatalytic CO2-to-CO performance. Appl Catal. B 309, 121232 (2022).
Shen, Y. et al. Programmable logic in metal–organic frameworks for catalysis. Adv. Mater. 33, e2007442 (2021).
Mahzari, M. Y., Ullah, S. & Assiri, M. A. Pre and post synthetic modifications of MOF-5 by graphene oxide and deep eutectic solvents for chemisorption and physisorption of CO2. Ceram. Int. 51, 10262–10272 (2025).
Yang, Z. et al. Tuning adsorption capacity of metal–organic frameworks with Al3+ for phosphorus removal: Kinetics, isotherm and regeneration. Inorg. Chem. Commun. 132, 108804 (2021).
Doherty, C. M. et al. Using functional nano- and microparticles for the preparation of metal–organic framework composites with novel properties. Acc. Chem. Res. 47, 396–405 (2014).
Kim, M. et al. MOF-derived nanoporous carbons with diverse tunable nanoarchitectures. Nat. Protoc. 17, 2990–3027 (2022).
Abdel-Mageed, A. M. & Rungtaweevoranit, B. Metal-organic frameworks-based heterogeneous single-atom catalysts (MOF-SACs) – assessment and future perspectives. Catal. Today 439, 114786 (2024).
Xu, K. et al. Recent progress of MOF-functionalized nanocomposites: from structure to properties. Adv. Colloid Interface Sci. 323, 103050 (2024).
Niu, X. et al. Defect-engineered chiral metal–organic frameworks. Microchim. Acta 191, 458 (2024).
Annamalai, J. et al. Synthesis of various dimensional metal organic frameworks (MOFs) and their hybrid composites for emerging applications – a review. Chemosphere 298, 134184 (2022).
Islam, I. U. et al. MOF and MOF-based membranes: promising solutions for pharmaceutical wastewater treatment. J. Mater. Sci. 60, 3634–3662 (2025).
Jiang, J. et al. Biomass-MOF composites in wastewater treatment, air purification, and electromagnetic radiation adsorption – a review. Chem. Eng. J. 494, 152932 (2024).
Manzoor, M. H. et al. Wastewater treatment using metal-organic frameworks (MOFs). Appl Mater. Today 40, 102358 (2024).
Shah, S. S. A. et al. Recent trends in wastewater treatment by using metal-organic frameworks (MOFs) and their composites: a critical view-point. Chemosphere 349, 140729 (2024).
Liu, X., Shan, Y., Zhang, S., Kong, Q. & Pang, H. Application of metal organic framework in wastewater treatment. Green. Energy Environ. 8, 698–721 (2023).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Batten, S. R. et al. Terminology of metal–organic frameworks and coordination polymers (IUPAC recommendations 2013). Pure Appl. Chem. 85, 1715–1724 (2013).
Chae, H. K. et al. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427, 523–527 (2004).
Yaghi, O. M., Li, G. & Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 378, 703–706 (1995).
Rowsell, J. L. C. & Yaghi, O. M. Metal–organic frameworks: a new class of porous materials. Microporous Mesoporous Mater. 73, 3–14 (2004).
Planas, N. et al. The mechanism of carbon dioxide adsorption in an alkylamine-functionalized metal–organic framework. J. Am. Chem. Soc. 135, 7402–7405 (2013).
He, Y., Li, B., O’Keeffe, M. & Chen, B. Multifunctional metal–organic frameworks constructed from meta-benzenedicarboxylate units. Chem. Soc. Rev. 43, 5618–5656 (2014).
Anastas, P. & Eghbali, N. Green chemistry: principles and practice. Chem. Soc. Rev. 39, 301–312 (2010).
Wang, W. et al. Metal–organic framework composites from a mechanochemical process. Mol. Syst. Des. Eng. 8, 560–579 (2023).
Xu, C., De, S., Balu, A. M., Ojeda, M. & Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 51, 6698–6713 (2015).
Jin, C. et al. Post-synthetic ligand exchange by mechanochemistry: toward green, efficient, and large-scale preparation of functional metal–organic frameworks. Chem. Mater. 35, 4489–4497 (2023).
Sun, N. et al. MOF-Based electrocatalysts: an overview from the perspective of structural design. Chem. Rev. 125, 2703–2792 (2025).
Wang, J. et al. Tunable reconstruction of metal-organic frameworks for advanced electrocatalytic degradation of antibiotics: key role of structural defects and pollutant properties. J. Hazard Mater. 494, 138456 (2025).
Shi, W. et al. Metal–organic framework with a redox-active bridge enables electrochemically highly selective removal of arsenic from water. Environ. Sci. Technol. 57, 6342–6352 (2023).
Kim, Y., Kim, K., Eom, H. H., Su, X. & Lee, J. W. Electrochemically-assisted removal of cadmium ions by redox active Cu-based metal-organic framework. Chem. Eng. J. 421, 129765 (2021).
Zhou, S.-H. et al. Multifunctional Ni-MOF (3D) with N-rich structures for self-generated reactive oxygen species and enhanced electron transfer for synergistic degradation of organic pollutants and removal of Hg(II). J. Clean. Prod. 476, 143807 (2024).
Stock, N. & Biswas, S. Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem. Rev. 112, 933–969 (2012).
Perry, J. J. IV, Perman, J. A. & Zaworotko, M. J. Design and synthesis of metal–organic frameworks using metal–organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 38, 1400 (2009).
Zhao, Y., Li, K. & Li, J. Review. Solvothermal synthesis of multifunctional coordination polymers. Z. für Naturforsch. B 65, 976–998 (2010).
Nanda, B., Sailaja, M., Mohapatra, P., Pradhan, R. K. & Nanda, B. B. Green solvents: a suitable alternative for sustainable chemistry. Mater. Today Proc. 47, 1234–1240 (2021).
Clarke, C. J., Tu, W.-C., Levers, O., Bröhl, A. & Hallett, J. P. Green and sustainable solvents in chemical processes. Chem. Rev. 118, 747–800 (2018).
Ren, J. et al. Review on the current practices and efforts towards pilot-scale production of metal-organic frameworks (MOFs). Coord. Chem. Rev. 352, 187–219 (2017).
Rubio-Martinez, M. et al. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 46, 3453–3480 (2017).
Liu, Z., Zhu, J., Peng, C., Wakihara, T. & Okubo, T. Continuous flow synthesis of ordered porous materials: from zeolites to metal–organic frameworks and mesoporous silica. React. Chem. Eng. 4, 1699–1720 (2019).
Ma, M., Lu, L., Li, H., Xiong, Y. & Dong, F. Functional metal organic framework/SiO2 nanocomposites: from versatile synthesis to advanced applications. Polymers11, 1823 (2019).
Chen, J. E. et al. Current progress and scalable approach toward the synthesis of 2D metal–organic frameworks. Adv. Mater. Interfaces 9, 2102560 (2022).
Bagi, S., Yuan, S., Rojas-Buzo, S., Shao-Horn, Y. & Román-Leshkov, Y. A continuous flow chemistry approach for the ultrafast and low-cost synthesis of MOF-808. Green. Chem. 23, 9982–9991 (2021).
Bagi, S. D., Myerson, A. S. & Román-Leshkov, Y. Solvothermal crystallization kinetics and control of crystal size distribution of MOF-808 in a continuous flow reactor. Cryst. Growth Des. 21, 6529–6536 (2021).
Guan, Y. et al. Monodispersed ZIF-8 particles with enhanced performance for CO2 adsorption and heterogeneous catalysis. Appl Surf. Sci. 423, 349–353 (2017).
Chang, C.-C. et al. Nanoparticle delivery of MnO 2 and antiangiogenic therapy to overcome hypoxia-driven tumor escape and suppress hepatocellular carcinoma. ACS Appl. Mater. Interfaces 12, 44407–44419 (2020).
Wu, H.-Y. et al. Continuous and ultrafast MOF synthesis using droplet microfluidic nanoarchitectonics. J. Mater. Chem. A Mater. 11, 9427–9435 (2023).
Razavi Bazaz, S. et al. Obstacle-free planar hybrid micromixer with low pressure drop. Microfluid Nanofluidics 24, 61 (2020).
Amar, K., Embarek, D. & Sofiane, K. Parametric study of the crossing elongation effect on the mixing performances using short two-layer crossing channels micromixer (tlccm) geometry. Chem. Eng. Res. Des. 158, 33–43 (2020).
Tan, Z., Shi, H., Zheng, Y. & Cao, Y. A 3D homogeneous microreactor with high mixing intensity at wide Re range for MOF preparation and POCT application. Chem. Eng. J. 476, 146481 (2023).
Rasmussen, E. G., Kramlich, J. & Novosselov, I. V. Scalable continuous flow metal–organic framework (MOF) synthesis using supercritical CO 2. ACS Sustain Chem. Eng. 8, 9680–9689 (2020).
Arfelis, S. et al. Linking mechanochemistry with the green chemistry principles: review article. Heliyon 10, e34655 (2024).
Jin, C. et al. Green and large-scale preparation of chiral metal–organic frameworks via mechanochemistry. Inorg. Chem. 61, 12190–12196 (2022).
Tegudeer, Z., Davenport, L. C., Kordesch, M. E. & Gao, W.-Y. Harnessing mechanochemistry for direct synthesis of imine-based metal–organic frameworks. J. Am. Chem. Soc. 147, 13522–13530 (2025).
Głowniak, S., Szczęśniak, B., Choma, J. & Jaroniec, M. Advances in microwave synthesis of nanoporous materials. Adv. Mater. 33, e2103477 (2021).
Phan, P. T., Hong, J., Tran, N. & Le, T. H. The properties of microwave-assisted synthesis of metal–organic frameworks and their applications. Nanomaterials 13, 352 (2023).
Nguyen, V. H., Pham, A. L. H., Nguyen, V.-H., Lee, T. & Nguyen, T. D. Facile synthesis of bismuth(III) based metal-organic framework with difference ligands using microwave irradiation method. Chem. Eng. Res. Des. 177, 321–330 (2022).
Yang, B. et al. Microwave synthesis of Fe–Cu diatomic active center MOF: synergistic cyclic catalysis of persulfate for degrading norfloxacin. Environ. Sci. Nano 10, 2778–2789 (2023).
Jung, W.-T. et al. Rapid microwave synthesis of MOF microrods: dispersive SPE coupled with UHPLC–MS/MS to determine fluoroquinolones in honey. Talanta 263, 124733 (2023).
Taddei, M., Steitz, D. A., van Bokhoven, J. A. & Ranocchiari, M. Continuous-flow microwave synthesis of metal–organic frameworks: a highly efficient method for large-scale production. Chem. A. Eur. J. 22, 3245–3249 (2016).
He, T., Kong, X.-J. & Li, J.-R. Chemically stable metal–organic frameworks: rational construction and application expansion. Acc. Chem. Res. 54, 3083–3094 (2021).
Yuan, S. et al. Stable metal–organic frameworks: design, synthesis, and applications. Adv. Mater. 30, e1704303 (2018).
Wang, L. et al. The chemical stability of metal-organic frameworks in water treatments: fundamentals, effect of water matrix and judging methods. Chem. Eng. J. 450, 138215 (2022).
Li, Y.-X. et al. Loosening metal nodes in metal-organic frameworks to facilitate the regulation of valence. Fund. Res. 5, 158–164 (2025).
Qi, S. et al. Generation of hierarchical porosity in metal–organic frameworks by the modulation of cation valence. Angew. Chem. Int. Ed. 58, 10104–10109 (2019).
Cruz-Navarro, J. A., Hernandez-Garcia, F. & Alvarez Romero, G. A. Novel applications of metal-organic frameworks (MOFs) as redox-active materials for elaboration of carbon-based electrodes with electroanalytical uses. Coord. Chem. Rev. 412, 213263 (2020).
Shen, N. et al. 99TcO4− removal from legacy defense nuclear waste by an alkaline-stable 2D cationic metal organic framework. Nat. Commun. 11, 5571 (2020).
Zhang, X. et al. Palladium(II) modulation enhances the water stability and aqueous 99 TcO 4 – /ReO 4 – removal performance of metal–organic frameworks. Inorg. Chem. 63, 16726–16732 (2024).
Huang, Y. et al. Novel metal-organic framework hydrogel for enhanced selective removal of uranyl ions from nuclear wastewater. Environ. Res. 266, 120470 (2025).
Fang, H. et al. A cobalt ferricyanide/ZIF-67/composted humus gel composite for improved cesium ion removal from wastewater. J. Water Process Eng. 75, 107752 (2025).
Li, J. et al. Revolutionary MOF-enhanced anion exchange membrane for precise monovalent anion separation through structural optimization and doping. Desalination 576, 117352 (2024).
Zeng, X. et al. Anionic MOFs embedded in anion-exchange membranes for the separation of lithium/magnesium cations. ACS Sustain Chem. Eng. 11, 12877–12887 (2023).
Saeed, F., Anil Reddy, K. & Sridhar, S. Efficient removal of environmentally hazardous heavy metal ions from water by NH2-MIL-101-Fe and MOF-808-EDTA based polyether sulfone adsorbents. Chem. Eng. J. 497, 154688 (2024).
Gai, S. et al. From precious metal recovery to value-added MOF synthesis: stable ZIF-8-hydrogel beads for sustainable multistage pollutant treatment. Sep. Purif. Technol. 367, 132900 (2025).
Al-Omari, M. H. et al. Optimized Congo red dye adsorption using ZnCuCr-based MOF for sustainable wastewater treatment. Langmuir 41, 5947–5961 (2025).
Song, Q. et al. Catalytic degradation of carbamazepine by metal organic frameworks (MOFs) derived magnetic catalyst Fe@PC in an electro-Fenton coupled membrane filtration system: performance, pathway, and mechanism. Sep. Purif. Technol. 309, 122988 (2023).
Duan, L., Jiang, H., Wu, W., Lin, D. & Yang, K. Defective iron based metal-organic frameworks derived from zero-valent iron for highly efficient Fenton-like catalysis. J. Hazard Mater. 445, 130426 (2023).
Li, M. et al. Iron-organic frameworks as effective fenton-like catalysts for peroxymonosulfate decomposition in advanced oxidation processes. NPJ Clean. Water 6, 37 (2023).
Wu, Y. et al. Boosting the degradation of antibiotics via peroxymonosulfate activation with a Cu-based metal–organic framework. Chem. Sci. 15, 9733–9741 (2024).
Younes, H. A., Taha, M., Khaled, R., Mahmoud, H. M. & Abdelhameed, R. M. Perovskite/metal-organic framework photocatalyst: a novel nominee for eco-friendly uptake of pharmaceuticals from wastewater. J. Alloy. Compd. 930, 167322 (2023).
Essam, A., Eldek, S. I. & Shehata, N. Management of caffeine in wastewater using MOF and perovskite materials: optimization, kinetics, and adsorption isotherm modelling. J. Environ. Health Sci. Eng. 22, 345–360 (2024).
Xu, H. et al. Efficient degradation of sulfonamides by introducing sulfur to magnetic Prussian blue analog in photo-assisted persulfate oxidation system. Chemosphere 357, 141938 (2024).
Zhou, J. et al. 2D/2D Ni-MOF/BiOCl S-scheme heterojunction with boosted charge transfer and photocatalytic degradation of tetracycline. Sustain. Mater. Technol. 39, e00825 (2024).
Bose, S. et al. Suitability of a diamine functionalized metal–organic framework for direct air capture. Chem. Sci. 14, 9380–9388 (2023).
O’Keefe, C. A. et al. NMR-Enhanced crystallography aids open metal–organic framework discovery using solvent-free accelerated aging. Chem. Mater. 32, 4273–4281 (2020).
Hosseini, S. E., Mohammadi, M. K., Hayati, P., Tavakkoli, H. & Rayatzadeh, A. Controlled morphology of a new 3D Co(II) metal–organic framework (Co-MOF) via green sonochemical synthesis: crystallography, Hirshfeld surface analysis. J. Nanopart. Res. 26, 100 (2024).
Vaitsis, C. et al. Sonochemical synthesis of zinc adipate Metal-Organic Framework (MOF) for the electrochemical reduction of CO2: MOF and circular economy potential. Sustain. Chem. Pharm. 29, 100786 (2022).
Liu, Y. et al. Electrochemical synthesis of large area two-dimensional metal–organic framework films on copper anodes. Angew. Chem. Int. Ed. 60, 2887–2891 (2021).
Zhang, Y. et al. An ultra-sensitive electrochemical aptasensor based on Co-MOF/ZIF-8 nano-thin-film by the in-situ electrochemical synthesis for simultaneous detection of multiple biomarkers of breast cancer. Microchem. J. 187, 108316 (2023).
Nisa, Z., Ashashi, N. A. & Sheikh, H. N. Synthesis of metal–organic frameworks with ionic liquids. In Synthesis of Metal-Organic Frameworks Via Water-based Routes (eds. Yasser Azim, Y., Rather, S. U., Bhatt, P. M.) 143–158 (Elsevier, 2024).
Teixeira, M., Maia, R. A., Karmazin, L., Louis, B. & Baudron, S. A. Ionothermal synthesis of calcium-based metal–organic frameworks in a deep eutectic solvent. CrystEngComm 24, 601–608 (2022).
Liu, H. et al. Role of supercritical carbon dioxide (scCO 2) in fabrication of inorganic-based materials: a green and unique route. Sci. Technol. Adv. Mater. 22, 695–717 (2021).
Fu, J. & Wu, Y. A showcase of green chemistry: sustainable synthetic approach of zirconium-based MOF materials. Chem. A Eur. J. 27, 9967–9987 (2021).
Gao, Z., Wang, H., Hu, Y. & Sun, J. Bimetallic MnZn-MOF-74 with enhanced percentage of MnIII: efficiently catalytic activity for direct oxidative carboxylation of olefins to cyclic carbonates under mild and solvent-free condition. J. Colloid Interface Sci. 671, 232–247 (2024).
Sun, Q., Duan, Z., Deng, H., Li, R. & Li, J.-R. Green and rapid synthesis of a two-dimensional Zn-MOF with Lewis acid–base sites via a solvent-free strategy. J. Mater. Chem. A Mater. 13, 12911–12916 (2025).
Zhi, T., Li, D., Li, Y., Yi, Z., Zhu, S. & Zhang, L. Spray-dried MOF-derived bimetallic oxide/carbon hybrids with superior electron transfercapability for catalyzing ammonium perchlorate decomposition. Def. Technol. (2025). https://doi.org/10.1016/j.dt.2025.08.007.
Perbet, M. et al. Upscaled al-fumarate synthesis and shaping by spray drying. Ind. Eng. Chem. Res. 64, 6541–6549 (2025).
Bhakat, P., Nigam, A. & Jagtap, S. Green synthesis of MOF nanostructures: environmental benefits and applications. Nanotechnol. Environ. Eng. 8, 815–827 (2023).
Shaiful Bahari, A. M. et al. Facile synthesis of Zr-based metal-organic gel (Zr-MOG) using “green” sol-gel approach. Surf. Interfaces 27, 101469 (2021).
Pan, S.-Y., Hsiao, Y.-Y., Negi, S., Matsagar, B. M. & Wu, K. C.-W. Green synthesis of waste-derived metal–organic frameworks for organic substance extraction from piggery wastewater as biofertilizers. ACS Sustain. Chem. Eng. 12, 17793–17805 (2024).
Liu, J., Li, Y. & Lou, Z. Recent advancements in MOF/biomass and bio-MOF multifunctional materials: a review. Sustainability 14, 5768 (2022).
Author information
Authors and Affiliations
Contributions
Authors’ Contributions:Austine Ofondu Chinomso Iroegbu (AOCI): Conceptualization, methodology, literature investigation, formal analysis, original draft preparation, visualization, and critical revisions. Moipone Linda Teffo (MLT): Supervision, validation, critical revisions and editing. Emmanuel Rotimi Sadiku (ERS): Supervision, validation, critical revisions and language editing. Reinout Meijboom (RM): Literature investigation, critical revisions and language editing. Shanganyane Percy Hlangothi (SPH): Literature investigation, ciritcal revisions and langauge editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Iroegbu, A.O.C., Teffo, M.L., Sadiku, E.R. et al. Advancing wastewater treatment with green and scalable metal–organic frameworks: from synthetic strategies to real-world deployment. npj Clean Water 8, 85 (2025). https://doi.org/10.1038/s41545-025-00514-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41545-025-00514-x