Abstract
Time-stamped cross-sectional data, which lack linkage across time points, are commonly generated in single-cell transcriptional profiling. Many previous methods for inferring gene regulatory networks (GRNs) driving cell-state transitions relied on constructing single-cell temporal ordering. Introducing COSLIR (COvariance restricted Sparse LInear Regression), we presented a direct approach to reconstructing GRNs that govern cell-state transitions, utilizing only the first and second moments of samples between two consecutive time points. Simulations validated COSLIR’s perfect accuracy in the oracle case and demonstrated its robust performance in real-world scenarios. When applied to single-cell RT-PCR and RNAseq datasets in developmental biology, COSLIR competed favorably with existing methods. Notably, its running time remained nearly independent of the number of cells. Therefore, COSLIR emerges as a promising addition to GRN reconstruction methods under cell-state transitions, bypassing the single-cell temporal ordering to enhance accuracy and efficiency in single-cell transcriptional profiling.
Similar content being viewed by others
Introduction
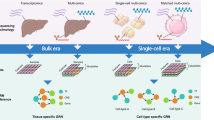
Over the past decade, the development of single-cell omics measurements has been a major breakthrough in experimental biotechnology. This innovation has generated an immense amount of data, providing critical insights into biological systems and complex diseases1,2,3,4,5,6. However, the sacrifice of individual cells in each assay is an inherent trade-off in single-cell omics experiments. As a result, cells measured at different times or developmental stages are from independent batches, precluding the acquisition of true longitudinal time series data. As shown in Fig. 1A, the current paradigm is limited to the generation of time-stamped cross-sectional data (TSCS) rather than comprehensive longitudinal data sets with real-time information7.
Despite extensive efforts over the past two decades, including the application of classical autoregressive models, differential equations, dynamic Bayesian networks, and various other dynamical models to fit both bulk and single-cell gene expression data8,9,10, challenges remain. Experimentalists have sometimes employed techniques such as cell-state synchronization or repeated sampling from larger cell cultures to generate data where trends in mean expression over time can be reasonably viewed as a time series8,11,12.
Even with bulk gene expression data, several computational temporal-ordering methods have been proposed13,14,15. And with the increasing of single-cell transcriptomic data, the field of reconstructing temporal information is rapidly advancing10,16. However, recent findings highlighted the sensitivity of gene regulatory network (GRN) inference methods to the precision of pseudo-time series construction, compromising their stability17. Several alternative approaches that do not rely on single-cell temporal ordering have been proposed to infer GRNs from time-stamped cross-sectional (TSCS) single-cell expression data18,19,20,21,22. However, most of these methods were limited to a single cell stage or time point, and the inferred network primarily represented the mechanism that maintains the current cell stage rather than the mechanism that drives the cell state transition along the cell lineage.
Furthermore, differential expression gene (DEG) analysis typically captures a plethora of potential upstream regulatory genes, resulting in a densely populated gene regulatory network (GRN) responsible for maintaining a single cell state, reflecting the inherent biological complexity. Conversely, GRNs that drive cell state transitions are considered sparse23. By identifying this smaller subset of potential upstream regulatory genes and discerning the possible regulatory relationships between them, subsequent biological functional analysis becomes more straightforward.
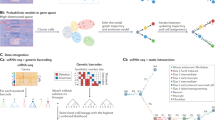
Hence, this paper took a direct modeling approach to characterize the Gene Regulatory Network (GRN) governing the temporal evolution of gene expression. We introduced a novel method, Covariance Restricted Sparse Linear Regression (COSLIR), which relies exclusively on the first and second moments of the measured sample (Fig. 1). Linear regression models as well as the assumption of sparsity are widely used in statistical modeling of time-series data, spanning fields such as economics, Kalman filtering, and systems biology24,25,26,27,28,29,30,31,32,33,34,35,36. The COSLIR model also employed the linear regression structure, but only used the estimated first two moments of single-cell samples at two consecutive time points.
COSLIR yielded a directed GRN, providing both signs and weights for each gene-gene interaction (Fig. 1D). The optimization problem was addressed using the alternative direction method of multipliers algorithm (ADMM37) (Fig. 1C). To refine the precision and stability of the estimator, bootstrapping38 and clipping thresholding techniques were implemented to select significant gene-gene interactions. The evaluation of COSLIR utilized published single-cell RT-PCR and RNA-seq gene expression datasets. Remarkably, COSLIR demonstrated performance on par with the best previous methods but with fewer assumptions and requirements. Furthermore, COSLIR’s running time remained nearly independent of the number of cells, ensuring its adaptability to large, recently generated datasets.
Methods
Models and assumptions
Let \(X_t\) and \(X_{t+1}\) denote two p-dimensional vectors representing the expression values of p genes within the same single cell at stages t and \(t+1\), respectively. To model the dynamic evolution from \(X_t\) to \(X_{t+1}\), we introduced a \(p \times p\) matrix \(A_t\), where the element \((A_t)_{ij}\) in the i-th row and j-th column represents the regulatory strength from gene j to gene i.
For simplicity, we used the following linear regression model, where the dependent variable is the difference between \(X_{t+1}\) and \(X_t\), and the explanatory variable is \(X_t\):
where the noise term \(\varepsilon _t\) is a p-dimensional random vector, independent of \(X_t\), with mean \(l_t\) and finite covariance matrix \(D_t\). Here, \(l_t\) represents some deterministic influence induced by the environment.
However, as pointed out in the introduction, the challenge arises from the fact that once we measure \(X_t\) or \(X_{t+1}\), the other becomes inaccessible. There is no correspondence between the cross-sectional single-cell data collected at stages t and \(t+1\), rendering traditional least-squares approaches ineffective. Even with an infinite sample size, the ultimate information obtainable remains limited to the exact distributions of \(X_t\) and \(X_{t+1}\), without providing any temporal information.
In COSLIR, the estimation of \(A_t\) relies solely on the first and second moments of \(X_t\) and \(X_{t+1}\), and the samples are not constrained to follow a normal distribution. Let \(\mu _t\) and \(\Sigma _t\) denote the mean and covariance of the p-dimensional random vector \(X_t\). The following equations can be derived from (1):
where I is the identity matrix.
Although the general methods of moments to infer parameters have been widely used in statistics, one always needs the covariance between the predictor and response when estimating parameters in linear regression model, which is absent in time-stamped cross-sectional data. Therefore, we only had equation (2) , which is underdetermined with respect to \(A_t\), implying infinitely many solutions for \(A_t\) even when \(\Sigma _{\cdot }\), \(\mu _{\cdot }\), \(l_{\cdot }\), and \(D_{\cdot }\) are known. Then we assumed sparsity in \(A_t\), and small values for both \(l_t\) and \(D_t\), indicating a dominance of linear relationships in this scenario. Sparsity is a common assumption in statistical learning24,25,27, aligning with observations in single-cell biology23. Following the principles of compressed sensing with nonlinear observations28 and applying the penalty method, we obtained the sparse matrix \(A_t\) by solving the non-convex optimization problem outlined in (3) (Fig. 1C, Supplementary Material and Methods):
where \(\hat{\Sigma }_{\cdot }\) and \(\hat{\mu }_{\cdot }\) are the estimates of the covariance and mean of \(X\) (Fig. 1B). \(||\cdot ||_F\) denotes the Frobenius norm, and \(||\cdot ||_1\) denotes the sum of the absolute values of all elements in a matrix.
In practical scenarios, we utilized the estimators \(\hat{\mu }\) and \(\hat{\Sigma }\) when the exact mean and covariance are unknown. For relatively large sample sizes, the naive sample mean and covariance estimators are recommended. However, in cases where the sample size is small compared to the number of components, resulting in potentially high variance, the sample mean and covariance may yield inaccurate estimators for \(A_t\). In such situations, the use of high-dimensional techniques39,40,41,42 was recommended to obtain more accurate estimates of mean and covariance. In the context of single-cell RNA-seq data, preliminary imputation techniques can be used to remove technical noise, such as dropout or batch effects43, before estimating the mean and covariance.
For simplicity, in the subsequent numerical experiments, we exclusively utilized the sample estimators \(\hat{\mu }\) and \(\hat{\Sigma }\). However, a correction was applied to the covariance matrix using
where I is an identity matrix, and \(\alpha =0.01\) ensures positive definiteness.
Two tuning parameters, \(\eta\) and \(\lambda\), play crucial roles. The first two terms in (3) regulate the quantitative relationships between the mean and covariance of \(X_t\) and \(X_{t+1}\) based on (1), while \(\lambda\) controls the sparsity of \(A_t\).
It is important to note that COSLIR should be applied to each pair of consecutive cell stages or time points and does not presuppose the gene regulatory network to be invariant with respect to time t.
Solving the optimization problem using ADMM
We employed an efficient numerical algorithm, the Alternating Direction Method of Multipliers (ADMM37), to solve (3). Initially, we introduced two constraints, \(A+I=(B+C)/2\) and \(B=C\), and subsequently, we partitioned the variables into three components for the application of ADMM. Introducing B and C is a common technique that significantly simplifies each substep in the ADMM algorithm.
The optimization problem can then be reformulated as follows:
In each iteration using ADMM, we simply minimised and updated A, B, C separately. Such an iteration will lead to a reasonably converged state (with an adaptively tuned step size). More explicitly, the algorithm is written in Algorithm (1).
Importantly, for the subproblems involving the update of A, B, C, the objective function is at most quadratic (and hence convex) when focusing on a single variable. This characteristic implies that, under the optimality condition for each subproblem, we only need to solve linear equations and can consistently obtain closed-form solutions. This property significantly contributes to the efficiency of our algorithm.
Furthermore, we have incorporated optimization techniques into our published code to accelerate the convergence rate of ADMM. These techniques, proven to be effective in both simulation studies and real data experiments44,45, enhance the performance of the algorithm.
Upon solving the optimization problem, the non-zero elements in \(A_t\) denote the regulatory relationships between genes (Fig. 1D). It is noteworthy that (3) constitutes a non-convex optimization problem, potentially leading to multiple local optima. However, we observed that initiating the ADMM algorithm with a zero matrix consistently yields a reasonable solution.
Bootstrapping and clipping thresholding
Estimating the mean and covariance introduces potential imprecision in predicting \(A_t\). To enhance both robustness and precision-of greater interest to experimentalists17-we employed the nonparametric bootstrapping technique46. In essence, we repeated the following procedure multiple times and aggregate all assembled estimators for \(A_t\) into an ensemble: initially, perform random sampling with replacement from the two collections of samples at different stages or times, forming two new collections of observations; subsequently, apply COSLIR to the new sample collections to acquire a fresh estimator for \(A_t\). Finally, only those non-zero elements with a confidence level (repetition ratio with the same sign) surpassing a specified threshold were retained in the ultimate estimator of the interaction matrix \(A_t\).
Furthermore, in practical scenarios, we implemented an additional clipping thresholding procedure on the output to discard non-zero entries with very small absolute values below a certain threshold (entries with negligible values in \(\hat{A}_t\) are likely due to noise). Following clipping thresholding and bootstrapping, we regarded the remaining non-zero entries as statistically significant, with their mean constituting the final estimator.
Model selection and evaluation
There are four hyperparameters to determine in the aforementioned process: two (\(\lambda\) and \(\eta\)) in the optimization problem and two (confidence threshold and clipping threshold) in the bootstrapping process.
\(\lambda\) controls the sparsity of \(A_t\), while \(\eta\) governs the noise term in (3). As our model is unsupervised due to the absence of simultaneously measured \(X_t\) and \(X_{t+1}\), cross-validation is impractical. To address this challenge, we empirically relied on three indices as criteria for model selection:
Here, \(e_{\Sigma }\) and \(e_{\mu }\) quantify the small noise terms in (2), while \(s_0(\hat{A})\) gauges the sparsity of \(\hat{A_t}\). We selected the model with all three indices minimized, indicating the sparsest matrix \(A_t\) with \(e_{\Sigma }\) and \(e_{\mu }\) below a reasonable level. Our simulation study demonstrated the effectiveness of this proposed criterion (see Supplementary Results, Supplementary Fig. S2, S3, Supplementary Tables S2, S3). To simplify the process, during bootstrapping, we initially determined the hyperparameters \(\lambda\) and \(\eta\) using the entire sample and then used only these determined values for subsequent analyses. Throughout the paper, we typically set \(\lambda = 10^{-6}\) and \(\eta =5\).
In the oracle case of the simulation study, a substantial gap always exists between the values of the non-zero elements, making the determination of the clipping threshold straightforward. However, in the sample case, it is less clear which value of the clipping threshold to choose. We proposed determining the clipping threshold based on the trade-off between the three indices of the criterion in (7). Specifically, we let \(\hat{A_t}(\varepsilon )\) be the adjusted estimator after taking \(\varepsilon\) as the clipping threshold. We determined the threshold \(\varepsilon\) by choosing the sparsest \(\hat{A_t}(\varepsilon )\) with \(e_{\Sigma }(\hat{A_t}(\varepsilon )) + \eta e_{\mu }(\hat{A_t}(\varepsilon ))\) below a reasonable level. Similar to the \(\lambda\) and \(\eta\) hyperparameters, this threshold was determined before bootstrapping on the full sample.
For the confidence threshold, it can be tuned, but we additionally required it to be greater than 0.5 for it to make sense.
In the simulation studies, we consider the non-zero elements with the correct sign as correctly recovered. To evaluate performance, we adopted the commonly used criteria precision and recall, i.e.,
In real datasets, when comparing COSLIR with other methods, instead of using the previous precision and recall, we used a slightly modified Early Precision from BEELINE17 for evaluation. Early precision is the number of true positives in the most significant k edges (which is also the precision of this top-k network).
Simulation settings
In the simulation studies, we first generated the mean and covariance of \(X_t\), the ground-truth interacting matrix A, as well as the mean and covariance of the noise term. Subsequently, using (2), we calculated the mean and covariance of \(X_{t+1}\). The sample data was then generated from the normal distribution based solely on the mean and covariance of \(X_t\) and \(X_{t+1}\). Furthermore, in the oracle case, we only input the mean and covariance at the two stages into our algorithm. In contrast, in the sample case, we provided the algorithm with different samples at the two stages without any time-series correspondence.
In order to make sure the generated covariance matrix \(\Sigma _t\) of \(X_t\) is positive-definite, we used the formula \(\Sigma _t = P \Lambda P^T\), where \(\Lambda \in \mathbb {R}^{p \times p}\) is a diagonal matrix with \(\Lambda _{ii} = \exp {\left( \frac{i}{p}\right) }\), \(i=1,2,\cdots ,p\), and \(P = I + R\), where I is the identity matrix. For each round of simulation, the elements of R were sampled independently from standard normal distributions. The true interacting matrix \(A_t \in \mathbb {R}^{p\times p}\) is a sparse matrix, where only \(10\%\) of the elements were non-zero and randomly generated from \(\mathcal {N}(0, 1)\) independently. The elements of the mean \(\mu _t \in \mathbb {R}^p\) of \(X_t\) were independently generated from \(\mathcal {N}(0, 100)\). The mean of each element of the noise term \(\varepsilon _t \in \mathbb {R}^p\) was set to 0.1, and the covariance matrix \(D_t\) of \(\varepsilon _t\) was set to be a diagonal matrix with \((D_t)_{ii}=0.01\) for each i. Subsequently, \(\mu _{t+1}\) and \(\Sigma _{t+1}\) were calculated using (2).
The numerical experiments were repeated 100 times in the oracle case and 50 times in the sample case. The bootstrapping procedure was repeated 50 times in each sample experiment. The obtained values of \(\eta\), \(\lambda\), and the clipping threshold were summarized in Supplementary Table S1.
Real datasets
The RT-PCR dataset47 included 442 single cells selectively collected from early mouse embryonic development. The dataset comprised mRNA expression levels of 48 genes, including 27 transcription factors, 19 known marker genes, and 2 housekeeping genes for normalization.
We used two experimental single-cell RNA sequencing (scRNA-seq) datasets: one involving human embryonic stem cell (hESC) differentiation to definitive endoderm48, and the other focused on mouse embryonic stem cell (mESC) differentiation to primitive endoderm49. Genes within the RNA-seq datasets were selected following the same strategy employed in BEELINE17. For the mESC datasets, we identified 970 genes, respectively. The datasets comprised 90 (0h), 68 (12h), 90 (24h), 82 (48h), and 91 (72h) cells across 5 time points. In the case of hESC datasets, with 814 genes, respectively, the dataset consisted of 92 (0h), 102 (12h), 66 (24h), 172 (36h), 138 (72h), and 188 (96h) cells spanning 6 time points. It’s essential to note that data from BEELINE were already normalized; thus, we utilized it post gene selection. The COSLIR bootstrapping procedure was repeated 50 times.
The single-cell human blood atlas dataset was from the official Peripheral Blood Mononuclear Cell Multiome dataset50. Wo focused on the three lineages of HSPC cells: HSPC\(\rightarrow\)B, HSPC\(\rightarrow\)Erythrocyte and HSPC\(\rightarrow\)Monocyte. There were 6044 cells in total, with 1909 HSPC cells, 1448 B cells, 1747 Erythrocyte cells and 940 Monocyte cells. For gene selection, we first selected top 2000 high variable genes. Then for each developmental lineage we determined DEGs using Seurat with default parameter. Finally we selected 623 genes during HSPC\(\rightarrow\)B, 893 genes in HSPC\(\rightarrow\)Erythrocyte and 949 genes in HSPC\(\rightarrow\)Monocyte. We took the union of them, 1475 genes in total.
We did not have any human or animal involved in this study.
Re-scaled values for selecting the most influential regulatory interactions
We recommended an additional processing step for real data analysis using COSLIR. In the analysis of single-cell expression data, one might be particularly interested in interactions that contribute more to changes in mean expression, i.e., differentially expressed genes (DEGs). Therefore, we introduced a rescaled value
to select the most influential regulatory gene-gene interactions. This technique was applied solely to the final estimator obtained after the bootstrapping procedure.
Results
Simulation study
In this study, we evaluated the performance of COSLIR through simulation studies, examining both the oracle and sample cases. The oracle case assumes knowledge of the true mean and covariance matrix, while the sample case involves working with random samples only (see simulation settings in Methods).
We did not intend to evaluate COSLIR on simulated single-cell expression data. What we really want to validate here is the optimization algorithm (3). Even when the data is generated by the linear model (1), there is no theoretical guarantee that the solution of the optimization algorithm (3) recovers the ground-truth matrix A in the absence of the cross-talk between the data at time t and time \(t+1\).
Simulation results
In the oracle cases, we evaluated three indices proposed in Eq. (7) across different values of \(\lambda\) and \(\eta\), including \(e_{\Sigma }(\hat{A})\) for the error of estimated covariance matrix, \(e_{\mu }(\hat{A})\) for the error of estimated mean, and \(s_0(\hat{A})\) for sparsity. The results exhibited robustness to variations in \(\eta\), and our criteria effectively guided the determination of optimal or suboptimal values for \(\lambda\) (see Supplementary Figures S1, S3, Table S1, S3).
Fig. 2A vividly demonstrated the almost exact recovery of the estimator obtained by COSLIR in oracle cases. This was true even when the data dimension reaches 500 and the number of gene-gene interactions in \(A_t\) to be inferred increases to 2.5 \(\times\) \(10^5\). Notably, the precision, recall and exact values of the estimator closely mirrored the ground truth values (see Supplementary Table S2). This robust performance provided confidence in the applicability of the method to simulated sample data, where separate estimation of \(\Sigma _t\), \(\Sigma _{t+1}\), \(\mu _t\) and \(\mu _{t+1}\) is mandatory.
Fig. 2B-D illustrated how COSLIR’s performance varied with the number of genes, cells and confidence threshold in the sample cases. Even with a large number of genes, high precision can be achieved by setting a sufficiently high bootstrapping confidence threshold (Fig. 2B,D). Although this comes at the cost of a lower recall compared to the scenario with a smaller number of genes (already lower than in the oracle case), as shown in Fig. 2B, it’s crucial to recognise that the matrix \(A_t\) contains an exceptionally high number of gene-gene interactions (square of the dimension). Despite the reduced recall, the actual number of successfully recovered gene interactions remained substantial.
Sample size significantly influenced COSLIR’s performance, with increasing precision and recall as sample size grows (Fig. 2C). The precision-recall trade-off when tuning the bootstrapping confidence threshold (Fig. 2D, Supplementary Fig. S4) can be tailored to specific needs in real applications. Generally, a higher confidence threshold is recommended for precision-focused studies, typical in most experimental science studies. In addition, the sparser the true matrix \(A_t\), the better the performance, as fewer samples are required (Supplementary Fig. S3).
We compared COSLIR with four established regression-based algorithms for Gene Regulatory Network (GRN) reconstruction: SINCERITIES, SCODE, SCRIBE, and SINGE, as summarized in BEELINE17. The precision and AUC of precision-recall curve of COSLIR was much higher than those of the four existing algorithms (Fig.2E, F, Supplementary Figure S5). It was not surprising, since the simulation data was generated based on Eq. 1. Although the four other algorithms are all based on similar regression models, the detailed assumptions and expressions are not the same.
Results on real datasets during early embryo development
Revealing gene expression patterns in early embryos is crucial for understanding cellular developmental processes47,51,52,53,54. However, due to the limitations of real-time experimental measurements, the detailed regulatory mechanisms that control this nascent stage of life remain unknown. In this study, we used COSLIR to infer the gene regulatory networks that govern cell fate decisions.
Single-cell RT-PCR dataset
We began our analysis by examining a dataset of published single-cell gene expression data during mouse embryonic development, obtained using the RT-PCR technique47. This dataset included 442 single cells selectively collected from early mouse embryonic development, spanning 7 developmental stages: Zygote, 2-cell stage, 4-cell stage, 8-cell stage, 16-cell stage, morula stage, and blastocyst stage. Of particular note is the blastocyst stage, where embryos manifest three distinct cell types-trophectoderm (TE), primitive endoderm (PE), and epiblast (EPI)-each characterized by unique gene markers and expression patterns55. In our analysis of the data from the 8-cell stage to the blastocyst stage (Fig. 3A and B, Supplementary Table S5), we used the non-linear dimension reduction method ISOmap56 instead of the linear dimension reduction method PCA used in Guo et al.47. This approach revealed two crucial cell fate decisions in the dataset: (1) the segregation of inner and outer cells at the 16-cell stage, leading to the formation of inner cell mass (ICM) and trophectoderm (TE), and (2) the subsequent differentiation of the ICM into primitive endoderm and epiblast.
The dataset comprised mRNA expression levels of 48 genes, including 27 transcription factors, 19 known marker genes, and 2 housekeeping genes for normalization, during the first two cell fate decisions of the early mouse embryo. Our analysis focused on the data of 46 non-housekeeping genes, and the raw data were rescaled through log transformation. Due to the prevailing dominance of maternal gene degradation in gene expression variation during the initial cell fate decision, in contravention of COSLIR’s requirement, our analysis using COSLIR was limited to the second cell fate decision.
Fig. 3C,D provided an overview of the two inferred gene regulatory networks (GRNs) using Cytoscape57. Using COSLIR, we inferred a set of directed gene regulatory relationships corresponding to the positive or negative elements in the \(A_t\) matrices, indicating activated or inhibited regulatory links between genes. The identified upstream regulatory genes and major regulatory relationships were consistent with existing knowledge about the second cell fate decision during early embryonic development51,58,59,60. Key upstream regulators such as Sall4, Sox2, Pou5f1, Gata6, and Tcfap2c played critical roles in the inferred GRN, driving ICM cells toward the EPI fate, activating EPI markers and inhibiting PE markers (Fig. 3C). Similarly, in the inferred network that directs ICM cells toward the PE fate, key upstream regulators such as Sall4, Sox2, Pou5f1, Gata4, Tcfap2c, and Nanog acted to inhibit EPI and activate PE markers (Fig. 3D). All identified upstream regulatory genes and their target markers were well established for their roles in early embryonic development47,61. In addition, through literature and database searches (ChIP-atlas(ESC), BioGRID, and TRRUST), we found that nearly half of the inferred regulatory relationships were reported experimentally (Supplementary Figures S6, S7), with many validated by multiple databases.
Early precision serves as the metric for evaluating these algorithms, quantified as the count of correctly inferred gene regulatory relationships among the top k relationships with the highest weights. For COSLIR, we utilized rescaled values, indicative of the significance of each inferred gene regulatory relationship for the differential expression of the target gene (refer to Methods for detailed information). Fig. 3E-F compared COSLIR with the four existing algorithms, using early precision. It illustrates that COSLIR competed favorably with existing methods using pseudo-time construction. The p-values were calculated by Wilcoxon signed rank test, using all the early precision at each \(k=1,2,\cdots ,40\).
The inferred regulatory relationships not cataloged in the three databases may still be accurate predictions (Supplementary Figure S7). For example, the regulatory link from Sox17 to Gata6 reported by Niakan et al.62 was not included in the three databases, but was successfully predicted by COSLIR. It’s important to note that we used only the ICM and EPI or ICM and PE datasets to infer the gene regulatory networks independently. For example, despite the modest fold change of Gata6 from the ICM stage to the PE stage (only 1.03), it was recognized as a crucial marker gene for the PE stage compared to its expression in the EPI stage. Remarkably, COSLIR accurately inferred this relationship without reference to gene expression data from the EPI stage, underscoring the robust and independent predictive power of COSLIR.
Single-cell RNA-seq datasets
We conducted a comprehensive comparison of COSLIR with four established regression-based algorithms for Gene Regulatory Network (GRN) reconstruction: SINCERITIES, SCODE, SCRIBE, and SINGE, as summarized in BEELINE17. Our analysis encompassed two experimental single-cell RNA sequencing (scRNA-seq) datasets: one involving human embryonic stem cell (hESC) differentiation to definitive endoderm48, and the other focused on mouse embryonic stem cell (mESC) differentiation to primitive endoderm49. Given the multiple time points within these datasets, we reconstructed GRNs for each pair of consecutive time points.
To evaluate the reconstructed networks, we employed cell-type-specific networks17 as the ground truth networks (Fig. 4). For a more in-depth comparison, including functional interaction networks (STRING) and additional cell-type-specific ChIP-Seq data, refer to Supplementary Figures S8-S11.
In Fig. 4A-B, the early precision of various methods at different values of k was presented for both datasets, using the cell type-specific ground truth in BEELINE17. The mean and error bar of early precision were computed from the outputs of each algorithm for each pair of consecutive time points. Notably, it was evident from the results that COSLIR’s performance competed favorably with the four existing methods. The p-values were calculated by Wilcoxon signed rank test, using all the early precision at \(k=1,\cdots ,100\) for each two consecutive time points.
Furthermore, the sets of gene-gene regulatory interactions inferred by various methods on high-dimensional single-cell RNAseq data exhibited minimal overlap with each other (Fig. 4C, D, and Supplementary Figures S12-S13). This suggested that the individual accuracy of any existing method was notably limited, and COSLIR emerged as a valuable addition to the existing toolkit of methods.
Scalability of COSLIR via single-cell Human blood atlas
The throughput of single-cell experiments is experiencing a remarkable increase. To effectively handle the surge in data throughput, computational methods must be designed with scalability at the forefront. Fortunately, COSLIR is inherently scalable, relying solely on the estimation of the first and second moments of the samples. This inherent scalability ensures the method’s applicability at a large scale.
We evaluated COSLIR using the official Peripheral Blood Mononuclear Cell Multiome dataset50. The majority of gene-regulatory interactions, identified by COSLIR with the highest rescaled values, corresponded to significant biomarkers and pathways implicated in blood development (Fig. 5A, B, Supplementary Tables S6-S7, Supplementary Figure S14). Notably, COSLIR demonstrated near independence of computation time from the cell number (Fig. 5C), showcasing its scalability. While the computational time of COSLIR exhibited an exponential increase with the number of genes (Fig. 5D), it’s important to note that such behavior is a common trait shared by almost all methods for inferring gene regulatory relationships17.
Conclusion and Discussion
In the current era, the convergence of machine learning and network biology presents both invaluable opportunities and challenges, especially in the field of gene regulatory network inference63. In this paper, we presented COSLIR, an optimization-based model designed for gene regulatory network inference. A distinctive feature of our approach is its minimal input requirements, relying only on the estimated mean and covariances of the samples at consecutive time points in single-cell expression data. Despite its simplicity, COSLIR produces directed gene regulatory networks with weights and signs, making it a versatile tool applicable to diverse sample distributions. In simulation studies, COSLIR demonstrated remarkable accuracy in recovering true networks under oracle conditions, and its performance remained robust in sample cases, particularly with the aid of bootstrapping to achieve near-perfect precision. In real data analyses, COSLIR successfully identified important upstream regulatory genes and gene-gene interactions in single-cell RT-PCR and RNA-seq datasets, shedding light on early mouse and human embryonic development.
However, in practical applications, several factors may affect the performance of COSLIR. Multi-scale data across genes may require normalization before applying COSLIR. While it is recommended to use a correlation matrix rather than a covariance matrix, this introduces some inherent bias. Gene selection is another challenge, as the computational time for COSLIR becomes significant when the number of genes exceeds 100017. Common strategies involve the selection of highly variable genes, often in conjunction with transcription factors. Also in our formulation modeling the difference between the stage t and \(t+1\), we assumed that the cells at stage t and \(t+1\) are homogeneous. Hence, COSLIR should be applied after the cells being clustered into homogenous populations, just as we have done in this paper when analyzing the real single-cell data along each developmental lineage.
Another important consideration is whether the data strictly adhere to the linear model (1). While the linear regression model is the predominant choice for gene regulatory network (GRN) reconstruction from single-cell expression data32,33,34,64,65, it’s worth noting that linearity justification is inherently missing from time-stamped cross-sectional data. In statistical learning, the default inclination is to choose the linear model unless there is compelling evidence of high nonlinearity. Empirically, as long as the data don’t deviate significantly from linearity, the performance of statistical inference by the linear model remains robust66. In addition, the linear model offers a distinct advantage in terms of interpretability, following the principle that simplicity is often synonymous with effectiveness.
To improve accuracy, the GRN reconstruction method can be synergistically integrated with existing prior knowledge by incorporating additional databases or information67. Recent advances, such as RNA velocity analysis, have demonstrated the potential to recover some temporal information68. Therefore, our future research path is to explore the merging of COSLIR with existing database knowledge and bioinformatic methods, leveraging these complementary approaches for improved precision and insight.
Overview of the COSLIR method for reconstructing Gene Regulatory Networks (GRNs) using time-stamped cross-sectional single-cell expression data. (A) Depiction of time-stamped cross-sectional data generated in single-cell experiments. Only data within the solid red rectangles were measured. (B) Estimation of sample mean and covariance matrix. (C) Solving the optimization problem in COSLIR using ADMM. (D) Inferred gene regulatory network among the genes represented by the estimator \(\hat{A}\).
The performance of COSLIR is rigorously evaluated through a simulation study that includes both oracle cases (A) with known true mean and covariance matrix, and sample cases (B–D) where only random samples are available. In the oracle case, precision and recall were shown (A). In the sample case, precision and recall vary with the number of genes (B), the number of cells (C) and the confidence threshold (D). The number of cells in (B, D) is 5000. The number of genes in (C, D) is 100. The precision of COSLIR is compared to four existing algorithms and random guess (E, F). The confidence threshold is set to 0.9 in (B, C, E, F).The clipping threshold is always 0.01. More detailed results can be found in Supplementary Figs. S2–S4 and Tables S1–S5.
Overview of the analysis of real single-cell RT-PCR expression data using COSLIR. (A) Developmental lineage tree representing early mouse embryonic development. (B) Data visualization using Isomap. (C) Inferred gene regulatory networks (GRNs) directing ICM cells towards the EPI fate, accompanied by a corresponding sketch map. (D) Inferred GRNs directing ICM cells to the fate of PE, accompanied by its sketch map. (E, F) Early precision comparison of COSLIR with four existing algorithms during the two cell lineages: ICM cells towards the EPI fate (E) and the PE fate (F). Here, the clipping threshold is 0.01, the confidence threshold is 0.8, and the threshold of the rescaled values we chose is 0.25 for ICM to EPI and 0.1 for ICM to PE. More details can be found in Supplementary Figures S6–S7 and Table S5.
Comparative analysis of various methods on mESC and hESC single-cell RNA-seq datasets. (A, B) Display of early precision across different methods and various values of k in mESC (A) and hESC (B) datasets. (C–F) Pie charts illustrating the distribution of database-supported gene regulatory relationships predicted by each method among all database-validated predictions between consecutive time points during mESC and hESC development. Notably, in (C, E), the SINGE method does not predict any gene regulatory relationships supported by the database. We use the cell type-specific ground truth in BEELINE17.
COSLIR being applied on single-cell Human blood atlas. (A) UMAP visualizations of human blood cell embeddings, with colors indicating distinct cell types. (B) COSLIR-inferred major upstream regulatory transcription factors (TFs) and downstream cell-type markers. (C) Time costs of different methods applied to subsampled atlases with varying cell numbers. (D) Time costs of COSLIR applied to subsampled atlases with different gene numbers. Further comprehensive verification with relevant literature can be found in Supplementary Tables S7–S10.
Data availability
The proposed algorithm and dataset are available at https://github.com/Ge-lab-pku/COSLIR.
References
Lu, W. & Tang, F. Single-cell sequencing in stem cell biology. Genome Biol. 17, 71 (2016).
Stegle, O., Teichmann, S. & Marioni, J. C. Computational and analytical challenges in single-cell transcriptomics. Nat. Rev. Genet. 16, 133–145 (2015).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865 (2017).
Papalexi, E. & Satija, R. Single-cell rna sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol. 18, 35 (2018).
Kolodziejczyk, A. A., Kim, J. K., Svensson, V., Marioni, J. C. & Teichmann, S. A. The technology and biology of single-cell rna sequencing. Mol. Cell 58, 610–620 (2015).
Tusi, B. K. et al. Population snapshots predict early haematopoietic and erythroid hierarchies. Nature 555, 54–60 (2018).
Huang, W., Cao, X., Biase, F. H., Yu, P. & Zhong, S. Time-variant clustering model for understanding cell fate decisions. Proc. Nat. Acad. Sci. 111, E4797–E4806 (2014).
Barjoseph, Z., Gitter, A. & Simon, I. Studying and modelling dynamic biological processes using time series gene expression data. Nat. Rev. Genet. 13, 552–564 (2012).
Oh, V. & Li, R. W. Temporal dynamic methods for bulk rna-seq time series data. Genes 12, 352 (2021).
Ding, J., Sharon, N. & Bar-Joseph, Z. Temporal modelling using single-cell transciptomics. Nat. Rev. Genet. 23, 355–368 (2022).
Whitfield, M., Sherlock, G. & Saldanha, A. E. A. Identification of genes periodically expressed in the human cell cycle and their expressio in tumors. Mol. Biol. Cell 13, 1977–2000 (2002).
Muskovic, W. et al. High temporal resolution rna-seq time course data reveals widespead synchronous activation between mammalian lncrnas and neighboring protein-coding genes. Genome Res. 32, 1463–1473 (2022).
Magwene, P., Lizardi, P. & Kim, J. Reconstructing the temporal ordering of biological samples using microarray data. Bioinformatics 19, 842 (2003).
Gupta, A. & Bar-Joseph, Z. Extracting dynamics from static cancer expression data. IEEE/ACM Trans. Comput. Biol. Bioinf. 5, 172 (2008).
Zeisel, A., K\(\ddot{o}\)stler, W. & Moloski, e. a., N. Coupled pre-mrna and mrna dynamics unveil operational strategies underlying transcriptional responses to stimuli. Mol. Syst. Biol.7, 529 (2011).
Saelens, W., Cannoodt, R., Todorov, H. & Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 39, 547–554 (2019).
Pratapa, A., Jalihal, A. P., Law, J. N., Bharadwaj, A. & Murali, T. M. Benchmarking algorithms for gene regulatory network inference from single-cell transcriptomic data. Nat. Methods 17, 147–154 (2020).
Langfelder, P. & Horvath, S. Wgcna: An r package for weighted correlation network analysis. BMC Bioinform. 9, 559 (2008).
Chan, T., Stumpf, M. & Babtie, A. Gene regulatory network inference from single-cell data using multivariate information measures. Cell Syst. 5, 251–267 (2017).
Kim, S. ppcor: An r package for a fast calculation to semi-partial correlation coefficients. Commun. Stat. Appl. Methods 22, 665–674 (2015).
Huynh-Thu, V., Irrthum, A., Wehenke, L. & Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE 5, e12776 (2010).
Moerman, T. et al. Grnboost2 and arboreto: Efficient and scalable inference of gene regulatory networks. Bioinformatics 35, 2159–2161 (2019).
Furchtgott, L. S. M., Menon, V. & Ramanathan, S. Discovering sparse transcription factor codes for cell states and state transitions during development. Elife 6, e20488 (2017).
Candes, E. J. & Tao, T. Decoding by linear programming. IEEE Trans. Inf. Theory 51, 4203–4215 (2005).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. Royal Statist. Soc. Series B (Methodological) 267–288 (1996).
Stock, J. & Watson, M. Vector autoregressions. J. Econ. Perspect. 15, 101–115 (2001).
Friedman, J., Hastie, T. & Tibshirani, R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics 9, 432–441 (2008).
Blumensath, T. Compressed sensing with nonlinear observations and related nonlinear optimization problems. IEEE Trans. Inf. Theory 59, 3466–3474 (2013).
Chatfield, C. & Xing, H. The Analysis of Time Series: An Introduction with R. 7th edition (Chapman and Hall/CRC, 2019).
Garrett Fitzmaurice, N. L. . J. W. Applied Longitudinal Analysis, 2nd Edition (Chapman and Hall/CRC, 2011).
Chui, C. & Chen, G. Kalman Filtering with Real-Time Applications (Springer, 2017).
Matsumoto, H. et al. Scode: An efficient regulatory network inference algorithm from single-cell rna-seq during differentiation. Bioinformatics 33, 2314–2321 (2017).
Deshpande, A., Chu, L., Stewart, R. & Gitter, A. Network inference with granger causality ensembles on single-cell transcriptomic data. Preprint at https://doi.org/10.1101/534834 (2020+).
Aubin-Frankowski, P. & Vert, J. Gene regulation inference from single-cell rna-seq data with linear differential equations and velocity inference. Preprint at https://doi.org/10.1101/464479 (2020+).
Carrizosa, E., Olivares-Nadal, A. & Ramírez-Cobo, P. A sparsity-controlled vector autoregressive model. Biostatistics 18, 244–259 (2017).
Wilms, I., Basu, S., Bien, J. & Matteson, c. Sparse identification and estimation of large-scale vector autoregressive moving averages. J. Am. Stat. Assoc.118:541, 571–582 (2023).
Boyd, S. et al. Distributed optimization and statistical learning via the alternating direction method of multipliers. Found. Trends Mach. Learn. 3, 1–122 (2011).
Mooney, C. Z., Duval, R. D. & Duvall, R. Bootstrapping: A nonparametric approach to statistical inference. 94-95 (Sage, 1993).
Bickel, P. J. et al. Covariance regularization by thresholding. Ann. Stat. 36, 2577–2604 (2008).
Cai, T. & Liu, W. Adaptive thresholding for sparse covariance matrix estimation. J. Am. Stat. Assoc. 106, 672–684 (2011).
Liu, H., Wang, L. & Zhao, T. Sparse covariance matrix estimation with eigenvalue constraints. J. Comput. Graph. Stat. 23, 439–459 (2014).
Fan, J., Liao, Y. & Mincheva, M. Large covariance estimation by thresholding principal orthogonal complements. J. Royal Stat. Soc: Series B (Stat. Methodol.) 75, 603–680 (2013).
Tian, L. et al. Benchmarking single cell rna-sequencing analysis pipelines using mixture control experiments. Nat. Methods 16, 479–487 (2020).
Bingsheng, H., Hai, Y. & Shengli, W. Alternating direction method with self-adaptive penalty parameters for monotone variational inequalities. J. Optim. Theory Appl. 106, 337–356 (2000).
Shengli, W. & Lizhi, L. Decomposition method with a variable parameter for a class of monotone variational inequality problems. J. Optim. Theory Appl. 109, 415–429 (2001).
Efron, B. Bootstrap methods: Another look at the jackknife. Ann. Stat. 7, 1–26 (1979).
Guo, G. et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell 18, 675–685 (2010).
Chu, L. F. et al. Single-cell rna-seq reveals novel regulators of human embryonic stem cell differentiation to definitive endoderm. Genome Biol. 17, 173 (2016).
Hayashi, T. et al. Single-cell full-length total rna sequencing uncovers dynamics of recursive splicing and enhancer rnas. Nat. Commun. 9, 90 (2018).
Xie, X. et al. Single-cell transcriptomic landscape of human blood cells. Natl. Sci. Rev. 8, 180 (2021).
Saiz, N. & Plusa, B. Early cell fate decisions in the mouse embryo. Reproduction 145, R65-80 (2013).
Nakai-Futatsugi, Y. & Niwa, H. Epiblast and primitive endoderm differentiation: Fragile specification ensures stable commitment. Stem Cell 16, 346–347 (2015).
Chazaud, C. & Yamanaka, Y. Lineage specification in the mouse preimplantation embryo. Development 143, 1063–1074 (2016).
Sozen, B., Can, A. & Demir, N. Cell fate regulation during preimplantation development: A view of adhesion-linked molecular interactions. Dev. Biol. 395, 73–83 (2014).
Rossant, J. & Tam, P. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136, 701–713 (2009).
Tenenbaum, J. B., de Silva, V. & Langford, J. C. A global geometric framework for nonlinear dimensionality reduction. Science 290, 2319–2323 (2000).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–504 (2003).
Frum, T. & Ralston, A. Cell signaling and transcription factors regulating cell fate during formation of the mouse blastocyst. Trends Genet. 31, 402–410 (2015).
Menchero, S., Rayon, T., Andreu, M. & Manzanares, M. Signaling pathways in mammalian preimplantation development: Linking cellular phenotypes to lineage decisions. Dev. Dyn. 246, 245–261 (2017).
Goolam, M. et al. Heterogeneity in oct4 and sox2 targets biases cell fate in 4-cell mouse embryos. Cell 165, 61–74 (2016).
Zernicka-Goetz, M., Morris, S. & Bruce, A. Making a firm decision: multifaceted regulation of cell fate in the early mouse embryo. Nat. Rev. Genet. 10, 467–477 (2009).
Niakan et al. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev. 312–326 (2010).
Camacho, D. M., Collins, K. M., Powers, R. K., Costello, J. C. & Collins, J. J. Next-generation machine learning for biological networks. Cell 173, 1581–1592 (2018).
Gao, N., MinhazUd-Dean, S., Gandrillon, O. & Gunawan, R. Sincerities: Inferring gene regulatory networks from time-stamped single cell transcriptional expression profiles. Bioinformatics 34, 258–266 (2018).
Sanchez-Castillo, M., Blanco, D., Tienda-Luna, I., Carrion, M. C. & Huang, Y. A bayesian framework for the inference of gene regulatory networks from time and pseudo-time series data. Bioinformatics 34, 964–970 (2018).
Hastie, T., Tibshirani, R. & Friedman, J. The elements of statistical learning: data mining, inference, and prediction (Springer Science & Business Media, 2009).
Aibar, S. et al. Scenic: Single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Qiu, X. et al. Inferring causal gene regulatory networks from coupled single-cell expression dynamics using scribe. Cell Syst. 10(3), 265–274 (2020).
Acknowledgements
We would like to thank Yunuo Mao, Xiaojie Qiu, Yu Xue, Zaiwen Wen, Wei Lin, Ruibin Xi and Xiaoliang S. Xie for helpful discussions. This work was supported by the NSFC T2225001 and 11971037 (to H. Ge).
Author information
Authors and Affiliations
Contributions
H. Ge conceived the project. R.S. Wan and Y.H. Zhang proposed the method under the supervision of H. Ge, J.Z. Jia, and F.C. Tang. R.S. Wan, Y.H. Zhang, and Y.L. Peng conducted the analysis and simulations with assistance from F. Tian and G. Gao. H. Ge, R.S. Wan, Y.H. Zhang, Y.L. Peng, and J.Z. Jia contributed to writing the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan, R., Zhang, Y., Peng, Y. et al. Unveiling gene regulatory networks during cellular state transitions without linkage across time points. Sci Rep 14, 12355 (2024). https://doi.org/10.1038/s41598-024-62850-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-62850-1