Abstract
Parkinson’s disease (PD) is frequently accompanied by mood and chronic fatigue syndrome (CFS) symptoms. The aim of this study is to examine whether the affective and CFS symptoms due to PD are associated with serum biomarkers of neuronal injury in association with immune activation and insulin resistance. Using a case (70 PD patients) control (60 healthy controls) study design, we assessed affective and CFS symptoms, measured the peripheral immune-inflammatory response system (IRS) using interleukin-6 (IL-6), IL-10, zinc, and calcium levels, the Homeostasis Model Assessment 2 insulin resistance (HOMA2IR) index, and serum brain injury markers including S100 calcium-binding protein B (S100B), neuron-specific enolase (NSE), phosphorylated tau217 (pTau217), and glial fibrillary acidic protein (GFAP). The aim is to examine whether immune, IR, and/or brain injury biomarkers determine affective and CFS symptoms due to PD. PD patients showed increased affective and CFS scores, IRS activation, HOMA2IR, NSE, GFAP, pTau217, and S100B levels as compared to controls. A large part (52.5%) of the variance in the mood + CFS score was explained by the regression on NSE, S100B, HOMA2IR index, IL-10 (all positively), and calcium (inversely). The HOMA2IR and IRS indices were significantly associated with all 4 brain injury biomarkers. A large part of the variance in the latter markers (37.0%) was explained by the cumulative effects of the IRS and HOMA2IR indices. IRS activation and IR in patients with PD contribute to damage to glial cell projections and type III intermediate filament, which in turn contribute to affective and CFS symptoms.
Similar content being viewed by others
Introduction
Parkinson’s disease is a neurodegenerative disorder that affects a significant number of individuals aged 60 years and above. In fact, it is considered the most prevalent movement disorder globally1. Parkinson’s disease is characterized by alterations in motor function, such as tremors, rigidity, bradykinesia, and postural instability2. Additional symptoms that may arise include loss of smell, decline in cognitive function, alterations in personality, feelings of sadness, increased anxiety, persistent fatigue, and dysautonomic symptoms3.
Parkinson’s disease is a complex condition characterized by neurodegeneration of dopaminergic neurons, microglial activation and neuroinflammation in the nervous system4,5. Activated immune-inflammatory pathways in the peripheral system play a significant role in this disorder6. In addition, Braak et al.‘s (2003)7 posits that the onset of sporadic Parkinson’s disease may be caused by a pathogen (virus or bacterium) in the intestines. Therefore, the neuroinflammation observed in this disease may be the result of the peripheral immune-inflammatory response induced by microorganisms. Both neuroinflammatory and neurodegenerative processes cause cellular structure damages8 and these processes may be accompanied by elevated levels of neuronal and astroglial damage biomarkers in serum9,10,11. Brain injury or neurodegenerative conditions may cause increases in serum neuronal injury markers, such as S100 calcium-binding protein B (S100B), neuron-specific enolase (NSE), phosphorylated tau217 (pTau217), and glial fibrillary acidic protein (GFAP)12,13,14,15. In Parkinson’s disease, S100B may serve as a possible marker for the progression of the illness16. NFL and GFAP exhibit a correlation with baseline assessments of motor and non-motor severity, whilst NFL proves to be a reliable predictor of disease progression17.
Non-motor symptoms of Parkinson’s disease, including neuropsychiatric symptoms, such as depression, anxiety, fatigue, cognitive impairments, and hallucinations, cause increased disease burden and impaired health-related quality of life4,5,18. Forty to fifty% of individuals diagnosed with Parkinson’s disease experience symptoms of depression, with around 50% of them being diagnosed with major depression (MDD)19. Chronic fatigue is a common neuropsychiatric symptom complex in Parkinson’s disease that impacts over 50% of patients. It significantly reduces their quality of life and ability to conduct daily tasks20. Chronic fatigue is one of the most distressing symptoms experienced by patients with Parkinson’s disease, which is frequently reported in the literature21.
These non-motor symptoms in Parkinson’s disease may be generated via immune-inflammatory mechanisms22. For example, aberrations in peripheral humoral immune responses are associated with the non-motor symptoms of Parkinson’s disease23. Both patients with major depression and chronic fatigue syndrome (CFS) exhibit peripheral IRS activation, leading to microglial activation24,25. Elevated serum levels of proinflammatory cytokines, such as interleukin (IL)-6, and immunoregulatory cytokines, like IL-10, have been found not only in Parkinson’s disease, but also in MDD, and CFS25,26,27. Elevated levels of biomarkers indicating neuronal or astroglial (projection) damage are not only observed in Parkinson’s disease but also in MDD and CFS28,29,30,31,32,33,34.
There is evidence from epidemiological studies suggesting a potential link between diabetes and an elevated risk of Parkinson’s disease, and that insulin resistance (IR) may play a role in the progression of the disease35,36. IR has been found to have a negative impact on the progression of Parkinson’s disease37MDD38and symptoms related to affective disorders and CFS caused by Long COVID39,40. However, it is currently unknown whether IRS activation and increased IR are associated with signs of brain injuries (elevated serum levels of S100B, NSE, pTau217, and GFAP) in Parkinson’s disease and whether elevated serum levels of these brain injury markers impact the severity of affective and CFS symptoms.
Hence, the current study has been conducted to examine whether serum levels of S100B, NSE, pTau217, and GFAP are associated with the affective and CFS symptoms due to Parkinson’s disease and whether activation of immune-inflammatory pathways and increased IR are associated with these biomarkers.
Subjects and methods
Participants
This case-control study examined a group of seventy patients diagnosed with Parkinson’s disease, as well as a control group of sixty individuals who were in good health. The patients were registered at the Middle Euphrates Center for Neurological Sciences, located in Najaf City, Iraq. The samples were collected during the period from February to April 2024. The diagnosis of Parkinson’s disease in all patients was made based on the clinical diagnostic criteria from the UK Parkinson’s Disease Brain Bank, as determined by two experienced neurologists41,42. The study did not include individuals with diabetes mellitus type 1 or other systemic autoimmune diseases, neurodegenerative or neuroinflammatory disorders other than Parkinson’s disease, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, liver or renal disease, or any other medical conditions. Individuals with axis-1 disorders, such as bipolar disorder, anxiety disorder, schizophrenia, autism, and substance use disorders, were not eligible to participate in the study. Furthermore, this study did not include patients who had experienced any affective disorder before developing Parkinson’s disease. These disorders encompass MDD, dysthymia, chronic fatigue syndrome, fibromyalgia, and psychotic disorders like delirium.
Prior to their involvement in the study, both control and patient participants were required to give written consent. They were provided with detailed information beforehand. The study was granted approval by the institutional ethics committee of the University of Kufa (2109/2023). The study followed ethical and privacy laws both in Iraq and internationally. It complied with various guidelines and declarations, such as the International Conference on Harmonization of Good Clinical Practice, the Belmont Report, the CIOMS Guidelines, and the World Medical Association’s Declaration of Helsinki. In addition, our institutional review board adheres to the International Guidelines for Human Research Safety (ICH-GCP).
Clinical measurements
An experienced neurologist conducted a semi-structured interview to evaluate and gather socio-demographic and clinical information from both control subjects and patients. An expert in the field of neurology evaluated the extent of the motor and non-motor symptoms associated with Parkinson’s disease by employing the Movement Disorders Society Revision of Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) as outlined in the studies conducted by Goetz et al. in 2007 and 2008 43,44. The MDS-UPDRS is divided into four parts; part I: non-motor Experiences of Daily Living (nM-EDL), part II: motor Experiences of Daily Living (m-EDL), part III: motor examination, and part IV: motor complications44. Neurologists conducted the MDS-UPDRS assessment on both patients and controls, utilizing the ratings from the 4 domains for statistical analysis.
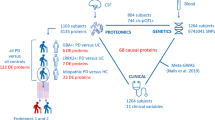
In addition, we have calculated two subdomains using the nM-EDL subdomain: mood symptoms (MOOD) and chronic fatigue syndrome (CFS) symptoms. MOOD was formulated as the summed combination of depressed mood, anxious mood, apathy, sleep problems, and fatigue. The concept of CFS involves the summed combination of cognitive impairment, sleep problems, pain and other sensations, daytime sleepiness, constipation problems, lightheadedness, and fatigue. As a result, we calculated the total of several factors such as depressed mood, anxious mood, apathy, sleep problems, fatigue, cognitive impairment, pain and other sensations, daytime sleepiness, constipation problems, and light-headedness. This total sum is labeled as MOOD + CFS. By utilizing the summation of MOOD + CFS scores, the patients were categorized into two distinct groups, employing a cut-off value of 14.5. Consequently, the patients were dichotomized into two subgroups: one consisting of individuals with low MOOD + CFS scores (n = 40, range: 4–14), and the other consisting of those with higher MOOD + CFS symptoms (n = 30, range 15–32). Figure 1 shows the flow of the subjects through the study and shows how the patients were divided into a group with low versus high MOOD + CFS scores.
Flow chart showing the flow of the healthy controls (HC) and patients with Parkinson’s disease (PD) through the study. PD patients were divided using affective (MOOD) and chronic fatigue syndrome (CFS) symptoms into those with low versus high MOOD + CFS scores. The latter were based on part I or non-motor Experiences of Daily Living. Mg: magnesium, Ca: calcium, Cu: copper, Zn zinc.
Therefore, we divided the patients with Parkinson’s disease into two subgroups and conducted a comparison of clinical and biomarker measurements among three groups: normal controls, and the two subgroups of Parkinson’s patients. The BMI is determined by dividing the weight in kilograms by the square of the height in meters. Tobacco use disorder (TUD) was diagnosed using the DSM-5 criteria.
Assays
A volume of 5 milliliters of fasting blood samples was collected at approximately 9:00 a.m. After complete clotting, the blood samples were centrifuged at 1,200 × g for five minutes at room temperature. Subsequently, the serum was carefully divided and distributed among three Eppendorf tubes. Excluded from the study were samples that had undergone hemolysis. The tubes were subsequently frozen at -80 °C and remained in this state until they were thawed for the assays.
We utilized sandwich ELISA techniques to quantify the levels of serum human GFAP, IL-6, IL-10, insulin, NSE, pTau217, and S100B. These measurements were conducted using pre-made ELISA kits provided by Nanjing Pars Biochem Co., Ltd. (Nanjing, China). The coefficient of variation (CV) for all ELISA kits was less than 10.0%. We employed sample dilutions for samples that contained analytes with elevated concentrations by 1:5 with the sample diluent provided with the kit. The spectrophotometric measurement of serum glucose, calcium, magnesium, copper, and zinc was performed using kits provided by Spectrum Diagnostics Co., (Cairo, Egypt). After analyzing the blood samples, we have calculated two indices: the first is the activation of the immune-inflammatory response system (IRS), which involves the z transformation of IL-6 (z IL-6) + z IL-10, - z zinc - z calcium (labeled as Comp_IRS). The second index that was examined is glial projection toxicity, which is computed as z NSE + z S100B + z GFAP + z pTau (labeled as Comp_GPT). The Homeostasis Model Assessment 2 (HOMA2) calculator, developed by the Diabetes Trials Unit at the University of Oxford, is a valuable tool for assessing homeostasis (https://www.dtu.ox.ac.uk/homacalculator/download.php). The HOMA2%S and HOMA2IR calculations were employed to determine insulin sensitivity and IR, respectively, based on the fasting serum insulin and glucose levels.
Statistical analysis
In order to examine the distribution types of the results group, the Kolmogorov-Smirnov test was implemented. The researchers employed analysis of variance (ANOVA) or the Kruskal-Wallis test (KWT) to assess variations in continuous variables across different groups. The researchers utilized protected pairwise post-hoc analyses in order to investigate the variations among group means. To examine associations between nominal variables, the χ2-test or Fisher exact probability test was utilized in conjunction with contingency tables. Utilizing Pearson’s product-moment correlation coefficients, the correlations between variables were examined. Using multivariate general linear model (GLM) analysis (followed by tests of between-subject effects), the associations between categories and biomarkers were investigated, accounting for confounding variables including age, sex, and body mass index (BMI). By employing manual and automatic stepwise multiple regression analyses with significance levels of biomarkers (p-to-enter was 0.05 and p-to-remove was 0.06), we identified the biomarkers that were statistically significant in predicting the scores on the neuropsychiatric rating scale. An analysis was conducted on the impacts of each biomarker in both the entire study population and the subset of patients diagnosed with Parkinson’s disease. The standardized beta coefficients for each significant explanatory variable were computed utilizing the t statistic with the precise value of p, in addition to the model F statistic and total variance explained (R2). The latter was utilized to estimate the effect size of the model. In this study, statistical significance was established at a p-value of 0.05 using two-tailed tests. IBM SPSS 28 for Windows was utilized to analyze the data. The primary statistical analysis is the multiple regression analysis with the MOOD + CFS score as the dependent variable and the biomarkers as explanatory variables. G*Power 3.1.9.7 showed that the a priori estimated sample size was 96 given a power of 0.8, alpha = 0.005, effect size = 0.142 (corresponding to 12.5% explained variance), and 5 predictors.
Protein-protein interaction (PPI) analysis was conducted using String (STRING: functional protein association networks; string-db.org). The organism under consideration was Homo sapiens, and the minimal interaction score was 0.7. In our case, we exclusively used the initial shell, with no further interactions occurring.
Results
Sociodemographic and clinical data
Table 1 contains the demographic and clinical information of Parkinson’s disease (PD) patients, categorized by the severity of their MOOD + CFS symptoms into high or low subgroups, in addition to the control subjects. Age, sex, employment status, rural-to-urban ratio, marital status, TUD, BMI, and years of education did not differ significantly between the study groups, according to the findings. In terms of age of onset and disease duration, there were no significant differences between the low and high MOOD + CFS subgroups. Patients who presented with high levels of MOOD + CFS symptoms exhibited higher EDL-Part III scores than those who had low MOOD + CFS scores. No substantial disparities were observed in the motor complications score or m-EDL between the two subgroups of patients.
Regarding Parkinson’s disease patients, no significant correlations were observed among the scores of EDL parts I, II , III, and IV. No statistically significant correlations were observed between the CFS scores and any of the MDS-UPDRS scores. A significant correlation was observed solely between the MOOD score and the MDS-UPDRS-Part III score (r = 0.274, p = 0.022, n = 70).
Differences in the biomarkers between PD patients’ groups and controls
The serum biomarkers of Parkinson’s disease patients, categorized by those with high or low MOOD + CFS symptoms, are presented in Table 2. A comparison with healthy controls is also provided. The findings indicated that the concentrations of magnesium, copper, zinc, and IL-6 did not differ significantly among the study groups. The levels of HOMA2IR, pTau217, and GFAP in both patient groups exhibited substantial increases when compared to the control group. Both patient groups exhibit reduced concentrations of calcium and HOMA2%S in comparison to the control group. There are significant differences in serum concentrations of NSE, S100B, and IL-10 among the three study groups, with concentrations rising from the control group to the low MOOD + CFS subgroup to the high MOOD-CFS subgroup. A notable distinction was observed among the three study groups in terms of the Comp_GPT and Comp_IRS indices, which exhibited an upward trend from the control group to the low and high MOOD-CFS groups.
Correlations between physio-affective symptoms and biomarkers in all subjects
Table 3 displays the intercorrelation matrix among serum biomarkers and the nM-EDL, MOOD, CFS, and MOOD + CSF scores for all study subjects (patients subgroups, and controls). Correlations between all neuropsychiatric scores and HOMA2IR, NSE, S100B, pTau217, GFAP, and IL-10 are statistically significant. All neuropsychiatric disorders exhibited noteworthy inverse correlations with calcium and HOMA2%S.
The outcomes of the multiple regression analysis are presented in Table 4. The dependent variables in this analysis were the neuropsychiatric scores, while the explanatory variables were biomarkers. The analyses allowed for the effects of age, sex, education, TUD, and BMI. Regression #1 demonstrates that calcium (inversely associated), NSE, IL-10, and HOMA2IR (all three significantly and positively associated), accounted for 55.5% of the variance in the nM-EDL score. In the second regression analysis, calcium (inversely associated), S100B, IL-10, NSE, and HOMA2IR (all significantly and positively associated), accounted for 48.8% of the variance in the MOOD score. As shown in Regression No. 3, we determined that the regression on NSE, IL-10, HOMA2IR (which is positively associated), and calcium (which is inversely associated) could account for 54.8% of the variance in the CFS score. It is demonstrated in Regression #4 that the regression on NSE, IL-10, HOMA2IR, S100B (all positively correlated), and calcium (inversely associated) could account for 52.5% of the variance in the MOOD + CFS score. 31.0% of the variance in the MOOD + CFS score was accounted for by the regression on Comp_IRS (β = 0.199, t = 2.38, p = 0.019) and Comp_GPT (β = 0.457, t = 5.46, p < 0.001) (F = 32.34, df = 2/127, p < 0.001).
Correlation between physio-affective symptom and serum biomarkers in PD group
Correlations between biomarkers and clinical rating scales were calculated for the restricted patient population. There were significant correlations between the total nM-EDL score and the following variables: HOMA2IR (r = 0.274, p = 0.022, n = 70), NSE (r = 0.480, p < 0.001), S100B (r = 0.282, p = 0.018), and IL-10 (r = 0.492, p < 0.001). The MOOD + CFS score demonstrated a significant association with HOMA2IR (r = 0.252, p = 0.036), NSE (r = 0.408, p < 0.001), S100B (r = 0.272, p = 0.023, and IL-10 (r = 0.460, p < 0.001).
The outcomes of various stepwise multiple regression analyses utilizing biomarkers as independent variables and clinical neuropsychiatric scores as dependent variables in the restricted patient subgroup are presented in Table 5. 32.7% of the variance in the nM-EDL score was accounted for by IL-10 and NSE (both positively associated), according to Regression #1. Regression #2 indicates that 9.0% of the variance in the MOOD score was accounted for by the model utilizing IL-10. IL-10 and NSE accounted for a substantial proportion of the variance (31.9%) in CFS (Regression #3) and the sum of MOOD + CSF (26.3%) in Regression #4. According to the findings of Regression #5, GFAP and HOMA2IR collectively accounted for a substantial proportion (26.3%) of the overall m-EDL variance.
Associations between neuronal and immune markers
In the total study group, we found significant correlations between Comp_IRS and NSE (r = 0.413, p < 0.001, n = 130), S100B (r = 0.326, p < 0.001), pTau217 (r = 0.419, p < 0.001), GFAP (r = 0.311, p < 0.001), and Comp_GPT (r = 0.507, p < 0.001). Figure 2 shows the partial regression of NSE on Comp_IRS after adjusting for age and sex. The Comp_GPT index was significantly associated with IL-6 (r = 0.497, p < 0.001), IL-10 (r = 0.526, p < 0.001), HOMA2%S (r=-0.375, p < 0.001), and HOMA2IR (r = 0.405, p < 0.001). The HOMA2IR was correlated with NSE (r = 0.245, p = 0.005), S100B (r = 0.371, p < 0.001), pTau217 (r = 0.313, p < 0.001), GFAP (r = 0.246, p = 0.005). We found that 37.0% of the variance (F = 37.31, df = 2/127, p < 0.001) in Comp_GPT is explained by the combined effects of Comp_IRS (β = 0.459, t = 6.45, p < 0.001) and HOMA2IR (β = 0.340, t = 4.78, p < 0.001). Figures 3 and 4 show the partial regressions of Comp_GPT on Comp_IRS and HOMA2IR, respectively.
In the restricted patient group, we found significant correlations between Comp_IRS and NSE (r = 0.319, p = 0.007, n = 70), S100B (r = 0.264, p = 0.027), pTau217 (r = 0.432, p < 0.001), GFAP (r = 0.333, p = 0.005), and Comp_GPT (r = 0.467, p < 0.001). In the patients, we found that the HOMA2IR was significantly correlated with NSE (r = 0.289, p = 0.015), S100B (r = 0.377, p = 0.001), pTau217 (r = 0.343, p = 0.004), GFAP (r = 0.243, p = 0.043). In patients, up to 36.3% of the variance in Comp_GPT (F = 19.09, df = 2/67, p < 0.001) was explained by the regression on Comp_IRS (β = 0.422, t = 4.29, p < 0.001) and HOMA2IR (β = 0.383, t = 3.90, p < 0.001).
PPI analysis using String showed that these 4 genes shaped a tight network (number of nodes = 4, edges = 6, average clustering coefficient = 1, the expected number of edges = 0; interaction score = 0.7). Figure 5 shows the gene network as well as the results of Gene Ontology cellular component annotation analysis. This network was enriched in glial cell projections (FDR p = 0.0246) and neuronal cell bodies (FDR p = 0.0448), according to cellular component GO analysis (all false discovery rate p values). This network was found to be associated with neurofilament (FDR p = 0.0047), type III intermediate filament (FDR p = 0.0061), glial cell projections (p = 0.0071) and polymeric cytoskeletal fiber (FDR p = 0.0168), according to compartment subcellular analysis (see Fig. 4).
Results of gene network analysis performed on 4 genes, namely MAPT (tau), S100 calcium-binding protein B (S100B), ENO2 (neuron-specific enolase or NSE), and glial fibrillary acidic protein (GFAP).
Left figure: the gene network; right figure: results of subcellular localization compartments enrichment (terms are sorted by strength).
Discussion
Neuronal biomarker of mood and CFS symptoms due to parkison’s disease
The first major finding of this study is that the neuronal damage biomarkers NSE, S100B, pTau217, and GFAP are significantly elevated in Parkinson’s patients compared to the control group. Furthermore, these biomarkers are significantly associated with depression and CFS scores derived from the nM-EDL score. These results indicate an association between the severity of neuronal damage markers and affective and CFS symptoms, suggesting that worsening neuronal damage in Parkinson’s patients may lead to the development of affective and CFS symptoms.
The most significant neuronal injury biomarker associated with MOOD and CFS symptoms in Parkinson’s disease is NSE. Increased NSE in the cerebrospinal fluid has been suggested to be a potential specific biomarker for Parkinson’s disease30. Neuronal damage45,46,47, neurodegenerative disorders, such as stroke and Alzheimer’s disease,46,48,49,50 traumatic brain injury,51,52and hypoxic encephalopathy53 are all accompanied elevated levels of NSE in serum and cerebrospinal fluid. Elevated NSE levels were found to be correlated with more severe depressive symptoms in children diagnosed with transfusion-dependent thalassemia30,34.
Previous research showed that patients with Parkinson’s disease exhibit a notable upregulation of S100B expression in brain tissues29, substantial increases in S100B in the substantia nigra54, and higher serum antibody levels directed to S100B than controls55. Furthermore, a correlation has been observed between elevated nocturnal S100B levels and both sleep disruption and the severity of Parkinson’s disease56. Another study reported an association between S100B polymorphisms and the age at onset of Parkinson’s disease57. A systematic review found no significant correlations between the severity of depression and serum S100B levels, and no alterations in these levels were associated with major depression58.
S100B is a protein that is found in significant amounts in astroglial and oligodendroglial cells in the central nervous system. Consequently, the release of S100B by these cells could potentially indicate a glial reaction to inflammation, ischemia, and metabolic stress15. In addition, inflammation can disrupt the blood-brain barrier, which in turn triggers the activation of astrocytes. This activation results in an elevated release of S100B59. Serum levels of S100B are typically quite low; however, if these levels are elevated, it could suggest a disturbance in the blood-brain barrier and activation of astrocytes60. Interestingly, the presence of S100B triggers activation of inducible nitric oxide synthase (iNOS) and proinflammatory cytokines including IL-6 and IL1β, leading to oxidative stress and inflammation61,62. It is worth noting that decreased levels of S100B have been associated with potential benefits for the brain, such as supporting the survival of neurons63, promoting neurogenesis64, and enhancing neuroplasticity65. These effects may contribute to improved memory and learning processes66.
A study conducted by Andersson et al. (2011) has found that the presence of Tau protein is a strong indicator of cognitive decline in individuals with Parkinson’s disease28. In the latter patients, the percentage of patients with an elevated t-tau/Aβ index increased from 15 to 29% when cognitive impairment is present, and this percentage further rises to 45% in patients with dementia caused by Parkinson’s disease67. People who experience depression are more prone to early tau accumulation in areas of the brain important for emotions and memory31.
The pTau217 protein is widely observed in the microtubule system within cortical and hippocampal neurons and, to a lesser degree, in astrocytes and oligodendrocytes. The importance of tau proteins is found in their involvement in cell signaling, synaptic plasticity, and the maintenance of genomic stability68. Plasma levels of this marker show an increase following neuronal damage, stress, or cognitive decline, as observed in studies by Luebke et al. (2023). and Green et al. (2023)69.
Increased serum GFAP is a potential biomarker for tracking the Parkinson’s disease and its progression33,68,69. In addition, increased GFAP and pTau217 levels are detected in MDD70. For example, serum GFAP may serve as a potential marker for astroglial pathology in MDD71. When astrocytes are injured, they release GFAP and its breakdown products into the interstitial/extracellular fluid72,73.
Based on the findings, it can be concluded that Parkinson’s disease is characterized by the impairment of glial cell (astrocyte) projections, neurofilament, and specifically the type III intermediate filament. It appears that these impairments are closely linked to the development of affective and CFS symptoms in individuals with Parkinson’s disease. Previously, it was observed that MDD is accompanied by damage to neuronal and astroglial projections70.
Immune activation and neuronal damage in parkinson’s disease
Another significant discovery from this study reveals that the peripheral IRS activation in Parkinson’s disease patients is closely linked to heightened levels of all four glial cell damage markers and their composite score as well. Importantly, the detected associations were observed both in the combined group of controls and patients, as well as in the more specific subgroup of patients. These findings underscore the importance of the connections between peripheral immune-inflammatory activation and damage to glial projections and neurofilaments. Furthermore, both glial damage and IRS activation appear to play a significant role in explaining the variability in MOOD + CFS symptoms.
Activation of the peripheral IRS is involved in the pathogenesis of both Parkinson’s disease74 and MDD25. In end-stage renal disease, the presence of affective and CFS-like symptoms is predicted by immune-inflammatory markers such as CRP and IL-10, as well as neuron damage biomarkers like serum neurofilament light chain protein, myelin basic protein, and nestin75. According to a study conducted by Ridhaa et al. (2023), the severity of affective and CFS-like symptoms in children with transfusion-dependent thalassemia can be predicted by assessing IRS activation (measured through IL-6, IL-10, CRP, zinc, and calcium levels) and neuronal damage (measured through serum NSE, GFAP, neurofilament light chain protein)34. In Long COVID, both immune activation and associated oxidative stress and increasing neuronal damage (as assessed with autoimmune response to myelin basic protein, synapsin, tubulin, myelin oligodendrocyte glycoprotein, and neurofilament chain protein) are associated with affective and CFS symptoms76.
Insulin resistance and neuronal damage in parkinson’s disease
This study found that the HOMA2IR index had a significant impact on the physio-affective phenome of Parkinson’s disease, beyond the effects of IRS activation and glial damage markers. These findings suggest that IR plays a role in the development of affective and CFS symptoms in individuals with Parkinson’s disease, as well as the damage to glial projections.
Recent research suggests an association between heightened insulin resistance and Parkinson’s disease and its progression (Parkinson’s Foundation, 2024, https://www.parkinson.org/blog/science-news/insulin-resistance. There are multiple mechanistic explanations for this correlation. Thus, disrupted insulin signaling may have a negative impact on the neurodegenerative processes in Parkinson’s disease77. Furthermore, impaired insulin signaling may disrupt dopamine homeostasis78,79, whilst hyperglycemia may negatively impact dopaminergic neurons, leading to programmed cell death via oxidative damage80,81. Patients exhibiting depressive symptoms may show signs of increased IR38, and IR plays a crucial role in the development of affective and CFS symptoms associated with MDD70, Long COVID39,40, type-2 diabetes mellitus82, and atherosclerosis83. Several studies have found associations between fatigue scores in Parkinson’s disease and various biomarkers, including inflammatory and anti-inflammatory markers84,85IR86and biomarkers indicating neuronal damage84,87.
Limitations
This study would have been more interesting if we had measured immune profiles based on cytokine/chemokines/growth factor assessments using a multiplex assay. Moreover, neuroimaging methodologies could provide additional insights into the associations between the serum biomarkers and the brain disorders in Parkinson’s disease. The results deserve replication in other countries and cultures.
Conclusion
Compared to controls, Parkinson’s disease patients have higher levels of S100B, GFAP, NSE, pTau217, and HOMA2IR indicating damage to glial cell projection and neurofilaments. Moreover, Parkinson’s disease is characterized by peripheral IRS activation, a significant increase in IR, and increased affective and CFS symptoms. NSE, SB100, HOMA2IR index, IL-10 (all positively), and calcium (inversely) accounted for a significant portion (52.5%) of the variance in the affective and CFS scores. All brain injury biomarkers measured in the current study showed a strong correlation with the HOMA2IR and IRS indices. Affective and CFS symptoms due to Parkinson’s disease are a result of damage to glial cell projections and type III intermediate filaments, whilst IRS activation and IR. may cause the latter It follows that IRS activation and IR are new drug targets to treat affective and CFS symptoms due to Parkinson’s disease.
Future research should examine post-mortem brain tissues of patients with depression, anxiety and CFS due to Parkinson’s disease and examine neurodegenerative processes, and neuroinflammation. Furthermore, using brain magnetic resonance imaging, future research should examine the grey and white matter integrity and degenerative patterns in affective symptoms and CFS due to Parkinson’s disease in association with peripheral IRS activation and insulin resistance.
Data availability
The database created during this investigation will be provided by the corresponding author (MM) upon a reasonable request once the authors have thoroughly used the data set.
References
Uwishema, O. et al. The Understanding of parkinson’s disease through genetics and new therapies. Brain Behav. 12, e2577. https://doi.org/10.1002/brb3.2577 (2022).
Saini, N. et al. Motor and non-motor symptoms, drugs, and their mode of action in parkinson’s disease (PD): a review. Med. Chem. Res., 1–20 (2024).
Silva, A. B. R. L. et al. Premotor, nonmotor and motor symptoms of parkinson’s disease: a new clinical state of the Art. Ageing Res. Rev. 84, 101834 (2023).
Hirsch, E. C. & Hunot, S. Neuroinflammation in parkinson’s disease: a target for neuroprotection? Lancet Neurol. 8, 382–397 (2009).
Butkovich, L. M., Houser, M. C. & Tansey, M. G. α-synuclein and noradrenergic modulation of immune cells in parkinson’s disease pathogenesis. Front. NeuroSci. 12, 626 (2018).
Chikatimalla, R. et al. Depression in parkinson’s disease: A narrative review. Cureus 14, e27750. https://doi.org/10.7759/cureus.27750 (2022).
Braak, H., Rüb, U., Gai, W. P. & Del Tredici, K. Idiopathic parkinson’s disease: possible routes by which vulnerable neuronal types May be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 110, 517–536 (2003).
Manev, H., Favaron, M., Guidotti, A. & Costa, E. Delayed increase of Ca2 + influx elicited by glutamate: role in neuronal death. Mol. Pharmacol. 36, 106–112 (1989).
Wang, K. K., Zhang, Z. & Moghieb, A. In Neural Regeneration401–410 (Elsevier, 2015).
Huibregtse, M. E., Bazarian, J. J., Shultz, S. R. & Kawata, K. The biological significance and clinical utility of emerging blood biomarkers for traumatic brain injury. Neurosci. Biobehav. Rev.. 130, 433–447 (2021).
Silvestro, S., Raffaele, I., Quartarone, A. & Mazzon, E. Innovative insights into traumatic brain injuries: biomarkers and new Pharmacological targets. Int. J. Mol. Sci. 25, 2372 (2024).
Palumbo, B., Sabalich, I. & Tranfaglia, C. & Lucilla Parnetti, M. J. F. n. Cerebrospinal fluid neuron-specific enolase: a further marker of Alzheimer’s disease? 23, 93 (2008).
Brenner, M. Role of GFAP in CNS injuries. Neurosci. Lett. 565, 7–13 (2014).
Palmqvist, S. et al. Discriminative accuracy of plasma phospho-tau217 for alzheimer disease vs other neurodegenerative disorders. Jama 324, 772–781 (2020).
das Neves, S. P., Sousa, J. C., Sousa, N., Cerqueira, J. J. & Marques, F. J. G. Altered astrocytic function in experimental neuroinflammation and multiple sclerosis. 69, 1341–1368 (2021).
Schaf, D. V. et al. S100B and NSE serum levels in patients with parkinson’s disease. Parkinsonism Relat. Disord. 11, 39–43 (2005).
Pilotto, A. et al. Plasma nfl, GFAP, amyloid, and p-tau species as prognostic biomarkers in parkinson’s disease. J. Neurol. 271, 7537–7546. https://doi.org/10.1007/s00415-024-12669-7 (2024).
Peña-Zelayeta, L. et al. In Preprints (Preprints, 2025).
Marsh, L. Depression and parkinson’s disease: current knowledge. Curr. Neurol. Neurosci. Rep. 13, 1–9 (2013).
Siciliano, M. et al. Fatigue in parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 33, 1712–1723 (2018).
Müller, B., Assmus, J., Herlofson, K., Larsen, J. P. & Tysnes, O. B. Importance of motor vs. non-motor symptoms for health-related quality of life in early parkinson’s disease. Parkinsonism Relat. Disord. 19, 1027–1032 (2013).
Lindqvist, D. et al. Non-motor symptoms in patients with parkinson’s disease–correlations with inflammatory cytokines in serum. (2012).
Sun, C. et al. Peripheral humoral immune response is associated with the Non-motor symptoms of parkinson’s disease. Front. Neurosci. Volume 13–2019 (2019).
Twisk, F. N. & Maes, M. A review on cognitive behavorial therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS. Neuroendocrinol. Lett. 30, 284–299 (2009).
Maes, M. & Carvalho, A. F. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol. Neurobiol. 55, 8885–8903 (2018).
Qin, X. Y., Zhang, S. P., Cao, C., Loh, Y. P. & Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 73, 1316–1324 (2016).
Morris, H. R., Spillantini, M. G., Sue, C. M. & Williams-Gray, C. H. The pathogenesis of parkinson’s disease. Lancet 403, 293–304 (2024).
Andersson, M., Zetterberg, H., Minthon, L., Blennow, K. & Londos, E. The cognitive profile and CSF biomarkers in dementia with lewy bodies and parkinson’s disease dementia. Int. J. Geriatr. Psychiatry. 26, 100–105 (2011).
Rydbirk, R. et al. Cytokine profiling in the prefrontal cortex of parkinson’s disease and multiple system atrophy patients. Neurobiol. Dis. 106, 269–278. https://doi.org/10.1016/j.nbd.2017.07.014 (2017).
Dutta, R. Role of neuron specific enolase as a biomarker in parkinson’s disease. J. Neurosci. Neurol. Disorders. 5, 061–068 (2021).
Gonzales, M. M. et al. Association of midlife depressive symptoms with regional Amyloid-β and Tau in the Framingham heart study. J. Alzheimer’s Dis., 1–12 (2021).
Al-Hakeim, H. K., Al-Rubaye, H. T., Almulla, A. F., Al-Hadrawi, D. S. & Maes, M. Chronic fatigue, depression and anxiety symptoms in long COVID are strongly predicted by neuroimmune and neuro-oxidative pathways which are caused by the inflammation during acute infection. J. Clin. Med. 12, 511 (2023).
Lin, J. et al. Plasma glial fibrillary acidic protein as a biomarker of disease progression in parkinson’s disease: a prospective cohort study. BMC Med. 21, 420 (2023).
Ridhaa, M. A. S., Al-Hakeim, H. K., Kahlol, M. K., Al-Naqeeb, T. H. & Maes, M. In children with transfusion-dependent thalassemia, inflammation and neuronal damage biomarkers are associated with affective and chronic fatigue symptoms. MedRxiv, 2023 – 2011 (2023).
Liu, W. & Tang, J. Association between diabetes mellitus and risk of parkinson’s disease: a prisma-compliant meta‐analysis. Brain Behav. 11, e02082 (2021).
Chohan, H. et al. Type 2 diabetes as a determinant of parkinson’s disease risk and progression. Mov. Disord. 36, 1420–1429 (2021).
Ruiz-Pozo, V. A. et al. The molecular mechanisms of the relationship between insulin resistance and parkinson’s disease pathogenesis. Nutrients 15, 3585 (2023).
Morelli, N. R. et al. Increased nitro-oxidative toxicity in association with metabolic syndrome, atherogenicity and insulin resistance in patients with affective disorders. J. Affect. Disord. 294, 410–419 (2021).
Al-Hakeim, H. K., Khairi Abed, A., Rouf Moustafa, S., Almulla, A. F. & Maes, M. Tryptophan catabolites, inflammation, and insulin resistance as determinants of chronic fatigue syndrome and affective symptoms in long COVID. Front. Mol. Neurosci. 16, 1194769 (2023).
Vojdani, A., Almulla, A. F., Zhou, B., Al-Hakeim, H. K. & Maes, M. Reactivation of herpesvirus type 6 and IgA/IgM-mediated responses to activin-A underpin long COVID, including affective symptoms and chronic fatigue syndrome. Acta Neuropsychiatrica. 36, 172–184 (2024).
Hughes, A. J., Daniel, S. E., Kilford, L. & Lees, A. J. Accuracy of clinical diagnosis of idiopathic parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 55, 181–184 (1992).
Perlmutter, J. S. Assessment of Parkinson disease manifestations. Current Protocols Neurosci. Chap 10. Unit10.11 (2009).
Goetz, C. G. et al. Movement disorder Society-sponsored revision of the unified parkinson’s disease rating scale (MDS‐UPDRS): process, format, and clinimetric testing plan. Mov. Disord. 22, 41–47 (2007).
Goetz, C. G. et al. Movement disorder Society-sponsored revision of the unified parkinson’s disease rating scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov. Disorders: Official J. Mov. Disorder Soc. 23, 2129–2170 (2008).
Herrmann, M. et al. Protein S-100B and neuron specific enolase as early neurobiochemical markers of the severity of traumatic brain injury. Restor. Neurol. Neurosci. 14, 109–114 (1999).
Isgrò, M. A., Bottoni, P. & Scatena, R. J. A. I. C. B. F. B. T. C. F. A. C. R. Neuron-specific enolase A. A.B.omarker: B.ochemical A.d C.inical A.pects. Advances Cancer Biomarkers, 125–143 (2015).
Constantinescu, R., Zetterberg, H., Holmberg, B. & Rosengren, L. J. P. & disorders, r. Levels of brain related proteins in cerebrospinal fluid: an aid in the differential diagnosis of parkinsonian disorders. 15, 205–212 (2009).
Chaves, M. L. et al. Serum levels of S100B and NSE proteins in Alzheimer’s disease patients. J. Neuroinflamm. 7 https://doi.org/10.1186/1742-2094-7-6 (2010).
Papuć, E. & Rejdak, K. Increased cerebrospinal fluid S100B and NSE reflect neuronal and glial damage in parkinson’s disease. Front. Aging Neurosci. 12, 156 (2020).
Christl, J. et al. Association of cerebrospinal fluid S100B protein with core biomarkers and cognitive deficits in prodromal and mild alzheimer’s disease. 72, 1119–1127 (2019).
Streitbürger, D. P. et al. Validating serum S100B and neuron-specific enolase as biomarkers for the human brain–a combined serum, gene expression and MRI study. (2012).
Kim, H. J., Tsao, J. W. & Stanfill, A. G. The current state of biomarkers of mild traumatic brain injury. JCI Insight. 3 https://doi.org/10.1172/jci.insight.97105 (2018).
León-Lozano, M. Z. et al. Cerebrospinal fluid levels of neuron-specific enolase predict the severity of brain damage in newborns with neonatal hypoxic-ischemic encephalopathy treated with hypothermia. PloS One. 15, e0234082 (2020).
Sathe, K. et al. S100B is increased in parkinson’s disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-α pathway. Brain 135, 3336–3347 (2012).
Wilhelm, K. R. et al. Immune reactivity towards insulin, its amyloid and protein S100B in blood Sera of parkinson’s disease patients. Eur. J. Neurol. 14, 327–334 (2007).
Carvalho, D. et al. Overnight S100B in parkinson’s disease: A glimpse into sleep-related neuroinflammation. 608, 57–63 (2015).
Fardell, C. et al. S100B polymorphisms are associated with age of onset of parkinson’s disease. BMC Med. Genet. 19, 1–9 (2018).
Kozlowski, T., Bargiel, W., Grabarczyk, M. & Skibinska, M. Peripheral S100B protein levels in five major psychiatric disorders: a systematic review. Brain Sci. 13, 1334 (2023).
Zhang, W. et al. The blood brain barrier in cerebral ischemic injury–Disruption and repair. 1, 34–53 (2020).
Yu, Y. et al. Perfluorooctane sulfonate disrupts the blood brain barrier through the crosstalk between endothelial cells and astrocytes in mice. 256, 113429 (2020).
Hu, J., Ferreira, A. & Van Eldik, L. J. S100β induces neuronal cell death through nitric oxide release from astrocytes. J. Neurochem. 69, 2294–2301 (1997).
Langeh, U. & Singh, S. Targeting S100B protein as a surrogate biomarker and its role in various neurological disorders. Curr. Neuropharmacol. 19, 265–277 (2021).
Bianchi, R., Adami, C., Giambanco, I. & Donato, R. S100B binding to RAGE in microglia stimulates COX-2 expression. J. Leucocyte Biology. 81, 108–118 (2007).
Baecker, J. et al. Treatment with the neurotrophic protein S100B increases synaptogenesis after traumatic brain injury. J. Neurotrauma. 37, 1097–1107 (2020).
Nishiyama, H., Knopfel, T., Endo, S. & Itohara, S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc. Natl. Acad. Sci. U S A. 99, 4037–4042. https://doi.org/10.1073/pnas.052020999 (2002).
Kleindienst, A., Grunbeck, F., Buslei, R., Emtmann, I. & Buchfelder, M. Intraperitoneal treatment with S100B enhances hippocampal neurogenesis in juvenile mice and after experimental brain injury. Acta Neurochir. (Wien). 155, 1351–1360. https://doi.org/10.1007/s00701-013-1720-2 (2013).
Montine, T. J. et al. CSF Aβ42 and Tau in parkinson’s disease with cognitive impairment. Mov. Disord. 25, 2682–2685 (2010).
Guo, T., Noble, W. & Hanger, D. P. J. A. N. Roles of Tau protein in health and disease. Acta Neuropathol. 133, 665–704 (2017).
Green, Z. D., Kueck, P. J., John, C. S., Burns, J. M. & Morris, J. K. Blood biomarkers discriminate cerebral amyloid status and cognitive diagnosis when collected with ACD-A anticoagulant. Curr. Alzheimer Res. 20, 557 (2023).
Al-Hakeim, H. K., Al-Naqeeb, T. H., Almulla, A. F. & Maes, M. The physio-affective phenome of major depression is strongly associated with biomarkers of astroglial and neuronal projection toxicity which in turn are associated with peripheral inflammation, insulin resistance and Lowered calcium. J. Affect. Disord. 331, 300–312 (2023).
Steinacker, P. et al. Glial fibrillary acidic protein as blood biomarker for differential diagnosis and severity of major depressive disorder. J. Psychiatr. Res. 144, 54–58 (2021).
Lotankar, S., Prabhavalkar, K. S. & Bhatt, L. K. Biomarkers for parkinson’s disease: recent advancement. Neurosci. Bull. 33, 585–597 (2017).
Luger, S. et al. Glial fibrillary acidic protein serum levels distinguish between intracerebral hemorrhage and cerebral ischemia in the early phase of stroke. 63, 377–385 (2017).
Muñoz-Delgado, L. et al. Peripheral inflammatory immune response differs among sporadic and Familial parkinson’s disease. Npj Parkinson’s Disease. 9, 12 (2023).
Al-Hakeim, H. K., Twaij, B. A. A. R., Al-Naqeeb, T. H., Moustafa, S. R. & Maes, M. Neuronal damage and inflammatory biomarkers are associated with the affective and chronic fatigue-like symptoms due to end-stage renal disease. J. Affect. Disord. 347, 220–229 (2024).
Almulla, A. F., Maes, M., Zhou, B., Al-Hakeim, H. K. & Vojdani, A. Brain-targeted autoimmunity is strongly associated with long COVID and its chronic fatigue syndrome as well as its affective symptoms. Journal Adv. Research (2024).
Nowell, J., Blunt, E. & Edison, P. Incretin and insulin signaling as novel therapeutic targets for alzheimer’s and parkinson’s disease. Mol. Psychiatry. 28, 217–229. https://doi.org/10.1038/s41380-022-01792-4 (2023).
Bassil, F. et al. Impaired brain insulin signalling in parkinson’s disease. Neuropathol. Appl. Neurobiol. 48, e12760 (2022).
Gruber, J. et al. Impact of insulin and insulin resistance on brain dopamine signalling and reward processing–an underexplored mechanism in the pathophysiology of depression? Neuroscience & Biobehavioral Reviews, 105179 (2023).
Renaud, J., Bournival, J., Zottig, X. & Martinoli, M. G. Resveratrol protects daergic PC12 cells from high glucose-induced oxidative stress and apoptosis: effect on p53 and GRP75 localization. Neurotox. Res. 25, 110–123 (2014).
Su, C. J. et al. Thioredoxin-interacting protein (TXNIP) regulates Parkin/PINK1-mediated mitophagy in dopaminergic neurons under high-glucose conditions: implications for molecular links between parkinson’s disease and diabetes. Neurosci. Bull. 36, 346–358 (2020).
Al-Hakeim, H. K., Hadi, H. H., Jawad, G. A. & Maes, M. Intersections between copper, β-arrestin-1, calcium, FBXW7, CD17, insulin resistance and atherogenicity mediate depression and anxiety due to type 2 diabetes mellitus: a nomothetic network approach. J. Personalized Med. 12, 23 (2022).
Mousa, R. F., Smesam, H. N., Qazmooz, H. A., Al-Hakeim, H. K. & Maes, M. A pathway phenotype linking metabolic, immune, oxidative, and opioid pathways with comorbid depression, atherosclerosis, and unstable angina. CNS Spectr. 27, 676–690 (2022).
Wang, H. et al. Possible inflammatory mechanisms and predictors of parkinson’s disease patients with fatigue (brief review). Clin. Neurol. Neurosurg. 208, 106844 (2021).
Herlofson, K. et al. Inflammation and fatigue in early, untreated parkinson’s disease. Acta Neurol. Scand. 138, 394–399 (2018).
Özer, G., Adıgüzel, H., Kırmacı, Z. İ. K. & Ergun, N. Is insulin resistance associated with fatigue, the severity of the disease and motor disability in idiopathic parkinson’s disease? J. Surg. Med. 3, 437–440 (2019).
De Dreu, M., Schouwenaars, I., Rutten, G., Ramsey, N. & Jansma, J. Fatigue in brain tumor patients, towards a neuronal biomarker. NeuroImage: Clin. 28, 102406 (2020).
Acknowledgements
We acknowledge the assistance of the workers at the Middle Euphrates Center for Neurological Sciences, Najaf City, Iraq in the sample collection and lab measurements.
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Contributions
Hussein Kadhem Al-Hakeim: Conceptualization, Supervision, Roles/Writing - original draft. Hayder Naji Khudhair: Data curation, Investigation, Project administration, Writing - review & editing. Sayed-Omid Ranaei-Siadat: Data curation, Investigation, Project administration, Writing - review & editing. Fataneh Fatemi: Writing - review & editing. Fateme Mirzajani: Writing - review & editing. Mengqi Niu: Writing - review & editing. Michael Maes: Formal analysis, Supervision, Visualization, Roles/Writing - original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study has been approved by the institutional ethics committee of the University of Kufa (2109/2023). The study adhered to both Iraqi and international ethical and privacy laws, including the International Conference on Harmonization of Good Clinical Practice, the Belmont Report, the CIOMS Guidelines, and the World Medical Association’s Declaration of Helsinki. Before participating in this study, each subject provided written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Al-Hakeim, H.K., Khudhair, H.N., Ranaei-Siadat, SO. et al. Affective and chronic fatigue symptoms are associated with serum neuronal damage markers in Parkinson’s disease. Sci Rep 15, 20647 (2025). https://doi.org/10.1038/s41598-025-07735-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07735-7
Keywords
This article is cited by
-
Neuroimmune, metabolic and oxidative stress pathways in major depressive disorder
Nature Reviews Neurology (2025)