Abstract
Multiple organ dysfunction syndrome (MODS) claims a life every few seconds worldwide and is a severe condition that can affect people of all ages. Despite the availability of various treatments, the unmet medical needs remain high. Nuclear factor erythroid 2-related factor 2 (NRF2) is a key molecule involved in biological protection and is expected to be beneficial for MODS, but its role in ameliorating MODS remains unknown. We identified a novel kelch-like ECH-associated protein 1 (KEAP1)-NRF2 protein–protein interaction inhibitor, CH7450924, and evaluated its efficacy in lipopolysaccharide (LPS)-induced MODS models. To characterize our compound, we analyzed CH7450924 binding to KEAP1 by crystallography. Comprehensive binding and inhibition experiments were conducted to evaluate selectivity. Subsequently, we assessed its therapeutic effects using an LPS-induced MODS mouse model. In the MODS model, survival rate, inflammatory markers, organ function, microcirculation, and histopathology were assessed. The results demonstrated that CH7450924 bound non-covalently to KEAP1 and competitively inhibited the KEAP1-NRF2 binding with high selectivity. In the MODS model, CH7450924 significantly ameliorated the mortality rate. CH7450924 significantly decreased plasma IL-6, IL-1β, and TNFα and suppressed inflammatory cytokine mRNA expression in kidney and liver. CH7450924 improved kidney function and liver injury as indicated by plasma biochemistry. While not affecting blood pressure or heart rate, CH7450924 significantly improved peripheral blood flow in the ear. CH7450924 also significantly increased platelet count and reduced plasma PAI-1. Endothelial damage markers were also reduced in kidney and liver. In a lung injury model induced by intratracheal LPS injection, CH7450924 significantly reduced inflammation, prevented structural damage, and improved respiratory function. In conclusion, this study reveals that NRF2 activation is critical for the treatment for MODS. CH7450924, a highly potent and selective NRF2 activator, ameliorated MODS by protecting kidney, liver, and lung through its anti-inflammatory properties and its ability to protect against endothelial damage and organ dysfunction.
Similar content being viewed by others
Introduction
Multiple organ dysfunction syndrome (MODS) is a life-threatening condition that claims a life every few seconds worldwide. MODS occurs in response to infection, trauma, or after major surgeries, and is characterized by the simultaneous dysfunction of several organs1. The initial inflammatory response becomes uncontrollable, leading to systemic inflammatory response syndrome (SIRS), which can progress to multiple organ failure2. In this condition, oxidative stress and excessive production of inflammatory cytokines play central roles, with interactions between organs exacerbating symptoms3,4. Current treatments for multi-organ failure primarily focus on symptomatic management tailored to each affected organ. These include fluid therapy, mechanical ventilation for respiratory dysfunction, and hemodialysis for renal dysfunction. Therefore, there is a growing demand for more fundamental therapeutic agents capable of improving the function of multiple organs5.
Nuclear factor erythroid 2-related factor 2 (NRF2) is a transcription factor that plays a central role in cellular defense mechanisms against oxidative stress and in the regulation of inflammation. Kelch-like ECH-associated protein 1 (KEAP1) functions as a negative regulator of NRF2. Under physiological conditions, KEAP1 interacts with NRF2 via its kelch domain and facilitates the ubiquitination and subsequent proteasomal degradation of NRF2. The dissociation of NRF2 from the KEAP1 complex results in the stabilization and nuclear translocation of NRF2, where it activates the transcription of numerous antioxidant molecules and detoxifying enzymes6. Considering the diverse range of biological functions mediated by NRF2, it has been hypothesized that NRF2 may exert multi-organ protective effects on multiple cell types. Numerous preclinical studies have demonstrated that NRF2 knockout mice are vulnerable to multi-organ injury7,8, while the administration of NRF2 activators or genetic engineering techniques to activate NRF2 in mice show multi-organ protective effects7,9,10,11,12,13. These findings suggest that NRF2 activation may be an attractive therapeutic approach for MODS. However, many previously reported NRF2 activators lack selectivity for NRF2, raising concerns about potential side effects. Furthermore, the genetic engineering approaches used to activate NRF2 present difficult challenges for medical application in humans.
Therefore, we have synthesized a novel NRF2 activator, CH7450924, a small molecule compound with high selectivity and potent activity. In this study, we demonstrated the protective effects of CH7450924 against multi-organ damage induced by lipopolysaccharide (LPS) administration in mice.
Materials and methods
Reagents
CH7450924 [4-[3-[2-chloro-4-[(2R,5R)-2,4,5-trimethylpiperazin-1-yl]benzoyl]-2,4-dihydro-1,3-benzoxazin-8-yl]-5-fluoro-2-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)benzoic acid hydrate] (Fig. 1A) and Bardoxolone methyl (BARD) was synthesized by Chugai Pharmaceutical Co., Ltd. (Kanagawa, Japan). Human KEAP1 Kelch domain (321–609) and E540A/E542A mutant were manufactured by Chiome Bioscience Inc. (Tokyo, Japan). Biotin-NRF2 (69–84) was purchased from Cambridge Research Biochemicals Ltd. (Billingham, UK). LPS and rifampicin were purchased from Sigma-Aldrich/MerckMillipore (St. Louis, MO). All other chemicals were of the highest purity available.
(A) Chemical structure of CH7450924. (B) (C) Overall structures of human KEAP1 in complex with CH7450924 and NRF2 ETGE peptide shown as diagrams in light-pink (PDB ID: 9M24) and pale-cyan (PDB ID: 5WFV), respectively. CH7450924, ETGE peptide, and interacting residues defined as having at least one non-hydrogen atom within 4.2 Å of the respective ligand, are shown as colored stick models (C, magenta/light-pink in Fig. 1B or marine/cyan in Fig. 1C; O, red; N, blue; F, pale-cyan; Cl, green). Common amino acid residues involved in binding both CH7450924 and the ETGE peptide are highlighted with a yellow surface representation. (D) KEAP1-NRF2 interaction inhibitory activity. Data are represented as mean + SD of three measurement points.
Crystallization and structural determination of human KEAP1 in complex with CH7450924
The purification of the kelch domain of human KEAP1 (residues 321–609, E540A/E542A mutation) was performed as described in several reports14. The obtained crystals were soaked in a solution containing CH7450924. Diffraction data were collected at Photon Factory BL1A and processed with autoPROC. The structure was determined by molecular replacement using Phaser, refined by Coot and Buster, and deposited in the PDB (ID: 9M24). Figure 1B and C were generated using PyMOL. Detailed methods are available in the supplementary material 1 and the supplementary material 2: Supplemental Table 1.
Binding activity of KEAP1 and NRF2
The binding activity of KEAP1 and NRF2 was evaluated by an AlphaScreen assay as previously reported15. Briefly, various concentrations of CH7450924 were added to the solution containing 12 nmol/L Biotin-conjugated NRF2 peptide fragment (69–84) and 4 nmol/L His-tagged KEAP1 kelch domain (321–609). The binding of KEAP1 and NRF2 was carried out with a one-hour incubation at room temperature. The binding activity was measured using an AlphaScreen® Histidine (Nickel Chelate) Detection Kit (PerkinElmer, Inc., MA) according to the manufacturer’s instructions.
NRF2 activity in vitro
NAD(P)H quinone oxidoreductase 1 (NQO1) mRNA was measured to evaluate the effect of CH7450924 on NRF2 activity in 293, COS-7, NRK-52E, and SV40 Mes13 cells derived from human, monkey, rat, and mouse kidney, respectively (American Type Culture Collection, Manassas, VA). Each cell line was cultured according to the manufacturer’s instructions. Cells were incubated with medium containing various concentrations of CH7450924 for 24 h.
Cerep assays
The selectivity of CH7450924 and BARD, a covalent binder of NRF2, for various molecular targets (100 binding assays using radioligand, 30 enzyme assays, 6 cellular assays) was examined using Cerep (Celle L’Evescault, France). CH7450924 and BARD were tested at 10 μmol/L and at several concentrations for IC50 or EC50 determination. All studies were internally controlled with reference ligands, and further details of the methodologies for each assay can be found at http://www.cerep.fr.
Animal experiments
BALB/c mice were selected for this LPS-induced sepsis model based on previous studies demonstrating their heightened susceptibility to LPS compared to C57BL/6 mice16. This strain has been well-documented in both intraperitoneal and intratracheal LPS administration models17,18. Five or 9-week-old male BALB/cCrSlc mice were purchased from Japan SLC, Inc. (Shizuoka, Japan). and acclimated for at least 4 days before being used in experiments. Animals were maintained under standard laboratory conditions with a 12-h light/dark cycle. Mice were housed in groups of four per cage for the majority of experiments. For telemetry studies measuring cardiovascular parameters and experiments monitoring oxygen saturation (SpO2), mice were individually housed to ensure accurate data collection. Throughout all experimental conditions, animals had unrestricted access to standard chow and water ad libitum. The group composition and number of animals for each test are shown in supplementary material 2: Supplemental Table 2. In all tests, group allocation was performed randomly based on body weight measured before the start of the experiment. A negative control group (NC: Normal Control) was established in which mice were administered saline intraperitoneally or intratracheally instead of LPS. Animals that did not meet the grouping criteria were excluded from the study and humanely euthanized. There were no exclusions of animals during the course of the experiment.
To evaluate systemic injury caused by MODS, LPS was administered intraperitoneally to 6-week-old mice at 10 mg/kg. CH7450924 was initially administered intraperitoneally at 20 mg/kg 7 h prior to LPS injection, with the second dose given immediately before LPS injection. Sixteen hours after LPS administration whole blood was collected from the posterior vena cava and centrifuged to obtain plasma. Kidneys and livers were rapidly frozen in liquid nitrogen. In addition to the main study, a separate experiment was conducted to assess the effect of the drug on peripheral blood flow. Using the same protocol, ear blood flow was measured under isoflurane anesthesia using the 2D laser blood flow imager Omegazone OZ-1 (OMEGAWAVE, INC., Tokyo, Japan) 16 h after LPS administration.
To examine the effects of 10 mg/kg of CH7450924 on systemic hemodynamics, blood pressure and heart rate were measured in 12-week-old conscious animals using a telemetry system (Data Sciences International, St. Paul, MN) as reported previously19. Briefly, under isoflurane anesthesia, telemetry probes were implanted in the right carotid artery, and analgesics were administered for 2 days post-operation. Mice were used for experiments two weeks after the operation. Administration of LPS and CH7450924 was performed as described above. Blood pressure and heart rate were monitored from 24 h before CH7450924 administration until 16 h after LPS administration.
For the evaluation of survival rate, 10 mg/kg of CH7450924 was initially administered to 6-week-old mice 7 h prior to LPS injection, with the second dose given immediately before LPS injection and was administered twice daily, with observations continuing up to 72 h post-LPS injection. Animals that became moribund or were determined to require humane intervention based on animal welfare considerations were euthanized by carbon dioxide inhalation. Euthanasia criteria followed the Guidelines for the Care and Use of Laboratory Animals at Chugai Pharmaceutical Co. Ltd..
To assess the impact on lung injury, 100 μg of LPS was administered intratracheally to 6-week-old mice. CH7450924 was administered intraperitoneally twice daily, starting approximately 7 h after LPS administration. Three days post-LPS administration, animals were euthanized, and lungs were harvested and weighed. The lungs were then either fixed in phosphate-buffered 10% formalin or frozen in liquid nitrogen. SpO2 was measured using a pulse oximeter MouseOx® PLUS (STARR Life Science Corp., Oakmont, PA) before and 3 days after LPS administration.
In all experiments, mice were orally administered rifampicin at a dose of 100 mg/kg approximately 1 h prior to CH7450924 administration to prolong the blood exposure of CH7450924.
mRNA measurement and biochemical analysis
Total RNA was purified from cell lysates and the frozen kidneys and livers using the RNeasy kit (QIAGEN, Hilden, Germany), and the total RNA concentration was measured. PrimeTime® qPCR Assays (IDT, Coralville, IA) or TaqMan Gene Expression Assays (Thermo Fisher Scientific Inc., MA) were mixed with the purified total RNA, and quantitative real-time PCR was performed. mRNA expression levels are given as ratios to Mapk1 mRNA.
Biochemical parameters in plasma were measured using an autoanalyzer (TBA-120FR; Canon Medical Systems Corporation, Tochigi, Japan). Complete blood count was performed using an automated blood cell analyzer (XN-3100 V; Sysmex Corp., Hyogo, Japan). Plasma cytokines were measured with MESO QuickPlex SQ 120 (Meso Scale Diagnostics LLC, MD). Plasma plasminogen activator inhibitor-1 (PAI-1) and Syndecan-1 were measured with Mouse PAI-1 ELISA Kit and Mouse Syndecan-1 ELISA Kit, respectively (abcam plc, Cambridge, UK).
Histopathology
The left lung and right anterior lung robe were injected with phosphate-buffered 10% formalin through the trachea and preserved in phosphate-buffered 10% formalin. These lungs were embedded in paraffin, sectioned, stained with hematoxylin and eosin (HE), and examined microscopically. Histopathological findings and criteria were modified from the American Thoracic Society report20. Briefly, semi-quantitative analysis of histopathological findings was scored and evaluated based on the following criteria. Neutrophil infiltration of alveoli were scored using a 6-point scale: 0 (not present), 0.5 (≦ 1/8 area), 1 (≦ 1/4 area), 2 (≦ 2/4 area), 3 (≦ 3/4 area), 4 (> 3/4 area). Thickening of alveolar wall was evaluated using a 5-point scale: 0 (not present), 1 (≦ 1/4 area), 2 (≦ 2/4 area), 3 (≦ 3/4 area), 4 (> 3/4 area). For each finding, the percentage of affected areas in the right anterior lung lobe and the left lung was separately scored. The total score was then calculated by adding these two percentages and dividing by two.
Statistical analysis
For the analysis of histopathological scores, SAS Release 9.1.3 was used (SAS Institute Inc., Cary, NC). Wilcoxon’s rank sum test was used to compare Normal control group and Disease control group, and the Shirley-Williams test was applied to compare Disease control group and CH7450924 groups. EC50 in each cell line was calculated by nonlinear regression analysis using JMP® 15.0.0 (SAS Institute Inc.). For the rest of the analysis, GraphPad Prism v10.1.2 was used. Survival curves were created using the Kaplan–Meier method, and survival rates were compared using the log-rank test. For the biochemical, mRNA measurements, and lung wet weight, Student’s t-test or William’s t-test was used. The significance level was set at 0.05.
Results
Structural analysis of CH7450924’s binding domain to KEAP1 and its inhibitory activity against KEAP1-NRF2 binding
The chemical structure of CH7450924 is shown in Fig. 1A. Crystal structure analysis of the kelch domain of KEAP1 complexed with CH7450924 revealed that CH7450924 bound to the NRF2 binding site of KEAP1 (Fig. 1B, C). Additionally, there were no cysteine residues at the binding site of CH7450924 (supplementary material 2: Supplemental Fig. 1). A binding assay using the kelch domain of KEAP1 and a partial peptide of NRF2 revealed that CH7450924 inhibited the interaction between KEAP1 and NRF2 in a concentration-dependent manner (Fig. 1D). These results suggest that CH7450924 binds non-covalently to KEAP1 and competitively inhibits the binding between NRF2 and KEAP1.
Activity and selectivity of CH7450924 for NRF2
CH7450924 increased the expression of NQO1 mRNA, a target gene of NRF2, in human kidney cell line 293 in a concentration-dependent manner (supplementary material 2: Supplemental Fig. 2A). The EC50 was 2.5 ± 0.1 nmol/L. Similar effects were observed in kidney-derived cell lines from monkey, rat, and mouse (COS-7, NRK-52E, Mes13) to the same extent as in 293 cells (Table 1, supplementary material 2: Supplemental Fig. 2B–D).
The inhibitory effects of CH7450924 or BARD on ligand binding and molecular function against 130 molecules are shown in supplementary material 2: Supplemental Table 3. In these assays, inhibition or stimulation exceeding 50% are considered to represent significant effects of the test compound. CH7450924 showed significant effects on 4 molecules, while BARD significantly affected 16 molecules. To examine the precise involvement of the 4 molecules (α2c, NK1, 5-HT1B, and UT) that were significantly inhibited by CH7450924, additional cellular assays were conducted. Results revealed inhibitory activity only against 5-HT1B at doses up to 10 μmol/L, indicating that CH7450924 has a very high selectivity for NRF2 (supplementary material 2: Supplemental Table 4).
Effects of CH7450924 in MODS model induced by intraperitoneal LPS injection
Effects of CH7450924 on MODS were evaluated using mice injected with LPS intraperitoneally. CH7450924 significantly ameliorated the survival rate (Fig. 2A). To investigate the mechanism underlying this improved survival, examinations were conducted in animals 16 h after intraperitoneal LPS injection. Plasma interleukin 6 (IL-6), interleukin 1β (IL-1β), and tumor necrosis factor alpha (TNFα) in disease control mice significantly increased compared with normal control mice (Fig. 2B). CH7450924 significantly attenuated these increases. The mRNA expression of IL-6, IL-1β, and TNFα in kidney (Fig. 2C) and liver (Fig. 2D) was also significantly increased by LPS injection. CH7450924 significantly reduced IL-6 and IL-1β mRNA in both organs, as well as TNFα mRNA in the kidney. Although the reduction of TNFα mRNA in the liver did not reach statistical significance, a clear downward trend was observed following CH7450924 administration (Fig. 2C, D).
Effects of CH7450924 in MODS model induced by intraperitoneal LPS injection. (A) 72-h survival rate. CH7450924 was administered at 10 mg/kg twice a day before and after LPS injection. (B) IL-6, IL-1β, and TNFα concentration in plasma, (C) mRNA expression of IL-6, IL-1β, and TNFα in the kidney and (D) liver, (E) plasma creatinine and BUN levels, (F) mRNA expression of KIM-1 and NGAL in the kidney, and (G) plasma AST, ALT, and T-BIL levels. CH7450924 was administered at 20 mg/kg before LPS injection. The actual values are represented as dots, and the bars represent mean ± SE (n = 8). #p < 0.05, significant difference between NC and DC (Student’s t-test). *p < 0.05, significant difference between DC and CH7450924 (Student’s t-test). NC; Normal control, DC; Disease control, CH0924; CH7450924.
Plasma creatinine and blood urea nitrogen (BUN) significantly increased in disease control mice compared with normal control mice and significantly decreased in the CH7450924 20 mg/kg group (Fig. 2E). Similar to plasma creatinine and BUN, the renal injury markers kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) mRNA in kidney tended to be improved by CH7450924 (Fig. 2F).
Plasma liver injury markers, total bilirubin (T-BIL), aspartate aminotransferase (AST), and alanine aminotransferase (ALT), were significantly increased by LPS injection. Treatment with CH7450924 significantly inhibited the elevation of T-BIL. While the suppression of AST and ALT levels did not reach statistical significance due to high variability, a clear inhibitory trend was observed with CH7450924 treatment (Fig. 2G).
These results revealed that in the LPS-induced MODS model, CH7450924 suppressed systemic inflammation as well as inflammation in the kidney and liver, demonstrated protective effects on both the kidney and liver, and markedly improved the overall condition of the animals.
Effects of CH7450924 on systemic circulatory injury in MODS model induced by intraperitoneal LPS injection
To further analyze the mechanism of CH7450924 in the MODS model, we evaluated its effects on blood pressure and heart rate using a telemetry system and on peripheral blood flow using a laser speckle imaging system. In the MODS model, mean blood pressure (MBP) and heart rate (HR) decreased dramatically, but CH7450924 had no effect on these parameters (supplementary material 2: Supplemental Fig. 3).
Skin blood flow on the surface of the ear was dramatically reduced in disease control mice compared with normal control mice and CH7450924 showed a significant improvement (Fig. 3A, B).
Effects of CH7450924 on systemic circulation in MODS model induced by intraperitoneal LPS injection. (A) Representative images of blood flow captured using Omegazone in ears, (B) cutaneous blood flow in ears, (C) blood platelet count, (D) plasma PAI-1 concentration, (E) plasma Syndecan-1 concentration, and (F) mRNA expression of ICAM-1 and VCAM-1 in the kidney and (G) liver. CH7450924 was administered at 20 mg/kg before LPS injection. The actual values are represented as dots, and the bars represent mean ± SE (n = 8). #p < 0.05, significant difference between NC and DC (Student’s t-test). *p < 0.05, significant difference between DC and CH7450924 (Student’s t-test). NC; Normal control, DC; Disease control, CH0924; CH7450924.
In addition, disease control mice exhibited significantly decreased platelet counts and elevated plasma PAI-1 protein levels. CH7450924 significantly ameliorated both platelet depletion and plasma PAI-1 elevation (Fig. 3C, D). Given that endothelial damage and activation are closely associated with inflammation and coagulation, Syndecan-1 in plasma and the mRNA expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in the kidney and liver were measured. Plasma Syndecan-1 levels and ICAM-1 and VCAM-1 mRNA expression in the kidney and liver were significantly reduced in the CH7450924-treated mice compared to disease control mice. (Fig. 3E–G).
These results suggest that CH7450924 improves peripheral blood flow not through the improvement of circulatory dynamics, such as MBP and HR, but by inhibiting the enhancement of blood coagulation caused by endothelial damage and activation.
Effects of CH7450924 in lung injury model induced by intratracheal LPS injection
As the MODS model induced by intraperitoneal LPS injection did not result in observable pathological or functional damage in the lungs (data not shown), an intratracheal LPS administration model was employed to examine the lung-protective effects of CH7450924.
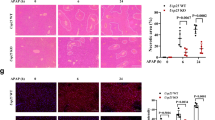
Intratracheal injection of LPS induced significant increases in IL-6 and IL-1β mRNA expression in lungs, which CH7450924 significantly suppressed. The inhibitory effect of CH7450924 was approximately equivalent across doses of 5, 10, and 20 mg/kg (Fig. 4A). Disease control mice exhibited a significant increase in lung wet weight, and CH7450924 significantly suppressed this increase at all tested doses (Fig. 4B). Representative histopathological images are shown in Fig. 4C. CH7450924 significantly and dose-dependently inhibited the marked neutrophil infiltration into lung tissue and alveolar wall thickening, which represents damage to alveolar epithelial cells, induced by intratracheal LPS injection (Fig. 4D). Moreover, decreased mRNA expression of surfactant protein C (SFTPC), an alveolar epithelial cell marker, was recovered by CH7450924 treatment (Fig. 4E).
Effects of CH7450924 on lung injury in MODS model induced by intratracheal LPS injection. (A) mRNA expression of IL-6, IL-1β in the lung, (B) lung wet weight, (C) representative HE-stained lung sections from NC, DC, and CH0924 (20 mg/kg) groups, (D) semi-quantitative analysis of neutrophil infiltration of alveoli and thickening of alveolar wall, (E) mRNA expression of SFTPC in lung, and (F) SpO2 monitored using infrared pulse oximetry. CH7450924 was administered at 5, 10, or 20 mg/kg twice a day before and after LPS injection. The actual values are represented as dots, and the bars represent mean ± SE (NC: n = 3 or 4, others: n = 8 or 9). *p < 0.05, significant difference versus vehicle-treated LPS mice (Student’s t-test). #p < 0.05, significant difference between NC and DC (Student’s t-test). *p < 0.05, significant difference between DC and CH7450924 (William’s t-test). BR, Bronchiole, NC; Normal control, DC; Disease control, CH0924; CH7450924.
To assess respiratory function, SpO2 was measured in conscious mice. Intratracheal LPS injection significantly lowered SpO2, and CH7450924 significantly recovered it (Fig. 4F).
Discussion
In this study, we identified a novel compound, CH7450924, as a potent and selective KEAP1-NRF2 protein–protein interaction inhibitor. X-ray structural analysis suggests that CH7450924 binds non-covalently to KEAP1 and competitively inhibits the binding between NRF2 and KEAP1. In contrast, BARD, a well-known NRF2 activator, has been reported to covalently bind to KEAP121. According to Cerep binding and functional assays, CH7450924 bound to 4 targets out of 130, whereas BARD bound to 16 out of 130. Furthermore, CH7450924 showed inhibitory activity only against 5-HT1B, one of the four targets, at up to 10 µmol/L. These results indicate that CH7450924 is a highly selective inhibitor of KEAP1-NRF2 binding. In diseases such as MODS, where various systemic abnormalities coexist, and the risk of off-target side effects with non-selective drugs is high. Therefore, the high selectivity of CH7450924 is particularly advantageous for treating MODS.
In the intraperitoneal LPS-induced MODS model, CH7450924 ameliorated the impairment of kidney function, as evidenced by reductions in plasma creatinine and BUN. Moreover, CH7450924 demonstrated significant or trending reduction of plasma and tissue inflammatory cytokines (IL-6, IL-1β or TNFα), suggesting that its anti-inflammatory effect may have contributed to functional improvement. It is reported that NRF2 binds to genes upstream of inflammatory cytokines such as IL-6 and IL-1β and that the induction of these genes by LPS is suppressed by NRF2 activators22. Our observation of increased inflammatory factors following LPS administration and their suppression by CH7450924 is consistent with this previous report.
BARD has been reported to increase the glomerular filtration rate (GFR)23,24, and although the underlying mechanism remains unknown, mesangial cell expansion is thought to be involved25. To elucidate the mechanism of the reno-protective effect of CH7450924 in the MODS model, we measured MBP and HR using telemetry and peripheral blood flow using a laser speckle imaging system. CH7450924 did not affect the increases in MBP and HR in the MODS model. Because the significant hypotension and bradycardia shown in the MODS model was not ameliorated by CH7450924, it was considered unlikely that CH7450924 had any significant effect on systemic blood pressure or cardiac function. In contrast, CH7450924 dramatically improved ear blood flow, which had been significantly reduced by LPS injection. These results suggest that CH7450924 affects peripheral blood circulation without affecting cardiac function or blood pressure.
In MODS caused by conditions such as sepsis or SIRS, inflammatory cytokines injure vascular endothelial cells, resulting in the formation of microthrombi within blood vessels and leading to disseminated intravascular coagulation (DIC)26,27,28. DIC leads to the failure of microcirculation and is thought to be one of the main reasons for multiple organ failure in sepsis29,30. In our study, LPS administration resulted in a significant decrease in platelets along with increases in plasma PAI-1 and Syndecan-1 protein levels. These changes are consistent with clinical DIC guidelines31 and previous DIC model reports32,33,34. The apparent recovery of platelet counts and significant reduction in plasma PAI-1 levels observed with CH7450924 administration suggest that NRF2 activation may inhibit thrombus formation in microvessels, thereby improving peripheral blood flow.
In addition, inflammatory cytokines increase vascular permeability, leading to the movement of fluid from the circulatory vessels into peripheral tissues, resulting in a decrease in circulating plasma volume in MODS, such as in the case of septic shock35. In this experiment, LPS administration resulted in a marked increase in hematocrit (supplementary material 2: Supplemental Fig. 4), indicative of a fluid shift to tissues. CH7450924 significantly mitigated this effect, likely through its anti-inflammatory action in preserving circulating plasma volume and promoting better peripheral blood flow.
IL-6, IL-1β, and TNFα mRNA in kidney were also significantly increased in the MODS model and they were significantly decreased by CH7450924 administration, indicating that its anti-inflammatory effects extend to the kidney. LPS administration not only reduces renal plasma flow via inflammation but also reduces the GFR through severe hypotension36,37. In addition, LPS administration directly induces renal tubular injury via inflammatory cytokines and ischemia38,39,40,41. The NRF2 activator BARD is reported to increase GFR in both preclinical and clinical settings23,24,25. CH7450924 also increased GFR in normal animals (data not shown). In this study CH7450924 significantly improved renal function as indicated by plasma creatinine and tended to improve markers of proximal tubular injury. This is thought to be due to its additive effects of direct GFR increase and anti-inflammatory action. However, it is important to note that changes of proximal tubular injury markers are preliminary and limited to mRNA expression analysis. Further investigation of protein expression levels through techniques such as Western blot or immunohistochemical staining would be necessary to fully characterize the renoprotective effects of CH7450924.
CH7450924 significantly improved plasma T-BIL, and tended to improve AST and ALT, which are markers of liver injury. Several reports have reported the hepatoprotective effects of NRF2 activators in sepsis-induced liver injury42,43,44. In our study, CH7450924 showed significant or trending reduction of the hepatic mRNA expression of IL-6, IL-1β, and TNFα, mirroring the responses observed systemically and in the kidney. Thus, the hepatoprotective benefit of CH7450924 is likely mediated by NRF2 activation, which reduces inflammation and may inhibit coagulation cascades.
Organ damage in MODS is commonly observed in the lungs, as well as in the kidneys and liver45,46,47. Because lung injury was not observed in the MODS model induced by intraperitoneal LPS injection, we employed an intratracheal LPS model to verify the pulmonary effect of CH7450924. LPS administration is known to stimulate alveolar macrophages and lung epithelial cells to produce IL-1β and TNFα via TLR4 and CD1448. In our lung model, LPS induced a significant increase in such inflammatory cytokines along with histopathological evidence of neutrophil infiltration and damage to alveolar epithelial cells. These results indicate that the enhanced production of inflammatory cytokines induced by LPS led to lung epithelial cell damage and decreased lung function. CH7450924 dramatically reduced the mRNA expression of IL-6 and IL-1β, decreased neutrophil infiltration into lung tissue, and recovered respiratory function as shown by improved SpO2. Taken together, these findings suggest that CH7450924 would be effective against LPS-induced lung injury.
Finally, we will mention the limitations of this study. First, although CH7450924 demonstrated protective effects against MODS in an LPS-induced model primarily via anti-inflammatory mechanisms, we did not evaluate its efficacy in a cecal ligation and puncture (CLP) model, which is generally considered to have the highest translatability to humans49. It is not yet clear to what extent CH7450924 will be effective against human MODS. Thus, further studies are needed to verify whether CH7450924 also has a protective effect in the CLP model. Second, while NRF2 activation confers both anti-inflammatory and anti-oxidative effects6,50, our study focused on the anti-inflammatory effects of CH7450924 without directly addressing its impact on oxidative stress. Given that oxidative stress is involved in MODS51,52,53,54, future investigations should explore how the anti-oxidative effect of CH7450924 contributes to its therapeutic efficacy.
Conclusions
CH7450924, a novel, potent, and selective NRF2 activator, demonstrated robust efficacy in both systemic and pulmonary LPS-induced MODS models by ameliorating inflammatory responses, improving peripheral microcirculation, and protecting kidney, liver, and lung function. CH7450924 shows promise as a novel therapeutic approach for MODS.
Data availability
The datasets generated and/or analysed during the current study are available in the PDB repository, ID: 9M24.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BARD:
-
Bardoxolone methyl
- BUN:
-
Blood urea nitrogen
- CLP:
-
Cecal ligation and puncture
- DIC:
-
Disseminated intravascular coagulation
- GFR:
-
Glomerular filtration rate
- HR:
-
Heart rate
- ICAM-1:
-
Intercellular adhesion molecule 1
- IL:
-
Interleukin
- KIM-1:
-
Kidney injury molecule-1
- KEAP1:
-
Kelch-like ECH-associated protein 1
- LPS:
-
Lipopolysaccharide
- MBP:
-
Mean blood pressure
- MODS:
-
Multiple organ dysfunction syndrome
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- NQO1:
-
NAD(P)H quinone oxidoreductase 1
- NRF2:
-
Nuclear factor erythroid 2-related factor 2
- PAI-1:
-
Plasminogen activator inhibitor-1
- SIRS:
-
Systemic inflammatory response syndrome
- SpO2 :
-
Oxygen saturation
- SFTPC:
-
Surfactant protein C
- T-BIL:
-
Total bilirubin
- TNFα:
-
Tumor necrosis factor alpha
- VCAM-1:
-
Vascular cell adhesion molecule 1
References
Gourd, N. M. & Nikitas, N. Multiple organ dysfunction syndrome. J. Intensive Care Med. 35, 1564–1575 (2020).
Wang, H. & Ma, S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am. J. Emerg. Med. 26, 711–715 (2008).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet 395, 200–211 (2020).
Abraham, E. & Singer, M. Mechanisms of sepsis-induced organ dysfunction. Crit. Care Med. 35, 2408–2416 (2007).
Evans, L. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 49, e1063–e1143 (2021).
Yamamoto, M., Kensler, T. W. & Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 98, 1169–1203 (2018).
Thimmulappa, R. K. et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem. Biophys. Res. Commun. 351, 883–889 (2006).
Thimmulappa, R. K. et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Invest. 116, 984–995 (2006).
Wang, Y. et al. A small-molecule inhibitor of Keap1-Nrf2 interaction attenuates sepsis by selectively augmenting the antibacterial defence of macrophages at infection sites. EBioMedicine 90, 104480 (2023).
Giustina, A. D. et al. Dimethyl fumarate modulates oxidative stress and inflammation in organs after sepsis in rats. Inflammation 41, 315–327 (2018).
Qi, T., Xu, F., Yan, X., Li, S. & Li, H. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int. J. Mol. Med. 37, 182–188 (2016).
Kong, X. et al. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am. J. Respir. Crit. Care Med. 184, 928–938 (2011).
Osburn, W. O. et al. Genetic or pharmacologic amplification of Nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol. Sci. 104, 218–227 (2008).
Hörer, S., Reinert, D., Ostmann, K., Hoevels, Y. & Nar, H. Crystal-contact engineering to obtain a crystal form of the kelch domain of human keap1 suitable for ligand-soaking experiments. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 69, 592–596 (2013).
Yasgar, A., Jadhav, A., Simeonov, A. & Coussens, N. P. Alphascreen-based assays: Ultra-high-throughput screening for small-molecule inhibitors of challenging enzymes and protein-protein interactions. Methods Mol. Biol. 1439, 77–98 (2016).
Matute-Bello, G., Frevert, C. W. & Martin, T. R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L379–L399 (2008).
Remick, D. G., Newcomb, D. E., Bolgos, G. L. & Call, D. R. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13, 110–116 (2000).
Liaudet, L. et al. Activation of poly(ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am. J. Respir. Crit. Care Med. 165, 372–377 (2002).
Bupp, S. et al. A novel murine in vivo model for acute hereditary angioedema attacks. Sci. Rep. 11, 15924 (2021).
Aeffner, F., Bolon, B. & Davis, I. C. Mouse models of acute respiratory distress syndrome: A review of analytical approaches, pathologic features, and common measurements. Toxicol. Pathol. 43, 1074–1092 (2015).
Magesh, S., Chen, Y. & Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 32, 687–726 (2012).
Ando, M. et al. The gut lactic acid bacteria metabolite, 10-oxo-cis-6,trans-11-octadecadienoic acid, suppresses inflammatory bowel disease in mice by modulating the NRF2 pathway and GPCR-signaling. Front. Immunol. 15, 1374425 (2024).
Ikejiri, K. et al. Effects of NRF2 polymorphisms on safety and efficacy of bardoxolone methyl: Subanalysis of TSUBAKI study. Clin. Exp. Nephrol. 28, 225–234 (2024).
Nangaku, M. et al. Randomized, double-blind, placebo-controlled phase 3 study of bardoxolone methyl in patients with diabetic kidney disease: design and baseline characteristics of the AYAME study. Nephrol. Dial. Transpl. 38, 1204–1216 (2023).
Ding, Y. et al. The synthetic triterpenoid, RTA 405, increases the glomerular filtration rate and reduces angiotensin II-induced contraction of glomerular mesangial cells. Kidney Int. 83, 845–854 (2013).
Hukkanen, R. R., Liggitt, H. D., Murnane, R. D. & Frevert, C. W. Systemic inflammatory response syndrome in nonhuman primates culminating in multiple organ failure, acute lung injury, and disseminated intravascular coagulation. Toxicol. Pathol. 37, 799–804 (2009).
Walborn, A., Hoppensteadt, D., Syed, D., Mosier, M. & Fareed, J. Biomarker profile of sepsis-associated coagulopathy using biochip assay for inflammatory cytokines. Clin. Appl. Thromb. Hemost. 24, 625–632 (2018).
Matsumoto, H. et al. Enhanced expression of cell-specific surface antigens on endothelial microparticles in sepsis-induced disseminated intravascular coagulation. Shock 43, 443–449 (2015).
Wang, G. et al. Exploring the mediating role of multiple organ dysfunction in sepsis-induced disseminated intravascular coagulation and its impact on worsening prognosis. Clin. Appl. Thromb. Hemost. 30, 10760296241271358 (2024).
Helms, J. et al. Disseminated intravascular coagulation is strongly associated with severe acute kidney injury in patients with septic shock. Ann. Intensive Care. 13, 119 (2023).
Iba, T., Helms, J. & Levy, J. H. Sepsis-induced coagulopathy (SIC) in the management of sepsis. Ann. Intensive Care. 14, 148 (2024).
Sun, Y. et al. Fibroblast growth factor 2 (FGF2) ameliorates the coagulation abnormalities in sepsis. Toxicol. Appl. Pharmacol. 460, 116364 (2023).
Shi, J. et al. NLRP3 inflammasome contributes to endotoxin-induced coagulation. Thromb. Res. 214, 8–15 (2022).
Tanaka, T. et al. Sepsis model with reproducible manifestations of multiple organ failure (MOF) and disseminated intravascular coagulation (DIC). Thromb. Res. 54, 53–61 (1989).
Goldenberg, N. M., Steinberg, B. E., Slutsky, A. S. & Lee, W. L. Broken barriers: A new take on sepsis pathogenesis. Sci. Transl. Med. 3, 8825 (2011).
Knotek, M. et al. Endotoxemic renal failure in mice: Role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int. 59, 2243–2249 (2001).
Bansal, S., Wang, W., Falk, S. & Schrier, R. Combination therapy with albumin and pentoxifylline protects against acute kidney injury during endotoxemic shock in mice. Ren. Fail. 31, 848–854 (2009).
Cunningham, P. N. et al. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J. Immunol. 168, 5817–5823 (2002).
Zager, R. A., Johnson, A. C., Lund, S. & Hanson, S. Acute renal failure: Determinants and characteristics of the injury-induced hyperinflammatory response. Am. J. Physiol. Renal. Physiol. 291, F546–F556 (2006).
Zager, R. A., Johnson, A. C., Lund, S., Hanson, S. Y. & Abrass, C. K. Levosimendan protects against experimental endotoxemic acute renal failure. Am. J. Physiol. Ren. Physiol. 290, F1453–F1462 (2006).
Wu, L. et al. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am. J. Physiol. Ren. Physiol. 292, F261–F268 (2007).
Zhang, X., Yuan, S., Fan, H., Zhang, W. & Zhang, H. Liensinine alleviates sepsis-induced acute liver injury by inhibiting the NF-kappaB and MAPK pathways in an Nrf2-dependent manner. Chem. Biol. Interact. 396, 111030 (2024).
Zhong, W. et al. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-kappaB related signaling. Biomed. Pharmacother. 83, 302–313 (2016).
Sun, Y. et al. Astragaloside IV attenuates lipopolysaccharide induced liver injury by modulating Nrf2-mediated oxidative stress and NLRP3-mediated inflammation. Heliyon. 9, e15436 (2023).
Lekander, B. J. & Cerra, F. B. The syndrome of multiple organ failure. Crit. Care Nurs. Clin. N. Am. 2, 331–342 (1990).
Borges, A. & Bento, L. Organ crosstalk and dysfunction in sepsis. Ann. Intensive Care. 14, 147 (2024).
Jiang, Y. et al. Pyroptosis in septic lung injury: Interactions with other types of cell death. Biomed. Pharmacother. 169, 115914 (2023).
Li, S., Lei, Y., Lei, J. & Li, H. All-trans retinoic acid promotes macrophage phagocytosis and decreases inflammation via inhibiting CD14/TLR4 in acute lung injury. Mol. Med. Rep. 24, 868 (2021).
Rittirsch, D., Huber-Lang, M. S., Flierl, M. A. & Ward, P. A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36 (2009).
Kobayashi, E. H. et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 7, 11624 (2016).
Motoyama, T. et al. Possible role of increased oxidant stress in multiple organ failure after systemic inflammatory response syndrome. Crit. Care Med. 31, 1048–1052 (2003).
Galley, H. F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 107, 57–64 (2011).
Andrades, M. et al. Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J. Surg. Res. 167, e307–e313 (2011).
Mantzarlis, K., Tsolaki, V. & Zakynthinos, E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid. Med. Cell Longev. 2017, 5985209 (2017).
Acknowledgements
We thank Hitoshi Hagita, Hironori Yamagishi, Yukihito Miura (Chugai Research Institute for Medical Science, Inc.), and Misaki Mori (Chugai Pharmaceutical Co., Ltd.) for their assistance; Jacob Davis (Chugai Pharmaceutical Co., Ltd.) for his assistance with English usage; Masayuki Yamamoto (Tohoku Medical Megabank Organization, Tohoku University, Sendai, Miyagi, Japan, Advanced Research Center for Innovations in Next-Generation Medicine, Tohoku University, Sendai, Miyagi, Japan) and Yuu Okura (Chugai Pharmaceutical Co., Ltd.) for their helpful discussions.
Author information
Authors and Affiliations
Contributions
M.H., Y.Y., and N.H. conceived and designed the research, edited and revised the manuscript and approved the final version. A.K. created the new compound. M.H., Y.Y., S.M., Y.I., H.K., and R.K. performed the experiments and analyzed data. M.H., Y.Y., S.M., Y.I., H.K., R.K., and N.H. interpreted the results of the experiments. M.H., Y.Y., Y.I., H.K., and N.H. prepared the figures and drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
Atsushi Kimbara is an inventor on a patent application (WO2023-JP211) related to the compound CH7450924 described in this study. All authors are employees of Chugai Pharmaceutical Co., Ltd.
Ethics approval and consent to participate
Animal procedures and protocols were in accordance with the Guidelines for the Care and Use of Laboratory Animals at Chugai Pharmaceutical Co. Ltd. and approved by the Institutional Animal Care and Use Committee (approval No.21–174, 23–145, 24–164, 24–182, 24–186, 24–284) and were also compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hoshino, M., Yasui, Y., Mitsumata, S. et al. CH7450924, a KEAP1-NRF2 interaction inhibitor, ameliorates LPS-induced multiple organ dysfunction via inflammatory pathway inhibition. Sci Rep 15, 36413 (2025). https://doi.org/10.1038/s41598-025-20350-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20350-w