Abstract
The fat mass and obesity-associated (FTO) gene has been widely implicated in the regulation of energy balance in mammals. Whether FTO has similar roles in non-mammals remains unexplored. The goal of this research was to determine if FTO inhibition affects feeding, metabolism and lipid homeostasis in zebrafish (Danio rerio). Rhein, a pharmacological inhibitor of FTO was used to examine if FTO suppression modulates metabolic pathways in zebrafish liver and brain in vivo, and in zebrafish liver (ZFL) cells in vitro. Rhein treatment resulted in a significant reduction in food intake, accompanied by corresponding downregulation of FTO and its downstream effector IRX3 at both the mRNA and protein levels. Inhibiting FTO upregulated lipid oxidation genes and downregulated lipogenic pathways, indicating enhanced lipid catabolism. Moreover, rhein administration elevated key metabolic regulators AMPK and PPARα while suppressing gluconeogenic genes, suggesting improved glucose homeostasis. These findings suggest that FTO plays an important role in zebrafish metabolic regulation and that its inhibition modulates energy balance via the STAT3 signaling pathway. This research provides insights into the evolutionary conservation of FTO function and further supports FTO as a key molecule in zebrafish metabolic regulation.
Similar content being viewed by others
Introduction
The global incidence of eating disorders and metabolic syndrome is on the rise and there is renewed interest in the neural and endocrine systems that regulate feeding and energy balance1,2. The brain, particularly the hypothalamus, in conjunction with peripheral organs including the gastrointestinal tract engages in a bidirectional communication to regulate energy homeostasis3,4,5. Impaired crosstalk between the brain and peripheral metabolic tissues is seen in type 2 diabetes and obesity4.
Fat mass and obesity-associated (FTO) gene belongs to the Fe (II)- and oxoglutarate-dependent AlkB oxygenase family. FTO polymorphisms are strongly associated with obesity and type 2 diabetes6,7. This link is largely attributed to their role in modifying mRNA, particularly through N6-methyladenosine (m6A) demethylation8. These epigenetic modifications influence mRNA stability, alternative splicing, and the translation of essential metabolic genes9,10. Altered FTO expression has been associated with excessive food intake due to impaired satiety signaling, as well as disturbances in hepatic gluconeogenesis and lipid metabolism11,12. FTO protein is primarily detected in the liver, hypothalamus, pancreas, and adipose tissue, and the FTO gene is highly conserved in vertebrates, including fish13. Within the liver, FTO expression is modulated by metabolic signals including circulating glucose and lipid12,13,14. Research using murine hepatocyte cell lines has shown that increased FTO levels are associated with the upregulation of key gluconeogenic genes glucose-6-phosphatase (G6PC)12 and phosphoenolpyruvate carboxykinase (PCK1), which in turn promotes elevated hepatic glucose production and reduced insulin sensitivity15,16. These findings suggest that FTO functions as a positive regulator of hepatic gluconeogenesis15. The role of FTO on lipid metabolism is significant, particularly within the liver, where it regulates the dynamics of lipid accumulation. Increased FTO expression has been associated with elevated lipid accumulation and the development of non-alcoholic fatty liver disease (NAFLD)17. Mechanistically, FTO modulates lipid homeostasis by promoting fatty acid synthesis and inhibiting triglyceride hydrolysis via RNA demethylation both in vivo and in vitro12. This results in the upregulation of essential lipogenic genes (FASN, SCD1, and MGAT1), and simultaneous downregulation of lipid transport-associated genes (MTTP, APOB, and LIPC)18. These findings demonstrate that FTO helps maintain lipid balance in the liver10,18.
The role of the FTO gene in regulating food intake is not yet fully understood19. In rats, fasting increases hypothalamic FTO mRNA13, while in mice, fasting decreases FTO abundance in the arcuate nucleus20. Recently, we have identified that FTO and its associated transcription factor, iroquois homeobox 3 (IRX3), are widely expressed in zebrafish metabolic tissues, including the brain, liver, muscle, and gut21. Both FTO and IRX3 exhibit dynamic expression changes in response to feeding and food deprivation in zebrafish, suggesting their role as nutrient-sensitive genes21. Specifically, fasting led to an increase in FTO and IRX3 mRNA in zebrafish metabolic tissues, which was reversed by refeeding. These findings suggest a role for FTO and IRX3 in appetite regulation and metabolic homeostasis in zebrafish, providing new insights into their evolutionarily conserved role in energy balance across vertebrates21,22. We hypothesized that the inhibition of FTO will affect feeding, appetite regulatory peptides and hepatic enzymes regulating gluconeogenesis and lipogenesis in zebrafish. This hypothesis was tested using zebrafish liver cells in vitro, and zebrafish in vivo, by employing rhein (4,5-dihydroxyanthraquinone‐2‐carboxylic acid), a FTO inhibitor purified from rhubarb extract23,24. As a natural inhibitor of FTO, rhein competes for binding to the enzyme’s catalytic domain, thereby preventing FTO from accessing single-stranded RNA substrates and effectively inhibiting its demethylase activity25.
Materials and methods
Animals
Adult male zebrafish (Danio rerio), with an approximate body weight of ~ 0.85 g, were purchased from the breeding colony maintained at the University of Saskatchewan. The fish were kept in 10 L aquaria with a continuous flow of temperature-regulated water maintained at 28 ± 1 °C with a photoperiod of 12 h of light and 12 h of darkness (12 L:12 D; lights on at 07:00 h). They were fed commercial slow-sinking pellets (2% body weight; GEMMA Micro 500, Skretting, USA) daily at 11:00 h. All experimental procedures adhered with the guidelines set by the Canadian Council for Animal Care and were approved by the Animal Research Ethics Board of the University of Saskatchewan (Protocol #2022-0045). The study adhered to the ARRIVE guidelines version 2.
Zebrafish liver (ZFL) cells
Zebrafish liver cells (Catalog # CRL-2643) were purchased from ATCC (USA). The cells were maintained at 28 °C in a 100% air atmosphere using a complete culture medium comprised of 50% Leibovitz’s L-15 (ATCC 30–2008), 35% Dulbecco’s Modified Eagle Medium (high glucose, Gibco 12100), and 15% Ham’s F-12 (Gibco 21700). The medium was supplemented with 0.15 g/L sodium bicarbonate, 15 mM HEPES, 10 µg/mL human insulin, 50 ng/mL mouse epidermal growth factor (EGF), 5% heat-inactivated fetal bovine serum, and 0.5% trout serum, following the protocol provided by ATCC. At 80% confluency, ZFL cells were sub-cultured and seeded into 96-well plates (2 × 104 cells/well), 24-well plates (at 5 × 105 cells/well) or 6-well plates (1 × 106 cells/well) for experiments (as detailed below).
Tissue distribution
To examine the tissue distribution of FTO and IRX3 and protein mRNA abundance, zebrafish liver and brain samples were collected and rapidly frozen in liquid nitrogen. ZFL cells were harvested using RiboZol™ RNA Isolation Reagent (VWR, USA) or RIPA Lysis (Thermo Scientific, Canada) and kept at − 80 °C for further analysis.
Cell viability
Cell viability was evaluated using MTT [3-(4,5-dimethylthiazolyl)−2,5-diphenyl-tetrazolium bromide] test (Catalog no. 475989, Sigma, Canada), a colorimetric method that quantifies mitochondrial dehydrogenase activity. ZFL cells were incubated in 96-well plates (density of 2 × 104 cells/well) overnight in a complete growth medium as described above. Cells (2 × 104 cells/well) were treated for 24 h with various concentrations (5, 10, 20, 40, 60, 80 and 100 µM) of rhein (Catalog no. 275611, Sigma, Canada), solubilized in dimethyl sulfoxide (DMSO). After a 24 h incubation, the cells were rinsed twice with phosphate-buffered saline (PBS), then MTT solution (0.5 mg/mL PBS) was added to each well and incubated at 37 °C for 3 h. To solubilize the formazan crystals, 100 µL of DMSO was added to each well and absorbance was measured at 570 nm using a microplate reader (Varioskan LUX Multimode Microplate Reader, Thermo Fisher Scientific, Canada), and the percentage of cell viability was calculated.
In vitro effects of rhein on the expression of genes associated with lipid and glucose metabolism
To test whether FTO inhibition affect lipid and glucose regulating enzyme mRNA expression, ZFL cells (passage 8) were plated at densities of 5 × 10⁵ cells per well in 24-well plates and 1 × 10⁶ cells per well in 6-well plates, then grown to 70–80% confluency. Rhein concentrations used for both in vivo and in vitro experiments were determined based on previous studies in zebrafish embryos, mouse hepatocytes and HepaRG cells15,26. The culture medium was replaced with 1 mL of fresh medium alone (control, 6 wells) or medium containing 0.0001, 0.001, 0.01, 0.1, 1, or 10 µM rhein. The plates were incubated for 2–24 h. After each incubation period, the medium was aspirated, and 500 µL of PureZOL™ RNA Isolation Reagent (Bio-Rad, Mississauga, ON, Canada) was added to each 24-well plate well, while 200 µL of RIPA lysis and extraction buffer (cat. no. 89901, Thermo Fisher Scientific, USA) was added to each 6-well plate well. The cells were then scraped, collected, and total RNA and protein were extracted and stored at − 80 °C until quantitative PCR and western blot analysis were conducted. The study was repeated twice to obtain the n of 6 wells/treatment or control.
Effect of rhein on food intake
Zebrafish were housed in four experimental groups (3 zebrafish per tank, n = 6 fish per group): (i) a control group receiving 1% DMSO, (ii) a group administered 1 ng/g body weight (bw) of rhein, (iii) a group administered 10 ng/g bw of rhein, and (iv) a group administered 100 ng/g bw of rhein. During a three-week acclimation period, food intake was monitored daily for one week prior to treatment to establish baseline levels of food consumption. A pre-quantified amount of food (equivalent to 4% of the body weight) was provided daily to each tank at a scheduled feeding time (11:00 am). Uneaten food was collected after one hour (12:00 pm), dried overnight at 65 °C, and weighed. The quantity of food eaten in each tank was determined by subtracting the dry weight of the leftover food from the total amount of food originally given to the zebrafish. The acclimation ensured consistency in feeding across groups of zebrafish used. On the experimental day, the fish were anesthetized (using 0.5% TMS-222) at the scheduled feeding time and intraperitoneally injected with either 1% DMSO (control) or rhein at doses of 1, 10, or 100 ng/g bw. After the injection, the fish were given 5 min to recover before being provided with a pre-measured amount of food. Any remaining food pellets were collected 1 h post-feeding, and food intake was determined as outlined above.
In vivo effects of rhein on the expression of genes involved in metabolism (lipid and glucose) and appetite regulation
To determine whether intraperitoneal (IP) injection of rhein affects the expression of genes involved in lipid and glucose metabolism, zebrafish were acclimated for 2 weeks under standard rearing conditions and subsequently divided into eight experimental groups (12 fish per group, with n = 6 allocated for qPCR and n = 6 for western blot). The experimental setup was comprised of four distinct treatment groups: (i) a control group receiving 1% DMSO (vehicle), (ii) a group administered 1 ng/g bw of rhein, (iii) a group administered 10 ng/g bw of rhein, and (iv) a group administered 100 ng/g bw of rhein. Following acclimation, fish were anesthetized at the scheduled feeding time and injected with vehicle or rhein as detailed above. Following the injections, the fish were allowed to recover and were fed. One hour post-injection, fish were anesthetized and euthanized via spinal transection. The whole brain and liver were collected and stored at − 80 °C until subsequent analysis for western blot and quantitative PCR.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from stored tissues using RiboZol reagent (Cat. No. N580, VWR, USA) following the manufacturer’s protocol. The absorbance ratios at 260 and 280 nm (A260/A280) were measured using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Vantaa, Finland) to determine RNA purity and concentration. Complementary DNA (cDNA) was synthesized from 1 µg of total RNA with the iScript Reverse Transcription Supermix (Bio-Rad, Canada) as per the manufacturer’s guidelines. Primer sequences were designed using Primer3 software (https://primer3.sourceforge.net) and synthesized by Integrated DNA Technologies (IDT, Toronto, ON, Canada). Primer sequences are provided in Supplementary Material - Table 1. RT-qPCR was performed in 96-well plates using iQ™ SYBR® Green Supermix (Bio-Rad) with 1 µL of cDNA and 500 nM of each primer in a final reaction volume of 10 µL. Primer specificity and annealing temperatures were optimized prior to experimental runs. Thermal cycling settings included an initial denaturation step of 95 °C for 3 min, followed by 35 cycles of denaturation (95 °C for 10 s), annealing (gene-specific temperatures for 30 s), and extension (72 °C for 20 s for primers with annealing temperatures < 55 °C). Post-amplification, a melt curve analysis (65–95 °C, 0.5 °C increments every 5 s) was conducted to confirm single-product amplification. Negative controls (nuclease-free water replacing cDNA) were included in each run. All reactions were done in triplicate using a CFX Connect Real-Time System (Bio-Rad). The 2⁻ΔΔCt technique was used to quantify relative mRNA abundance, which was normalized to β-actin as an internal control reference27.
Western blot analysis
Western blot analysis was performed following previously reported protocols28. Total protein was extracted using RIPA lysis and extraction buffer (Cat. No. 89901, Thermo Fisher Scientific, USA), and protein concentrations were measured using the Pierce™ BCA Protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein (35 µg) were separated by electrophoresis on 8–16% Mini-Protean TGX gels (Cat. No. 456–1104, Bio-Rad, USA) and subsequently transferred to nitrocellulose membranes (Cat. No. 1704158, Bio-Rad, USA) using the Trans-Blot Turbo Transfer System (Bio-Rad, USA). Following membrane blocking with a 1X rapid blocking buffer (catalog number M325, VWR, USA) for 15 min at room temperature, the membrane was incubated overnight at 4 °C with primary antibodies. The membrane was subsequently subjected to three washes using TBST (0.1% Tween-20 in TBS. Afterwards, membranes were incubated with a secondary antibody for 1 h at 37 °C, followed by final washing steps. Visualization was performed using a ChemiDoc MP imaging system (Bio-Rad, USA), and band intensities were analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, USA). All original uncut blot images are provided in Supplementary Material - Figs. 1, 2, 3, 4 and 5.
Relative mRNA abundance of FTO and IRX3 in ZFL cells, liver and brain (A), Representative blots for FTO and IRX3 in ZFL cells, liver and brain (B), FTO and IRX3 protein levels in ZFL cells, liver and brain expression were normalized to the amount of β-actin. (C). The corresponding full, uncut blots are presented in Supplementary Material - Fig. 2. Data were assessed using one-way analysis of variance (ANOVA), and differences between means were analyzed with Tukey’s test. Results are expressed as mean ± SEM. Bars sharing the same lowercase letter are not significantly different from each other, whereas different lowercase letters indicate statistically significant differences (p ≤ 0.05). Each group consisted of six samples, derived from two independent studies. Fat mass and obesity associated (FTO), Iroquois homeobox protein (IRX3).
In vitro effects of the exposure to rhein on the expression of FTO, IRX3, PPARα and AMPKα in ZFL cells after 2 h and 24 h of DMSO 1% (control) or containing 0. 1, 1 or 10 µM of Rhein (A-D). Representative blots for AMPKα, FTO, IRX3 and PPARα in ZFL cells (E) and protein expression of AMPKα, FTO, IRX3 and, PPARα (F-I) after 24 h exposure to 1% DMSO (control) or 0. 1, 1 or 10 µM rhein. The corresponding full, uncut blots are presented in Supplementary Material - Fig. 3. Data were assessed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test. Results are expressed as mean ± SEM. Asterisks indicate significant differences between the control and treated groups * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Black lines under the asterisks cluster groups with same significance when compared to controls. Each group consisted of six samples, derived from two independent studies. Fat mass and obesity associated (FTO), Iroquois homeobox protein (IRX3), proliferator-activated receptor alpha (PPARα) and AMP‐activated protein kinase (AMPKα).
Primary antibodies employed were mouse monoclonal anti-β-actin (1:1000, RRID: AB_528068, Catalog No. JLA20, Developmental Studies Hybridoma Bank, DSHB, University of Iowa, USA), rabbit polyclonal anti-FTO (1:1000, NB110-60935, Novus Biologicals, USA), rabbit polyclonal anti-IRX3 (1:1000, ab25703, Abcam, USA), rabbit polyclonal anti-neuropeptide Y (1:1000, ab30914, Abcam, USA), rabbit polyclonal anti- PPARα (1:1000, ab209350, Abcam, USA), Rabbit polyclonal anti-orexin-A (1:1000, AB3704, Sigma, USA), and rabbit polyclonal anti-AMPKα (1:1000, RRID: AB_330331, Catalog No. 2532 S, Cell Signaling Technology, USA. Secondary antibodies used were goat anti-mouse IgG (H + L)-HRP conjugate (1:5000, RRID: AB_11125547, Catalog No. 170–6516, Bio-Rad, USA), and goat anti-rabbit IgG (H + L)-HRP conjugate (1:5000, RRID: AB_11125142, Catalog No. 170–6515, Bio-Rad, USA).
Statistical analysis
Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test (compare between control and treatments, for both in vivo and invitro studies) or Tukey’s multiple comparisons test (for pairwise comparisons) were used. Assumptions of normality and homogeneity of variance were assessed using the Brown–Forsythe test. Results are reported as mean ± SEM and statistical significance was set at p < 0.05. All graphical illustrations were produced using GraphPad Prism (version 10.1.2 GraphPad Inc., USA).
Results
Rhein causes cytotoxicity and reduces FTO mRNA abundance in ZFL cells
The MTT assay revealed a significant reduction in cell viability at 20, 40, 60, 80, and 100 µM compared to vehicle (1% DMSO) treated controls (Supplementary Material - Fig. 1A). The IC50 value of rhein for 24 h of treatment in ZFL cells was determined as 48.79 µM. We chose 10 µM rhein that had no adverse effects on cell viability yet caused the most significant reduction in FTO mRNA at 2 h (Supplementary Material - Fig. 1B) and 24 h (Supplementary Material - Fig. 1C) for the rest of the studies.
FTO and IRX3 mRNA and protein abundance in ZFL cells and zebrafish liver and brain
FTO and IRX3 mRNA was detected in ZFL cells, and zebrafish brain and liver (Fig. 1A), with relatively higher abundance in the liver and brain when compared to ZFL cells. Western blot detected FTO (55 kDa) and IRX3 (55 kDa) in ZFL cells, liver, and brain (Fig. 1B-C), with more abundance in the brain. Full, uncut images of these blots are provided in Supplementary Material - Fig. 2.
Rhein modulated FTO, IRX3, AMPKα and PPARα in zebrafish liver
Treatment with rhein at concentrations of 0. 1, 1, or 10 µM for 2 or 24 h resulted in a significant (p ≤ 0.01) dose-dependent reduction in FTO abundance compared to control (vehicle-treated; 0.1% DMSO) (Fig. 2A). Immunoblot analysis further confirmed that FTO protein levels were significantly (p ≤ 0.01, p ≤ 0.001) reduced 24 h after treatment (Fig. 2E). Full, uncut images of these blots are provided in Supplementary Material - Fig. 3. Given the significant inhibition of FTO expression, we next evaluated the impact of rhein on the IRX3, proliferator-activated receptor alpha (PPARα) and AMP‐activated protein kinase (AMPKα) signaling pathways. Rhein treatment markedly decreased (p ≤ 0.01, p ≤ 0.001, p ≤ 0.0001) IRX3 mRNA abundance (Fig. 2B), while a significant, concentration-dependent increase in PPARα mRNA (Fig. 2C) and AMPKα mRNA levels (Fig. 2D) were observed after both 2 and 24 h (p ≤ 0.01, p ≤ 0.001, p ≤ 0.0001). Western blot analysis corroborated these findings, revealing a reduction in FTO (Fig. 2F) and IRX3 protein (Fig. 2G) levels alongside an increase in AMPKα protein (Fig. 2H) and PPARα (Fig. 2I) 24 h after treatment of rhein at doses of 1, 10, or 100 ng/g body weight. In zebrafish liver, both FTO protein and mRNA were inhibited (p ≤ 0.0001) in a concentration‐dependent manner (Fig. 3A-C). Full, uncut images of these blots are provided in Supplementary Material - Fig. 4. Additionally, downregulation (p ≤ 0.0001) of IRX3 mRNA (Fig. 3D), and upregulation (p ≤ 0.01, p ≤ 0.001) of PPARα mRNA (Fig. 3E), AMPKα1 mRNA (p ≤ 0.0001) (Fig. 3F) and leptin receptor isoform b (LEPRb) (p ≤ 0.001, p ≤ 0.0001) (Fig. 3G) were observed 1 h after rhein administration.
In vivo effects of rhein on FTO, PPARα and AMPKα. Representative western blot images showing FTO and β-actin in the liver of zebrafish 1 h after the intraperitoneal administration of 1% DMSO (control) or 1, 10 or 100 ng/g bw of rhein (A). The corresponding full, uncut blots are presented in Supplementary Material - Fig. 4. Quantitative data from the analysis of the above blots showing FTO protein (B). FTO mRNA, IRX3 mRNA, PPARα mRNA, AMPKα mRNA and LEPRb abundance in zebrafish liver (C-G) 1 h after intraperitoneal administration of 1% DMSO (control) or 1, 10 or 100 ng g-1 body weight rhein. Data are expressed as mean + SEM (n = 6). Data were assessed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test., * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001. Black lines under the asterisks cluster groups with same significance when compared to controls. Fat mass and obesity associated (FTO), Iroquois homeobox protein (IRX3), proliferator-activated receptor alpha (PPARα) and AMP‐activated protein kinase (AMPKα).
Rhein altered the expression of genes involved in lipid metabolism in vitro and in vivo
Rhein treatment significantly decreased fatty acid synthesis related genes in ZFL cells, after 2 h and 24 h in concentration-dependant manner. Sterol regulatory element binding transcription factor 1 (SREBF1), cAMP Response Element-Binding protein (CREB) (Fig. 4A-C), fatty acid synthase (FAS) (Fig. 4E), stearoyl-CoA desaturase (SCD) (Fig. 4G) and acetyl-CoA acetyltransferase 1 (ACAT1) (Fig. 4I) were markedly downregulated after both 2 h and 24 h in all rhein treated groups (p ≤ 0.05, p ≤ 0.01, p ≤ 0.001). A marked increase in fatty acid β-oxidation gene aldehyde oxidase 1 (AOX1) (Fig. 4K; p ≤ 0.001) and upregulation of adipose triglyceride lipase (ATGL) (Fig. 4M; p ≤ 0.05) was found after 2 h and 24 h in cells treated with 1 and 10 µM rhein treated cells. Carnitine palmitoyltransferase I (CPT-1) was significantly increased in the 10 µM rhein-treated group after 2 h (p ≤ 0.05) and in 1 µM (p ≤ 0.01) after 24 h (Fig. 4O). IP administration of rhein downregulated (p ≤ 0.0001) mRNAs involved in fatty acid synthesis, SREBF1(Fig. 4B), CREB (Fig. 4D), FAS (Fig. 4F), SCD (Fig. 4H) and ACAT1 (Fig. 4J). Meanwhile, a significant increase (p ≤ 0.001) was found in CPT1A (Fig. 4L) (p ≤ 0.01, p ≤ 0.0001), AOX1(Fig. 4N), (p ≤ 0.01, p ≤ 0.0001) and ATGL (Fig. 4P) abundance at 1 h post-IP administration of rhein in a concentration-dependent manner.
ZFL cells treated with rhein significantly affected cholesterol synthesis genes and an increase was found in ATP-binding cassette transporter A1 (ABCA1b) (Fig. 4Q) at 1 µM (p ≤ 0.01) and 10 µM (p ≤ 0.001) after 2 h and 24 h. The abundance of hydroxymethylglutaryl-CoA lyase (HMGCL1) was significantly (p ≤ 0.05) reduced (Fig. 4S). In zebrafish liver, similar changes were observed (Fig. 4R). ABCA1b mRNA was significantly upregulated (p ≤ 0.05) after 1 h IP-administration (Fig. 4T), while HMGCL1 mRNA showed a notable decrease in all treatment groups (p ≤ 0.001).
Rhein changed genes involved in endoplasmic reticulum (ER) stress -related transcription factors in vitro and in vivo
In ZFL cells, activating transcription factor 4 (ATF4) abundance significantly (p ≤ 0.05, p ≤ 0.01) decreased in all rhein treated groups after 2 h, with further downregulation observed at 24 h (p ≤ 0.001) in 0. 1 and 1 µM rhein groups, but not with the highest concentration of rhein tested (Fig. 4U). Activating transcription factor 6 (ATF6) ATF6 mRNA was also upregulated in a time and concentration-dependent manner, with significant increase ((p ≤ 0.0001) in 10 µM after both 2 h and 24 h (Fig. 4W). IP administration of rhein showed similar results, with a significant downregulation in ATF4 mRNA abundance in 1 and 10 µM rhein groups (p ≤ 0.05) after 24 h (Fig. 4V), and a significant increase in ATF6 mRNA (p ≤ 0.0001) after 24 h in all treatment groups (Fig. 4X).
Rhein regulated the expression of genes involved in glucose metabolism in vitro and in vivo
In ZFL cells, a significant decrease (p ≤ 0.05) was observed in glucose-6-phosphatase (G6PC) (Fig. 5A) at 1 and 10 µM of rhein after 24 h compared with controls. Significant reduction (p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, p ≤ 0.0001) also recorded phosphoenolpyruvate carboxykinase 1 (PCK1 (Fig. 5C), phosphoenolpyruvate carboxykinase 2 (Fig. 5E) (PCK2) and fructose 1,6-bisphosphatase 1a (FBP1a) (Fig. 5G) mRNAs in concentration-dependent manner. Similar downregulation (p ≤ 0.001, p ≤ 0.0001) was observed in glucokinase (GCK) (Fig. 5I) and pyruvate kinase (PKLR) (Fig. 5K) mRNAs at all doses after 24 h treatment with rhein. Both phosphofructokinase liver isoform A (PFKLa) and phosphofructokinase liver isoform B (PFKLb) mRNAs were significantly downregulated (p ≤ 0.01, p ≤ 0.0001) in higher concentrations (1 and 10 µM) of rhein after 24 h (Fig. 5M-O). In zebrafish liver, G6PC (Fig. 5B), PCK1 (Fig. 5D), PCK2 (Fig. 5F), FBP1a (Fig. 5H), GCK (Fig. 5J) PFKLa and PFKLb (Fig. 5N-P) were downregulated in a concentration-dependent manner at 1 h post-administration while there was no significant change in mRNA abundance of PKLR (Fig. 5L). The sodium-glucose cotransporter 1 (SGLT1) (Fig. 5Q) and glucose transporter 2 (GLUT2) (Fig. 5S) were downregulated by rhein in ZFL at 0. 1 µM (p ≤ 0.0001) after 24 h incubation. A significant decrease (p ≤ 0.0001) was recorded in both SGLT1(Fig. 5R) and GLUT2 (Fig. 5T) mRNAs at 1 h post-IP injection in all rhein treated groups.
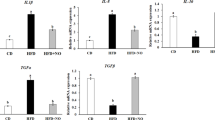
Effects of rhein on lipogenesis-related factors (SREBF1, CREB, FAS, SCD and ACAT1) in the ZFL cells (A, C, E, G, I; labeled in green letters) and zebrafish liver (B, D, F, H, J; labeled in red letters). Fatty acid β-oxidation (CPT1, AOX1, ATGL) in the ZFL cells (K, M, O) and zebrafish liver (L, N, P). Cholesterol synthesis and excretion genes (ABCA1b, HMGCL) in the ZFL cells (Q, S) and zebrafish liver (R, T). ER stress -related transcription factor in the ZFL cells (U, W) and zebrafish liver (V, X). Data were assessed using a one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test. Results are expressed as mean ± SEM. Asterisks indicate significant differences between the control and treated groups * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Black lines under the asterisks cluster groups with same significance when compared to controls. Each group consisted of six samples, derived from two independent studies. Sterol regulatory element binding transcription factor 1 (SREBF1), cAMP Response Element-Binding protein (CREB), stearoyl-CoA desaturase (SCD), acetyl-CoA acetyltransferase 1 (ACAT1), aldehyde oxidase 1 (AOX1), adipose triglyceride lipase (ATGL), carnitine palmitoyltransferase I (CPT-1), Fatty acid synthase (FAS), ATP-binding cassette transporter A1 (ABCA1b), hydroxymethylglutaryl-CoA lyase (HMGCL1), Activating transcription factor 4 (ATF4) and activating transcription factor 6 (ATF6).
Effects of rhein on gluconeogenesis related genes (G6PC, PCK1, PCK2, and FBP1a) in the ZFL cells (A, C, E, G; labeled in green letters) and zebrafish liver (B, D, F, H; labeled in red letters). Glycolysis (GCK, PKLR, PFKLa and PFKLb) in the ZFL cells (I, K, M, O) and zebrafish liver (J, L, N, P). Glucose transport (SGLT1, GLUT2) in the ZFL cells (Q, S) and zebrafish liver (R, T). Data were assessed using a one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test. Results are expressed as mean ± SEM. Asterisks indicate significant differences between the control and treated groups * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Black lines under the asterisks cluster groups with same significance when compared to controls. Each group consisted of six samples, derived from two independent studies. Glucose-6-phosphatase (G6PC), phosphoenolpyruvate carboxykinase 1(PCK1), phosphoenolpyruvate carboxykinase 2 (PCK2), fructose 1,6-bisphosphatase 1a (FBP1a), glucokinase (GCK), pyruvate kinase (PKLR), phosphofructokinase liver isoform A (PFKLa) and phosphofructokinase liver isoform B(PFKLb), sodium-glucose cotransporter 1 (SGLT1) and glucose transporter 2 (GLUT2).
IP administration of Rhein affected FTO, food intake and feeding regulatory genes in zebrafish brain
At 1 h post-administration, rhein (p ≤ 0.0001) caused marked downregulation of FTO mRNA in all doses tested (Fig. 6A). Furthermore, western blot analysis revealed that the FTO protein showed a similar significant reduction following intraperitoneal injection of rhein (Fig. 6F-G). Full, uncut images of these blots are provided in Supplementary Material - Fig. 5. A significant decrease (p ≤ 0.05) in food intake was observed at 1, 10 and 100 ng/g bw rhein at 1 h post-administration (Fig. 6B). In Fig. 6B, the “pre-injection” data show the average daily baseline food intake over the one-week acclimation period when feeding was monitored, whereas the “post-injection” data represent the amount of food intake within 1 h post-injection on the experimental day. A significant, concentration-dependant (p ≤ 0.0001) decrease was recorded in IRX3 mRNA and protein at 1 h post-administration of rhein (Fig. 6C-H). At 24 h post-IP administration, all rhein-treated groups exhibited a significant decrease in the abundance of orexin A and neuropeptide Y (NPY) (p ≤ 0.01, p ≤ 0.001, p ≤ 0.0001) (Fig. 6D-E). This reduction was also reflected at the protein level, with orexin A and NPY significantly diminished in the treated groups (Fig. 6I-J).
Effects of rhein on feeding regulation in zebrafish. Relative FTO mRNA abundance in brain of zebrafish (a), Food intake (b) 1 h after the intraperitoneal administration of 1% DMSO (control) or containing 1, 10 or 100 ng g−1 body weight rhein. The pre-injection period is presented as the mean daily food intake derived from the one-week baseline monitoring of feeding prior to treatment, while the injection-day values indicate the amount of food intake within 1 h after injection on the day of administration. IRX3 mRNA, Orexin mRNA and NPY mRNA abundance (C-E), Representative blots for FTO, IRX3, Orexin and NPY in the brain of zebrafish (F) and protein expression of FTO, IRX3, Orexin A and NPY in the brain of zebrafish (G-J) 1 h after intraperitoneal administration of 1% DMSO (control) or containing 1, 10 or 100 ng/g bw rhein. The corresponding full, uncut blots are presented in Supplementary Material - Fig. 5. Data were assessed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisonstest. Results are expressed as mean + SEM. Asterisks indicate significant differences between the control and treated groups * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Black lines under the asterisks cluster groups with same significance when compared to controls. Each group consisted of six samples, derived from two independent studies. Fat mass and obesity associated (FTO), Iroquois homeobox protein (IRX3) and Neuropeptide Y (NPY).
IP administration of rhein resulted in a significant upregulation of cocaine- and amphetamine-regulated transcript (CART) and proopiomelanocortin (POMC) mRNAs (p ≤ 0.001) in all treated groups (p ≤ 0.001, p ≤ 0.0001) (Fig. 7A-B). Additionally, there was a significant increase in agouti-related protein (AGRP) (p ≤ 0.001) (Fig. 7C), nucleobindin 2a (NUCB2a) (p ≤ 0.001) (Fig. 7D), nucleobindin 2b (NUCB2b) (p ≤ 0.01) (Fig. 7E) and sirtuin 6 (SIRT6) (p ≤ 0.01) (Fig. 7F) mRNAs in the brain after at 1 ng/g body weight rhein (p ≤ 0.01) compared to the control group. Melanocortin 4 receptor (MC4R) was also significantly increased (p ≤ 0.01) following the administration of rhein at a dose of 100 ng/g body weight (Fig. 7G). Additionally, leptin mRNA abundance was elevated (p ≤ 0.001, p ≤ 0.0001) after the injection of both 10 and 100 ng/g body weight of rhein (Fig. 7H). Rhein at all doses tested also caused a significant decrease in cannabinoid receptor 1 (CB1) (p ≤ 0.0001) (Fig. 7I), sirtuin 1 (SIRT1) (p ≤ 0.05) (Fig. 7J), galanin receptor 2a (GALR2) (p ≤ 0.01, p ≤ 0.0001) (Fig. 7K), and galanin receptor 2b (GALR2b) (p ≤ 0.0001) (Fig. 7L) mRNAs in a dose-dependent manner at 1 h post injection.
Effects of rhein on the relative expression of appetite-regulating peptide mRNAs (CART, POMC, AGRP, NUCB 2a, NUCB 2b, SIRT6, MC4R, Leptin, CB1, SIRT 1, GALR2a, and GALR2b) in the zebrafish brain (A-L)1 h after intraperitoneal administration of 1% DMSO (control) or 1, 10 or 100 ng/g bw rhein. Data were assessed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test. Results are expressed as mean ± SEM. Asterisks indicate significant differences between the control and treated groups * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Black lines under the asterisks cluster groups with same significance when compared to controls. Each group consisted of six samples, derived from two independent studies. Cocaine- and amphetamine-regulated transcript (CART), proopiomelanocortin (POMC) agouti-related protein (AGRP), nucleobindin 2a (NUCB2a), nucleobindin 2b (NUCB2b), sirtuin 1 (SIRT1), sirtuin 6 (SIRT6), melanocortin 4 receptor (MC4R), cannabinoid receptor 1 (CB1), galanin receptor 2a (GALR2) and galanin receptor 2b (GALR2b).
Discussion
Recent evidences have implicated the FTO gene in various physiological processes, notably in the regulation of energy homeostasis and food intake29,30,31. FTO gene is responsible for an enzyme that functions as a nucleic acid demethylase, influencing several genes involved in metabolic pathways10,32. A recent article from our group reported that both FTO and a related gene, IRX3, are expressed in zebrafish brain and liver, and in ZFL cells. It was also found that nutritional states such as fasting and refeeding modulate FTO expression in zebrafish21. We hypothesized that FTO suppression by rhein leads to downstream changes in gene expression, potentially affecting key metabolic and regulatory pathways. Rhein has been shown to bind directly to FTO, inhibiting its m6A demethylase activity33,34. Supporting this, treatment of ZFL cells with rhein at 0.1 and 10 µM significantly downregulated both FTO mRNA and protein levels in a concentration-dependent manner. Given that the changes in FTO gene directly influence IRX3 expression34,35, rhein treatment also reduced both IRX3 mRNA and protein levels. Furthermore, intraperitoneal administration of rhein caused similar changes in FTO and IRX3 mRNA abundance, which is consistent with our in vitro findings.
To explore whether FTO regulates lipid and glucose metabolism, we exposed ZFL cells or injected zebrafish with varying concentrations of rhein and quantified genes involved in these metabolic pathways. In ZFL cells, rhein treatment significantly increased PPARα expression and protein levels in a concentration-dependent manner compared to controls. Similarly, in the zebrafish liver, PPARα mRNA upregulation was observed post-rhein administration. In line with these findings, previous research has shown that microinjection of FTO morpholino in zebrafish disrupts lipid metabolism, leading to reduced fat accumulation and lower triglyceride levels36. PPARα is a critical regulator of lipid metabolism, governing key processes including fatty acid synthesis, transport, oxidation, glycolysis, and gluconeogenesis37,38. These findings also agree with prior studies in which FTO overexpression in HepG2 cells was shown to suppress both PPARα mRNA and protein levels, thereby promoting triglyceride accumulation. In contrast, FTO knockdown was observed to enhance PPARα protein expression, indicating that PPARα acts downstream of FTO in the regulation of hepatic lipid metabolism37. Although the exact mechanism by which FTO regulates PPARα remains unclear, it has been proposed that demethylase activity of FTO may directly influence PPARα mRNA methylation and stability18. The loss of FTO function disrupts these effects, further supporting its role in modulating PPARα expression37. Additionally, PPARα activation has been shown to induce SREBP-1c expression, which in turn upregulates lipogenic genes, contributing to hepatic steatosis38. These findings suggest a complex regulatory relationship between FTO and PPARα in zebrafish lipid metabolism. The activation of PPARα has been shown to induce AMPK, a key downstream target of PPARs that plays a crucial role in sensing and regulating cellular energy homeostasis. The observed increase in AMPK protein levels and mRNA abundance following rhein treatment suggests activation of the AMPK signaling pathway in zebrafish. Additionally, AMPK activation downregulates the expression of lipogenic genes such as sterol regulatory element-binding protein-1c (SREBP-1c) while upregulating genes involved in fatty acid oxidation, including CPT1) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARγ)39. The downregulation of key genes involved in fatty acid synthesis, including SREBF1, fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD), and adipose triglyceride lipase (ATGL) in zebrafish, along with the increased expression of β-oxidation-associated genes such as CPT1 and aldehyde oxidase 1 (AOX1) in response to rhein6,12, suggesting a metabolic shift toward fatty acid oxidation40. The lipogenic gene expression suppression in the zebrafish liver, and the upregulation of CPT1 results in the transport of long-chain fatty acids into mitochondria, thereby promoting β-oxidation41,42. These findings are consistent with research conducted in C57BL/6 mice and HepG2 cells, which demonstrated that rhein decreases the expression of genes related to fatty acid synthesis, leading to reduced lipid accumulation. Notably, the upregulation of genes associated with fatty acid oxidation, such as CPT1α and PGC-1α, reduces lipid storage43. The possible role of rhein in regulating fatty acid β-oxidation may be suggested by the association between FTO splice variants and decreased CPT1 expression44. The inactivation of FTO by rhein in our study led to a significant increase in CPT1 levels. This finding suggests that FTO, functioning as an RNA demethylase, may regulate fatty acid oxidation through m6A demethylation. Furthermore, previous studies have shown that a high-fat diet feeding in mice results in elevated hepatic FTO levels and alterations in the expression of both lipogenic and lipolytic genes45,46. We report here that FTO is negatively correlated with SREBF1, a transcription factor that positively regulates cholesterol biosynthesis through HMGCR, and ABCA1 expression47,48, which are essential for cholesterol efflux. The observed downregulation of HMGCR and ABCA1 suggests that FTO is involved in cholesterol synthesis in zebrafish liver.
Although additional research is required to confirm, the results presented here suggest that rhein inhibition of FTO may influence glucose homeostasis by reducing hepatic glucose uptake, gluconeogenesis, and glycogenolysis. This is supported by the observed decrease in glucose transporters Glut2 and Sglt1 in ZFL cells and the liver of zebrafish following rhein treatment. The role of rhein in alleviating increased glucose uptake and elevated Glut2 expression in tumor cells has been documented49. However, the precise mechanism underlying this effect remains unclear. We also observed that rhein downregulated activating transcription factor 4 (ATF4). ATF4 is a multifunctional transcriptional regulator involved in diverse cellular stress responses and the control of energy balance and glucose metabolism50. ATF4 knockout mice exhibit reduced fat mass, increased thermogenesis, improved insulin sensitivity, and resistance to diet-induced hyperglycemia. For instance, ATF4 knockout mice are lean and resistant to high-fat diet-induced obesity and hyperglycemia51. Mizuno et al. reported that pharmacological inhibition of FTO by rhein led to a reduction in ATF4 mRNA and protein, whereas FTO overexpression resulted in increased ATF4 mRNA expression in mouse hepatocyte AML12 cells15. Mechanistically, FTO functions as an N⁶-methyladenosine (m⁶A) demethylase, and its activity appears to enhance the stability and/or translation of ATF4 transcripts by demethylating specific m⁶A sites within the ATF4 mRNA52. Rhein-induced inhibition of FTO likely preserves these m⁶A modifications, leading to reduced ATF4 expression. This regulatory mechanism is further supported by the fact that the inhibition of FTO (by entacapone) in hepatocytes not only decreased the expression of key gluconeogenic genes but also improved glucose tolerance in mice53. The ATF4 decrease following rhein treatment suggests that ATF4 is a downstream target of FTO activity in the regulation of hepatic gluconeogenesis in zebrafish. In line with the observed downregulation of ATF4, rhein significantly downregulated key gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase (PEPCK), fructose-1,6-bisphosphatase (FBPase), and glucose-6-phosphatase (G6Pase), in ZFL cells and zebrafish liver. Rhein also upregulated leptin-b mRNA in the zebrafish liver, aligning with the observed increase in LepRb expression. FTO expression is modulated by leptin and IL-6, which concurrently induce STAT3 tyrosine phosphorylation in LepRb-expressing HuH7 cells14. Overexpression of FTO in vitro disrupts leptin-induced phosphorylation of STAT3 at both Y705 and S727, leading to altered glucose-6-phosphatase expression. Additionally, there was a notable reduction in the expression of cAMP Response Element-Binding protein (CREB), a critical transcriptional regulator of G6PC44. CREB has the ability to interact with the promoters of gluconeogenic genes, thereby influencing their expression44,54. In mammals, FTO functions as a co-activator for CREB, facilitating its binding to the promoter of PPARγ, and FTO mediated gluconeogenesis by influencing the expression of G6PC and PEPCK[44,55. Furthermore, the downregulation of CREB induced by rhein appears to contribute to the suppression of gluconeogenic enzymes, which subsequently impacts glucose metabolism in zebrafish. This observation is consistent with existing evidence in mammals, suggesting that ATF4 and CREB work collaboratively to regulate glucose metabolism through the modulation of FTO-mediated gluconeogenesis15,56. The downregulation of key glycolytic genes (PFK-A, PFK-B, PKLR, and GCK) following rhein treatment indicates a metabolic shift from glycolysis, perhaps by affecting alternate energy pathways such as gluconeogenesis and fatty acid oxidation. Furthermore, the activation of AMPK could promote fatty acid oxidation as an alternative energy source when glycolysis is suppressed57.
IP administration of rhein decreased FTO and IRX3 protein and mRNA in the zebrafish brain. This reduction was associated with a decrease in food intake, which was observed at 1-hour post-administration. The suppression of IRX3 mRNA abundance and protein expression following rhein administration further reinforces the role of FTO in appetite regulation58. IRX3 is known to be functionally linked to FTO, with evidence suggesting that in mice with a deletion of the IRX3 gene an increased number of leptin-responsive neurons was observed in the arcuate nucleus59. This neuronal increase is associated with reduced food intake and a leaner body composition. These findings highlight the significant role of IRX3 in regulating energy balance through its influence on hypothalamic neurons and that its expression is critical for the orexigenic effect of FTO58. This orexigenic function is in accordance with our recent report on FTO and IRX3 mRNA being upregulated during a 7-day fasting period in different metabolic tissues of zebrafish21. A similar reduction in food intake was reported following the inhibition of FTO by rhein in mice, where hypothalamic ERK1/2 inactivation blocked FTO inhibition-induced STAT3-S727 phosphorylation and suppressed feeding60, contrary to this alteration of FTO by adenoviral infection in the arcuate nucleus of rats increased food intake45. A reason for this variability might be the different methods used for FTO inactivation (pharmacological inhibition or siRNA knockdown) and the existence of species-specific effects. Considering the orexigenic role of FTO in zebrafish, we investigated whether intraperitoneal administration of rhein influences the expression of appetite-regulating peptides. The most significant findings include a reduction in orexin and NPY at both mRNA and protein levels, along with an increase in leptin mRNA expression. The role of NPY and orexin in stimulating food intake in mammals and fish is well-known61,62,63,64. The deletion of FTO in rats resulted in an increase in food intake, while AgRP, POMC, and NPY mRNAs remained unchanged65. While the results on NPY and orexin support the food intake effects seen in this research, there appears to be species- or model-specific differences on FTO effects on food intake as seen in the above study. Moreover, as the western blot showed some non-specific bands, especially for orexin and IRX3, these findings would benefit from repetition and validation employing further optimized primary antibodies against zebrafish endogenous orexin or IRX3.Under energy-restricted conditions, both FTO mRNA and protein declined in the hypothalamus and brainstem regions where FTO is co-expressed with the long isoform of the leptin receptor (LepRb)66. Leptin promotes the formation of STAT3 dimers that bind to the promoters of POMC and AgRP in the nucleus. This binding increases POMC expression while suppressing AgRP, leading to reduced food intake and enhanced energy expenditure. In wild-type mice and in hypothalamic neuronal culture, leptin-induced STAT3 phosphorylation is crucial for downregulating FTO, although this process is disrupted in db/db mice60,67. Moreover, in zebrafish, an upregulation of leptin-b, POMC, and MC4R mRNA levels has been observed, suggesting the activation of anorexigenic pathways that contribute to decreased food intake68. Leptin-b is abundantly expressed in the hypothalamus and colocalizes with POMC mRNA, confirming that POMC neurons are direct targets of leptin signaling69. Conversely, leptin deficiency triggers NPY activation and melanocortin inhibition, leading to increased food intake and weight gain70. In addition, the observed reduction in SIRT1 expression implies potential alterations in hypothalamic energy balance regulation, given that SIRT1 modulates both AgRP and POMC neuronal activity71. Overexpression of SIRT1 in AgRP neurons enhances leptin sensitivity and suppresses feeding behavior in mice72. Further supporting this cascade, studies using hypothalamic N43/5 cells have demonstrated that rhein-induced inhibition of FTO promotes the phosphorylation of STAT3 at S727. This phosphorylation not only enhances POMC expression but also activates ERK1/260. Thus, our findings revealed rhein-induced inhibition of FTO may initiate a cascade of downstream events via enhanced STAT3 signaling14. This integrated network, characterized by reduced expression of both FTO and SIRT1 in conjunction with enhanced leptin signaling, ultimately contributes to decreased food intake in zebrafish. Therefore, this evidence supports our findings in which rhein-induced inhibition of FTO might trigger a cascade of downstream events via enhanced STAT3 signaling that activates leptin-dependent anorexigenic pathways. This integrated network, characterized by decreased FTO and SIRT1 expression along with increased leptin signaling, could subsequently trigger decreased food intake in zebrafish.
Protein-level results, particularly western blots for IRX3 and orexin, showed non-specific bands and could not be optimized or replicated due to limited samples and non-availability of primary antibodies against these endogenous proteins in zebrafish, rendering these findings preliminary. Future research should validate protein-level findings with optimized assays, integrate metabolite flux and omics analyses, and assess the impact of selective FTO inhibitors on m6A modification patterns to clarify functional consequences. To gain a deeper understanding on this association, future work should focus on AMPKα inhibition (via compound C and AMPKα2 knockdown), PPARα pathway modulation (agonists/antagonists), and FTO knockdown, together with enhanced AMPK activity assays. These mechanistic studies will unravel whether the observed associations truly reflect direct regulatory relationships.
Conclusions
This study revealed a very important role for the FTO gene in regulating feeding and metabolism in zebrafish. The expression of FTO and IRX3 in key metabolism regulatory tissues, including the brain, liver underscores their involvement in metabolic processes. The observed modulation of several appetite-regulatory and metabolic genes following FTO inhibition using rhein is likely associated with substantial metabolic alterations including enhanced lipid oxidation and improved glucose metabolism (summarized in Fig. 8). These effects are likely mediated through the activation of AMPK and PPARα, coupled with the suppression of gluconeogenic processes. These outcomes also associate FTO with AMPKα and PPARα signaling. Despite the limitations discussed earlier, our findings supported by both transcript and protein-level data provide a robust foundation for further investigation into how FTO suppression influences lipid and glucose metabolism. The possible role of AMPKα-PPARα in this process warrants consideration. The concurrent reduction in food intake after rhein treatment furthers the role of FTO in appetite regulation. These findings align with previous studies in mammals, suggesting a conserved function for FTO on feeding regulation across species. Future research should prioritize elucidating precise molecular mechanisms underlying FTO on energy homeostasis and explore the development of FTO inhibitors for metabolic diseases.
Scheme summarizing aspects of zebrafish feeding and metabolism modulated when FTO was inhibited with rhein. It suppresses orexigenic mediators including NPY and orexin, while enhancing satiety-related signals including POMC, CART, IRX3, and MC4R, thereby reducing food intake. In hepatic tissue or hepatocytes, rhein downregulates gluconeogenic genes (G6PC, PCK1, ATF4), resulting in decreased glucose production. Concurrently, activation of AMPK promotes phosphorylation of ACC, alleviates CPT1 inhibition, and enhances β-oxidation, with PPARα and its downstream targets (CPT1A, ACOX1), further supporting fatty acid catabolism. Lipogenic processes are attenuated through suppression of SREBF1 and its downstream effectors (FAS, SCD), together with downregulation of CREB. Collectively, these findings suggest that inhibition of FTO by rhein may contribute to improved metabolic homeostasis by shifting cellular energy metabolism away from gluconeogenesis and lipid synthesis toward oxidative and catabolic pathways. Acronyms used and their full forms: Proopiomelanocortin (POMC), amphetamine-regulated transcript (CART), agouti-related melanocortin 4 receptor (MC4R) Iroquois homeobox protein (IRX3), proliferator-activated receptor alpha (PPARα) and AMP‐activated protein kinase (AMPKα). Sterol regulatory element binding transcription factor 1 (SREBF1), cAMP Response Element-Binding protein (CREB) stearoyl-CoA desaturase (SCD), carnitine palmitoyltransferase I (CPT-1), Fatty acid synthase (FAS), Activating transcription factor 4 (ATF4), Glucose-6-phosphatase (G6PC), phosphoenolpyruvate carboxykinase 1(PCK1).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Liu, C. M. & Kanoski, S. E. Homeostatic and non-homeostatic controls of feeding behavior: distinct vs. common neural systems. Physiol. Behav. 193, 223–231 (2018).
Yin, D. et al. FTO : a critical role in obesity and obesity-related diseases. Br. J. Nutr. 130, 1657–1664 (2023).
Clarke, G. S., Page, A. J. & Eldeghaidy, S. The gut–brain axis in appetite, satiety, food intake, and eating behavior: insights from animal models and human studies. Pharmacol. Res. Perspect. 12, e70027 (2024).
Roh, E., Song, D. K. & Kim, M. S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 48, e216–e216 (2016).
Wee, C. L. et al. A bidirectional network for appetite control in larval zebrafish. eLife 8, e43775 (2019).
Huang, C., Chen, W. & Wang, X. Studies on the fat mass and obesity-associated (FTO) gene and its impact on obesity-associated diseases. Genes Dis. 10, 2351–2365 (2023).
Lan, Q. et al. The critical role of RNA m6A methylation in cancer. Cancer Res. 79, 1285–1292 (2019).
Jia, G. et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887 (2011).
Wu, J. et al. Emerging role of m6 A RNA methylation in nutritional physiology and metabolism. Obes. Rev. 21, e12942 (2020).
Yang, H., Li, Y., Huang, L., Fang, M. & Xu, S. The epigenetic regulation of RNA N6-Methyladenosine methylation in glycolipid metabolism. Biomolecules 13, 273 (2023).
Li, Y., Su, R., Deng, X., Chen, Y. & Chen, J. FTO in cancer: functions, molecular mechanisms, and therapeutic implications. Trends Cancer. 8, 598–614 (2022).
Mizuno, T. M. Fat mass and obesity associated (FTO) gene and hepatic glucose and lipid metabolism. Nutrients 10, 1600 (2018).
Fredriksson, R. et al. The obesity Gene, FTO, is of ancient Origin, Up-Regulated during food deprivation and expressed in neurons of Feeding-Related nuclei of the brain. Endocrinology 149, 2062–2071 (2008).
Bravard, A. et al. FTO contributes to hepatic metabolism regulation through regulation of leptin action and STAT3 signalling in liver. Cell. Commun. Signal. 12, 4 (2014).
Mizuno, T. Regulation of activating transcription factor 4 (Atf4) expression by fat mass and Obesity-Associated (Fto) in mouse hepatocyte cells. Acta Endocrinol. Buchar. 17, 26–32 (2021).
Mizuno, T. M., Lew, P. S., Luo, Y. & Leckstrom, A. Negative regulation of hepatic fat mass and obesity associated (Fto) gene expression by insulin. Life Sci. 170, 50–55 (2017).
Guo, J. et al. Fat mass and Obesity-Associated gene enhances oxidative stress and lipogenesis in nonalcoholic fatty liver disease. Dig. Dis. Sci. 58, 1004–1009 (2013).
Kang, H. et al. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. J. Cell. Biochem. 119, 5676–5685 (2018).
Churcher, A. M. et al. Changes in the gene expression profiles of the brains of male European Eels (Anguilla anguilla) during sexual maturation. BMC Genom. 15, 799 (2014).
Gerken, T. et al. The Obesity-Associated FTO gene encodes a 2-Oxoglutarate-Dependent nucleic acid demethylase. Science 318, 1469–1472 (2007).
Karimzadeh, K., Uju, C., Zahmatkesh, A. & Unniappan, S. Fat mass and obesity associated gene and homeobox transcription factor iriquois-3 mRNA profiles in the metabolic tissues of zebrafish are modulated by feeding and food deprivation. Gen. Comp. Endocrinol. 360, 114621 (2025).
Olszewski, P. K. et al. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci. 10, 129 (2009).
Bati, B. et al. Effect of Isgin (Rheum Ribes L.) on biochemical parameters, antioxidant activity and DNA damage in rats with obesity induced with high-calorie diet. Arch. Physiol. Biochem. 129, 298–306 (2023).
Deng, T. et al. Rhein for treating diabetes mellitus: A Pharmacological and mechanistic overview. Front. Pharmacol. 13, 1106260 (2023).
Dong, S., Wu, Y., Liu, Y., Weng, H. & Huang, H. N6 -methyladenosine steers RNA metabolism and regulation in cancer. Cancer Commun. 41, 538–559 (2021).
You, L. et al. Rhein induces cell death in HepaRG cells through cell cycle arrest and apoptotic pathway. Int. J. Mol. Sci. 19, 1060 (2018).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2 – ∆∆CT method. Methods 25, 402–408 (2001).
Nasri, A. & Unniappan, S. Nucleobindin-derived nesfatin-1 and nesfatin-1-like peptide stimulate pro-opiomelanocortin synthesis in murine AtT-20 corticotrophs through the cAMP/PKA/CREB signaling pathway. Mol. Cell. Endocrinol. 536, 111401 (2021).
Li, J., Pei, Y., Zhou, R., Tang, Z. & Yang, Y. Regulation of RNA N 6 -methyladenosine modification and its emerging roles in skeletal muscle development. Int. J. Biol. Sci. 17, 1682–1692 (2021).
Mayman, N. et al. Case report: A novel biallelic FTO variant causing multisystem anomalies with severe epilepsy, widening the spectrum of FTO syndrome. SAGE Open. Med. Case Rep. 11, 2050313X231188883 (2023).
Fang, M., Ye, L., Zhu, Y., Huang, L. & Xu, S. M6A demethylase ALKBH5 in human diseases: from structure to mechanisms. Biomolecules 15, 157 (2025).
Li, R., Cao, Y., Wu, W., Liu, H. & Xu, S. Inhibitor of FTO, Rhein, restrains the differentiation of myoblasts and delays skeletal muscle regeneration. Animals 14, 2434 (2024).
Yang, D. et al. Rhein promotes skin wound healing by activating the PI3K/AKT signaling pathway. Open. Med. 19, 20241116 (2024).
De Araujo, T. M. et al. The partial Inhibition of hypothalamic IRX3 exacerbates obesity. EBioMedicine 39, 448–460 (2019).
Ma, Y. et al. IRX3 plays an important role in the pathogenesis of metabolic-associated fatty liver disease by regulating hepatic lipid metabolism. Front. Endocrinol. 13, 895593 (2022).
Sun, L. et al. Triclosan-induced abnormal expression of miR-30b regulates fto-mediated m6A methylation level to cause lipid metabolism disorder in zebrafish. Sci. Total Environ. 770, 145285 (2021).
Wei, X. et al. Fat mass and obesity–associated protein promotes liver steatosis by targeting PPARα. Lipids Health Dis. 21, 29 (2022).
Yan, F. et al. Peroxisome Proliferator-Activated receptor α activation induces hepatic Steatosis, suggesting an adverse effect. PLoS ONE. 9, e99245 (2014).
Ferré, P., Phan, F. & Foufelle, F. SREBP-1c and lipogenesis in the liver: an update. Biochem. J. 478, 3723–3739 (2021).
Zhelev, Z. et al. A Weird Mitochondrial Fatty Acid Oxidation as a Metabolic Secret of Cancer. Oxid. Med. Cell. Longev. 1–38 (2022). (2022).
Adeva-Andany, M. M., Carneiro-Freire, N., Seco-Filgueira, M. & Fernández-Fernández, C. Mouriño-Bayolo, D. Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion 46, 73–90 (2019).
Talari, N. K. et al. Lipid-droplet associated mitochondria promote fatty-acid oxidation through a distinct bioenergetic pattern in male Wistar rats. Nat. Commun. 14, 766 (2023).
Sheng, X. et al. Rhein ameliorates fatty liver disease through negative energy balance, hepatic lipogenic regulation, and Immunomodulation in diet-induced obese mice. Am. J. Physiol. -Endocrinol Metab. 300, E886–E893 (2011).
Guo, F. et al. Fat mass and obesity associated (FTO) gene regulates gluconeogenesis in chicken embryo fibroblast cells. Comp. Biochem. Physiol. Mol. Integr. Physiol. 179, 149–156 (2015).
Church, C. et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 42, 1086–1092 (2010).
Irisarri, A. et al. FTO Inhibition mitigates high-fat diet-induced metabolic disturbances and cognitive decline in SAMP8 mice. Mol. Med. 31, 73 (2025).
Duan, Y. et al. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal. Transduct. Target. Ther. 7, 265 (2022).
Basso, F. et al. Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J. Lipid Res. 44, 296–302 (2003).
Liu, Z. H., Li, Y. J., Chen, Z. H., Liu, D. & Li, L. S. Glucose transporter in human glomerular mesangial cells modulated by transforming growth factor-beta and Rhein. Acta Pharmacol. Sin. 22, 169–175 (2001).
Ebert, S. M. et al. Biology of activating transcription factor 4 (ATF4) and its role in skeletal muscle atrophy. J. Nutr. 152, 926–938 (2022).
Zhang, Q. et al. Central activating transcription factor 4 (ATF4) regulates hepatic insulin resistance in mice via S6K1 signaling and the vagus nerve. Diabetes 62, 2230–2239 (2013).
Zhou, J. et al. N6-Methyladenosine guides mRNA alternative translation during integrated stress response. Mol. Cell. 69, 636–647e7 (2018).
Peng, S. et al. Identification of Entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1. Sci. Transl Med. 11, eaau7116 (2019).
Gautier-Stein, A., Zitoun, C., Lalli, E., Mithieux, G. & Rajas, F. Transcriptional regulation of the Glucose-6-phosphatase gene by cAMP/Vasoactive intestinal peptide in the intestine. J. Biol. Chem. 281, 31268–31278 (2006).
Wu, Q., Saunders, R. A., Szkudlarek-Mikho, M., Serna, I. D. L. & Chin, K. V. The obesity-associated Fto gene is a transcriptional coactivator. Biochem. Biophys. Res. Commun. 401, 390–395 (2010).
Mizuno, T. Fat mass and obesity associated (FTO) gene and hepatic glucose and lipid metabolism. Nutrients 10, 1600 (2018).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell. Biol. 13, 251–262 (2012).
Smemo, S. et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507, 371–375 (2014).
Dou, Z., Son, J. E. & Hui, C. Irx3 and Irx5 - Novel regulatory factors of postnatal hypothalamic neurogenesis. Front. Neurosci. 15, 763856 (2021).
Hu, F., Yan, H. J., Gao, C. X., Sun, W. W. & Long, Y. S. Inhibition of hypothalamic FTO activates STAT3 signal through ERK1/2 associated with reductions in food intake and body weight. Neuroendocrinology 113, 80–91 (2023).
Blais, A. et al. Impact of Orexin-A treatment on food Intake, energy metabolism and body weight in mice. PLOS ONE. 12, e0169908 (2017).
Blanco, A. M., Bertucci, J. I., Hatef, A. & Unniappan, S. Feeding and food availability modulate brain-derived neurotrophic factor, an orexigen with metabolic roles in zebrafish. Sci. Rep. 10, 10727 (2020).
Mercer, R. E., Chee, M. J. S. & Colmers, W. F. The role of NPY in hypothalamic mediated food intake. Front. Neuroendocrinol. 32, 398–415 (2011).
Volkoff, H. & Peter, R. E. Interactions between orexin A, NPY and Galanin in the control of food intake of the goldfish, Carassius auratus. Regul. Pept. 101, 59–72 (2001).
Qi, Y. et al. Agrp-negative arcuate NPY neurons drive feeding under positive energy balance via altering leptin responsiveness in POMC neurons. Cell. Metab. 35, 979–995e7 (2023).
Wang, P. et al. Involvement of leptin receptor long isoform (LepRb)-STAT3 signaling pathway in brain fat mass- and obesity-associated (FTO) downregulation during energy restriction. Mol. Med. Camb. Mass. 17, 523–532 (2011).
Liu, H., Du, T., Li, C. & Yang, G. STAT3 phosphorylation in central leptin resistance. Nutr. Metab. 18, 39 (2021).
Baldini, G. & Phelan, K. D. The melanocortin pathway and control of appetite-progress and therapeutic implications. J. Endocrinol. 241, R1–R33 (2019).
Cheung, C. C., Clifton, D. K. & Steiner, R. A. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138, 4489–4492 (1997).
Oswal, A. & Yeo, G. S. H. The leptin melanocortin pathway and the control of body weight: lessons from human and murine genetics. Obes. Rev. 8, 293–306 (2007).
Al-Massadi, O. et al. MCH regulates SIRT1/FoxO1 and reduces POMC neuronal activity to induce Hyperphagia, Adiposity, and glucose intolerance. Diabetes 68, 2210–2222 (2019).
Sasaki, T. et al. Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia 57, 819–831 (2014).
Acknowledgements
This research was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to Suraj Unniappan (SU). SU is the University of Saskatchewan Centennial Enhancement Chair in Comparative Endocrinology. Chinelo Uju (CU) was supported by a Devolved Scholarship from the Department of Veterinary Biomedical Sciences, and the Dean’s Doctoral Scholarship for International students from the University of Saskatchewan.
Author information
Authors and Affiliations
Contributions
KK conducted the experiments, analyzed and interpreted the data and prepared the draft of the manuscript and revised the manuscript for submission. CU assisted with experiments and helped interpret data. AZ helped with data analysis and graphing. SU provided the original ideas, secured funding for the project, provided space and facilities to conduct the research, helped with data interpretations, and reviewed draft manuscripts and revised and finalized it for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Karimzadeh, K., Uju, C., Zahmatkesh, A. et al. Regulation of feeding and metabolism by fat mass and obesity-associated protein in zebrafish. Sci Rep 15, 40979 (2025). https://doi.org/10.1038/s41598-025-24733-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-24733-x