Abstract
Colorectal cancer (CRC) is one of the most prevalent malignancies worldwide. Fusobacterium nucleatum (F. nucleatum) has been strongly implicated in CRC metastasis. However, the contribution of laminin γ2 chain (LAMC2) to F. nucleatum-induced CRC metastasis remains elusive. We investigated the role of LAMC2 in F. nucleatum-induced CRC cell migration through a series of in vitro assays, including transmission electron microscopy, wound-healing, and transwell migration experiments, as well as in vivo animal models. Additionally, we explored the expression profile of LAMC2 in CRC tissues and assessed its prognostic relevance using immunohistochemistry, bulk transcriptome, and single-cell transcriptomics analyses. We found that F. nucleatum upregulates LAMC2 expression, thereby promoting CRC cell migration. Mechanistic analyses revealed that F. nucleatum downregulates hsa-miRNA-7977, releasing its transcriptional repression of LAMC2. Additionally, LAMC2 activates the FAK-PI3K-AKT signaling pathway and modulates H3K27 histone acetylation. Immunohistochemistry, together with single-cell and bulk transcriptome analyses, demonstrated that LAMC2 is highly expressed in metastatic CRC tissues, and is significantly associated with poor prognosis. Moreover, a Cox regression-based prognostic model constructed using LAMC2-associated genes exhibited robust predictive performance across multiple independent cohorts. Our findings identify LAMC2 as a key mediator of F. nucleatum-induced CRC metastasis and highlight it as both a prognostic biomarker and a potential therapeutic target.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) remains a leading cause of cancer-related morbidity and mortality, characterized by poor responses to current therapies. Approximately 25% of CRC patients present with synchronous metastasis, while an additional 50% develop metastasis during disease progression1. The prognosis of CRC patients with distant metastasis remains dismal, with a 5-year survival rate reduced by nearly 10%2. Metastasis is a multi-step and tightly regulated process, involving factors such as epithelial-mesenchymal transition (EMT), cancer stem cells (CSCs), and dysbiosis of intestinal microbiota3,4,5. Therefore, there is an urgent need to explore the mechanisms underlying CRC metastasis and to identify effective strategies to prevent metastasis and improve patients outcomes.
Mounting evidence highlights a critical role for the gut microbiota in CRC initiation and progression6,7, with F. nucleatum receiving particular attention. This Gram-negative anaerobic bacterium exhibits invasive, adhesive, and pro-inflammatory properties and is markedly enriched in the feces and tumor tissues of CRC patients8,9,10. Notably, elevated F. nucleatum abundance is associated with adverse prognostic outcomes, including tumor recurrence and distant metastasis11,12,13. Recent studies further indicate that F. nucleatum can drive CRC metastasis by reprogramming the tumor microenvironment, and modulating EMT, non-coding RNA expression, and chemokine signaling14,15,16. Despite these insights, the precise mechanisms by which F. nucleatum promotes CRC metastasis remain largely unclear, necessitating further investigation.
The laminin subunit gamma-2, encoded by the LAMC2 on chromosome 1q25-q3117, is a component of the heterotrimeric glycoprotein laminin-332 (LAM-332, formerly known as laminin-5), consisting of α3, β3, and γ2 chains18,19. Accumulating evidence demonstrates that LAMC2 is upregulated across multiple malignancies, including gastric cancer20, esophageal squamous cell carcinoma21, and pancreatic ductal adenocarcinoma22,23. Additionally, elevated LAMC2 expression has been linked to tumor metastasis, recurrence, and patient mortality rates24,25,26. However, the specific mechanisms through which LAMC2 promoted CRC metastasis remain poorly understood.
Here, we investigated the molecular mechanisms and clinical relevance of F. nucleatum-induced CRC metastasis by regulating LAMC2 expression. In vitro and in vivo experiments demonstrated that F. nucleatum specifically upregulates LAMC2 expression to promote CRC metastasis. Mechanistically, F. nucleatum suppressed hsa-miRNA-7977, thereby releasing its transcriptional repression of LAMC2, which in turn activated the FAK-PI3K-AKT signaling pathway and modulated histone H3K27 acetylation. Moreover, LAMC2 was highly expressed in metastatic CRC tissues, and associated with poor prognosis. A prognostic model constructed based on LAMC2-related genes exhibited robust predictive performance in multiple independent cohorts. Together, these findings identified a critical F. nucleatum-LAMC2 axis in CRC metastasis, providing new insights for targeted interventions.
Materials and methods
Bacterial strain and cell culture
F. nucleatum (ATCC 25,586) was purchased from Guangdong Microbial Culture Collection Center (GDMCC), and grown in Brain Heart Infusion Broth (hopebio, China) supplemented with hemin, K2HPO4, Vitamin K1, and L-Cysteine in a round bottom vertical anaerobic culture bag (hopebio, China) at 37 ℃. Escherichia coli strain DH5α (E.coli) (obtained from Genomics Research Center, College of Pharmacy, Harbin Medical University) was cultured in Luria-Bertani medium10. Human CRC cell lines (HCT116, LOVO, HCT8, SW480 and SW620) were purchased from the American Type Culture Collection (ATCC, USA). Cells were cultured in RPMI 1640, DMEM, or F-12 K Nutrient Mixture (Gibco, USA) supplemented with 10% fetal bovine serum. All cell lines were cultured at 37 ℃ in humidified 5% CO2 atmosphere.
F. nucleatum–cancer cell co-culture protocol
To determine the optimal multiplicity of infection (MOI) for F. nucleatum, we drew on our prior publication on F. nucleatum10, and tested a dose range of 0, 50, 100, 200, 300 and 500 bacteria per cell. MOIs below 100 produced insufficient stimulation of tumour cells, whereas MOIs above 100 induced extensive cell death. Consequently, an MOI of 100 was selected for all experiments.
Cell transfection
The LAMC2 shRNA lentivirus and its control shRNA lentivirus were purchased from GenePharma (Suzhou, China) Company, while the LAMC2 oe-RNA lentivirus and its control oe-RNA lentivirus were purchased from Genechem (Shanghai, China). Transfection of lentiviral constructs was performed according to the manufacturer’s instructions. Following transfection, cells were selected for stable expression of shRNA and oe-RNA in the presence of puromycin (Beyotime, China). The efficiency of knockdown and overexpression was confirmed by qPCR and Western blot analysis.
The sequences of the LAMC2 shRNAs were GCCTCAACTGCAATGACAACA (Sh1), GCAGGTGTTTGAAGTGTATCC (Sh2), GGAGCAACTGACAAGGGAAAC (Sh3), and the sequence of the negative control shRNA was GTTCTCCGAACGTGTCACGT (ShCtl).
RNA extraction and quantitative real time PCR
Total RNAs were extracted from CRC cell lines by using TRIzol™ Reagent (Invitrogen, USA), and then reverse transcribed to cDNA using PrimeScript™ RT Master Mix (Takara, Japan). For miRNA, reverse transcriptions were carried out using a commercial miRNA reverse transcription PCR kit (Takara, Japan). Quantitative real-time PCR was performed in ABI StepOne™ Real-Time PCR System with PowerUp SYBR Master Mix (Applied biosystems, USA). Relative abundance of mRNA was calculated by 2 − ΔΔCt method. All primer sequences are shown in Supplementary Data 1.
Protein extraction and western blotting
Total protein was extracted from CRC cells using RIPA lysis buffer supplemented with PMSF and phosphatase inhibitors. Use the BCA protein concentration assay kit to quantify the protein concentration of the samples. Proteins were electrophoresed through an 8–12% SDS polyacrylamide gel and then transferred to a PVDF membrane. The membrane was blocked with 5% skim milk powder solution for 2 h and then incubated with primary antibody overnight at 4 °C. The next day, the membrane was incubated with secondary antibodies labeled with HRP for 2 h at room temperature, and the signal was detected using an ECL kit. anti-LAMC2, anti-FAK, anti-p-FAK, anti-AKT, anti-p-AKT and anti-H3K27ac were purchased from Abcam. anti-PI3K and anti-p-PI3K were purchased from CST. GAPDH was purchased from Abclona.
Wound healing assays
CRC cells, including LOVO and HCT116, were seeded in 6-well plates to form a confluent monolayer of cells. Each well of cells was infected with F. nucleatum or E.coli (DH5α) at a Multiplicity of Infection (MOI) of 100:1 or with an equal volume of phosphate-buffered saline(PBS) for 2 h. Subsequently, the monolayer of cells was scratched in a straight line using a 10 µl pipette tip to create “wounds”. After washing twice with PBS, the cells were incubated in medium supplemented with streptomycin, metronidazole, and 1% serum for 24 h. Cell images were captured under a microscope at 0 and 24 h. The scratch area was measured and calculated using ImageJ software. Cell migration rate (%) was determined as follows: Migration rate (%) = (scratch area at 0 h - scratch area after 24 h migration) / scratch area at 0 h × 100%.
Transwell migration assays
After pre-incubation of CRC cells LOVO and HCT116 with PBS, F. nucleatum or E.coli (DH5α) for 2 h, the cells were washed twice with PBS, trypsinized, centrifuged to remove the supernatant, and resuspended in medium containing 1% serum. Then, 200 µl of medium containing 1 × 10^5 cells (1% serum) was seeded into the upper chamber of a transwell insert (8 μm pore size, Corning, USA), and 800 µl of fresh medium (containing 10% serum) was added to the lower chamber. After incubation at 37 °C for 24 h, the cells in the upper chamber were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Carefully remove the cells from the upper chamber using a cotton swab. Five random fields were observed under an optical microscope (100× magnification).
Dual-luciferase reporter system
The wild-type (WT) and mutant (mut) LAMC2 vectors were constructed in PsiCHECK2 vector 3`- UTR. The oligonucleotide sequences used for luciferase analysis were as follows:
LAMC2-WT 3’UTR 5’-GGCACUUCCACCUUGGCUGGGAA-3ʹ,
LAMC2-mut 3’UTR 5’-GGCACUUCCACCGGAAUGAAACA-3ʹ,
hsa-miR-7977 3’-ACCACGCAACCGACCCUU-5’.
Transfection in 293T cells was performed by adding 1 µL of microRNA (20 µM) or microRNA inhibitor, together with 0.5 µg of plasmid, per well. The luciferase signal was measured through the Dual Luciferase Assay Kit (Promega, Madison, USA) after transfection for 48 h.
In vivo assay
BALB/c nude mice (6 weeks old) were selected to establish a lung metastasis model and were housed in SPF facilities (25 °C, appropriate humidity typically around 50%, 12-hour light/dark cycle). F. nucleatum (MOI of 100:1) was incubated with LAMC2 knockdown or control knockdown LOVO cells for 24 h. Afterward, the cells were injected into the tail vein of nude mice (1 × 10^6 cells per mouse). Eight weeks later, the mice were euthanized, and lung tissues were harvested to evaluate tumor metastasis. Mice were deeply anesthetized by intraperitoneal injection of sodium pentobarbital (100 mg/kg). Loss of consciousness was verified by the absence of toe-pinch and corneal reflexes. Death was then confirmed by permanent cessation of heartbeat and respiration, observed for ≥ 3 min. Only after confirmation of death were tissues harvested. All procedures were approved by the Research Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (Approval No.YJSDW2022-197).
Public bulk transcriptome data acquisition and processing
Transcriptome data and corresponding clinical data for the TCGA COAD cohort were obtained from the UCSC Xena browser27. Counts were normalized to log2(TPM + 1) to enable cross-sample comparisons. Additionally, six independent colorectal cancer datasets (GSE41258, GSE17536, GSE39582, GSE90944, GSE102573, GSE141805) were retrieved from the GEO database, while the GEO combined cohort was sourced from our prior study28. Data processing followed established protocols from our previous work29.
Survival analysis
To ensure the robustness of survival analysis, samples with a follow-up duration of less than one month were excluded. Gene expression values were treated as continuous variables, and optimal cutoff thresholds were determined using the survminer R package to stratify patients into high- and low-expression groups. Kaplan-Meier (KM) plots were generated to visualize survival differences, and statistical significance was assessed using the log-rank test.
Single-cell sequencing analysis workflow
To investigate the expression of LAMC2, we performed in silico analyses using the publicly available CRC single-cell RNA sequencing dataset GSE13246530. After filtering out low-quality cells from the raw data, a total of 63,689 high-quality cells were retained for further analysis. The single-cell RNA sequencing data were processed and annotated following the methods detailed in our previous study29. Subsequently, the cells were classified into seven distinct clusters, namely T cells, B cells, myeloid cells, mast cells, epithelial cells, endothelial cells, and mesenchymal cells (Supplementary Fig. 1). The sample tissues included two groups: adjacent normal tissue and tumor tissue of CRC. We then analyzed the expression and distribution of LAMC2 across these different tissue types.
RNA sequencing after LAMC2 knockdown
Control and LAMC2-sh LOVO cells were co-cultured with F. nucleatum and subsequently subjected to RNA sequencing. The RNA secequencing data have been uploaded and can be accessed in Supplementary Data 2. Total RNA was extracted from the samples using the TRIzol method, followed by treatment with DNase I (TaKara) to remove genomic DNA. Subsequently, the samples were subjected to transcriptome sequencing. Differential gene analysis between samples was conducted using edgeR. Genes with p-value < 0.05 and |log2(FC)| ≥ 1 were considered significantly differentially expressed.
Differential expression analysis
The differential expression analysis was conducted using limma and edgeR31. The criteria for selecting differentially expressed genes (DEGs) was as follows:
1) p-value < = 0.05.
2) |log2(fold change)| >= 1.
To visualize the DEGs, volcano plots and heatmaps were utilized.
Functional enrichment analysis
In silico analyses, such as Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG), were performed using the R package “clusterProfiler”32. GO analysis included three terms, such as biological process (BP), cellular component (CC) and molecular function (MF). Additionally, Gene Set Enrichment Analysis (GSEA) was conducted using reference gene sets from the MSigDB website33. The DEGs ranked by fold change were used as the input list for GSEA analysis.
MiRNAs prediction
For predicting potential miRNAs that may bind to LAMC2, the following online databases were used, including TargetScan (version 8.0, http://www.TargetScan.org/ ), MirDIP (version 5.2.3.1, http://ophid.utoronto.ca/mirDIP/ ) and MiRDB (version 1.0, http://mirdb.org/ )34,35,36. We first predicted candidate miRNAs targeting LAMC2 using miRDB (87 candidates) and TargetScan (335 candidates). Prediction with mirDIP under the Minimum Score: High criterion identified 155 additional candidates. Intersection across the three databases yielded 14 shared miRNAs. To refine these candidates, we compared them with the set of downregulated miRNAs identified in CRC cells co-cultured with F. nucleatum derived from supplementary data in Yang et al.37. This analysis converged on miR-7977, which was subsequently selected for experimental validation.
Immunohistochemistry staining
A total of 15 paired CRC and normal tissues were collected from patients who underwent surgical procedures at the Department of CRC Surgery, the Second Affiliated Hospital of Harbin Medical University, from December 2022 to July 2023, under the ethical approval from the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (Approval No. KY2022-087). And all patients signed informed consent. This cohort consisted of 11 males and 4 females, with a median age of 69 years, ranging from 48 to 81 years (Supplementary Data 3). The primary antibodies used in the immunohistochemistry (IHC) analysis were anti-LAMC2 (Proteintech Group, Catalog Number:19698-1-AP). The experiment was conducted as described in our previous study29. The IHC results underwent independent analysis by two proficient pathologists. Regarding tumor tissues, the expression of LAMC2 was evaluated using the Immunoreactive Score (IRS) derived from IHC.
Statistical analysis
All bioinformatic analyses were performed in R 4.0.3. Statistical tests for in vivo and in vitro experiments were performed using GraphPad Prism Software. Data were shown as mean ± standard deviation (SD). P value less than 0.05 was considered statistically significant. The symbols * (P < 0.05), ** (P < 0.01), *** (P < 0.001), and **** (P < 0.0001) denote increasing levels of significance, while “ns” indicates no statistical significance. All the procedures done within the current study were shown in the flow chart (Supplementary Fig. 2).
Results
F. nucleatum promotes the migration ability of CRC cells
To investigate the interaction between F. nucleatum and CRC cells, we performed co-culture experiments. We observed that F. nucleatum extensively adhered to the surface of HCT116 cells and invaded the intracellular space by electron microscopy (Fig. 1A and B), which was consistent with a previous study38. To further elucidate the impact of F. nucleatum infection on the migratory capacity of CRC cells, we co-cultured two CRC cell lines (LOVO and HCT116) with either F. nucleatum or Escherichia coli, and assessed cell migration at 24 h using wound healing and Transwell assays. Compared with E. coli and phosphate-buffered saline (PBS) controls, F. nucleatum infection significantly promoted the migration of LOVO cells (Fig. 1C-F). Consistent results were also observed in HCT116 cells (Fig. 1G-J). Collectively, these findings demonstrate that F. nucleatum can promote the migration of CRC cells in vitro.
F. nucleatum promotes the migration of CRC cells in vitro. (A) Scanning electron microscopy and (B) transmission electron microscopy images show rod-shaped bacteria (arrows) penetrating and invading HCT116 cells. (C-J) LOVO and HCT116 cells were incubation with PBS control, E.coli, or F. nucleatum. Cell migration ability was evaluated through wound healing assays and Transwell assays (n = 4 per group, One-way ANOVA test). In the Transwell assays, migrated cells were stained with crystal violet, and images were randomly captured under a microscope, with 5 random fields per sample counted. Statistical analysis was performed using one-way ANOVA. Data are shown as mean ± SD.* represents p < 0 0.05, ** represents p < 0 0.01, *** represents p < 0.001, and **** represents p < 0.0001, ns represents no statistical significant.
F. nucleatum upregulates the expresson of LAMC2 with increasing infection time and MOI
To further investigate the potential mechanisms that F. nucleatum promoted CRC cells migration, we analyzed the transcriptome data of CRC cells infected by F. nucleatum in GSE173549. We found that F. nucleatum treatment significantly upregulated 304 genes and downregulated 213 genes (Fig. 2A). Subsequently, we intersected these DEGs with metastasis genes from the CancerSEA database, and found that MMP7, LAMC2, TYMP, PLAU, TGM2, FN1, JAG1, and GLI1 were common genes, among which MMP7 showing the highest expression (Fig. 2B). The roles of MMP7 in F. nucleatum-induced CRC metastasis have been deeply studied in our previous work, but the relationship between LAMC2 and F. nucleatum-induced CRC metastasis was still unclear. Our in-house transcriptome data revealed that F. nucleatum could upregulate the expression of LAMC2 in LOVO cells, as well as other CRC cells (Supplementary Fig. 3A-D). To identify the primary cell types expressing LAMC2, we analyzed the single-cell transcriptome data of CRC and found that LAMC2 was predominantly expressed in epithelial cells. Moreover, the expression level of LAMC2 in epithelial cells derived from tumor tissues was significantly higher than that in epithelial cells from adjacent normal tissues (Fig. 2C and D). Based on these findings, subsequent cell experiments were primarily conducted in tumor epithelial cells.
F. nucleatum upregulates LAMC2 in CRC cells. (A) Volcano plot showing the expression profile of differentially expressed genes (DEGs) in LOVO cells between F. nucleatum infection and PBS control treatment (n = 3 per group, p < 0.05 and |log2 (fold change)| ≥ 1). (B) Intersection genes between the up DEGs from the GSE173549 dataset and the metastasis-related genes from the CancerSEA database. (C and D) The expression of LAMC2 was analyzed by single-cell RNA sequencing data GSE132465. The cells were classified into seven distinct clusters, including T, B, myeloid, mast, epithelial, endothelial, and mesenchymal cells. The statistical significance between two groups was evaluated by wilcox.test. Normal: adjacent normal tissues of CRC, Tumor: tumor tissues of CRC. (E and F)The mRNA and protein expression of LAMC2 after F. nucleatum or PBS treatment in CRC cells (n = 4 per group, One-way ANOVA test). ( Gand H) The mRNA expression of LAMC2 after F. nucleatum, E. coli or PBS treatment in CRC cells (n = 4 per group, One-way ANOVA test). ( Iand K) The protein expression of LAMC2 after PBS treatment, F. nucleatum infection of MOI = 100 or 500, or E. coli infection of MOI = 100 in CRC cells (repeated three times). (J and L) The protein expression of LAMC2 at 0, 4, 12, 24 and 48 h after F. nucleatum infection in CRC cells (n = 4 per group, One-way ANOVA test). Data are shown as mean ± SD.* represents p < 0 0.05, ** represents p < 0 0.01, *** represents p < 0.001, and **** represents p < 0.0001, ns represents no statistical significant.
To validate the transcriptome results, we examined LAMC2 expression in HCT116, SW480, SW620, HCT8, and LOVO cell lines after F. nucleatum infection by qPCR and western blot. LAMC2 expression was significantly upregulated in F. nucleatum-infected CRC cells (Fig. 2E and F). Then we selected LOVO and HCT116 cells for further investigation. In addition, F. nucleatum induced a greater increase in LAMC2 expression compared to E. coli (Fig. 2G and H). Notably, F. nucleatum upregulated LAMC2 expression in a manner dependent on infection time and MOI (Fig. 2I-L). Taken together, these results suggest that F. nucleatum infection may increase LAMC2 expression to affect the migration ability of CRC cells .
F. nucleatum promotes the metastasis of CRC through LAMC2
To validate whether F. nucleatum promoted CRC metastasis through LAMC2, we performed loss-of-function and gain-of-function experiments in LOVO and HCT116 cells, respectively. We observed the transfection efficiency in LOVO cells under fluorescence microscopy (Supplementary Fig. 3E) and validated the knockdown efficiency at both the mRNA and protein levels (Fig. 3A and B). The LAMC2-Sh1 and LAMC2-Sh3 were selected for subsequent experiments due to the better knockdown efficiency. Additionally, we found that the expression of LAMC2 induced by F. nucleatum was successfully knocked down by the lentivirus (Fig. 3C and D). Furthermore, we observed that knockdown of LAMC2 attenuated the pro-migratory effect of F. nucleatum on LOVO cells (Fig. 3E-H). To further elucidate the role of LAMC2 in F. nucleatum-induced CRC metastasis, we constructed CRC lung metastasis mouse model by tail vein injection of LOVO cells (Fig. 3I). F. nucleatum significantly increased the number and size of tumor nodules in CRC lung metastases, while LAMC2 knockdown effectively reversed the pro-metastatic effects of F. nucleatum (Fig. 3J and K). We also validated the overexpression efficiency of LAMC2 at both the mRNA and protein levels in HCT116 cells (Fig. 4A-C). Overexpression of LAMC2 promoted HCT116 cells migration (Fig. 4D-G). Collectively, these results suggested that F. nucleatum promoted CRC metastasis by upregulating the expression of LAMC2.
F. nucleatum promotes metastasis of CRC cells in a LAMC2 dependent manner. (A and B) Detection of the knockdown efficiency of LAMC2 by different sequences of lentiviral vectors at the mRNA (n = 4 per group, One-way ANOVA test) and protein (repeated three times) levels. LOVO cell were transfected with LAMC2 shRNA lentivirus or control lentivirus, and then incubated with F. nucleatum or PBS. (C and D) The mRNA expression and protein expression of LAMC2 were measured by qPCR (n = 4, One-way ANOVA test) and western blot (repeated three times). (E and G) The motility was detected by wound-healing assay (n = 6, One-way ANOVA test) or (F and H) transwell assay(n = 5, One-way ANOVA test). (I) LOVO cells constructed with lentivirus were incubated with F. nucleatum or PBS and then injected into the tail vein of nude mice (5 mice per group). (J and K) HE staining represents the images and the number of metastatic lesions (Student’s t-test) in each group. Data are shown as mean ± SD. * represents p < 0 0.05, ** represents p < 0 0.01, *** represents p < 0.001, and **** represents p < 0.0001, ns represents no statistical significant.
F.nucleatum upregulates the expression of LAMC2 through hsa-miRNA-7977. (A) western blot (repeated three times) was used to detect the overexpression efficiency of LAMC2. HCT116 cell were transfected with LAMC2 oe-RNA lentivirus or control lentivirus, and then incubated with F. nucleatum or PBS. (B and C) The protein expression and mRNA expression of LAMC2 were measured by western blot (repeated three times) and qPCR (n = 4, One-way ANOVA test). ( D and F) The motility was detected by wound-healing assay (n = 6, One-way ANOVA test) or ( E and G) transwell assay (n = 5, One-way ANOVA test). (H) Prediction of miRNA binding to LAMC2 using public databases, with a Venn diagram showing overlapping genes from the MiRDB, MirDIP, and Targetscan databases. (I) A Venn diagram showing candidate miRNAs binding to LAMC2 predicted by three public databases (miRNA-LAMC2) and downregulated miRNAs after F. nucleatum infection in CRC cells (Fn-miRNA). (J) Detection of miR-7977 expression using qPCR in CRC cells after F. nucleatum infection (n = 4, One-way ANOVA test). (K)Prediction of the binding site of has-miR-7977 to the 3’-UTR of LAMC2 using the Targetscan database.
F. nucleatum upregulates the expression of LAMC2 through hsa-miRNA-7977
The miRNAs play important roles in the development of cancers by regulating gene expression39. To determine whether F. nucleatum induced LAMC2 expression by regulating miRNAs, we utilized three databases (MiRDB, MirDIP, and Targetscan) to predict the potential miRNAs targeting LAMC2, and finally identified 14 candidates (Fig. 4H). Next, we intersected these candidate miRNAs with the downregulated differentially expressed miRNA influenced by F. nucleatum in CRC cells (with the criteria p < 0.05 and log2 (fold change) ≤ -1)37, and further identified has-miRNA-7977 as a targeting miRNA for LAMC2 (Fig. 4I). Subsequently, qRT-PCR assays were performed to detect the expression of hsa-miRNA-7977 in LOVO and HCT116 cells infected by F. nucleatum. A specific forward primer for hsa-miR-7977 and the universal reverse primer were used. And we found that the expression of hsa-miRNA-7977 was downregulated compared to the control group (Fig. 4J). Furthermore, we predicted the binding sites between hsa-miRNA-7977 and LAMC2 using the Targetscan database (Fig. 4K). We also performed a luciferase reporter assay to validate LAMC2 as a direct target of hsa-miR-7977. As shown in Supplementary Fig. 3F, luciferase activity in the hsa-miR-7977 inhibitor group was markedly higher than in the hsa-miR-7977 mimic group, whereas no significant changes were observed in the other groups. This result demonstrated that inhibition of hsa-miR-7977 attenuates its interaction with the LAMC2 3’-UTR, thereby derepressing luciferase expression and confirming the specific targeting relationship between hsa-miR-7977 and LAMC2. Based on these results, we hypothesized that F. nucleatum upregulates LAMC2 expression by downregulating the expression of hsa-miRNA-7977, thereby promoting the migration ability of CRC cells.
LAMC2 activates the FAK-PI3K-AKT pathway to promote F. nucleatum-induced CRC metastasis
To further elucidate the potential mechanism of LAMC2 promoting F. nucleatum-induced CRC metastasis, we conducted RNA sequencing analysis on shCtrl and LAMC2-Sh3 LOVO cells, and both the two groups were infected by F. nucleatum. We observed that 189 genes were upregulated and 209 genes were downregulated after LAMC2 knockdown (Fig. 5A and B). GO analysis showed that the DEGs were enriched in cell extracellular matrix disassembly and response to interferon-alpha. KEGG analysis revealed that DEGs induced by LAMC2 knockdown were enriched in the pathways crucial for cancer metastasis, such as the ECM-receptor interaction, PI3K-Akt signaling pathway, and TCA cycle (Fig. 5C and D). KEGG Maper analysis demonstrated that LAMC2 could activate the FAK-PI3K-AKT pathway (Fig. 5E). Additionally, increasing evidence suggests that the TCA cycle, besides existing in mitochondria, also involves some proteins present in the cell nucleus. Various metabolites produced by the TCA cycle are required for epigenetic modifications such as histone acetylation, succinylation, and methylation40,41,42. The RNA sequencing results suggested that LAMC2 might regulate tumor cell metastasis by affecting nuclear TCA cycle and thereby influencing epigenetic modifications. To validate whether LAMC2 in F. nucleatum-treated CRC cells could affect epigenetic modifications and activation of the FAK-PI3K-AKT pathway, we detected H3K27ac and proteins related to the FAK-PI3K-AKT pathway. The results showed that knockdown of LAMC2 reduced the expression of H3K27ac, p-FAK, p-PI3K, and p-AKT in LOVO cells (Fig. 5F). GSEA analysis revealed that LAMC2 knockdown was associated not only with EMT, but also with the interferon-alpha response and interferon-gamma response, which indicated the roles of LAMC2 in tumor immune (Fig. 5G and Supplementary Fig. 3G). Furthermore, we found LAMC2 was significantly correlated with common immune molecules using the GEPIA 2 database, and validated the genes (such as, CD47, TNFRSF9, TNFRSF14, PVR, and HLA-DMA) using qPCR (Supplementary Fig. 4A-H). Collectively, F. nucleatum-induced LAMC2 can influence CRC metastasis, epigenetic modifications, and tumor immune.
Knockdown of LAMC2 inhibits the FAK-PI3K-AKT pathway upon F. nucleatum infection. (A and B) Heat map and volcano plot represent DEGs after LAMC2 knockdown. (C-D) The representative enrichment terms of GO (C) and KEGG (D) analyses based on DEGs. (E) The detail information of PI3K-AKT signaling pathway from KEGG Maper. (F) LOVO cells transfected with LAMC2 shRNA lentivirus or control lentivirus, were incubated with or without F. nucleatum. The protein expression was measured by western blot (repeated three times). (G) The representative enrichment terms of GSEA (E) analysis based on DEGs. Data are shown as mean ± SD.* represents p < 0 0.05, ** represents p < 0 0.01, *** represents p < 0.001, and **** represents p < 0.0001, ns represents no statistical significant.
LAMC2 is upregulated in CRC and associated with a poor prognosis
To clarify the clinical role of LAMC2, we analyzed the correlation between LAMC2 and clinicopathological parameters in multiple cohorts. We found that in patients with colon adenocarcinoma, LAMC2 expression was significantly elevated in those with stage III and IV, T3 and T4, lymphatic invasion, and venous invasion (Fig. 6A-E). Moreover, the expression levels of LAMC2 in liver and lung metastases were significantly higher than those in the primary CRC lesions (Fig. 6F and G). Additionally, CRC patients with high LAMC2 expression had significantly poorer overall survival (OS), disease-free survival (DFS), disease-specific survival (DSS), recurrence-free survival (RFS), and progression-free survival (PFS) compared with those with low LAMC2 expression (Fig. 6H and Supplementary Fig. 4I-L). We also collected 15 pairs of CRC tissues and adjacent normal tissues from our center and evaluated the expression of LAMC2 by immunohistochemistry. The results showed that LAMC2 was mainly expressed in epithelial cells, and the protein level of LAMC2 in tumor epithelial cells was significantly higher than that in normal epithelial cells (Fig. 6I and J).
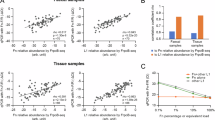
LAMC2 is upregulated in CRC patients and associated with a poor prognosis. (A-E) LAMC2 expression in different TNM, T and N stages, as well as in groups with different lymphatic and vascular invasion statuses (TCGA COAD cohort, Wilcox test). (F and G) Analysis of LAMC2 expression in CRC patients with liver and lung metastasis in GSE41258 dataset (Wilcox test). (H)The OS analysis of LAMC2 in the GSE17536 cohort (log-rank test). (I) Representative IHC images of LAMC2 in CRC tissues and normal tissues (15 paired samples). (J) Immunohistochemistry IRS scoring results of LAMC2 (n = 15, Paired t-test). (K-M) Analysis of the relationship between LAMC2 and CMS classification in GEO_combined, TCGA_COAD, and GSE39582 cohorts. Data are shown as mean ± SD.* represents p < 0 0.05, ** represents p < 0 0.01, *** represents p < 0.001, and **** represents p < 0.0001, ns represents no statistical significant.
The consensus molecular subtypes (CMS) classification is one of the most widely used classification systems in clinical management of CRC, comprising four subtypes: CMS1 (microsatellite instability immune subtype), CMS2 (canonical subtype), CMS3 (metabolic subtype), and CMS4 (mesenchymal subtype). Patients with different subtypes exhibit distinct molecular characteristics, prognoses, and treatment choices. Specifically, patients of the CMS4 subtype are characterized by significant EMT, activation of the TGF-β signaling pathway, stromal invasion, and angiogenesis, leading to poor overall survival and recurrence-free survival43. According to previous studies, F. nucleatum infection has been identified as a poor prognostic risk factor for CMS4 subtype patients44. To investigate the potential association between LAMC2 and the CMS classification, we utilized the CMScaller package to evaluate CMS classification of CRC patients45. And then we analyzed the relationship between LAMC2 and CMS classification in the GEO_combined, TCGA_COAD, and GSE39582 cohorts. The results revealed that LAMC2 expression was significantly higher in CMS4 subtype patients compared to the other three subtypes(Fig. 6K-M). The proportion of CMS4 subtype was significantly higher in patients with high LAMC2 expression than in those with low LAMC2 expression. In summary, these results indicated that LAMC2 was closely related to the metastasis and poor prognosis of CRC.
Patients with a high LAMC2-related signature exhibit poor prognosis
To construct a prognostic model based on LAMC2-related genes, we conducted Spearman correlation analysis between LAMC2 and all other genes to identify genes most strongly correlated with LAMC2 in the TCGA-COAD dataset. These genes with an absolute correlation coefficient greater than 0.3 (Spearman |r| > 0.3) and a p-value less than 0.05 (p-value < 0.05) were defined as LAMC2-associated genes. And we selected the top 100 genes with the smallest p-values (including LAMC2) to construct the prognostic model. Next, we performed univariate and multivariate Cox regression analysis on these genes, identifying a total of 14 genes (LAMB3, C1orf106, MYOF, CTNNA1, PIAS3, PPL, EMP1, ECM1, ETV3, TPM4, TRIM16, IQGAP1, SERPINB8, LAMC2) with p-values less than 0.05. We observed that the majority of LAMC2-associated genes were altered in LOVO cells upon F. nucleatum infection in the GSE173549 dataset (Supplementary Fig. 5A). Furthermore, transcriptomic data of F. nucleatum-infected LOVO cells from our own laboratory also confirmed that most LAMC2-associated genes were influenced by F. nucleatum infection (Supplementary Fig. 5B). These genes, along with their regression coefficients, were used to construct the LAMC2-related signature referring to our previous publication29.
The Kaplan-Meier analysis revealed that patients with high LAMC2-related signature had poorer prognosis in the TCGA COAD, GSE17536, and GEO combined datasets (Fig. 7A-C). ROC analysis indicated that the predictive efficacy of LAMC2-related signature combined with tumor staging for patient survival was significantly better than that of tumor staging (Fig. 7D-F). The C-index analysis also demonstrated that the predictive efficacy of LAMC2-related signature combined with tumor staging for survival was significantly higher than tumor staging alone (Fig. 7G). Additonally, LAMC2-related signature provided prognostic value beyond MSI status as revealed by ROC analysis (Supplementary Fig. 6). Subgroup analysis showed that the LAMC2-related signature was a risk factor (HR > 1) within subgroups of age, gender, and tumor staging (Fig. 7H). Furthermore, multivariate Cox regression analysis of these clinical parameters indicated that the LAMC2-related signature, age, and tumor staging were still robust risk factors after adjusting for age, gender, and tumor staging (Fig. 7I). For clinical applicability, we constructed a nomogram using age, tumor staging, and LAMC2-related signature to predict patient survival at 1, 3, and 5 years (Fig. 7J). Collectively, these results suggested LAMC2-related signature was a promising predictor for CRC prognosis. A schematic diagram illustrates the mechanisms by which F. nucleatum promotes CRC cell metastasis (Fig. 8).
Construction of prognostic model based on LAMC2-related genes. (A-C) Kaplan–Meier plots exhibit the prognosis between CRC patients with low and high level of LAMC2 in the GEO-combined, TCGA-COAD, and GSE17536 datasets (time unit: months, the log-rank test). (D-F) Area under the ROC curve assessing the predictive efficacy of LAMC2-related signature + Stage for overall survival. (G) C-index assessing the predictive efficacy of LAMC2-related signature + Stage. (H) Assessment of LAMC2-related signature as a risk factor in different subgroups. (I) Multivariate Cox regression analysis of LAMC2-related signature after incorporating different clinical parameters. (J) Construction of a nomogram using age, tumor stage, and LAMC2-related signature for predicting patient survival at 1, 3, and 5 years.
Discussion
Tumor metastasis is one of the hallmark malignant features of CRC and a major contributor to poor patient prognosis46. F. nucleatum plays a crucial role in promoting CRC metastasis. Studies have shown that F. nucleatum levels are higher in the tissues and fecal samples of patients with advanced CRC (T1b–T3) compared to those with early-stage CRC (Tis and T1a)47. Notably, F. nucleatum abundance is positively correlated with lymph node metastasis in CRC patients48. Understanding the mechanisms by which F. nucleatum drives CRC metastasis is essential for improving CRC treatment and prognosis.
LAMC2, a key component of the heterotrimeric glycoprotein laminin-332, regulates cell adhesion, differentiation, migration, and invasion24,25,26. In this study, we identified that F. nucleatum infection significantly upregulates LAMC2 expression in CRC cells, and that LAMC2 plays a critical role in F. nucleatum-induced CRC metastasis, which are uniquely discovered. Additionally, we found that LAMC2 is highly expressed in CRC tissues and is associated with poor patient prognosis. At present, we have only demonstrated that LAMC2 is up-regulated in clinical CRC tissues by immunohistochemistry. Elucidating the associations among F. nucleatum abundance, LAMC2 expression, miRNA profiles, and downstream signaling cascades in clinical specimens is essential for enhancing the translational value of our study. Future work will therefore systematically delineate the relationship between F. nucleatum and LAMC2 within well-annotated patient cohorts. Mechanistic investigations revealed for the first time that F. nucleatum regulated LAMC2 expression by suppressing hsa-miRNA-7977. However, the molecular mechanism underlying this regulation remains insufficiently explored. F. nucleatum modulates host miRNA expression through multiple pathways, thereby driving the progression of CRC. Yang et al. demonstrated that F. nucleatum activates the Toll-like receptor 4 (TLR4)/MYD88 signaling axis, induces nuclear factor-κB (NF-κB) activation, and upregulates miR-21 expression, thereby promoting the development of CRC in mice37. At the epigenetic level, Xu et al. confirmed that F. nucleatum facilitates pri-miR-4717 maturation by METTL3-dependent m⁶A modification, and then positively regulates CRC cell proliferation49. In addition, studies have shown that gut microbiota-derived metabolites (e.g., short-chain fatty acids, bile acid derivatives) and structural components (e.g., lipopolysaccharides, peptidoglycans) can directly or indirectly affect host epigenetic regulatory mechanisms, including the expression of noncoding RNAs (such as miRNAs and lncRNAs) and histone acetylation levels50. These mechanisms provide new insights for future research into F. nucleatum-induced regulation of hsa-miRNA-7977.
It has been known that LAMC2 activates the FAK–PI3K–AKT signaling pathway21. Here, we demonstrate that F. nucleatum can also trigger this LAMC2-mediated FAK–PI3K–AKT signaling axis, thereby promoting CRC cell metastasis. Notably, growing evidence suggests that various TCA cycle metabolites are essential for epigenetic modifications, such as histone acetylation40,41,42. RNA sequencing results indicate that LAMC2 influences the TCA cycle. To determine whether F. nucleatum-induced LAMC2 upregulation further affects histone acetylation, we examined H3K27ac expression. Our findings show that LAMC2 knockdown reduces H3K27ac levels in LOVO cells, suggesting a potential role of epigenetic regulation in LAMC2-mediated CRC metastasis. A schematic diagram illustrates the potential mechanism by which F. nucleatum promotes CRC cell metastasis (Fig. 8).
RNA sequencing of multiple cohorts revealed that LAMC2 is highly expressed in CRC liver and lung metastases and is strongly correlated with the CMS4 (mesenchymal) molecular subtype, which is characterized by high invasive and metastatic potential43. We hypothesize that upregulation of LAMC2 induced by F. nucleatum may be a key molecule leading to the poor prognosis of CMS4 patients and may serve as a target for improving the prognosis of CMS4 patients. Additionally, elevated LAMC2 expression in CRC patients was strongly associated with markedly inferior OS, DFS, DSS, RFS, and PFS. Moreover, subgroup analysis showed that the LAMC2-related signature was a significant risk factor (HR > 1) within subgroups of age, gender, and tumor staging. Integrating LAMC2-related signature with tumor stage significantly improved the predictive accuracy of overall survival in CRC patients, highlighting its potential clinical utility.
However, our study still has some limitations. Although the correlation between LAMC2 and epigenetic modifications and immune function has been identified, a comprehensive understanding of the specific regulatory mechanisms is still lacking. While our data demonstrate that LAMC2 knockdown reduces H3K27ac levels, the upstream regulatory mechanisms remain to be fully elucidated. It is possible that LAMC2 influences histone acetylation through modulation of histone acetyltransferases (HATs) or by affecting nuclear metabolite availability, such as acetyl-CoA, produced via the nuclear TCA cycle. Additionally, it remains unclear whether LAMC2 regulates gene expression by influencing the activity or expression of H3K27ac-modifying enzymes. Therefore, future research should focus on elucidating the detailed roles of LAMC2 in epigenetic modifications and immune function, thereby influencing the development and treatment of CRC.
Overall, our study provides insights into the mechanisms underlying the metastasis of CRC. And we, for the first time, identified that F. nucleatum infection significantly upregulates LAMC2 expression to promote CRC metastasis. F. nucleatum and LAMC2 may emerge as potential therapeutic targets for inhibiting CRC metastasis. Moreover, our study implicates histone acetylation as a potential post-translational modification underlying the F. nucleatum–LAMC2-CRC metastasis.This study will further expand our knowledge in this field and provide more effective strategies for the clinical treatment of CRC.
Conclusion
Our study demonstrates that F. nucleatum promotes CRC metastasis by upregulating LAMC2 expression. F. nucleatum and LAMC2 hold therapeutic potential for inhibiting CRC metastasis.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Van Cutsem, E., Cervantes, A., Nordlinger, B. & Arnold, D. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals Oncology: Official J. Eur. Soc. Med. Oncol. 25 (Suppl 3), iii1–9 (2014).
Dahan, L., Sadok, A., Formento, J. L., Seitz, J. F. & Kovacic, H. Modulation of cellular redox state underlies antagonism between oxaliplatin and cetuximab in human colorectal cancer cell lines. Br. J. Pharmacol. 158, 610–620 (2009).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Fu, A. et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 185, 1356–1372e1326 (2022).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell. Host Microbe. 14, 207–215 (2013).
Qu, R. et al. Role of the gut microbiota and Its metabolites in tumorigenesis or development of colorectal cancer. Adv. Sci. (Weinheim, Baden-Wurttemberg, Germany) 10 e2205563, (2023).
Karpiński, T. M., Ożarowski, M. & Stasiewicz, M. Carcinogenic microbiota and its role in colorectal cancer development. Sem. Cancer Biol. 86, 420–430 (2022).
Brennan, C. A. & Garrett, W. S. Fusobacterium nucleatum - symbiont, opportunist and Oncobacterium. Nat. Rev. Microbiol. 17, 156–166 (2019).
Ou, S. et al. Fusobacterium nucleatum and colorectal cancer: from phenomenon to mechanism. Front. Cell. Infect. Microbiol. 12, 1020583 (2022).
Ou, S. et al. Fusobacterium nucleatum upregulates MMP7 to promote metastasis-related characteristics of colorectal cancer cell via activating MAPK(JNK)-AP1 axis. J. Translational Med. 21, 704 (2023).
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980 (2016).
Zhang, Y. et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis. Gut Microbes. 14, 2038852 (2022).
Chen, S. et al. Fusobacterium nucleatum reduces METTL3-mediated m(6)A modification and contributes to colorectal cancer metastasis. Nat. Commun. 13, 1248 (2022).
Guo, S. et al. Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut, (2020).
Yu, M. R., Kim, H. J. & Park, H. R. Fusobacterium nucleatum accelerates the progression of colitis-associated colorectal cancer by promoting EMT. Cancers 12, (2020).
Xu, C. et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes. 13, 1980347 (2021).
Garg, M. et al. Laminin-5γ-2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration, and invasion by modulating signaling of EGFR. J. clinical Endocrinol. Metab. 99 E62-72, (2014).
Colognato, H. & Yurchenco, P. D. Form and function: the laminin family of heterotrimers. Dev. Dynamics: Official Publication Am. Association Anatomists. 218, 213–234 (2000).
Miyazaki, K. Laminin-5 (laminin-332): unique biological activity and role in tumor growth and invasion. Cancer Sci. 97, 91–98 (2006).
Xu, L. et al. Nuclear drosha enhances cell invasion via an EGFR-ERK1/2-MMP7 signaling pathway induced by dysregulated miRNA-622/197 and their targets LAMC2 and CD82 in gastric cancer. Cell Death Dis. 8, e2642 (2017).
Liang, Y. et al. LncRNA CASC9 promotes esophageal squamous cell carcinoma metastasis through upregulating LAMC2 expression by interacting with the CREB-binding protein. Cell Death Differ. 25, 1980–1995 (2018).
Erice, O. et al. LAMC2 regulates key transcriptional and targetable effectors to support pancreatic cancer growth. Clin. Cancer Research: Official J. Am. Association Cancer Res. 29, 1137–1154 (2023).
Kirtonia, A. et al. Overexpression of laminin-5 gamma-2 promotes tumorigenesis of pancreatic ductal adenocarcinoma through EGFR/ERK1/2/AKT/mTOR cascade. Cell. Mol. Life Sci. 79, 362 (2022).
Lorusso, G. et al. Connexins orchestrate progression of breast cancer metastasis to the brain by promoting FAK activation. Sci. Transl. Med. 14, eaax8933 (2022).
Kamal, M. A. et al. Oncogenic KRAS-Induced protein signature in the tumor secretome identifies Laminin-C2 and Pentraxin-3 as useful biomarkers for the early diagnosis of pancreatic cancer. Cancers 14, (2022).
Islam, S. et al. ITGA2, LAMB3, and LAMC2 May be the potential therapeutic targets in pancreatic ductal adenocarcinoma: an integrated bioinformatics analysis. Sci. Rep. 11, 10563 (2021).
Navarro Gonzalez, J. et al. The UCSC genome browser database: 2021 update. Nucleic Acids Res. 49, D1046–d1057 (2021).
Wang, H. et al. Tumor microenvironment Heterogeneity-Based score system predicts clinical prognosis and response to immune checkpoint Blockade in multiple colorectal cancer cohorts. Front. Mol. Biosci. 9, 884839 (2022).
Wang, H. et al. Characterization of Endoplasmic reticulum stress unveils ZNF703 as a promising target for colorectal cancer immunotherapy. J. Translational Med. 21, 713 (2023).
Lee, H. O. et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 52, 594–603 (2020).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. EdgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinf. (Oxford England). 26, 139–140 (2010).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. Omics: J. Integr. Biology. 16, 284–287 (2012).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005).
Chen, Y. & Wang, X. miRDB: an online database for prediction of functional microRNA targets. Nucleic acids Res. 48 D127-d131, (2020).
Agarwal, V., Bell, G. W., Nam, J. W. & Bartel, D. P. Predicting effective MicroRNA target sites in mammalian mRNAs. eLife 4, (2015).
Tokar, T. et al. MirDIP 4.1-integrative database of human MicroRNA target predictions. Nucleic Acids Res. 46, D360–d370 (2018).
Yang, Y. et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating Toll-Like receptor 4 signaling to nuclear Factor-κB, and Up-regulating expression of MicroRNA-21. Gastroenterology 152, 851–866e824 (2017).
Galeano Niño, J. L. et al. Effect of the intratumoral microbiota on Spatial and cellular heterogeneity in cancer. Nature 611, 810–817 (2022).
Niu, L. et al. Biological implications and clinical potential of Metastasis-Related MiRNA in colorectal Cancer. Molecular therapy. Nucleic Acids. 23, 42–54 (2021).
Li, W. et al. Nuclear localization of mitochondrial TCA cycle enzymes modulates pluripotency via histone acetylation. Nat. Commun. 13, 7414 (2022).
Lauterbach, M. A. et al. Toll-like receptor signaling rewires macrophage metabolism and promotes histone acetylation via ATP-Citrate lyase. Immunity 51, 997–1011e1017 (2019).
Nagaraj, R. et al. Nuclear localization of mitochondrial TCA cycle enzymes as a critical step in mammalian zygotic genome activation. Cell 168, 210–223e211 (2017).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Salvucci, M. et al. Patients with mesenchymal tumours and high fusobacteriales prevalence have worse prognosis in colorectal cancer (CRC). Gut 71, 1600–1612 (2022).
Eide, P. W., Bruun, J., Lothe, R. A. & Sveen, A. CMScaller: an R package for consensus molecular subtyping of colorectal cancer pre-clinical models. Sci. Rep. 7, 16618 (2017).
Wong, S. H. & Yu, J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 16, 690–704 (2019).
Zorron, C. T. et al. Microbiota profile is different for early and invasive colorectal cancer and is consistent throughout the colon. J. Gastroenterol. Hepatol. 35, 433–437 (2020).
Li, Y. Y. et al. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J. Gastroenterol. 22, 3227–3233 (2016).
Xu, Q. et al. Fusobacterium nucleatum induces excess methyltransferase-like 3-mediated microRNA-4717-3p maturation to promote colorectal cancer cell proliferation. Cancer Sci. 113, 3787–3800 (2022).
Zhang, Q. et al. Implications of gut microbiota-mediated epigenetic modifications in intestinal diseases. Gut Microbes. 17, 2508426 (2025).
Funding
This work was supported by National Natural Science Foundation of China (No. 81872034), Natural Science Foundation of Heilongjiang Province (No. H2017016), Heilongjiang Natural Science Foundation of China (No. LH2020H120), Haiyan Research Fund of Harbin Medical University Cancer Hospital (No. JJZD2020-04), Wu Jieping Medical Foundation (No. 320.6750.19092-41), Chen Xiao-ping Foundation for The Development of Science and Technology of Hubei Province (No. CXPJJH12000002-2020025), Scientific Research Foundation of Heilongjiang Provincial Health and Family Planning Commission (No. 2018249), Beijing Medical Foundation (No. MDK2022-1001).
Author information
Authors and Affiliations
Contributions
All authors contributed to this study. Songlin Ran was responsible for conceptualization, experimental design, implementation of experiments, and manuscript writing. Jinhua Ye curated the data and performed visualization. Hufei Wang conducted bioinformatics analysis, while Fangzhou Liu performed the animal experiments. Suwen Ou contributed to formal analysis, and Zhiyong Chen reviewed the manuscript. Yanni Song edited the original draft, and Shulin Liu reviewed and revised the manuscript. Rui Huang contributed to project administration, validation, and funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All the authors agree for the publication.
Patient consent for publication
The study involving human participants was subject to rigorous scrutiny and was granted approval by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (Approval No. YJSKY2022-182). Written informed consent was obtained from all participants, who explicitly confirmed their voluntary involvement in this research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ran, S., Ye, J., Wang, H. et al. Single-cell and bulk transcriptomics reveal Fusobacterium nucleatum promotes colorectal cancer metastasis by upregulating LAMC2 in in vitro and in vivo models. Sci Rep 15, 42100 (2025). https://doi.org/10.1038/s41598-025-26249-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26249-w