Abstract
The relationship between glycemic variability (GV) and mortality in critically ill trauma patients remains unclear. We evaluated whether GV, quantified by the coefficient of variation (CV) of glucose during the ICU stay, is associated with short- and long-term mortality in this population. Using the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, we identified 4,009 adult trauma patients admitted to the intensive care unit and assessed the association between CV and 30-day and 1-year mortality with Cox proportional hazards models, Kaplan–Meier analysis, restricted cubic splines, subgroup analyses, and mediation models to explore the potential mediating role of sepsis. Higher GV was independently associated with increased 30-day (adjusted HR 1.50, P < 0.001) and 1-year mortality (adjusted HR 1.29, P < 0.001). Nonlinear analyses showed a J-shaped relationship between CV and mortality, with risk rising steeply above a CV of approximately 12.2%, particularly in younger patients and those without diabetes. Mediation analyses suggested that sepsis accounted for about 50.7% of the association with 30-day mortality and 70.5% with 1-year mortality. In critically ill trauma patients, higher GV is independently associated with both short- and long-term mortality, with an apparent threshold effect at mid-teen CV values, and sepsis appears to be a key pathway linking GV to mortality, although these mediation findings should be interpreted as exploratory and hypothesis-generating.
Similar content being viewed by others

Introduction
Trauma remains a major contributor to global morbidity and mortality. It causes more than five million deaths each year and is the leading cause of death among people younger than 45 years1. Critically injured patients who require admission to an intensive care unit (ICU) experience particularly high case-fatality rates, owing to the interplay of hemodynamic instability, systemic inflammation and multi-organ dysfunction2. Although modern pre-hospital systems, damage-control resuscitation and advanced critical care techniques have improved short-term survival, overall mortality in severe trauma has plateaued3. Furthermore, trauma patients have persistently elevated long-term mortality rates that exceed those of age-matched populations. This suggests that acute care improvements have not fully addressed the broader impact of traumatic injury on survival4. This underscores the need to identify modifiable physiological targets that could further enhance outcomes5.
Glycemic variability (GV)—the degree of fluctuation in blood-glucose concentrations over time—has emerged as one such target. In the critically ill, sympathetic over-activation, counter-regulatory hormone surges (glucagon, catecholamines, growth hormone, cortisol) and stress-induced insulin resistance combine to destabilize glucose homeostasis6,7. Among the several indices proposed to quantify GV, the coefficient of variation (CV) is favored for its simplicity, unit-free nature and proven prognostic value8,9.
Accumulating evidence suggests that GV is more deleterious than sustained hyperglycemia alone. Across diverse ICU cohorts—sepsis, heart failure, stroke and acute kidney injury—high GV independently predicts mortality, nosocomial infection and organ failure10,11,12,13. Mechanistic studies indicate that oscillating glucose levels amplify endothelial dysfunction, oxidative stress and inflammatory cytokine release beyond that seen with stable hyperglycemia14. Clinically, greater GV has also been linked to malignant arrhythmias, septic complications and progression to multi-organ failure15.
Whether these observations hold true in trauma, however, remains unclear. The metabolic milieu of trauma is unique, characterized by massive catecholamine surges, acute blood-loss anemia, hyper-coagulability and a pronounced systemic inflammatory response that may alter glucose handling relative to other critical-illness states16,17. In addition, major tissue injury, hemorrhagic shock, transfusion, and repeated operative interventions lead to large fluctuations in hemodynamics, nutritional delivery, and insulin requirements. Together, these factors may amplify glycemic variability beyond that observed in non-trauma ICU cohorts. Hyperglycemia in trauma patients appears to be “an entity of its own” with distinct pathophysiology compared with other ICU populations18. Nevertheless, current glycemic-control recommendations for trauma patients are largely extrapolated from mixed ICU populations, despite the wide heterogeneity of injury patterns, resuscitation strategies, and operative interventions in trauma care19. Whether GV provides trauma-specific prognostic information in this setting has not been systematically evaluated.
Against this backdrop, we undertook a retrospective cohort study using the Medical Information Mart for Intensive Care IV (MIMIC-IV) database. Our aim was to evaluate the relationship between GV and both short-term (30-day) and long-term (1-year) all-cause mortality in adult ICU trauma patients. We hypothesized that higher CV would be independently associated with increased mortality and that a portion of this risk would be mediated through downstream complications such as sepsis. Clarifying these associations could inform evidence-based glucose-management protocols and help clinicians identify high-risk trauma patients who may benefit from tighter glycemic monitoring and targeted intervention.
Results
Baseline characteristics
The final cohort consisted of 4,009 critically ill trauma patients (mean age ± SD, 61.84 ± 21.75 years), of whom 2,529 (63.08%) were male. Overall, 616 (15.37%) patients died within 30 days and 937 (23.37%) died within 1 year of ICU admission. Patients were stratified into quartiles based on their glucose coefficient of variation (CV) (Q1: n = 1,005; Q2: n = 1,001; Q3: n = 1,001; Q4: n = 1,002). As detailed in Table 1 and Supplementary Table S1, baseline characteristics differed markedly across these CV quartiles.
Compared with Q1, individuals in Q4 were older (65.59 vs. 58.16 years) and displayed greater physiological derangement, reflected by higher mean scores for OASIS (32.73 vs. 29.43), median SOFA (5.00 [IQR 3.00–7.00] vs. 3.00 [IQR 2.00–5.00]) and Charlson Comorbidity Index (4.22 vs. 2.71) (all P < 0.001). The highest-CV quartile also had lower arterial pressures, lower hemoglobin concentrations (10.16 vs. 10.92 g/dL), and worse renal biochemistry—creatinine 1.10 mg/dL (IQR 0.80–1.50) versus 0.90 mg/dL (IQR 0.80–1.10) and BUN 21.00 mg/dL (IQR 15.00–30.00) versus 16.00 mg/dL (IQR 12.00–22.00) (all P < 0.001).
Comorbid illness was more prevalent in Q4, particularly diabetes mellitus (37.62% vs. 8.06%), myocardial infarction (10.18% vs. 4.88%) and congestive heart failure (18.36% vs. 9.45%). Correspondingly, high-CV patients required more intensive therapeutic support: vasoactive drugs (31.64% vs. 11.44%), insulin therapy (37.33% vs. 19.60%) and blood transfusion (39.52% vs. 22.89%) (all P < 0.001). Complication rates followed a similar pattern, with sepsis present in 52.59% of Q4 versus 29.35% of Q1 and acute kidney injury in 78.94% versus 61.00% (both P < 0.001).
Mortality increased stepwise across quartiles: 30-day death rose from 9.65% in Q1 to 22.75% in Q4, and 1-year death from 16.02% to 31.44% (P < 0.001 for trend), underscoring a strong unadjusted association between higher glycemic variability and adverse outcomes.
The relationship between glycemic variability and mortality
Cox proportional-hazards models confirmed that higher glycemic variability was independently associated with both early and late death (Table 2). In the crude model, each one-unit increase in log-transformed CV doubled the hazard of 30-day mortality (HR 2.07, 95% CI 1.77–2.43; P < 0.001) and raised the hazard of 1-year mortality by 79% (HR 1.79, 95% CI 1.57–2.03; P < 0.001). After comprehensive adjustment for demographic factors, physiology, comorbidities, laboratory indices, severity scores, trauma burden, and therapeutic interventions, the associations remained robust: HR 1.50 (95% CI 1.24–1.83; P < 0.001) for 30-day death and HR 1.29 (95% CI 1.11–1.49; P < 0.001) for 1-year death.
Treating CV as a categorical variable revealed a clear dose–response pattern. Relative to the lowest quartile (Q1), fully adjusted hazards for 30-day death were 0.80 (95% CI 0.61–1.05) in Q2, 0.90 (95% CI 0.69–1.17) in Q3 and 1.33 (95% CI 1.02–1.73) in Q4 (P for trend = 0.002). For 1-year death, the corresponding HRs were 0.88 (95% CI 0.71–1.09) in Q2, 1.01 (95% CI 0.82–1.24) in Q3 and 1.25 (95% CI 1.02–1.54) in Q4, respectively (P for trend = 0.004) (Fig. 1).
Kaplan–Meier curves stratified by quartile corroborated these findings (log-rank P < 0.001 for both time horizons). Survival probabilities diverged early and remained separated, with the greatest gap between Q1 and Q4 (Fig. 2).
Model discrimination improved markedly with covariate adjustment. For 30-day death, the area under the ROC curve (AUC) increased from 0.614 (95% CI 0.516–0.712) in the unadjusted model to 0.845 (95% CI 0.747–0.943) in the fully adjusted model - a 37.6% relative gain (Supplementary Fig. S2A). For 1-year death, the AUC rose from 0.597 (95% CI 0.499–0.695) to 0.831 (95% CI 0.733–0.929), representing a 39.4% improvement (Supplementary Fig. S2B).
Restricted cubic-spline and threshold analyses
To investigate the shape of the association between glycemic variability and mortality, we fitted a series of multivariable restricted cubic splines (RCS) with progressively greater adjustment (Supplementary Fig. S3). Interestingly, the J-shaped relationship only became evident after comprehensive multivariable adjustment. In the crude and partially adjusted models (Models 1–3), the association appeared consistent with linearity (all P for non-linearity > 0.05). However, in the fully adjusted model (Model 4), a significant non-linear, J-shaped relationship emerged for both 30-day (P for non-linearity = 0.004) and 1-year mortality (P for non-linearity = 0.010) (Fig. 3). The spline curves revealed that mortality risk was lowest at a log(CV) of 2.498, corresponding to a CV of approximately 12.2%, and increased sharply at values above this nadir. In exploratory stratified spline analyses by diabetes status, injury pattern, and tertiles of glucose-sampling intensity, a similar J-shaped pattern with nadirs in the mid-teens was most apparent among non-diabetic patients, those with multiple trauma, and patients with high sampling intensity. In contrast, among patients with diabetes, isolated TBI, or lower sampling intensity, the non-linear component was not statistically significant and the estimated inflection points were unstable (Supplementary Fig. S4).
Dose-response association of log(CV) with 30-day and 1-year mortality in critically ill trauma patients, analyzed using restricted cubic splines (RCS). The RCS models used 4 knots, placed at the 5th, 35th, 65th, and 95th percentiles of the log(CV) distribution. The models were adjusted for demographics, vital signs, laboratory results, comorbidities, severity scores, trauma variables, and treatments. Reference values for estimation were set at the inflection point of lowest risk. The solid lines show adjusted hazard ratios, shaded areas indicate 95% confidence intervals, and the vertical dashed line indicates the inflection point. (A) RCS curve for 30-day mortality. (B) RCS curve for 1-year mortality.
Moreover, based on the nadir identified by the RCS analysis, we formally tested this threshold effect using a pre-specified two-piecewise Cox regression model with a knot at a CV of 12.2% (Table 3). For 30-day mortality, no significant association was found below this threshold (HR 0.82, 95% CI 0.50–1.33; P = 0.420). However, above a CV of 12.2%, higher glycemic variability was strongly associated with increased mortality (HR 1.75, 95% CI 1.39–2.21; P < 0.001). A nearly identical pattern was observed for 1-year mortality, with a significant increase in risk only above the 12.2% threshold (HR 1.49, 95% CI 1.24–1.80; P < 0.001), while the association below it was non-significant (HR 0.83, 95% CI 0.59–1.17; P = 0.294). The likelihood ratio test confirmed that this two-piecewise model provided a significantly better fit than a standard linear model for both 30-day (P = 0.019) and 1-year mortality (P = 0.013), supporting the presence of a distinct threshold effect at the population level.
Subgroup analyses
Prespecified subgroup analyses assessed whether the GV–mortality association differed across twelve clinically relevant strata (Fig. 4). Subgroups were defined by demographics (age < 60 vs. ≥ 60 years; sex), injury profile (traumatic brain injury, multiple trauma, ISS ≥ 16), baseline health status (diabetes mellitus, hypoglycemia, Charlson Comorbidity Index ≥ 3), critical-care interventions (mechanical ventilation, vasoactive-drug infusion, blood transfusion) and complications, including Sepsis-3–defined sepsis.
Subgroup analyses of the association between glycemic variability and mortality in critically ill trauma patients. Forest plots display the adjusted hazard ratios (HRs) for 30-day (A) and 1-year (B) all-cause mortality. The HRs and 95% confidence intervals (CIs) represent the risk associated with a one-unit increase in the log-transformed coefficient of variation (log(CV)). The P for interaction was calculated to assess for significant effect modification across the strata of each subgroup. TBI (traumatic brain injury), ISS (Injury Severity Score).
For 30-day mortality, significant effect modification was detected for age, diabetes, hypoglycemia, Charlson score and vasoactive-drug use. The impact of glycemic variability was more than three-fold stronger in younger patients (HR 4.18, 95% CI 2.98–5.87) than in those ≥ 60 years (HR 1.53, 95% CI 1.28–1.83; P < 0.001 for interaction). A larger effect was also observed in participants without diabetes (HR 2.31, 95% CI 1.92–2.77) versus those with diabetes (HR 1.35, 95% CI 0.95–1.90; P = 0.007) and in patients who experienced hypoglycemia (HR 3.23, 95% CI 1.91–5.45 vs. HR 1.86, 95% CI 1.57–2.21; P = 0.047). Lower Charlson scores and vasoactive-drug therapy likewise intensified risk (both P < 0.001). For sepsis, log(CV) was associated with higher 30-day mortality in both non-septic and septic trauma patients (HR 1.66, 95% CI 1.32–2.09 and 2.29, 95% CI 1.84–2.87, respectively), with a borderline P for interaction of 0.052.
For 1-year mortality, the interaction pattern persisted. Log(CV) conferred a greater hazard in patients < 60 years (HR 3.19, 95% CI 2.35–4.32) than in older individuals (HR 1.37, 95% CI 1.19–1.58; P < 0.001) and in those receiving vasoactive drugs (HR 2.52, 95% CI 1.96–3.25 vs. HR 1.41, 95% CI 1.22–1.64; P < 0.001). A lower comorbidity burden (Charlson < 3) was paradoxically associated with a steeper gradient (P < 0.001). When stratified by sepsis status, log(CV) remained associated with 1-year mortality in both non-septic and septic trauma patients (HR 1.49, 95% CI 1.25–1.78 and 1.95, 95% CI 1.62–2.35, respectively; P for interaction = 0.040). No significant interactions were noted for sex, mechanical ventilation, transfusion, TBI, multiple trauma or ISS ≥ 16.
Mediation analyses
To probe pathways linking glycemic variability to death, we performed a bootstrap-based mediation analysis with sepsis and acute kidney injury (AKI) as potential ICU-acquired mediators of the association between log(CV) and mortality (Supplementary Table S2 and Fig. 5). After adjustment for all prespecified covariates, sepsis accounted for 50.7% of the total effect on 30-day mortality and 70.5% of the effect on 1-year mortality (both P < 0.001), whereas the mediating role of AKI was smaller but directionally similar. Because GV was summarized from admission-anchored ICU windows and sepsis and AKI were diagnosed during the ICU stay, most variability is expected to precede but not invariably occur before these complications. Accordingly, these mediation estimates should be viewed as exploratory and as consistent with, rather than proof of, a pathway in which GV contributes to sepsis-related organ dysfunction and death.
Path diagrams illustrating the mediating effect of sepsis on the association between glycemic variability and mortality. Glycemic variability was quantified as the log-transformed coefficient of variation (log(CV)). Panel (A) shows the model for 30-day mortality, and Panel (B) shows the model for 1-year mortality. All effects are presented as Hazard Ratios (HRs) with their corresponding 95% confidence intervals (CIs) and P-values, derived from the multivariable-adjusted Cox mediation model.
Sensitivity analyses
Multiple sensitivity checks corroborated the primary findings. First, we recalculated the coefficient of variation over five admission-anchored ICU windows—the first 3, 7, 14, and 21 days, as well as the entire hospital stay—and re-ran the fully adjusted Cox model. As shown in Fig. 6, the association between log(CV) and mortality was consistent across all intervals. It was already evident when GV was quantified using only the first 3 ICU days (approximately 0–72 h). For 30-day death, adjusted hazard ratios (HRs) ranged from 1.40 (95% CI 1.18–1.67) to 1.49 (1.23–1.81); for 1-year death, HRs ranged from 1.22 (1.07–1.40) to 1.29 (1.11–1.49). Every estimate remained statistically significant (P ≤ 0.003), indicating that the prognostic value of glycemic variability is not driven by a particular sampling frame or restricted to a late ICU phase.
Sensitivity analysis of the association between glycemic variability and mortality in critically ill trauma patients, using different time windows. The plots show the fully adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for 30-day (A) and 1-year (B) all-cause mortality. The coefficient of variation (CV) was calculated over the first 3, 7, 14, and 21 days of the ICU stay, as well as for the entire stay.
Second, we evaluated three data-handling strategies—complete-case analysis, multiple imputation, and exclusion of extreme CV values (n = 36, 0.9%)—using the same covariate set. Effect estimates changed little: for 30-day death, HRs varied between 1.44 and 1.50; for 1-year death, between 1.22 and 1.29. Quartile-based trend tests remained significant throughout. Together, these analyses show that the observed CV–mortality relationship is robust to alternative exposure definitions, missing-data procedures, and outlier treatment.
Third, to address potential measurement frequency bias, we adjusted for glucose sampling frequency in addition to all Model 4 covariates. The association between glycemic variability and mortality remained statistically significant for both 30-day mortality (adjusted HR 1.35, 95% CI 1.11–1.65, P = 0.003) and 1-year mortality (adjusted HR 1.19, 95% CI 1.03–1.39, P = 0.023), though somewhat attenuated (Supplementary Table S3). Sampling frequency itself was independently associated with mortality (HR 1.09 per measurement/day, P < 0.001), indicating it serves as a marker of illness severity.
Discussion
In this retrospective study using the MIMIC-IV database, we found that glycemic variability during the ICU stay was independently associated with both short- and long-term mortality in critically ill trauma patients. Among 4,009 patients, we observed a robust, nonlinear J-shaped relationship between the coefficient of variation (CV) of glucose and mortality, with relatively stable risk at lower CV values and progressively higher risk at greater variability. Restricted cubic spline models suggested an approximate population-level nadir around a CV of 12.2% for both 30-day and 1-year mortality, beyond which mortality increased more steeply. Subgroup analyses further indicated that younger patients (< 60 years), those without diabetes, and individuals receiving vasoactive support were particularly susceptible to the adverse associations between higher GV and mortality. In exploratory mediation analyses, sepsis emerged as a major pathway through which GV was linked to mortality.
To our knowledge, this is the largest dedicated analysis of glycemic variability in a general trauma ICU population, and it addresses an important gap in a literature largely focused on mixed medical–surgical or purely septic cohorts. Trauma patients exhibit distinct metabolic characteristics compared with general critically ill patients. Major tissue injury, hemorrhagic shock, transfusion, and repeated operative interventions trigger massive catecholamine release, cortisol elevation, and cytokine activation, creating a highly hypermetabolic and hemodynamically unstable state6,20. In patients with shock, plasma epinephrine and norepinephrine concentrations can increase up to 50-fold and 10-fold, respectively, and cortisol output may rise ten-fold with severe stress21,22. These abrupt physiological perturbations, superimposed on trauma-specific interventions, are likely to produce more pronounced and rapid glucose oscillations than those typically observed in non-trauma ICU populations and may help explain why we observed apparent GV thresholds at relatively low CV values, which should be interpreted at the population level rather than as strict treatment targets23,24,25,26.
When we compare our findings to previous research in mixed ICU populations, we observe both similarities and important differences. Earlier studies have consistently demonstrated associations between glycemic variability and mortality, though the magnitude of risk and optimal thresholds have varied considerably. Krinsley, for instance, reported that patients with CV > 20% had significantly higher mortality rates in a mixed ICU population27. Similarly, studies in septic patients identified CV > 31.5% as cutoff points for increased mortality risk28. What is noteworthy about our results is that the CV threshold we identified (12.2%) is considerably lower than what has been reported in mixed or septic populations. In stratified spline analyses, a similar J-shaped pattern with nadirs in the mid-teens was most clearly expressed in non-diabetic patients, those with multiple trauma, and patients with high glucose-sampling intensity, whereas in patients with diabetes or less intensive monitoring the curves were flatter and the estimated inflection points were imprecise. This suggests that trauma patients as a group may be more sensitive to glycemic fluctuations than previously recognized. However, subgroup-specific thresholds should be interpreted cautiously rather than as a single universal cutoff. The J-shaped relationship we observed is consistent with previous findings in various ICU populations29, indicating that mortality risk remains relatively stable at lower CV values but increases sharply beyond specific thresholds.
Our mediation analysis provides insights into the pathophysiological pathways, though we acknowledge some uncertainty remains regarding the underlying mechanisms. The literature indicates that glucose oscillations generate reactive oxygen species more potently than sustained hyperglycemia30. We suspect this oxidative stress burden may be particularly detrimental in trauma patients who are already managing significant inflammatory challenges. The finding that sepsis mediated 50.7% of the 30-day mortality association and an even more substantial 70.5% of the 1-year mortality association suggests that glycemic variability may predispose patients to infectious complications. Higher GV is independently associated with increased mortality in sepsis patients, even after adjusting for illness severity and hypoglycemia31. A meta-analysis of 10 studies further confirms that septic patients with higher GV have significantly higher mortality, with immune dysfunction, such as impaired phagocytosis, as a proposed mechanism32. Glucose fluctuations impair neutrophil and macrophage function, increasing susceptibility to bacterial infections, including nosocomial infections like ventilator-associated pneumonia33,34. Does glycemic variability precipitate sepsis through immune dysfunction, or does incipient sepsis drive glucose instability—or both? Given the retrospective design and the likely bidirectional interplay between sepsis and GV, our mediation analysis cannot definitively establish temporal ordering. Some of the estimated “indirect” effect may also reflect sepsis-induced glycemic instability. Nevertheless, in our data elevated CV typically preceded sepsis diagnosis. The adverse association between GV and mortality was already apparent when GV was restricted to early ICU windows, suggesting that variability may be more than simply a passive marker of illness severity13. Moreover, the persistence of the GV–mortality association in fully adjusted models and across multiple early ICU time windows in our sensitivity analyses further supports this interpretation, although residual confounding cannot be excluded.
Our subgroup findings complement these mediation results. When patients were stratified by Sepsis-3 status, higher glycemic variability was associated with increased short- and long-term mortality in both non-septic and septic trauma patients, with a numerically stronger association among septic trauma patients. These findings suggest that, in addition to mediating part of the GV–mortality relationship, sepsis may amplify the prognostic impact of glycemic variability within the broader trauma-related physiological stress response.
The differential impact of glycemic variability based on diabetes status represents a noteworthy finding. Non-diabetic patients demonstrated substantially higher mortality risk (HR 2.31, 95% CI 1.92–2.77) compared to diabetic patients (HR 1.35, 95% CI 0.95–1.90) when experiencing elevated CV. This paradoxical tolerance in diabetic patients, consistently reported in critical care literature27,35,36, may stem from chronic hyperglycemia conferring adaptive metabolic responses, such as downregulation of glucose transporters37. This finding has important implications for clinical practice. Non-diabetic patients—who, based on our data, represent approximately 80% of trauma admissions—may require more intensive glycemic monitoring, challenging a “one-size-fits-all” approach to glycemic control27. In addition, the age-related differences in vulnerability to glycemic variability warrant further discussion. Younger patients (age < 60) demonstrated markedly greater susceptibility to the harmful effects of GV (HR 4.18, 95% CI 2.98–5.87) compared to older patients (HR 1.53, 95% CI 1.28–1.83). This age-related pattern is consistent with findings from previous studies38,39. A proposed mechanism is that elderly patients may have greater adaptability and tolerance to oxidative-stress-related damage, because oxidative stress levels naturally increase with age39,40.
The clinical application of these findings faces several challenges. While we identified specific CV thresholds associated with increased mortality, the optimal intervention strategy remains uncertain. Current glucose management protocols focus primarily on absolute glucose targets without considering variability19. Attempting to reduce CV through intensive insulin therapy carries significant risks, particularly hypoglycemia, which independently increases mortality in critically ill patients41. Furthermore, no prospective studies have demonstrated that specifically targeting glycemic variability improves patient outcomes. Most interventions that reduce variability also lower mean glucose levels, making it difficult to isolate the beneficial effects8. At present, there is no established intervention that selectively reduces glycemic variability without increasing the risk of hypoglycemia, particularly in the unstable trauma ICU setting. Therefore, our findings support the use of GV primarily as a tool for risk stratification and prognostic enrichment rather than as an immediate target for aggressive therapeutic manipulation.
This raises fundamental questions about the nature of glycemic variability in trauma. Is elevated CV a modifiable risk factor that directly contributes to poor outcomes, or merely an epiphenomenon reflecting illness severity? Our mediation findings and the strong links between GV, sepsis, and organ dysfunction suggest that variability is closely intertwined with pathways leading to adverse outcomes, but they cannot disentangle cause and effect in this retrospective setting. Until such evidence emerges from prospective interventional or continuous glucose monitoring–based studies, CV and related GV metrics should be regarded as prognostic markers and tools for risk enrichment, rather than as proven therapeutic targets42.
Strengths and limitations
This study has several notable strengths. First, we utilized a large, well-characterized trauma cohort from the validated MIMIC-IV database, which provided comprehensive clinical data and robust long-term follow-up. Second, our approach employed advanced statistical methods, including restricted cubic spline analysis to identify nonlinear relationships and threshold effects, as well as bootstrap-based mediation analysis to explore causal pathways. Third, we evaluated both short-term and long-term mortality outcomes with extensive covariate adjustment across multiple domains. Finally, this represents the first dedicated analysis of glycemic variability specifically in trauma patients, which addresses an important gap in the current literature that has predominantly focused on mixed ICU populations.
However, several limitations merit consideration. First, due to the retrospective observational design, we cannot establish causal relationships despite extensive adjustment for potential confounders. Second, glucose monitoring frequency varied according to clinical indication, which may have introduced ascertainment bias, although our sensitivity analysis adjusting for sampling frequency showed that glycemic variability retained independent prognostic significance and residual confounding is still possible. Third, we lacked detailed information on insulin protocols, nutritional support timing, and corticosteroid administration, and autoimmune comorbidities—factors that may influence both glycemic patterns and outcomes43,44. Fourth, we did not perform formal landmark analyses around surgery or sepsis onset; in this heterogeneous retrospective cohort, operative timing, repeated procedures, and the often gradual evolution of sepsis made such models difficult to interpret, so we instead relied on admission-anchored time-window analyses to approximate early and later ICU phases. Finally, the single-center nature of the MIMIC-IV database may limit generalizability, although the large sample size and comprehensive clinical data strengthen the internal validity of our findings.
Conclusion
In conclusion, glycemic variability emerges as an important prognostic factor in trauma patients, with particular relevance for young, non-diabetic individuals who show greatest vulnerability. While the association with mortality is clear, translating this knowledge into improved outcomes requires further research to establish causality and develop safe, effective interventions. Until then, clinicians should consider CV as one of several factors in comprehensive risk assessment, recognizing that our current ability to modify glycemic variability remains limited.
Methods
Data source
This retrospective cohort study used data from the MIMIC-IV database, version 3.1 (https://mimic.mit.edu/). MIMIC-IV is a publicly available, de-identified repository that contains granular clinical information on hospital and intensive-care admissions to Beth Israel Deaconess Medical Center (BIDMC; Boston, MA, USA) between 2008 and 202245. The current release comprises more than 65,000 ICU admissions, and captures demographics, high-frequency vital signs, laboratory results, medications, imaging and procedure reports, and longitudinal mortality data. One author of the present study (LB) completed the Collaborative Institutional Training Initiative (CITI) “Data or Specimens Only Research” course, signed the data-use agreement, and was granted access to the database (certification number: 41254964). The use of de-identified data in MIMIC-IV has been approved by the Institutional Review Boards of BIDMC and the Massachusetts Institute of Technology, with a waiver of informed consent under the Health Insurance Portability and Accountability Act (HIPAA) safe-harbor provisions. Accordingly, no additional ethical approval was required for the present analysis. All study procedures adhered to the principles of the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines46.
Study population
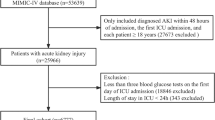
Trauma cases were extracted from the MIMIC-IV database. To ensure a single baseline record per individual, we kept only each patient’s earliest hospital admission and the first ICU stay within that admission, yielding 65,366 distinct ICU encounters. Patients whose primary diagnosis was trauma, as defined by International Classification of Diseases (ICD)-9/10 codes, were screened for inclusion (n = 6,257). Exclusion criteria were: (1) age < 18 years (n = 0); (2) discharge or death within 24 h of ICU admission (n = 731); (3) fewer than three recorded blood glucose measurements during the ICU stay (n = 517). The final study cohort comprised 4,009 trauma patients (Fig. 1). These patients were subsequently stratified into quartiles of the glucose coefficient of variation (CV): Q1 (n = 1,005), Q2 (n = 1,001), Q3 (n = 1,001), and Q4 (n = 1,002).
Data extraction
Data were extracted from MIMIC-IV database with Structured Query Language (SQL) scripts run in Navicat 16.3.3 on the native PostgreSQL server and cleaned in R 4.5.0. We captured eight variable blocks: (1) Demographics: age, sex, race; (2) Vital signs: heart rate (HR), respiratory rate (RR), systolic, diastolic and mean arterial pressures (SBP, DBP, MAP), body temperature (BT), peripheral oxygen saturation (SpO₂); (3) Laboratory indices: hemoglobin (Hb), white-blood-cell count (WBC), platelet count (PLT), prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), creatinine (Cr), blood urea nitrogen (BUN), sodium (Na), potassium (K), chloride (Cl), calcium (Ca); (4) Comorbidities: diabetes mellitus (DM), myocardial infarction (MI), congestive heart failure (CHF), cerebrovascular disease (CVD), chronic pulmonary disease (COPD), chronic kidney disease (CKD), malignant cancer; (5) Trauma descriptors: anatomical injury flags for brain, chest, abdominal, spinal, extremity and multiple trauma, trauma-mechanism category, and Injury Severity Score (ISS) according ICD-9/10 code diagnosis; (6) Severity scores: Oxford Acute Severity of Illness Score (OASIS), Sequential Organ Failure Assessment (SOFA), Glasgow Coma Scale (GCS); (7) Therapeutic measures: mechanical ventilation (MV), vasoactive-drug infusion, continuous renal-replacement therapy (CRRT), insulin therapy, emergency surgery, blood transfusion; (8) Complications: sepsis (Sepsis-3), and acute kidney injury (AKI). At the same time, survival information was also collected.
Vital signs and laboratory values were extracted from the first 24 h after ICU admission. Glycemic variability was calculated using all glucose measurements throughout the ICU stay, obtained from bedside testing, serum chemistry, or whole-blood analyzers, for the entire stay and rolling 3-, 7-, 14-, and 21-day intervals. The minimum glucose value was used to identify hypoglycemia (glucose < 70 mg/dL). Trauma features were parsed from ICD-9/10 codes, and ISS was approximated with the icdpicr package47. Variables with > 20% missingness were excluded; those with ≤ 20% missing data were imputed via multiple imputation by chained equations (five iterations, mice package) to limit bias48.
Glycemic variability
Glycemic variability can be characterized using several indices, including dynamic metrics such as the mean amplitude of glycemic excursions (MAGE) and the mean absolute glucose change per unit time (MAG). These indices capture the magnitude and rate of glucose swings when dense or continuous glucose monitoring is available. In the present study, GV was quantified with the coefficient of variation (CV), a dimensionless metric that is well suited to retrospective datasets with variable timing and frequency of glucose measurements across patients. For each individual, we collated every plasma, serum, or whole-blood glucose result obtained between ICU admission and the earlier of discharge or death. CV was then calculated as
.
Only patients who contributed at least three valid glucose measurements met the reliability threshold for inclusion in the analysis, ensuring that the CV captured true within-stay fluctuation rather than random assay noise.
Study endpoint
Two all-cause mortality endpoints were examined: short-term mortality, defined as death within 30 days of the index ICU admission, and long-term mortality, defined as death within 365 days of that admission. Survival time was measured from the moment of first ICU entry; patients still alive at the end of each window were censored at 30 days or 1 year, respectively. Mortality dates were taken from the state and hospital vital-status files embedded in MIMIC-IV, which capture deaths both in-hospital and after discharge and provide at least one year of follow-up for every patient.
Statistical analysis
The study population was divided into four groups based on CV quartiles (Q1–Q4). Normality was assessed with the Kolmogorov–Smirnov test. Continuous variables are reported as mean ± SD when normally distributed or median (interquartile range) otherwise; categorical variables are shown as n (%). Group comparisons used one-way ANOVA or the Kruskal–Wallis H test for continuous data and Pearson’s χ2 or Fisher’s exact test for categorical data, as appropriate. The distribution of CV was found to be highly right-skewed (Supplementary Fig. S1); consequently, it was natural-log–transformed (log(CV)) for use in regression models and was also categorized into quartiles (Q1–Q4) for analysis. Linear trends across quartiles were assessed by treating quartile categories as ordinal variables in Cox regression models.
The association between glycemic variability and mortality was examined with Cox proportional-hazards models for 30-day and 1-year all-cause death. The proportional hazards assumption was verified using Schoenfeld residuals. Four nested models were fitted: Model 1, unadjusted; Model 2, adjusted for age, sex and race; Model 3, further adjusted for vital signs, comorbidities and laboratory indices; and Model 4, additionally adjusted for trauma descriptors, severity scores, and therapeutic measures. Hazard ratios (HRs) and 95% confidence intervals (CIs) are reported, and multicollinearity was excluded by verifying variance-inflation factors < 5. Survival curves were generated with the Kaplan–Meier method and compared with the log-rank test. Model discrimination was quantified with time-dependent receiver-operating-characteristic (ROC) curves and corresponding areas under the curve (AUC).
Potential non-linear relations between log-transformed CV and mortality were explored using restricted cubic splines (RCS) in multivariable-adjusted Cox proportional hazards models. Four knots were placed at the 5%, 35%, 65%, and 95% percentiles of each index’s distribution. Non-linearity was assessed using likelihood-ratio tests comparing spline and linear models. The value of log(CV) at the nadir of the curve (point of lowest risk) was set as the reference (HR = 1). To formally test the threshold effect suggested by the RCS curve, we conducted a two-piecewise Cox regression analysis. The knot for this model was pre-specified at the nadir value. We then compared the hazard ratios across the two segments defined by this knot to quantify the change in association.
Prespecified subgroup analyses evaluated effect modification by demographics (age < 60 vs. ≥ 60 years, sex), trauma characteristics (traumatic brain injury, multiple trauma, ISS ≥ 16), clinical conditions (diabetes, hypoglycemia, Charlson comorbidity score ≥ 3), critical care interventions (mechanical ventilation, vasoactive drugs, blood transfusion), and complications (sepsis). Interaction p-values were derived from multiplicative interaction terms in Cox proportional hazards models. To explore potential causal pathways, bootstrap-based mediation analysis (1000 replicates) assessed whether sepsis mediated the GV–mortality relationship.
All statistics were performed using the R programming environment (version 4.5.0, R Foundation for Statistical Computing, Vienna, Austria). Two-sided p-values < 0.05 were deemed statistically significant.
Continuous numerical variables are expressed as means ± standard deviations or medians (interquartile range), and categorical variables are presented as numbers (percentages). ISS: Injury Severity Score, GCS: Glasgow Coma Scale, TBI: traumatic brain injury, AKI: acute kidney injury.
Data availability
The datasets analyzed in the current study are available in the MIMIC-IV database. (https://physionet.org/content/mimiciv/3.1/)
Abbreviations
- GV:
-
Glycemic variability
- CV:
-
Coefficient of variation
- ICU:
-
Intensive care unit
- MIMIC-IV:
-
Medical Information Mart for Intensive Care IV
- HRs:
-
Hazard ratios
- CIs:
-
Confidence intervals
- ISS:
-
Injury Severity Score
- SOFA:
-
Sequential Organ Failure Assessment
- GCS:
-
Glasgow Coma Scale
- TBI:
-
Traumatic brain injury
- DM:
-
Diabetes mellitus
- AKI:
-
Acute kidney injury
- MV:
-
Mechanical ventilation
- RCS:
-
Restricted cubic splines
- ROC:
-
Receiver-operating-characteristic
- AUC:
-
Areas under the curve
- CRRT:
-
Continuous renal-replacement therapy
- CHF:
-
Congestive heart failure
- MI:
-
Myocardial infarction
- CVD:
-
Cerebrovascular disease
- COPD:
-
Chronic pulmonary disease
- CKD:
-
Chronic kidney disease
- OASIS:
-
Oxford Acute Severity of Illness Score
- HR:
-
Heart rate
- RR:
-
Respiratory rate
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- MAP:
-
Mean arterial pressure
- BT:
-
Body temperature
- SpO₂:
-
Peripheral oxygen saturation
- Hb:
-
Hemoglobin
- WBC:
-
White-blood-cell count
- PLT:
-
Blood platelet count
- PT:
-
Prothrombin time
- APTT:
-
Activated partial thromboplastin time
- INR:
-
International normalized ratio
- Cr:
-
Creatinine
- BUN:
-
Blood urea nitrogen
- Na:
-
Sodium
- K:
-
Potassium
- Cl:
-
Chloride
- Ca:
-
Calcium
- ICD:
-
International Classification of Diseases
- SD:
-
Standard deviation
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- AIC:
-
Akaike information criterion
- SQL:
-
Structured Query Language
- CITI:
-
Collaborative Institutional Training Initiative
- HIPAA:
-
Health Insurance Portability and Accountability Act
References
World Health Organization. Injuries and violence. https://www.who.int/news-room/fact-sheets/detail/injuries-and-violence
van Breugel, J. M. M. et al. Global changes in mortality rates in polytrauma patients admitted to the ICU—a systematic review. World J. Emerg. Surg. 15, 55. https://doi.org/10.1186/s13017-020-00330-3 (2020).
Frydrych, L. M. et al. Short-term versus long-term trauma mortality: A systematic review. J. Trauma. Acute Care Surg. 87, 990–997. https://doi.org/10.1097/TA.0000000000002430 (2019).
Davidson, G. H. Long-term survival of adult trauma patients. JAMA 305, 1001. https://doi.org/10.1001/jama.2011.259 (2011).
Oyeniyi, B. T. et al. Trends in 1029 trauma deaths at a level 1 trauma center: impact of a bleeding control bundle of care. Injury 48, 5–12. https://doi.org/10.1016/j.injury.2016.10.037 (2017).
Marik, P. E. & Bellomo, R. Stress hyperglycemia: an essential survival response! Crit. Care. 17, 305. https://doi.org/10.1186/cc12514 (2013).
Deng, H. & Chai, J. The effects and mechanisms of insulin on systemic inflammatory response and immune cells in severe trauma, burn injury, and sepsis. Int. Immunopharmacol. 9, 1251–1259. https://doi.org/10.1016/j.intimp.2009.07.009 (2009).
Lanspa, M. J. et al. Coefficient of glucose variation is independently associated with mortality in critically ill patients receiving intravenous insulin. Crit. Care. 18, R86. https://doi.org/10.1186/cc13851 (2014).
Zhou, Z., Sun, B., Huang, S., Zhu, C. & Bian, M. Glycemic variability: adverse clinical outcomes and how to improve it? Cardiovasc. Diabetol. 19, 102. https://doi.org/10.1186/s12933-020-01085-6 (2020).
Shuai, W. L. et al. Association of glycemic variability with short and long-term mortality among critically ill patients with heart failure: analysis of the MIMIC-IV database. Diabetes Res. Clin. Pr. 221, 112009. https://doi.org/10.1016/j.diabres.2025.112009 (2025).
Liu, Y. et al. U – shaped association between the glycemic variability and prognosis in hemorrhagic stroke patients: a retrospective cohort study from the MIMIC-IV database. Front. Endocrinol. 16, 1546164. https://doi.org/10.3389/fendo.2025.1546164 (2025).
Guo, Y. et al. Association between glycemic variability and short-term mortality in patients with acute kidney injury: a retrospective cohort study of the MIMIC-IV database. Sci. Rep. 14, 5945. https://doi.org/10.1038/s41598-024-56564-7 (2024).
Chao, W. C. et al. Higher glycemic variability within the first day of ICU admission is associated with increased 30-day mortality in ICU patients with sepsis. Ann. Intensive Care. 10, 17. https://doi.org/10.1186/s13613-020-0635-3 (2020).
Papachristoforou, E., Lambadiari, V., Maratou, E. & Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 1–17 (2020). https://doi.org/10.1155/2020/7489795. (2020).
Du, Y. et al. Glycemic variability: an independent predictor of mortality and the impact of age in pediatric intensive care unit. Front. Pediatr. 8, 403. https://doi.org/10.3389/fped.2020.00403 (2020).
Kreutziger, J. et al. Absence of stress hyperglycemia indicates the most severe form of blunt liver trauma. Diagnostics 11, 1667. https://doi.org/10.3390/diagnostics11091667 (2021).
Tsai, Y. W. et al. Impact of stress-induced hyperglycemia on the outcome of children with trauma: A cross-sectional analysis based on propensity score-matched population. Sci. Rep. 9, 16311. https://doi.org/10.1038/s41598-019-52928-6 (2019).
Eakins, J. Blood glucose control in the trauma patient. J. Diabetes Sci. Technol. 3, 1373–1376. https://doi.org/10.1177/193229680900300617 (2009).
Honarmand, K. et al. Society of critical care medicine guidelines on glycemic control for critically ill children and adults. Crit. Care Med. 52, e161–e181. https://doi.org/10.1097/CCM.0000000000006174 (2024). (2024).
Dungan, K. M., Braithwaite, S. S. & Preiser, J. C. Stress hyperglycaemia. Lancet 373, 1798–1807. https://doi.org/10.1016/S0140-6736(09)60553-5 (2009).
Marik, P. E. Critical Illness-Related corticosteroid insufficiency. Chest 135, 181–193. https://doi.org/10.1378/chest.08-1149 (2009).
CHERNOW, B., LAKE, C. R. & RAINEY, T. G. & Endogenous and exogenous catecholamines in critical care medicine. Crit. Care Med. 10, 409–416. https://doi.org/10.1097/00003246-198206000-00019 (1982).
Lazzeri, C. et al. The prognostic role of peak glycemia and glucose variability in trauma: a single-center investigation. Acta Diabetol. 57, 931–935. https://doi.org/10.1007/s00592-020-01493-w (2020).
Qi, L. et al. Association of glycemic variability and prognosis in patients with traumatic brain injury: A retrospective study from the MIMIC-IV database. Diabetes Res. Clin. Pr. 217, 111869. https://doi.org/10.1016/j.diabres.2024.111869 (2024).
Bochicchio, G. V., Bochicchio, K. M., Joshi, M., Ilahi, O. & Scalea, T. M. Acute glucose elevation is highly predictive of infection and outcome in critically injured trauma patients. Ann. Surg. 252, 597–602. https://doi.org/10.1097/SLA.0b013e3181f4e499 (2010).
Zhang, Y., Yan, Y., Sun, L. & Wang, Y. Stress hyperglycemia ratio is a risk factor for mortality in trauma and surgical intensive care patients: a retrospective cohort study from the MIMIC-IV. Eur. J. Med. Res. 29, 558. https://doi.org/10.1186/s40001-024-02160-4 (2024).
Krinsley, J. S. et al. Diabetic status and the relation of the three domains of glycemic control tomortality in critically ill patients: an international multicenter cohort study. Crit. Care. 17, R37. https://doi.org/10.1186/cc12547 (2013).
Lu, Z. et al. Association of blood glucose level and glycemic variability with mortality in sepsis patients during ICU hospitalization. Front. Public. Health. 10, 857368. https://doi.org/10.3389/fpubh.2022.857368 (2022).
Zhou, J., Chen, Z., Huang, H. N., Ou, C. Q. & Li, X. Association between various blood glucose variability-related indicators during early ICU admission and 28-day mortality in non-diabetic patients with sepsis. Diabetol. Metab. Syndr. 17, 22. https://doi.org/10.1186/s13098-025-01580-4 (2025).
Ceriello, A. et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Nestle Nutr. Works Se. 57, 1349–1354. https://doi.org/10.2337/db08-0063 (2008).
Kumar, A. et al. Association of Glycemic Variability with Outcomes in Non-diabetic Sepsis Patients: A Prospective Observational Study. Indian J. Crit. Care Med.: Peer-rev. Off. Publ. Indian Soc. Crit. Care Med. 29, 27–35 (2024). https://doi.org/10.5005/jp-journals-10071-24873
Li, X. et al. Acute glycemic variability and risk of mortality in patients with sepsis: a meta-analysis. Diabetol. Metab. Syndr. 14 https://doi.org/10.1186/s13098-022-00819-8 (2022).
Monnier, L. et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295, 1681. https://doi.org/10.1001/jama.295.14.1681 (2006).
Donati, A. et al. Glycaemic variability, infections and mortality in a medical–surgical intensive care unit. Crit. Care Resusc. : J. Australas Acad. Crit. Care Med. 16, 13–23. https://doi.org/10.1016/S1441-2772(23)01437-0 (2014).
Fong, K. M., Au, S. Y. & Ng, G. W. Y. Glycemic control in critically ill patients with or without diabetes. BMC Anesthesiol. 22, 227. https://doi.org/10.1186/s12871-022-01769-4 (2022).
Hsu, C. W., Sun, S. F. & Lin, H. S. Glycemic variability in critically ill patients: risk factors and association with mortality. Chin. Med. J. (Engl). 133, 1255–1256. https://doi.org/10.1097/CM9.0000000000000686 (2020).
Greco, G., Kirkwood, K. A., Gelijns, A. C., Moskowitz, A. J. & Lam, D. W. Diabetes is associated with reduced stress hyperlactatemia in cardiac surgery. Diabetes Care. 41, 469–477. https://doi.org/10.2337/dc17-1554 (2018).
Su, Y., Fan, W., Liu, Y. & Hong, K. Glycemic variability and in-hospital death of critically ill patients and the role of ventricular arrhythmias. Cardiovasc. Diabetol. 22, 134. https://doi.org/10.1186/s12933-023-01861-0 (2023).
Lu, L., Lu, Y., Gao, C. & Zhang, N. Age moderates the relationships between obesity, glucose variability, and intensive care unit mortality: a retrospective cohort study. J. Intensive Care. 9, 68. https://doi.org/10.1186/s40560-021-00582-4 (2021).
Liguori, I. et al. Oxidative stress, aging, and diseases. Clin. Interv Aging. 13, 757–772. https://doi.org/10.2147/CIA.S158513 (2018).
The NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl. J. Med. 367, 1108–1118. https://doi.org/10.1056/NEJMoa1204942 (2012).
Hryciw, B. N. et al. Glycemic variability as a prognostic factor for mortality in patients with critical illness: A systematic review and Meta-Analysis. Crit. Care Explor. 6, e1025. https://doi.org/10.1097/CCE.0000000000001025 (2024).
Barker, H. L., Morrison, D., Llano, A., Sainsbury, C. A. R. & Jones, G. C. Practical guide to glucocorticoid induced hyperglycaemia and diabetes. Diabetes Ther. 14, 937–945. https://doi.org/10.1007/s13300-023-01393-6 (2023).
Gunst, J. et al. Tight Blood-Glucose control without early parenteral nutrition in the ICU. N Engl. J. Med. 389, 1180–1190. https://doi.org/10.1056/NEJMoa2304855 (2023).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data. 10, 1–9. https://doi.org/10.1038/s41597-022-01899-x (2023).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (lond Engl). 370, 1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X (2007).
Clark, D. E. & Black, A. W. ICDPIC-R Version 1.0.0. Injury 52, 3545 (2021). https://doi.org/10.1016/j.injury.2021.02.079
White, I. R., Royston, P. & Wood, A. M. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 30, 377–399. https://doi.org/10.1002/sim.4067 (2011).
Acknowledgements
We thank the contributors to the Medical Information Mart for Intensive Care-IV (MIMIC-IV) database and acknowledge the efforts of all personnel who made this research possible.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
BL, GHJ, JHZ, CYS, JJM, XWL, JW, RCL, XMW and XNL contributed to the study conception and design. Material preparation, data collection and analysis were performed by BL, JHZ, CYS and JW. Data visualization was conducted by BL, JHZ, CYS and RCL. The first draft of the manuscript was written by BL, JHZ, CYS and RCL, and all authors commented on previous versions of the manuscript. Project administration and supervision were provided by GHJ, JJM and XWL. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The MIMIC protocol was approved by the review boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. As the data were publicly available, the study was exempt from the requirements of an ethics approval statement and informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, B., Zhang, J., Song, C. et al. Association of glycemic variability with short- and long-term mortality in critically ill patients with trauma. Sci Rep 16, 2588 (2026). https://doi.org/10.1038/s41598-025-32464-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32464-2