Abstract
Group 2 innate lymphoid cells (ILC2s) are highly heterogeneous tissue-resident lymphocytes that regulate inflammation and tissue homeostasis in health and disease. However, how these cells integrate into the tissue microenvironment to perform tissue-specific functions is unclear. Here, we show neuropilin-1 (Nrp1), which is induced postnatally and sustained by lung-derived transforming growth factor beta-1 (TGFβ1), is a tissue-specific marker of lung ILC2s. Genetic ablation or pharmacological inhibition of Nrp1 suppresses IL-5 and IL-13 production by ILC2s and protects mice from the development of pulmonary fibrosis. Mechanistically, TGFβ1–Nrp1 signaling enhances ILC2 function and type 2 immunity by upregulating IL-33 receptor ST2 expression. These findings identify Nrp1 as a tissue-specific regulator of lung-resident ILC2s and highlight Nrp1 as a potential therapeutic target for pulmonary fibrosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-sequencing data have been deposited in GEO under the primary accession code GSE168809. Published ILC2 scRNA-seq datasets were accessed in GEO under the accession code GSE117568. Published ILC2 bulk RNA-seq dataset were accessed in GEO under the accession code GSE126107. The raw reads of RNA-seq were aligned to the mouse reference genome (version mm10). All other data are available in the article and supplementary files or from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Hoyler, T. et al. The transcription factor GATA3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37, 634–648 (2012).
Kim, B. S. et al. TSLP Elicits IL-33-Independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, 170 (2013).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Ricardo-Gonzalez, R. R. et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 19, 1093–1099 (2018).
von Moltke, J., Ji, M., Liang, H.-E. & Locksley, R. M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016).
Dahlgren, M. W. et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity 50, 707–722 (2019).
Cardoso, V. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017).
Schneider, C. et al. Tissue-resident group 2 Innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity 50, 1425–1438 (2019).
Robinette, M. L. et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 16, 306–317 (2015).
Simoni, Y. et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 46, 148–161 (2017).
Ricardo-Gonzalez, R. R. et al. Tissue-specific pathways extrude activated ILC2s to disseminate type 2 immunity. J. Exp. Med. 217, e20191172 (2020).
Yudanin, N. A. et al. Spatial and temporal mapping of human innate lymphoid cells reveals elements of tissue specificity. Immunity 50, 505–519 (2019).
Rana, B. M. J. et al. A stromal cell niche sustains ILC2-mediated type-2 conditioning in adipose tissue. J. Exp. Med. 216, 1999–2009 (2019).
Martinez-Gonzalez, I. et al. Allergen-experienced group 2 innate lymphoid cells acquire memory-like properties and enhance allergic lung inflammation. Immunity 45, 198–208 (2016).
Huang, Y. et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359, 114–119 (2018).
Huang, Y. et al. IL-25-responsive, lineage-negative KLRG1hi cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 16, 161–169 (2015).
Evren, E. et al. Distinct developmental pathways from blood monocytes generate human lung macrophage diversity. Immunity 54, 259–275 (2021).
Seidman, J. S. et al. Niche-specific reprogramming of epigenetic landscapes drives myeloid cell diversity in nonalcoholic steatohepatitis. Immunity 52, 1057–1074 (2020).
Qiu, J. et al. Tissue signals imprint Aiolos expression in ILC2s to modulate type 2 immunity. Mucosal Immunol. 14, 1306–1322 (2021).
Kolodkin, A. L. et al. Neuropilin is a semaphorin III receptor. Cell 90, 753–762 (1997).
Soker, S., Takashima, S., Miao, H. Q., Neufeld, G. & Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735–745 (1998).
Glinka, Y. & Prud’homme, G. J. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 84, 302–310 (2008).
Delgoffe, G. M. et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256 (2013).
Leclerc, M. et al. Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by neuropilin-1. Nat. Commun. 10, 3345 (2019).
Liu, C. et al. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat. Immunol. 21, 1010–1021 (2020).
Shikhagaie, M. M. et al. Neuropilin-1 Is expressed on lymphoid tissue residing LTi-like group 3 innate lymphoid cells and associated with ectopic lymphoid aggregates. Cell Rep. 18, 1761–1773 (2017).
Heldin, C. H., Miyazono, K. & tenDijke, P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390, 465–471 (1997).
Seillet, C. et al. Deciphering the innate lymphoid cell transcriptional program. Cell Rep. 17, 436–447 (2016).
Miyazaki, M. et al. Id2 and Id3 maintain the regulatory T cell pool to suppress inflammatory disease. Nat. Immunol. 15, 767–776 (2014).
Hacker, C. et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat. Immunol. 4, 380–386 (2003).
Buitenhuis, M. et al. Differential regulation of granulopoiesis by the basic helix-loop-helix transcriptional inhibitors Id1 and Id2. Blood 105, 4272–4281 (2005).
Li, D. et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 134, 1422–1432 (2014).
Hams, E. et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl Acad. Sci. USA 111, 367–372 (2014).
Bi, J. et al. NK cells alleviate lung inflammation by negatively regulating group 2 innate lymphoid cells. J. Immunol. 198, 3336–3344 (2017).
Meng, X.-m, Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Passalacqua, G. et al. IL-13 and idiopathic pulmonary fibrosis: Possible links and new therapeutic strategies. Pulm. Pharmacol. Therapeutics 45, 95–100 (2017).
Lichtman, M. K., Otero-Vinas, M. & Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regeneration 24, 215–222 (2016).
Gieseck, R. L. 3rd, Wilson, M. S. & Wynn, T. A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 18, 62–76 (2018).
Daly, J. L. et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 370, 861–865 (2020).
Denney, L. et al. Pulmonary epithelial cell-derived cytokine TGF-β1 Is a critical cofactor for enhanced innate lymphoid cell function. Immunity 43, 945–958 (2015).
de Boer, W. I. et al. Transforming growth factor beta(1) and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am. J. Respiratory Crit. Care Med. 158, 1951–1957 (1998).
Sullivan, D. E., Ferris, M., Nguyen, H., Abboud, E. & Brody, A. R. TNF-α induces TGF-β(1) expression in lung fibroblasts at the transcriptional level via AP-1 activation. J. Cell. Mol. Med. 13, 1866–1876 (2009).
Jones, C. P., Gregory, L. G., Causton, B., Campbell, G. A. & Lloyd, C. M. Activin A and TGF-β promote T(H)9 cell-mediated pulmonary allergic pathology. J. Allergy Clin. Immunol. 129, 1000–1010 (2012).
Laouar, Y., Sutterwala, F. S., Gorelik, L. & Flavell, R. A. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat. Immunol. 6, 600–607 (2005).
Wang, L. et al. TGF-β induces ST2 and programs ILC2 development. Nat. Commun. 11, 35 (2020).
Khalil, N., O’Connor, R. N., Flanders, K. C. & Unruh, H. TGF-β-1, but not TGF-β-2 or TGF-β-3, is differentially present in epithelial cells of advanced pulmonary fibrosis: An immunohistochemical study. Am. J. Respir. Cell Mol. Biol. 14, 131–138 (1996).
Cantuti-Castelvetri, L. et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860 (2020).
Acknowledgements
We thank members of L. Shen’s laboratory for their help and suggestions on this project. We thank the National Institute of Parasitic Diseases at Chinese Center for Disease Control and Prevention for help with N. brasiliensis infection experiments. We thank the Flow Cytometry Facility and Animal Facility at Shanghai Jiao Tong University School of Medicine for service and assistance. We thank J. Zhou and T. Hong for helping with human samples collection. We thank E. Engleman for editing the manuscript. This study was supported by grant 2020YFA0509200 (to L.S.) and 2020YFA0509103 (to J.Q.) from the Ministry of Science and Technology of China, grant 81971487 (to L.S.), 32022027 and 31970860 (to J.Q.) from the National Natural Science Foundation of China, grant 20ZR1430200, 20142202300 (to L.S.) and 20ZR1466900 (to J.Q.) from Science and Technology Commission of Shanghai Municipality, and Shanghai Jiao Tong University School of Medicine Innovation Team on Pediatric Research (to L.S.).
Author information
Authors and Affiliations
Contributions
J.Z. and J.X.Q. performed the experiments and analyzed the data. W.Z. and X.H. contributed to human samples collection and experiment design. J.C. supervised worm infection experiments. W.M. and B.S. contributed to discussion. J.Z. performed bioinformatics analyses. L.S., J.Q., and J.Z. wrote the manuscript. L.S., J.Q., and B.H. conceived, designed, and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Ralph Stadhouders and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. N. Bernard was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
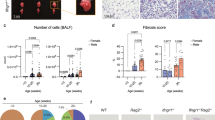
Extended Data Fig. 1 Nrp1 is a tissue-specific marker of lung ILC2s.
(a) Box plot compared the expression of NRP1 in human ILC2s from lung (n = 6) and spleen (n = 5). The bulk RNA-seq data were obtained from Gene Expression Omnibus (GEO) under accession code GSE126107. The center line, lower whisker, and upper whisker stand for median, minima, and maxima, respectively. Bounds of box are 25-75th percentile. (b) Gating strategy of ILC2s. ILC2s were gated on live and single cells with lin−(CD3−B220−CD11b−CD11c−CD5−) CD45+CD127+GATA3+. (c) The compensation of 15-color (including 19 markers) flow cytometric panel was shown by 14×14 view. Cells were gated on single lymphocytes. (d) ILC2s sorted from large intestine were cultured with TGFβ1 at 1 ng/ml for 48 h. mRNA expression level of Nrp1 was analyzed by quantitative RT-PCR (n = 3/group). Each symbol represents an individual mouse (d). Data are representative of 3 independent experiments. Two-tailed unpaired Student’s t test.
Extended Data Fig. 2 Loss of Nrp1 does not alter lung ILC2 homeostasis in Id2cre-ERT2Nrp1f/f mice under steady state.
a-m Id2cre-ERT2Nrp1f/+ and Id2cre-ERT2Nrp1f/f mice were injected intraperitoneally with tamoxifen (2 mg/day per mouse) for 5 days to knock out Nrp1 expression in the ILC lineage. (a-c) The expression level of Nrp1 on Treg, DCs, and neutrophils from lung were analyzed by flow cytometry. (a) The top row was gated on CD45+ live cells. (b) The top row was gated on CD45+CD11b+F4/80−Ly6G− live cells. (c) The top row was gated on CD45+ live cells. (d-f) Absolute cell counts of lung Treg, DCs, and neutrophils (n = 3/group). (g) The expression level of Nrp1 on lung ILC2s were analyzed by flow cytometry. Cells were gated on lin−CD45+CD127+ lymphocytes. (h, i) H&E staining and Masson’s trichrome staining of lung tissue. (j) Absolute cell counts of lung ILC2s (n = 11/group). (k) Percentage of IL-5+ ILC2s and IL-13+ ILC2s. Cells were gated on total ILC2s. (l, m) Frequency and total number of IL-5+ ILC2s (n = 10/group) and IL-13+ ILC2s (n = 7/group) Each symbol represents an individual mouse (d-f, j, l, and m). Data are representative of 3-4 independent experiments and are presented as mean ± SEM.
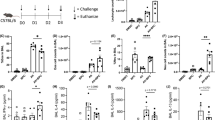
Extended Data Fig. 3 Ablation of Nrp1 impairs anti-helminth immunity in Id2cre-ERT2Nrp1f/f mice.
a-i Id2cre-ERT2Nrp1f/+ and Id2cre-ERT2Nrp1f/f mice were injected intraperitoneally with tamoxifen (2 mg/day per mouse) for 5 days to knock out Nrp1 expression in the ILC lineage. 3 days later, these mice were infected with 500 L3 N brasiliensis. Lung tissues were collected and analyzed 5 days after worm infection. (a) Intracellular staining of IL-5 and IL-13 in lung ILC2s. (b, c) Frequency and absolute account of IL-5+ ILC2s in lung. (d, e) Frequency and absolute account of IL-13+ ILC2s in lung. (f) Total number of lung ILC2s. (n = 7 for black group and n = 8 for red group in b-f) (g) Flow cytometric analysis of eosinophils (CD45+Ly6G−CD11b+SiglecF+) in lung. Cells were gated on CD45+Ly6G− live single cells. (h) Absolute count of lung eosinophils. (i) Worm burden in the small intestine collected on day 7 post infection. (n = 8 for black group and n = 9 for red group in h-i) Each symbol represents an individual mouse (b-f, h, and i). Data are representative of 3 independent experiments and are presented as mean ± SEM. Two-tailed unpaired Student’s t test.
Extended Data Fig. 4 Nrp1 deficiency does not affect ILC2 compartment in R5/ + Nrp1f/f mice under steady state.
(a)The expression of Nrp1 on lung ILC2s in R5/ + and R5/ + Nrp1f/f mice was analyzed by flow cytometry. Cells was gated on lin-CD45 + CD127 + lymphocytes. (b, c) Frequency and absolute number of IL-5+ILC2s in lung (n = 4 for black group and n = 7 for red group). (d, e) Frequency and absolute number of IL-13+ILC2s in lung. (f) Absolute count of total ILC2s in lung. (n = 5 for black group and n = 7 for red group in d-f) Each symbol represents an individual mouse (b-f). Data are representative of 3 independent experiments and are presented as mean ± SEM.
Extended Data Fig. 5 Loss of Nrp1 reduces ILC2 function and anti-helminth immunity in R5/ + Nrp1f/f mice.
a-i R5/ + and R5/ + Nrp1f/f mice were infected with 500 L3 N brasiliensis subcutaneously. Lung tissues were collected and analyzed 5 days after worm infection. (a) Intracellular staining of IL-5 and IL-13 in lung ILC2s. (b, c) Frequency and absolute account of IL-5+ ILC2s in lung (n = 5/group). (d, e) Frequency and absolute account of IL-13+ ILC2s in lung (n = 5/group). (f) Total number of lung ILC2s (n = 5/group). (g) Flow cytometric analysis of eosinophils in lung. Cells were gated on CD45+ CD11b+Ly6G− live single cells. (h) Absolute count of lung eosinophils (n = 5/group). (i) Worm burden in the small intestine collected on day 7 post infection (n = 5/group). Each symbol represents an individual mouse (b-f, h, and i). Data are representative of 3 independent experiments and are presented as mean ± SEM. Two-tailed unpaired Student’s t test.
Extended Data Fig. 6 Nrp1 deficiency leads to reduced ILC2 function during pulmonary fibrosis.
(a) R5/ + and R5/ + Nrp1f/f mice were challenged with bleomycin to induce pulmonary fibrosis. Mice were sacrificed on day 21 and lung tissues were collected. Flow cytometric analysis of IL-5+ lymphocytes. Cells were gated on live CD45+ lymphocytes. b-e ILC2s isolated from lung and large intestine of R5/ + or R5/ + Nrp1f/f mice were transferred intravenously into Rag2–/–Il2rg–/– mice, respectively. After 7 days, the recipient mice were given bleomycin intranasally. Lung tissues were collected and analyzed on day 21 of fibrosis. (b, c) Frequency and absolute number of lung ILC2s (n = 4/group). (d, e) Frequency and absolute number of IL-5+ ILC2s in lung (n = 4/group). Each symbol represents an individual mouse (b-e). Data are representative of 2-3 independent experiments and are presented as mean ± SEM. Two-tailed unpaired Student’s t test.
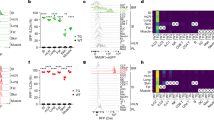
Extended Data Fig. 7 The expression profile of Nrp1 in lung immune cells.
a, c-e Wild-type mice were challenged with bleomycin (2 mg/kg body weight) intranasally to induce fibrosis, and subsequently were treated with EG00229 or DMSO control for three weeks. (a) H&E staining of the representative lung tissue. (b) Nrp1 expression in lung immune cells from WT mice was analyzed by flow cytometry. (c-e) Absolute numbers of lung macrophages (n = 14 for DMSO, n = 11 for EG00229 in c), DCs and Treg cells (n = 5 for DMSO, n = 4 for EG00229 in d-e) in each group of mice. Each symbol represents an individual mouse (c-e). Data are representative of at least 3 independent experiments and are presented as mean ± SEM.
Extended Data Fig. 8 Treatment with EG00229 intranasally alleviates pulmonary fibrosis.
a-i Bleomycin-challenged mice were treated with EG00229 (2.5 mg/kg body weight in 20μl PBS) or DMSO control intranasally three times weekly for three weeks. (a) The survival rate of mice treated with EG00229 or DMSO control was observed for a period of 21 days (n = 16/group). (b, c) H&E staining and Masson’s trichrome staining of lung tissues. (d) mRNA expression of the fibrotic genes in lung (n = 3, 6, 6 for PBS, DMSO, and EG00229 group). (e, f) Frequency and absolute count of IL-13+ ILC2s (n = 6/group). (g, h) Frequency and absolute count of IL-5+ ILC2s (n = 6/group). (i) Total number of α-SMA+ myofibroblast (n = 6/group). Each symbol represents an individual mouse (d-i). Data are representative of at least 3 independent experiments and are presented as mean ± SEM. (a) Log-rank test. (d-i) Two-tailed unpaired Student’s t test.
Extended Data Fig. 9 The therapeutic effect of EG00229 on pulmonary fibrosis is dependent on lung ILC2s.
(a) Bleomycin-challenged Rag1−/− mice were treated with EG00229 (10 mg/kg body weight) or DMSO control intraperitoneally for three weeks. Each group of mice were also received 200 μg of anti-Thy1.2 or isotype control antibodies every 3 days throughout the treatment. Masson’s trichrome staining for each group was shown. Data are representative of 3 independent experiments.
Supplementary information
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 4
Unprocessed Western Blots
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 5
Unprocessed Western Blots
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Fig. 7
Unprocessed Western Blots
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Rights and permissions
About this article
Cite this article
Zhang, J., Qiu, J., Zhou, W. et al. Neuropilin-1 mediates lung tissue-specific control of ILC2 function in type 2 immunity. Nat Immunol 23, 237–250 (2022). https://doi.org/10.1038/s41590-021-01097-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-021-01097-8
This article is cited by
-
Obesity disrupts ILC2 metabolic and functional homeostasis by inhibiting mTORC1 signaling
Cellular & Molecular Immunology (2026)
-
Neuropilin-1-target self-assembled peptide nanoparticles contribute to tumor treatment by inducing pyroptosis
BMC Cancer (2025)
-
Fibrogenesis-driven tumor progression in clear cell renal cell carcinoma: prognostic, therapeutic implications and the dual role of neuropilin-1
Cancer Cell International (2025)
-
NRP1 instructs IL-17-producing ILC3s to drive colitis progression
Cellular & Molecular Immunology (2025)
-
Piezo1-mediated mechanotransduction regulates the translational activity, function and lung pathogenicity of group 2 innate lymphoid cells
Signal Transduction and Targeted Therapy (2025)