Abstract
CD4+ T follicular helper (TFH) cells support tailored B cell responses against multiple classes of pathogens. To reveal how diverse TFH phenotypes are established, we profiled mouse TFH cells in response to viral, helminth and bacterial infection. We identified a core TFH signature that is distinct from CD4+ T follicular regulatory and effector cells and identified pathogen-specific transcriptional modules that shape TFH function. Cytokine-transcriptional TFH programming demonstrated that type I interferon and TGFβ signaling direct individual TFH phenotypes to instruct B cell output. Cytokine-directed TFH transcriptional phenotypes are shared within human germinal centers, but distinct TFH phenotypes dominate between donors and following immune challenge or in antibody-mediated disease. Finally, we identified new cell surface markers that align with distinct TFH phenotypes. Thus, we provide a comprehensive resource of TFH diversity in humans and mice to enable immune monitoring during infection and disease and to inform the development of context-specific vaccines.
Similar content being viewed by others
Main
Flexibility of the immune system ensures the clearance of, and generation of memory against a wide range of pathogen classes, such as viral, bacterial, fungal and helminth infections1,2. Environmental cues, such as infection route, expression of pathogen-associated molecular pattern molecules and antigen affinity and availability, are integrated to impact the generation of specialized CD4+ T helper (TH) cells that enable pathogen-specific adaptive immunity3,4,5,6,7. Accordingly, TH1 cells produce interferon (IFN)γ and tumor necrosis factor (TNF) to assist in antiviral responses, TH2 cells mediate protection against helminth infection via the production of interleukin (IL)-4, IL-5 and IL-13, and TH17 cells secrete IL-17 family cytokines to protect against bacteria and fungi1,8. In addition to the differentiation of these CD4+ TH effector (Teff) cell subsets, CD4+ TFH cells are generated to instruct humoral immunity by promoting the germinal center (GC) reaction and differentiation of T-dependent memory B cells and plasma cells, a process dependent on the cardinal TFH cell cytokines IL-21 and IL-4 (refs. 9,10,11). In contrast to Teff cells, which, despite their plasticity, are honed to defend against particular classes of pathogens, TFH cells drive B cell responses across multiple settings while retaining the ability for context-specific tailoring1,8,9,12. Thus, TFH cells are induced by all categories of pathogens and licensed vaccines, and influence the pathogenesis of diseases such as immunodeficiency, autoimmunity, asthma, allergies and cancer9,12. How this exceptional array of TFH functions is orchestrated is not clear.
Much of the knowledge on TFH differentiation has focused on the bifurcation model, where TFH differentiation contrasts with that of TH1, TH2 or TH17 cells9,13,14; however, this model has been questioned and additional complexity has been proposed to incorporate TFH functional diversity12,15,16. While multiple cytokine signaling pathways regulate TFH fate, the role these mediators play in tailoring pathogen-specific TFH phenotypes beyond this branch point is lacking17,18,19,20,21,22,23,24.
Evidence of TFH heterogeneity has been assessed by the distinct expression of transcriptional regulators and varied production of TH cytokines and chemokine receptor surface expression12,15. The TFH lineage-defining transcription factor B cell lymphoma (Bcl)-6 can be coexpressed with transcription factors that define TH1, TH2 and TH17 differentiation, namely, T-bet, GATA3 and RORγt9,12,15,17,25. Aligned with these borrowed transcription factors, TFH cells can produce the cytokines IFN-γ, IL-4, IL-10 and IL-17 to tailor B cell responses and dictate the class-switch isoform of high-affinity antibodies11,26. In humans, circulating memory TFH (cTFH) cells are identified via the expression of chemokine receptors, where CXCR3+ cTFH cells resemble TH1 cells, CXCR3−CCR6− cells resemble TH2 cells and CCR6+ cells resemble TH17 cells12,27,28,29. Immune profiling of specific cTFH populations is correlated with disease and protective vaccine outcomes9,30,31,32. Although TFH cells can parallel Teff cells at multiple levels, the extent of TFH heterogeneity or how distinct TFH phenotypes are established remains unclear.
In this study, we transcriptionally profiled a spectrum of TFH phenotypic states induced by multiple pathogens, assessing viral, helminth and bacterial infections. By combining TFH cell profiles across pathogens, we established a core TFH signature that was separate from that of T follicular regulatory (TFR) cells and Teff cells. In addition to TFH identity, distinct TFH phenotypes were directed by cytokine pathways to enable tailoring of B cell responses. These cytokine-directed transcriptional modules were evident in human tonsils and discriminated TFH phenotypes present in clinical datasets. In combination, we present a resource to understand TFH phenotype heterogeneity and plasticity; monitor TFH dynamics during infection, vaccination, antibody deficiency and antibody-mediated disease; and reveal opportunities to fine-tune humoral responses for vaccination.
Results
Diverse pathogen challenges induce heterogeneous TFH cells
We hypothesized that pathogen-specific cues result in functionally distinct TFH phenotypes with the capacity to tailor B cell responses. To test this, we investigated TFH and Teff differentiation and function during viral, helminth and bacterial infections. As TFH cells evolve over time11,33, we assessed the early peak of TFH and GC B cell accumulation for each infection, defined by TFH and GC B cell frequency, in polyclonal TFH (CD3+CD4+CD44+Ly6C−CD162−CXCR5+PD-1+Bcl-6+) and Teff (CD3+CD4+CD44+Ly6C+CD162+CXCR5−PD-1−Bcl-6−) cell compartments after viral (acute Armstrong lymphocytic choriomeningitis virus (LCMV) or influenza A HKx31 H3N2 strain), helminth (Trichuris muris7 or Heligmosomoides polygyrus), and bacterial (Citrobacter rodentium) infections (Fig. 1a and Extended Data Fig. 1a,b). To maximize the influence of environmental cues, we analyzed the natural infection route and draining lymph nodes for each infection (inguinal, brachial and axillary lymph nodes for LCMV; mediastinal and cervical lymph nodes for influenza A; and mesenteric lymph nodes for the remaining pathogens) to investigate the breadth of TFH phenotypes. TFH cells were generated to varying degrees in all infections, as reflected by their frequency and the ratio between the TFH and Teff populations and compared to steady-state lymph nodes (Fig. 1b,c and Extended Data Fig. 1c,d).
a–i, Analysis of draining lymph node cells from wild-type (a–c,h,i) and ZsGreen_T-bet reporter (d–g) mice infected with the indicated pathogens at the early peak GC response (LCMV day 12, influenza day 10, T. muris day 21, H. polygyrus day 12 and C. rodentium day 12). Representative plots of CD4+CD44+Ly6C+CD162+ Teff cells and CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells with histograms displaying Bcl-6 expression (a). Frequencies of TFH cells in CD4+CD44+ gate (b) and the ratio of TFH:Teff cells (LCMV n = 8, influenza n = 8, T. muris n = 6, H. polygyrus n = 9, C. rodentium n = 10 mice per group) (c). Representative plots of ZsGreen_T-bet reporter expression (TFH cells are blue, Teff cells are red) (d). ZsGreen_T-bet reporter+ frequency and gMFI of CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells (LCMV n = 8, influenza n = 8, T. muris n = 6, H. polygyrus n = 10, C. rodentium n = 7 mice per group) (e). Immunofluorescence staining of draining lymph node GCs. Red arrows indicate ZsGreen_T-bet reporter+ TFH cells. Yellow, CD4; blue, IgD; magenta, GL7; green, ZsGreen_T-bet reporter. Scale bar, 200 μm (f). Correlation of ZsGreen_T-bet reporter expression with the Teff:TFH cell ratio across infections (g). Frequency of TFH cells that produced IFNγ, IL-4 or IL-17A (h). IFNγ+ TFH1, IL-4+ TFH2, and IL-17+ TFH17 cells are included in the entire TFH population (i). The inner slice displays cytokine coexpression. The outer ring (green) indicates the proportion of ZsGreen_T-bet+ TFH cells expressed. The data are from 6–10 mice per group and are presented as the mean ± s.e.m. Statistical tests included one-way analysis of variance (ANOVA) for multiple comparisons and Pearson correlation with two-tailed P values. ****P < 0.0001 or otherwise indicated.
The TH1 lineage-defining transcription factor T-bet (encoded by Tbx21) plays a context-specific role in TFH bifurcation and function in response to viral infections17,34. We therefore sought to investigate the expression of the ZsGreen-T-bet reporter across diverse pathogen classes35. Teff cells reported higher T-bet than TFH cells in all infections (Fig. 1d,e and Extended Data Fig. 1e). The T-bet expression level was graded within the TFH cell compartment (Fig. 1d,e). Confocal analysis revealed conserved draining lymph node and GC (GL7+IgD−) morphology (Extended Data Fig. 2). Within GC structures, we identified T-bet+ TFH cells in LCMV-, influenza- and T. muris-infected tissue but not in H. polygyrus or C. rodentium, consistent with the increased level of TFH T-bet expression in these infections (Fig. 1f). T-bet expression correlated with TFH:Teff bifurcation (r = 0.7416), demonstrating that the context-specific role of T-bet extends beyond viral pathogens to helminth and bacterial infections (Fig. 1g)7,19,34.
As cytokine production defines TFH cell function15, each pathogen-induced TFH population displayed a distinct cytokine profile (Fig. 1h and Extended Data Fig. 3a). This mirrored Teff cytokine production for each infection (Extended Data Fig. 3b). The majority of TFH cells demonstrated cytokine specialization, with few cells producing IFNγ, IL-4 or IL-17 in combination (Fig. 1i, inner pie slice). Intracellular cytokine expression was confirmed via the use of IFNγ-GFPx4C13R reporter mice for T. muris infection which, in addition to similar IFNγ and IL-4 expression, indicated the presence of an IL-13-expressing TFH population (Extended Data Fig. 3c). TFH cytokine production largely reflected the serum cytokine milieu (Extended Data Fig. 3d). Furthermore, overlaying TFH T-bet expression with TFH cytokine production revealed that beyond the TFH:TH1 bifurcation, T-bet underlies the IFNγ+ TFH1 phenotype across all infections (Fig. 1i).
TFH phenotypes correlate with tailored B cell responses
We next investigated how the variation in TFH cytokine expression correlated with GC B cell and antibody production. GC B (B220+CD138−IgDloCD38−CD95+) cells were quantified, with H. polygyrus generating the largest GC B cell response (Fig. 2a and Extended Data Fig. 4a). The frequency of GC B cells and the Teff:TFH ratio were not statistically correlated across infections (r = −0.3025) (Extended Data Fig. 4b). In contrast, the relative frequency of memory B cells (MBCs; B220+CD138−IgDloCD38+CD95−) correlated with a high Teff:TFH ratio (r = 0.6608) as well as high TFH T-bet+ expression (r = 0.7835) (Fig. 2b,c). Given that IL-4 progressively impacts MBC formation, these observed correlations may alter throughout GC formation and collapse11. Furthermore, serum antibody analysis demonstrated distinct IgG class-switch isotype expression, which was consistent with each infection producing a unique combination of TFH cytokines (Fig. 2d). In combination, diverse pathogen infections induce distinct populations of TFH cells that exhibit distinct cytokine profiles and correlate with GC and MBC output and with distinct antibody isotype usage.
a–d, Analysis of draining lymph node cells (a–c) and serum (d) from wild-type (a,b) and ZsGreen_T-bet (c) reporter mice infected with the indicated pathogens at the early peak GC response. Analysis of B220+IgDloCD95+CD38− GC B cells (a) and B220+IgDloCD95−CD38+ MBCs (LCMV n = 8; influenza n = 8; T. muris n = 6; H. polygyrus n = 9; and C. rodentium n = 8 mice per group) (b). Correlation of the frequency of MBC with the ratio of Teff:TFH cells and ZsGreen_T-bet reporter expression gMFI across infections (c). Serum IgG isotype concentration (d). The data are from 6–10 mice per group and are presented as the mean ± s.e.m. Statistical tests included one-way ANOVA for multiple comparisons and Pearson correlation with two-tailed P values. ****P < 0.0001 or otherwise indicated.
Core TFH and TFR transcriptional signatures
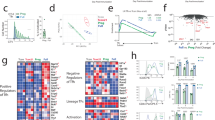
We next performed RNA sequencing (RNA-seq) on GC CD4+ T cell populations to define a core TFH transcriptional program that is centrally induced regardless of the pathogen or TFH phenotype. For this, we analyzed TFH (CD4+CD44+CXCR5+PD-1+) cells compared to Teff (CD4+CD44+CXCR5−PD-1−) cells following LCMV, influenza, T. muris, H. polygyrus and C. rodentium infection (Extended Data Fig. 5a). As IL-21+ cells have been shown to exhibit increased GC residency (referred to as GC TFH) with distinct transcriptional profiles, we used the IL-21GFP transcriptional reporter to profile both IL-21+ and IL-21− TFH cells10,36. Furthermore, we isolated TFR (CD4+CD44+FoxP3+CXCR5+PD-1+) cells, a subset that represses GC size and facilitates the emergence of high-affinity GC B cells37,38. Principal-component analysis (PCA) revealed that all samples were separated by cell type on PC1 (32.13%) and PC2 (21.9%) (Extended Data Fig. 5b). IL-21+ TFH and IL-21− TFH cells were transcriptionally distinct (Extended Data Fig. 6a); however, across infections, the intersection of similarly regulated genes revealed only Ly6c2, Il7r (encoding CD127), and Sell (encoding CD62L), were downregulated in the IL-21+ TFH population in four of five infections (Extended Data Fig. 6a). Cell surface staining confirmed the regulation of these factors at the protein level in LCMV and influenza (Extended Data Fig. 6b–g). To define the core TFH transcription program, we combined all TFH samples and performed differential expression (DE) analysis relative to Teff from each infection (Fig. 3a). Validating this approach, known TFH genes (Bcl6, Cxcr5, Pdcd1 (encoding PD-1) and Cd200) were upregulated, and known Teff genes (Klf2, Ccr7, Il7r, Ly6c1 and Sell) were downregulated (Fig. 3a). While Tbx21, Gata3 and Rorc encode transcription factors that mediate the bifurcation between TFH cells and their respective TH subsets, the core TFH signature was independent of these genes (Fig. 3a). In contrast, Foxo1, Bach2 and Foxp1 were present in the Teff core signature, indicating their potential to act in opposition to the TFH program regardless of the pathogen encountered39,40,41,42. We next performed pairwise DE analysis for each infection to establish a core TFH signature that contained common significant directional regulation across three or more infections (608 upregulated and 635 downregulated genes) (Fig. 3b and Supplementary Table 1).
a–j, Bulk RNA-seq of sorted CD4+CD44+PD-1+CXCR5+FoxP3-RFP−IL-21–GFP+ TFH, CD4+CD44+PD-1+CXCR5+FoxP3-RFP−IL-21–GFP− TFH, CD4+CD44+PD-1+CXCR5+FoxP3-RFP+IL-21–GFP+ TFR, and CD4+CD44+PD-1−CXCR5−FoxP3-RFP−IL-21–GFP− Teff cells from the draining lymph nodes of the mice infected with pathogens described in Fig. 1. MA plot visualizing the log fold change (FC) in the mean expression of genes expressed differentially between the TFH and Teff populations for the five infections (a). UpSet plot showing intersections of genes differentially expressed between the TFH and Teff populations for each infection (b). Common DE genes across three or more infections were used to derive the core TFH signature. False discovery rate (FDR) < 0.05. Heatmap of core TFH signature genes (row-based z scores of normalized log2 counts per million) for cytokine and cell surface receptor genes (c) and transcriptional regulator genes (d). GSEA of differentially expressed genes in TFH (TFH versus Teff comparison), TFR (TFR versus Teff), and Teff cells (Teff versus TFH) for all infections (e,f). NES of TFH, non-TFH, GC TFH, GC B, human TFH and cancer-associated TFH cell programs (e) and precursors of exhausted (TPEX), exhausted progenitor (TPROG) and long-term hematopoietic stem cell (HSC-LT) gene programs in the TFH, TFR and Teff populations (f). The NES score represents the enrichment of genes (sets) relative to each comparison, correcting for multiple testing. UpSet plot of the TFR signature showing intersections of upregulated genes expressed differentially between the TFR versus TFH cell contrast population and the TFR versus Teff cell contrast population for five infections (g). FDR < 0.05. Heatmap of TFR signature genes shared across contrasts (TFR versus TFH and TFR versus Teff) (row-based z score of normalized log2 counts per million) for cytokine and cell surface receptor genes (h) and transcriptional regulator genes (i). Bubble plot of Bcl-6 network transcriptional regulator genes in the TFH core showing log2FC differences in the TFH (TFH versus Teff contrast) and TFR (TFR versus Teff contrast) populations (j). The size of the bubble represents the −log10(FDR), and the color indicates the log2FC compared to Teff cells. The colored bar indicates genes present in the TFH core and Bcl-6 gene sets from Extended Data Fig. 5g. Novel genes proposed to be independent of the Bcl-6 network are indicated with gray bars. The data represent independent samples of 2–3 per cell type per infection.
Analysis of genes encoding cell surface proteins, cytokine signaling components and transcription factors revealed that the core TFH signature fell into four groups (1) TFH core genes downregulated by both Teff and TFR cells; (2) TFH genes coexpressed in TFR cells; (3) Teff core genes downregulated by TFH and TFR cells; and (4) Teff genes coexpressed in TFR cells (Fig. 3c,d) (Supplementary Table 1). Group 1 highlighted genes required for TFH function and receptors that promote GC positioning and B cell interactions (Btla and Il4)11,43, genes that assist in the transition from TFH to TFR (Sostdc1)44, transcriptional and epigenetic regulators of cell fate (Ascl2 and Ezh2)9 and genes not previously associated with TFH identity or function (Hmgb1, Padi4 and Lmo4). Group 2 contained genes required for TFH and TFR identity (Bcl6, Cxcr5, Pdcd1, Id3, Icos, Cd200, Hif1a and Tnfrsf4 (encoding OX40)), along with genes suggestive of memory potential (Tox, Tox2 and Mxd4)45. In contrast, the Teff core signature (groups 3 and 4) included antagonists of TFH lineage commitment (Foxo1, Bach2 and Foxp1)39,40,41,42, T cell zone positioning receptors (Ccr7, Sell and S1pr1), inflammatory mediators and signaling (Ly6c1, Ltb, Ifngr2, Oasl1 and Smad7) and proliferation and apoptosis inhibitors (Mcl1, Bcl2 and Myc). Core upregulated (CD200 and BTLA) and downregulated (CCR7) cell surface molecules were confirmed at the protein level across multiple infections (Extended Data Fig. 5c). The core TFH signature featured genes associated with GC TFH cells (Tigit, Maf and Plekho1)46 (Fig. 3c,d). Furthermore, gene set enrichment analyses (GSEAs) with published datasets with normalized enrichment score (NES) confirmed that the TFH cell core aligns with GC TFH cells, GC B cells and additional infection-, vaccine- and cancer-induced TFH cells in mice and humans (Fig. 3e). In line with the observation that both TFH and TFR cells (group 2 genes) expressed checkpoint receptors (Pdcd1, Ctla4, Lag3 and Tigit) and the aforementioned drivers of long-term memory, GSEA-NES analysis revealed alignment with gene signatures of CD8+ T cell precursors and progenitors of exhausted cells from chronic infection and cancer and signatures associated with long-term hematopoietic stem cell memory relative to Teff cells (Fig. 3f). Thus, the core TFH signature is suggestive of increased memory potential, similar to that observed for stem-like memory CD8+ T cells47.
We next performed differential gene analysis to identify a TFR cell transcriptional signature that was distinct from that of TFH and Teff cells (Fig. 3g and Extended Data Fig. 5d–f). This highlighted the effector status of TFR cells coordinated by Prdm1 (encoding BLIMP) and the expression of suppression genes (Foxp3, Il10, Ctla4 and Entpd1 (encoding CD39); Fig. 3h,i)48,49. Furthermore, this identified cytokine receptor (Il2ra, Il2b and Il1rl1) pathways, co-stimulation markers (Tnfrsf18 (encoding GITR), Tnfrsf4 (encoding OX40), Cd80 and CD83) and chemokines and integrins that distinguish TFR cells (Fig. 3h).
As Bcl-6 promotes multiple aspects of TFH cell fate and function9,50,51,52, we next investigated how individual transcription factors expressed in both the TFH and TFR core signatures are influenced by Bcl-6. Aligned with the role of Bcl-6 as an obligate repressor, direct Bcl-6 targets were decreased in TFH and TFR cells relative to Teff cells (Fig. 3j)50,52,53,54. Genes indirectly regulated by Bcl-6 through repressor-of-repressor (Bcl-6-rr) circuits showed increased expression in TFH and TFR cells (Fig. 3j and Extended Data Fig. 5g)53. Additionally, the Bcl-6-rr genes overlapped with genes known to be upstream inducers of Bcl-6. While a small group of transcriptional regulators were separate from known Bcl-6 circuits, the majority were within the Bcl-6 network, emphasizing that Bcl-6 underpins TFH and TFR differentiation independent of the pathogen class.
Pathogen-specific TFH transcriptional signatures
While T-bet expression correlated with the number of IFNγ+ TFH1 cells (Fig. 1i), other regulators of TFH heterogeneity are undefined. We therefore examined the pathogen-specific transcriptional programs of TFH phenotypes. Multidimensional scaling (MDS) and PCA revealed that pathogen-directed TFH cells were separated by infection into PC3 (6.94%) and PC4 (4.44%) (Fig. 4a and Extended Data Fig. 5b). DE analysis compared samples from each infection to the combined transcriptomes of all others for both the TFH and Teff populations. For influenza and H. polygyrus, a large proportion of pathogen-specific genes were shared between TFH and Teff cells, whereas this was not the case for LCMV or T. muris (Fig. 4b). We therefore defined pathogen-specific TFH signatures to include the shared and TFH distinct differentially regulated genes. Analysis of cell surface and cytokine genes revealed distinct cytokines and chemokines (Ifng, Cxcl10, Il6, Ccl1, Il4, Il22 and Il17) and inhibitory and co-stimulatory molecules (Cd80, Cd274 (encoding PD-L1), Cd40 and Havcr2 (encoding TIM3)) (Fig. 4c,e). Cell surface molecules (CXCR3, CCR5, LAG3 and CCR4) that indicated distinct pathogen-specific TFH phenotypes were confirmed at the protein level across multiple infections (Fig. 4h). TFH cells arising from each infection also expressed a distinct set of transcriptional and epigenetic regulators (Fig. 4d). We observed that Tbx21, Gata3 and Rorc were differentially expressed within the pathogen-specific signatures (Fig. 4d,f). Tbx21 and Gata3 showed graded, reciprocal expression between infections, whereas C. rodentium was the only infection that induced TFH cell Rorc expression (Fig. 4f).
a–g, Bulk RNA-seq of sorted CD4+CD44+PD-1+CXCR5+FoxP3-RFP−IL-21–GFP+ TFH, CD4+CD44+PD-1+CXCR5+FoxP3-RFP−IL-21–GFP− TFH cells from the draining lymph nodes of mice infected with the indicated pathogens (as in Fig. 1). MDS plot of CD4+CD44+PD-1+CXCR5+FoxP3-RFP−IL-21–GFP+ TFH transcriptomes showing the separation of samples by infection type along dims 1 and 2 (a). Genes expressed differentially for TFH (single infection vs. all other infections) and Teff cells (single infection versus all other infections) (FDR < 0.05) (b). Orange, common pathogen-specific DE genes in both TFH and Teff cells; blue, pathogen-specific signatures unique to TFH cells; red, pathogen-specific signatures unique to Teff cells. Heatmaps of pathogen-specific TFH signature genes (average row-based z score of normalized log2 counts per million) for selected cytokine and cell surface receptor genes (c) and transcriptional regulator genes (d). Cytokine pathway genes from Mouse Genome Informatic (MGI) database gene sets indicated in colored circles. IFN (red), TNF (purple), IL-1 (pink), TGFβ (blue), IL-4 (green), IL-6 (yellow) and IL-17 (orange). Heatmap of pathogen-specific TFH signature genes (average row-based z score of normalized log2 counts per million) for selected signature-defining cytokines and chemokines for each infection (e) and lineage-defining transcription factors (f). GSEA of pathogen-specific signatures via MGI MSigDB ‘Hallmark’ gene sets for cytokine signaling and response (g). Bubble size represents the -log10(FDR), and color indicates the NES. The data represent independent samples of 2–3 per cell type per infection. h, Analysis of draining lymph node CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells from wild-type mice infected with indicated pathogens (as in Fig. 1) displaying gMFI of pathogen-specific TFH signature marker (LCMV n = 7; T. muris n = 5; and C. rodentium n = 7 mice per group). Data show experiments of 5–7 mice per group and mean ± s.e.m. Statistical tests were one-way ANOVA of multiple comparisons and Pearson correlation with two-tailed P values. ****P < 0.0001 or otherwise indicated.
Cytokine signaling pathways instruct TFH phenotypes
Pathogen-specific TFH signatures indicated multiple genes that aligned to distinct cytokine signaling pathways (Fig. 4c,d). Therefore, we performed GSEA on the cytokine hallmark gene sets of the MSigDB database to understand the cytokine signaling pathways that may direct different TFH phenotypes. Type I and type II IFN (IFN-I and IFN-II, respectively) signaling genes were positively enriched in TFH cells from LCMV and T. muris (Fig. 4g). In contrast, these pathway genes were negatively enriched for H. polygyrus and C. rodentium, indicating IFNs as drivers of pathogen-specific TFH phenotypes. Notably, despite being a viral infection the cytokine signaling observed in TFH cells from influenza was distinct from those in LCMV-stimulated cells (Fig. 4c,d,g). Instead, TFH cells in influenza were enriched for TGFβ signaling. These data support the concept that cytokine pathways are a stronger driver of specific heterogeneous TFH cell phenotypes over pathogen class (Fig. 4c–e,g). We next investigated the role of TGFβ and IFN-I signaling in infection models dominated by the cytokine pathways of influenza and LCMV, respectively. For this purpose, the TFH phenotypes were categorized into TFH1, TFH2 and TFH17 cell subpopulations according to CXCR3 and CCR6 chemokine expression, which aligns with differential cytokine production (Extended Data Fig. 7a–c). Consistent with previous work, targeted T cell deletion of TGFβR2 (Tgfbr2fl/flCreLCK) decreased the TFH:Teff cell ratio (Extended Data Fig. 7d). In addition, the TFH cell phenotype composition was altered, with increased frequency of TFH1 and decreased TFH2 and TFH17 cell populations (Fig. 5a). Mixed (50:50 wild-type:Tgfbr2fl/flCreLCK) bone marrow chimeras were generated to demonstrate that TGFβ modified the TFH cell phenotype in a cell-specific manner (Fig. 5b and Extended Data Fig. 7e). An altered TFH cell phenotype impacted the B cell response, with decreased frequency of cell surface IgG1+ and reciprocally increased IgG2c+ GC B cells (Fig. 5c,d). Additionally, GC cycling was dysregulated (Fig. 5c and Extended Data Fig. 7l). IFNAR deficiency (Ifnar−/−) resulted in reciprocal alterations in TFH:Teff generation compared to TGFβR2 deficiency (Extended Data Fig. 7f). Within TFH cells, the loss of IFN-I signaling decreased the frequency of TFH1 and TFH17 cells and increased the TFH2 cell phenotype (Fig. 5e). Mixed (50:50 wild-type:Ifnar−/−) bone marrow chimeras confirmed that the altered TFH phenotypes were cell intrinsic (Fig. 5f and Extended Data Fig. 7g). Furthermore, these altered TFH cell phenotypes were reflected in the B cell response, with increased IgG1+ GC B cells, decreased dark zone GC B cells and increased serum IgG1 concentrations (Fig. 5g,h). To investigate how context-specific cytokine signals drive TFH phenotypes, we tested each mixed chimera with the alternative infection. This demonstrated that both TGFβ and IFN-I mediate the context-dependent regulation of TFH:Teff cell bifurcation (Extended Data Fig. 7h). TGFβR2 deficiency altered TFH cell composition in both influenza and LCMV infection (Extended Data Fig. 7i–k). In contrast, IFN-I had a more dominant role for TFH composition in LCMV indicating it is a pathogen-specific factor that alters TFH phenotype, function and B cell outcomes. Combined, this provides new insight into how cytokine signaling networks integrate to shape the functional TFH cell response to direct B cell and GC output.
a–d, Analysis of draining lymph nodes from Tgfbr2-LckCre and LckCre control mice infected with influenza A virus in intact mice (a,c,d) and 50:50 bone marrow chimera mice (b). CXCR3+ TFH1, CXCR3−CCR6− TFH2, and CCR6+ TFH17 cells within CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells are displayed as parts of the whole population (a,b). Frequency within TFH population, and representative histograms of CXCR3 and CCR6 expression on TFH cells (n = 9–10 mice per group) (a). The inner slice of the pie chart displays CXCR3+CCR6+ coexpression. Wild-type and Tgfbr2-LckCre cells were identified by congenic marker expression (b). Frequency of CXCR3+ TFH1, CXCR3−CCR6− TFH2, and CCR6+ TFH17 cells and frequency of IFNγ+ TFH1 cells within CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells (n = 10 mice per group). The inner slice of the pie chart displays CXCR3+CCR6+ coexpression. IgG1 or IgG2c class switched B220+IgDloCD95+ GC B cells and CD86−CXCR4+ dark zone (DZ) or CD86+CXCR4− light zone (LZ) B cells on B220+IgDloCD95+ GC B cells (n = 7–10 mice per group) (c). Serum IgG isotype concentration (d). e–h Draining lymph node analysis of Ifnar−/− and Ifnar +/+ control mice infected with LCMV in intact mice (e,g,h) and 50:50 bone marrow chimera mice (f). CXCR3+ TFH1, CXCR3−CCR6− TFH2, and CCR6+ TFH17 cells within CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells are displayed as parts of the whole population (e,f). Frequency within TFH population, and representative histograms of CXCR3 and CCR6 expression on TFH cells (n = 7 mice per group) (e). The inner slice of the pie chart displays CXCR3+CCR6+ coexpression. Wild-type and Ifnar−/− cells were identified by congenic marker expression (f). Frequency of CXCR3+ TFH1, CXCR3−CCR6− TFH2, and CCR6+ TFH17 cells and frequency of IFNγ+ TFH1 cells within CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells (n = 8 mice per group). The inner slice of the pie chart displays CXCR3+CCR6+ coexpression. IgG1 or IgG2c class switched B220+IgDloCD95+ GC B cells and CD86−CXCR4+ DZ or CD86+CXCR4− LZ B cells from B220+IgDloCD95+ GC B cells (n = 7 mice per group) (g). Serum IgG isotype concentration (h). The data are from 7–10 mice per group and are presented as the mean ± s.e.m. Statistical tests were a two-tailed unpaired Student’s t-test for the intact system and a two-tailed paired Student’s t-test for the bone marrow chimera model. ****P < 0.0001 or otherwise indicated.
Heterogeneous TFH phenotypes in human tissue
Human tonsils are secondary lymphoid organs that are constantly exposed to the upper respiratory tract. Despite this, minimal tonsillar TFH heterogeneity exists due to a lack of both transcriptional heterogeneity and identification of CXCR3+ and CCR6+ populations9,55. We hypothesized that our derived pathogen-specific TFH signatures may provide new insight into TFH heterogeneity within human lymphoid tissue. We performed paired single-cell RNA-seq (scRNA-seq) and surface protein sequencing (CITE-seq) analysis on TFH cells (CD3+CD4+CD45RA−CD45RO+CXCR5+) from tonsils of adults with sleep apnea but otherwise ostensibly healthy individuals (Extended Data Fig. 8a,b and Supplementary Table 2; Donor Table 1). Unsupervised Louvain clustering of 24,393 TFH cells identified 11 transcriptionally distinct TFH cell clusters (C) (Fig. 6a). C4 disproportionally consisted of cells from the female donor; however, removing X-chromosome genes from the dataset had minimal effect on cluster distribution and DE gene analysis (Extended Data Fig. 8c and Supplementary Table 3). The ranked scores for pathogen-specific signatures (identified in Fig. 4) were overlaid onto Uniform Manifold Approximation and Projection (UMAP) pseudobulked clusters to indicate the proportion of pathogen-driven TFH cell signatures evident within human TFH cell clusters (Fig. 6b and Extended Data Fig. 8d). Overlap between mouse and human datasets was considerable considering the experimental differences in acquiring cells from acute pathogen infection and healthy human lymphoid tissue (Extended Data Fig. 8d). Further, the core Teff (TFH downregulated) cell signature was not evident in our human TFH cell dataset (Extended Data Fig. 8b). Consistent with the overall cytokine milieu directing signatures, Teff cell pathogen-specific signatures were present on the TFH cell dataset, although these were less aligned with distinct human TFH cell clusters (Extended Data Fig. 8e). For all three individual tonsil samples, the LCMV and influenza signatures aligned to C7 and C8, respectively (Fig. 6b–d). In addition to LCMV, T. muris also scored high on C7, whereas the H. polygyrus and C. rodentium signatures highlighted C2, C5 and C11, and C3 and C9, respectively (Fig. 6b–d). Dichotomy between clusters was observed, with inverse alignment between C8 and C7 when ranked on the basis of the influenza and LCMV or T. muris signatures, with C2 and C7 when ranked on the basis of H. polygrus and T. muris, and with C9 and C11 when ranked on the basis of the C. rodentium and H. polygyrus signatures (Fig. 6b,d). As C7 and C8 were the most separated from other TFH populations and were the most polarized by pathogen-specific signatures, we performed GSEA and visualized the results via vissE analysis. Consistent with cytokine-guided differentiation, C7 was enriched in gene networks related to IFN signaling, whereas C8 was enriched in gene networks related to TGFβ, IL-23, IL-6, IL-21 and IL-27 signaling (Fig. 6e). Similarly, GSEA-vissE analysis of C1 revealed enrichment of cytokine signaling gene networks for TH17 cell and IL-2 family signaling responses, whereas C2 was enriched in TH2 cell pathway genes (Extended Data Fig. 8f). Thus, distinct cytokine pathways are evident in both mouse and human TFH cell phenotypes.
a–e, scRNA-seq of sorted CD3+CD4+CD45RA−CD45RO+CXCR5+CD27+ human tonsillar TFH cells from three healthy adult donors. UMAP dimensional reduction of data depicting 11 clusters on the basis of Louvain clustering via the Jaccard similarity index (k = 9) (a). Overlay of the mean ranked scores of each pathogen-specific signature onto UMAP clusters (b). Mean of the ‘TotalScore’ from the singscore: simpleScore function collated for each cluster. Pathogen-specific upregulated signature genes used. Rank scores of pathogen-specific signatures for each pseudosample on the basis of cluster and individual donors (c). Influenza A versus LCMV (left); LCMV versus T. muris (middle); influenza A versus H. polygyrus (right). Heatmap of the mean rank scores of pathogen-specific signatures for each cluster (row-based z score of the mean rank score) (d). GSEA-vissE analysis for the comparison of C7 versus C8 (e). Top gene sets of selected clusters as bar plots of DE gene counts with corresponding gene statistics (FDR as color shade) from the DE analysis. GSEA was performed via the Hallmarks c2 (‘CP:REACTOME’, ‘CP:PID’, ‘CP:BIOCARTA’,‘CP:KEGG’) and c5 (‘GO:BP’,‘GO:MF’) collections from the MSigDB using a two-tailed approach correcting for multiple testing with the FDR adjusted to P ≤ 0.05. f, Ranked score of pathogen-specific signatures in human dataset TFH cells from donors in SARS-CoV-2 vaccine (n = 5), influenza vaccine (n = 1), malaria infection (adult n = 3, child n = 3), peanut allergy (n = 3), asthma (n = 4) and autoimmune contexts (healthy donor n = 6, systemic lupus erythematosus n = 8). Ranked scores normalized to the first time point for signatures. Data are presented as mean ± s.e.m. g, Visium spatial tonsil data displaying a ranked score of pathogen-specific signatures onto GCs highlighted by the core TFH cell signature. h, Xenium spatial human lymph node data displaying a ranked score of pathogen-specific signatures in TFH cells and GCs highlighted by the core TFH cell signature.
To test the utility of cytokine-directed pathogen-induced TFH transcriptional signatures, signatures were overlayed onto existing human datasets via a ranked score approach. Ranking context-specific transcriptional signatures onto longitudinal vaccine fine-needle aspirate scRNA-seq data following either SARS-CoV-2 or influenza vaccination demonstrated that distinct TFH phenotypes dominate each response (Fig. 6f and Extended Data Fig. 8g). Furthermore, distinct TFH phenotypes dominated cTFH responses during adult and child malaria infection, during oral exposure to food antigen-tolerant immunotherapy, during allergy challenge, or in lupus patients compared to those in healthy donors (Fig. 6f and Extended Data Fig. 8g). Across these distinct immune-modifying conditions, the induction of the LCMV TFH phenotype dominated in highly inflammatory settings (such as SARS-CoV-2 vaccination, allergic asthma and lupus), whereas the C. rodentium TFH cell phenotype emerged in malaria infection in children and oral tolerance therapy and was specifically lost in non-allergic asthma and lupus disease (Fig. 6f).
Visium human tonsil atlas and publicly available Xenium steady-state human lymph node data showed that TFH cell signatures were shared within GC structures (Fig. 6g,h and Extended Data Fig. 8h,i). While GCs within each tissue biopsy sample highlighted similar TFH cell signature rankings, heterogeneity was observed when tonsillar tissue from different donors was compared (Extended Data Fig. 8j). These data suggest that cells exhibiting distinct TFH cell phenotypes are capable of mixing within GC structures, but lymphoid tissue displays a dominant TFH cell phenotype. Thus, identified TFH cell signatures can be used to probe TFH cell function following immune challenge or in antibody-mediated diseases.
Cell surface identification of TFH phenotypes
We next sought to identify a tractable method to identify cytokine-directed TFH cell phenotypes within human lymphoid tissue. Using surface protein analysis in our scRNA-seq dataset, key markers of cell identity (CD4 and CD45RO) and TFH (CD279 and CD278) and Teff (CD62L and KLRG1) cell core signatures were identified (Extended Data Fig. 9a and Supplementary Table 2; Donor Table 2). CXCR3, CCR4 and CCR6 expression were minimally detected and did not indicate distinct TFH cell clusters (Fig. 7a and Extended Data Fig. 9b)9,55. Instead, several markers discriminated either individual or groups of TFH cell transcriptional phenotypes (Extended Data Fig. 9c). We next examined whether these markers could identify populations in human tonsils and peripheral blood mononuclear cells (PBMCs) from healthy adult donors. Consistent with previous studies, CD57+ TFH cells were exclusively found in tonsillar samples, aligning them with a GC TFH cell population with high CXCR5, ICOS and CCR4 expression to engage B cells (Fig. 7b and Extended Data Fig. 9d,e)9,56,57,58. Similarly, PD-1, CD69 and CD82 were differentially expressed between tonsil TFH and cTFH cells (Fig. 7c). In contrast, CD127 (IL-7Rα), CD99, CD71 and CD151 exhibited considerable overlap, albeit with different frequencies, between tonsillar PD-1+CD57+, PD-1+CD57−, PD-1−CD57− TFH cells and PD-1−CD57− cTFH cells (Fig. 7d,e and Extended Data Fig. 9f,g). TFH phenotype marker expression did not align with the expression of CXCR3, CCR4 or CCR6, and their expression was largely stable following PMA/ionomycin stimulation (Extended Data Fig. 9g,h). Matching markers onto scRNA-seq clusters (C3, C7, C9: CD57; C1: CD99; C8: CD71, CD151; and C10: CD127) identified the dominant TFH cell phenotype represented by each marker (Fig. 7f and Extended Data Fig. 9c). We next assessed how these markers of TFH cell heterogeneity track across different tissue sites, comparing donor-matched tonsil, adenoid and PBMCs in five juvenile donors (Supplementary Table 2; Donor Table 3). While some cell surface markers (PD-1, CD57 and CD69) were restricted to lymphoid organs, CD151-, CD99-, ICOS-, CD71- and CD43-expressing TFH cells had counterparts represented in each site, potentially indicating shared origins of populations (Fig. 7g). Tonsil TFH cell populations defined by CD151 and CD99 exhibited distinct expression of activation and co-stimulatory molecules, suggesting distinct functional potential (Extended Data Fig. 10a). Therefore, we next explored CD151 and CD99 expression longitudinally on SARS-CoV-2 Spike tetramer+ antigen-specific cTFH cells following either infection or mRNA-LNP (Comirnaty) vaccination (Extended Data Fig. 10b–d and Supplementary Table 2; Donor Tables 4 and 5). cTFH PD-1 expression decreased following antigen clearance in convalescent infection and 3 months after vaccination (Fig. 7h,i). While no significant differences were observed using CXCR3 and CCR6, CD99− (both Q3 CD151+ and Q4 CD151−) cTFH cells were increased during acute SARS-CoV-2 infection compared to convalescence, which showed a shift toward Q1 (CD151−CD99+) (Fig. 7h). In comparison, Spike tetramer+ cTFH cells 7 days after vaccination increased Q2 (CD151+CD99+), demonstrating the ability to identify distinct cTFH populations following different immune challenges (Fig. 7h,i). Combined, this paired cell surface and transcriptional resource of TFH phenotypes paves the way for investigating how functional TFH heterogeneity arises and shapes B cell responses in human health and antibody-mediated disease.
a–e, Flow cytometry analysis of CD3+CD4+CD45RA−CD45RO+CXCR5+ TFH cells from five PBMC and six tonsil healthy adult donors. t-distributed stochastic neighbor embedding (t-SNE) and heatmap expression of TFH cell markers of PD-1, CXCR3, CCR6 and CCR4 (a). TFH cell populations identified by PD-1 and CD57 with expression of the TFH cell markers CXCR5, ICOS, OX40 and CCR4 on each population (b). Histograms of PD-1, CD57, CD69 and CD82 expression on TFH cells (c). Histograms of CD127, CD99, CD71 and CD151 expression on TFH cells (d). TFH populations separated into quadrants (Q1–Q4) based on expression of CD99 and CD151 markers (e). f, scCITEseq surface protein expression (log counts) of sorted CD3+CD4+CD45RA−CD45RO+CXCR5+CD27+ human tonsillar TFH cells from three healthy adult donors (as in a–e) of cluster-identifying markers overlaid onto the scRNA-seq UMAP. g, TFH cell surface and activation markers in CD3+CD4+CD45RA−CD45RO+CXCR5+ human TFH cells from matched tonsil, adenoid tissue and PBMC from five healthy juvenile donors. h,i, SARS-CoV-2 Spike tetramer+ CD3+CD4+CD45RA−CXCR5+ cTFH cells from SARS-CoV-2 infected donors (h) at time of infection (11 of 11 donors; n = 11) and 6 months convalescence (10 of 11 donors; n = 10) and nine COVID-19 mRNA vaccinated donors (n = 9) (i) at 7 days post-vaccine and 3 months post-vaccine. Frequency of Spike tetramer+PD-1+ TFH cells, gMFI expression for CXCR3 and CCR6, and frequencies and dot plots of CD151/CD99 quadrant-separated TFH cell populations. Data show experiments of 8–11 samples per group. Statistical test was a two-tailed paired Student’s t-test. P values are indicated.
Discussion
Here, we interrogated the TFH cell phenotypes that emerged following diverse pathogen challenge to understand how functional TFH cell flexibility is established. Our data are consistent with an initial bifurcation between TFH and Teff cells (comprising TH1, TH2 and TH17 cell populations) to establish a core TFH cell transcriptional signature irrespective of the pathogen class13. While graded T-bet expression correlated with Teff:TFH cell ratios, the TFH cell core signature was independent of Tbx21, Gata3 and Rorc. Instead, Foxo1, Bach2 and Foxp1 opposed the TFH program independently of pathogen class39,40,41,42. We identified several new core transcriptional regulators that may direct TFH cell differentiation or function independently from the Bcl6 transcriptional network50,51,52,53,54,59. Our data highlight a secondary differentiation branching that occurs after, or parallel with, the initial bifurcation, which directs the functional diversification of TFH cells. Here, Bcl-6 was coexpressed with distinct lineage-defining transcription factors that superimpose a stimulus-specific program to direct the function and tailoring of B cell responses. Of note, some TFH cell core signature genes were also found within specific TFH cell phenotypes, suggesting these factors are expressed in a spectrum (rather than on/off switches). In addition to Teff and TFH cells, equivalent transcriptional modules led by T-bet, GATA3 and RORγt exist for T regulatory, innate lymphoid and dendritic cells60,61,62. Thus, our results highlight the dual transcriptional processes of lineage identity and functional transcriptional modules as central processes that underpin how the immune system adapts to diverse environmental challenges.
The concentrations of cytokines, namely, IL-2, IL-6 and IL-12, mediate the balance of Bcl-6, T-bet and GATA3 to direct differentiation to either TFH or Teff cell fates14,17,18,21,63. We extend this concept to show that local cytokine exposure also specifies functional TFH cell phenotypes. TGFβ or IFN-I signaling altered TFH cell phenotype differentiation in a context-dependent manner, and in turn, shapes B cell output. Notably, cytokine signaling was a stronger driver of TFH cell differentiation than pathogen class, as TFH cells in LCMV and T. muris featured IFN-I signaling pathways, whereas TFH cells in influenza did not. Our mouse resource was generated using the C57BL/6 strain; however, genetic background will impact the cytokine milieu that alter TFH cell specification. Nevertheless, these cytokine signaling pathways were confirmed in human TFH cell populations, indicating their potential to drive the ontogeny of TFH cell heterogeneity in both species. Accordingly, IFNAR deficiency mirrored T-bet deletion in mice and human T-bet loss-of-function mutations34,64. There are likely other cytokines that also shape TFH cell function. Indeed, H. polygyrus TFH cells were not enriched for either IFN-I or TGFβ signaling, suggesting that an alternative cytokine or receptor‒ligand pathway performs TFH cell functions in this context. In human TFH cells, we identified clusters enriched for IL-23, IL-6, IL-2 and IL-1 signaling. Thus, the local cytokine environment guides the generation of functionally distinct TFH cell phenotypes. These data rationalize multiple studies that have identified conflicting or context-dependent cytokines and transcription factors that modulate TFH cell differentiation, suggesting that these factors may modify the differentiation of specific TFH cell phenotypes rather than the overall TFH cell fate17,22,34,65,66.
To gain an expansive understanding of TFH cell heterogeneity, we analyzed pathogen infections that differ by infection route, lymph node drainage and varied antigen load and affinity. Each of these factors likely plays a role in generating the cytokine milieu that determines the TFH cell phenotype and function. Still, pathogens (C. rodentium, T. muris and H. polygyrus) infected via the same route yielded distinct TFH cell phenotypes suggesting TFH cell diversity is more directed by distinct pathogens and cytokine signaling than infection route alone. Even so, our dataset provides a resource for these aspects to be investigated individually. Similarly, pathogen-induced TFH cell phenotypes were compared at a single time point corresponding to early GC establishment. As TFH cell phenotypes may change over time11,33, how the stability and plasticity of TFH cell signatures is maintained over the course of GC duration, memory and rechallenge responses remains to be determined. The combination of our mouse and human transcriptional dataset and human cell surface resources provides new tools to address these questions.
Although the cytokine signatures overlapped in our mouse and human TFH cell datasets, there are some important differences. We showed that CD99 is a new marker for human TFH cell heterogeneity, which is differentially expressed in vaccine and infection. While there is a proposed mouse homologue for CD99, this is encoded on a different chromosome (chromosome 4, rather than X and Y in humans) and was not detected in our mouse dataset. Similarly, B3GAT1 (encoding CD57) which is expressed on GC TFH cells in humans56 did not appear in our mouse dataset. Future work will investigate the functional relevance of human TFH cell phenotypes that are defined by these new markers.
We demonstrated that cytokine-mediated alterations in the TFH cell phenotype influenced GC B cell and antibody production. Evidence of cytokine-biased TFH cell phenotypes in human and mouse systems enables opportunities to target these signaling pathways to direct B cell and antibody responses for the development of optimal humoral immunity in response to vaccines against a diverse range of pathogens. Furthermore, our approach to match transcriptional TFH cell phenotypes with novel cell surface markers of TFH cell heterogeneity unlocks avenues to understand and treat antibody-mediated diseases such as immunodeficiency, allergy, asthma and autoimmunity.
Methods
Mice
The mice were bred and maintained on a C57BL/6 background under specific-pathogen-free conditions in-house at the Walter and Eliza Hall Institute of Medical Research (WEHI) at 19–24 °C, 45–65% humidity on a 12-h light–dark cycle. The T-bet-ZsGreen reporter35, IL-4–AmCyan–IL-13–DsRed–IFN-γ–GFP reporter7,67, IL-21–GFP reporter10, FoxP3–RFP reporter68, Tgfbr2-LckCre (ref. 69) and Ifnar−/− (ref. 70) mice used have been previously described. Mixed chimeras were generated via lethal irradiation of Ly5.1xC57BL/6 mice (two doses of 0.55 Cy), reconstituted with Tgfbr2-LckCre or Ifnar−/− and Ly5.1/2 bone marrow at a 1:1 ratio and left for 8 weeks before infection. All experiments were conducted in compliance with the guidelines of the Walter and Eliza Hall Institute Animal Ethics Committee and performed on sex-matched 6–10-week-old mice.
Infections
Mice inoculated intravenously with 3 × 103 plaque-forming units (p.f.u.) of LCMV-Armstrong were collected 12 days post-infection. Mice intranasally infected with 1 × 104 p.f.u. influenza A virus strain HKx31 were collected 10 days post-infection. Mice infected via oral gavage with 2 × 109 colony-forming units of C. rodentium, 200 embryonated T. muris eggs, or 200 L3 stage H. polygyrus larvae were collected 12, 21 and 12 days after each infection, respectively.
Human tonsil, adenoid tissue and PBMC samples
Details of cryopreserved tonsil, adenoid tissue and PBMCs used in scRNA-seq and flow cytometric analyses provided in Supplementary Tables 1–5. Juvenile tonsil, adenoid tissue and PBMC sample collection were approved by the Tasmanian Human Research Ethics Committee. SARS-CoV-2 study protocols were approved by the University of Melbourne Human Research Ethics Committee (approval nos. 2056689, 13793 and 23497) and Royal Melbourne Hospital Ethics Committee (study no. 2021/272) and carried out in accordance with the approved guidelines71. Patients recovered from SARS-CoV-2 infection and/or been vaccinated with Moderna BA.1 bivalent messenger RNA vaccine were recruited through contacts with the investigators and invited to provide blood samples. No statistical methods were used to predetermine sample sizes. Whole blood was collected with sodium heparin anticoagulant. PBMCs were isolated via Ficoll-Paque (Sigma), cryopreserved in 90% fetal calf serum (FCS)/10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen. All procedures involving human participants were approved by and in accordance with the ethical standards of the Human Research Ethics Committee at WEHI and the 1964 Helsinki Declaration and its later amendments.
Method details
Flow cytometry
All mice were collected at the early peak of TFH and GC B cell accumulation in the respective draining lymph nodes for each infection. Single-cell suspensions of the appropriate draining lymph nodes were stained for surface antigen expression via the indicated antibodies for 20 min at 4 °C, followed by viability dye staining for 10 min at 4 °C. For cytokine detection, single-cell suspensions were stimulated in round-bottom tubes at 37 °C + 5% CO2 in RPMI with 100 ng ml−1 PMA (Sigma), 500 ng ml−1 ionomycin (Sigma), 100 ng ml−1 brefeldin A (BD), 100 ng ml−1 Monesin (BD) and 10% FCS for 4 h. Cytokine staining was performed using BD Cytofix/Cytoperm kit (BD). Transcription factor staining was performed using Invitrogen Foxp3 Transcription Factor Staining kit (Thermo Fisher). Flow cytometry analysis was performed on a BD LSRFortessa X-20, BD FACSymphony A3 Cell Analyzers (BD) and Cytek Aurora (Cytek Biosciences) and analyzed using FlowJo v.10 (FlowJo).
Generation of peptide MHC II tetramers
Human DPB1*04:01 SSANNCTFEYVSQPFLMDLE (SARS-CoV-2 S167–180) biotinylated monomers were generated by NIH Tetramer Core Facility. DRB1*15:01 NLLLQYGSFCTQLNRAL (SARS-CoV-2 S751–767), DRB1*04:01 YQTSNFRVQPTESIVRFPNI (SARS-CoV-2 S313–332), DRB1*04:01 NFSQILPDPSKPSKRSFIED (SARS-CoV-2 S801–820) biotinylated monomers were produced by ProImmune. Biotinylated monomers were tetramerized by sequential addition of streptavidin-APC (Thermo Fisher).
Tetramer staining
Cryopreserved PBMCs were thawed in RPMI-1640 (Thermo Fisher) with 10% FCS and 2% penicillin–streptomycin (RF10). Up to 1 × 106 PBMCs were washed in 2% FCS/PBS before incubation with 50 nM dasatinib (in 2% FCS/PBS) at 37 °C for 30 min. Tetramers were added at 4 µg ml−1 at 37 °C for 60 min. A cocktail of chemokine receptors comprising of CXCR5-BB515, CXCR3-Pacific Blue and CCR6-BV785 was added during the last 30 min of tetramer staining. Cells were washed in PBS, stained with viability dye, and incubated for 30 min at 4 °C with the indicated surface stain antibodies. Cells were washed with 2% FCS/PBS and fixed with 1% Cytofix (BD). Data were acquired on a FACS Symphony A5 SE (BD).
Immunofluorescence staining and confocal microscopy
Lymph nodes were collected and fixed in 4% paraformaldehyde (Sigma) for 8 h at 4 °C, immersed in 30% sucrose overnight at 4 °C, and embedded in OCT compound (Tissue-Tek). The tissues were cut via a microtome (Leica) into 12–20-μm sections and mounted onto Superfrost Plus slides. The slides were incubated in blocking buffer containing 2% normal rat serum (Jackson ImmunoResearch) and 0.1% Triton X-100 (Sigma) in PBS for 24 h at 4 °C. Sections were stained with antibodies in buffer containing 0.2% normal rat serum (Jackson ImmunoResearch) and 0.01% Triton X-100 (Sigma) in PBS overnight at 4 °C. The slides were washed by immersion in PBS containing 0.1% Triton X-100 (Sigma), and the coverslips were mounted with Prolong Diamond (Thermo Fisher). Images were acquired on an LSM980 confocal microscope (Carl Zeiss MicroImaging) and processed via Zen Black (Zeiss) software.
Serum cytokine bead array
Blood was collected from infected mice and centrifuged at 20,000g for 15 min. Serum supernatant was collected and loaded onto a BD Mouse TH1/TH2/TH17 CBA kit (BD) according to the manufacturer’s instructions. In brief, serum and standards were added to cytokine capture beads and PE detection reagent in a 96-well plate for 2 h at 20 °C in the dark. The plate was washed, resuspended and acquired on a BD LSRFortessa X-20 cell analyzer (BD). Data analysis and standard curve generation were performed with FCAP Array Software v.3.0 (BD).
ELISA
High-binding 96-well ELISA plates (Sarstedt) were coated with anti-IgG unconjugated antibodies (Southern Biotech) and incubated overnight at 4 °C, followed by incubation with blocking buffer containing 1% bovine serum albumin (BSA) in PBS for 1 h at 20 °C. The supernatant was removed, and the plates were washed with PBS containing 0.04% Tween 20 (Thermo Fisher) followed by incubation with dH2O. Serum samples were diluted in 1% BSA in PBS, serially diluted threefold and incubated for 3 h at 37 °C. The plates were washed and incubated with secondary antibody conjugated to horseradish peroxidase (Southern Biotech) for 1.5 h at 37 °C. The plates were washed and the OPD color reaction mixture (Sigma) was added to each well. The absorbance was measured at 450 nm via a FLUOstar Omega Microplate reader (BMG LABTECH).
Antibodies and dyes for staining
Mouse T cell analysis
Single-cell suspensions were stained with fixable viability stain 700 (1:1,000 dilution; BD; cat. no. 564997); anti-CD4 (1:600 dilution; clone GK1.5; BD); anti-CD3 (1:200 dilution; clone 145-2C11; BD, cat. no. 564298); anti-CD44 (1:200 dilution; clone IM7; BD; cat. no. 560568); anti-Ly6C (1:400 dilution; clone HK1.4; BioLegend; cat. no. 128033); anti-CXCR5 (1:200 dilution; clone L138D7; BioLegend; cat. no. 145513); anti-CXCR5 (1:200 dilution; clone L138D7; BioLegend; cat. no. 145517); anti-CD162 (1:800 dilution; clone 2PH1; BD; cat. no. 740746); anti-PD-1 (1:200 dilution; clone RMP1-30; BioLegend; cat. no. 109116); anti-CD62L (1:400 dilution, clone MEL-14; Thermo Fisher; cat. no. 25-0621-82); anti-CD127 (1:200 dilution; clone SB/199; BD; cat. no. 612841); anti-IFNγ (1:400 dilution; clone XMG1.2; BD; cat. no. 557649); anti-IL-4 (1:400 dilution; clone 11B11; BD; cat. no. 554436); anti-IL-17A (1:400; clone TC11-18H10; BD; cat. no. 560220); anti-Bcl-6 (1:400 dilution; clone K112-91; BD; cat. no. 563363); anti-CXCR3 (1:200 dilution; clone CXCR3-173; BioLegend; cat. no. 126531) and anti-CCR6 (1:200 dilution; clone 29-2L17; BioLegend; cat. no. 129814).
Human T cell analysis
Single-cell suspensions were stained with fixable viability stain Zombie UV (1:1,000 dilution; BioLegend; cat. no. 423107); anti-CD4 (1:20 dilution; clone SK3; BD; cat. no. 612749); anti-CD4 (1:20 dilution; clone SK3; BD; cat. no. 341095); anti-CD3 (1:20; clone SK7; BD; cat. no. 564001); anti-CD8 (1:20 dilution; clone SK1; BD; cat. no. 664530); anti-CD45RA (1:50 dilution; clone HI100; BD; cat. no. 750258); anti-CD45RA (1:50 dilution; clone HI100; BD; cat. no. 560675); anti-CD45RO (1:20 dilution; clone UCHL1; BioLegend; cat. no. 304210); anti-CD45RO (1:20 dilution; clone UCHL1; BioLegend; cat. no. 304226); anti-CD27 (1:20 dilution; clone I128; BD; cat. no. 562656); anti-PD-1 (1:20 dilution; clone EH12.2H7; BioLegend; cat. no. 329928); anti-CXCR5 (1:20 dilution; clone RF8B2; BD; cat. no. 564624); anti-ICOS (1:20 dilution; clone ISA-3; Invitrogen; cat. no. 46-9948-42); anti-OX40 (1:20 dilution; clone ACT35; BioLegend; cat. no. 350025); anti-CD25 (1:20 dilution, clone BC96; BioLegend; cat. no. 302631); anti-CD127 (1:50 dilution, clone A018D5; BioLegend; cat. no. 135043); anti-CD162 (1:20 dilution, clone KPL-1; BioLegend; cat. no. 328813); anti-CXCR3 (1:20 dilution; clone G025H7; BioLegend; cat. no. 353723); anti-CCR6 (1:20 dilution; clone G034E3; BioLegend; cat. no. 353405); anti-CCR4 (1:20 dilution; clone L291H4; BioLegend; cat. no. 359439); anti-CD57 (1:20 dilution; clone QA17A04; BioLegend; cat. no. 393329); anti-CD151 (1:20 dilution; clone 50-6; BioLegend; cat. no. 350407); anti-CD71 (1:20 dilution; clone CY1G4; BioLegend; cat. no. 334119); anti-CD69 (1:20 dilution; clone FN50; BioLegend; cat. no. 310907); anti-CD82 (1:20 dilution; clone ASL-24; BioLegend; cat. no. 342109); anti-CD43 (1:20 dilution; clone CD43-10G7; BioLegend; cat. no. 343205); anti-TGFBR2 (1:20 dilution; clone FAB2411N; R&D Systems; cat. no. FAB2411N-025) and anti-CD99 (1:20 dilution; clone 3B2/TA8; BioLegend; cat. no. 371311).
B cell analysis
Single-cell suspensions were stained with fixable viability stain 700 (1:1,000 dilution; BD; cat. no. 564997); anti-B220 (1:800 dilution; clone RA3-6B2; BD; cat. no. 563103); anti-CD138 (1:400 dilution; clone 281-2; BD; cat. no. 563193); anti-CD95 (1:600 dilution; clone JO2; BD; cat. no. 557653); anti-IgD (1:200 dilution; clone 11-26c; WEHI Antibody Facility); anti-CD38 (1:600 dilution; clone 90; eBioscience; cat. no. 46038182); anti-CD86 (1:200 dilution; clone GL1; BD; cat. no. 563055); anti-CXCR4 (1:200 dilution; clone 2B11; Invitrogen; cat. no. 12-9991-82); anti-IgG1 (1:200 dilution; clone X56; BD; cat. no. 742480) and anti-IgG2a/2b (1:200 dilution; clone R2-40; BD; cat. no. 553399).
Single-cell sorting for mouse bulk RNA-seq
Single-cell suspensions were stained with fixable viability stain (1:1,000 dilution; BD, cat. no. 564406), anti-CD4 (1:600 dilution; clone GK1.5; BD; cat. no. 569845), anti-CD44 (1:200 dilution; clone IM7; BD; cat. no. 560568), anti-CXCR5 (1:200 dilution; clone L138D7; BioLegend; cat. no. 145513) and anti-PD-1 (1:200 dilution; clone RMP1-30; BioLegend; cat. no. 109121) antibodies.
Single-cell sorting of human tonsils
Single-cell suspensions were stained with fixable viability stain (1:1,000 dilution; BD; cat. no. 564997); anti-CD3 (1:20; clone SK7; BD; cat. no. 564001); anti-CD4 (1:20 dilution; clone SK3; BioLegend; cat. no. 344615); anti-CD8 (1:20 dilution; clone SK1; BioLegend; cat. no. 344739); anti-CD45RA (1:50 dilution; clone HI100; Invitrogen; cat. no. 25-0458-42); anti-CD45RO (1:20 dilution; clone UCHL1; BioLegend; cat. no. 304210); anti-CXCR5 (1:20 dilution; clone RF8B2; BD; cat. no. 564624); and anti-CD27 (1:20 dilution; clone I128; BD; cat. no. 562656).
Confocal
Lymph node tissue sections were stained with anti-CD4 (1:100 dilution; clone GK1.5–7; WEHI Antibody Facility), anti-IgD (1:200 dilution; clone 11-26c; eBioscience; cat. no. 48-5993-82) and anti-GL7 (1:100 dilution; clone GL7; BioLegend; cat. no.144606).
Preparation of mouse lymph node samples for bulk RNA sequencing
Sample preparation and cell sorting
Single-cell suspensions from draining lymph nodes of female IL-21–GFP–FoxP3–RFP reporter mice were stained for surface antigen expression via the indicated antibodies for 30 min at 4 °C, followed by viability dye staining for 15 min at 4 °C. Sample suspensions were sorted via a BD FACSAria Fusion Flow Cytometer (BD) to isolate CD4+CD44+PD-1+CXCR5+FoxP3–RFP−IL-21–GFP+ TFH, CD4+CD44+PD-1+CXCR5+FoxP3–RFP−IL-21–GFP− TFH, CD4+CD44+PD-1+CXCR5+FoxP3–RFP+IL-21–GFP+ TFR, and CD4+CD44+PD-1−CXCR5−FoxP3–RFP−IL-21–GFP− TEFF cells. Biological replicates for each infection consisted of 2–3 independent experiments of 7–10 mice pooled per replicate, sorted for TFH, TFR and Teff populations.
Bulk RNA sequencing
RNA was isolated via a RNeasyPlusMicro kit (QIAGEN) and library was prepared via a SMART-seqPLUS Kit (Takarabio). The RNA was sequenced via the Illumina NextSeq 500 System on a HiSeq paired-end run. Sequencing reads were aligned to the GRCm39 Mus musculus reference genome release v.103, and featureCounts72 was subsequently used to quantify transcripts per gene. All samples were sequenced at the same time, avoiding the need for batch correction.
Data processing
Counts from two technical replicates (sequencing runs) were summed. Genes with low expression were determined via the edgeR::filterByExpr function (v.3.34.0)73 and samples with small library sizes (<1 million reads) were discarded. Relative log expression plots and PCA were used to identify variations in the data and identify batch effects. Outliers and samples with poor RNA quality were subsequently removed. Normalization was performed via the trimmed mean of log expression ratios (TMM) method74 with no batch effects identified.
Differential expression analysis
DE analysis was performed via the voom-limma pipeline75,76 from the limma R/Bioconductor package (v.3.48.0)76. Linear models were fit via limma::lmfit to a design matrix accounting for biological factors of interest against the log expression of each transcript to identify DE between contrasts. A t-test relative to a threshold (TREAT) criterion was then applied77 (with a minimal fold change threshold of 1.1) to perform statistical tests. The decideTests function was used to determine the DE genes with statistical significance P ≤ 0.05 and multiple testing adjustments were calculated using the Benjamini–Hochberg method with FDR at 5%.
Deriving the core TFH cell signature
Differential expressed genes between the TFH and Teff cells from all the infections was derived and differential expressed genes between the TFH and Teff cells for each of the five individual infections was obtained. The core TFH signatures were defined as the DE genes occurring in three or more infections and were present in the TFH versus Teff comparison from all the infections.
Deriving the TFR cell signature
The TFR signature was derived from the common differential expressed genes between the contrasts TFR versus TFH cells and TFR versus Teff cells from all infections.
Deriving the pathogen-specific TFH cell signatures
Pathogen-specific TFH signatures were derived from DE analysis results of only TFH cells; specifically, the differential expressed genes between individual infections (for example LCMV) versus all other infections (influenza, T. muris, H. polygyrus and C. rodentium).
Deriving the pathogen-specific Teff cell signatures
Pathogen-specific Teff cell signatures were derived from DE analysis results of only Teff cells; specifically, the differential expressed genes between individual infections (for example LCMV) versus all other infections (influenza, T. muris, H. polygyrus and C. rodentium).
Gene set enrichment analysis
The enrichment of marker gene sets from published TFH and GC B cell datasets36,78,79,80,81,82,83,84 and precursors of exhausted and memory T cell85,86,87,88 datasets in the DE results was calculated using the fgsea package89 and visualized as NES dot plots and barcode plots for the specified gene sets53,54,59,78,90.
Visualization of signatures
DE between contrasts was visualized with MA Bland–Altman plots via the ggplot2 R package (https://ggplot2.tidyverse.org). Visualization of the intersection of DE between contrasts was performed via the UpSetR package91. Signatures were visualized via heatmaps via the ComplexHeatmap R package92 for cell surface receptors and transcriptional regulators. Cell surface receptor genes were identified on the basis of gene sets downloaded from the Mouse Genome Database93 for the cell surface (GO:0009986) and cell surface receptor signaling pathways (GO:0007166). Transcriptional regulator genes included those related to transcription factors annotated in the Immunological Genome Project (ImmGen) database94, the mouse tissue transcription factor atlas95, and gene sets downloaded from the Mouse Genome Database for transcription factor activity (GO:0003700), transcription activator activity (GO:0001216), transcription repressor activity (GO:0001217) and transcription factor binding (GO:0008134). Bcl-6 network transcriptional regulator genes in the TFH cell core (without cell cycle genes) were visualized for TFH versus TEFF cell and TFR versus TEFF cell contrasts via the ggplot2 R package.
Preparation of human tonsil samples for cell sorting, scRNA-seq and CITE-seq
Sample preparation and cell sorting
The cryopreserved mononuclear cell suspensions from the tonsil samples were thawed and processed to form single-cell suspensions. The samples were stained for surface antigen expression via the indicated antibodies and TotalSeq HashTags (BioLegend) for 30 min at 4 °C, followed by viability dye staining for 15 min at 4 °C. Sample suspensions were sorted via a BD FACSAria Fusion Flow Cytometer (BD) to isolate CD3+CD4+CD45RA−CD45RO+CXCR5+CD27+ TFH cells.
scRNA-seq and CITE-seq
Equal numbers of sorted cells from each tonsil sample were pooled and stained with TotalSeq-A Human Universal Cocktail (BioLegend) in accordance with the manufacturer’s protocols. Sequence tags of DNA-barcoded mAbs for CITE-seq were incorporated into existing scRNA-seq methods to enable simultaneous quantification of protein and gene expression by single cells. A total of 40,000 cells from the stained TFH pool were subjected to 3′-tag capture via the 10x Genomics Chromium Controller. Library preparation was conducted for sequencing according to the manufacturer’s protocols. Next-generation sequencing was performed using the Illumina NovaSeq 6000 System. All samples were sequenced at the same time, avoiding the need for batch correction.
Data processing and demultiplexing of scRNA-seq data
Reads from each capture were processed via 10x Genomics Cell Ranger software (v.7.0.0). First, cellranger::mkfastq and bcl2fastq (v.2.19.1) were used to convert to FASTQ files for the gene expression (GEX), antibody-derived tag and hashtag oligonucleotide (HTO) libraries and cellranger::multi was used to generate count matrices. The GEX data were mapped to the GRCh38 human reference genome. The DropletUtils R/Bioconductor package (v.1.18.1) was used to load the Cell Ranger output files into R (v.4.2.1)96. For demultiplexing, the demuxmix (v.1.0.0) R/Bioconductor package97 was applied to the HTO data, with the ‘naive’ model and default parameters.
Analysis of scRNA-seq data
Quality control, clustering and phenotype scoring
The demultiplexed scRNA-seq data were assessed with low-quality cells, cells with high mitochondrial genes (≥20%), doublets and cells with unknown HTO tags removed. The unstimulated samples were extracted, leaving 27,022 cells and were normalized using scran98 and scuttle99 R packages and converted to log-normalized counts. The top 2,000 highly variable genes were used to perform PCA dimension reduction. The data were clustered via the Louvain method for k = 9 nearest neighbors and the Jaccard weighting scheme (using igraph and scran), resulting in 11 clusters. The ranked scores for the TFH cell signatures were calculated using singscore100,101 R package. Ranked scores were visualized by overlaying across the UMAP using the scater::plotReducedDim function.
Differential expression analysis
DE analysis was conducted using a pseudobulked approach based on ‘clusters’ and ‘samples’ via the aggregateAcrossCells function in Scuttle99, after which samples with fewer than 10 cells were filtered out, and gene-level quality control was performed via edgeR::filterByExpr, leaving 33 pseudosamples and 10,595 genes for analysis. DE analyses were conducted via a voom-limma-duplicate correlation pipeline using the edgeR::voomLmFit function to fit a linear model with the cluster labels as the covariate and to estimate the consensus correlation across donors and account for donor variation as a random effect73. An empirical Bayes moderated t statistic was generated with multiple testing adjustments carried out via the Benjamini–Hochberg procedure to identify statistically significant genes (adjusted P < 0.05).
Gene set enrichment analysis
GSEA was performed using the fry approach from the limma package on gene sets from the Molecular Signature Database102, which includes the following categories: hallmark, curated (c2: Reactome, PID, Biocarta and KEGG) and Ontology (c5: BP and MF). Gene sets with an FDR < 0.05 were considered significant. Significant gene sets were analyzed via the vissE103 R package, clustering similar processes and identifying overarching biological themes. The gene set networks were generated via vissE by applying overlap coefficient thresholds of 0.25 and 0.15 (up- and downregulated, respectively) for both the C7 versus C8 and C1 versus C2 comparisons.
Data processing and visualization of CITE-seq data
CITE-seq data were extracted and normalized by applying the CLR (centered log ratio transformation) method across cells using from the Seurat::NormalizeData function104. The log count expression for selected markers was visualized by overlaying over the scRNA-seq generated UMAPs of the tonsil TFH cells.
Significance score ranking of the human datasets
Data download and processing for scRNA-seq data
Publicly available scRNA-seq data from the following datasets were downloaded:
-
Nehar-Belaid et al. 2020 (ref. 105) processed Seurat objects obtained from the authors.
-
Alladina et al. 2023 (ref. 106), from the GSE193816 repository.
-
Monian et al. 2022 (ref. 107), from the GSE158667 repository.
-
Schattgen et al. 2024 (ref. 108) extracted the h5 object and metadata for 2-year T cells from https://zenodo.org/records/6476021 (ref. 109) to rebuild the Seurat object.
-
Borcherding et al. 2024 (ref. 110) downloaded the Seurat object for Fig. 2 from the interactive online tool CellPilot (https://cellpilot.emed.wustl.edu) provided by the authors.
-
Dooley et al. 2023 (ref. 111) download the Seurat object from https://zenodo.org/records/6973241 (ref. 112).
For preprocessing, cells were filtered via functions perCellQCMetrics and quickPerCellQC from the Scuttle R package. For the data object of Schattgen et al., scran normalization was applied (functions quickCluster, computeSumFactors and logNormCounts)109. The filtered cells were then pseudobulked on relevant factors including cell type. The pseudobulked samples were then filtered via edgeR::filterByExpr. Pseudobulked samples with low cell counts were removed (cell count cutoff ranges between 10 and 30 cells, specific to each dataset) and the remaining pseudobulked samples were normalized using edgeR::calcNormFactors. The pseudobulked samples were ranked via the R package singscore on the basis of the expression of genes upregulated in each of the refined pathogen-specific signatures. The TFH cell-associated samples were then extracted for each dataset for visualization.
Data download and processing for Visium spatial tonsil data
Visium spatial tonsil data from eight donors were downloaded from the atlas of cells in human tonsils113 (https://zenodo.org/records/8373756; ref. 114). The core TFH cell signature was ranked using singscore for the expression of these genes across the whole tonsil spots. The spots in the regions annotated as ‘Germinal Center’ and ‘Proliferating Follicle’ were extracted and similarly rank scored based on the expression of genes in each of the refined pathogen-specific signatures.
Data download and processing for Xenium spatial lymph node data
Xenium FFPE 5k lymph node data were downloaded from https://www.10xgenomics.com/datasets/preview-data-xenium-prime-gene-expression. Spatial Experiment objects were built from the transcript, metadata and cell-based data. Quality control steps were applied, including filtering out empty cells and removing cells with either total transcript counts or detected gene counts less than 10% of the quantile of the data. The TFH-like cells were identified by first extracting cells annotated (by 10x Genomics) as MBCs, effector CD4+ T cells, T cells and CD4+ memory T cells and then selecting cells with a positive ranked score (scored using singscore based on the expression of genes in the core up- and downregulated signatures). These cells were then rank scored using a singscore based on the expression of genes in each of the refined pathogen-specific upregulated signatures.
Quantification and statistical analysis
Quantification and statistical analysis
Statistical differences between groups in datasets with one categorical variable were evaluated by unpaired t-tests (two groups) or one-way ANOVA (more than two groups) corrected for multiple comparisons. Statistical differences between groups in datasets with two categorical variables were evaluated by two-way ANOVA corrected for multiple comparisons. P < 0.05 was considered statistically significant. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in previous publications115. Data distribution was assumed to be normal but this was not formally tested. Data collection and analysis were not performed blind to the conditions of the experiments. All experimental data are presented as mean ± s.e.m. with statistical analysis performed via Prism v.9 (GraphPad Software).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All experimental models and reagents will be made available upon completion of a material transfer agreement. The bulk RNA-seq data (GSE302862) and scRNA-seq data and single-cell CITE-seq data (GSE302645) have been deposited to the Gene Expression Omnibus database and are publicly available. Any additional information required to reanalyze the data reported in this paper is available from the corresponding author upon request. Source data are provided with this paper.
References
O’Shea, J. J. & Paul, W. E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327, 1098–1102 (2010).
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Keck, S. et al. Antigen affinity and antigen dose exert distinct influences on CD4 T-cell differentiation. Proc. Natl Acad. Sci. USA 111, 14852–14857 (2014).
Pepper, M. et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 11, 83–89 (2010).
Becattini, S. et al. T cell immunity. Functional heterogeneity of human memory CD4(+) T cell clones primed by pathogens or vaccines. Science 347, 400–406 (2015).
Zaini, A. et al. Heterogeneous Tfh cell populations that develop during enteric helminth infection predict the quality of type 2 protective response. Mucosal Immunol. 16, 642–657 (2023).
Tuzlak, S. et al. Repositioning T(H) cell polarization from single cytokines to complex help. Nat. Immunol. 22, 1210–1217 (2021).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019).
Luthje, K. et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 13, 491–498 (2012).
Shehata, L. et al. Interleukin-4 downregulates transcription factor BCL6 to promote memory B cell selection in germinal centers. Immunity 57, 843–858 e845 (2024).
Ueno, H., Banchereau, J. & Vinuesa, C. G. Pathophysiology of T follicular helper cells in humans and mice. Nat. Immunol. 16, 142–152 (2015).
Osum, K. C. & Jenkins, M. K. Toward a general model of CD4(+) T cell subset specification and memory cell formation. Immunity 56, 475–484 (2023).
Nakayamada, S. et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35, 919–931 (2011).
Eisenbarth, S. C. et al. CD4(+) T cells that help B cells - a proposal for uniform nomenclature. Trends Immunol. 42, 658–669 (2021).
Cannons, J. L., Lu, K. T. & Schwartzberg, P. L. T follicular helper cell diversity and plasticity. Trends Immunol. 34, 200–207 (2013).
Sheikh, A. A. & Groom, J. R. Transcription tipping points for T follicular helper cell and T-helper 1 cell fate commitment. Cell Mol. Immunol. 18, 528–538 (2020).
DiToro, D. et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 361, eaao2933 (2018).
Pepper, M., Pagan, A. J., Igyarto, B. Z., Taylor, J. J. & Jenkins, M. K. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity 35, 583–595 (2011).
Ray, J. P. et al. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity 40, 367–377 (2014).
Ballesteros-Tato, A. et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36, 847–856 (2012).
McCarron, M. J. & Marie, J. C. TGF-β prevents T follicular helper cell accumulation and B cell autoreactivity. J. Clin. Invest. 124, 4375–4386 (2014).
Schmitt, N. et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat. Immunol. 15, 856–865 (2014).
Chang, Y. et al. TGF-β specifies T(FH) versus T(H)17 cell fates in murine CD4(+) T cells through c-Maf. Sci. Immunol. 9, eadd4818 (2024).
Oestreich, K. J., Huang, A. C. & Weinmann, A. S. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J. Exp. Med. 208, 1001–1013 (2011).
Reinhardt, R. L., Liang, H. E. & Locksley, R. M. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10, 385–393 (2009).
Morita, R. et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2011).
He, J. et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 39, 770–781 (2013).
Ma, C. S. et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J. Allergy Clin. Immunol. 136, 993–1006 e1001 (2015).
Juno, J. A. et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 26, 1428–1434 (2020).
Locci, M. et al. Human circulating PD-1+CXCR3−CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39, 758–769 (2013).
Bentebibel, S. E. et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 5, 176ra132 (2013).
Weinstein, J. S. et al. TFH cells progressively differentiate to regulate the germinal center response. Nat. Immunol. 17, 1197–1205 (2016).
Sheikh, A. A. et al. Context-dependent role for T-bet in T follicular helper differentiation and germinal center function following viral infection. Cell Rep. 28, 1758–1772 e1754 (2019).
Zhu, J. et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 37, 660–673 (2012).
Suan, D. et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity 42, 704–718 (2015).
Linterman, M. A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Chung, Y. et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17, 983–988 (2011).
Stone, E. L. et al. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity 42, 239–251 (2015).
Geng, J. et al. Bach2 negatively regulates t follicular helper cell differentiation and is critical for CD4(+) T cell memory. J. Immunol. 202, 2991–2998 (2019).
Lahmann, A. et al. Bach2 controls T follicular helper cells by direct repression of Bcl-6. J. Immunol. 202, 2229–2239 (2019).
Wang, H. et al. The transcription factor Foxp1 is a critical negative regulator of the differentiation of follicular helper T cells. Nat. Immunol. 15, 667–675 (2014).
Mintz, M. A. et al. The HVEM–BTLA axis restrains T cell help to germinal center B cells and functions as a cell-extrinsic suppressor in lymphomagenesis. Immunity 51, 310–323 e317 (2019).
Wu, X. et al. SOSTDC1-producing follicular helper T cells promote regulatory follicular T cell differentiation. Science 369, 984–988 (2020).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Zhu, F. et al. Spatiotemporal resolution of germinal center Tfh cell differentiation and divergence from central memory CD4(+) T cell fate. Nat. Commun. 14, 3611 (2023).
Broomfield, B. J. et al. Transient inhibition of type I interferon enhances CD8+ T cell stemness and vaccine protection. J. Exp. Med. 222, e20241148 (2025).
Cretney, E. et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–311 (2011).
Sage, P. T. et al. Suppression by T(FR) cells leads to durable and selective inhibition of B cell effector function. Nat. Immunol. 17, 1436–1446 (2016).
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
Yu, D. et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31, 457–468 (2009).
Nurieva, R. I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Choi, J. et al. Bcl-6 is the nexus transcription factor of T follicular helper cells via repressor-of-repressor circuits. Nat. Immunol. 21, 777–789 (2020).
Hatzi, K. et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J. Exp. Med. 212, 539–553 (2015).
Vella, L. A. et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J. Clin. Invest. 129, 3185–3200 (2019).
Kim, J. R., Lim, H. W., Kang, S. G., Hillsamer, P. & Kim, C. H. Human CD57+ germinal center-T cells are the major helpers for GC-B cells and induce class switch recombination. BMC Immunol. 6, 3 (2005).
Liu, B. et al. Affinity-coupled CCL22 promotes positive selection in germinal centres. Nature 592, 133–137 (2021).
Liu, D. et al. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature 517, 214–218 (2015).
Liu, X. et al. Genome-wide analysis identifies Bcl6-controlled regulatory networks during T follicular helper cell differentiation. Cell Rep. 14, 1735–1747 (2016).
Fang, D. & Zhu, J. Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate lymphoid cell subsets. J. Exp. Med. 214, 1861–1876 (2017).
Feuerer, M., Hill, J. A., Mathis, D. & Benoist, C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat. Immunol. 10, 689–695 (2009).
Brown, C. C. et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 179, 846–863 e824 (2019).
Choi, Y. S., Eto, D., Yang, J. A., Lao, C. & Crotty, S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 190, 3049–3053 (2013).
Yang, R. et al. Human T-bet governs innate and innate-like adaptive IFN-γ immunity against mycobacteria. Cell 183, 1826–1847 e1831 (2020).
Weinstein, J. S. et al. STAT4 and T-bet control follicular helper T cell development in viral infections. J. Exp. Med. 215, 337–355 (2018).
Marshall, H. D. et al. The transforming growth factor β signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. eLife 4, e04851 (2015).
Scheer, S. et al. The methyltransferase DOT1L controls activation and lineage integrity in CD4(+) T cells during infection and inflammation. Cell Rep. 33, 108505 (2020).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007).
Zhang, N. & Bevan, M. J. TGF-β signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat. Immunol. 13, 667–673 (2012).
Hwang, S. Y. et al. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons α and β and alters macrophage responses. Proc. Natl Acad. Sci. USA 92, 11284–11288 (1995).
Lee, W. S. et al. Randomized controlled trial reveals no benefit to a 3-month delay in COVID-19 mRNA booster vaccine. J. Clin. Invest. 134, e181244 (2024).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
McCarthy, D. J. & Smyth, G. K. Testing significance relative to a fold-change threshold is a TREAT. Bioinformatics 25, 765–771 (2009).
Liu, X. et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J. Exp. Med. 209, S1841–1824 (2012).
Andreatta, M. et al. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 12, 2965 (2021).
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
Weinstein, J. S. et al. Global transcriptome analysis and enhancer landscape of human primary T follicular helper and T effector lymphocytes. Blood 124, 3719–3729 (2014).
Ciucci, T. et al. The emergence and functional fitness of memory CD4(+) T cells require the transcription factor Thpok. Immunity 50, 91–105 e104 (2019).
Yeh, C. H., Finney, J., Okada, T., Kurosaki, T. & Kelsoe, G. Primary germinal center-resident T follicular helper cells are a physiologically distinct subset of CXCR5(hi)PD-1(hi) T follicular helper cells. Immunity 55, 272–289 e277 (2022).
Gloury, R. et al. Dynamic changes in Id3 and E-protein activity orchestrate germinal center and plasma cell development. J. Exp. Med. 213, 1095–1111 (2016).
Utzschneider, D. T. et al. T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427 (2016).
Chen, Z. et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity 51, 840–855 e845 (2019).
Siddiqui, I. et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211 e110 (2019).
Ivanova, N. B. et al. A stem cell molecular signature. Science 298, 601–604 (2002).
Korotkevich, G. et al. Fast gene set enrichment analysis. Preprint at bioRxiv https://doi.org/10.1101/060012 (2021).
Sun, Q. et al. BCL6 promotes a stem-like CD8(+) T cell program in cancer via antagonizing BLIMP1. Sci. Immunol. 8, eadh1306 (2023).
Conway, J. R., Lex, A. & Gehlenborg, N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics 33, 2938–2940 (2017).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Blake, J. A. et al. Mouse Genome Database (MGD): knowledgebase for mouse-human comparative biology. Nucleic Acids Res. 49, D981–D987 (2021).
Heng, T. S. & Painter, M. W. The Immunological Genome Project. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Zhou, Q. et al. A mouse tissue transcription factor atlas. Nat. Commun. 8, 15089 (2017).
Lun, A. T. L. et al. EmptyDrops: distinguishing cells from empty droplets in droplet-based single-cell RNA sequencing data. Genome Biol. 20, 63 (2019).
Klein, H. U. demuxmix: demultiplexing oligonucleotide-barcoded single-cell RNA sequencing data with regression mixture models. Bioinformatics 39, btad481 (2023).
Lun, A. T., McCarthy, D. J. & Marioni, J. C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res. 5, 2122 (2016).
McCarthy, D. J., Campbell, K. R., Lun, A. T. & Wills, Q. F. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 33, 1179–1186 (2017).
Bhuva, D. D., Cursons, J. & Davis, M. J. Stable gene expression for normalisation and single-sample scoring. Nucleic Acids Res. 48, e113 (2020).
Foroutan, M. et al. Single sample scoring of molecular phenotypes. BMC Bioinform. 19, 404 (2018).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Bhuva, D. D. et al. vissE: a versatile tool to identify and visualise higher-order molecular phenotypes from functional enrichment analysis. BMC Bioinform. 25, 64 (2024).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 e1821 (2019).
Nehar-Belaid, D. et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat. Immunol. 21, 1094–1106 (2020).
Alladina, J. et al. A human model of asthma exacerbation reveals transcriptional programs and cell circuits specific to allergic asthma. Sci. Immunol. 8, eabq6352 (2023).
Monian, B. et al. Peanut oral immunotherapy differentially suppresses clonally distinct subsets of T helper cells. J. Clin. Invest. 132, e150634 (2022).
Schattgen, S. A. et al. Influenza vaccination stimulates maturation of the human T follicular helper cell response. Nat. Immunol. 25, 1742–1753 (2024).
Schattgen, S. et al. Spatiotemporal development of the human T follicular helper cell response to Influenza vaccination. Zenodo https://doi.org/10.5281/zenodo.12611325 (2022).
Borcherding, N. et al. CD4(+) T cells exhibit distinct transcriptional phenotypes in the lymph nodes and blood following mRNA vaccination in humans. Nat. Immunol. 25, 1731–1741 (2024).
Dooley, N. L. et al. Single cell transcriptomics shows that malaria promotes unique regulatory responses across multiple immune cell subsets. Nat. Commun. 14, 7387 (2023).
Boyle, M. et al. Malaria drives unique regulatory responses across multiple immune cells during human infection. Zenodo https://doi.org/10.5281/zenodo.6973240 (2024).
Massoni-Badosa, R. et al. An atlas of cells in the human tonsil. Immunity 57, 379–399 e318 (2024).
Massoni-Badosa, R. Seurat objects associated with the tonsil cell atlas. Zenodo https://doi.org/10.5281/zenodo.6340173 (2022).
Tellier, J. et al. Unraveling the diversity and functions of tissue-resident plasma cells. Nat. Immunol. 25, 330–342 (2024).
Acknowledgements
We acknowledge the Wurundjeri people of the Kulin nation as the traditional owners and guardians of the land on which the majority of the work was performed. Cryopreserved tonsil samples were provided by C. Ma (Garvan Institute). scRNA-seq experiments were technically assisted by the WEHI Genomics Facility. This research was funded in part by the National Health and Medical Research Council of Australia (NHMRC), for purposes of open access, the authors have applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission. This work was supported by an NHMRC project grant (GNT1137989) to J.R.G. and K.L.G.-J. L.D. was supported by a Melbourne research scholarship. J.R.G. is an NHMRC Investigator (GNT2007812). V.L.B. is supported by a Sir Clive McPherson Family Fellowship and Rae Foundation. K.L.G.-J. was supported by the Bellberry-Viertel Senior Medical Research Fellowship and is supported by NHMRC Investigator Grant (GNT2033037). S.L.N. is supported by the NHMRC (GNT1155342) and the Jacques and Margaret Miller Fellowship. J.A.J. is supported by Charles and Sylvia Viertel Foundation and NHMRC Investigator Grant (GNT2009308). K.K. is supported by an NHMRC Investigator Grant (GNT2033783) and University of Melbourne Dame Kate Campbell Fellowship. C.E.S. is supported by an NHMRC EL2 Investigator Fellowship (GNT2033880). L.J.H. is supported by an NHMRC Investigator Grant (GNT2007884). This work was supported by Clifford Craig Foundation to K.K. and K.L.F. This work was supported by the Norman, Ann and Graeme Atkins Charitable Trust. This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.R.G. Methodology: L.D., C.W.T., A.A.S. and R.M. Investigation: L.D., C.W.T., A.A.S., R.M., L.J.H., C.A., T.H., A.Z., L.C., A.K., L.H., A.N., B.E.W. and M.Z.M.Z. Software and Formal Analysis: L.D. and C.W.T. Visualization: L.D. and C.W.T. Resources: C.E.S., L.M., K.L.F., K.K., N.H., J.A.J., C.Z., N.L.L.G., M.J.D., S.L.N., K.L.G.-J., V.L.B. and J.R.G. Writing – Original draft and amended manuscript: L.D. and J.R.G. Funding Acquisition: J.R.G. Supervision: K.L.G.-J., V.L.B. and J.R.G.
Corresponding author
Ethics declarations
Competing interests
No authors declare competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Induction of TFH response following diverse infection compared to steady state homeostasis.
a, Gating strategy to identify CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH and CD4+CD44+Ly6C+CD162+ TEFF cells and Bcl-6 expression. b, Timecourse analysis of draining lymph node GC cells from wild-type mice infected with indicated pathogens displaying frequencies and counts of CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells (closed circle) and B220+IgDloCD95+ GC B cells (open circle) (LCMV n = 3, influenza n = 4, H. polygyrus n = 4, C. rodentium n = 3 mice per group). T.muris data in7. c, Steady state analysis of indicated lymph nodes from uninfected wild-type mice. CD4+CD44+ cell counts, frequency of CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells and frequency of TFH cells produced IFNγ, IL-4, and IL-17 (n = 10 mice per group). d,e Analysis of draining lymph node cells from (d) wild-type and (e) ZsGreen_T-bet reporter mice infected with indicated pathogens at early peak GC response. (d) Total lymphocyte counts, CD4+CD44+ cell counts and frequency of CD4+CD44+ cells of live cells (LCMV n = 8, influenza n = 8, T. muris n = 6, H. polygyrus n = 9, C. rodentium n = 11 mice per group). (e) ZsGreen_T-bet reporter+ frequency and gMFI of CD4+CD44+Ly6C+CD162+ TEFF cells (LCMV n = 8, influenza n = 8, T. muris n = 6, H. polygyrus n = 10, C. rodentium n = 7 mice per group). Data show experiments of 6–10 mice per group and mean ± SEM. Statistical tests: one-way ANOVA of multiple comparisons. **** P values are <0.0001 or otherwise indicated.
Extended Data Fig. 2 Conserved GC morphology in draining lymph nodes following infection with diverse pathogens.
Immunofluorescent staining of draining lymph node of infected ZsGreen_T-bet reporter mice. Boxes indicate zoom of GC shown in Fig. 1f. Data are representative of 2–3 mice per group. Yellow: CD4, blue: IgD, magenta. Scale bar consistent for all images, 200μm.
Extended Data Fig. 3 Heterogenous TFH phenotypes display diverse cytokine profiles, similar to that of TEFF and systemic cytokine milieu.
a–d Analysis of (a–c) draining lymph node cells and (d) serum from (a,b,d) wild-type and (c) IFNγ-GFPx4C13R reporter mice infected with indicated pathogens at early peak GC response. (a) Representative plots of CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cell IFNγ, IL-4, and IL-17 cytokine production. (b) Frequency of CD4+CD44+Ly6C+CD162+ TEFF cell produced IFNg, IL-4, IL-17A. (c) IFNγ-GFPx4C13R reporter expression in CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells following T. muris infection. Representative plots, frequency of cytokine reporter with TFH population and displayed as parts of whole TFH population. Inner slice displays cytokine coexpression. (d) Serum cytokine concentration (pg/mL) in serum (n = 10 mice per group). Cytokines displayed as parts of total cytokines analyzed. Data show experiments of 7–10 mice per group and mean ± SEM. Statistical tests: one-way ANOVA of multiple comparisons. **** P values are <0.0001 or otherwise indicated.
Extended Data Fig. 4 Pathogen-induced TFH phenotypes correlate tailored B cell responses.
a, Gating strategy to identify GC and MBC populations. b, Analysis of B220+IgDloCD95+CD38− GC B cells from ZsGreen_T-bet reporter mice infected with indicated pathogens at early peak GC response. (b) Correlation of frequency of GC B to the ratio of TEFF/TFH cells and to ZsGreen_T-bet reporter expression gMFI across infections. Data show experiments of 6–10 mice per group. Statistical tests: Pearson correlation of two-tailed P value.
Extended Data Fig. 5 Pathogen-specific transcriptional programs separate by cell type and infection and establishment of TFH and TFR core signatures.
RNA-seq of CD4+CD44+PD-1+CXCR5+FoxP3-RFP−IL-21–GFP+ TFH, CD4+CD44+PD-1+CXCR5+FoxP3-RFP−IL-21–GFP− TFH, CD4+CD44+PD-1+CXCR5+FoxP3-RFP+IL-21–GFP+ TFR, and CD4+CD44+PD-1−CXCR5−FoxP3-RFP−IL-21–GFP− TEFF cells from draining lymph nodes of wild-type mice infected with indicated pathogens at early peak GC response. a, Gating strategy of sorted populations. b, Principal Component Analysis (PCA) plots of sorted IL-21+ TFH, IL-21− TFH, TFR, and TEFF cell transcriptomes stratified by either (top) cell type or (bottom) infection type. Data show independent samples of 2–3 per cell type per infection. c, Analysis of draining lymph node cells from wild-type mice infected with indicated pathogens. gMFI of upregulated (CD200 and BTLA) and downregulated (CCR7) core TFH signature markers for CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH cells. Data show experiments of 5–7 mice per group and mean ± SEM. d, UpSet plot of TFR signature showing intersections of downregulated genes expressed differentially between TFR vs. TFH contrast and TFR vs. TEFF contrast for five infections. False discovery rate (FDR) < 0.05. e,f, MA plot visualizing log fold change against the mean expression of genes expressed differentially between (e) TFR and TFH cells and (f) TFR and TEFF cells for five infections. g, GSEA of differentially expressed genes in TFH (TFH vs. TEFF contrast) and TFR cells (TFR vs. TEFF contrast) combined for five infections. Barcode and ES plots displaying enrichment of genes (ES and NES) upregulated in core TFH signature (TFH Core Up), TFH genes, genes indirectly regulated by Bcl-6 through repressor-of-repressor circuits (Indirect Bcl-6-rr), genes directly repressed by Bcl-6 (Direct Bcl-6-r), and Bcl-6 target genes (Bcl-6 targets) in TFH cells (TFH vs. TEFF) contrast53,54,59,78,90. Statistical tests: one-way ANOVA of multiple comparisons and Pearson correlation with two-tailed P value. **** P values are <0.0001 or otherwise indicated.
Extended Data Fig. 6 IL-21+ TFH and IL-21− TFH cells are transcriptionally similar across diverse infections.
a, UpSet plot displaying intersects of genes expressed differentially between IL-21+ TFH and IL-21− TFH cells from separate infections in bulk RNA-seq analysis of CD4+CD44+PD-1+CXCR5+FoxP3-RFP−IL-21–GFP+ TFH and CD4+CD44+PD-1+CXCR5+ FoxP3-RFP−IL-21–GFP− TFH cells as in Fig. 3. Three genes intersecting across 4 infections indicated with arrow. b-g, Analysis of CD4+CD44+PD-1+CXCR5+CD162− TFH cells and CD4+CD44+CD162+ TEFF cells from draining lymph nodes from wild-type mice infected with indicated pathogens, LCMV or influenza A at early peak GC response. (b) Gating strategy to identify TEFF, IL-21− TFH, and IL-21+ TFH cells. (c) Representative plots and histograms of CD62L, CD127, and Ly6C staining on IL-21− TFH, IL-21+ TFH cells and TEFF cells in LCMV infection. (d-g) Frequency and gMFI of CD62L+, CD127+, and Ly6C+ on (d,f) IL-21− TFH, IL-21+ TFH cells, frequency of total TFH population (e,g) TFH and TEFF, frequency of CD4+CD44+ population. (d-e) in LCMV infection (n = 8 mice per group), (f-g) in influenza A infection (n = 6 mice per group). Data show experiments of 4–6 mice per group and mean ± SEM. Statistical tests: Two-tailed unpaired Student’s t test. **** P values are <0.0001 or otherwise indicated.
Extended Data Fig. 7 Pathogen-induced cytokine signaling influence TFH and TEFF bifurcation, TFH subpopulations, and TFH cells capacity to direct B cell and antibody output.
a, Gating strategy to identify CD4+CD44+CXCR5+PD-1+Ly6C−CD162− TFH1, TFH2, TFH17, and TFH17.1 phenotypes based on expression of CXCR3 and CCR6 chemokine receptors. b-k, Analysis of draining lymph nodes from (b,c) wild-type, (d) Tgfbr2-LckCre and LckCre mice, (f) Ifnar−/− and Ifnar+/+ mice, (e,h,j,k) wild-type and Tgfbr2-LckCre 50:50 bone marrow chimeras, or (g-i,k) wild-type and Ifnar−/− 50:50 bone marrow chimera mice infected with (b,d,e,h,i,k) influenza or (c,f,g,h,j,k) LCMV. (b,c) gMFI expression of IFNg, IL-4, and IL-17 in TFH1 (CXCR3+CCR6−), TFH2 (CXCR3−CCR6−), TFH17 (CXCR3−CCR6+) and TFH17.1 (CXCR3+CCR6+) populations (influenza n = 10, LCMV n = 6 mice per group). (d,f) TFH and TEFF cell counts, TFH and TEFF cell frequencies, and TFH/TEFF cell ratio (influenza n = 9-10, LCMV n = 7 mice per group). (e,g) TFH and TEFF cell frequencies and TFH/TEFF cell ratio (influenza n = 10, LCMV n = 8 mice per group). (h) Comparison of effect of Tgfbr2-LckCre and Ifnar −/− on TFH frequency in chimeras. (i-j) Frequency of TFH1, TFH2, TFH17 and TFH17.1 cells within TFH population in chimeric mice (influenza n = 8, LCMV n = 7 mice per group). (k) Comparison of effect of Tgfbr2-LckCre and Ifnar −/− on TFH1, TFH2 and TFH17 cell frequency in influenza and LCMV infection in chimeras (Tgfbr2-LckCre influenza n = 10, Tgfbr2-LckCre LCMV n = 7, Ifnar −/− influenza n = 8, Ifnar −/− LCMV n = 8 mice per group). l, Gating strategy to identify IgG1 and IgG2 class switched GC B cells, and dark zone (DZ) and light zone (LZ) GC B cells. Data show experiments of 6–10 mice per group and mean ± SEM. Statistical tests: Two-tailed unpaired Student’s t test for intact system; two-tailed paired Student’s t test for bone marrow chimera model. **** P values are <0.0001 or otherwise indicated.
Extended Data Fig. 8 Human lymphoid tissue TFH phenotypes identified by core and pathogen-specific TFH signatures.
a, Gating strategy to sort CD3+CD4+CD45RA−CD45RO+CXCR5+CD27+ human tonsillar TFH cells from three healthy adult donors for paired scRNA-seq and CITE-seq. b, Overlay of mean ranked scores of core TFH and TEFF core signatures onto UMAP clusters. Mean of the ‘TotalScore’ from singscore::simpleScore function collated for each cluster. c, Distribution of TFH cells across 11 clusters by donor. d, Proportion of pathogen-specific TFH signatures overlapping with human cluster genes expressed differentially (one cluster vs. all other clusters). e, Overlay of mean ranked scores of each TEFF cell pathogen-specific signature onto UMAP clusters. Mean of the ‘TotalScore’ from singscore::simpleScore function collated for each cluster. TEFF cell pathogen-specific upregulated signature genes used. f, Gene set Enrichment and vissE analyses for the comparison Cluster 1 vs. Cluster 2. Top gene sets clusters of interest shown as barplot of DE gene counts with corresponding gene statistics (FDR as color shade) from the DE analysis. GSEA was performed using Hallmarks c2 (‘CP:REACTOME’, ‘CP:PID’, ‘CP:BIOCARTA’,‘CP:KEGG’) and c5 (‘GO:BP’,‘GO:MF’) collections from the MsigDB using a two-tailed approach correcting for multiple testing with false discovery rate (FDR) adjusted p ≤ 0.05. g, Ranked score of pathogen-specific signatures onto human dataset TFH cells from donors in SARS-CoV-2 vaccine (n = 5), influenza vaccine (n = 1), malaria infection (adult n = 3, child n = 3), peanut allergy (n = 3), asthma (n = 4) and autoimmune contexts (healthy donor n = 6, systemic lupus erythematosus n = 8). Data are presented as mean values ± SEMs. h, Visium spatial tonsil data113 displaying ranked score of core TFH signature. i, Xenium spatial human lymph node data displaying ranked score of core TFH signature. j, Visium spatial tonsil data113 displaying ranked score of pathogen-specific signatures onto germinal centers highlighted by core TFH signature.
Extended Data Fig. 9 Novel markers identify distinct human TFH subpopulations across tonsil and PBMC.
a–c, scCITEseq surface protein expression of sorted CD3+CD4+CD45RA−CD45RO+CXCR5+CD27+ human tonsillar TFH cells from three healthy adult donors (as in Fig. 7). scCITEseq surface protein expression (log counts) of (a) T cell identity markers, (b) chemokine receptors, and (c) cluster-identifying markers, overlaid onto scRNA-seq UMAP. d-h, CD3+CD4+CD45RA−CD45RO+CXCR5+ human TFH cells from five PBMC samples and six tonsil samples from healthy adult donors. (d), Gating strategy to identify TFH cells in human tonsil, adenoid, and PBMCs with PD-1+ expression in tonsil and PBMCs displayed in dot plots. (e) TFH surface marker frequency in tonsil CD57+ or CD57− TFH cells (n = 6 samples per group). (f) CD151/CD99 TFH populations (Q1-Q4) in TFH cells distinguished by CD57 and PD-1 from tonsil (n = 6 tonsils per group) and PBMCs (n = 5 PBMCs per group). (g) CXCR3, CCR6, and CCR4 expression in CD57/PD-1 and CD151/CD99 TFH populations with key to identify TFH populations distinguished by CD57 and PD-1, and TFH populations (Q1-Q4) distinguished by CD151 and CD99. (h) gMFI expression of TFH markers (PD-1, ICOS, OX40), chemokine receptor markers (CXCR3, CCR6, CCR4), and novel markers (CD127, CD99, CD71, CD151, CD43, CD69) with (+) and without (-) 4 hr PMA and ionomycin stimulation. Data show experiments of 5 PBMC samples per group and 6 tonsil samples per group and mean ± SEM. Statistical tests: one-way ANOVA of multiple comparisons. **** P values are <0.0001 or otherwise indicated.
Extended Data Fig. 10 Novel markers identify distinct human TFH subpopulations in spike tetramer-specific PBMCs of SARS-CoV-2 infected and mRNA vaccinated donors.
a, CD3+CD4+CD45RA−CD45RO+CXCR5+ TFH cells from six tonsil samples (n = 6) from healthy adult donors displaying TFH surface marker frequencies in TFH populations (Q1-Q4) distinguished by CD151 and CD99. b, Gating strategy to identify SARS-CoV-2 spike tetramer+ cTFH cells. c,d Spike tetramer+ cells in (c) SARS-CoV-2 infection (11/11 donors; n = 11) or 6 months convalescence (10/11 donors; n = 10), or (d) nine COVID-19 mRNA vaccinated donors (n = 9) at 7 days post-vaccine and 3 months post-vaccine. Frequency Spike tetramer+ CD3+CD4+CD45RA− T cells and CD3+CD4+CD45RA−CXCR5+ TFH cells. Data show experiments of 8-11 samples per group. Statistical test: Two-tailed paired Student’s t test. **** P values are <0.0001 or otherwise indicated.
Supplementary information
Supplementary Table 1
Supplementary Tables 1–3.
Source data
Source Data for all figures
Statistical Source Data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dalit, L., Tan, C.W., Sheikh, A.A. et al. Divergent cytokine and transcriptional signatures control functional T follicular helper cell heterogeneity. Nat Immunol 26, 1821–1835 (2025). https://doi.org/10.1038/s41590-025-02258-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41590-025-02258-9
This article is cited by
-
TFH cell programs by pathogen and species
Nature Immunology (2025)